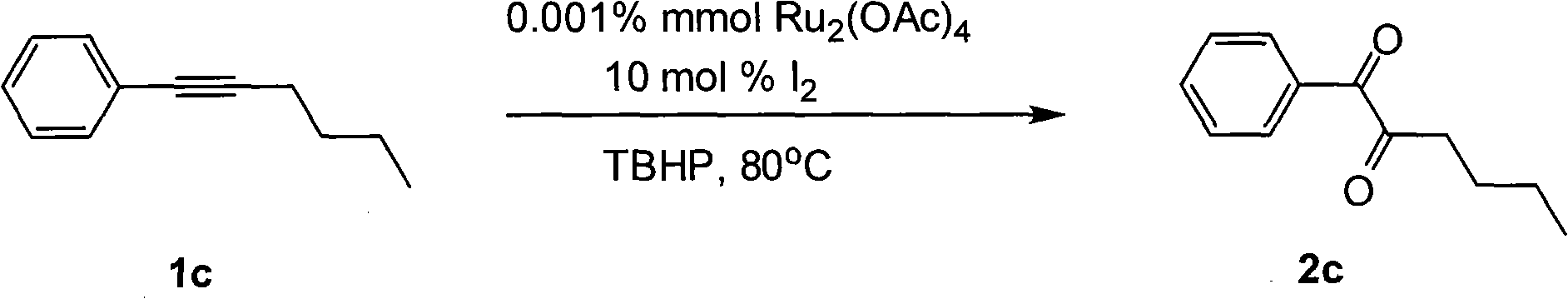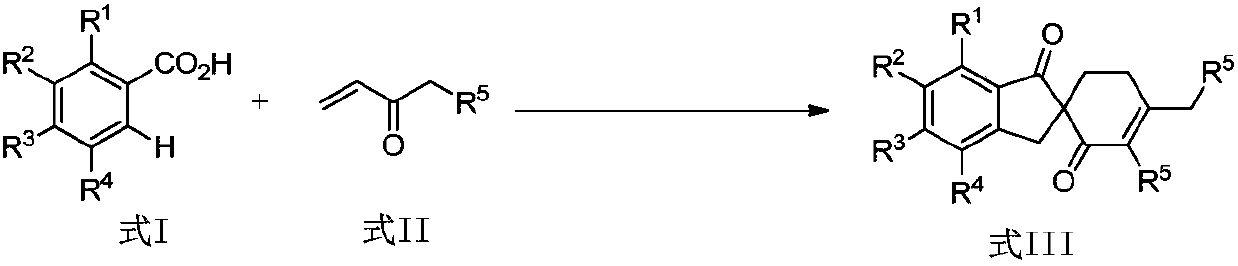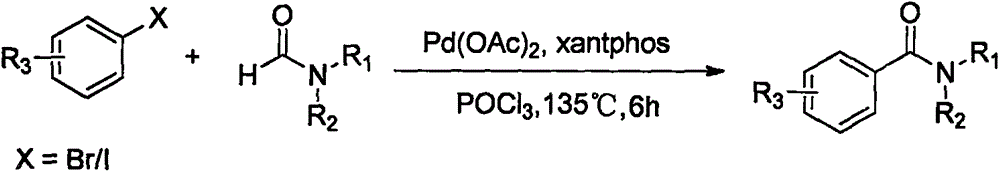Patents
Literature
101 results about "P-Cymene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
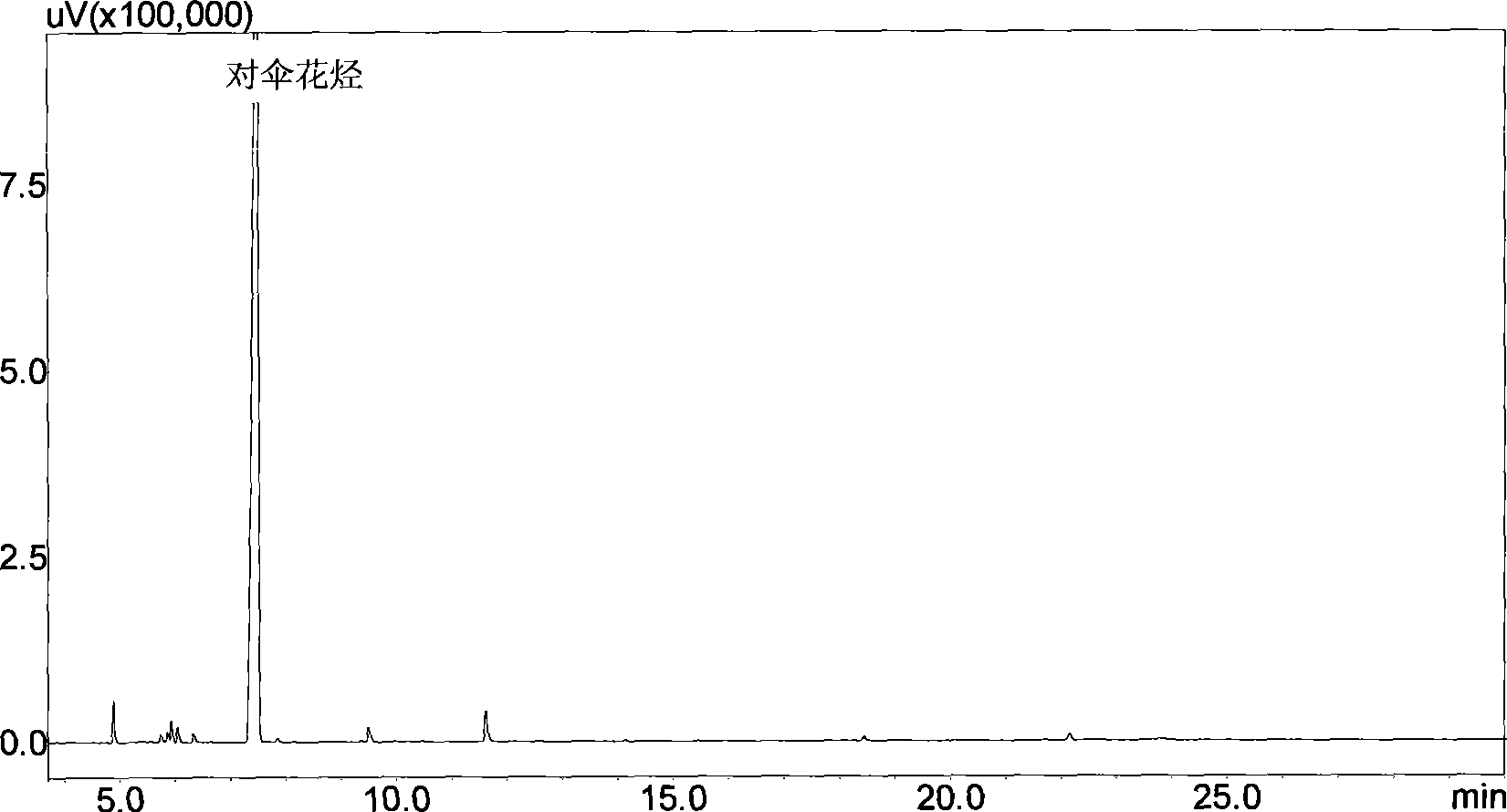
P-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. p-Cymene is insoluble in water, but miscible with organic solvents.
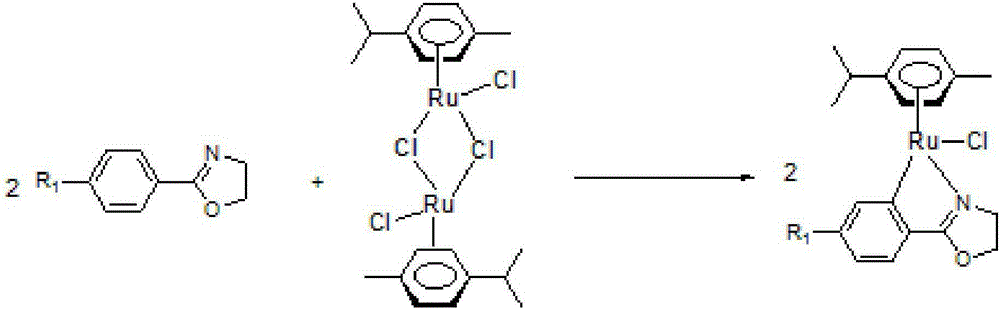
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Pest control using natural pest control agent blends
ActiveUS20110135764A1Increase volumeBiocideHydroxy compound active ingredientsMethyl salicylateBULK ACTIVE INGREDIENT
Embodiments of the invention relate to a composition for controlling a target pest, wherein the composition includes at least two active ingredients selected from the group consisting of thymyl acetate, linalyl acetate, amyl butyrate, anise star oil, black seed oil, p-cymene, geraniol, isopropyl myristate, d-limonene, linalool, lilac flower oil, methyl salicylate, alpha-pinene, piperonal, piperonyl alcohol, tetrahydrolinalool, thyme oil white, thyme oil red, thymol, vanillin, and winter-green oil, wherein the composition causes synergistic control of the target pest.
Owner:TYRATECH +1
Polysilazane-Treating Solvent and Method for Treating Polysilazane by Using Such Solvent
InactiveUS20080102211A1Good edge cut part shapeImprove solubilityOrganic chemistryChemical paints/ink removersTetralinAlicyclic Hydrocarbons
Owner:MERCK PATENT GMBH
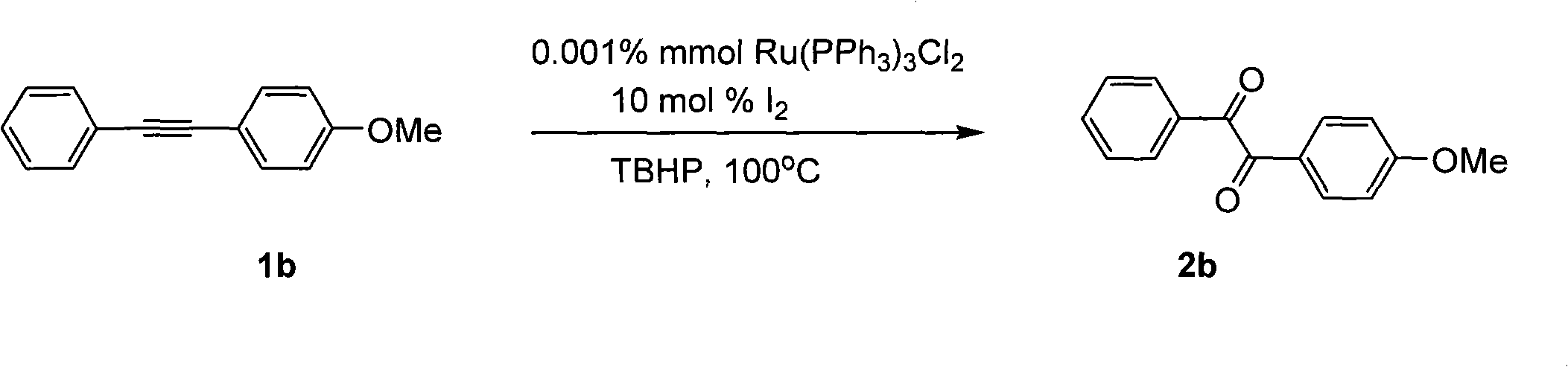
Method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes
InactiveCN101624322AMild reaction conditionsMeet the requirementsCarboxylic acid nitrile preparationOrganic compound preparationCatalytic oxidationKetone
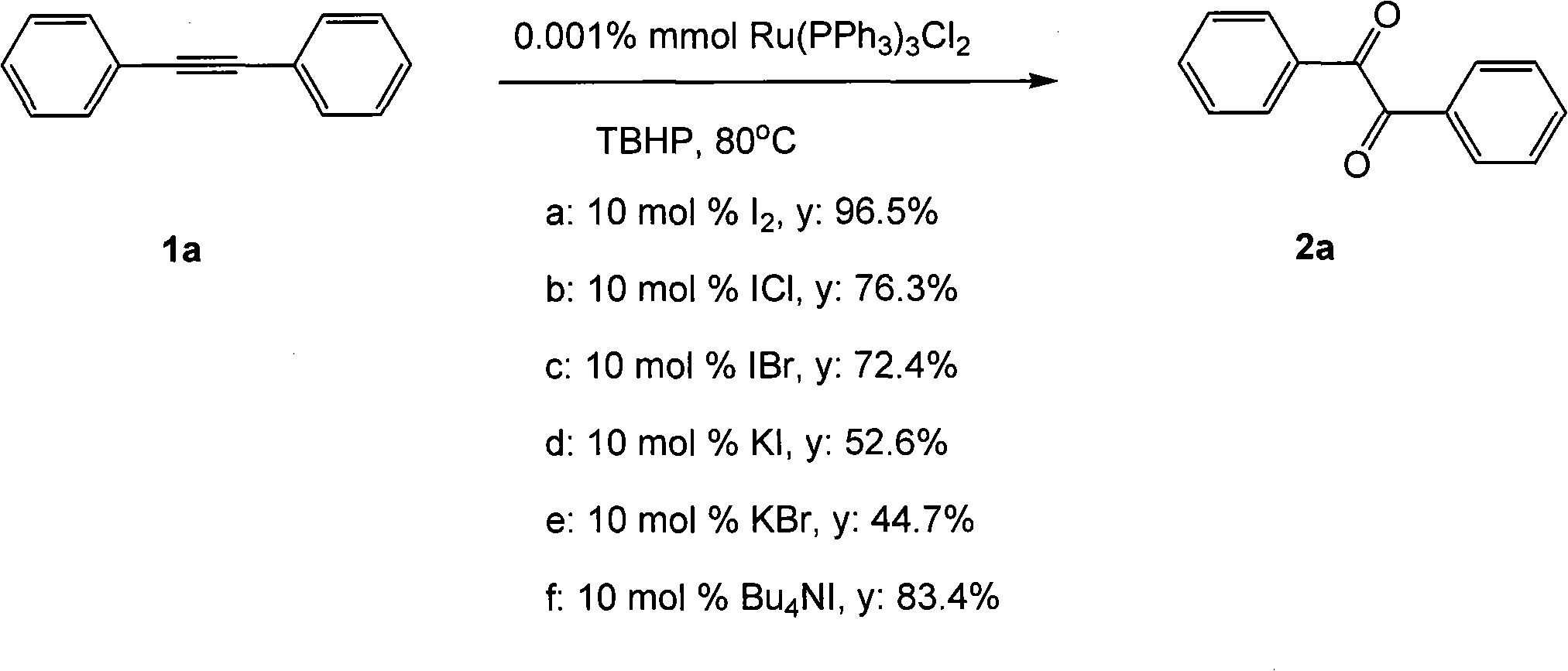
The invention belongs to the field of catalysis and oxidization, and particularly discloses a method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes. The method comprises the following steps: taking alkynes R1-C-C-R2 (acetylenic link exists between C and C) as a reaction substrate, taking one of TBHP, m-chloroperoxybenzoic acid and p-benzoquinone as a oxidant, taking one of dichloro (p-cymene) ruthenium (II) dimer, tri (triphenylphosphine) ruthenous chloride, ruthenium acetate, ruthenium dichlorophenyl (II) dimer, ruthenium trichloride, BINAP ruthenous chloride, dodecacarbonyltriruthenium and tricarbonyldichlororuthenium (II) dimer as a catalyst, taking one of iodine, iodine chloride, iodine bromide, potassium iodide, tetrabutyl ammonium iodide and potassium bromide as a cocatalyst, and taking 1, 4-dioxane as a solvent to react under 40 DEG C to 100 DEG C for 4 to 24 h to prepare the 1, 2-diketone. The method is economic, environmental-friendly and mild.
