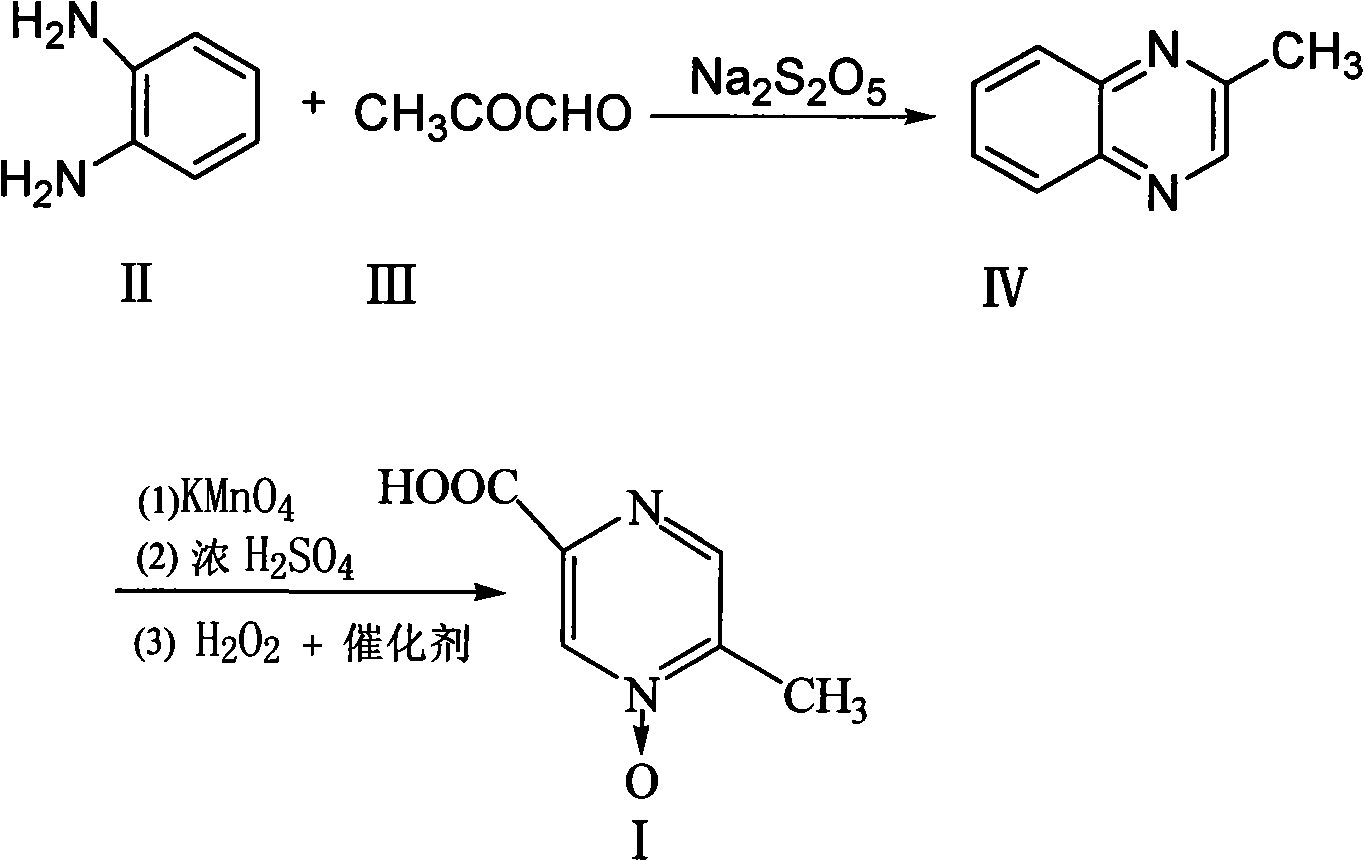
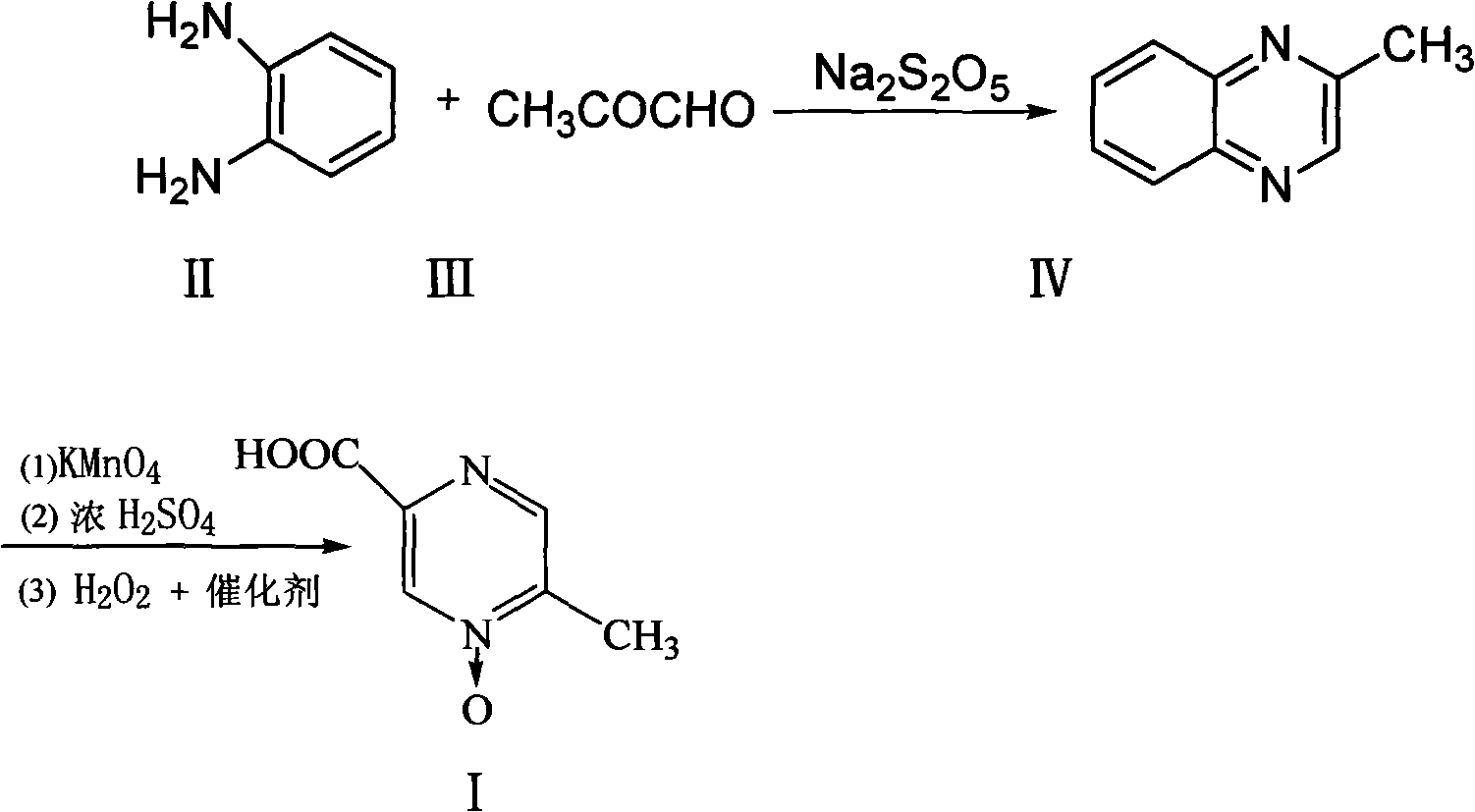
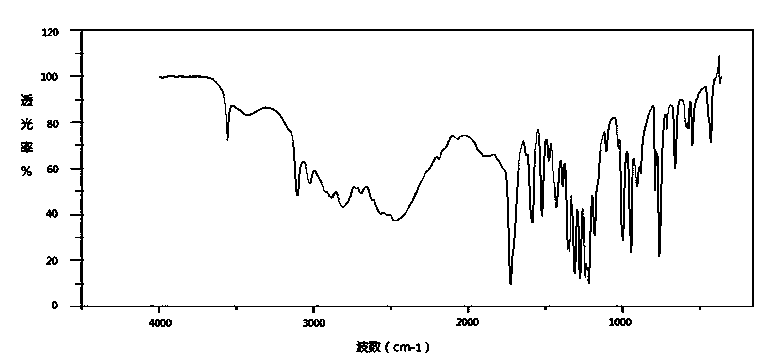
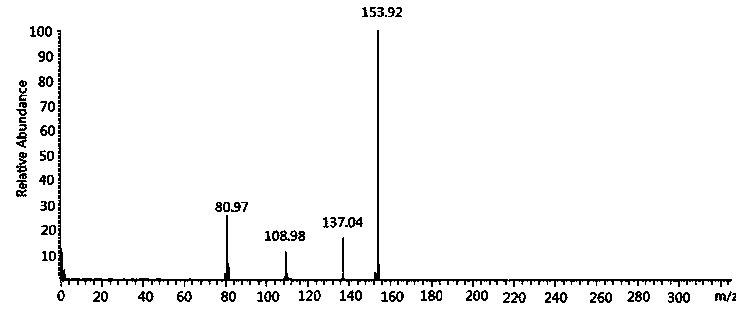
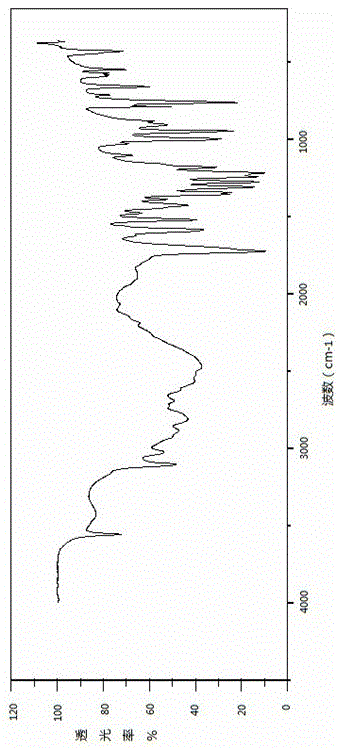
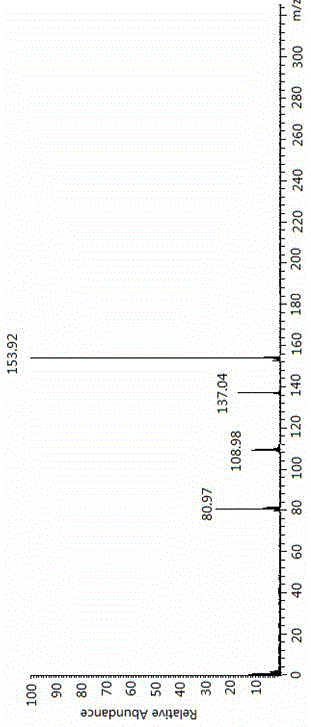
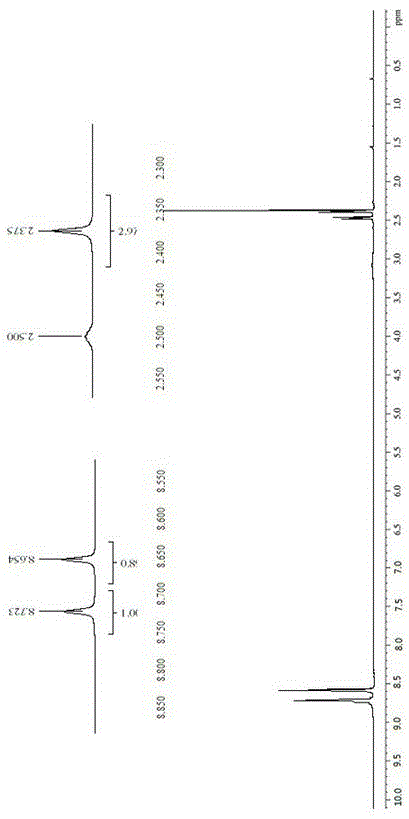
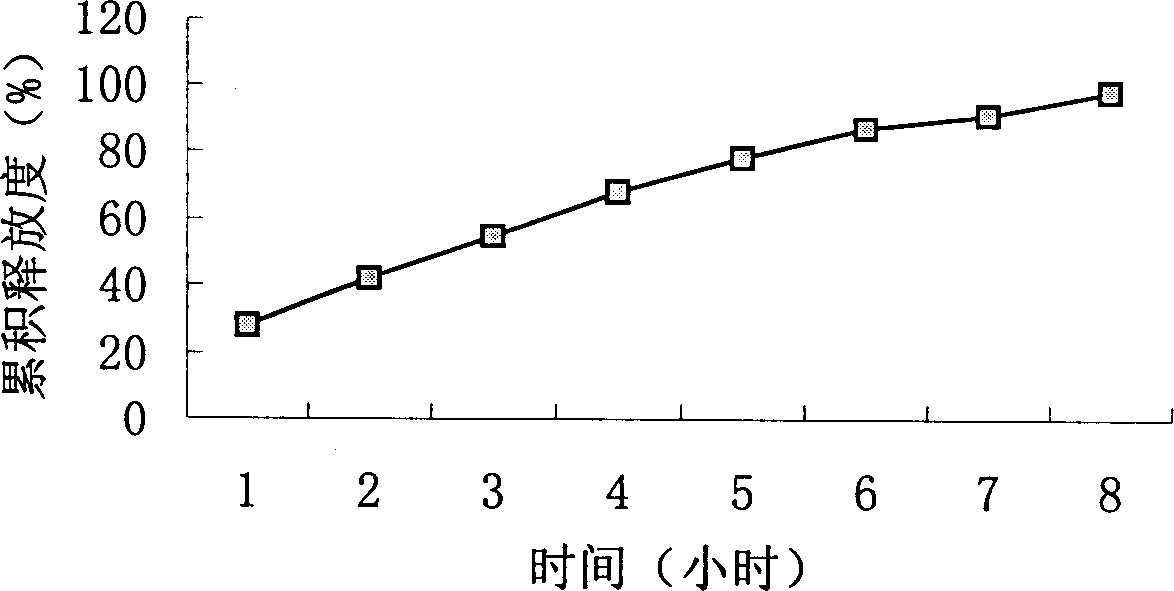
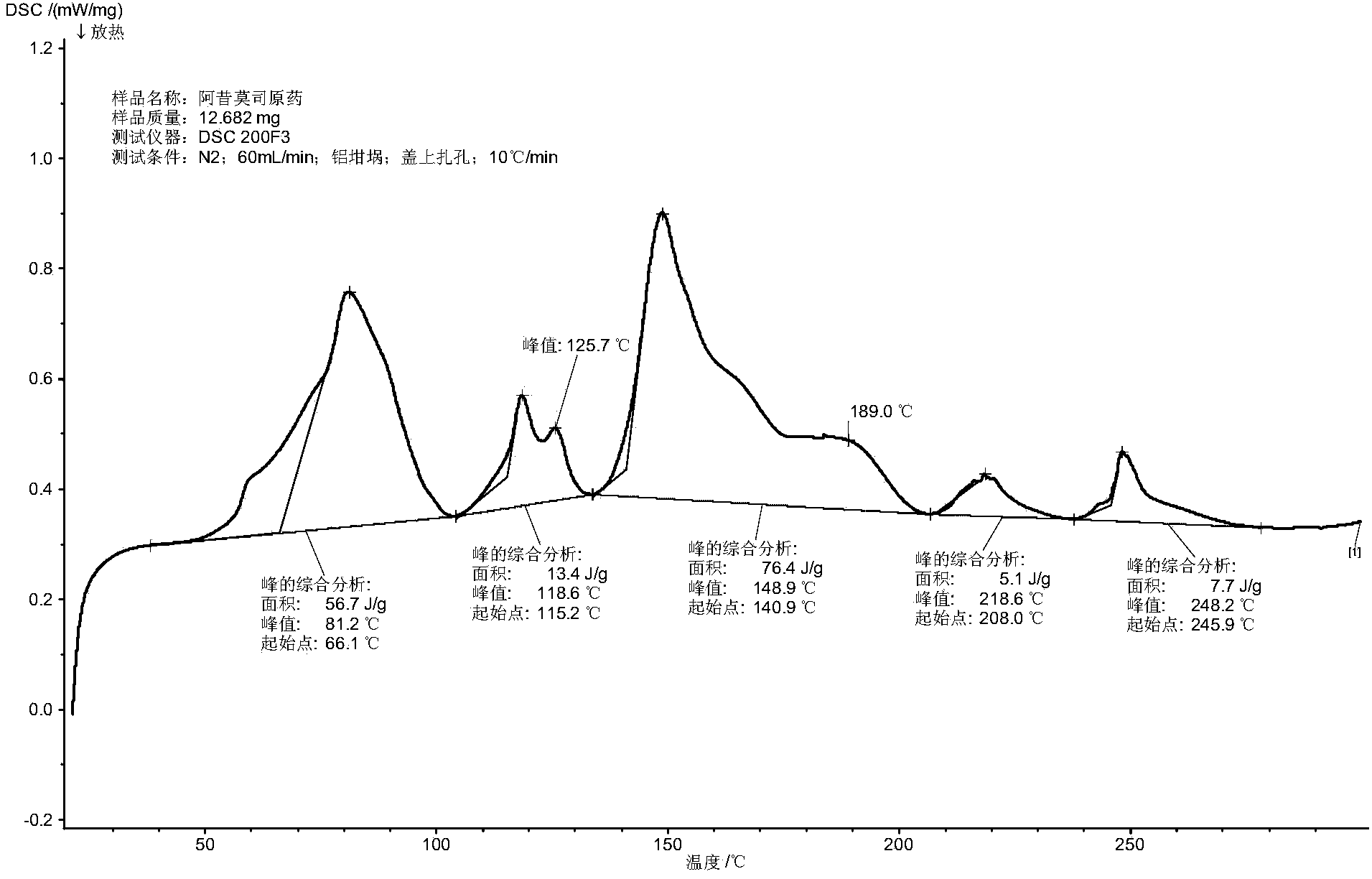
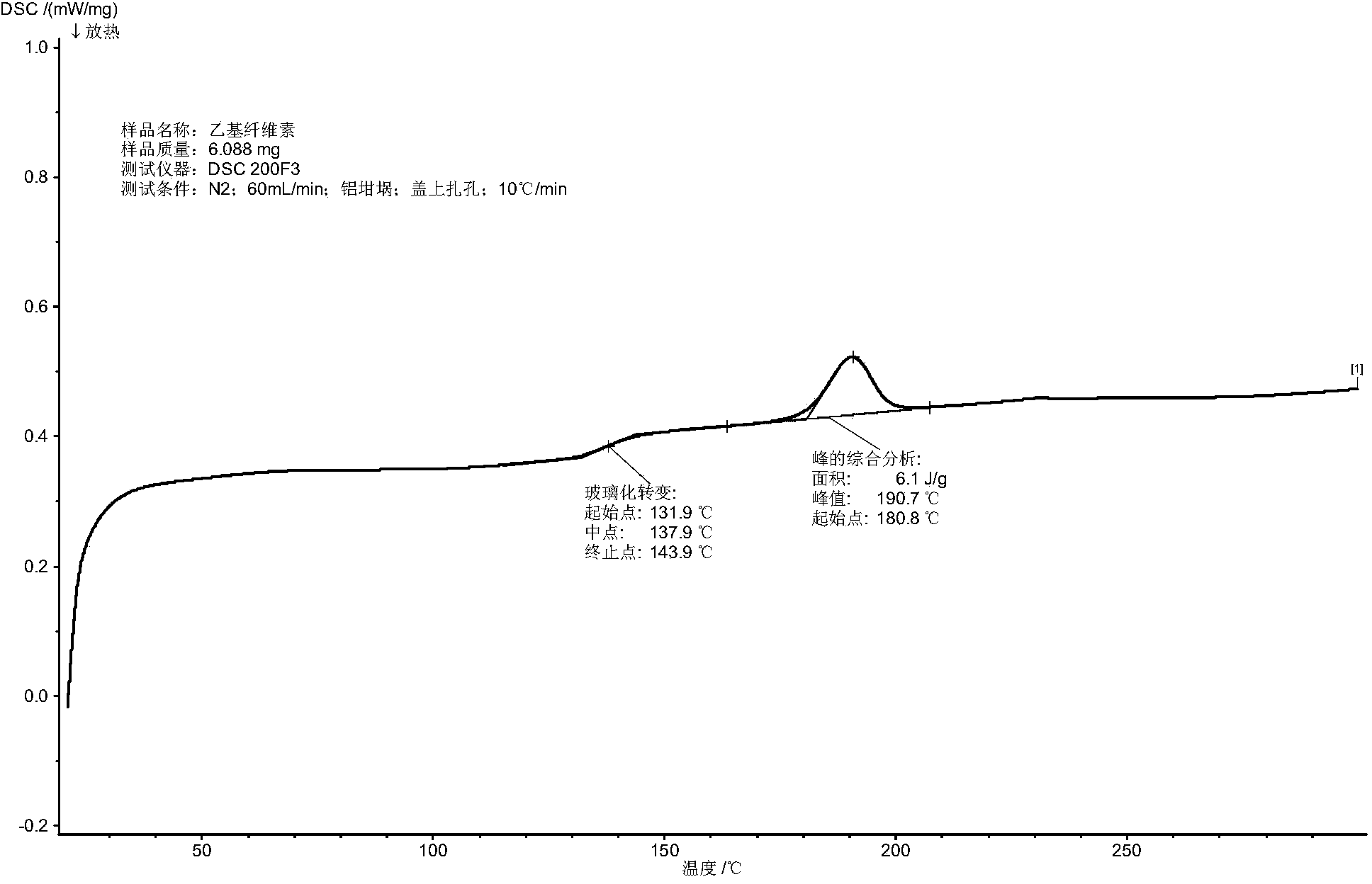
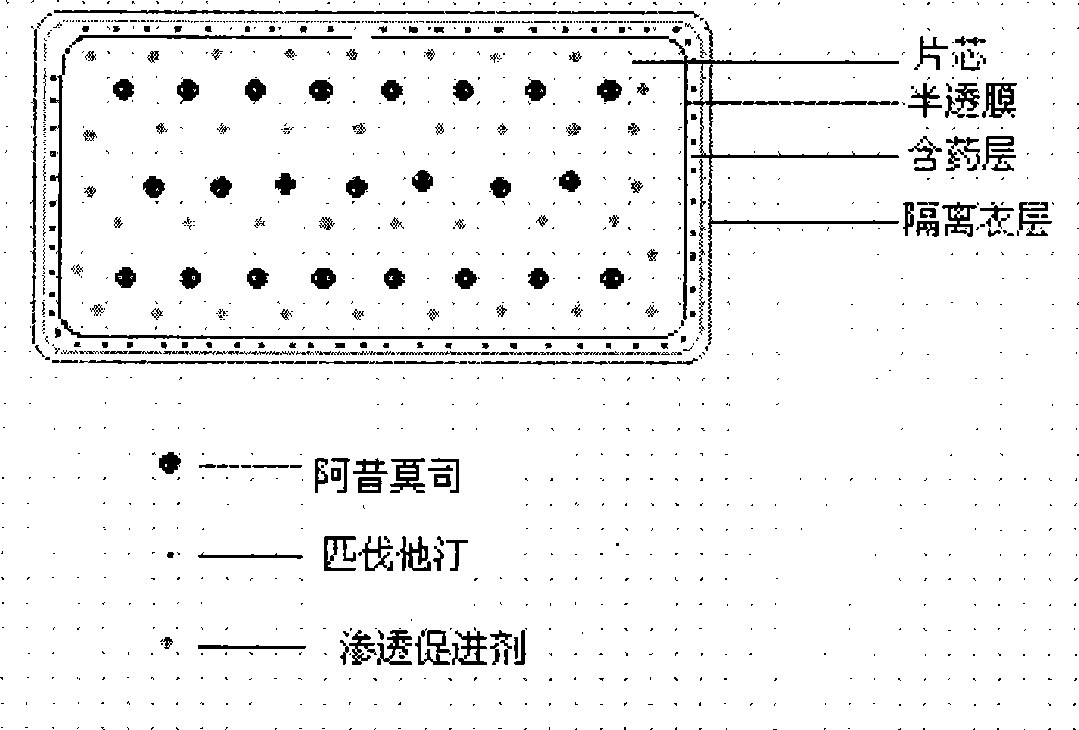
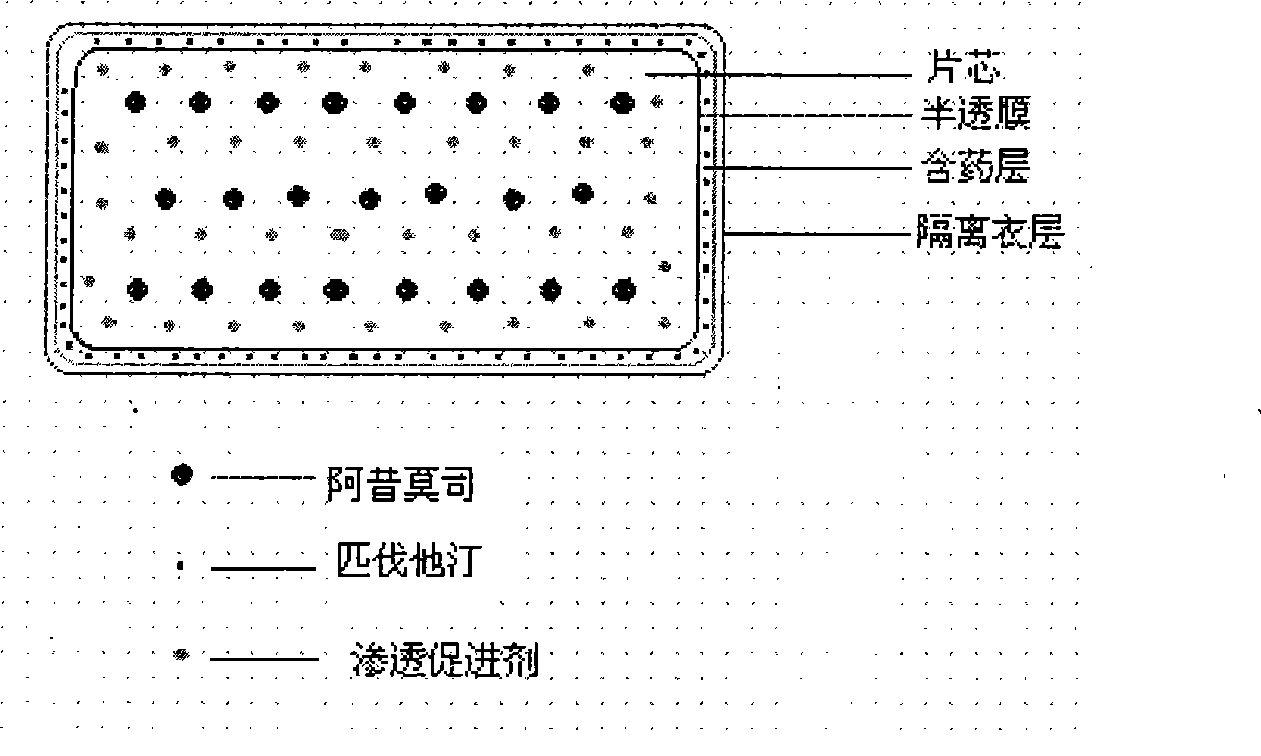
Patents
Literature
69 results about "Acipimox" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
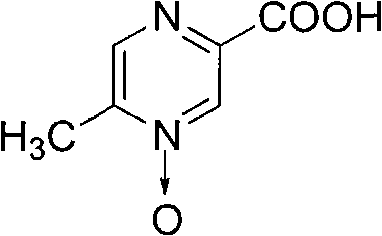
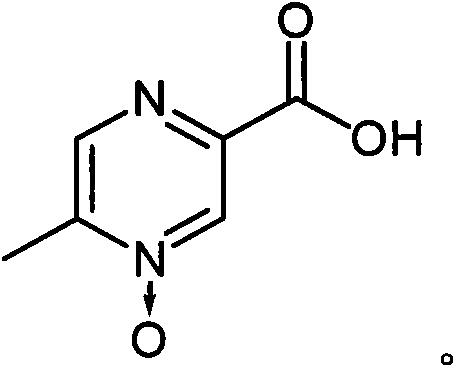
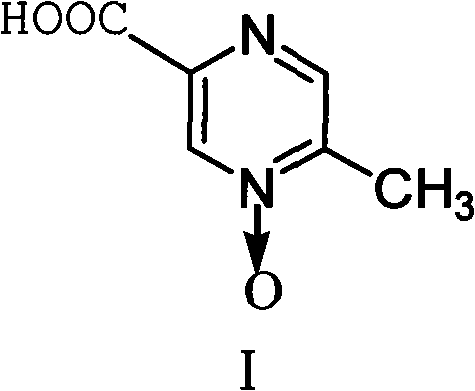
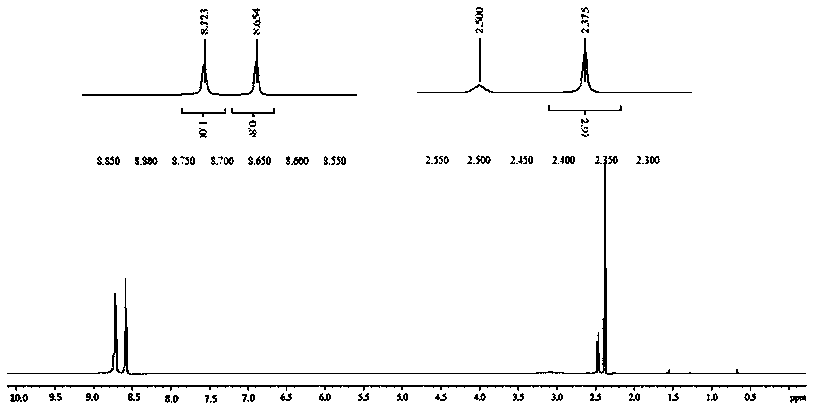
Acipimox (trade name Olbetam in Europe) is a niacin derivative used as a lipid-lowering agent. It reduces triglyceride levels and increases HDL cholesterol. It may have less marked adverse effects than niacin, although it is unclear whether the recommended dose is as effective as standard doses of niacin.
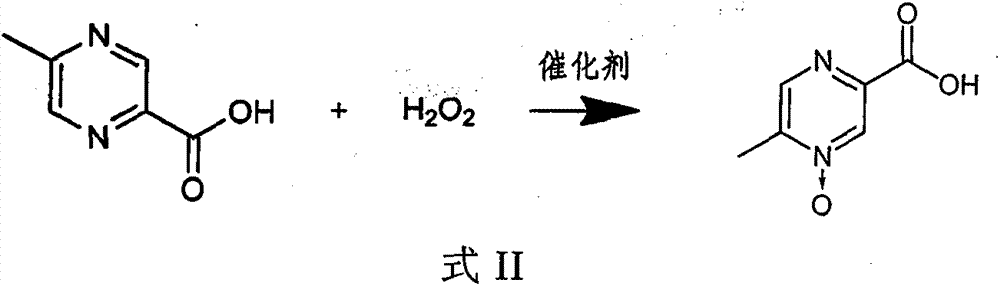
Preparation method of acipimox
