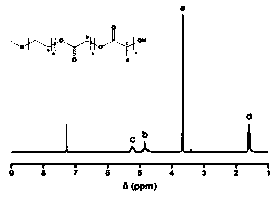
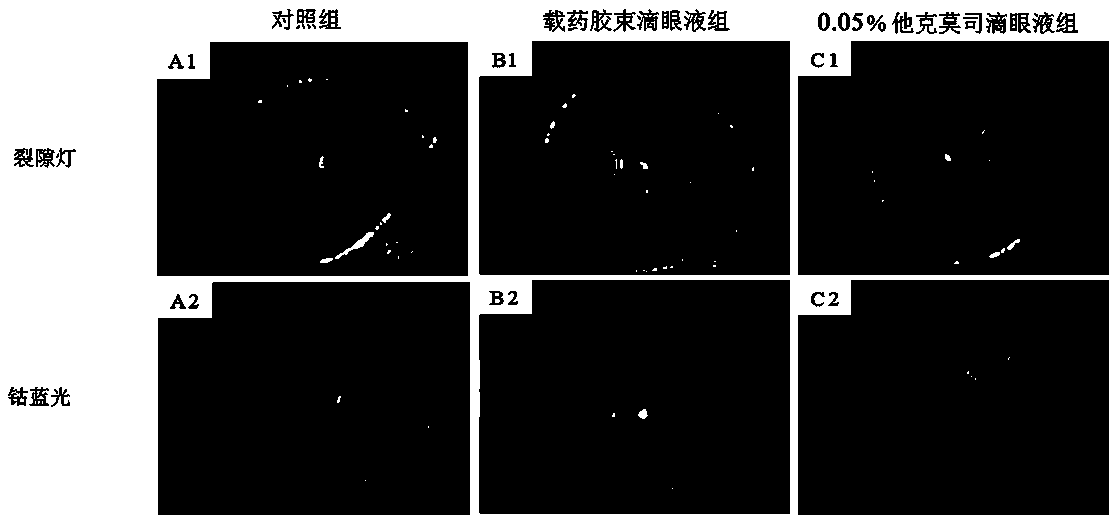
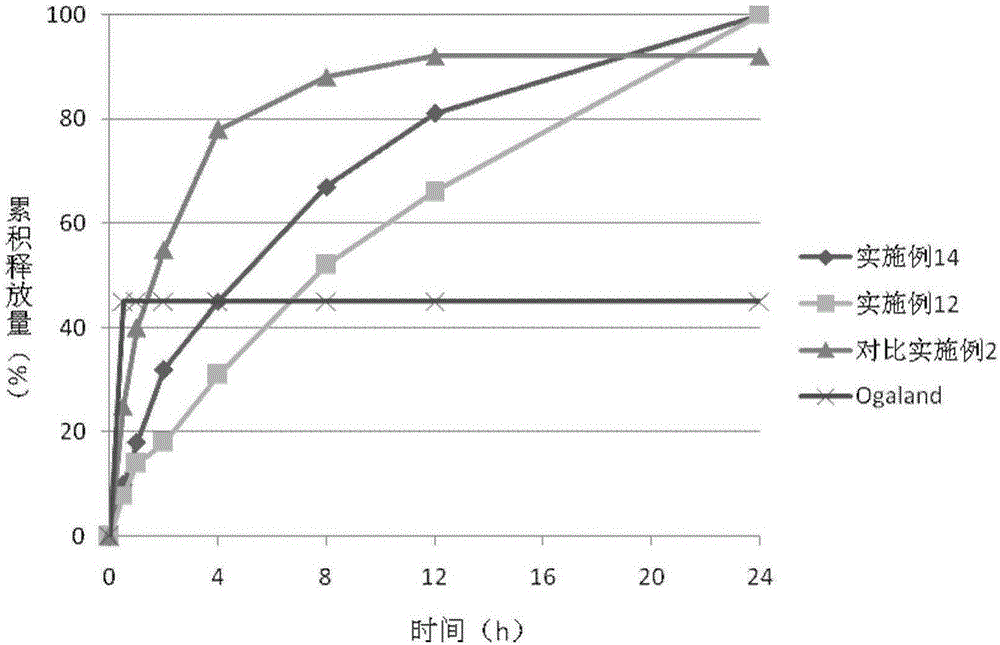
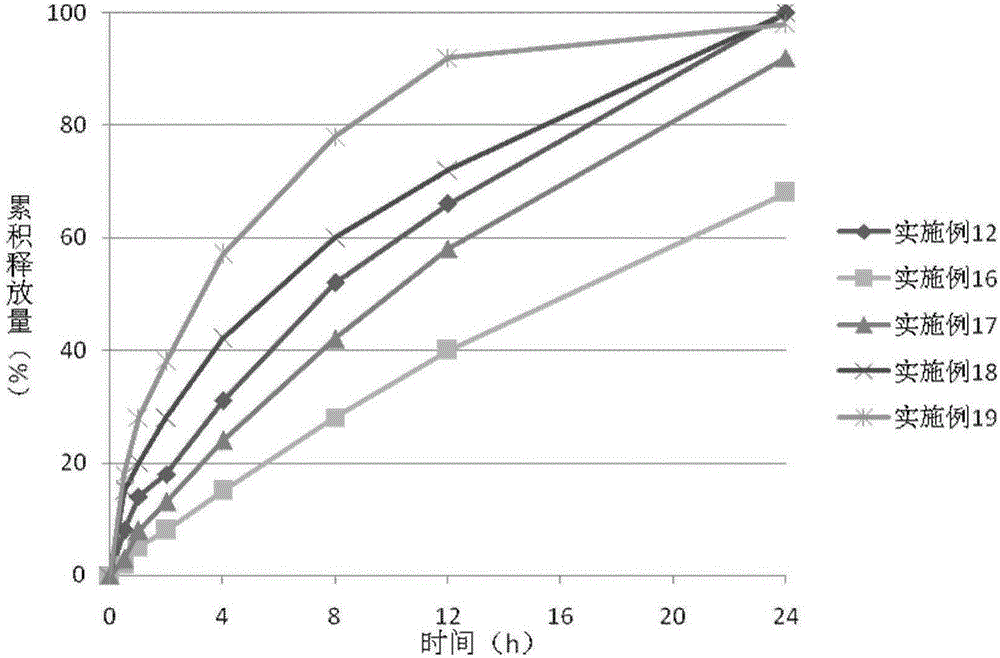
Patents
Literature
78results about How to "Reduce the frequency of medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal patch containing pramipexole
ActiveCN103432104AGood solubilityImprove uniformityOrganic active ingredientsNervous disorderSolventMicrogram
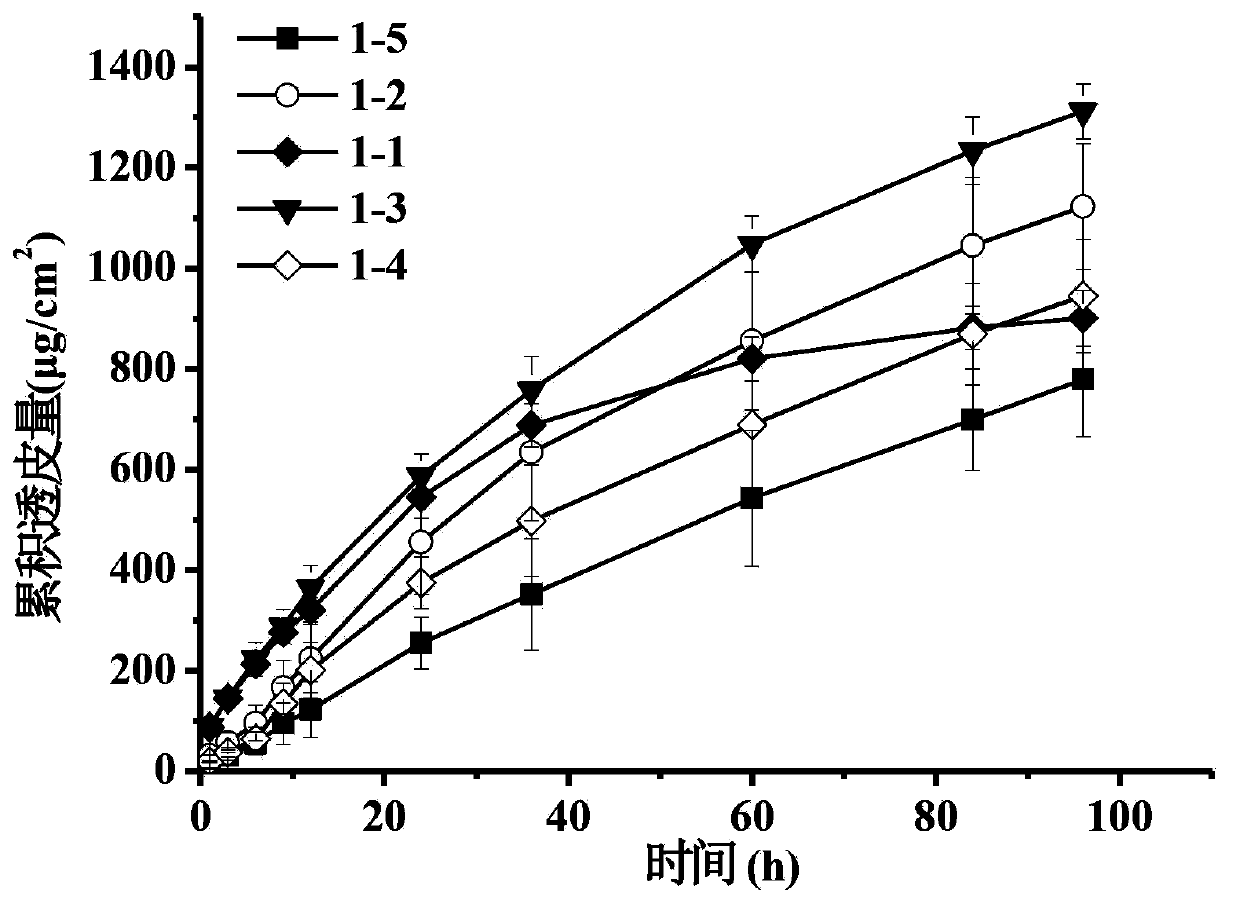
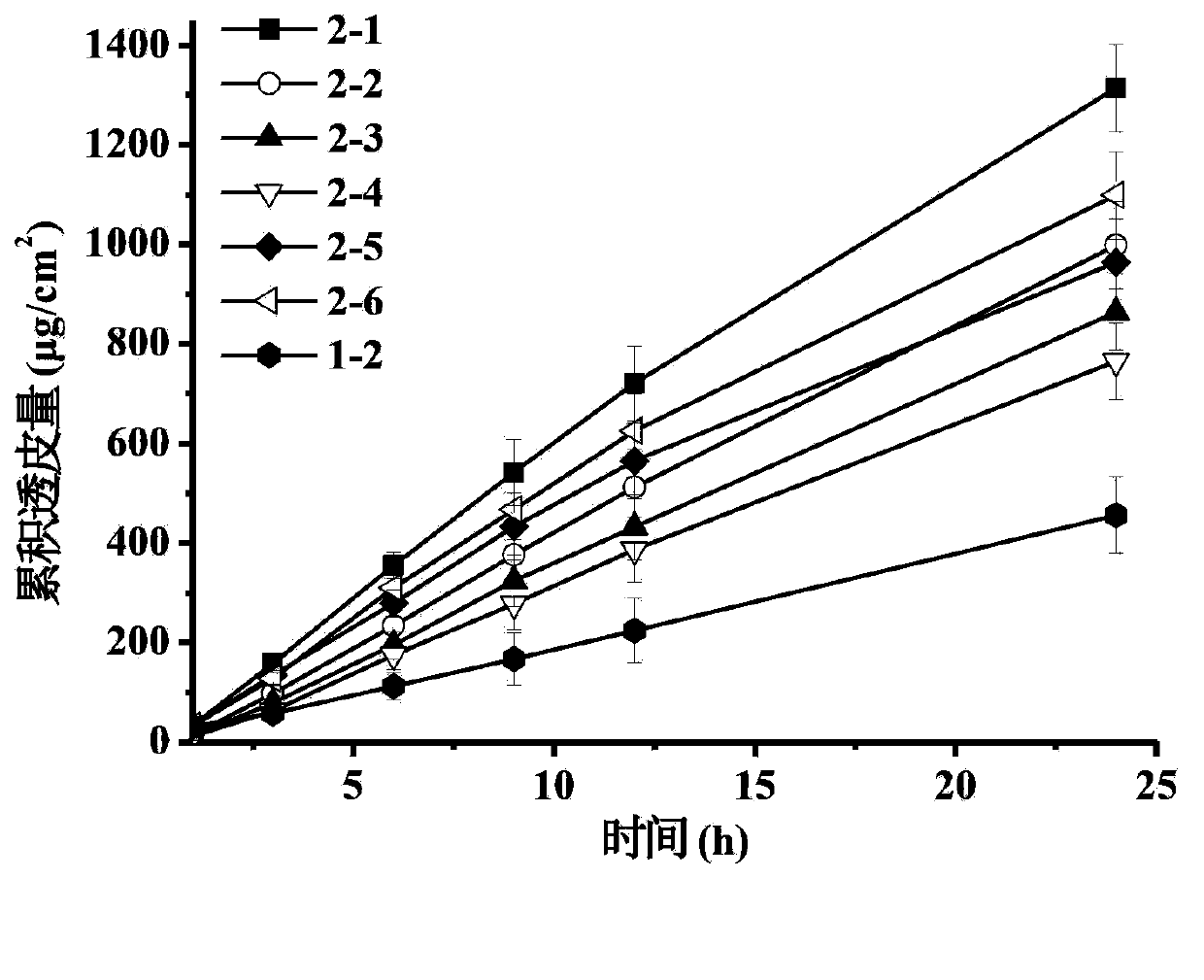
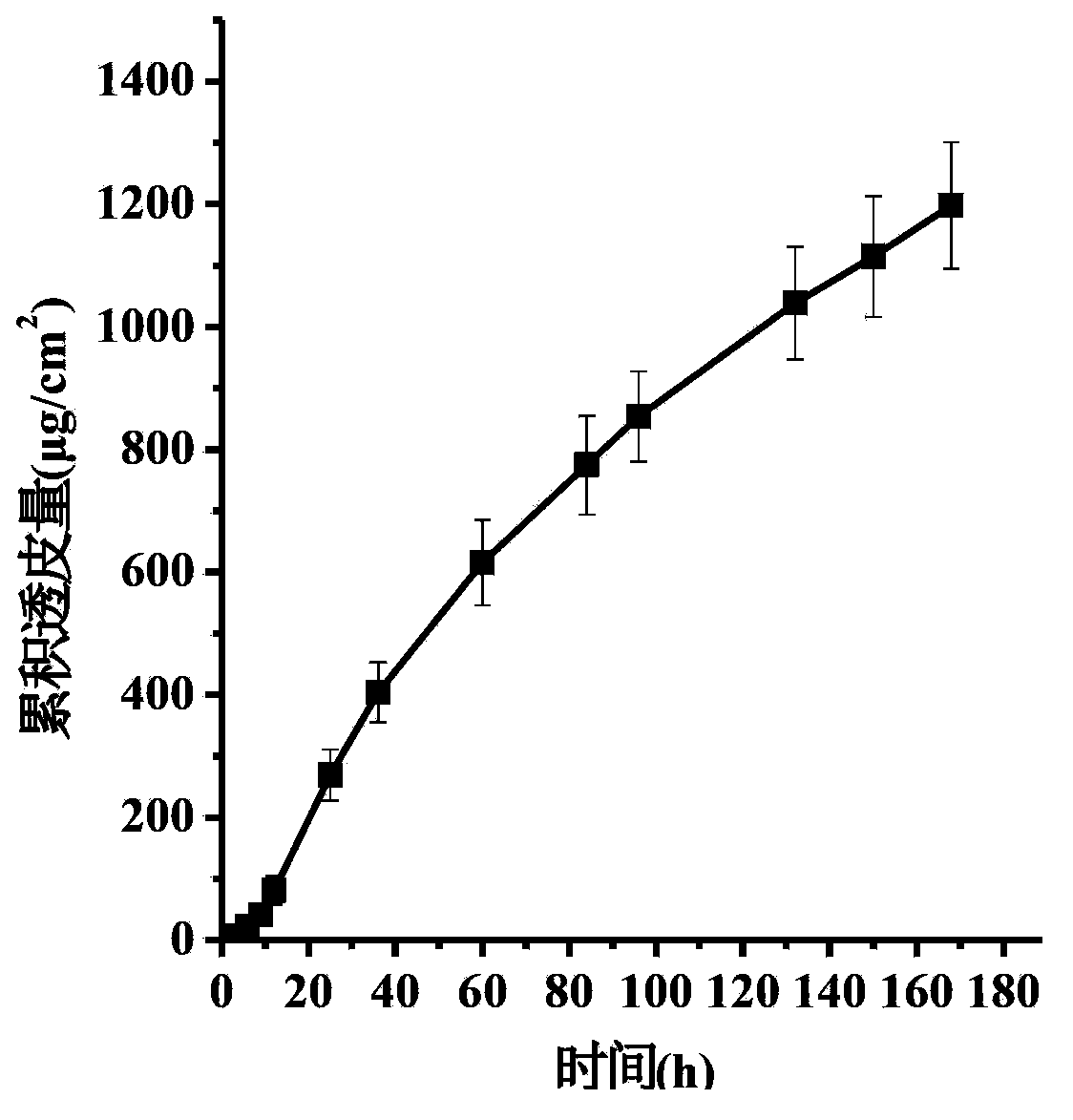
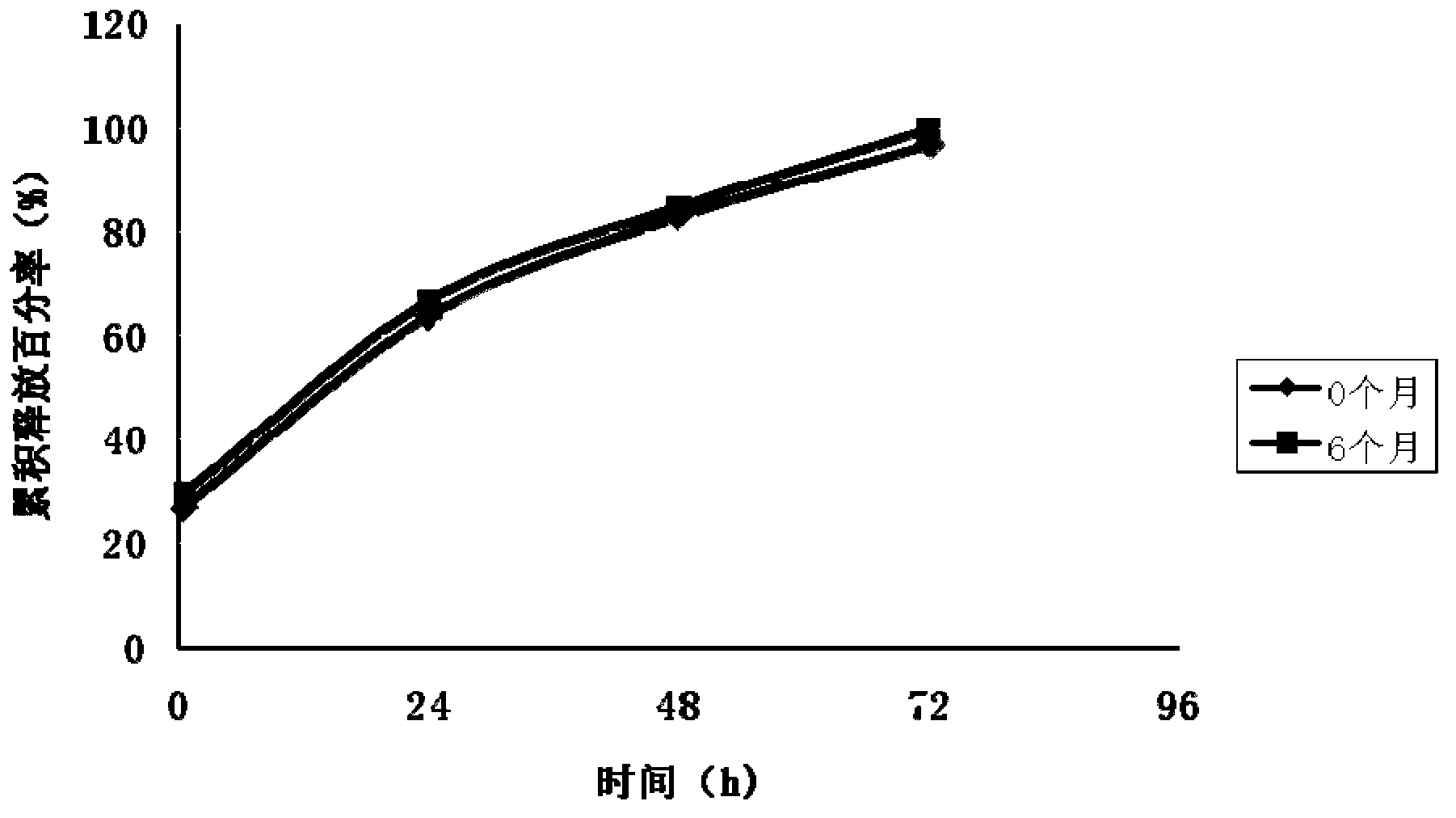
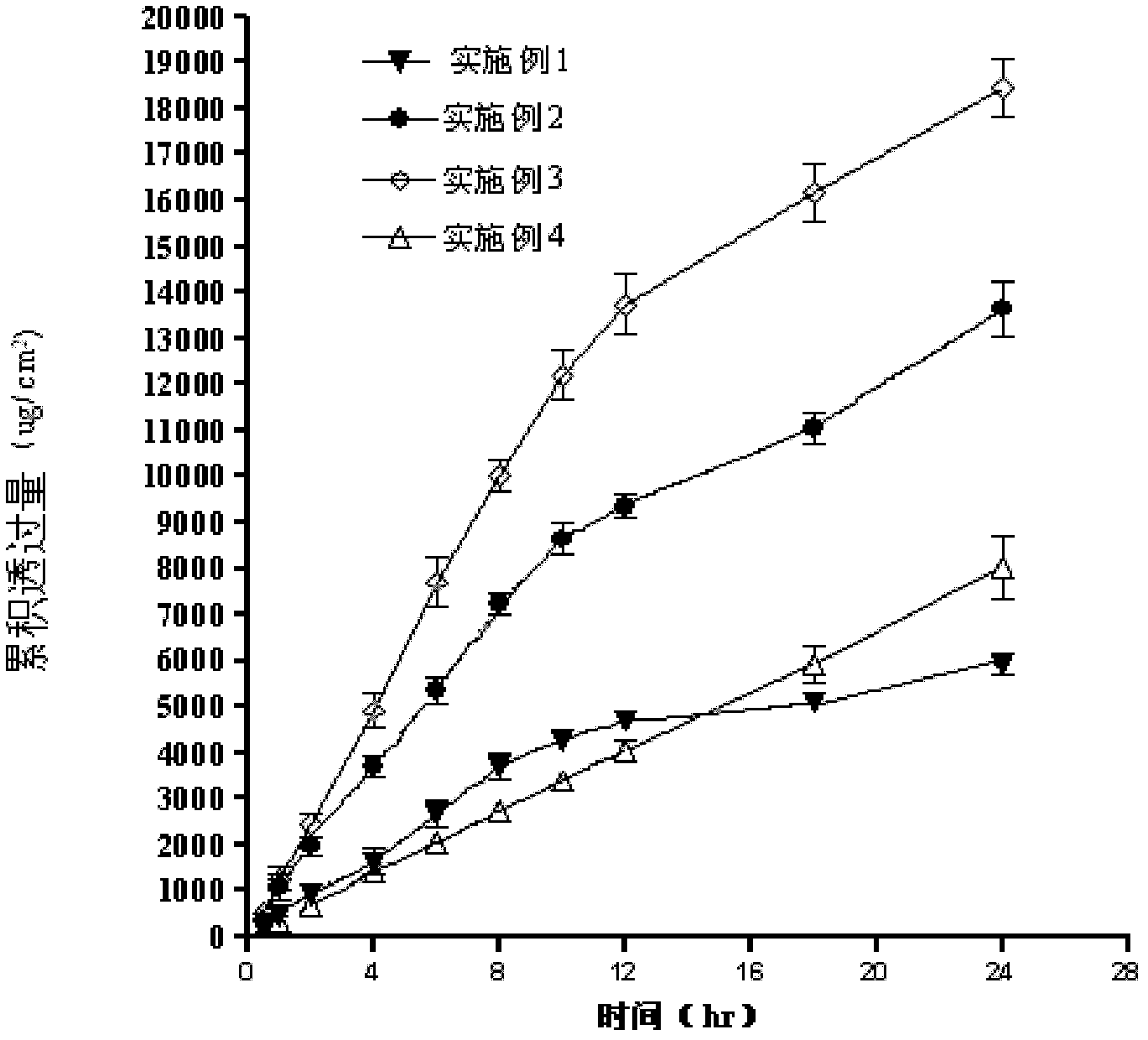
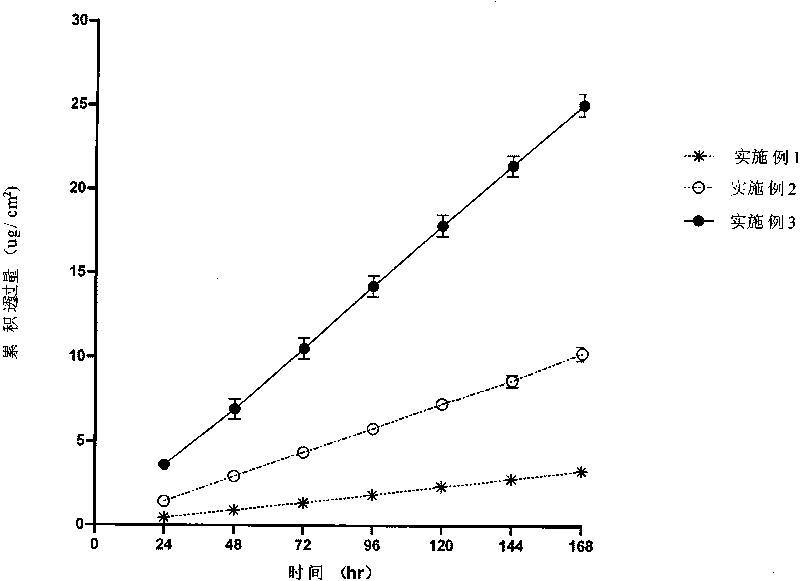
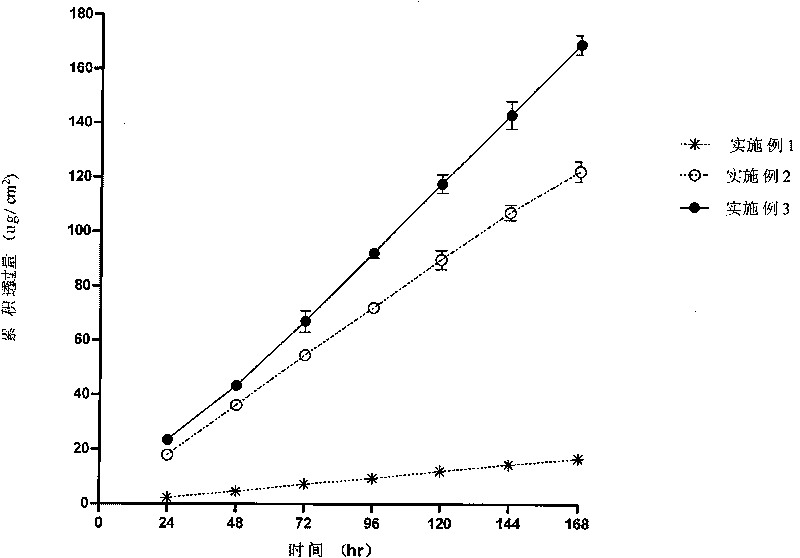
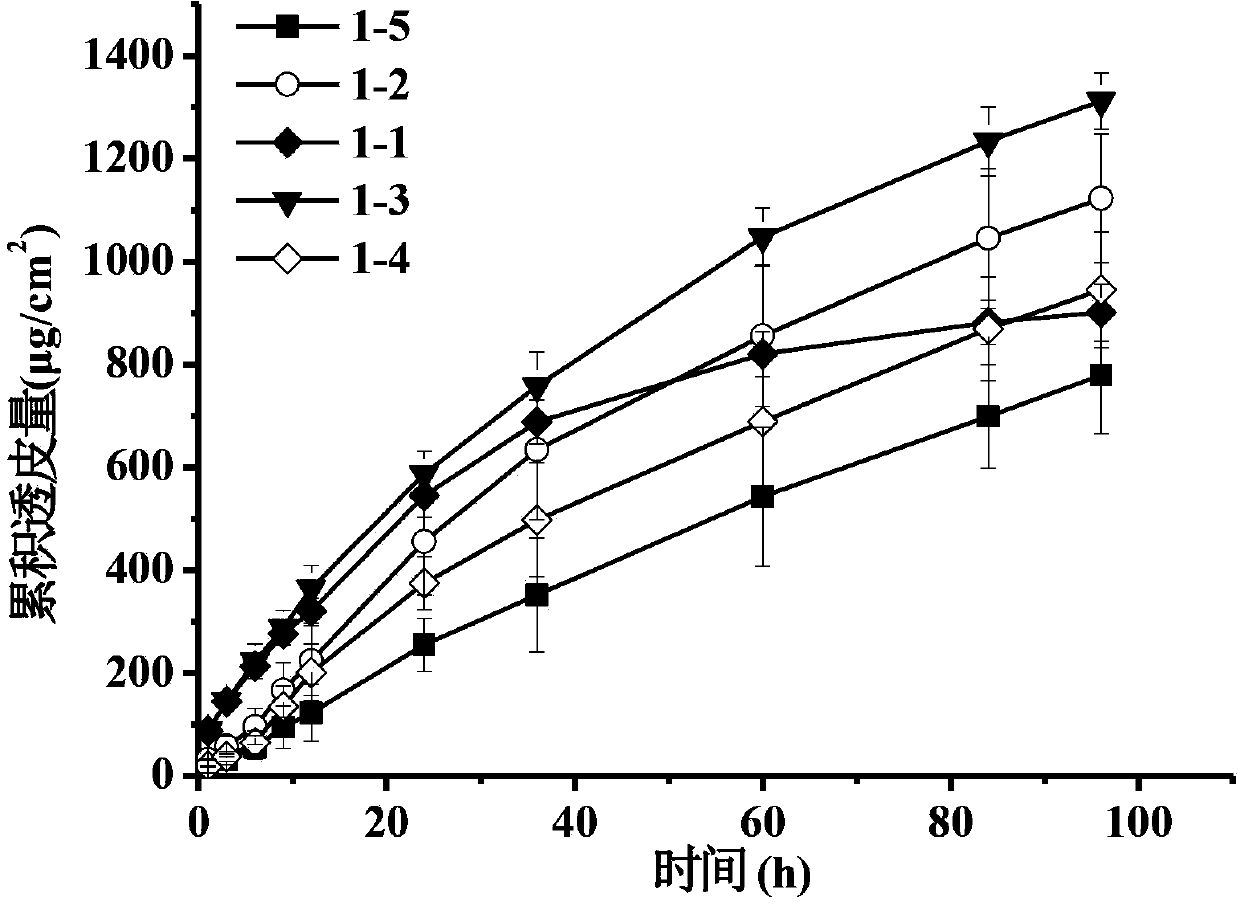
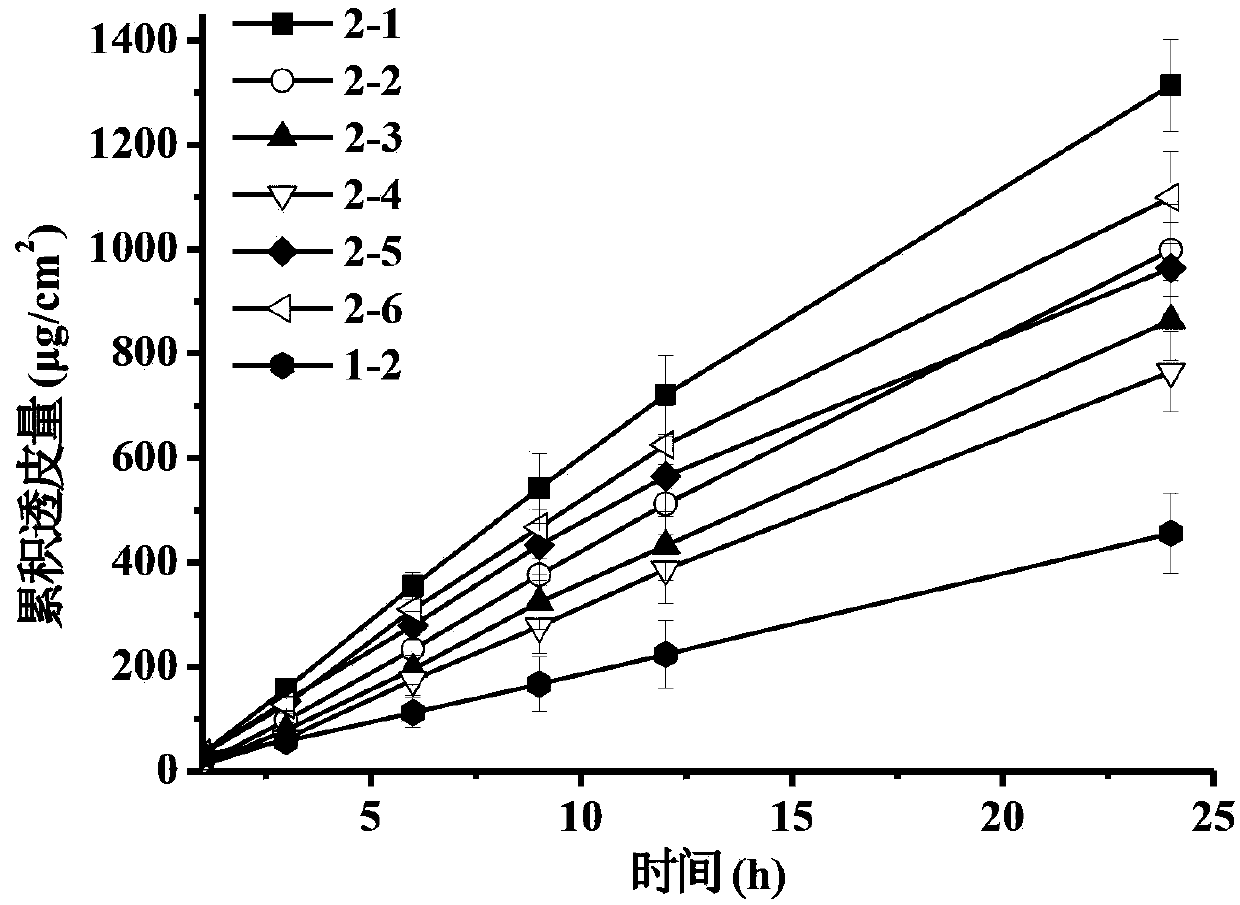
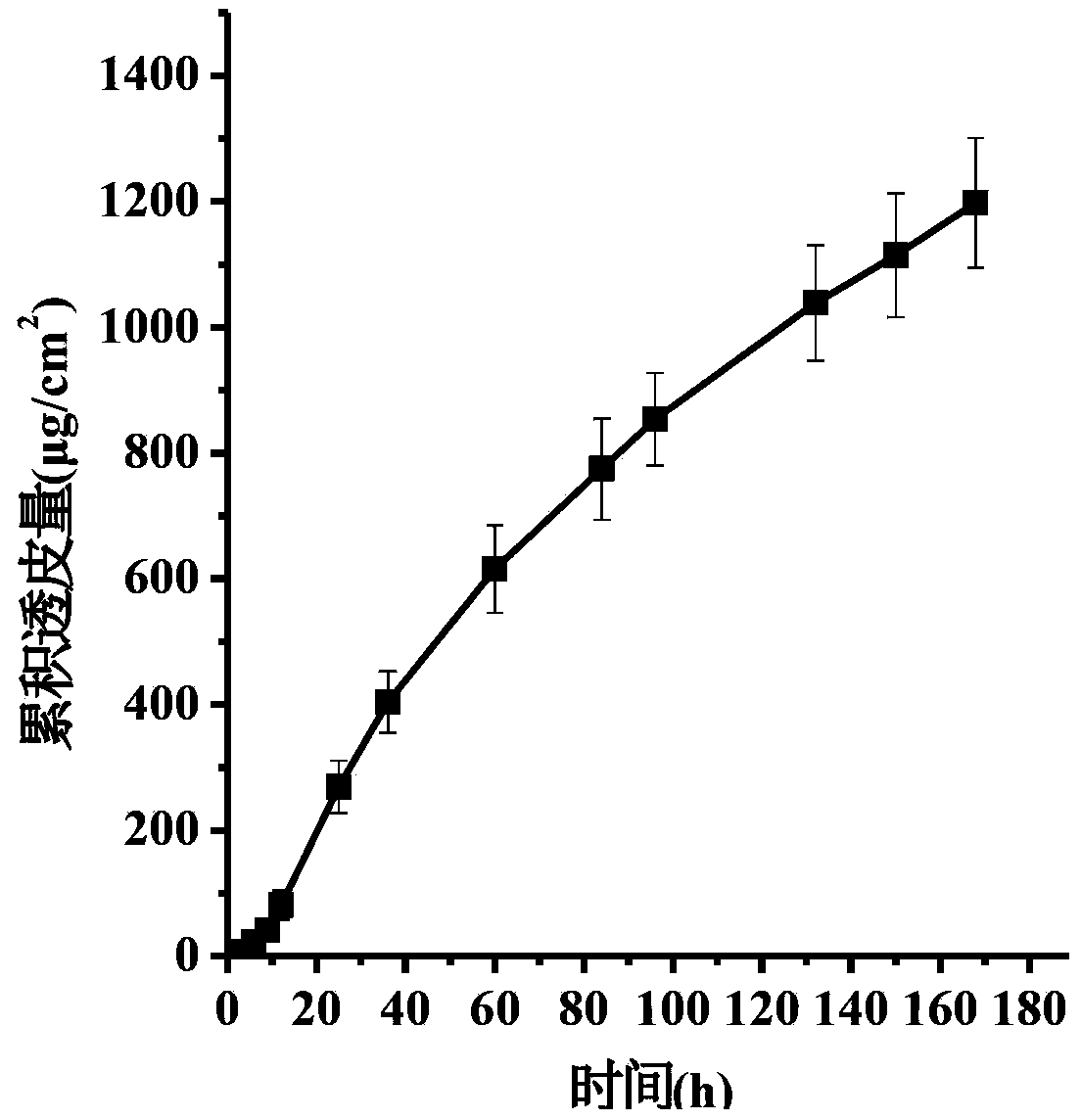
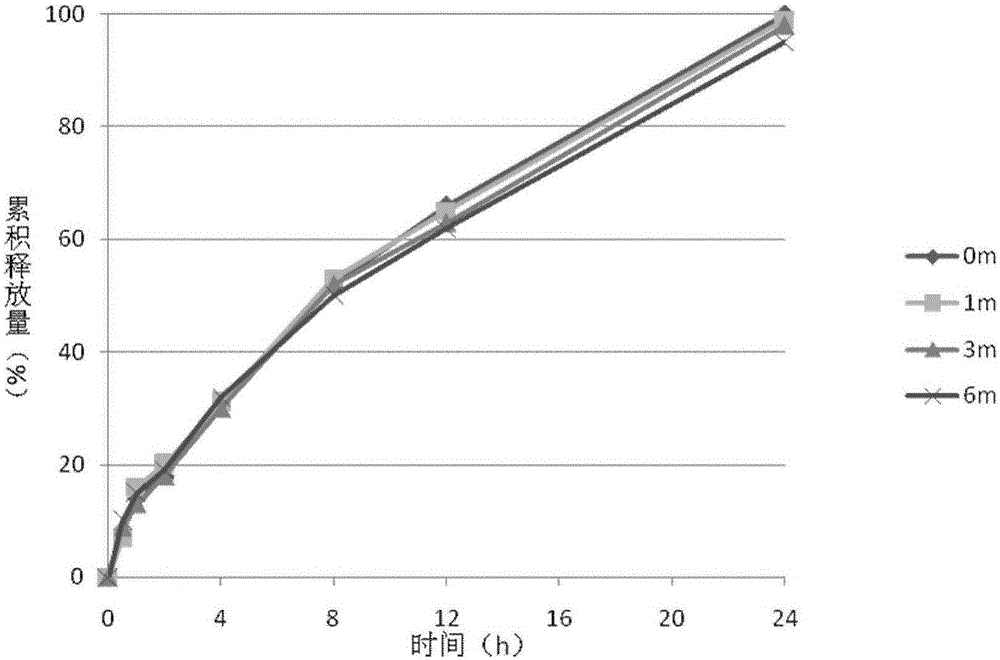
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Grain prolamin microsphere and preparation method
InactiveCN1476825AExtended stayLittle side effectsGranular deliveryMacromolecular non-active ingredientsAlcoholMicrosphere
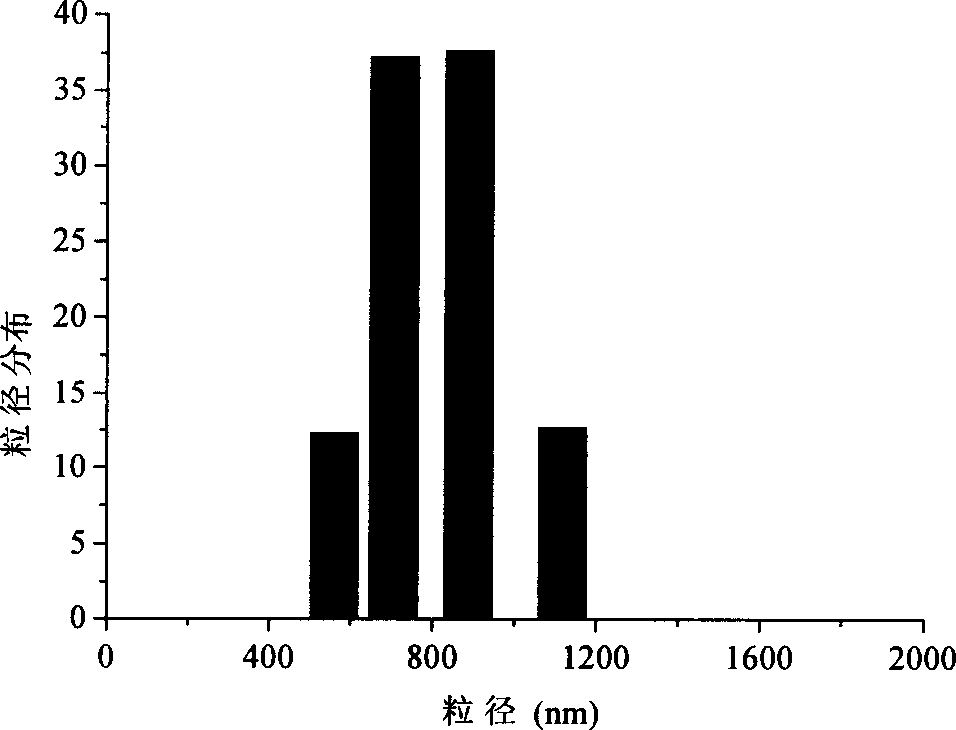
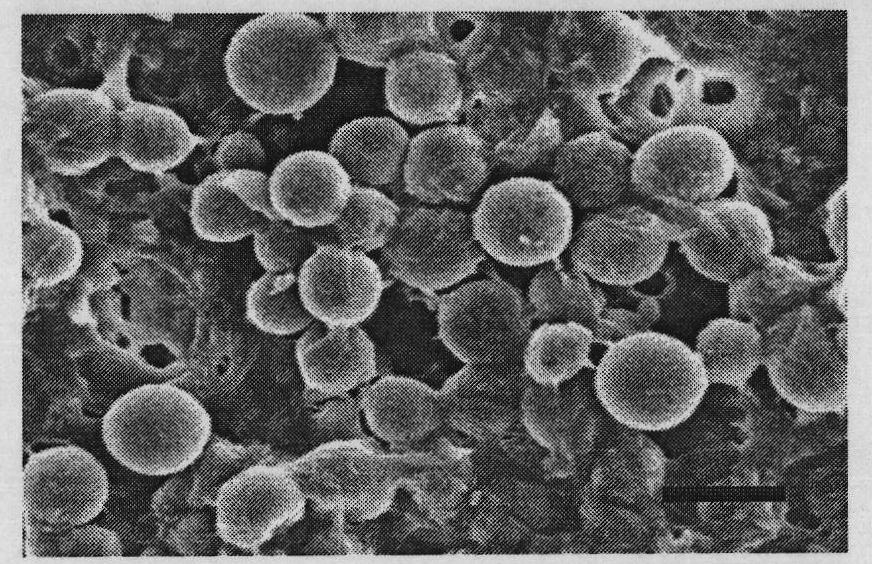
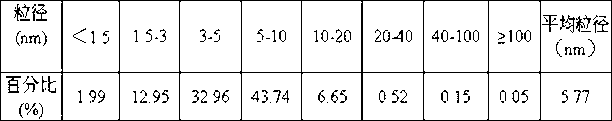
The present invention relates to a cereal alcohol soluble protein microsphere. Its grain size distribution is in 100-1500 nm. It is a method for preparing microsphere by utilizing phase separation method and using cereal alcohol soluble protein as membrane material. Said prepared microsphere can be used as base material for preparing passive target preparation.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Transdermal medicament delivery system containing donepezil compound, preparation and preparation method
