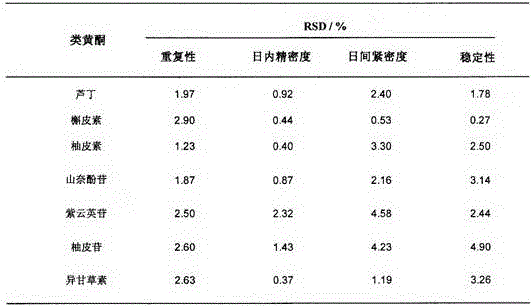
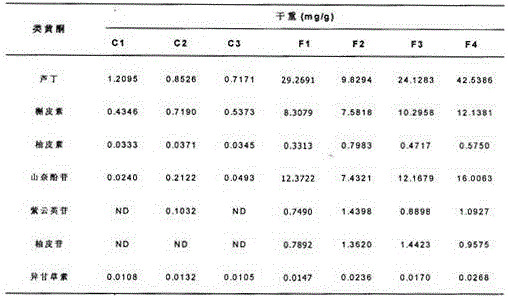
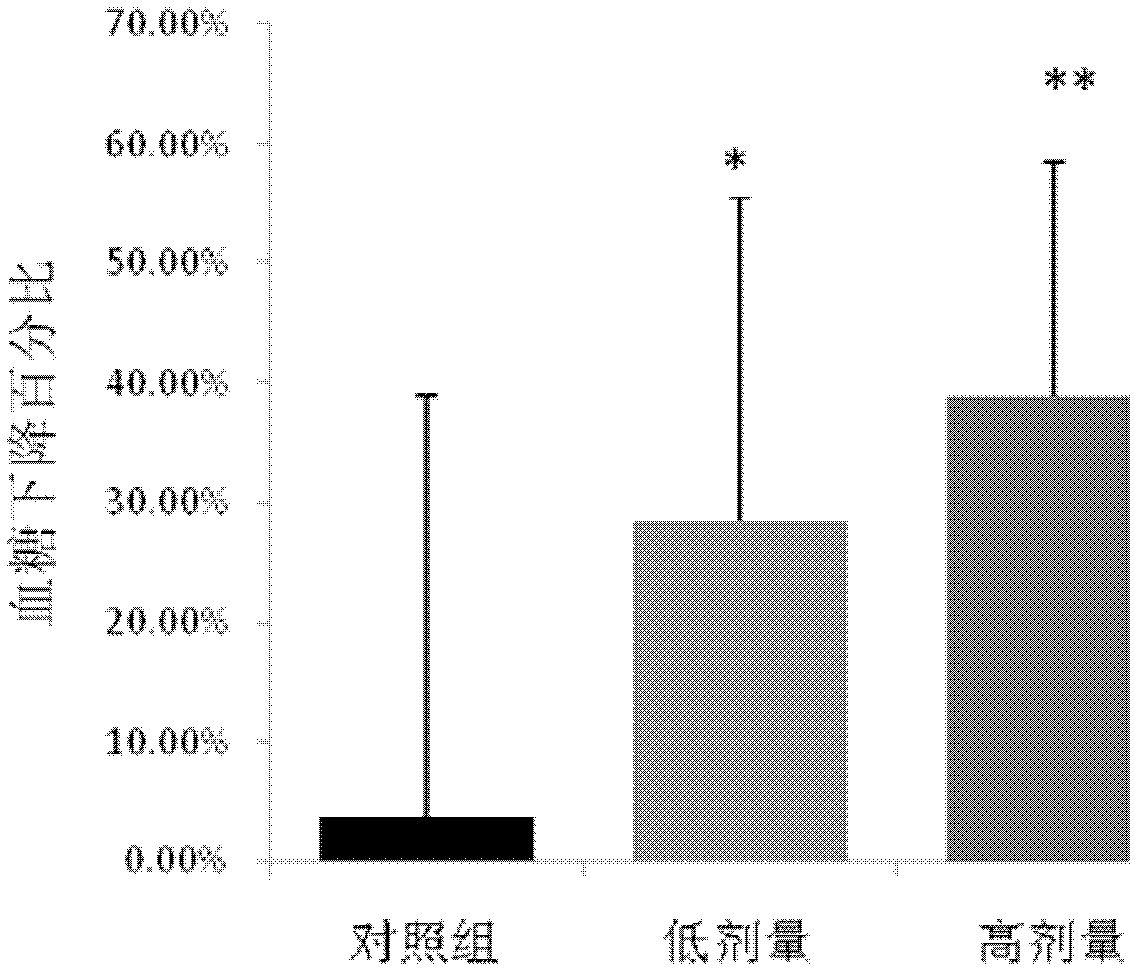Patents
Literature
297 results about "Naringenin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
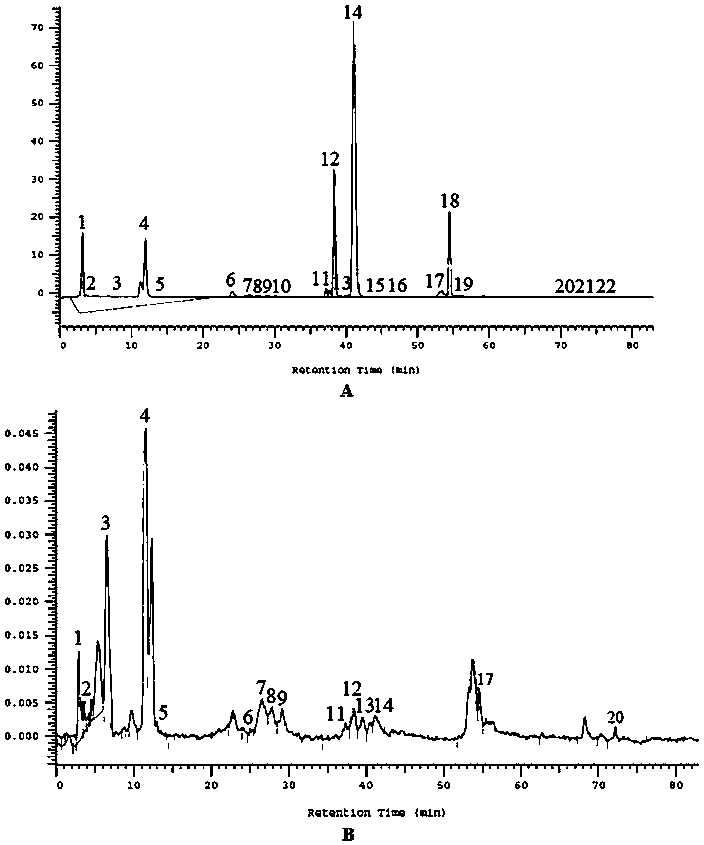
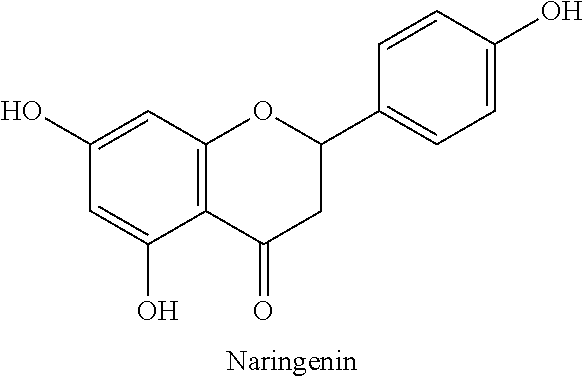
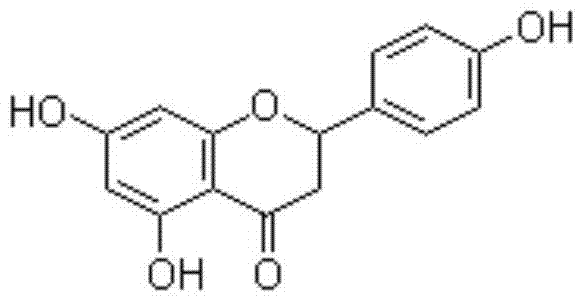
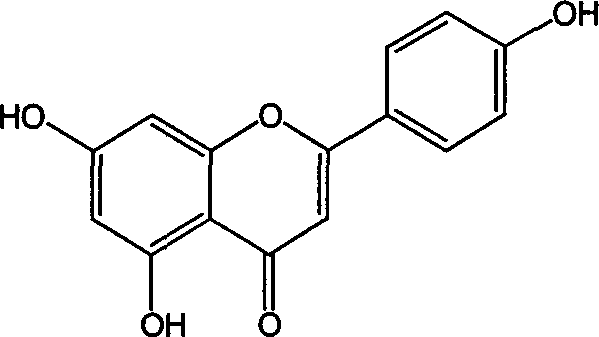
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.
Anti-aging composition containing resveratrol and method of administration
InactiveUS20090163580A1Improve athletic performanceBiocideNervous disorderActive agentExercise performance
Formulations and methods of treatment and putative prevention for aging (anti-aging composition) and for diseases or conditions of all reactive oxygen species-dependant illnesses, such as Alzheimer's disease, Parkinson's disease, diabetes mellitus, cardiovascular disease, cancer, hepatitis, and disorders associated with estrogen deficiencies including osteoporosis and breast cancer and for improving athletic performance of humans include resveratrol and two (2) or more of the following features or additional active ingredients: (1) slow release formulation of resveratrol; (2) pterostilbene; (3) quercetin; (4) fisetin, and (5) naringenin. Slow release is defined for the purposes of the present invention as releasing 95% of the active agent or agents in eight (8) hours through normal human gastrointestinal absorption.
Owner:NATROL
Naringin and its salt used for preparing cough suppressing phlegm tramsforming medicine
ActiveCN1555793AGood cough and phlegm effectQuality improvementOrganic active ingredientsPill deliveryNaringinChemical synthesis
An application of naringenin and its salt in preparing the medicines for treating cough and resolving phlegm is disclosed. Said naringenin is prepared from naringin through hydrolysis or from the natural medicinal materials through extracting, or by chemical synthesis. Its advantages are high curative effect and no toxic by-effect.
Owner:广东伯克生物医药有限公司
Method for hydrolytic preparing biological tangeritin by enzyme
InactiveCN101089187AControl the hydrolysis processEasy to separate and purifyFermentationNaringinCitrus Bioflavonoids
In the invented method, citrus bioflavonoid and corresponding enzyme are used as raw materials, being dissolved in organic solvent, adding glucoside solution at concentration of 1-100g / L, under pH value of 3.0-7.0 and temperature of 20-65 deg.C for 20-60 min. After separation and purification, obtained is citrus bioflavonoid monoglycoside. With this invention, naringoside enzymolysis and cirmtim enzymolysis method for production of naringen in monoglycoside and hesperetin monoglycoside, compared with chemical method, has advantages of:easily to be controlled of enzymolysis, mild reaction, less by-products and easy to be separated of products. By using this invented method, the enzymolysis of naringoside and cirmtim for production of naringenin monocoside and hesperetin monoglycoside can be effectively controlled, and only the first glycosidic bond is broken, so obtained is highest yield of naringenin monoglycoside, hesperetin monoglycoside and rhamnose.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing naringenin/hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process
ActiveCN106265596AImprove solubilityHigh dissolution rateOrganic active ingredientsAntinoxious agentsSupercritical anti solventOrganic solvent
The invention discloses a method for preparing naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process, comprising the steps of S1, dissolving naringenin and hydroxypropyl-Beta-cyclodextrin in an organic solvent to obtain a sample solution; S2, introducing CO2 into a crystallizer, and adjusting temperature and pressure in the crystallizer; S3, continually introducing CO2 at a flow speed, maintaining the temperature and pressure in the crystallizer constant, and introducing the sample solution of step S1 into the crystallizer; S4, after introduction of the sample solution, continuously introducing CO2, maintaining the temperature and pressure in the crystallizer constant, and relieving the pressure over a period of time; when the pressure in the crystallizer drops to atmospheric pressure, opening the crystallizer to collect the naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules. The method provided herein wraps naringenin in HP-Beta-CD by using supercritical anti-solvent process, dissolving property of the naringenin in an aqueous solution is greatly improved, dissolvability is significantly improved, and bioavailability of the naringenin can be improved.
