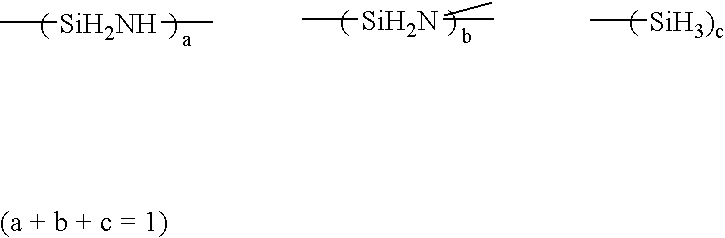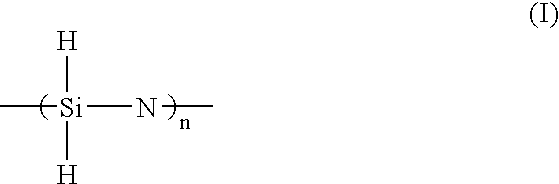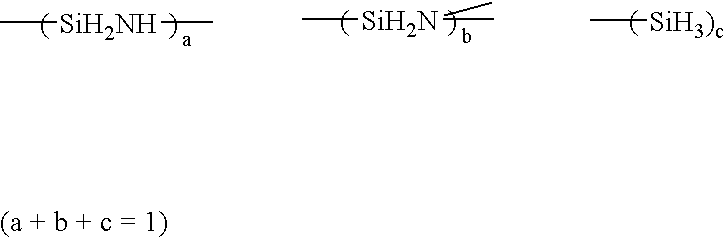Patents
Literature
377 results about "Limonene oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
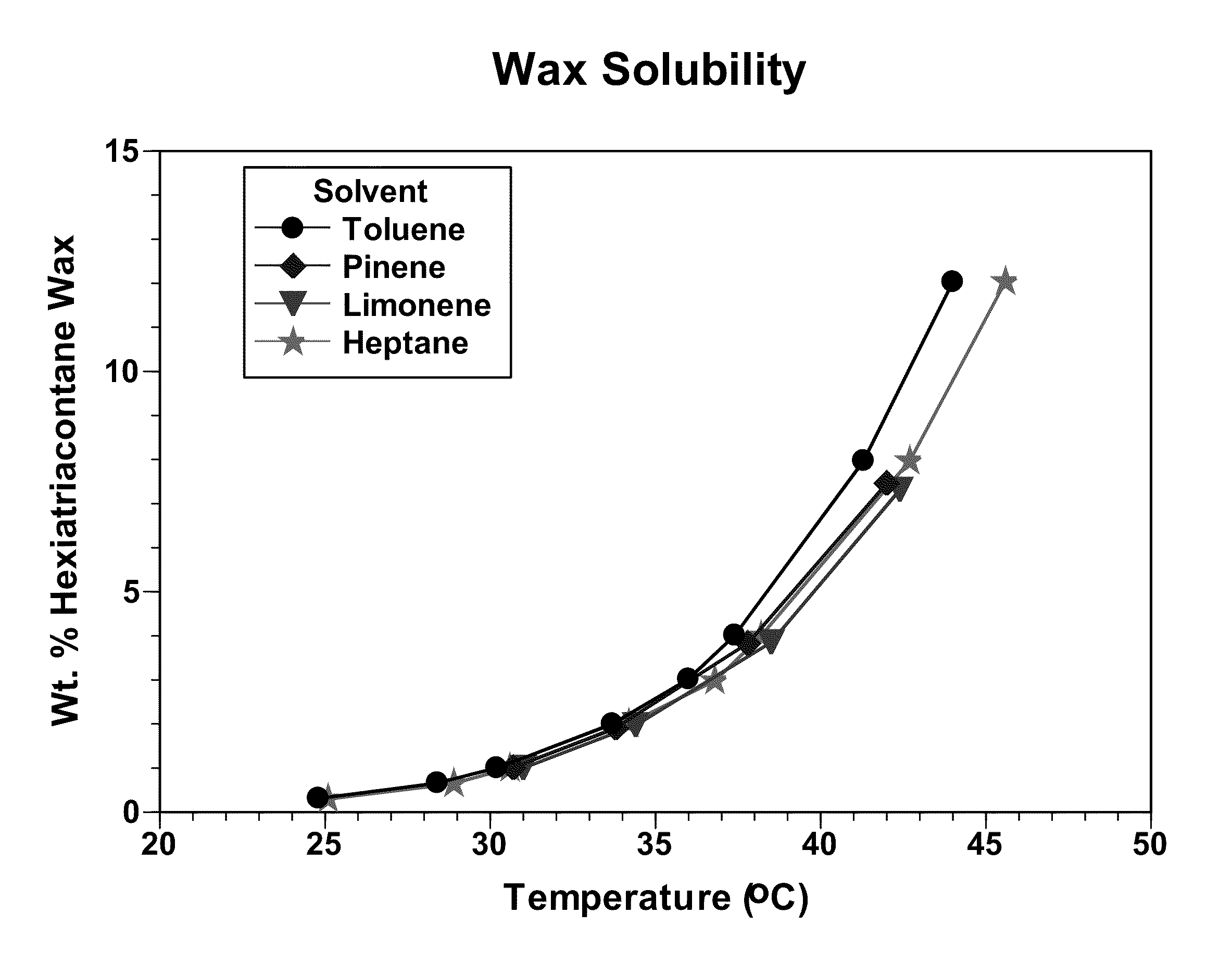
Chemical reactions. Limonene is a relatively stable monoterpene and can be distilled without decomposition, although at elevated temperatures it cracks to form isoprene. It oxidizes easily in moist air to produce carveol, carvone, and limonene oxide. With sulfur, it undergoes dehydrogenation to p-cymene.
Foamable interpolymer resin particles containing limonene as a blowing aid
ActiveUS20040152795A1Lower Level RequirementsImprove acceleration performanceFoundry mouldsFoundry coresPolyolefinFoaming agent
Interpolymer resin particles comprised of 20% to 80% by weight polyolefin, e.g. polyethylene and 80% to 20% by weight of an in situ polymerized vinyl aromatic resin, e.g. polystyrene or poly(styrene-butyl acrylate) and forming an interpenetrating network of polyolefin and vinyl aromatic resin particles. The interpolymer particles are impregnated with a volatile hydrocarbon blowing agent, and limonene, e.g. d-limonene, ranging from about 0.1 to about 5 parts, preferably 0.1 to 1 part by weight, based on 100 parts by weight of the interpolymer particles, for improved expandability and a pleasant fragrance.
Owner:BVPV STYRENICS LLC
Foamable interpolymer resin particles containing limonene as a blowing aid
ActiveUS6908949B2Improve acceleration performanceLower Level RequirementsFoundry mouldsFoundry coresPolyolefinFoaming agent
Interpolymer resin particles comprised of 20% to 80% by weight polyolefin, e.g. polyethylene and 80% to 20% by weight of an in situ polymerized vinyl aromatic resin, e.g. polystyrene or poly(styrene-butyl acrylate) and forming an interpenetrating network of polyolefin and vinyl aromatic resin particles. The interpolymer particles are impregnated with a volatile hydrocarbon blowing agent, and limonene, e.g. d-limonene, ranging from about 0.1 to about 5 parts, preferably 0.1 to 1 part by weight, based on 100 parts by weight of the interpolymer particles, for improved expandability and a pleasant fragrance.
Owner:BVPV STYRENICS LLC
Corrosion inhibitor intensifier and method of using the same
An aqueous organic acid composition containing a terpene as corrosion inhibitor intensifier is especially suitable for use in acidizing subterranean formations and wellbores. The composition substantially reduces the corrosive effects of the acidic solution on metals in contact with the acidic solution. Suitable terpenes include carotene, limonene, pinene, farnesene, camphor, cymene and menthol.
Owner:BAKER HUGHES INC
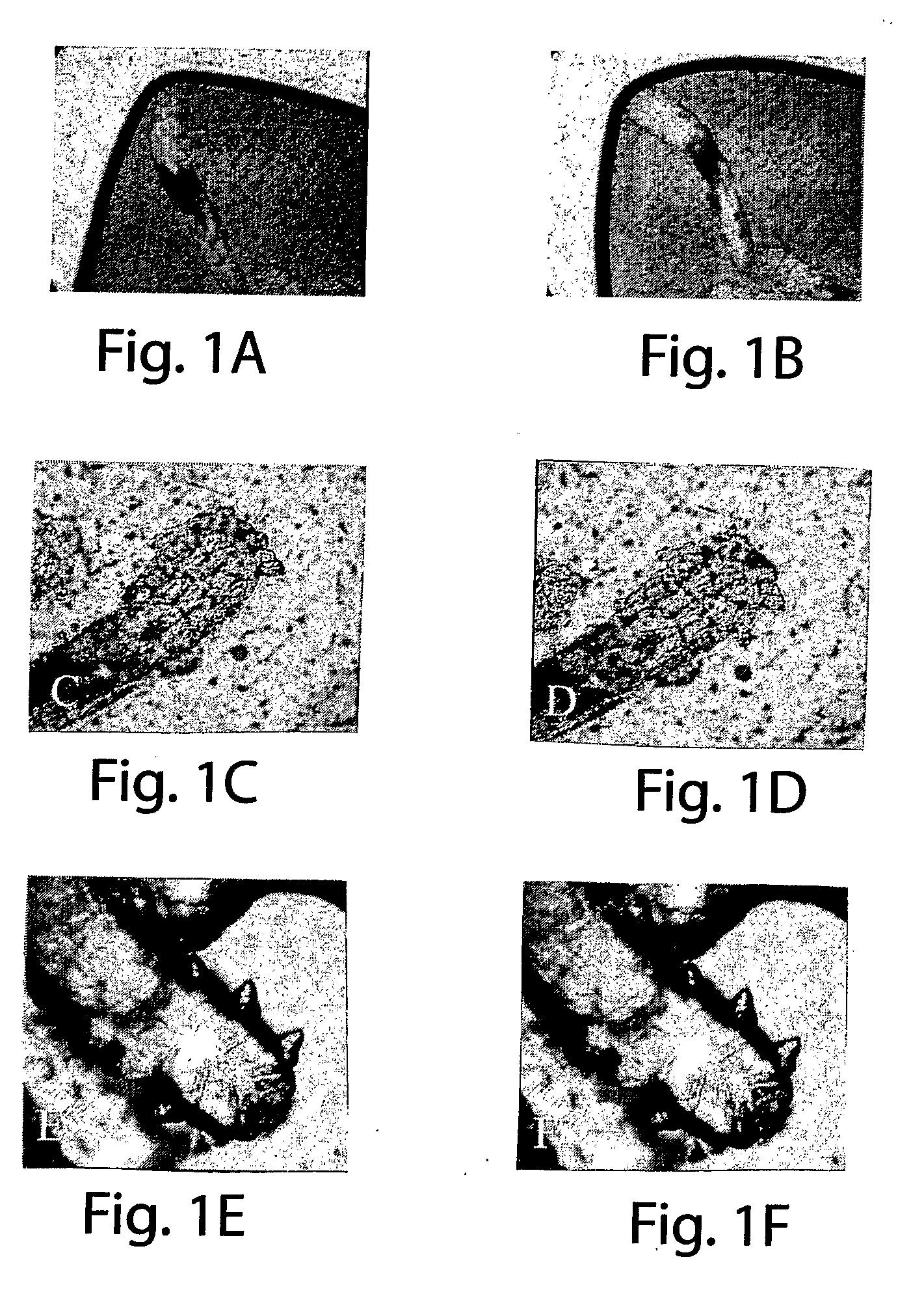
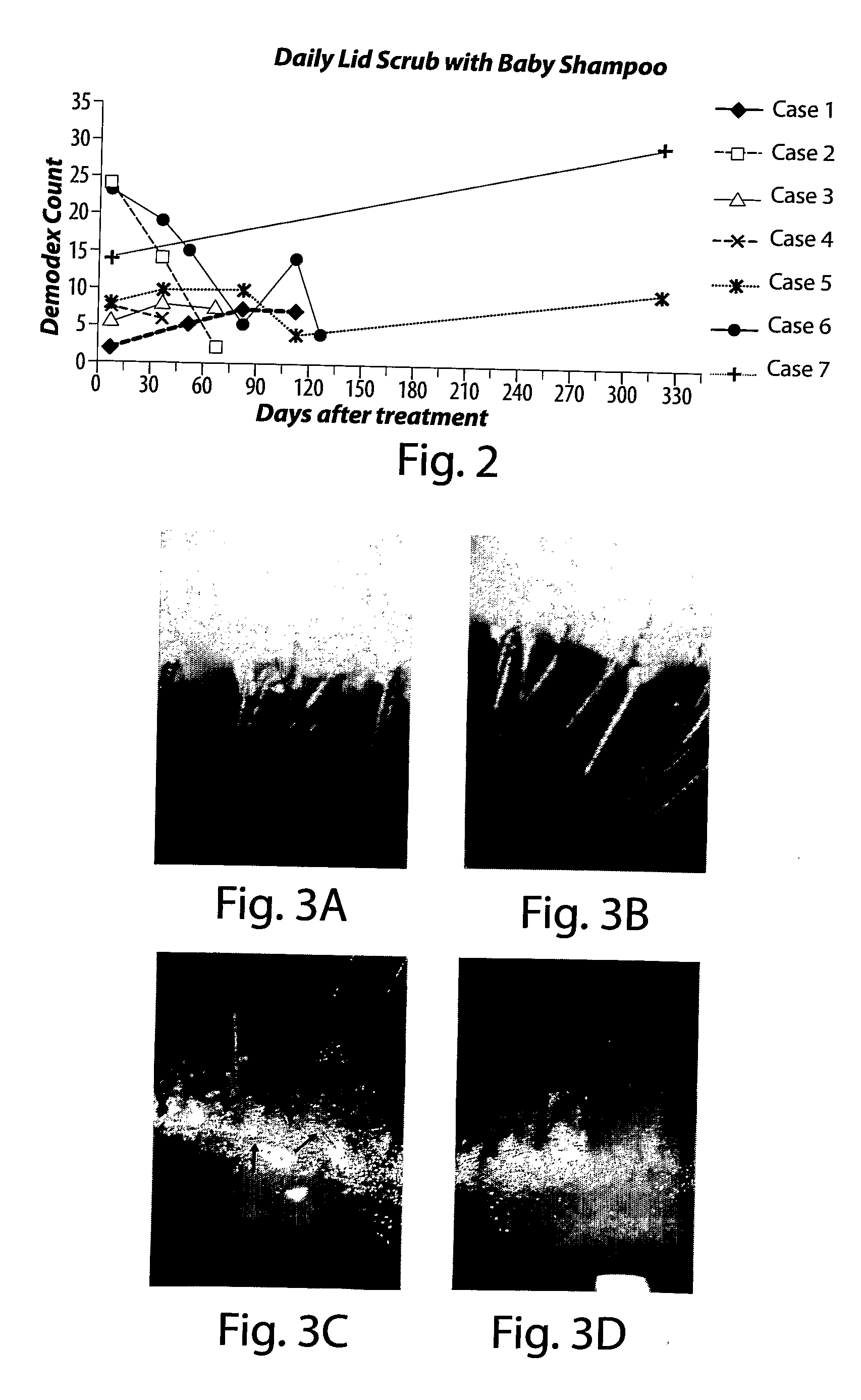
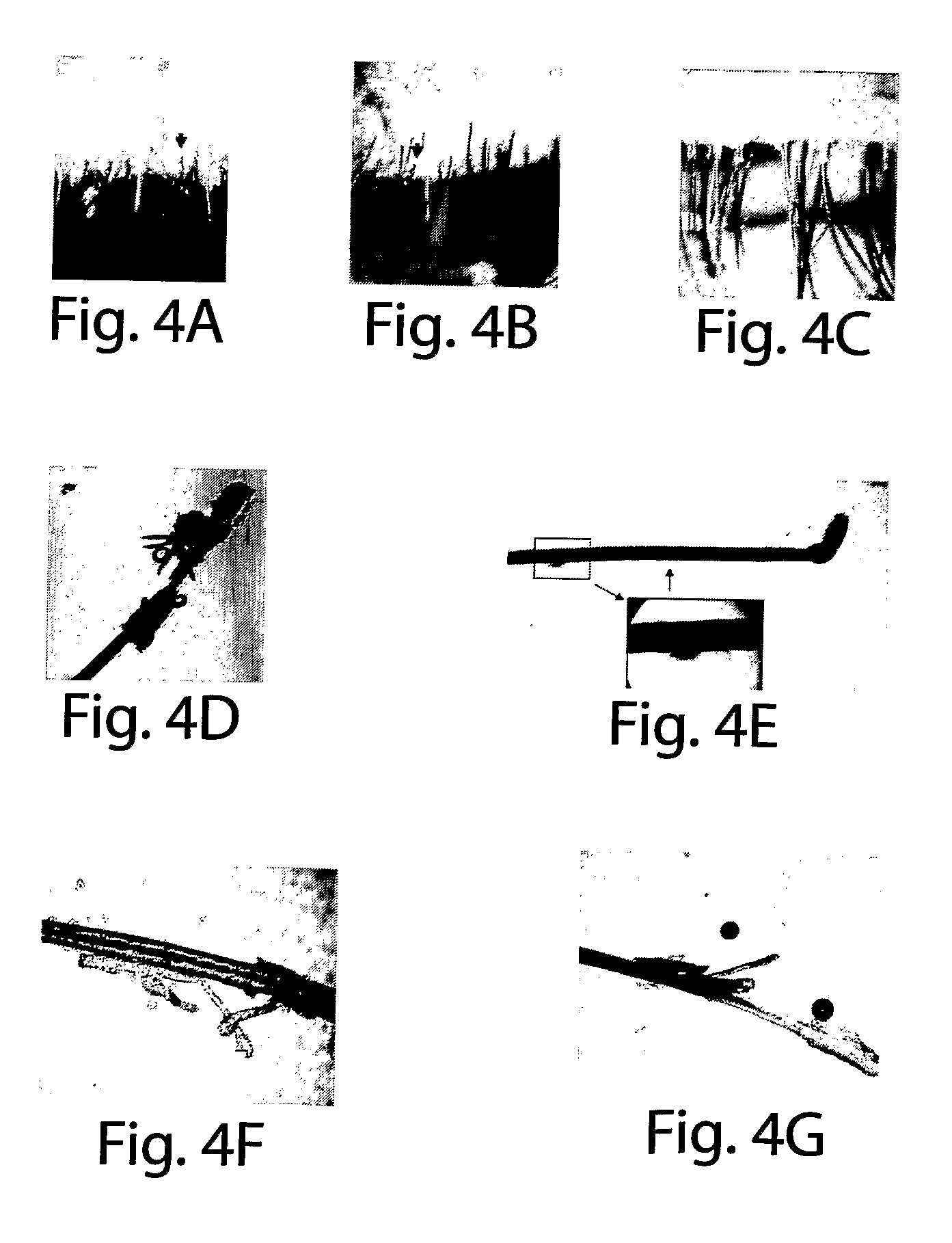
Method for treating ocular demodex
InactiveUS20090214676A1Equally distributedFacilitate eradicationBiocideSenses disorderDiseaseDemodex
The present invention relates to a method for treating a disorder chosen from ocular Demodex, Demodex-induced blepharitis, rosacea, acne, and meibomian gland dysfunction in a patient in need thereof, comprising administering to the patient a composition comprising a therapeutically effective amount of a substance chosen from at least one of an isoprenoidal essential oil such as Tea Tree Oil; Terpinen-4-ol; (+)-Carvone; alpha-Terpineol; Cardinene; d-Carvone; l-Carvone; gamma-Terpinene; alpha-Terpinene; 1,8-Cineole; alpha-Terpineol; para-Cimene; alpha-Pinene; Limonene; (R)-(+)-Limonene; alpha-Thugene; Eucalyptol; (+)-Ledene; Cuminic Aldehyde; and Myrcene; the administration comprising contacting or scrubbing an affected area of skin or hair, or eyelid margin and lashes of the patient with the composition; also disclosed are a method for treating mange and mite infestations on a mammalian animal; and kits for in-office and at home treatments of the disorders.