ActiveCN102188363AImprove complianceAvoid first pass effectNervous disorderMacromolecular non-active ingredientsDonepezilCross linker
The invention discloses a transdermal medicament delivery system containing a donepezil compound, a transdermal preparation and a preparation method. The transdermal medicament delivery system comprises the following components in percentage by weight: 0.1 to 50 percent of donepezil or acid radical salt thereof, 1 to 95 percent of skeleton polymer, 0.1 to 60 percent of transdermal penetration enhancer, 0 to 10 percent of cross linker, 0.5 to 60 percent of humectant, 0.02 to 10 percent of bacteriostatic agent, 0.02 to 30 percent of pH regulator and 0 to 90 percent of solvent. The system is used for treating light, medium and severe senile dementia, can maintain long-time stable medicament delivery of at least 3 days, has better performance, is convenient for medicament delivery, and can reduce the administration frequency and increase the compliance of patients; and meanwhile, the transdermal path avoids first-pass effect on gastrointestinal tracts and liver due to oral administration of medicaments, and the system has higher bioavailability and obvious advantages in medicinal application.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Acetic acid copaxone microsphere and preparation method thereof
ActiveCN103169670AHigh encapsulation efficiencyQuality improvementNervous disorderPeptide/protein ingredientsAcetic acidMicrosphere
The invention relates to an acetic acid copaxone microsphere and a preparation method thereof. The preparation method is simple in process, and the encapsulation efficiency of the prepared acetic acid copaxone microsphere is high and stable in quality.