Owner:CHINA PHARM UNIV
Anti-bacterial high-viscosity medical glue and preparation method thereof
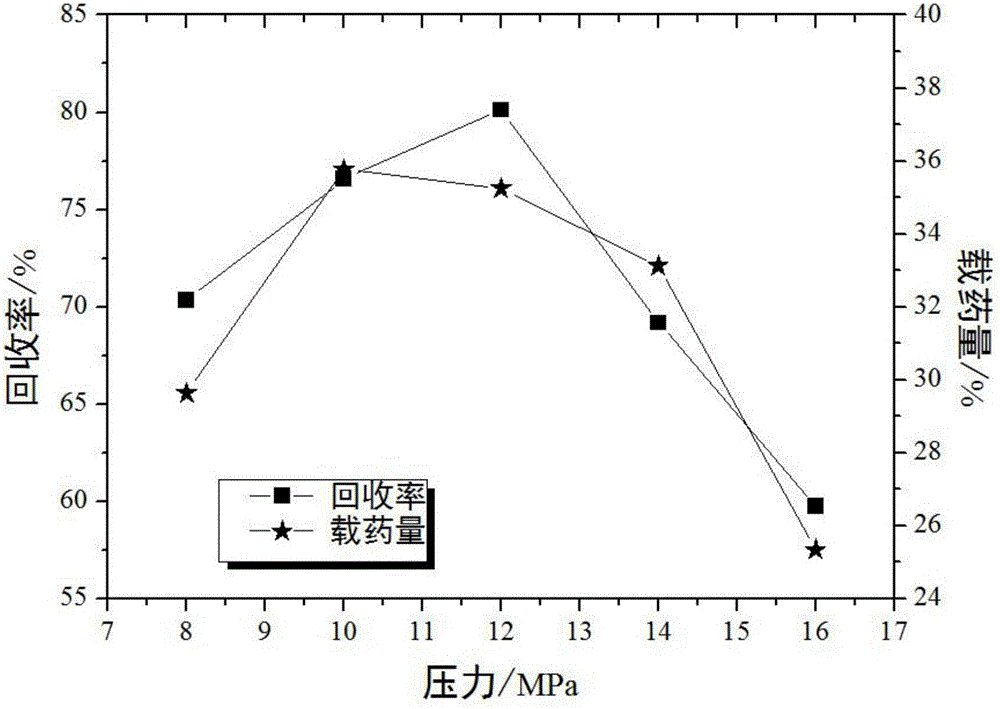
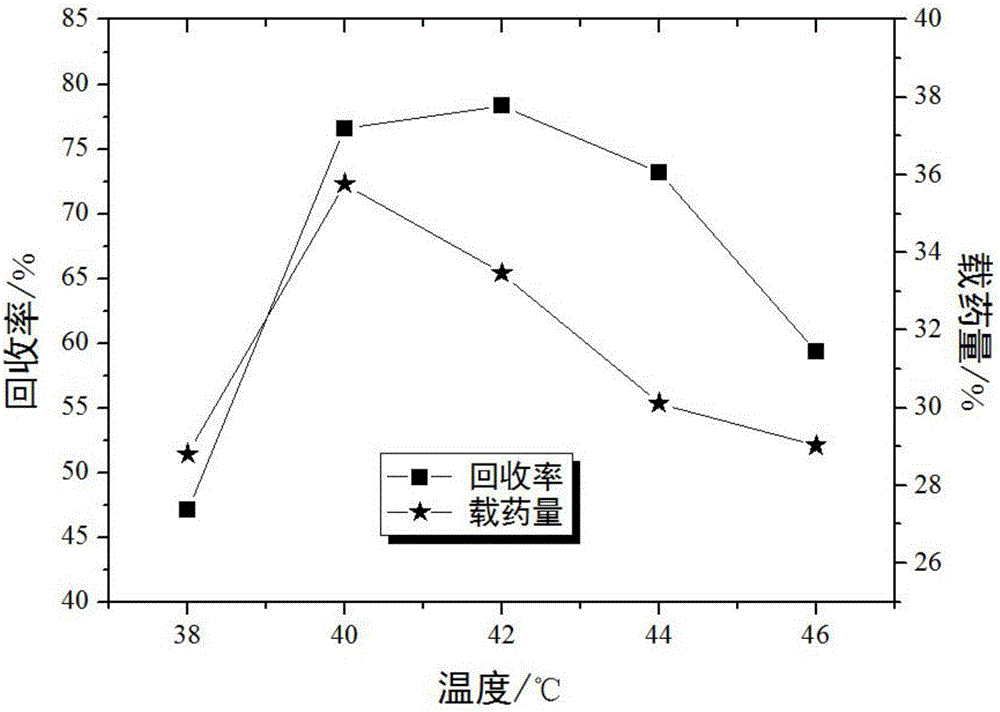
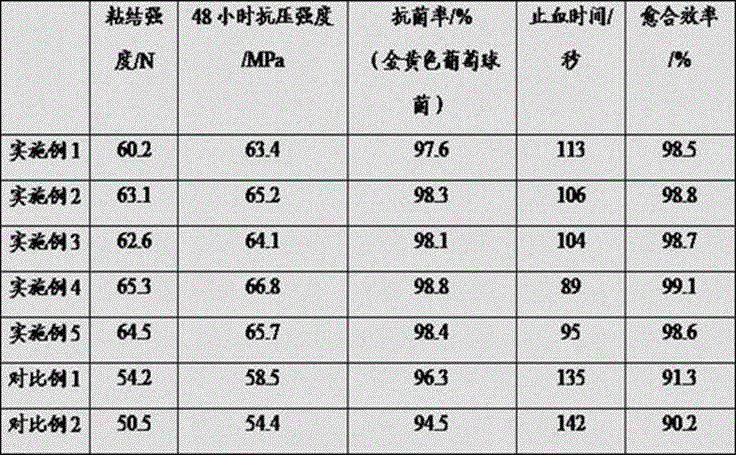
InactiveCN106039391AMeet application requirementsHigh antibacterial rateSurgical adhesivesPharmaceutical delivery mechanismIrritationAdditive ingredient
Owner:林春梅
Preparation method of high-purity naringenin
The invention provides a preparation method of naringenin. The preparation method comprises the following steps: (1) preparing solution of naringin in lower alcohol or lower ketone solvent, leading the naringin in the prepared solution to be subjected to acid hydrolysis in an acidic condition to obtain naringenin, distilling under reduced pressure to remove solvent to obtain a naringenin crude product; (2) dissolving the naringenin crude product in absolute ethanol or absolute methanol, adding active carbon, stirring to decolor; and (3) filtering the active carbon, separating and purifying the prepared solution to obtain the naringenin. The preparation method has the advantages of few reaction steps, less used solvent, less emission of the 'three wastes', mild reaction condition and complete reaction and easy operation, is suitable for industrial production, and has higher practical value; and the prepared product has more stable quality, the purity is higher than 99 percent, which is higher than the drug standard.
Owner:HENAN TOPFOND PHARMA
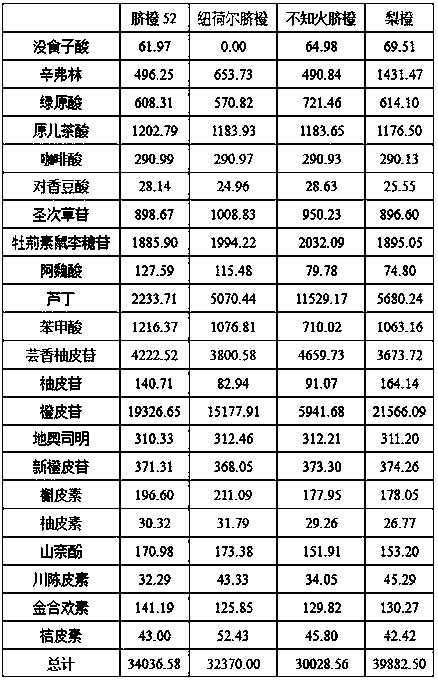
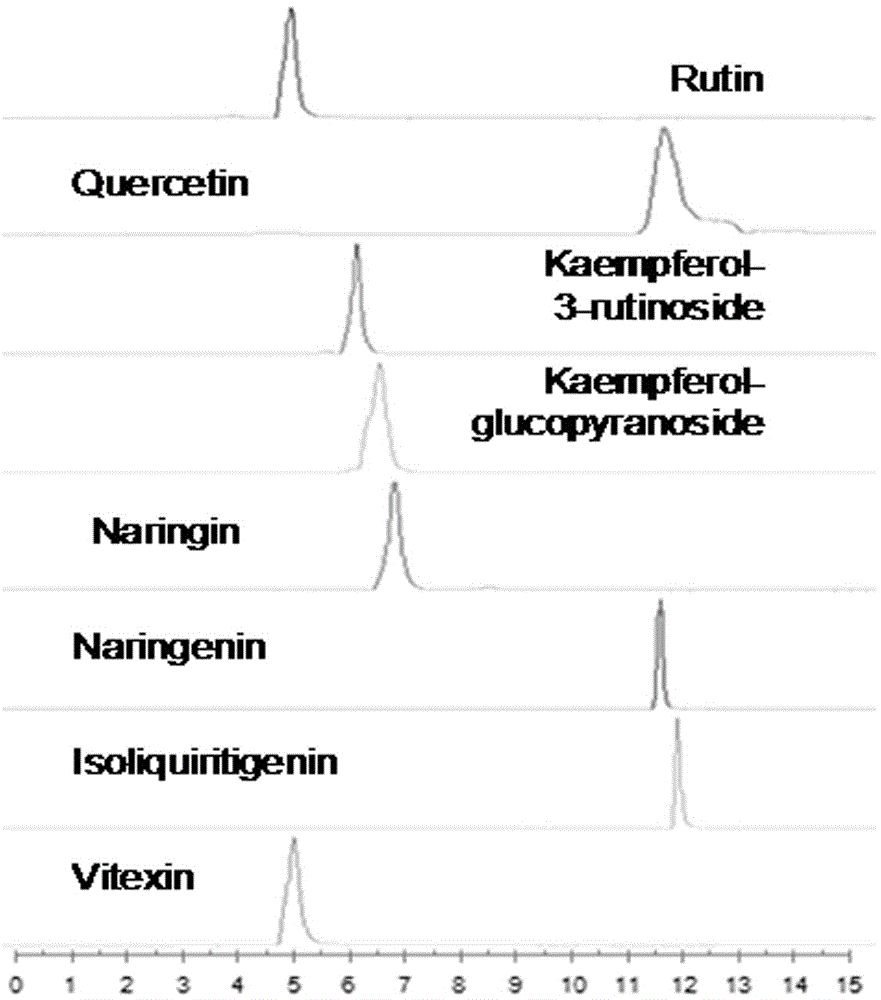
Method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits
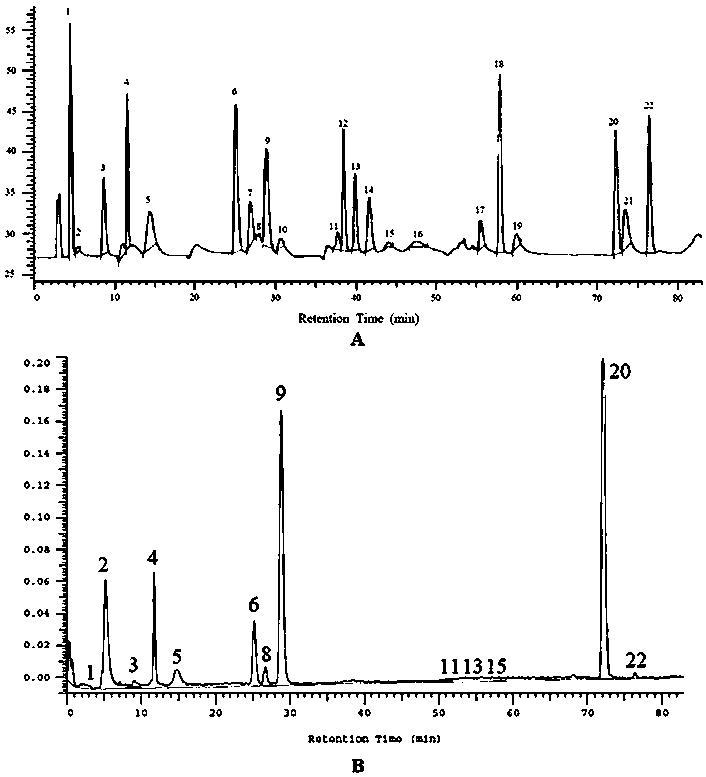
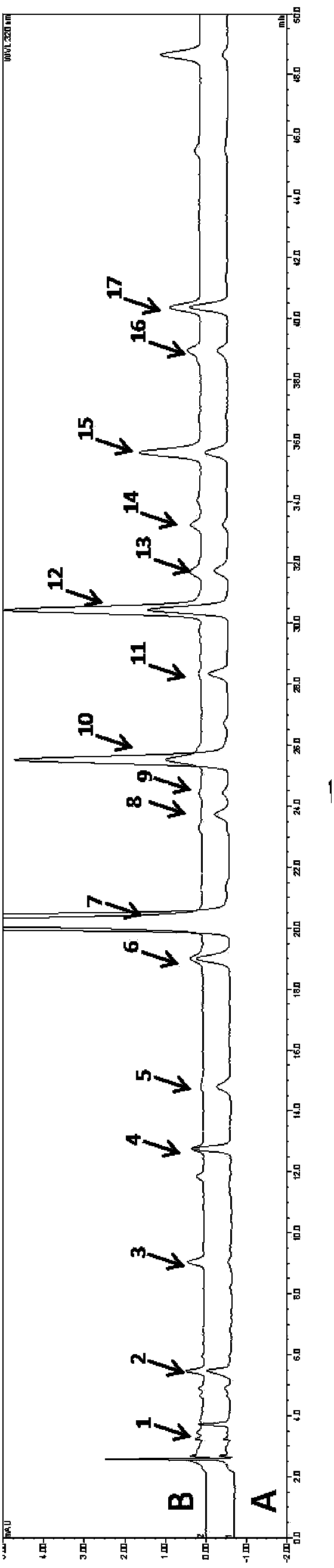
The invention discloses a method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits by adopting high performance liquid chromatography-diode array detector-fluorescence detector (HPLC-DAD-FLD). The method is capable of simultaneously determining twenty-two phenolic compounds in citrus fruits such as gallic acid, synephrine, chlorogenic acid, protocatechuic acid,caffeic acid, p-coumaric acid, rhamnosylvitexin, eriocitrin, ferulic acid, rutin, benzoic acid, narirutin, naringin, hesperidin, diosmin, neohesperidin, quercetin, naringenin, kaempferol, nobiletin,hesperetin, acacetin and the like, derivatization is not needed, and the method is high in accuracy, high in sensitivity and excellent in repeatability.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
Quality control method for effective parts of pomelo flavedo and pomelo flavedo preparation
InactiveCN103364506AGuarantee normal productionMonitor stabilityComponent separationActive ingredientNaringenin
The present invention discloses a quality control method for effective parts of pomelo flavedo and a pomelo flavedo preparation. According to the present invention, a methanol solution containing neoeriocitrin, prunin, naringin, rhoifolin, meranzin hydrate, 7-(2''-alpha-rhamnose-6''-(3''''-hydroxy-3''-methylglutaryl)-beta-D-glucosyl)naringenin, and naringenin is used as a reference; a methanol extract of pomelo flavedo effective parts or the pomelo flavedo preparation is used as a test sample; in particular chromatographic conditions, the high performance liquid chromatographic (HPLC) fingerprint analysis method is used to establish fingerprints and control fingerprints of a reference HPLC and a test sample HPLC, whether a drug is qualified is determined by comparing peaks of the test sample with corresponding peaks of the reference, and similarity evaluation results with the control fingerprint; and contents of various active ingredients in the test sample are simultaneously determined by a method of multi-component quantitive by one marker, and the quality of the drug is evaluated according to the contents of the active ingredients. The method can achieve scientific, comprehensive, rapid and accurate monitoring of the quality of effective parts and the preparation in production. The production process stability is controlled, the quality is ensured to be stable, uniform and controllable, and the method has the advantages of being advanced, good stability and reproducibility, and high operability.
Owner:SUN YAT SEN UNIV
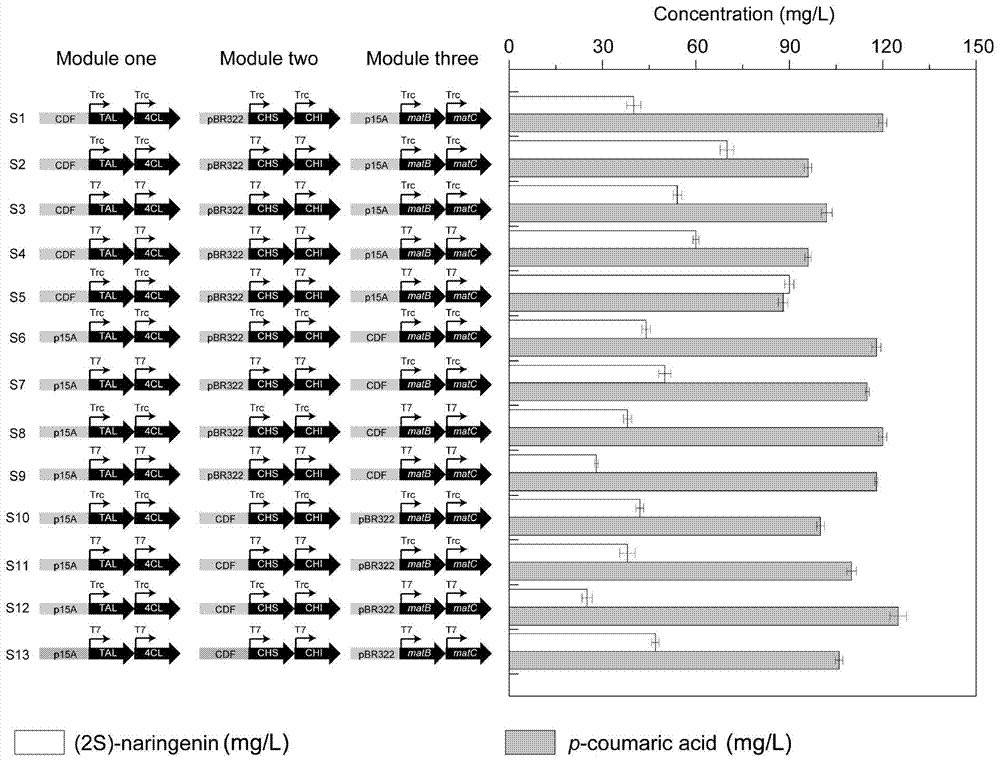
Genetic engineering strain taking tyrosine as substrate to synthesize naringenin and construction method thereof
The invention discloses a genetic engineering stain taking tyrosine as a substrate to synthesize naringenin and a construction method thereof, belonging to the field of synthetic biology or metabolic engineering. According to the construction method disclosed by the invention, a synthesis route constituted by six genes, namely a gene matB encoding a malonic acid transporting enzyme, a gene matC encoding a malonic acid absorption route, a gene encoding a tyrosine ammonialyase (TAL), the gene encoding a 4-cinnamic acid: coenzyme A ligase (4CL), the gene encoding a chalcone synthase (CHS) and the gene encoding a chalcone isomerase (CHI) is introduced into Escherichia coli BL21 to obtain a recombinant strain capable of taking the tyrosine as the substrate to synthesize the naringenin, the synthesis route is further optimized through a modular transformation theory, the strain is finally fermented in a shaking flask for 72 hours by taking the tyrosine as the substrate, and the yield of the naringenin achieves 90mg / L. A strategy adopted by the construction method disclosed by the invention provides certain reference significance for future production of natural small molecular substances by a microbiological method.
