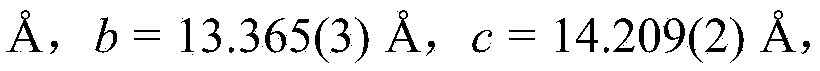Patents
Literature
54 results about "Isonicotinamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
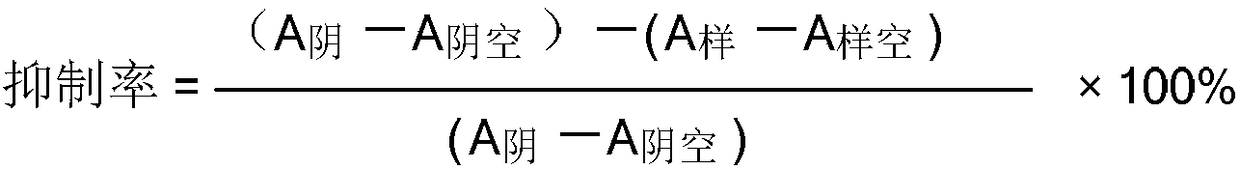
Isonicotinamide (pyridine-4-carboxamide) is the amide form of isonicotinic acid. It is an isomer of nicotinamide, which has the carboxamide group in the 3-position. It is soluble in water at 191 gm/L, also in ethanol, DMSO, methanol, chloroform, chloroform methanol mixture, and in dioxane 10 mg/L. This compound is used for material synthesis and is generally recognised as safe.
A cyanide-free lytic reagent compositiona nd method for hemoglobin and cell analysis
The present invention relates to a cyanide-free lytic reagent composition and method for measuring the total hemoglobin concentration in a blood sample, for counting the number of leukocytes and for differential counting of three leukocyte subpopulations including lymphocytes, monocytes, and granulocytes. The cyanide-free lytic reagent composition comprises a hemolytic surfactant selected from the group consisting of quaternary ammonium salts, pyridinium salts, organic phosphate esters, and alkyl sulfonates to lyse erythrocytes and release hemoglobin, and an organic ligand selected from the group consisting of triazole and its derivatives, tetrazole and its derivatives, alkaline metal salts of oxonic acid, melamine, aniline-2-sulfonic acid, quinaldic acid, 2-amino-1,3,4-thiadiazole, triazine and its derivatives, urazole, DL-pipecolinic acid, isonicotinamide, anthranilonitrile, 6-aza-2-thiothymine, adenine, 3-(2-thienyl)acrylic acid, benzoic acid and alkali metal and ammonium salts of benzoic acid, and pyrazine and its derivatives to form a stable chromogen with hemoglobin. Alternatively, the organic ligands can be added to a suitable blood diluent for hemoglobin and leukocyte analysis.
Owner:COULTER INTERNATIONAL CORPORATION
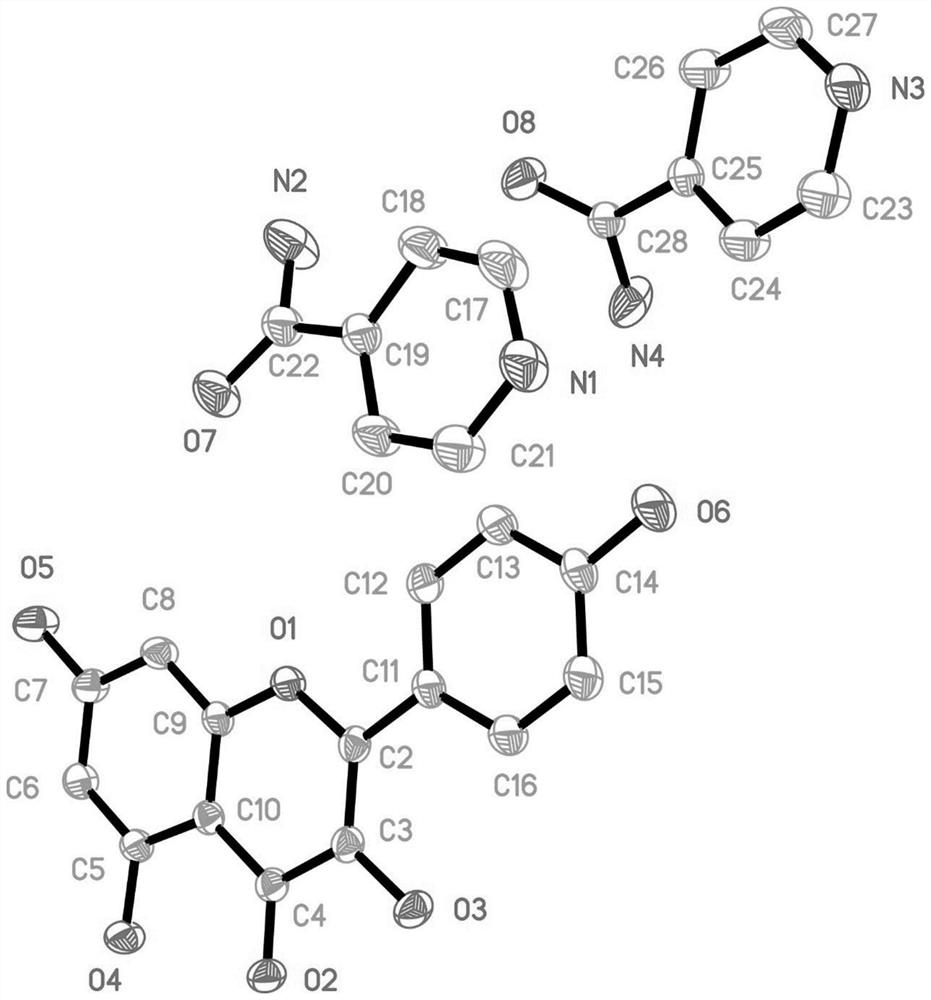
Naringenin isonicotinamide co-crystal
The invention belongs to the field of a medical technology and specifically relates to a co-crystal formed by naringenin and isonicotinamide, wherein stoichiometric ratio of naringenin to isonicotinamide in the co-crystal is 1:2. The co-crystal has an X-ray powder diffraction pattern, a differential scanning calorimetry pattern and an infrared spectrogram which are different from those of naringenin crystals and a physical mixture of naringenin and isonicotinamide, is a novel crystalline form completely different from naringenin, and has remarkably-enhanced solubility.
Owner:CHINA PHARM UNIV
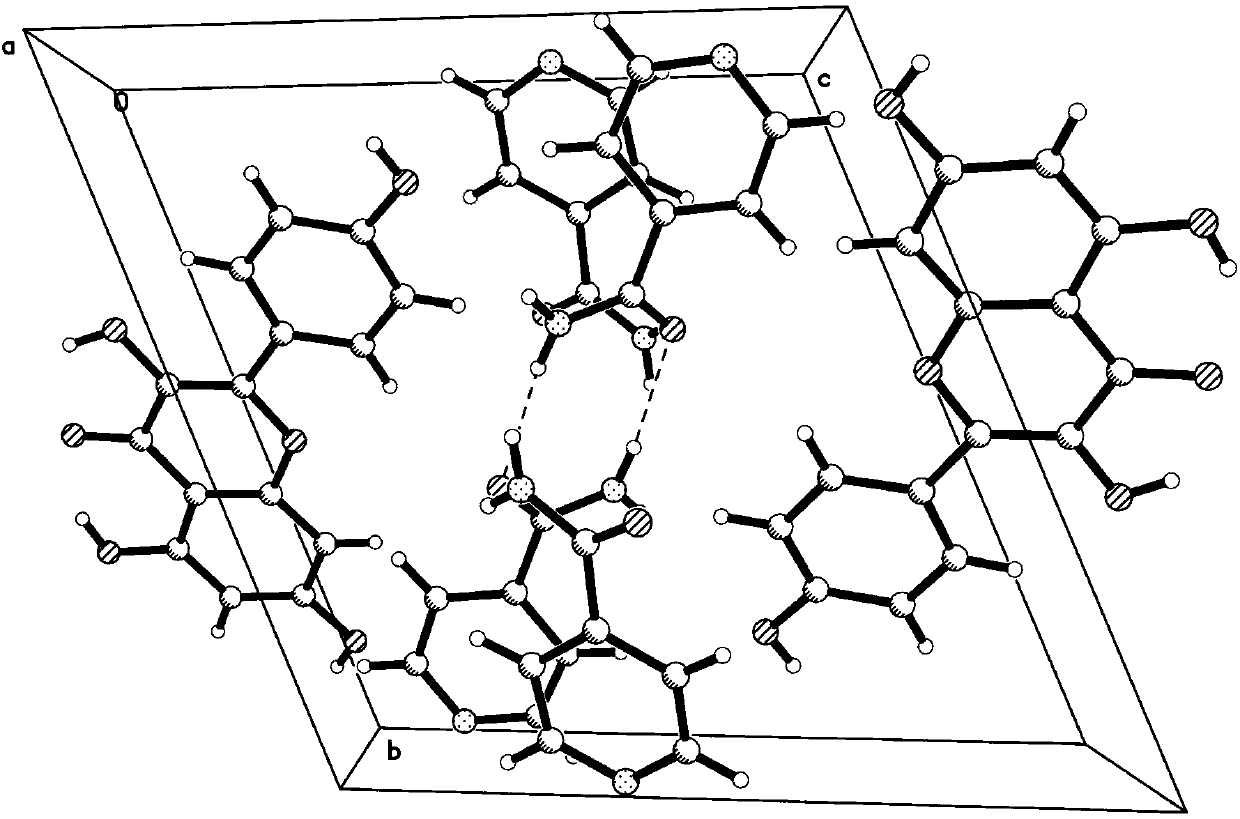
Isonicotinamide eutectic crystal of 17beta estradiol, and preparation method and application thereof