Owner:TISSUETECH INC
Liquid nutritional compositions containing unsaturated fatty acids
ActiveUS20070048405A1Improve oxidation stabilityAdd flavorFood ingredient as taste affecting agentFood preparationLipid formationTotal protein
Disclosed are liquid nutritional compositions comprising: carbohydrate; lipid having from about 0.1% to about 20% of an n-3 polyunsaturated fatty acid, n-6 polyunsaturated fatty acid, or combinations thereof by weight of the composition; a protein matrix having from about 15% to about 50% of a whey protein fraction by weight of the total protein in the composition; and a limonene-containing material, cranberry oil, or combinations thereof. The compositions are preferably aseptically packaged, and provide enhanced oxidative stability, flavor, and aroma, especially when formulated with relative high polyunsaturated fatty acid concentrations.
Owner:ABBOTT LAB INC
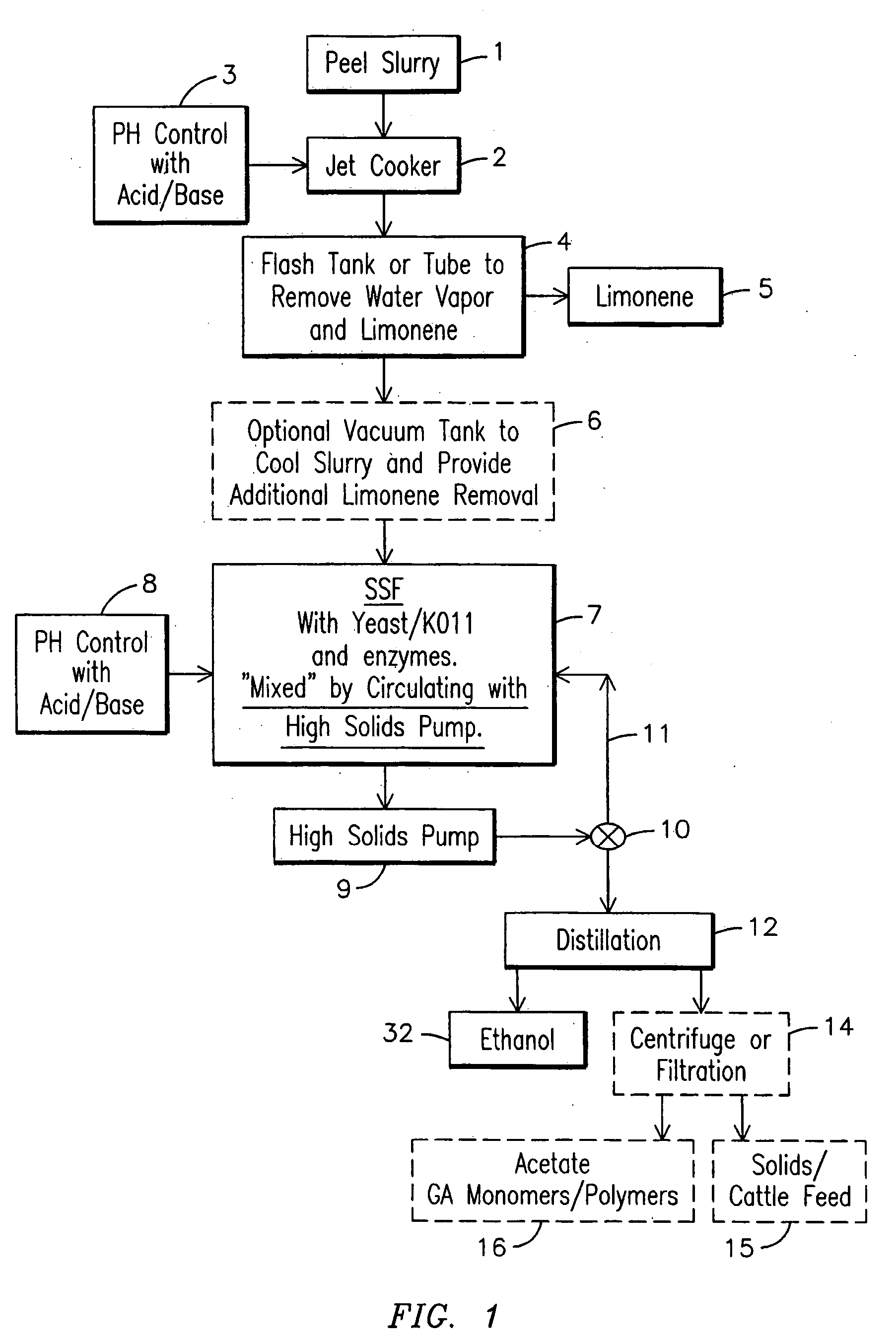
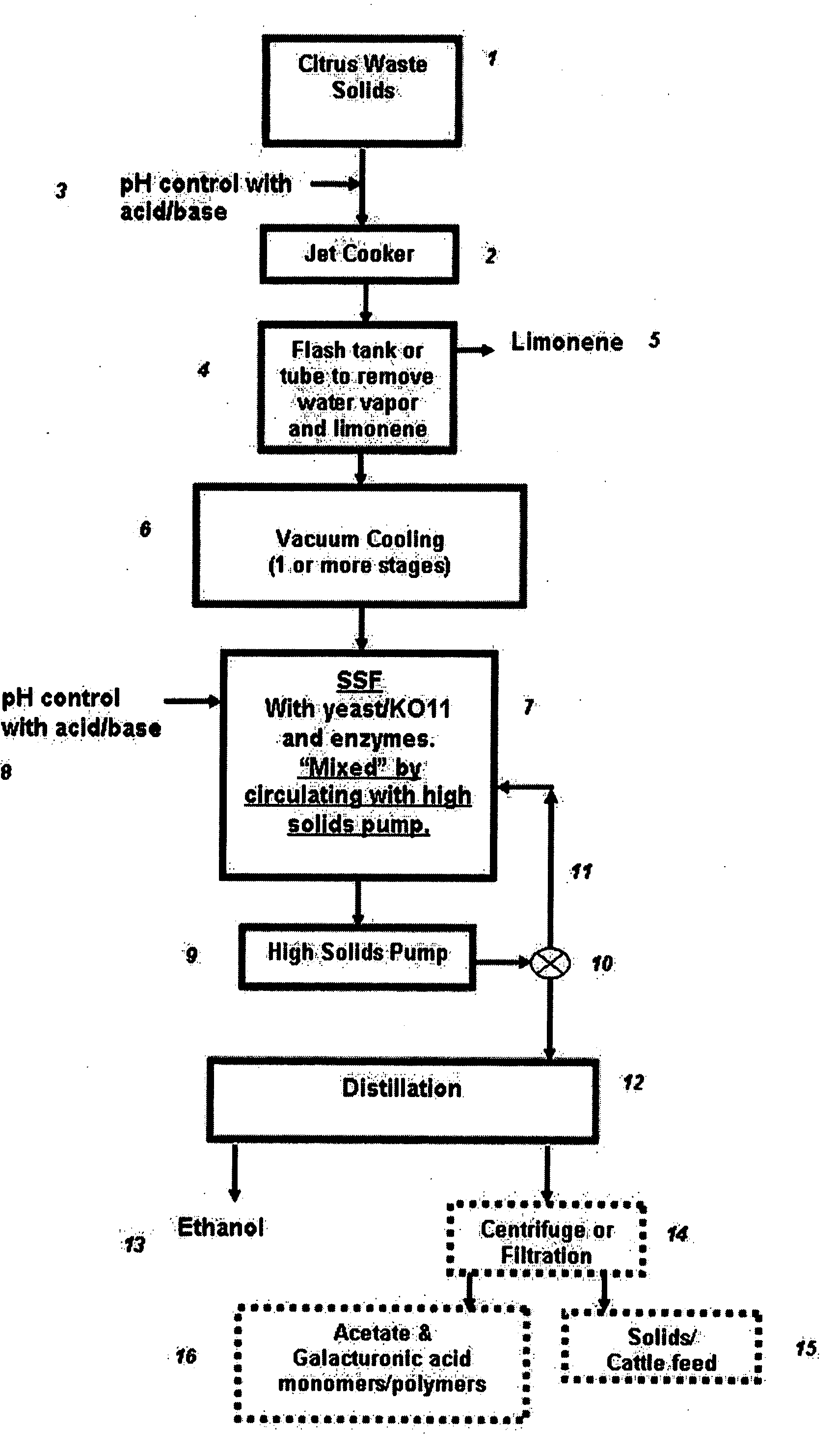
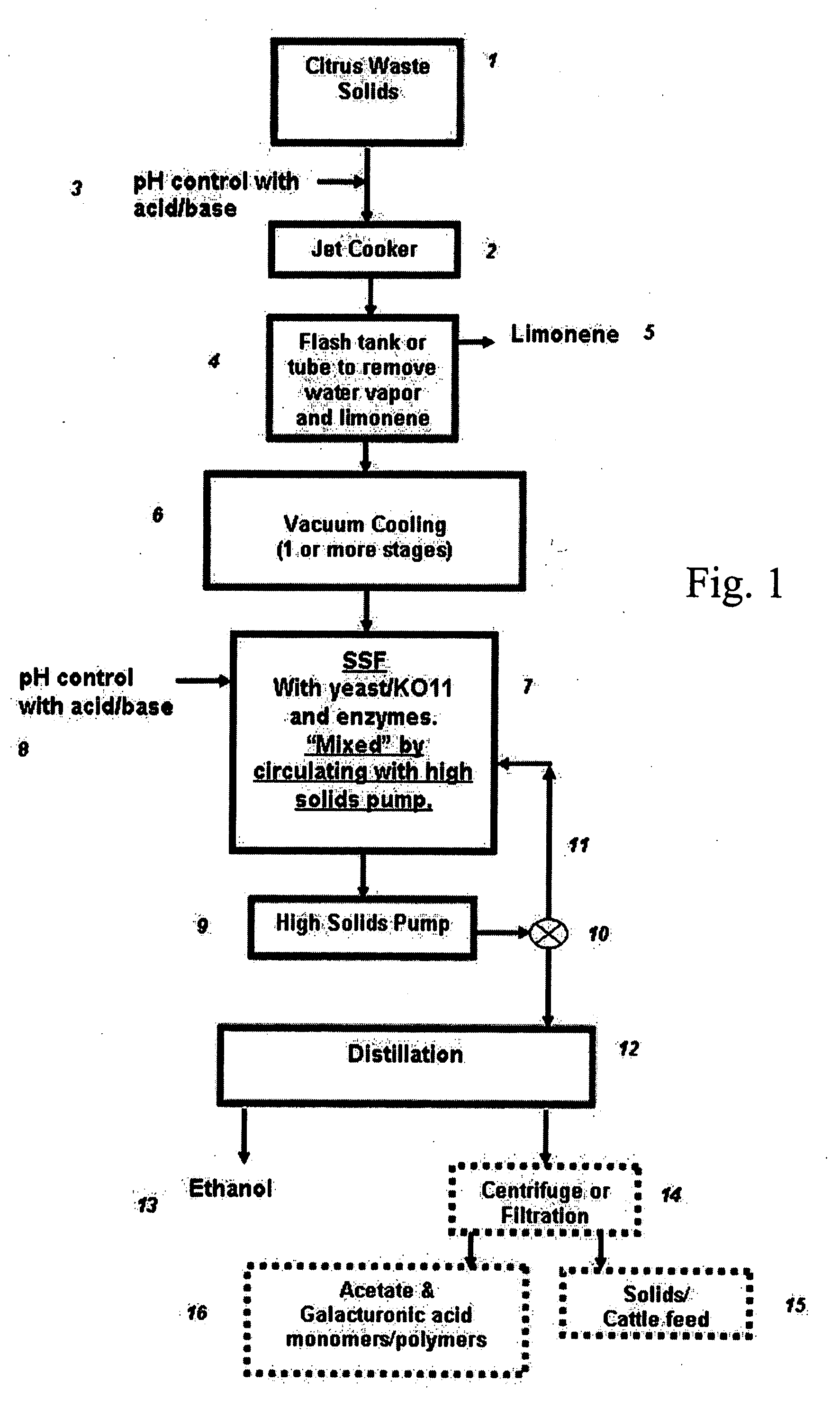
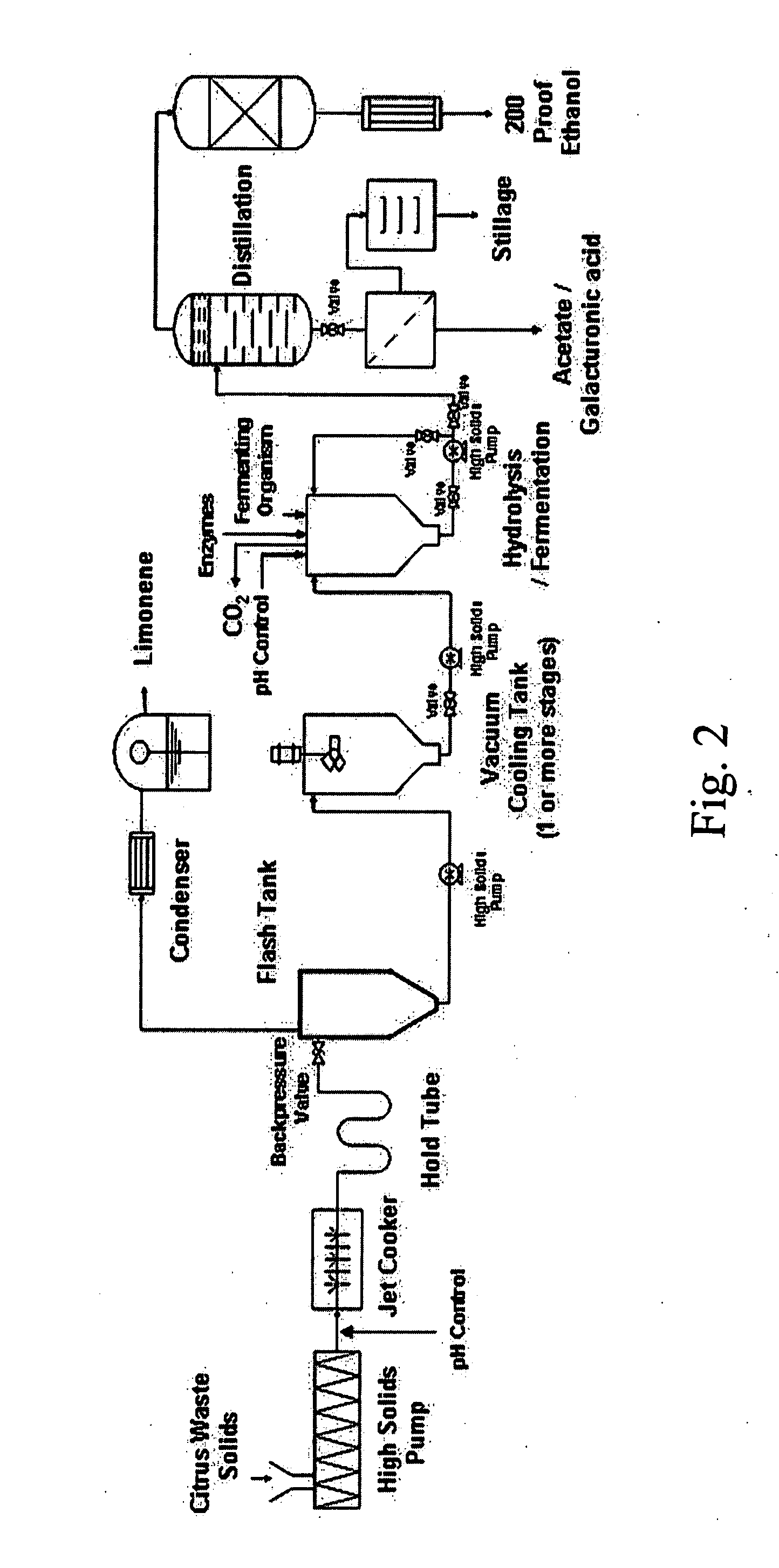
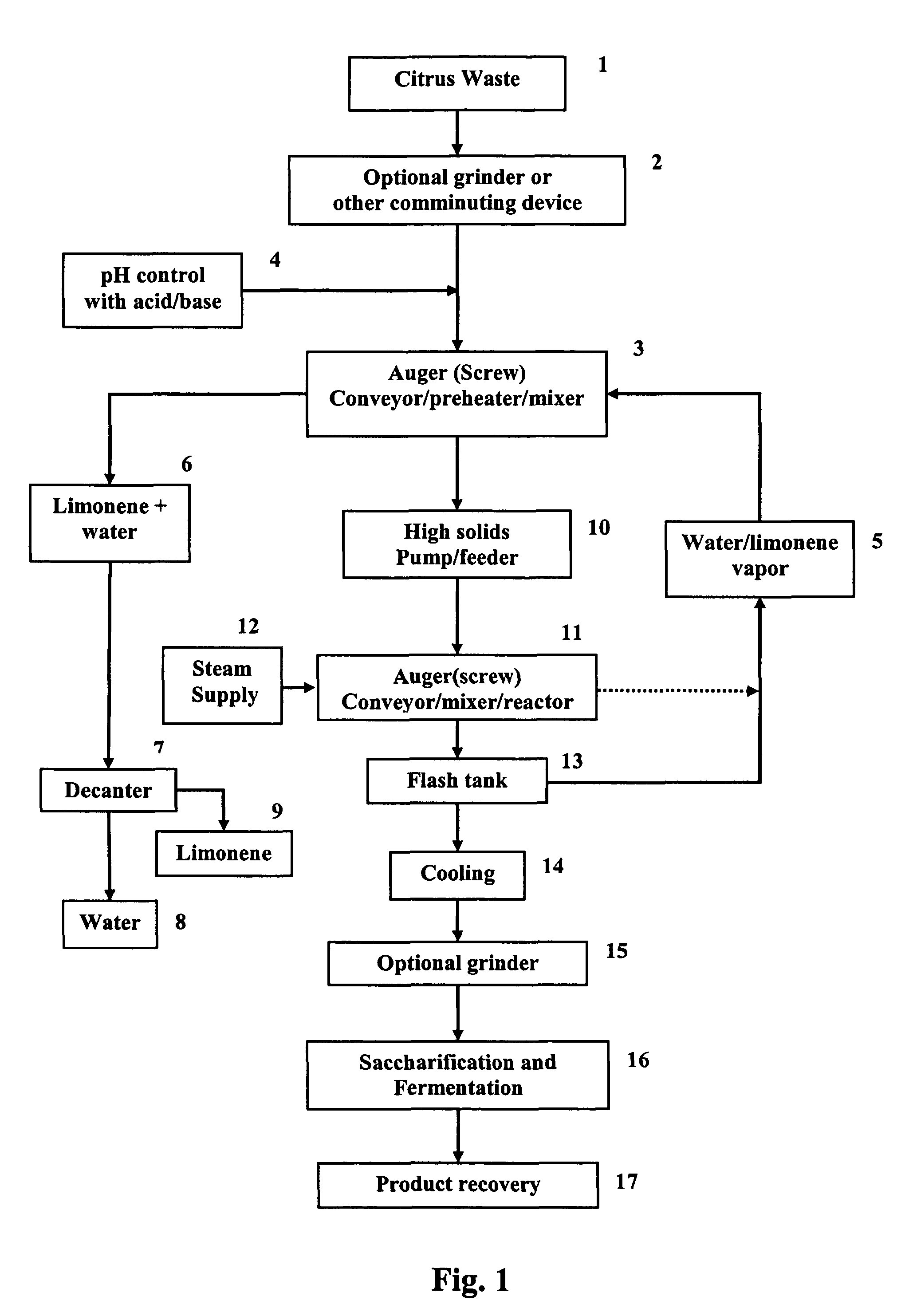
Ethanol production from citrus processing waste
InactiveUS20060177916A1Promote recoveryReduce pollutionFood processingBiofuelsSlurryAlternative methods
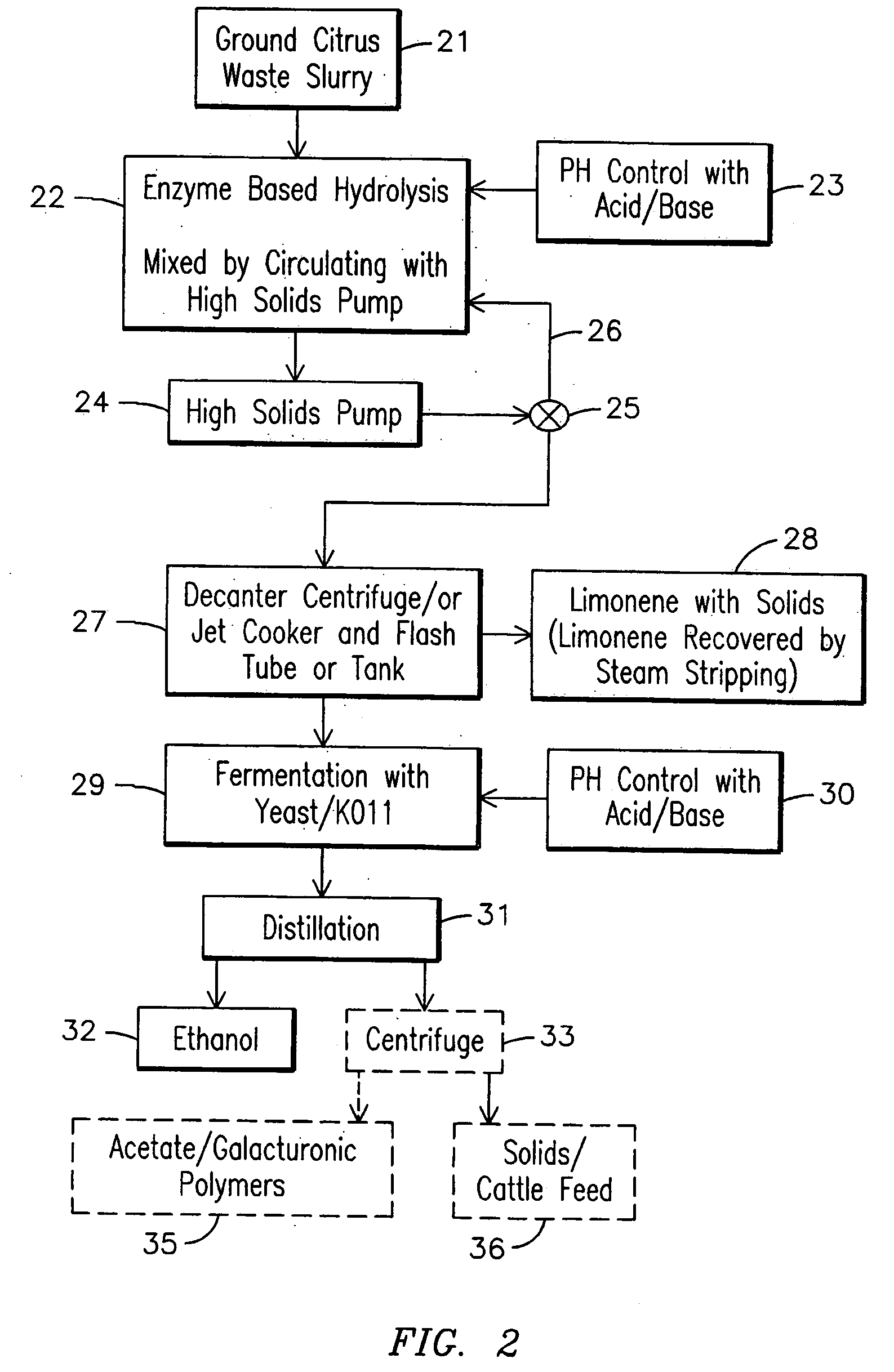
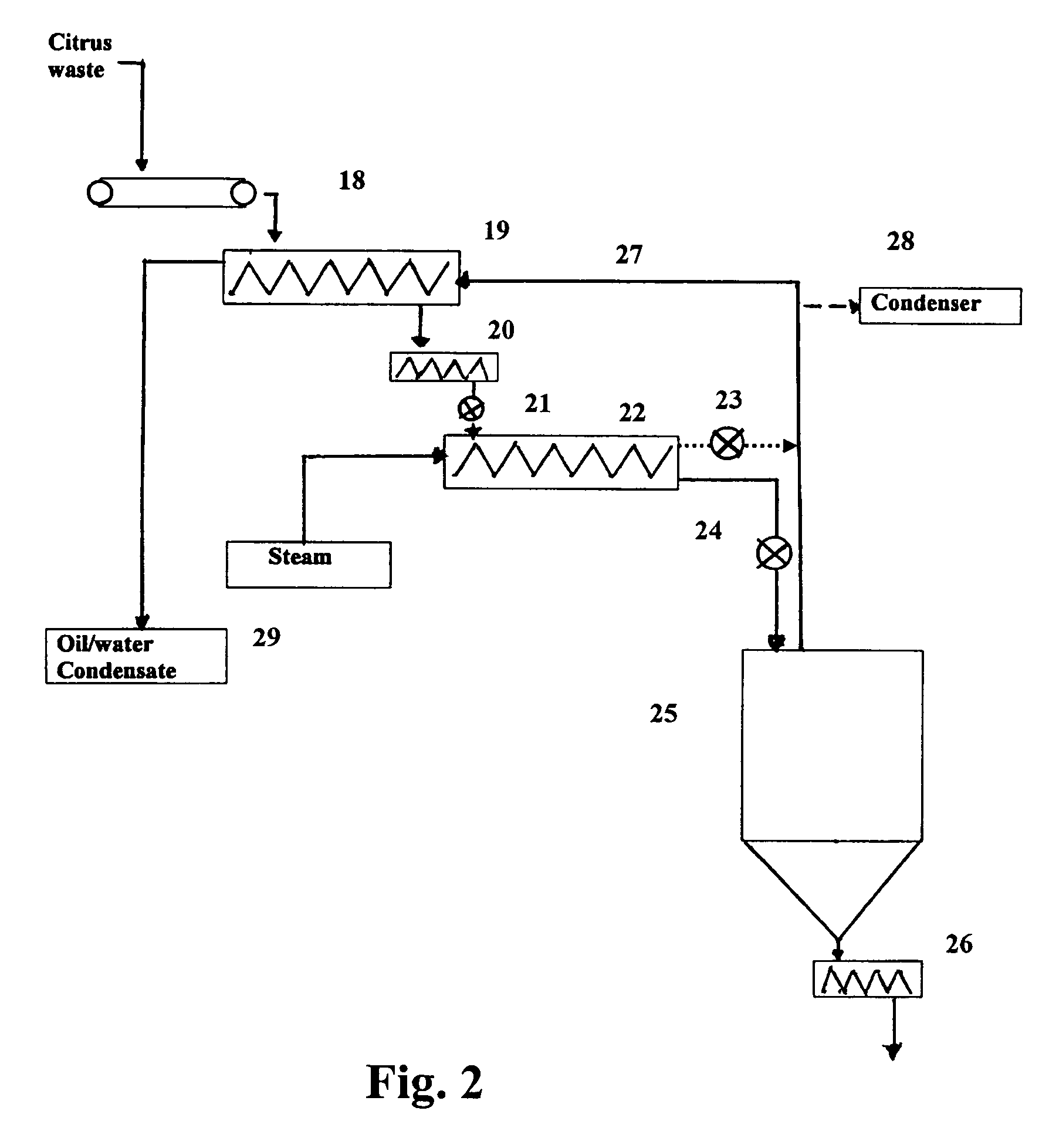
Processes for producing ethanol from citrus waste by reducing the concentration of limonene in citrus waste to allow fermentation are disclosed. In one embodiment a slurry of ground citrus waste 1 is partially hydrolyzed by heating using a jet cooker 2 and then injected into a flash tank 4 to remove limonene 5. The heated citrus