Owner:湖南鸿健生物科技有限公司
Naringenin and salts thereof for sweetness enhancement
The use of naringenin and a salt thereof to enhance the sweetness of a sweetness modifier and to decrease the amount of a sweetness modifier used in a consumable is provided.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Extraction method of dendrobium officinale leave, extract of dendrobium officinale leave and application of extract of dendrobium officinale leave
ActiveCN107184823AFacilitate polysaccharidePromote absorptionCosmetic preparationsToilet preparationsAdditive ingredientLignan
The invention relates to the technical field of dendrobium officinale, and particularly relates to an extraction method of dendrobium officinale leave, an extract of the dendrobium officinale leave and application of the extract of the dendrobium officinale leave. The extraction method jointly uses a repeated freezing and thawing wall breaking technology and an enzymolysis technology, so that the extraction rate of the dendrobium officinale leave is 60% or more, the purity of effective components such as protein, polysaccharide and total flavone reaches 90%, the obtained extract of the dendrobium officinale leave also contains rich antioxidant components such as anthocyanidin, naringenin, lignan, dihydroisoflavone and the like, indicating that the extract of the dendrobium officinale leave has a bright application prospect in the field of skin care products.
Owner:连南瑶族自治县欣胜堂生物科技有限公司
Method for enzymatically synthesizing astragalin
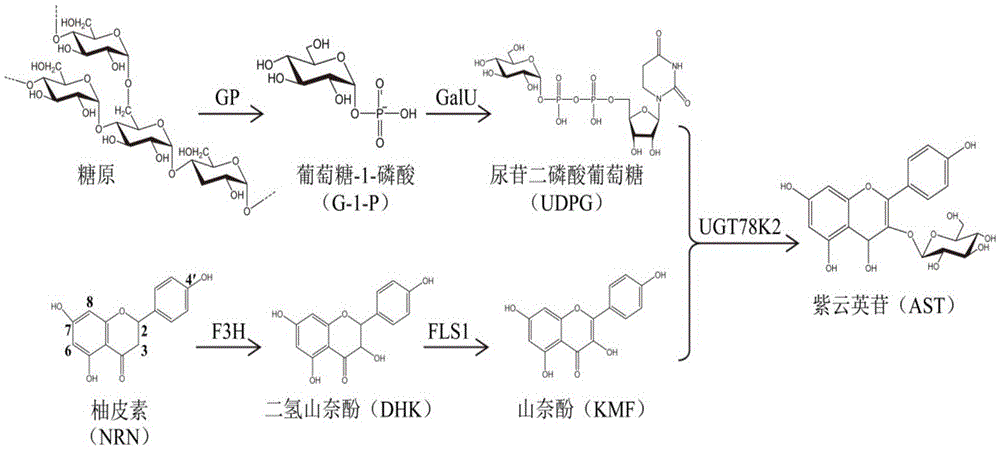
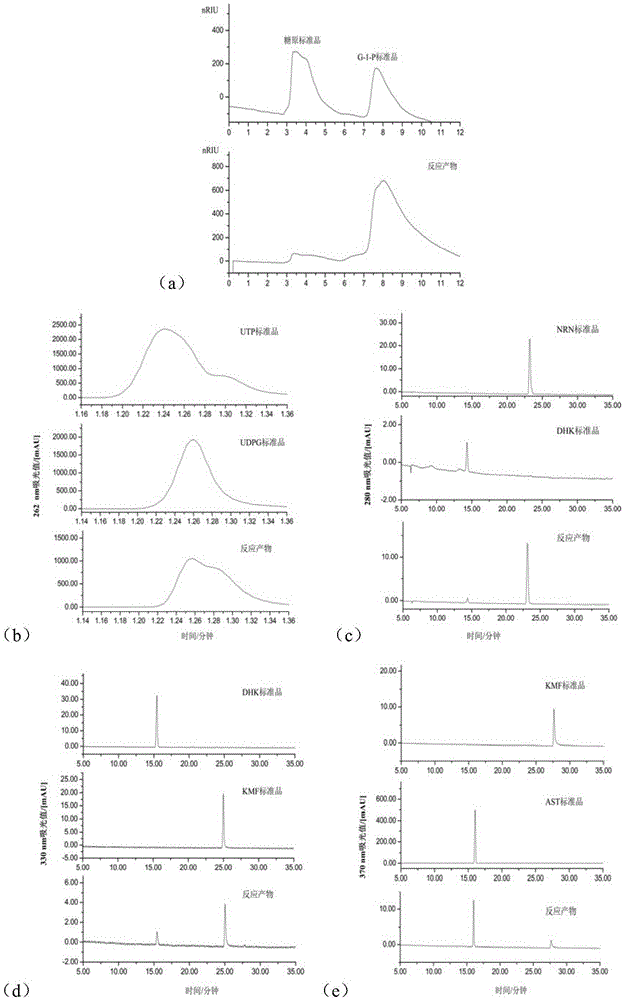
InactiveCN105463044AFew stepsMild operating conditionsFermentationEnzymatic synthesisDihydro-kaempferol
The invention provides a method for enzymatically synthesizing astragalin. The method comprises the following steps that 1, key enzymes needed in the synthesizing process of astragalin are cloned, expressed and purified, wherein the key enzymes comprise glycogen phosphorylase GP, glucose pyrophosphorylase GalU, flavanone-3-hydroxylase F3H, flavanone synthase FLS1 and flavonoid3-O-glucanotransferase UGT78K2; 2, glycogen Gn is synthesized into glucose-1-phosphoric acid G-1-P under the GP effect; 3, G-1-P is synthesized into uridine diphosphate UDPG under the GalU effect; 4, naringenin NRN is synthesized into dihydro kaempferol DHK under the F3H effect; 5, DHK is synthesized into kaempferol KMF under the FLS1 effect; 6, KMF and UDPG are synthesized into astragalin under the UGT78K2 effect. According to the method, few steps are adopted, the operation condition is mild, few side products are generated, the yield is large, pollution is avoided, and the production cost is remarkably reduced.
Owner:YANGZHOU UNIV
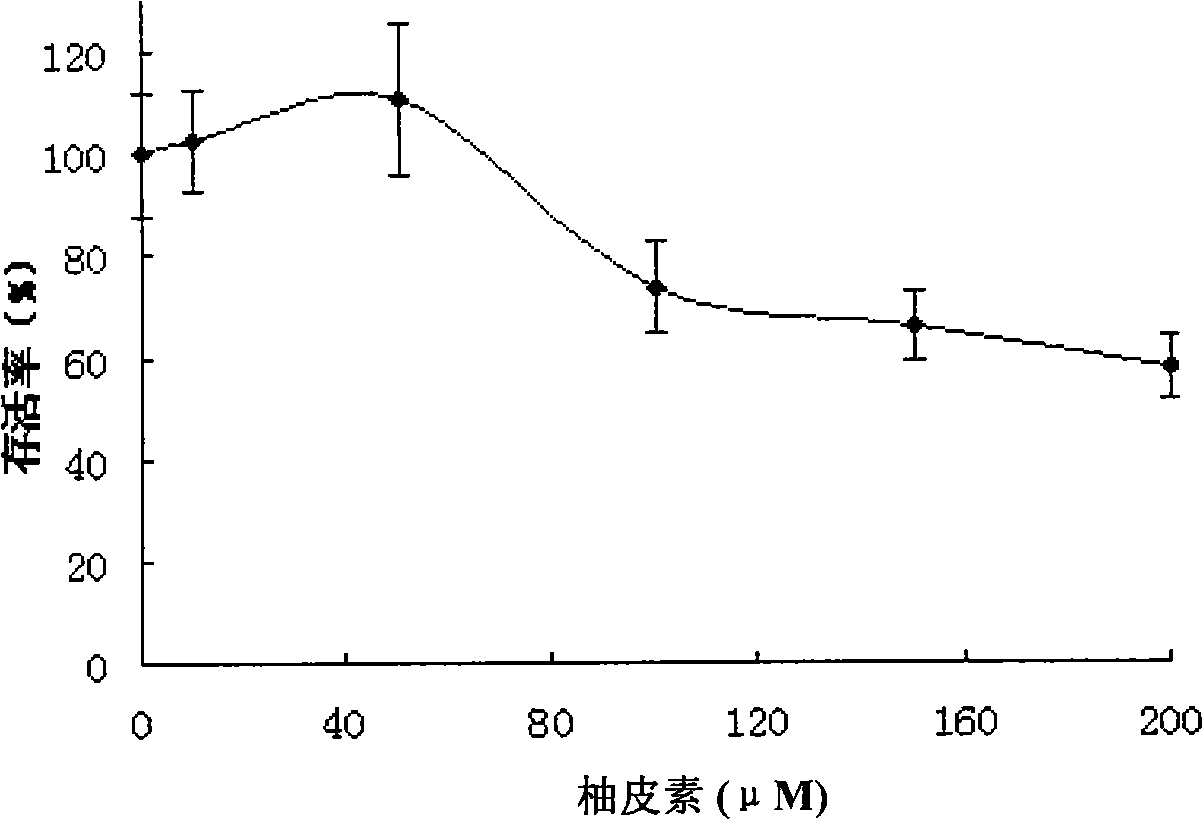
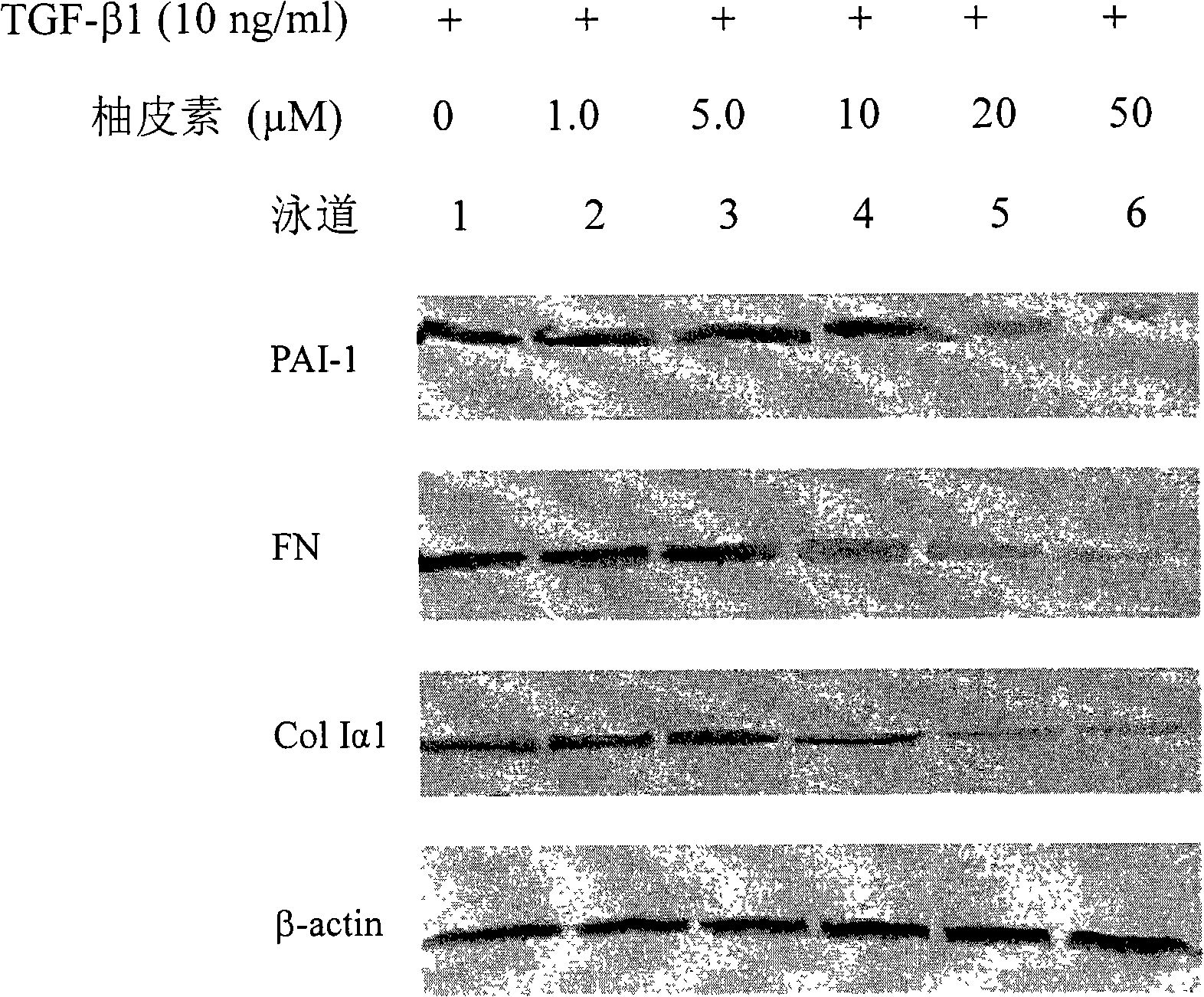
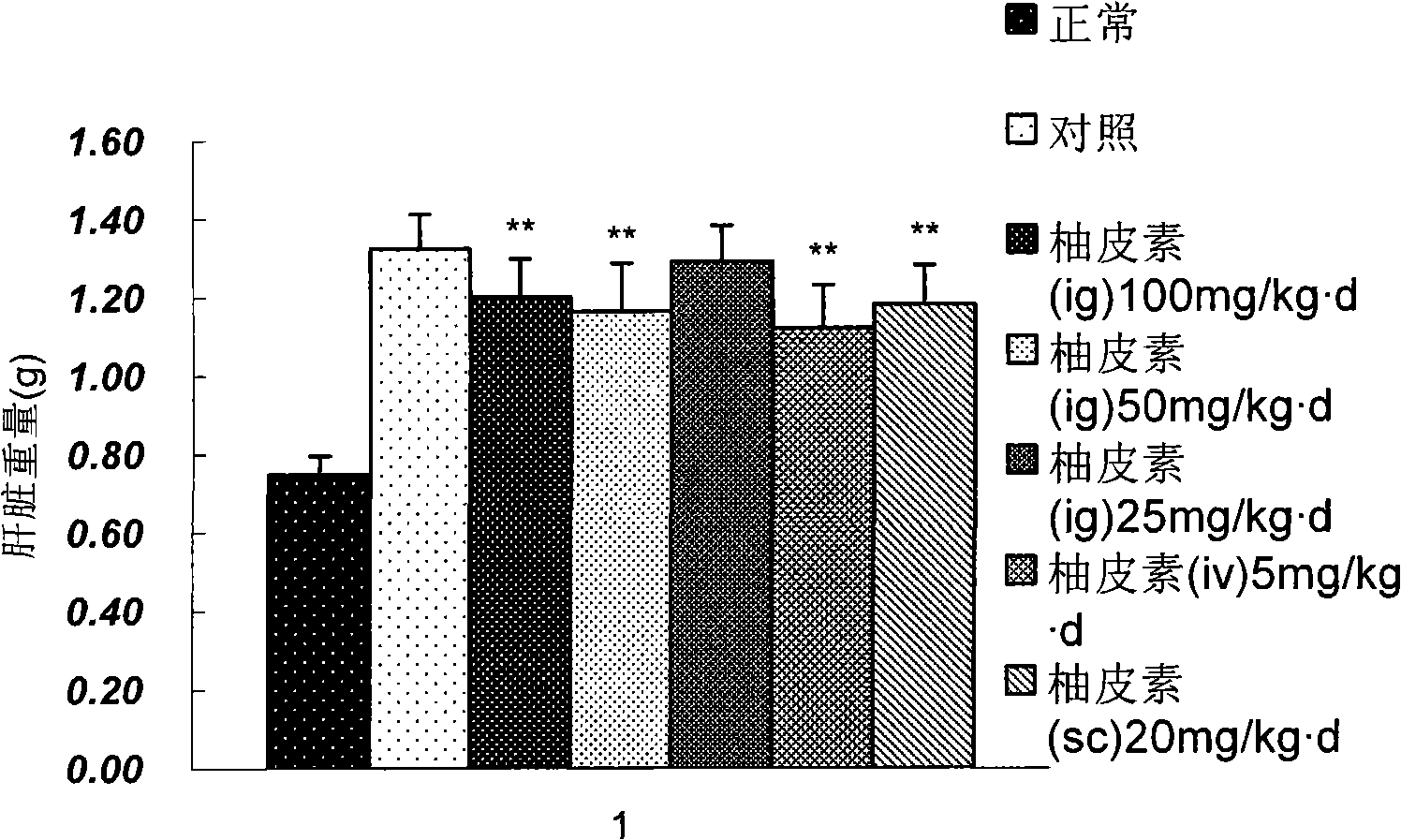
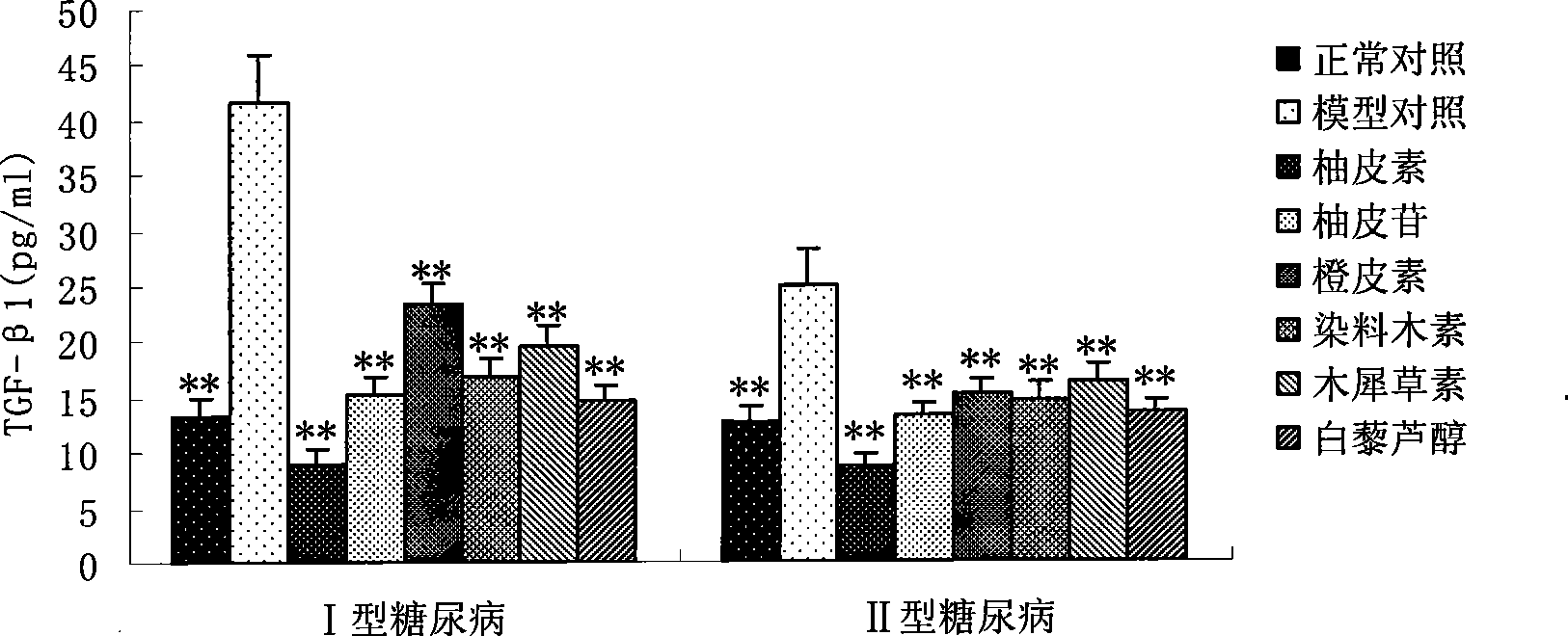
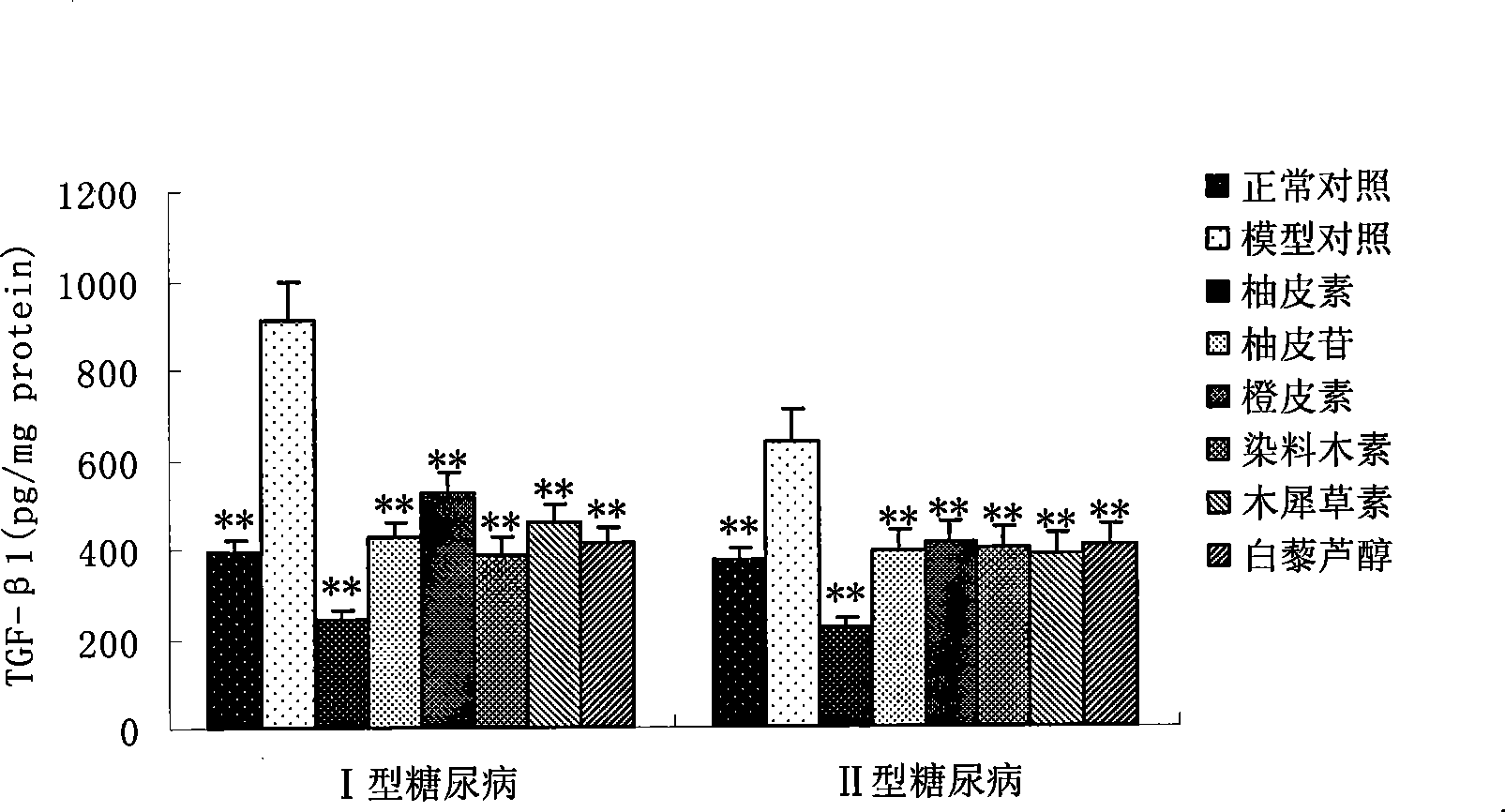
Applications of naringenin and naringin as signal pathway inhibitor of transforming growth factor-beta 1
ActiveCN101322700AReduce liver fibrosisReduce pulmonary fibrosisOrganic active ingredientsDigestive systemNaringinFibrosis
The invention discloses the application of naringenin and aurantiin as the inhibitor of the signal passage of transforming growth factor-Beta1, in particular the application to the treatment or prevention of fibrosis and tumors.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES +1
Metabonomics method for detecting multiple flavonoids in fresh tobacco leaves
