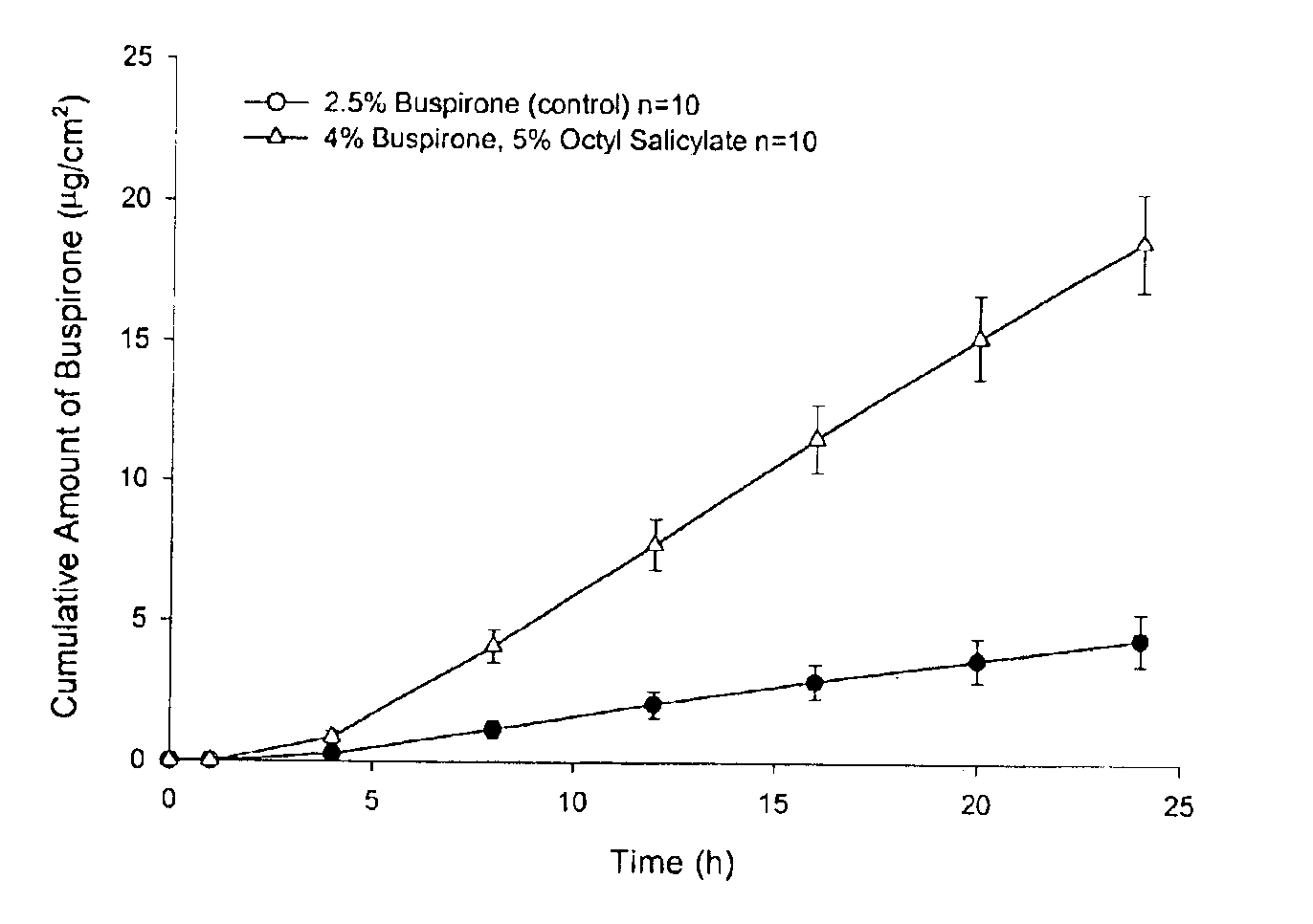
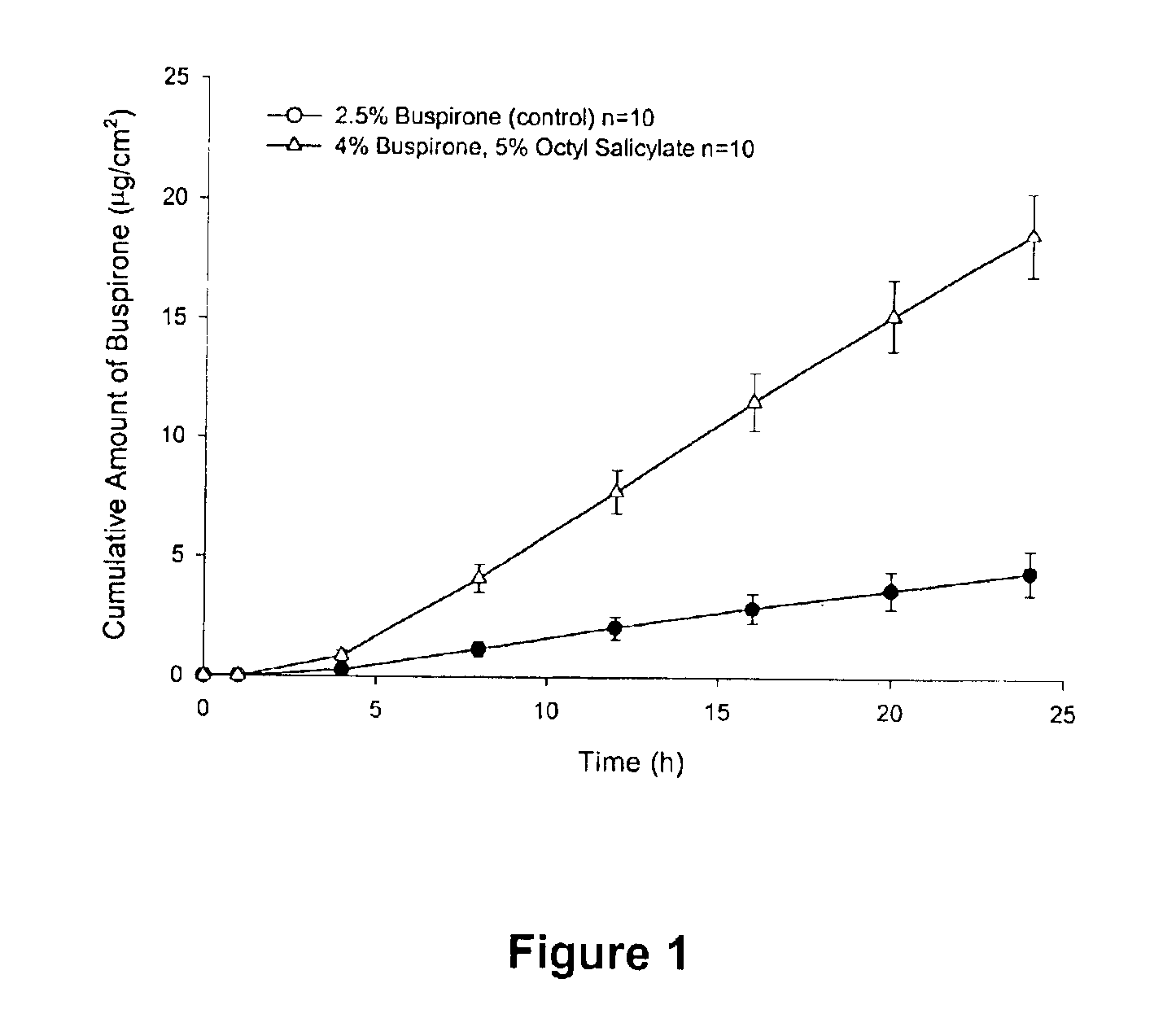
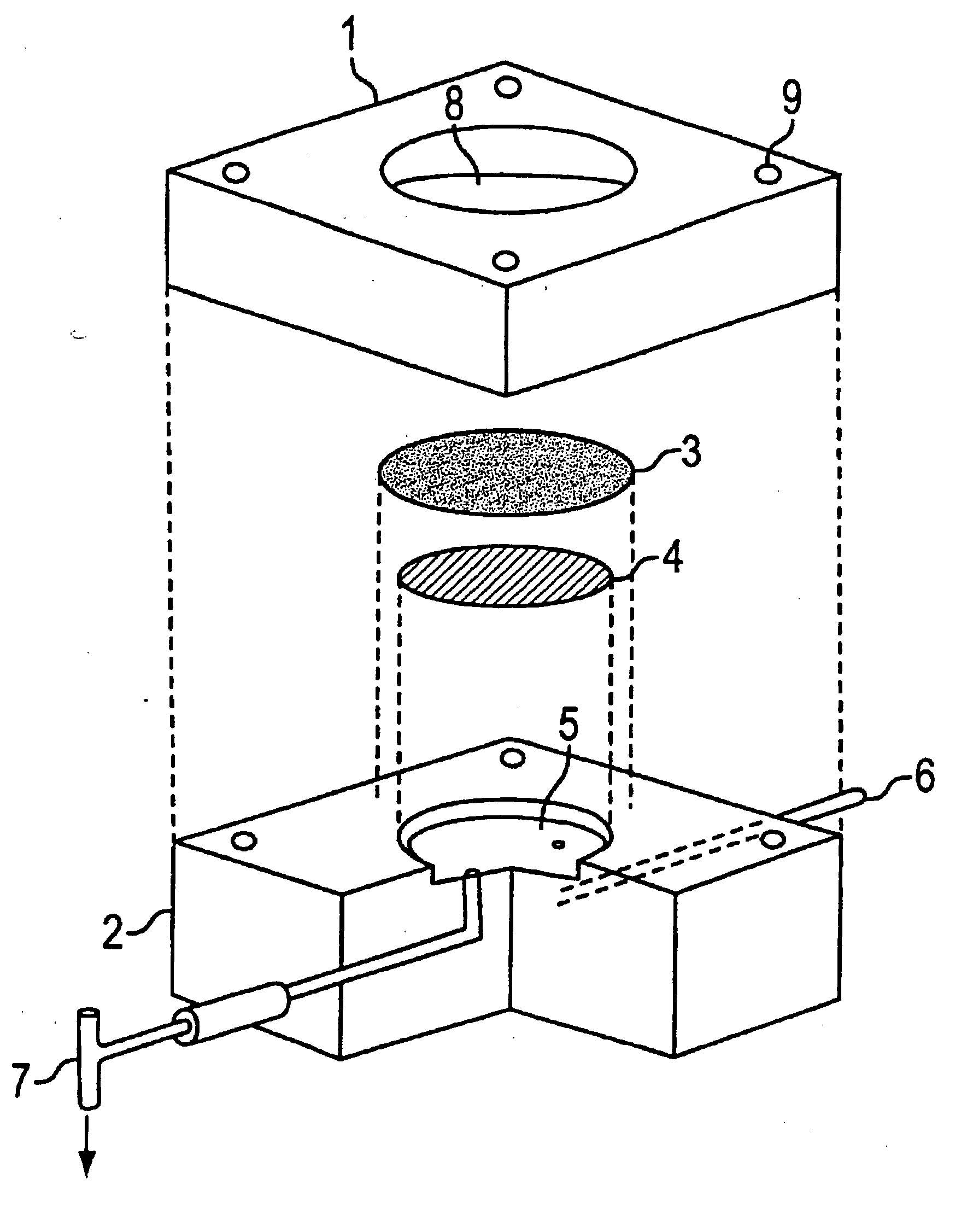
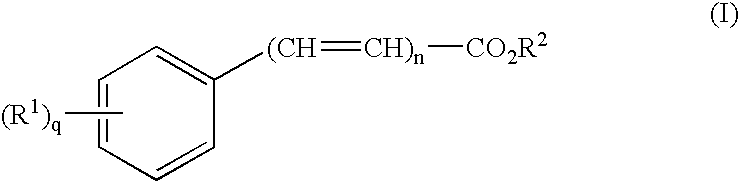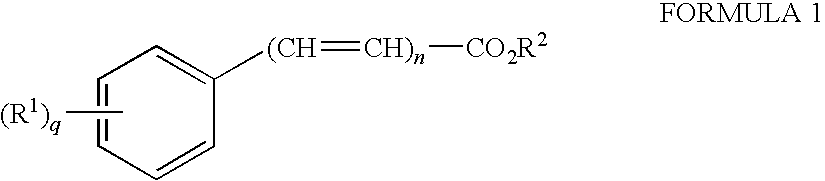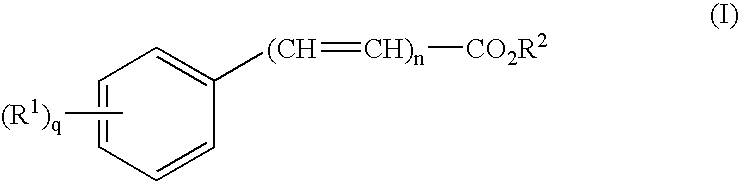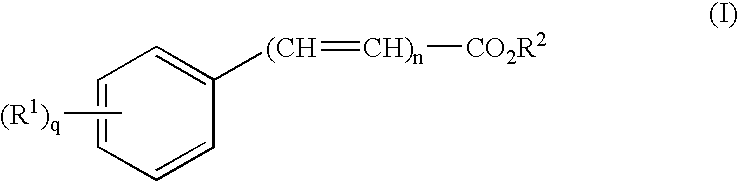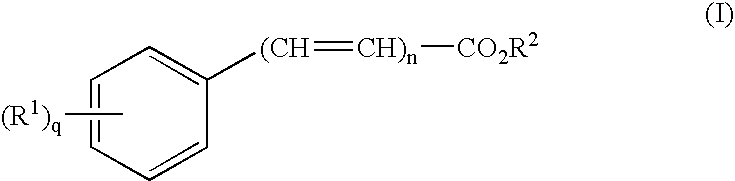Patents
Literature
175 results about "Skin penetration enhancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal delivery of antiparkinson agents
InactiveUS6929801B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyMedicine
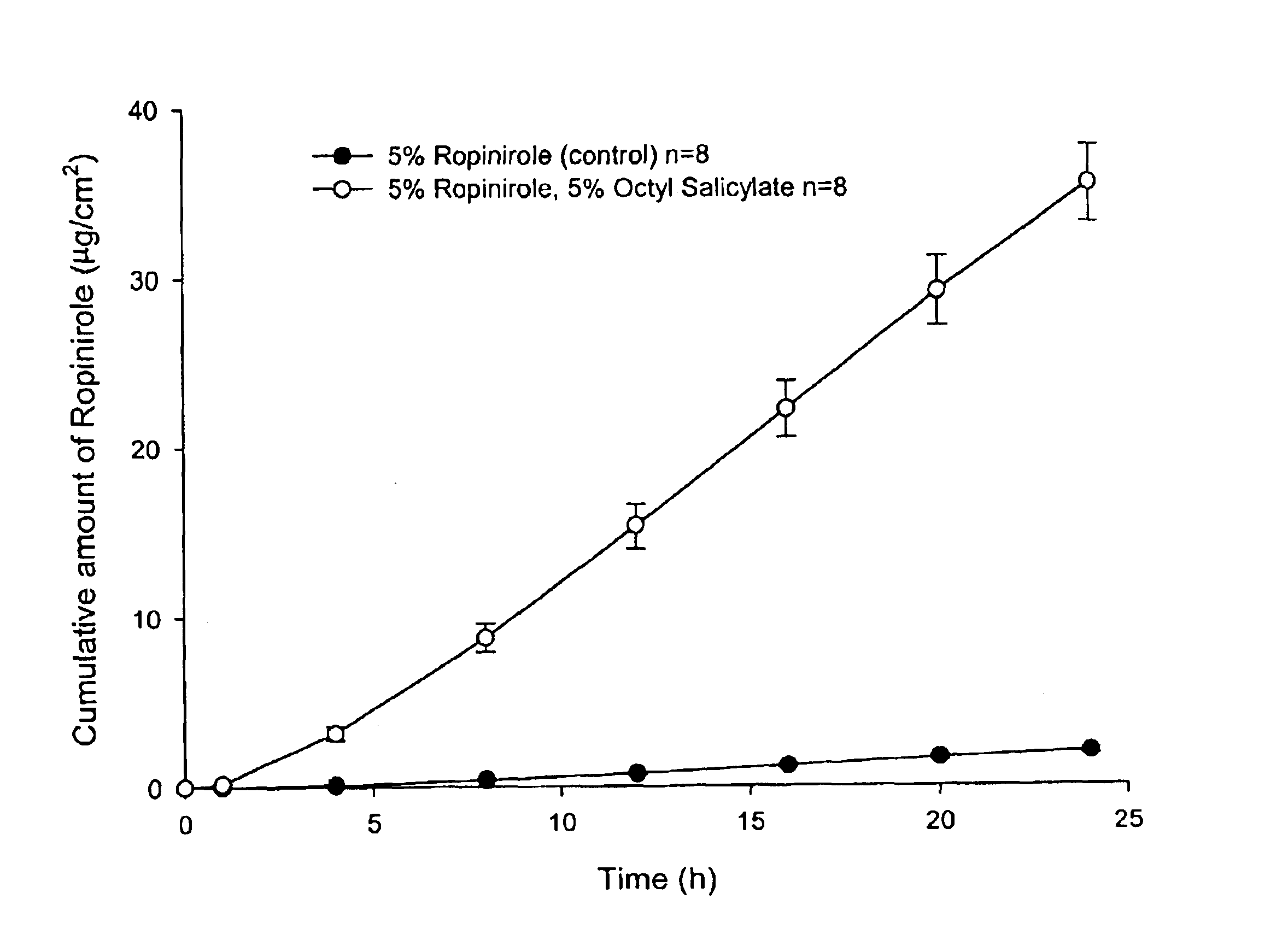
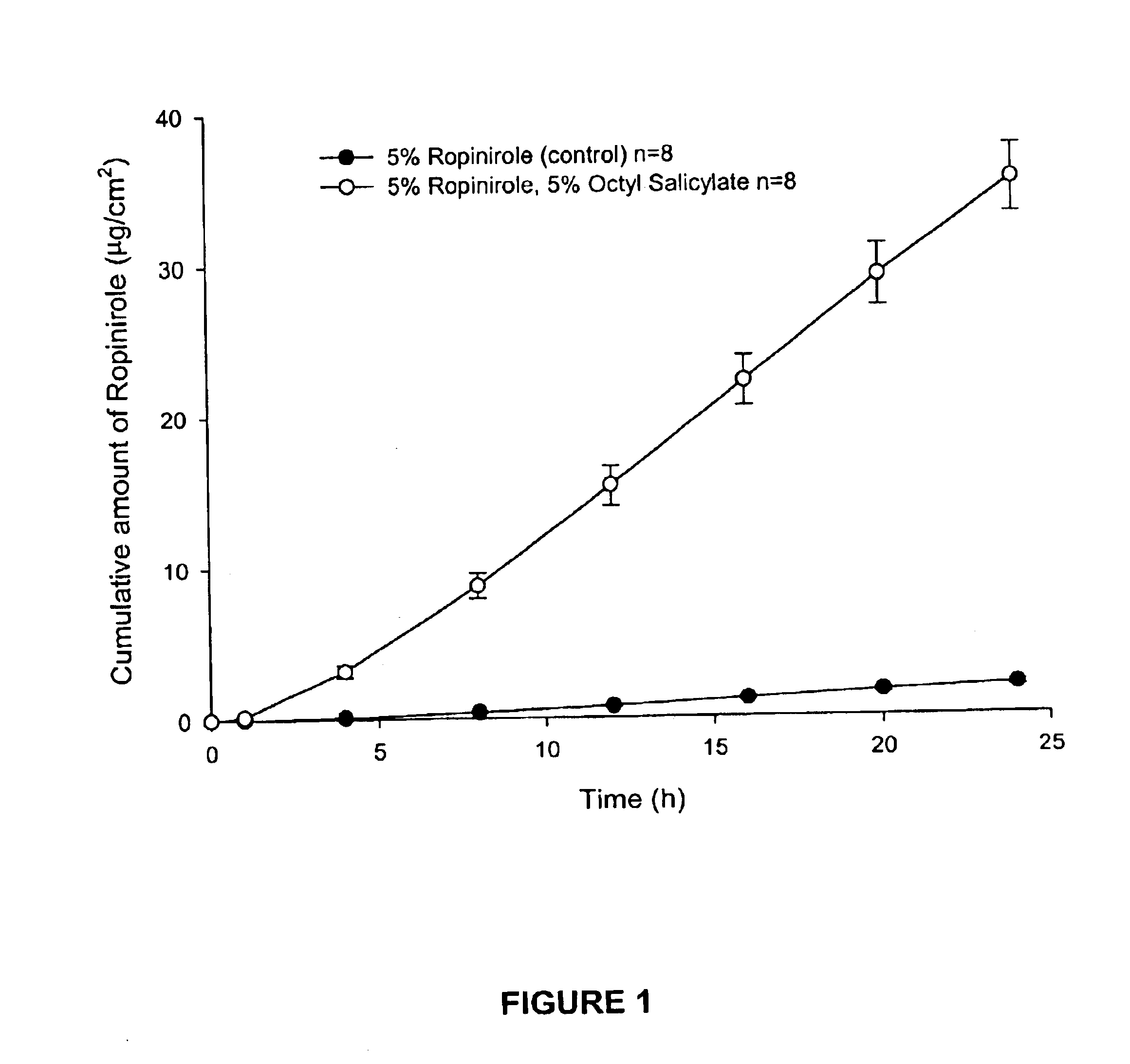
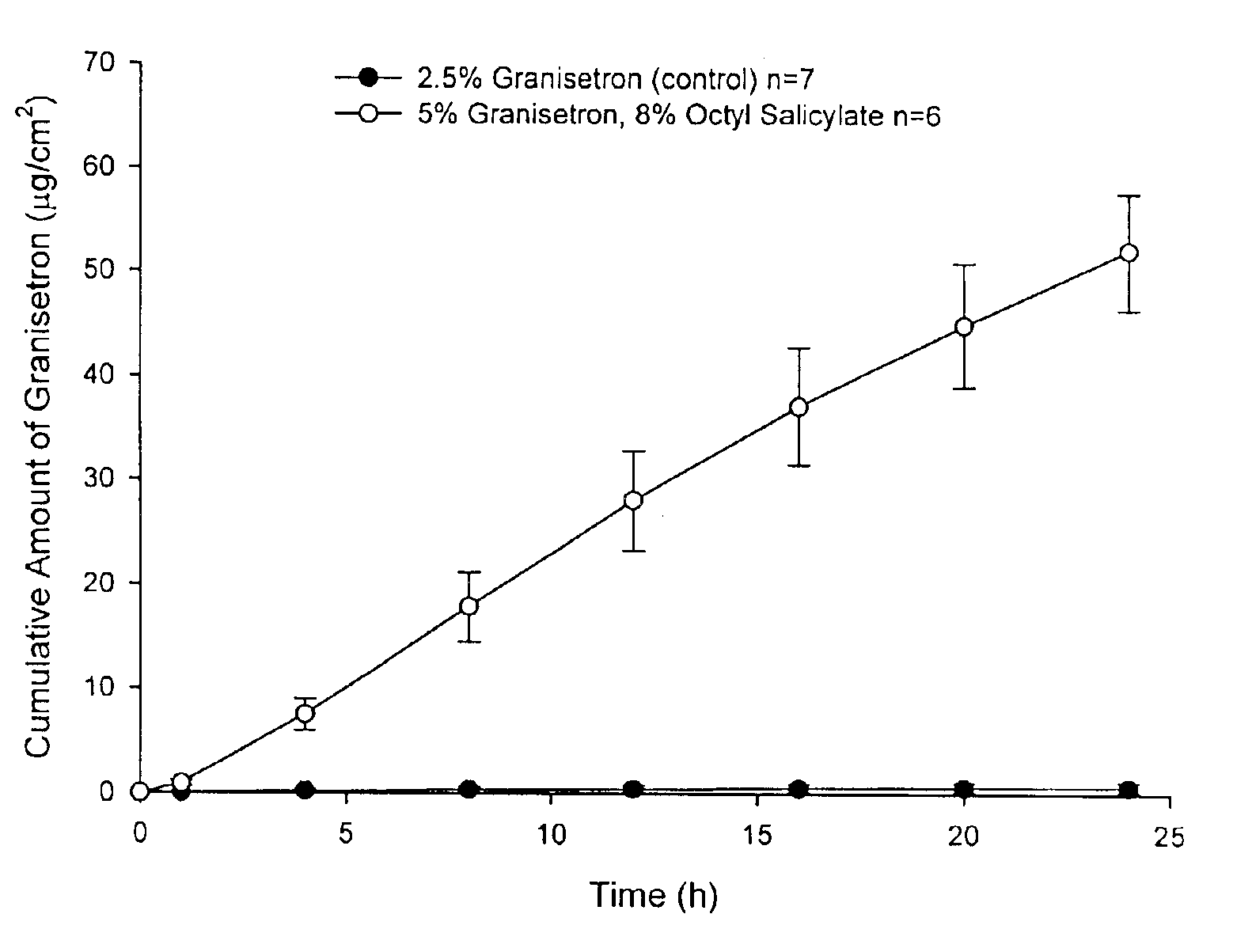
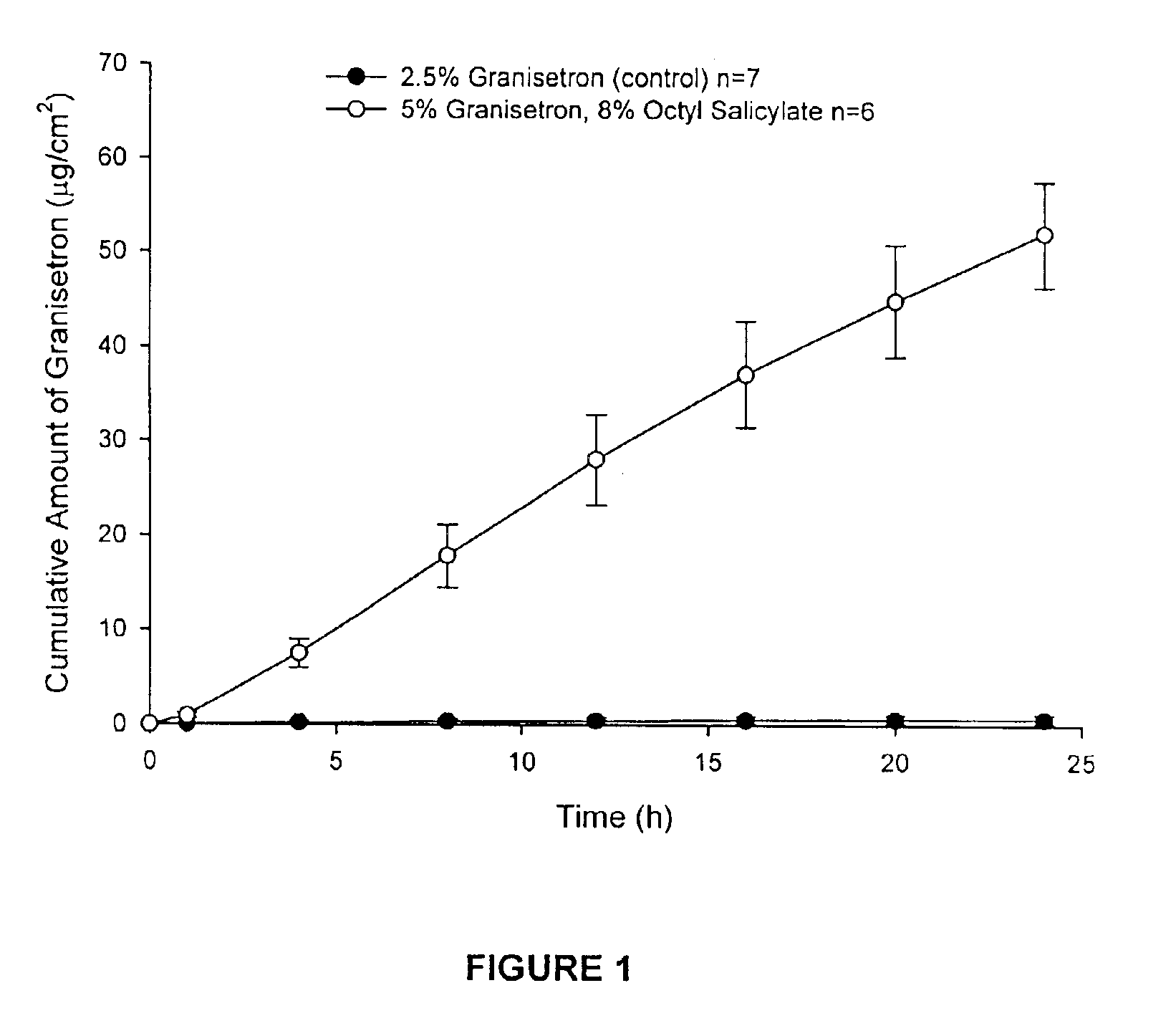
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antiParkinson agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antiParkinson agent to an animal which comprises applying an effective amount of the antiParkinson agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