Owner:HYBIO PHARMA
Synergistic treatment type multi-material sustained-release eye drop and preparation method
ActiveCN101816627AWith controlled releaseDry fastSenses disorderPharmaceutical delivery mechanismHYDROSOLHigh pressure
The invention discloses a synergistic treatment type multi-material sustained-release eye drop and a preparation method. The preparation method comprises the following steps of: instantaneously forming chitosan aqueous solution which is blended with therapeutic materials into superfine gel particles under the action of direct current high voltage static; receiving the particles by using hyaluronic acid hydrosol or hyaluronic acid hydrogel; and coating the surface of chitosan particles with a hyaluronic acid gel crust layer to obtain a medicament sustained-release eye drop, wherein the size of the chitosan gel particles can be accurately controlled by controlling voltage, the concentration of the chitosan solution and the distance from a jet orifice to the liquid level of the hyaluronic acid hydrosol, and the flowability of the eye drop can be regulated by controlling the concentration of the hyaluronic acid, so that the adhesive capacity of the eye drop to the eye surface and a long-term treatment effect are improved. The preparation method has simple and practicable process, high repeatability, and clean and pollution-free whole preparation process flow; and as the provided multi-therapeutic material sustained-release eye drop for synergistic treatment on eye diseases does not need complicated process, and the proportion of each component and the release rate are convenient to control, so the preparation method has a good application prospect.