The invention relates to detection of flavonoids in tobacco, in particular to a metabonomics method for detecting multiple flavonoids in fresh tobacco leaves.The method is characterized in that flavonoids in the tobacco leaves are extracted through an ultrasonic extraction method, and rutin, quercetin, naringenin, kaempferol glucoside, astragalin, naringin and isoliquiritigenin in the fresh tobacco leaves are detected through UPLC-QqQ-MS / MS.Compared with the prior art, the method has the advantages that accuracy and sensitivity are high, repeatability is good, and the method is suitable for simultaneously and quantitatively detecting the seven flavonoid compounds in plants and can be used for research on metabolic pathways and gene functions of plant flavonoid matter.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Method for extracting naringenin from grapefruits
Owner:GUILIN NATURAL INGREDIENTS CORP
Method for preparing scutellarin and analogues thereof
ActiveCN102746351AEasy to getImprove bioavailabilitySugar derivativesSugar derivatives preparationUronic acidBioavailability
The invention discloses a method for preparing scutellarin and analogues thereof. The method comprises the following steps of: preparing a 7-OH receptor compound from naringenin; preparing a uronic acid donor compound from glucuronic acid 3,6 lactone; and reacting the 7-OH receptor compound with the uronic acid donor compound to obtain scutellarin or analogues thereof finally. In the method, naringenin is taken as a raw material, and is cheap and readily available; a reaction route is flexible, and a series of analogues of scutellarin are convenient to obtain; and the obtained analogues of scutellarin have higher bioavailability.
Owner:ABIOCHEM BIOTECH CO LTD
Preparation method of naringenin molecular imprinting electrochemical sensor
InactiveCN107064256AHigh affinityHigh selectivityMaterial electrochemical variablesTetrafluoroborateCarbon nanotube
The invention discloses a preparation method of a naringenin molecular imprinting electrochemical sensor. The preparation method is characterized in that firstly, a glassy carbon electrode is modified with a nano silver / carbon nanotube, and a nano silver / carbon nanotube modified electrode is obtained; 50%-60% of ethanol, 15%-20% of dipentaerythritol triacrylate, 4%-8% of methacrylic acid, 12%-18% of 1-vinyl-3-ethyl imidazole tetrafluoroborate, 1.0%-2.0% of azo-bis-iso-heptonitrile and 3.0%-6.0% of naringenin are added to a reactor, stirred, dissolved and stirred to react at the temperature of 65+ / -2 DEG C for 12-15 h in an oxygen-free atmosphere, template molecules are removed through a methanol and acetic acid mixed solution, and naringenin molecular imprinting polymers are obtained; the naringenin molecular imprinting polymers are dispensed to the nano silver / carbon nanotube modified electrode, and the naringenin molecular imprinting electrochemical sensor is obtained. The sensor has high affinity, selectivity and sensitivity and good specificity, can realize rapid detection and can be repeatedly used.
Owner:UNIV OF JINAN
Method for preparing naringenin, hesperetin and mono-glucoside mixtures of naringenin and hesperetin
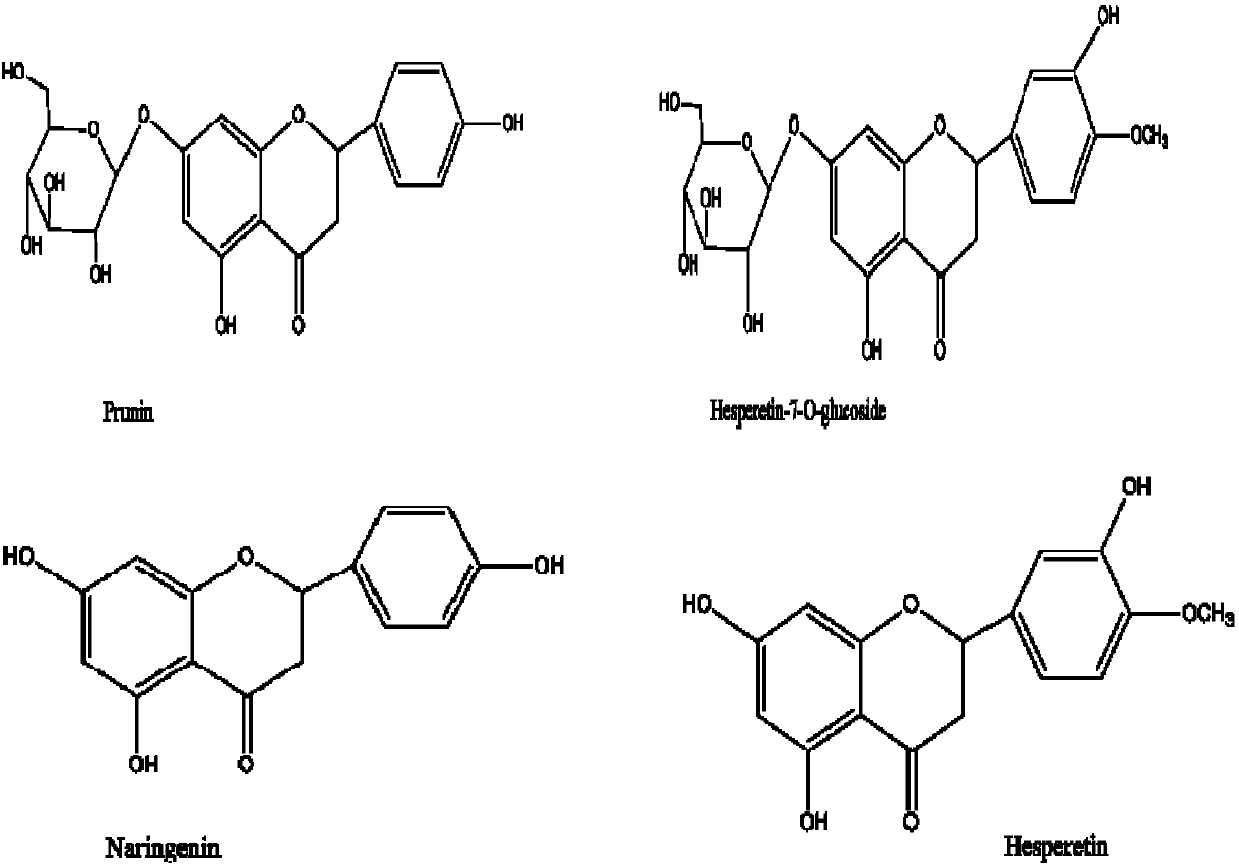
The invention provides a medicinal composition mainly containing naringenin, Prunin, hesperetin and hesperetin-7-0-glucoside. A medicine containing naringin, narirutin, hesperidin and neohesperidin isutilized as a raw material, an extract of the medicine is hydrolyzed, or extracting and hydrolyzing are conducted simultaneously on the extract of the medicine, and the plant extract can be obtained.The medicinal composition can be used for development and production of foods, health foods and new traditional Chinese medicines.
