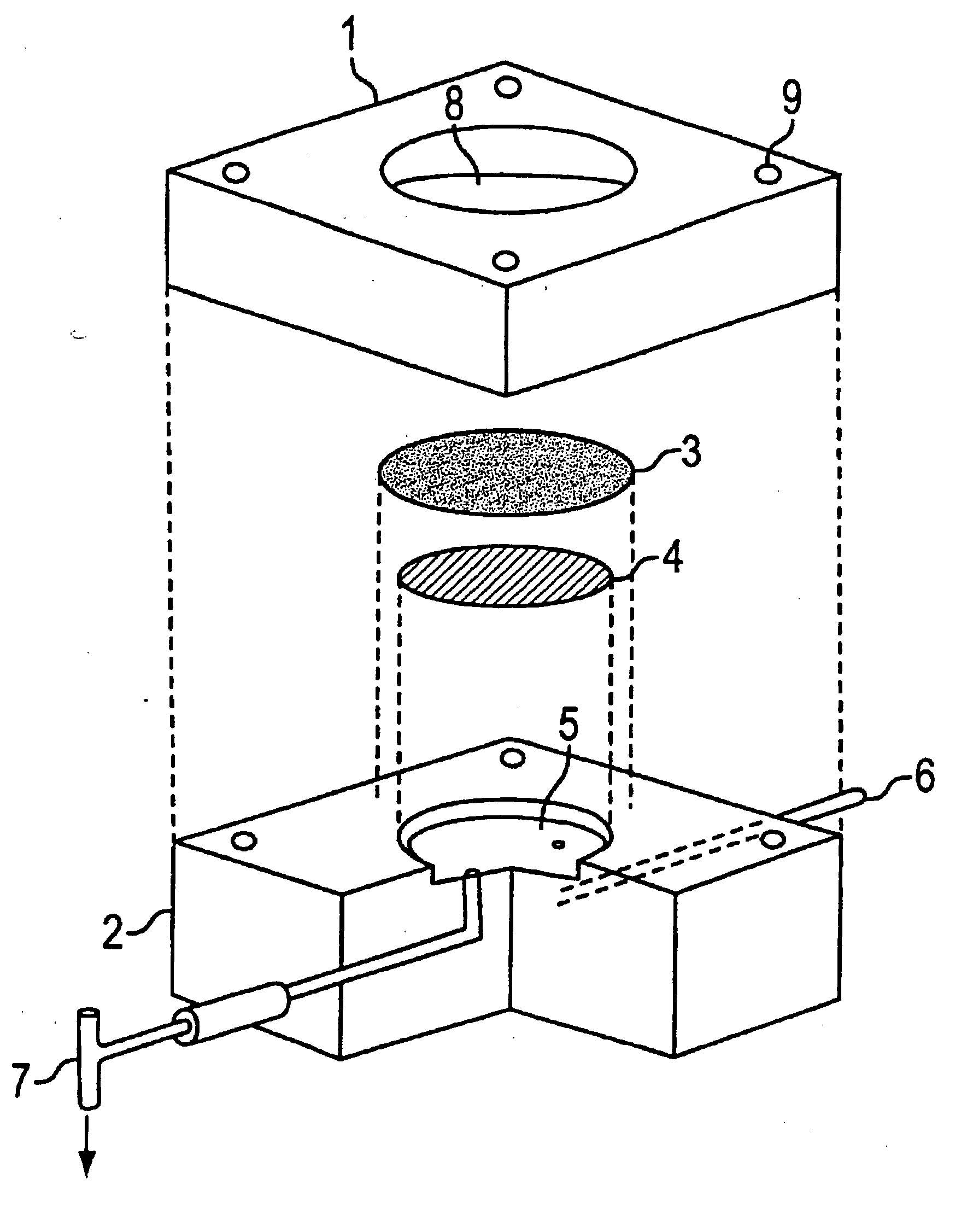
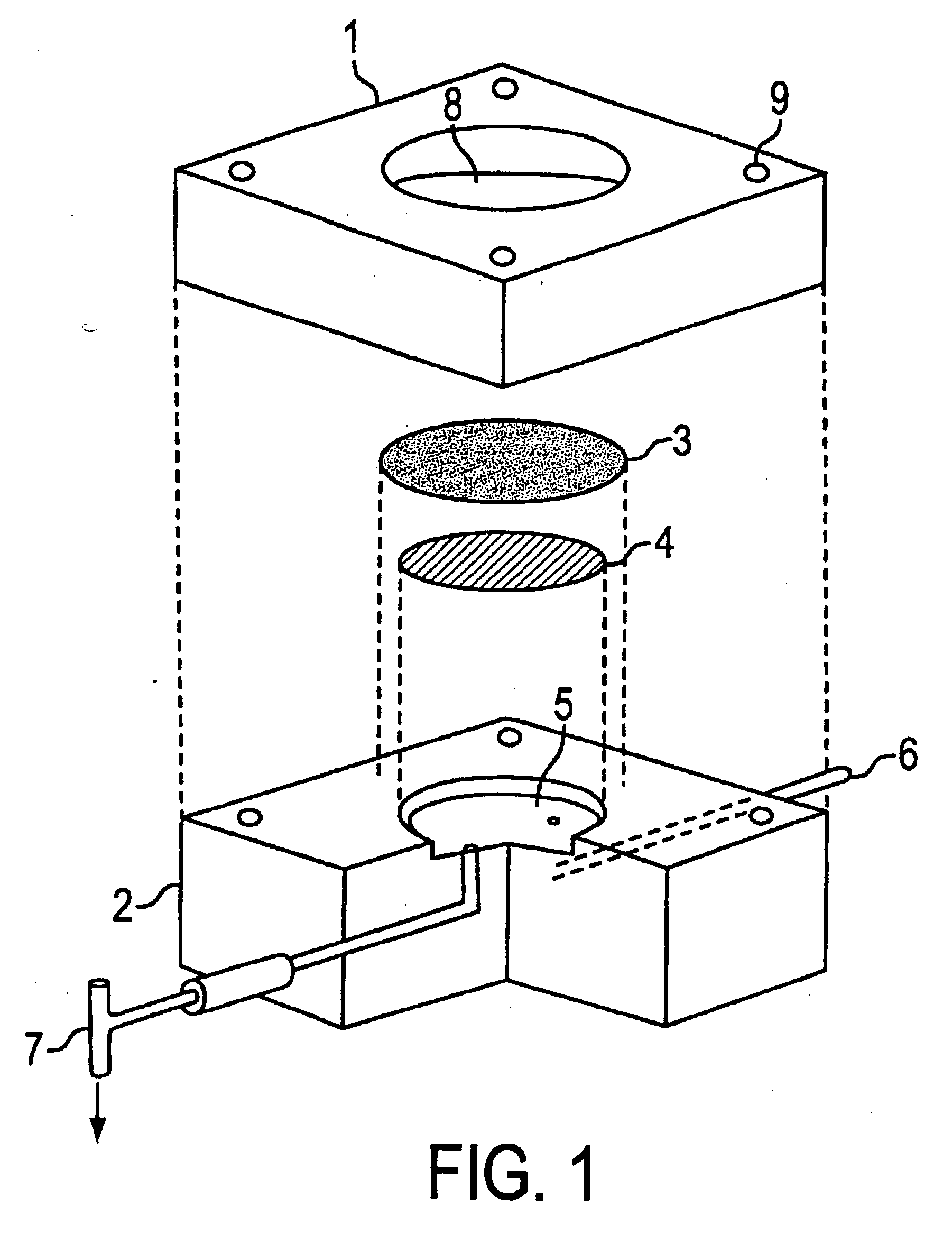
Dermal penetration enhancers and drug delivery systems involving same
a technology of penetration enhancer and drug delivery system, which is applied in the direction of antivirals, organic active ingredients, and delivery of aerosols, etc., can solve the problems of lack of systemic drug efficacy, general inapplicability of systemic drugs to this type of administration, and inability to effectively treat the effects of systemic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
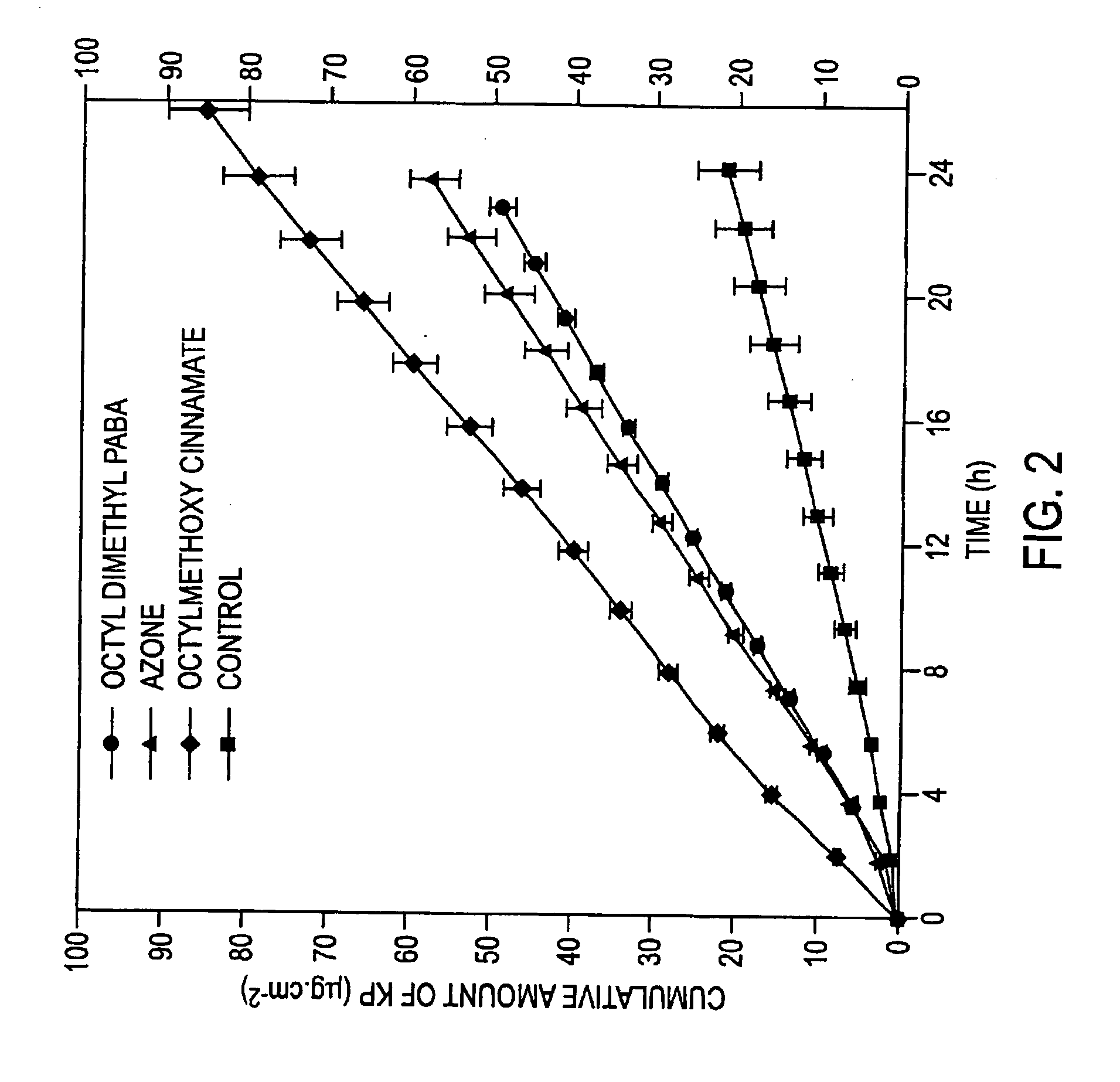
[0188] The in vitro diffusion cell method described above was used to compare the penetration of 400 microL of 2% w / v ketoprofen in 70% v / v aqueous ethanol applied to the shed snake skin following the application of 400 microL of the different dermal penetration enhancers in a 2% v / v solution in 70% ethanol, 2 hours prior to the application of the ketoprofen. The control experiment involved application of 400 microL of 70% aqueous ethanol alone for 2 hours, followed by application of 400 microL of the 2% ketoprofen solution. Samples were assayed according to the method described previously. The detection wavelength was 255 nm and the mobile phase consisted of