Owner:SUZHOU UNIV
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS20070270593A1High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Polysilazane-treating solvent and method for treating polysilazane by using such solvent
ActiveCN101111575AImprove solubilityPerformance is not affectedLiquid surface applicatorsNitrogen compoundsTetralinAliphatic hydrocarbon
The present invention relates to a polysilazane treatment solvent which has excellent solvency and stability, has no influence on the performance of the substrate and polysilazane as the bottom layer, has a good cutting edge shape, and is highly safe to the human body of. The processing solvent contains a solvent selected from the group consisting of tetralin, p-menthane, p-cumylmethane, α-pinene, 1,8-cineole and mixtures thereof, and also relates to a process using the solvent Polysilazane treatment method. The solvent may also contain a solvent selected from the group consisting of aliphatic hydrocarbons, alicyclic hydrocarbons, and mixtures thereof.
Owner:MERCK PATENT GMBH
Shape-selective alkylation catalyst for synthesizing p-cymene with toluene and propylene as raw materials
InactiveCN101940943AHigh selectivityReduce loss rateMolecular sieve catalystsHydrocarbonsIsomerizationAlkaline earth metal
The invention relates to a shape-selective alkylation catalyst for synthesizing p-cymene with toluene and propylene as the raw materials. The catalyst adopts the following components in 100 parts by weight: 40-90 parts of one or mixture of any two of ZSM-35 zeolite, Beta zeolite or MCM-22 zeolite, 1-15 parts of at least one metal oxide of rare-earth metals or alkaline earth metals and the balance oxide binders, wherein the mole ratio of SiO2 to Al2O3 in ZSM-35 zeolite is 20-100; and the ZSM-35 zeolite, Beta zeolite or MCM-22 zeolite is subjected to surface modification by liquid Si deposition. The reaction conditions for synthesizing p-cymene are as follows: reaction temperature: 340-460 DEG C, pressure: 0-3.0MPa, benzene / alkene ratio: 2.0-10.0, and space velocity: 0.5-8h<-1>. The catalyst has good catalytic reaction effect after being applied to the toluene alkylation process for synthesizing p-cymene with toluene and propylene as the raw materials. The invention solves the following problems: the conversion rate of toluene is low, such secondary reactions as disproportionation reaction and isomerisation reaction are serious and the catalyst is fast in deactivation.
Owner:TONGJI UNIV
Pest control using natural pest control agent blends
Embodiments of the invention relate to a composition for controlling a target pest, wherein the composition includes at least two active ingredients selected from the group consisting of thymyl acetate, linalyl acetate, amyl butyrate, anise star oil, black seed oil, p-cymene, geraniol, isopropyl myristate, d-limonene, linalool, lilac flower oil, methyl salicylate, alpha-pinene, piperonal, piperonyl alcohol, tetrahydrolinalool, thyme oil white, thyme oil red, thymol, vanillin, and winter-green oil, wherein the composition causes synergistic control of the target pest.
Owner:TYRATECH +1
Manufacturing method of optical activity alcohol
InactiveCN1926083AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenAlcohol
Owner:KANTO CHEM CO INC +1
Preparation method of L-carnitine
ActiveCN102633664AReduce pollutionRaw materials are easy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSpecific rotationEthyl Chloride
The invention relates to a method for preparing L-carnitine by asymmetric catalytic hydrogenation reduction. Alkyl 4-chloroacetoacetate is used as a raw material, [RuCl(cymene)(S-BINAP)]Cl[chloro[(S)-(-)-2,2'-di(diphenylphosphine)-1,1'-binaphthyl](p-cymene)ruthenium chloride(II))] is used as a catalyst, and amination and hydrolysis reaction are carried out to obtain the L-carnitine product. The amination reaction does not need any solvent or virulent cyanide. The chemical purity of the L-carnitine product is higher than 97%, and the specific rotation [alpha]20D is -29 -32. The invention has the advantages of high reaction speed, stable catalytic system, low pressure required by the reaction process, high conversion rate, no need of solvent in the amination reaction, and is convenient to operate, thereby lowering the cost, reducing the environmental pollution and being convenient for large-scale production.
Owner:GUANGXI XINJING TECH +2
Ethylbenzene dehydrogenating catalyst and preparation method thereof
ActiveCN101829576AHigh selectivityHigh activityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsPolymer science4-isopropenyltoluene
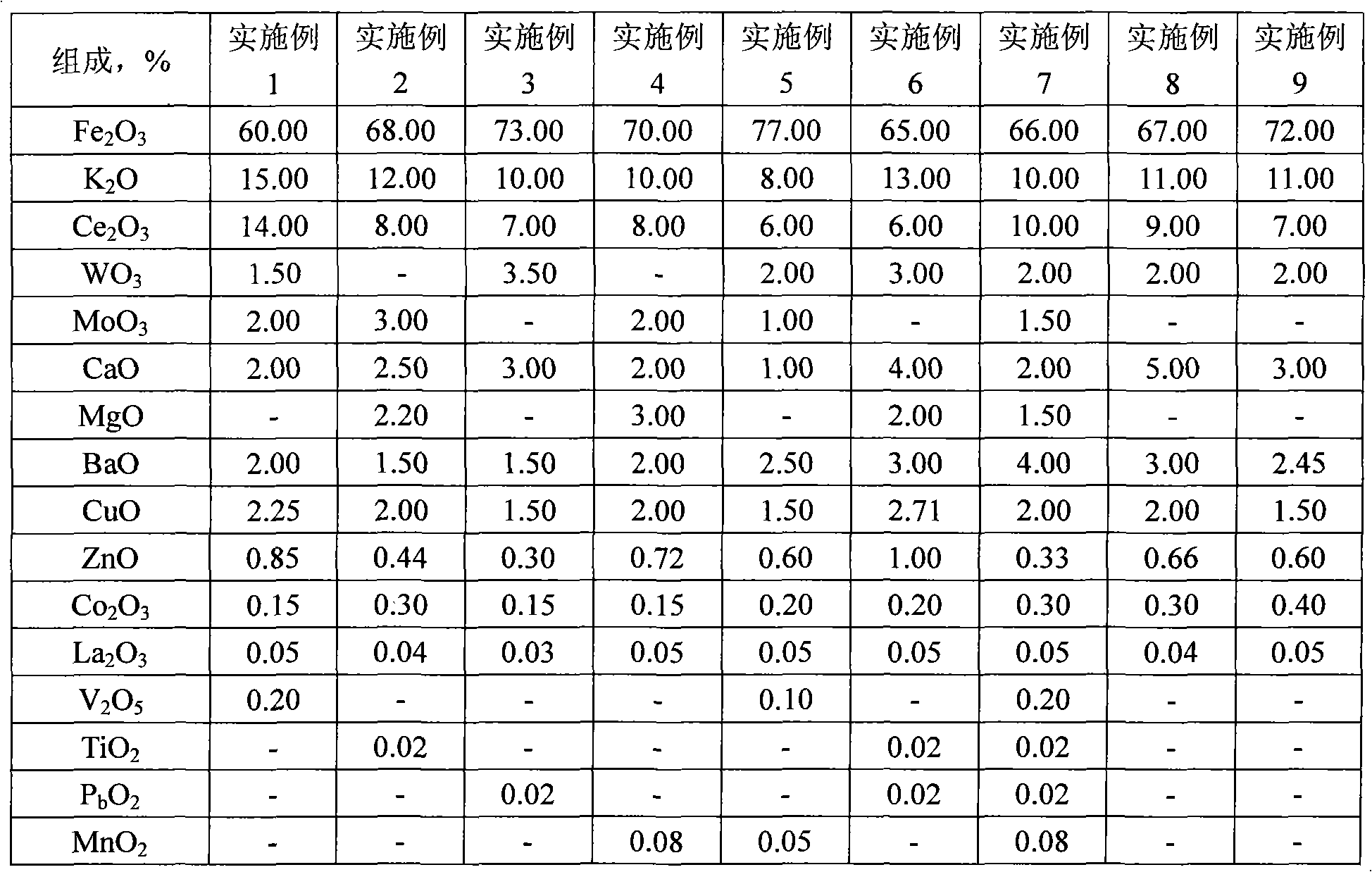
The invention relates to an ethylbenzene dehydrogenating catalyst and a preparation method thereof. A plurality of metallic oxide stabilizing additives of WO3 and / or MoO3, CaO, BaO, CuO, ZnO2, Co2O3 and La2O3 are added into a catalyst which uses Fe-K-Ce-Mo (or W or Mo-W) as the main system. The catalyst has higher activity and selectivity, low inactivation speed and high stability, is suitable for preparing stryrene by dehydrogenating ethylbenzene, and is also suitable for preparing isopropenylbenzene by dehydrogenating isopropyl benzene, and preparing 4-isopropenyltoluene by dehydrogenating p-cymene. The catalyst is prepared by adopting a kneading method.