InactiveCN103508963AReduce usageEasy to get materialOrganic chemistryAcetic anhydrideCarboxylic acid
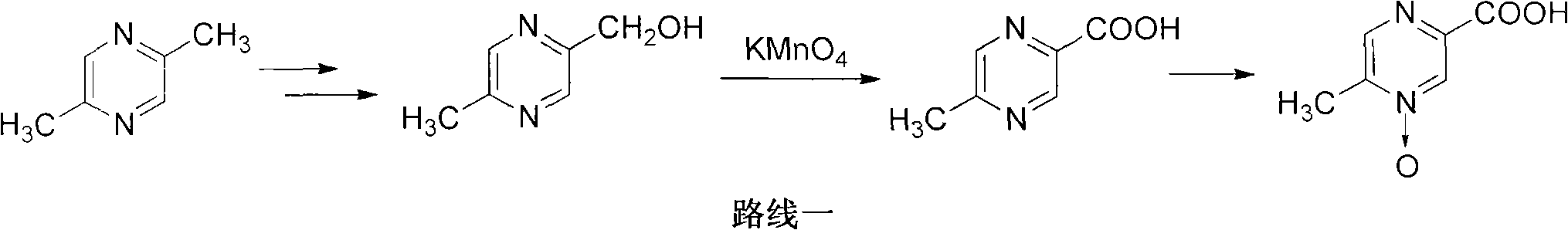
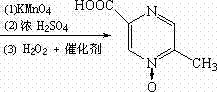
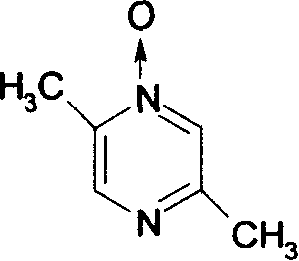
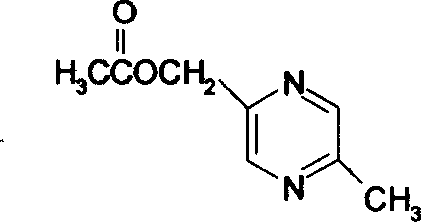
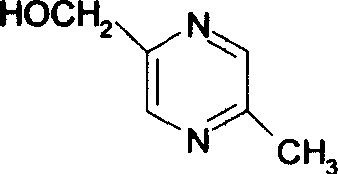
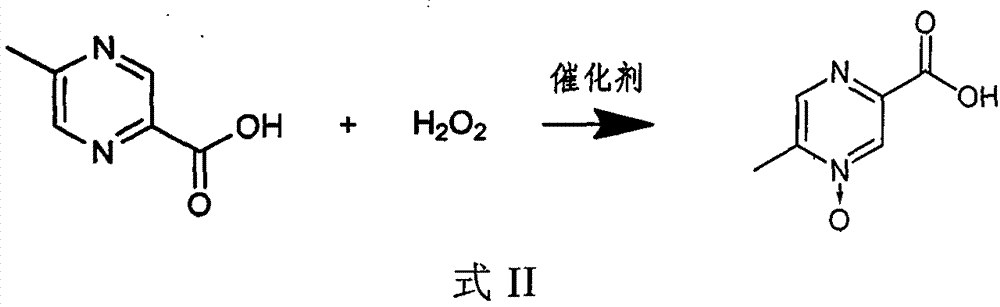
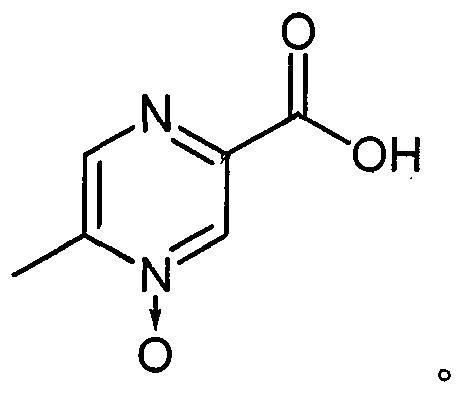
The invention relates to a preparation method of acipimox for treating hyperlipidemia, belonging to the field of medicines. The invention provides an acipimox synthesis process which is simple and easy to operate. The preparation method comprises steps of with 2, 5-dimethylpyrazine as a raw material, carrying out nitrogen oxidation, reaction with acetic anhydride and alkaline hydrolysis to obtain 2-hydroxymethyl-5-methylpyrazine; directly oxidizing a post-treatment fluid by using 2,2,6,6-tetramethyl-1-piperidinyloxy / sodium hypochlorite / potassium bromide to obtain 5-methylpyrazine-2-carboxylic acid, wherein the post-treatment fluid is not needed to be dried and concentrated; oxidizing 5-methylpyrazine-2-carboxylic acid by using hydrogen peroxide / sodium tungstate to obtain acipimox.