waste is then cooled, hydrolyzed with enzymes and fermented to ethanol. An alternative method of limonene removal uses enzymatic hydrolysis followed by centrifugation 27 to separate sugar-containing liquid from residual citrus waste solids containing limonene. Sugars are fermented and ethanol is distilled from the fermented mixture / beer. The remaining solids and liquids may be processed further to yield other byproducts. More particularly, the solids may be dried and pressed for use in cattle feed and the liquids may be further fermented or processed to yield additional ethanol, acetate, galacturonic acid monomers and polymers, five carbon sugars and other products.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE +1
Coccidiosis and clostridial disease prophylactic and/or therapeutic feed for coccidiosis and clostridial disease
InactiveUS20080160000A1Decrease in breeding yieldDeterioration in productivity caused by coccidiosis or clostridial disease can be preventedAntibacterial agentsBiocideDiseaseTreatment effect
There is provided an animal feed, an anticoccidial agent, and an anticlostridial agent having less harm and an excellent prophylactic and / or therapeutic effect for coccidiosis and clostridial disease. Also provided is a prophylactic and / or therapeutic feed for coccidiosis containing pinene, thymol, eugenol, and limonene; an anticoccidial agent containing pinene, thymol, eugenol, and limonene as active ingredients; a clostridial disease prophylactic and / or therapeutic feed containing pinene, thymol, eugenol, and limonene; and an anticlostridial agent containing pinene, thymol, eugenol, and limonene as active ingredients.