Transdermal delivery of hormones
InactiveUS6923983B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyHormones regulation
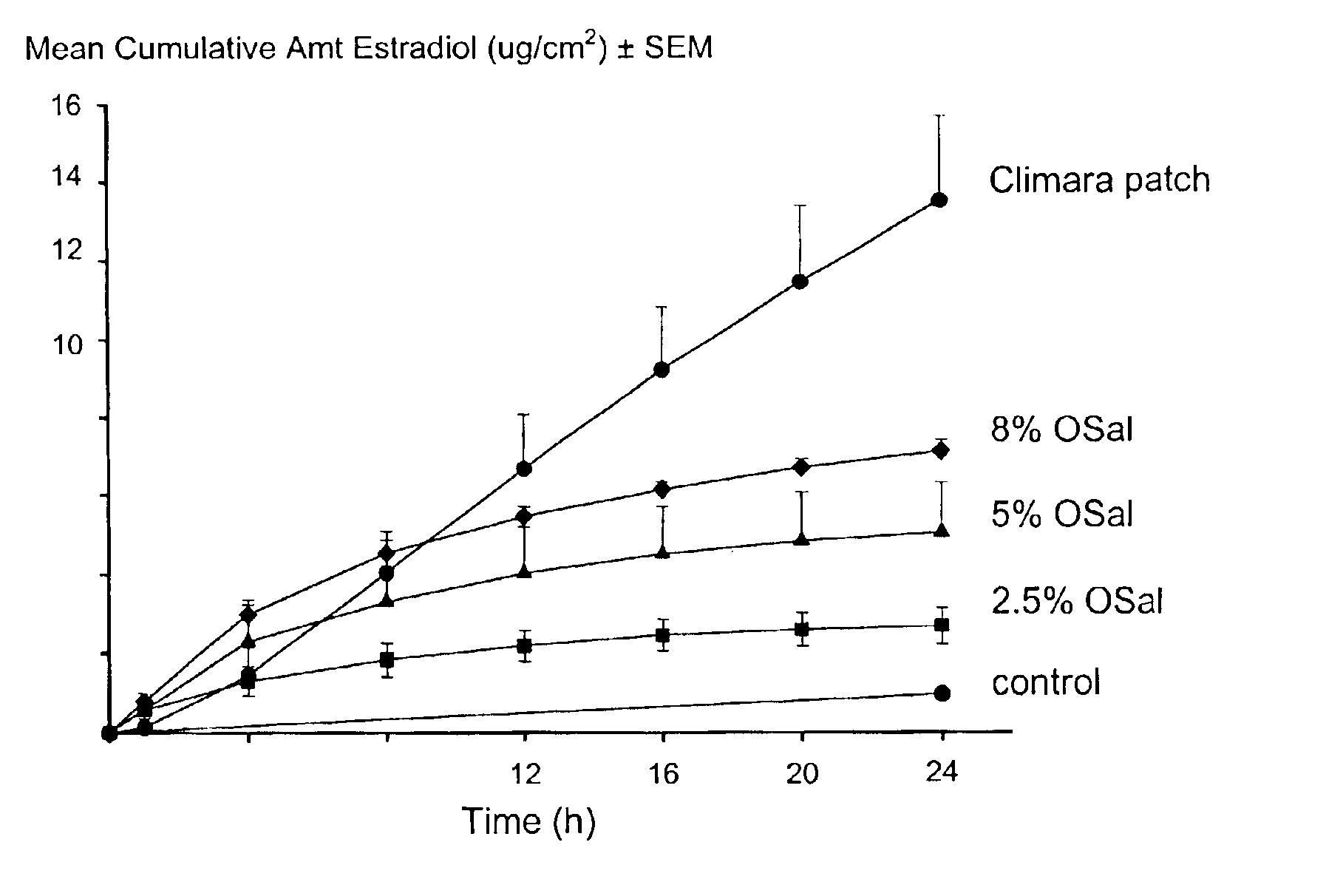
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of a hormone; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting hormone to an animal which comprises applying an effective amount of the hormone in the form of the drug delivery system of the present invention
Owner:ACRUX DDS
Anti-infection augmentation foamable compositions and kit and uses thereof
Anti-infective foamable composition and kits include a foamable carrier; a therapeutically safe and effective concentration of an anti-infective agent; an augmenting agent selected from the group consisting of a keratolytic agent and a skin penetration enhancer; and a propellant. The composition is housed in a container and upon release is expandable to form a breakable foam. The foamable carrier is selected to generate a foam of good or excellent quality in the presence of the augmenting agent and anti-infective agent. Methods for treating, alleviating or preventing a disorder of the skin, a body cavity or mucosal surface, wherein the disorder involves a fungal, bacterial or viral infection as one of its etiological factors, is described.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Topical and transdermal delivery system utilizing submicron oil spheres
The present invention relates to a delivery system which includes a bioactive drug or cosmetic substance presented in the form of submicron oil spheres alone, or drugs or cosmetic substances in a combination with the oil spheres in an aqueous suspension or emulsion. Optionally, a skin penetration enhancer may be included in such formulations. Such preparations achieve improved bioavailability and exert larger pharmacological effects than an equivalent dose of the drug or cosmetic formulated in conventional creams, lotions or oleaginous bases.