Owner:JILIN UNIV
Electronic cigarette liquid
PendingCN110353298APlay a role in health careSuppress coughTobacco treatmentLiquid smokeElectronic cigarette
The invention relates to an electronic cigarette liquid. The embodiment of the invention provides the electronic tobacco liquid. The electronic tobacco liquid comprises naringenin, wherein the contentof the naringenin in the electronic cigarette liquid is 0.09%-10% of the total weight of the electronic cigarette liquid. The electronic cigarette liquid is atomized, wherein the naringenin containedin the electronic cigarette liquid can be atomized together with the tobacco liquid and absorbed by a human body, so that the effects of diminishing inflammation, relieving cough and eliminating phlegm, inhibiting oral cavity and respiratory tract bacteria, and enabling the electronic cigarette liquid to be more comfortable in taste after being atomized can be achieved, the fragrance of the cigarettes is relatively mild, the fragrance of the cigarettes is enriched, and the smoking quality is improved.
Owner:SHENZHEN RELX TECH CO LTD
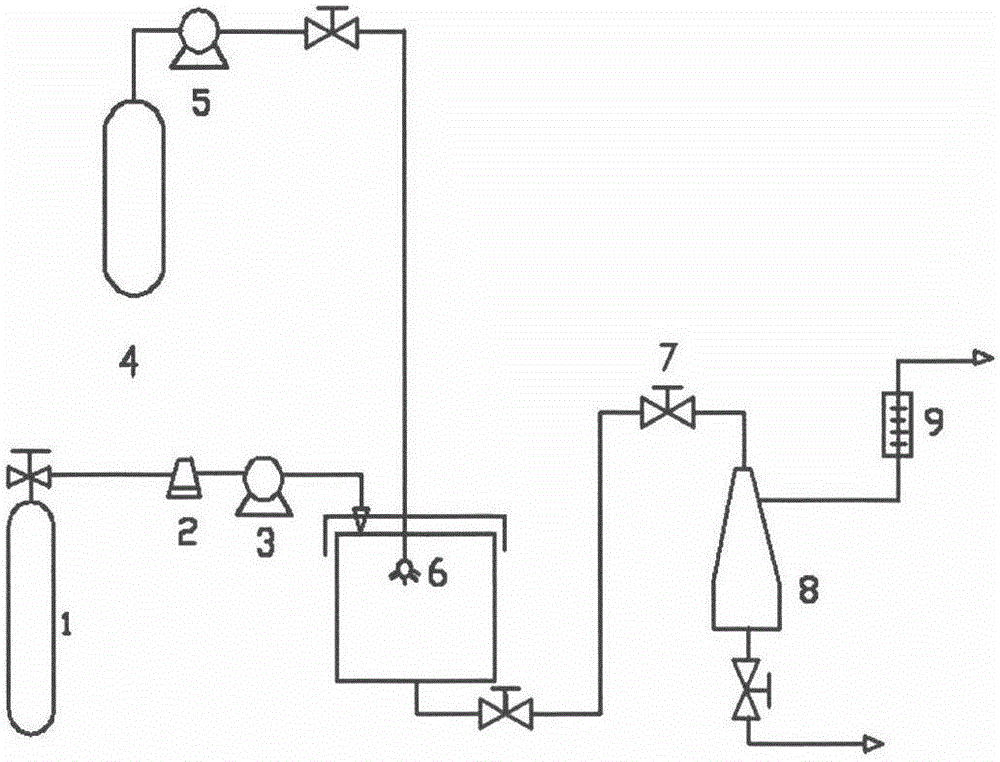
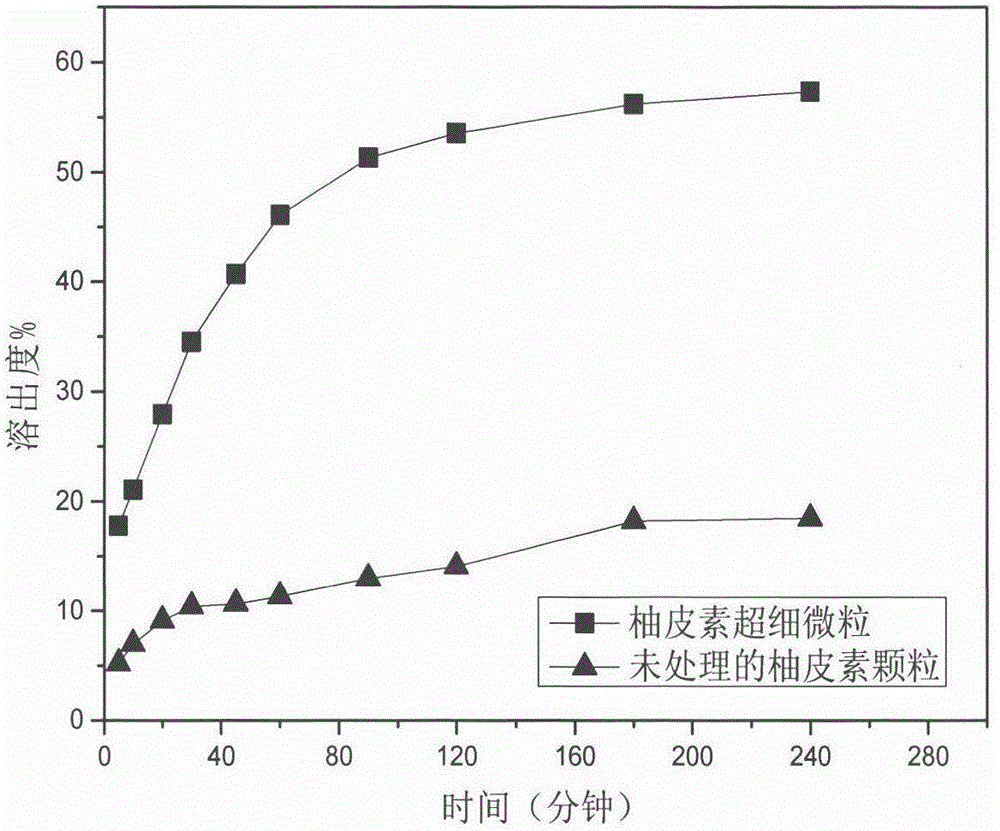
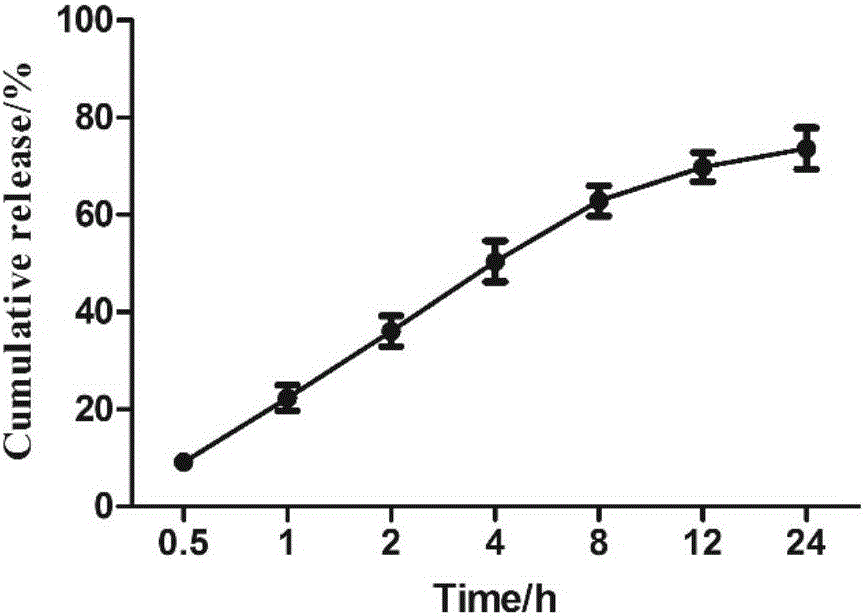
Method for preparing naringenin ultrafine particle by using supercritical compressed fluid anti-solvent precipitation process
InactiveCN104650021AImprove solubilityReasonable workmanshipOrganic chemistryBulk chemical productionAnti solventCompressed fluid
The invention relates to a method for preparing solid naringenin and particularly relates to a method for preparing naringenin ultrafine particle by using a supercritical compressed fluid anti-solvent precipitation process, so as to produce the naringenin ultrafine particle with an improved dissolution rate. By regulating pressure, temperature, solution concentration, CO2 flow rate and the like, the particle size of the naringenin ultrafine particle can be controlled effectively; the particle size of the prepared naringenin ultrafine particle is in a range of 0.5mu m to 6mu m and the product yield is above 85%. The accumulative release rate of the prepared naringenin ultrafine particle within 240min is increased by 3.2 times in comparison with that of a naringenin raw material.
Owner:CHINA PHARM UNIV
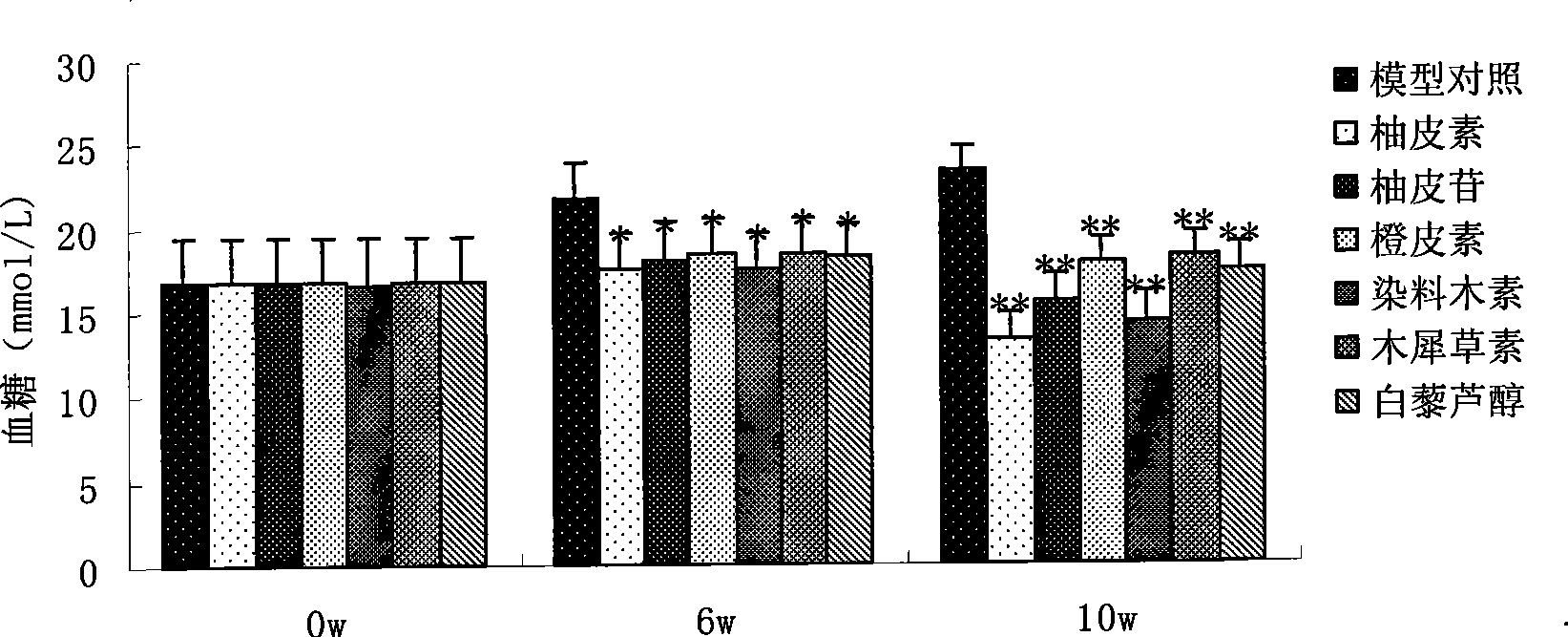
Use of resveratrol or naringenin in preventing and treating diabetic nephropathy
The invention discloses the application of resveratrol and naringenin, particularly the application of the resveratrol and the naringenin in restraining the level increasing of the transforming growth factor Beta 1 in the human body and preparing medicines used for preventing or treating diabetes and nephropathy.
