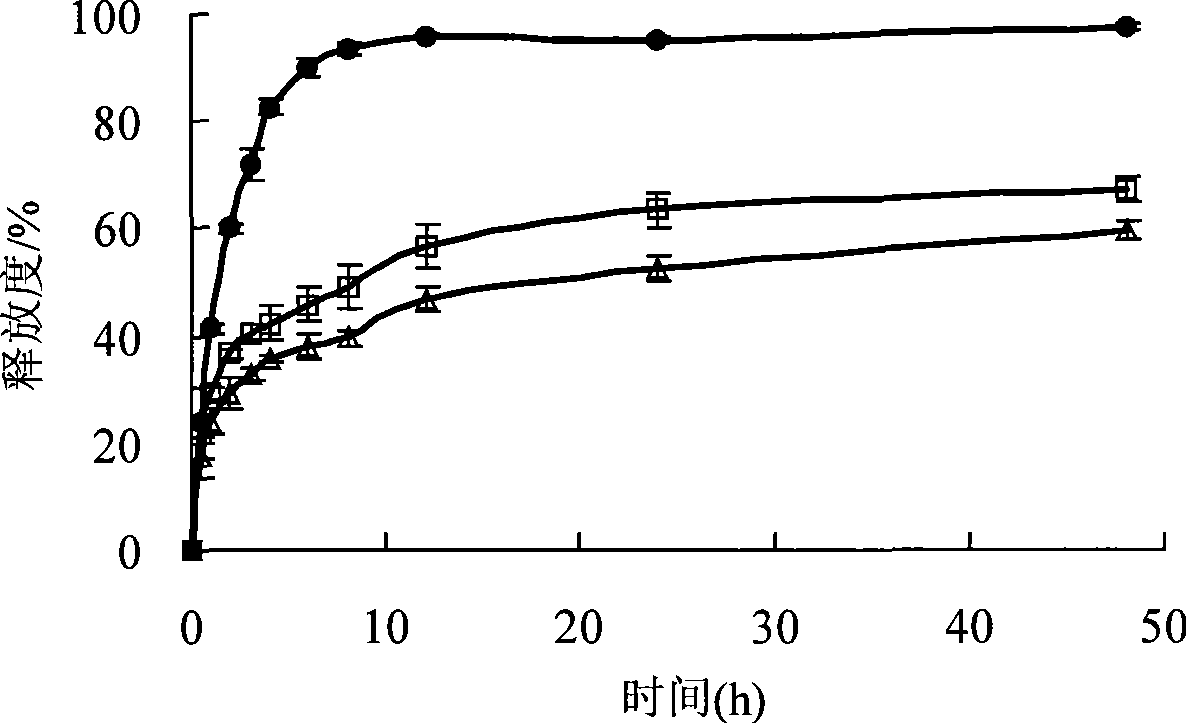
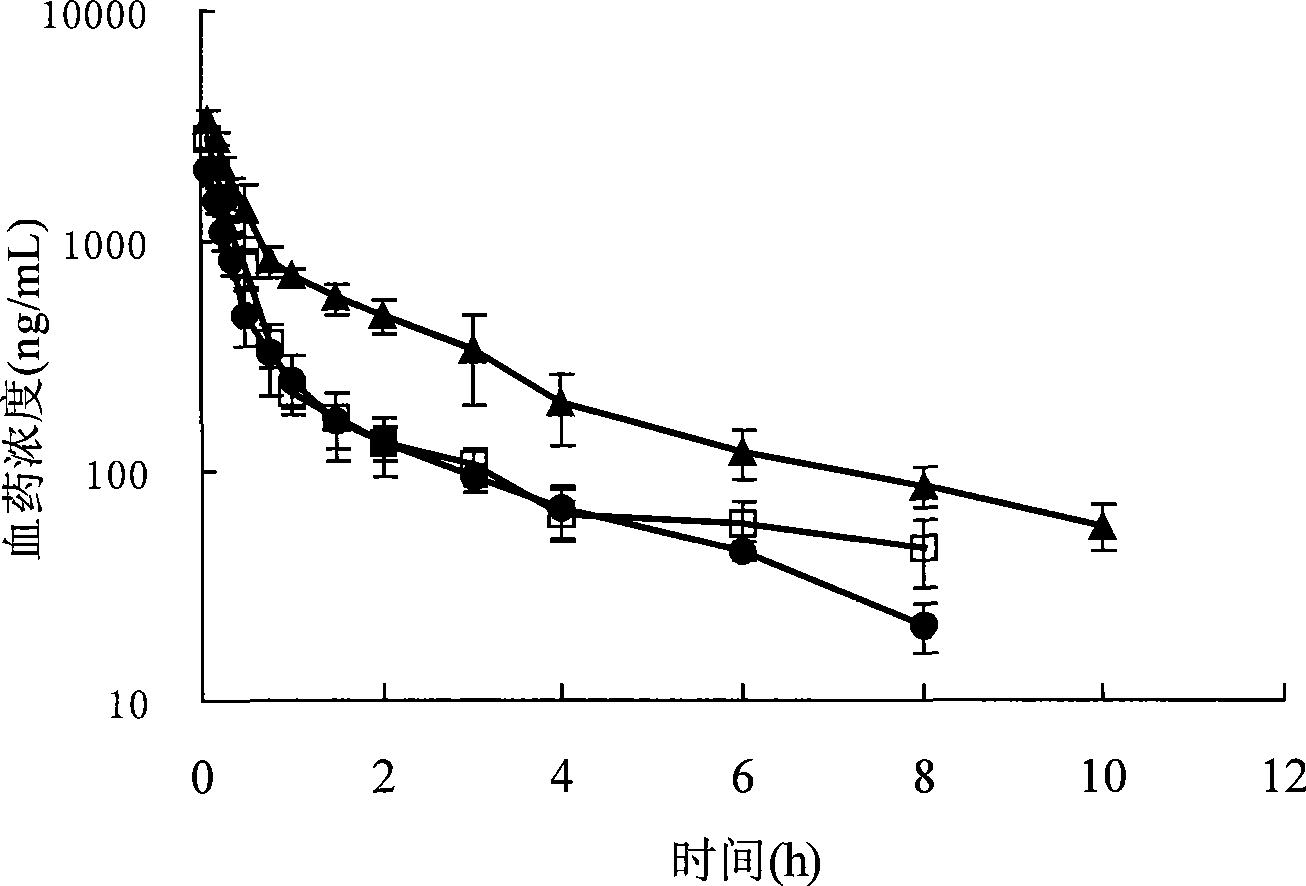
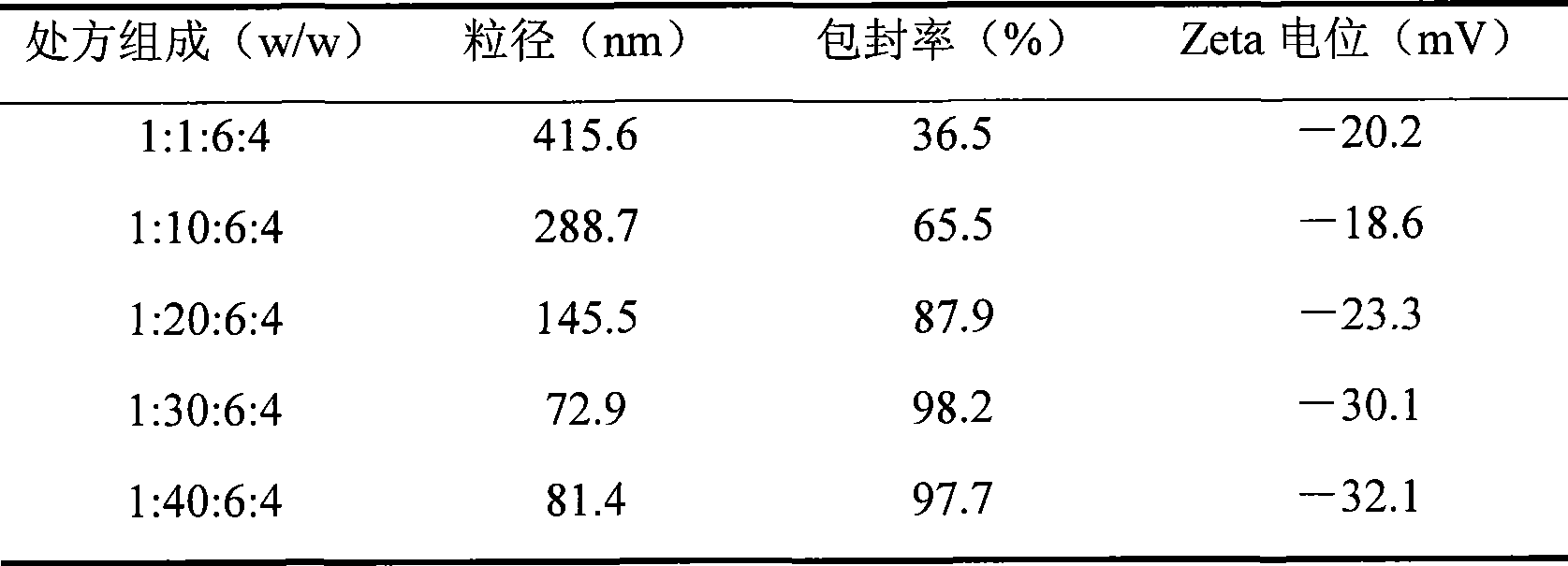
Biogastrone acid prosome liposome with long circulation function and preparation method thereof
A technology of proliposome and glycyrrhetic acid, which is applied in the directions of liposome delivery, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems such as the lack of proliposomes , to achieve the effect of simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Synthesis of intermediate lactide
[0054] Put quantitative lactic acid into the three-necked bottle, pass N 2 Gas, add zinc oxide under mechanical stirring, heat to 120°C, and evaporate the water contained in the lactic acid. Then the temperature was raised to about 140°C, and the reaction was performed for 1 hour under reduced pressure to distill off the water generated during the reaction. Repeat many times until no water evaporates. Then the temperature was raised to 180°C, the fractions were collected, and recrystallized twice with ethyl acetate to obtain white flaky crystals.
[0055]
[0056] (2) Synthesis of mPEG2000-PLA block copolymer