Owner:PETROCHINA CO LTD
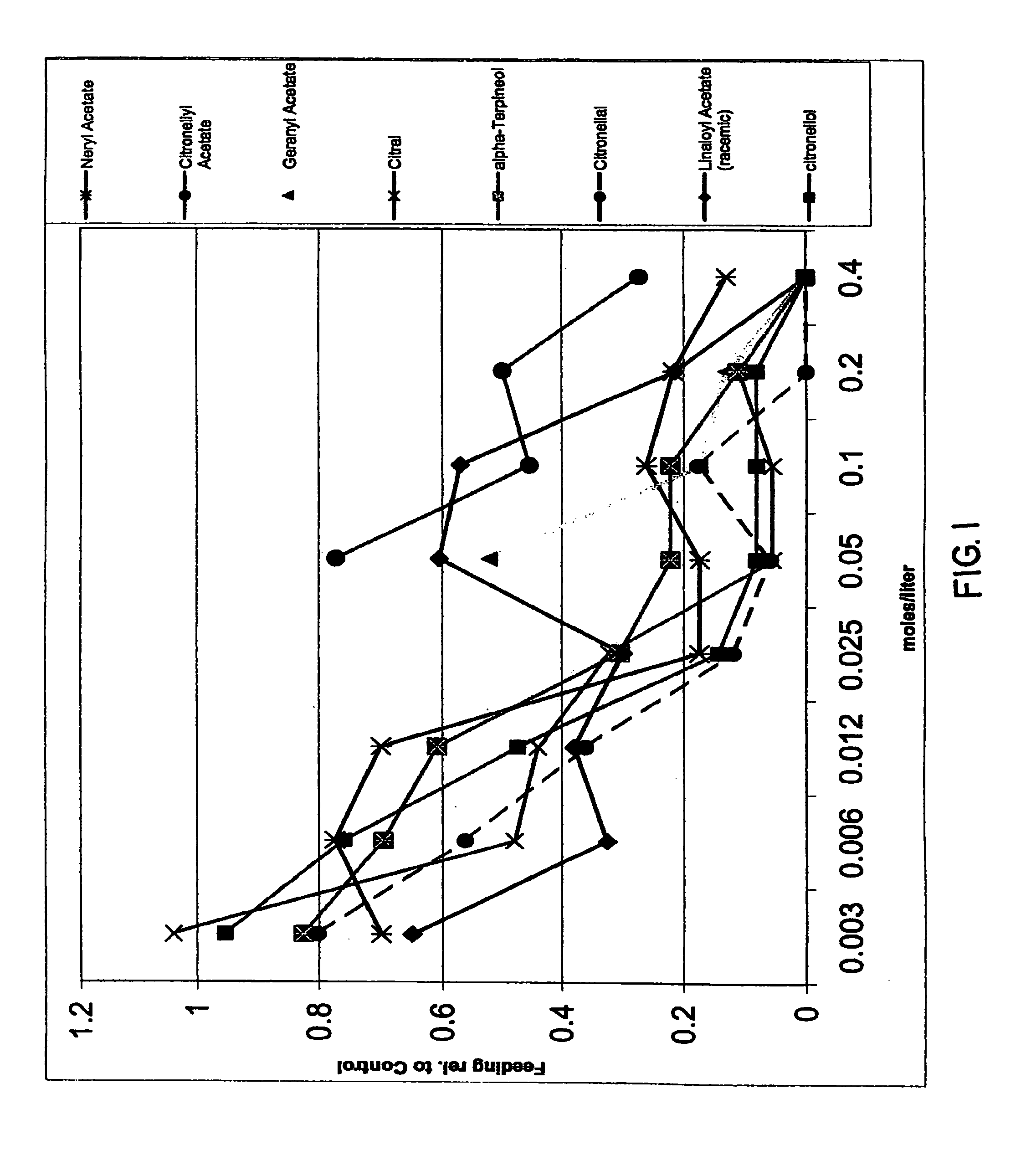
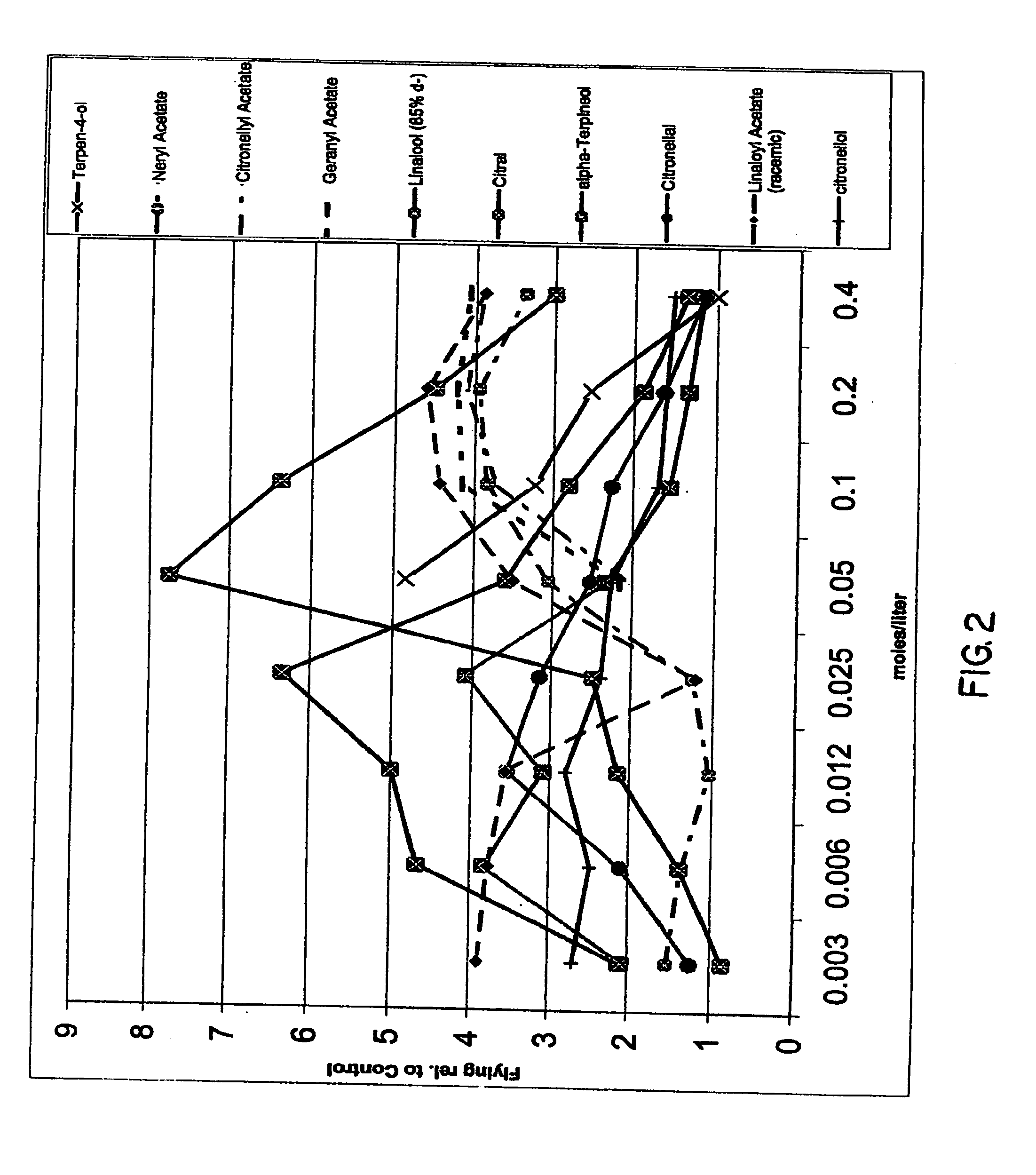
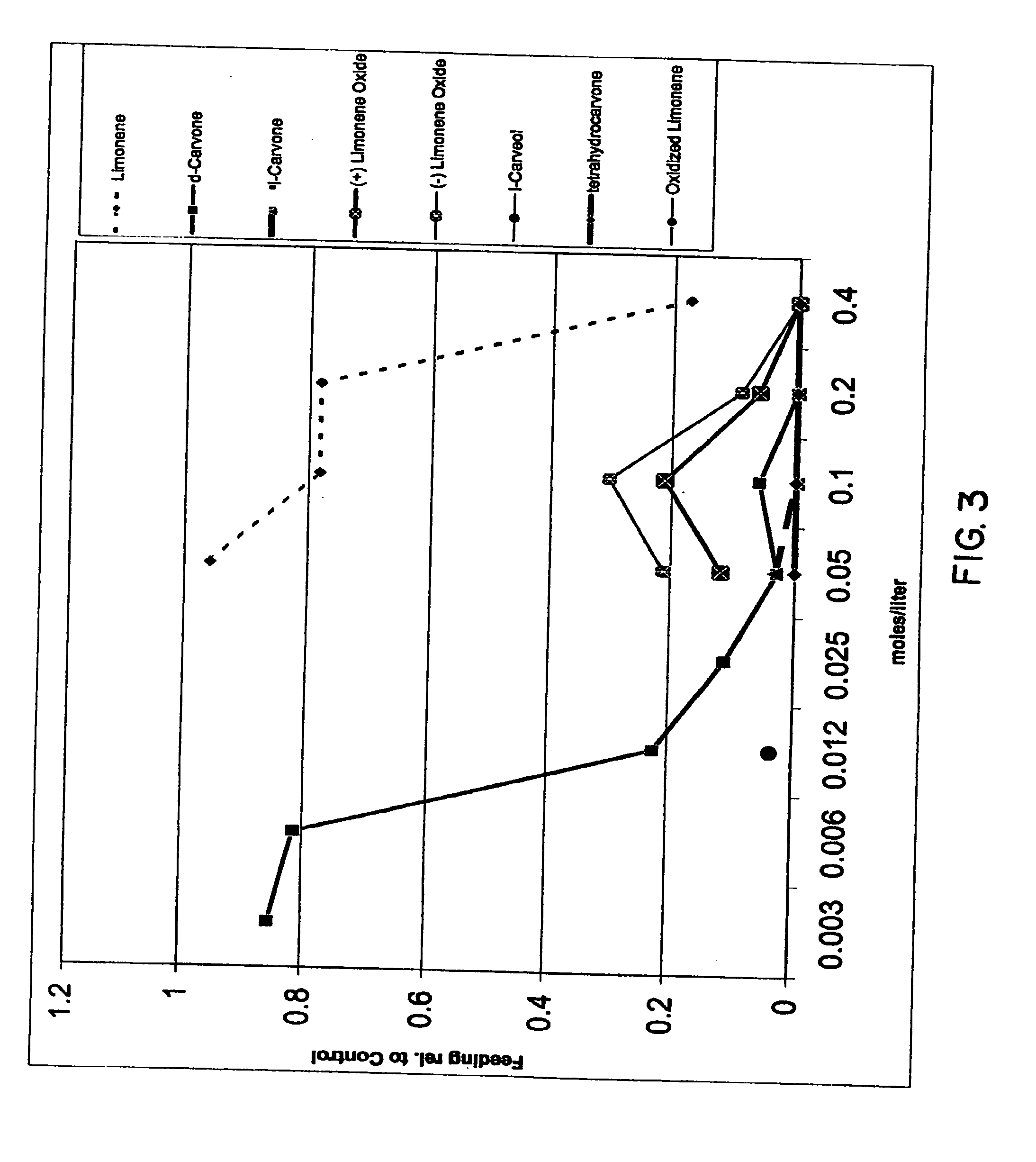
Spatial inhibitors, deterrents and repellents for mosquitoes and midges
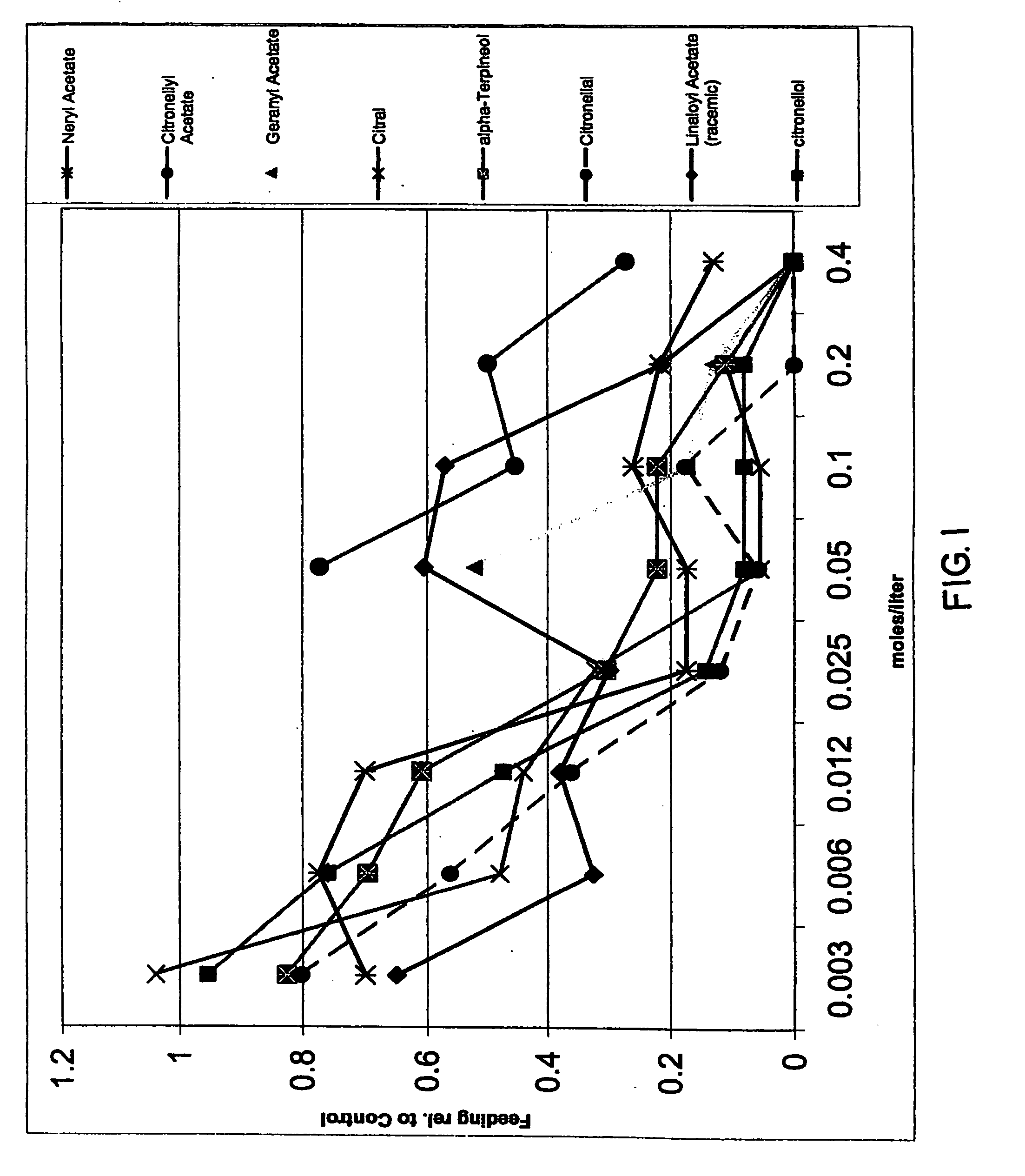
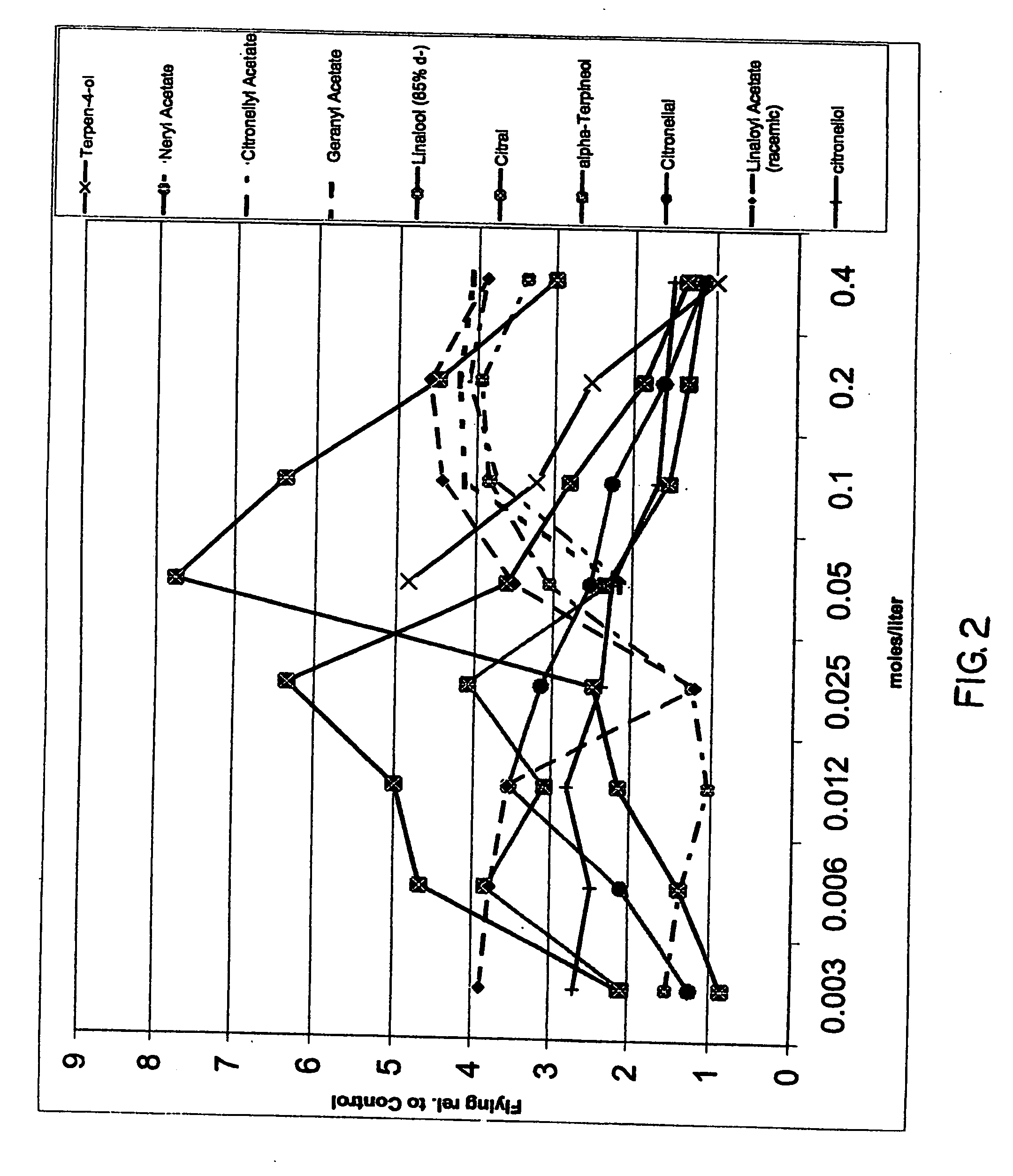
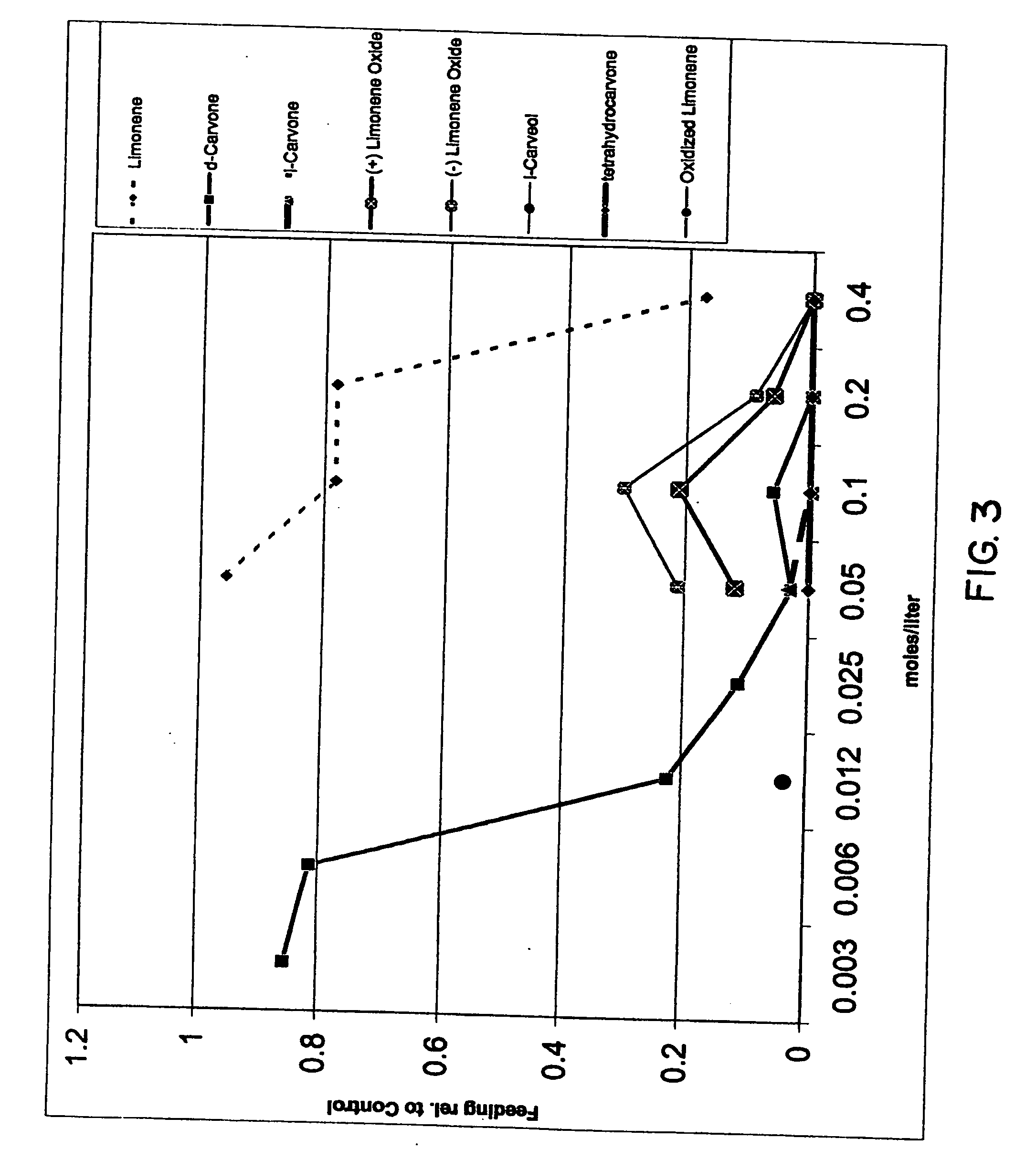
InactiveUS20070049644A1Effective contactBiocideHydroxy compound active ingredientsCarveolNeryl acetate
Certain components of citrus fruits and oxidation products of limonene are effective deterrents, repellents and / or spatial inhibitors for mosquitoes and biting midges. The compounds that have been found to be deterrents, repellents and infibitors for mosquitoes and biting midges are neryl acetate, citronellyl acetate, geranyl acetate, hydroxy-p-cymene, citral, α-terpineol, citronellal, linaloyl acetate, citronellol, terpen-4-ol, tetrahydrocarvone, products of oxidized oxidized limonene inclusive of d- and l-carvone, (+) limonene oxide, (−) limonene oxide, cis and tran carveol, a diol and an aldehyde, and mixtures thereof.
Owner:BEDOUKIAN RES
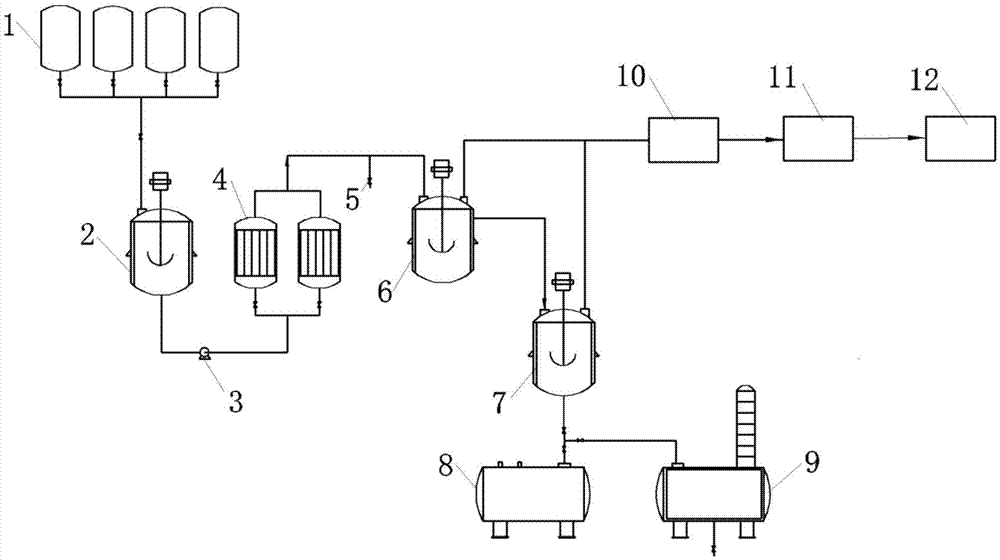
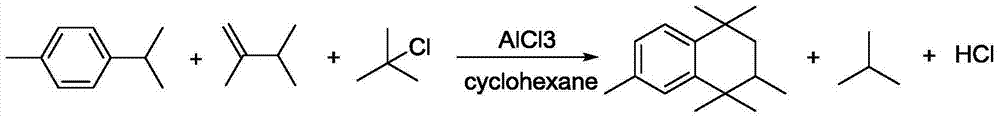
HMT continuous synthesis apparatus and method
ActiveCN105439789AKeep reaction yieldIncrease production capacityHydrocarbon from saturated and unsaturated hydrocarbon additionP-CymeneProcess safety
The present invention provides an HMT continuous synthesis apparatus and method. The method comprises: taking p-cymene, 2,3-dimethyl-1-butylene, tert-butylchloride cyclohexane as raw materials to prepare a liquid mixture; stirring the mixture uniformly and then placing the mixture into a tubular reactor containing aluminium trichloride; obtaining an HMT reaction liquid in the tubular reactor; carrying out heat insulation while stirring in a transfer kettle and then overflowing the liquid into a hydrolysis kettle; after water washing and alkaline washing, obtaining an HMT crude product by rectification; and obtaining HMT with high purity degree by recrystallization. According to the method provided by the present invention, the process safety is good, the reaction conditions are easy to control, and the product quality is stable, so that HMT continuous large-scale production can be achieved.