Owner:PHARMOS
Transdermal, alcohol-free, pharmaceutical compositions
An alcohol-free, transdermal drug delivery composition administered via a metered spray drug delivery device is described herein. The non-occlusive transdermal drug delivery composition includes a therapeutically effective amount of at least one physiologically active agent or prodrug thereof, an effective amount of at least one dermal penetration enhancer; and at least one non-volatile liquid. The transdermal drug delivery composition is administered to a dermal or mucosal surface of an animal needing the same using a metered spray device capable of delivering a fine spray of substantially uniform particle size to minimize the required drying time therefor.
Owner:LUMARA HEALTH IP
Transdermal delivery of analgesics
InactiveUS6916486B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyAnalgesic agents
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an analgesic; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting analgesic to an animal which comprises applying an effective amount of the analgesic in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
Transdermal delivery rate control using amorphous pharmaceutical compositions
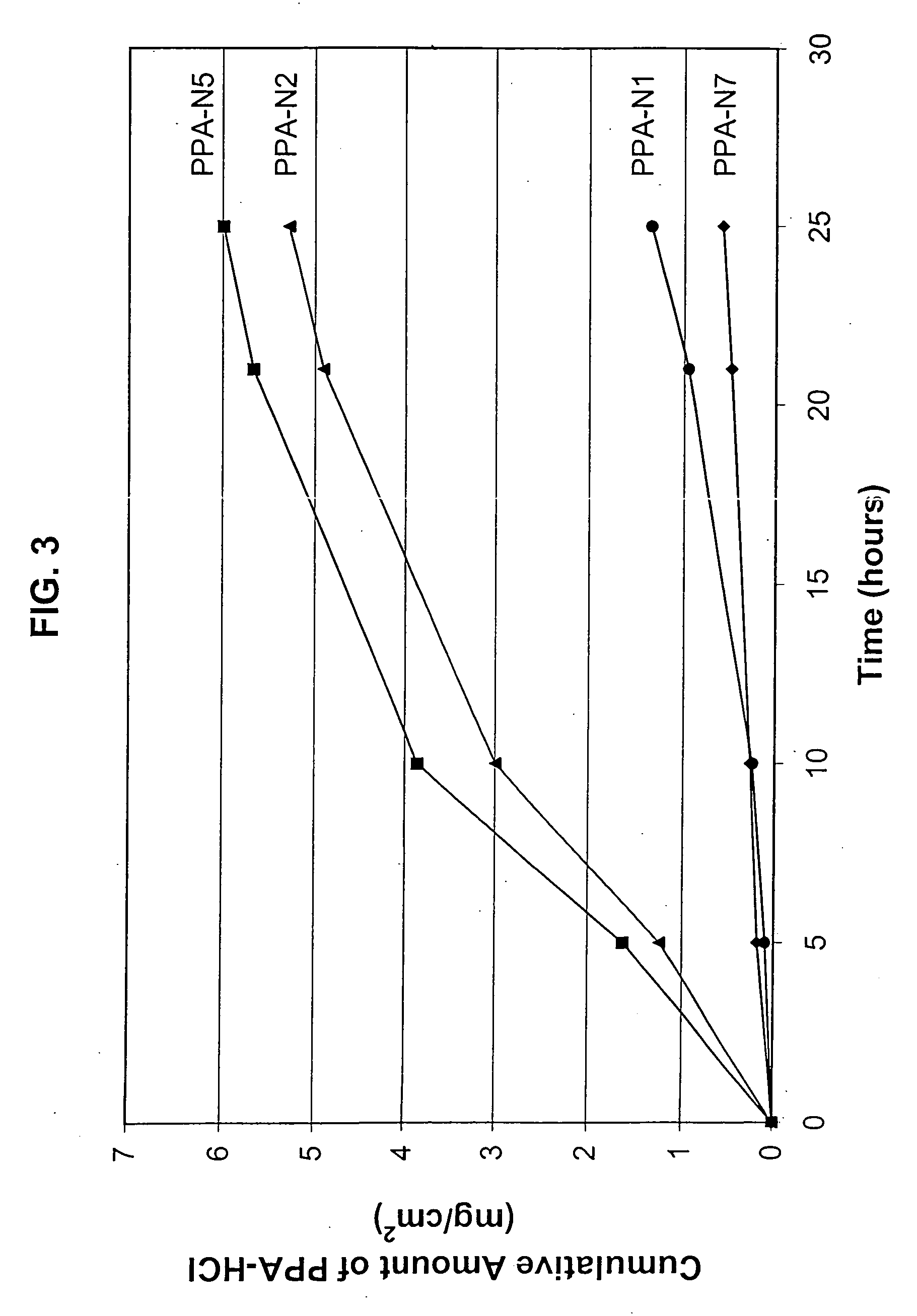
InactiveUS20050175680A1High propensity toward skin irritationDecrease in percutaneous absorption efficiencyOrganic active ingredientsNervous disorderMedicineActive agent
A pharmaceutical composition for transdermal delivery comprising one or more physiologically active agents; one or more dermal penetration enhancers; and a volatile pharmaceutically acceptable carrier comprising a volatile solvent; and wherein the physiologically active agent and dermal penetration enhancer form an amorphous deposit upon evaporation of the volatile carrier, said amorphous deposit forming a reservoir within the stratum corneum; and (A) wherein the composition has a release rate profile of physiologically active agent so as to provide a ratio of the maximum concentration (Cmax) to the average concentration (Cavg) for the physiologically active agent over the dosage interval within the range of 1 to 10.