[0057] Add a certain proportion of lactide and mPEG2000 into the three-necked bottle, seal it, N 2 Air was removed under flow, and the heating mantle was heated to 160°C. After the contents were completely melted, 0.5 wt% stannous octoate was added, and the reaction was stopped for 8 hours under magnetic stirring. T...
Embodiment 2
[0070] Prescription: Percentage (%)
[0071] Glycyrrhetinic acid 2.50
[0072] Hydrogenated soybean lecithin 80.0
[0073] Cholesterol 7.50
[0074] Sodium chenodeoxycholate 10.0
[0075] Glycyrrhetinic acid, hydrogenated soybean phosphatidylcholine, cholesterol and sodium chenodeoxycholate were sonicated with 50mL of absolute ethanol; another 50mL of pH7.4 phosphate buffer was placed in a pear-shaped bottle, heated and stirred at 60°C for uniformity. Slowly inject the ethanol solution into the phosphate buffer, sonicate for 20 minutes to form a lipid solution, remove the ethanol by rotary evaporating the lipid solution at 45°C, pass through a 0.22 μm filter membrane three times, and remove the pyrogen to obtain glycyrrhetinic acid liposome suspension. The above-mentioned liposome is hydrated and reconstituted with 5% glucose aqueous solution to obtain the glycyrrhetinic acid liposome suspension for intravenous injection. The particle d...
Embodiment 3
[0077] Prescription: Percentage (%)
[0078] Glycyrrhetinic acid 1.12
[0079] Egg yolk lecithin 40.45
[0080] Cholesterol 6.74
[0081] Sodium glycinoxycholate 2.25
[0082] mPEG2000-PLA 4.49
[0083] Lactose 44.94
[0084] Glycyrrhetinic acid, phospholipids, cholesterol, mPEG2000-PLA and sodium glycoxycholate were ultrasonically dissolved in 50 mL of ethanol, and the ethanol was removed by rotary evaporation at 45°C to form a colloidal solution. Lactose was dissolved in an appropriate amount of purified water, and pH 7.4 phosphoric acid was added. Dilute the colloidal solution with 50 mL of salt buffer solution, sonicate for 20 minutes, pass through 0.8, 0.45 and 0.22 μm filter membranes successively, filter the membrane three times repeatedly, remove pyrogen, and obtain glycyrrhetinic acid by freeze-drying method. Body liposome preparations. Hydrate and reconstitute the above proliposome with 0.9% sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com