Owner:ZHEJIANG UNIV
Active substance-contained gel composite based on multilayer liquid crystal framework and method for producing same
ActiveCN102614109AIncrease concentrationImprove stabilityNervous disorderPharmaceutical delivery mechanismChemistryLiquid crystal
The invention discloses an active substance-contained gel composite based on a multilayer liquid crystal framework and a method for producing the same, wherein the gel composite comprises the following components in percentage by weight: 0.1-35% of liquid crystal gel surfactant, 0.2-30% of permeable skin penetration enhancer, 0.5-30% of liquid paraffin, 0.1-20% of gel host material, 3-90% of water, 0.1-15% of active substance and 3-90% of ethanol solution. The active substance-contained gel composite based on the multilayer liquid crystal framework not only has better efficiency, but also can reduce the medicine taking frequency, so the compliance of a user is increased; and meanwhile, a permeable skin way avoids the first-pass effect after the medicine is orally taken and then passes by gastrointestinal tract and liver, so higher bioavailability is obtained.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
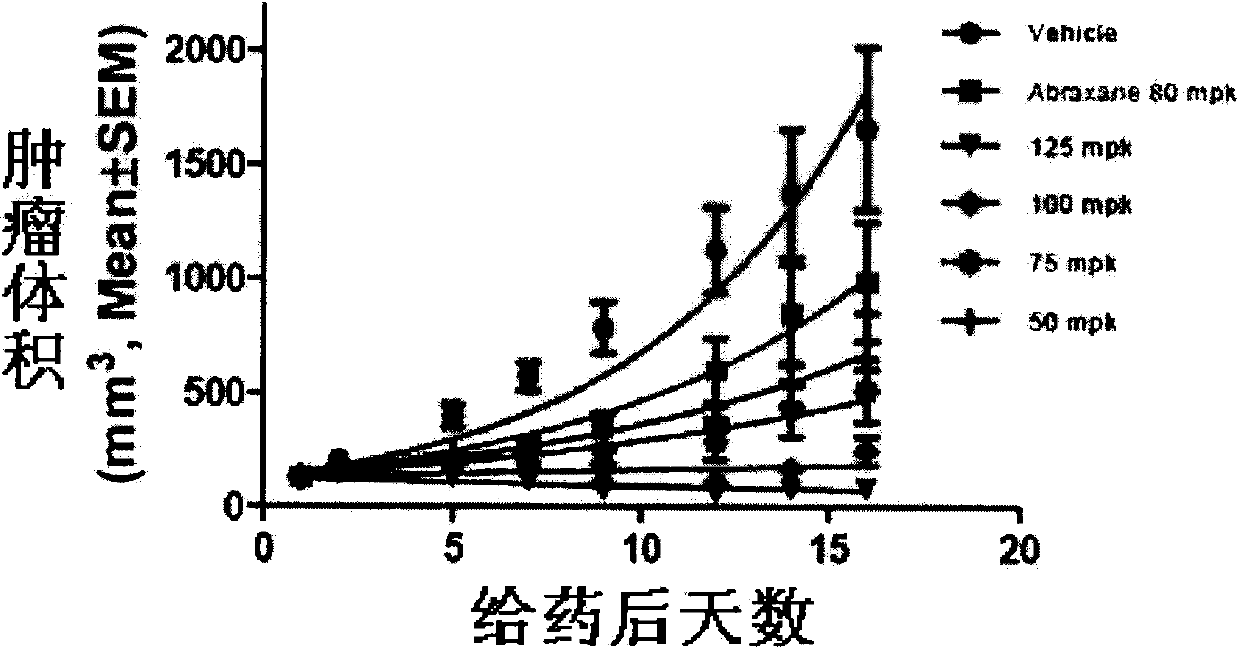
Taxol long-circulating nanoparticle preparation and preparation method thereof
InactiveCN102772368AReduce the frequency of medicationFacilitated releaseOrganic active ingredientsPowder deliverySolventPaclitaxel
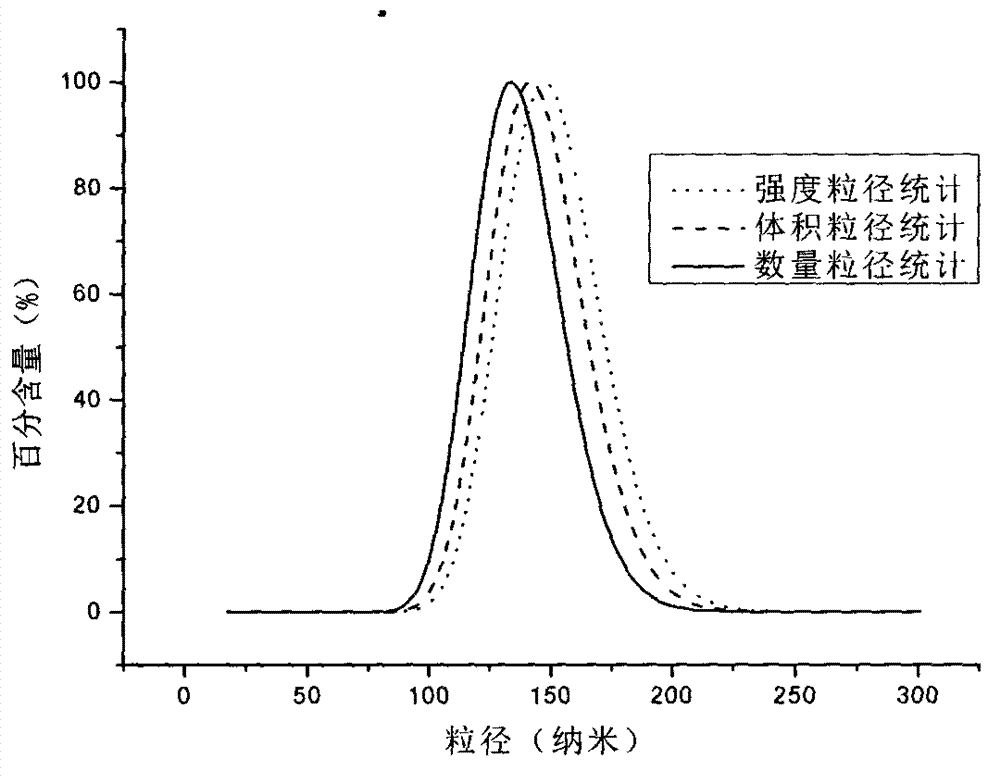
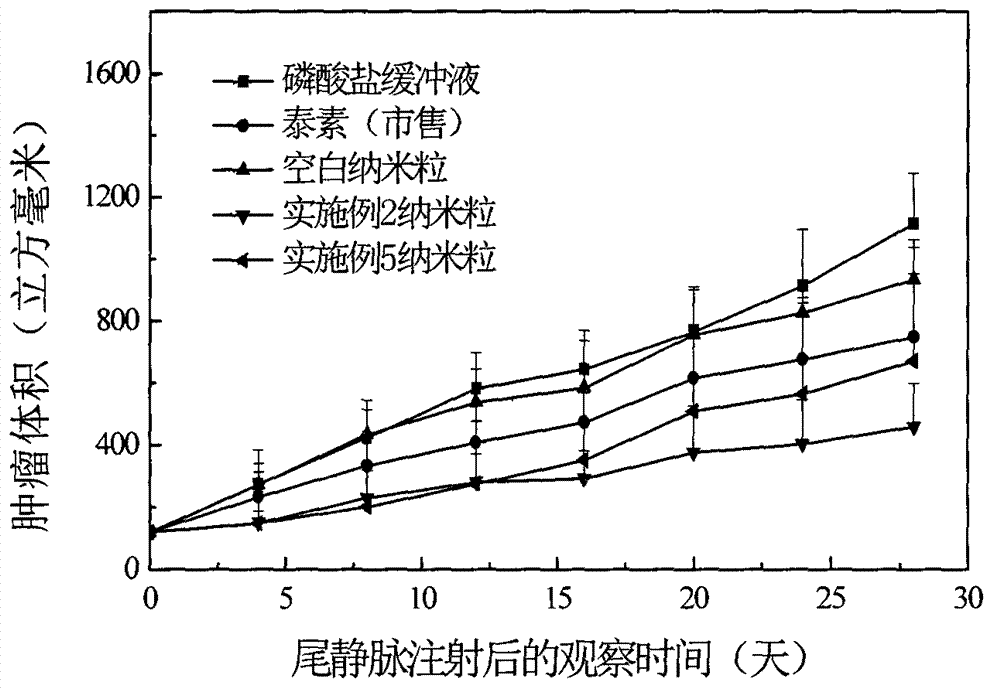
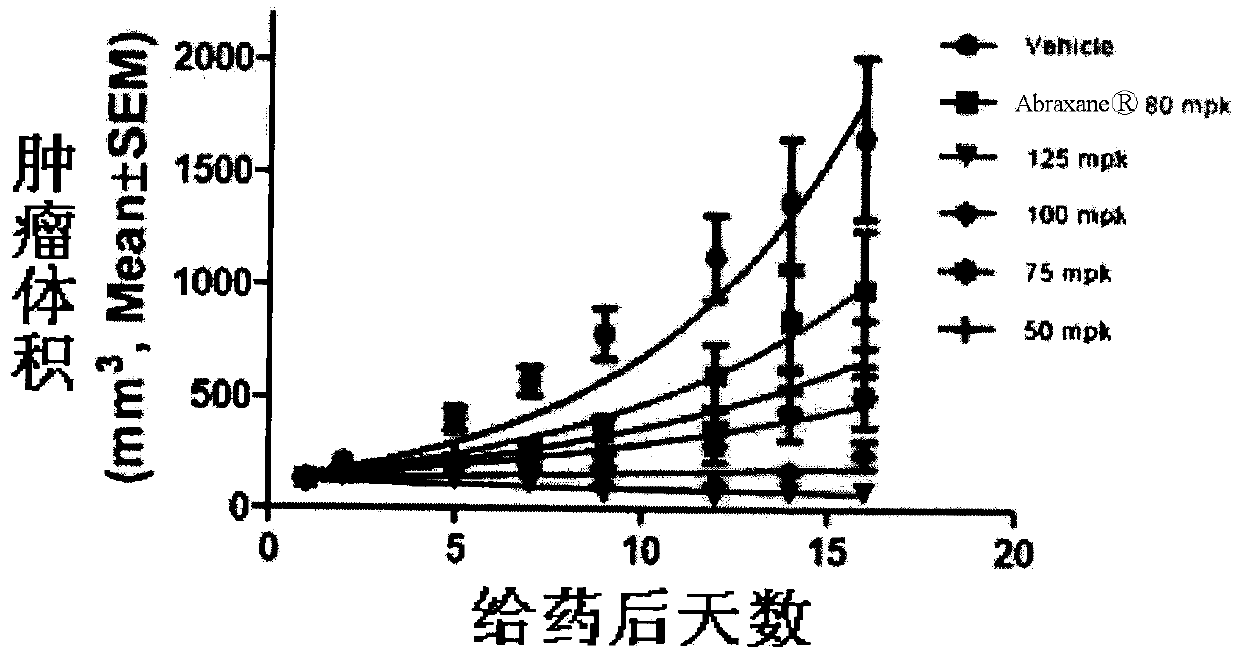
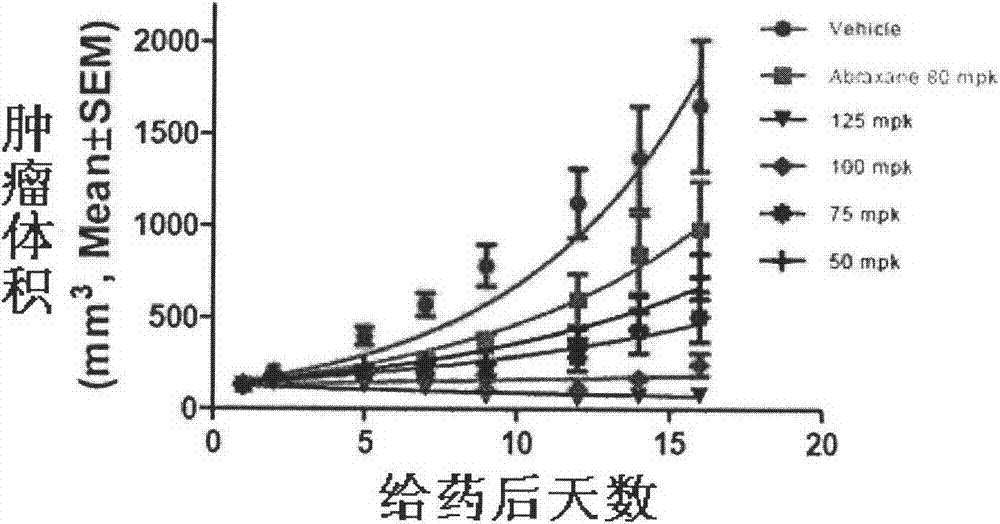
The invention discloses taxol long-circulating nanoparticles. The nanoparticle preparation comprises taxol, a copolymer, a cosolvent, water for injection and the like, wherein according to the nanoparticles, the taxol is 5 to 20 percent of the weight of the copolymer. The invention also discloses a method for preparing the taxol long-circulating nanoparticles. A water phase with cosolvent water soluble vitamin E (TPGS) is dropwise added into an oil phase with the taxol and the copolymer by adopting a method for reversely dropwise adding the solvent, and the nanoparticle preparation with uniform particle size, high entrapment efficiency and high stability can be obtained, and the average particle size is less than 200 nm. The nanoparticle preparation can be used for treating malignant tumors; and an animal experiment shows that the nanoparticle preparation is relatively high in safety and effectiveness.
Owner:HANGZHOU PUSH KANG BIOTECH CO LTD
Compound contraceptive composition, contraceptive transdermal patch containing composition and preparation method
InactiveCN101700246AEffective regulated releasePrevent crystallizationOrganic active ingredientsSexual disorderTransdermal patchActive component
The invention provides a compound contraceptive composition, a contraceptive transdermal patch containing the composition and a preparation method. The compound contraceptive composition comprises effective contraceptive quantity of active components dispersed in a skeleton polymer substrate: etonogestrel and estrogen with a contraceptive cooperative function. Drugs are carried out bidirectional regulation by distribution and dispersal in two phases of the skeleton polymer substrate and a pressure sensitive adhesive substrate for drug transdermal release; and a crystallization inhibiting agent is added to a drug warehouse or the pressure sensitive adhesive substrate in order to effectively inhibit the drugs from crystallizing and maintain the long-time continuous transdermal high activity and power of the drugs in the substrates. The contraceptive transdermal patch prepared from the compound contraceptive composition maintains longer (1 to 7 days) stable drug release and blood drug concentration, has better effect, reduces administration frequency, adds user compliance and has obvious advantages in medical application.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
A transdermal patch containing pramipexole
ActiveCN103432104BImprove uniformityImprove solubilityOrganic active ingredientsNervous disorderHigh concentrationTransdermal patch
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Pyridostigmine bromide coated sustained-release pellets and preparation method thereof
InactiveCN105434403AIdeal sustained release rateReduce the frequency of medicationOrganic active ingredientsMuscular disorderSustained release pelletsSocial benefits
The invention provides pyridostigmine bromide coated sustained-release pellets. The pyridostigmine bromide coated sustained-release pellets are characterized by comprising 57-63 parts of pellets prepared from pyridostigmine bromide serving as an active ingredient and 12-17 parts of sustained-release coating layers by weight. The invention further provides a preparation method of the pyridostigmine bromide coated sustained-release pellets. The pyridostigmine bromide coated sustained-release pellets have an ideal sustained-release rate, the medicine taking frequency is reduced, the compliance of patients for long-time medicine taking is improved, adverse reactions caused by fluctuation of peaks and valleys are reduced, a novel dosage form is provided for clinical use of pyridostigmine bromide, research blank is filled up, an efficient and safe medicine suitable for being taken for a long time is provided for clinical treatment of myasthenia gravis, and the pyridostigmine bromide coated sustained-release pellets have very good clinical application prospect and social benefits.