The invention provides an isonicotinamide eutectic crystal of 17beta estradiol, and a preparation method and application thereof. Particularly, the invention provides the isonicotinamide eutectic crystal of 17beta estradiol. X-ray powder diffraction analysis, thermogravimetic analysis, differential scanning thermometric analysis and other analysis indicate that the eutectic crystal provided by the invention has more excellent physicochemical property and pharmaceutical property. The invention provides the preparation method of the eutectic crystal. The method is simple to operate, easy to control and favorable in reproducibility, and can stably obtain the target eutectic crystal.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Synthesis of combinatorial libraries of compounds reminiscent of natural products
InactiveUS7109377B2High densityIncrease diversitySequential/parallel process reactionsOrganic compound preparationNatural productHigh density
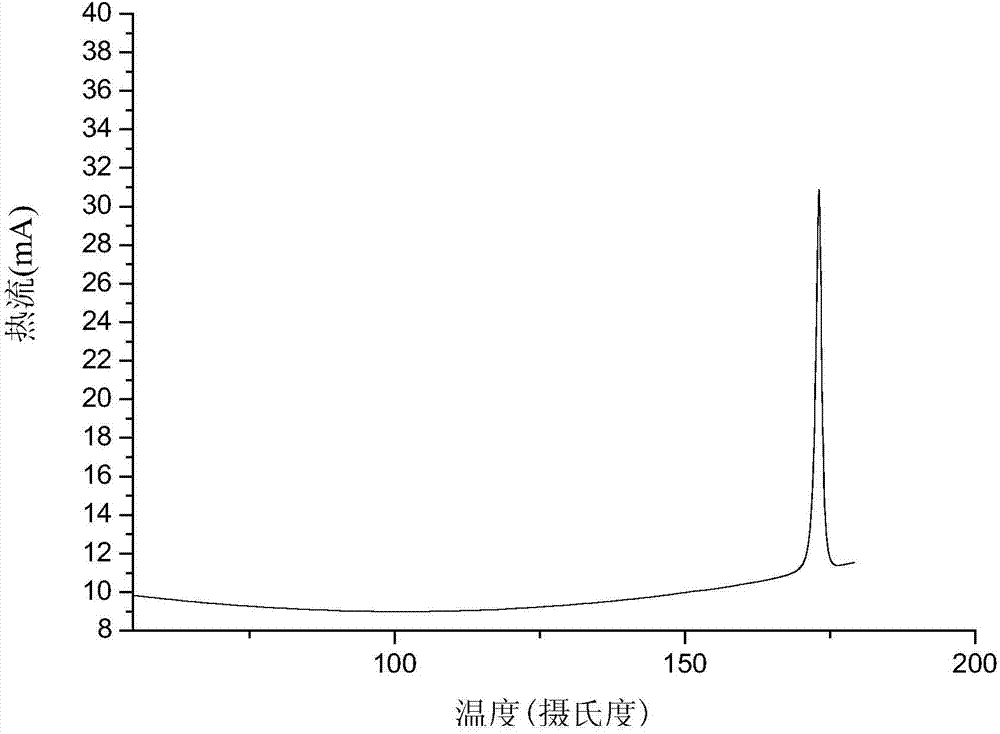
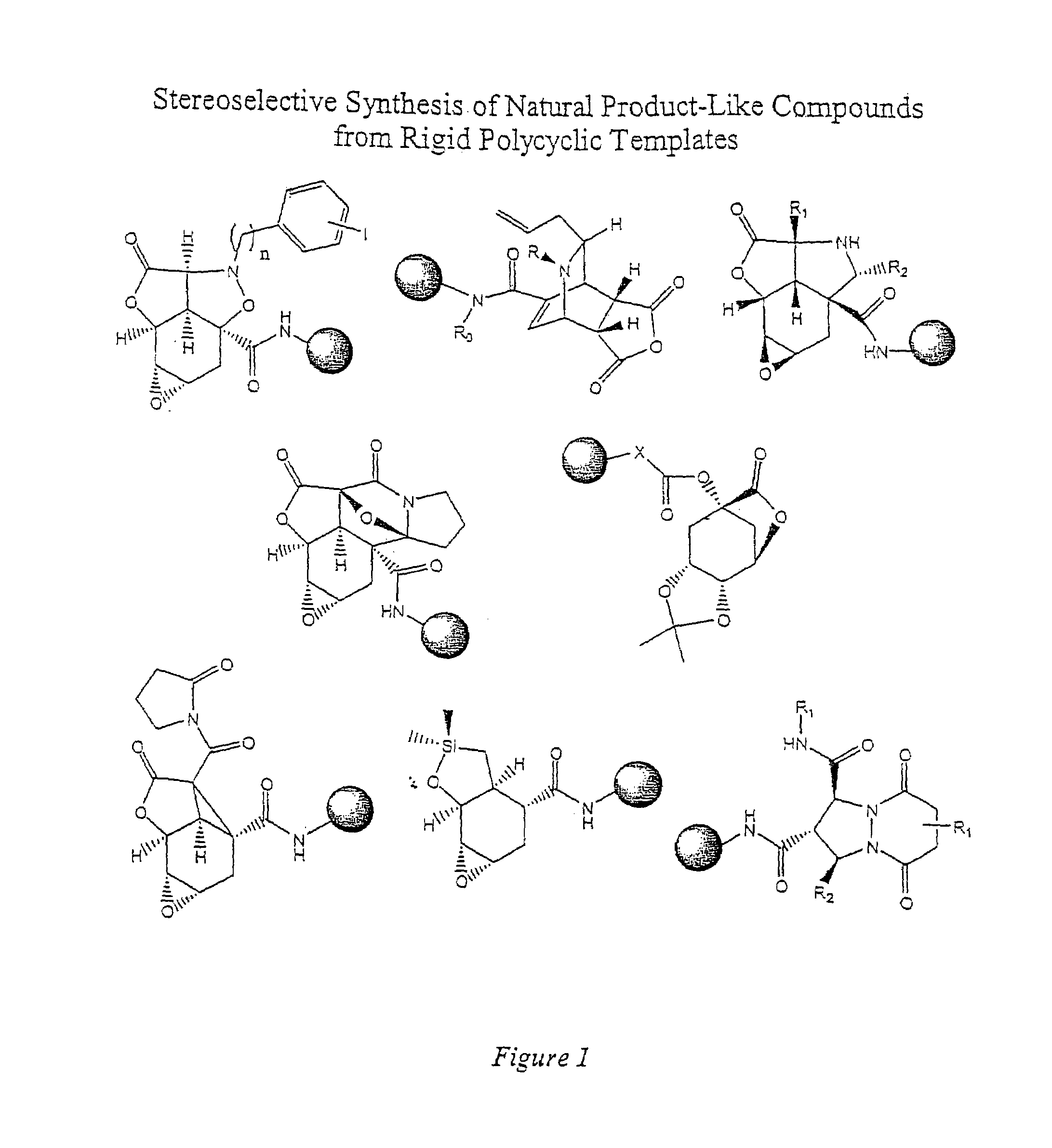
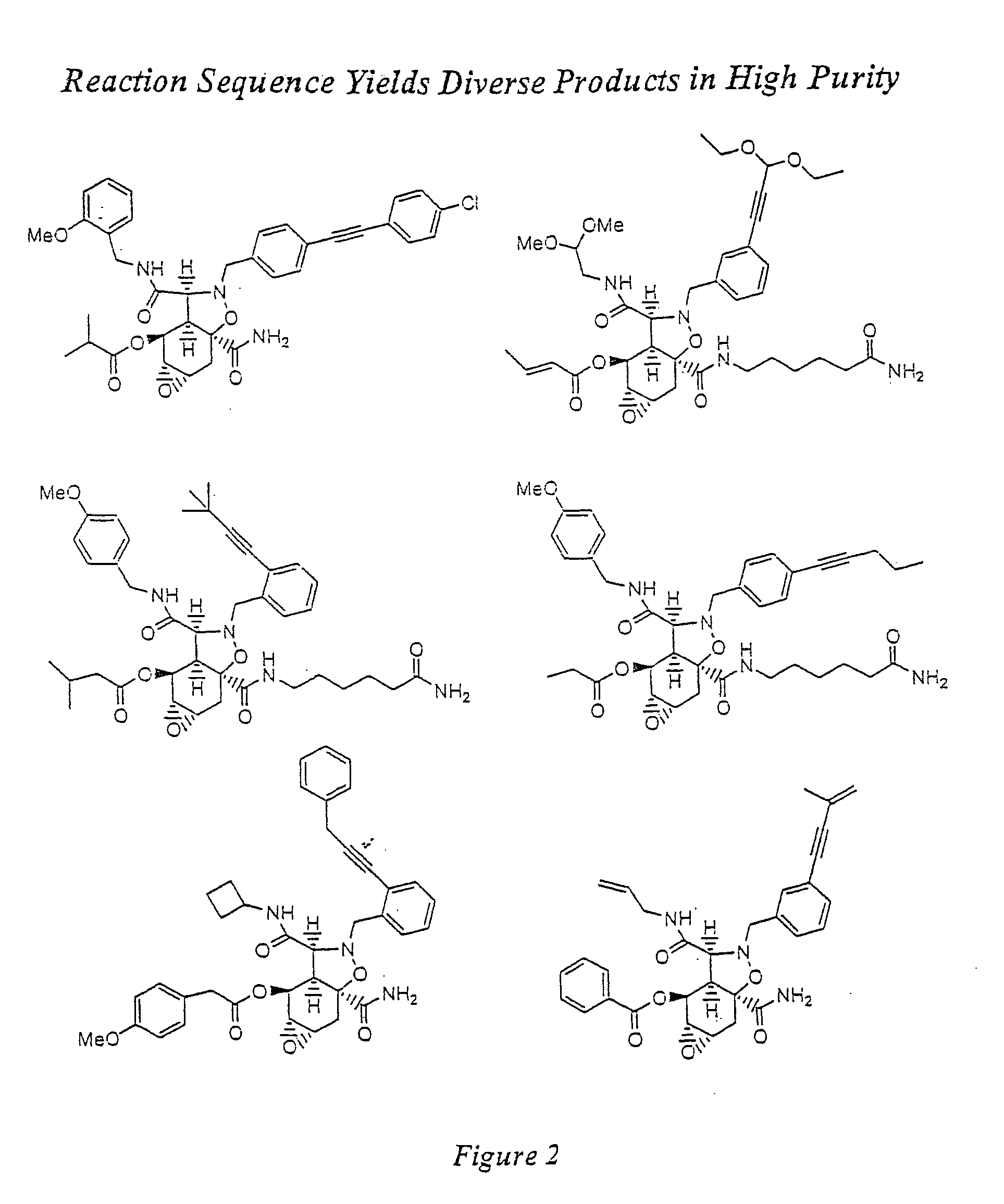
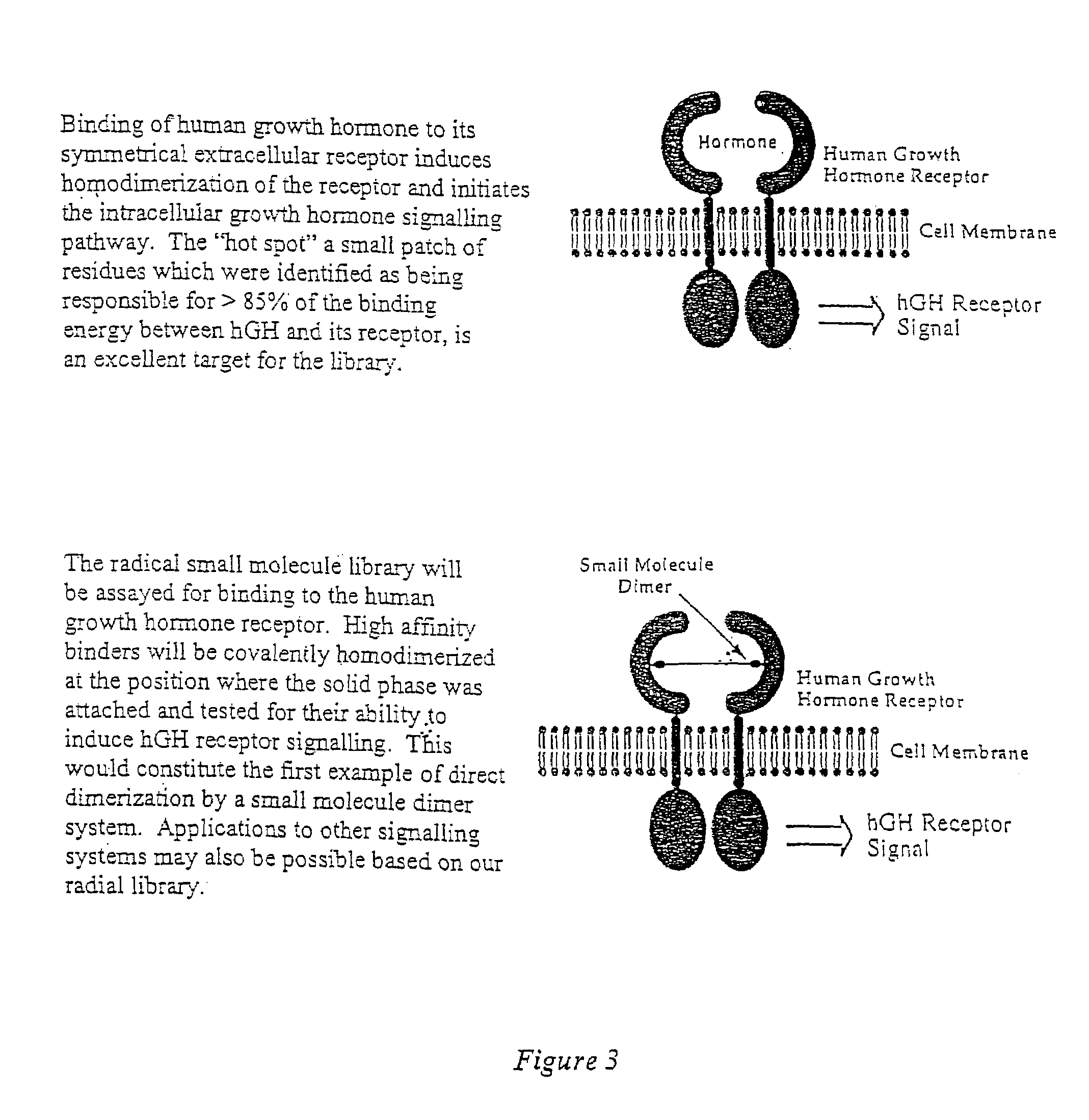
The present invention provides complex compounds reminiscent of natural products and libraries thereof, as well as methods for their production. The inventive compounds and libraries of compounds are reminiscent of natural products in that they contain one or more stereocenters, and a high density and diversity of functionality. In general, the inventive libraries are synthesized from diversifiable scaffold structures, which are synthesized from readily available or easily synthesizable template structures. In certain embodiments, the inventive compounds and libraries are generated from diversifiable scaffolds synthesized from a shikimic acid based epoxyol template. In other embodiments, the inventive compounds and libraries are generated from diversifiable scaffolds synthesized from the pyridine-based template isonicotinamide. The present invention also provides a novel ortho-nitrobenzyl photolinker and a method for its synthesis. Furthermore, the present invention provides methods and kits for determining one or more biological activities of members of the inventive libraries. Additionally, the present invention provides pharmaceutical compositions containing one or more library members.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for converting cyanopyridine compounds into niacinamide compounds, its catalyst and the catalyst preparation
InactiveCN101239311AExclude generationEasy and economical separationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNiacinamideVitamin B12
The present invention provides a corrective method for converting cyano-group pyridines compound to niacinamide compound. In particular, the invention relates to the preparation of the niacinamide compound and the isonicotinamide compound, it is used for preparing anti-TB medicine-retozide, and an intermediate of vitamin B12. The invention also relates to catalyst preparing method by niacinamide and isonicotinamide.
