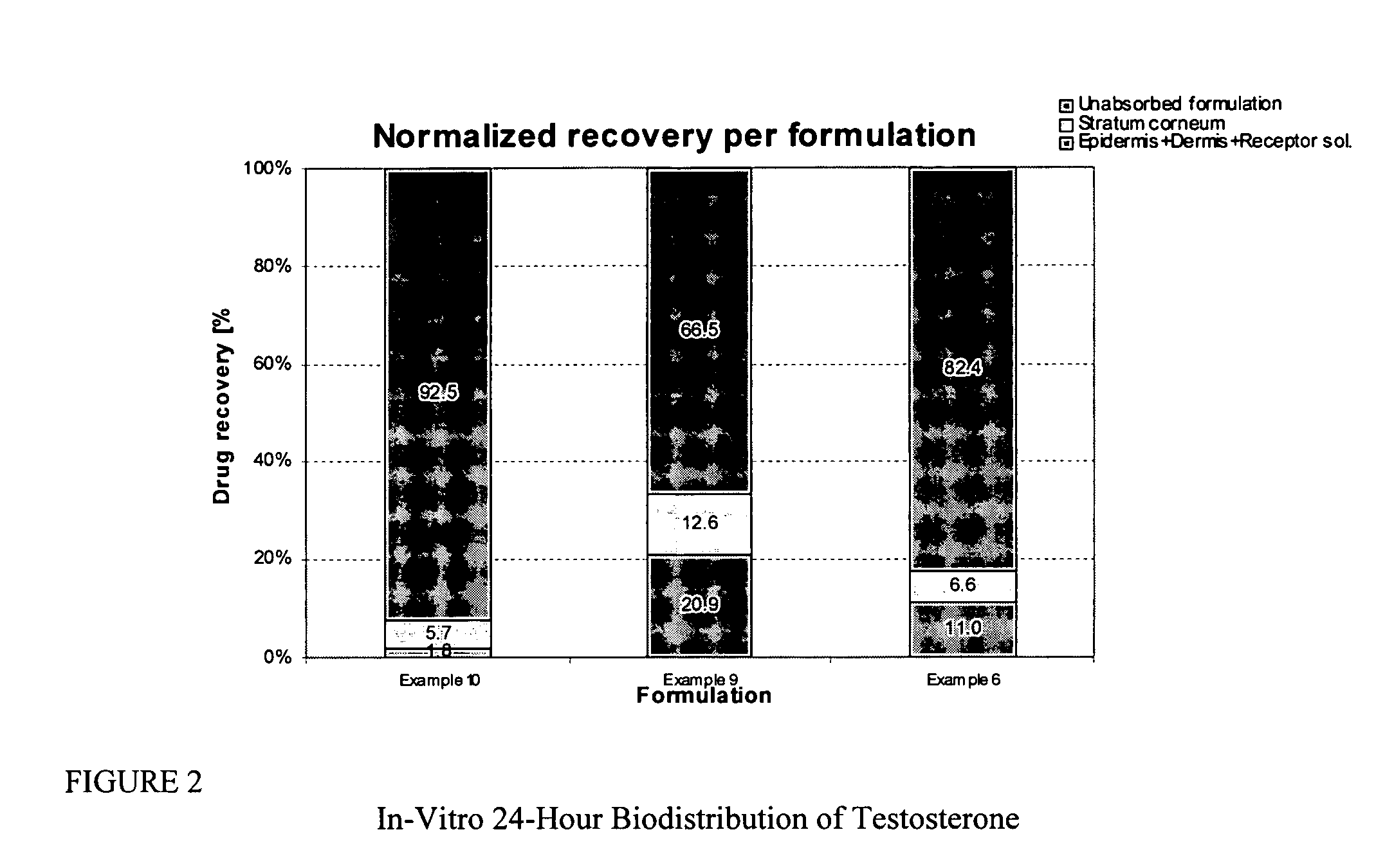
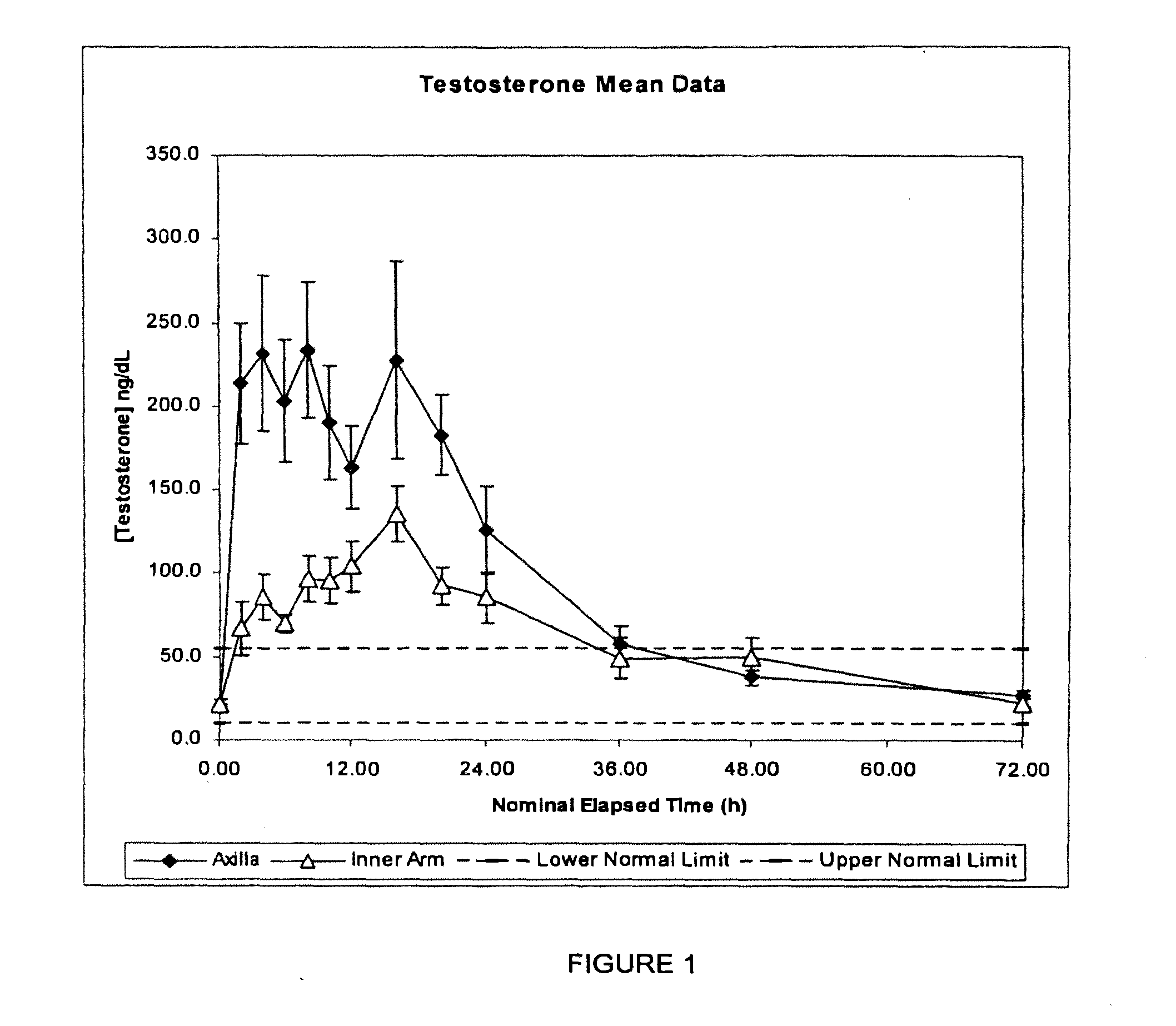
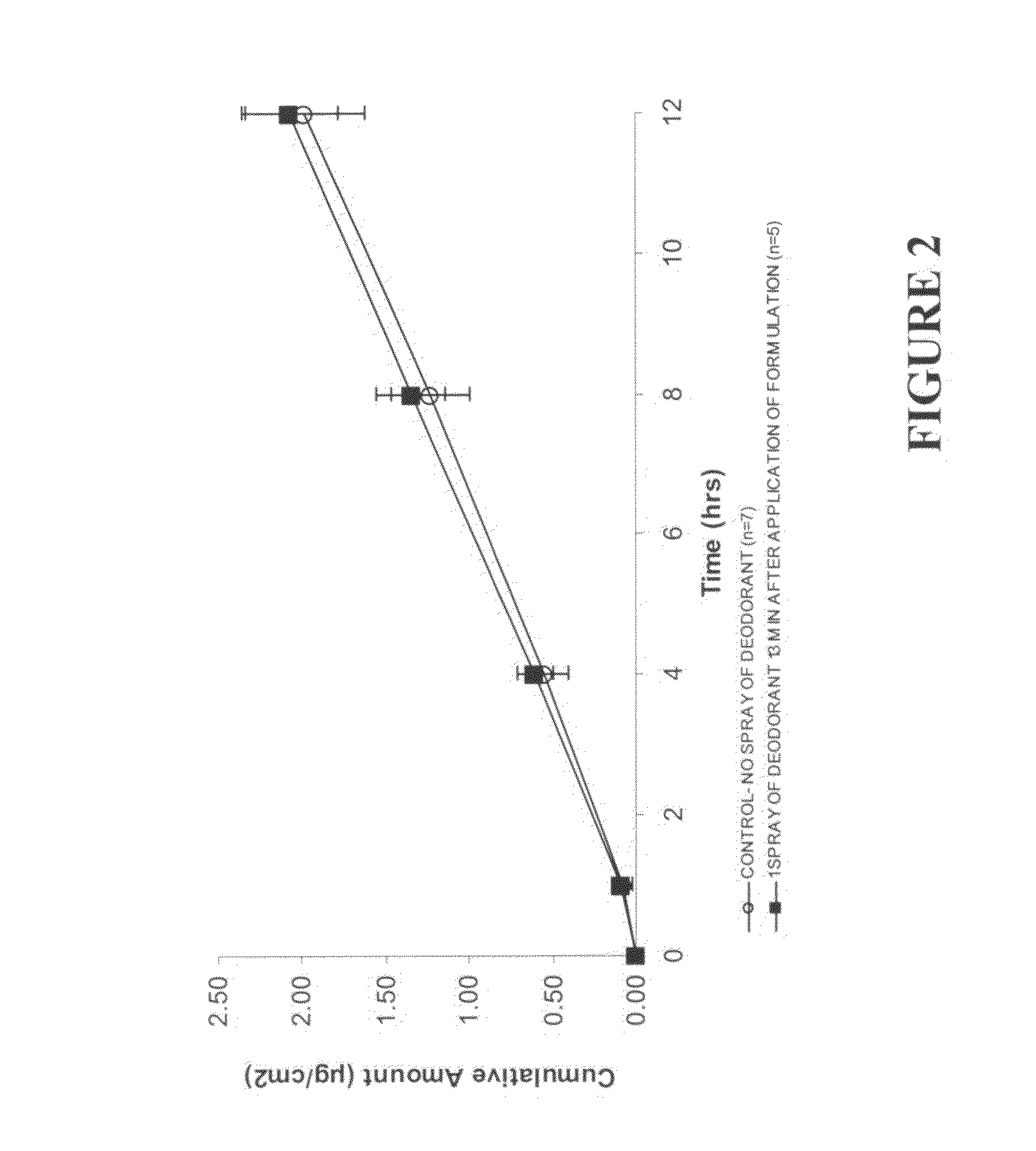
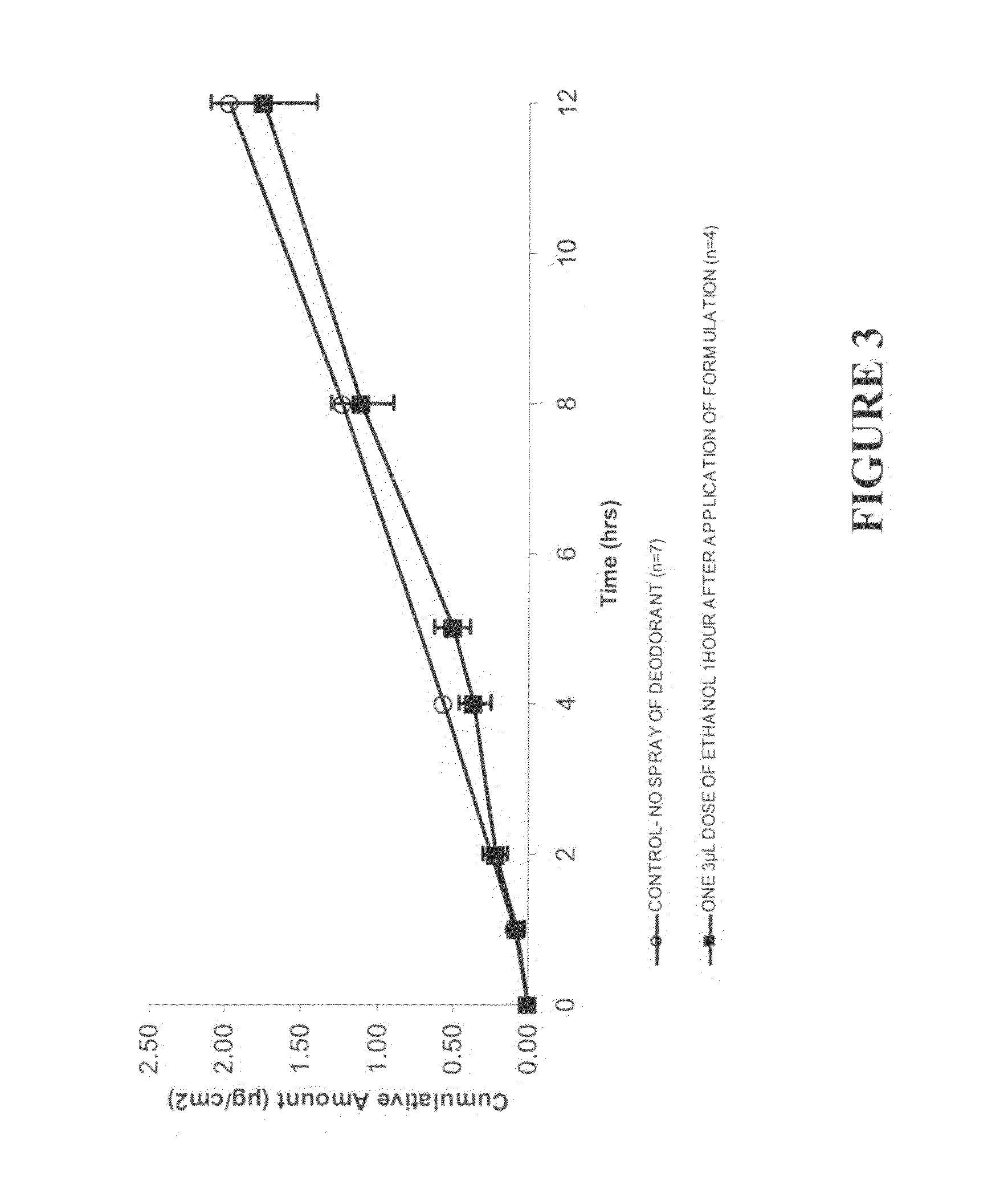
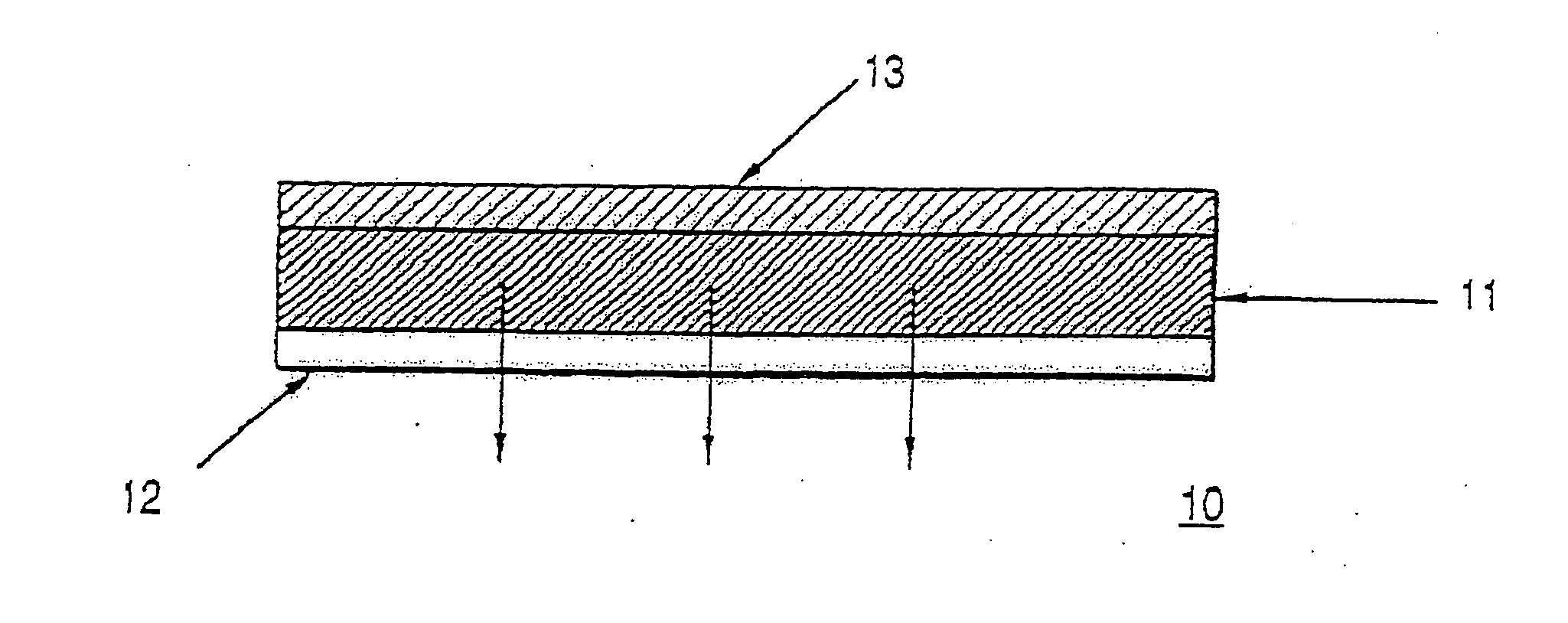
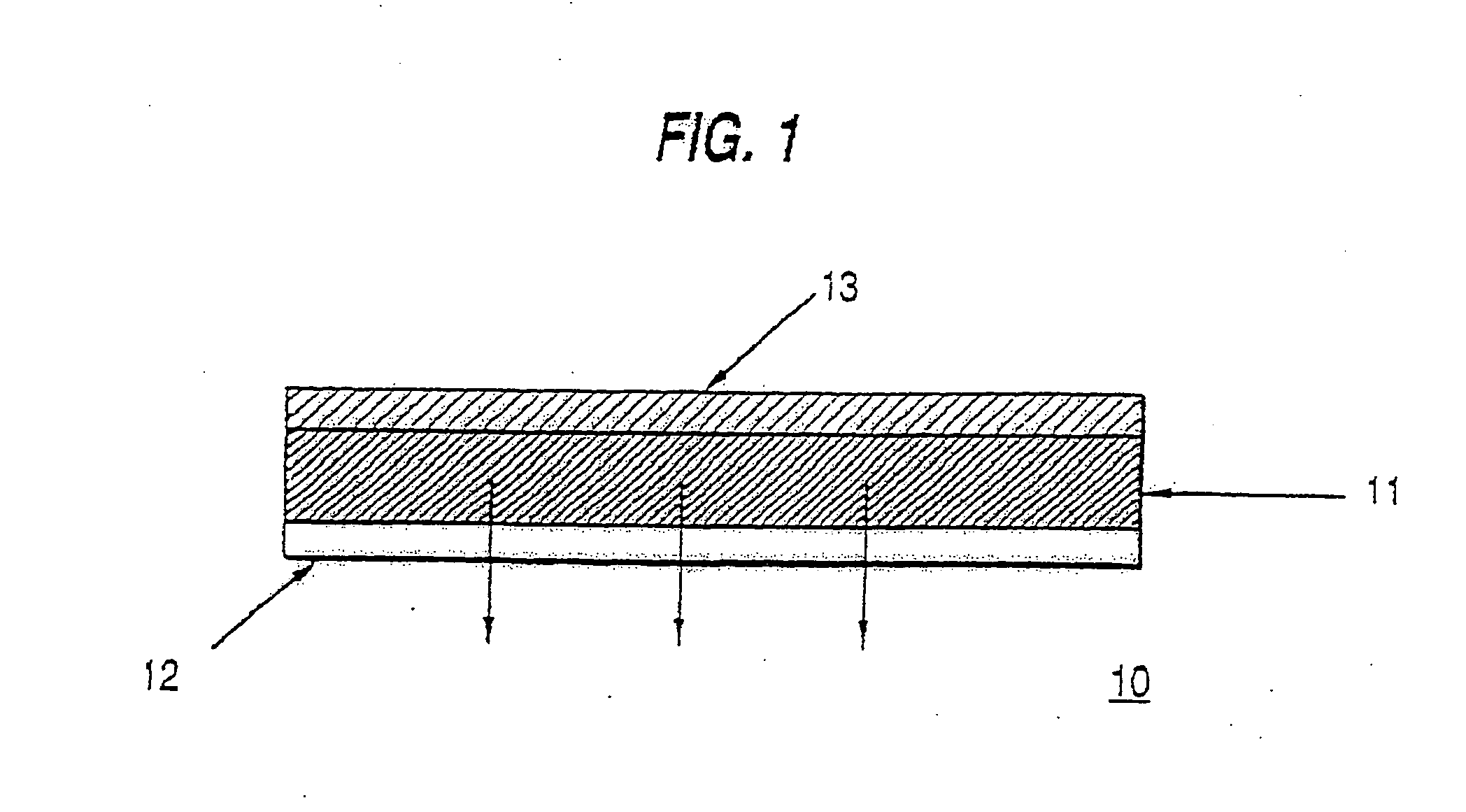
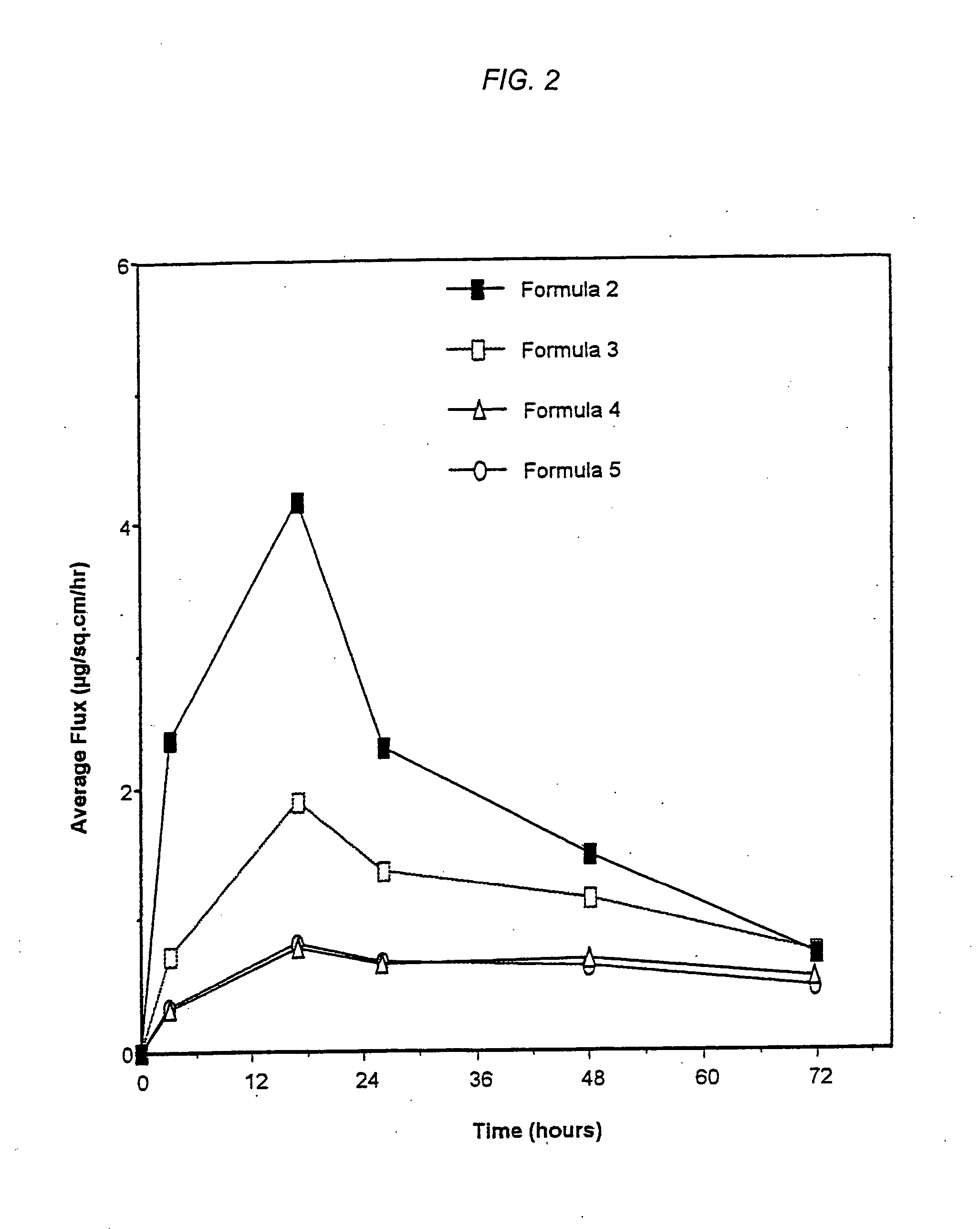