Owner:MARUBENI NISSHIN FEED CO LTD
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Plasma display panel manufacturing method for manufacturing a plasma display panel with superior picture quality, a manufacturing apparatus and a phosphor ink
InactiveUS6547617B1Improve liquidityStrong force FVessels or leading-in conductors manufactureLuminescent coatings applicationManufactured apparatusEngineering
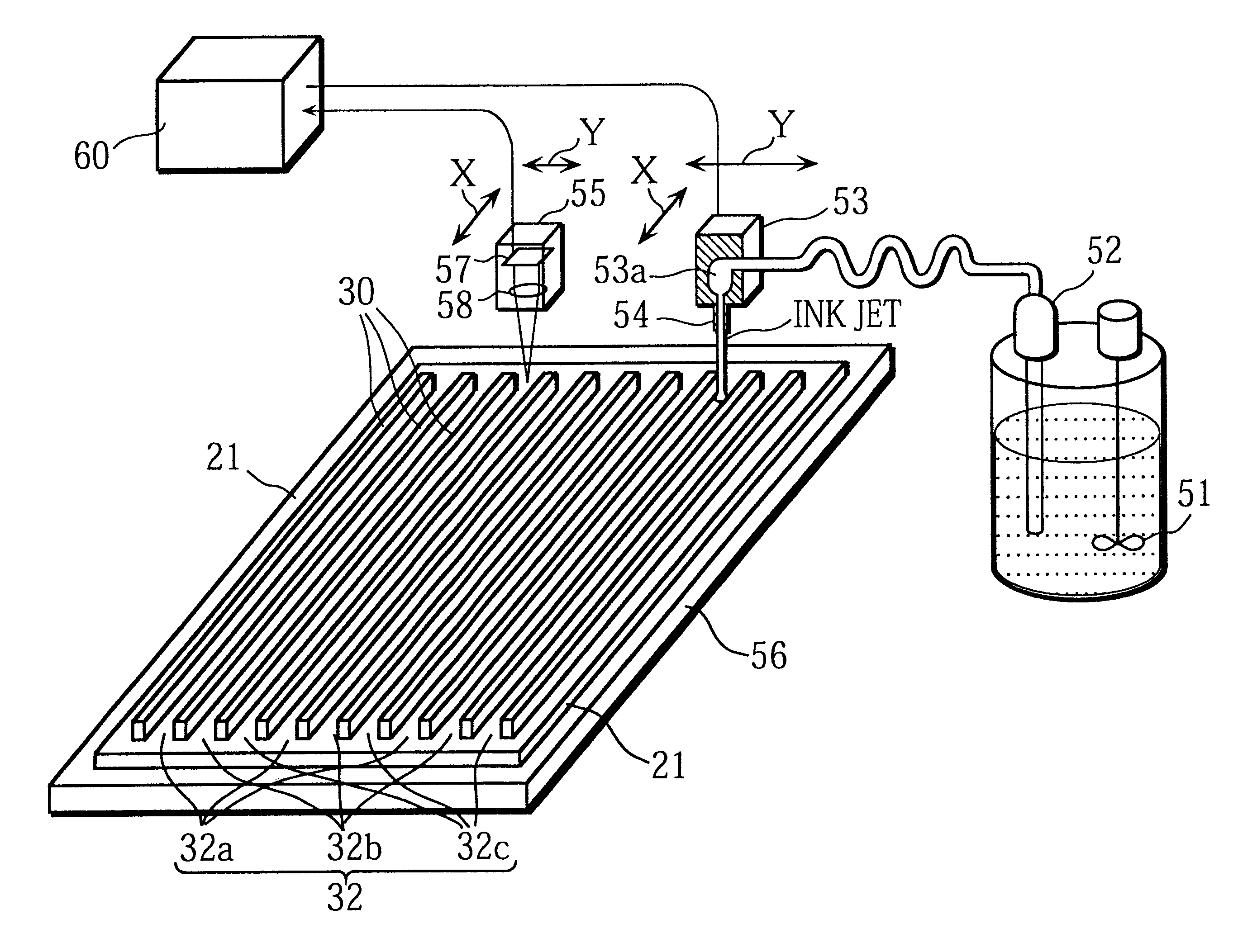
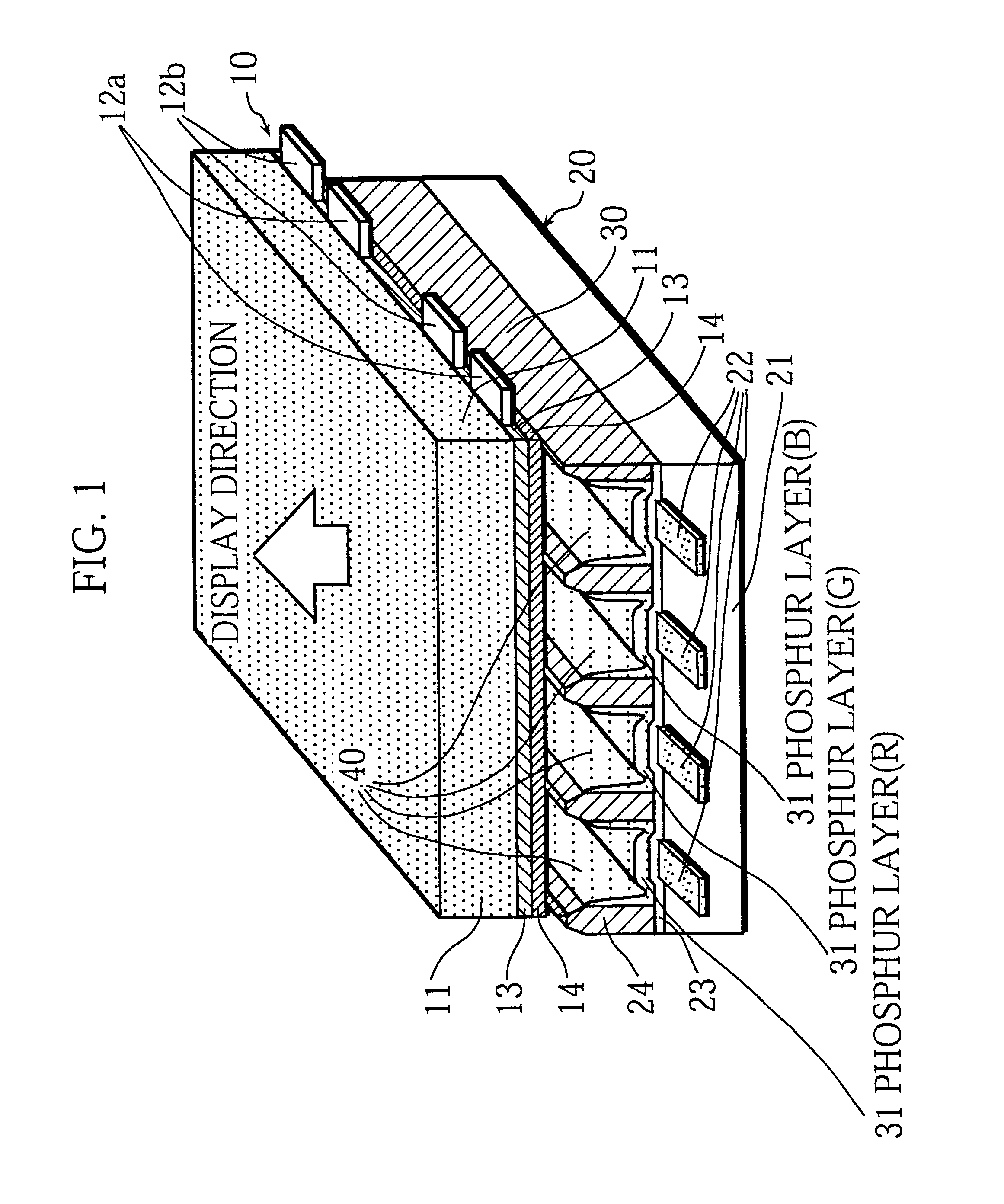
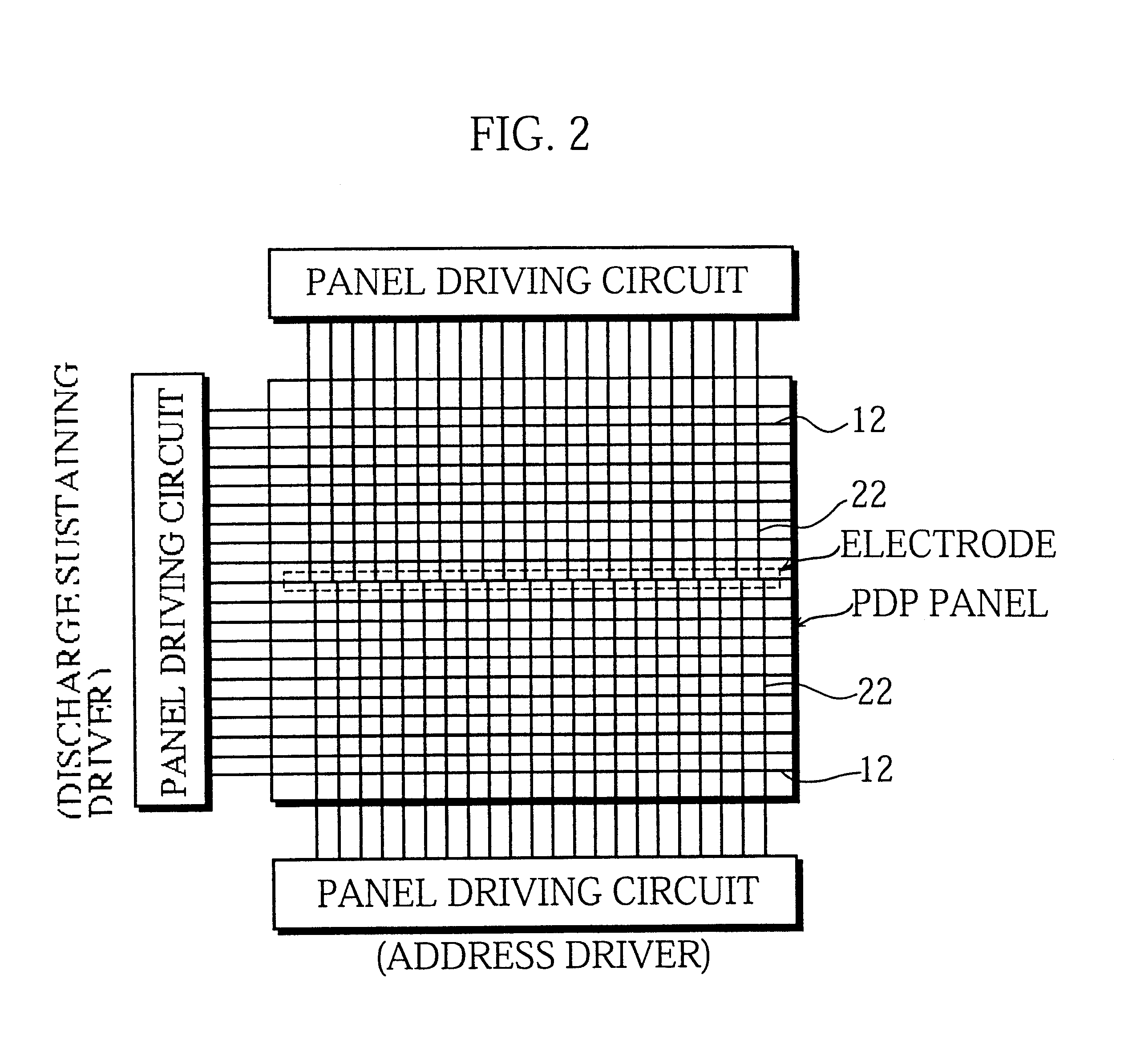
The present invention intends to provide a manufacturing method for a PDP that can continuously apply phosphor ink for a long time and can accurately and evenly produce phosphor layers even when the cell construction is very fine. To do so, phosphor ink is continuously expelled from a nozzle while the nozzle moves relative to channels between partition walls formed on a plate so as to scan and apply phosphor ink to the channels. While doing so the path taken by the nozzle within each channel between a pair of partition walls is adjusted based on position information for the channel. When phosphor particles is successively applied to a plurality of channels, phosphor ink is continuously expelled from the nozzle even when the nozzle is positioned away from the channels. The phosphor ink is composed of: phosphor particles that have an average particle diameter of 0.5 to 5 mum; a mixed solvent in which materials selected from a group consisting of terpineol, butyl carbitol acetate, butyl carbitol, pentandiol, and limonene are mixed; and a binder that is an ethylene group polymer or ethyl cellulose containing at least 49% of ethoxy group (-OC2H5) cellulose molecules. After dispersion a charge-removing material is added to the phosphor ink.
Owner:PANASONIC CORP
Heat Generation Process for Treating Oilfield Deposits
ActiveUS20110114323A1Increase temperatureIncreased deposit solvencyCleaning apparatusHollow article cleaningOil processingFuran
Generating heat within a combination solvent / acid system removes undesirable deposits from petroleum reservoir formations (especially the near well-bore region), oilfield equipment, and petroleum processing equipment. An exothermic reaction occurs between the solvent and the acid and the heat evolved helps remove organic solid deposits. The acids may include organic acid compounds, such as sulfonic acids, sulfuric acid and nitric acid. The solvents may include terpene- and terpene-derivative-containing solvents, including, but not necessarily limited to, limonene, pinene, dipentene, myrcene, turpentines and compounds having at least one double bond, such as methyl furan, dienes, styrene, vinyl acetate and the like. The exothermic reaction produces a great amount of heat, and together with using certain acids and solvents already known as effective to remove paraffin and asphaltene deposition, removing such deposits is improved.
Owner:BAKER HUGHES INC
Device and process for the recovery of increased volumes of pure terpenes and terpenoids from scrap polymers and elastomers
Owner:BEAVER EARL R +1
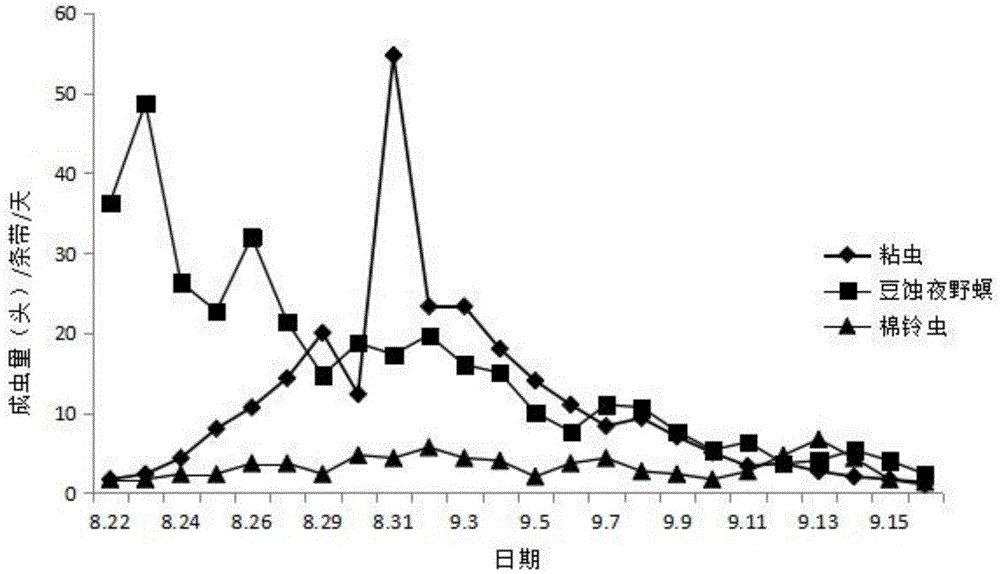
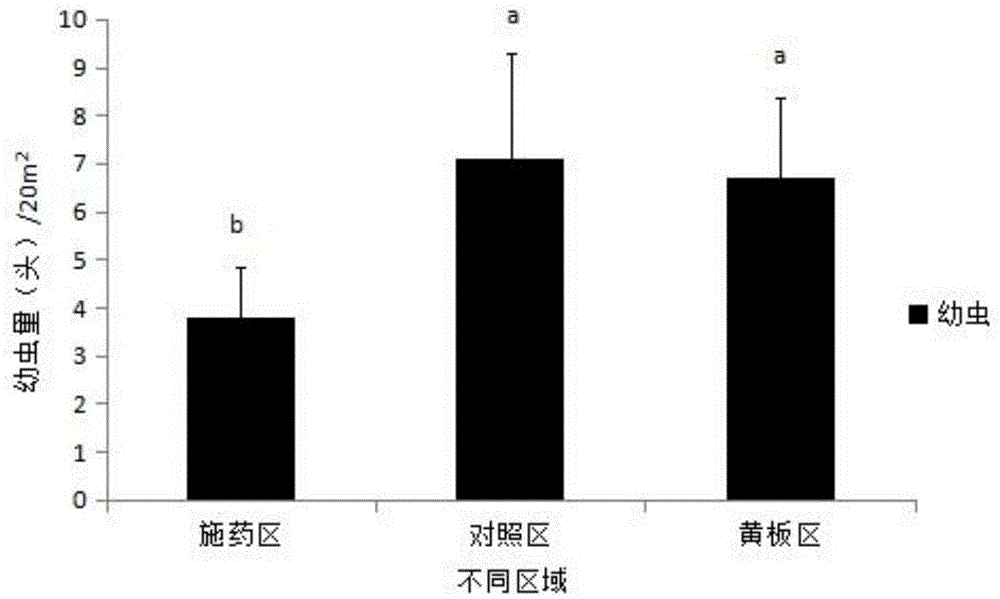
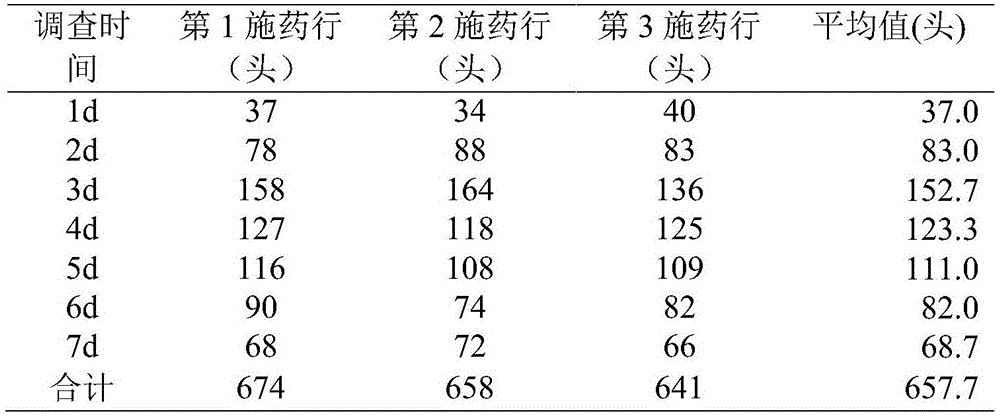
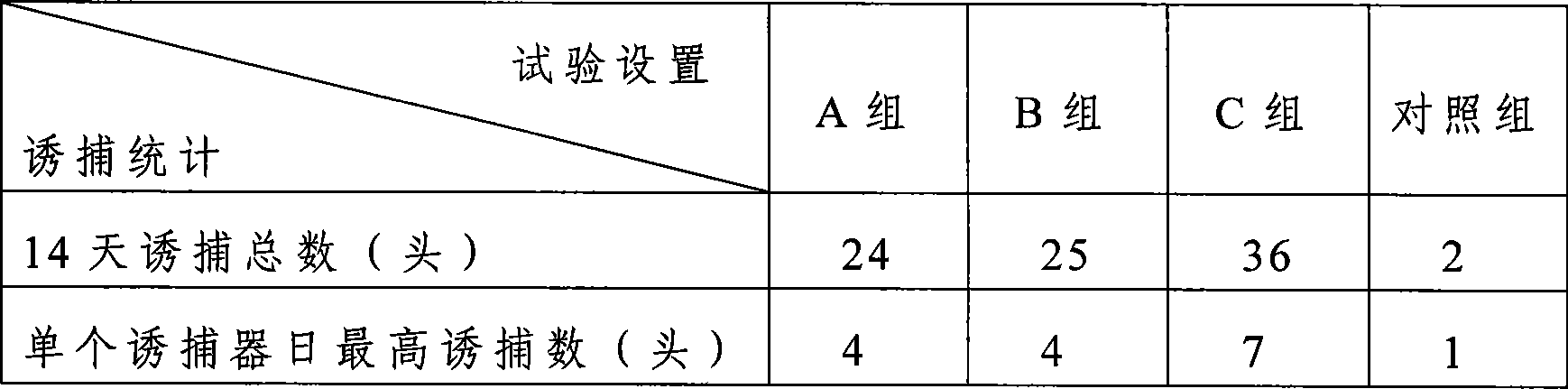
Noctuidae pest attractant and preparation method and application thereof
InactiveCN105613495AStrong trapping effectGood control effectBiocidePest attractantsO-methoxybenzoic acidPlusia agnata
The invention discloses a noctuidae pest attractant. The noctuidae pest attractant comprises, by weight, 0.5-5 parts of phenylacetaldehyde, 0.5-5 parts of methyl o-anisate, 0.5-5 parts of methyl salicylate, 0.5-5 parts of cis-hexenyl acetate, 0.5-5 parts of limonene, 0.5-20 parts of feed attractant, 10-90 parts of slow-release carrier and 30-80 parts of water. The invention further discloses a preparation method and application of the attractant. The attractant prepared by the method is stable in performance, long in efficacy lasting time, wide in pest attracting spectrum and capable of attracting female and male imagoes. The attractant prepared by the method is a wide-spectrum attractant, has good attracting effect on various noctuidae pests like armyworm, Spodoptera litura, Spodoptera exigua, cotton bollworm, Helicoverpa assulta, cutworm and Plusia agnata and can attract and Lamprosema indicata fabricius and several Coleopterous pests. The noctuidae pest attractant can effectively attract and kill noctuidae pests when cooperating with a tiny amount of stomach-toxicity pesticides. The noctuidae pest attractant can be directly applied on crops and can be used with the help of various traps.
