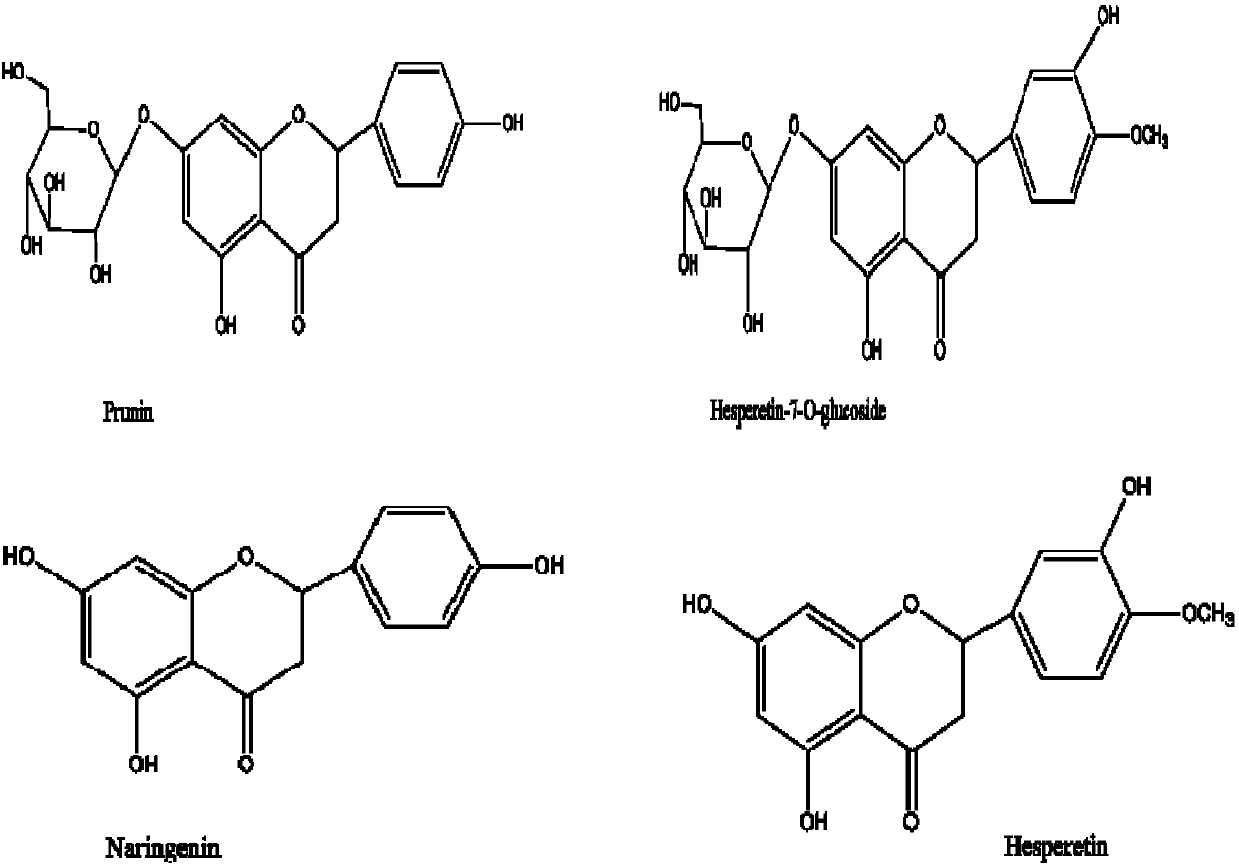
Method for preparing naringenin, hesperetin and mono-glucoside mixtures of naringenin and hesperetin
A technology for hesperetin and naringenin, which is applied in directions such as pharmaceutical combinations, pharmaceutical formulations, and medical preparations containing active ingredients, can solve the problem that there is no literature report on the preparation method of plant extracts, it is difficult to obtain extracts, and there is no discovery. problems, to achieve the effect of saving the use of organic solvents, high yield and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 10g of Citrus aurantium, crush, sieve, dissolve in 200mL of 85% ethanol, add hydrochloric acid to make the concentration reach 1.6mol / L, stir in a water bath at a temperature of 75°C, extract and hydrolyze, react for 2.5h; recover ethanol, Obtained 4.8 g of a brown solid. As determined by HPLC, the contents of the four compounds in the mixture are 6.3% naringenin monoglycoside, 4.1% hesperetin monoglycoside, 3.9% naringenin and 2.4% hesperetin.
Embodiment 2
[0039] Take 10g of Citrus aurantium, crush, sieve, dissolve in 200mL of 85% ethanol, add hydrochloric acid to make the concentration reach 1.6mol / L, stir in a water bath at a temperature of 75°C, extract and hydrolyze, react for 2.5h; recover ethanol, Extract with ethyl acetate (200mL×4), recover ethyl acetate under reduced pressure to obtain 3.49g of a yellow solid, as determined by HPLC, the contents of the four compounds in this mixture are respectively 13% of naringenin monoglycoside, 13% of hesperetin monoglycoside 11%, naringenin 7%, hesperetin 5%. The conversion rate of prunin was 46.0%; the conversion rate of hesperetin monoglycoside was 39.0%; the conversion rate of naringenin was 40.1%; the conversion rate of hesperetin was 26.3%.
Embodiment 3
[0041] Take 10g of Citrus aurantium, crush, sieve, dissolve in 200mL of 85% ethanol, add hydrochloric acid to make the concentration reach 1.6mol / L, stir in a water bath at a temperature of 75°C, extract and hydrolyze, react for 2.5h; recover ethanol, Extract with ethyl acetate (200mL×4), recover ethyl acetate under reduced pressure to obtain 3.40g of yellow solid, take this mixture as mobile phase and carry out silica gel column chromatography, collect naringenin, naringenin monoglycoside, hesperetin 1. The components of hesperetin monoglycoside were combined, and the solvent was recovered under reduced pressure to obtain 2.4 grams of the plant extract with the highest content. As determined by HPLC, the contents of the four compounds in the mixture are 18.0% of naringenin monoglycoside, 15.4% of hesperetin monoglycoside, 10.2% of naringenin and 7.6% of hesperetin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com