Active substance-contained gel composite based on multilayer liquid crystal framework and method for producing same
A technology of gel composition and active substances, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, drug delivery, etc., and can solve problems such as accelerated transdermal water loss, skin vulnerable to external attacks, and low surfactant content , to achieve the effect of reducing skin irritation, improving moisturizing effect, and reducing the area of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
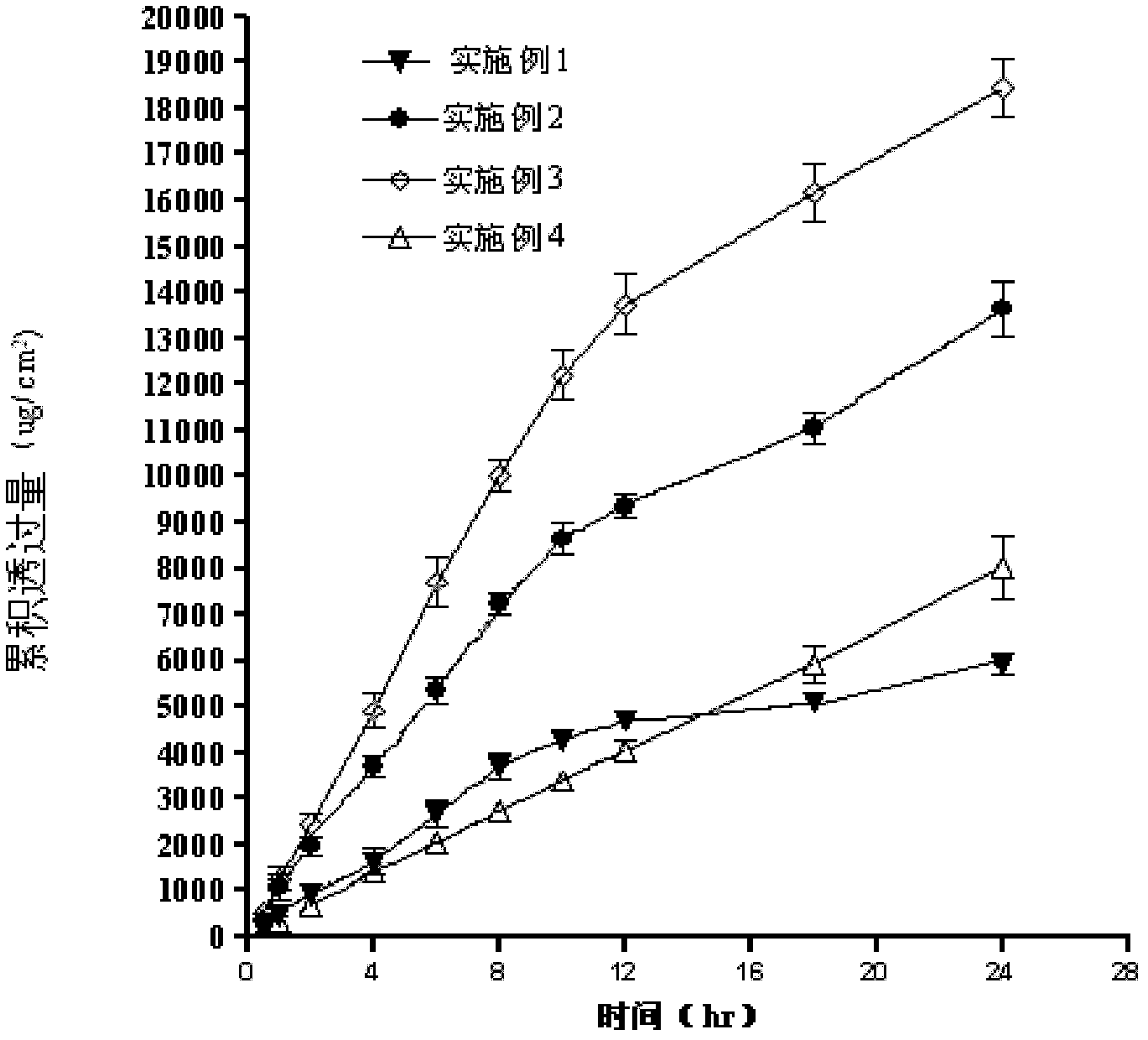
Examples
Embodiment 1
[0050] Prescription: (percentage by weight)
[0051]
[0052]
[0053] Preparation:
[0054] (1) Heat and melt polyoxyethylene polyoxypropylene block polymer, triacetin, and liquid paraffin at 75°C to obtain phase A;
[0055] (2) Swell PEO in water for 24 hours to obtain phase B;
[0056] (3) Dissolving donepezil hydrochloride in aqueous ethanol solution and mixing to obtain phase C;
[0057] (4) pyridoxamine hydrochloride, methylparaben and sodium hydroxide solution are mixed to obtain phase D;
[0058] At 25°C, add phase C to phase B, stir at 5000 rpm for 2 hours, mix and disperse to obtain a drug-containing hydrogel;
[0059] When the temperature drops to 35°C, add phase A to the above drug-containing hydrogel, stir at 20 rpm for 1 hour, and emulsify to form a liquid crystal gel;
[0060] Then add phase D and stir at 20 rpm for 30 minutes to obtain a gel composition based on a multilayer liquid crystal skeleton and containing active substances.
Embodiment 2
[0062] Prescription: (percentage by weight)
[0063]
[0064] Preparation:
[0065] (1) Heat and melt OW 340B, lauryl lactate, and liquid paraffin at 75°C to obtain phase A;
[0066] (2) Carbopol was swelled in water for 10 hours to obtain phase B;
[0067] (3) Dissolving donepezil hydrochloride in aqueous ethanol solution and mixing to obtain phase C;
[0068] (4) Sodium metabisulfite, ethylparaben and triethanolamine are mixed to obtain phase D;
[0069] At 35°C, add phase C to phase B, stir at 500rpm for 0.5 hours, mix and disperse to obtain a drug-containing hydrogel;
[0070] When the temperature drops to 20°C, add Phase A to the above drug-containing hydrogel, stir at 60 rpm for 0.5 hours, and emulsify to form a liquid crystal gel;
[0071] Then add phase D and stir at 60 rpm for 10 to obtain a gel composition based on a multilayer liquid crystal skeleton and containing active substances.
Embodiment 3
[0073] Prescription: (percentage by weight)
[0074] Arlatone LC 15%, isopropyl myristate 0.5%, liquid paraffin 15%, E-INSPIRE 343 (acrylate and acrylate copolymer) 1.5%, water 27%, donepezil hydrochloride 10%, absolute ethanol 27%, Sodium metabisulfite 1%, ethylparaben 1%, 2% sodium hydroxide solution 2%. The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com