Patents
Literature
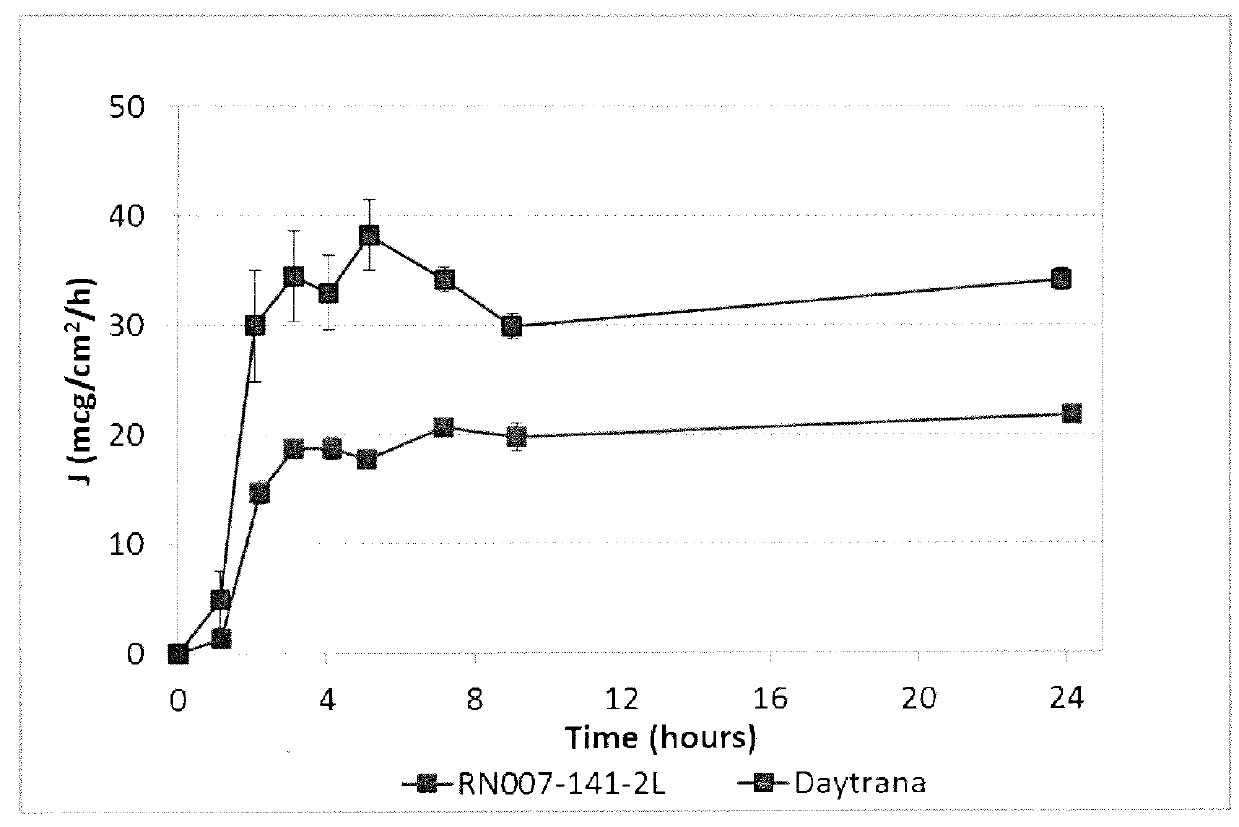
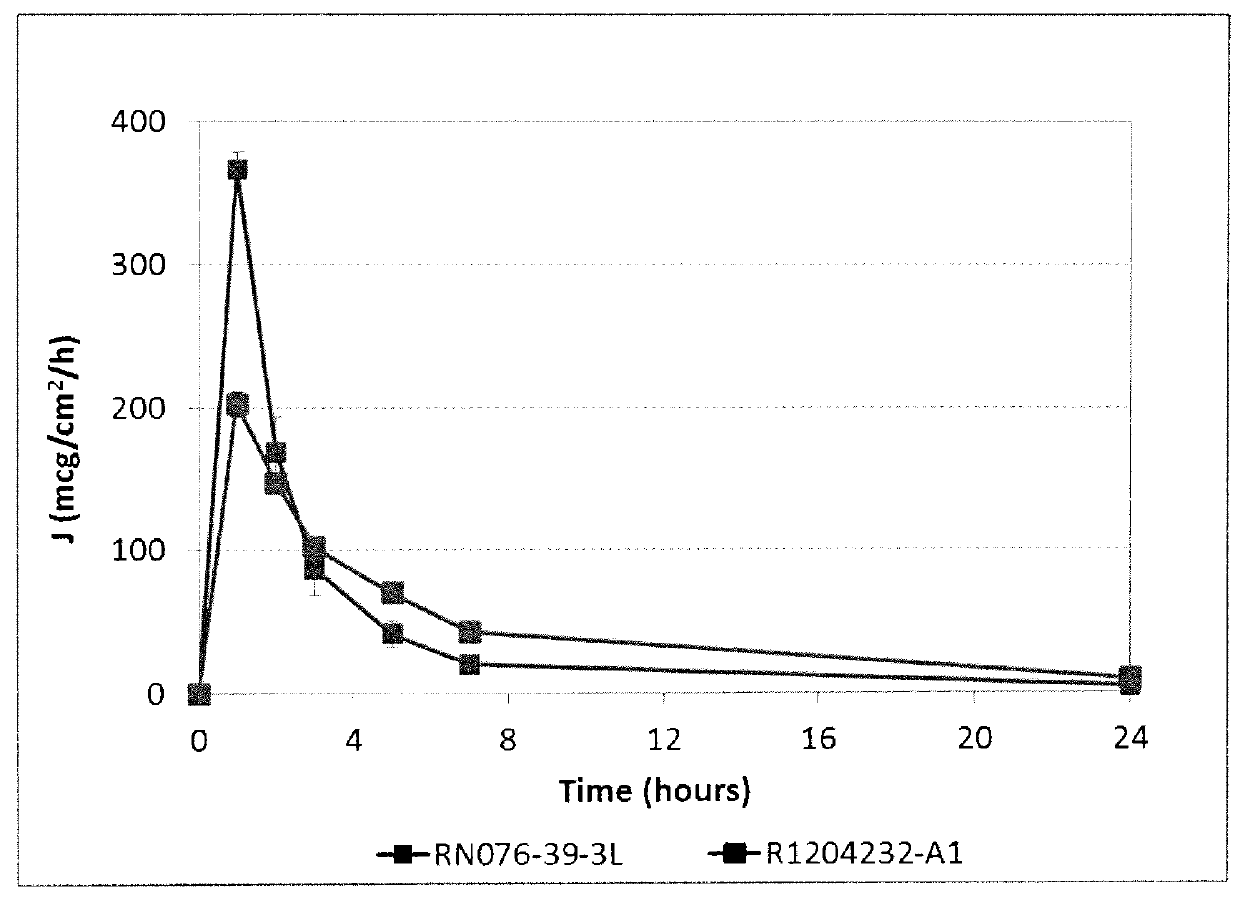
155 results about "Transdermal medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
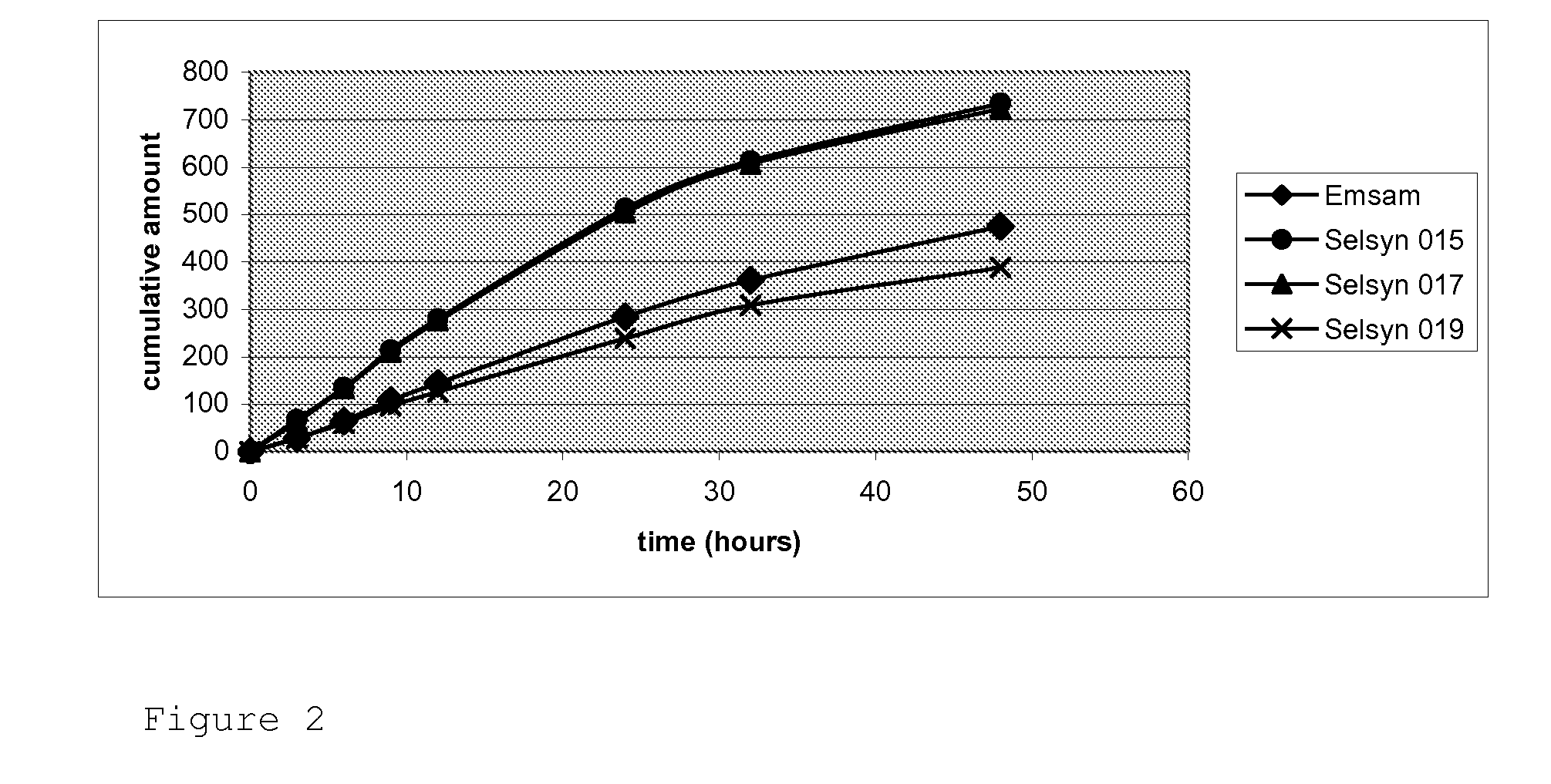
There are various drugs manufactured for transdermal delivery. The most well known is fentanyl, a medication used to control pain. Fentanyl is available as a "patch.". Other drugs, like methimazole, ketoprofen, thyroid supplements, phenobarbital, insulin and metoclopramide, are dissolved in a transdermal gel.