Owner:WEIHAI WEITAI PHARMA TECH DEV
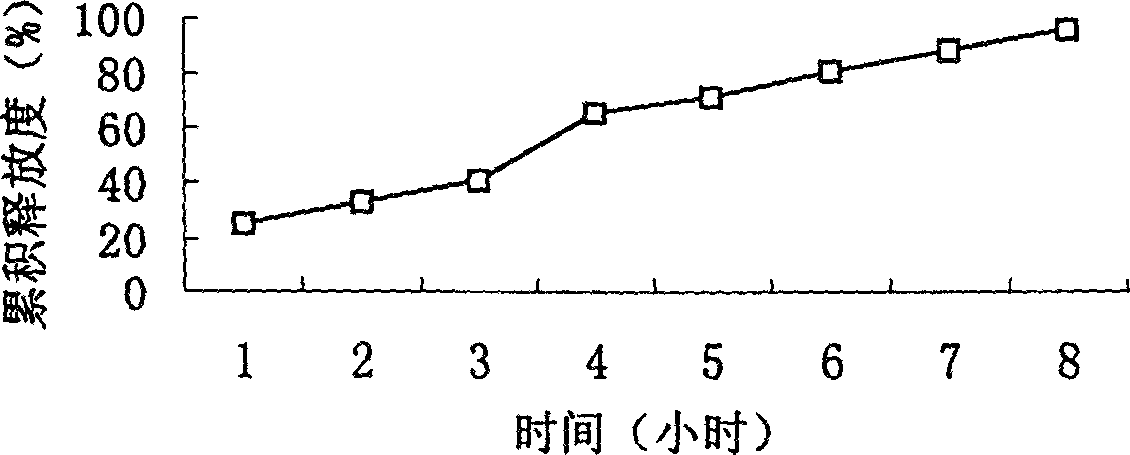
Osmotic pump controlled release preparation composition and preparation thereof
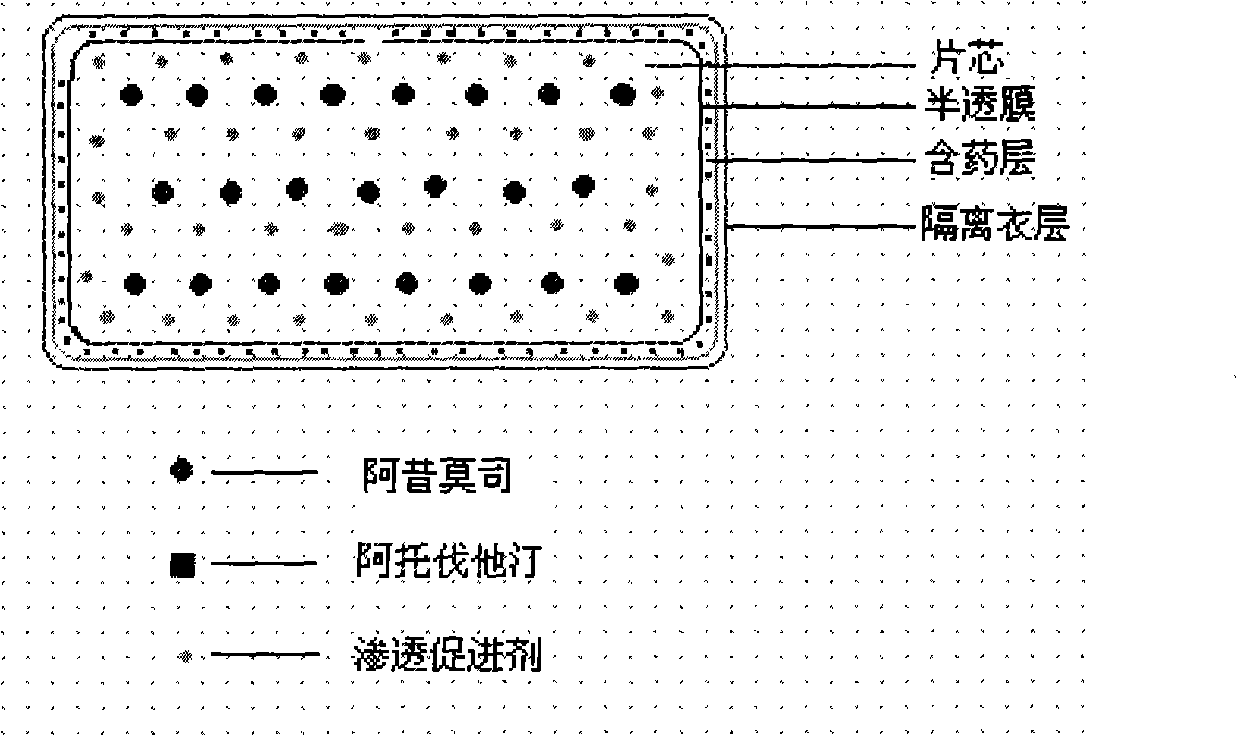
ActiveCN101352426AFully functionalSmall toxicityMetabolism disorderPharmaceutical delivery mechanismSide effectBULK ACTIVE INGREDIENT
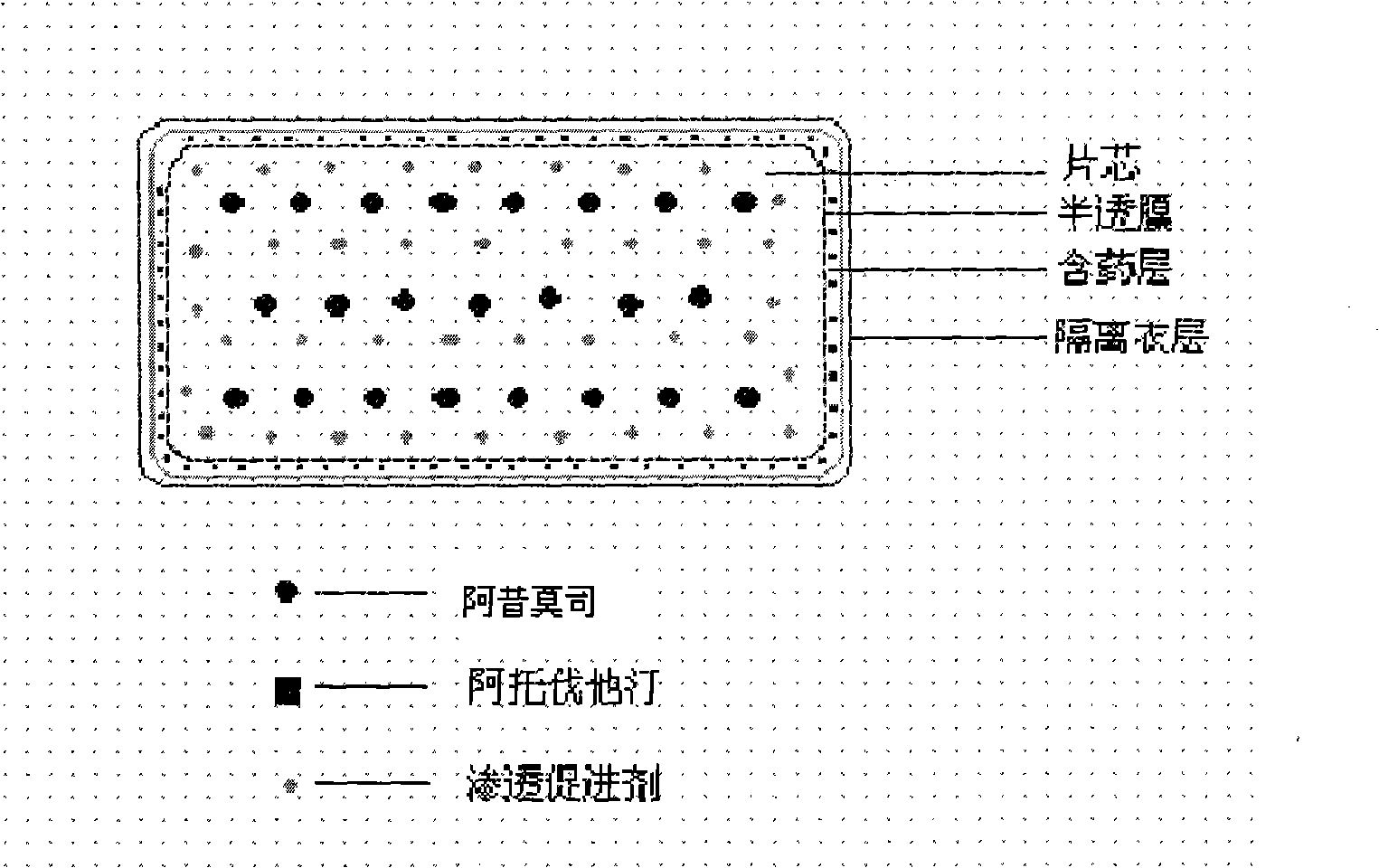
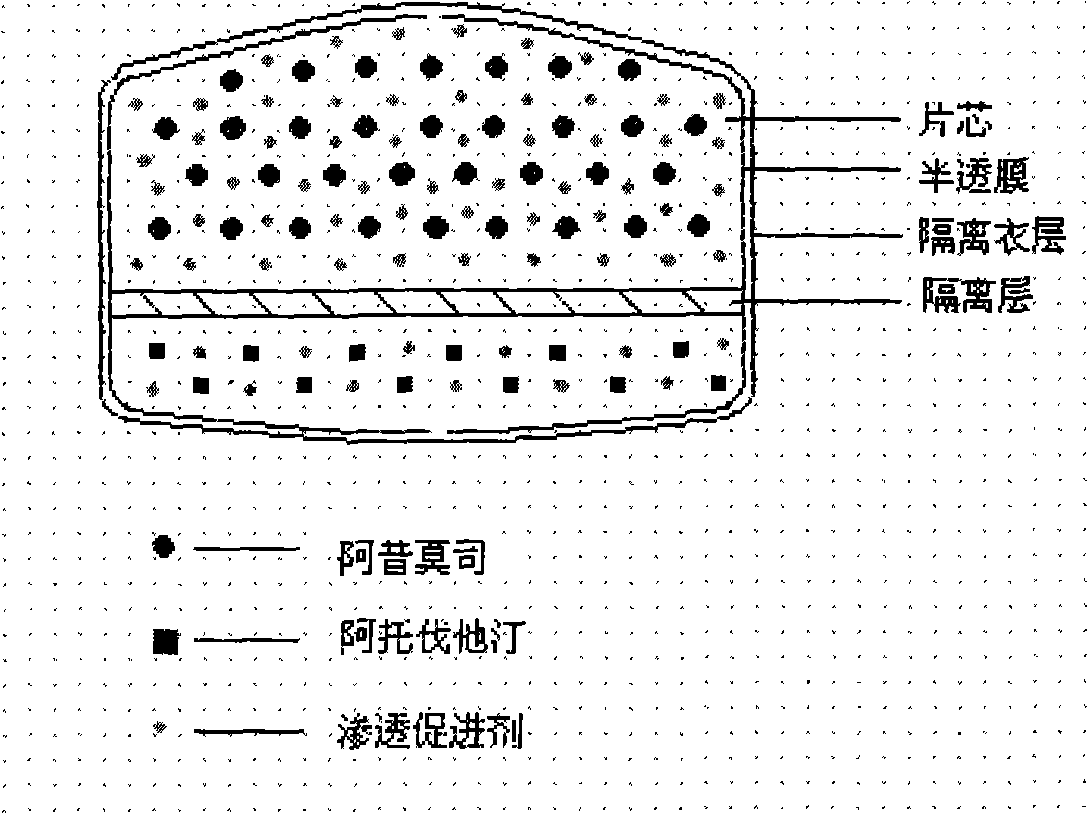
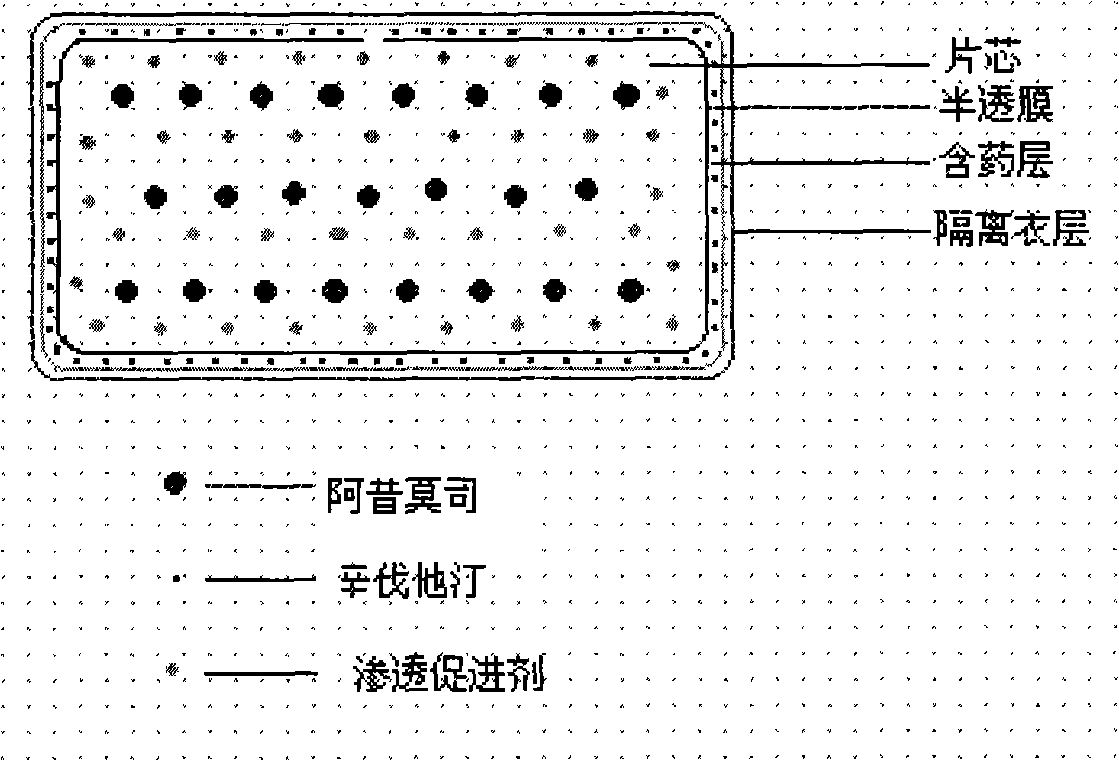
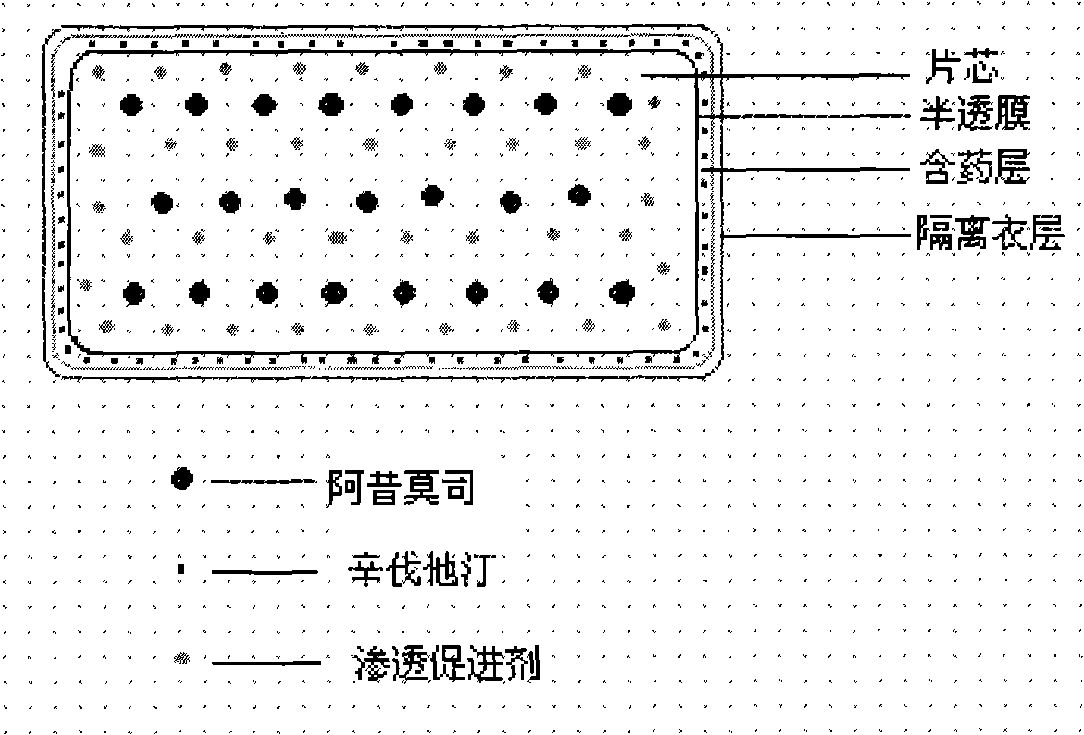
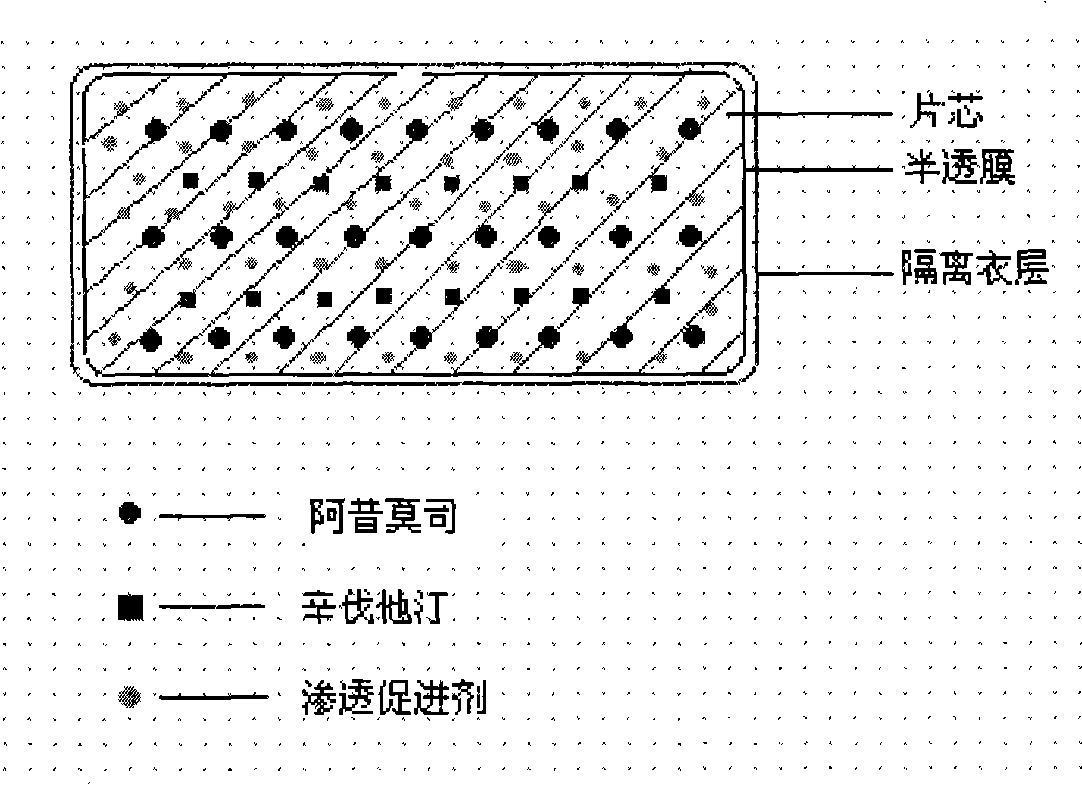
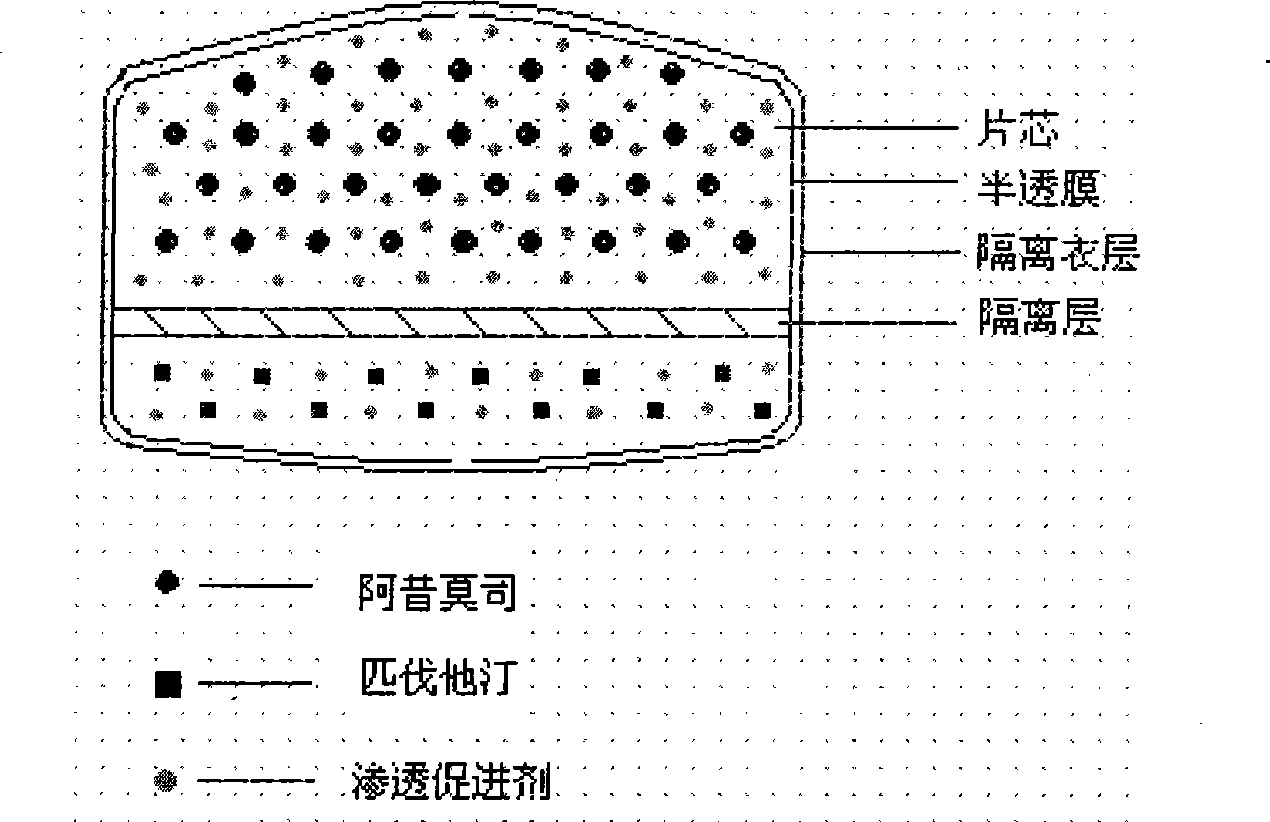
The invention relates to an osmotic pump controlled release pharmaceutical composition which contains two medicine active ingredients and is used for treating hyperlipoidemia and a preparation method thereof, the pharmaceutical composition has a nicotinic acids medicine such as acipimox and the other one estatina medicine such as atorvastatin; wherein, the nicotinic acids medicine such as acipimox is a controlled release part, and the estatina medicine such as atorvastatin is a quick release part; or both the acipimox and the atorvastatin are the controlled release part. The compound osmotic pump preparation of the invention has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
Refining method of acipimox
The invention belongs to the field of medicine synthesis, and particularly relates to a refining method of acipimox. The acipimox is refined by adopting a method of dissolving in hot water, removing impurities by active carbon, and dropping acetone to perform secondary cooling and re-crystallization under the condition of controlling the cooling speed. According to the technical scheme, through controlling crystallization conditions, the acipimox which is higher in purity, smaller in maximum individual impurity, slighter in color and suitable for preparation can be obtained through once refining. The refining method has the advantages of simplicity and convenience in operation, high yield and low cost, and is beneficial to industrialized production.
Owner:SHANDONG NEWTIME PHARMA
Process of selective synthesizing 5-methyl pyrazine-2-carboxylic acid using 2,5-dimethyl pyrazine
