Sodium heparin loaded hydrogel slow-release plaster
A technology of hydrogel and heparin sodium, which is applied in medical science, absorbent pads, bandages, etc., can solve the problems of fast water loss, inability to continue releasing active ingredients, loose molecular structure, etc., and achieve tight molecular structure and high water content , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
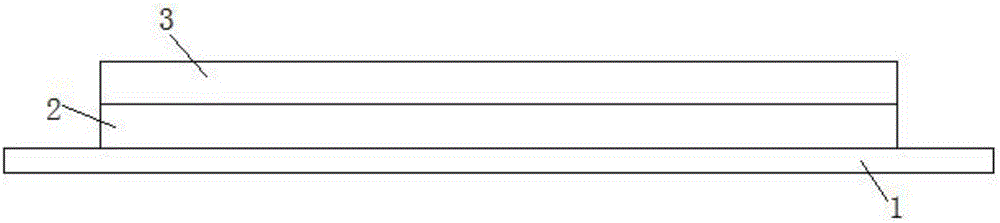
[0022] Embodiment one: if figure 1 As shown, the hydrogel sustained-release patch loaded with heparin sodium in this embodiment includes a fixed layer 1, a backing layer 2 and a hydrogel functional layer 3 that are sequentially arranged on the fixed layer 1, wherein the hydrogel functional layer is It is prepared by freeze-thaw cycle synthesis after fully dissolving 10% polyvinyl alcohol, 0.07% heparin sodium, 1% laurocapram and 88.93% water; the backing layer is made of semi Permeable membrane with a water vapor transmission rate of 500g / (m 2 ·24h), the backing layer 2 is closely bonded to the hydrogel functional layer 3;
[0023] The above-mentioned hydrogel sustained-release patch was applied to the abdomen of the human body. After using it for 12 hours, the content of heparin sodium in the hydrogel functional layer 3 and the four items of blood coagulation in the human body were detected, and the results showed that the absorption of heparin sodium by the human body accou...
Embodiment 2
[0024] Embodiment 2: The hydrogel sustained-release patch loaded with heparin sodium in this embodiment includes a fixed layer 1, a backing layer 2 and a hydrogel functional layer 3 sequentially arranged on the fixed layer 1, wherein the hydrogel functional layer It is made of 10% polyvinyl alcohol, 2% polyvinyl pyrrolidone, 1% heparin sodium, 5% laurocaprazine and 82% water, and then synthesized by high-energy electron beam radiation Obtained; its backing layer is made of non-woven fabric, and its water vapor transmission rate is 800g / (m 2 24h), the backing layer 2 is closely combined with the hydrogel functional layer 3; the material of the fixing layer 1 is medical adhesive tape, and the fixing layer 1 is separated from the slow-release patch, and the hydrogel is slowed down by the fixing layer 1 during use. The patch is fixed on the affected area.
[0025] The above-mentioned hydrogel sustained-release patch was applied to the abdomen of the human body. After 24 hours of ...
Embodiment 3
[0026] Embodiment 3: The hydrogel sustained-release patch loaded with heparin sodium in this embodiment includes a fixed layer 1, a backing layer 2 and a hydrogel functional layer 3 arranged on the fixed layer 1 in sequence, wherein the hydrogel functional layer It is prepared by gamma ray radiation synthesis after fully dissolving 20% polyacrylic acid, 25% starch, 3% heparin sodium, 2% laurocaprazine and 50% water; its backing The material of the layer is non-woven fabric, and its water vapor transmission rate is 650g / (m 2 ·24h), the backing layer 2 is closely connected with the hydrogel functional layer 3; the fixing layer 1 is made of medical tape, and is tightly connected with the backing layer 2.
[0027] The above-mentioned hydrogel sustained-release patch was applied to the abdomen of the human body. After 24 hours of use, the content of heparin sodium in the hydrogel functional layer 3 and the four items of blood coagulation in the human body were tested. The results...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com