Owner:TIANMEN DEYUAN CHEM TECH CO LTD
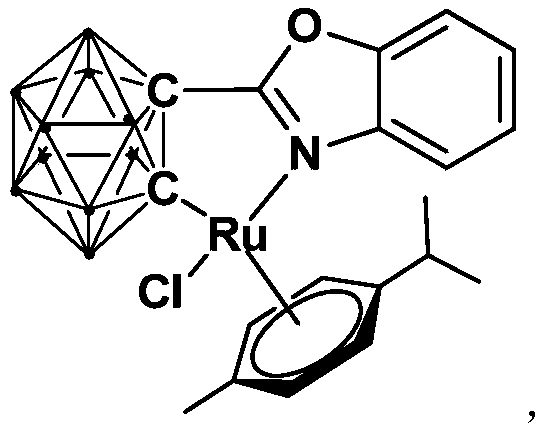
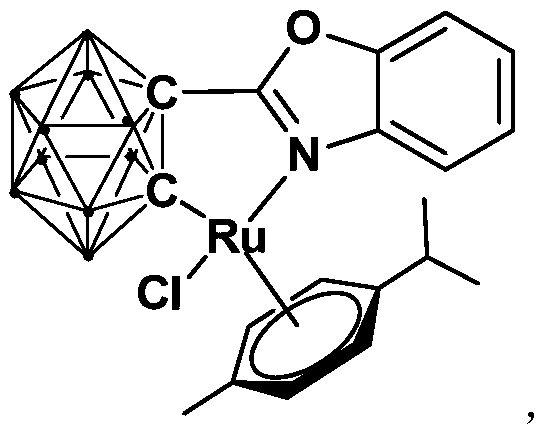
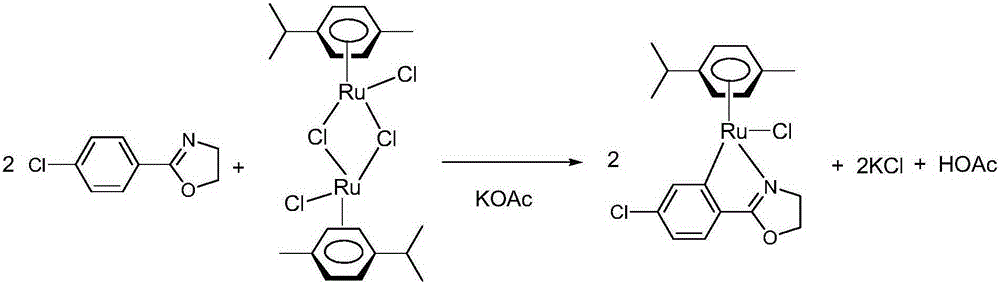
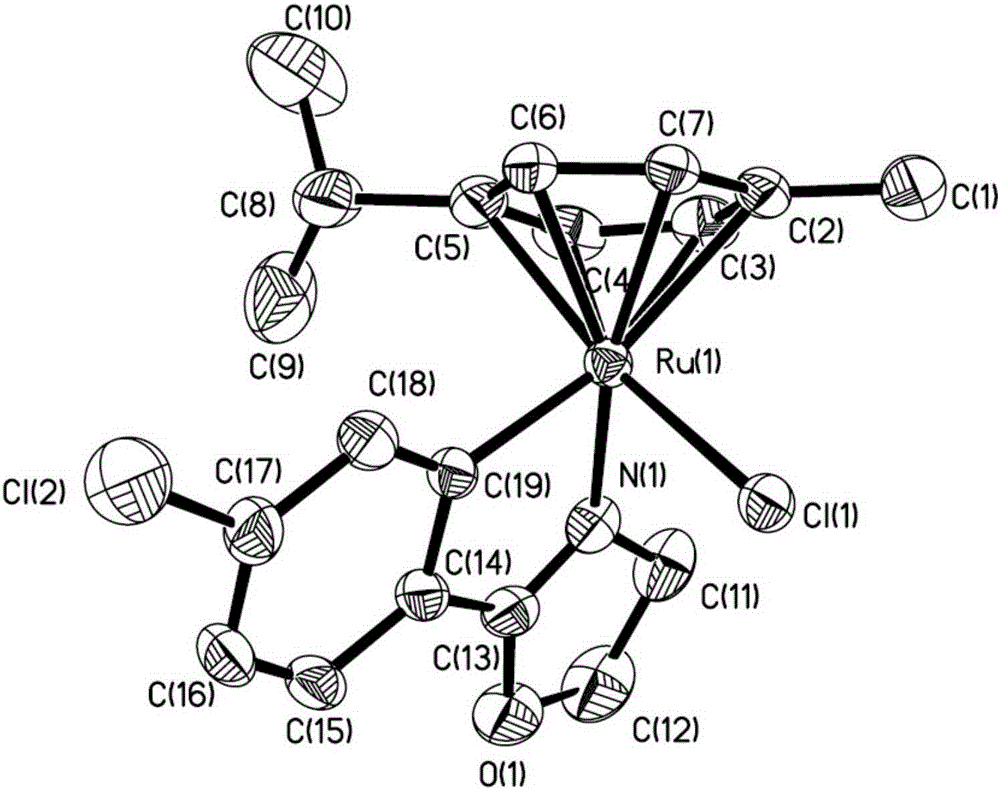
Ruthenium complex containing ortho-carbonboryl benzoxazole structure and preparation and application thereof
ActiveCN110105404AHigh catalytic activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsMetallocenesBenzoxazoleOrtho position
The invention relates to a ruthenium complex containing an ortho-carbonboryl benzoxazole structure and preparation and application thereof. A preparation method of the ruthenium complex comprises thefollowing steps that 1, an n-BuLi solution is added into an ortho-carborane solution for a reaction at the room temperature for 30-60 minutes; 2, bromobenzoxazole is added for a reaction at the room temperature for 6-8 hours; 3, [(p-cymene)RuCl2]2 is added for a reaction at the room temperature for 3-5 hours, and post-treatment is carried out to obtain the ruthenium complex; the ruthenium complexis used for catalyzing auto-oxidation coupling of primary amines to prepare imine compounds. Compared with the prior art, the prepared bivalent semi-sandwich ruthenium complex containing the ortho-carbonboryl benzoxazole structure has the advantages that the ruthenium complex has stable physical and chemical properties and thermal stability and is still stable at the high temperature of 300 DEG C;moreover, the preparation method is simple and environmentally friendly, and the ruthenium complex achieves excellent catalytic activity and a high yield in the reaction of catalyzing auto-oxidationcoupling of primary amines to prepare imine compounds.