Owner:ZHAOMING ZEKANG (BEIJING) BIO-PHARM TECH CO LTD
Extraction method and application of red peony synergy whitening extractive
ActiveCN105496846AGood whitening effectCosmetic preparationsToilet preparationsActivation functionAdditive ingredient
The invention belongs to the field of traditional Chinese medicine product extraction and separation, particularly relates to an extraction method and application of a red peony synergy whitening extractive, and is used for solving the problems of high content of impurities including pigment and the like and poor whitening effect since the traditional whitening cosmetics in which traditional Chinese medicine extractives are added are generally prepared from traditional Chinese medicine crude extractives. The extraction method of the red peony synergy whitening extractive comprises the following steps: dipping, and carrying out alcohol extraction, concentration, centrifugation and purification to obtain the product. The product contains seven peony monoterpenoid glycoside compounds and four flavonoid compounds, wherein the seven peony monoterpenoid glycoside compounds consist of paeoniflorin sulfurous ester, oxypaeoniflorin, albiflorin std, paeoniflorin, galloyl paeoniflorin, benzoyloxy paeoniflorin and benzoylpaeoniflorin, and the four flavonoid compounds consist of catechinic acid, dihydroquercetin, astragalin and naringenin. The product has a tyrosinase inhibition function, a blood activation function and an oxidation resistance function, and possesses the characteristics of traditional Chinese medicine cosmetic which has a diversity of functional components performing a synergy whitening function.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
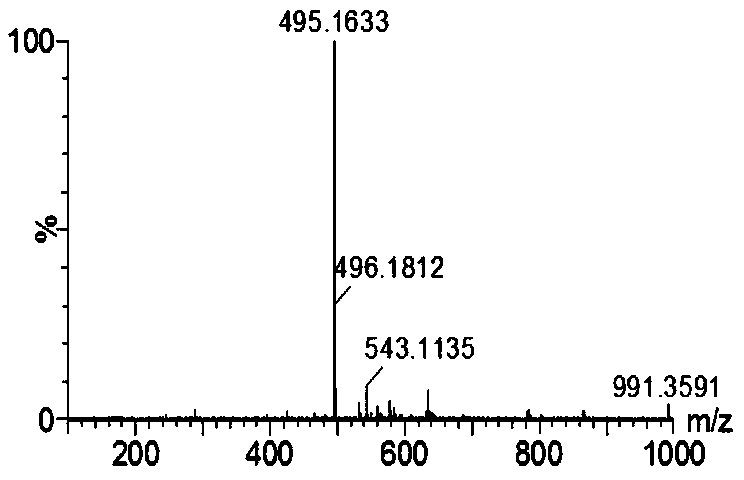
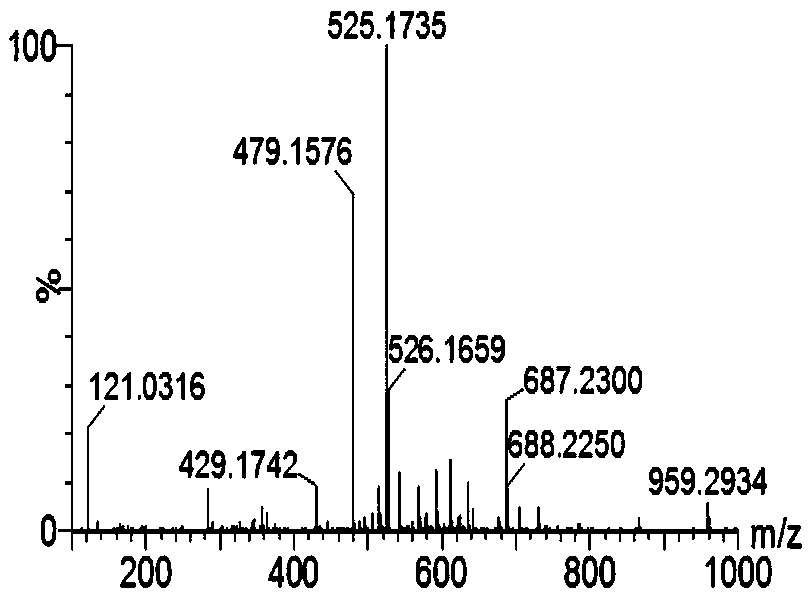
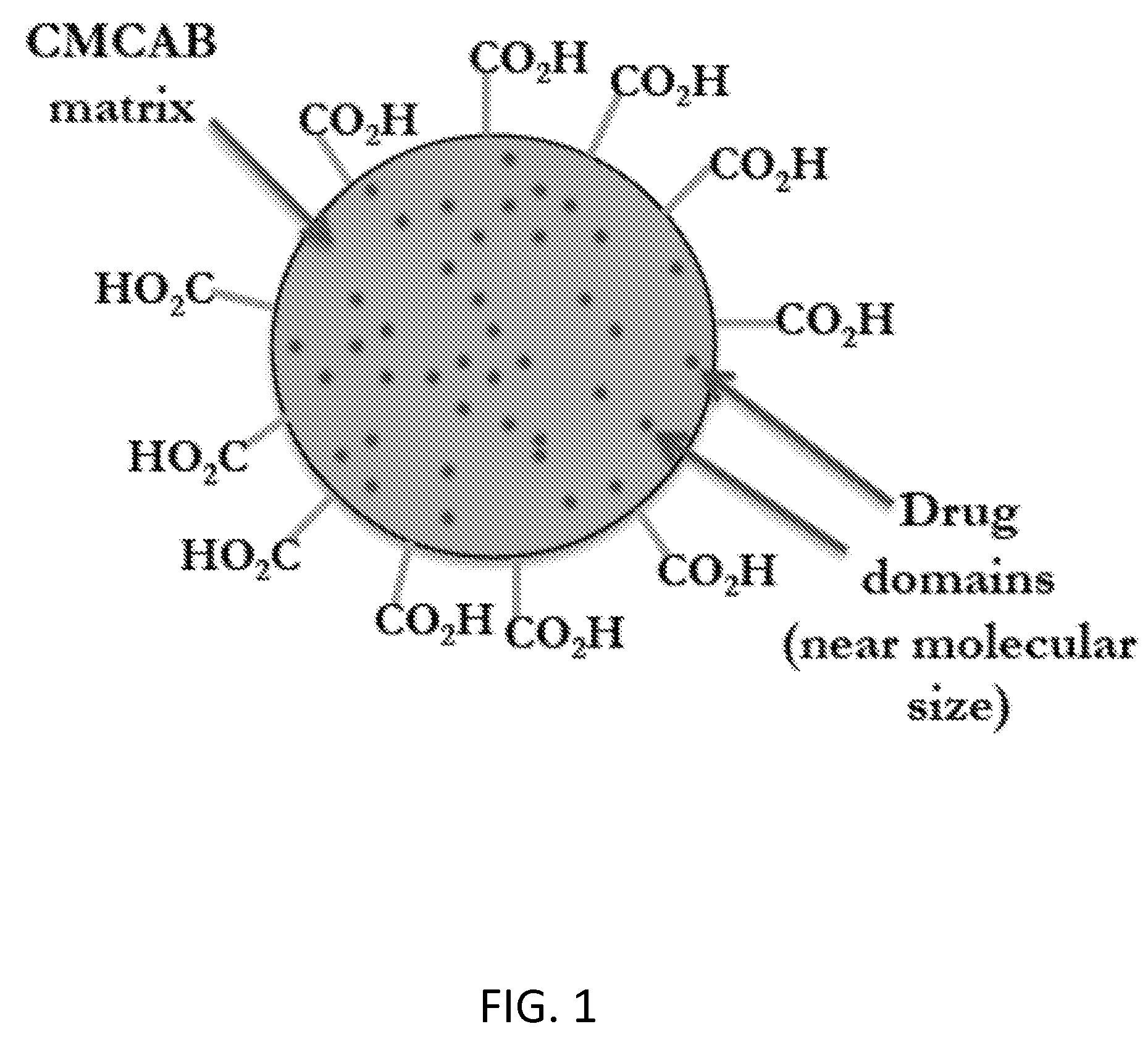
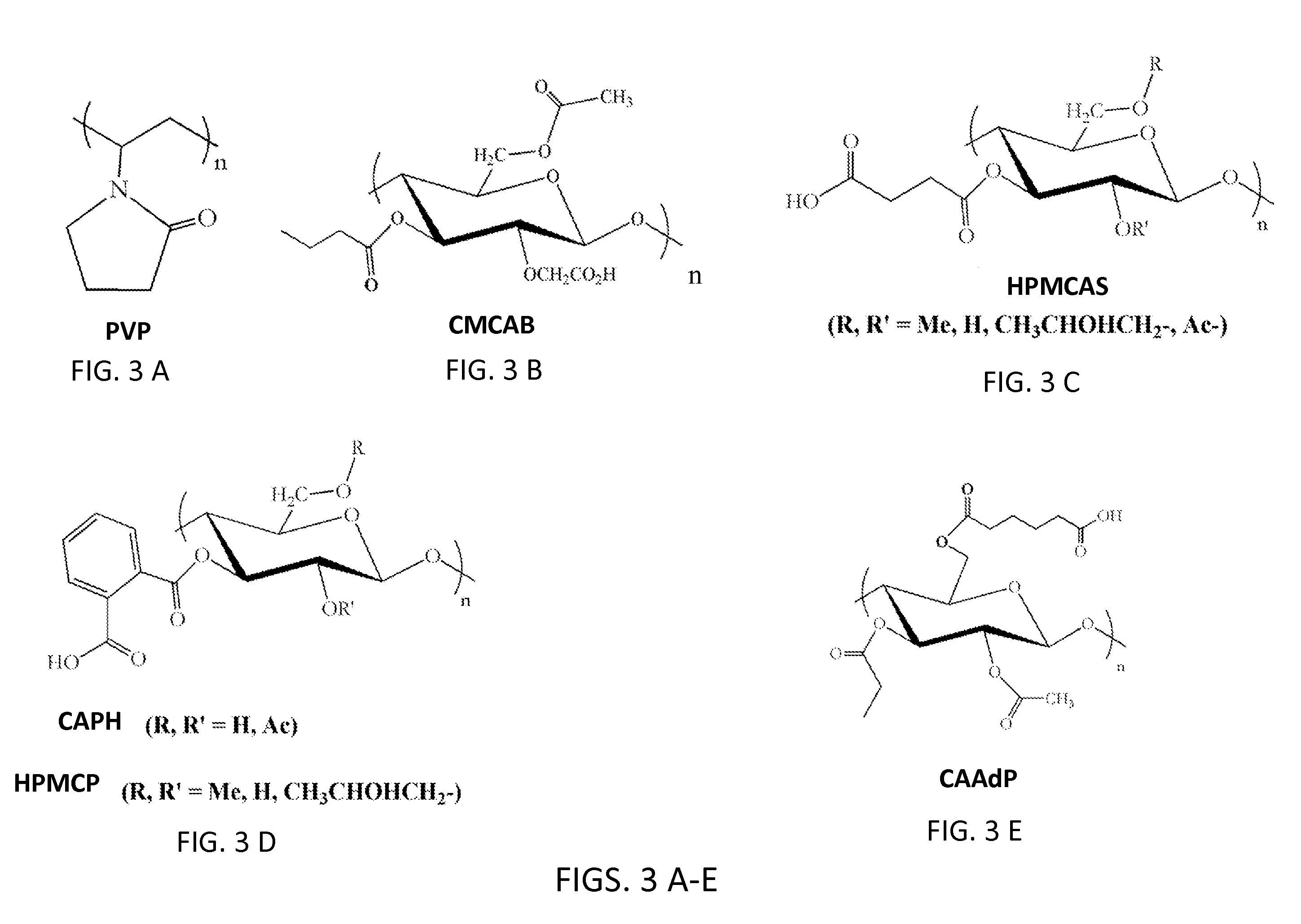
Cellulose derivatives for enhancing bioavailability of flavonoids
InactiveUS20130237609A1Increase the number ofImprove solubilityBiocideHydroxy compound active ingredientsHigh dosesHuman health
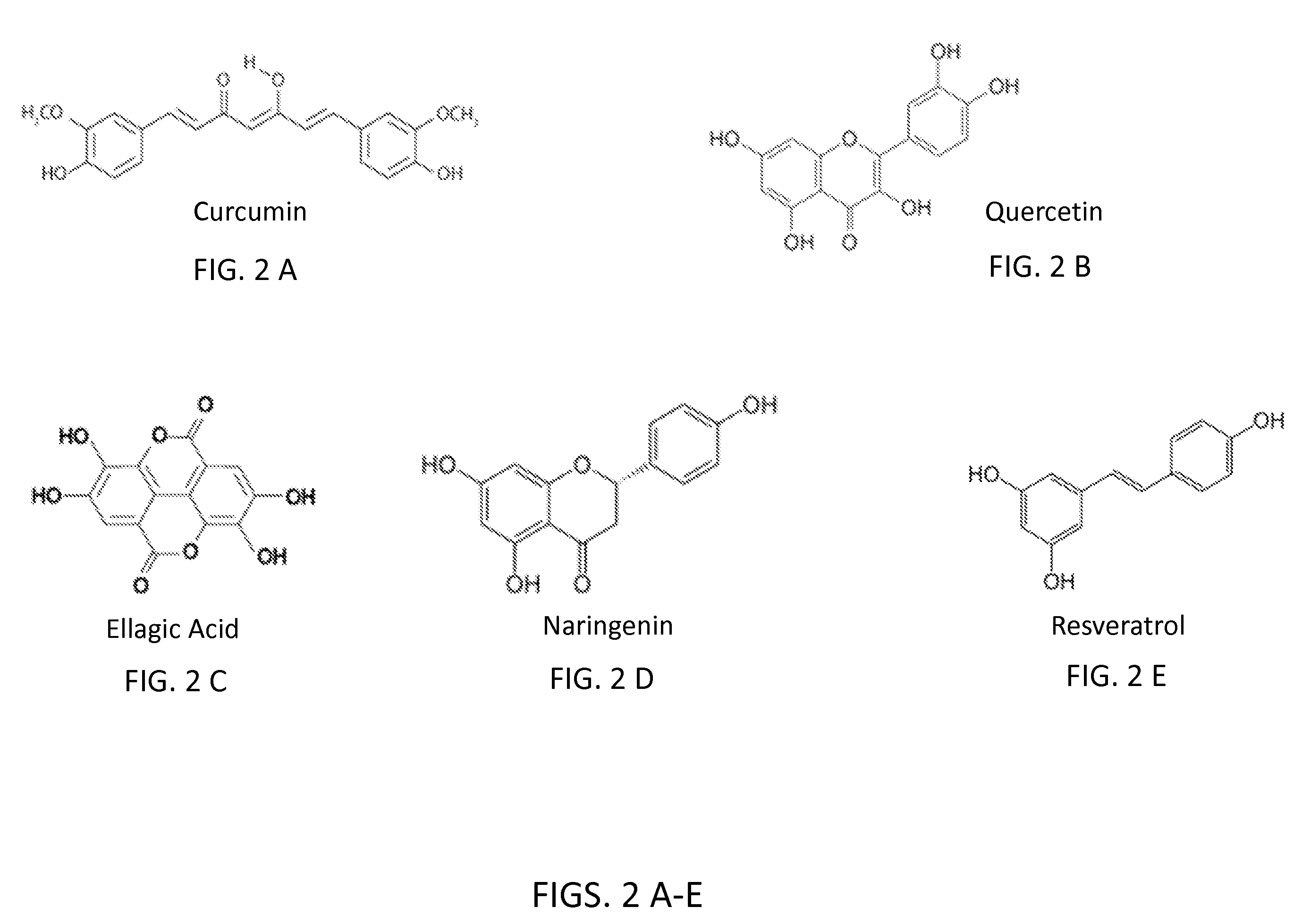
The present invention relates to delivery systems to enhance the bioavailability of flavonoids to improve human health. Flavonoids of interest include but are not limited to curcumin, resveratrol, ellagic acid, naringenin, and quercetin. Flavonoids are important in part because they are known to have beneficial effects on human health, including cardioprotective, antioxidant, and anticancer effects. Utility has been limited by low bioavailability, both in the sense of requiring high doses and in that it has been difficult to carry out proper dose-response studies in the absence of methods to control the actual dose administered. In addition to pharmaceutical applications, there are potential nutraceutical uses, for example in supplements that might be sold in health food stores and pharmacies.