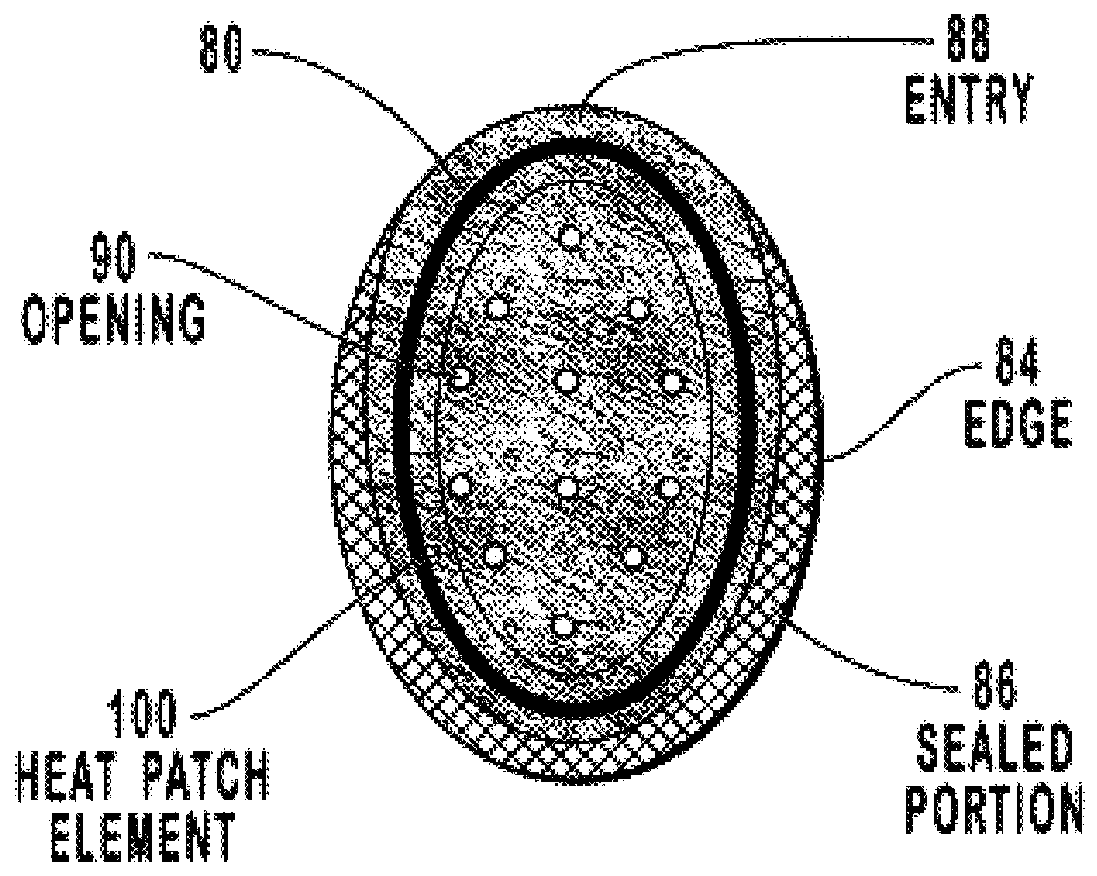
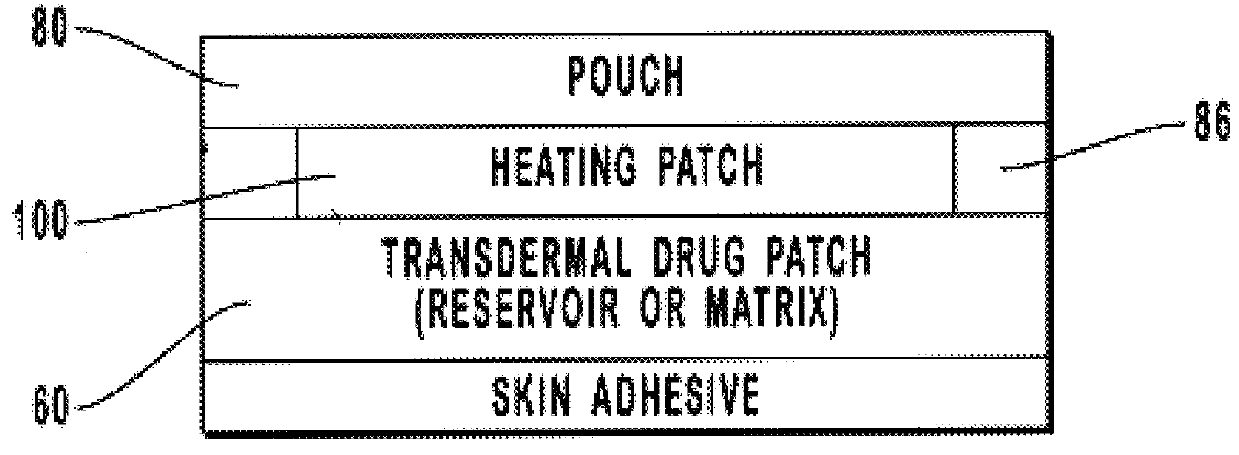
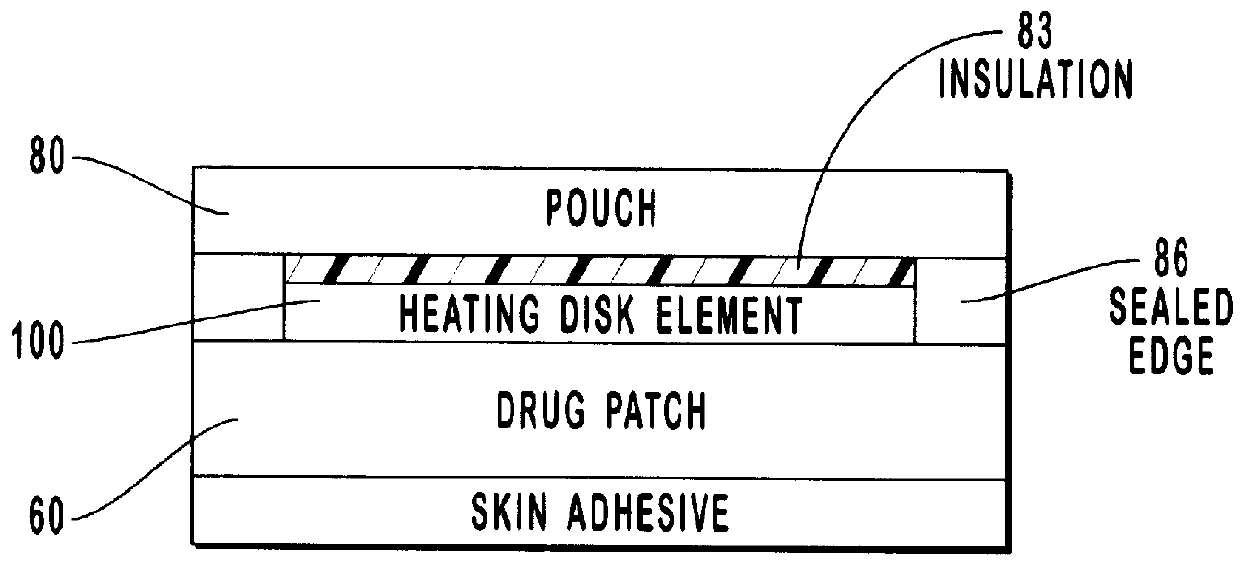
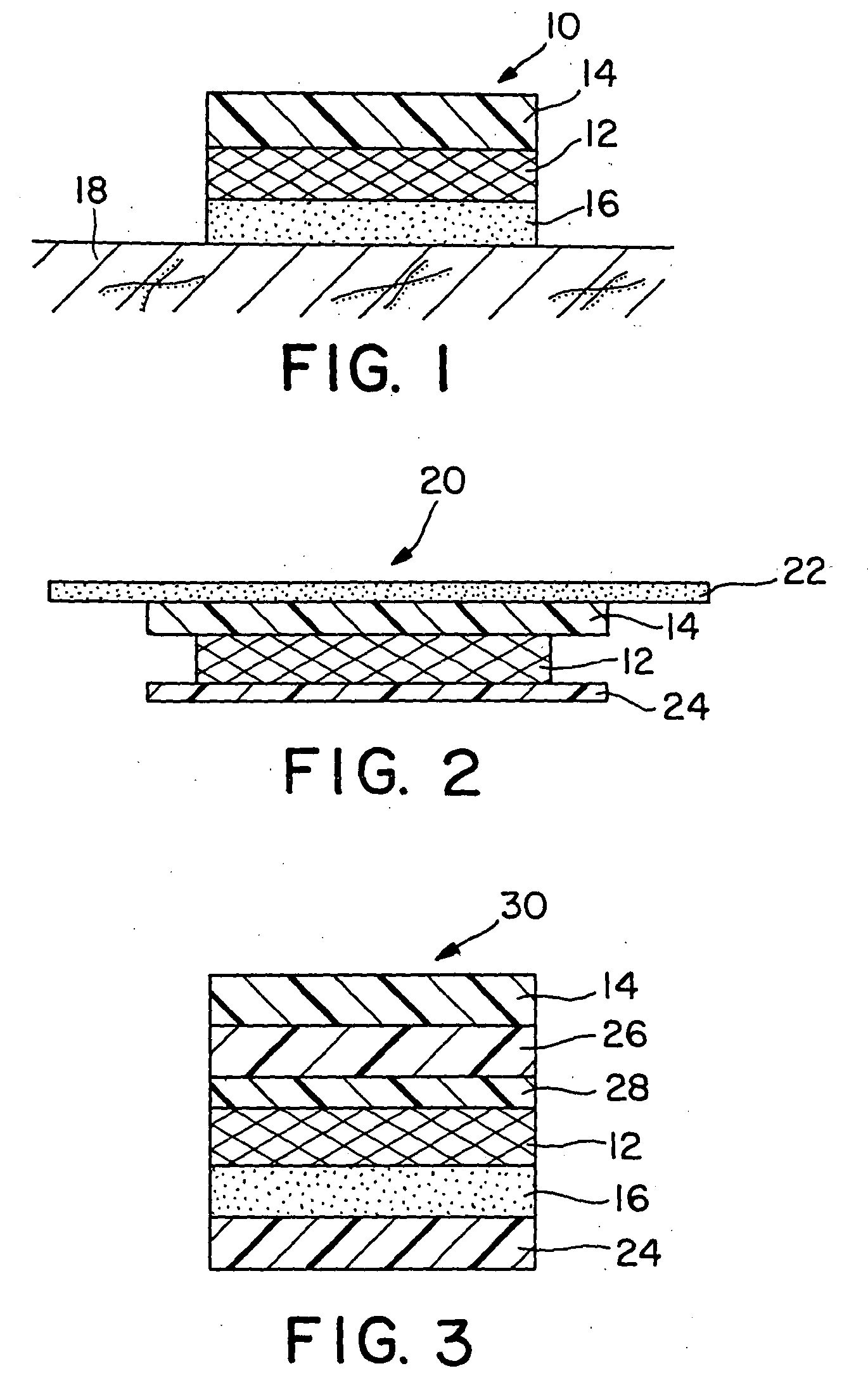
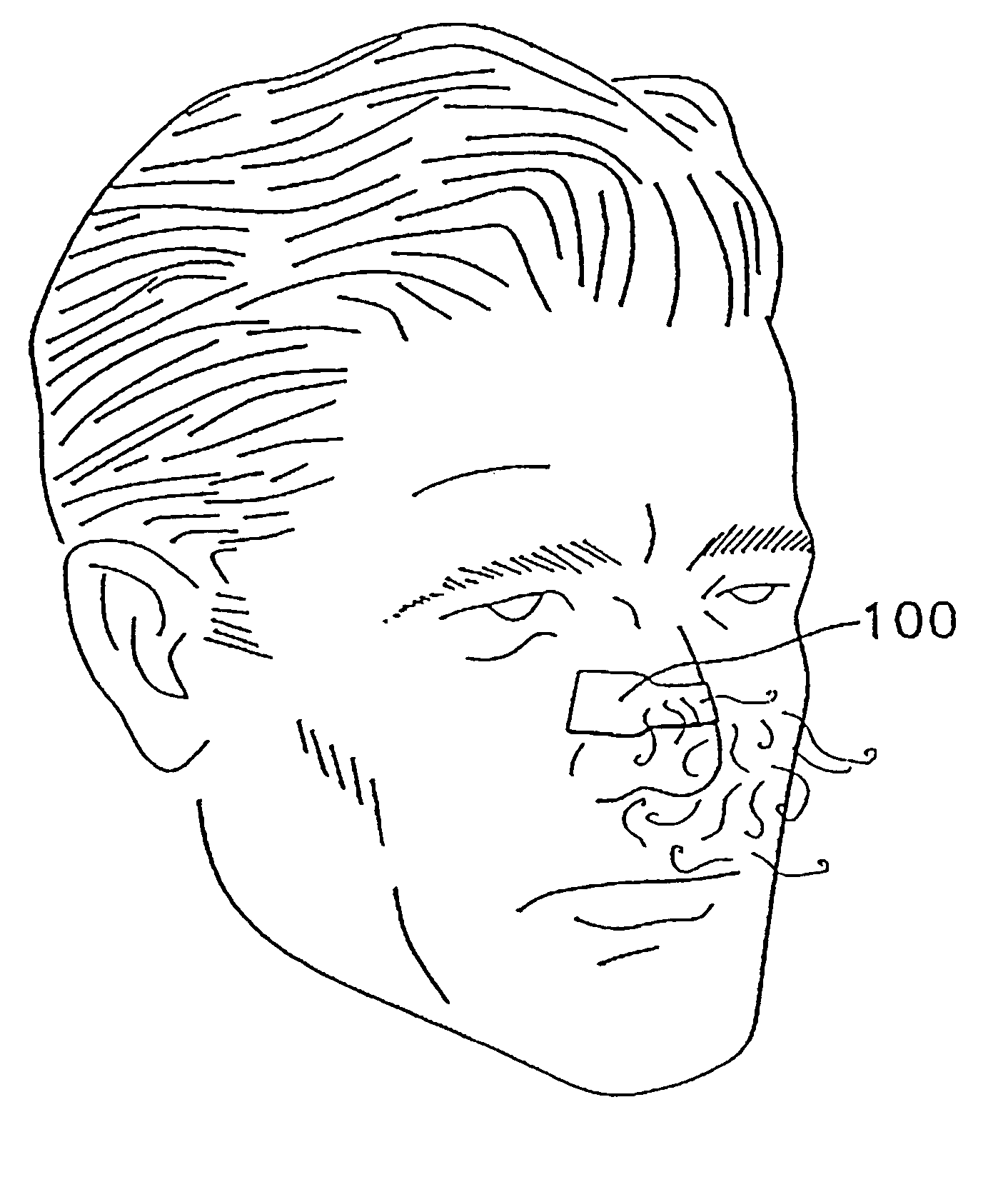
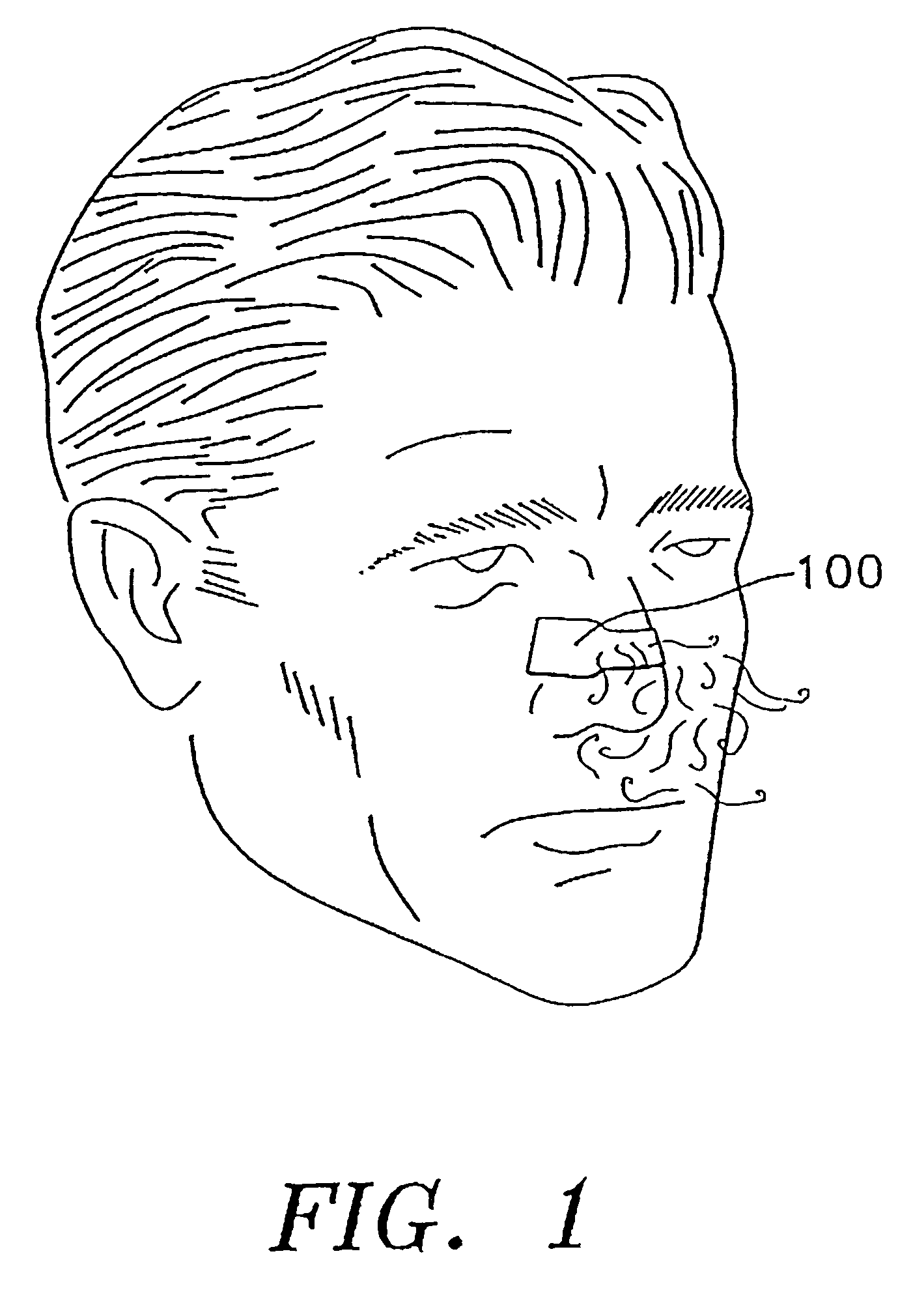
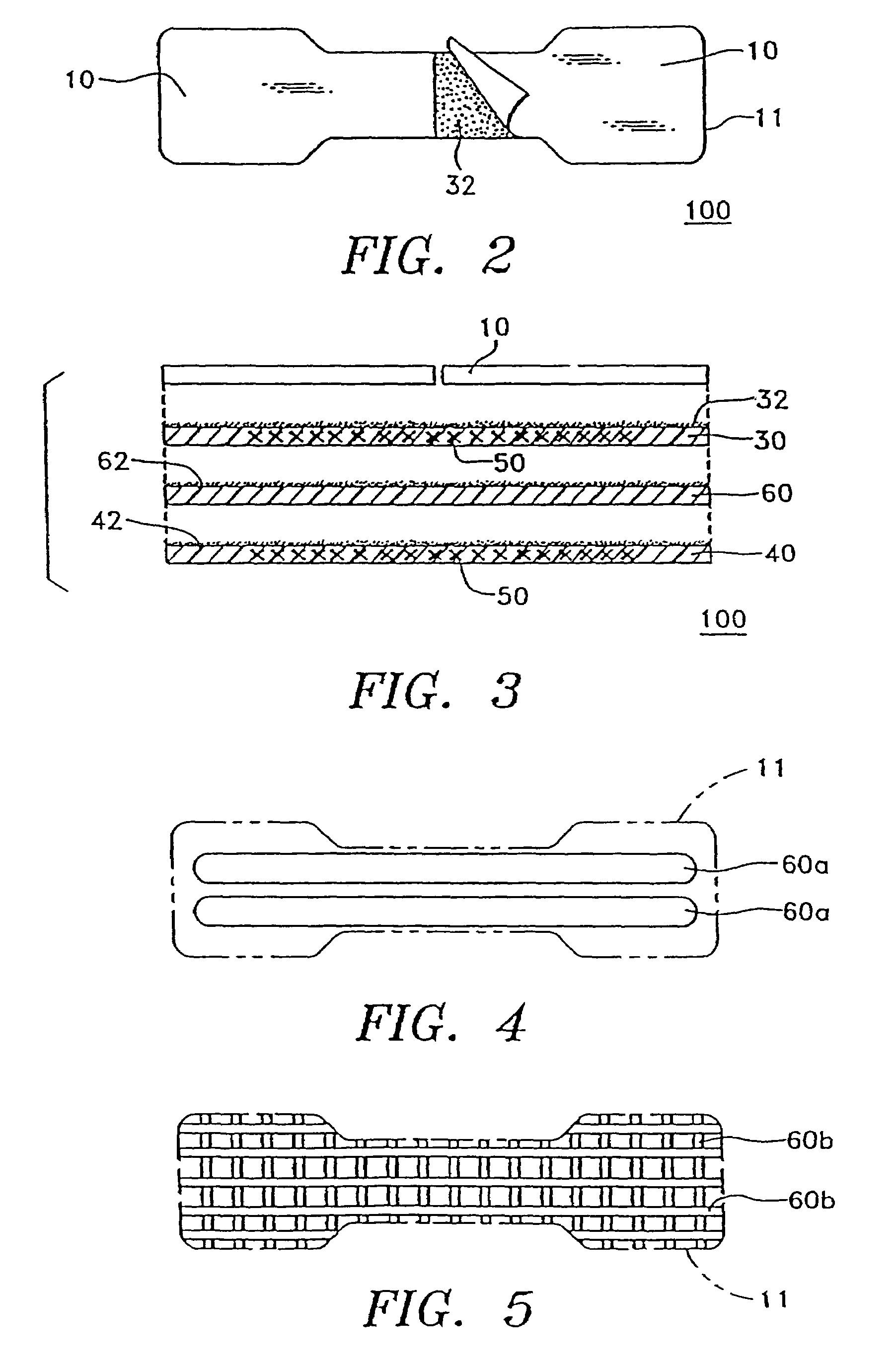
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050042268A1Improves transdermal penetrationSmall amountOrganic active ingredientsPharmaceutical delivery mechanismHormones regulationTransdermal medication
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes urea and / or a derivative thereof as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050020552A1Increase concentrationImproves transdermal penetrationOrganic active ingredientsAerosol deliveryIsostearic acidHormones regulation
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes isostearic acid as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Transdermal drug delivery device including an occlusive backing
InactiveUS20060078604A1Simple and inexpensive to manufactureDesirable in adhesiveOrganic active ingredientsNervous disorderActive agentMoisture vapor transmission rate
A transdermal drug delivery system for the topical application of one or more active agents contained in one or more polymeric and / or adhesive carrier layers, proximate to a non-drug containing polymeric backing layer which can control the delivery rate and profile of the transdermal drug delivery system by adjusting the moisture vapor transmission rate of the polymeric backing layer.
Owner:NOVEN PHARMA
Active transdermal medicament patch
InactiveUS20080214985A1Improve securityReduce technical difficultyElectrotherapySheet deliveryPlanar substrateTransdermal medication
An active transdermal medicament patch includes a planar substrate with a therapeutic face releasably retainable against the skin of a patient. A return electrode and a medicament matrix susceptible to permeation by medicament are secured at separated locations on the therapeutic face. Each electrically conductively engages the skin, when the substrate is retained thereon. A power source carried