Owner:CHENGDU MEDICAL COLLEGE
Lipofectin nanometer silver gel and preparation method thereof
ActiveCN103006561AImprove stabilityRelease stabilityInorganic active ingredientsAntisepticsCholesterolGlycerol
The invention relates to a lipofectin nanometer silver gel and a preparation method of the lipofectin nanometer silver gel. The lipofectin nanometer silver gel is prepared from the following raw materials in parts by weight: 1.5 to 4.5 parts of soya bean lecithin and cholesterol, 1 to 9 parts of chitosan, 1 to 20 parts of glycerol, 0.1 to 2 parts of oleum menthae, 30 to 70 parts of acetum, 20 to 60 parts of nanometer silver solution, and the balance of deionized water. According to the preparation method, the nanometer silver is prepared into lipofectin, most of the nanometer silvers are encapsulated in a bilayer formed by the lipofectin, and therefore the stability of the nanometer silver can be improved; and the proper macromolecular optimum formula is adopted to prepare the gel, thus the convenience is provided for paving medicine on the skin. The prepared preparation has the advantages of being stable in medicine release rate, convenience in medicine paving, low in frequency of using the medicine, free from toxicity and stimulation and high in compliance of a user, and can be used for preventing and treating skin intolerance caused by various pathogenic microorganisms.
Owner:ZHEJIANG SANHE NANOMETER SCI & TECH
Bactericidal composition, preparation and application of bactericidal composition
ActiveCN105707126AExtend the effectiveness of prevention and treatmentGood control effectBiocideDead animal preservationBacillus amyloliquefaciensAntibiotic Y
The invention provides bactericidal composition, a preparation and an application of the bactericidal composition. Bactericidal active ingredients of the bactericidal composition mainly comprise Bacillus amyloliquefaciens and an antibiotic in the weight ratio being 10:(0.5-20), wherein the antibiotic is any one of kasugamycin, polyoxin and Zhongshengmycin, and the number of live endospores of the Bacillus amyloliquefaciens is 5-50 billion / g. The Bacillus amyloliquefaciens and the antibiotic are compounded to have a synergistic effect, and the control effect on diseases is better than that of single chemicals.
Owner:LONGDENG CHEM XIAN
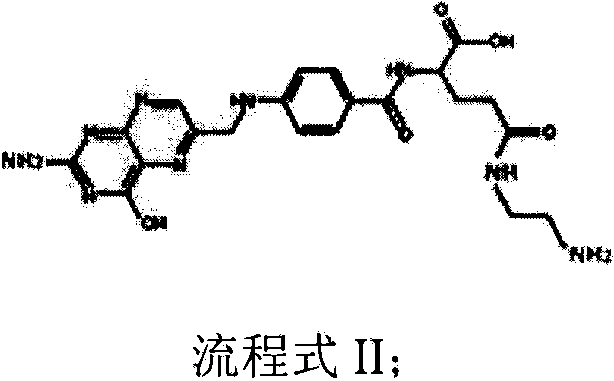
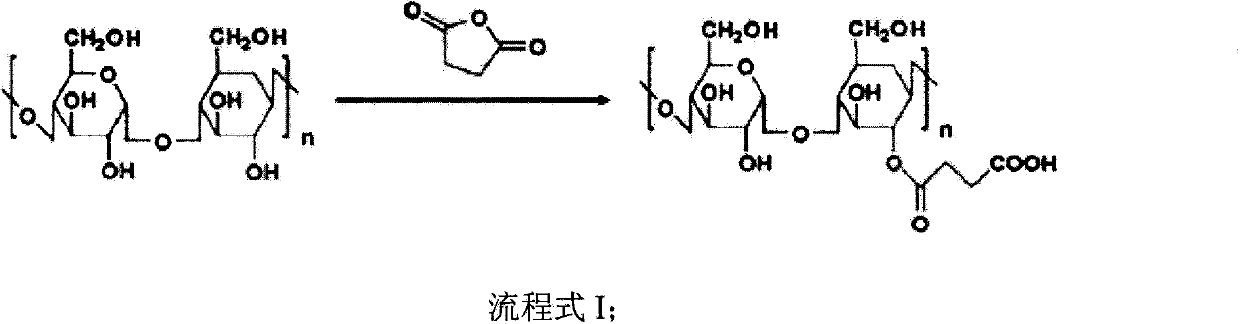
Accessory for coating medicinal compound and preparation method thereof
InactiveCN103992417AAdaptableImprove toleranceOrganic active ingredientsPharmaceutical non-active ingredientsDrug compoundCombinatorial chemistry
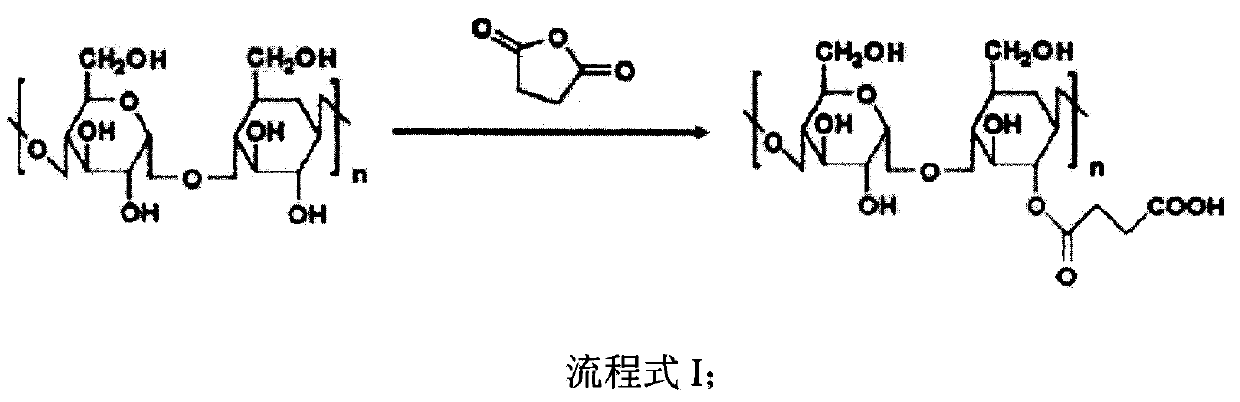
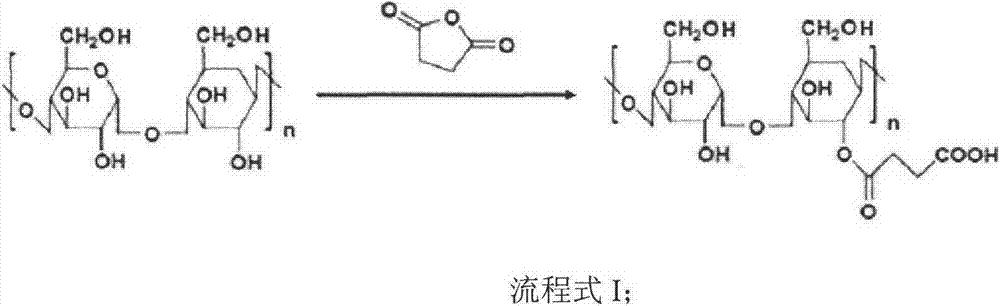
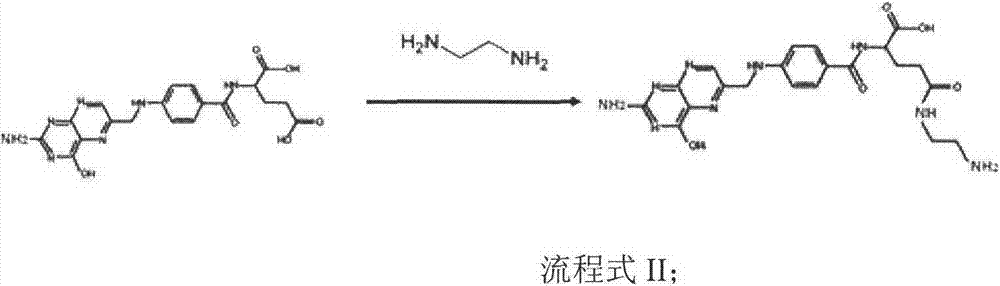
The invention discloses a preparation method of an accessory for coating a medicinal compound. The method comprises synthesis of succinylated glucan, synthesis of an aminated folacin analogue, and synthesis of a folacin-dextran conjugate namely the accessory. The invention also discloses the accessory produced by the method, and a medicinal compound containing the accessory and a preparation method thereof.
Owner:SUZHOU YOULIN BIO TECH
Piribedil sustained-release tablet and preparation method thereof
InactiveCN104721161ASimple processConducive to large-scale industrial productionOrganic active ingredientsNervous disorderSustained Release TabletAcrylic resin
The invention belongs to the field of pharmaceutical preparations and discloses a piribedil sustained-release tablet and a preparation method thereof. The piribedil sustained-release tablet mainly comprises the following components in percent by mass: 20-40% of piribedil, 10-60% of a framework material, 10-50% of a filling agent, 1-5% of a binding agent and 0.5-4% of a lubricating agent. The piribedil sustained-release tablet comprises a hydrophilic gel framework material and an acrylic resin-like framework material which are used for regulating release rate of main medicines. The prepared piribedil sustained-release tablet can be used for treating Parkinson's disease. The piribedil sustained-release tablet product has the advantages that administration frequency is reduced, medicine taking compliance of a patient is improved and pharmaceutical effect and medicine safety are improved, so that the piribedil sustained-release tablet is more applicable to the patient; and meanwhile, a technique of the piribedil sustained-release tablet is simple, and industrial large-scale production is benefited.
Owner:SUZHOU HOMESUN PHARMA
Tacrolimus loaded micelle and preparation method and applications thereof
InactiveCN108743531AUniform particle sizeSmall dispersion coefficientOrganic active ingredientsSenses disorderPolymer scienceBioavailability
The invention discloses a tacrolimus loaded micelle and a preparation method and applications thereof. The micelle comprises tacrolimus and a di-block copolymer. The di-block copolymer is mPEG-b-P(LA-co-GA) or mPEG-b-PLA, the structure of the di-block polymer is represented by the formula (I) or the formula (II), n is a positive integer (10-100), x is a positive integer (10-300), and y is a positive integer (30-300). Di-block biodegradable high molecular segmented copolymers with different molecular weights are designed and taken as a carrier to load tacrolimus so as to prepare the tacrolimusloaded micelle, which has the advantages of uniform particle size, low polydispersity index (PDI=0.1), and good stability. Eye drops prepared from the micelle can well relieve the rejection reactionsafter corneal transplantation, and compared with tacrolimus eye drops (0.05%) used in clinic, the bioavailability is much better.
Owner:SUN YAT SEN UNIV
Solid preparation containing capsaicin and preparation method thereof
InactiveCN106214655AReduce energy consumptionImprove stabilityOrganic active ingredientsMetabolism disorderChemistryGastrointestinal tract
The invention provides a solid preparation containing capsaicin.The solid preparation is prepared from capsaicin, menthol, a coating layer and auxiliary materials, wherein the coating layer is a double-layer coating and consists of an isolation coating layer and a slow release coating layer.The auxiliary materials include a filling agent and a lubricant. The solid preparation containing the capsaicin applies the menthol as a carrier for the first time, so that the in-water solubility of the capsaicin in the preparation is improved from 0.011 micro-g / mL to 40 micro-g / mL and is far higher than that ofJapan's commercially availablecapsaicin tablets (2.6micro-g / mL). Therefore, the taking dosage of a final product can be reduced, the irritation of the capsaicin to the gastrointestinal tract is reduced. In addition, double-layer coating design is conducted on the final product, the stability of the product is also ensured while release is controlled. Furthermore,attractive, complete and smooth slow-release coatings having a slow-release effect can be achieved without any plasticizer or anti-sticking agent.
Owner:杭州成邦医药科技有限公司
Fusion proteins and preparation method thereof, and application of fusion protein to preparation of medicines used for treating ophthalmic diseases and resisting inflammations and tumors
ActiveCN108623693AShort half-lifePromote proliferationAntibacterial agentsSenses disorderDiseaseHalf-life
The invention discloses fusion proteins and a preparation method thereof, and application of the fusion proteins to medicines used for treating ophthalmic diseases and resisting inflammations and tumors, belonging to the technical field of biopharmaceuticals. According to the invention, a flexible (F) or rigid (R) linker is used for fusing two polypeptides so as to obtain two bifunctional fusion proteins, respectively; that is, the two angiogenesis-inhibiting polypeptides HM-3 and IL-4 are linked with the Fc fragment of an immunoglobulin in virtue of the amino acid linker so as to form multi-functional fusion protein macromolecules. The fusion proteins can improve drug efficacy, prolong half-life and enhance stability, have the characteristics of strong action, low toxicity and the like, and is applicable to the prevention and treatment of solid tumors, various inflammations and neovascular ophthalmic diseases. The fusion proteins are expressed in eukaryotic cells by using a genetic engineering method and are obtained through affinity chromatographic purification.
Owner:徐寒梅
Dulaglutide injection and preparation method thereof
InactiveCN104173275ANot easy to degradeEffective regulationPeptide/protein ingredientsMetabolism disorderOsmotic pressureUrology
Disclosed are a dulaglutide injection and the preparation method thereof, the injection containing: dulaglutide, a pH regulator, a solubilizer, an antimicrobial agent, an osmotic pressure regulator, and water for injection, wherein, in addition to water for injection, the content of each component by weight ratio is: dulaglutide:the pH regulator:the solubilizer:the antimicrobial agent: the osmotic pressure regulator 1.5:(2.0-35):(2-5): 7-16:(180-420), and the ratio of the total weight of these five components and the volume of the water for injection in mg / ml is 0.6-1.6.
Owner:HYBIO PHARMA
Nanoscale particle-type auxiliary material
InactiveCN103936880AAdaptableImprove toleranceOrganic active ingredientsPowder deliveryDrug compoundCombinatorial chemistry
The invention discloses nanoscale particles, and a preparation method thereof. The preparation method comprises following steps: succinylated glucan is synthesized; an aminated folacin is synthesized, a folic acid-glucan conjugate is synthesized, and a medical compound is mixed with the folic acid-glucan conjugate. The invention also discloses applications of the nanoscale particles in preparation of pharmaceutical compositions which are suitable for fat soluble medical compounds, can be used for preventing tumor, and / or high in drug tolerance and low in toxicity.
Owner:SUZHOU YOULIN BIO TECH
In-situ gel containing cyclosporin micelle as sustained-release ophthalmic drug delivery system
PendingCN112516084AImproved membrane transportImprove stabilitySenses disorderAntipyreticOphthalmic drugOphthalmology
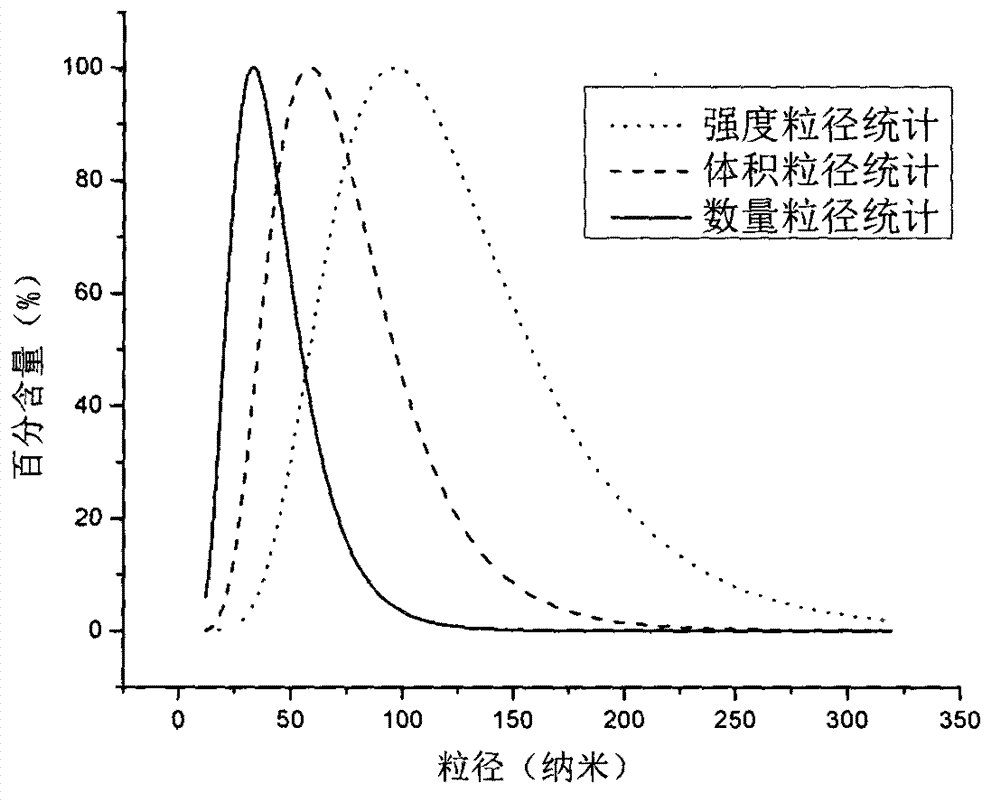
The invention provides an in-situ gel containing cyclosporin micelles as a sustained release ophthalmic drug delivery system. The in-situ gel contains 0.01 wt% to 5 wt% of an aqueous ophthalmic formulation of cyclosporin present in the form of a micelle with the particle size of no greater than 20 nm.
