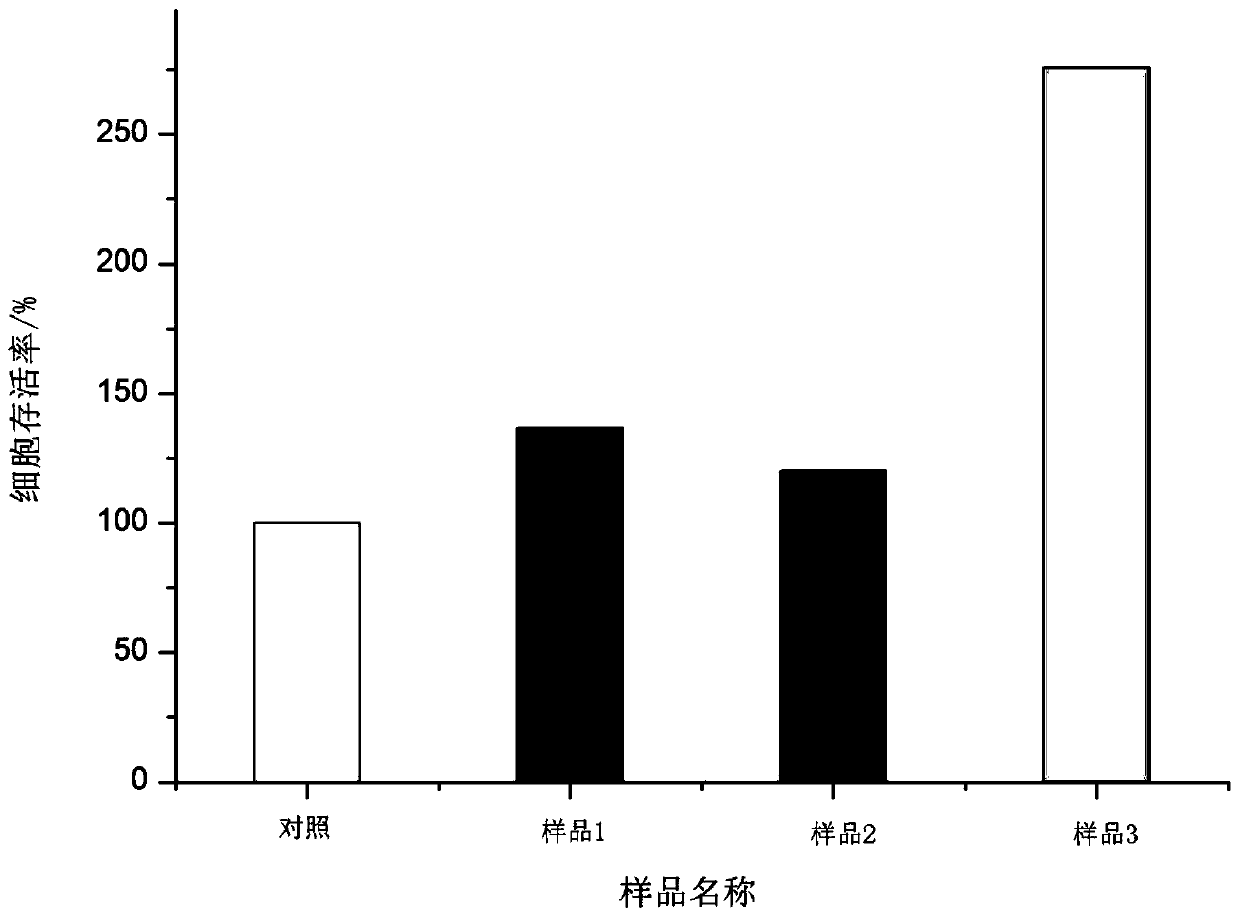
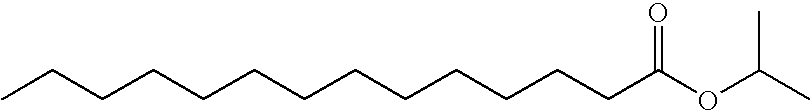
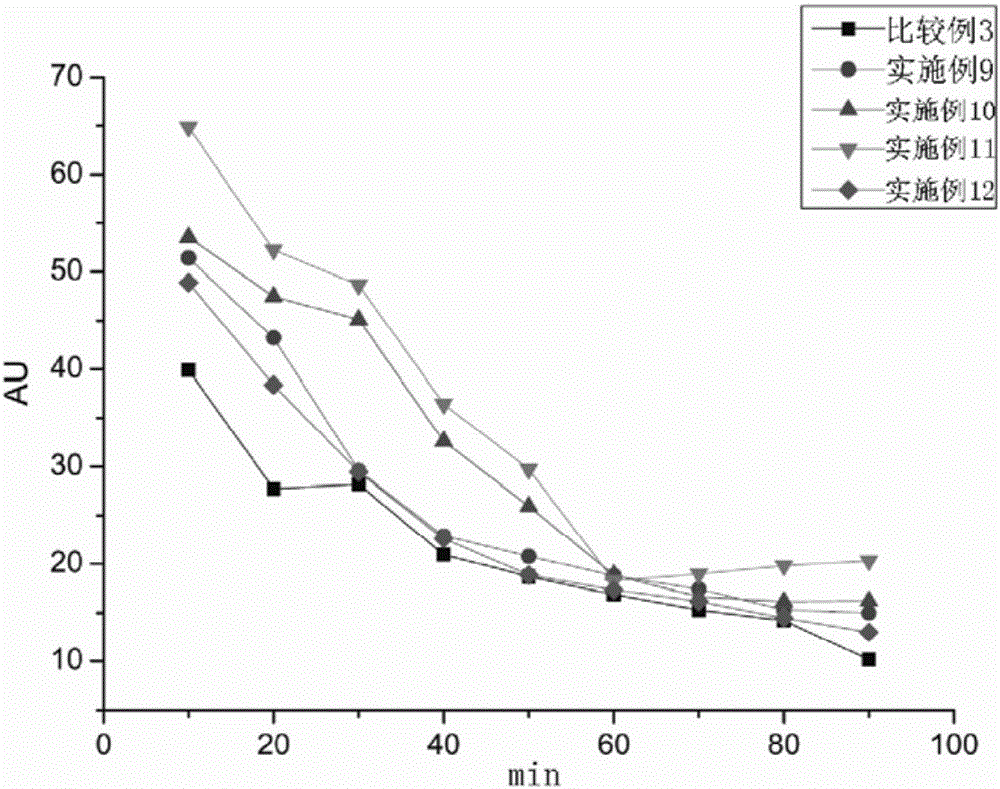
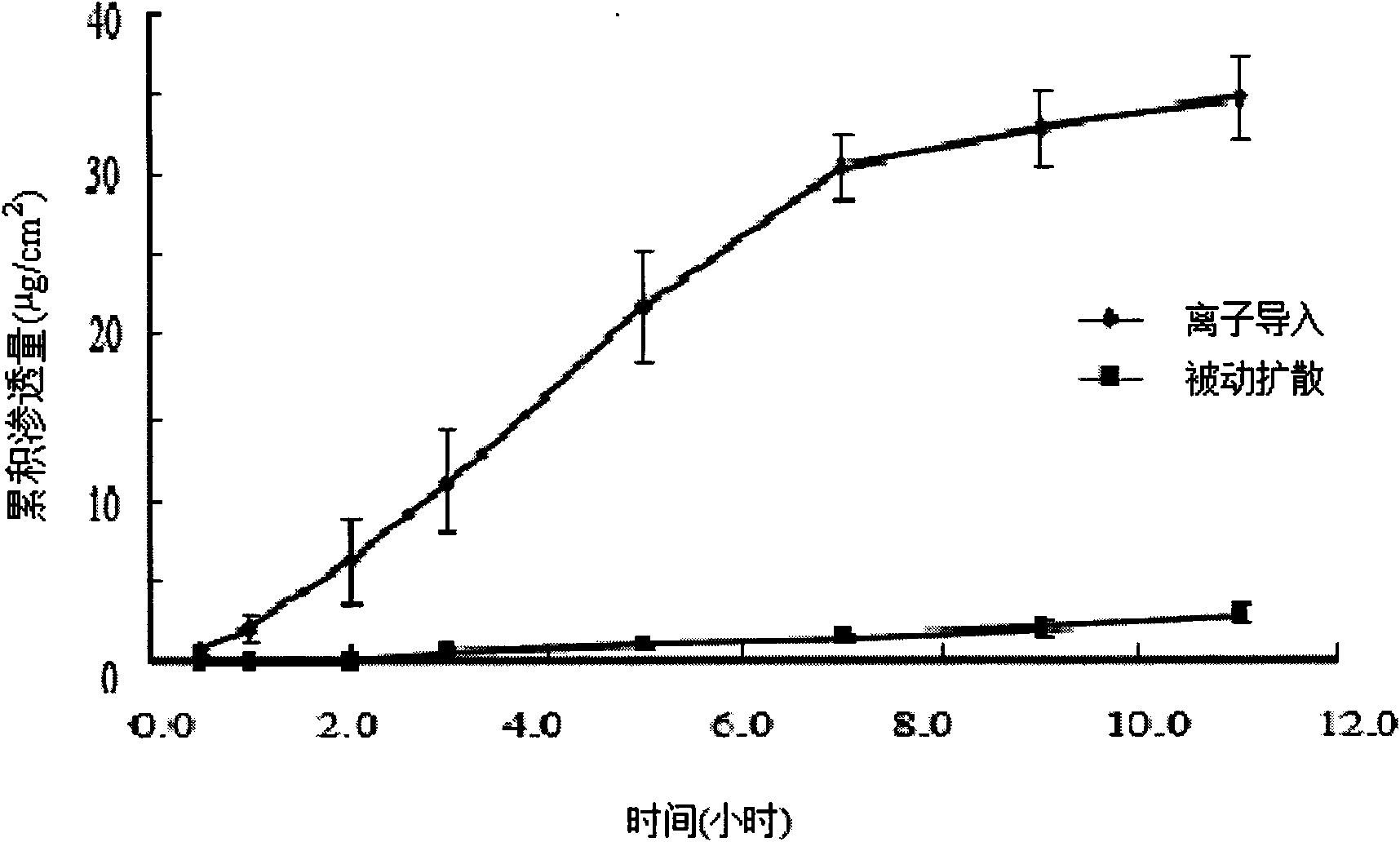
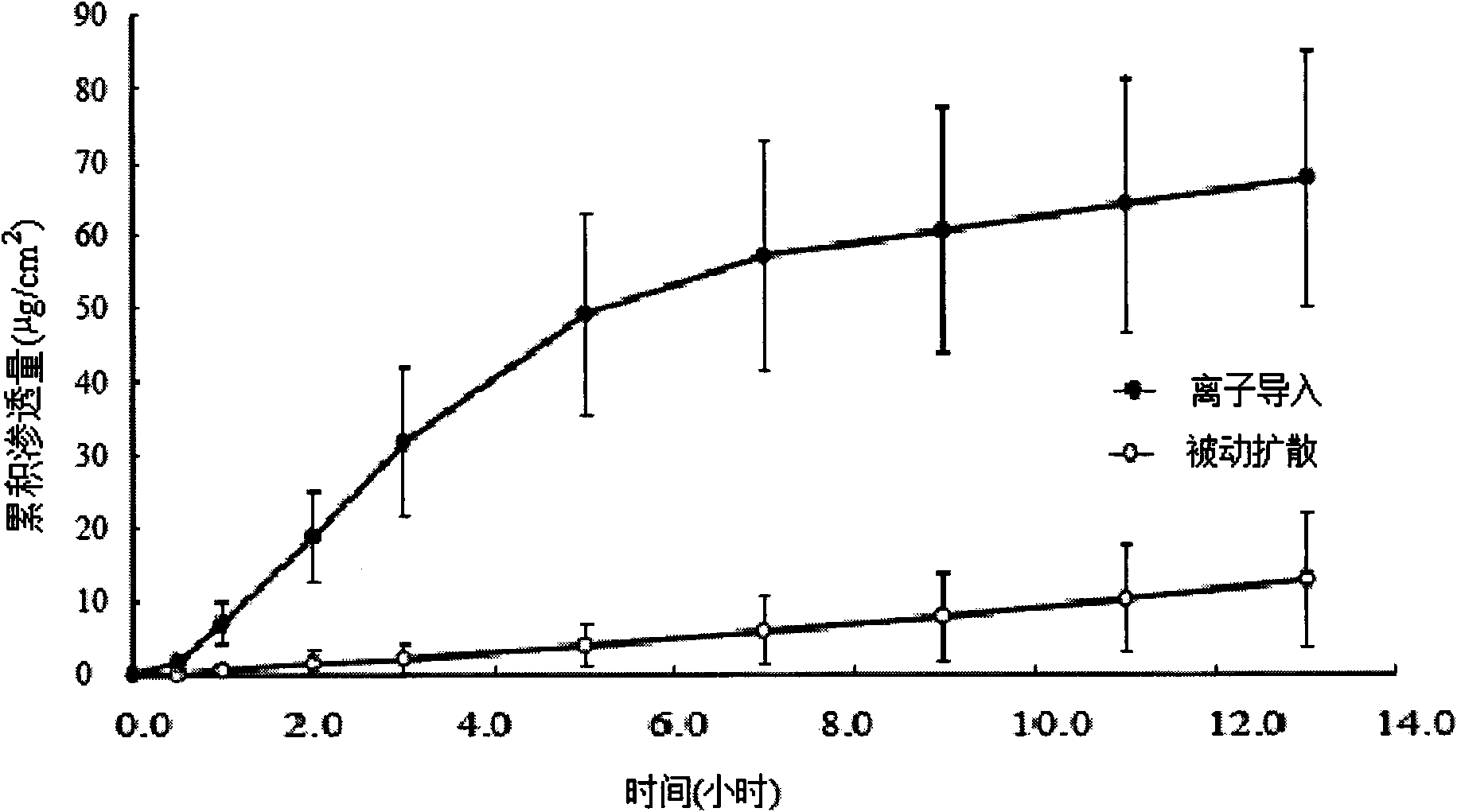
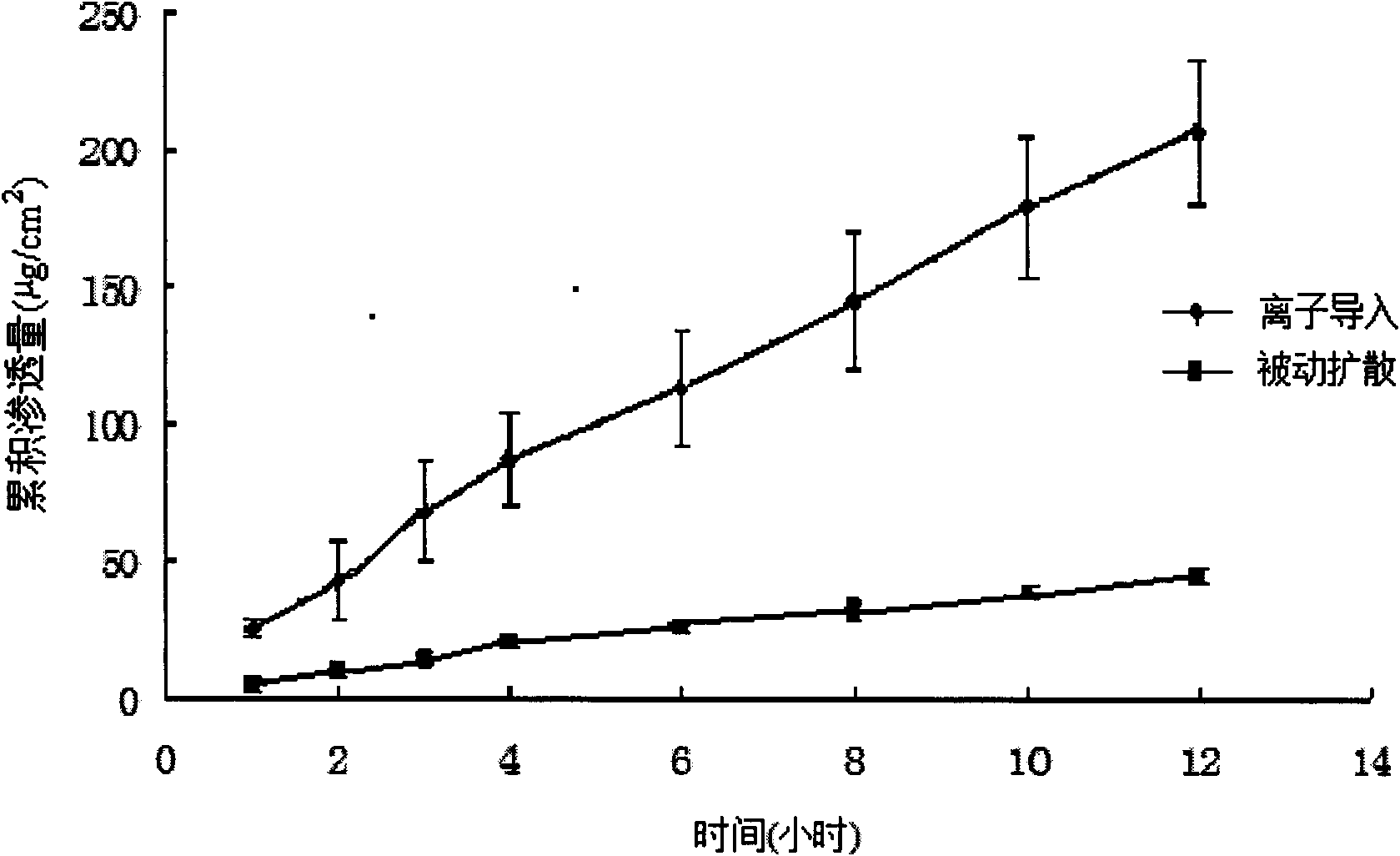
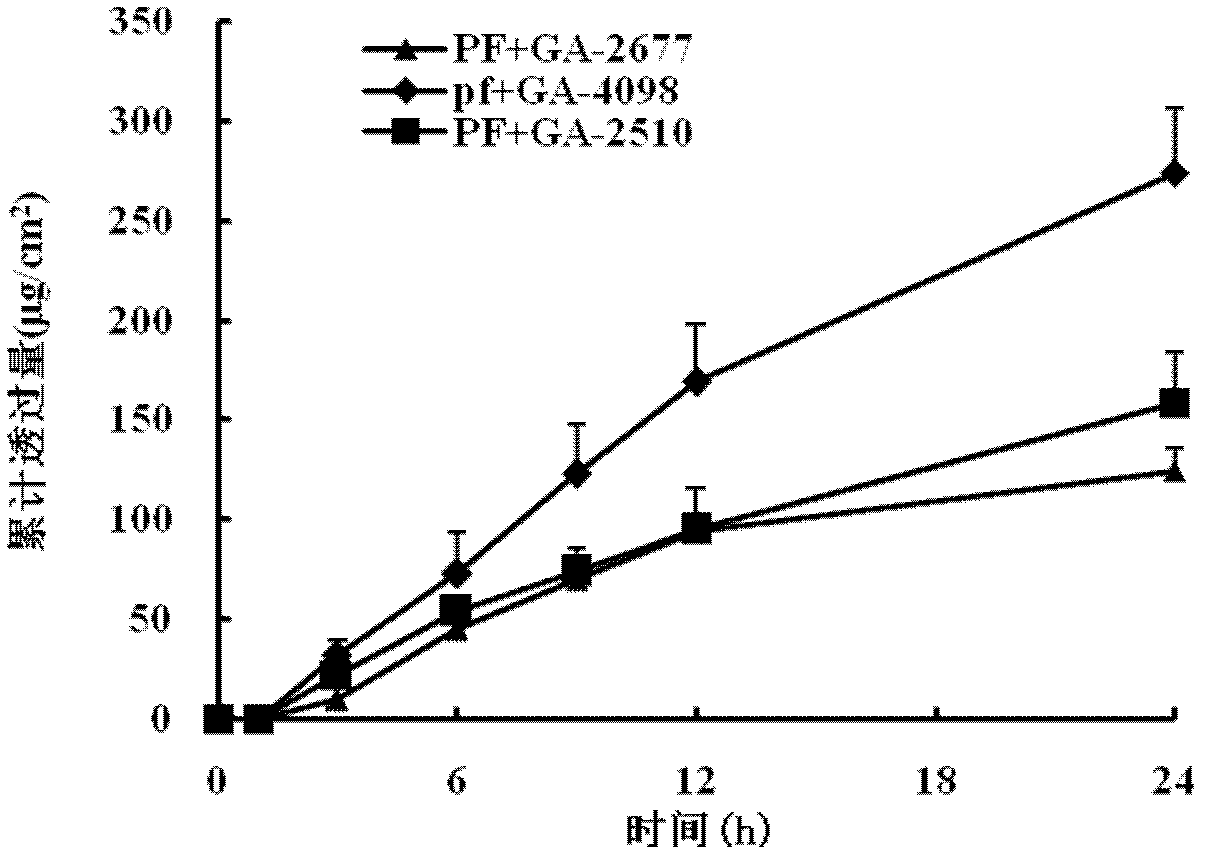
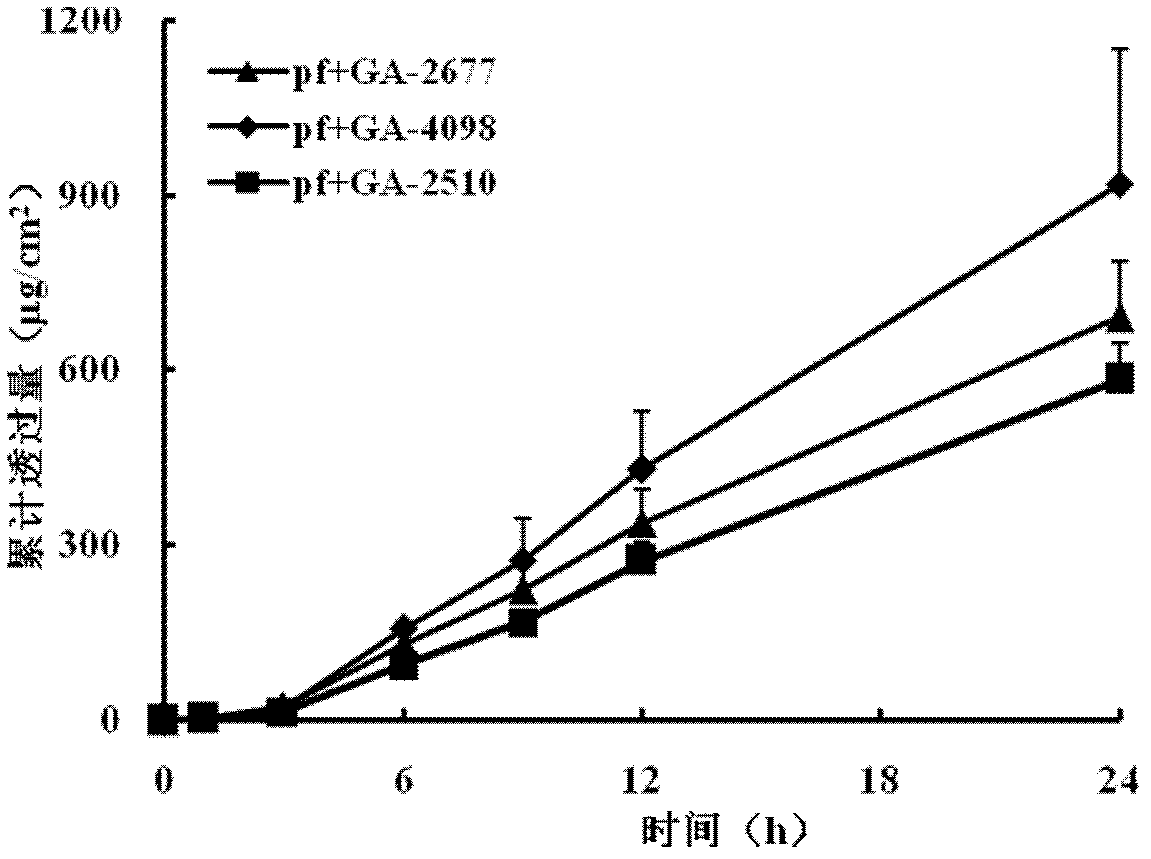
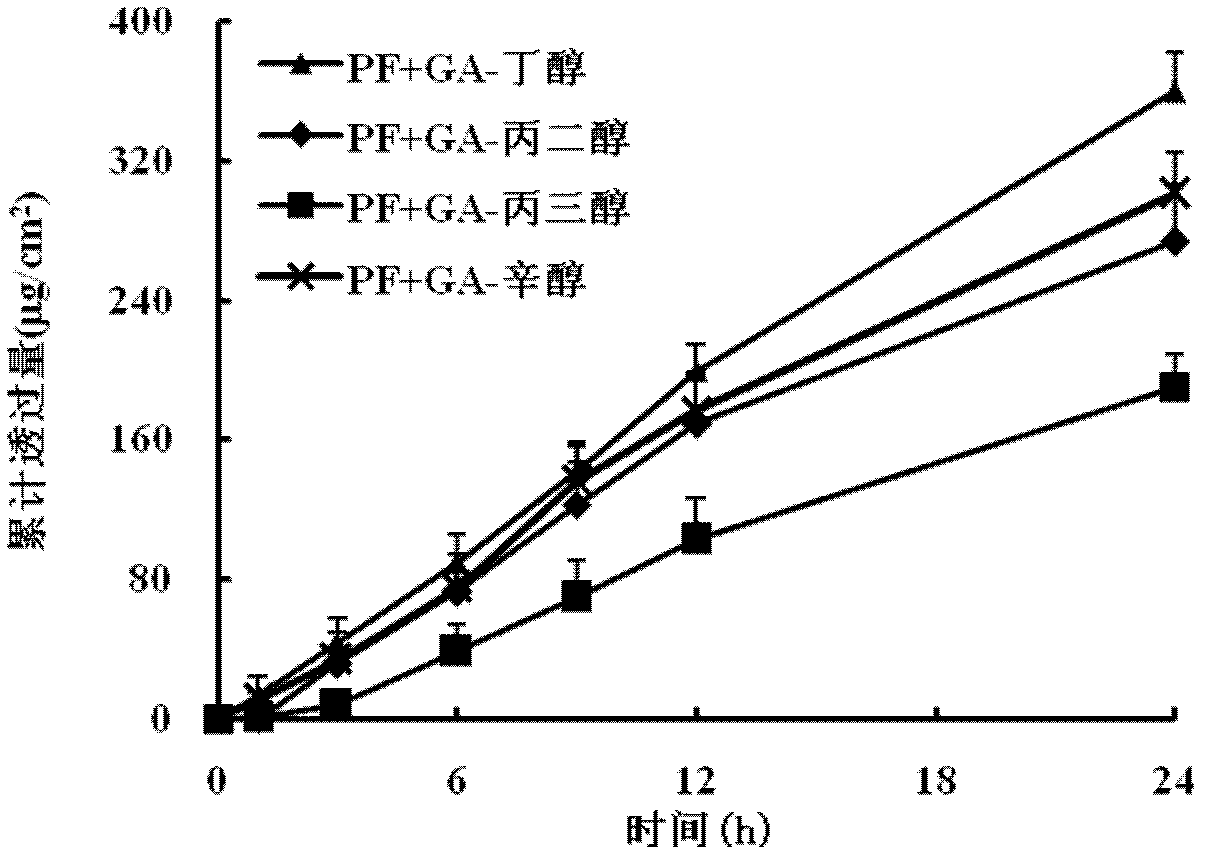
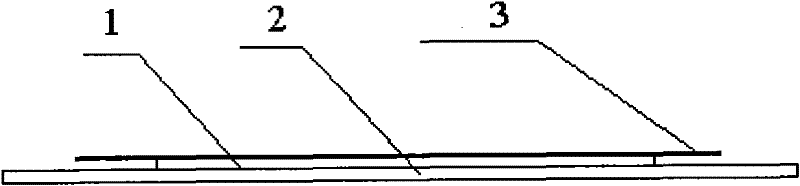
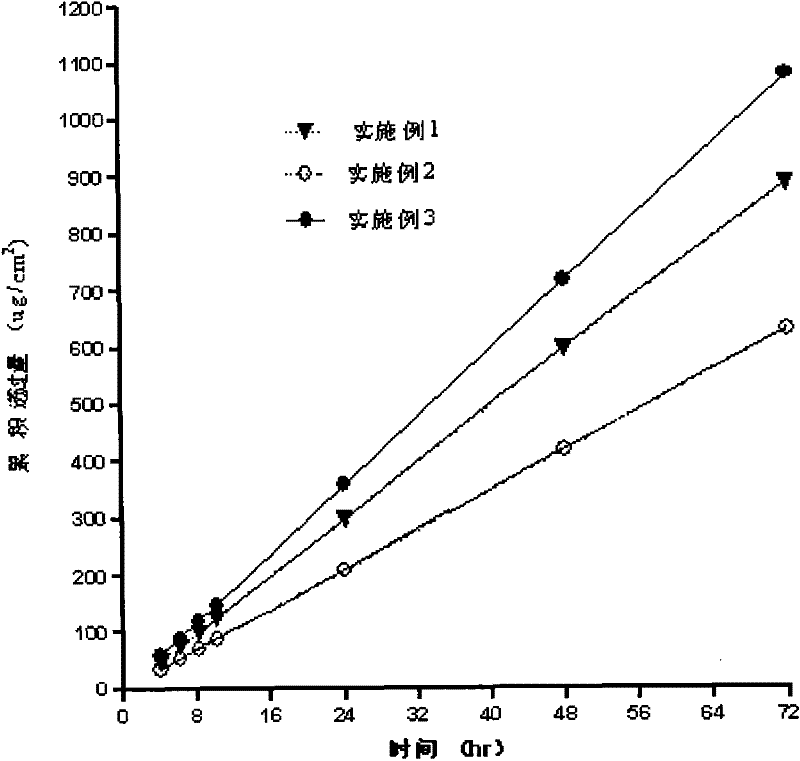
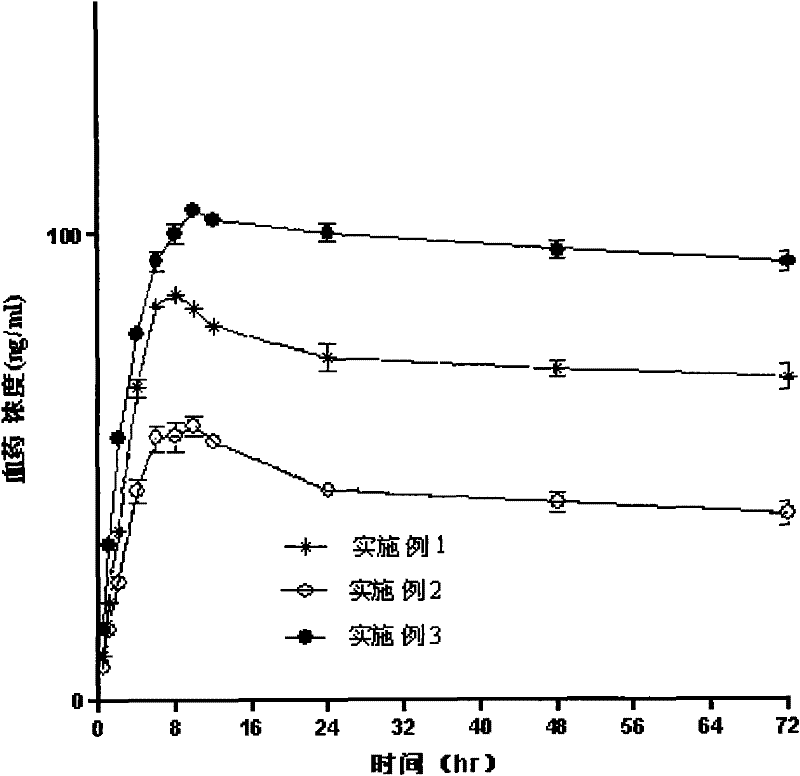
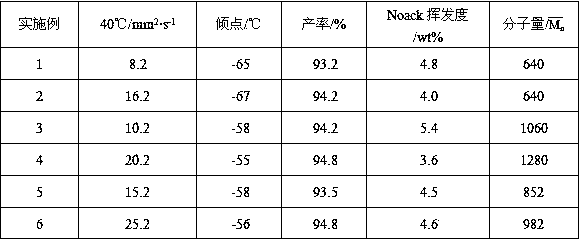
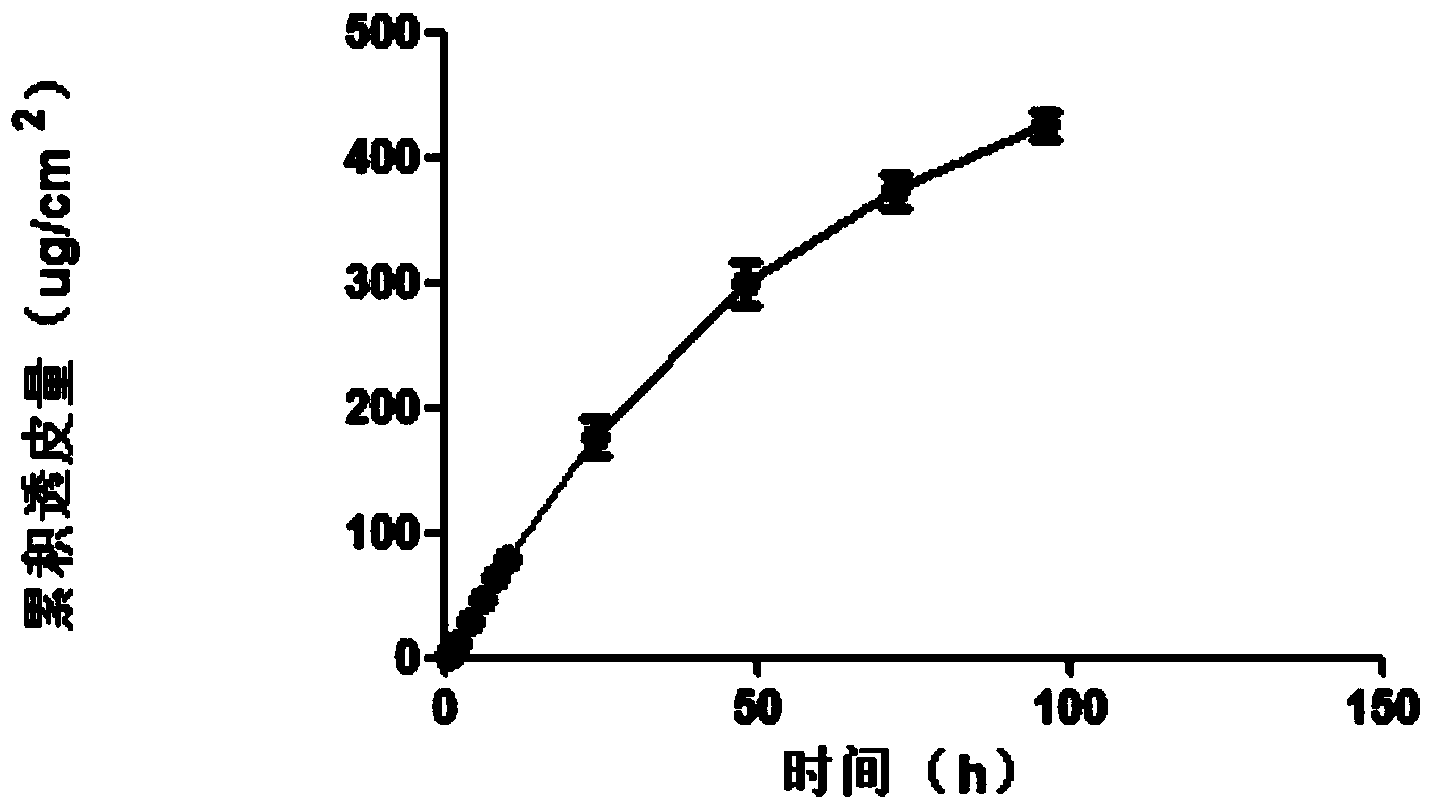
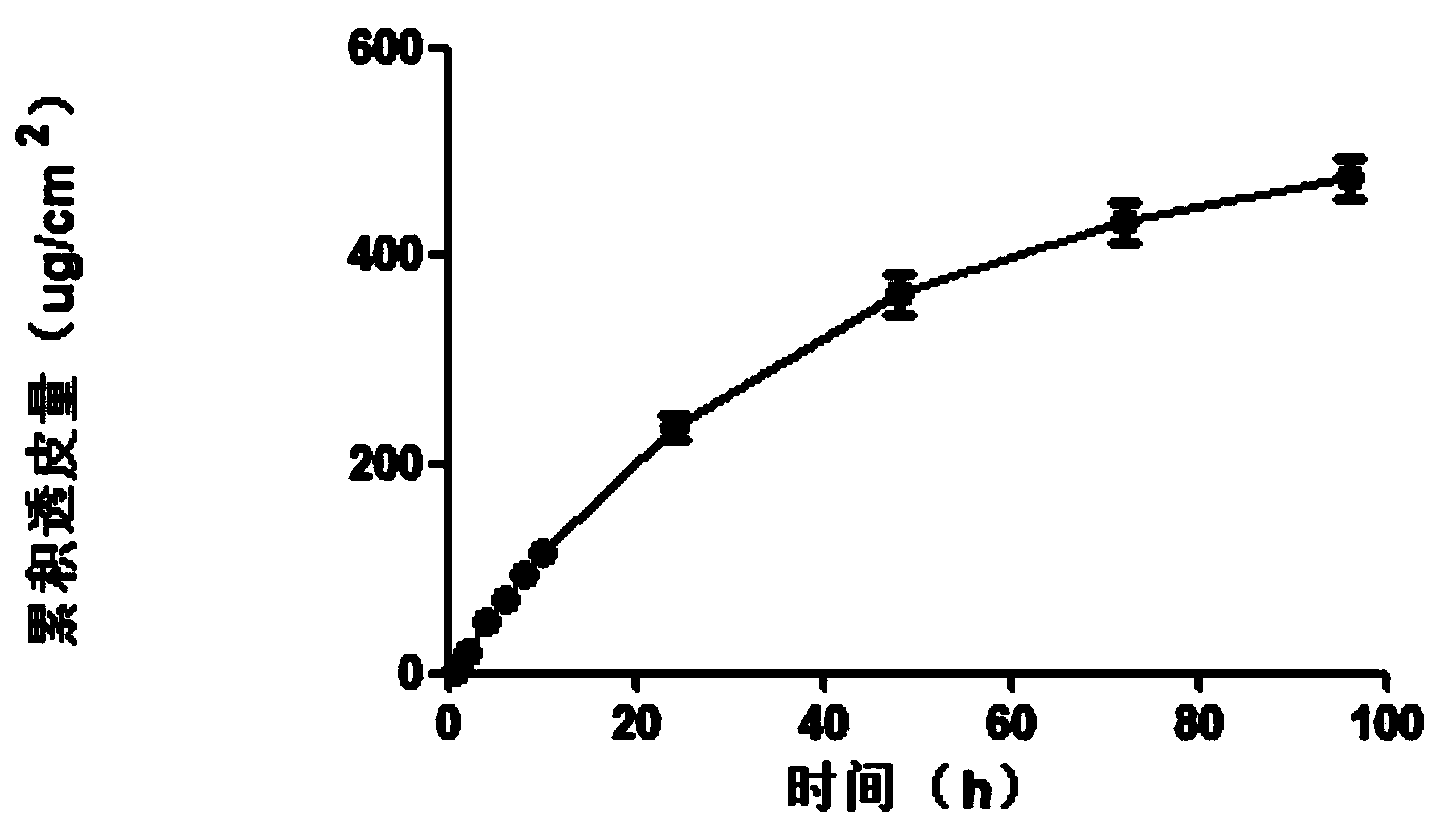
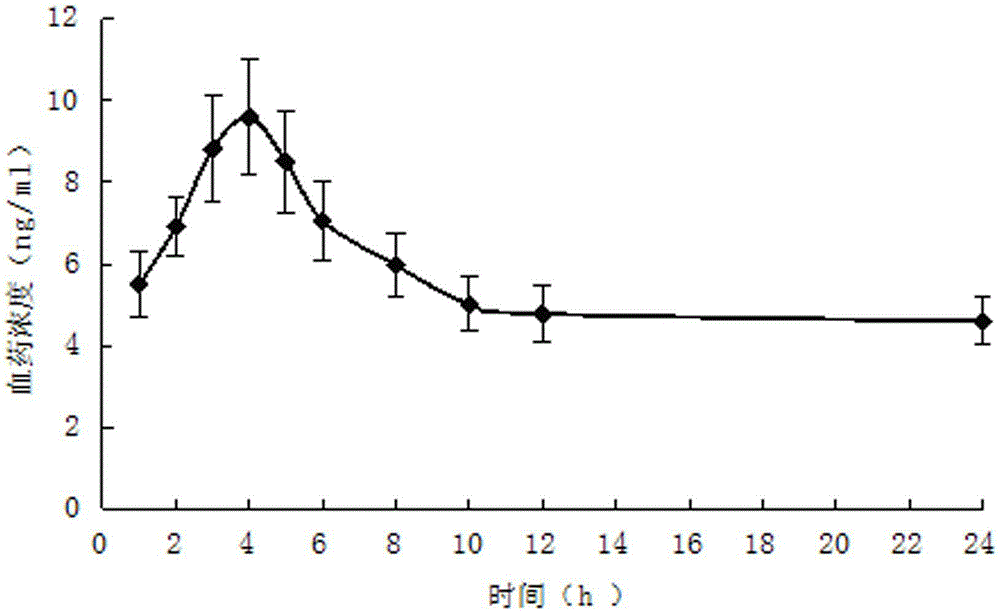
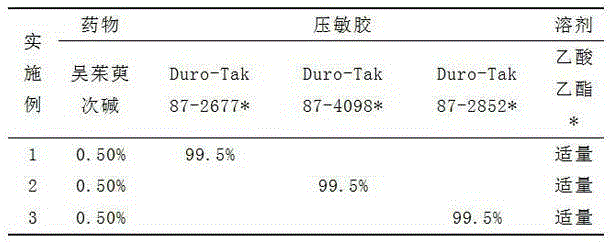
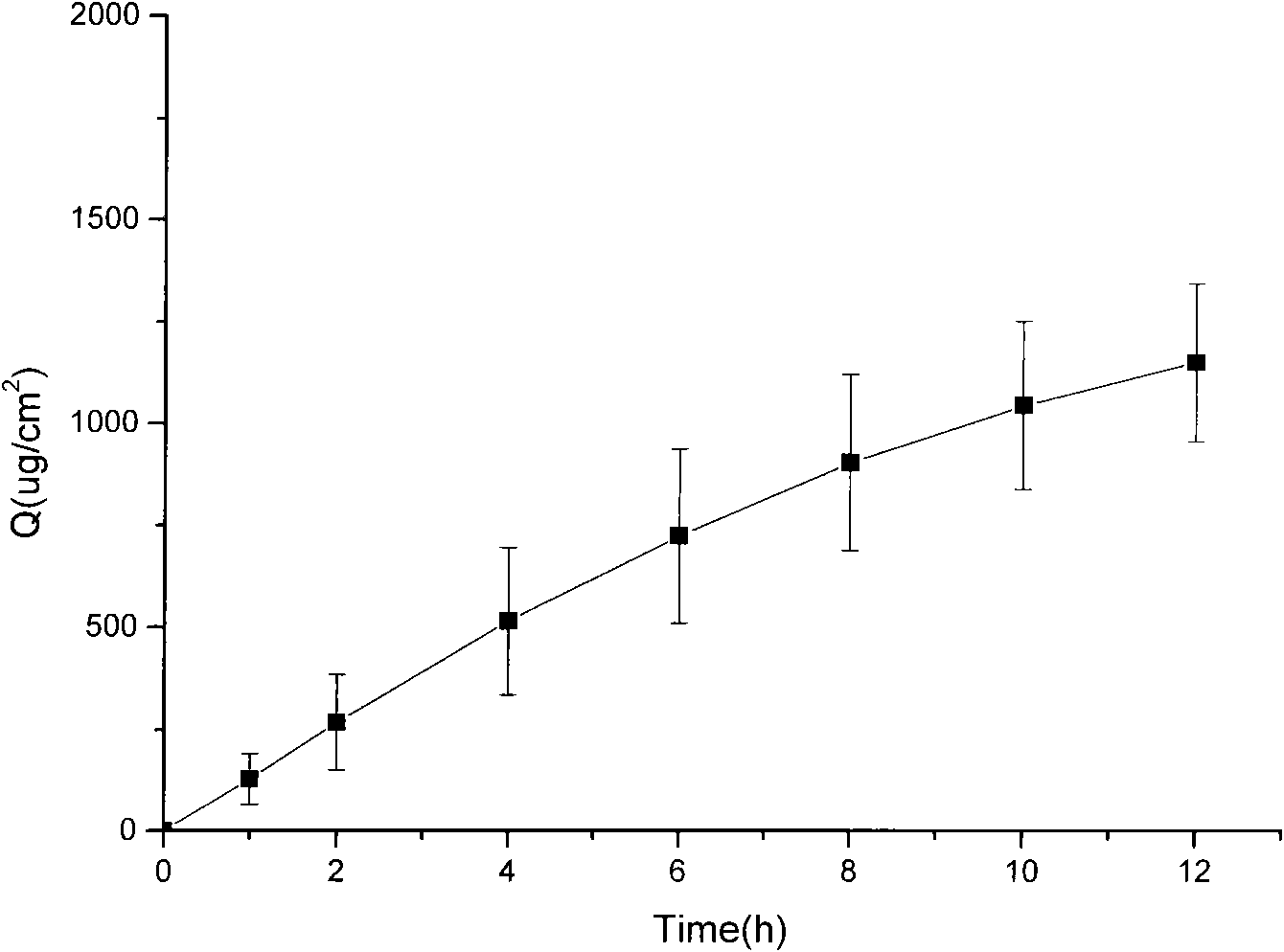
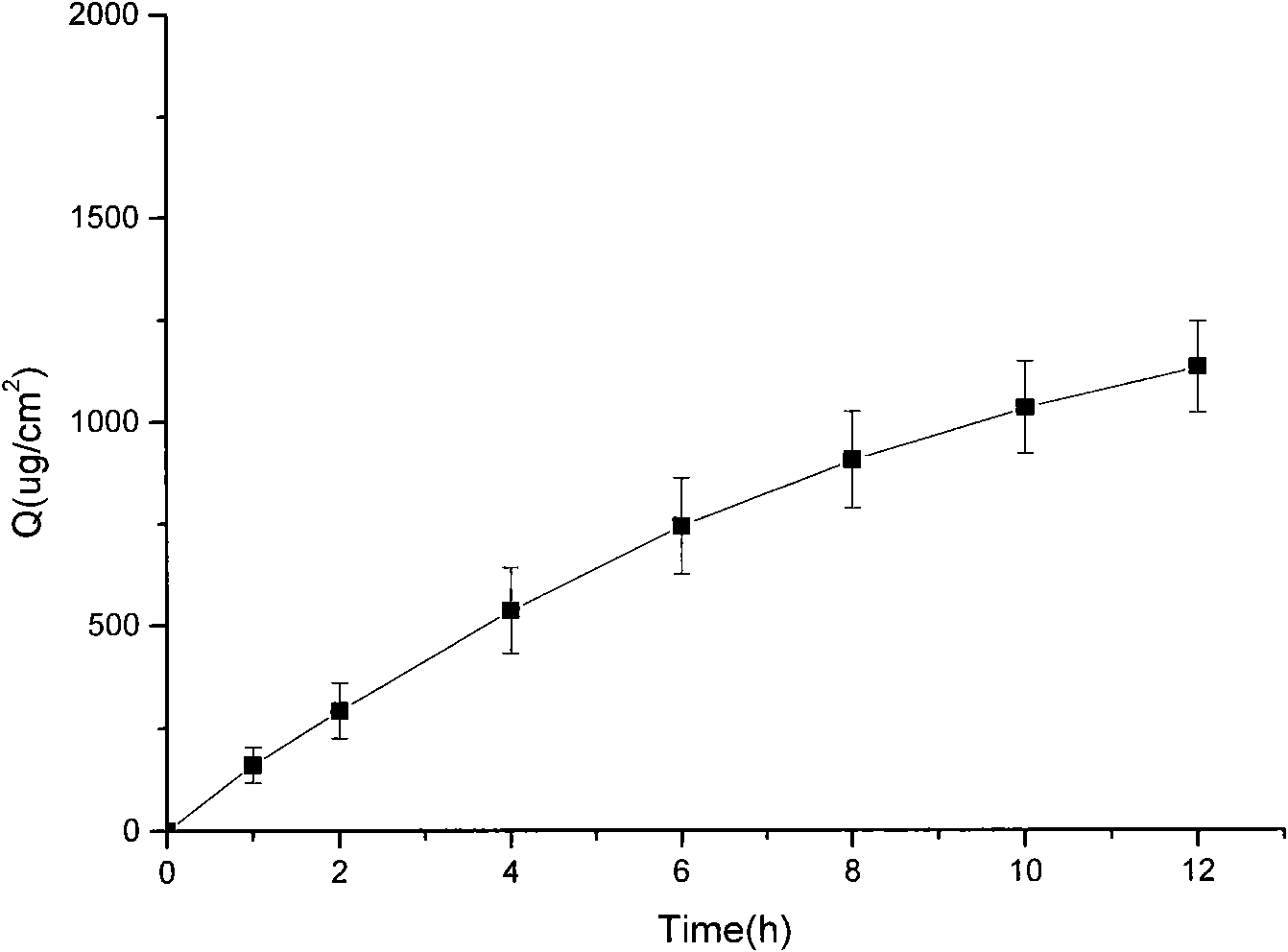
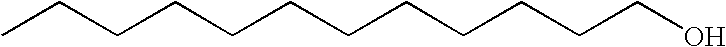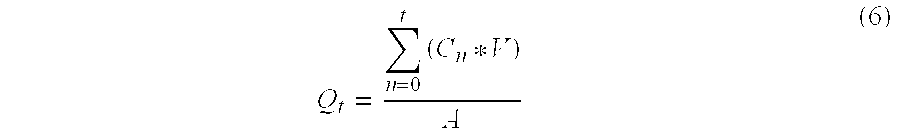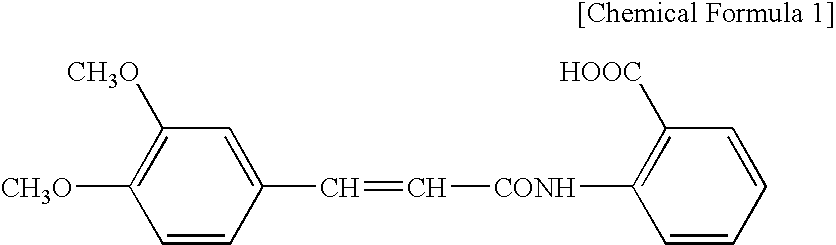Patents