This invention use 2,5- dimethyl pyrazine as raw materials, selectively synthesize 5-methyl pyrazine-2-carboxylic acid. Its technology is use 2,5 - dimethyl pyrazine as raw material, through oxidation of nitrogen and hydrogen peroxide, acetic anhydride acid oxidation, hydrolysis and oxidation process, gain 5-methyl pyrazine-2-carboxylic acid. HPLC analysis of their content is over 99%, with 0.1 mol / L of sodium hydroxide titration, content is not less 98%, melting point is not less than of 163 deg, the quality of medicine to meet glipizide, Acipimox requirements. The process is scheme simple, high-yield, low cost and suitable for industrial production.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Synthetic process for acipimox
InactiveCN105218464AReduce the number of crystallizationAvoid recrystallization operationsOrganic chemistryOXALIC ACID DIHYDRATECarboxylic acid
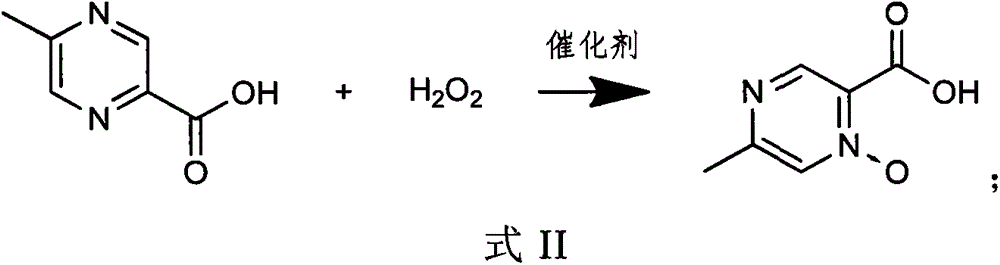
The invention provides a synthetic process for acipimox. The synthetic process comprises steps: firstly, 5-methylpyrazine-2-carboxylic acid and hydrogen peroxide are reacted under action of catalysts in an aqueous solution; secondly, an auxiliary agent is added into the solution obtained in the first step, and the auxiliary agent is one selected from sulfite, bisulfate, oxalic acid and oxalate; thirdly, active carbon is added in the solution obtained in the second step and filtering is carried out; fourthly, the solution obtained in the third step is subjected to cooling crystallization and drying, and an acipimox finshed product is obtained. The synthetic process can lower the crystallization frequency effectively, avoids recrystallization operation, raises the yield and lowers the production cost.
Owner:SICHUAN YIMING PHARMA CO LTD
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
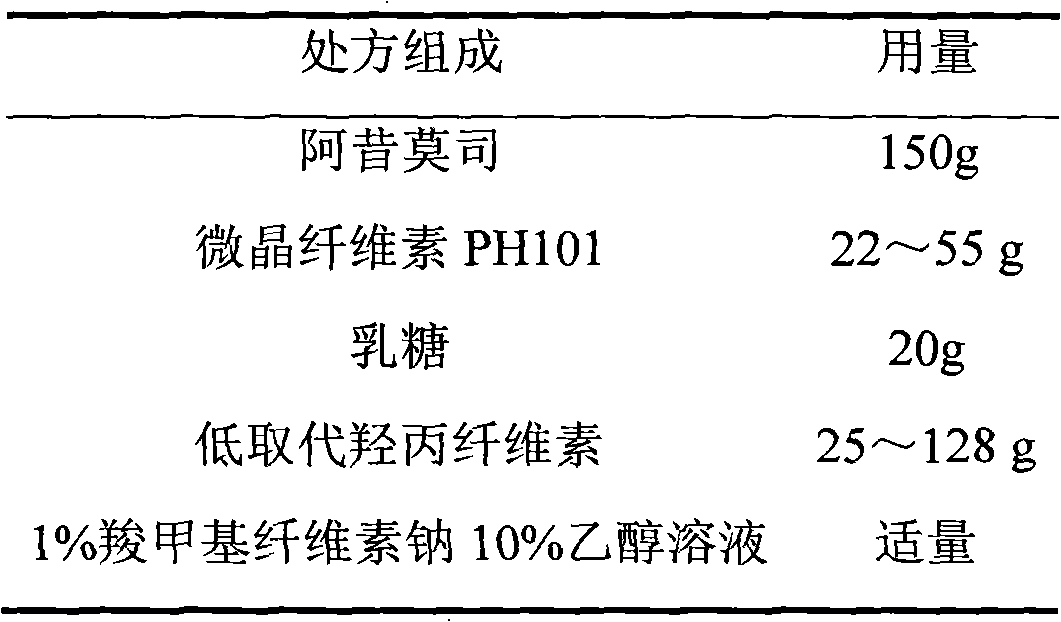
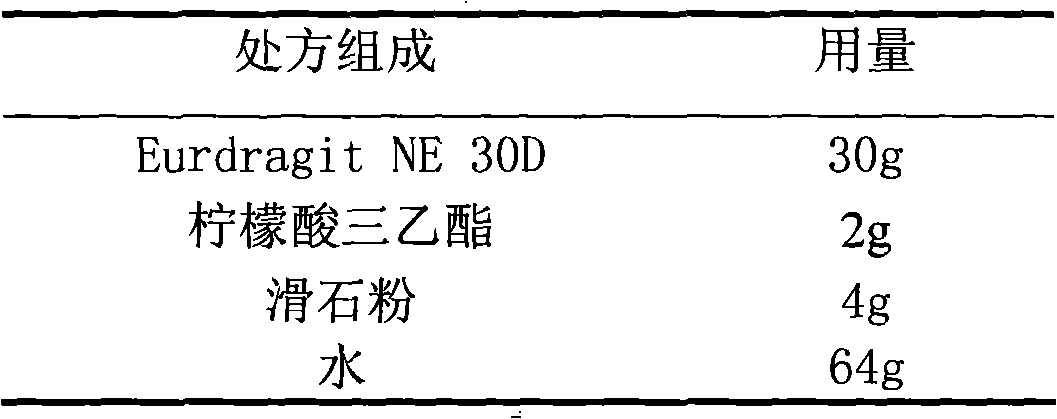
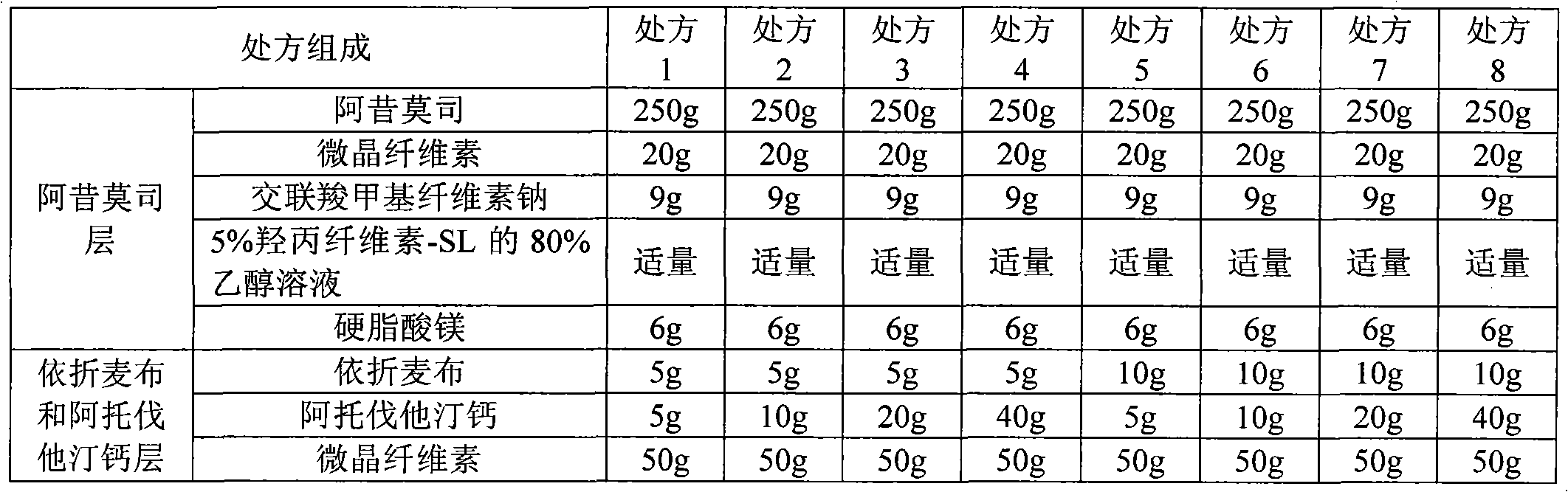
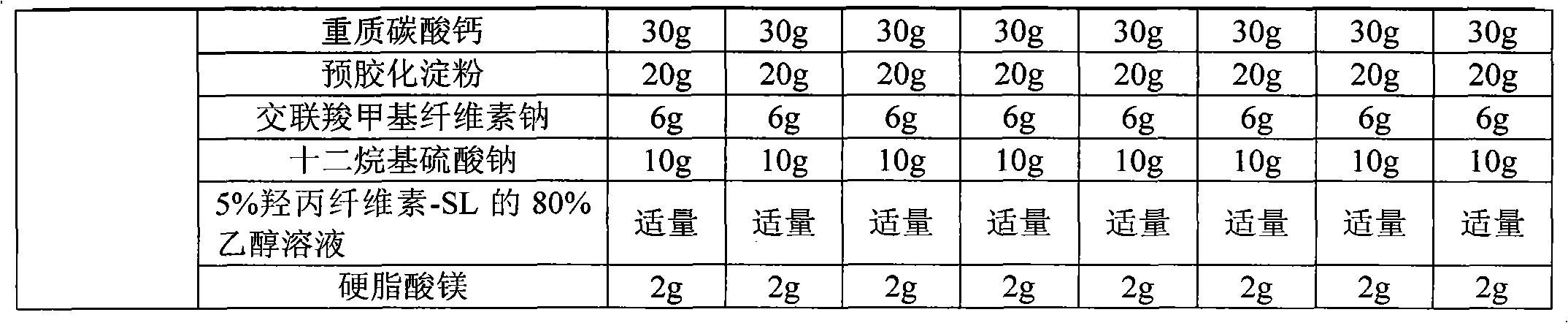
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
Composition for curing hyperlipemia
InactiveCN1457786AEffective treatmentGood curative effectOrganic active ingredientsMetabolism disorderActive componentCurative effect
The composition for treating hyperlipemia contains nicotinic acid or its derivative and rosuvastatin in effective amount as the active component. The said nicotinic acid derivative is inositol nicotinate, vitamin E nicotinate or Acipimox. Compared with the medicine with the active component nicotinic acid or its derivative or rosuvastatin, the composition has wonderful raised curative effect.