acetonitrile:water (55:45) made to pH 3.0 with orthophosphoric acid (BDH, Australia). Table 1 shows the mean flux of ketoprofen across the snake skin over 24 hours as determined by the linear regression of the cumulative amount of ketoprofen crossing the skin versus time (Units=microg / cm2·h). FIG. 2 shows the representative mea...
example 2
[0190] The in vitro diffusion cell method described above was used to compare the penetration of 30 microL of the commercial formulation Indospray™ (Rhone-Poulenc Rorer, Australia), which is a 1.0% w / w solution of indomethacin in 95% v / v ethanol when applied to the snake skin. 10 microL of increasing concentrations of Octyl dimethyl PABA in absolute ethanol were applied 30 mins prior to the application of the indomethacin formulation. The control experiment involved application of 10 microL of absolute ethanol alone 30 mins prior to the application of the indomethacin formulation. Samples were assayed according to the method described previously. The detection wavelength was 254 nm and the mobile phase consisted of acetonitrile:water (55% v / v:45% v / v) made to pH 3.0 with orthophosphoric acid. Table 2 shows the mean flux of indomethacin across the snake skin over 24 hours.
TABLE 2Mean flux + / −Enhancerstd errorp valueconc.(microg / relative toEnhancementEnhancer type(% v / v)cm2 · h)cont...
example 3
[0192] The same protocol as Example 1 was repeated, except the dermal penetration enhancers were included in the ketoprofen formulation, such that 400 microL of 2% w / v ketoprofen and 2% v / v dermal penetration enhancer in 70% v / v aqueous ethanol was applied to the skin from the start of the diffusion experiment.
[0193] Table 3 shows the mean flux of ketoprofen across the snake skin over 24 hours. FIG. 3 shows the representative mean cumulative amount versus time plots for ketoprofen.
TABLE 3Mean flux + / −std errorp value(microg / relative toEnhancementEnhancer typecm2 · h)controlratioControl - no0.78 ± 0.07——enhancer, n = 10Azone, n = 22.84 ± 0.113.6Octyl dimethyl2.71 ± 0.183.5PABA, n = 2Octylmethoxy2.08 ± 0.390.04132.7cinnamate, n = 2Octyl salicylate, n = 461.68 ± 14.8979.1
[0194] These results demonstrate the ability of the dermal penetration enhancers to be applied together with the physiologically active ingredient / s within the same formulation to achieve their percutaneous absorpti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| octanol-water partition coefficients | aaaaa | aaaaa |
| octanol-water partition coefficient | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com