on the substrate is electrically coupled between the medicament matrix and a programmed microprocessor also carried on the substrate. A substantially invariant voltage presented at an output contact of the microprocessor is applied during a predetermined therapy period across the skin between the medicament matrix and the return electrode, inducing transcutaneous migration of medicament into the skin at a substantially constant rate. A light-emitting diode carried on the substrate and coupled to the microprocessor communicates that the patch is operating.
Owner:ACTIVATEK
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050025833A1Increase concentrationImproves transdermal penetrationPowder deliveryOrganic active ingredientsAmmonium compoundsCompound (substance)
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes a quaternary ammonium compound as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
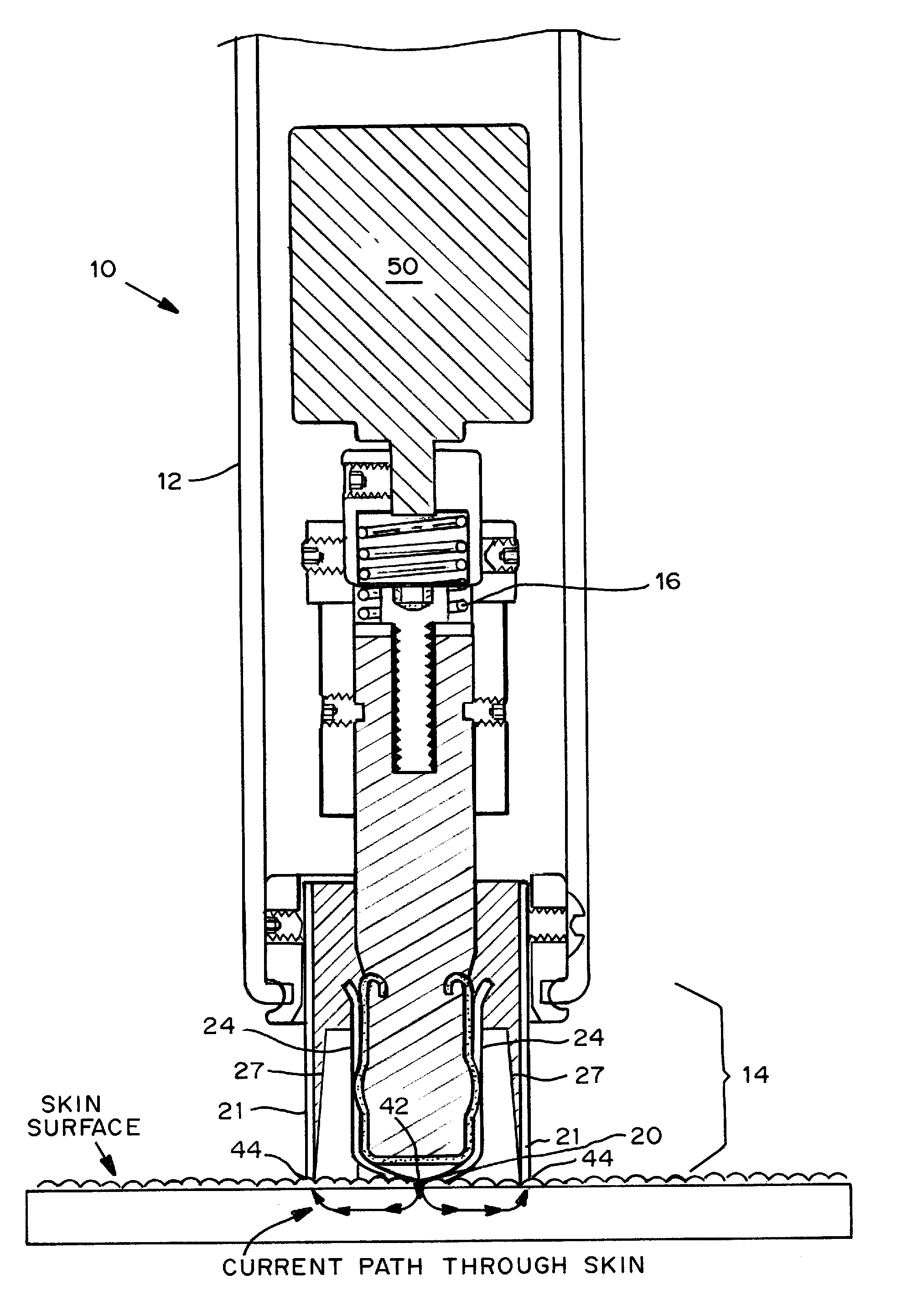
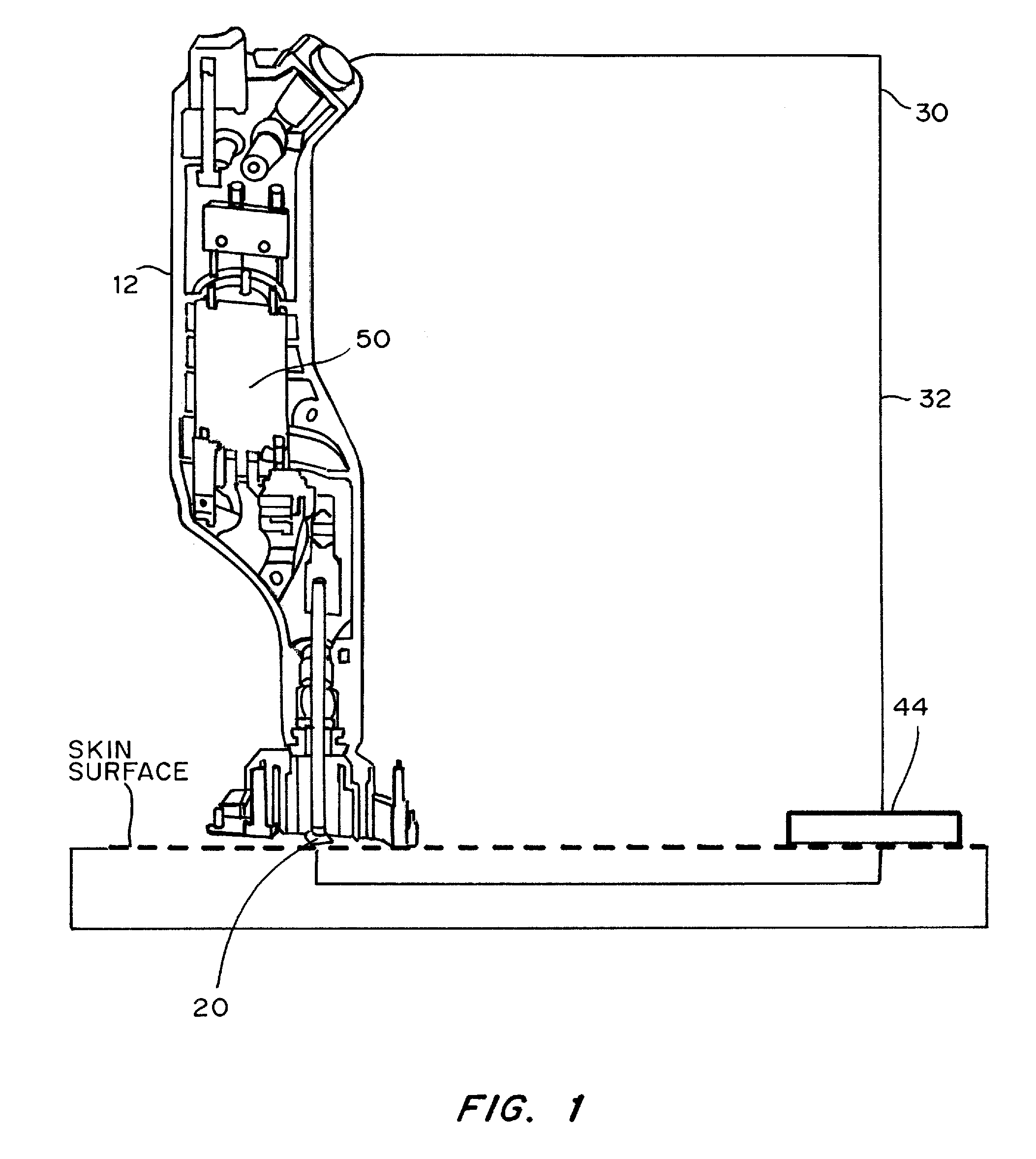
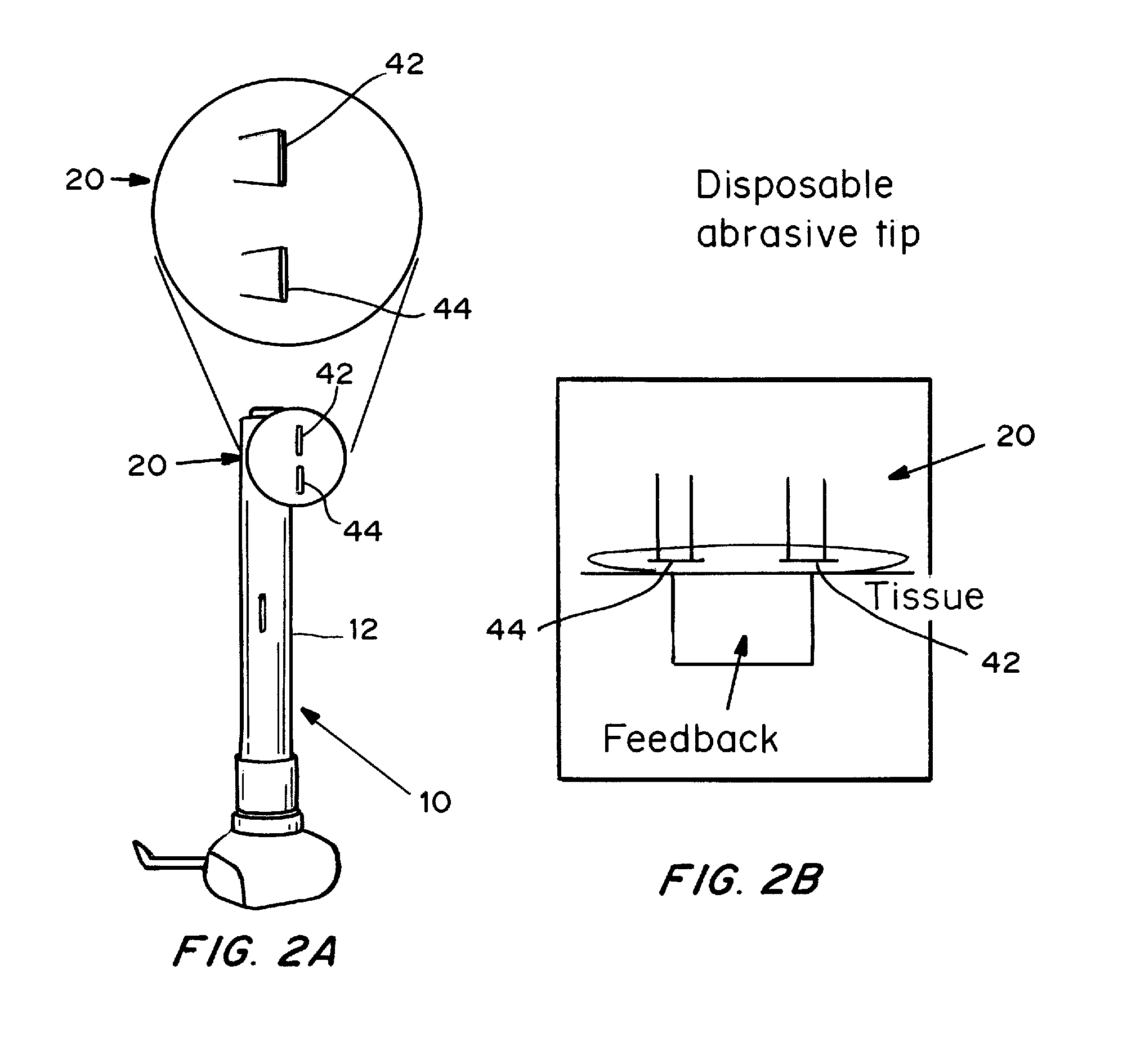
Skin permeation device for analyte sensing or transdermal drug delivery
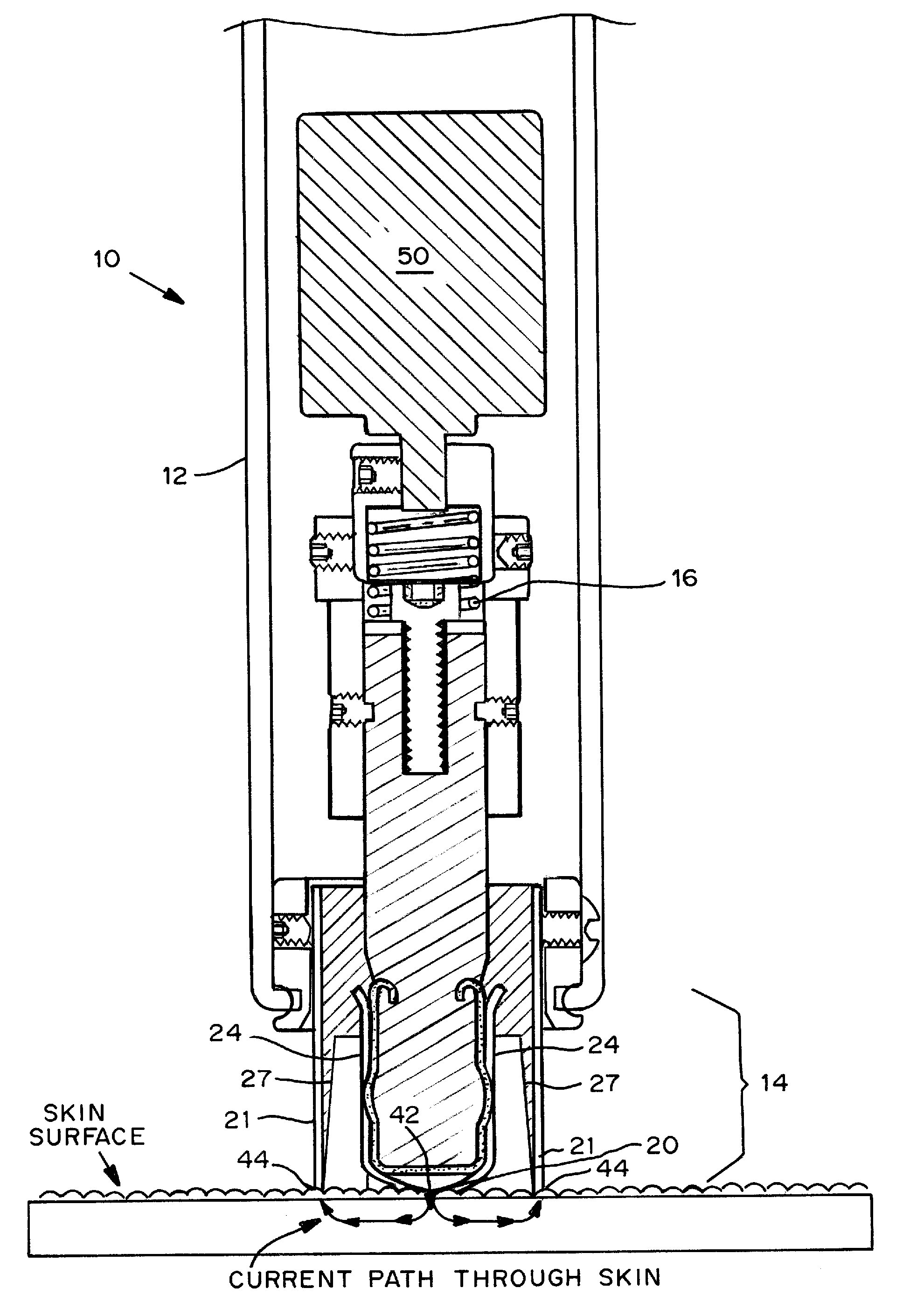
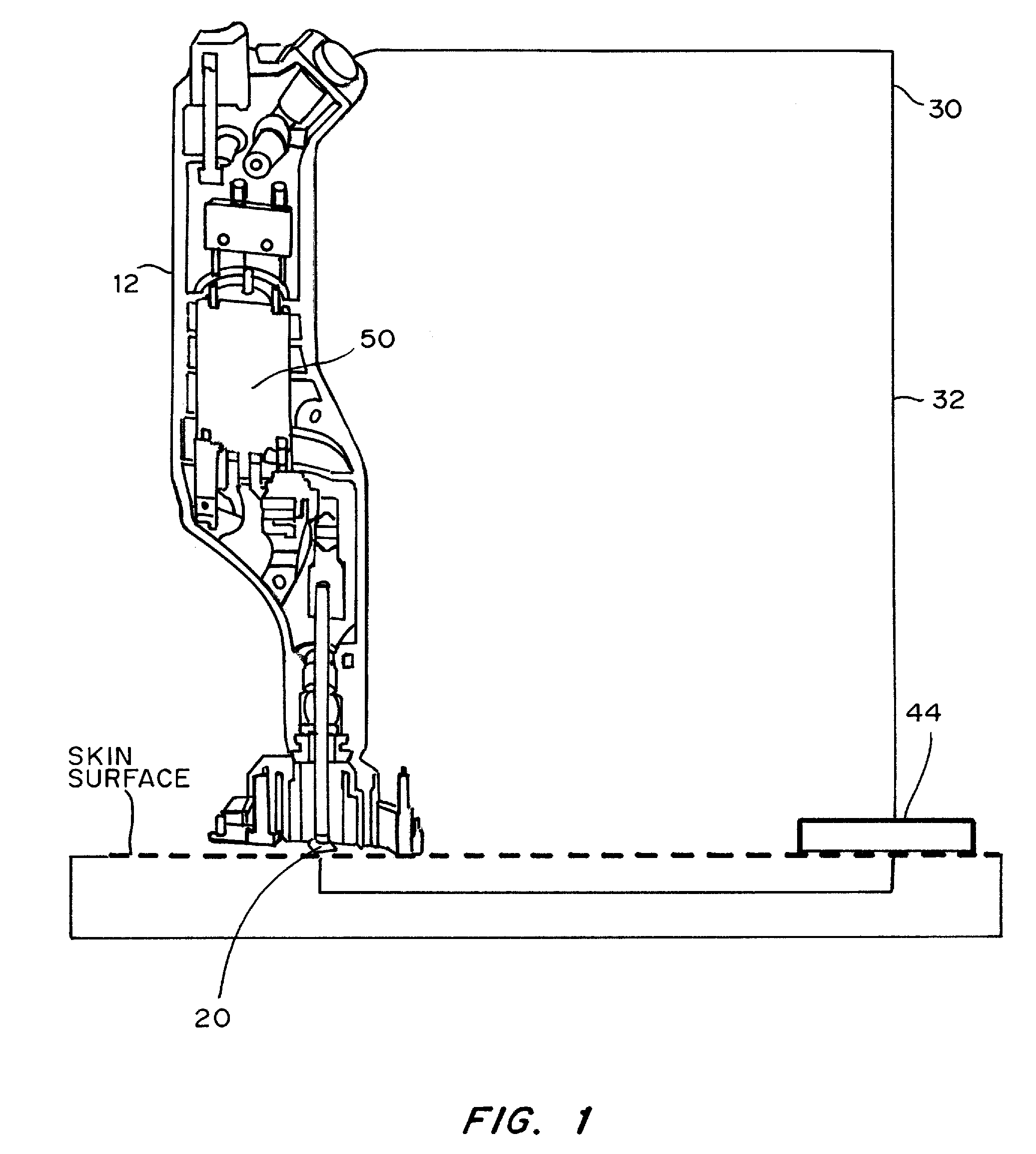
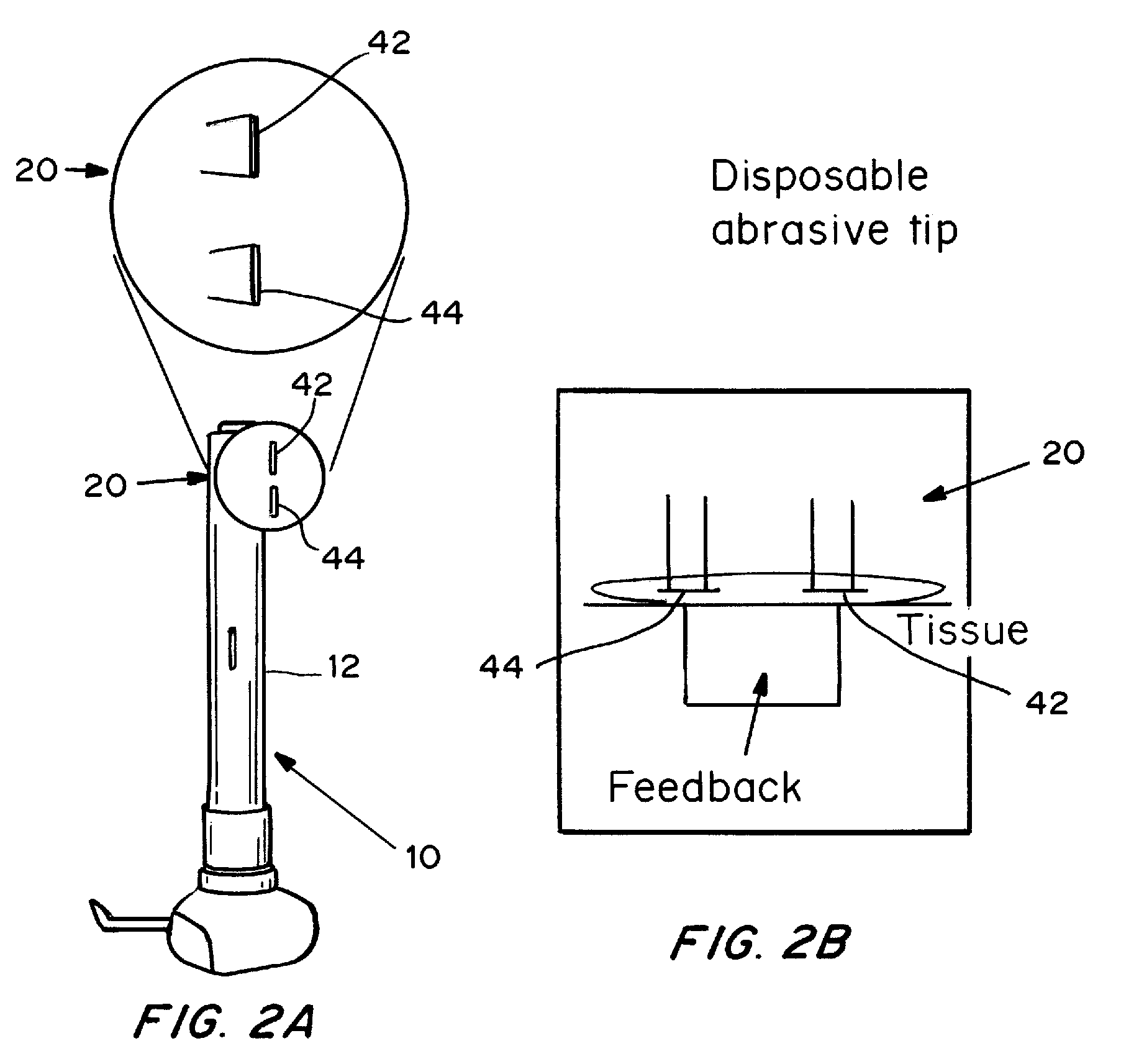
Devices, systems, kits and methods for increasing the skin's permeability controlled by measured skin electrical parameter are described herein. They may be used for transdermal drug delivery and / or analyte extraction or measurement. The controlled abrasion device contains (i) a hand piece, (ii) an abrasive tip, (iii) a feedback control mechanism, (iv) two or more electrodes, and (v) an electrical motor. The feedback control mechanism may be an internal feedback control mechanism or an external feedback control. The kit contains the controlled abrasion-device, one or more abrasive tips, optionally with a wetting fluid. The method for increasing the skin's permeability requires applying the controlled abrasion device to a portion of the skin's surface for a short period of time, until the desired level of permeability is reached. Then the abrasion device is removed, and a drug delivery composition or device or an analyte sensor is applied to the treated site.
