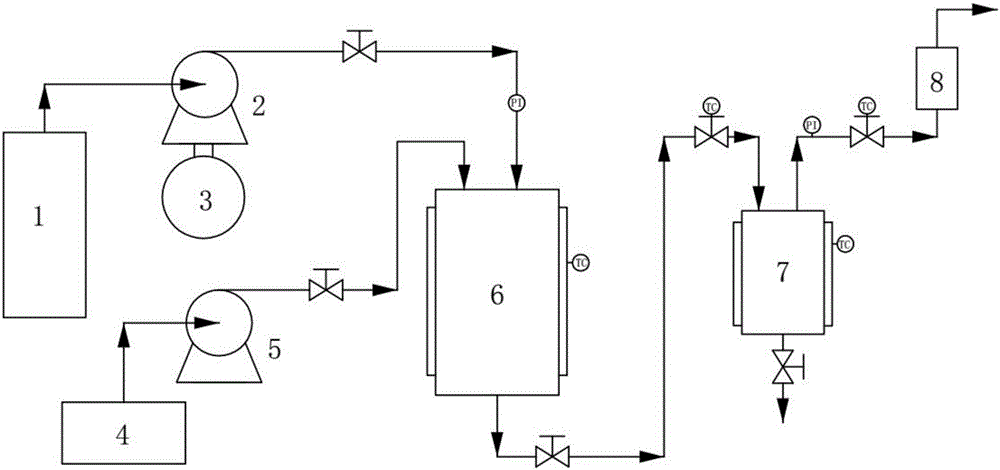
Method for preparing naringenin/hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process
A supercritical anti-solvent, naringenin technology, applied in microcapsules, antidote, capsule transportation and other directions, can solve the problems of slow dissolution rate, poor water solubility of naringenin, limited application, etc. Availability and the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
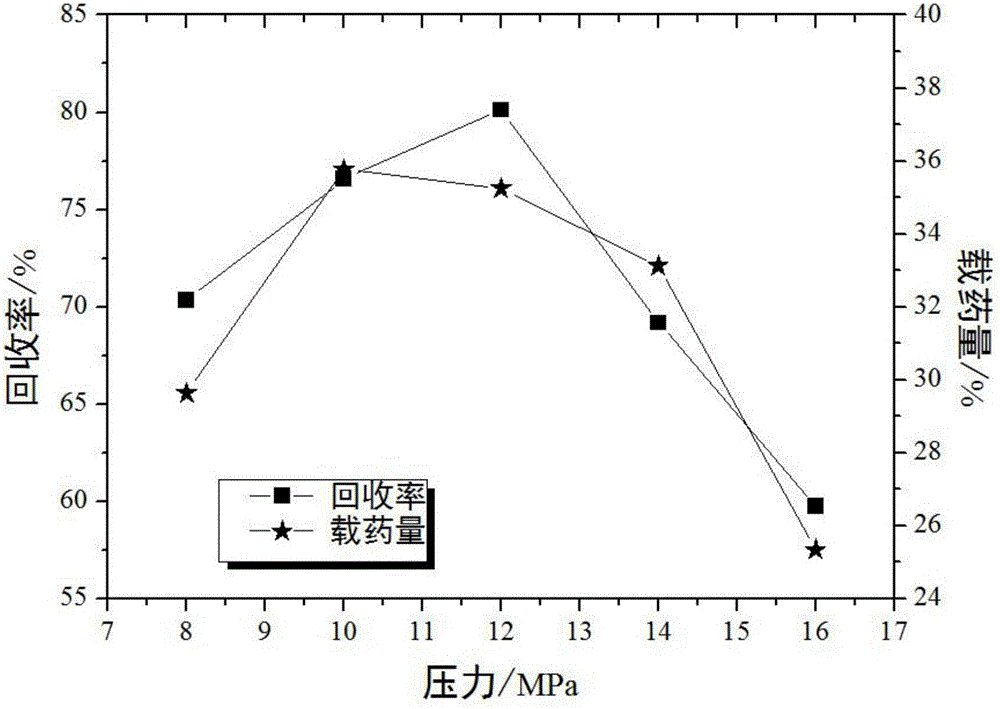
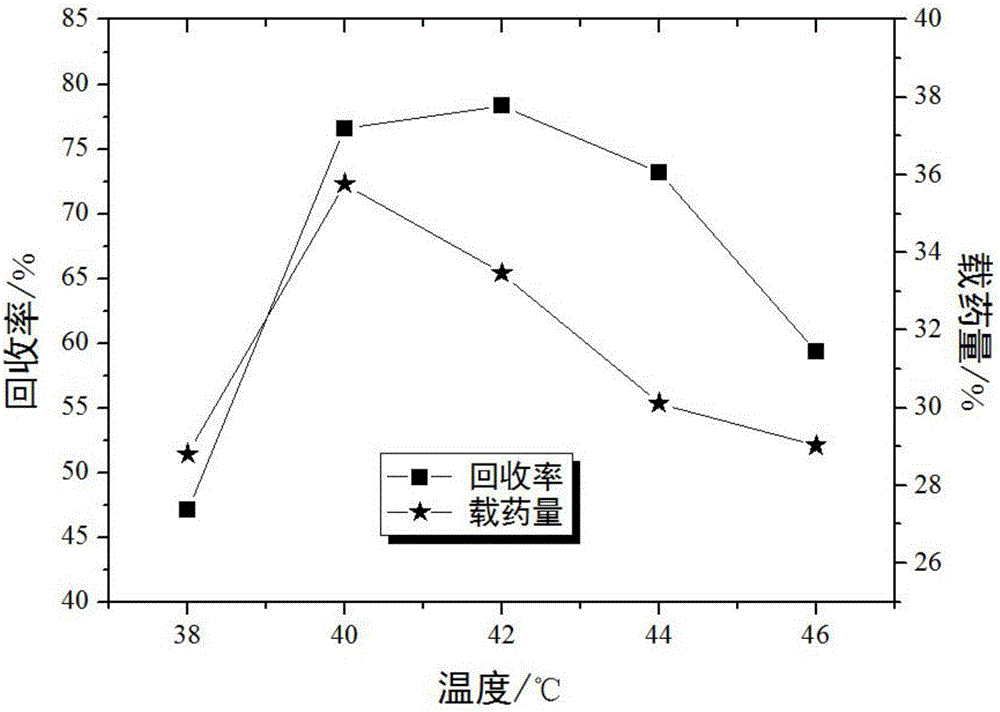
[0033] Embodiment 1: single factor method determines the preferred value range of each key parameter
[0034] Experimental Instruments and Materials
[0035] The main instruments used in the experiment are shown in Table 1, and the main raw materials and reagents are shown in Table 2.
[0036] Table 1 Main Instruments
[0037] device name model Manufacturer Supercritical Particle Preparation System Helix AppliedSeparations, USA Series1500 high pressure infusion pump Helix AppliedSeparations, USA air compressor pump TYW-2 Suzhou Tongtong Electromechanical Co., Ltd. Low temperature constant temperature bath SDC-6 Nanjing Xinchen Biotechnology Co., Ltd. UV-visible spectrophotometer UV-1800 Shimadzu Corporation Intelligent Dissolution Tester ZRS-8L Tianjin Tianda Tianfa Technology Co., Ltd. Analytical Balances BS124S Beijing Sartorius Instrument System Co., Ltd.
[0038] Table 2 Main raw materials ...
Embodiment 2
[0067] Embodiment 2: Orthogonal optimization of the best test parameters
[0068] Orthogonal experimental design and results
