Use of Polyethylene Glycol Derivatives-Small Molecule Drug Conjugate Polymer Micelle in Inflammation Targeted Drug Delivery System
A technology of drug conjugates and polyethylene glycol, which is applied in the field of medicine, can solve the problems of non-evaluable use, drug leakage, poor water solubility, etc., and achieve the effect of increasing accumulation and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
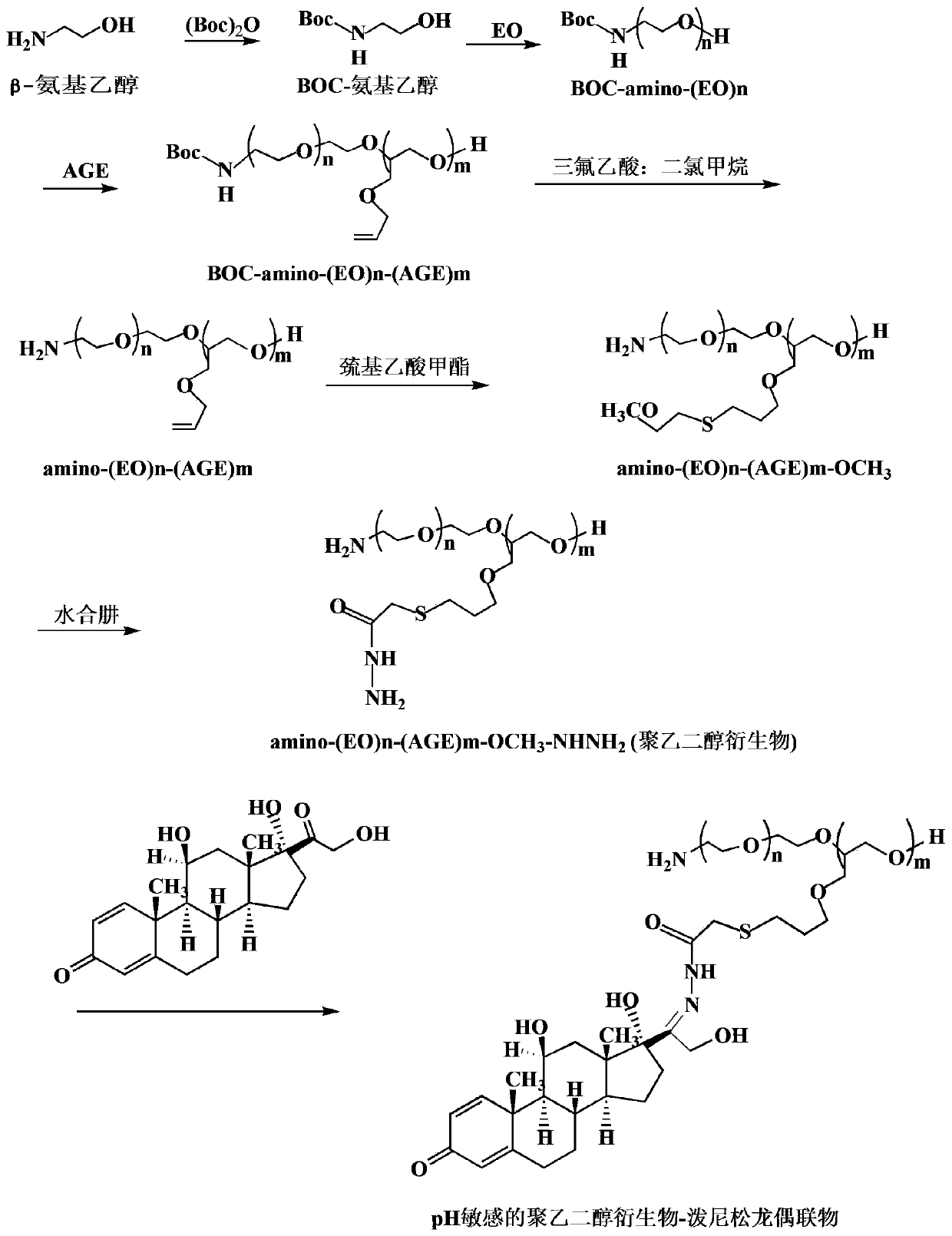
[0075] First, β-aminoethanol (7.37 g, 0.12 mol) was protected with BOC anhydride (32.72 g, 0.15 mol) to obtain BOC-aminoethanol. Next, BOC-aminoethanol (0.88 g, 5 mmol) was subjected to two-step ring-opening copolymerization with EO (100 mL, 1.98 mol) and AGE (30.1 g, 0.26 mol), respectively, to obtain BOC-N-(EO)n -(AGE)m. Then BOC-N-(EO)n-(AGE)m removes the BOC protecting group in trifluoroacetic acid / dichloromethane (v / v: 5 / 2) solution to obtain the product animo-(EO)n- (AGE)m. The above product animo-(EO)n-(AGE)m (6.72 g, 7.53 mmol C=C) was dissolved in THF, and further mixed with methyl thioglycolate under the catalysis of azobisisobutyronitrile (AIBN) in Reflux reaction under nitrogen protection for 5h, the product animo-(EO)n-(AGE)m-OCH was obtained after rotary evaporation 3 . Then all the above products were reacted with hydrazine hydrate (37.7g, 36.6 mL, 753 mmol) under reflux in THF solution for 7 h, and the product animo-(EO)n-(AGE)m-OCH was obtained by rotary e...