Owner:ECHO THERAPEUTICS INC
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS20060153905A1Reduce transferLoss of therapyOrganic active ingredientsNervous disorderActive agentBULK ACTIVE INGREDIENT
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Topical drug delivery using phosphatidylcholine
InactiveUS20060105955A1Topical deliveryEasier and pleasanterOrganic active ingredientsPeptide/protein ingredientsTopical drugTransdermal medication
The present invention relates to compositions and methods for transdermal drug delivery comprising formulating a phosphatidylcholine carrier composition containing the drug and applying the composition to the skin.
Owner:TRANSDERMAL BIOTECHNOLOGY INC
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS7335379B2Reducing and preventing transferMinimize contaminationOrganic active ingredientsNervous disorderAlcoholActive agent
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Method and composition for transdermal drug delivery
InactiveUS20100322884A1Minimising effectsMinimising inconvenienceOrganic active ingredientsBiocideActive agentTransdermal medication
The invention is directed to a transdermal drug delivery composition which includes at least one physiologically active agent; and at least one volatile solvent; and at least one viscosity modulating agent. The invention extends to methods of administering such a composition to a subject and treatment of subjects using the composition.
Owner:ACRUX DDS
Transdermal, alcohol-free, pharmaceutical compositions
An alcohol-free, transdermal drug delivery composition administered via a metered spray drug delivery device is described herein. The non-occlusive transdermal drug delivery composition includes a therapeutically effective amount of at least one physiologically active agent or prodrug thereof, an effective amount of at least one dermal penetration enhancer; and at least one non-volatile liquid. The transdermal drug delivery composition is administered to a dermal or mucosal surface of an animal needing the same using a metered spray device capable of delivering a fine spray of substantially uniform particle size to minimize the required drying time therefor.