Owner:SHENZHEN BIOGLOBAL AGRI SCI +1
Limonene, pinene, or other terpenes and their alcohols, aldehydes and ketones, as polymer solvents for conducting polymers in aqueous and non-aqueous coating formulations and their uses
A low-VOC (volatile organic compound) and / or low-toxicity coating formulation, including at least one non-halogenated solvent including terpene(s) or terpenoid(s), and at least one polymer including conducting polymers, electroactive polymers and / or conjugated polymers, wherein the polymers and non-halogenated solvent(s) are in non-aqueous form. In other embodiments, coating formulations, includes about 0.01% wt. to about 99.9% wt. of at least one non-halogenated solvent including a terpene or terpenoid, about 0.01% wt. to about 90% wt. of at least one polymer including conducting polymers, conjugated polymers, and electroactive polymers, and about 0.001% wt to about 90% wt. of at least one surfactant, wherein the polymers, solvents, and surfactants are in non-aqueous form. Also included are aqueous low VOC and / or toxicity coating formulations having at least one non-halogenated solvent including terpene(s) or terpenoid(s), and at least one conjugated, electroactive, or conductive polymer, copolymer, block polymer, and mixtures thereof.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
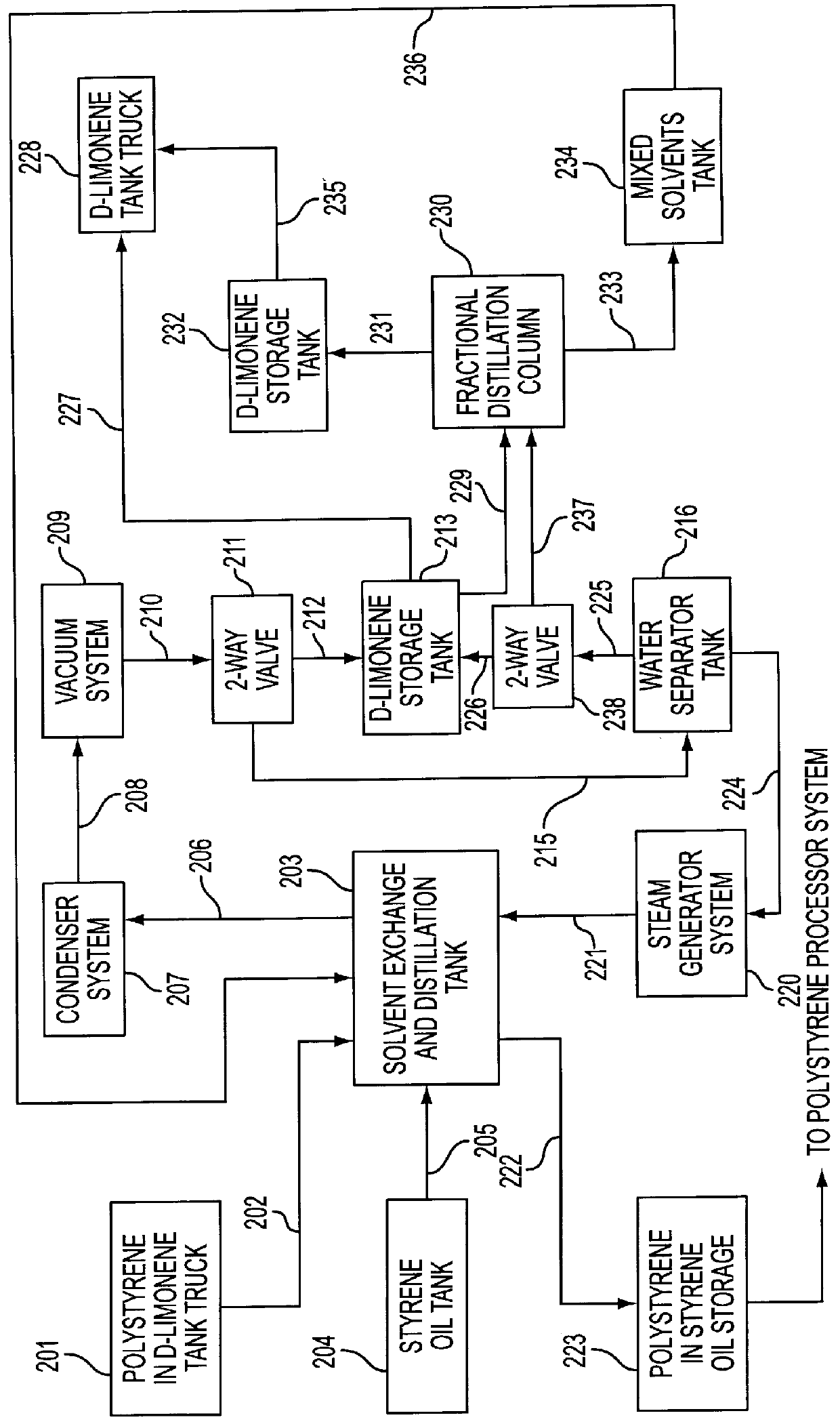
Alternative solvents for a method of reclaiming styrene and other products from polystyrene based materials
A method of pretreating polystyrene-containing materials to form a solution of polystyrene in a processing solvent from which the styrene in the polystyrene in the materials is reclaimed. The materials are mixed with an environmentally acceptable pretreating solvent having a lower boiling point than the processing solvent, typically at a location remote from the reclamation plant. The pretreating solvent is selected from the group consisting of d-limonene, l-limonene, dipentene, and blends thereof. Prior to actual processing to reclaim styrene, the pretreating solvent is substantially replaced with the processing solvent. The pretreating solvent may be recovered for reuse.
Owner:PONSFORD THOMAS E +1
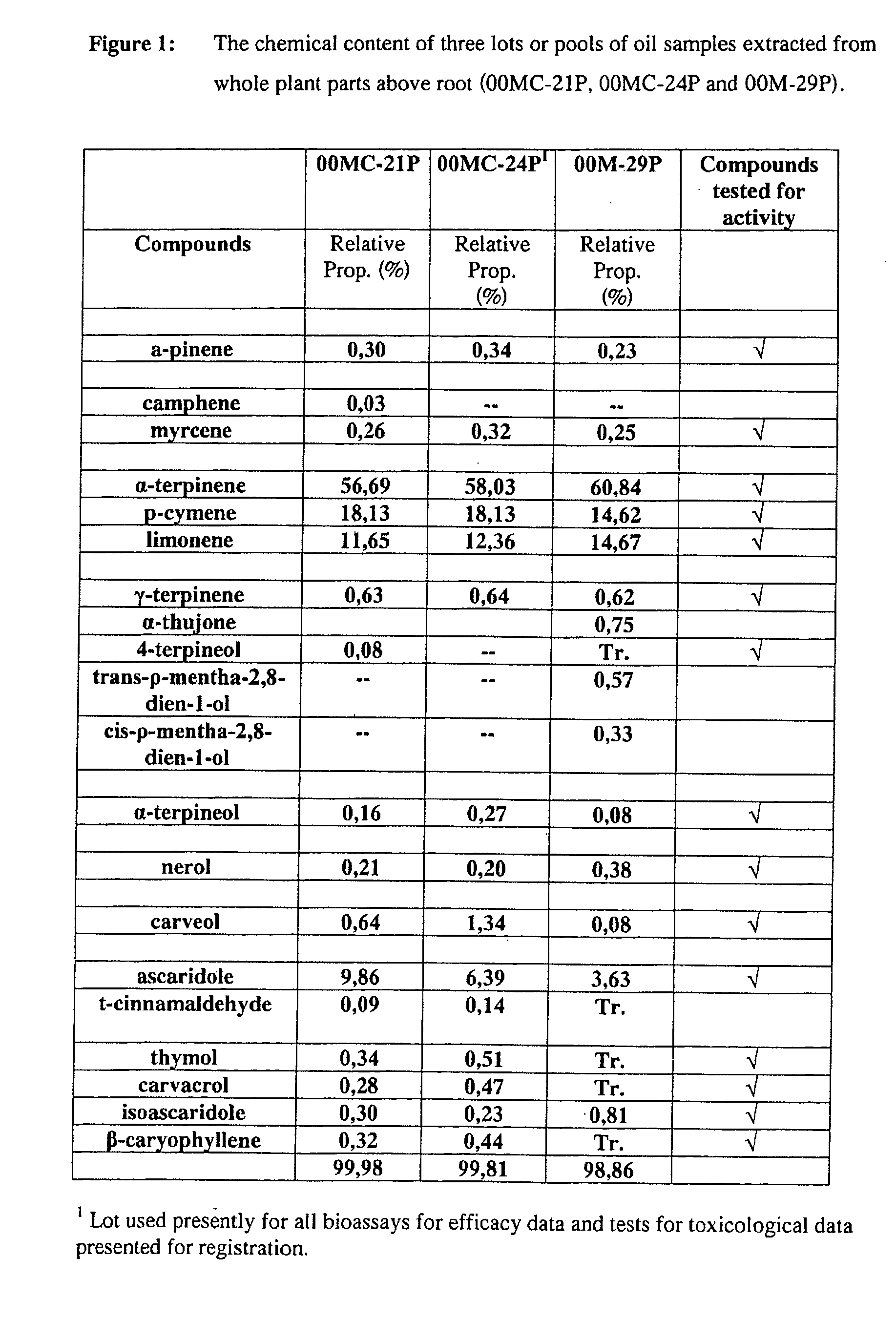
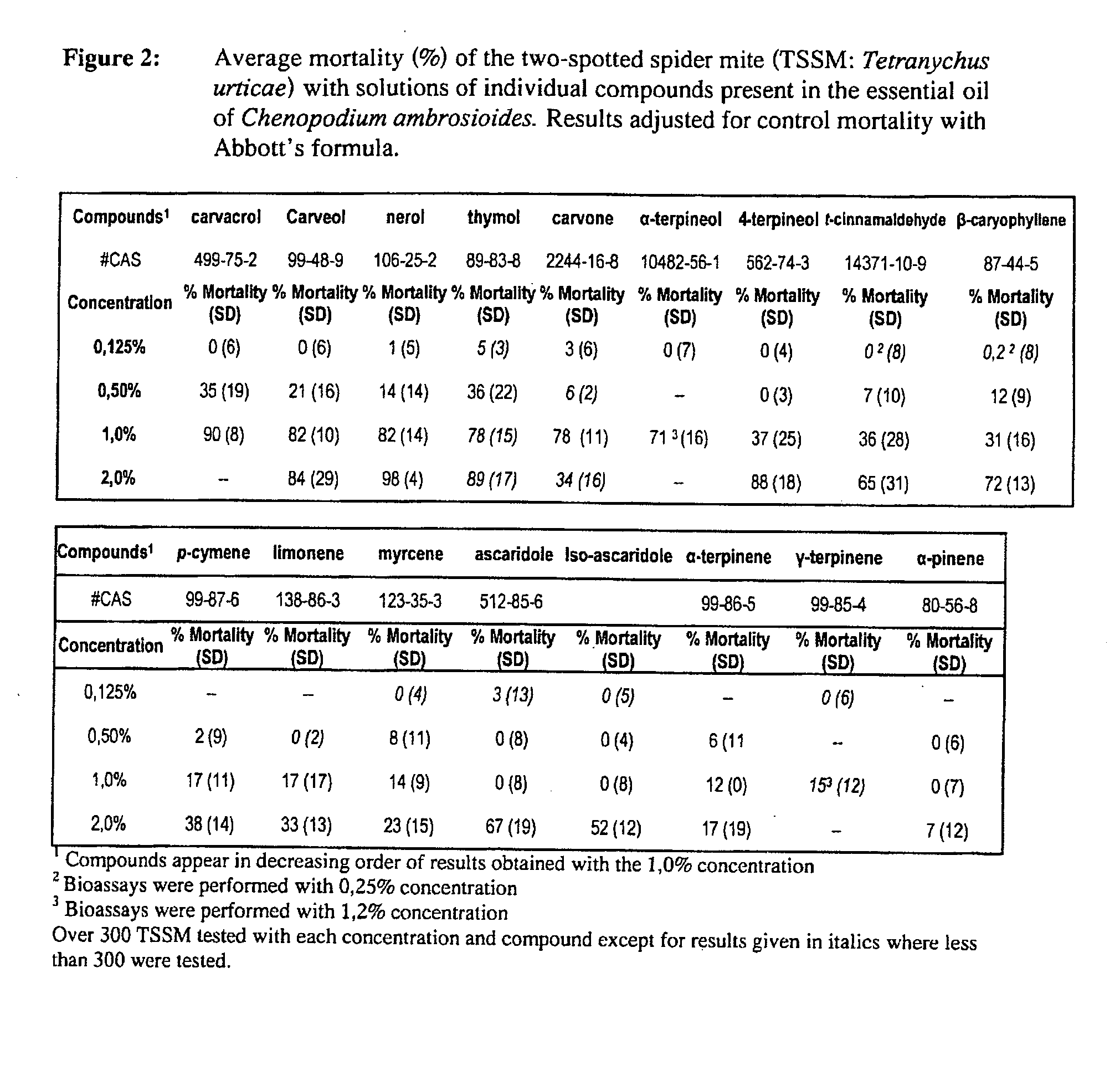
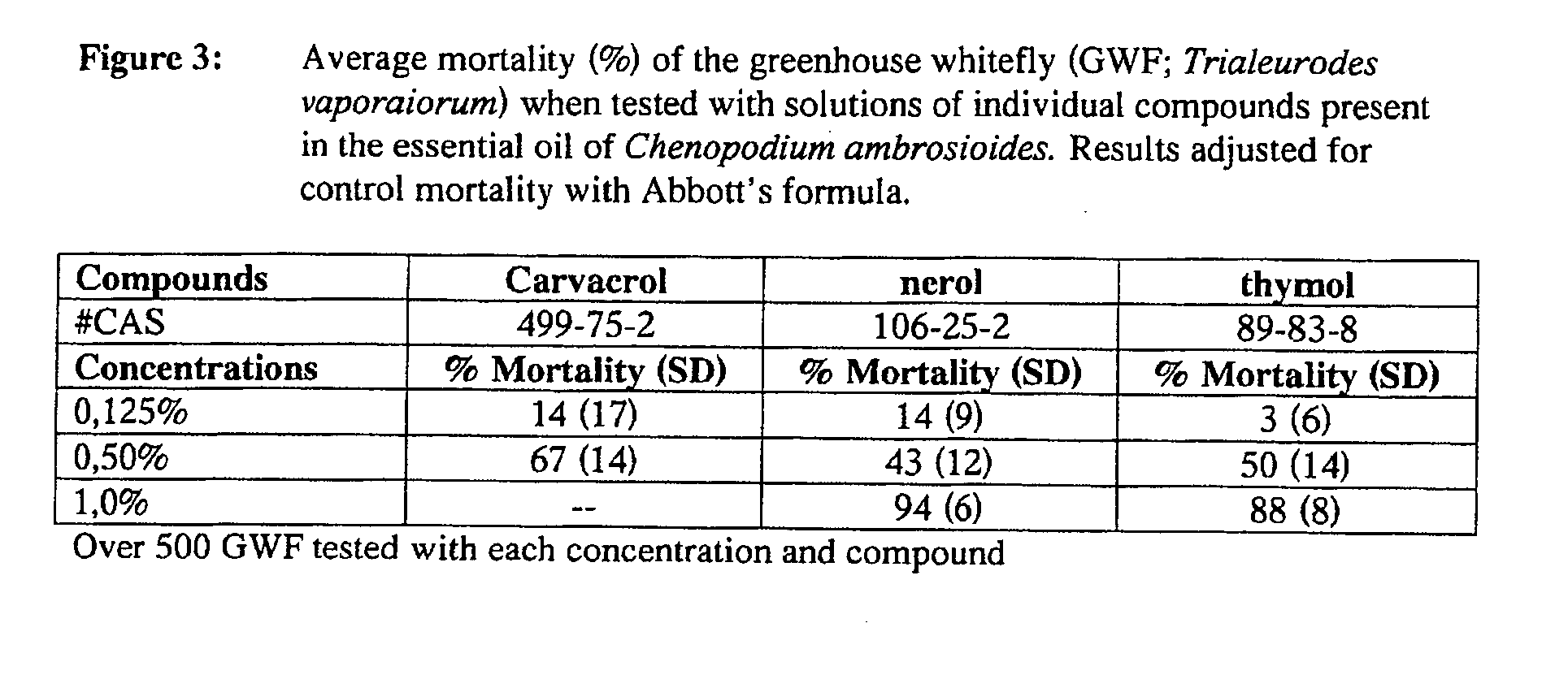
Extracts derived from chenopodium plants and uses thereof
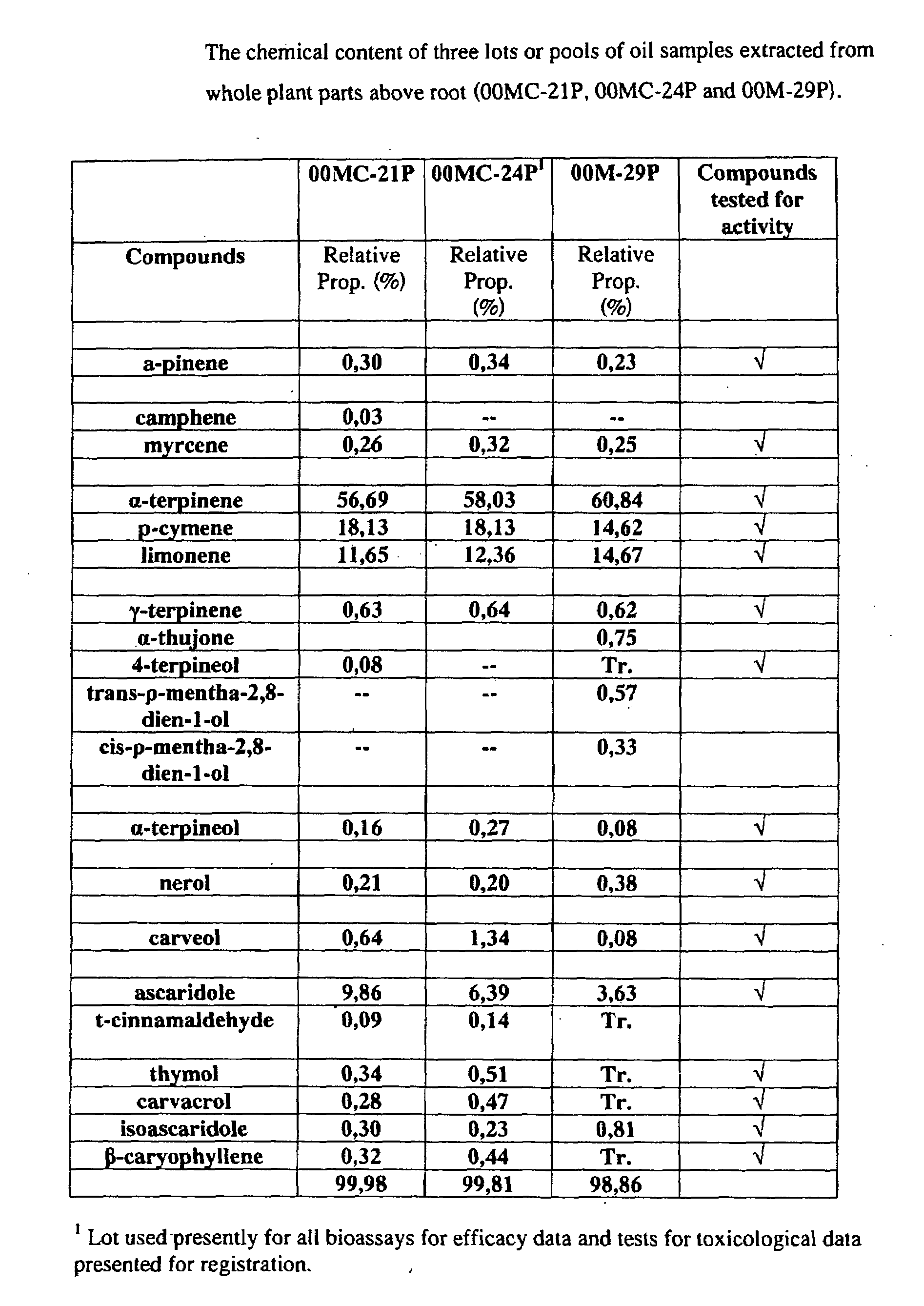
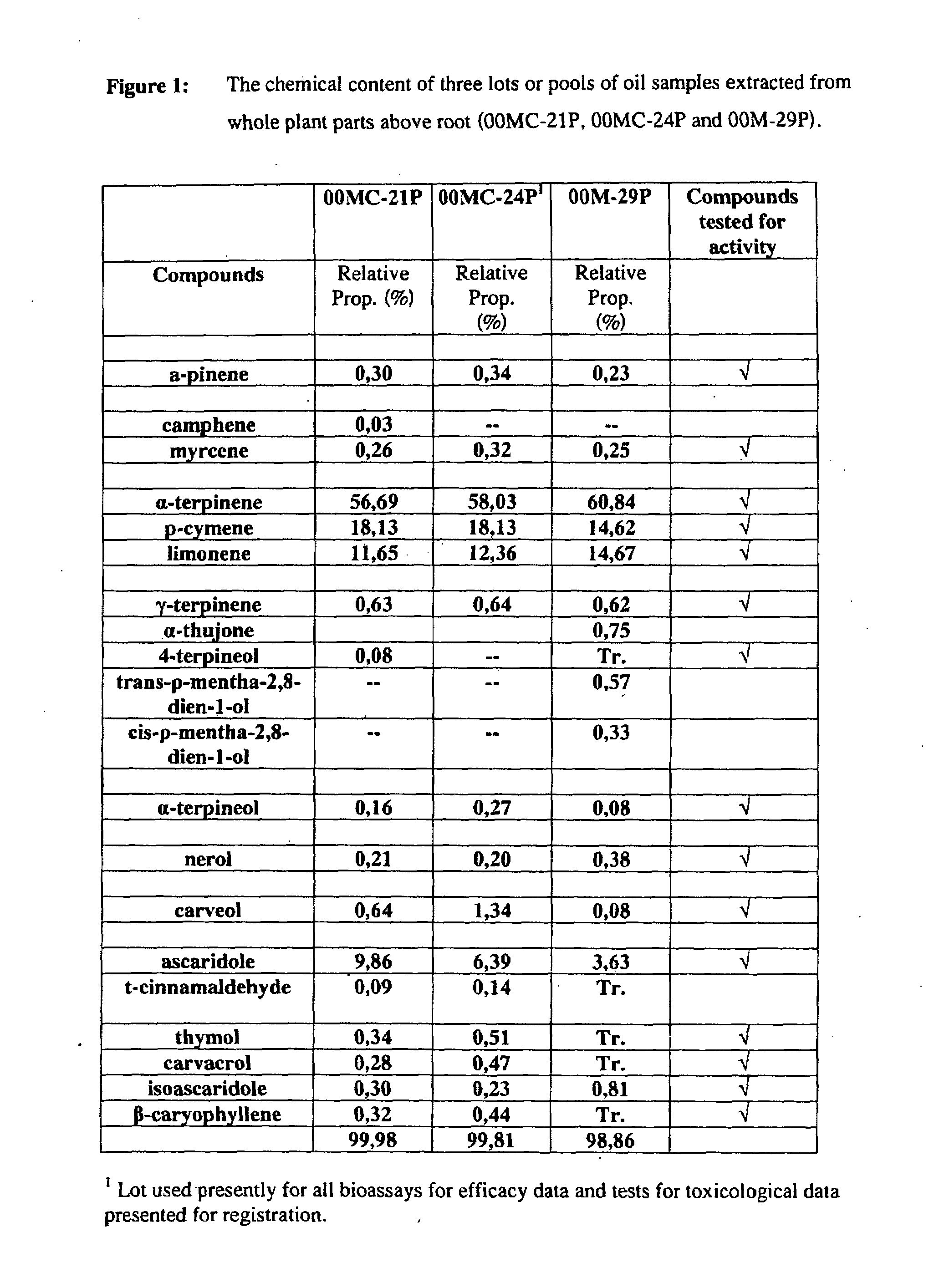
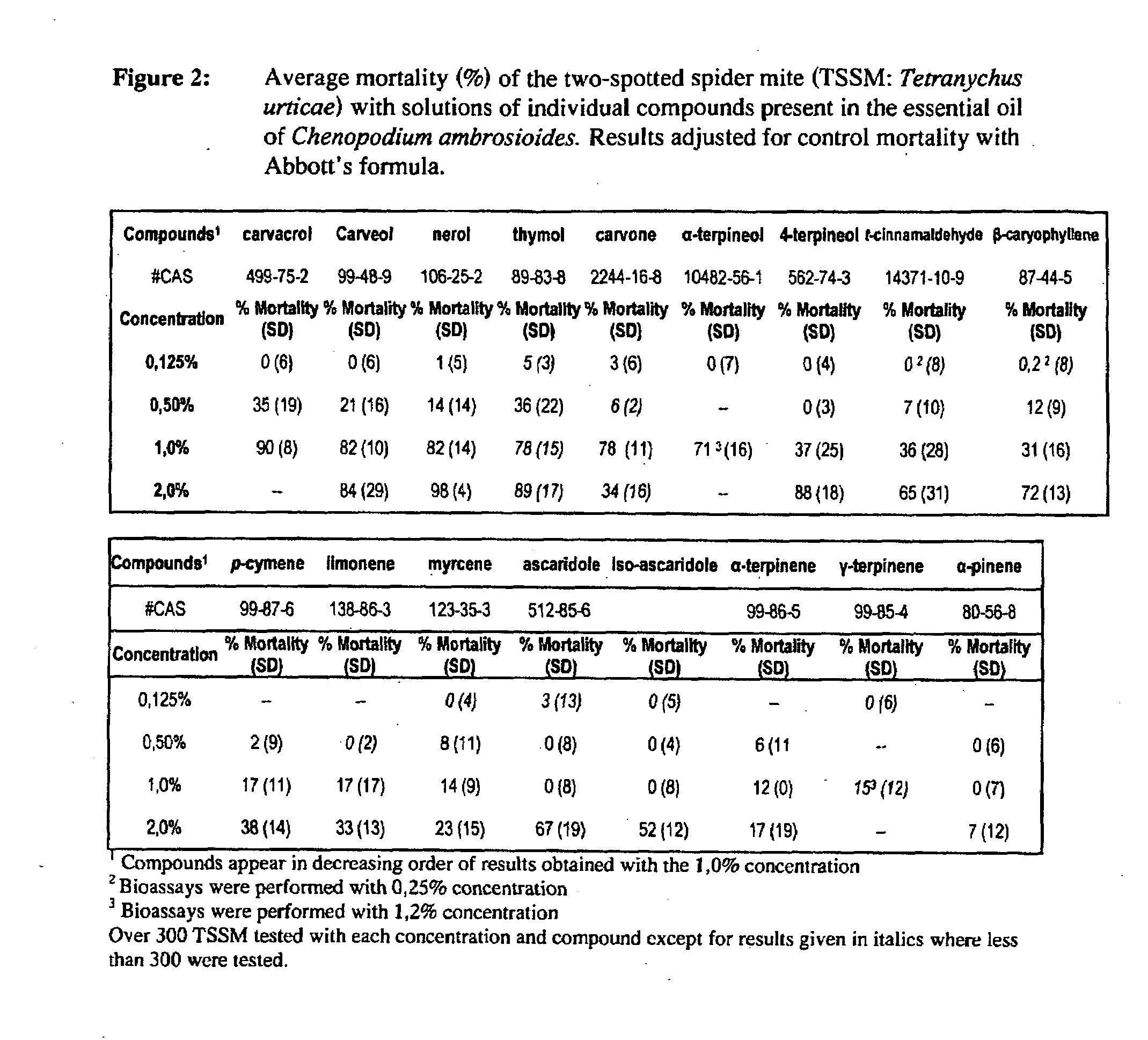
The present invention relates to pesticides. More particularly, the present invention relates to botanical pesticides. In particular, the present invention relates to compositions and methods for controlling plant-infesting pests with plant extracts and notably with compositions comprising oil extracts derived from Chenopodium sp. plant material. The invention further relates to compositions comprising such extracts as pesticidal compositions and providing the advantages of minimal development of resistance thereto, minimal toxicity to mammals, minimal residual activity and environmental compatibility. The pesticidal compositions of the present invention comprises α-terpinene, ρ-cymene, limonene, carvacrol, carveol, nerol, thymol, and carvone.
Owner:CHIASSON HELENE
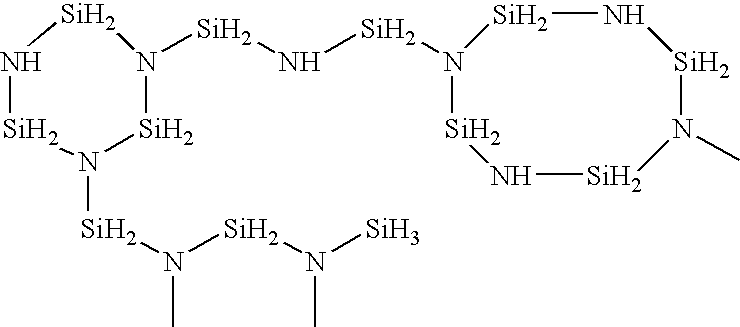
Solvent for treating polysilazane and method of treating polysilazane with the solvent
InactiveUS7344603B2Improve abilitiesAvoid adversely affecting the properties of a groundSemiconductor/solid-state device manufacturingDetergent compounding agentsAlkaneNonane