Literature
102 results about "Transdermal penetration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
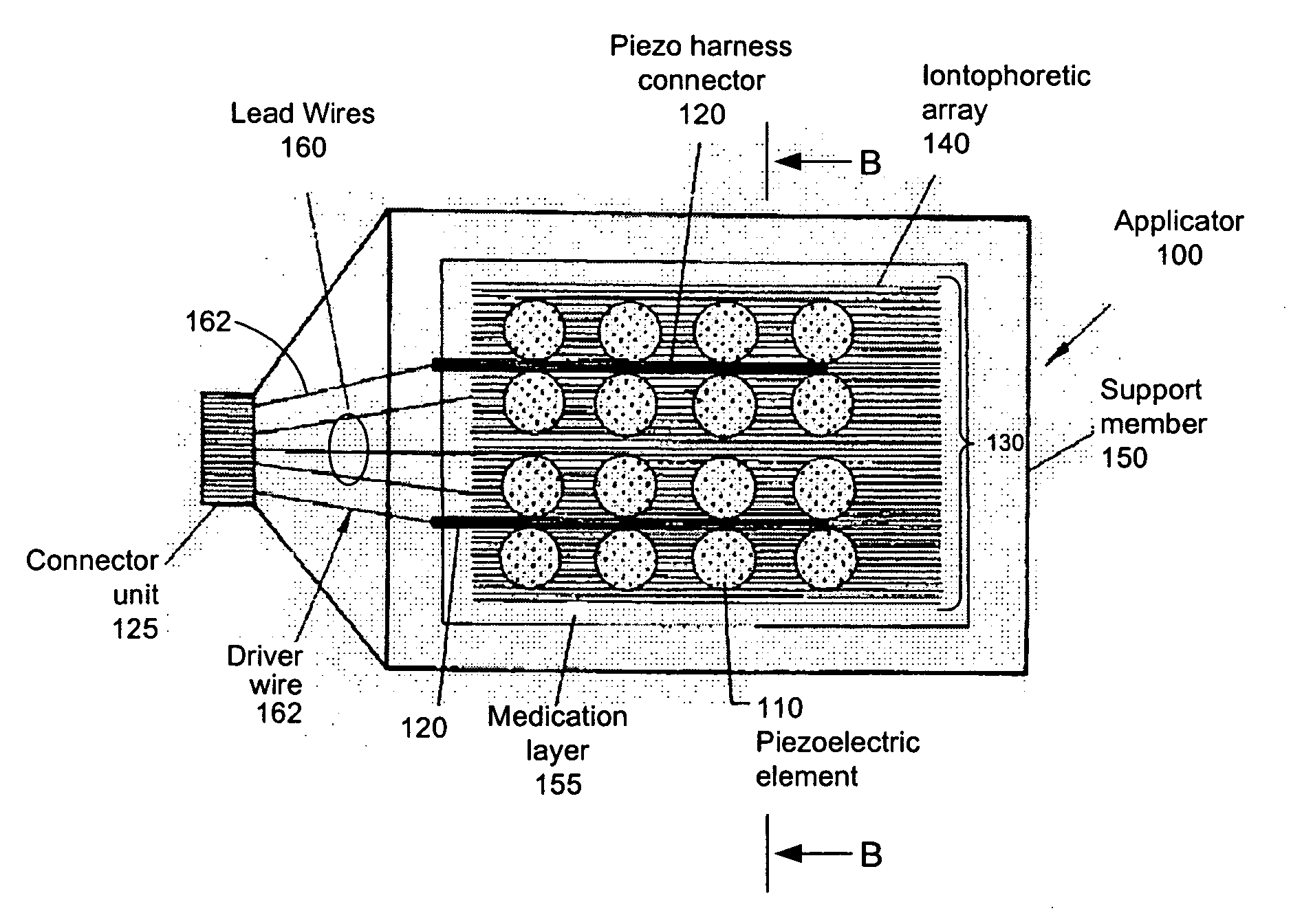
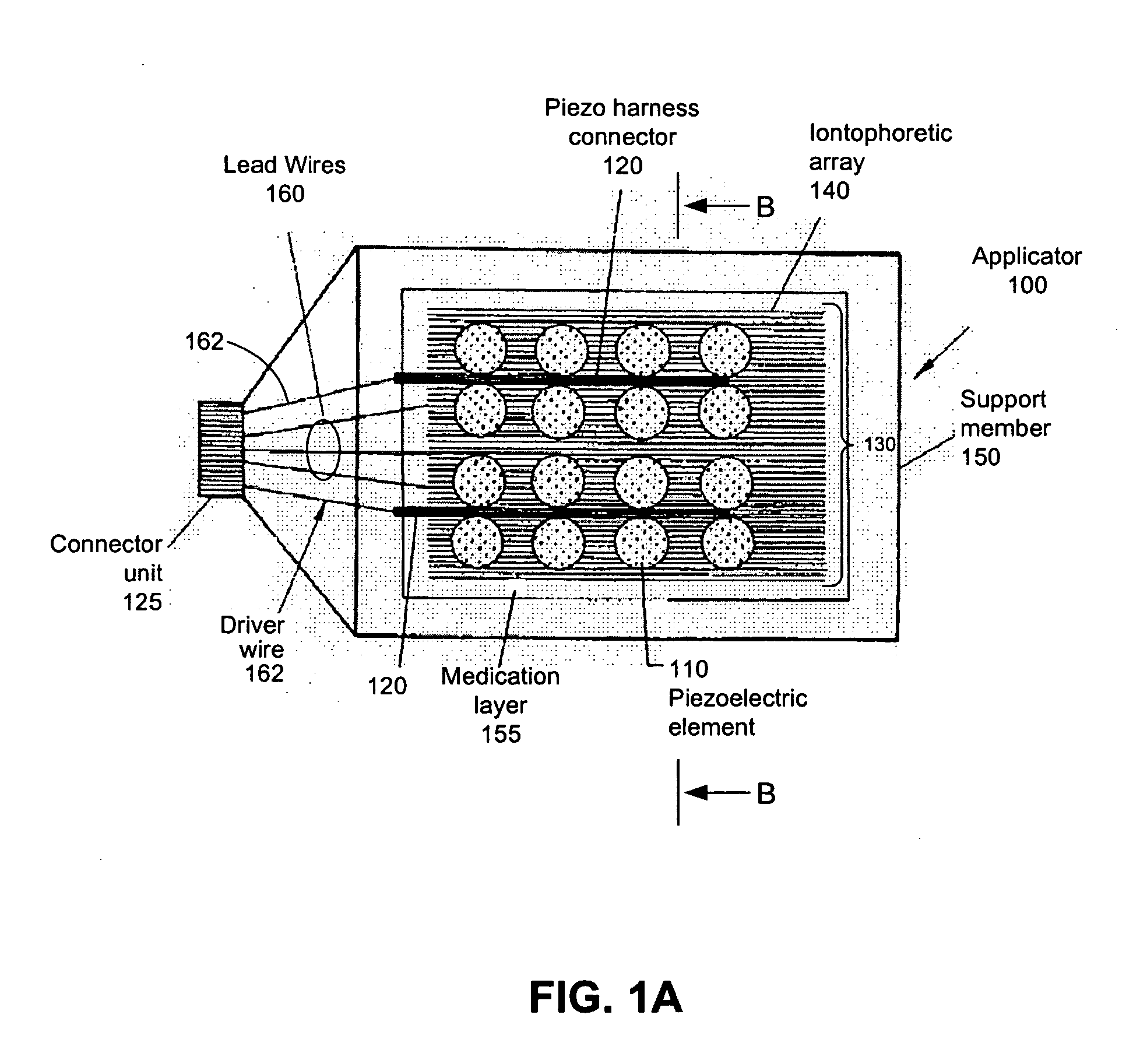
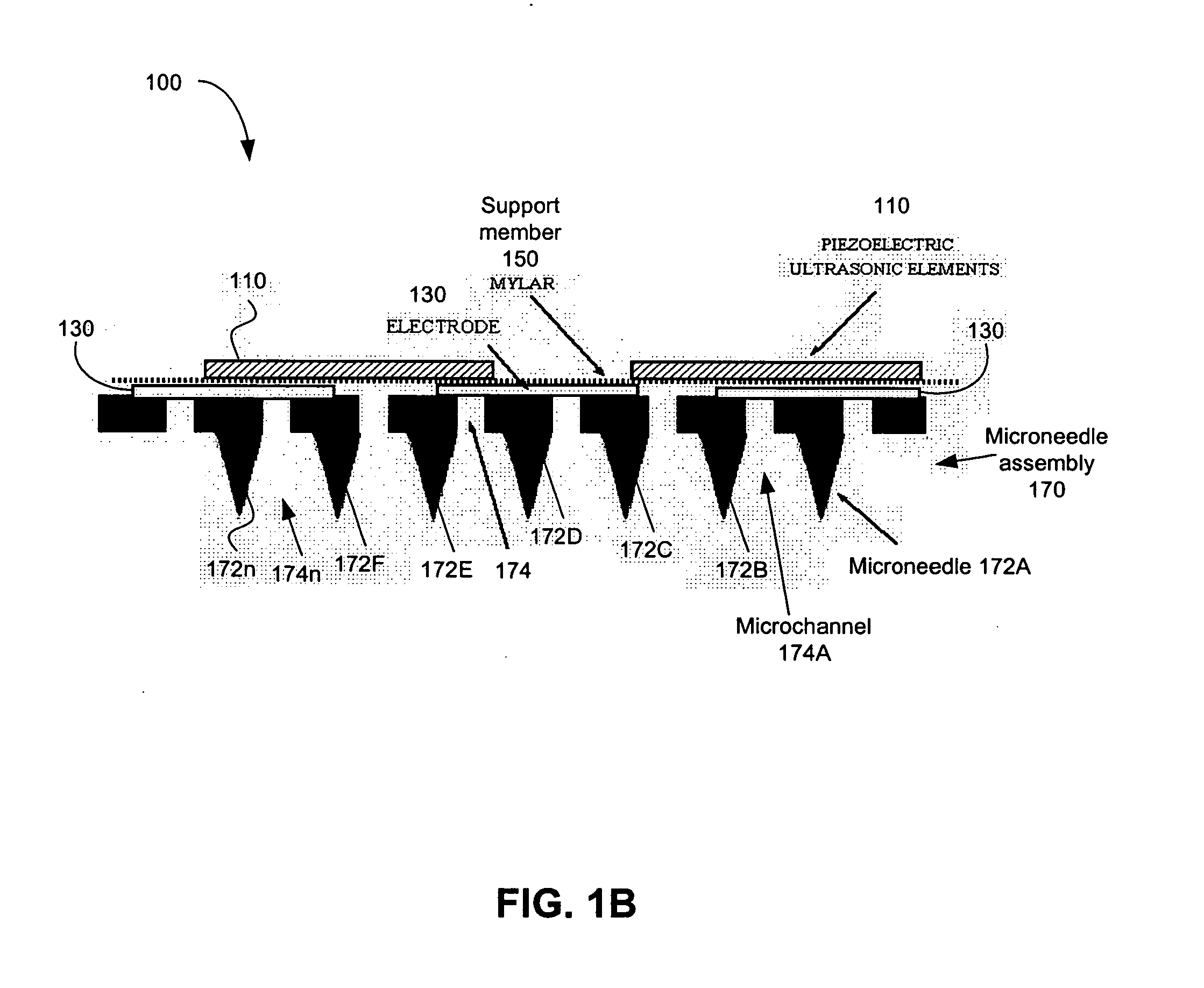
Iontosonic-microneedle applicator apparatus and methods
Novel multichannel ionosonic devices with microneedle arrays incorporated into the devices for transdermal and intradermal delivery are described. In an embodiment, the ionosonic device includes a multichannel ionophoretic driver used in combination with a multichannel dispersion electrode integrated with ultrasonic elements and microneedle array elements mounted on a single application electrode. The ionosonic-microneedle transdermal device can be configured in a variety of shapes and structural flexibility for the treatment of skin and systemic disorders through the intradermal and