Owner:SHANGHAI INST OF TECH
Spatial inhibitors, deterrents and repellents for mosquitoes and midges
Certain components of citrus fruits and oxidation products of limonene are effective deterrents, repellents and / or spatial inhibitors for mosquitoes and biting midges. The compounds that have been found to be deterrents, repellents and inhibitors for mosquitoes and biting midges are neryl acetate, citronellyl acetate, geranyl acetate, hydroxy-p-cymene, citral, α-terpineol, citronellal, linaloyl acetate, citronellol, terpen-4-ol, tetrahydrocarvone, products of oxidized oxidized limonene inclusive of d- and l-carvone, (+) limonene oxide, (−) limonene oxide, cis and trans carveol, a diol and an aldehyde, and mixtures thereof.
Owner:BEDOUKIAN RES
High density renewable fuels based on the selective dimerization of pinenes
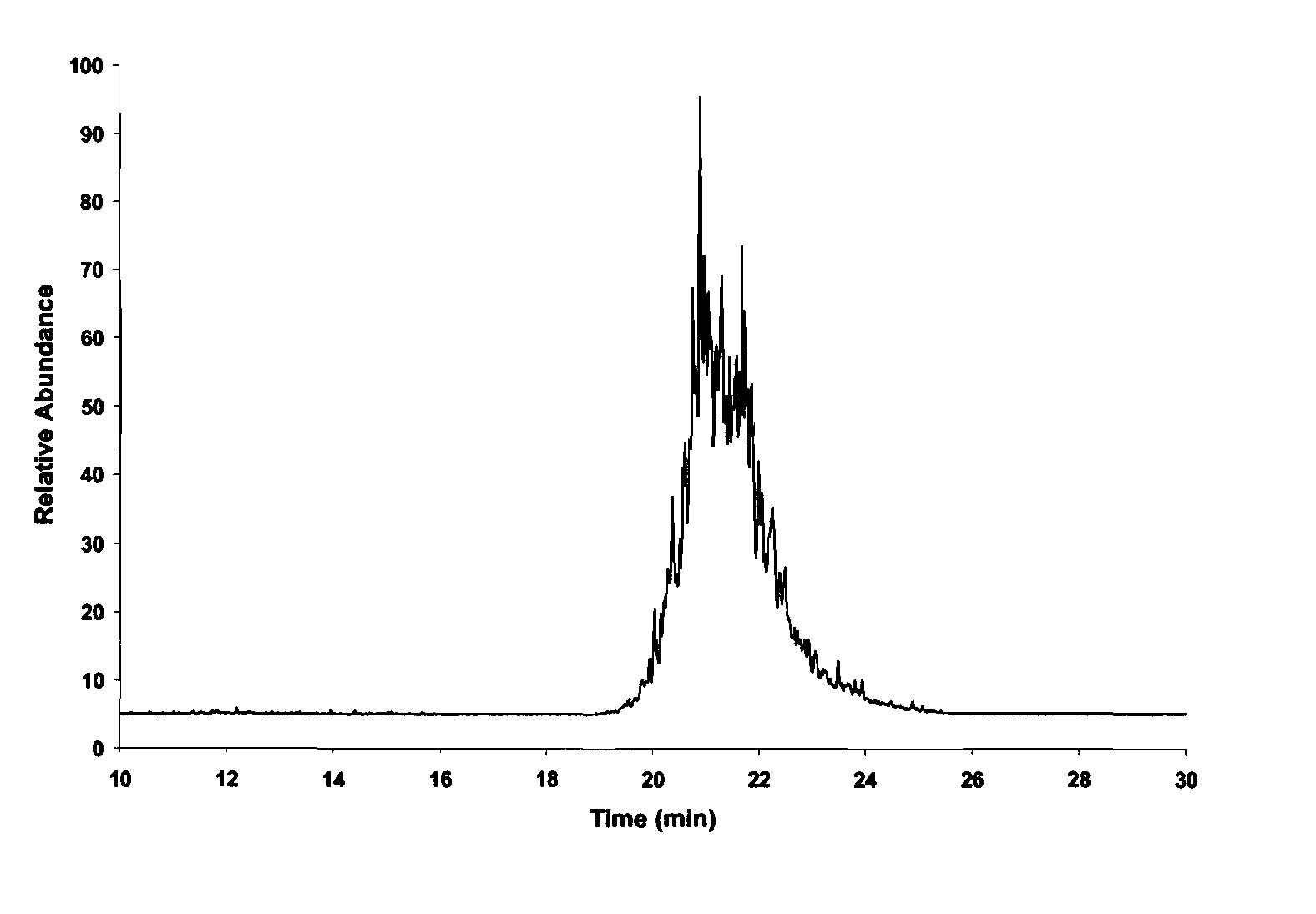
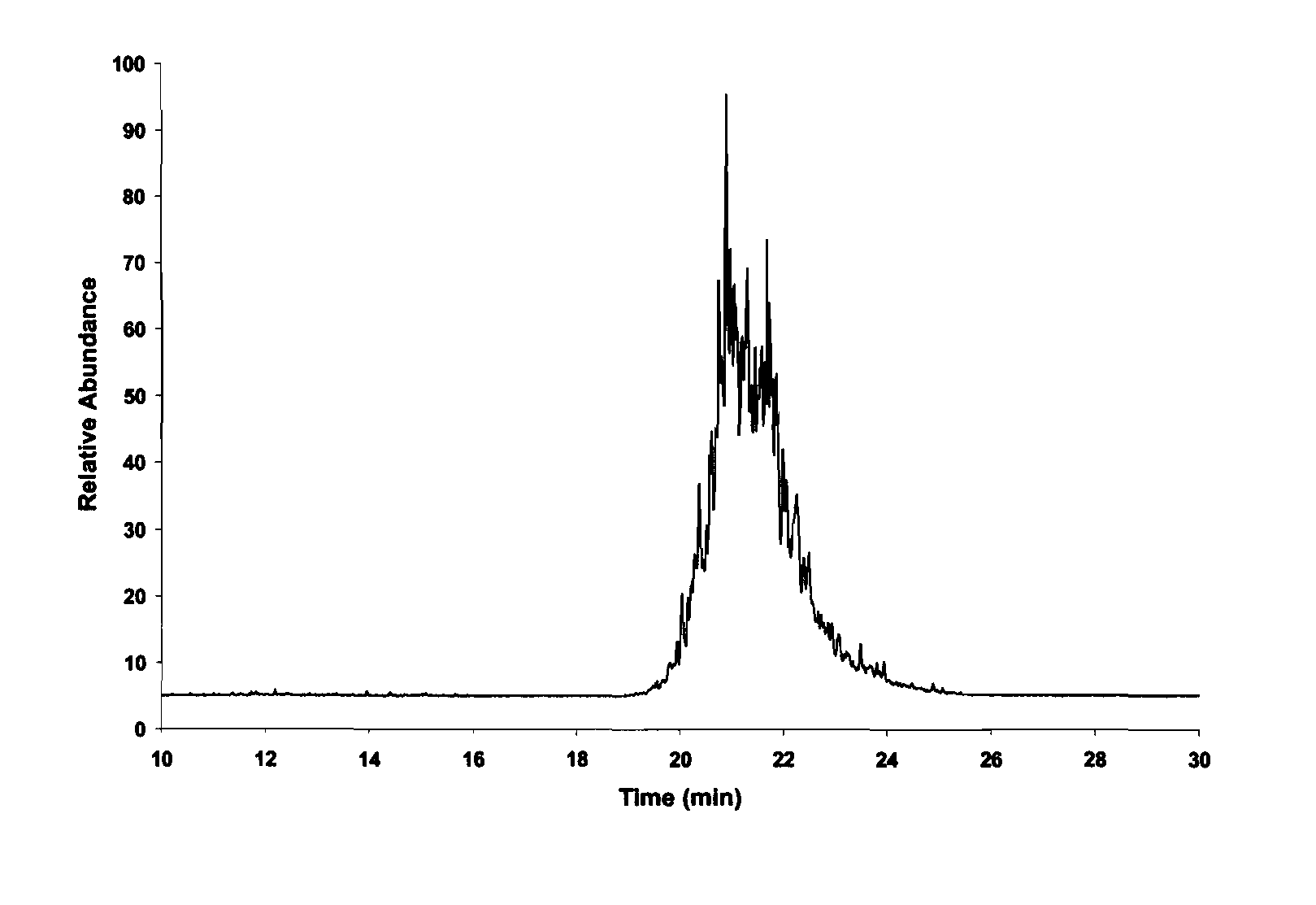
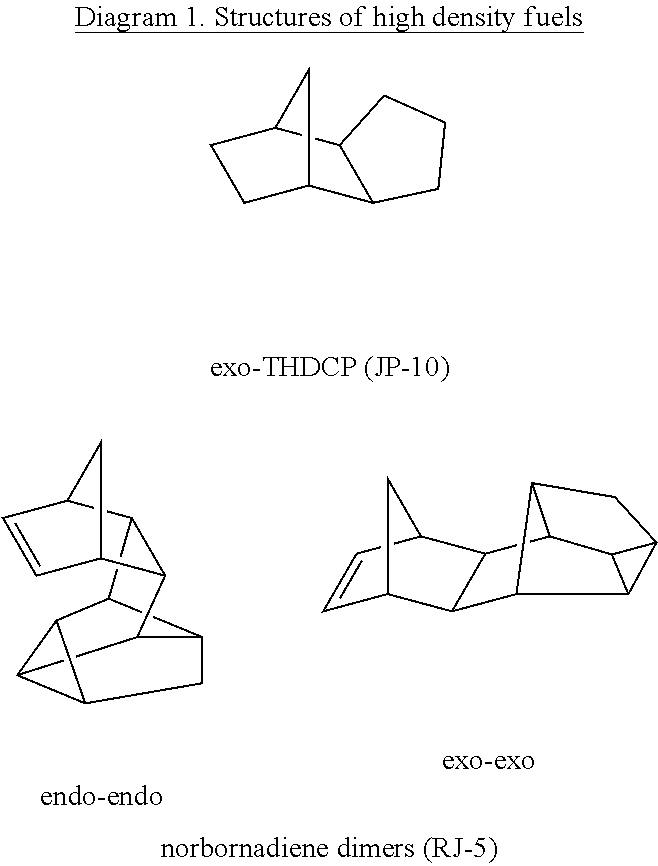
An effective method for producing high density fuel candidates from pinenes is provided. MMT-K10 is an efficient catalyst for the reaction, although significant amounts of p-cymene and camphene produced as byproducts limit the overall yield to about 80%. Nafion is also an excellent catalyst for pinene dimerization and was capable of producing dimers in up to 90% yield. Pinene dimers synthesized with these heterogenous catalysts have a density and net heat of combustion comparable to JP-10. It is emphasized that this abstract is provided to comply with the rules requiring an abstract that will allow a searcher or other reader to quickly ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope of the claims.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Half-sandwich cyclometalated ruthenium coordination compound, and preparation method and application thereof
ActiveCN105153237AReduce pollutionLow costOrganic compound preparationGroup 8/9/10/18 element organic compoundsRutheniumShielding gas
The invention discloses a half-sandwich cyclometalated ruthenium coordination compound, and a preparation method and application thereof. The structure of the half-sandwich cyclometalated ruthenium coordination compound is shown as a formula (A), and R1 is Cl, Br or CH3. The preparation method comprises under the condition of existence of a protection gas, mixing a compound shown as a formula (B), dichloro(p-cymene)ruthenium(II) dimer, an alkaline and a solvent at a temperature of 80-110 DEG C for 15-20 h, so as to prepare the half-sandwich cyclometalated ruthenium coordination compound, and R1 is Cl, Br or CH3. According to the technical scheme, the compound shown as the formula (B) and dichloro(p-cymene)ruthenium(II) dimer are subjected to one-step reaction for preparing the half-sandwich cyclometalated ruthenium coordination compound, and the half-sandwich cyclometalated ruthenium coordination compound is applied to catalytically reduce nitrobenzene compounds for preparing aniline and derivatives thereof, so that the effects of low cost, simple operation and small environment pollution are realized.