Owner:LUMARA HEALTH IP
Pharmaceutical composition and method for the transdermal delivery of magnesium
InactiveUS20050196434A1Reduce disadvantagesBiocideAerosol deliveryAutonomic bladder dysfunctionMagnesium salt
The present invention relates to a method and transdermal pharmaceutical composition for preventing magnesium deficiency or imbalances associated with magnesium deficiency including diabetes, hypertension, high cholesterol, cardiac arrhythmias, acute myocardial infarction, arteriosclerosis, atherosclerosis, preeclampsia, dysautonomia, mitral valve prolapse, asthma, constipation, irritable bowel syndrome, migraines, muscle spasms and cramping, premenstrual syndrome, osteoporosis, kidney stones, chronic fatigue syndrome, and fibromyalgia. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of magnesium and a pharmaceutically acceptable carrier. A therapeutically effective amount of a pharmaceutically acceptable salt of zinc a vitamin such as B-complex vitamin, a carotenoid, a mineral, or a combination thereof may also be included in the transdermal pharmaceutical composition. A therapeutically effective amount of progesterone may also be included in the transdermal pharmaceutical composition. The transdermal pharmaceutical composition may be topically administered to prevent magnesium deficiency or imbalances caused by magnesium deficiency.
Owner:BRIERRE BARBARA T
Transdermal drug delivery system for liquid active ingredient
InactiveUS20100087768A1Reduce lossesModerate shearPowder deliveryBiocideAdditive ingredientCross linker
A monolithic device for transdermal administration of an active pharmaceutical ingredient which is selected from propargylamines and rivastigmine and is liquid at 25° C., has an adhesive matrix layer which includes the active ingredient in an acrylic polymer pressure sensitive adhesive without cross-linker agent containing a metal atom, the adhesive having a shear value of