Owner:COUNCIL OF SCI & IND RES
Isonicotinamide methylpyrazine derivative eutectic I
ActiveCN109503475AHigh purityImprove stabilityOrganic active ingredientsMetabolism disorderX-rayANTILIPEMIC AGENTS
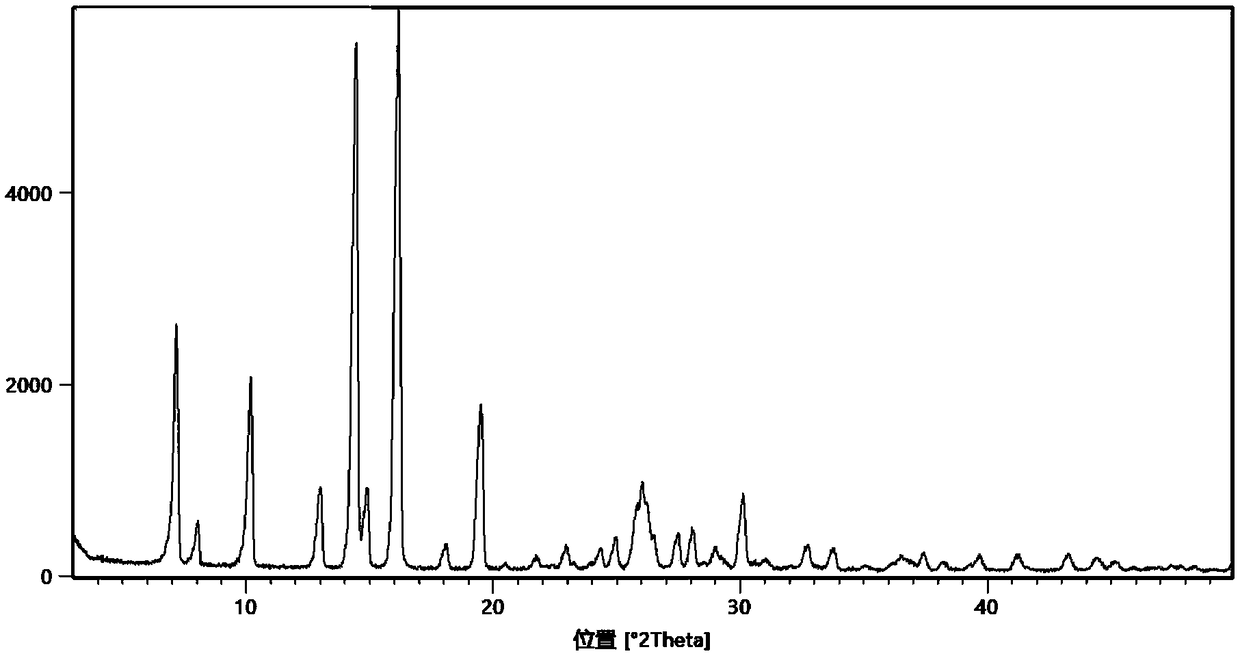
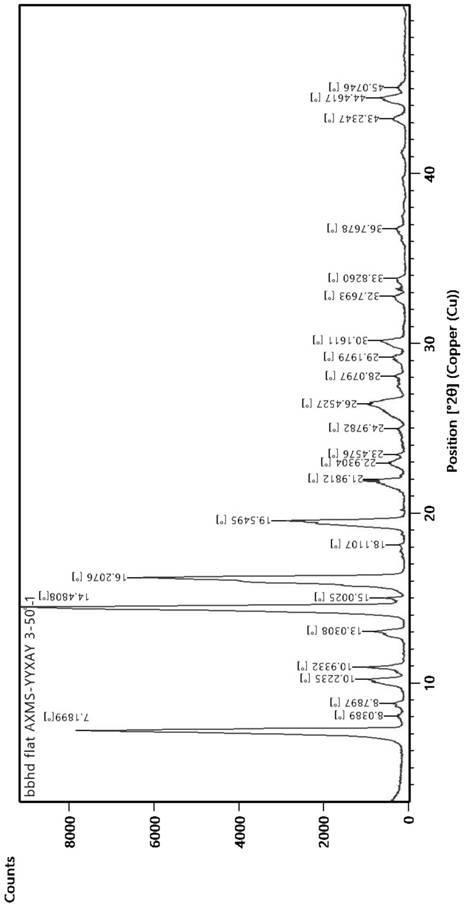
The invention belongs to the technical field of the medicine, and specifically provides isonicotinamide methylpyrazine derivative eutectic I, a preparation method thereof, and use thereof for preparing antilipemic agent. The isonicotinamide methylpyrazine derivative eutectic I prepared by the invention uses Cu-Kalpha radiation, an X-ray diffraction spectrum represented by 2theta has characteristicpeaks at 7.2+ / -0.2 degrees, 8.1+ / -0.2 degrees, 14.5+ / -0.2 degrees, and 16.2+ / -0.2 degrees. The prepared isonicotinamide methylpyrazine derivative eutectic prepared is good in stability in the medium,and is high in product purity and good in stability after being placed in a solid state; an animal experimental verifies that the bioavailability is high. The isonicotinamide methylpyrazine derivative eutectic I disclosed by the invention is simple in preparation process and has good industrial application prospect.
Owner:LUNAN BETTER PHARMA
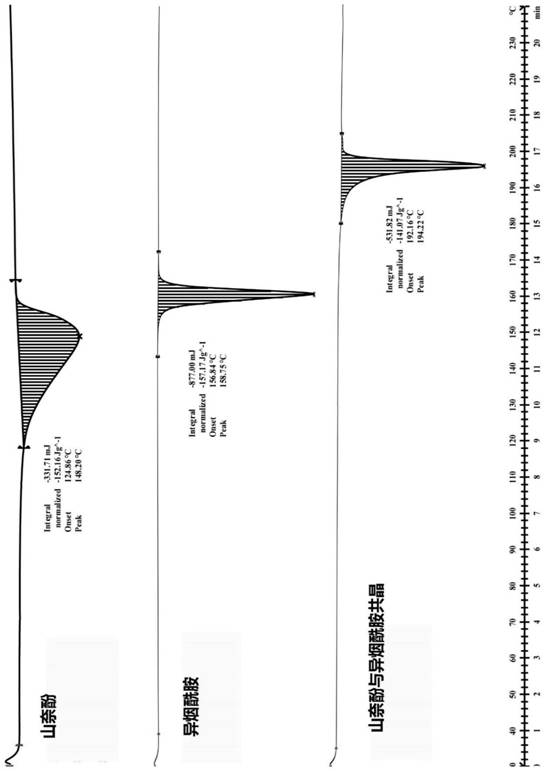
Kaempferol and isonicotinamide eutectic compound, preparation method, pharmaceutical composition thereof and application
ActiveCN109988104AAdvantages of good safety medicineImprove solubilityAntibacterial agentsOrganic active ingredientsAntiviral drugActive component
The invention discloses a kaempferol and isonicotinamide eutectic compound, a preparation method, a composition thereof and application. Specifically, the invention discloses a novel kaempferol and isonicotinamide eutectic compound which takes kaempferol (as shown in a formula a) as a medicine active component and isonicotinamide (as shown in a formula b) as a eutectic ligand, a preparation methodof the kaempferol and isonicotinamide eutectic Compound, and application of the kaempferol and isonicotinamide eutectic compound as a medicinal active ingredient to preparation of medicines for preventing and treating cancer, resisting cancer, resisting inflammation, resisting oxidation, resisting bacteria or resisting viruses.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Method for preparing cephalonium from 7-aminocephalosporanic acid at one step
The invention discloses a method for preparing cephalonium from 7-aminocephalosporanic acid at one step. The method comprises the following steps: (1) adding 7-aminocephalosporanic acid into water with the temperature of 0-10 DEG C; (2) regulating the pH value of the solution; (3) adding an organic solvent, tiophencacetyl chloride and ethyl acetate, and reacting for 1-3h while stirring at the temperature of 0-10 DEG C; (4) after the reaction is ended, standing for layering; (5) adding activated carbon into a water phase for decoloring, and carrying out suction filtration; (6) adjusting the pH value of the filtrate, then, adding isonicotinamide, and reacting at the temperature of 15-50 DEG C; (7) after the reaction is ended, growing the grain for over 1h; (8) filtering to obtain a crystal, and drying to obtain the cephalonium. The cephalonium is prepared at one step, and an intermediate is not needed to be separated and extracted in the reaction process, so that the method is simple and convenient in operation, simple in reaction step and high in yield; the 7-aminocephalosporanic acid is used as the raw material and is simple and easy to obtain and low in market price, so that the production cost is greatly reduced.
Owner:FUJIAN FUKANG PHARMA
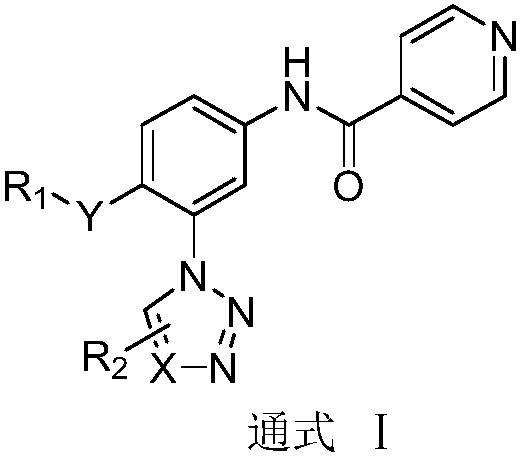
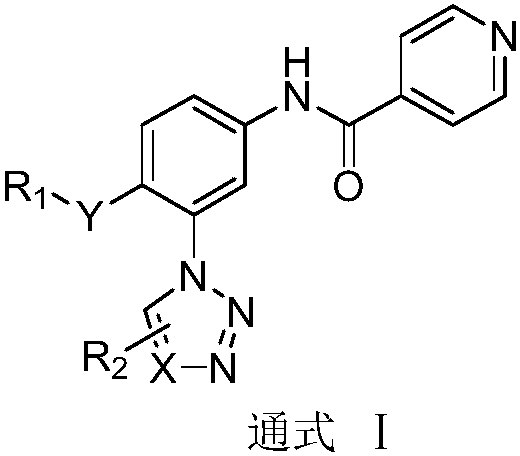
N-(3-azolylphenyl) isonicotinamide compounds as well as preparation method and application thereof
InactiveCN108997315ASimple manufacturing methodHigh yieldOrganic active ingredientsOrganic chemistryStereochemistryIsonicotinamide
The invention belongs to the field of medicine, and relates to N-(3-azolylphenyl) isonicotinamide compounds as well as a composition comprising the same and a preparation method of the N-(3-azolylphenyl) isonicotinamide compounds. The invention further relates to the application of the compounds and the compositions in anti-gout. The compounds have a general formula I as shown in the specification; the molecular structure of the compounds is more innovative and the activity is greatly enhanced. Moreover, the compounds with the general formula I provided by the invention are simple and feasiblein preparation method, high in yield and easy to be mass-produced.
Owner:中国医科大学
Process for preparing a catalyst for conversion of cyanopyridines to nicotinamides
InactiveUS7455827B2Increase conversionsHigh selectivityOrganic chemistryManganese oxides/hydroxidesIsoniazidAntituberculous drugs
Nicotinamides and isonicotinamides, used in the preparation of anti-TB drugs i.e. isoniazid and as an intermediate of vitamin B12 are prepared from cyanopyridines and nicotinamides. Catalysts useful for the preparation of nicotinamide and isonicotinamide.
Owner:COUNCIL OF SCI & IND RES
Preparation method of isoniazid
PendingCN111138354AShorten the hydrazinolysis reaction timeImprove production efficiencyOrganic chemistryNicotinuric acidIsonicotinic acid
The invention discloses a preparation method of isoniazide, comprising the following steps: carrying out an esterification reaction among isonicotinic acid, alcohol and an acylation reagent to generate isonicotinate, carrying out a reaction between isonicotinate and an ether reagent in an alcoholic solution of hydrogen chloride to generate isonicotinate hydrochloride, condensing with hydrazine hydrate to generate an isoniazide crude product, and refining to obtain the finished product. According to the method, by adding the step of reacting isonicotinate to generate isonicotinate hydrochloride, on one hand, the subsequent condensation hydrazinolysis reaction time can be greatly shortened and preparation efficiency is improved; on the other hand, isonicotinic acid which is not fully reacted, isonicotinamide introduced by an isonicotinic acid raw material and potential 2-picolinic acid impurities can be removed, the purity of the finally obtained isoniazide is as high as 99.99%, and thesingle impurity content is smaller than 0.10%. The preparation method is mild in reaction condition, easy to operate, good in product quality, high in yield, high in preparation speed and suitable forindustrial production.
Owner:沈阳双鼎制药有限公司
Preparation method of novel multifunctional Alaska pollock polypeptide modified by taurine
ActiveCN109354600AActiveAntioxidantPeptide preparation methodsBulk chemical productionBrain developmentSwelling ratio
The invention provides a preparation method of novel multifunctional Alaska pollock polypeptide modified by taurine. The multifunctional polypeptide includes natural polypeptide and a substituent group, wherein the amino acid sequence of the natural polypeptide is SEQ ID NO.1, and taurine is adopted as the substituent group which is combined with the natural polypeptide and introduced for modification. The preparation method of the polypeptide includes preparation of protective peptide fragments, a microwave condensation reaction and a deamination protection reaction. During preparation of theprotective peptide fragments, the natural polypeptide is dissolved in a sodium carbonate solution, an acetone solution containing amino-terminal protection groups and an accelerant is added, stirringis conducted for a reaction, and extraction and precipitation are carried out to obtain the fragments, wherein the accelerant comprises o-aminobenzoic sulfonic acid and isonicotinamide. The providednovel polypeptide has high activity and stability, no spiral structure, high fluidity and a high swelling ratio and can achieve biological activities such as oxidation resistance, removal of free radicals and promotion of brain development. The conversion rate and the yield are high during preparation. The time consumed for synthesis is short, the yield of by-products is low, products are easy topurify, and the production cost is low.
Owner:ZHEJIANG OCEAN UNIV
Mezlocillin sodium freeze-dried powder injection and preparation method thereof
InactiveCN106176628AAdvantages and Notable ImprovementsImprove stabilityAntibacterial agentsPowder deliveryFreeze-dryingPowder injection
The invention discloses a mezlocillin sodium freeze-dried powder injection. The freeze-dried powder injection is prepared from mezlocillin sodium, isonicotinamide and propylgallate according to a weight ratio of 10:(0.1-0.4):(0.01-0.05). The mezlocillin sodium freeze-dried powder injection is high in stability, high in safety due to few types of auxiliary materials, simple in process and suitable for mass production.
Owner:NANJING ZHENGKUAN MEDICAL TECH
A kind of preparation method for preparing ceflonine from 7-aminocephalosporanic acid one-step method
The invention discloses a method for preparing cephalonium from 7-aminocephalosporanic acid at one step. The method comprises the following steps: (1) adding 7-aminocephalosporanic acid into water with the temperature of 0-10 DEG C; (2) regulating the pH value of the solution; (3) adding an organic solvent, tiophencacetyl chloride and ethyl acetate, and reacting for 1-3h while stirring at the temperature of 0-10 DEG C; (4) after the reaction is ended, standing for layering; (5) adding activated carbon into a water phase for decoloring, and carrying out suction filtration; (6) adjusting the pH value of the filtrate, then, adding isonicotinamide, and reacting at the temperature of 15-50 DEG C; (7) after the reaction is ended, growing the grain for over 1h; (8) filtering to obtain a crystal, and drying to obtain the cephalonium. The cephalonium is prepared at one step, and an intermediate is not needed to be separated and extracted in the reaction process, so that the method is simple and convenient in operation, simple in reaction step and high in yield; the 7-aminocephalosporanic acid is used as the raw material and is simple and easy to obtain and low in market price, so that the production cost is greatly reduced.