Owner:ANHUI NORMAL UNIV
Preparation method for p-isoproplyl toluene
InactiveCN101215219AIncrease contentEasy to operateHydrocarbon by isomerisationDistillation purification/separationP-isopropyltolueneP-Cymene
The invention relates to a process for preparing p-Cymene, which takes dipentene as raw material and palladium carbon as catalyst to react at a temperature of 160DEG C-180DEG C for 1-12 hours to obtain mixture of p-Cymene and p-menthane, then the p-menthane is removed via fractional distillation to obtain crude products of p-Cymene, and lastly water containing soda is added in the crude products of p-Cymene which are then heated to 170DEG C-190DEG C and after heat preservation for 5-30 minutes charged in a fractionating tower for fractionating operation to obtain products of p-Cymene. The content of p-Cymene obtained through the preparing process of the invention reaches above 99% with low production cost.
Owner:TAICANG YONGJIA PHARMA
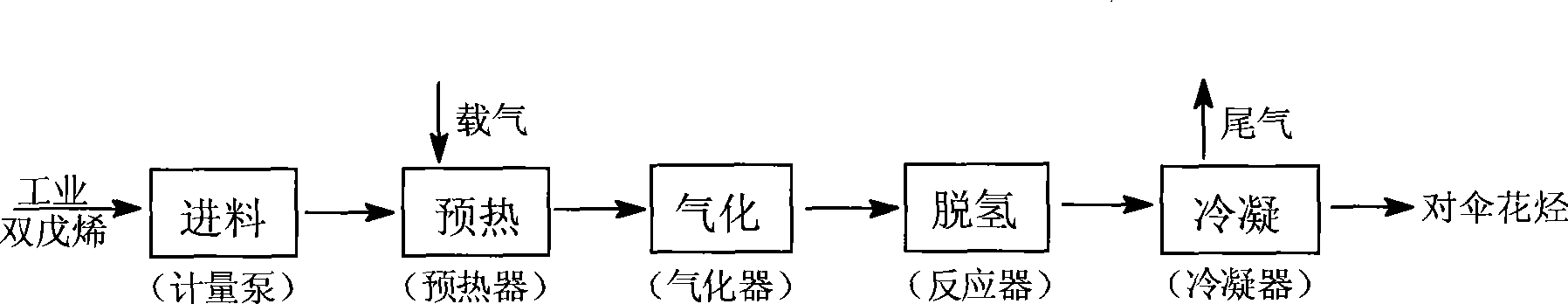
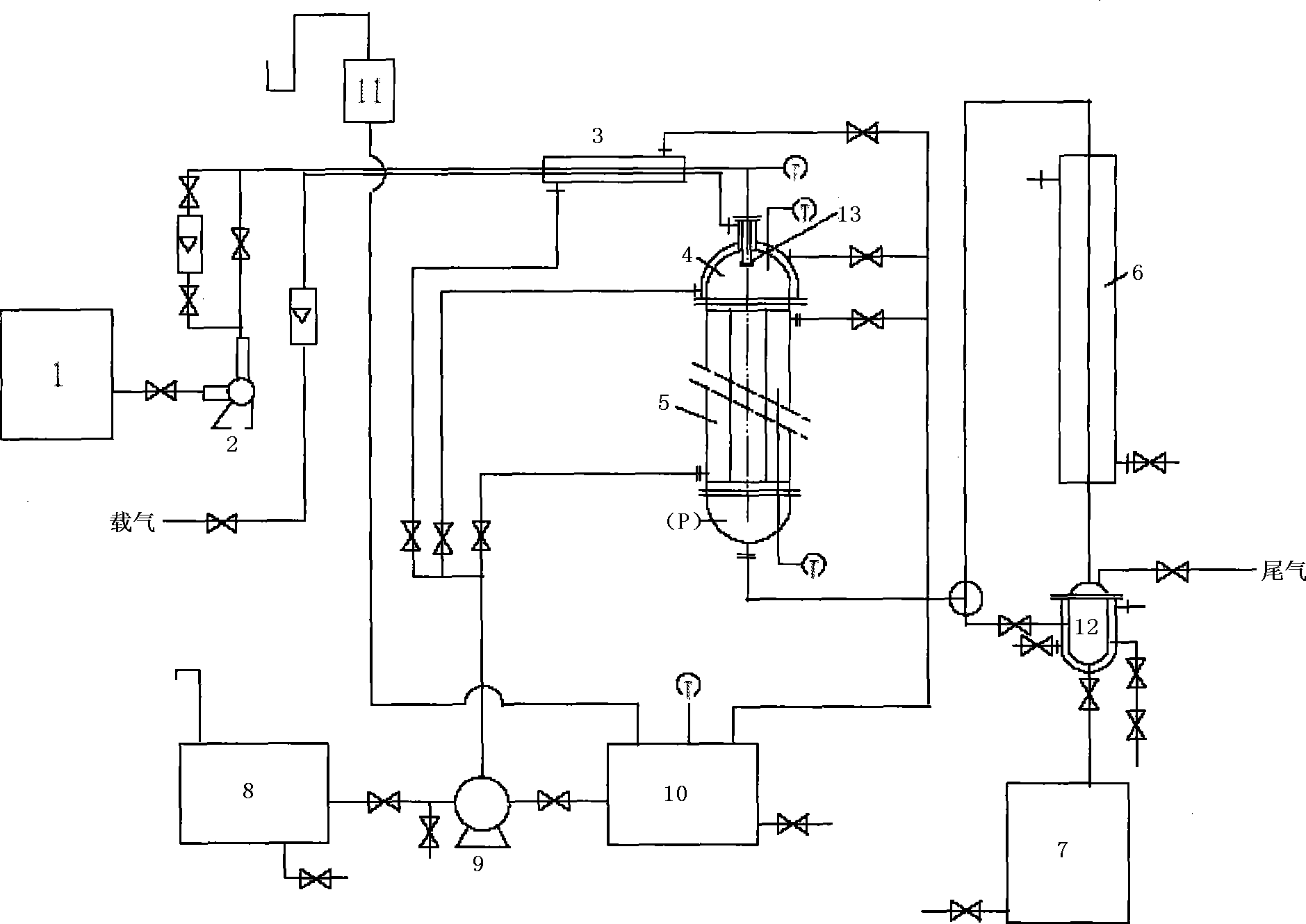
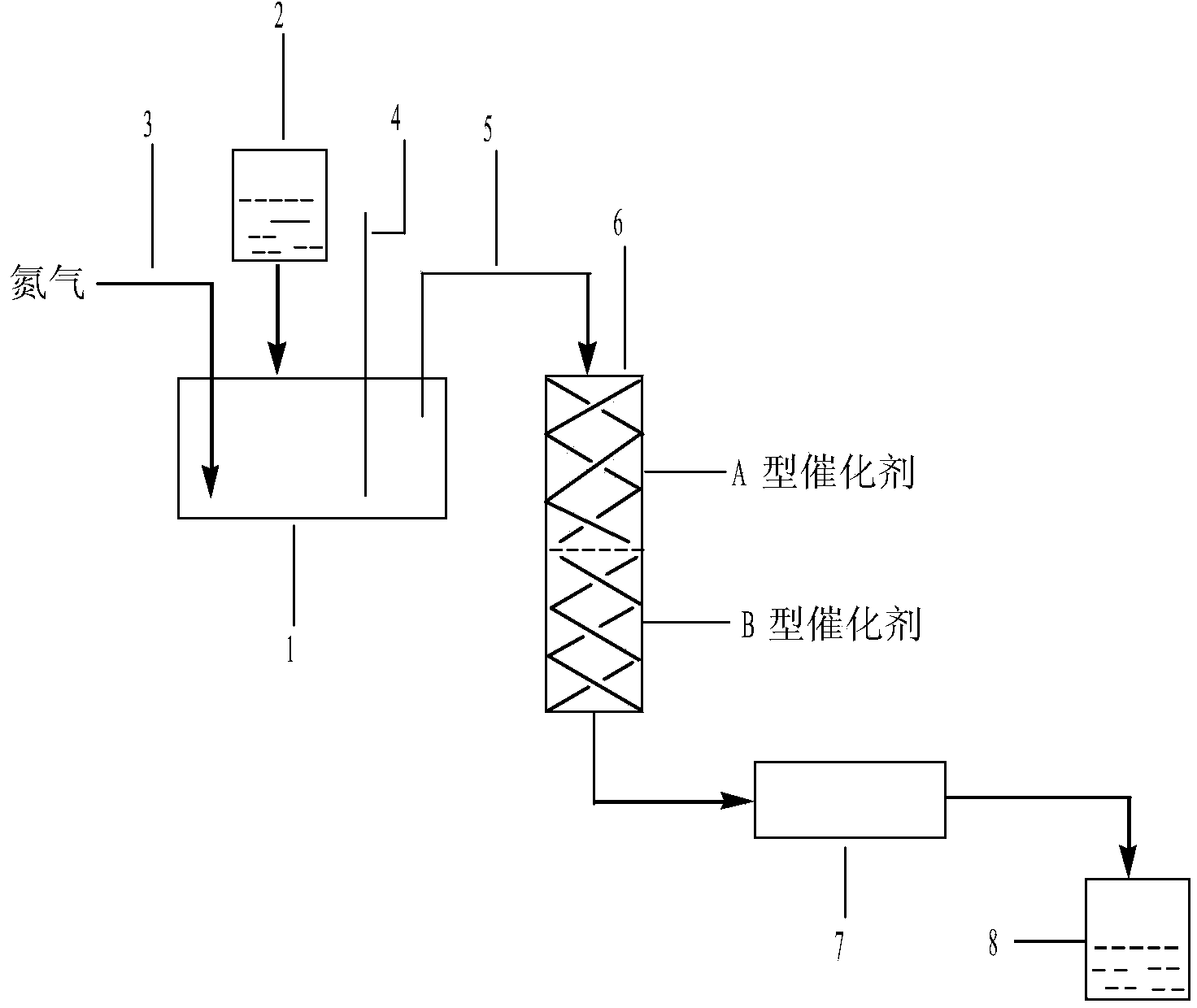
Method for producing p-cymene by continuous production and apparatus thereof
The invention discloses a method for continuously producing p-cymene and a device thereof. The method comprises the following steps: 0.1kg-50.0kg of catalyst is filled in a reactor, and the catalyst is Pd / C catalyst; inertia eluant gas is pumped into the catalyst and industrial dipentene raw material is continuously pumped in at the speed being equal to 0.5h<-1>-5.0h<-1> of the airspeed when the temperature of the reactor reaches 150-400 DEG C; the industrial dipentene raw material is preheated and gasified, and has contact reaction with the catalyst; the obtained reaction product continuously enters a condenser, and the p-cymene is obtained. The device consists of a raw material storage tank, a preheater, a gasifier, the reactor, the condenser and a product storage tank. A metering pump which can be used for continuous feeding with constant flow is arranged between the preheater and the raw material storage tank, and the inlet of the metering pump is communicated with the raw material storage tank; the metering pump is connected with the preheater and then is communicated with the top of the reactor through the gasifier, and the bottom of the reactor is connected with the product storage tank by the condenser. The invention has the advantages of simple operation, good controllability, no waste production, no solvent and other reaction reagents, recycled catalyst, purity and greening, etc.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Process for Producing Optically Active Alcohol
InactiveUS20070225528A1High yieldHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholHydrogen
Owner:KANTO CHEM CO INC
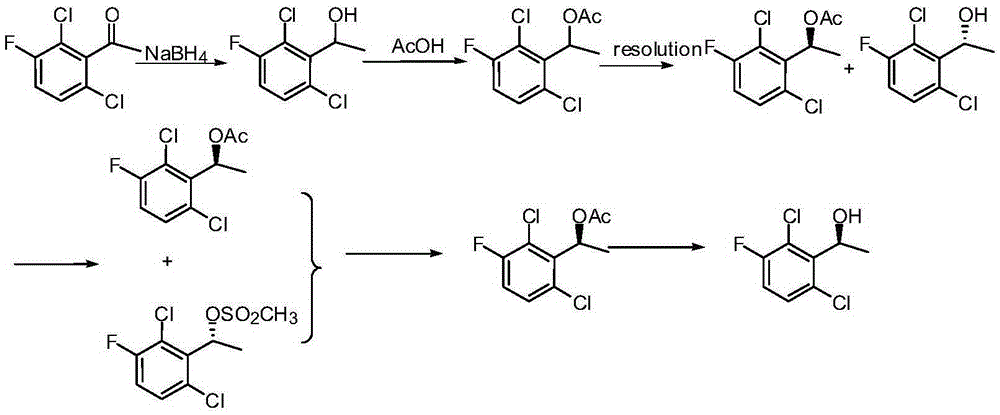
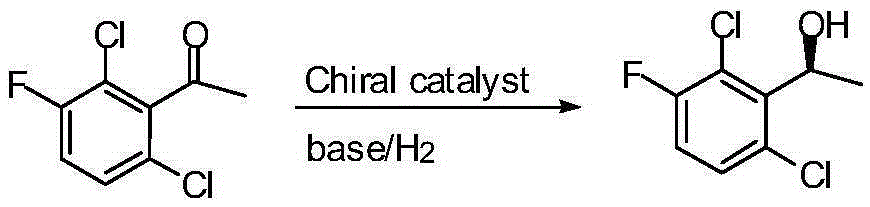
Preparation method of crizotinib intermediate (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol
InactiveCN105294401AGood catalyticReduce lossOrganic compound preparationHydroxy compound preparationPhenethyl alcoholDiphosphines
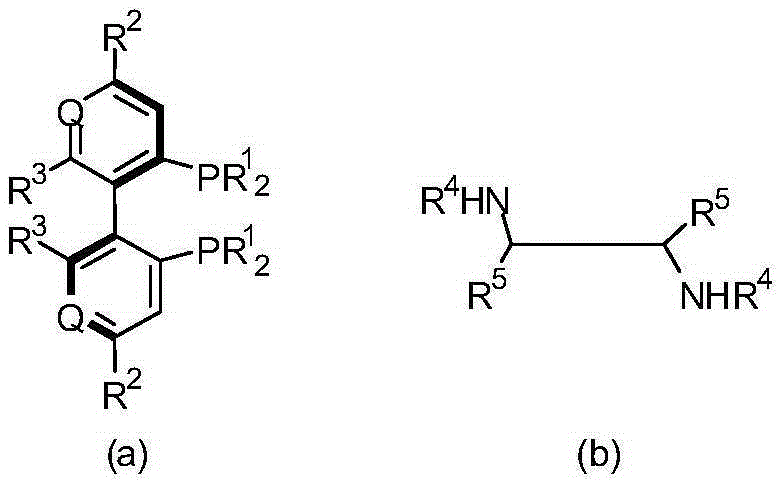
The invention belongs to the technical field of organic chemistry, also belongs to the technical field of medicinal chemistry, and in particular to a preparation method of a crizotinib intermediate (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol. The method uses 1-(2,6-dichloro-3-fluorophenyl) acetophenone as the raw material to obtain a crizotinib chiral intermediate S-1-(2,6- dichloro-3-fluoro) phenethyl alcohol under the effect of a chiral catalyst, alkali and hydrogen. The chiral catalyst is [MX2((S)-a)((R,R)-b)], which is composed of chiral diphosphine ligand compound a, a chiral nitrogen ligand compound b and a metal salt catalyst MX2(P-cymene) in coordination combination. The synthetic method provided by the invention can prepare crizotinib intermediate with high enantioselectivity; the intermediate has ee% reaching 98%; and the mole dosage of the catalyst is only 1 / 150000-1 / 50000 of a reaction substrate 1-(2,6-dichloro-3-fluorophenyl) acetophenone, and the substrate can be completely transformed.