Polysilazane is treated with a single or mixed solvent comprising one or more members selected from the group consisting of xylene, anisole, decalin, cyclohexane, cyclohexene, methylcyclohexane, ethylcyclohexane, limonene, hexane, octane, nonane, decane, a C8-C11 alkane mixture, a C8-C11 aromatic hydrocarbon mixture, an aliphatic / alicyclic hydrocarbon mixture containing 5 to 25% by weight of C8 or more aromatic hydrocarbons, and dibutyl ether, wherein the number of 0.5 micron or more fine particles contained in 1 ml of the solvent is 50 or less. As the treatment of polysilazane, there are illustrated, for example, edge-rinsing and back rinsing of a polysilazane film formed by spin coating polysilazane on a semiconductor substrate. The water content of the solvent is preferably 100 ppm or less.
Owner:MERCK PATENT GMBH
Cleaning composition with terpene and hydrogen peroxide
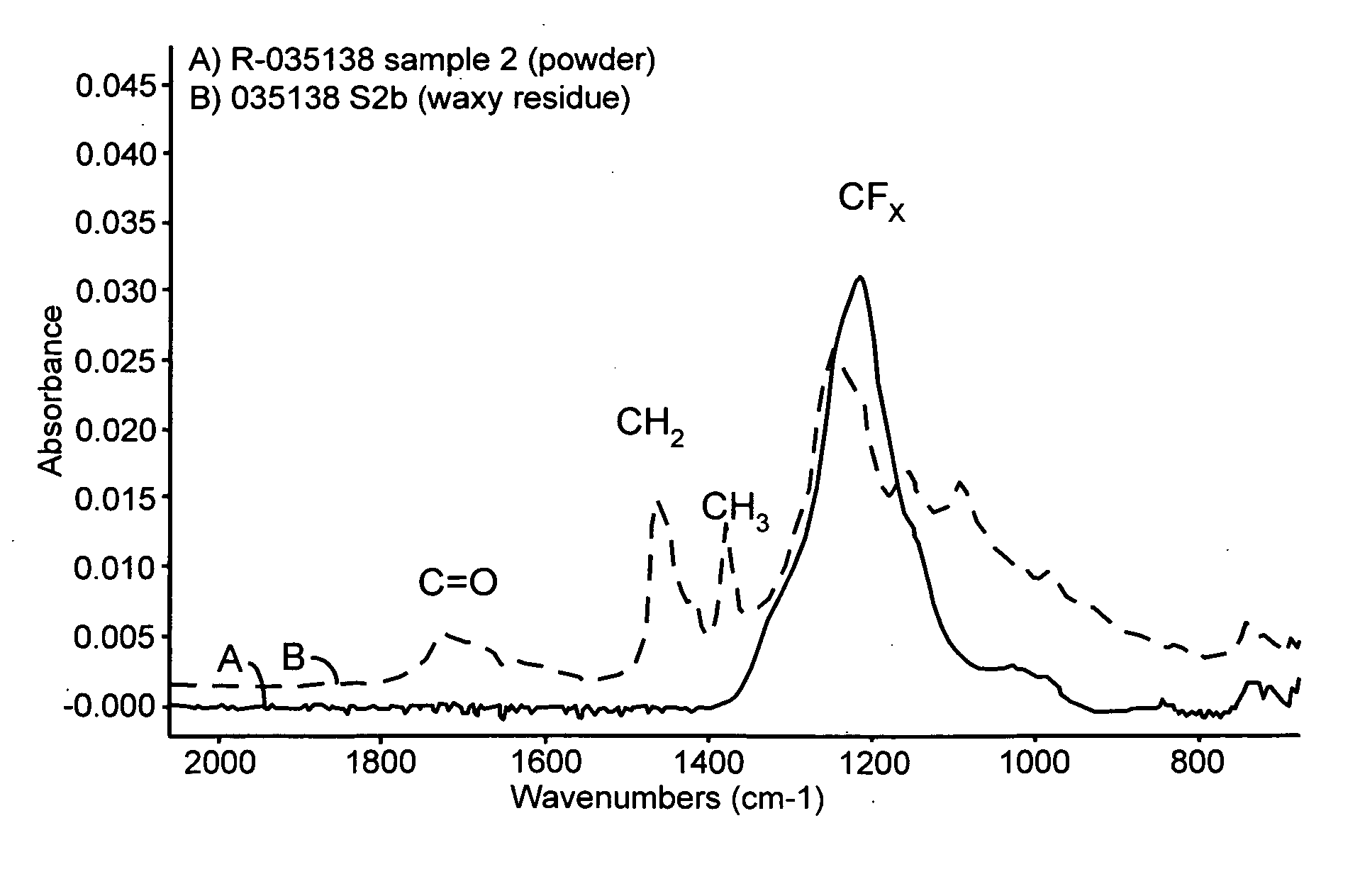
InactiveUS6939839B2Simple compositionOrganic detergent compounding agentsNon-ionic surface-active compoundsLimonene oxideSURFACTANT BLEND
A cleaning composition uses a terpene such as D-limonene or orange oil, a nonionic surfactant, a single anionic surfactant, an anti-oxidant, hydrogen peroxide, and the balance deionized water.
Owner:JOHNSON LOUIS B
Extracts derived from chenopodium plants and uses thereof
The present invention relates to pesticides. More particularly, the present invention relates to botanical pesticides. In particular, the present invention relates to compositions and methods for controlling plant-infesting pests with plant extracts and notably with compositions comprising oil extracts derived from Chenopodium sp. plant material. The invention further relates to compositions comprising such extracts as pesticidal compositions and providing the advantages of minimal development of resistance thereto, minimal toxicity to mammals, minimal residual activity and environmental compatibility. The pesticidal compositions of the present invention comprises α-terpinene, ρ-cymene, limonene, carvacrol, carveol, nerol, thymol, and carvone.
Owner:FORAGEN TECH MANAGEMENT
Formation of low K material utilizing process having readily cleaned by-products
InactiveUS20060160374A1Easy to disassembleSolid-state devicesSemiconductor/solid-state device manufacturingFuranCyclopentene
Nano-porous low dielectric constant films are deposited utilizing materials having reactive by-products readily removed from a processing chamber by plasma cleaning. In accordance with one embodiment, an oxidizable silicon containing compound is reacted with an oxidizable non-silicon component having thermally labile groups, in a reactive oxygen ambient and in the presence of a plasma. The deposited silicon oxide film is annealed to form dispersed microscopic voids or pores that remain in the nano-porous silicon. Oxidizable non-silicon components with thermally labile groups that leave by-products readily removed from the chamber, include but are not limited to, limonene, carene, cymene, fenchone, vinyl acetate, methyl methacrylate, ethyl vinyl ether, tetrahydrofuran, furan, 2,5 Norbornadiene, cyclopentene, cyclopentene oxide, methyl cyclopentene, 2-cyclopentene-1-one, and 1-butene.
Owner:APPLIED MATERIALS INC
Release layer paste and method of production of a multilayer type electronic device
InactiveUS20070190251A1Enable formationPrintabilityOther chemical processesFixed capacitor electrodesAdditive ingredientSolvent
A release layer paste used for producing a multilayer type electronic device and forming a release layer of a thickness of 0.05 to 0.1 μm, used in combination with an electrode layer paste including one or more solvents selected from limonene, dihydroterpinyl methyl ether, α-terpinyl acetate, terpinyl methyl ether, isobornyl acetate, caryophyllene, 1-dihydrocarvyl acetate, menthone, menthyl acetate, perillyl acetate, carvyl acetate, d-dihydrocarvyl acetate, and butyl carbitol acetate and a binder comprised of ethyl cellulose, including a ceramic powder, organic vehicle, plasticizer, and dispersion agent, the organic vehicle containing a binder having polyvinyl acetal as its main ingredient, a ratio (P / B) of the ceramic powder with respect to the binder and plasticizer being controlled to 1.33 to 5.56 (however, excluding 5.56).