Embodiment 2
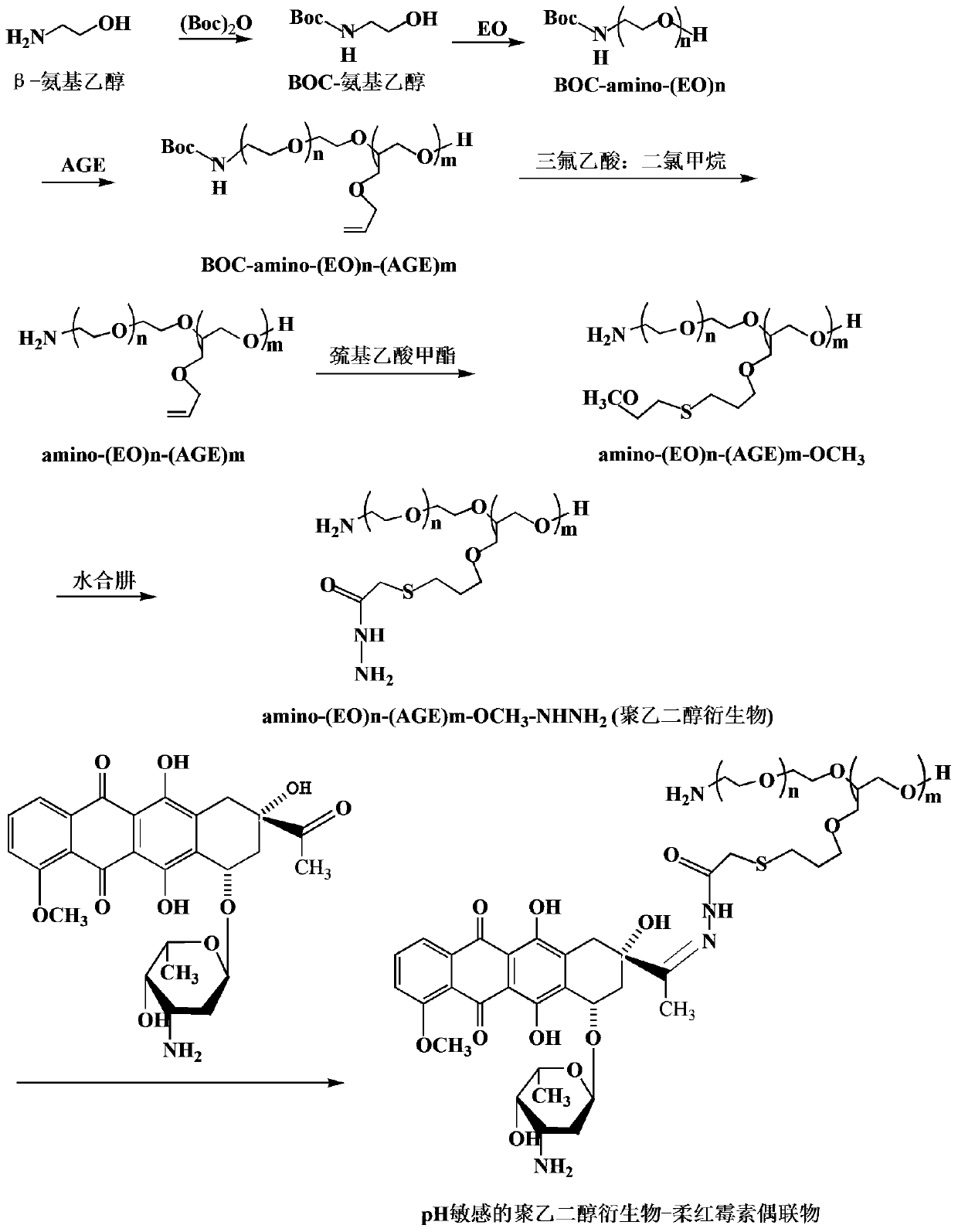
[0078] First, β-aminoethanol (7.37 g, 0.12 mol) was protected with BOC anhydride (32.72 g, 0.15 mol) to obtain BOC-aminoethanol. Next, BOC-aminoethanol (0.88 g, 5 mmol) was subjected to two-step ring-opening copolymerization with EO (100 mL, 1.98 mol) and AGE (30.1 g, 0.26 mol), respectively, to obtain BOC-N-(EO)n -(AGE)m. Then BOC-N-(EO)n-(AGE)m removes the BOC protecting group in trifluoroacetic acid / dichloromethane (v / v: 5 / 2) solution to obtain the product animo-(EO)n- (AGE)m. The above product animo-(EO)n-(AGE)m (6.72 g, 7.53 mmol C=C) was dissolved in THF, and further mixed with methyl thioglycolate under the catalysis of azobisisobutyronitrile (AIBN) in Reflux reaction under nitrogen protection for 5h, the product animo-(EO)n-(AGE)m-OCH was obtained after rotary evaporation 3 . Then all the above products were reacted with hydrazine hydrate (37.7g, 36.6 mL, 753 mmol) under reflux in THF solution for 7 h, and the product animo-(EO)n-(AGE)m-OCH was obtained by rotary e...