Owner:ACRUX DDS
Sterile polymerized covering dressing for wound surface
Owner:JIANGSU HUAYI CELL & TISSUE ENG CO LTD
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
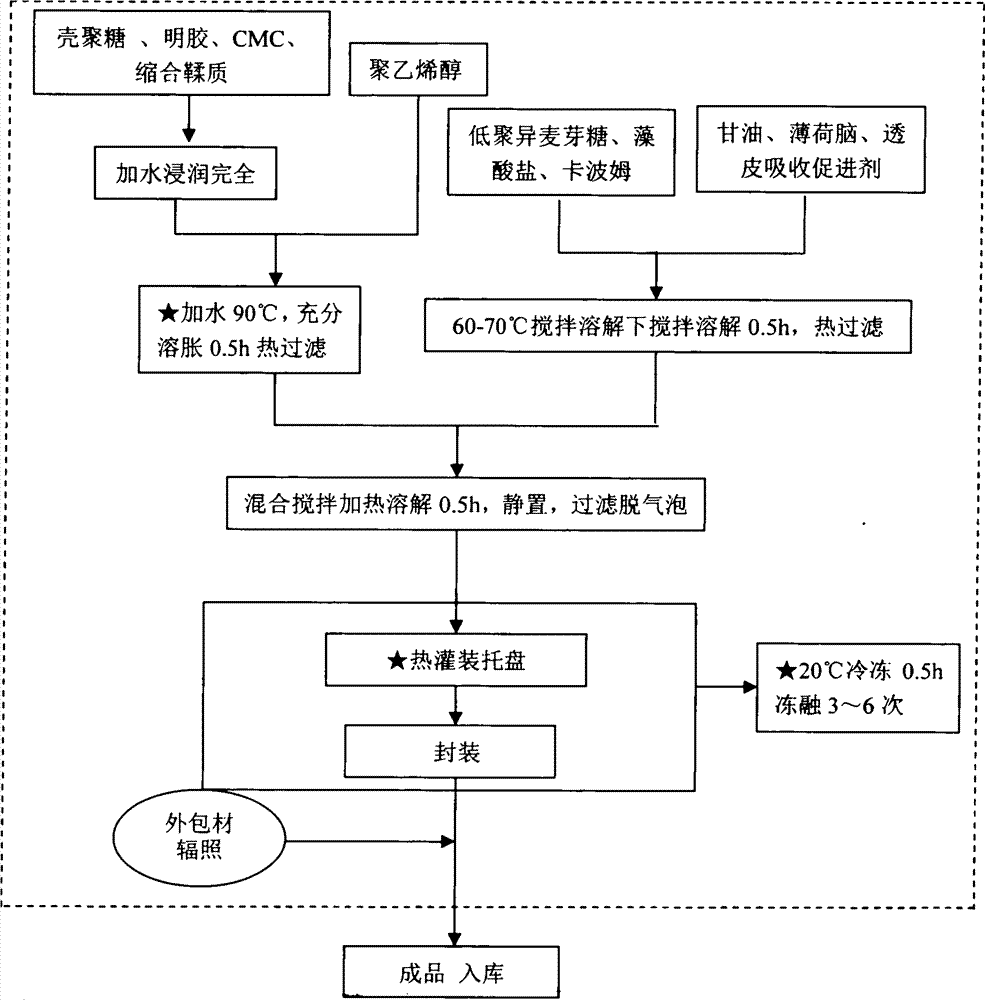
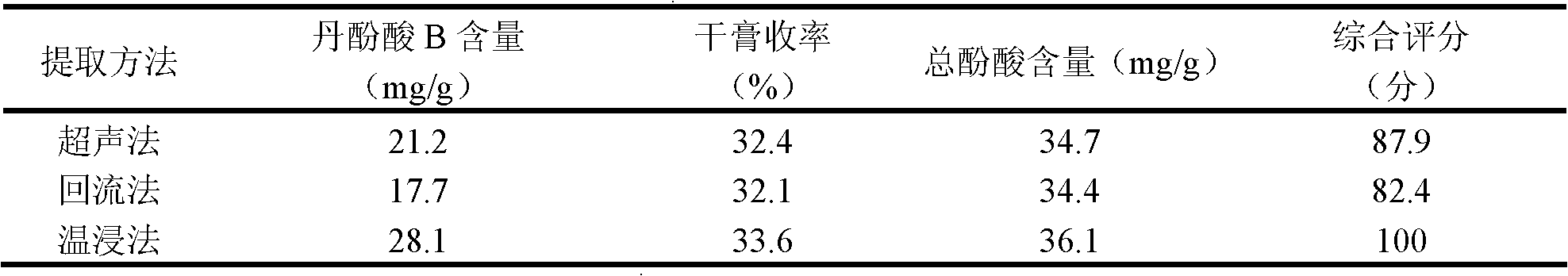
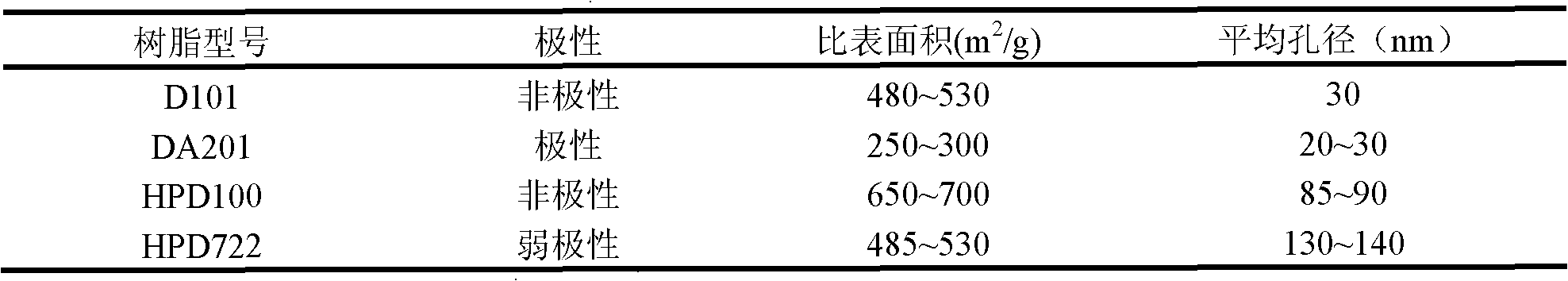
Red sage root mask and preparation method thereof
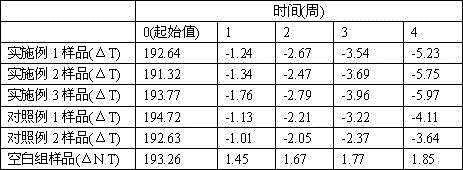
ActiveCN102198062AWith promoting blood circulation and removing blood stasisAnti-inflammatoryCosmetic preparationsToilet preparationsAntioxidantBULK ACTIVE INGREDIENT
The invention provides a red sage root mask. The red sage root mask is prepared by adding a mask matrix and water into total salvianolic acid utilized as an active ingredient, wherein, the matrix comprises the following components in parts by weight: 4.9-14.7 parts of the total salvianolic acid, 22.4-67.2 parts of a film-forming material, 7.8-23.4 parts of a thickening agent, 4.9-14.7 parts of a skin penetration enhancer, 0.25-0.75 parts of an antioxidant and 1-3 parts of a preservative. The invention further provides a preparation method of the red sage root mask. The red sage root mask is a peel-off mask, wherein, the total salvianolic acid has an effect on promoting body surface microcirculation, thus smoothening the body surface blood circulation, accelerating the blood flow velocity, fully moisturizing and nourishing skin and hair and ensuring the stability of water-soluble components of red sage root. Therefore, the red sage root mask can be widely used in health and skin care.
Owner:成都中医大华神医疗美容医院有限公司
Transdermal delivery of antiemetics
InactiveUS6916487B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyMedicine
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antiemetic; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antiemetic thereof to an animal which comprises applying an effective amount of the antiemetic in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
Pharmaceutical gel formulations
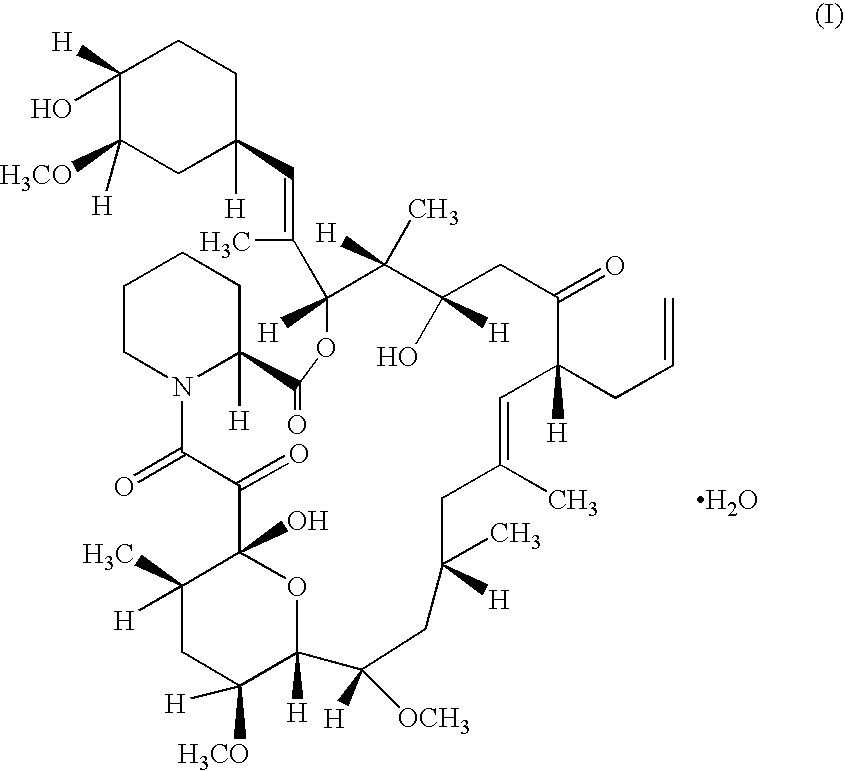
A pharmaceutical gel composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) one or more gel forming agents; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Pharmaceutical ointment formulations
A pharmaceutical ointment composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) an ointment base; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant or a pharmaceutically acceptable salt or ester thereof through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Topical stabilized prostaglandin E compound dosage forms
InactiveUS6841574B2Improve sexual dysfunctionBiocideCosmetic preparationsProstaglandin EProstaglandin PGE
Compounds of prostaglandin E group (PGE compounds) are stabilized as non-aqueous compositions that include the compound together with a bulking agent that can be a non-aqueous liquid or a solid in a sheet, film or powder form. The composition can optionally include a skin penetration enhancer. A non-aqueous, solid dosage form comprises a PGE compound substantially uniformly distributed in a carrier sheet or film.