Owner:TDK CORPARATION
Solvent for treating polysilazane and method of treating polysilazane with the solvent
InactiveUS20050027089A1Excellent propertyImprove trimming effectSemiconductor/solid-state device manufacturingDetergent compounding agentsAlkaneNonane
Polysilazane is treated with a single or mixed solvent comprising one or more members selected from the group consisting of xylene, anisole, decalin, cyclohexane, cyclohexene, methylcyclohexane, ethylcyclohexane, limonene, hexane, octane, nonane, decane, a C8-C11 alkane mixture, a C8-C11 aromatic hydrocarbon mixture, an aliphatic / alicyclic hydrocarbon mixture containing 5 to 25% by weight of C8 or more aromatic hydrocarbons, and dibutyl ether, wherein the number of 0.5 micron or more fine particles contained in 1 ml of the solvent is 50 or less. As the treatment of polysilazane, there are illustrated, for example, edge-rinsing and back rinsing of a polysilazane film formed by spin coating polysilazane on a semiconductor substrate. The water content of the solvent is preferably 100 ppm or less.
Owner:MERCK PATENT GMBH
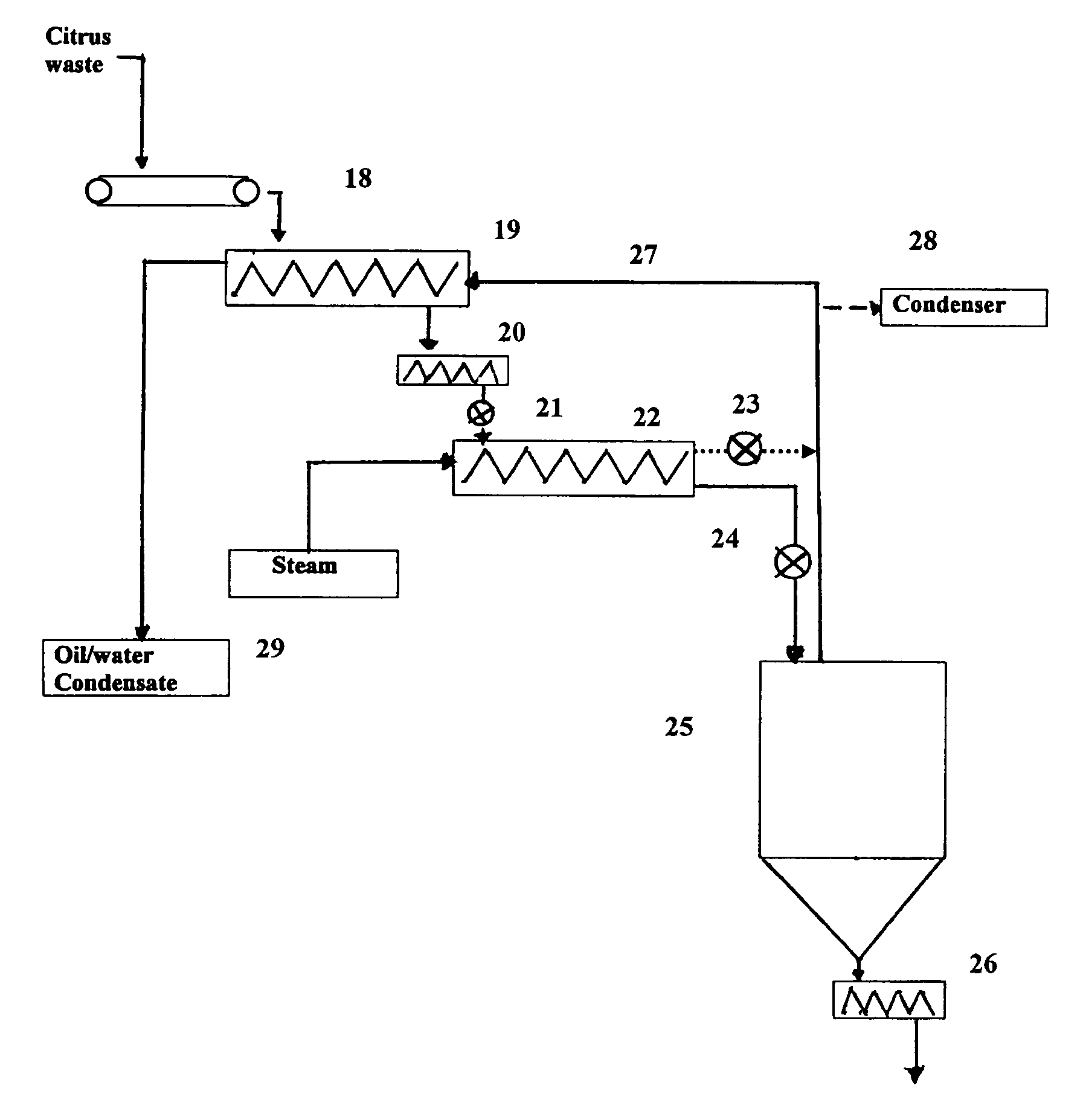
Ethanol production from solid citrus processing waste
InactiveUS20080213849A1Promote recoveryReduce pollutionOrganic compound preparationMicroorganismsSugarLimonene oxide
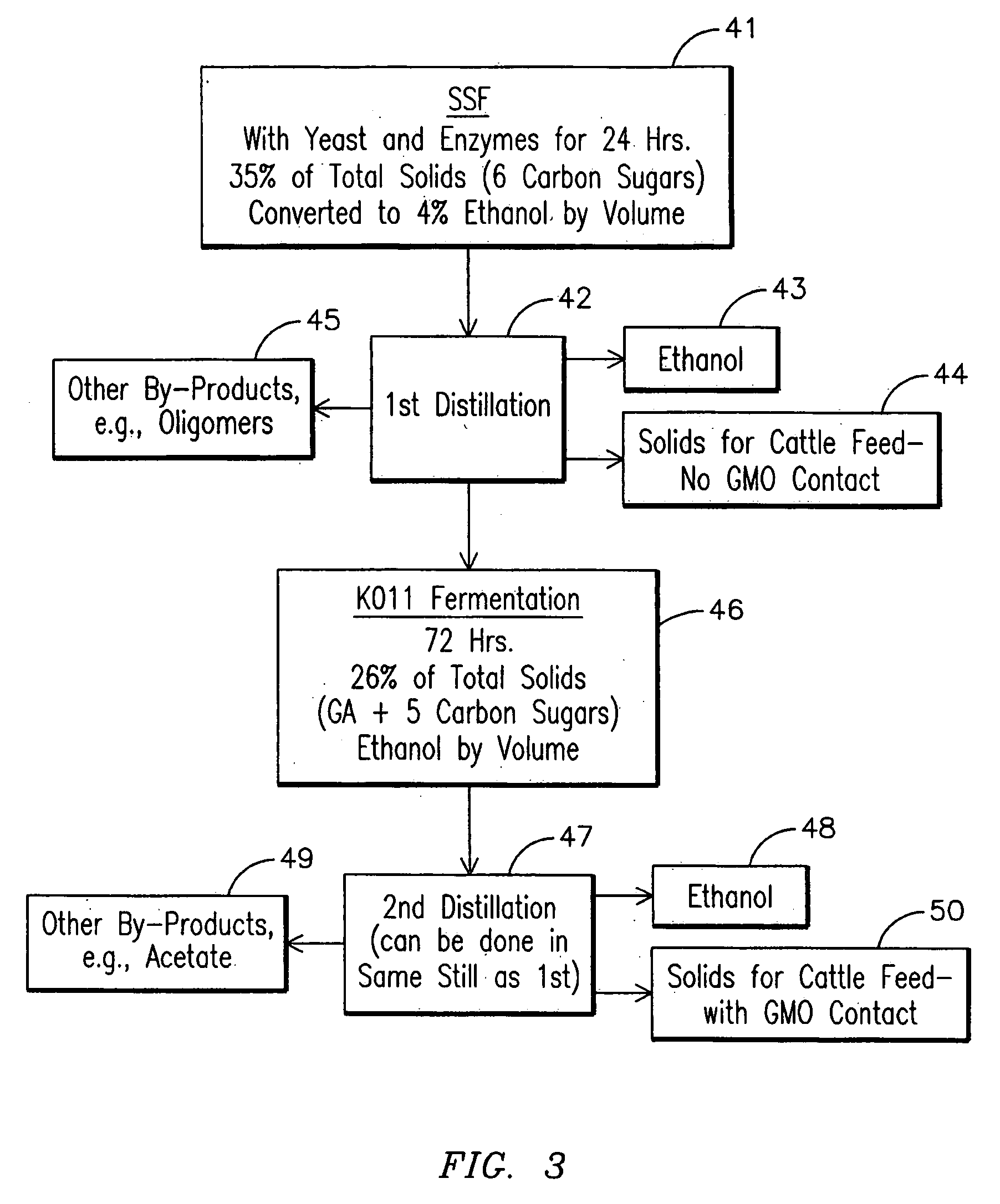
Method for producing ethanol from solid citrus waste by reducing the concentration of limonene in citrus waste to allow fermentation. In one embodiment ground solid citrus waste is partially hydrolyzed and pasteurized by heating using a jet cooker and then injected into a flash tank to remove limonene. The heated citrus waste is then cooled, hydrolyzed with enzymes and fermented to ethanol. The remaining solids and liquids may be processed further to yield other byproducts. More particularly, the solids may be dried and pressed for use in cattle feed and the liquids may be further fermented or processed to yield additional ethanol, acetate, galacturonic acid monomers and polymers, five carbon sugars and other products.
Owner:US SEC AGRI
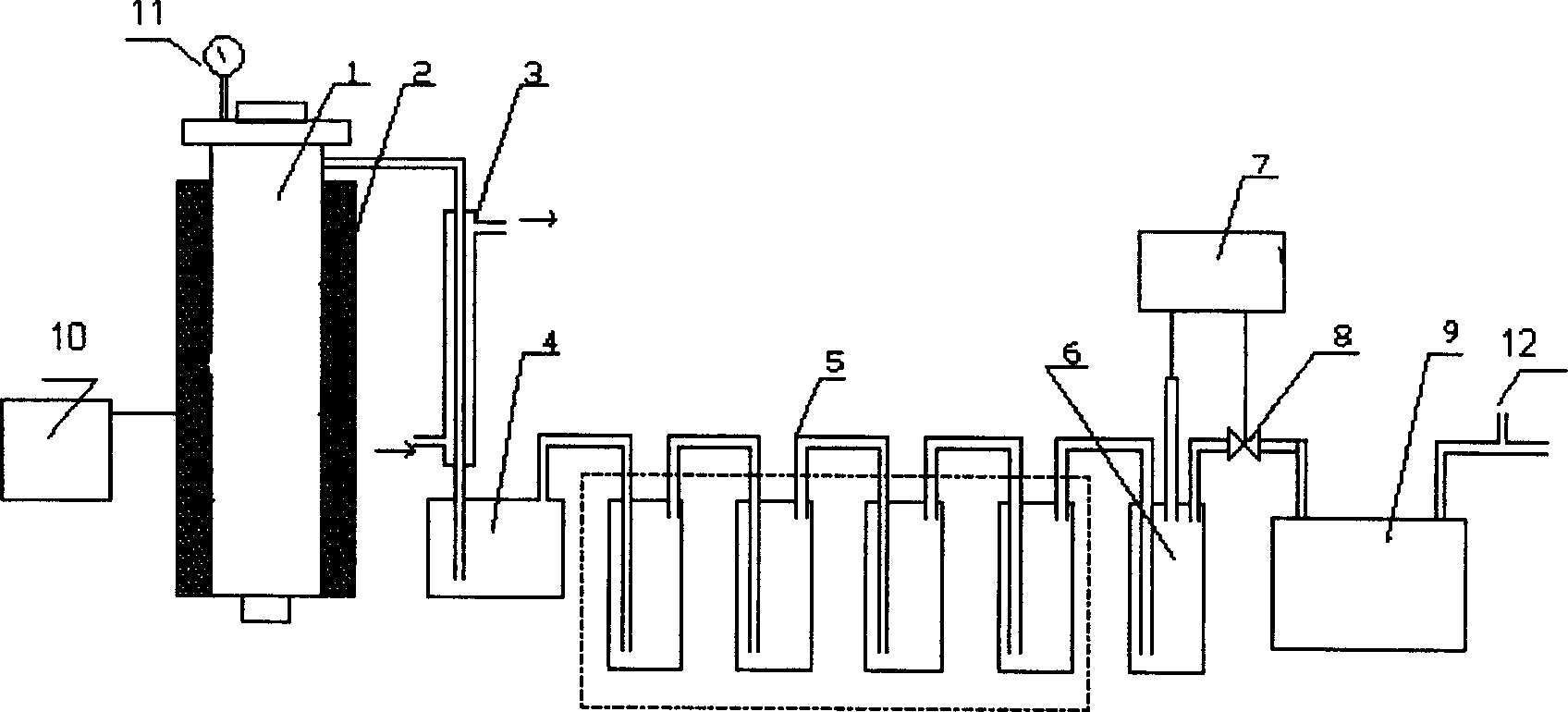
Method and equipment of vacuum catalytic cracking for preparing limonene, fuel oil and carbon black from scrap tire
InactiveCN1760333ANo emissionsDo not pose a hazardPigmenting treatmentPlastic recyclingLiquid productFuel oil
A process for preparing lemon oil extract, fuel oil and carbon black from used tyre by vacuum catalytic cracking includes such steps as proportionally mixing sodium hydroxide and zinc oxide to obtain catalyst, proportionally loading the catalyst and used tyre particles into vacuum catalytic cracking reactor, reacting to obtain gas mixture and carbon black residual in reactor, condensing the gas mixture to obtain the mixture of lemon oil extract, fuel and water, separating, and purifying. Its apparatus is also disclosed.
Owner:广州中科环能科技有限公司
Semanotus sinoauster attractant
InactiveCN101243835AAvoid pollutionThe effect is thoroughAnimal feeding stuffTrappingAdditive ingredient
The invention discloses an attractant of Semanotus bifasciatus, which comprises the components in weight ratio as follows: 1 Alpha-pinene, 2 to 5 Beta-pinene, 2 to 20 3-carene, 2 to 200 thujopsene and 2 to 5 limonene. The attractant of Semanotus bifasciatus has the advantages of simple preparation, high efficiency, environmental protection and low cost, and is mainly used for trapping of Semanotus bifasciatus as well as used for monitoring and control of Semanotus bifasciatus.
Owner:BEIJING FORESTRY UNIVERSITY +1
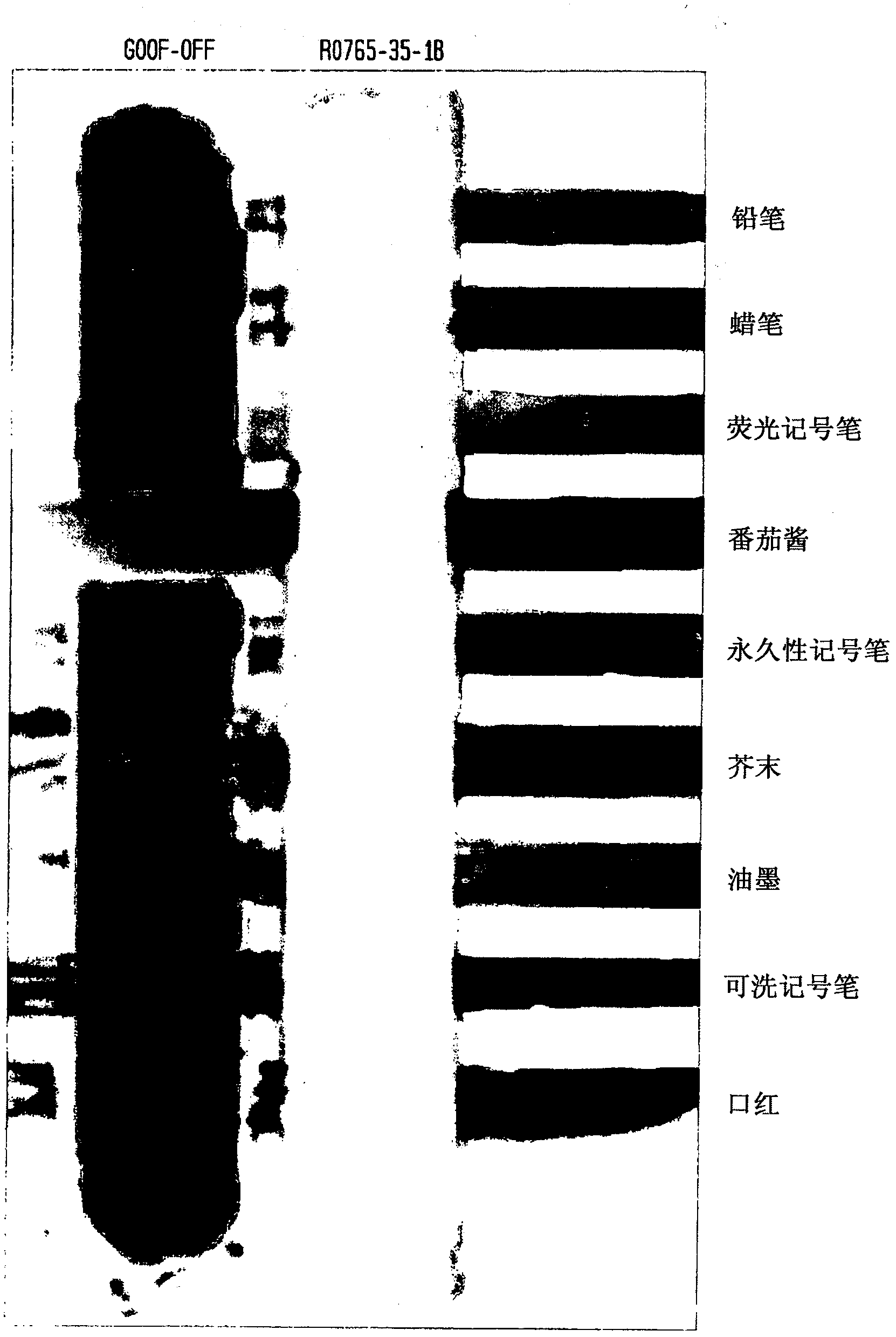
Ink cleaning composition and methods for use
InactiveCN102428165ANon-ionic surface-active compoundsDetergent mixture composition preparationDibasic esterPhenol
An environmentally-friendly cleaning composition for industrial and consumer applications comprising (a) a blend of dibasic esters, (b) one or more surfactants (c) and, optionally, (d) water or a solvent. The dibasic esters are be derived from a blend of adipic, glutaric, and succinic diacids, and, in one particular embodiment, the blend comprises dialkyl adipate, dialkyl methylglutarate and dialkyl ethylsuccinate, wherein the alkyl groups individually comprise a Q- Ci2 hydrocarbon group. The one or more surfactants are typically chosen from alcohol alkoxylate, an alkyl phenol ethoxylate, a terpene, a terpene alkoxylate or any derivates thereof. Optionally, additional components or additives including delaminates such as pinene and d- limonene, fragrances, whiteners, stabilizers, thickeners and the like can be added to the composition. The industrial or consumer application selected from the group consisting of a graffiti cleaner, a painted-substrate cleaner, an ink cleaner, a metal substrate cleaner, a plastic substrate cleaner, an environmentally friendly cleaner, a stain-spot cleaner, an industrial hand cleaner, a resin cleaner, a tar resin cleaner, a textile cleaner, a paint stripper and any combination thereof.