Owner:LUNAN PHARMA GROUP CORPORATION
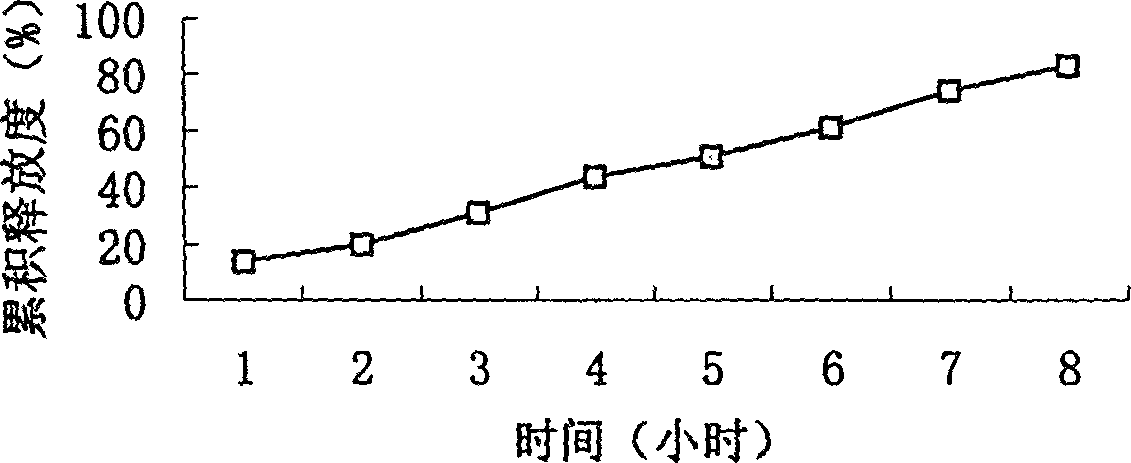
Compound osmotic pump controlled release preparation and preparation method thereof
ActiveCN101352425AObvious side effectsSmall toxicityOrganic active ingredientsMetabolism disorderSide effectOsmotic pump
The invention provides a new osmotic pump controlled release pharmaceutical composition used for treating hyperlipoidemia and a preparation method thereof, the pharmaceutical composition has a nicotinic acids medicine such as acipimox and the other one estatina medicine such as simvastatin; wherein, the nicotinic acids medicine such as acipimox is a controlled release part, and the estatina medicine such as simvastatin is a quick release part; or both the acipimox and the simvastatin are the controlled release part. The compound osmotic pump preparation of the invention has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for improving synthesis process of Acipimox
ActiveCN101899012AOvercome the defect of easy slow oxidationImprove responseOrganic chemistryCatalytic oxidationDecarboxylation
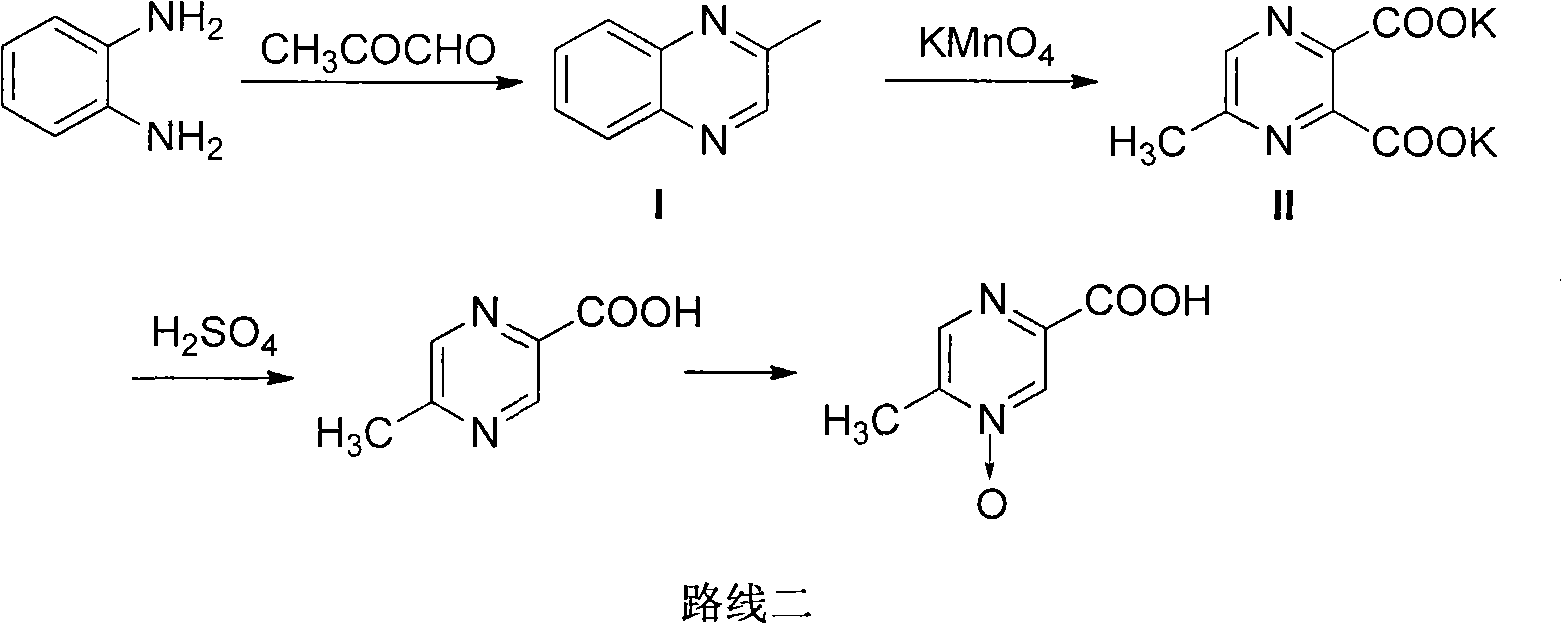
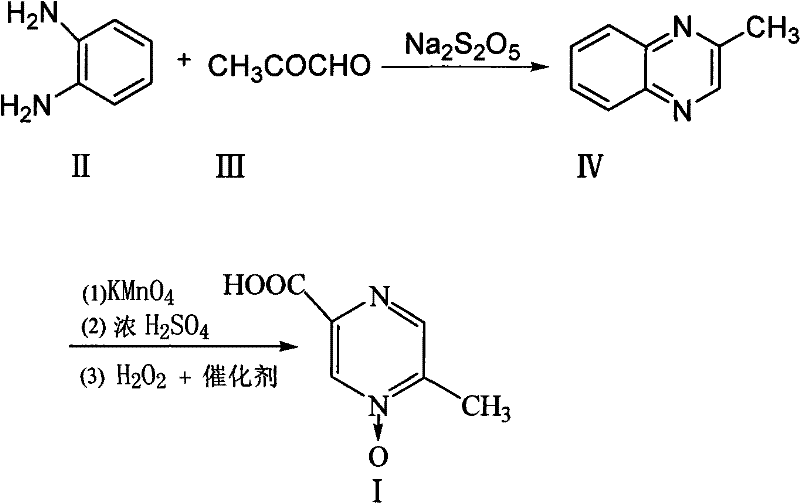
The invention provides a method for improving the synthesis process of Acipimox, which is characterized by carrying out one-pot method on the steps of oxidization, acidification, decarboxylation and oxidization after cyclizing methylglyoxal and o-phenylenediamine which are taken as the original raw materials to directly prepare Acipimox. The method dispenses with intermediate processing, thus greatly simplifying the process steps, and overcomes the defect that the intermediate is easy to oxidize slowly due to multi-step processing, simultaneously utilizes the catalyst for catalytic oxidation in the process of H2O2 oxidation so as to facilitate reaction, improves the reaction yield (66% of overall yield) and is more beneficial to industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing acipimox
InactiveCN103664805AImprove product qualitySuitable for industrialized mass productionOrganic chemistryOrganosolvSolvent
Owner:NORTH CHINA UNIV OF WATER RESOURCES & ELECTRIC POWER
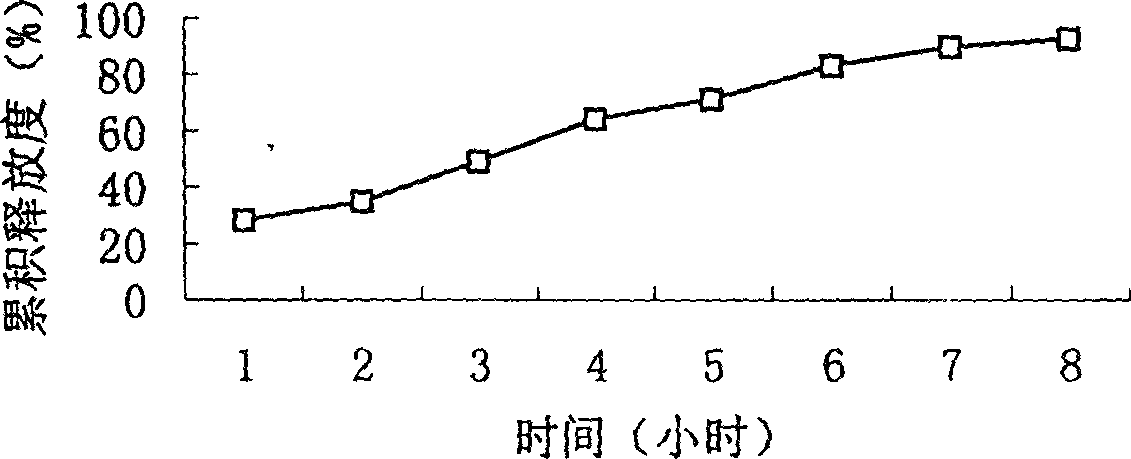
Osmotic pump controlled release preparation composition for treating hyperlipemia and preparation method thereof
ActiveCN101385729AFully functionalSmall toxicityOrganic active ingredientsMetabolism disorderControl releaseOsmotic pump
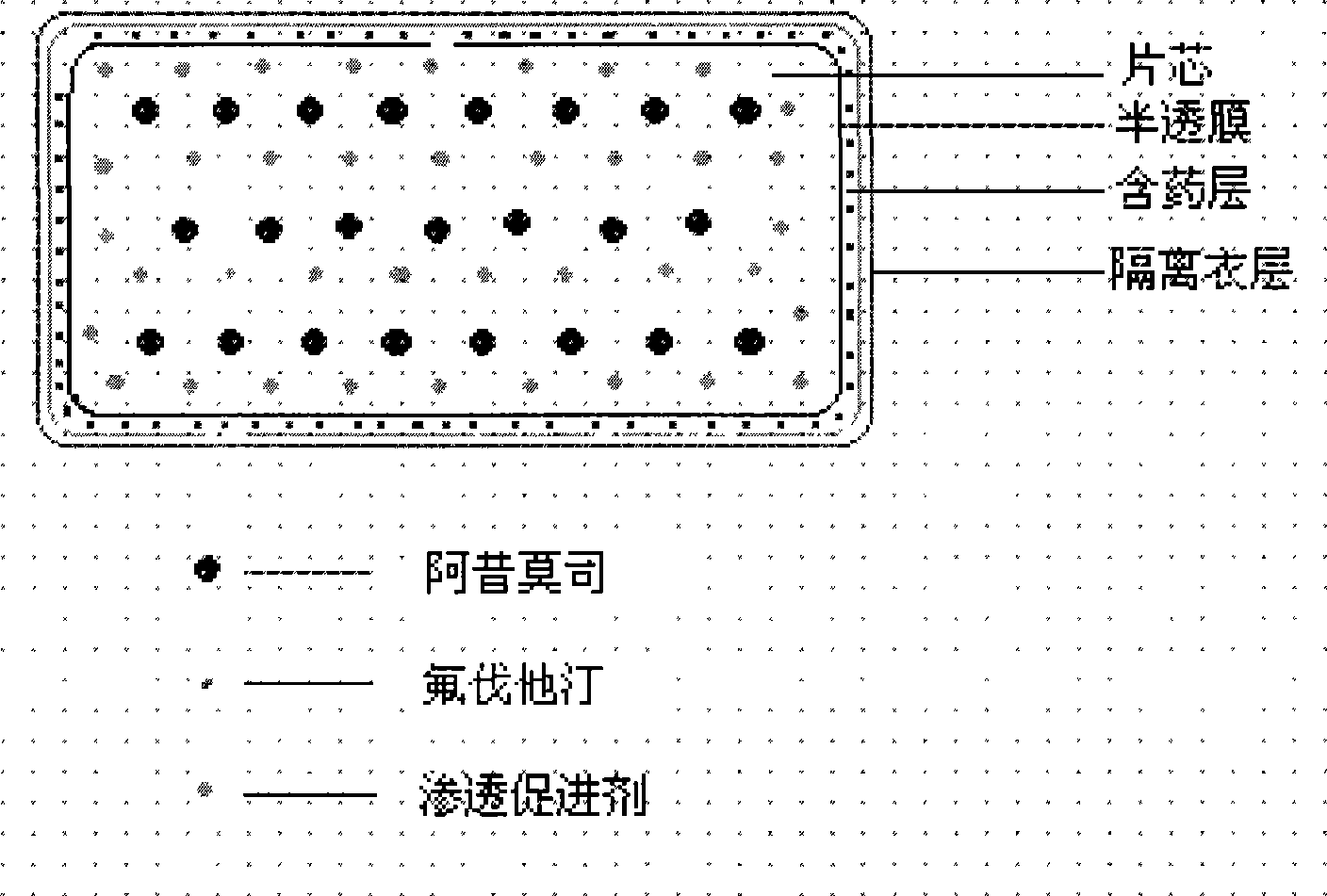
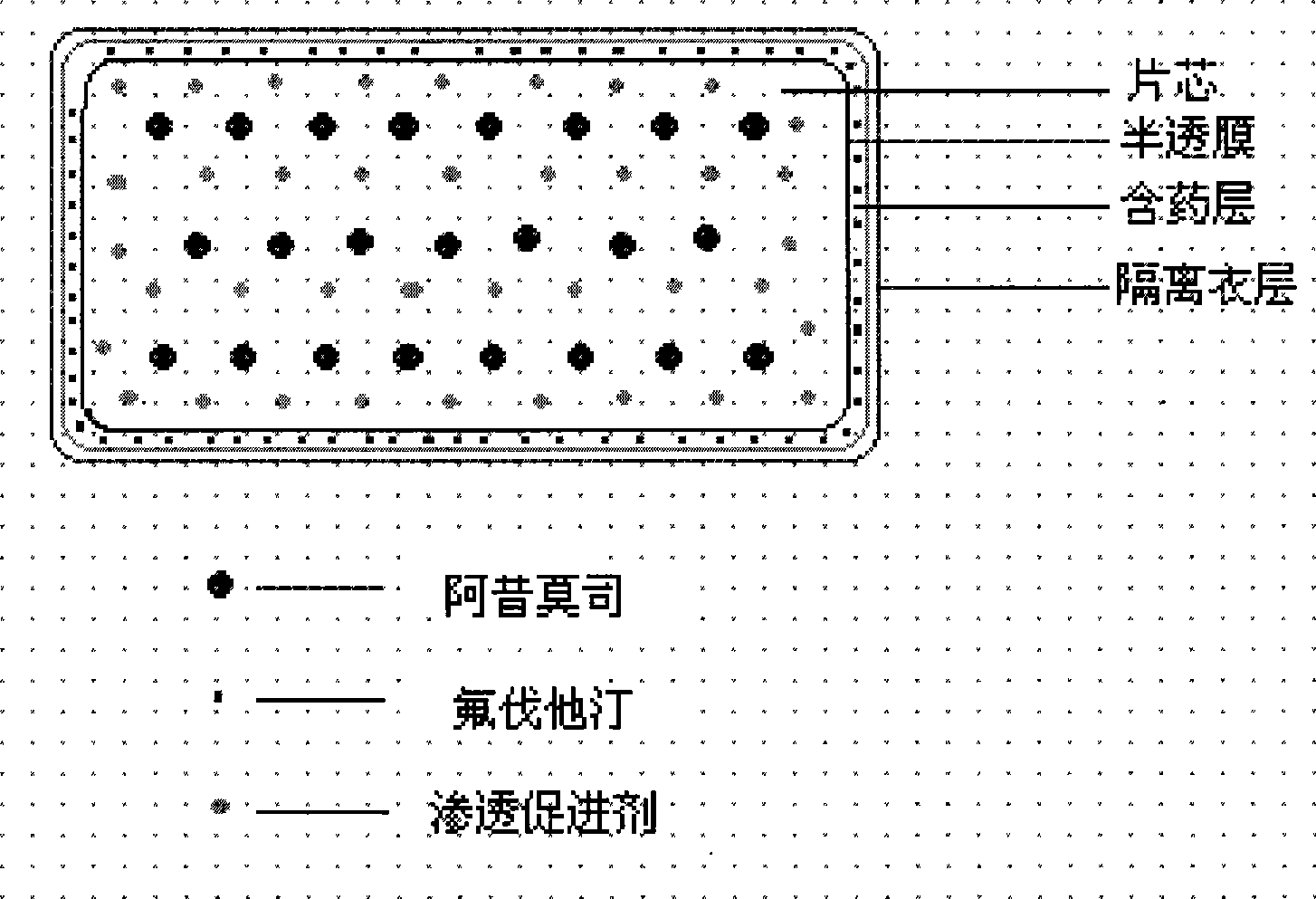
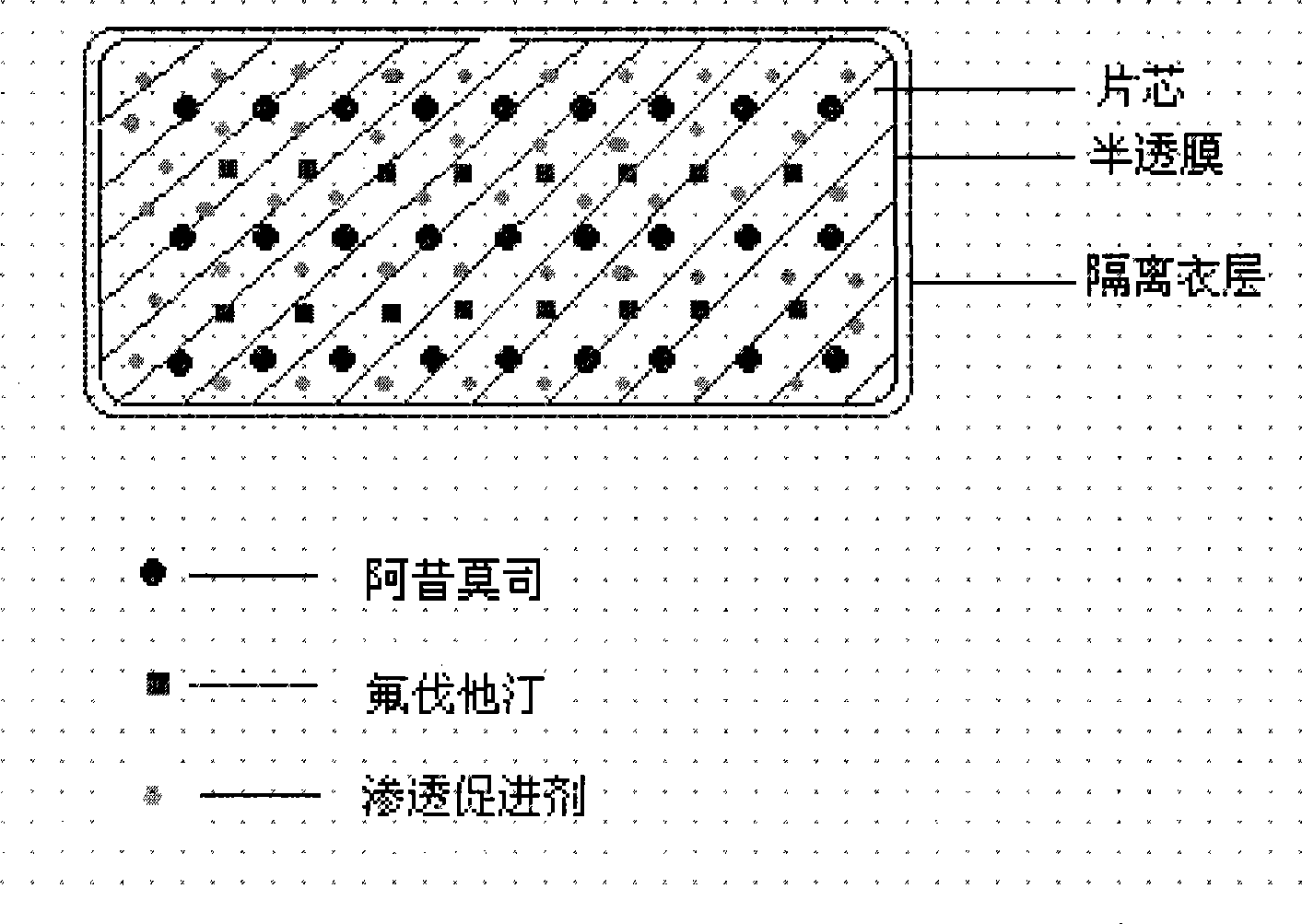
The invention relates to an osmotic pump preparation containing acipimox, fluvastatin and other pharmaceutical excipients, wherein, the acipimox is the controlled-release part, the fluvastatin is the rapid-release part; or the acipimox and the fluvastatin are both the controlled-release parts. In addition, the invention further discloses a preparation method of osmotic pump tablets of the composition, and the osmotic pump table which is prepared by applying the method can better bring the synergy of the acipimox and the fluvastatin into play.
Owner:LUNAN PHARMA GROUP CORPORATION
Composite for treating hyperlipidemia
InactiveCN1692906AFully functional and enhancedObvious side effectsMetabolism disorderAntibody ingredientsSecondary hyperlipidemiaMedicine
Owner:LUNAN PHARMA GROUP CORPORATION
Method for improving synthesis process of Acipimox
ActiveCN101899012BOvercome the defect of easy slow oxidationImprove responseOrganic chemistryCatalytic oxidationDecarboxylation