Owner:IVEW THERAPEUTICS (ZHUHAI) CO LTD
Diltiazem hydrochloride tablet and preparation method thereof
InactiveCN110464710AAvoid direct contactAvoid water contactOrganic active ingredientsPill deliveryOrganic solventAdhesive
The invention relates to a diltiazem hydrochloride tablet. The diltiazem hydrochloride tablet is prepared by the following steps: taking diltiazem hydrochloride, a filling agent, polyethylene glycol,hydrogenated castor oil, microcrystalline cellulose and the like as raw materials, performing melting and pelletizing, and performing tabletting so as to obtain the diltiazem hydrochloride tablet. Theinvention has the beneficial effects that the diltiazem hydrochloride tablet is molten and pelletized, water or an organic solvent does not need to be added to serve as an adhesive, no residue of theorganic solvent exists, and the prepared particle does not need a drying step; the microcrystalline cellulose in the raw materials is matched with the hydrogenated castor oil to achieve a synergisticeffect, a skeleton is formed by virtue of melting and pelletizing, the hydrogenated castor oil is promoted to well achieve a skeleton slow-release effect, a slow-release purpose of the diltiazem hydrochloride is achieved, and drug action time is prolonged.
Owner:XINHUA PHARMA GAOMI CO LTD
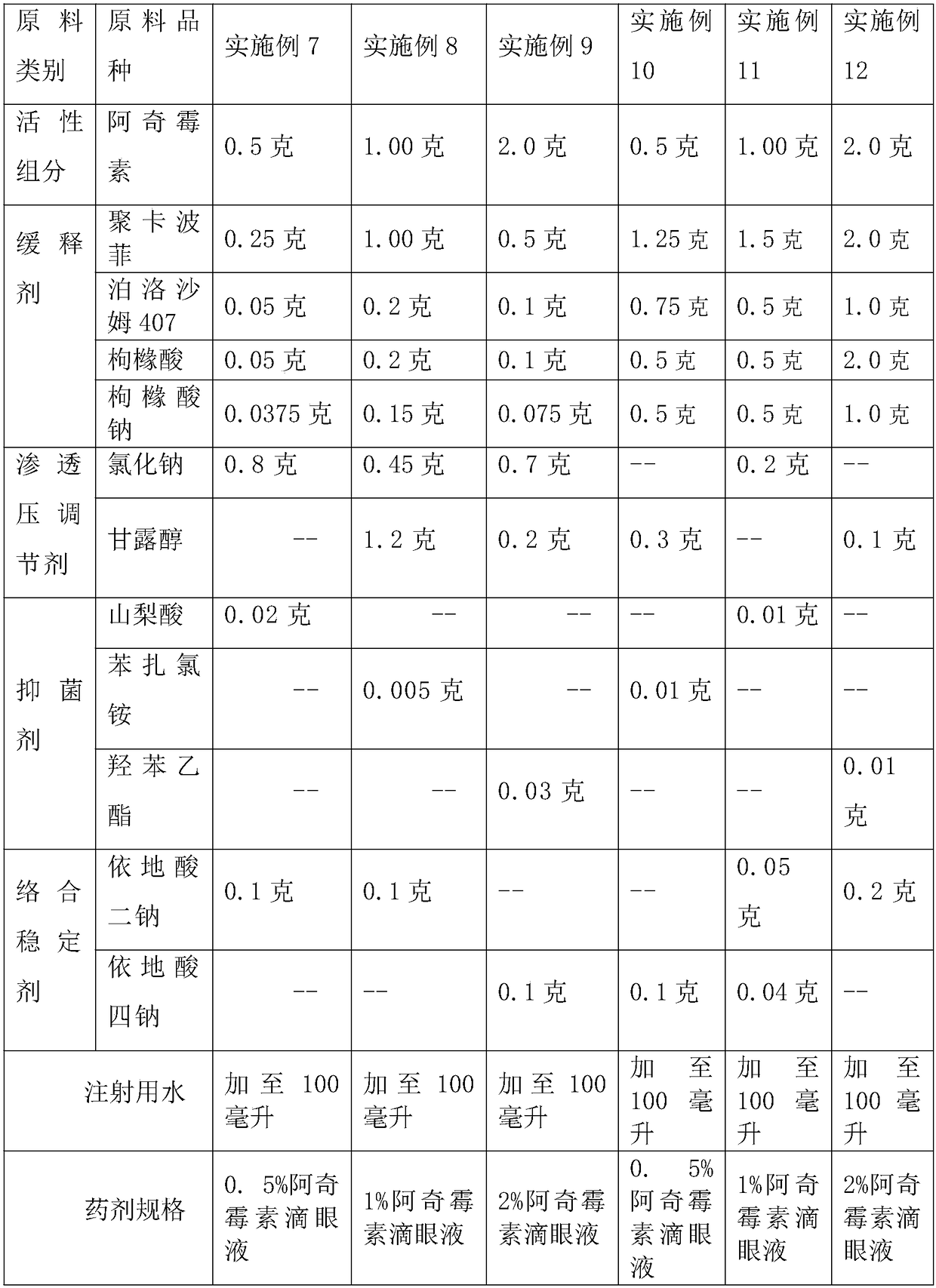
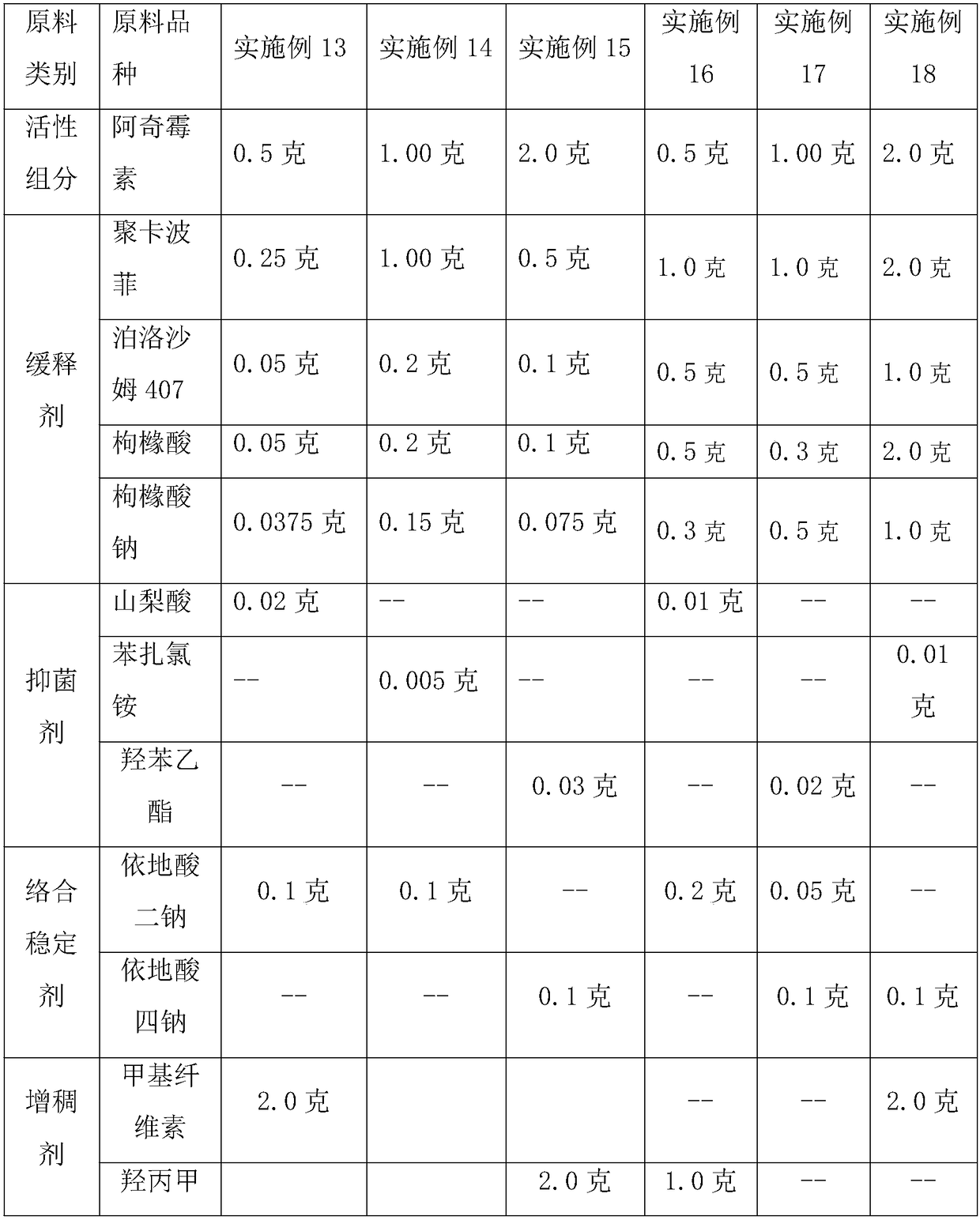
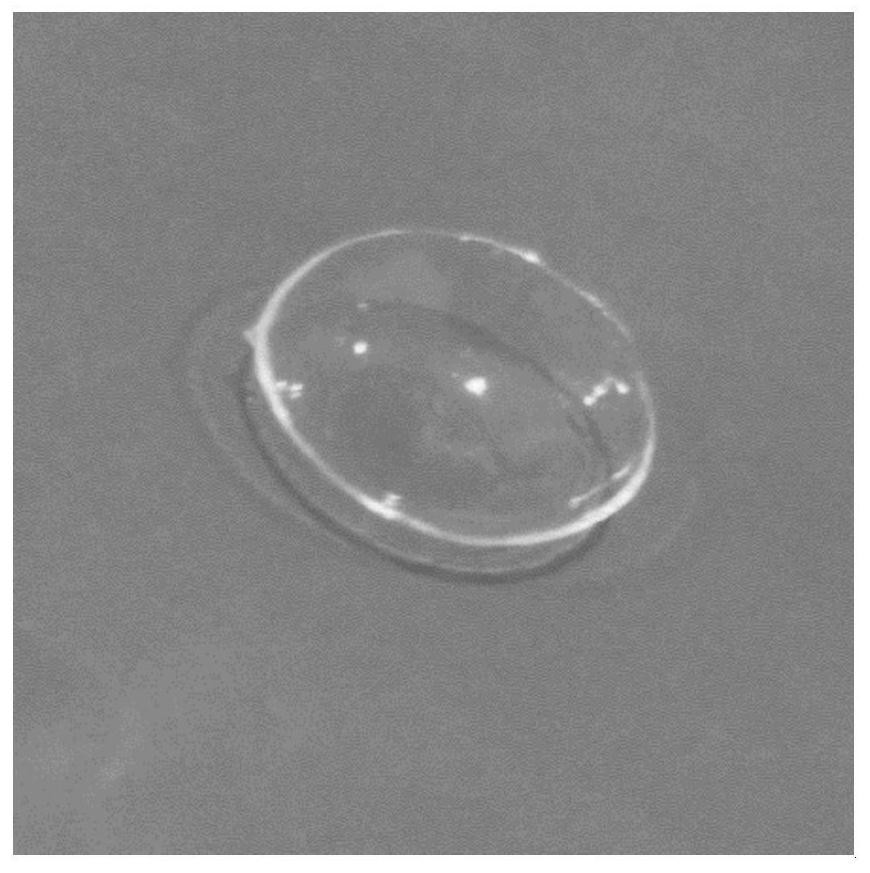
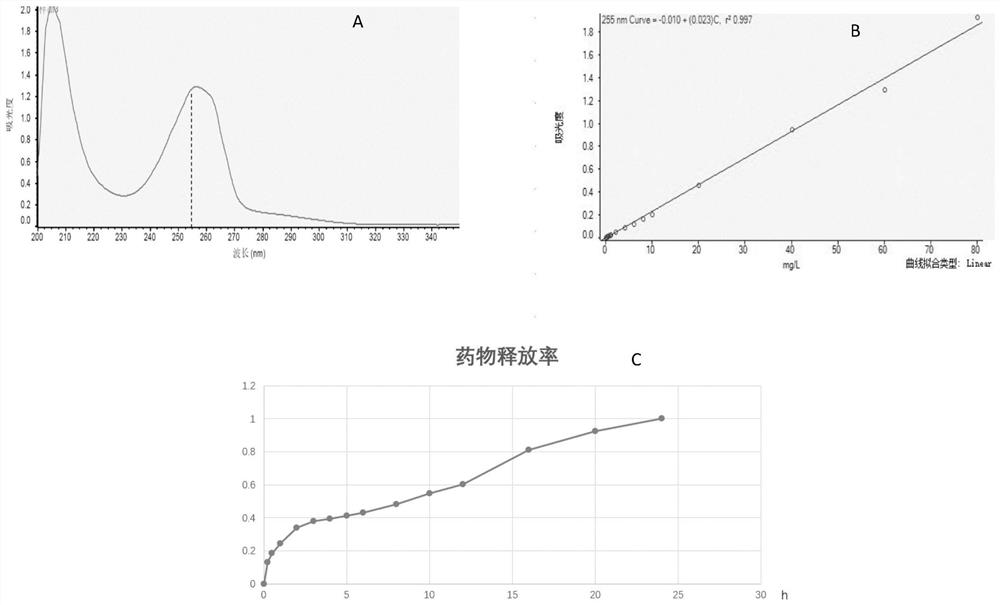
Drug release system, azithromycin eye preparation containing drug release system, and preparation method of drug release system
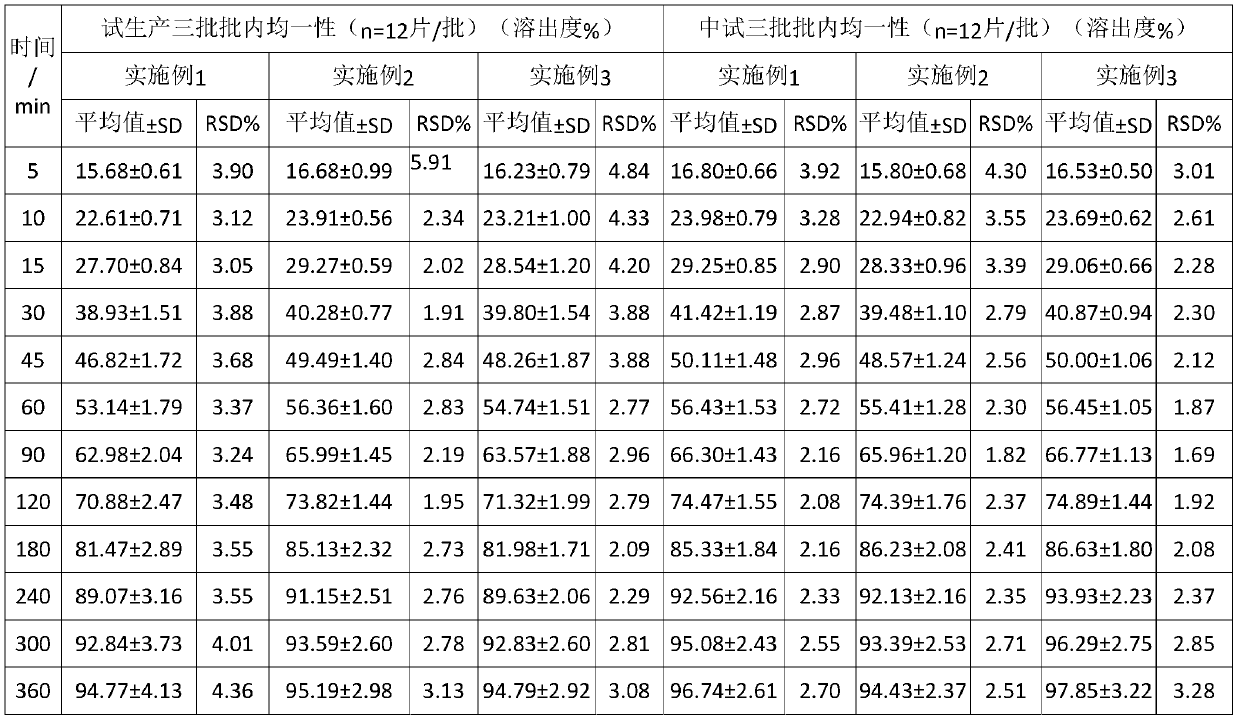
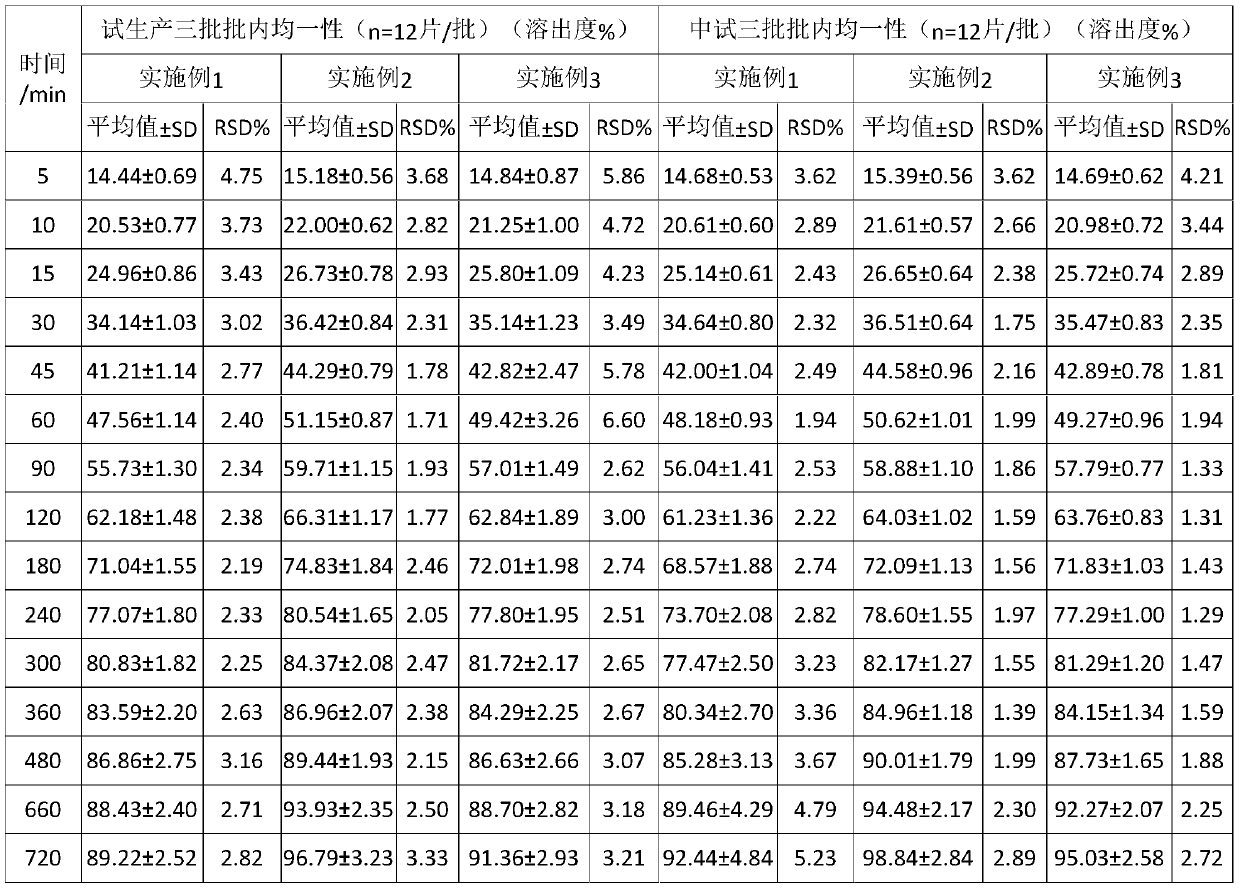
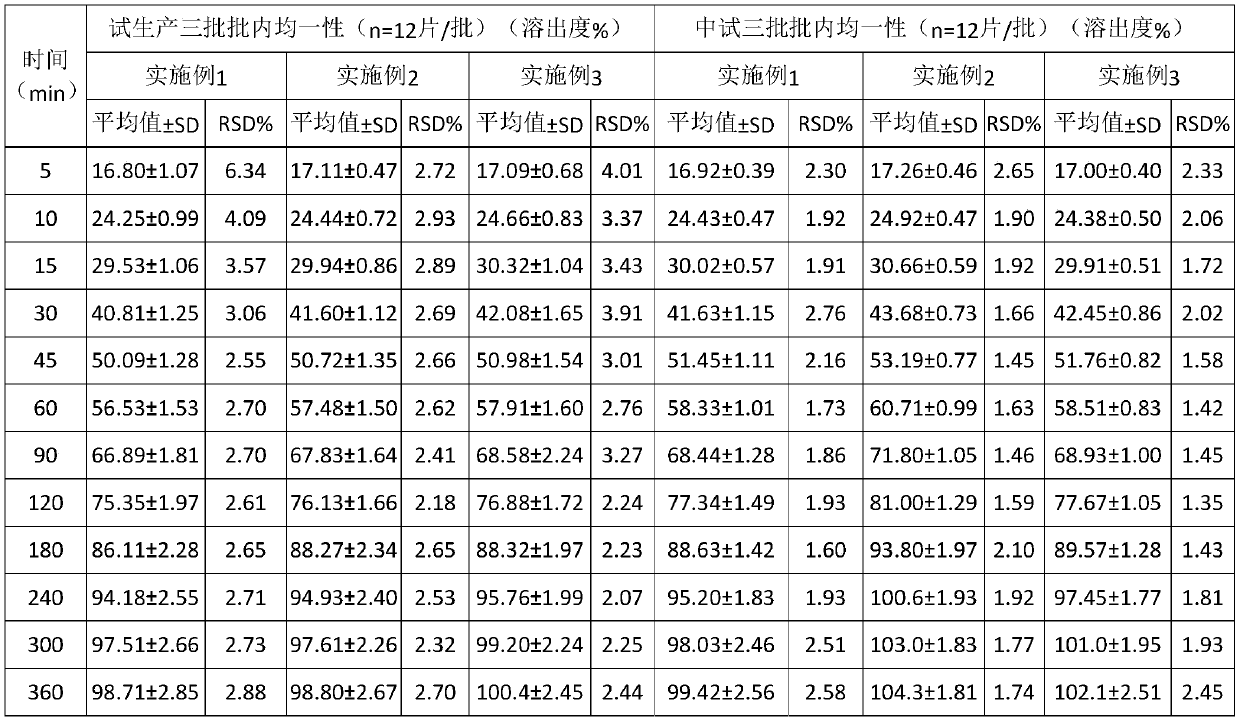
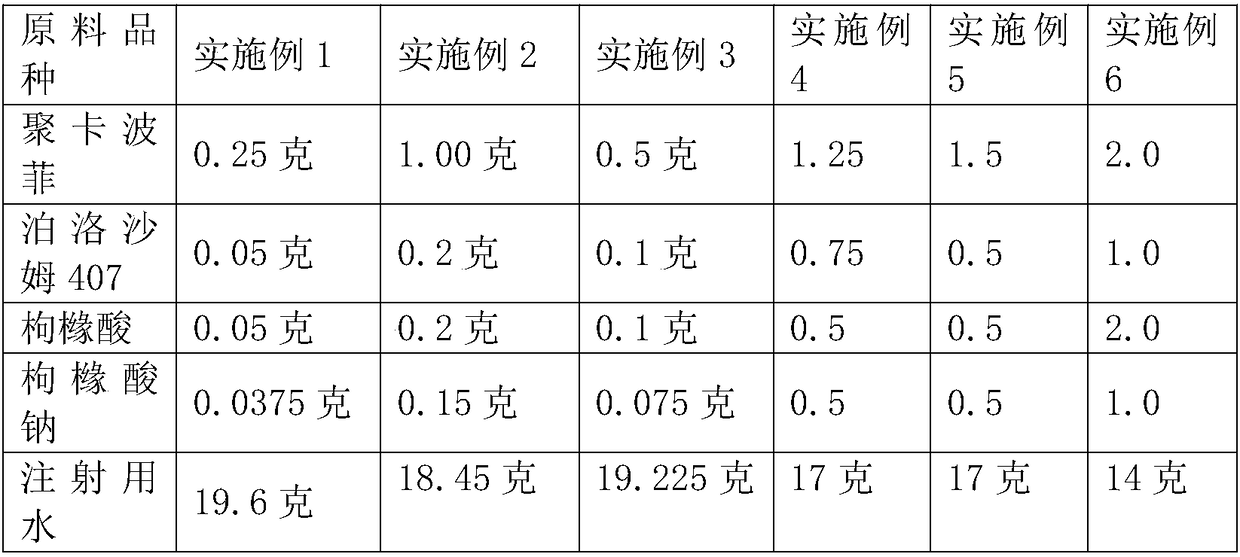
InactiveCN108210450ASolve solubilityFix stability issuesAntibacterial agentsOrganic active ingredientsSolubilityRetention time
The invention relates to a drug release system, an azithromycin eye preparation containing the drug release system, and a preparation method of the drug release system. According to the preparation method, the mucosa adhesive polymer drug release system is prepared taking Azithromycin as a drug effect raw material; the weight ratio of polycarbophil, poloxamer 407, citric acid, and sodium citrate is controlled to be 1:0.1-1.0:0.1-1.0:0.1-0.8; an eye local acceptable pharmaceutical accessory material is adopted to prepare the slow release preparation; the weight ratio of Azithromycin to the drugrelease system polymer is controlled to be 1:1-2. The azithromycin eye preparation can be prepared into eye drop, ophthalmic gel, eye ointment, or any pharmaceutical dosage form suitable for eye local external use. The preparation method is capable of solving problems of Azithromycin such as poor water dissolvability and stability, prolonging retention time of active drugs on eye surfaces, and increasing the antibacterial activity on target tissues.