Owner:FUJIAN FUKANG PHARMA
Process for the synthesis of isonicotinic acid hydrazide
InactiveUS6734309B1Simple stepsAvoiding harmful chemicalsOrganic chemistryHydrazine compoundIsonicotinic acid
The invention provides a process for the manufacture of isonicotinic acid hydrazide(INH) useful in the treatment of tuberculosis. The invention relates to the single step conversion of isonicotinamide by hydrazine hydrate to isonicotinic acid hydrazide (INH) of yield greater than 95% (w / w) and purity more than 99%.
Owner:COUNCIL OF SCI & IND RES
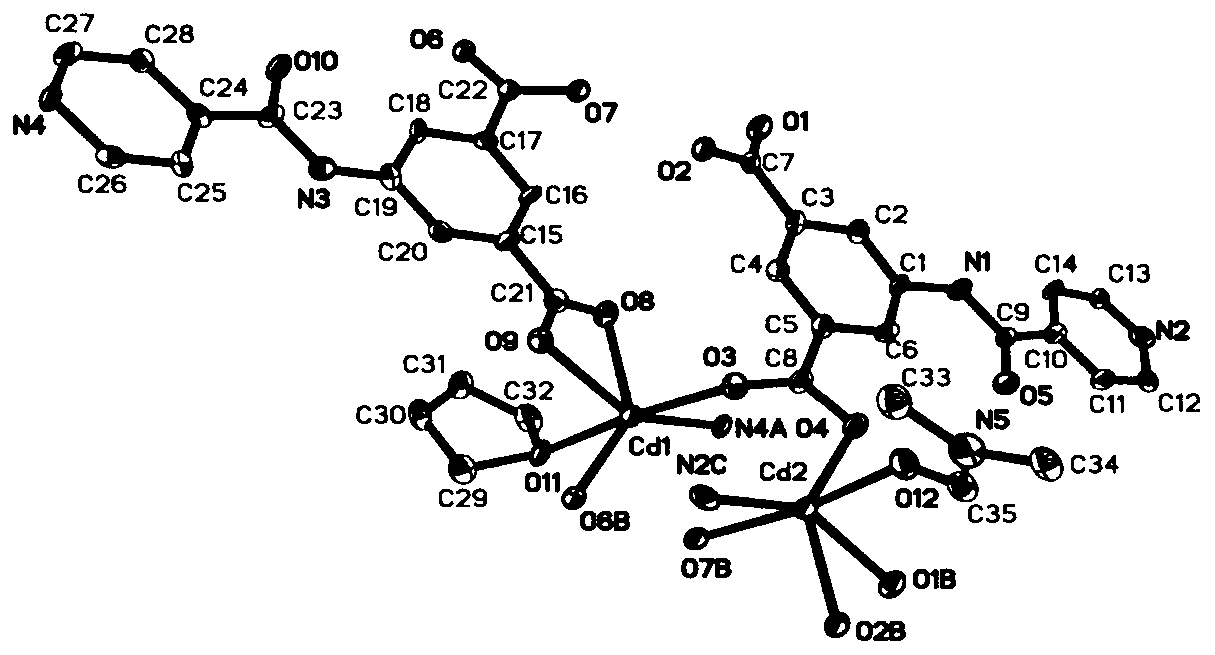
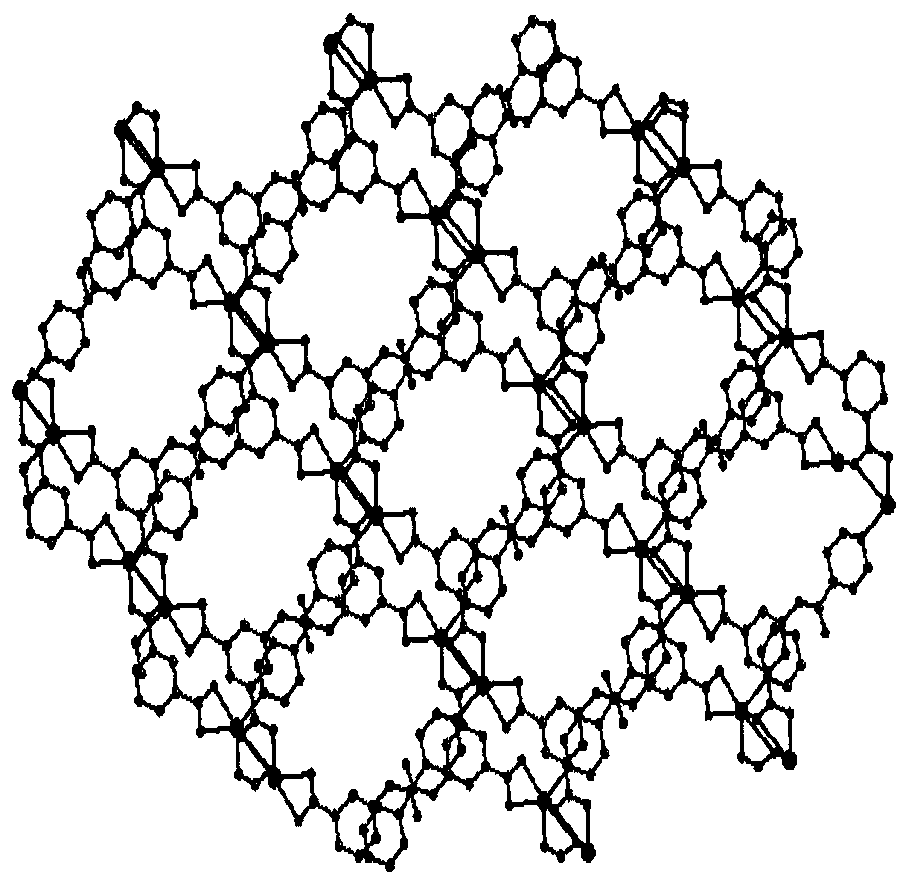
5-isonicotinamidopyridylisotitanate cadmium complex, and preparation method and application thereof
ActiveCN110922420AHigh catalytic activityHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationFluoProbesPermanganic acid
The invention discloses a 5-isonicotinamidopyridylisotitanate cadmium complex, and a preparation method and an application thereof, and belongs to the technical field of porous layered materials. Theporous complex has a two-dimensional double-layer network structure, the chemical expression of the porous complex is [Cd2(L)2DMFTHF].2DMF.THF, wherein L is a 5-isonicotinamidopyridylisotitanate ion.The preparation method comprises the following steps: carrying out a solvothermal reaction on 5-isonicotinamidopyridylisotitanic acid and a cadmium salt, and washing and drying the obtained reaction product to obtain the novel porous cadmium complex with a stable structure. The preparation method has the advantages of simple process, convenience in operation, and high yield; and the prepared 5-isonicotinamidopyridylisotitanate cadmium complex can selectively catalyze a Knoevenagel condensation reaction of p-tolualdehyde and malononitrile, has the characteristics of good selectivity and high catalytic activity as a catalyst, and can be used as a fluorescent probe for dichromate ions and permanganate ions.
Owner:HENGYANG NORMAL UNIV
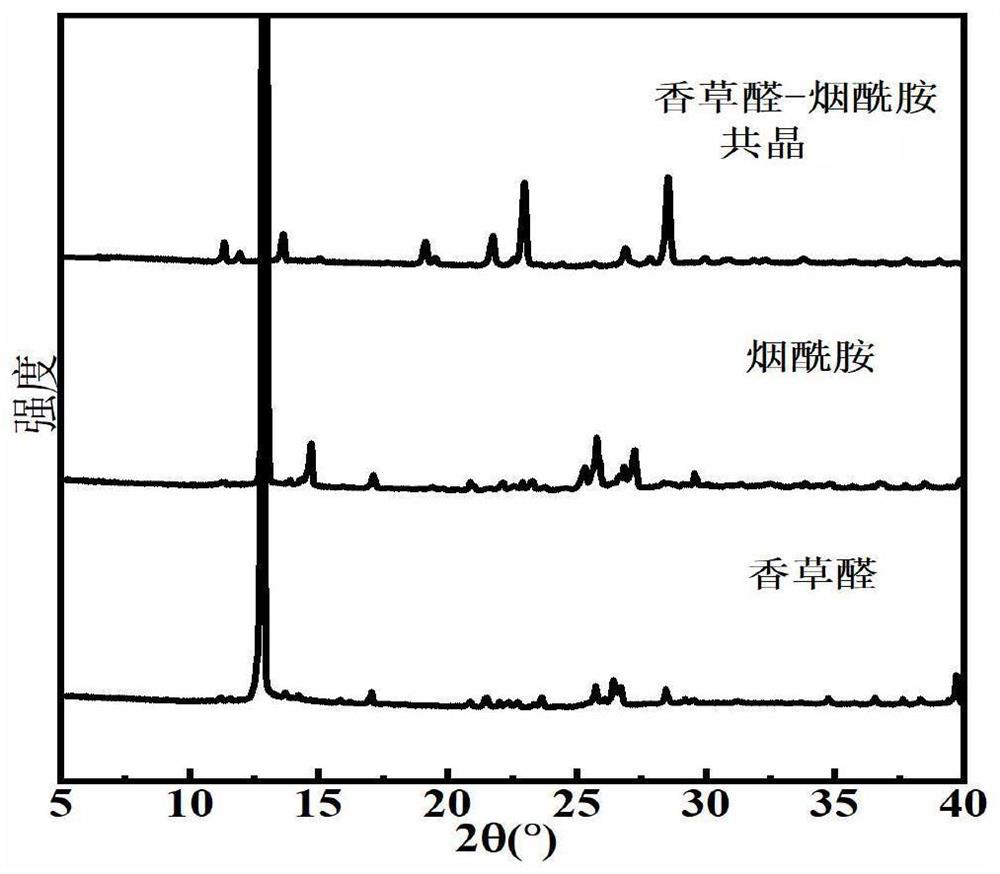
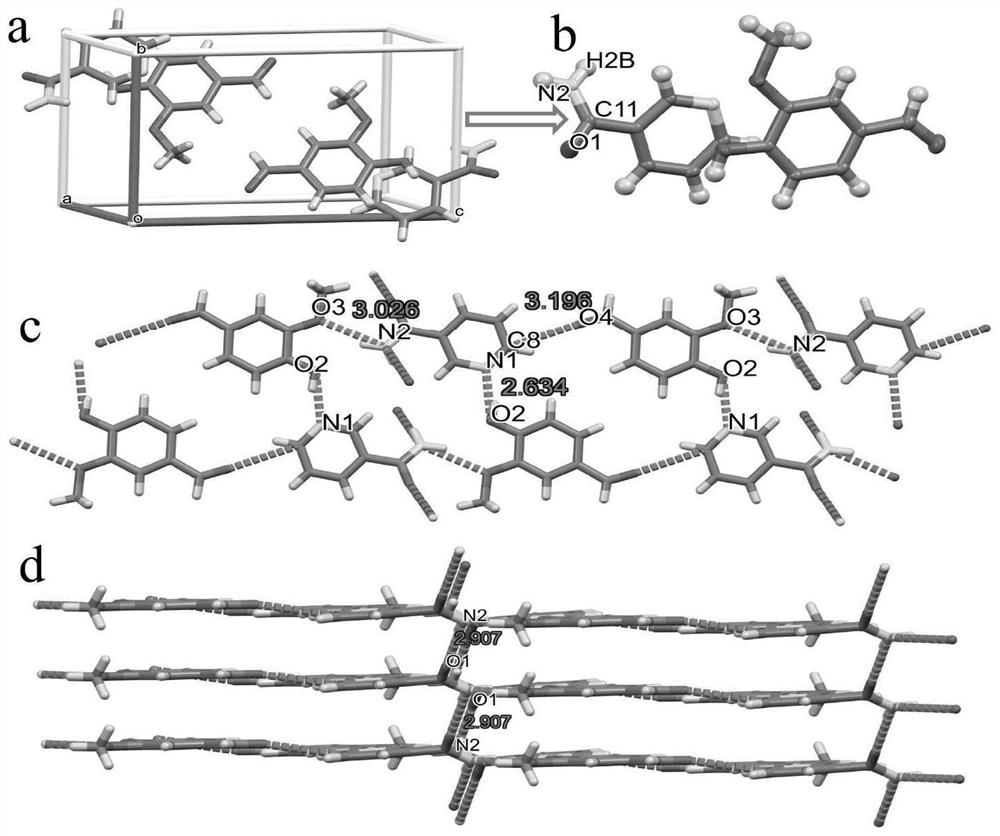
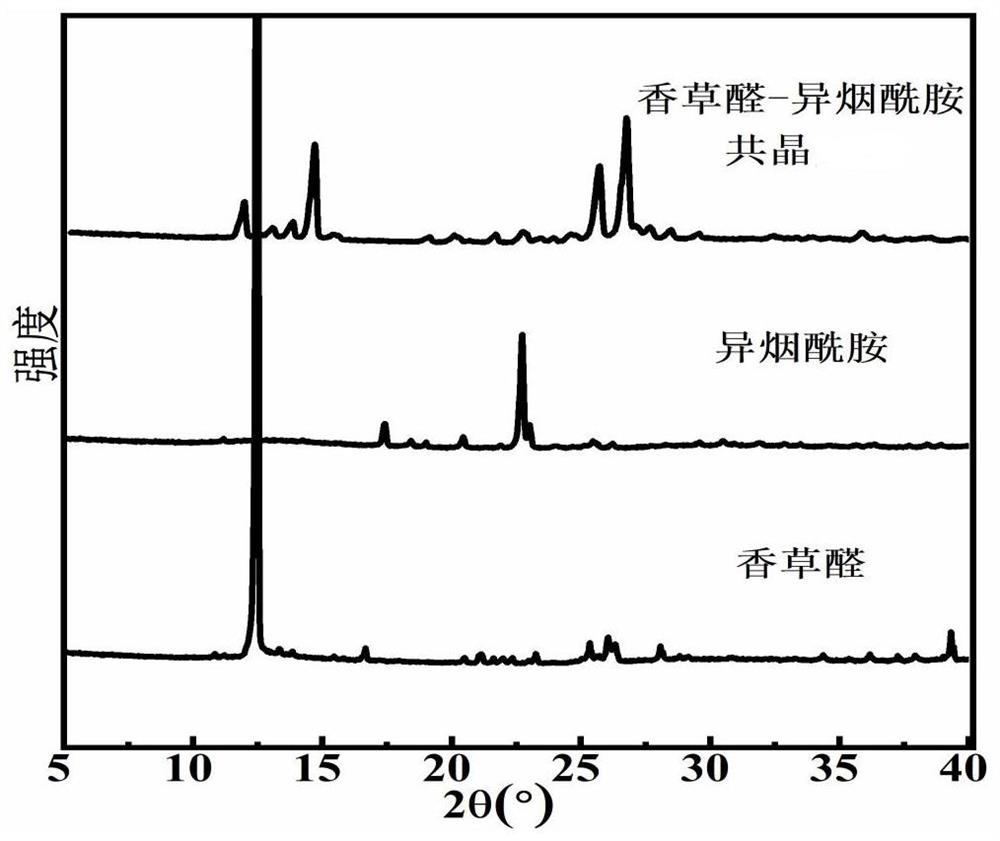
Eutectic structure of vanillin and amide compound and preparation method thereof
ActiveCN114805046AGood lookingImprove performancePolycrystalline material growthFrom normal temperature solutionsFreeze-dryingPhysical chemistry
The preparation method of the eutectic structure of the vanillin and the amide compound comprises the following steps: firstly, preparing a vanillin solution under a stirring condition, then adding nicotinamide or isonicotinamide which is equal to the vanillin in mole into the obtained vanillin solution, and keeping the stirring process until the nicotinamide or isonicotinamide is completely dissolved and becomes a clear solution; adding the silicon dioxide nanoparticles into the clear solution according to the mass ratio to adsorb vanillin, nicotinamide or isonicotinamide, and then separating, freeze-drying, heating, melting and drying to finally obtain a eutectic powder product of vanillin and nicotinamide or vanillin and isonicotinamide; at normal temperature, the eutectic powder product of vanillin and nicotinamide and the eutectic powder product of vanillin and isonicotinamide are dissolved in an organic solvent, after saturation is achieved, the mixture is placed in a constant-temperature and constant-humidity box to be slowly volatilized, the single crystal structure is determined, the yield is very high and can reach 90% or above, the purity reaches 98% or above, and the method is suitable for industrial production.