The invention provides a method for improving the synthesis process of Acipimox, which is characterized by carrying out one-pot method on the steps of oxidization, acidification, decarboxylation and oxidization after cyclizing methylglyoxal and o-phenylenediamine which are taken as the original raw materials to directly prepare Acipimox. The method dispenses with intermediate processing, thus greatly simplifying the process steps, and overcomes the defect that the intermediate is easy to oxidize slowly due to multi-step processing, simultaneously utilizes the catalyst for catalytic oxidation in the process of H2O2 oxidation so as to facilitate reaction, improves the reaction yield (66% of overall yield) and is more beneficial to industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of method for preparing acipimox
InactiveCN103664805BImprove product qualitySuitable for industrialized mass productionOrganic chemistryCarboxylic acidSolvent
The invention relates to a method for preparing lipid lowering medicament acipimox. Acipimox is prepared by synthetic reaction by using 5-methylpyrazine-2,3-dicarboxylic acid, water, acid, sodium tungstate and hydrogen peroxide as raw materials, wherein a mol ratio of 5-methylpyrazine-2,3-dicarboxylic acid, acid, hydrogen peroxide to sodium tungstate is 1:0.05-5:1-10:0.04-0.2. Acipimox is prepared by the method by one-step reaction, purity of the product exceeds 99.5% through once recrystallization, yield exceeds 80%, and the method is suitable for industrial batch production. The method has advantages of low cost of raw material, short synthetic route, simple technology, convenient operation, simple post-treatment, safety and environmental protection of the solvent, basic no pollution of the oxidizing agent, no need of organic solvent in the reaction process, greening, environmental protection, small pollution and wide market prospect, and the production cost is less than a half of that of the prior method.
Owner:NORTH CHINA UNIV OF WATER RESOURCES & ELECTRIC POWER
Slow-releasing acipimox tablet
InactiveCN1395928AStable blood concentrationProlong the action timeOrganic active ingredientsMetabolism disorderProlonged-release tabletBiochemistry
A slow-releasing acipimox tablet for regulating blood fat is prepared from acipimox, slow-releasing agent, adhesive and lubricant through proportioning, wet granulating, baking, mixing with lubricant, and tabletting. Its advantages are stable concentration of medicine in blood, durable acting and low toxic by-effect.
Owner:LUNAN PHARMA GROUP CORPORATION
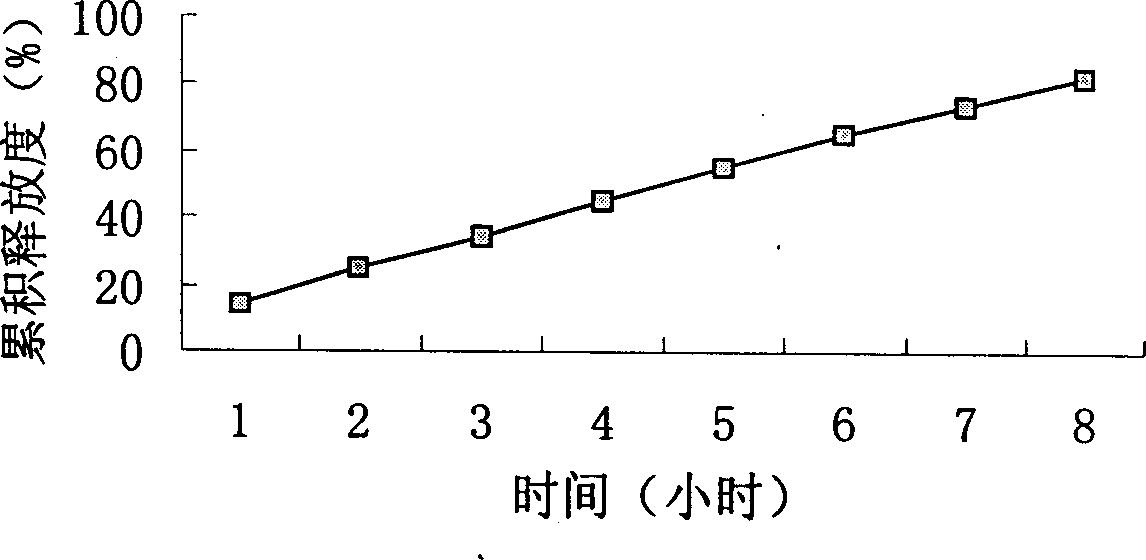
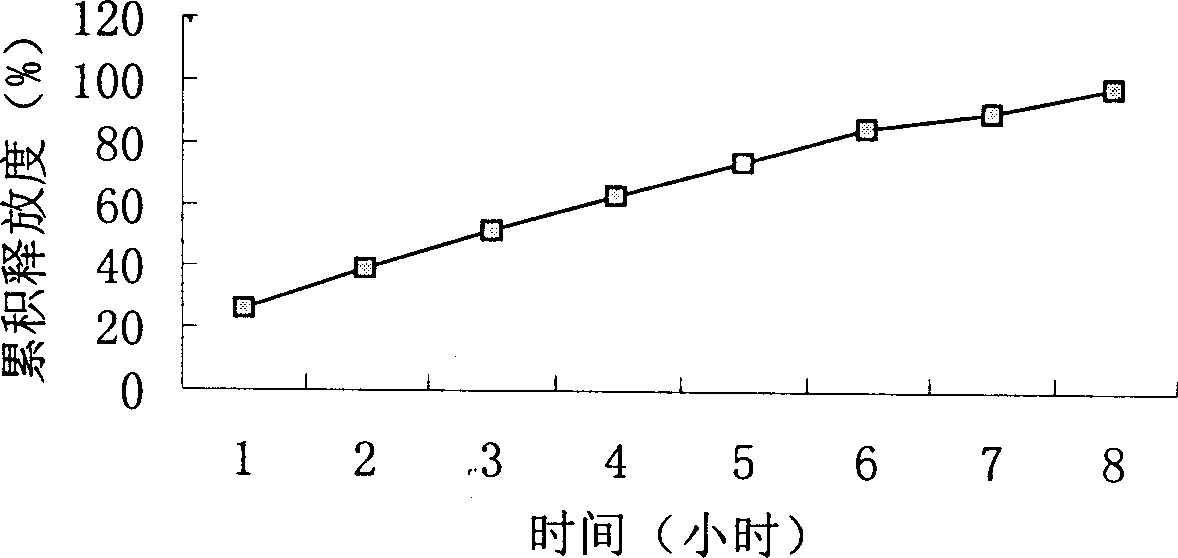
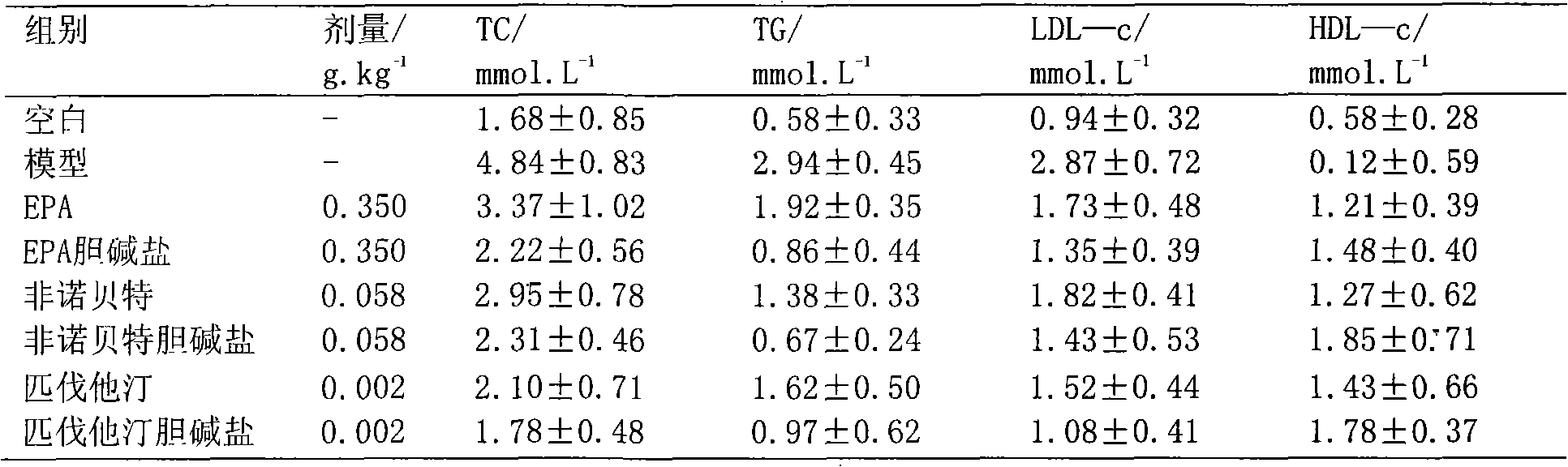
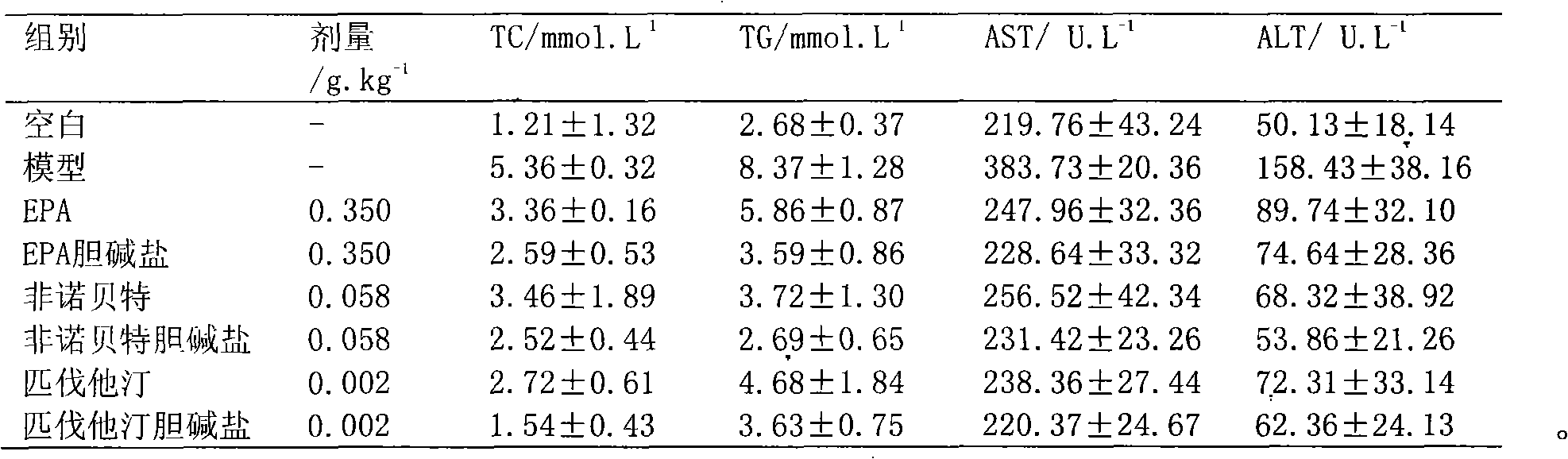
Choline salt of hypolipidemic drug and preparation method and pharmaceutical use thereof
InactiveCN101863780APromote absorptionEfficient use ofOrganic chemistryElcosanoid active ingredientsDocosahexaenoic acidPitavastatin
The invention relates to a choline salt of a hypolipidemic drug and a preparation method and a pharmaceutical use thereof. The invention provides a choline salt of a class of hypolipidemic drugs, and the hypolipidemic drugs include but not limited to clofibrate, libet, fenofibrate, ciprofibrate, gemfibrozil, acipimox, niacin, lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, pitavastatin, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and other unsaturated fatty acids. The choline salt of the hypolipidemic drug can be used for treating hyperlipidemia and other cardiovascular diseases. The invention also provides a preparation method of the choline salt of the hypolipidemic drug.