transdermal delivery of one or more of a variety of therapeutic and modulating agents. Because of enhanced transdermal penetration this device offers the transdermal delivery of therapeutic peptides is getting closer to reality. The devices described herein deliver the therapeutic agents to the targeted diseased area as well as systemic levels obviating the need for oral ingestion, the associated side effects and as in the case of peptides bypasses the hydrolyzing digestive enzymes that make such agents ineffective when taken by mouth.
Owner:MIT
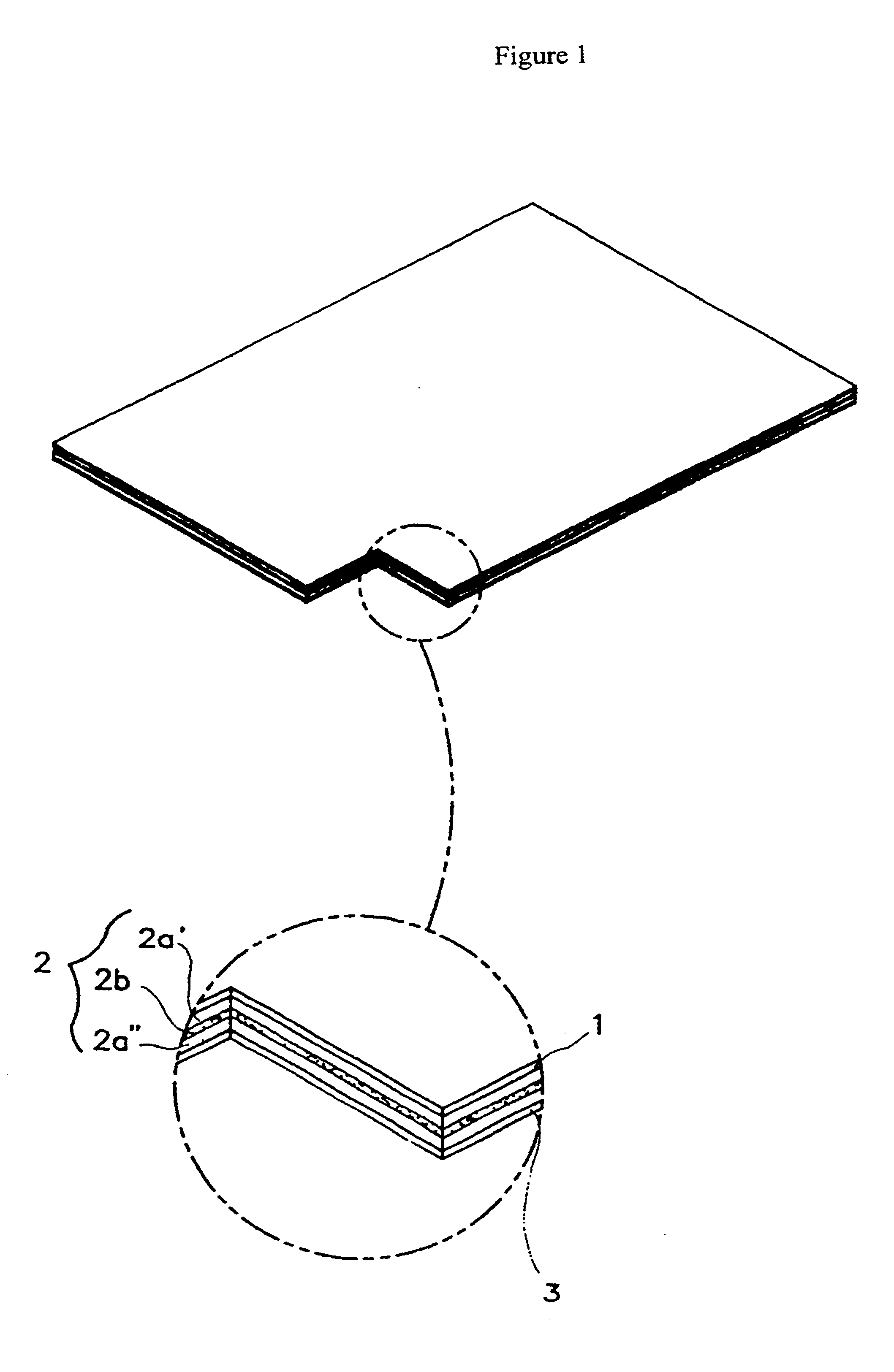
Transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonium salt, and the manufacturing method thereof
InactiveUS6723337B1Improve solubilityPromote absorptionPowder deliveryPeptide/protein ingredientsAdditive ingredientDissolution
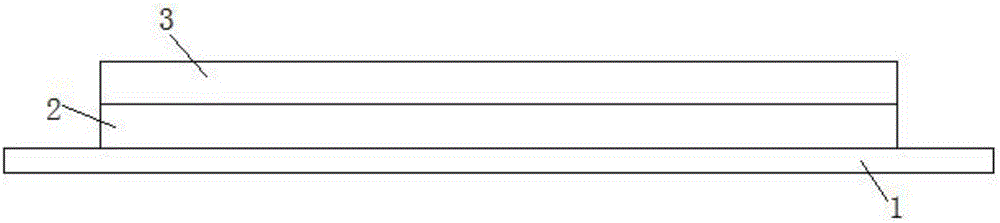
The invention herein relates to a transdermal drug delivery systern for anti-Inflammatory analgesic. agent comprising diclofenac diethylammonium salt, wherein a backing film (1), a matrix layer (2) containing active ingredients, a release liner (3) which is removed before application onto the skin are laminated therein. Mome particularly, the invention herein relates to a transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonum salt, wherein the transdermal penetration and adhesion of the patch to the body are enhanced by means of a matrix layer which comprises a diclofenac diethylammonum salt as active ingredient in addition to acrylic polymer as adhesive constituent, non-ionic surfacant as absorption enhancer, terpene and dissolution assistant, and the volatile and non-volatile constituents of the composition arc separately applied therein for significantly reducing the manufacturing time thereof.