Owner:WUHAN SINO SANTA CHEM TECH CO LTD
Sol-gel dissolved oxygen sensitive membrane, its preparation method and application
InactiveCN102398392AShort response timeEasy to operateSynthetic resin layered productsGlass/slag layered productsActive componentFluorescence
The invention relates to a preparation method of a sol-gel dissolved oxygen sensitive membrane and its application, and more specifically relates to a preparation method and application of a sol-gel dissolved oxygen sensitive membrane with tris(4,7-diphenyl-1,10-phenanthroline)ruthenium(II) as a main active component. The preparation method of the sol-gel dissolved oxygen sensitive membrane comprises: (1) preparation of tris(4,7-diphenyl-1,10-phenanthroline)ruthenium(II) by reacting dichloro(p-cymene)ruthenium with 4,7-diphenyl-1,10-phenanthroline; (2) preparation of the sol-gel dissolved oxygen sensitive membrane. The sol-gel dissolved oxygen sensitive membrane can be used in a fluorescent dissolved oxygen detector for quantitative detection of dissolved oxygen. The method of the invention can obtain the sol-gel dissolved oxygen sensitive membrane through a synthesis means and technology, and has the advantages of simple operation, low cost, and short membrane response time.
Owner:YANTAI DONGRUN INSTR
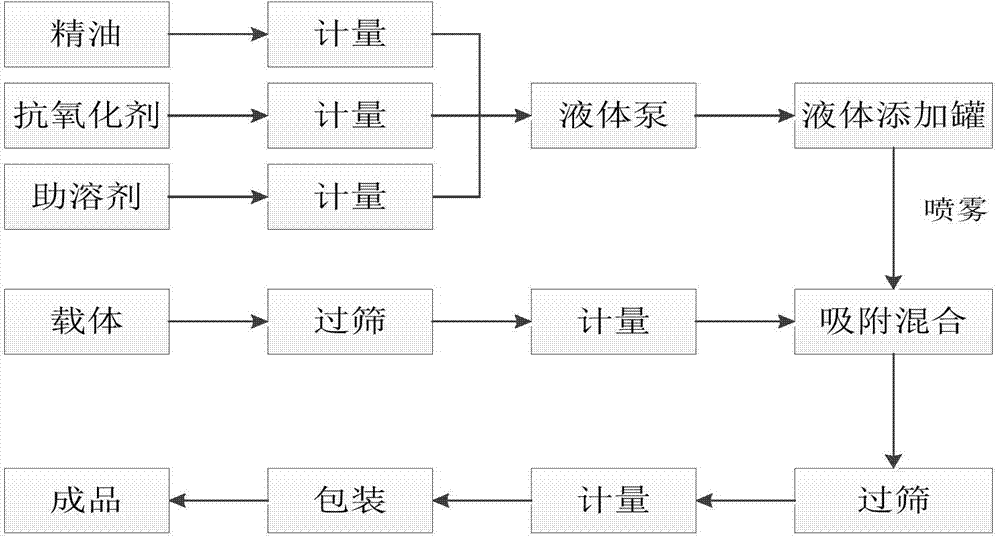
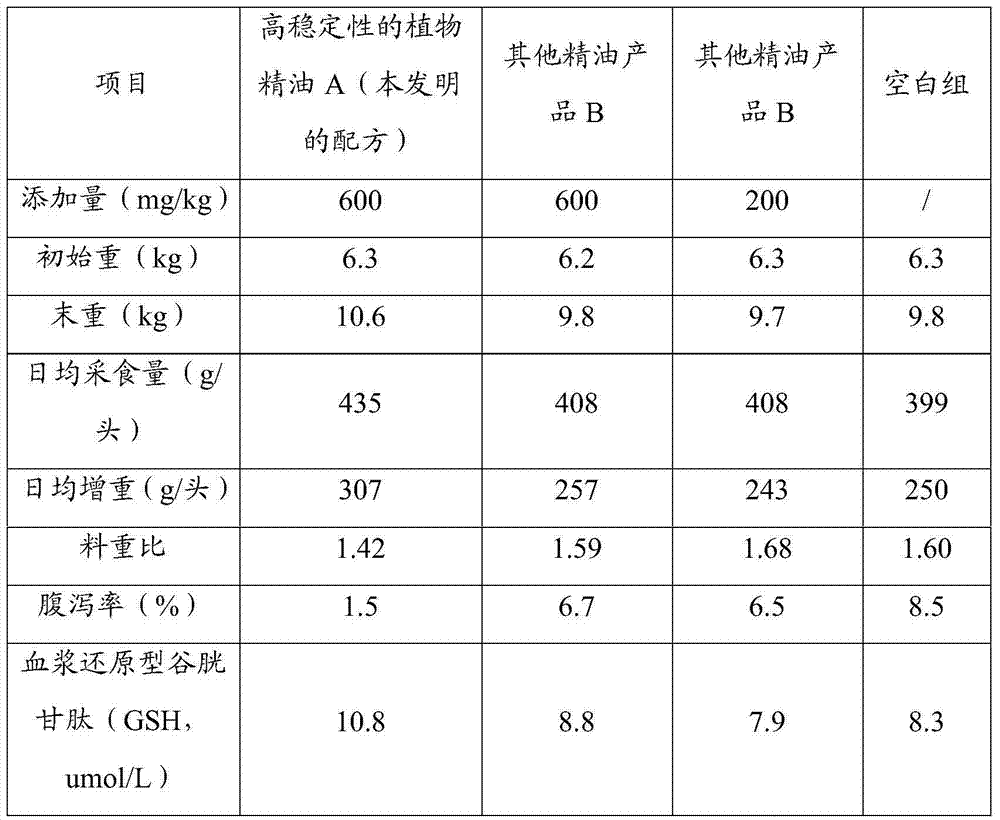
High-stability pig plant essential oil additive as well as preparation method and application thereof
The invention relates to a high-stability pig plant essential oil additive. The high-stability pig plant essential oil additive is characterized by comprising the following components in weight percent: 20%-25% of cinnamyl aldehyde, 13.5%-17.5% of thymol, 1.5%-3.5% of limonene, 1.5%-3.5% of gamma-terpinene, 0.5%-1.5% of p-cymene, 1.5%-5% of an antioxidant, 2%-6% of a cosolvent and 42%-47% of a carrier. The invention further provides a preparation method and application of the high-stability pig plant essential oil additive. The high-stability pig plant essential oil additive has an anti-oxidization function and high stability, and can easily play a role in digestive tracts of pigs.
Owner:上海美农生物科技股份有限公司
Mothproof antibacterial essential oil
InactiveCN104642405AGood antibacterialExcellent mothproofBiocidePest repellentsCampheneAlpha-humulene
The invention discloses mothproof antibacterial essential oil which is used for solving the problems that a mothproof function and an antibacterial function cannot be combined very well in the prior art, and the smell is pungent. The mothproof antibacterial essential oil comprises the following components in percentage by weight: 5%-70% of natural camphor, 0-10% of lavender essential oil, 0-10% of pyrethrin, 10%-40% of other components, and 10%-85% of 1,8 cineole and / or aromatic alcohol, based on 100% in total, wherein the other components comprise one or more of p-cymene, alpha-pinene, beta-pinene, geraniolene, alpha-terpene alcohol, camphene, limonene, caryophyllene, alpha-humulene, borneol and alpha-phellandrene. According to the technical scheme adopted by the invention, the problems in the prior art can be solved very well.
Owner:肖正君
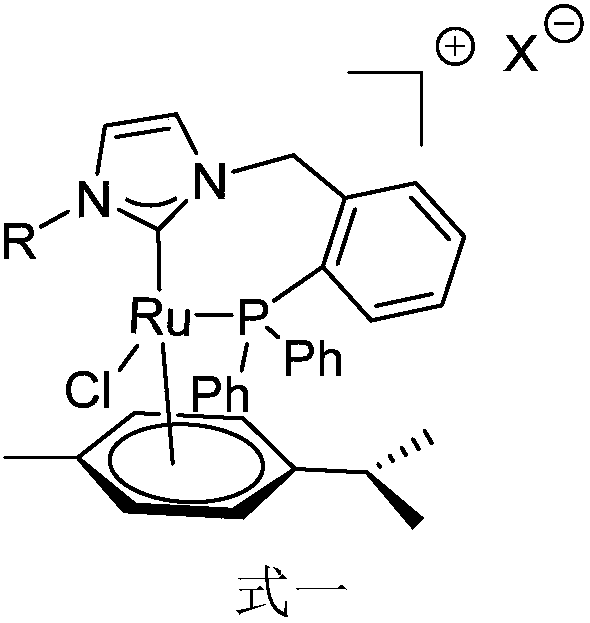
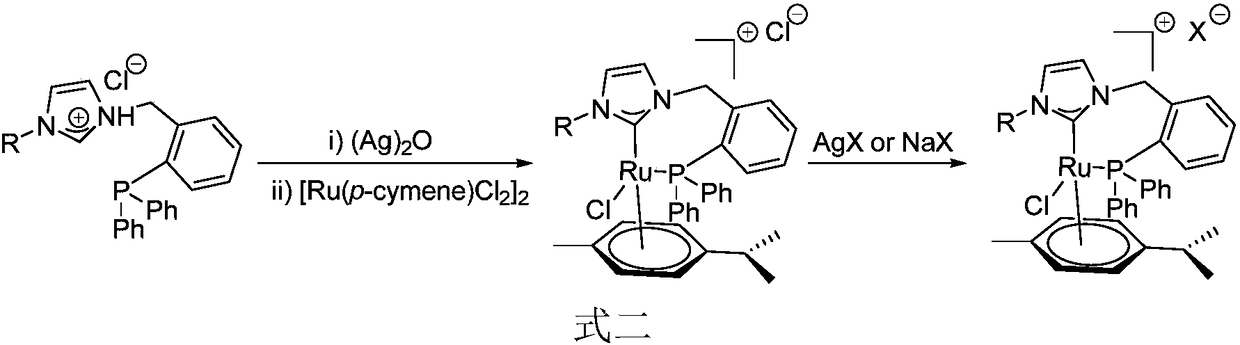
Novel bidentate phosphorus-N-heterocyclic carbine p-cymene type ruthenium complex catalyst as well as preparation method and synthetic application thereof
ActiveCN108380245ASynthetic raw materials are simpleEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFiltrationEquivalent weight
The invention discloses a novel bidentate phosphorus-N-heterocyclic carbine p-cymene type ruthenium complex catalyst as well as a preparation method and synthetic application thereof. The catalyst isprepared by the following steps: adding silver oxide and a corresponding bidentate phosphorus-aza-imidazole chloride salt ligand into a dichloromethane solution, and performing a reaction for 12-24 hunder the nitrogen protection conditions of avoiding light at room temperature; adding [Ru(p-cymene)Cl2]2 with an equivalent weight of 0.5 times of that of the ligand under nitrogen protection, and performing a reaction for 12-24 h at room temperature; and after the reaction is finished, performing filtration, and performing recrystallization or performing separation by using dichloromethane-methanol through a silica gel column, so as to obtain the bidentate-phosphorus-N-heterocyclic carbine p-cymene type ruthenium complex catalyst. The catalyst provided by the invention can catalyze a ''hydrogen borrowing coupling'' reaction of an alcohol and an aromatic primary amine under basic conditions to obtain a series of valuable N-alkyl compounds; the bidentate phosphorus-N-heterocyclic carbine p-cymene type ruthenium complex catalyst provided by the invention has simple synthetic raw materials, operation steps are easy to implement, and the stability is good; and the ruthenium complex catalyst has the advantages of a wide range of reaction substrates, mild conditions, high efficiency and practicality, and has important application value.