Owner:EDGAR KEVIN J +4
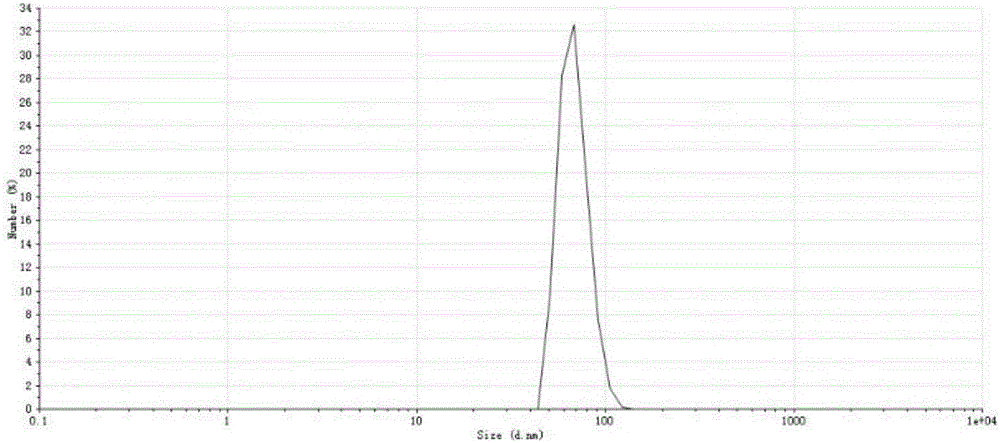
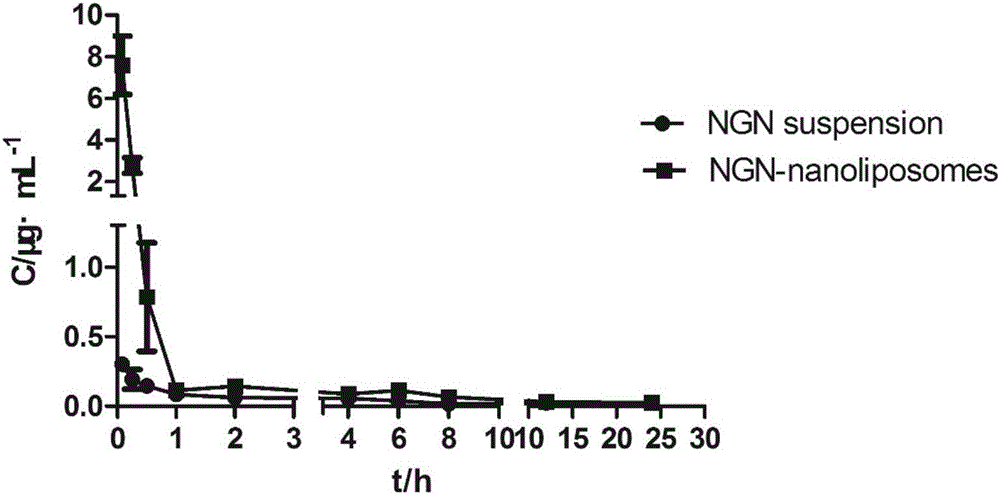
Purpose of naringenin, naringenin nanoliposome and preparation method and application thereof
ActiveCN107432874AEasy to prepareEasy to controlOrganic active ingredientsDigestive systemWater bathsCholesterol
The invention discloses purpose of naringenin, naringenin nanoliposome and a preparation method and application thereof. The naringenin is applied in the preparation of drugs for treating non-alcoholic fatty liver. The naringenin nanoliposome comprises naringenin and nanoliposome. The nanoliposome comprises phosphatide and cholesterol. The mass ratio of naringenin to phosphatide to cholesterol is 1:4-9:1-2. The preparation method comprises the following steps: 1) dissolving naringenin, phosphatide and cholesterol in a solvent, mixing, and removing the solvent to obtain a mixture; 2) hydrating the mixture obtained in the step 1) by the use of an aqueous medium to obtain a naringenin nanoliposome crude suspension; and 3) successively carrying out water-bath ultrasonic treatment and probe ultrasonic treatment on the naringenin nanoliposome crude suspension obtained in the step 2), so as to obtain the naringenin nanoliposome. The naringenin nanoliposome can raise oral bioavailability of naringenin and enhance the control effect of naringenin on non-alcoholic fatty liver.
Owner:PEKING UNIV
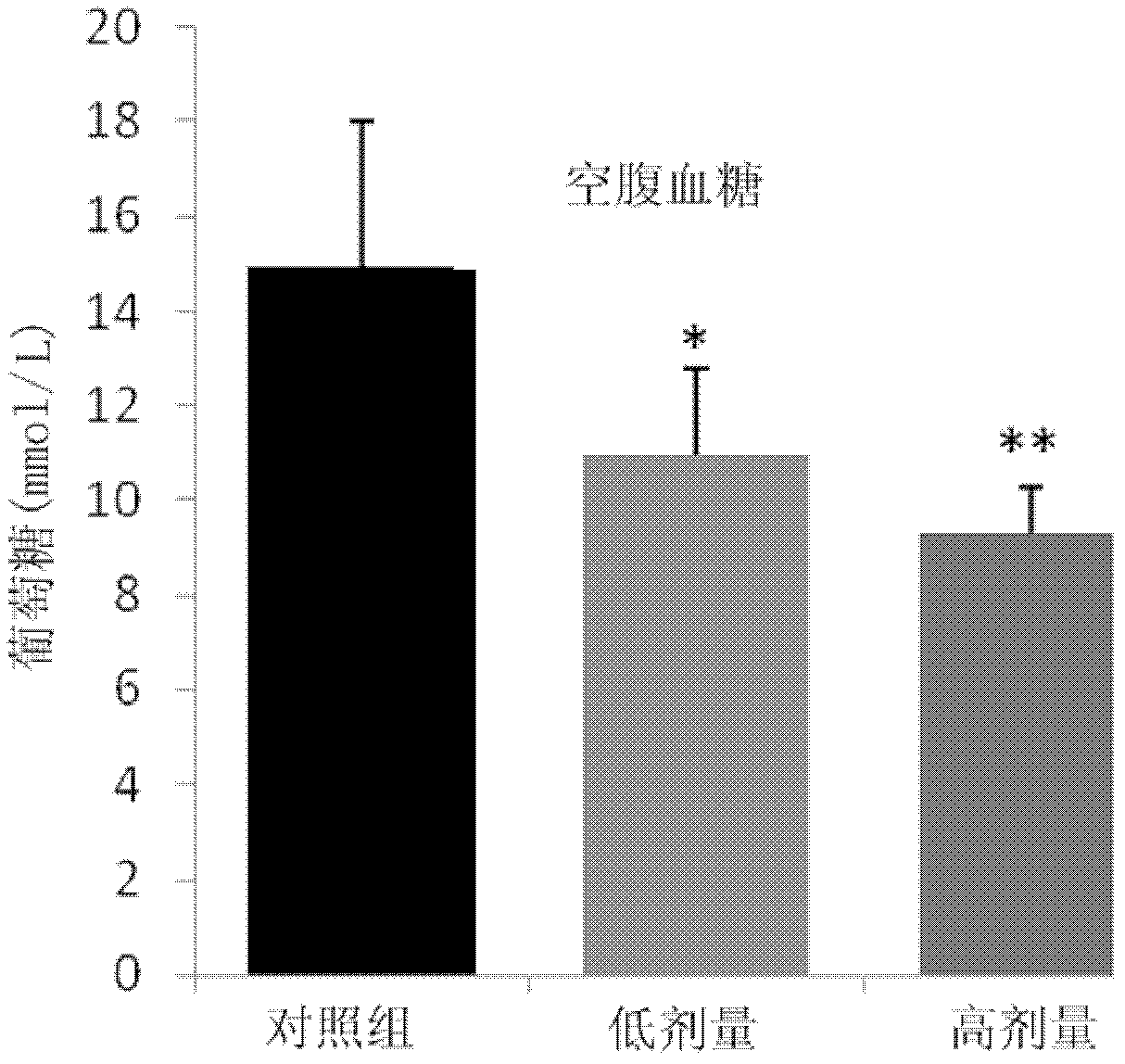
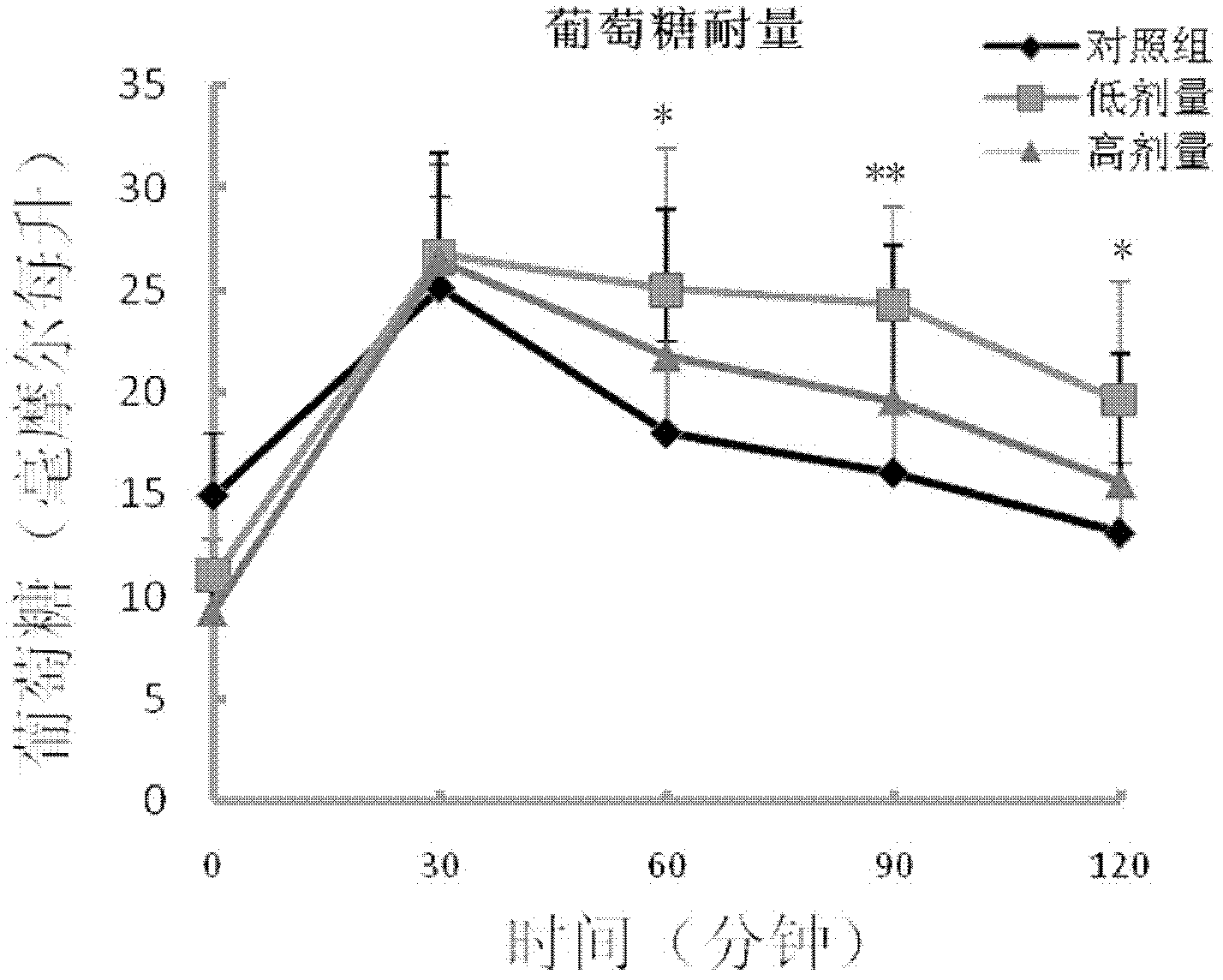
Blood sugar reducing composition and application thereof
ActiveCN111388461AGood synergySmall doseOrganic active ingredientsMetabolism disorderPharmaceutical drugQuercitrin
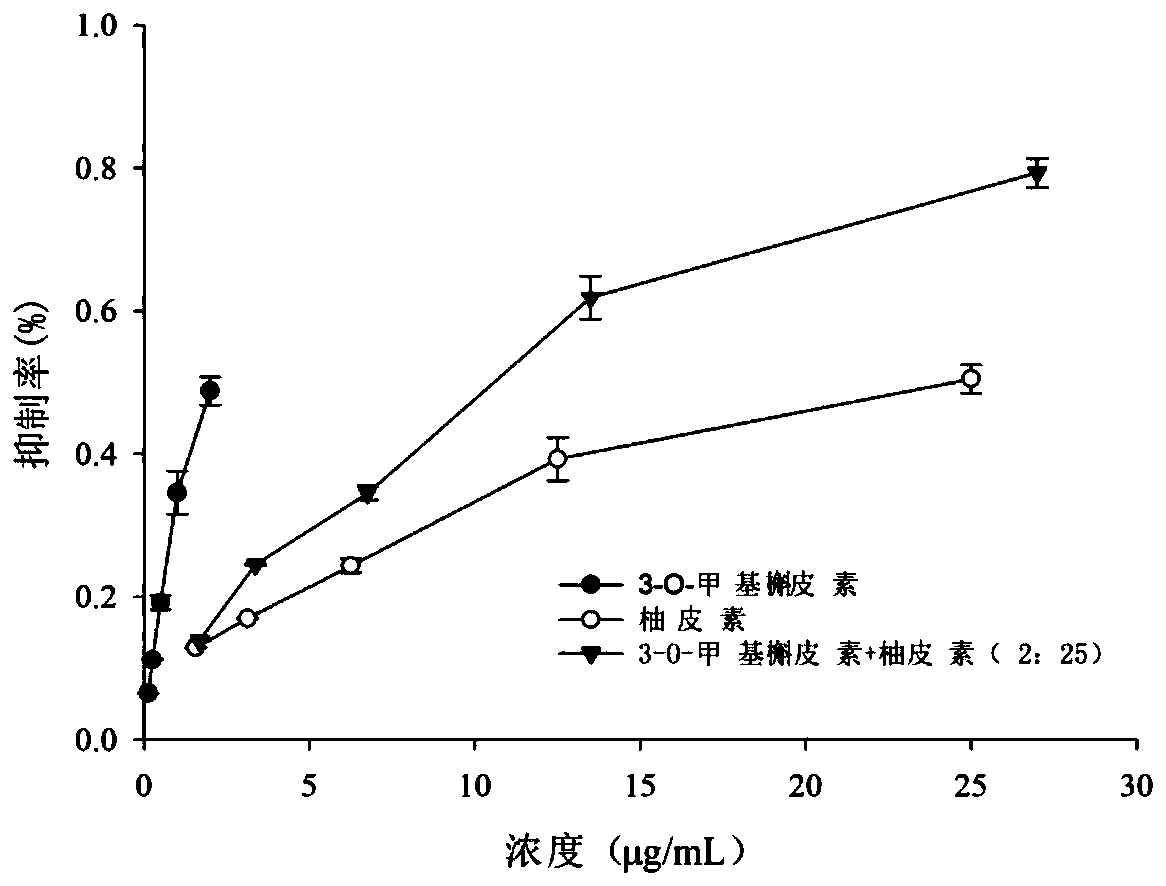
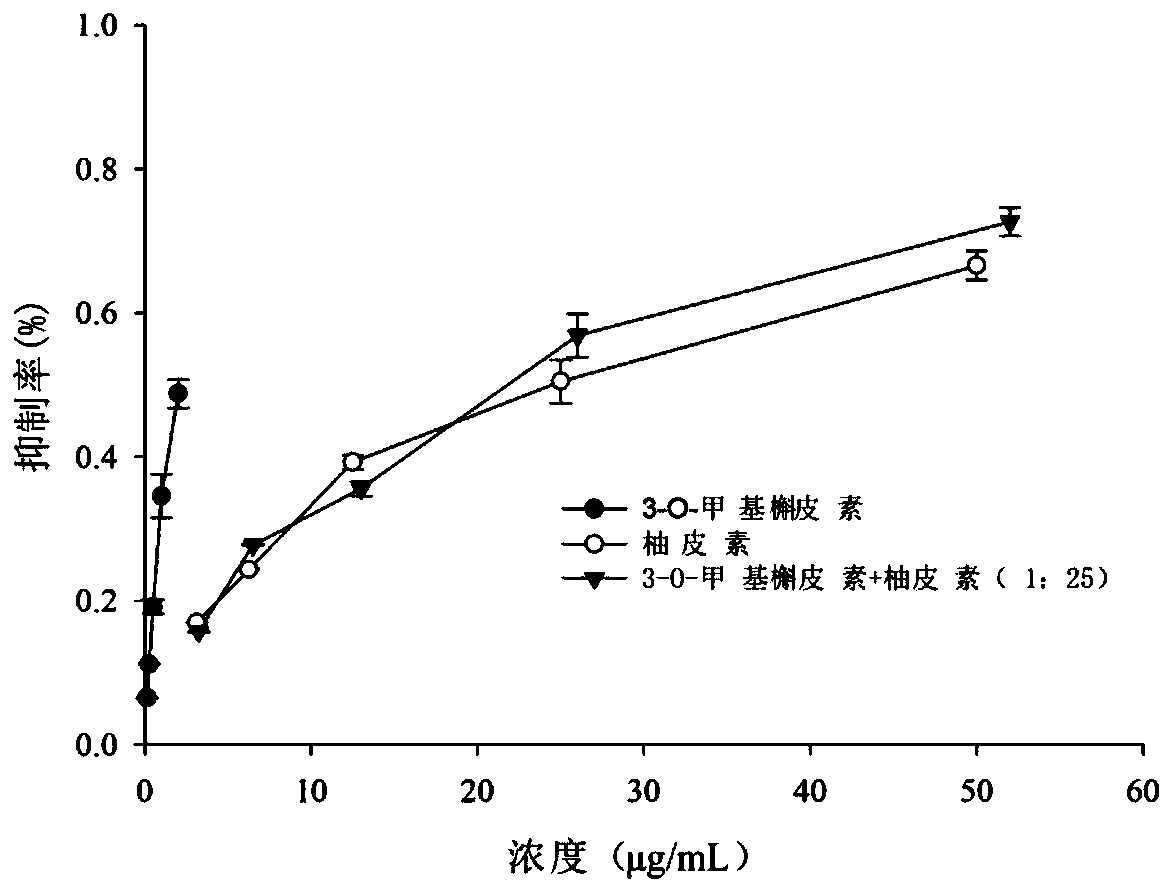
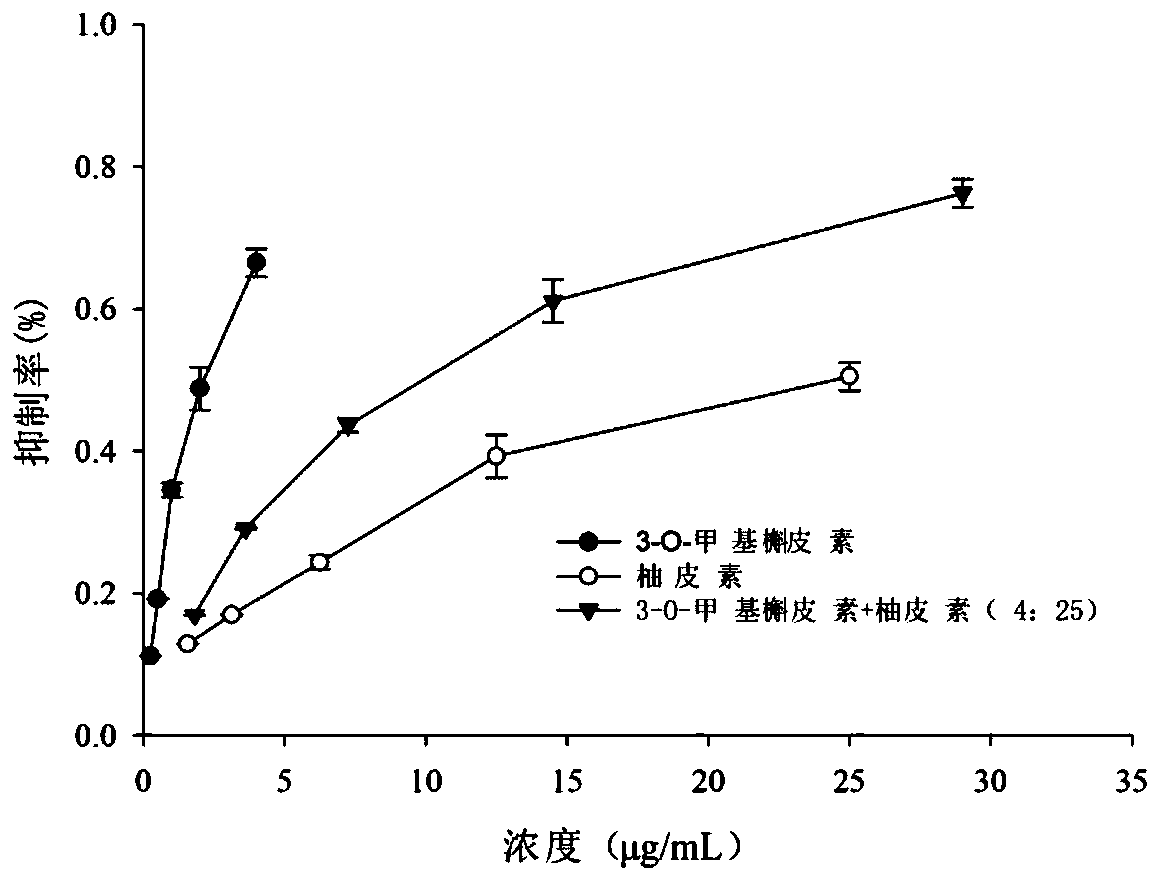
The present invention discloses a blood sugar reducing composition and an application thereof, and belongs to the technical field of medicines. The blood sugar reducing composition is prepared from 3-O-methyl quercetin and naringenin according to a mass concentration ratio of (1:25) to (6:25). Preferably, the mass concentration ratio of the 3-O-methyl quercetin to the naringenin is (2:25) to (6:25). The 3-O-methyl quercetin and naringenin composition has the obvious alpha-glucosidase inhibiting synergistic effect, the effect is superior to that of a flavone compound used alone, the medicine dosage can be reduced, and the occurrence of drug resistance is reduced; and the composition has the wide application prospect in the preparation of diabetes treatment medicines, health care products orfoods.
Owner:ZHENGZHOU FRUIT RES INST CHINESE ACADEMY OF AGRI SCI
Wikstroemia indica (L.) C.A.Mey extract as well as preparation method and application thereof
InactiveCN102344454AWide range of clinical applicationsEffective part is clearOrganic active ingredientsAntipyreticBenzoic acidCytotoxicity
The invention discloses a wikstroemia indica (L.) C.A.Mey extract as well as a preparation method and application thereof. The wikstroemia indica (L.) C.A.Mey extract has the effective compositions of: naringenin, 5, 6, 7- trihydroxy-4'-methoxyflavanone, p-hydroxybenzoic acid, benzoic acid, quercetin glycoside,genkwano1 B, daphnoretin, Lirioresinol, Sikokianin and the like. The invention simultaneously provides application of the wikstroemia indica (L.) C.A.Mey extract, in particular the favorable application in preparing anti-cancer or anti-inflammation analgesia drug. A cytotoxicity experiment shows that the wikstroemia indica (L.) C.A.Mey extract disclosed by the invention has cytotoxicity functions on breast cancer of human, breast cancer drugresistant cell strain, mouse breast adenocarcinoma, which are in linear relation.