Owner:SAMYANG BIOPHARMLS CORP
Freeze-dried powder, solvent and application of freeze-dried powder and solvent
PendingCN110237022AHigh activityGood effectCosmetic preparationsToilet preparationsWrinkle skinFreeze-drying
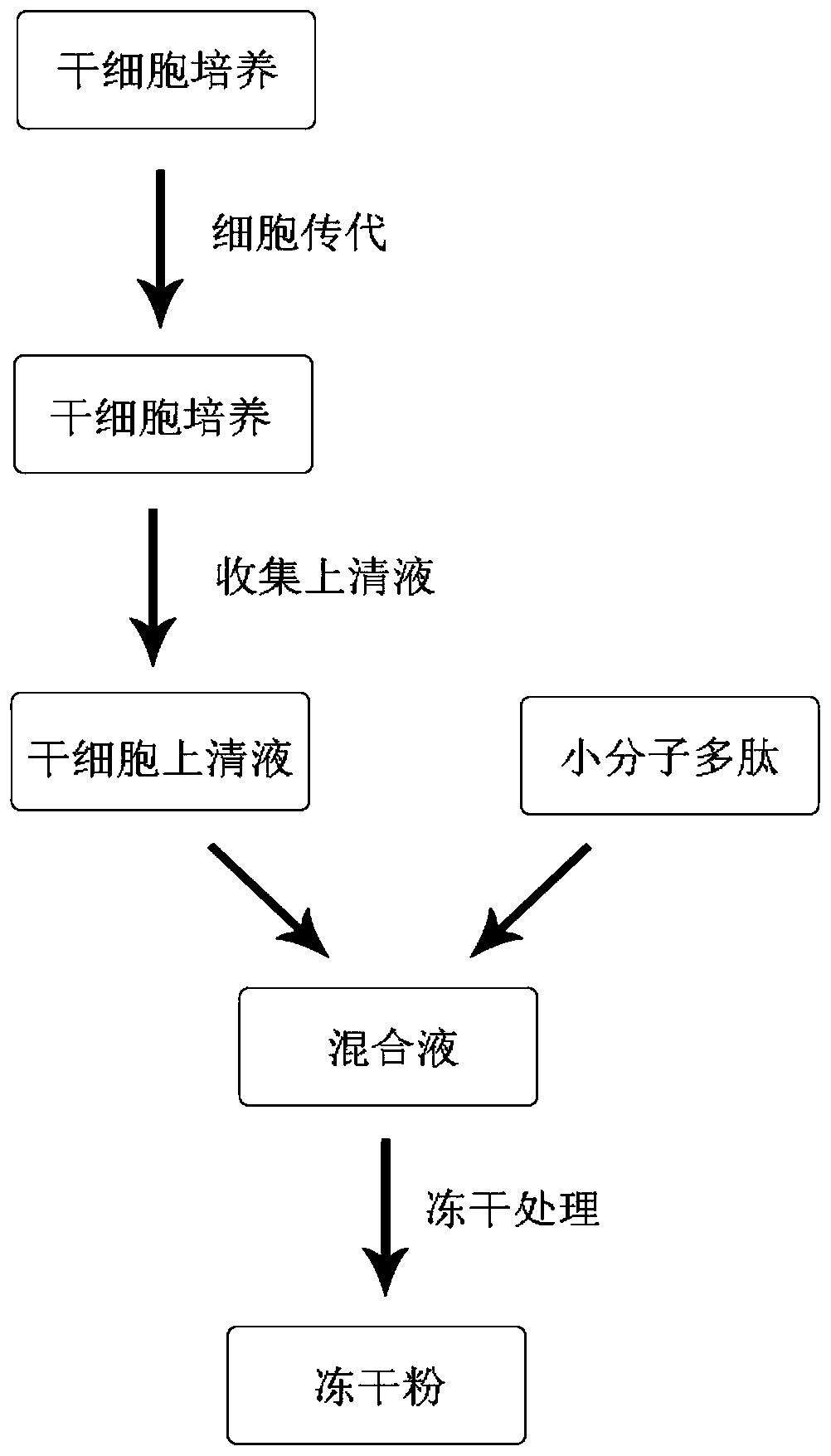
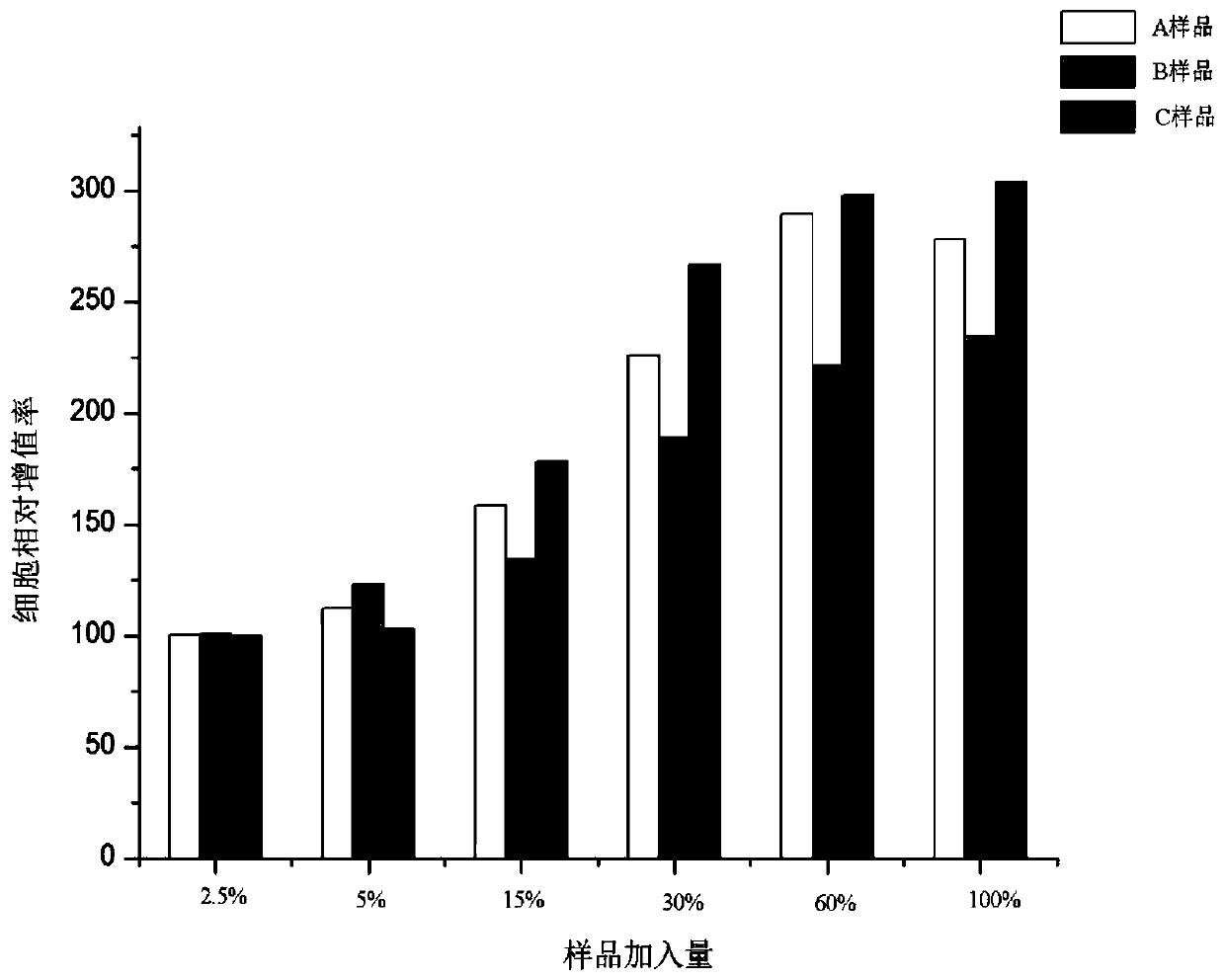
The invention belongs to the technical field of cosmetics, and particularly discloses a freeze-dried powder containing a stem cell supernate and micro-molecule polypeptide, a solvent containing a transdermal penetration enhancer and a cosmetic set containing the freeze-dried powder and the solvent. According to the prepared freeze-dried powder, the stem cell supernate and the micro-molecule polypeptide are compounded, so that the stem cell supernate and the micro-molecule polypeptide exert a synergistic effect, and the prepared freeze-dried powder has the functions of significantly delaying aging, reducing fine wrinkles, moisturizing and tendering the skin and the like; the solvent used for dissolving the freeze-dried powder specifically contains the transdermal penetration enhancer, the situation is promoted that active components of cell factors, micro-molecule polypeptide and the like in the freeze-dried powder permeate into the dermis through the transdermal penetration enhancer, and therefore the skin protection effect of the solvent is further improved. According to the freeze-dried powder, the solvent and the cosmetic set, the freeze-dried powder and the solvent are individually packaged, the quality guaranteeing period of the cosmetic set is prolonged, no preservative is added into the freeze-dried powder, and it is effectively ensured that the active components in the freeze-dried powder are not influenced.
Owner:STEMIRNA THERAPEUTICS CO LTD
Formulations and Methods for Enhancing the Transdermal Penetration of a Drug
InactiveUS20070065494A1Improve permeabilityIncrease the areaBiocidePharmaceutical non-active ingredientsPenetration enhancerLauryl Alcohol
Methods and formulations of enhancing the permeability the skin of a subject to a drug are disclosed. The method may include administering a combination of lauryl alcohol and isopropyl myristate as a penetration enhancer to the area of skin to provide synergistically enhanced penetration of the drug.
Owner:WATSON LAB INC
Transdermal penetration system and treatment for cellulite
InactiveUS20050182076A1Small sizeSmooth appearanceBiocideAnimal repellantsSkin surfaceAdditive ingredient
The present invention is a powerful vasodilatation and transdermal penetration system, which quickly penetrates the dermis of the skin and deposits beneficial ingredients at the subcutaneous layer, which then stimulates the capillary blood flow to fat cells and increases the metabolizing process of fat cells and cellulite, thereby reducing the size of fat cells, thus creating a smoother appearance to the surface of the skin as well as reducing the appearance of the size of the affected area.
Owner:PACHECO ADAM A +1
Insulin carrier transdermal drug delivery preparation and preparation method thereof
ActiveCN102178662AHighly deformablePromote absorptionPeptide/protein ingredientsMetabolism disorderCholesterolPhospholipid
The invention discloses an insulin carrier transdermal drug delivery preparation which comprises the following components of insulin, phospholipid, cholesterol, surfactant, transdermal penetration enhancer, triethanolamine-hydrochloric acid and gel. A preparation method of the insulin carrier transdermal drug delivery preparation comprises the steps of: 1, taking the phospholipid, the cholesterol, the surfactant and the transdermal penetration enhancer and then adding into alcohol for dissolving; 2, taking insulin, dissolving the insulin with a triethanolamine-hydrochloric acid buffer solution, adding the obtained solution in the solution obtained in the step 1, homogenizing and nanometerizing liquid through micro jet to obtain an insulin carrier solution; and 3, taking the gel, adding water to enable the gel to be swelled to prepare a gel substrate, slowly adding the insulin carrier solution in the step 2 into the gel substrate, and uniformly stirring to obtain the insulin carrier transdermal drug delivery preparation. The insulin carrier transdermal drug delivery preparation prepared by adopting the preparation method disclosed by the invention has the characteristics of uniformparticle size, higher entrapment rate, good stability and convenience for medicine delivery; and at least three days of medicine delivery time can be maintained, and compliance of patients is improved while the medicine delivery interval is greatly shortened.
Owner:王义明
Cosmetic and application of perilla extract in cosmetic as transdermal penetration enhancer
ActiveCN105012185APromote absorptionGood moisturizing effectCosmetic preparationsToilet preparationsFucus serratusSeaweed extract
The invention relates to a cosmetic and an application of a perilla extract in the cosmetic as a transdermal penetration enhancer. The cosmetic provided by the invention contains the perilla extract and a functional component, wherein the perilla extract is a perilla root, stem or leaf extract; the functional component comprises a deep-sea seaweed extract; and the deep-sea alga extract comprises an austria seaweed extract, a light black seaweed extract or a fucus serratus extract. The perilla extract is applied to the cosmetic as the transdermal penetration enhancer, so that absorption of the functional component in the cosmetic can be significantly promoted, so that the functional component in the cosmetic can be relatively well absorbed by the skin; and relatively good efficacies of the skin for moisturizing, replenishing water and locking water are promoted.
Owner:GUANGZHOU KENENG COSMETICS RES CO LTD +1
Topical administration carrier composition and therapeutic formulations comprising same
ActiveUS20070141004A1Reduce evaporative lossReduce the evaporative lossBiocideCosmetic preparationsWhole bodyBULK ACTIVE INGREDIENT
A topical administration carrier composition including water, glycerin and polysorbate, suitable for use with compositions containing active ingredients such as minoxidil that are susceptible to volatilization and transdermal penetration in application to the body. The carrier formulation retards the evaporative and systemic migration losses of the active ingredient composition, to provide sustained topical action, in relation to formulations lacking the components of the inventive composition.
Owner:CELMATRIX CORP
Plant extract-mediated drug transdermal introducing system and transdermal method thereof
InactiveCN102793639AGood treatment effectCosmetic preparationsToilet preparationsTransdermal patchPreparing skin
The present invention discloses a plant extract-mediated drug transdermal introducing system and a transdermal method thereof. The plant extract-mediated drug transdermal introducing system comprises a transdermal introducing agent prepared from at least one plant extract, and biological macromolecules or a preparation thereof. The transdermal method includes: using the transdermal introducing agent to promote penetration, then performing transdermal penetration for the biological macromolecules or the preparation thereof; or mixing the biological macromolecules or the preparation thereof with the transdermal introducing agent to prepare a composite formula preparation for transdermal penetration. The present invention also provides the application of the plant extract-mediated drug transdermal introducing system or the transdermal method thereof in the preparation of skin health products. Preparing skin health products by the system, the biological macromolecules can penetrate into the skin to achieve a better therapeutic effect.
Owner:百源科创分子医学研究所(南通)有限公司
Application of ionic type cyclodextrin derivative in preparation of medicine preparation for iontophoresis transdermal administration
InactiveCN101897977AImprove transdermal penetration rateImprove import efficiencyAerosol deliveryOintment deliveryCyclodextrin derivativePharmaceutical formulation
The invention discloses the application of ionic type cyclodextrin derivative in preparation of medicine preparation for iontophoresis transdermal administration. The ionic type cyclodextrin derivative comprises one or two of cationic cyclodextrin derivative and anionic cyclodextrin derivative; the medicine is selected from one or more of molecular type medicine, charged medicine with the dissolubility less than 1mg / ml, faintly acid charged medicine with the dissociation constant p Ka larger than 4, or alkalescent charged medicine with the dissociation constant p Ka less than 4. The ionic type cyclodextrin derivative applied to the iontophoresis transdermal administration improves the transdermal penetration rate of the molecular type medicinem and the charged medicine with low dissolubility or degree of dissociation.
Owner:ZHEJIANG UNIV
Transdermal patch containing paeoniflorin and glycyrrhetinic acid and method for preparing same
ActiveCN102218074AEffective percutaneous penetrationPlay a synergistic effectOrganic active ingredientsAntipyreticTransdermal patchAdditive ingredient
The invention discloses composition of a paeoniflorin- glycyrrhetinic acid transdermal patch and a method for preparing the same. The transdermal patch consists of a lining, a drug-carrying pressure sensitive adhesive layer and an anti-sticking layer, wherein the drug-carrying pressure sensitive adhesive layer comprises the following ingredients in part by weight: 1 to 20 parts of paeoniflorin and 1 to 20 parts of glycyrrhetinic acid or 5 to 30 parts of extract of paeoniflorin and 5 to 30 parts of extract of glycyrrhetinic acid, organic alkali, a penetration enhancer and butyl alcohol. The content of the paeoniflorin of the extract of paeoniflorin and the content of the glycyrrhetinic acid of the extract of glycyrrhetinic acid are 20 to 90 percent respectively. The main active ingredients of common peony and liquorice can enter skin and subcutaneous tissues by transdermal penetration to effectively alleviate and inhibit spasm and pain in local tissues.
Owner:DALIAN UNIV OF TECH
Transdermal medicament delivery system containing donepezil compound, preparation and preparation method
ActiveCN102188363AImprove complianceAvoid first pass effectNervous disorderMacromolecular non-active ingredientsDonepezilCross linker
The invention discloses a transdermal medicament delivery system containing a donepezil compound, a transdermal preparation and a preparation method. The transdermal medicament delivery system comprises the following components in percentage by weight: 0.1 to 50 percent of donepezil or acid radical salt thereof, 1 to 95 percent of skeleton polymer, 0.1 to 60 percent of transdermal penetration enhancer, 0 to 10 percent of cross linker, 0.5 to 60 percent of humectant, 0.02 to 10 percent of bacteriostatic agent, 0.02 to 30 percent of pH regulator and 0 to 90 percent of solvent. The system is used for treating light, medium and severe senile dementia, can maintain long-time stable medicament delivery of at least 3 days, has better performance, is convenient for medicament delivery, and can reduce the administration frequency and increase the compliance of patients; and meanwhile, the transdermal path avoids first-pass effect on gastrointestinal tracts and liver due to oral administration of medicaments, and the system has higher bioavailability and obvious advantages in medicinal application.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Compositions and Methods for Treating Hyperpigmentation
InactiveUS20100166689A1Reduce skin pigmentationEnhance transdermal transportCosmetic preparationsHair cosmeticsKojic acidHyperpigmented skin
This invention provides compositions and methods for reducing hyperpigmentation. In preferred embodiments, the compositions are topical compositions that contain kojic acid and a carrier molecule for enhancing the transdermal penetration of kojic acid. This invention also provides kits for treating hyperpigmentation.
Owner:REVANCE THERAPEUTICS INC
Preparation method of novel medicinal transdermal penetration enhancer
ActiveCN104277181AHigh catalytic activityExtended service lifePharmaceutical non-active ingredientsPolyolefinIrritation
The invention discloses a preparation method of a novel medicinal transdermal penetration enhancer, and relates to a synthesis method of liquid hydrogenated polyolefin. The method includes the steps of catalyst preparation, olefin catalytic polymerization and polyolefin hydrogenation. Polyolefin obtained through the method is colorless, tasteless and nontoxic, has the characteristics of high chemical stability, chemical inertness and high permeability, has no irritation or discomfort to skins as a transdermal absorption enhancer, has good compatibility with the skins, has no pharmacological activity, is suitable for medicines of all transdermal absorption dosage forms, and is a novel transdermal absorption enhancer.