Owner:RHODIA OPERATIONS SAS
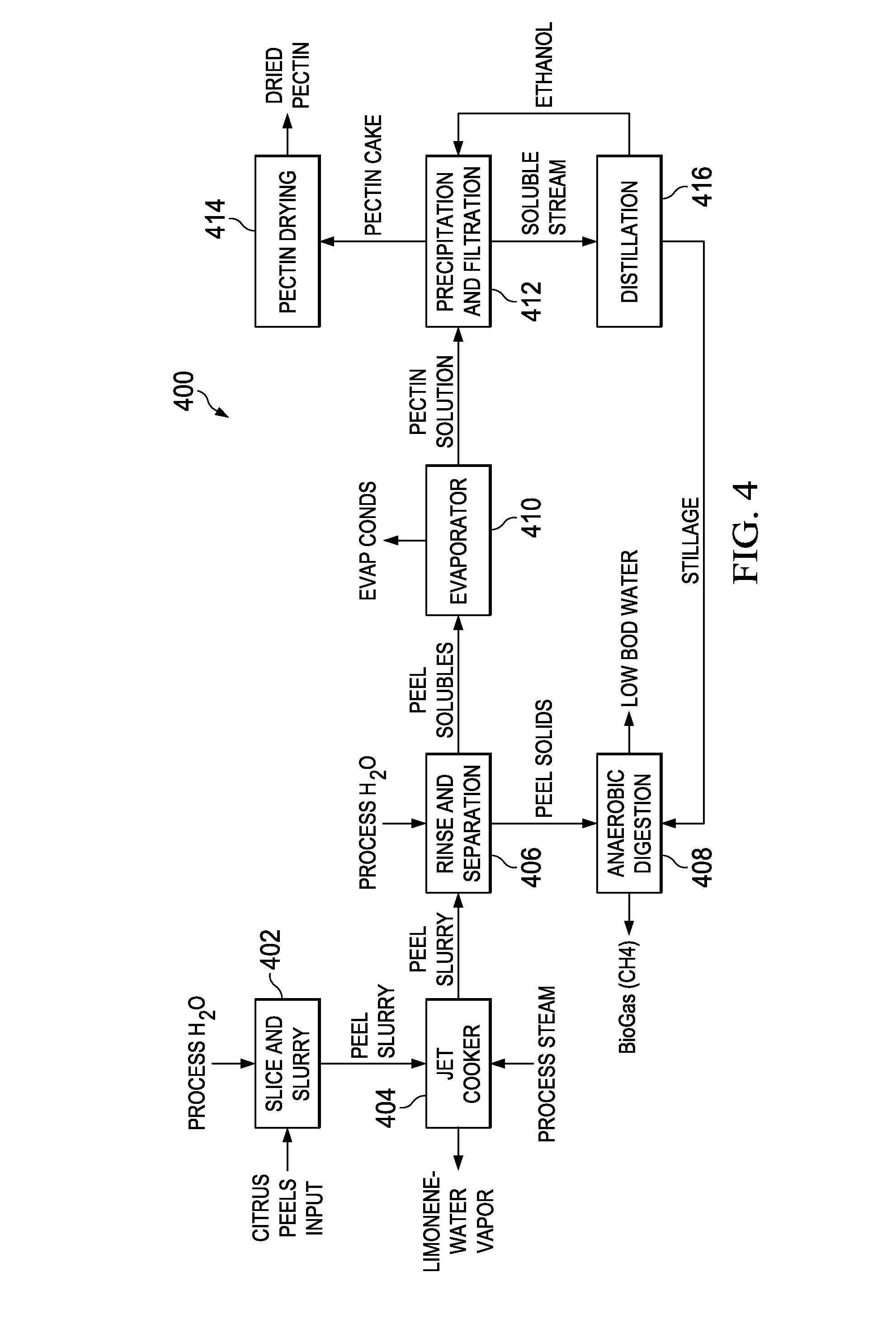
Sustainable conversion of citrus peel waste
ActiveUS20130109065A1Reduced pHQuality improvementLighting and heating apparatusFood processingWater vaporFiltration
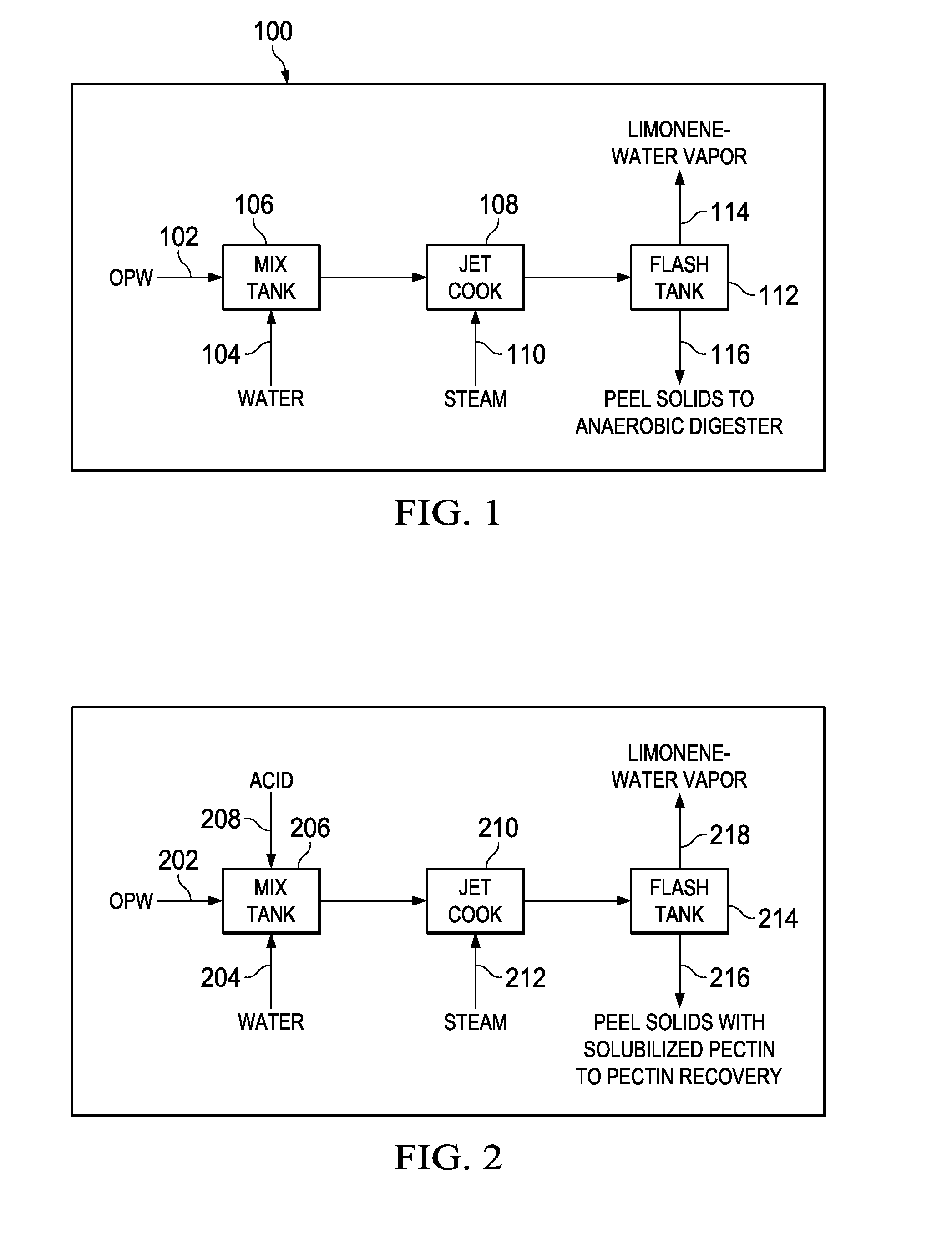
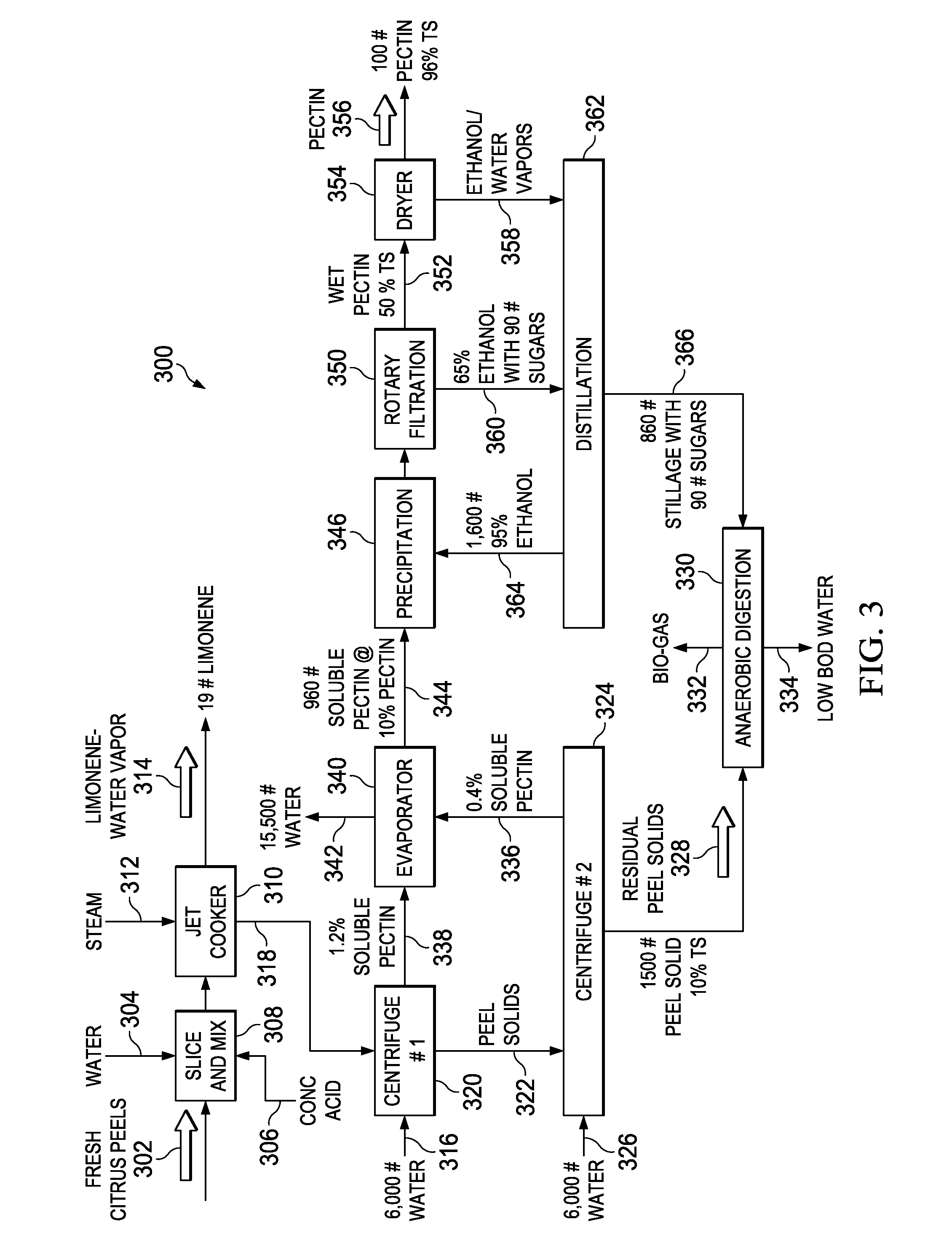
A sustainable method and system for producing bio-fuels from citrus peels and the recovery of limonene and pectin prior to fermentation of the peel solids. In one embodiment a sustainable method and system for the concurrent recovery of limonene and pectin from citrus peels is disclosed. The peels are optionally zested, mixed with water and an acid, and then exploded in a jet cooker or a pressurized extruder. The exploded peels are transferred to a flash vessel where the remaining limonene and water vapor are separated into water and limonene. The pectin fraction is removed from the flash tank and extracted by centrifuge, precipitation and / or filtration. The remaining peel solids are fermented in an anaerobic digester which produces methane, ethanol, acids, CO2 or other end products which can be used as fuel for power generation equipment sufficient to supply the processing system for sustainable operations as described herein.
Owner:FRITO LAY NORTH AMERICA INC
Electronic cigarette tar and preparation method thereof
ActiveCN107125803ARich aromaComfortable tasteTobacco treatmentEssential-oils/perfumesCinnamyl acetateLemon oil
The invention provides electronic cigarette tar which comprises the following components by mass percentage: 90-99% of atomized liquid and 1-10% of coca cola essence, wherein the coca cola essence comprises the following components in parts by weight: 0.1-2 parts of alpha pinene, 0.1-2 parts of beta pinene, 1-30 parts of limonene, 1-10 parts of sweet orange oil, 1-20 parts of lemon oil, 0.1-0.5 parts of myrcene, 0.1-2 parts of cinnamic aldehyde, 0.1-5 parts of cinnamon oil, 0.1-10 parts of citral, 0.1-5 parts of geranial, 1-10 parts of geranyl acetate, 0.1-5 parts of sinensal, 1-10 parts of citronellol, 1-10 parts of geranyl propionate, 0.1-5 parts of eugenol allylguajacol, 0.1-2 parts of ethyl maltol, 0.1-3 parts of furanone, 0.1-5 parts of raspberry ketone, 1-25 parts of cinnamyl alcohol, 1-10 parts of cinnamyl acetate, 1-15 parts of vanillin, 1-15 parts of ethyl vanillin and 0.1-2 parts of coffee extract. The electronic cigarette tar with a coca cola taste is rich and plump in fragrance, and can achieve an effect similar to a pleasant feeling brought by drinking coca cola. The invention further provides a preparation method of the electronic cigarette tar. The method is simple and easy to operate, and is suitable for industrial production.
Owner:SHENZHEN HANGSEN STAR TECH
Environment protection additive for welding and cutting natural gas
The green additive for welding and cutting natural gas consists of ethanol 5-10 wt%, 2-hexanol 5-10 wt%, 2-hexanone 5-10 wt%, limonene 10-15 wt%, organoplatinum 0.02-0.08 wt%, and C5-C7 isoalkane for the rest. It is liquid with high oil solubility, low boiling point and high solubility in natural gas, and may be added into liquid one gaseous natural gas in any ratio. It is non-toxic and environment friendly, and can raise the combustion temperature of natural gas greatly to 3450 deg.c. In addition, it can lower the use cost of natural gas obviously.
Owner:SANAN INDAL GAS ZHOUSHAN
Method of pretreating citrus waste
InactiveUS7879379B1Efficiently disrupts cell structureComponent can be removedOrganic chemistryAlcoholic beverage preparationPre treatmentDirect heating
Method of pretreating citrus waste to break cell structure, pasteurize or sterilize citrus waste solids and remove inhibitory peel oil components (e.g., limonene) involving optionally reducing the particle size of the citrus waste prior to preheating, preheating the citrus waste through indirect heating in a preheater reactor to form preheated citrus waste and conveying the preheated citrus waste to a main reactor, heating the citrus waste through a combination of (simultaneous) indirect heating and direct heating to produce treated citrus waste solids that are pasteurized and a vapor containing water and peel oil components, separating the pasteurized citrus waste solids and the vapor containing water and peel oil components, and collecting the separated vapor containing water and peel oil components by condensation. The method optionally includes cooling the pasteurized citrus waste solids followed by saccharifying with enzymes and fermentation to produce ethanol or other products.
Owner:RENEWABLE SPIRITS LLC +1
Medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and preparation method
ActiveCN101590033AActive ingredients are clearImprove bioavailabilityMetabolism disorderRespiratory disorderYolkGlycerol
The invention aims to develop medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and a preparation method. The preparation comprises the following components in percentage by weight: 0.0128 to 0.0320 percent of eucalyptol, 0.0084 to 0.0210 percent of limonene, 0.0028 to 0.0070 percent of alpha-pinene, 10 to 30 percent of soybean oil for injection, 1.0 to 1.5 percent of yolk lecithin for injection or 0.8 to 1.5 percent of soybean phospholipids for injection, 2.0 to 2.5 percent of glycerol for injection, and water for injection added to 100 milliliters. The preparation method has the characteristics of strong controllability of production quality, and definite treatment effect of a product. The invention fills up the blank of the medicinal composition fat emulsion injection preparation containing the eucalyptol, the limonene and the alpha-pinene.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com