Isonicotinamide methylpyrazine derivative eutectic I
A technology of isonicotinamide methyl pyrazine and methyl pyrazine, which is applied in the field of isonicotinamide methyl pyrazine derivative co-crystal I, can solve the problem of less crystal eutectic structure, low yield, and unimproved characterization parameters. and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
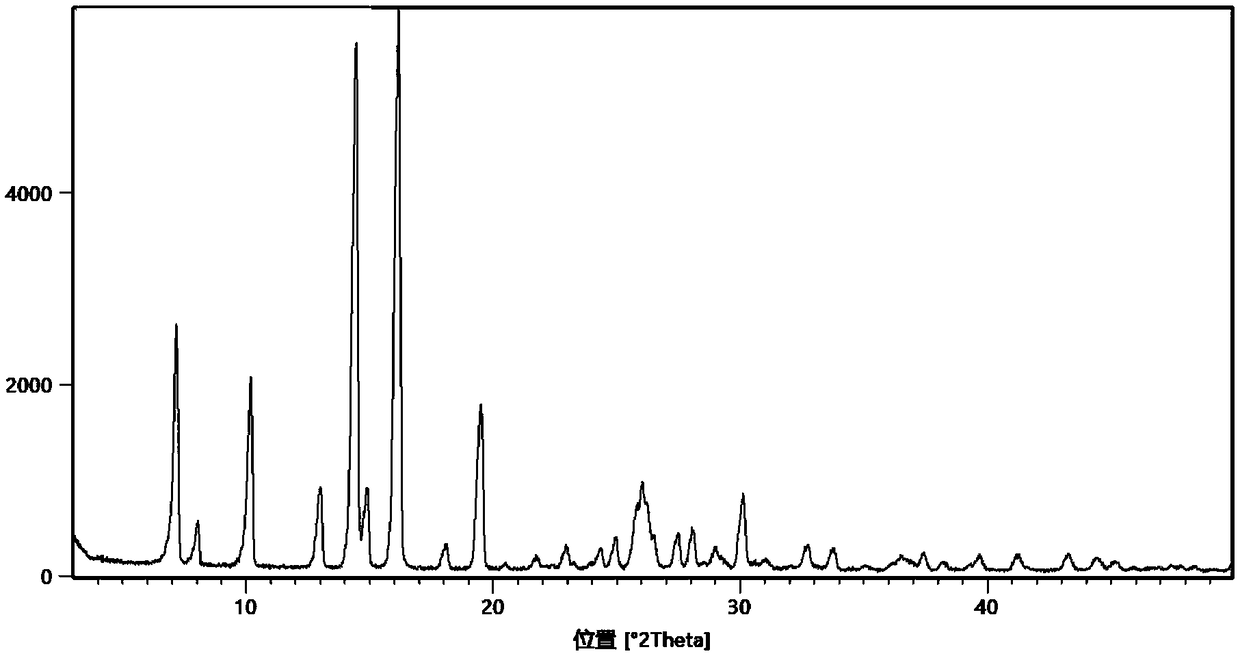
[0064] Dissolve 5.0g (32.4mmol) of methylpyrazine derivatives and 4.0g (32.4mmol) of isonicotinamide in 50ml of methanol, heat to 60°C to dissolve, after the solution is clear, cool down to 20°C, and statically crystallize for 48 hours. After filtration and drying, 8.6 g of eutectic form I of the isonicotinamide methylpyrazine derivative was obtained, with a yield of 96.5%, HPLC: 99.96%, and impurity I: 0.03%.
Embodiment 2
[0066] Dissolve 5.0g (32.4mmol) of methylpyrazine derivatives and 6.0g (48.6mmol) of isonicotinamide in 50ml of ethyl acetate, heat to 77°C to dissolve, after the solution is clear, cool down to 15°C, and statically crystallize for 52 hours, filtered and dried to obtain 8.8 g of co-crystal form I of isonicotinamide methylpyrazine derivative, yield 98.0%, HPLC: 99.93%, impurity I: 0.05%.
Embodiment 3
[0068] Dissolve 10.0g (64.8mmol) of methylpyrazine derivatives and 9.6g (77.8mmol) of isonicotinamide in 50ml of methanol, heat to 65°C to dissolve, after the solution is clear, cool down to 10°C, and statically crystallize for 36 hours. After filtration and drying, 17.2 g of the eutectic form I of the isonicotinamide methylpyrazine derivative was obtained, with a yield of 96.0%, HPLC: 99.91%, and impurity I: 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com