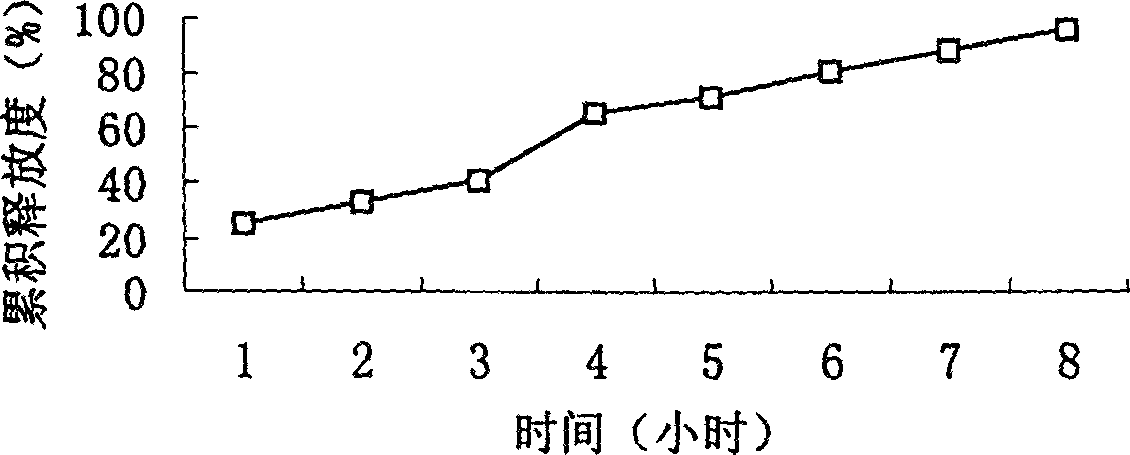
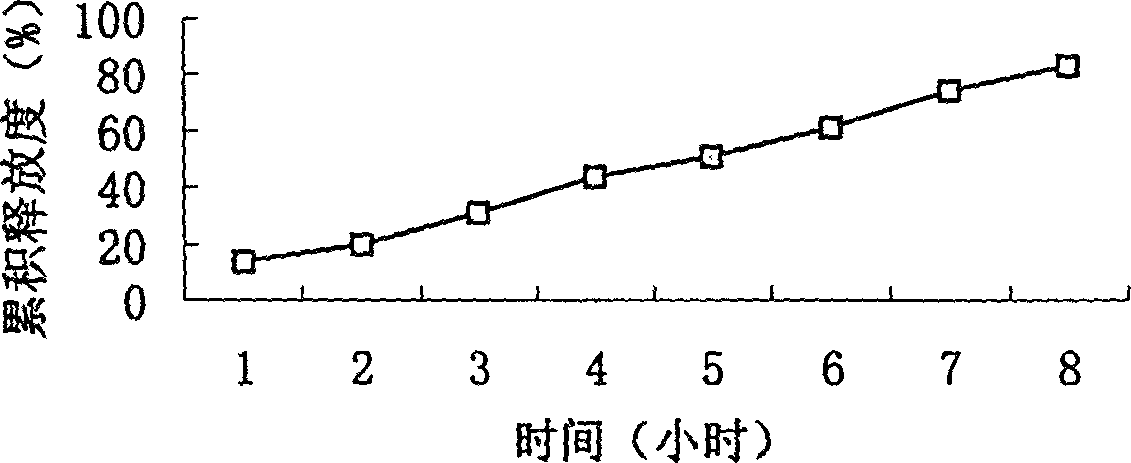
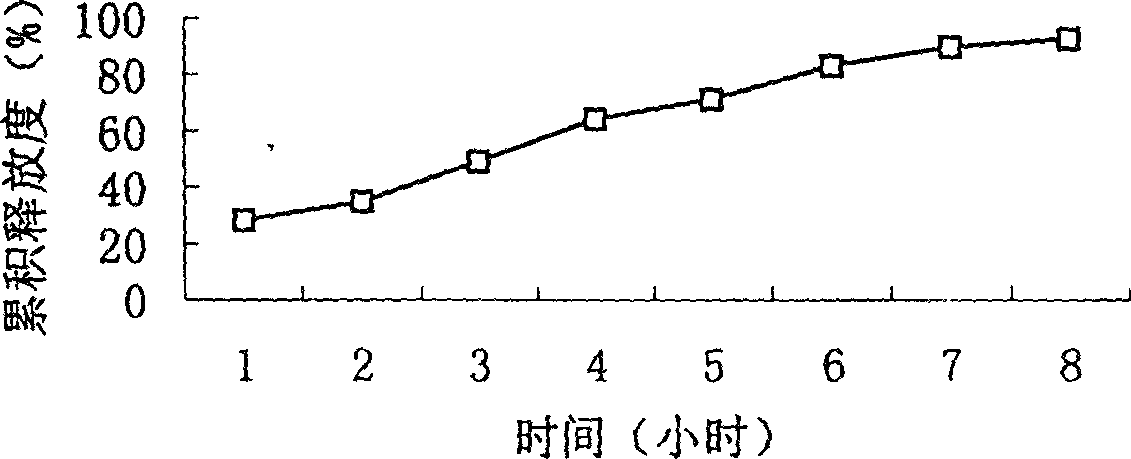
Slow-releasing acipimox capsule
A technology of slow-release capsules and slow-release materials, applied in the field of medicine, can solve the problems of diabetes and gout patients with aggravated conditions, poor safety, and influence on glucose and uric acid metabolism pathways, and achieve convenient administration, reduced toxic and side effects, and extensive social benefits and economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] (1) Preparation of pill-containing core
[0045] Acipimox 190g
[0046] Blank pellet core 300g
[0047] 7% PVP solution (90% ethanol solvent) 200g
[0048] Preparation Process:
[0049] Pass acipimox through a 160-mesh sieve, weigh the prescription, and pour it into the lower hopper. Open the granulation coating machine, enter the air pressure 0.5bar, CYL 3bar, CAP1 0.8bar, pour into the blank pellet core, and granulate. The feeding speed is 4rpm, the peristaltic pump is 12%, the rotating speed of the turntable is 145rpm, sprayed with 7% PVP solution (solvent is 90% ethanol), and dried at 50°C. The granulation is finished and 500g is discharged.
[0050](2) Eight specific prescriptions are as follows: (Determine the type and quantity of sustained-release agents)
[0051] Prescription A:
[0052] Containing pill core 500g
[0053] Ethyl cellulose 5g
[0054] Stearic acid 30g
[0055] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com