Owner:广州君博医药科技有限公司
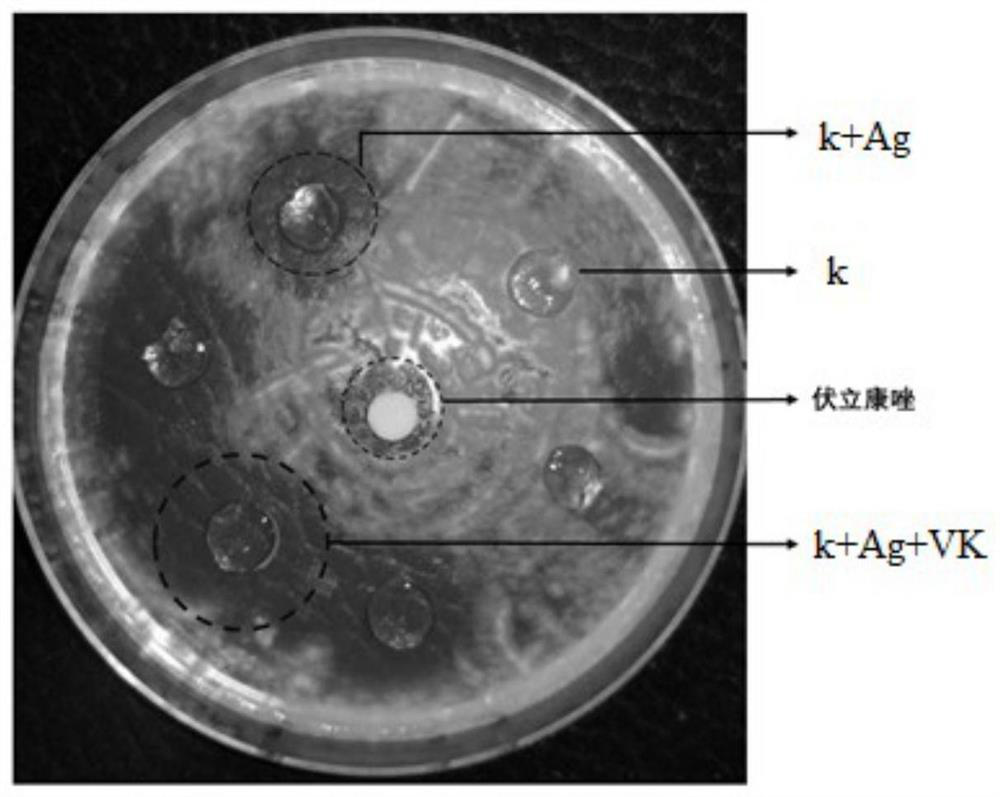
Voriconazole-coated carrageenan corneal contact lens and preparation method thereof
PendingCN112999354AGood tissue compatibilityNon-immunogenicOrganic active ingredientsSenses disorderCarrageenanOphthalmic Dosage Form
The invention relates to the technical field of ophthalmic dosage forms and medical materials, and relates to a voriconazole-coated carrageenan corneal contact lens which is prepared by the following components according to the content: sequentially adding a silver salt aqueous solution and a voriconazole solution into a carrageenan aqueous solution, wherein the mass volume ratio of the carrageenan aqueous solution to the silver salt aqueous solution to the voriconazole solution is 10 g: (5-15) microliter: (7-13) microliter, the mass percent concentration of the carrageenan aqueous solution is 0.99-10%, the voriconazole solution is a voriconazole DMSO solution with the mass concentration of 0.1-5 mg / microliter, and the silver salt aqueous solution is a silver nitrate aqueous solution with the molar concentration of 0.1 mol / liter; the added silver ions can promote gelation of carrageenan, and the silver elementary substance can kill fungi, so that the dosage of voriconazole is reduced, the drug release time can be prolonged to 24 hours, the frequency of eye administration is reduced, the local eye stimulation symptom is relieved and the treatment effect on fungal keratitis is improved.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof
The invention relates to the technical field of biology, and discloses a whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof. The invention adopts a whole human antibody technology and acquires an anti-human TNF alpha whole human antibody. Preliminary analyses on the physical, chemical and biological activities of the antibody indicate that the antibody has better affinity with TNF, and can effectively neutralize the killing effect of the TNF on L929 cells in vitro. The whole human TNF alpha monoclonal antibody disclosed by the invention minimizes the immunogenicity of a human body. A PEG (polyethylene glycol) surface modification technology is adopted, thus avoiding the defect of short half life of small molecule antibody, and the whole human TNF alpha monoclonal antibody is more suitable for application in vivo while keeping the anti-TNF alpha activity.