Owner:北京利乐生制药科技有限公司
Preparation method of acymose
ActiveCN1282647CHigh yieldPerfect process conditionsOrganic chemistryAcetic anhydridePermanganic acid
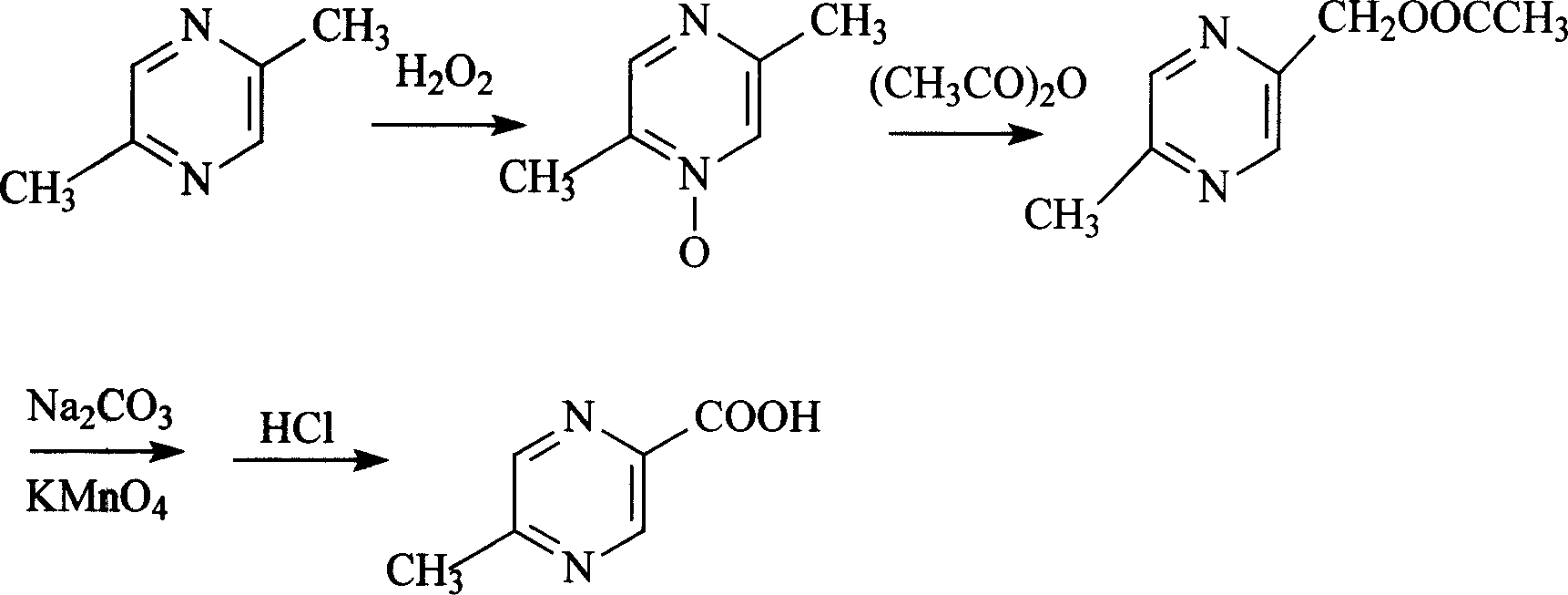
The invention discloses a preparation method of acilimus, which uses 2,5-dimethylpyrazine as a starting material, oxidizes with hydrogen peroxide, esterifies with acetic anhydride, and hydrolyzes it under alkaline conditions, then uses permanganic acid Potassium and hydrogen peroxide are oxidized to produce acipimox. The invention improves the yield of acipimox and greatly reduces the production cost by optimizing the reaction conditions and perfecting the synthesis process, and is suitable for large-scale industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing acipimox-ethylcellulose sustained release micro-capsule
InactiveCN103893152AExtended half-lifeProlong the action timeOrganic active ingredientsMetabolism disorderOrganic solventHalf-life
The invention discloses a method for preparing an acipimox-ethylcellulose sustained release micro-capsule. The method comprises the following steps: (1) dissolving ethyl cellulose powder into a volatile organic solvent to obtain a solution a; (2) adding the acipimox powder to the solution a to obtain a solution b under an agitation state, wherein the mass ratio of the acipimox powder to the ethyl cellulose powder is (1:1) to (3:1); (3) dissolving an emulsifier sodium dodecyl benzene sulfonate into nonvolatile high boiling liquid at 30-35 DEG C to obtain a solution c, wherein the volume ratio of the nonvolatile high boiling liquid to the volatile organic solvent is (3:1) to (5:1), and the mass percent concentration of the sodium dodecyl benzene sulfonate in the solution c is 1-2%; and (4) adding the solution b to the solution c at 30-35 DEG C, agitating for 3-6 hours, then carrying out suction filtration, washing the solid by water, and naturally drying at room temperature. By adopting the method disclosed by the invention, the half-life period of the acipimox medicine can be prolonged, the bioavailability of the medicine is improved, and the medicine action time is prolonged.
Owner:BEIJING UNION UNIVERSITY
Osmotic pump preparation composition for treating hyperlipemia
ActiveCN101385718AFully functionalEasy to useOrganic active ingredientsMetabolism disorderSide effectOsmotic pump
The invention provides a new osmotic pump controlled-release pharmaceutical composition for treating hyperlipemia and a preparation method thereof, one of which is a nicotinic acid drug of acipimox, and the other is a statin drug of pitavastatin calcium; wherein, the acipimox is the controlled-release part, the pitavastatin calcium is the rapid-release part; or the acipimox and the pitavastatin calcium are both the controlled-release parts. A compound osmotic pump preparation has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
Medicament for treating cardiac neurosis and preparation method thereof
InactiveCN104001162AImprove defenseGeneralized central depressionHeavy metal active ingredientsOrganic active ingredientsSide effectPalpitations
The invention discloses medicament for treating cardiac neurosis and a preparation method of the medicament. The medicament solves the problem that existing medicament is poor in effect on treatment of cardiac neurosis and has side effects. The medicament for treating cardiac neurosis comprises the following main materials of schisandra chinensis, manyprickle acanthopanax roots, albizzia julibrissin durazz, American ginseng, radix polygoni multiflori preparata, magnetit, fungus ganoderma lucidum, angelica sinensis, prepared dried rehmannia, fushen, agarwood and semen ziziphi spinosae. The medicine further comprises the following auxiliary materials of lumbrokinase, polypeptide, multivitamins, water-soluble dietary fibers, acipimox, phosphatidylcholine and honey. The medicament has the good curative effect on common cardiac neurosis in cardiovascular diseases, and has wide application prospect in the fields of treatment of palpitation, precardium area pains, dyspnea, panasthenia, anxiety, sinus arrhythmia and the like.
Owner:徐海年
Drug combination for treating or preventing fatty hyperlipidemia
InactiveCN105232554AFunction and effect enhancementLose weightOrganic active ingredientsMetabolism disorderLow density lipoprotein cholesterolObesity prevention
The invention relates to a drug combination for treating or preventing fatty hyperlipidemia, in particular to a drug combination containing orlistat or cetilistat and acipimox, and belongs to the field of pharmaceuticals. The drug combination can be solid preparations such as conventional tablets, dispersible tablets, sustained-release tablets, capsules and granules. Experiments accidentally show that a drug combination containing orlistat or cetilistat and rosuvastatin calcium has an obvious synergistic effect in the aspects of lowering serum total cholesterol, serum triglyceride and low-density lipoprotein cholesterol. A preparation method is simple, convenient to operate and suitable for industrialized production.
Owner:QINGDAO YUNTIAN BIOTECH
Slow-releasing acipimox capsule
InactiveCN1395927AStable blood concentrationProlong the action timeOrganic active ingredientsMetabolism disorderPlasticizerSustained Release Capsule
A slow-releasing acipimox capsule for regualting blood fat is prepared from acipimox, empty core, slow-releasing agent, plasticizer and antisticking agent through preparing medicine core, drying, then coating slow-releasing layer, drying by blowing and loading in capsule. Its advantages are stable concentration of medicine in blood, durable acting and low toxic by-effect.
Owner:LUNAN PHARMA GROUP CORPORATION
Slow-releasing acipimox capsule
InactiveCN1205933CStable blood concentrationProlong the action timeOrganic active ingredientsMetabolism disorderLipid formationSide effect
The invention belongs to medical technology, in particular to an acipimox sustained-release capsule. It is made up of acipimox, blank ball core, sustained-release agent, plasticizer, anti-tack agent, and the weight ratio of acipimox to blank ball core, slow-release agent anti-tack agent is 100:115:(0.4~ 40): (0~25), use ethanol to mix slurry, and the antisticking agent is 1% of ethanol weight. The preparation process adopts a granule coating mechanism to contain the pill core, and after drying, it is coated with a slow-release coating layer and blast-dried. Put it into a hard capsule shell. The invention provides an acipimox sustained-release capsule, which is a high-efficiency and safe blood lipid regulating drug, which can be released slowly to maintain a relatively stable blood drug concentration and a longer action time, and has the advantages of reduced toxic and side effects and more convenient administration .
Owner:LUNAN PHARMA GROUP CORPORATION
Composite for treating hyperlipidemia
InactiveCN1692905AFully functional and enhancedAct quicklyOrganic active ingredientsMetabolism disorderMedicineSecondary hyperlipidemia
The present invention provides a drug composite for treating hyperlipidemia, the characteristics of which lies in containing effective amount Acipimox and Fluvastatin natrium, the weight ratio is (5~30):1, the optimal selection weight ratio is (5~15):1, the further optimal selection weight ratio is 10:1. The lipid-lowering effect is superior to the simple recipe with same dose significantly, which shows that there is synergistic effect and no distinct toxicity function under taking the Acipimox and the Fluvastatin both.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthetic process of acipimox
InactiveCN105218464BReduce the number of crystallizationAvoid recrystallization operationsOrganic chemistryOXALIC ACID DIHYDRATEAdjuvant
Owner:SICHUAN YIMING PHARMA CO LTD
Production method of acipimox
The invention relates to a production method of acipimox, wherein the production method comprises the following specific steps: under cooling and stirring, dissolving 78 g of maleic anhydride in 60 ml of a chloroform solution, adding 40 ml of a 30% hydrogen peroxide, 2 h later, adding 4.8 g of 5-methylpyrazine-2-carboxylic acid, keeping for 2 d at the temperature of 0-5 DEG C, filtering to remove maleic acid, concentrating to leave small volume, adding ethyl acetate, and filtering to obtain 1.1 g of acipimox with the melting point of 178-180 DEG C. According to the production method of acipimox, the manufacturing cost is low, and the production is convenient.
Owner:储海燕
Composition capable of curing abnormal blood lipid
InactiveCN103127115AAvoid absorptionInhibit synthesisMetabolism disorderHeterocyclic compound active ingredientsSide effectBlood lipids
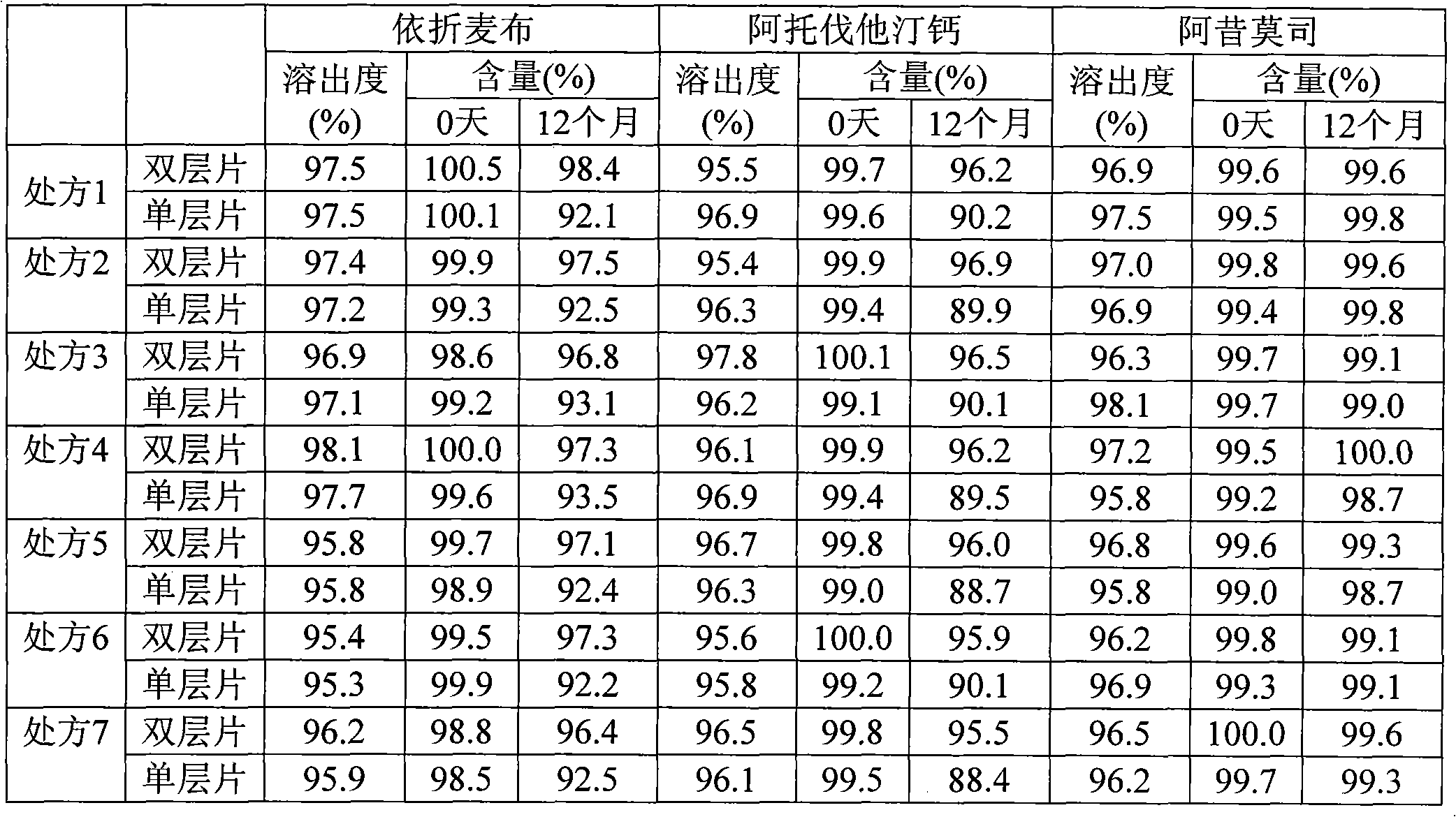
The invention relates to a composition capable of curing abnormal blood lipid. The composition capable of curing the abnormal blood lipid comprises ezetimibe, atorvastatin calcium and acipimox. The range of weight ratios of the ezetimibe, the atorvastatin calcium and the acipimox in the composition is 5-10: 5-80: 250-1000, preferably 10: 10-40: 250-750 and optimally, 10: 10-20; 500-750. The composition capable of curing the abnormal blood lipid is strong in blood lipid regulating effect, comprehensive in function and little in side effect.