Owner:EAST CHINA UNIV OF TECH
Composition and method for hemoglobin and cell analysis
A cyanide-free lytic reagent composition and method for measuring the total hemoglobin concentration in a blood sample, for counting the number of leukocytes and for deferential counting of leukocyte subpopulations are described. The cyanide-free lytic reagent composition comprises a quaternary ammonium salt or a pyridinium salt to lyse erythrocytes and release hemoglobin, and an organic ligand selected from the group consisting of triazole and its derivatives, tetrazole and its derivatives, alkaline metal salts of oxonic acid, melamine, aniline-2-sulfonic acid, quinaldic acid, 2-amino-1,3,4-thiadiazole, triazine and its derivatives, urazole, DL-pipecolinic acid, isonicotinamide, anthranilonitrile, 6-aza-2-thiothymine, 3-(2-thienyl)acrylic acid, benzoic acid and alkali metal and ammonium salts of benzoic acid, and pyrazine and its derivatives to form a stable chromogen with hemoglobin, and a salt to adjust conductivity of the reagent for impedance measurement. The reagent composition is mixed with a blood sample without pre-dilution and the UV absorption of the sample mixture is measured at the predetermined absorption wavelength. Counting the number of leukocytes and differential counting of leukocyte subpopulations are accomplished simultaneously on an automated cell counter utilizing DC impedance measurement.
Owner:COULTER INTERNATIONAL CORPORATION
Process for conversion of cyanopyridines to nicotinamides and catalyst therefor, process for preparing said catalyst
InactiveUS20040186297A1Procedure be complicateCumbersome procedureOrganic chemistryIsoniazidVitamin B12
The present invention relates to an improved process for conversion of cyanopyridines to nicotinamides More particularly the present invention relates to preparation of nicotinamides and isonicotinamides which finds its usage in the preparation of anti-TB drug i.e. isoniazid and as an intermediate of vitamin B12. The present invention also relates to a process for a catalyst useful for the preparation of nicotinamide and isonicotinamide.
Owner:COUNCIL OF SCI & IND RES
A topical composition
PendingUS20200315939A1Cosmetic preparationsToilet preparationsAnti microbial peptideAntimicrobial peptides
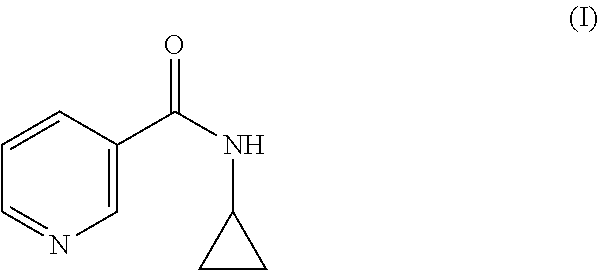
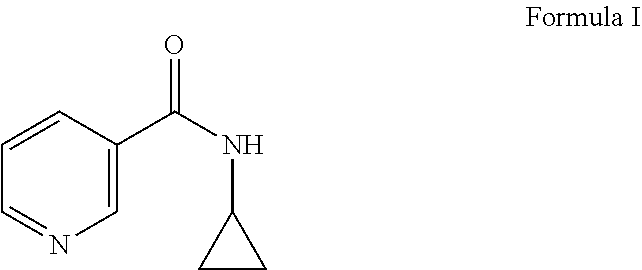
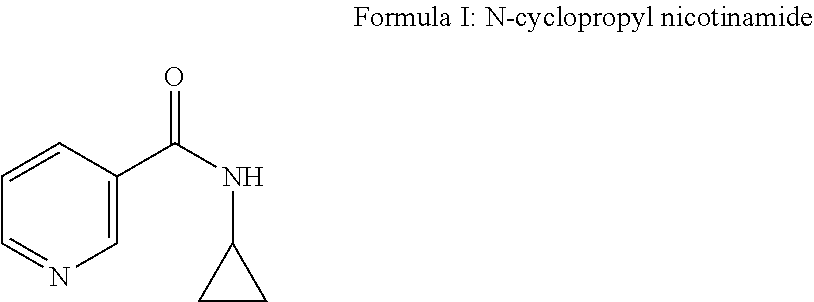
The present invention relates to a topical composition comprising from 0.001 to 10% by weight a compound of formula (I), i.e. N-cyclopropyl nicotinamide, that provides improved antimicrobial effect through generation of antimicrobial peptides when applied to an external surface of the human body. A combination of compound of formula I and at least one ingredient selected from niacinamide, picolinamide and iso-nicotinamide triggers generation of AMPs in a synergistic way.
Owner:CONOPCO INC D B A UNILEVER
