Compound contraceptive composition, contraceptive transdermal patch containing composition and preparation method
A technology of a composition and a transdermal penetration enhancer, which is applied in the field of contraceptive transdermal patches and its preparation, can solve the problems of no drug delivery system, poor compliance of users, and high contraceptive failure rate, so as to avoid the first-pass effect, High bioavailability, effect of reducing frequency of dosing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
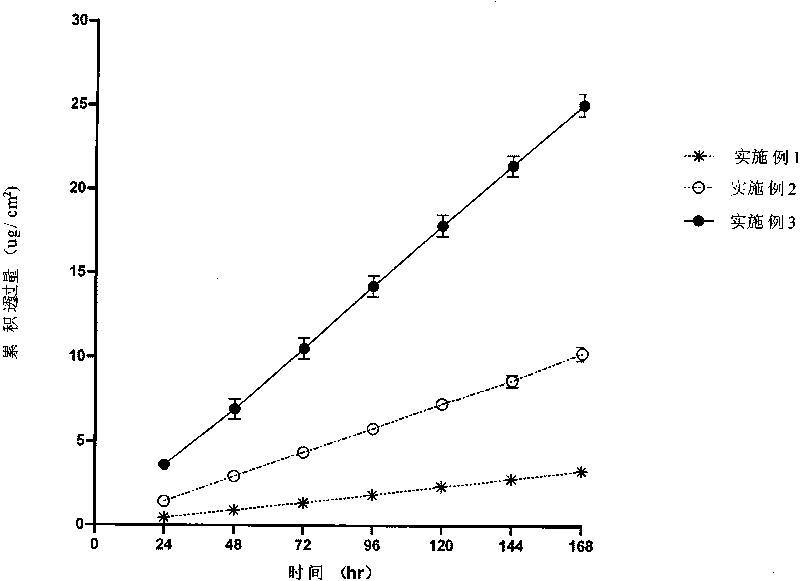
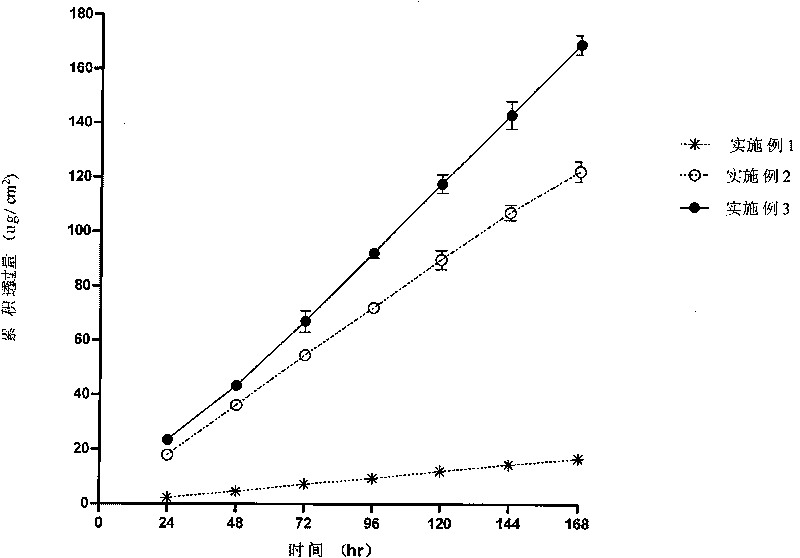
Embodiment 1
[0069] [Prescription] (based on 1000 tablets)
[0070] Etonogestrel 0.6g, ethinylestradiol 0.6g, polyacrylate pressure-sensitive adhesive 100g, sodium alginate 20g, lauric acid 1g, Eudragit RL (10% trimethylamine chloride methacrylic acid, Rohm, Germany) 3g;
[0071] Preparation:
[0072] Add the drug and sodium alginate to 20g of ethanol to dissolve, and stir at 5000rpm for 0.5 hours to obtain a skeleton storage system;
[0073] In addition, lauric acid and Eudragit RL were added to 5 g of acetone to dissolve, then polyacrylate pressure-sensitive adhesive was added, mixed evenly, and degassed to obtain a pressure-sensitive adhesive solution.
[0074] Then, mix the skeleton-type reservoir system obtained above with the pressure-sensitive adhesive solution, stir at 10,000 rpm for 1 hour, mix and disperse, and degas to obtain the drug-containing adhesive solution.
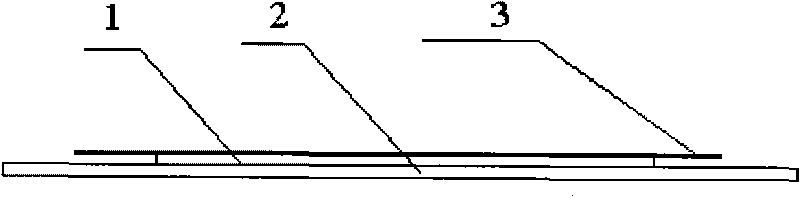
[0075] Apply the above-mentioned drug-containing viscose solution on the release layer, dry at 80°C for 1 hour, ...
Embodiment 2
[0080] [Prescription] (based on 1000 tablets)
[0081] Etonogestrel 3g, ethinyl estradiol 1g, silicone pressure sensitive adhesive 30g, hydroxypropyl cellulose 10g, azone 3g, β-cyclodextrin 1g;
[0082] Preparation method: Add drug and hydroxypropyl cellulose to 20g ethanol to dissolve, stir at 8000rpm for 1 hour to obtain a skeleton storage system;
[0083] In addition, add azone and β-cyclodextrin to 10 g of ethanol to dissolve, then add silicone pressure-sensitive adhesive, mix well, and degas to obtain a pressure-sensitive adhesive solution.
[0084] Then, mix the skeleton-type reservoir system obtained above with the pressure-sensitive adhesive solution, stir at 8000 rpm for 1 hour, mix and disperse, and degas to obtain the drug-containing adhesive solution.
[0085] Apply the above-mentioned drug-containing viscose solution on the release layer, dry at 90°C for 1 hour, and finally cover the adhesive surface with a backing layer to obtain the product.
[0086] The above...
Embodiment 3
[0090] [Prescription] (based on 1000 tablets)
[0091] Etonogestrel 10g, ethinyl estradiol 2g, polybutylene pressure-sensitive adhesive 30g, cross-linked polyvinylpyrrolidone 20g, oleic acid 3g, dimethyl sulfoxide 6g;
[0092] Preparation method: Add drug, dimethyl sulfoxide, and cross-linked polyvinylpyrrolidone to 50 g of dichloromethane to dissolve, stir at 5000 rpm for 0.5 hour, and obtain a skeleton storage system;
[0093] In addition, oleic acid is added to the polybutylene pressure-sensitive adhesive, mixed evenly, and degassed to obtain a pressure-sensitive adhesive solution.
[0094] Then, mix the skeleton-type storage system obtained above with the pressure-sensitive adhesive solution, stir at 12000 rpm for 1.5 hours, mix and disperse, and degas to obtain the drug-containing adhesive solution.
[0095] Apply the above-mentioned drug-containing viscose solution on the release layer, dry at 90°C for 1 hour, and finally cover the adhesive surface with a backing layer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com