Owner:GUANGDONG PHARMA UNIV
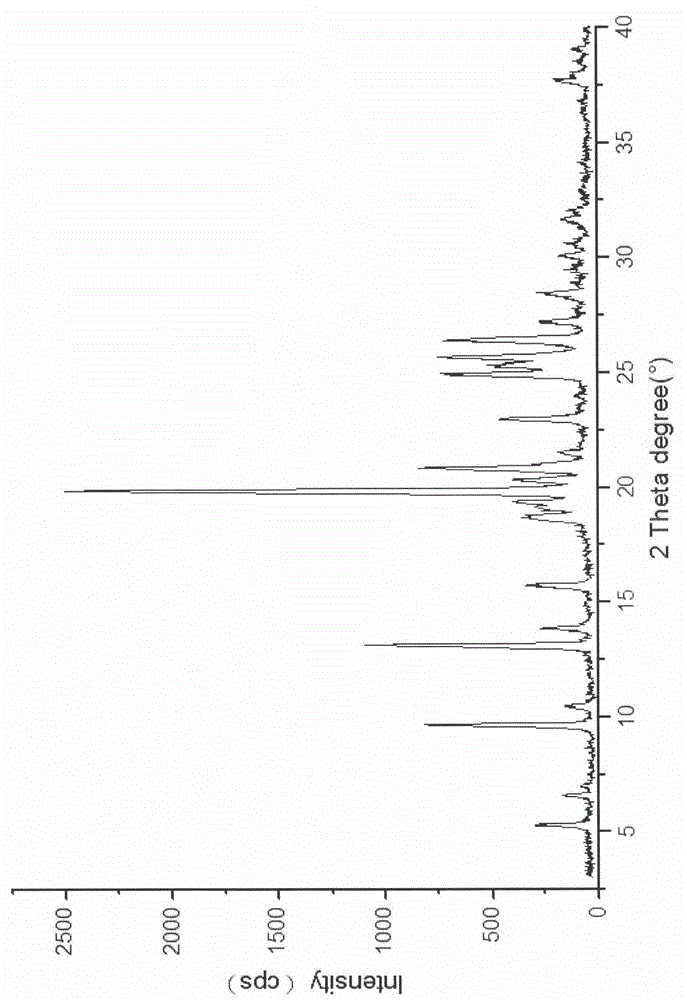
Naringenin isonicotinamide co-crystal
The invention belongs to the field of a medical technology and specifically relates to a co-crystal formed by naringenin and isonicotinamide, wherein stoichiometric ratio of naringenin to isonicotinamide in the co-crystal is 1:2. The co-crystal has an X-ray powder diffraction pattern, a differential scanning calorimetry pattern and an infrared spectrogram which are different from those of naringenin crystals and a physical mixture of naringenin and isonicotinamide, is a novel crystalline form completely different from naringenin, and has remarkably-enhanced solubility.
Owner:CHINA PHARM UNIV
Hypoglycemic compound, its preparation method and health food with hypoglycemic function
The invention relates to a hypoglycemic compound, its preparation method and a health food with hypoglycemic function. The compound contains the following components of: by weight, 300-600 parts of propolis, 300-600 parts of naringenin, 60-120 parts of anthocyanidin, 0.5-1.5 part of momordica polypeptide, 0.001-0.01 part of vitamin D3, 0.001-0.1 part of vitamin H, 8-15 parts of vitamin E, 0.01-0.2 part of chromium element and 1-20 parts of zinc element. The hypoglycemic compound can be prepared by directly mixing raw materials containing the above components. According to the invention, with the combination of the above components, a good synergistic effect is generated among all the components. The invention has advantages of obvious hypoglycemic function, easily-obtained raw materials, simple preparation and relatively low cost. In addition, the components of the hypoglycemic compound are all natural bioactive substances. Therefore, the compound has good security and no toxic and side-effect and is especially applicable to be used as a health food for adjunctive treatment of diabetic patients.
Owner:中哈福生物医药科技(上海)有限公司
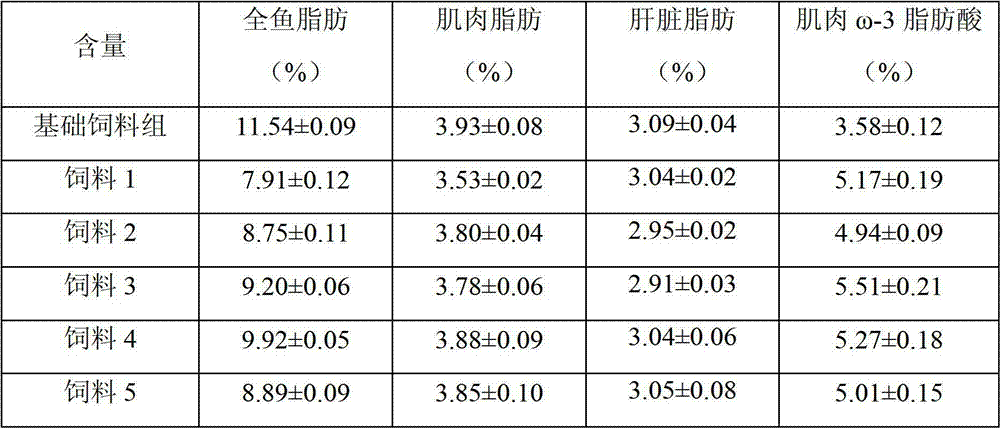
Salmon and trout feed based on peroxisome proliferator activated receptor (PPAR) and method for preparing salmon and trout feed
The invention provides a salmon and trout feed based on a peroxisome proliferator activated receptor (PPAR) and a method for preparing the salmon and trout feed. The salmon and trout feed comprises proteins, fat, trace elements and other basic substances which are required by the salmon and trout growth, and further contains natural PPAR alpha and / or PPAR beta binding active substances, such as daidzin, curcumin, isoflavone, arachidonic acid, berberine, naringenin, polydatin, kaempferol, isoflavoues aglycone, conjugated linoleic acid and the like. The salmon and trout feed can regulate the fat and fatty acid anabolism of the cultured salmon and trout, the fat content of the salmon and trout is lowered, the beneficial omega-3 fatty acid content is improved, and the salmon and trout quality is improved.
Owner:BEIJING FISHERIES RES INST
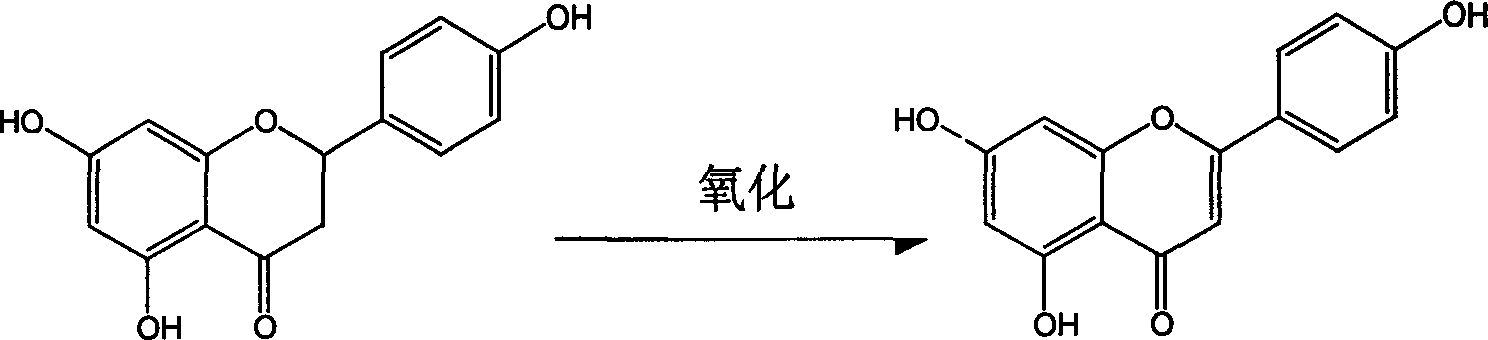
Process for semi-synthesizing of apiolin
The invention discloses a semi compounding method for apium celery element that uses natural extract as basic raw material. It uses pomelo peel element as raw material, 1, 4-dioxy cyclohexane as reaction solvent, taking oxidative dehydrogenation reaction with iodine to form raw apium celery element. Taking multi-steps recrystallizing, the refined apium celery element would be gained. The invention is easy to operate, stable reacting condition, easy to control. The yield would be 60-70% and the purity could be over 97%. And it is low cost, no harmful to the environment.
Owner:ZHEJIANG TIANCAO BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com