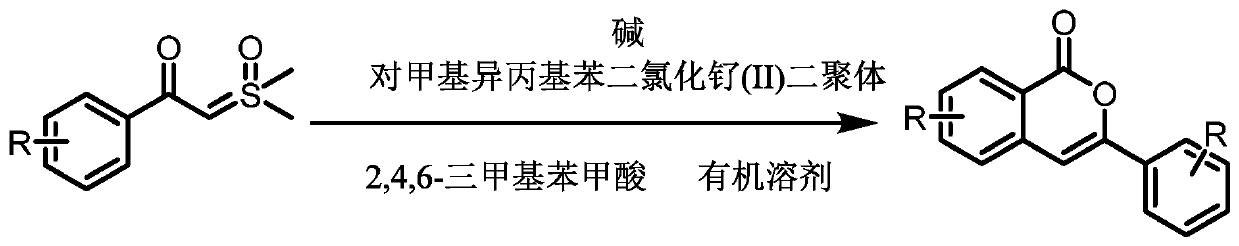
Preparation method for substituted isocoumarin derivatives
A technology of isocoumarin and derivatives, applied in organic chemistry and other fields, can solve the problems of low yield, long reaction time, and high requirements for reaction conditions, and achieve the effects of high yield, short reaction time, and strong reaction specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
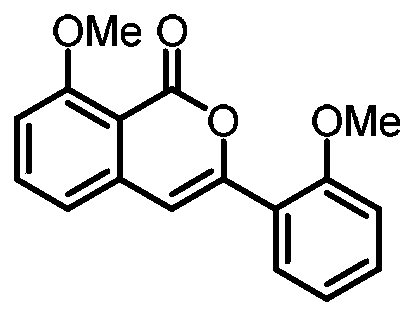
[0024] Preparation of 8-methoxy-3-(2-methoxyphenyl)-1H-isochromen-1-one
[0025]
[0026] Add 0.15 mmol of sodium phosphate, 0.005 mmol of p-methylcymene ruthenium (II) dichloride dimer, 0.15 mmol of 2,4,6-trimethylbenzoic acid, 2-(dimethyl (oxo) -λ 6 Sulfanyl)-1-(2-methoxyphenyl)ethan-1-one 0.2mmol, and 1,1,1,3,3,3-hexafluoro-2-propanol 1mL added to 15mL reaction tube, placed in an oil bath at 110°C, and reacted for 24 hours; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 13.2 mg of the target product with a yield of 48%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, Chloroform-d) 68.00(d, J=7.7Hz, 1H), 7.61(t, J=8.1Hz, 1H), 7.40-7.36(m, 1H), 7.30(s, 1H), 7.09 -7.03(m, 2H), 7.00(d, J=8.3Hz, 1H), 6.9...
Embodiment 2
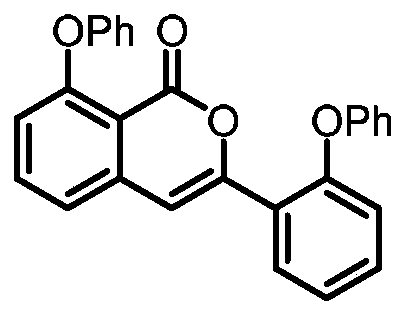
[0028] Preparation of 8-phenoxy-3-(2-phenoxyphenyl)-1H-isochromen-1-one
[0029]
[0030] Add 0.15 mmol of sodium phosphate, 0.005 mmol of p-methylcymene ruthenium (II) dichloride dimer, 0.15 mmol of 2,4,6-trimethylbenzoic acid, 2-(dimethyl (oxo) -λ 6 Sulfanyl)-1-(2-phenoxyphenyl)ethan-1-one 0.2mmol, and 1,1,1,3,3,3-hexafluoro-2-propanol 1mL added to 15mL reaction tube, placed in an oil bath at 110°C, and reacted for 24 hours; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 17.3 mg of the target product with a yield of 43%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, Chloroform-d) δ8.09-8.06(m, 1H), 7.50(t, J=8.0Hz, 1H), 7.40-7.31(m, 6H), 7.25-7.21(m, 1H), 7.18-7.13(m, 2H), 7.11(d, J=7.7Hz, 1H), 7.0...
Embodiment 3
[0032] Preparation of 8-fluoro-3-(2-fluorophenyl)-1H-isochromen-1-one
[0033]
[0034] Add 0.15 mmol of sodium phosphate, 0.005 mmol of p-methylcymene ruthenium (II) dichloride dimer, 0.15 mmol of 2,4,6-trimethylbenzoic acid, 2-(dimethyl (oxo) -λ 6 Add 0.2mmol of sulfanyl)-1-(2-fluorophenyl)ethan-1-one and 1mL of 1,1,1,3,3,3-hexafluoro-2-propanol into a 15mL reaction tube , placed in an oil bath at 110°C, reacted for 24h; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 17.1 mg of the target product, with a yield of 67%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, Chloroform-d) δ8.04-7.99(m, 1H), 7.72-7.67(m, 1H), 7.44-7.39(m, 1H), 7.32-7.26(, 2H), 7.21-7.15( ,3H); 13C NMR (126MHz, Chloroform-d) δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com