Co-crystal of nifedipine and isonicotinamide
ActiveCN108794383BPharmaceutically activeGood light stabilityOrganic active ingredientsOrganic chemistry methodsNifedipineSingle Crystal Diffraction
The invention relates to a co-crystal of nifedipine and isonicotinamide, its preparation method and application. The co-crystal of nifedipine and isonicotinamide was comprehensively characterized by means of X-ray single crystal diffraction analysis, X-ray powder diffraction analysis, thermogravimetric analysis, differential scanning calorimetry analysis, and infrared spectroscopy, and it was found that the Compared with nifedipine, the co-crystal has the advantage of higher light stability. The preparation method of the co-crystal of nifedipine and isonicotinamide is simple, easy to control and good in reproducibility, and stable co-crystal of nifedipine and isonicotinamide can be obtained.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Process for the manufacture of HI-6 dimethanesulfonate
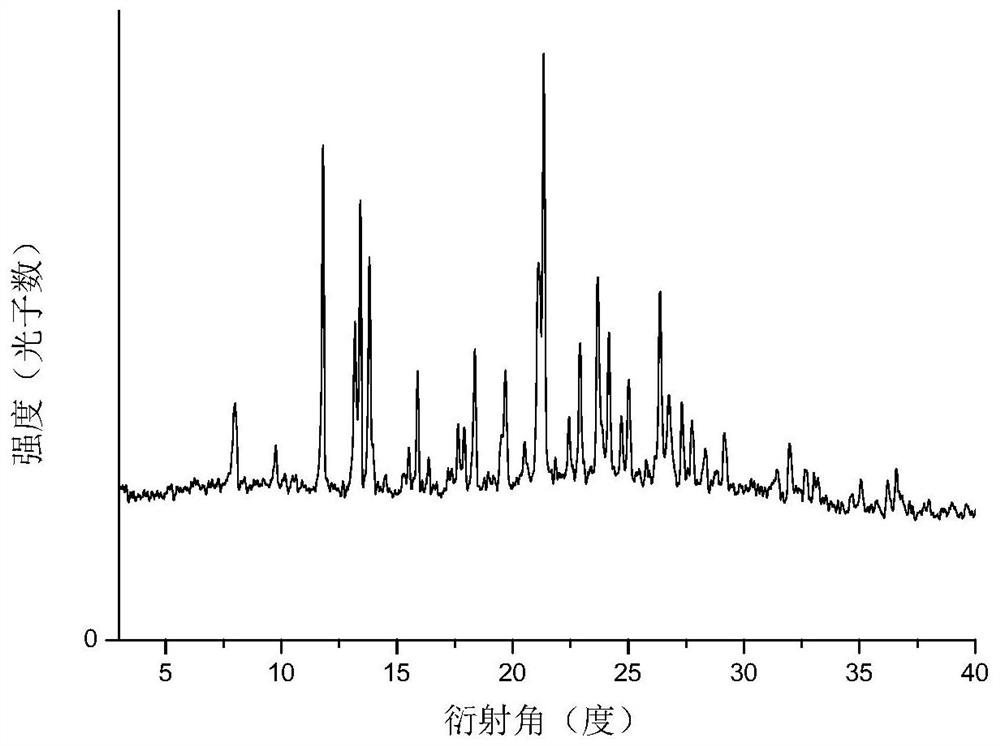
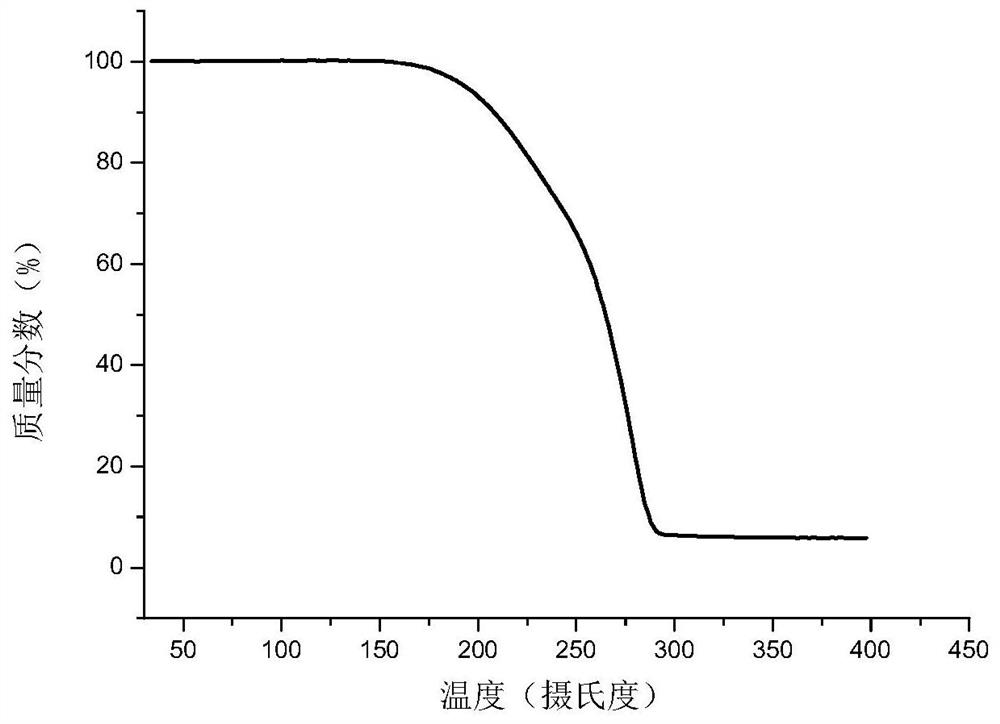
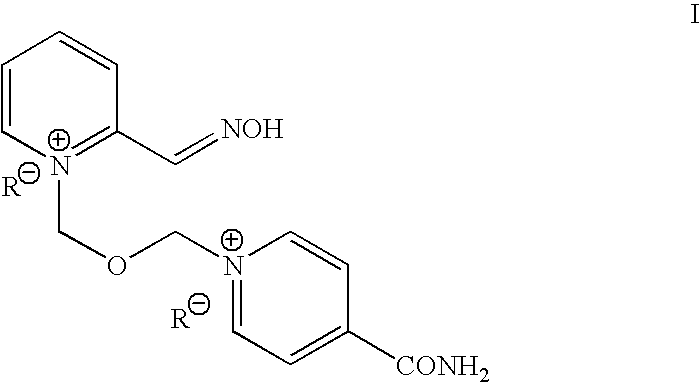
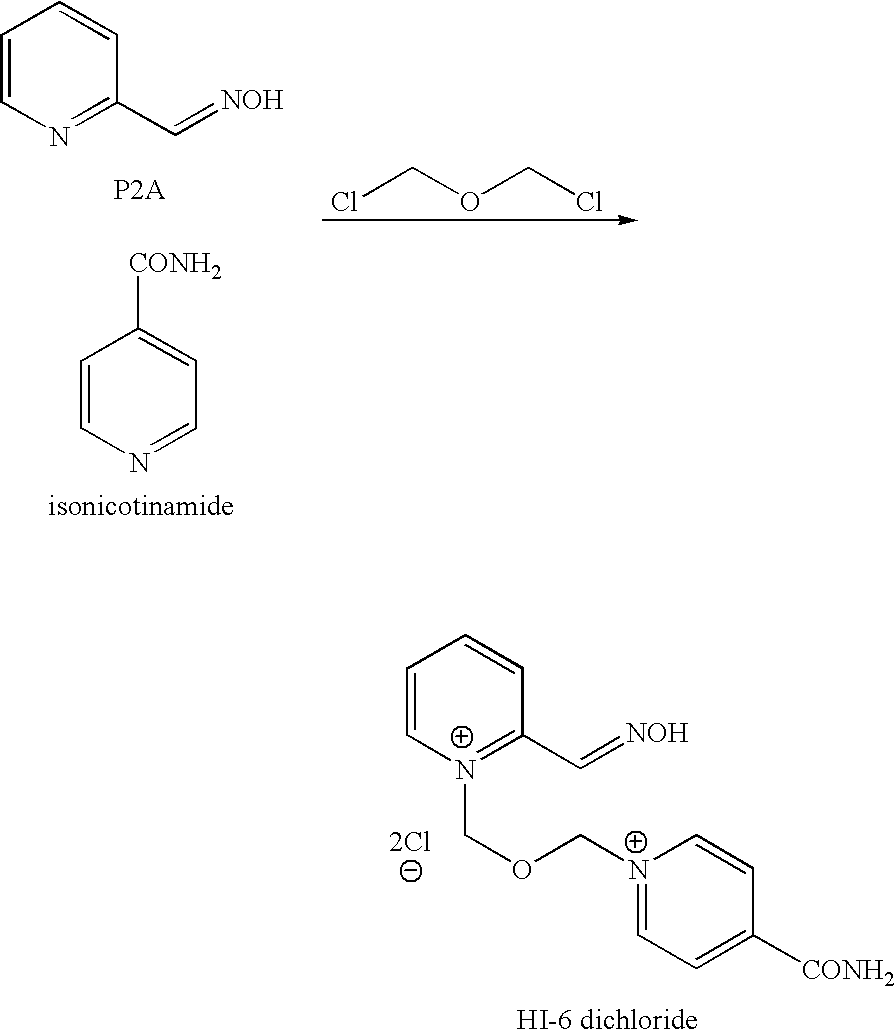
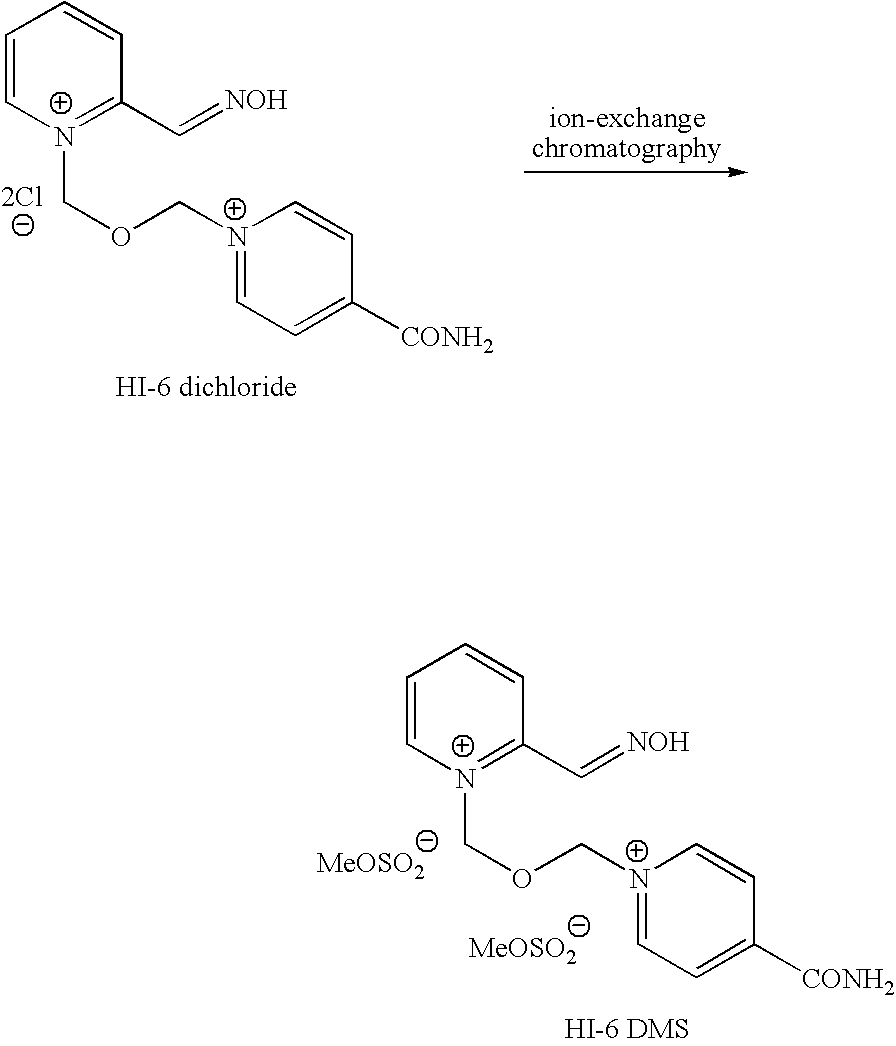
InactiveUS8143406B2Reduce usagePurity satisfactoryOrganic chemistryBulk chemical productionEtherPyridine
The invention provides a process for the manufacture of HI 6 dimethanesulfonate comprising contacting an O-protected pyridine aidoxime compound with bis(methylsulphonoxymethyl)ether in a suitable solvent to form an intermediate compound, contacting said intermediate compound with isonicotinamide to form an O-protected HI 6 product precursor, and de-protecting the precursor to form HI 6 dimethanesulfonate.
Owner:PHOENIX CHEM LTD (GB)
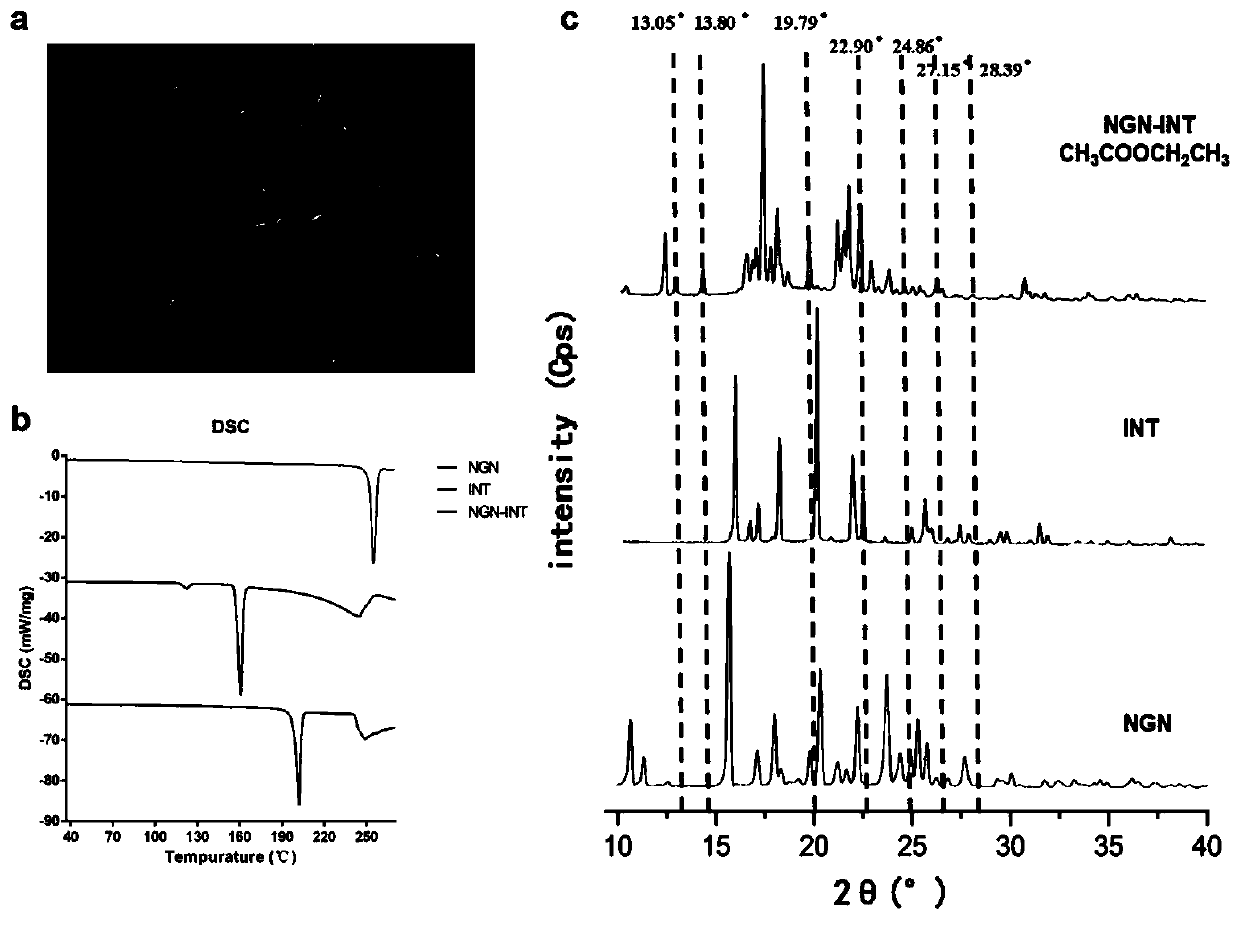
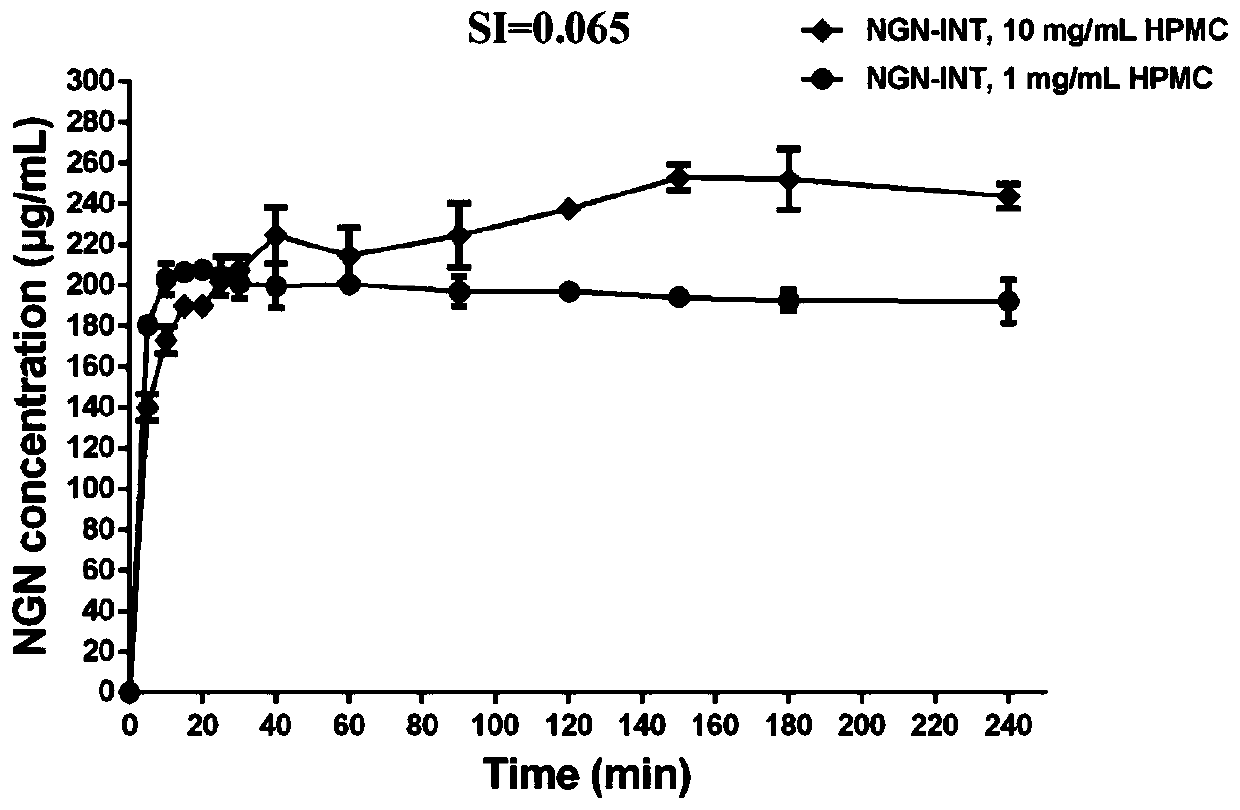
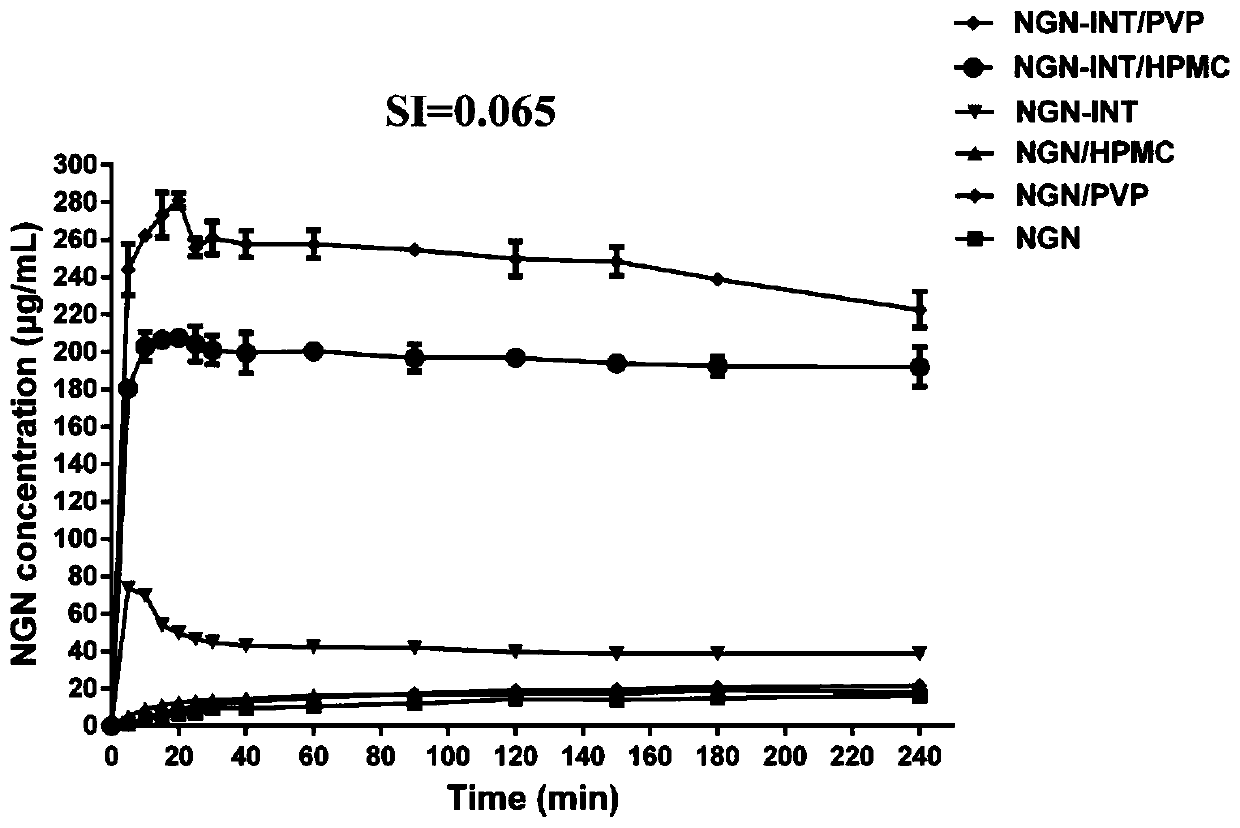
Application of HPMC and naringenin isonicotinamide co-crystal in preparation of medicine for preventing and treating abdominal aortic aneurysm
ActiveCN111184709AImprove bioavailabilityImprove efficacyPowder deliveryPharmaceutical non-active ingredientsEfficacyEngineering
The invention provides an application of an HPMC and naringenin (NGN) isonicotinamide (INT) co-crystal in preparation of a medicine for preventing and treating abdominal aortic aneurysm (AAA). According to the application, firstly the co-crystal (NGN-INT) of the NGN and the INT is prepared, HPMC and PVP are added to the NGN-INT co-crystal separately, the supersaturation effect of the NGN in the HPG / PVP extension co-crystal is investigated, and a blood concentration-time curve of different forms of the NGN in mice is investigated, and results show that the HPMC can significantly increase the oral bioavailability of the NGN in the NGN-INT co-crystal than that of the PVP, and significantly promote the anti-AAA efficacy of the NGN-INT.