Owner:XIAN IAMTECH POLYMER MATERIAL
Transdermal delivery preparation in three-dimensional netty spatial configuration of agomelatine and preparation method thereof
ActiveCN103830206AAvoid oral gastrointestinalAvoid liver first pass effectOrganic active ingredientsNervous disorderBlood concentrationSilicon dioxide
The invention discloses a transdermal delivery preparation in three-dimensional netty spatial configuration of agomelatine and a preparation method thereof. The transdermal delivery preparation consists of a backing layer, a medicine-loading system in a three-dimensional netty spatial configuration coated on the backing layer and an anti-sticking layer compounded on the system. The medicine-loading system in the three-dimensional netty spatial configuration comprises the following components in percentage by weight: 1-40% of agomelatine, 0-10% of nano-porous carbon dioxide, 40-90% of a pressure-sensitive adhesive, 1-30% of a transdermal penetration enhancer and 0-20% of a dispersant. The transdermal delivery preparation disclosed by the invention not only can continuously deliver the medicine in a transdermal manner for a longer time to maintain the constant blood concentration, but also is quick in transdermal absorption rate and high in transdermal absorptive amount, so that the preparation has the characteristics of stability and efficiency.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
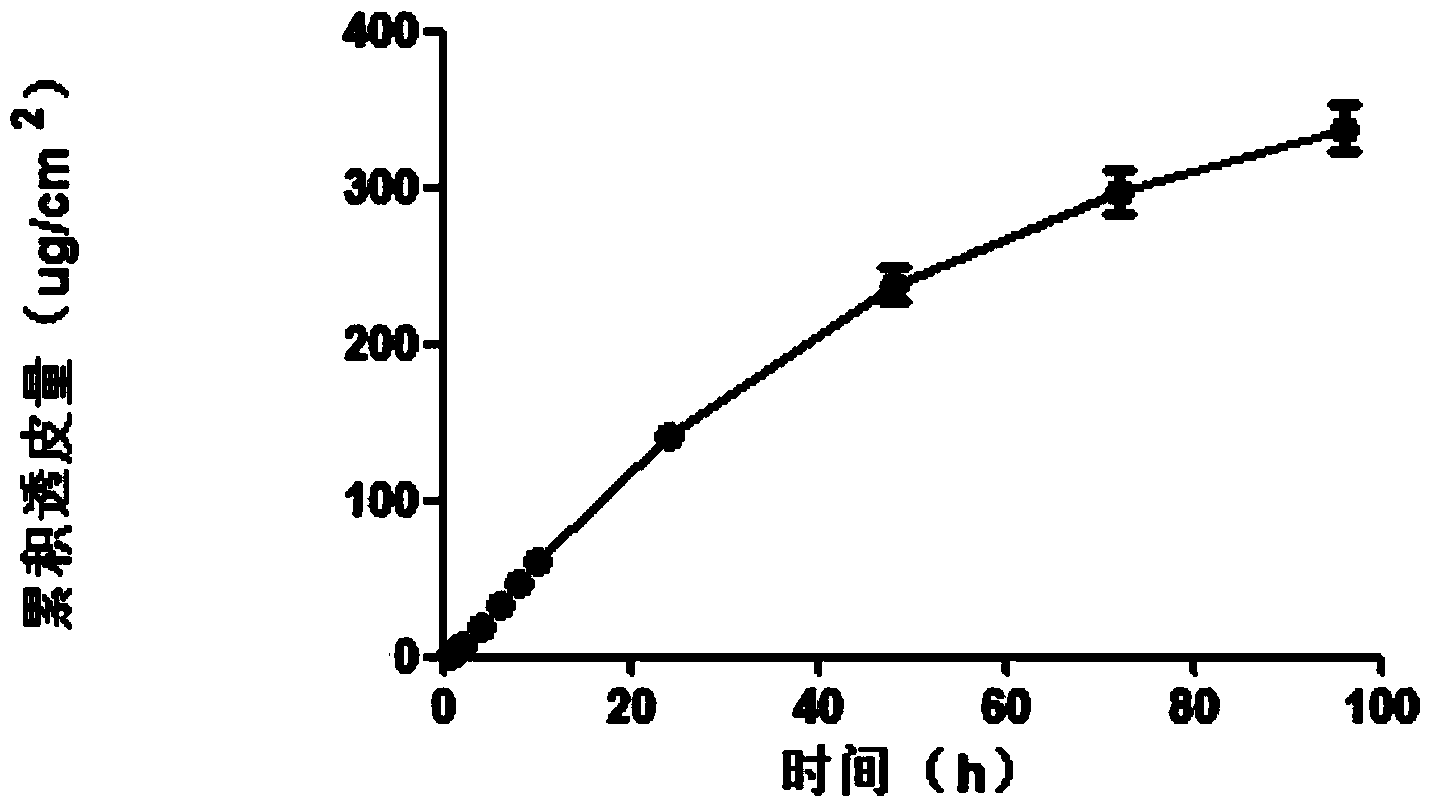
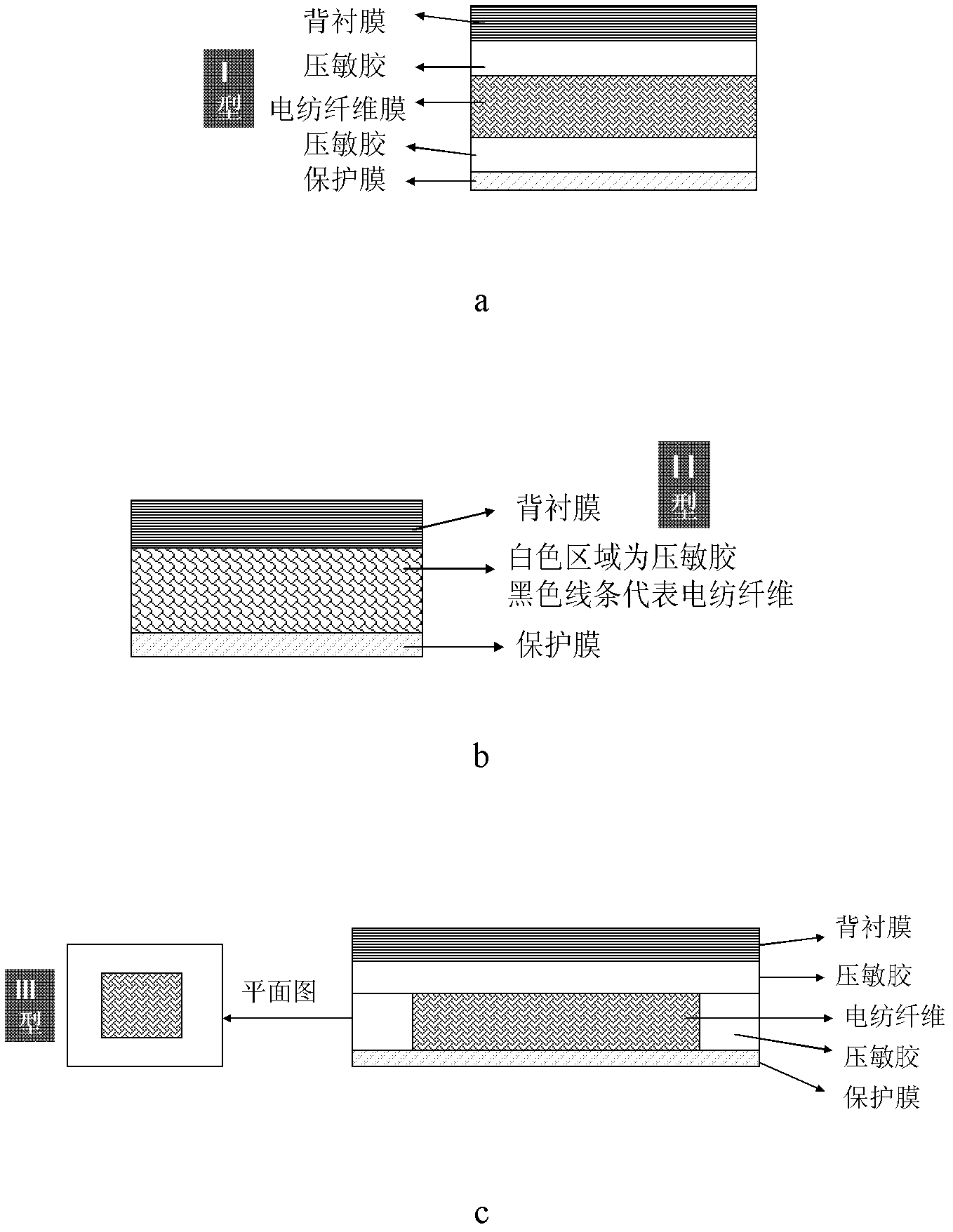
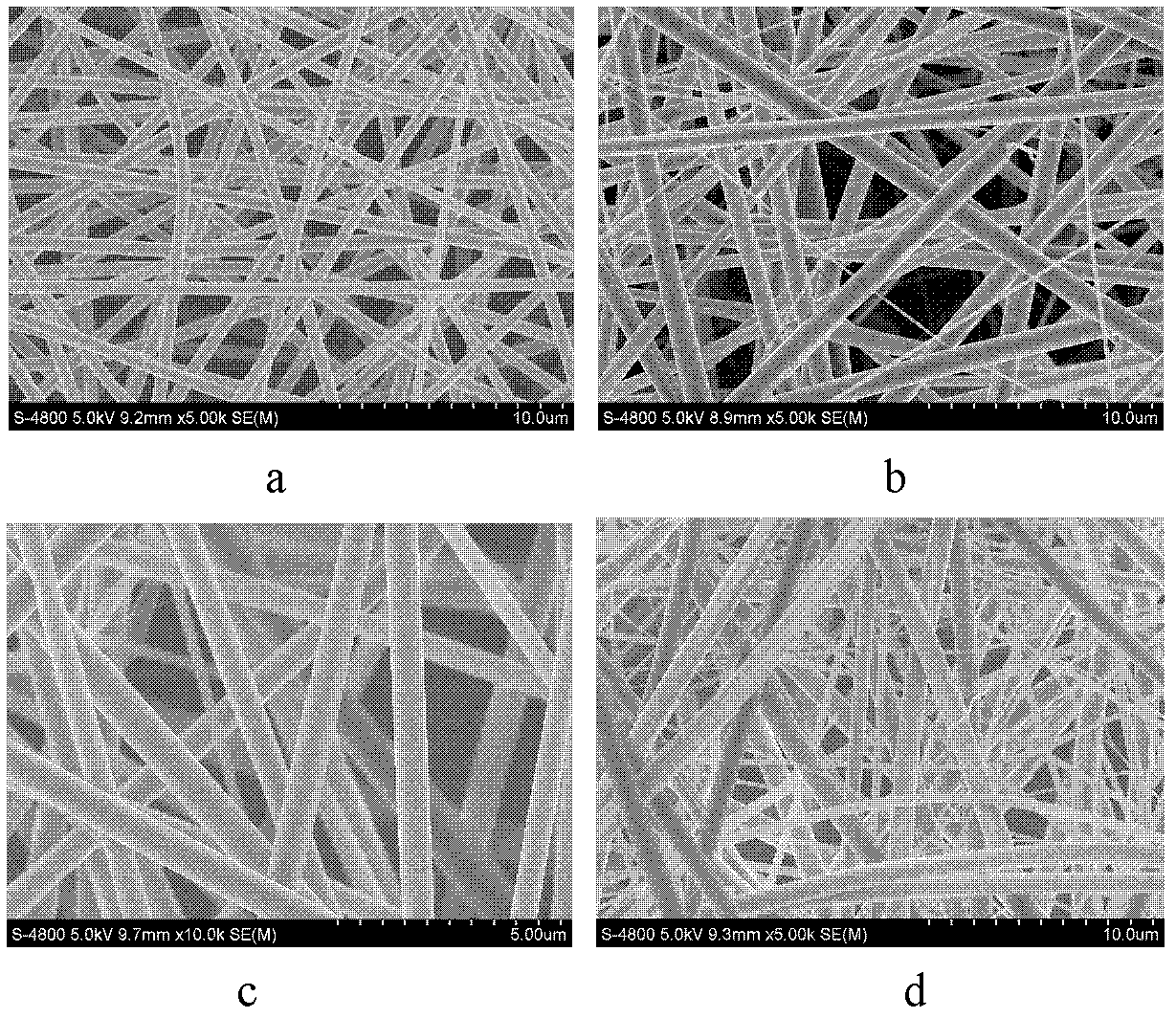
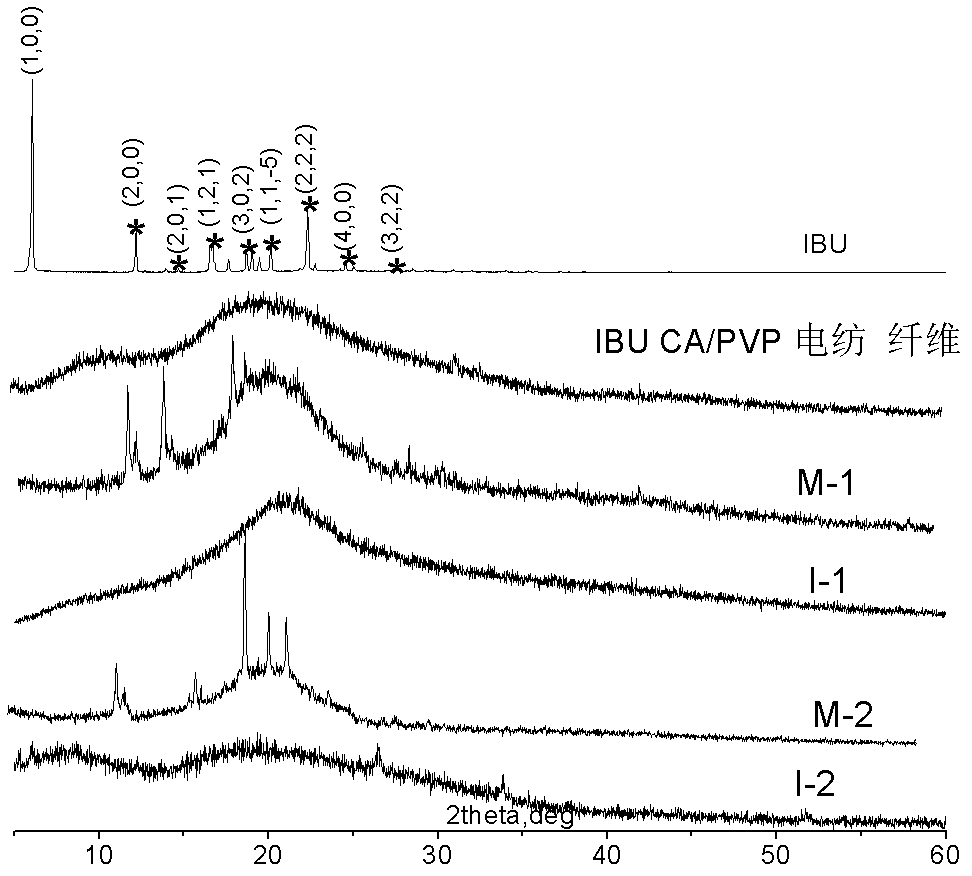
Method of preparing polymer electrospinning fiber and application in transdermal drug delivery patch
InactiveCN102552220AImprove sustained releaseAdhesion effectCosmetic preparationsToilet preparationsTectorial membraneFiber
The invention relates to a method of preparing polymer electrospinning fiber and application in a transdermal drug delivery patch. At least one drug or transdermal enhancer is loaded on polymer electrospinning fiber to form a polymer electrospinning fibrous membrane which is then combined with a backing membrane, pressure-sensitive adhesive and a protective membrane, thus forming a patch which can be adhered on the skin and used for transdermal penetration and drug delivery. The polymer electrospinning fiber transdermal drug delivery patch is combined by the polymer electrospinning fibrous membrane, the pressure-sensitive adhesive, the backing membrane and the protecting membrane; matters such as the patch, drug, transdermal enhancer and crystallization inhibitor can be dissolved or diffused into the polymer solution, and loaded on the fiber during the polymer fibration process, thus achieving the effects high loading amount of the drug, the transdermal enhancer and the like and crystallization inhibition effect, overcoming the problem of low loading amount of pressure-sensitive adhesive (or transdermal enhancer), and reducing the influence of components such as the drug and the transdermal enhancer on the adhesion of the pressure-sensitive adhesive.
Owner:TIANJIN UNIV
Gastrodia elata genin transdermal gel for central nervous system disease treatment
The invention provides a gastrodia elata genin transdermal gel for central nervous system disease treatment, which includes the components of (by weight percentage): 0.1-10% of gastrodia elata genin, 0.5-2% of transdermal sorbefacient, 0.03-1% of preservative, 5-10% of moisturizers, 1-4% of gel substrate, 5-35%alcohol solvent and water in the rest percentage. The gastrodia elata genin of transdermal gel is the main active component of gastrodia elata, has good transdermal penetration property, and can be absorbed through skin to enter blood circulation for having sedative and hypnotic medical efficacies; so the gastrodia elata genin transdermal gel is used for the treatment of central nervous system diseases such as insomnia, dizzy and neurasthenia, and has the advantages of durable efficacy, safety and easy production and preparation.
Owner:SOUTHERN MEDICAL UNIVERSITY
Transdermal drug delivery preparation with three-dimensional mesh stereoscopic configuration and preparation method of transdermal drug delivery preparation
InactiveCN104546804AImprove complianceFast absorption ratePharmaceutical non-active ingredientsSheet deliveryBlood concentrationMedicine
The invention discloses a transdermal drug delivery preparation with three-dimensional mesh stereoscopic configuration and a preparation method of the transdermal drug delivery preparation. The transdermal drug delivery preparation with the three-dimensional mesh stereoscopic configuration is composed of a transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration, a backing layer and an anti-sticking layer, wherein the backing layer is compounded on one side of the transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration; and the anti-sticking layer is compounded on the other side of the transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration. With nanoporous silica as a carrier, the defects that a preparation is low in drug loading capacity, medicines are easily separated out and crystallized to affect the transdermal absorption efficiency, the adhesion performance of a pressure-sensitive adhesive system is not ideal enough, the pressure-sensitive adhesive system is easy to age, and the medicine stability is poor are solved; the long-term lasting transdermal penetration of the medicine can be effectively achieved; the stable blood concentration can be maintained; and the preparation has the characteristics of high transdermal absorption rate, high transdermal absorption amount, stability and high efficiency.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
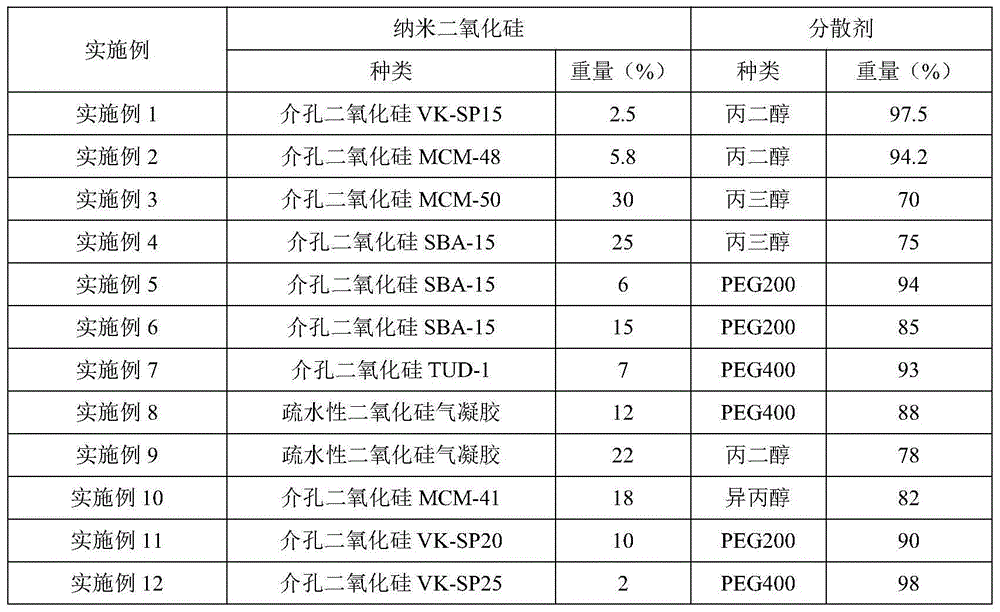
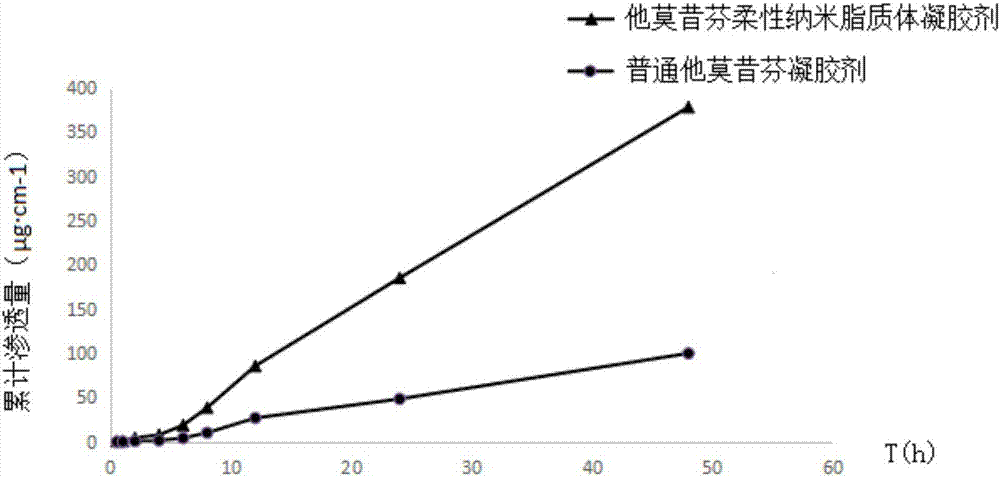
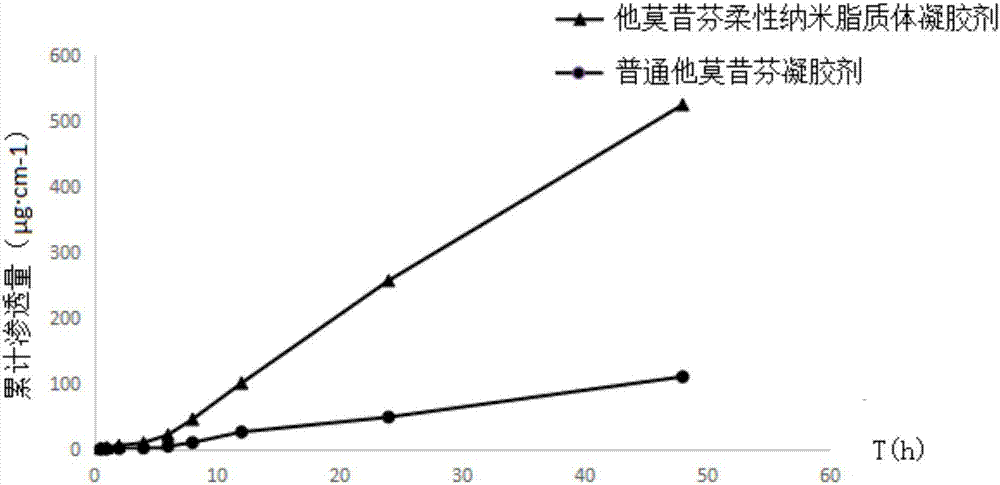
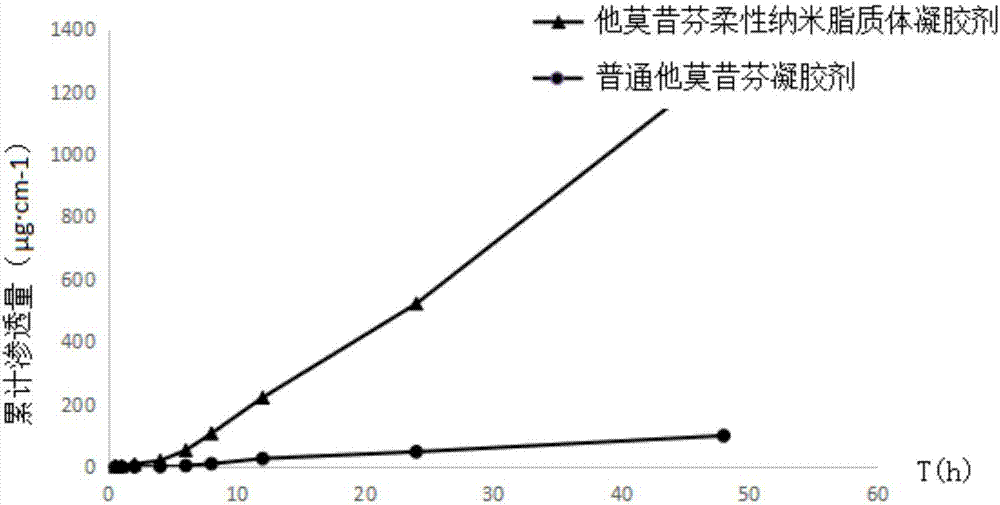
Tamoxifen flexible nano-liposome gel and preparation method thereof
ActiveCN106924176AEasy to prepareEasy to operateOrganic active ingredientsAerosol deliveryCholesterolPhospholipid
The invention discloses a tamoxifen flexible nano-liposome gel and a preparation method thereof. The gel is prepared from the following raw materials at a certain ratio: tamoxifen, cholesterol, phospholipid material, water-soluble gel substrate, transdermal penetration enhancer, surface active agent, preservative, humectants, pbs buffer solution, ammonia water, distilled water and ethyl alcohol. The preparation method comprises the following steps: 1) preparing a tamoxifen flexible nano-liposome suspension; 2) preparing a blank gel substrate; and 3) uniformly mixing the tamoxifen flexible nano-liposome suspension with the blank gel substrate, thereby acquiring the tamoxifen flexible nano-liposome gel. The gel has higher drug-carrying capacity and encapsulating effect to tamoxifen and has higher stability and transdermal permeability. The preparation method is simple, the operation is convenient and the preparation cost is low.