Owner:优锐生物医药科技(深圳)有限公司
Efinaconazole microemulsion composition
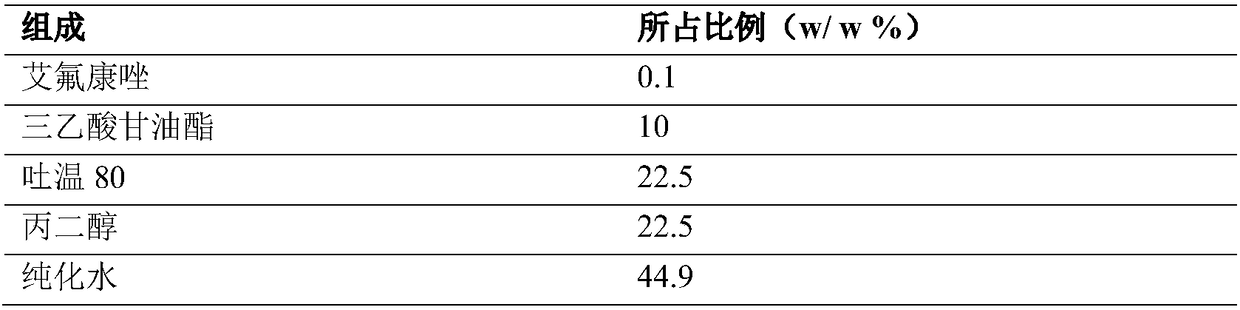
ActiveCN108721215AGood treatment effectImprove stabilityOrganic active ingredientsAntimycoticsGlycerolOil phase
The invention discloses an efinaconazole microemulsion composition. The efinaconazole microemulsion composition is an efinaconazole microemulsion solution or efinaconazole microemulsion gel. The efinaconazole microemulsion solution is prepared from the following raw materials: 0.01 to 1.0 percent of efinaconazole, 5 to 15 percent of oil phase, 15 to 30 percent of emulsifier, 15 to 30 percent of co-emulsifier and 30 to 60 percent of water; the oil phase is selected from glyceryl triacetate, triolein and isopropyl myristate; the emulsifier is selected from Tween 80, Tween 60 and lecithin; the co-emulsifier is selected from propylene glycol, ethanol and glycerol. The efinaconazole microemulsion gel comprises the efinaconazole microemulsion solution and a gel material. The efinaconazole microemulsion solution disclosed by the invention has the advantages of good stability, ideal microemulsion particle size and capability of effectively improving the skin permeability of drugs. Compared with the efinaconazole microemulsion solution, the efinaconazole microemulsion gel has the advantages that the skin permeability and retention volume can be further improved, the compliance of onychomycosis in clinical treatment can be improved and a slow release effect is achieved.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
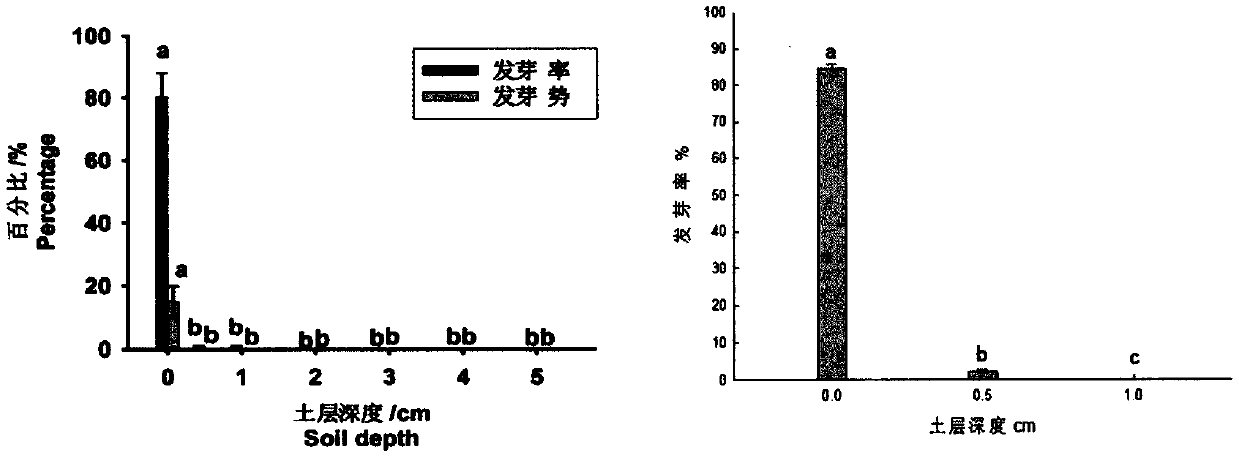
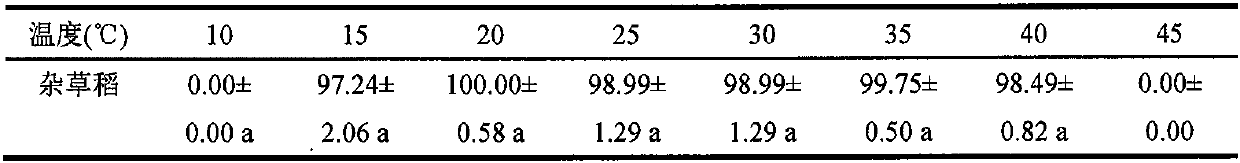
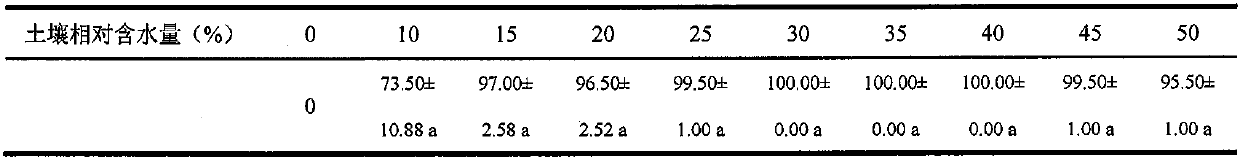
Comprehensive control and removal method for weeds in paddy fields
InactiveCN110495362AAvoid blind useReduce dosagePlant protectionRice cultivationSocial benefitsSlurry
The invention provides a comprehensive control and removal method capable of reducing the use amount of a herbicide in paddy fields and used for weeds in the paddy fields, and belongs to weed controland removal in the field of plant protection. By means of the comprehensive technology of taking a soil (green manure) deep ploughing-water slurry management-mechanical ploughing trinity agronomic andagricultural machinery weed control measure based on biological and ecological characteristics of dominant weeds in the paddy fields with the assistance of the guidance of ecological and economic control and removal thresholds of the main dominant weeds for reducing the use frequency of the herbicide and in combination with the compatibility of the herbicide and spray aids for reducing the use amount of the herbicide, the use frequency of the herbicide is reduced to 2-3 from 3-4 in the direct seeding paddy fields and reduced to 1-2 from 2-3 in the transplanting paddy fields, and the use amount of the herbicide per unit area is reduced by 30-70%. By means of the method, on the promise of not reducing the weed removal effect, the main dominant weeds in the current paddy fields are effectively controlled and removed, the generation of resistant weeds in the paddy fields is delayed, the input reduction and the efficiency improvement are achieved, and the method has obvious economic, ecological and social benefits.
Owner:SHANGHAI ACAD OF AGRI SCI
Folic acid-glucan conjugate and application thereof
InactiveCN103864955AAdaptableImprove toleranceOrganic active ingredientsPharmaceutical non-active ingredientsFolic acid analoguesGlucan
The invention discloses a folic acid-glucan conjugate and an application thereof. The method comprises the following steps: synthesizing amber acylation glucan; synthesizing amino folic acid analogues; and synthesizing the folic acid-glucan conjugate. The invention also discloses an application of the folic acid-glucan conjugate for preparing a drug composition, and the like.
Owner:SUZHOU YOULIN BIO TECH
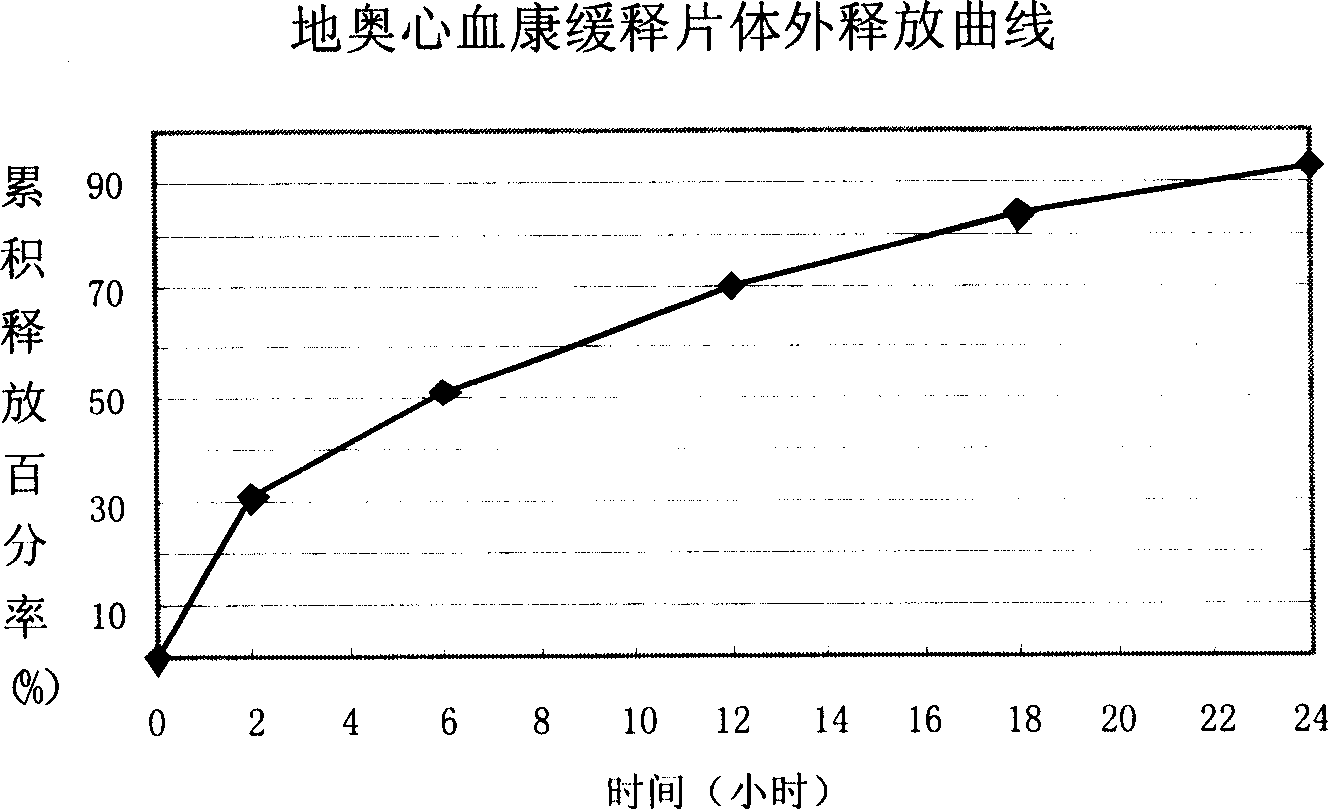
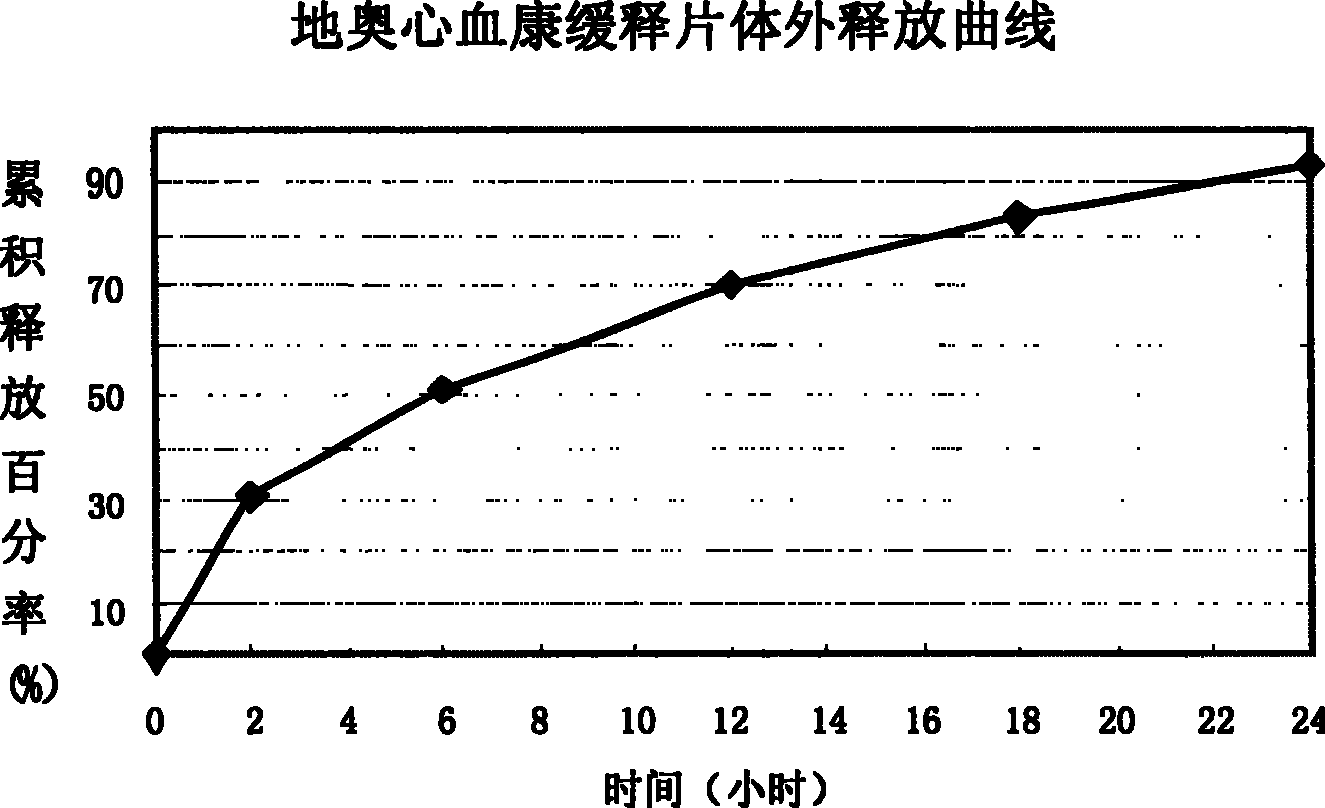
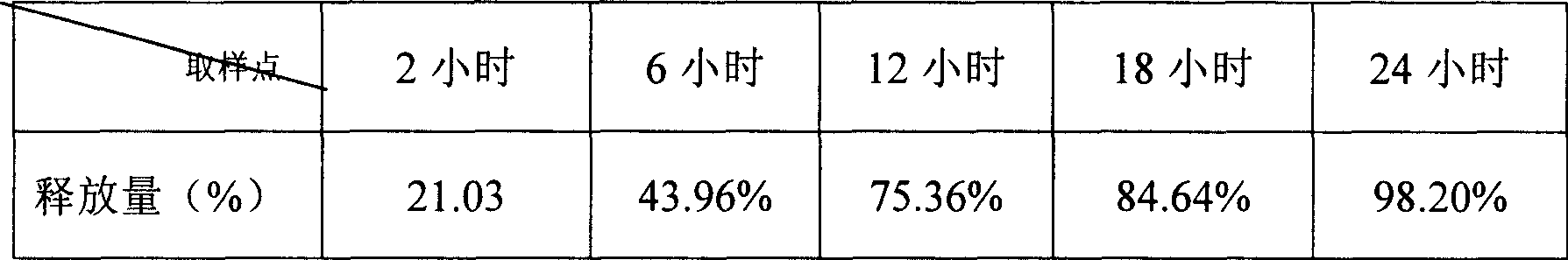
Di'ao Xinxuekang sustained-release preparation and its preparation method and use
ActiveCN101108230BImprove solubilitySolve slow-release technical problemsPharmaceutical delivery mechanismDiseaseIn vitro test
The invention provides a Di'ao Xinxuekang sustained-release preparation, which is composed of steroidal total saponins extracted from the rhizome of Chinese yam or / and Dioscorea punctatus as active ingredients, combined with solid dispersion technology and melting granulation technology or slow-release coating technology , that is, firstly mix the total steroidal saponins with a hydrophilic carrier material or / and a solubilizer to prepare a solid dispersion with excellent solubility, and then add a slow-release material to prepare the preparation. In vitro tests show that the sustained-release preparation is released continuously and smoothly within 24 hours, which provides a new option for the prevention and treatment of cardiovascular and cerebrovascular diseases.
Owner:CHENGDU DIAO PHARMA GROUP
Insecticidal composition containing methoprene
Owner:江苏功成生物科技有限公司
Azithromycin eye drops
InactiveCN102579335AAvoid abuseImprove complianceOrganic active ingredientsSenses disorderBacterial ConjunctivitisStaphylococcus aureus
The invention discloses azithromycin eye drops. The azithromycin eye drops are a stable eye preparation which is prepared from azithromycin serving as a main active ingredient and proper auxiliary materials. The long-acting eye drops with mucosa adhesiveness prepared from the latest auxiliary materials at home and abroad have a medicine controlled-release system, the active ingredient stops in eyes for several hours to enhance the antibacterial activity of target tissues, so that the using frequency of the eye drops can be reduced, and the eye drops are convenient to use. The azithromycin eye drops are suitable for treating bacterial conjunctivitis caused by microbial sensitive strains such as G group corynebacterium, haemophilus influenzae, staphylococcus aureus, streptococcus mitis groups and streptococcus pneumoniae.
Owner:GUANGDONG WHOLEWIN TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com