Owner:PEKING UNIV
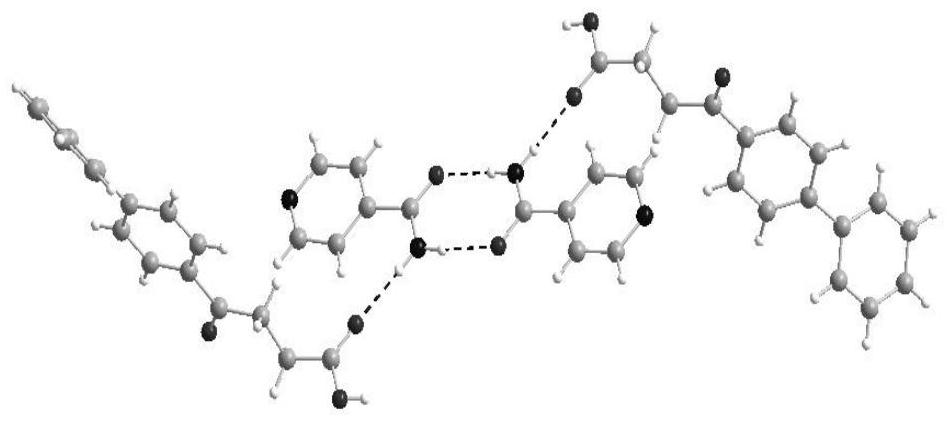
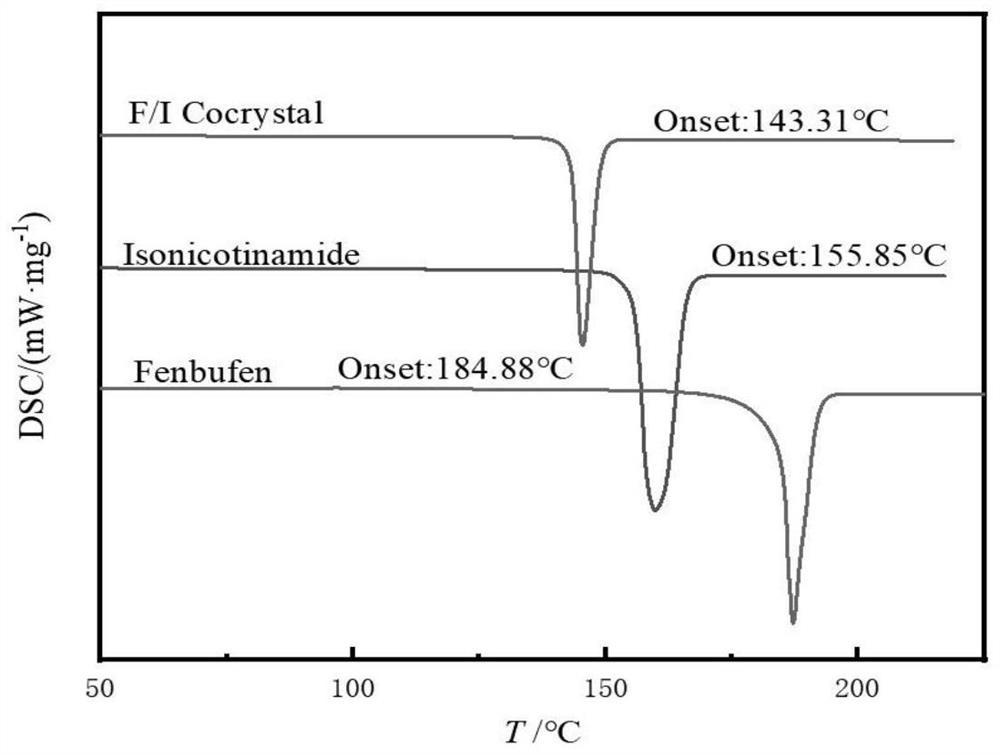
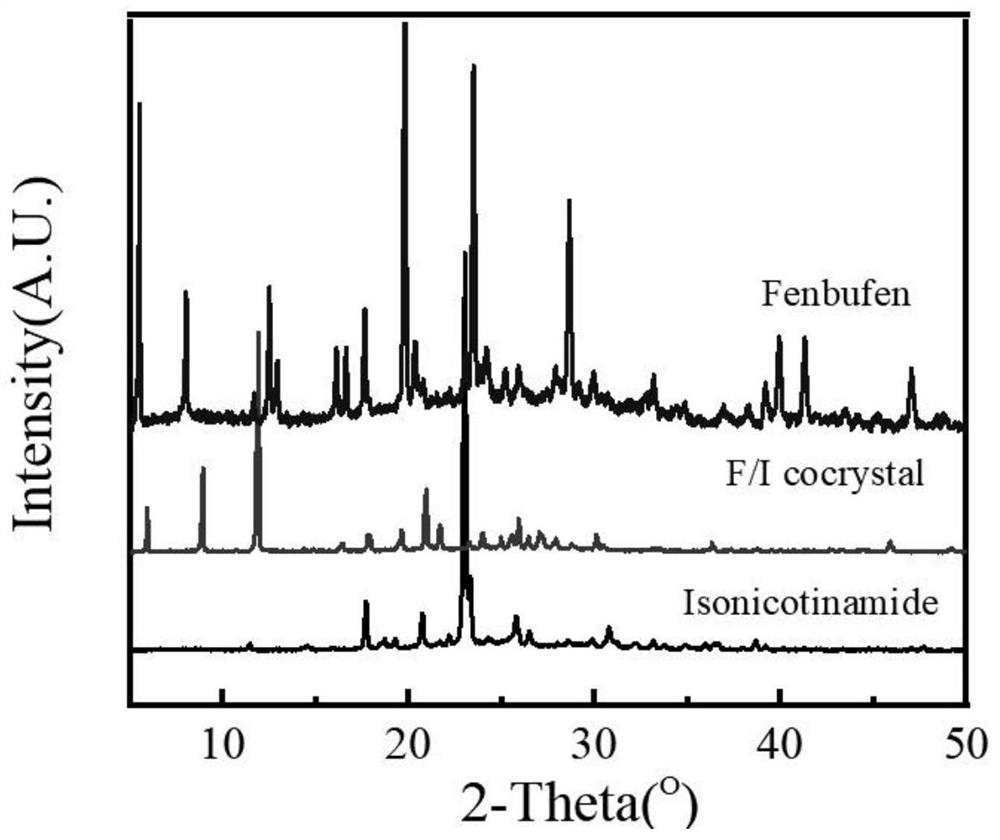
Fenbufen pharmaceutical co-crystal and preparation method thereof
PendingCN114436823AImprove stabilityImprove solubilityOrganic chemistry methodsCarboxylic compound separation/purificationSingle crystalBULK ACTIVE INGREDIENT
The invention discloses two fenbufen pharmaceutical co-crystals and a preparation method thereof, and the two co-crystals both take fenbufen as pharmaceutical active ingredients and take isonicotinamide or nicotinamide as co-crystal formations respectively. The two eutectic crystals are prepared by adopting a solvent evaporation method. The obtained eutectic crystal is characterized by differential scanning calorimetry, powder X-ray diffraction and single crystal X-ray diffraction, and formation of a new crystal form is proved. The solubility of the fenbufen medicine and the two eutectic crystals of the fenbufen medicine in water and a simulated duodenum solution is investigated, the two pharmaceutical eutectic crystals obviously improve the solubility of the fenbufen medicine, and the bioavailability of the fenbufen medicine is improved.
Owner:SOUTHEAST UNIV
A kind of 5-isonicotinamide pyridyl isotitanic acid porous copper complex and its preparation method and application
ActiveCN104072525BHigh catalytic activityHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystBenzaldehyde
The invention discloses a 5-isonicotinamide-pyridyl-isophthalic acid porous copper complex, as well as a preparation method and application thereof. The porous copper complex comprises a (3,6) dual-node three-dimensional topology lattice structure; the chemical formula of the porous copper complex is [Cu(L)].DMAc.H2O, wherein L is 5-isonicotinamide-pyridyl-isophthalic acid radical ion. The preparation method comprises the following steps: solvent thermal reaction between 5-isonicotinamide-pyridyl-isophthalic acid and nantokite, washing, and drying to obtain the novel porous copper complex of which the structure is stable. The preparation method is simple in process, convenient to operate and high in productivity. The 5-isonicotinamide-pyridyl-isophthalic acid porous copper complex obtained according to the method can catalyze the Knovevenagel condensation reaction between benzaldehyde and malononitrile, is high in catalysis selectivity and catalytic activity, and can be effectively recycled and used repeatedly.
