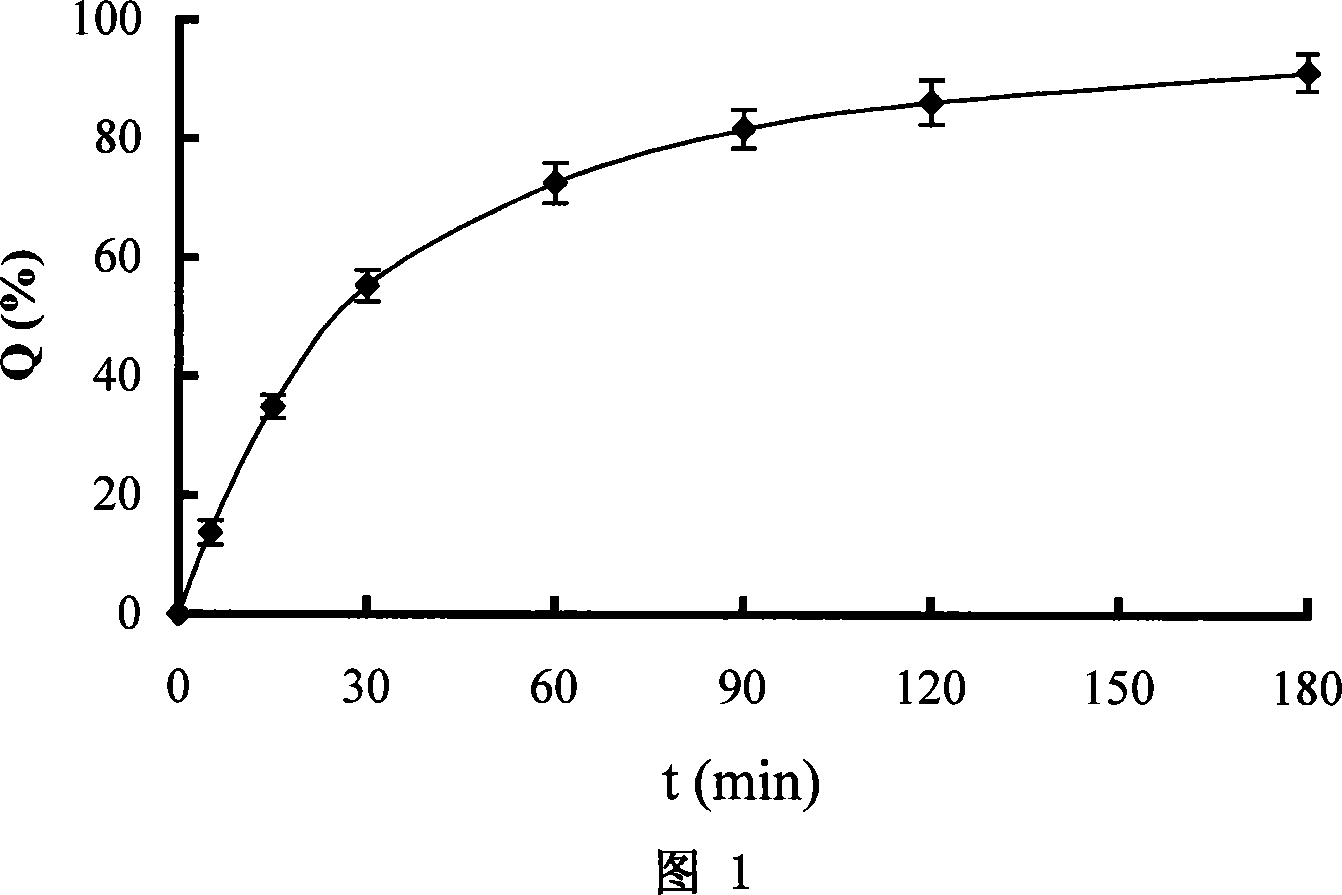
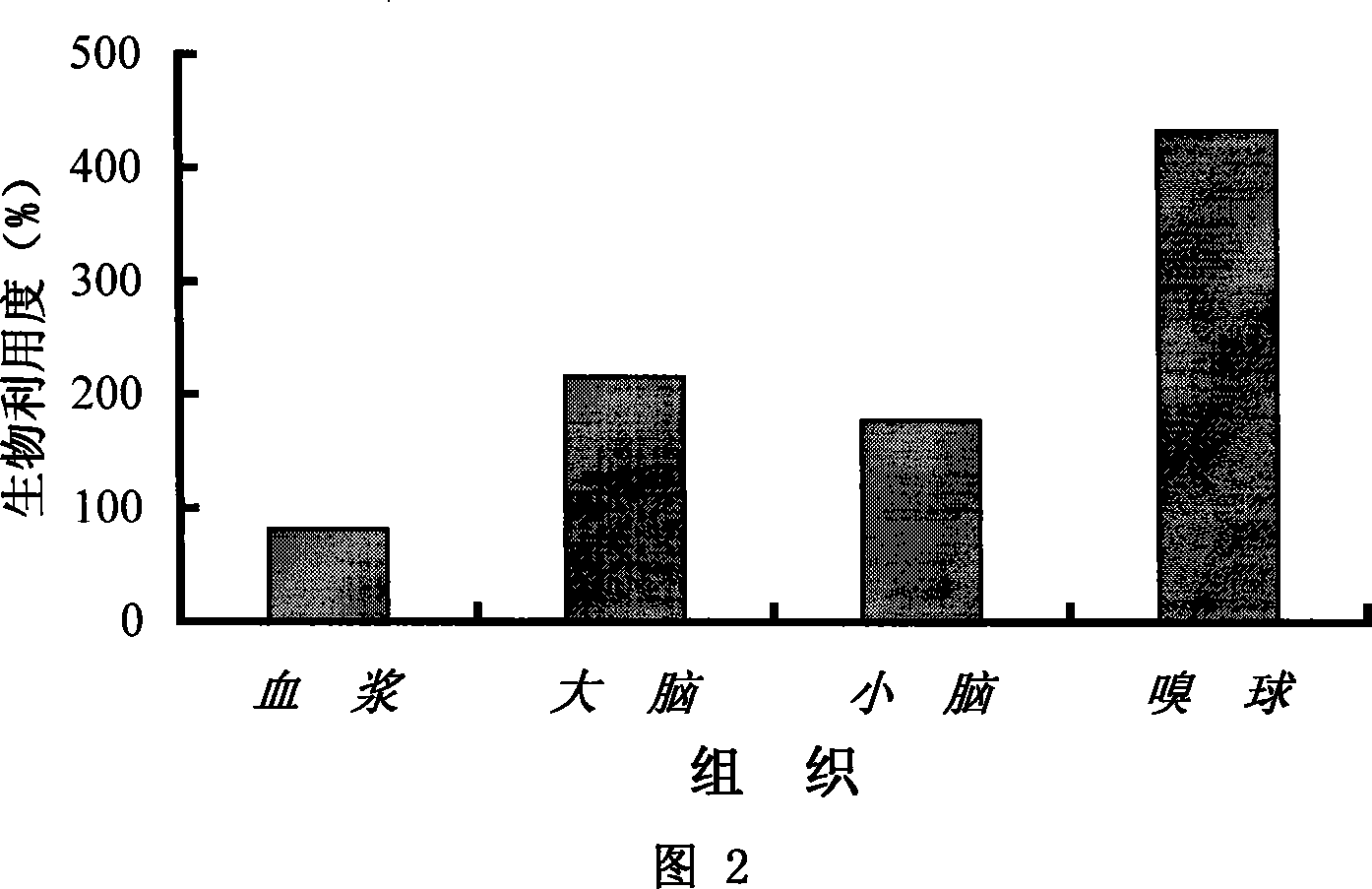
Rhizoma Gastrodiae nasal gel preparation
A gel and gastrodin technology, applied in the field of chemical pharmaceuticals, can solve the problems of aggravating the mental burden of patients with neurological diseases, and achieve the effects of avoiding the first-pass effect, delaying release, and good patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Drug formulation:
[0056] Gastrodin 15g
[0057] Carbomer 934 1g
[0058] 1mol / LNaOH to adjust the pH value to 6.5
[0059] Chlorobutanol 0.5g
[0060] Peppermint Oil 0.5ml
[0061] Purified water add water to 100g
[0062] Preparation method: Take Carbomer 934, add 50ml of purified water, stir to make it fully swell, adjust the pH to 6.5 with 1mol / L NaOH to obtain a gel, then add gastrodin, chlorobutanol and peppermint oil, and add water to 100g, stir well to obtain gastrodin nasal gel.
Embodiment 2
[0064] Drug formulation:
[0065] Gastrodin 40g
[0066] Sodium Carboxymethyl Cellulose 2g
[0067] borneol 0.2g
[0068] Thimerosal 0.01g
[0069] Add purified water to 100g
[0070] Preparation method: Take sodium carboxymethyl cellulose, add 50ml of purified water, stir to make it fully swell to obtain a gel, then add gastrodin, thimerosal and borneol, add water to 100g, stir well to obtain gastrodin nasal gel agent.
Embodiment 3
[0072] Drug formulation:
[0073] Gastrodin 10g
[0074] Deacetylated Gellan Gum 0.5g
[0075] Ethylparaben 0.03g
[0076] Add purified water to 100ml
[0077] Preparation method: remove acetylated gellan gum, add 80ml of purified water, dissolve at 90°C and let cool to room temperature, then add gastrodin and ethylparaben to dissolve, filter the solution through a vertical fusing glass funnel, add water to 100ml, stir well to get gastrodin ion-sensitive nasal in-situ gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com