Owner:JINGCHU UNIV OF TECH
Chinese wolfberry nutrition eye mask
The invention provides a Chinese wolfberry nutrition eye mask, which is characterized in that Chinese wolfberry nutrition powder, a Chinese wolfberry natural active component extract, Chinese wolfberry oil or wolfberry seed oil is taken as an adsorbent, non-woven fabrics, gel or nanometer far-infrared ceramic micropowder is taken as a carrier, 0.1%-0.5% sodium hyaluronate or its aqueous solution is added to prepare the Chinese wolfberry nutrition eye mask. According to the mechanisms of a penetration acupoint therapy and a transdermal penetration therapy, the nutrient composition of Chinese wolfberry can be directly effected on eyes, and is capable of supplementing the nutrition required by eyes, and improving the microcirculation of eyeground. The Chinese wolfberry nutrition eye mask has the characteristics of rapid take effect, comprehensive and abundant nutrition. The Chinese wolfberry nutrition eye mask has the substantial curative effects for treating eye acid bilge, ache, lachrymation, visual display terminals syndrome and the like caused by myopia, amblyopia, astigmatism and fatigue eyesight, and has the advantages of no toxicity, no side-effect, safe and convenient usage.
Owner:天津药食同源健康产业有限公司
Transdermal spray preparation for plastic mist membranization and preparation method of transdermal spray preparation
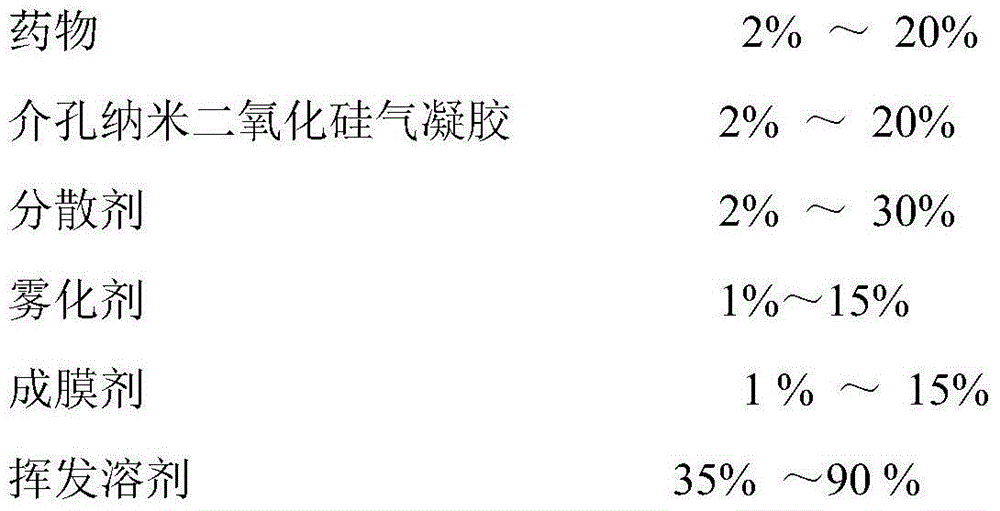
ActiveCN104414975APromote absorptionImprove complianceOrganic active ingredientsAntipyreticPorosityControlled release
The invention discloses a transdermal spray preparation for plastic mist membranization and a preparation method of the transdermal spray preparation. The transdermal spray preparation for plastic mist membranization comprises the following components in percentage by mass: 2%-20% of medicine, 2%-20% of mesoporous nano silica aerogel, 2%-30% of a dispersing agent, 1%-15% of atomizing agent, 1%-15% of a film-forming agent and 35-90% of a volatile solvent. The mesoporous nano silica aerogel is a controlled-release mesoporous nanometer carrier; the porosity of the mesoporous nano silica aerogel is 90%-99.8%; the aperture of the mesoporous nano silica aerogel is 20-100nm; the three-dimensional nano particle size is 2-70nm; the specific surface area is 100-1,000m<2> / g; the density is 0.003-30g / cm<3>; and the heat-conducting coefficient is 0.01-0.018w / m.k. According to the transdermal spray preparation, long-time continuous transdermal penetration of the medicine can be effectively achieved; the constant blood concentration is maintained; and the preparation has the characteristics of high transdermal absorption rate, high transdermal absorption amount and is stable and efficient.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Liquid woundplast, and preparation method thereof
The invention relates to a liquid woundplast, and a preparation method thereof. The liquid woundplast is prepared from, by mass, 1 to 5% of matrine, 1 to 5% of oxymatrine, 10 to 15% of circium japonicum, 1 to 2% of borneol, 0.5 to 2% of glycerin, 0.5 to 5% of a transdermal penetration enhancer, 3 to 8% of a film-forming material, and the balance ethanol. The liquid woundplast is capable of promoting wound healing, sterilizing, inhibiting bacteria, and relieving pain, is capable of forming a layer of thin film on the surface of human body, is convenient to use, and is capable of providing safer protection compared with common woundplast; and after coating, the thin film possesses water resistance, and wound surfaces are coated with the thin film firmly.
Owner:NINGXIA VOCATIONAL TECH COLLEGE
Method for electrically polymerizing water and oil body through bio-based ionic liquid
InactiveCN108079896AHigh affinityEvenly blendedCosmetic preparationsElectrolysis componentsElectricityCompound (substance)
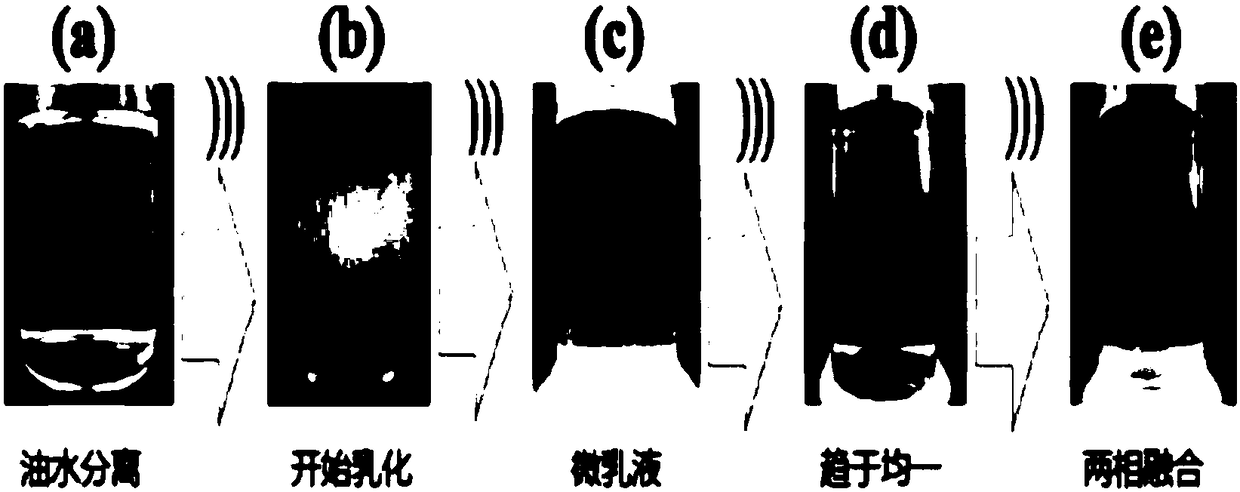
The invention discloses application of electrically polymerizing a water and oil body through bio-based ionic liquid. Ionic liquid is mainly prepared from plant acid and plant alkaline through one-step reaction; ionic liquid formed by an action between acid and alkaline has very high affinity with water; meanwhile, a hydrophobic group is introduced, so that the ionic liquid further generates interaction with hydrophobic molecules (an oil phase); and the ionic liquid supplements with the hydrophobic molecules, so that the ionic liquid can fuse a water phase and the oil phase; the ionic liquid is stirred for 20-60 minutes under an electric polymerization condition of 20 kHz-40kHz, so that the water and oil body is fused more uniformly, the use of chemical emulsifiers is also avoided, and thewater and oil body can be fused by the method safer. The method is beneficial for preparing substrate materials of safe, natural and gentle daily chemical products; and the formed fused molecular groups are small and are regularly arranged, so that hydrophilic-lipophilic properties are better, and transdermal penetration is realized easier.
Owner:中健医疗器械(广州)有限公司
Sodium heparin loaded hydrogel slow-release plaster
InactiveCN106421869AHigh biosecurityIncrease moisture contentAbsorbent padsBandagesDesorptionHigh energy
The invention relates to a medical dressing, in particular to sodium heparin loaded hydrogel slow-release plaster which comprises a fixed layer, a backing layer and a hydrogel functional layer, wherein the backing layer and the hydrogel functional layer are sequentially arranged on the fixed layer, and the hydrogel functional layer is prepared from hydrophilic high-molecular compounds, sodium heparin and transdermal penetration enhancer water solution through high-energy ray radiation or freezing and thawing cycle or combination of high-energy ray radiation and freezing and thawing cycle. The hydrogel slow-release plaster has the advantages that sheet solid hydrogel materials synthesized by the hydrophilic high-molecular compounds with high biological safety have high biological safety, water content and the like, a molecular structure is compact, water desorption rate can be controlled, service time is prolonged, in addition, the hydrogel slow-release plaster prepared from the sheet solid hydrogel materials serving as a medicine slow-release component is simple to operate and convenient to use and can be timely removed if discomfort and adverse reaction occur, and deterioration of adverse reaction is avoided.
Owner:长春吉原生物科技有限公司 +1
Anticancer analgesic percutaneous absorption preparation
InactiveCN101229229AReduce adverse effectsHarm reductionNervous disorderAnthropod material medical ingredientsCantharisLiver and kidney
The invention discloses an anticancer and analgesic percutaneous absorption preparation, which is prepared by the following method: (1) adopting the materials according to the following weight proportion: cantharis of 0.01 to 0.3 portion; nux vomica of 0.1 to 3 portions; (2) extracting the materials by ethanol solution and obtaining the extractant; (3) adding transdermal penetration enhancers. As adopting transdermal target medication system and being added with the auxiliary materials accepted by pharmacy, the anticancer and analgesic percutaneous absorption preparation of the invention can be made into adhesive plaster in emplastrum, cataplasma paste, and emplastrum; or the invention can be made into plaster, gelata, ointment, liniment, aerosol or spray agent, so the invention can deliver the medicine successively with long time, maintain necessary blood concentration, overcome the bad influence on human body when the plasma concentration reaches peak value after oral administration, and avoid the first-pass effect. The invention has little damage on liver and kidney and reaches the purpose of taking effect both from the symptom and the cause root.
Owner:崔建平
Transdermal patch containing proton pump inhibitor drug and preparation method of transdermal patch
InactiveCN107929268AAvoid oral gastrointestinalReduce the influence of gastrointestinal environmentOrganic active ingredientsDigestive systemTransdermal patchBlood concentration
The invention relates to a transdermal patch containing a proton pump inhibitor drug. The transdermal patch is characterized by mainly comprising a back lining layer, a drug library layer and an anti-sticking layer, wherein the drug library layer contains the following components in percentage by weight: 0.1%-40% of active components, 20%-90% of a drug library matrix, 1%-5% of a stabilizer, 1%-30%of a transdermal absorption enhancer and 0-20% of a dispersing agent. According to the transdermal patch, long-time continuous transdermal penetration of the drug can be effectively realized, a constant blood concentration can be maintained, and a preparation is high in transdermal absorption speed and transdermal absorbing capacity; and the transdermal patch has the characteristics of stability,high efficiency, relatively high pharmaceutical safety and the like.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Rutaecarpin transdermal patch and preparation method thereof
ActiveCN105395521AAvoid first pass effectCumulative penetration is highOrganic active ingredientsDigestive systemTransdermal patchCellulose
The invention provides a rutaecarpin transdermal patch which is composed of a backing layer, a medicine-containing adhesion layer and an anti-adhesion layer. The medicine-containing adhesion layer is composed of rutaecarpin, transdermal penetration enhancer and pressure-sensitive adhesive; the transdermal penetration enhancer is formed by mixing and using one or more of alcohol, fatty acid and esters of fatty acid, surface active agents, terpene, terpene, amino acid and ester or phospholipid compounds of amino acid, or combined penetration enhancer of oleic acid and azone; the pressure-sensitive adhesive is one of polyisobutylene class pressure-sensitive adhesive, silicone class pressure-sensitive adhesive, polyacrylic resin class pressure-sensitive adhesive or cellulose. The preparation method of the rutaecarpin transdermal patch includes the steps that medicine-containing adhesive is prepared; solvent is picked to disperse rutaecarpin, the pressure-sensitive adhesive is added and stirred to be uniform, then the penetration enhancer is added and stirred uniformly, and degassing is conducted for standby use; obtained medicine-containing adhesive liquid is arranged on the anti-adhesion layer in a coated mode, the coating thickness is controlled accurately, the anti-adhesion layer stands still at the room temperature to remove solvent and is heated, cured, coated with a backing film and cut, and the rutaecarpin transdermal patch is obtained.
Owner:ZUNYI MEDICAL UNIVERSITY
5-hydroxymethyl tolterodine gel preparation and preparation method thereof
InactiveCN101843579AAvoid or reduce adverse reactionsAvoid the disadvantages of taking awayOrganic active ingredientsPharmaceutical delivery mechanism5-hydroxymethyl tolterodineMoisture
The invention provides a 5-hydroxymethyl Tolterodine gel preparation. The 5-hydroxymethyl Tolterodine gel preparation is in external use through being applied and has the transdermal medication effect, and the defects of low bioavailability and many untoward effects of an oral preparation are overcome. The 5-hydroxymethyl Tolterodine gel preparation is mainly prepared from 5-hydroxymethyl Tolterodine, a gel substrate, a filming substrate, an organic silicon elastic body, a transdermal penetration promoting agent, a pH regulating agent, a moisture preserving agent and the like. When the 5-hydroxymethyl Tolterodine gel preparation is in use, the gel preparation contains the filming substrate capable of forming flexible films after being dried, the 5-hydroxymethyl Tolterodine gel preparation is in external use to be applied on the parts of the thigh, the abdomen, the upper arm or the shoulder and the like, can fast form flexible and wear-resistance films on the selected skin regions, and can prevent the gel from being brought away by clothes, at the same time, the medication parts are in a non-sealed state, and the ventilation effect is good. The contained organic silicon elastic body can obviously improve the performance such as the dispersivity, the softness, the wettability, the lustrousness and the like of the gel system, the prepared gel is smooth, translucent and exquisite, the skin has no foreign body sensation after use, the medicine is perpetually and slowly released, the transdermal absorption effect is good, the use is convenient, and the adaptability of the patients can be improved.