Owner:NEXMED HLDG INC
Transdermal delivery of antianxiety agents
InactiveUS6964777B2Less complicated manufactureImprove dose uniformityCosmetic preparationsNervous disorderWhole bodyAntianxiety Agent
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antianxiety agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antianxiety agent to an animal which comprises applying an effective amount of the antianxiety agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
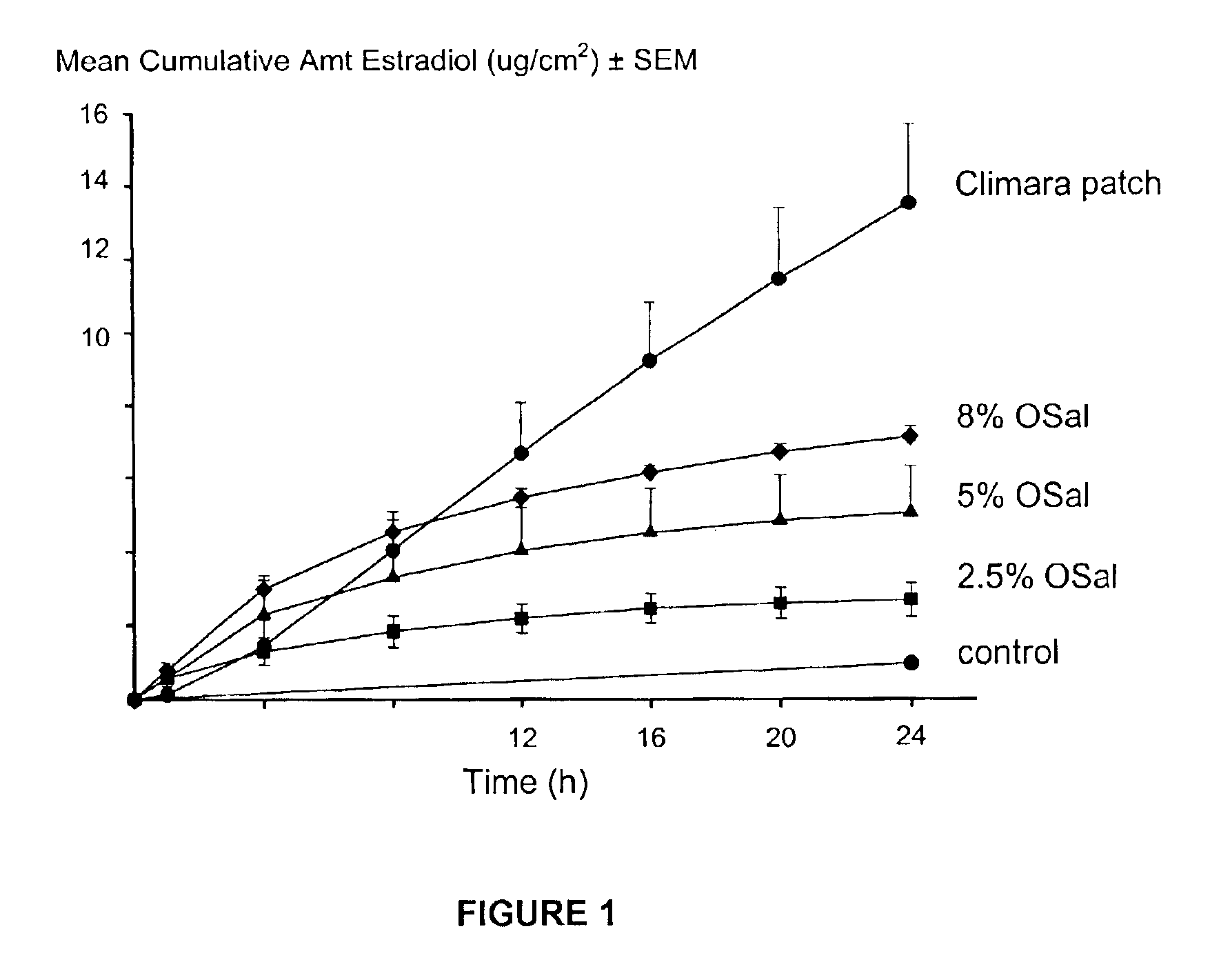
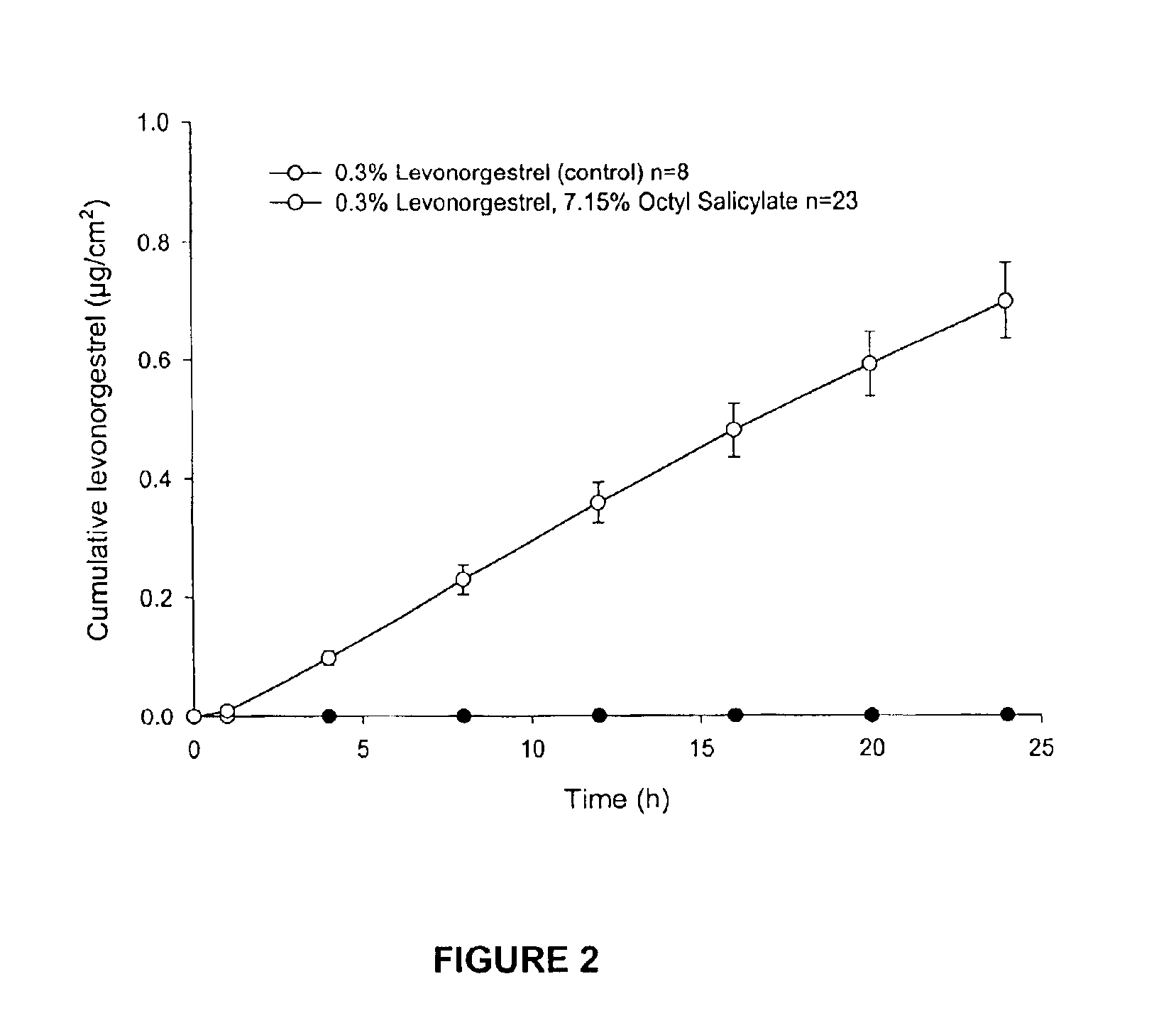
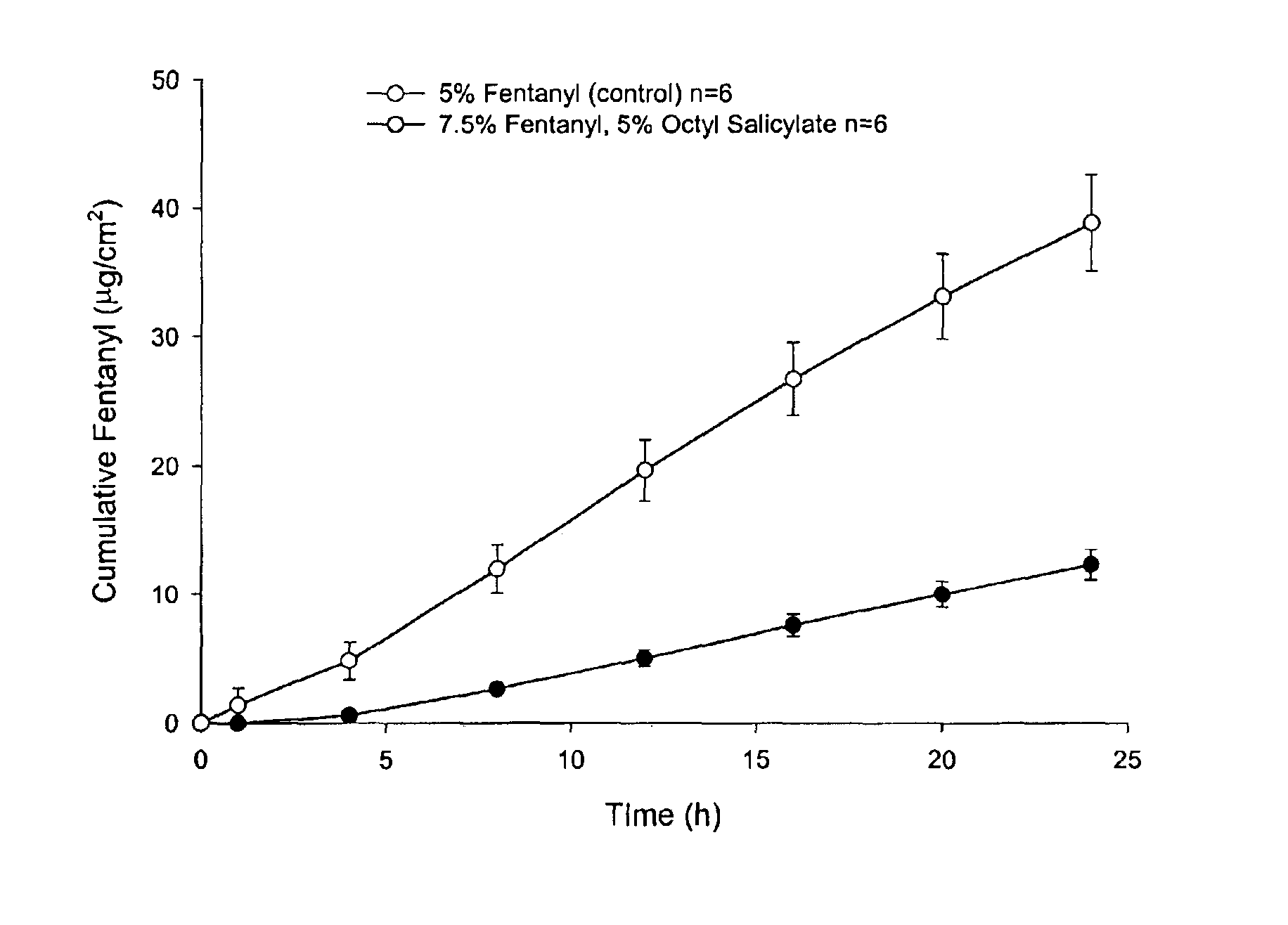
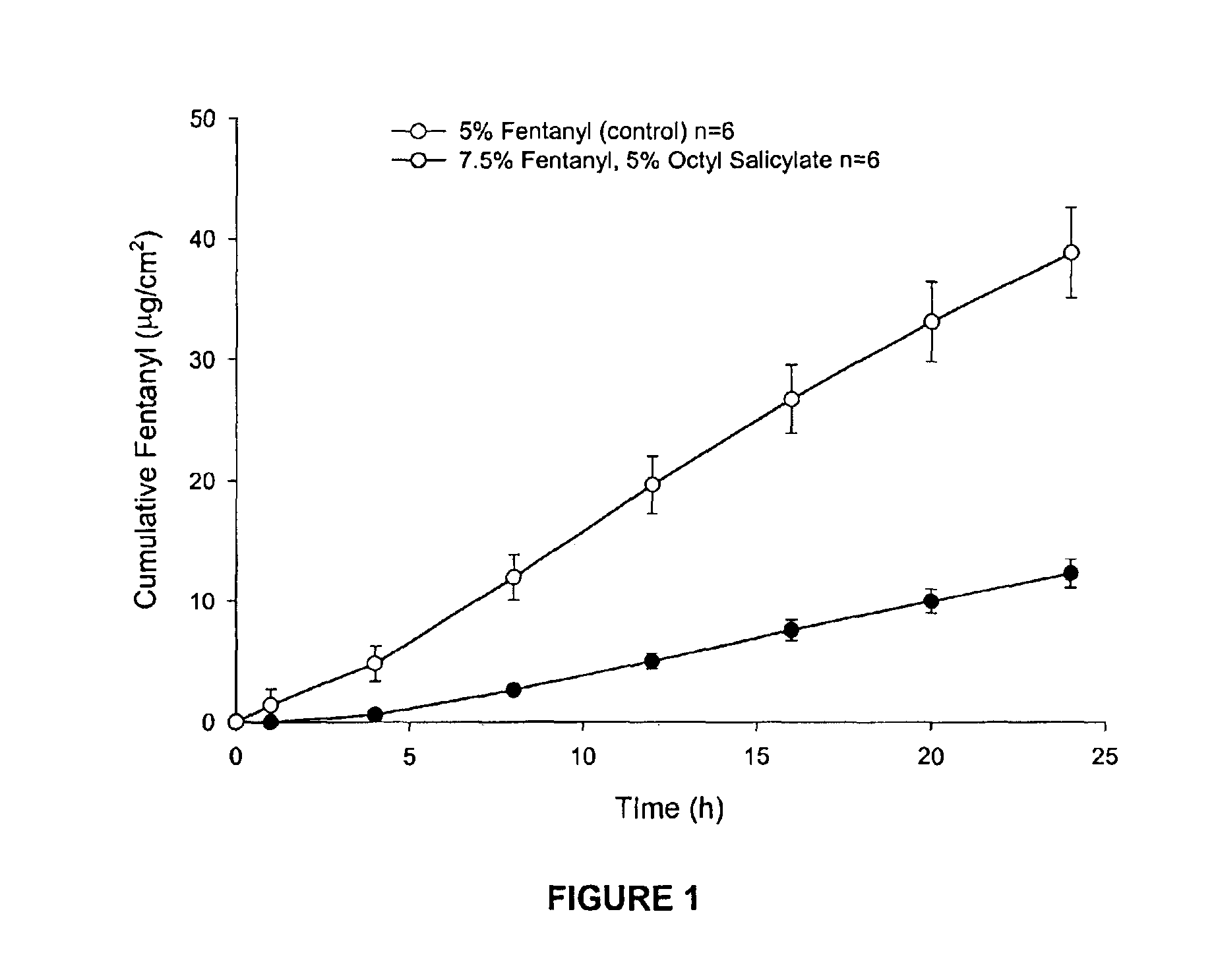
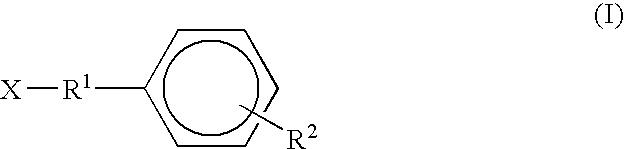
Dermal penetration enhancers and drug delivery systems involving same
InactiveUS20070071803A1Low toxicityComplicated to manufactureOrganic active ingredientsNervous disorderMedicineActive agent
The invention relates to a method for treatment or prophylaxis of a disease or condition in an animal comprising administering to a mucosal membrane of said animal in need of such treatment a therapeutically effective amount of a drug delivery system comprising at least one physiologically active agent or prodrug thereof and at least one penetration enhancer selected from safe ester sunscreens.
Owner:ACRUX DDS
Anti-infection augmentation foamable compositions and kit and uses thereof
Anti-infective foamable composition and kits include a foamable carrier; a therapeutically safe and effective concentration of an anti-infective agent; an augmenting agent selected from the group consisting of a keratolytic agent and a skin penetration enhancer; and a propellant. The composition is housed in a container and upon release is expandable to form a breakable foam. The foamable carrier is selected to generate