Owner:内蒙古天衡医院管理有限公司
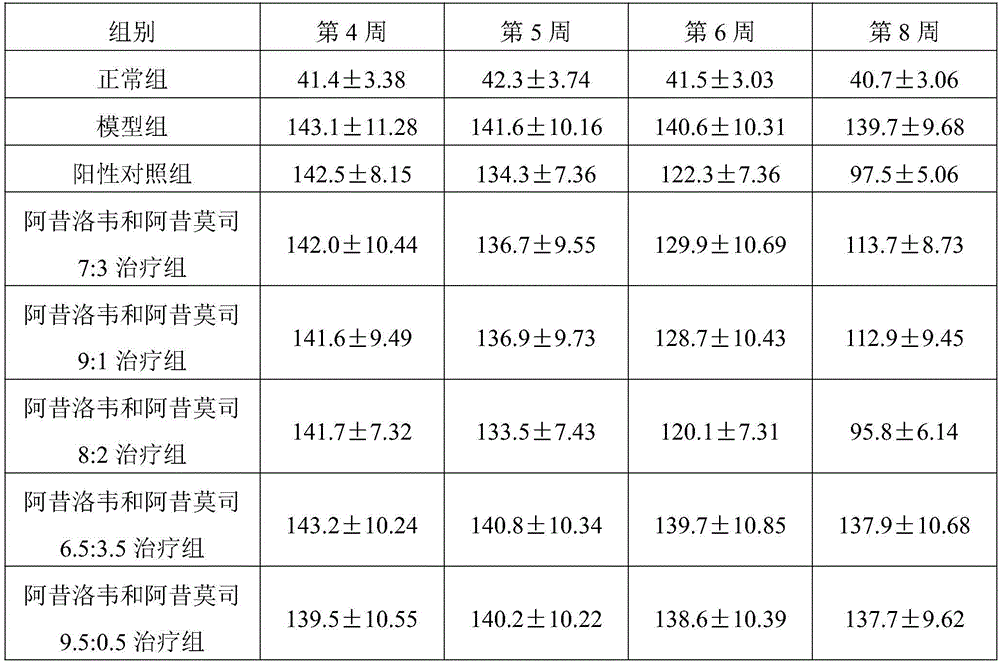
New application of pharmaceutical composition of acyclovir and acipimox in field of medicines
InactiveCN105748481AGood treatment effectSignificant progressAntipyreticAnalgesicsTherapeutic effectAcipimox
The invention discloses a new application of a pharmaceutical composition of acyclovir and acipimox in the field of medicines. The pharmaceutical composition provided by the invention contains acyclovir and acipimox, and weight part proportion of acyclovir to acipimox in the composition is 7:3 to 9:1. The pharmaceutical composition provided by the invention contains two chemicals, acyclovir and acipimox, commonly used in clinic, and study shows that treatment effect on chronic nephritis is obvious and is not significantly different from that of a positive medicine when acyclovir and acipimox jointly act and are in a certain weight part proportion ranging from 7:3 to 9:1, so that the pharmaceutical composition provided by the invention can be developed into a medicine used for treating the chronic nephritis. Compared with the prior art, the pharmaceutical composition provided by the invention has outstanding substantial characteristics and obvious progress.
Owner:李月升
Preparation method of acipimox
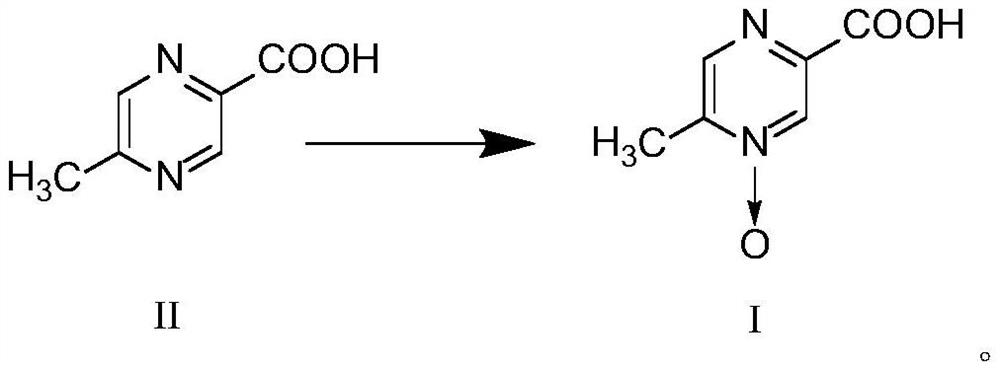
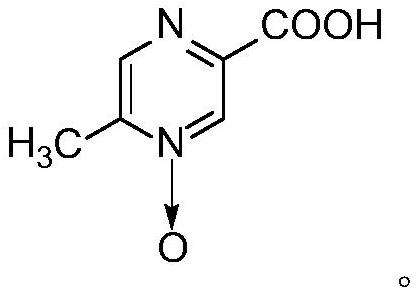
PendingCN112125857ALow reaction temperatureThorough responseOrganic chemistryPtru catalystNitrogen oxides
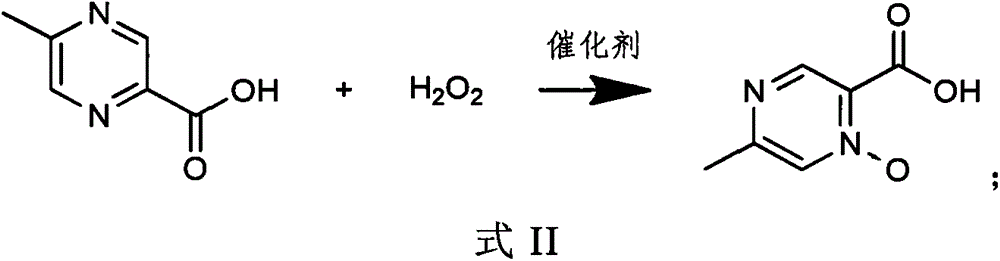
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a preparation method of acipimox. The method for preparing acipimox comprises the following steps of adding5-methylpyrazine-2-carboxylic acid into purified water, adding concentrated hydrochloric acid for salifying, adding an oxidant, cooling and crystallizing to obtain the acipimox. The method has the advantages of simple reaction conditions, high selectivity in the nitrogen oxide formation process, avoiding of the use of a metal catalyst, simplification of the preparation process, effective improvement of the yield and the purity of the acipimox, and suitableness for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Osmotic pump controlled release preparation composition for treating hyperlipemia and preparation method thereof
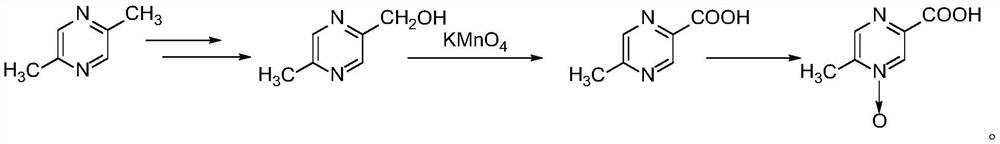
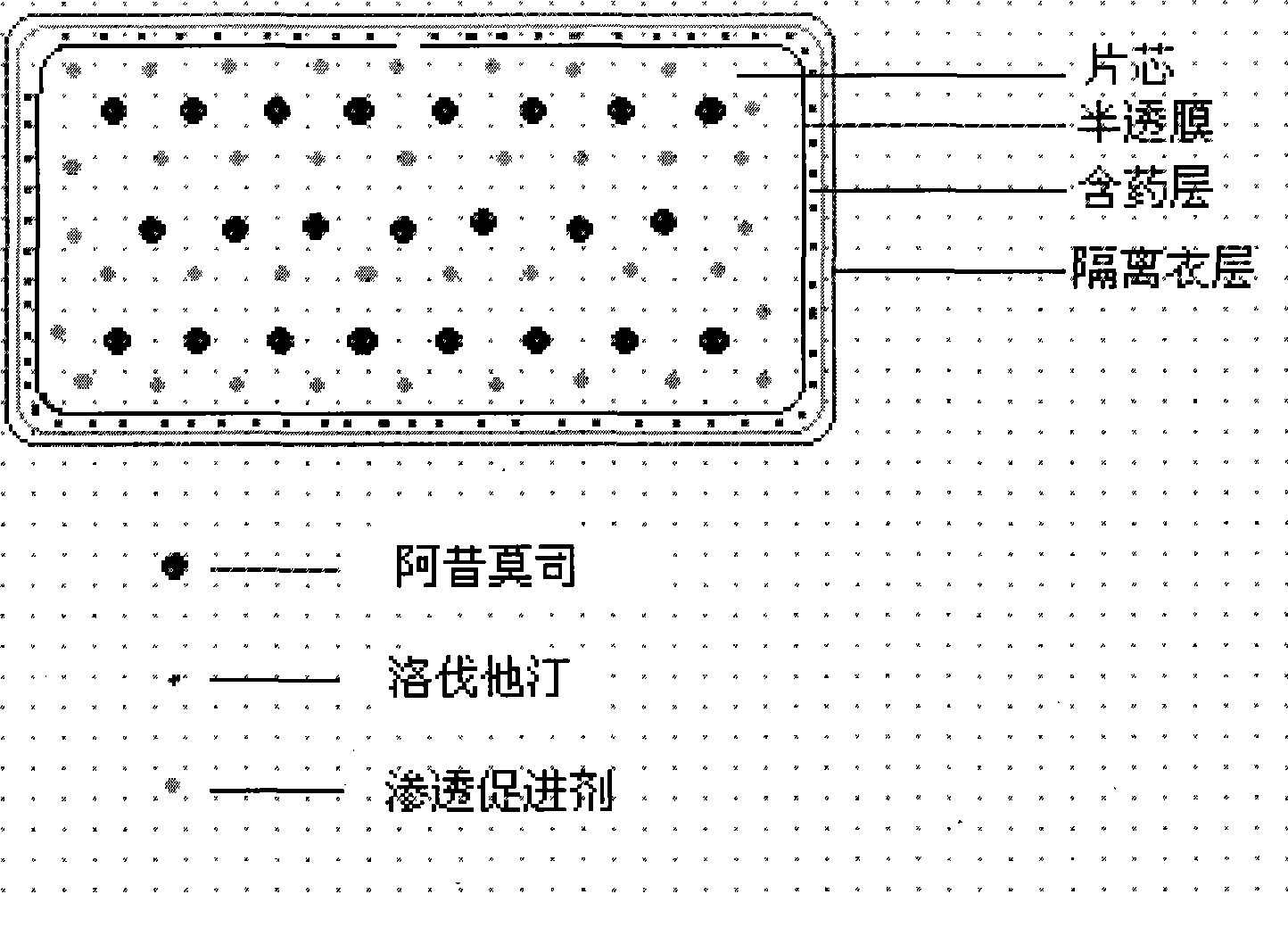
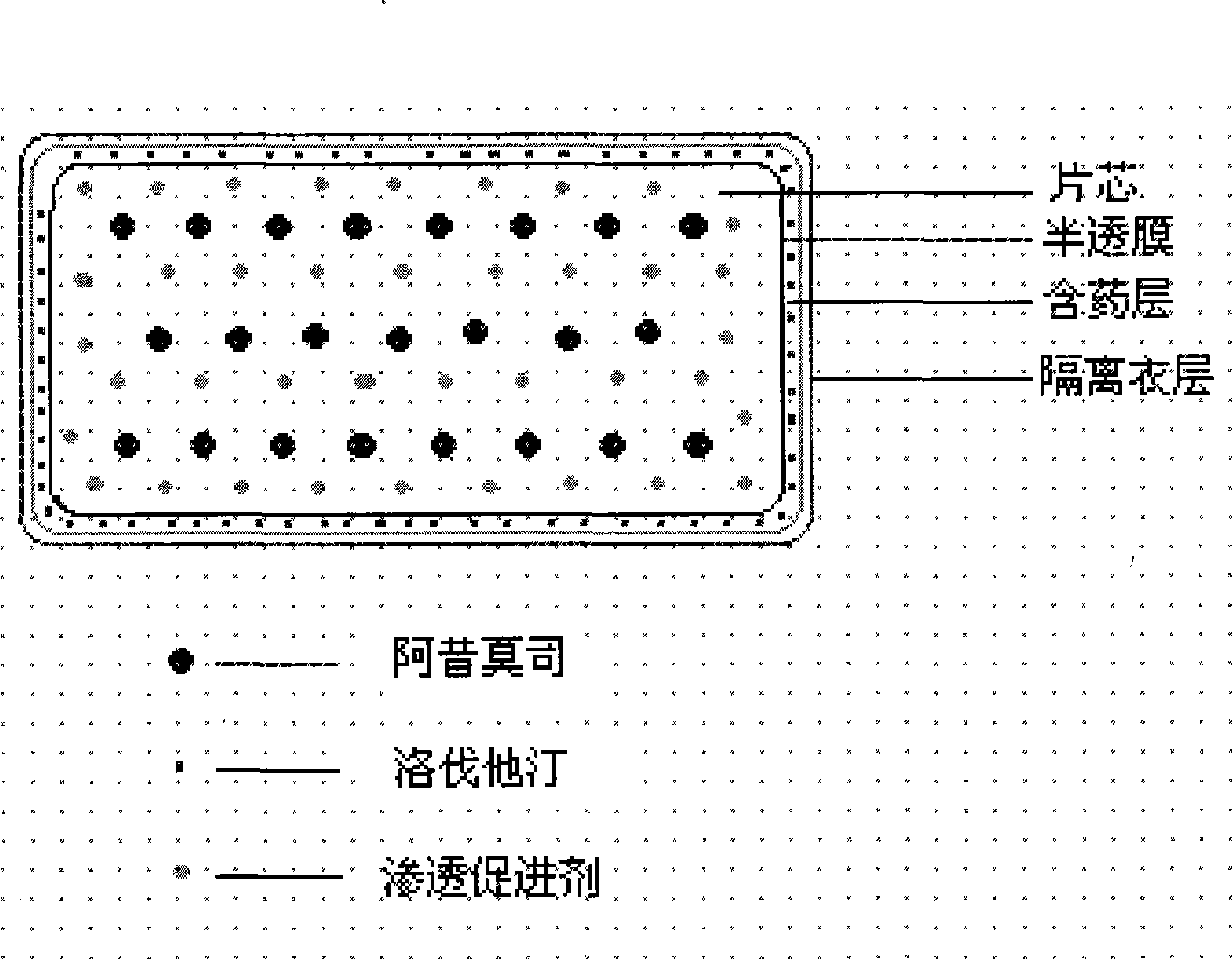
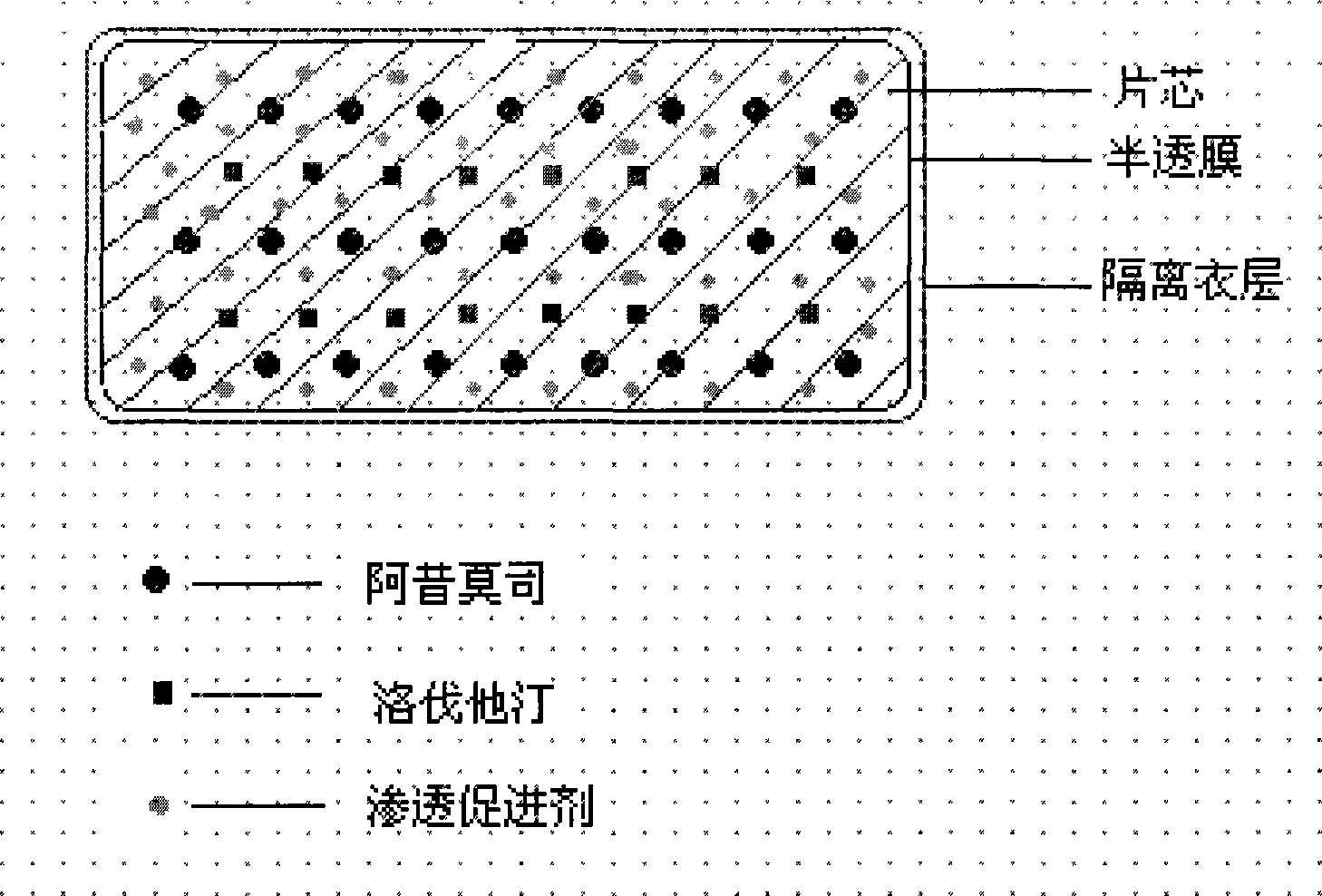
ActiveCN101385730AFully functionalSmall toxicityOrganic active ingredientsMetabolism disorderLovastatinOsmotic pump
The invention relates to an osmotic pump preparation containing acipimox, lovastatin and other pharmaceutical excipients, wherein, the acipimox is the controlled-release part, the lovastatin is the rapid-release part; or the acipimox and the lovastatin are both the controlled-release parts. In addition, the invention further discloses a preparation method of an osmotic pump tablet of the composition, and the osmotic pump table which is prepared by applying the method can better bring the synergy of the acipimox and the lovastatin into play.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com