Owner:李又欣 +2
Adaptive transdermal iontophoresis method introduction system for cosmetology field
PendingCN108853720AImprove beauty effectImprove securityExternal electrodesDosing regimenElectronic systems
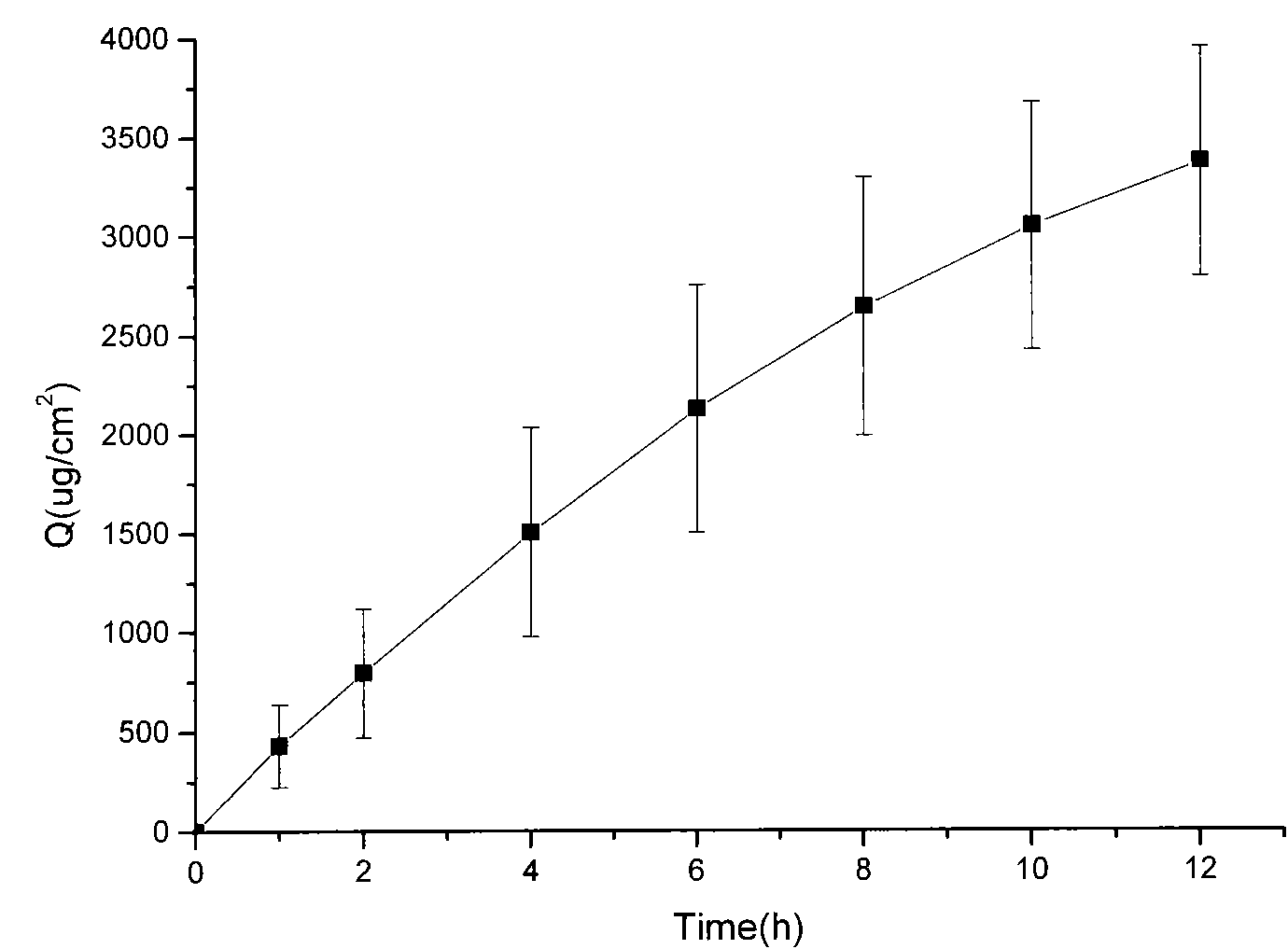
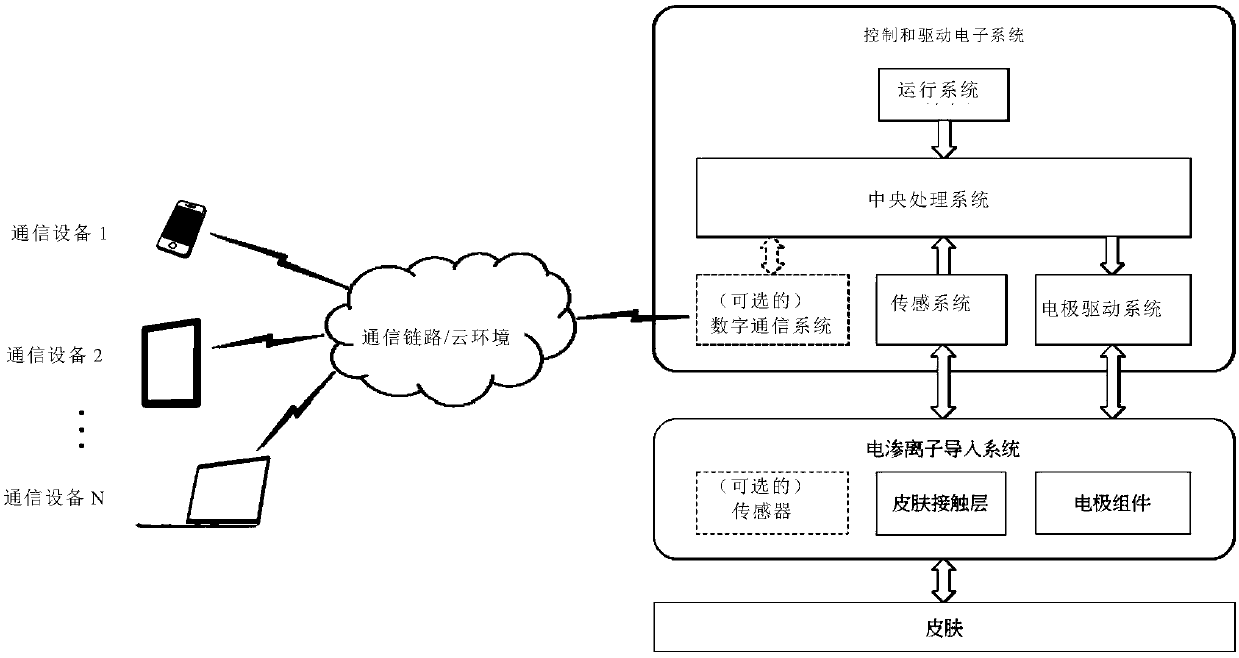
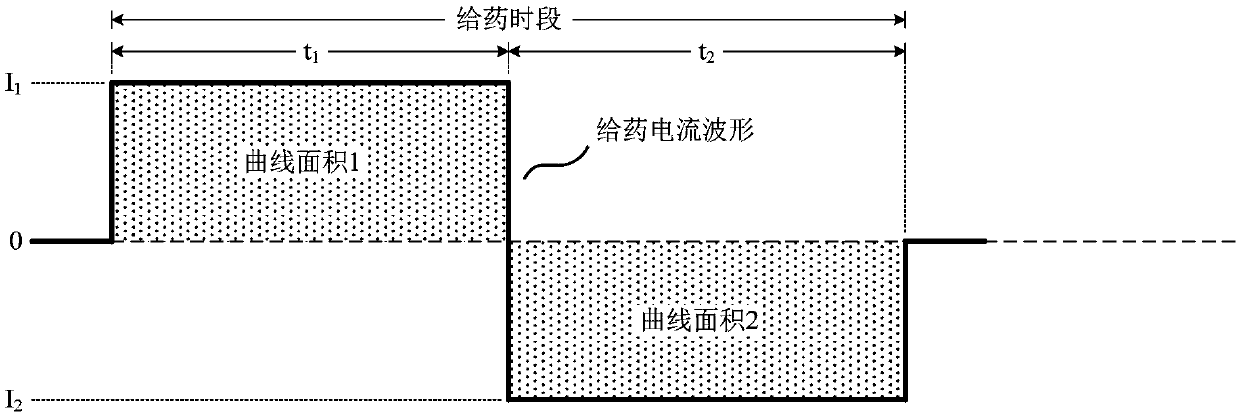
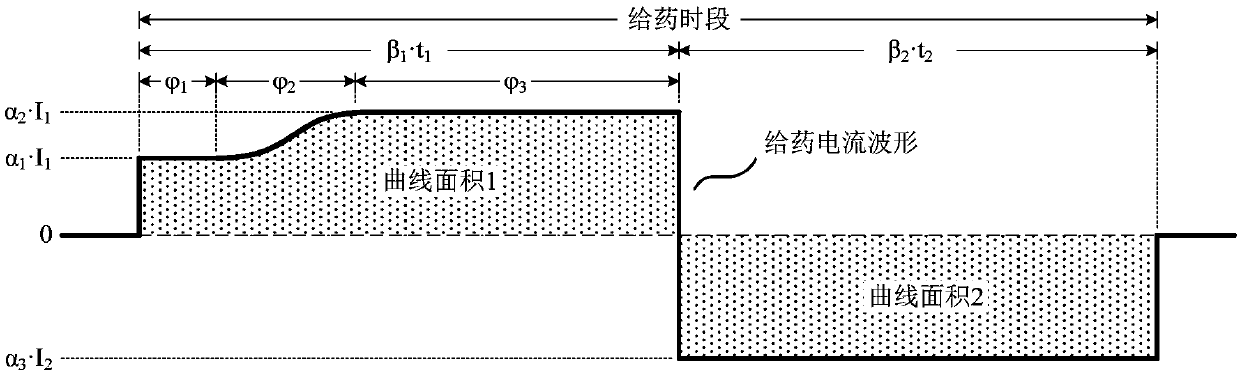
The invention relates to an adaptive transdermal iontophoresis method introduction system for the cosmetology field; specifically, the system comprises an iontophoresis introduction system and a control and drive electronic system; the control and drive electronic system comprises an operation system, a central processing system, an electrode drive system, a sensing system and a data communicationsystem; the adaptive transdermal iontophoresis method introduction system can customize personal transdermal dosage regimens according to user skin real conditions, can optimize the transdermal penetration dosage, depth and speed in real time, thus improving the product usage effect and safety in a maximum level.
Owner:SHANGHAI FUTAI TECH CO LTD
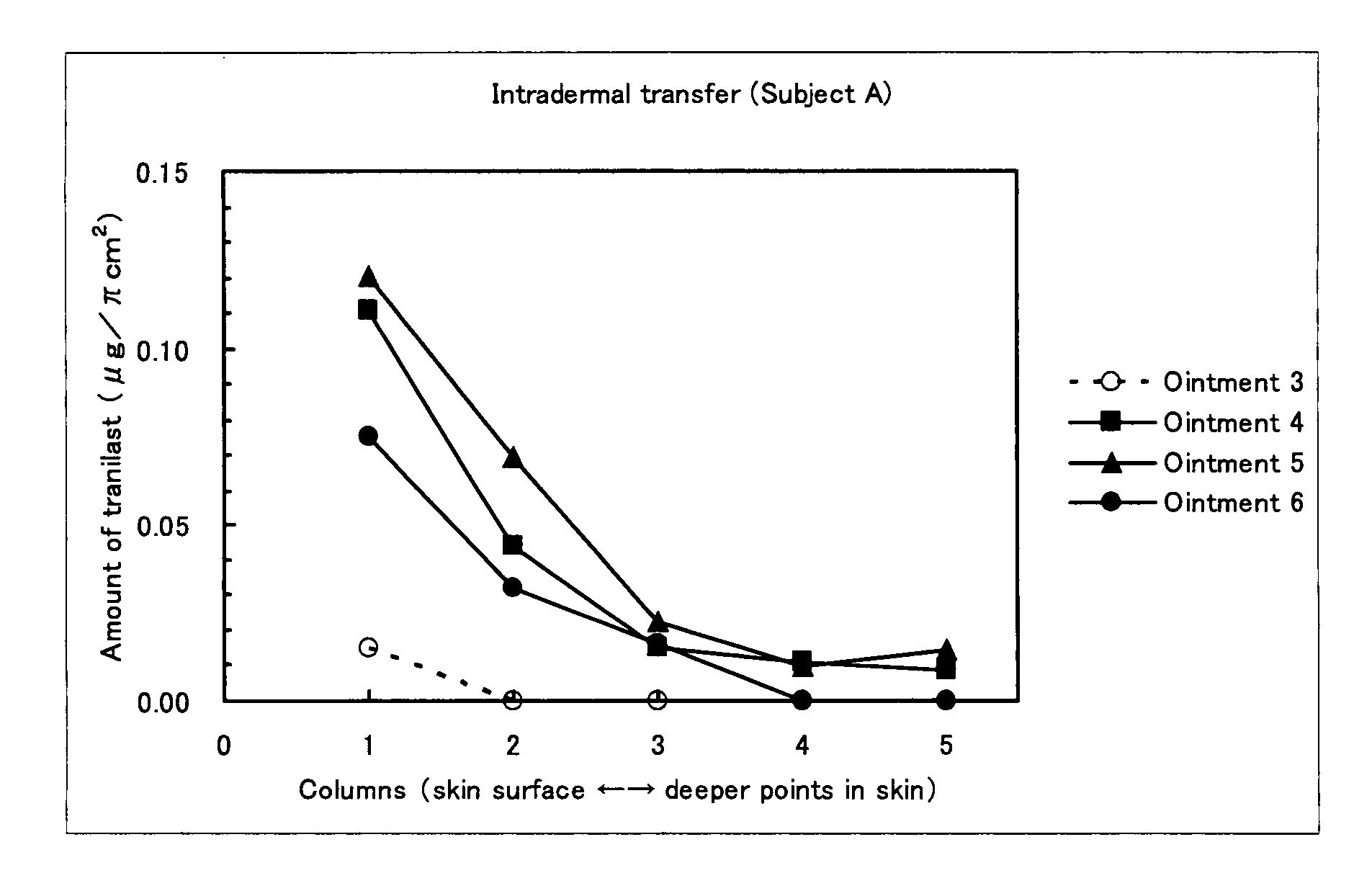
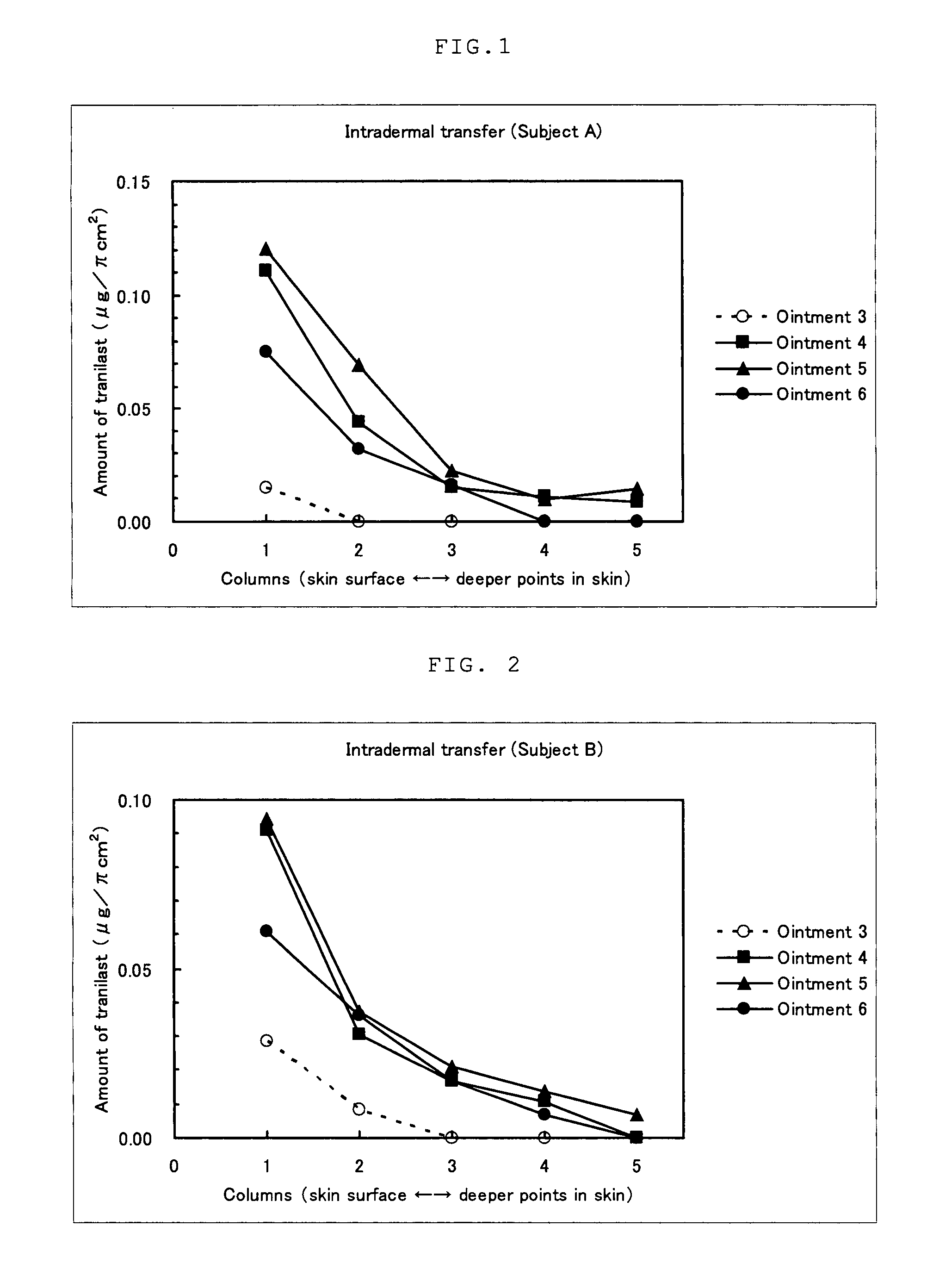
External Preparation
InactiveUS20080108700A1Improve light resistancePoor photostabilityBiocidePeptide/protein ingredientsMast cellPharmacology
It is an object of the present invention to provide an external preparation having enhanced transdermal penetration of a mast cell degranulation inhibitor. It is also an object of the present invention to provide a method for improving the photostability of a preparation containing a mast cell degranulation inhibitor. The present invention provides an external preparation containing a mast cell degranulation inhibitor and a topical anesthetic. Further, the method for enhancing the photostability of a preparation containing a mast cell degranulation inhibitor according to the present invention includes adding a topical anesthetic thereto.
Owner:MEDRX CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com