a foam of good or excellent quality in the presence of the augmenting agent and anti-infective agent. Methods for treating, alleviating or preventing a disorder of the skin, a body cavity or mucosal surface, wherein the disorder involves a fungal, bacterial or viral infection as one of its etiological factors, is described.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Antiperspirant and deodorant products and methods for their use
InactiveUS20020037264A1Improve efficiencySlow sedimentationCosmetic preparationsBiocidePhysiologyPerspiration
A new method for the inhibition of human sweating and body malodour is described. An agent capable of blocking calcium channels in the secretory coil cells of human sweat glands is topically applied; on reaching the secretory coil cells, the action of this agent results in reduced sweat production. Preferred agents are natural plant extracts. The transport of the agent to the secretory coil is preferably enhanced in some way, for example by iontophoresis or the use of a skin penetration enhancer. Antiperspirant products comprising a calcium channel blocking agent and a pore-blocking antiperspirant active are also claimed.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Composition for external use preparation with improved transdermal permeability
InactiveUS20160058723A1High skin permeationNo skin irritationOrganic active ingredientsCosmetic preparationsSkin permeabilityIrritation
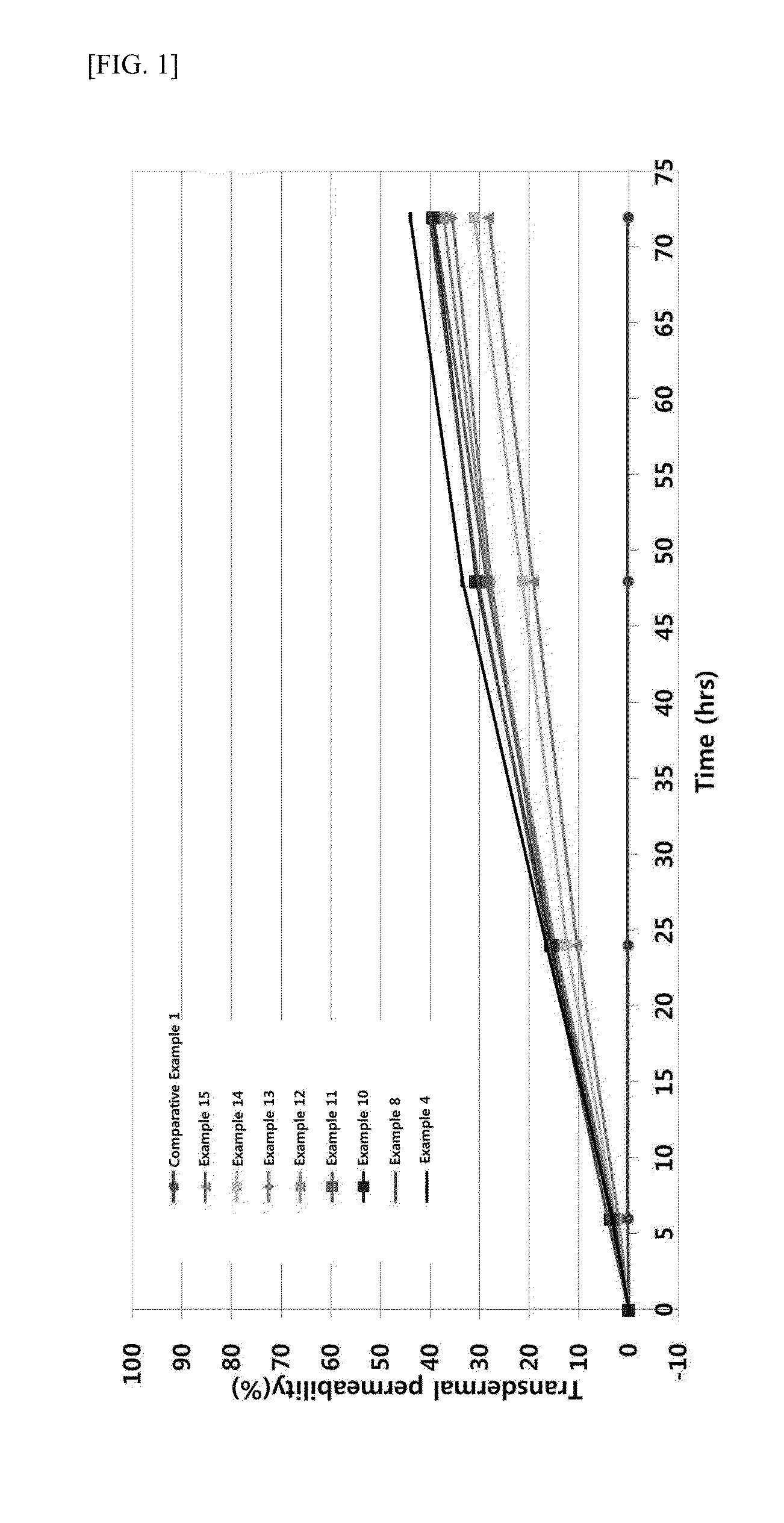
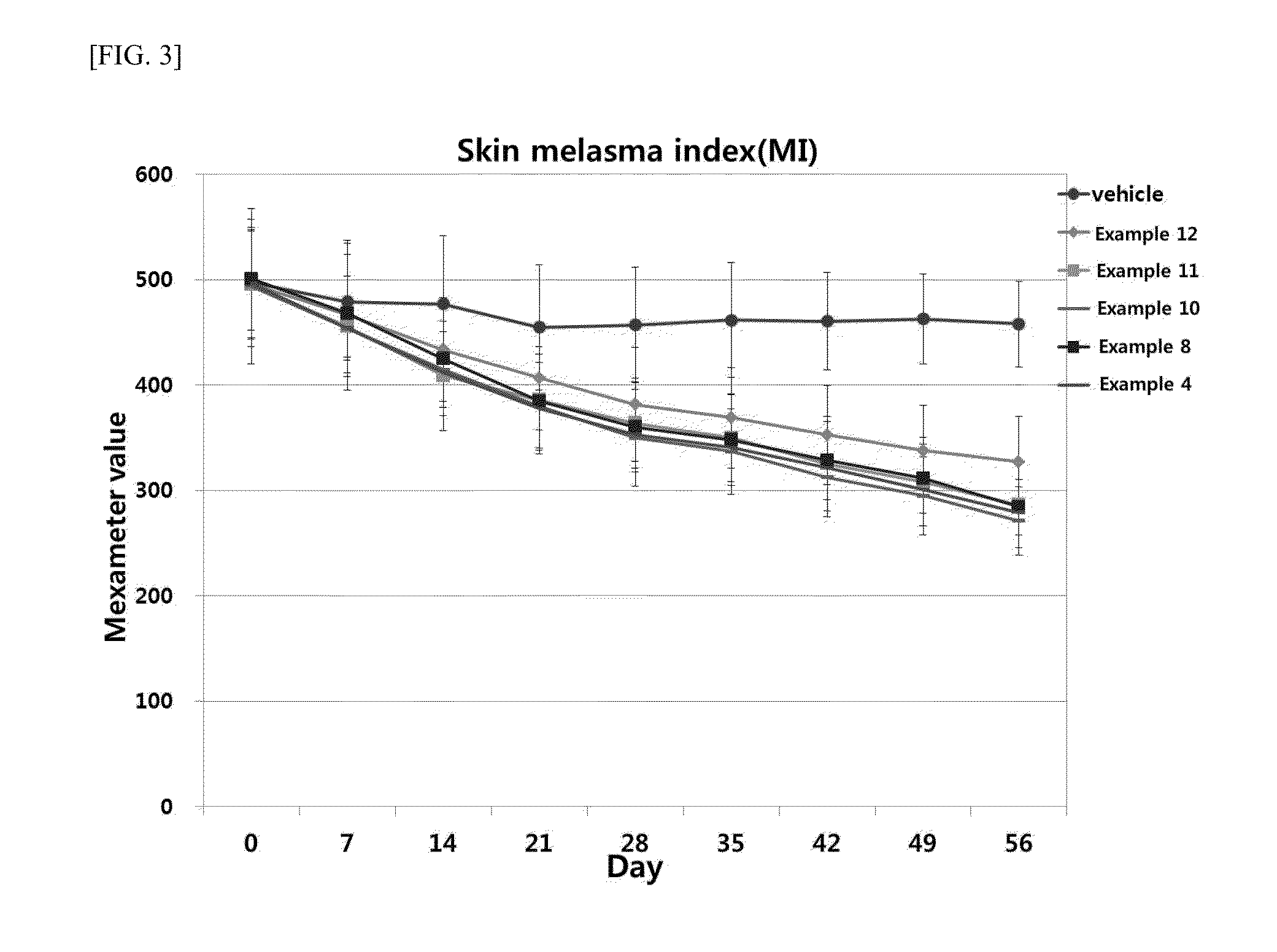
Provided is a skin external composition, which includes tranexamic acid or a salt thereof and a skin penetration enhancer, thereby showing remarkably increased skin permeability and improved sense of use, skin irritation, and storage stability.