Embodiment 3
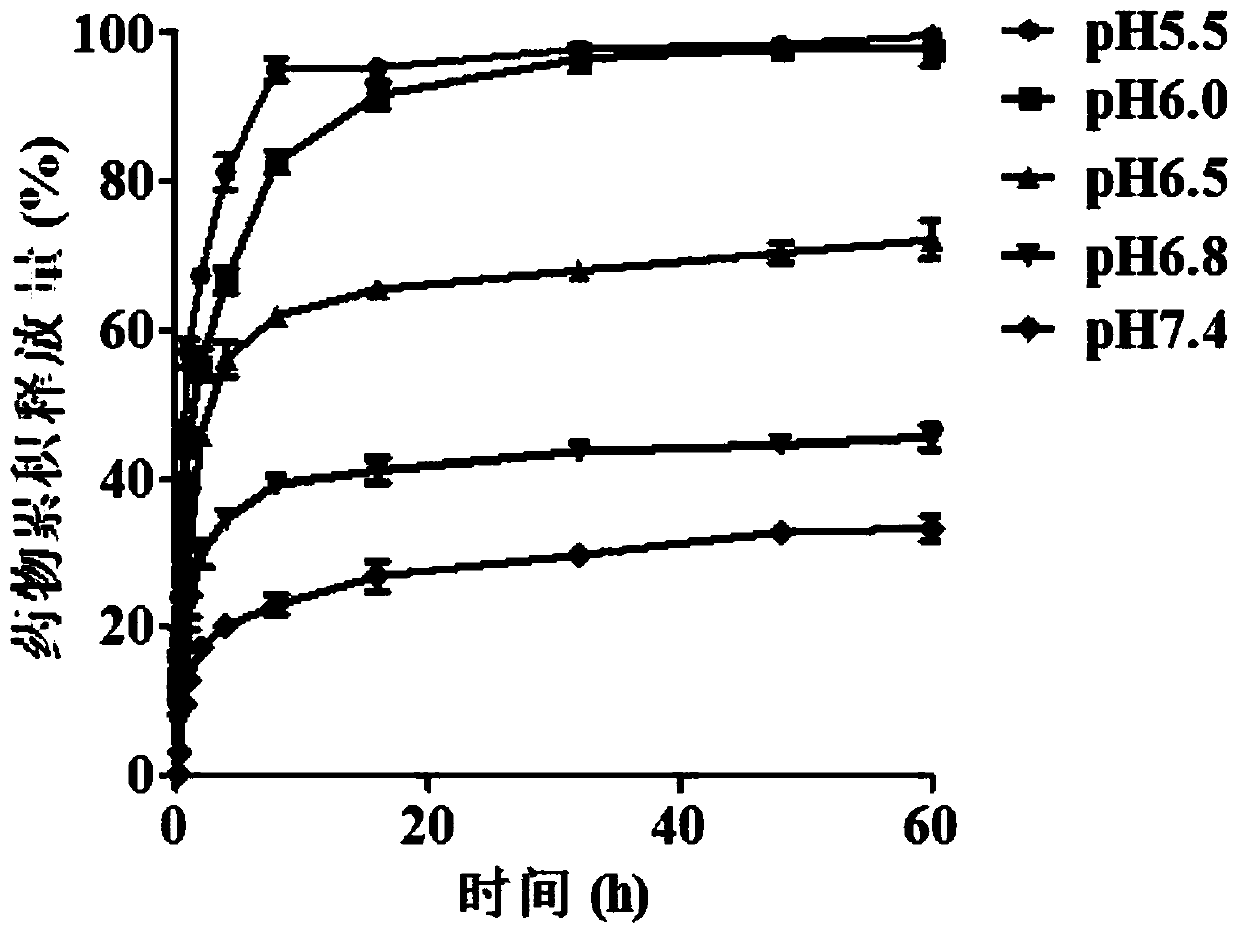
[0080] Accurately weigh 30 mg of the above two pH-sensitive polyethylene glycol derivatives-small molecule drug conjugates, dissolve them in 7.5 ml of double-distilled water, and then stir in the same direction at 25°C for 0.5 hours to obtain 4 Polymer micelles of mg / ml pH-sensitive polyethylene glycol derivative-small molecule drug conjugates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com