[0069] With the recovery rate as an index, an orthogonal experiment is designed to investigate crystallization pressure (A), crystallization temperature (B), solution concentration (C), and solution volume flow rate (D). Table 4 is a factor level design table, and Table 5 is an orthogonal experiment Design and Results.
[0070] Table 4 Factor level table
[0071]
[0072] Table 5 Orthogonal design and results
[0073] Test No. A / MPa B / ℃ C / g·L -1 D / mL·min -1 Recovery rate / % 1 1 1 1 1 75.43 2 1 2 2 2 68.16 3 1 3 3 3 65.58 4 2 1 2 3 84.76 5 2 2 3 1 83.24 6 2 3 1 2 78.23 7 3 1 3 2 72.38 8 3 2 1 3 58.25 9 3 3 2 1 79.34 K 1 69.723 77.523 70.637 79.337 K 2 82.077 69.883 77.420 72.923 K 3 69.990 74.383 73.733 69.530 ...
Embodiment 3
[0080] Example 3: Characterization analysis of naringenin / HP-β-CD microcapsules
[0081] DSC analysis
[0082] DSC analysis of naringenin / HP-β-CD microcapsules such as Figure 6 It can be seen from C that the melting point of naringenin is 251.7°C. It can be seen from A and B that the melting point peak of naringenin at around 251.7°C disappears, which proves that naringenin is successfully included in HP-β-CD.
[0083] Solubility test
[0084] Take the excess naringenin raw material drug and 3 parts of naringenin / HP-β-CD microcapsule samples prepared under the optimal process, put them in a 20mL conical flask with a stopper, and add 20mL of purified water. Shake at a constant temperature at 37°C for 48 hours, take the supernatant, filter it with a 0.45 μm microporous membrane, measure the absorbance value at a wavelength of 288 nm after appropriate dilution of the subsequent filtrate, and substitute the absorbance value into the standard curve equation to calculate The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com