Owner:HYUNDAI PHARMA
Pharmaceutical cream formulations
InactiveUS20050249757A1BiocidePharmaceutical delivery mechanismMacrolide resistanceMacrocyclic lactone
A pharmaceutical cream composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) one or more cream forming agents; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Compositions and Methods for Treating Acne
ActiveUS20100021571A1Reduce outflowDecreases redness and swellingCosmetic preparationsBiocideSunscreen agentsVasoconstrictor Agents
This invention provides anti-acne kits that are useful for treating acne, especially severe cases of acne. The anti-acne kits include a vasoconstrictor and an anti-acne agent, and optionally one or more of a a skin lightening therapeutic, a sealing layer, a skin cleanser, an astringent, a skin penetration enhancer, a sunscreen, and nutritional supplements that promote healing of acne lesions. This invention also provides methods for treating acne using a vasoconstrictor in conjunction with an anti-acne agent.
Owner:REVANCE THERAPEUTICS INC
Antiperspirant and deodorant products and methods for their use
InactiveUS6632422B2Improve efficiencySlow sedimentationBiocideCosmetic preparationsPhysiologyPerspiration
A new method for the inhibition of human sweating and body malodour is described. An agent capable of blocking calcium channels in the secretory coil cells of human sweat glands is topically applied; on reaching the secretory coil cells, the action of this agent results in reduced sweat production. Preferred agents are natural plant extracts. The transport of the agent to the secretory coil is preferably enhanced in some way, for example by iontophoresis or the use of a skin penetration enhancer. Antiperspirant products comprising a calcium channel blocking agent and a pore-blocking antiperspirant active are also claimed.< / PTEXT>
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Photodynamic Therapy For The Treatment Of Hyperactive Sebaceous Gland Disorders Using Topically Applied Hydrophobic Green Porphyrins
InactiveUS20080096857A1Small sizeReduced activityBiocideHydroxy compound active ingredientsDiseasePresent method
The present method involves the photodynamic treatment of hyperactive gland disorders. The method involves the topical administration of a photosensitizer composition comprising hydrophobic and / or lipophilic green porphyrins such as lemuteporfin, polyethylene glycol and skin penetration enhancers such as oleyl alcohol and TRANSCUTOL™ to affected skin and subsequent exposure of that skin to energy of a wavelength capable of activating the photosensitizer.
Owner:VALEANT PHARMA INT
Formula and preparing method of freeze-dried excipient preparation
The invention relates to a formula and a preparing method of a freeze-dried excipient preparation. The formula is new and simplified. By controlling the ratio of an adhesive and the ratio of active components in the freeze-dried excipient preparation, the freeze-dried excipient preparation can be free of a skeleton supporting agent system and only contains the adhesive and the active components. The skeleton supporting agent comprises, but not limited to, sugar, sugar alcohol, amino acids having 2-12 carbon atoms, inorganic salts, and the like. Based on this, an antioxidant, a corrigent, essence, a skin penetration enhancer, a pH modifier, and other auxiliary materials can be added into the preparation. By avoiding the use of the skeleton supporting agent, technical effects of reducing the moisture adsorption risk, simplifying the preparing process, reducing the cost and increasing the drug loading capacity per unit are achieved.
Owner:常州柚盾实业投资有限公司
Transdermal delivery rate control using amorphous pharmaceutical compositions
InactiveUS20100166674A1Increase and controlGood water solubilityBiocideNervous disorderMedicineActive agent
A pharmaceutical composition for transdermal delivery comprisingone or more physiologically active agents;one or more dermal penetration enhancers; anda volatile pharmaceutically acceptable carrier comprising a volatile solvent;and wherein the physiologically active agent and dermal penetration enhancer form an amorphous deposit upon evaporation of the volatile carrier, said amorphous deposit forming a reservoir within the stratum corneum; and(A) wherein the composition has a release rate profile of physiologically active agent so as to provide a ratio of the maximum concentration (Cmax) to the average concentration (Cavg) for the physiologically active agent over the dosage interval within the range of 1 to 10.
Owner:ACRUX DDS
Anti-parasitic ivermectin transdermal solution used for livestock and preparation method thereof
InactiveCN101579309AFully reflect the advantages of preparationsPromote percutaneous absorptionOrganic active ingredientsPharmaceutical delivery mechanismSide effectThird generation
The invention relates to an anti-parasitic ivermectin transdermal solution used for livestock and a preparation method thereof. The anti-parasitic ivermectin transdermal solution comprises the following components respectively according to parts by volume: 5 to 15 parts of ethyl acetate, 20 to 30 parts of dimethyl sulfoxide, 2 to 6 parts of azone, 1 to 3 parts of isopropanol and 50 to 70 parts of propanediol; and the content of ivermectin is 0.3g to 1g in a solution of 100ml, and the finished product is prepared by the processes of dissolving, mixing, filtering and filling. The azone related in the prescription of the invention is a novel skin penetration enhancer, can increase the transdermal absorption of the skin to various medicines of different types, effectively avoids the pass effect of hepar, avoids peak and valley phenomena in the absorption process of the medicine, reduces the toxic or side effect caused by the correlative dosage in the medicine, prolongs the effective effect time, changes the administration area, effectively regulates the administration dosage and reduces differences among individuals. The invention has the characteristic of simple, convenient and fast use, and is more suitable for the anti-parasitic prevention and treatment of a pasturing area compared with other preparation forms.
Owner:TIANJIN BIJIA PHARMA CO LTD
Composition for transdermal administration of non-steroidal Anti-flammatory drug
InactiveUS20120157536A1Good fluxImprove complianceOrganic active ingredientsBiocideAnalgesics drugsNon steroid anti inflammatory drug
This invention pertains to compositions and method for transdermal administration of non-steroidal anti-inflammatory and analgesic drugs (NSAID) for the treatment of inflammation and pain caused by conditions such as arthritis, degenerative joint disease, minor strains, pains, and contusions. This invention particularly relates to transdermal compositions comprising an NSAID, a bioadhesive graft copolymer, and a skin penetration enhancer, selected from pyrrolidone or its derivatives and dialkyl sulfoxides and combinations thereof.
Owner:POLYTHERAPEUTICS
Hydroxide-releasing agents as skin permeation enhancers
InactiveUS20060093659A1Increase ratingsImprove throughputOrganic active ingredientsPeptide/protein ingredientsActive agentIrritation
A method is provided for increasing the permeability of skin or mucosal tissue to topically or transdermally administered pharmacologically or cosmeceutically active agents. The method involves use of a specified amount of a hydroxide-releasing agent, the amount optimized to increase the flux of the active agent through a body surface while minimizing the likelihood of skin damage, irritation or sensitization. Topically applied formulations and drug delivery devices employing hydroxide-releasing agents as permeation enhancers are provided as well.
Owner:LUO ERIC C +2
Ivermectin transdermal liniment for animals
InactiveCN102440944APromote percutaneous absorptionReduce decomposition rateOrganic active ingredientsPharmaceutical delivery mechanismIrritationEthyl acetate
The invention discloses an ivermectin transdermal liniment for animals, which is prepared from medicinal liquor and ivermectin, wherein the medicinal liquor is prepared from the following components in percentage by volume: 20-40% of ethyl acetate, 2-5% of azone and 55-75% of propylene glycol; and the ratio of ivermectin to medicinal liquor is (0.5-1g):100mL. The novel transdermal accelerator selected in the formula can enhance the absorption of skin for medicines, does not have toxicity or irritation on the skin and body, does not have pharmacological action, and does not react with the medicines and other additives. The invention is simple to prepare and convenient to use, has high stability, and can achieve favorable anthelminthic effect.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Hydrogel mask with whitening effect and preparation method thereof
InactiveCN106236624AImprove function and effectGood whitening effectCosmetic preparationsToilet preparationsAdditive ingredientArginine
The invention relates to a hydrogel mask with a whitening effect and a preparation method thereof and belongs to the technical field of masks. The hydrogel mask contains a support layer and a gel layer. The gel layer contains the following ingredients (by weight): a cellulose derivative water-soluble polymer, an enzymatic bearberry leaf extract, a lemon extract, arginine, lavender essential oil, chamomile essential oil, an aloe extract, tartaric acid, a skin penetration enhancer, sodium chloride, vitamin E, carbomer, glycerin, essence, a preservative and water. By the use of natural medicinal materials and through enzymolysis of bearberry leaf, active materials are decomposed into micromolecules. Thus, effects are enhanced. The hydrogel mask has advantages of good whitening effect and skin maintenance.
Owner:刘德金
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com