Owner:SUN YAT SEN UNIV
Synthetic method of spirocyclic compound containing 1-indanone skeleton
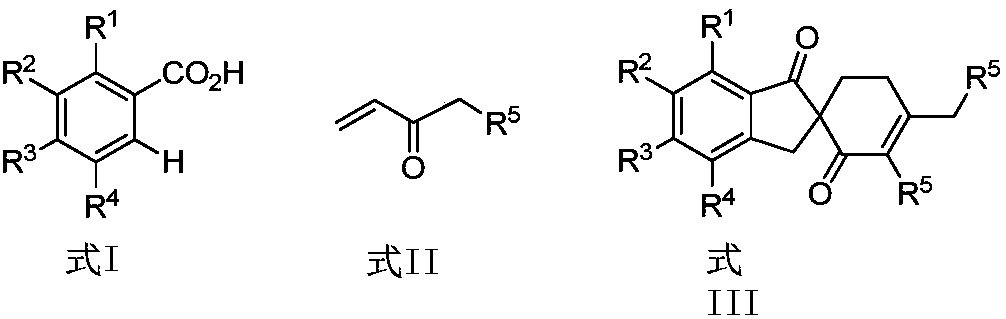
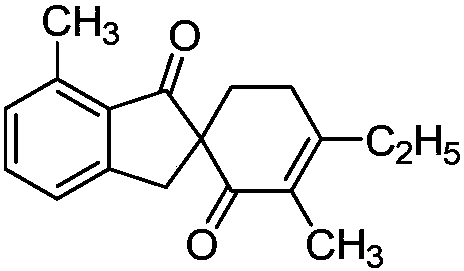
ActiveCN108047007AAtom utilization is highEasy to operateOrganic compound preparationCarbonyl group formation/introductionPentamethylcyclopentadieneMANGANESE ACETATE
The invention discloses a synthetic method of a spirocyclic compound containing 1-indanone skeleton. The method comprises the following steps: taking aromatic carboxylic acid and alpha, beta-unsaturated ketone as raw materials, taking any of p-cymene ruthenium chloride dimer, pentamethylcyclopetadienyl rhodium chloride dimmer, tris(acetonitrile)(pentamethylcyclopentadienyl)rhodium bis(hexafluoroantimonate) as a catalyst, taking any of anhydrous manganese acetate, manganese acetate tetrahydrate, anhydrous zinc acetate and zinc acetate as an additive, and thus synthesizing the spirocyclic compound containing 1-indanone skeleton by adopting a one-step method. The reaction comprises four steps such as conjugate addition reaction of aromatic carboxylic acid ortho-position C-H bond and alpha, beta-unsaturated ketone, intramolecular dehydration, Michael addition with second molecular alpha,beta-unsaturated ketone and intramolecular aldol condensation. The synthetic method has the characteristics that the raw materials are low in price and easy to obtain, the efficiency is high, the atom utilization rate is high, the reaction operation is simple and four new C-C bonds are constructed by adopting the one-step method.
Owner:SHAANXI NORMAL UNIV
Essence suitable for low-temperature non-combustion smoke products, preparation method and application thereof
InactiveCN109852476APlay a role in flavoringGreat tasteTobacco preparationTobacco treatmentPraeruptorin CGuaiacol
The invention provides an essence suitable for low-temperature non-combustion smoke products, a preparation method and an application thereof, and relates to the technical field of low-temperature non-combustion smoke products. The essence comprises components with antitussive and expectorant effect and the components with antitussive and expectorant effect is prepared from at least one of foliumArtemisiae Argyi oil, daurian rhododendron oil, oleum viticis negundo, alpha-asarone, Germacrone, P-Cymene, guaiacol sulfonic acid potassium, acetylcysteine, isofraxidine, esculetin, ligustilide, stachydrine, ligustrazine, imperatorin, isoimperatorin, Praeruptorin A, Praeruptorin B, Praeruptorin C, hygrine, and Cuscohygrine. The antitussive and expectorant active ingredient is natural active ingredient, which can volatilize and transfer to the total particulate matter of the smoke at atomization temperature, enrich the smoke and enhance the mouthfeel, and at the same time is partly absorbed bythe human body, so that the low-temperature non-combustible smoking product has the function of regulating the respiratory system function to a certain extent.
Owner:NEW FLAME INTELLIGENT MFG (SHENZHEN) CO LTD
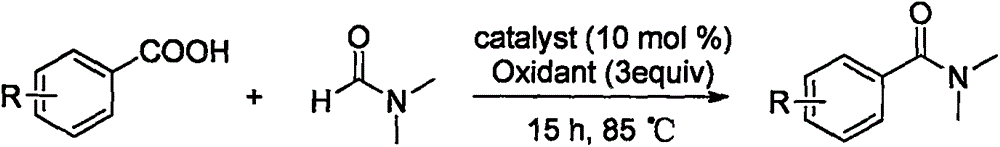
Synthesis method of amide aryl compound
InactiveCN106496056AWidely applicable preparation methodStable in natureCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsCarboxylic acid
The invention relates to a synthesis method of an amide aryl compounds. According to the method, Ru-(p-cymene) C12 is taken as a catalyst, K2S2O8 is taken as an oxidizing agent, Xantphos is taken as a ligand, one reactant (N, N-dialkyl formamide) is taken as a solvent, and a substrate aryl carboxylic acid and the N, N-dialkyl formamide are subjected to coupling reaction, so that the amide aryl compound is obtained. The reaction substrate is low in cost and easy to get, stable in performance, small in toxicity and mild in reaction conditions, and has a wide applicability for substrate with different functional groups. The amide aryl compound efficiently established through the synthesis method belongs to an important molecular skeleton of various medicines, bioactive molecules and natural products, and the synthesis method provides a widely applicable preparation method for the synthesis of the compounds.
Owner:中国人民解放军63975部队
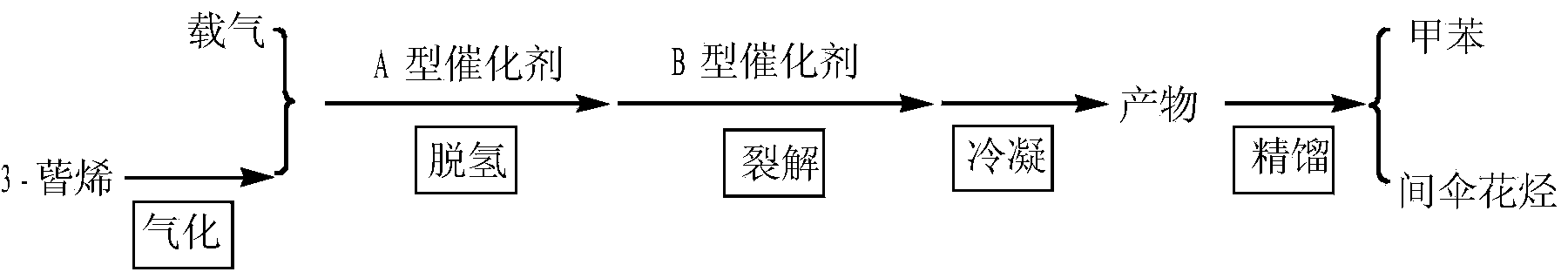
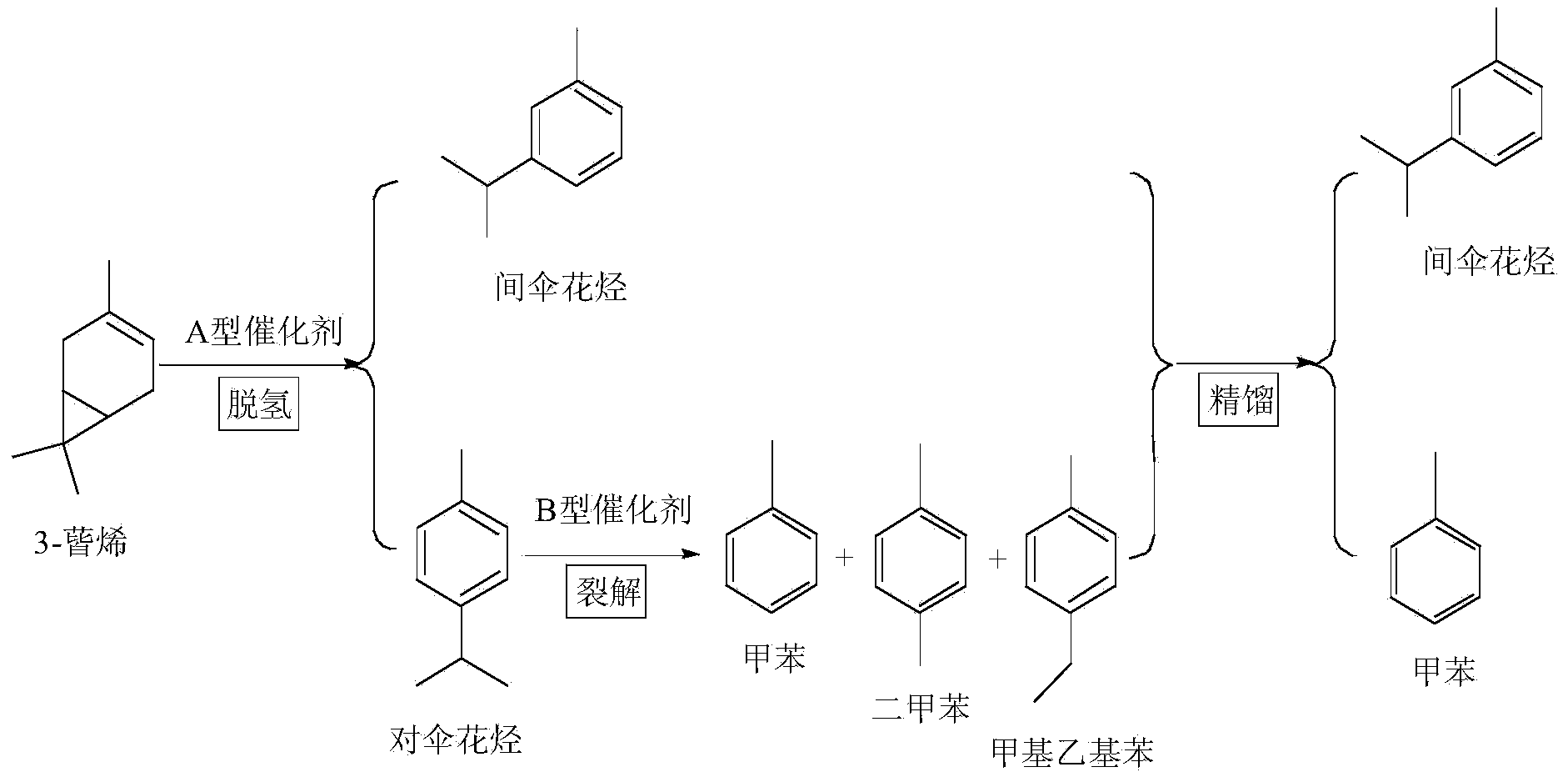
Method for preparing methylbenzene and m-cymene from 3-carene and device thereof
ActiveCN103524289AA new way to useEfficient way to useHydrocarbon by isomerisationHydrocarbonsCracking reactionCymenes
The invention discloses a method for preparing methylbenzene and m-cymene from 3-carene and a device of the method. 3-carene is subjected to isomerism to be a mixture of p-cymene and m-cymene under catalysis of an A-type catalyst, wherein the A-type catalyst is one or a mixture compounded from more of supported catalysts of platinum, palladium and nickel; the mixture of p-cymene and m-cymene conducts splitting reaction on p-cymene in the mixture on presence of a B-type catalyst so as to obtain a mixture with methylbenzene and m-cymene; the mixture is rectified, separated and purified to obtain methylbenzene and m-cymene; the B-type catalyst is one or a mixture compounded from more of ZSM-5 sodium type molecular sieve and ZSM-5 hydrogen type molecular sieve of various silica-alumina ratios. The method is green, simple and efficient, raw materials are natural and reproducible, and a product has property equivalent to natural products.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
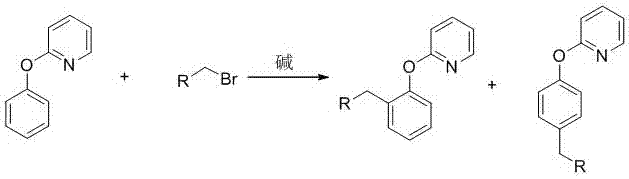
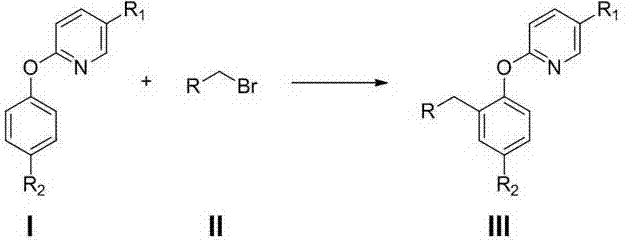
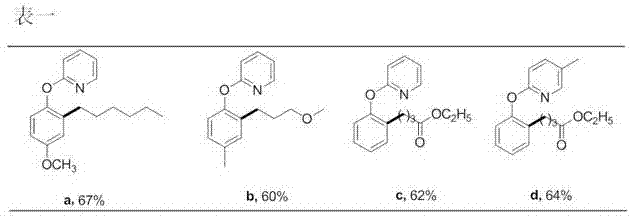
Preparation method of 2-(2-alkylphenoxy)pyridine derivative
InactiveCN107043350AHigh puritySynthetic raw materials are readily availableOrganic chemistryCyclohexanecarboxylic acidGlycol synthesis
A preparation method of a 2-(2-alkylphenoxy)pyridine derivative adopts a reaction of a 2-phenoxypyridine derivative and a primary bromoalkane compound. The method concretely comprises the following steps: directly adding the 2-phenoxypyridine derivative, the primary bromoalkane compound, a catalyst, an additive, an alkali and a solvent into a reaction device, stirring and heating the above added substances until the temperature is 90-130 DEG C, carrying out a reaction for 18-36 h, and separating the obtained product to obtain the 2-(2-alkylphenoxy)pyridine derivative, wherein the catalyst is dichloro(p-cymene)ruthenium dimer; the alkali is potassium carbonate or lithium carbonate; the additive is 1-adamantanecarboxylic acid or naphthenic acid; and the solvent is benzene or DMF or ethylene glycol dimethyl ether. The method has the advantages of easiness in obtaining of synthesis raw materials, simple process, and very good specificity of the reaction.
Owner:ANYANG NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com