Owner:HENGYANG NORMAL UNIV
Kaempferol and isonicotinamide co-crystal and preparation method and pharmaceutical composition and use thereof
ActiveCN109988104BAdvantages of good safety medicineImprove solubilityAntibacterial agentsOrganic active ingredientsAntiviral drugPharmaceutical drug
The invention discloses a co-crystal of kaempferol and isonicotinamide, a preparation method, a composition and an application thereof. Specifically, the present invention discloses a new kaempferol and isonicotinamide co-crystal with kaempferol (such as formula a) as the active ingredient of medicine and isonicotinamide (such as formula b) as the eutectic ligand; kaempferol The preparation method of the co-crystal with isonicotinamide; the application of the co-crystal of kaempferol and isonicotinamide as active ingredients in the preparation of anti-cancer, anti-cancer, anti-inflammatory, anti-oxidation, antibacterial or antiviral drugs.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Method for converting cyanopyridine compounds into niacinamide compounds, its catalyst and the catalyst preparation
InactiveCN101239311BEasy and economical separationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNiacinamideVitamin B12
Owner:COUNCIL OF SCI & IND RES
Isonicotinamide derivative, its preparation method and application
ActiveCN104788365BMEK inhibitory activity inhibitionHas inhibitory activityOrganic chemistryAntineoplastic agentsDiseaseIsonicotinic acid
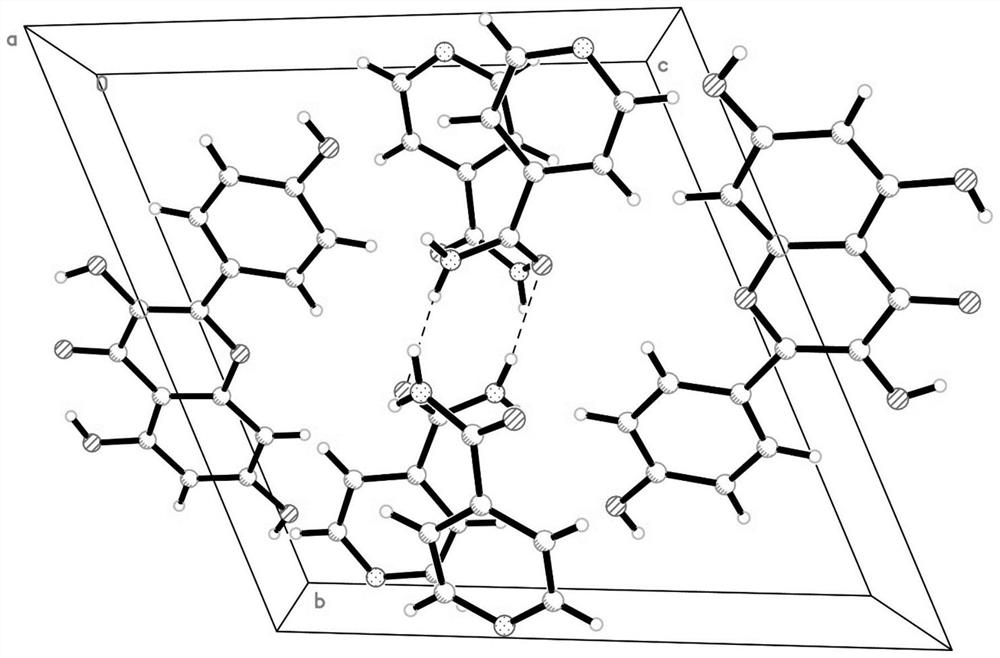
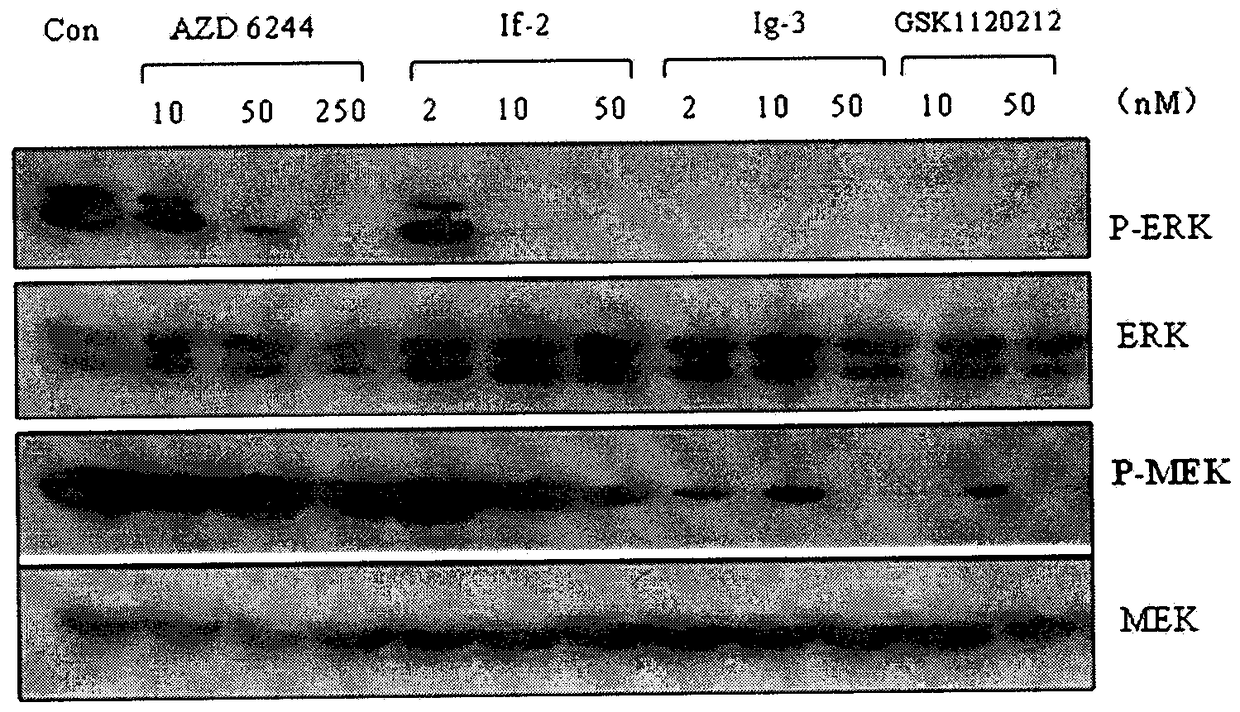
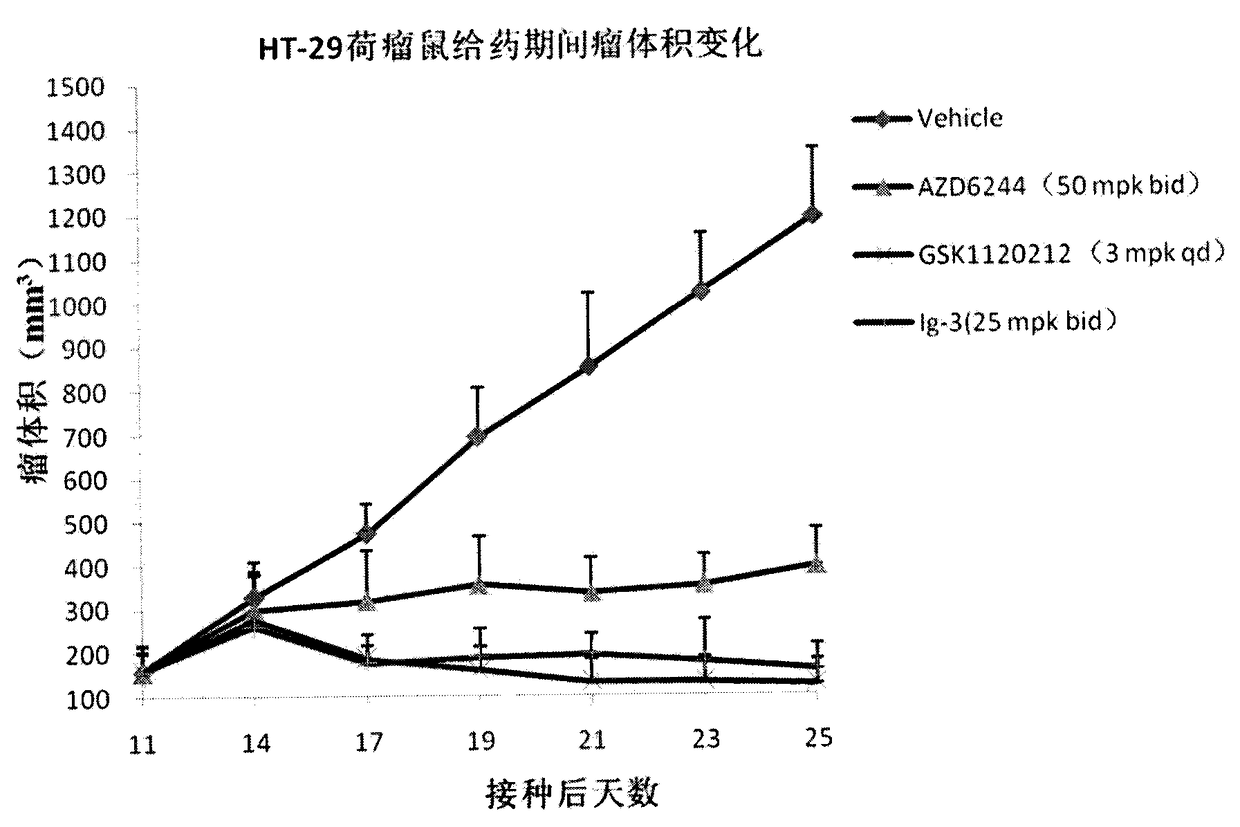
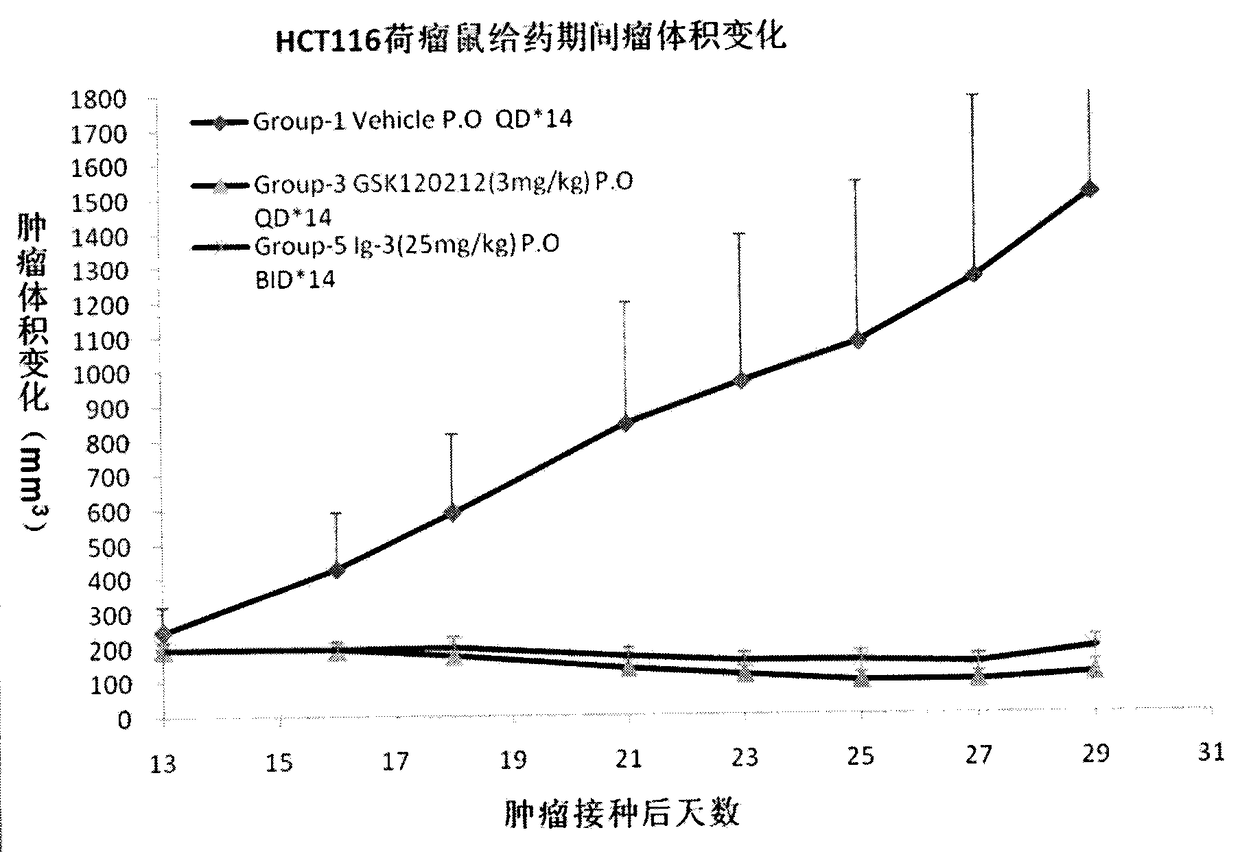
The present invention discloses an isonicotinamide derivative represented by a formula (I) and a pharmaceutically acceptable salt thereof, wherein R1, R2, R3 and A are defined in the specification. The present invention further discloses a preparation method of the compounds, a drug composition containing the compound, and applications of the compounds in treatment of mammal, especially human overproliferation diseases and in preparation of drugs for treatment of mammal, especially human overproliferation diseases. The formula (I) is defined in the specification.
Owner:SHANGHAI ALLIST PHARM CO LTD
Process for conversion of cyanopyridines to nicotinamides and catalyst therefor, process for preparing said catalyst
The present invention relates to an improved process for conversion of cyanopyridines to nicotinamides. More particularly the present invention relates to preparation of nicotinamides and isonicotinamides which finds its usage in the preparation of anti-TB drug i.e. isoniazid and as an intermediate of vitamin B12. The present invention also relates to a process for a catalyst useful for the preparation of nicotinamide and isonicotinamide.
Owner:COUNCIL OF SCI & IND RES
Isonicotinamide and acipimox eutectic crystal II and preparation method thereof
ActiveCN112110865AImprove solubilityImprove stabilityOrganic active ingredientsMetabolism disorderCrystallographyMetallurgy
The invention belongs to the technical field of medicines, and particularly provides an isonicotinamide and acipimox eutectic crystal II, a preparation method thereof and application thereof in preparation of hypolipidemic drugs. The isonicotinamide and acipimox eutectic crystal II prepared by the method is radiated by Cu-K alpha, and an X-ray diffraction pattern expressed by 2theta has characteristic peaks at 10.9 + / -0.2 degrees, 22.0 + / -0.2 degrees, 23.5 + / -0.2 degrees and 26.5 + / -0.2 degrees. The isonicotinamide and acipimox eutectic crystal prepared by the preparation method disclosed by the invention is good in stability in a medium, high in product purity, high in bioavailability and better in drug effect. The method is simple in preparation process and has a good industrial application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com