between 1.5 and 15 hours, and further includes a non-volatile coadjuvant selected from squalene and triethylcitrate present in the layer in an amount of 1 to 15 wt %. The combination provides good release of the drug in use, reduces loss of the drug during a drying step in manufacture, reduces chemical interaction of the layer with the drug and achieves low level of skin irritation.
Owner:AMARIN TECH
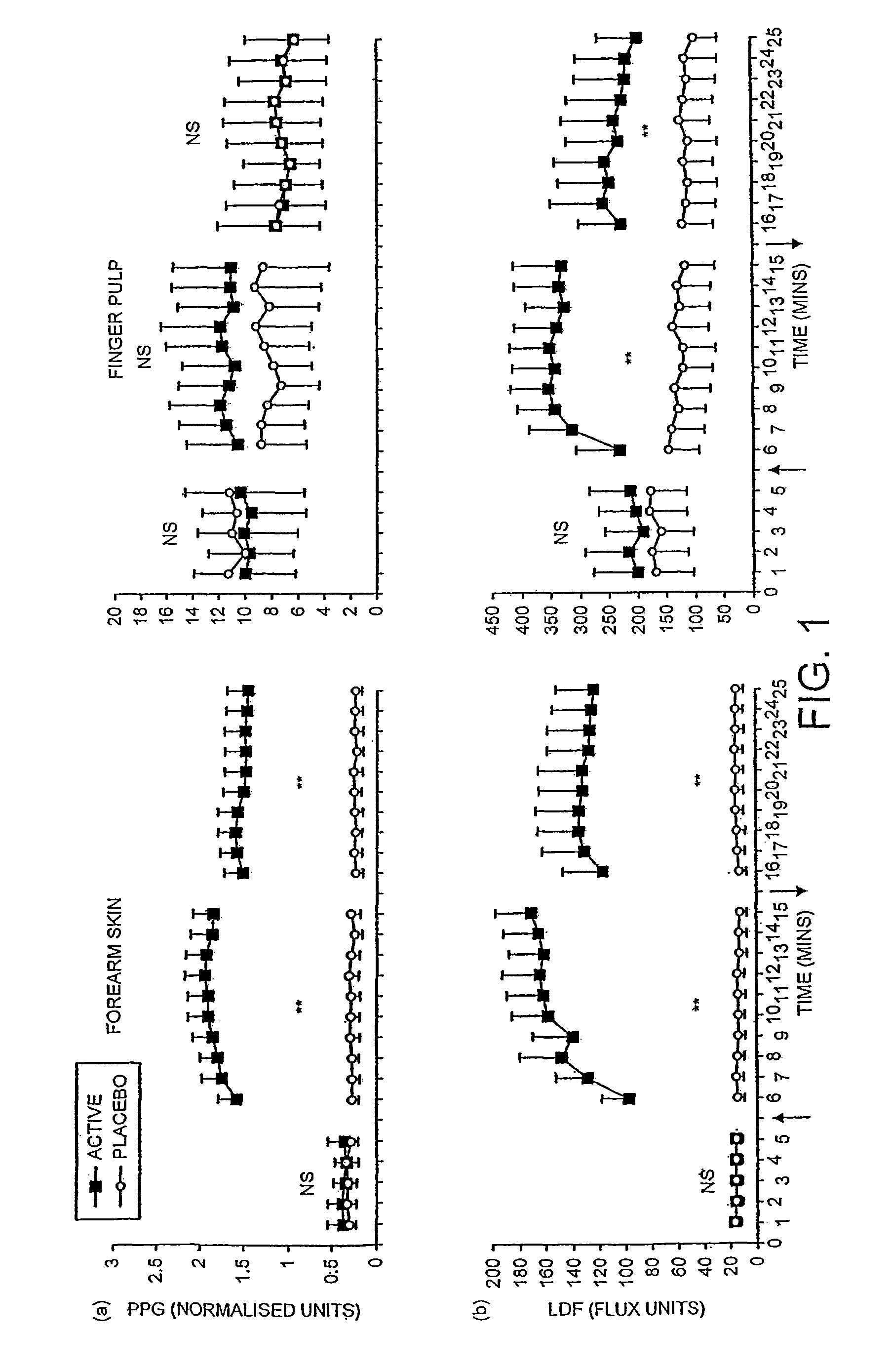
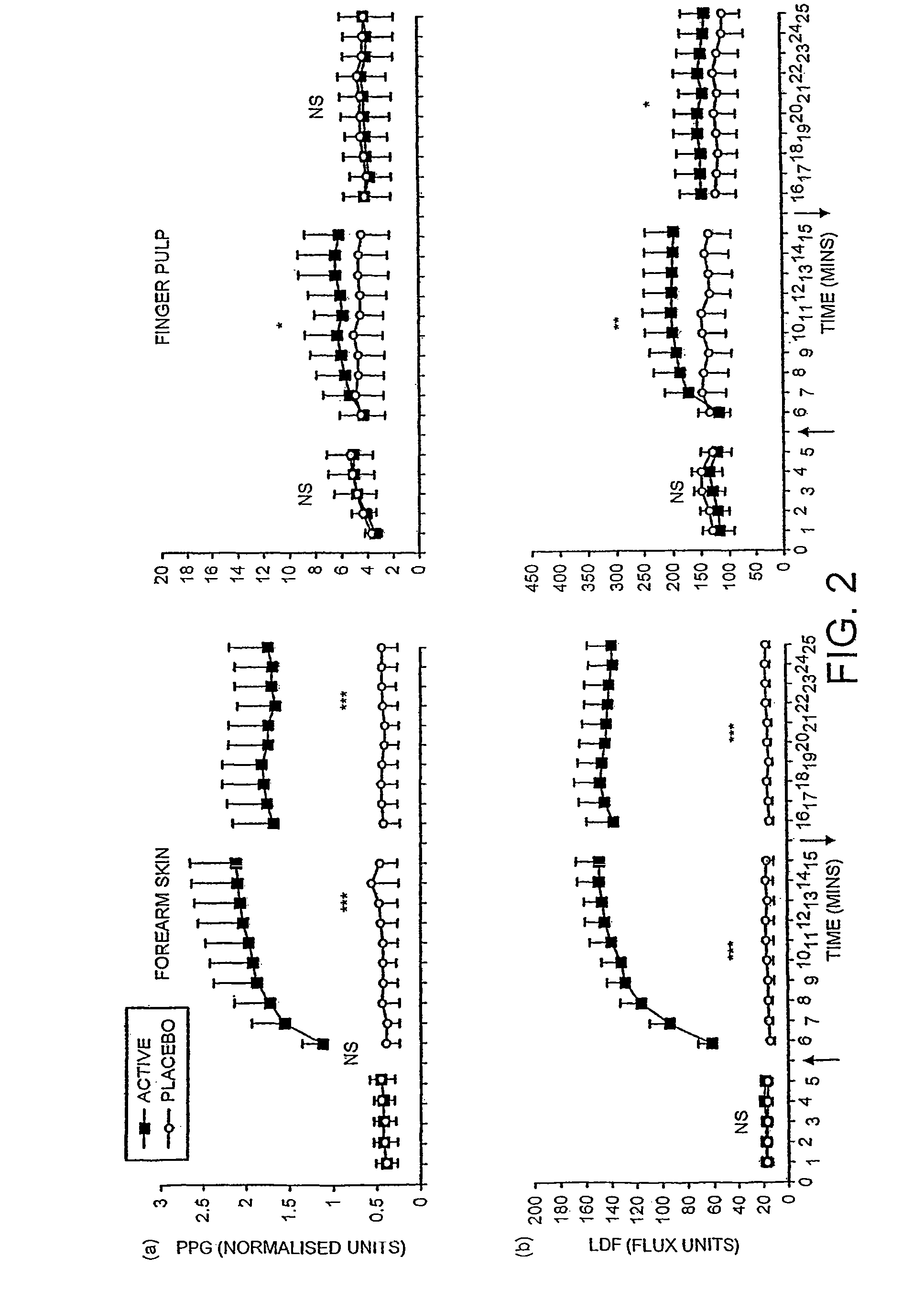
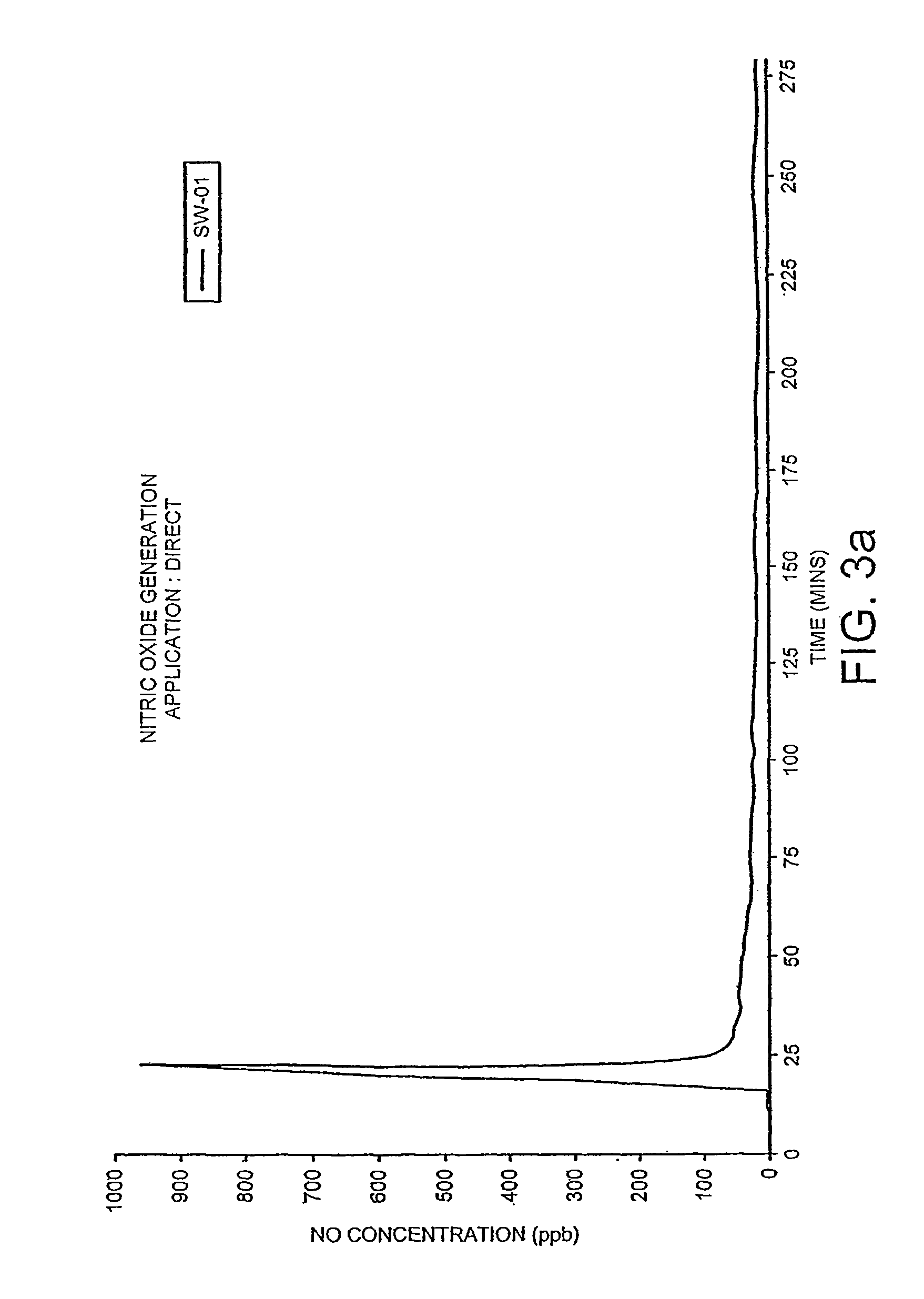
Transdermal pharmaceutical delivery compositions
A pharmaceutically delivery system and method of use therefor are described comprising a pharmaceutically active agent and acidified nitrite as an agent to produce local production of nitric oxide at the skin surface. The dosage form may be in any pharmaceutically acceptable carrier means and comprises an acidifying agent adapted to reduce the pH at the environment. In one embodiment, a barrier consisting of a membrane allows diffusions of the anaesthetic and nitrite ions while preventing direct contact of the skin and acidifying agent.
Owner:QUEEN MARY UNIV OF LONDON
Composition and method for controlling drug delivery from silicone adhesive blends
InactiveUS20050019385A1Reduce concentrationPrevent/inhibit crystallizationSheet deliveryBandagesControlled drugsSolubility
Compositions and methods for controlling transdermal drug delivery, particularly of amine-functional and basic drugs, comprising a blend of a first silicone-based polymer having a reduced silanol concentration and a second silicone-based polymer have a substantial or high silanol concentration. The blend of such silicone-based polymers, particularly pressure-sensitive silicone adhesives, provides sufficient drug solubility and reduced initial drug delivery onset to permit a prolonged delivery duration at a substantially zero-order rate of delivery.
Owner:NOVEN PHARMA
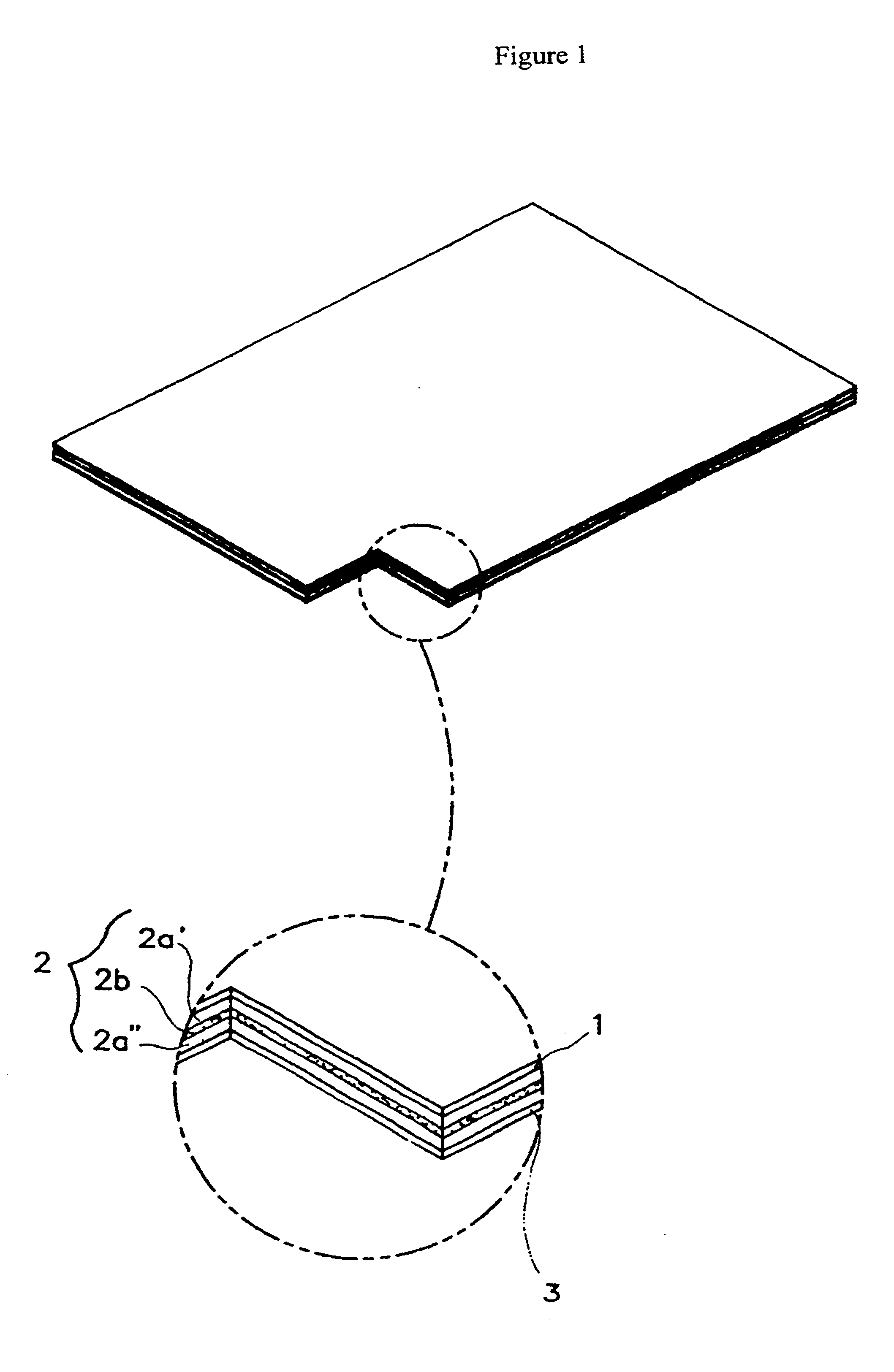
Transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonium salt, and the manufacturing method thereof
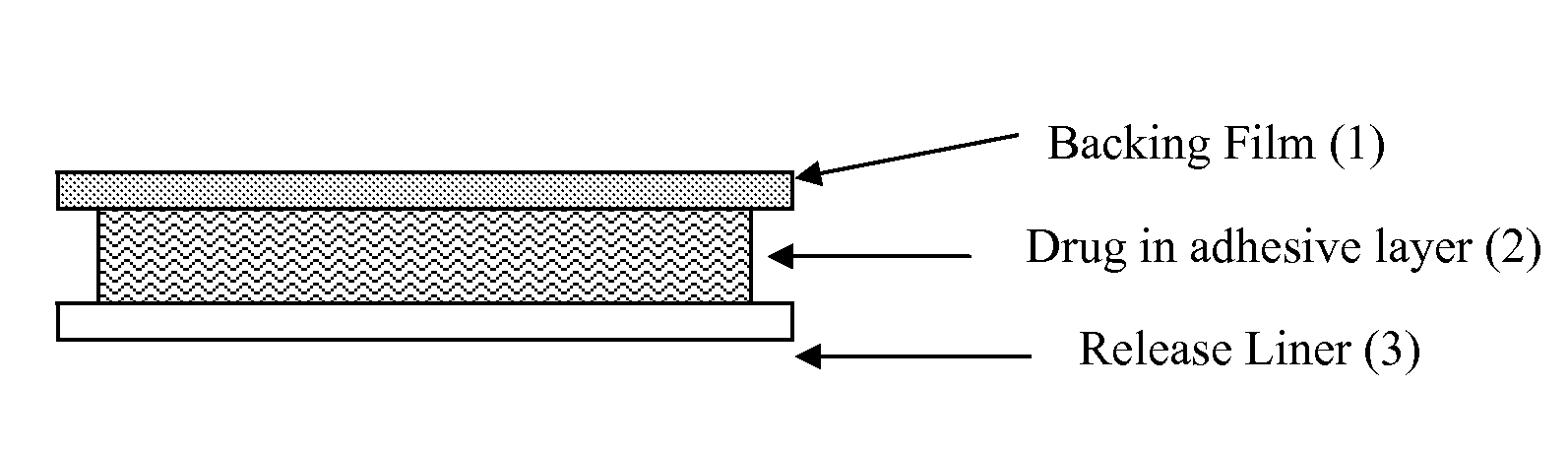
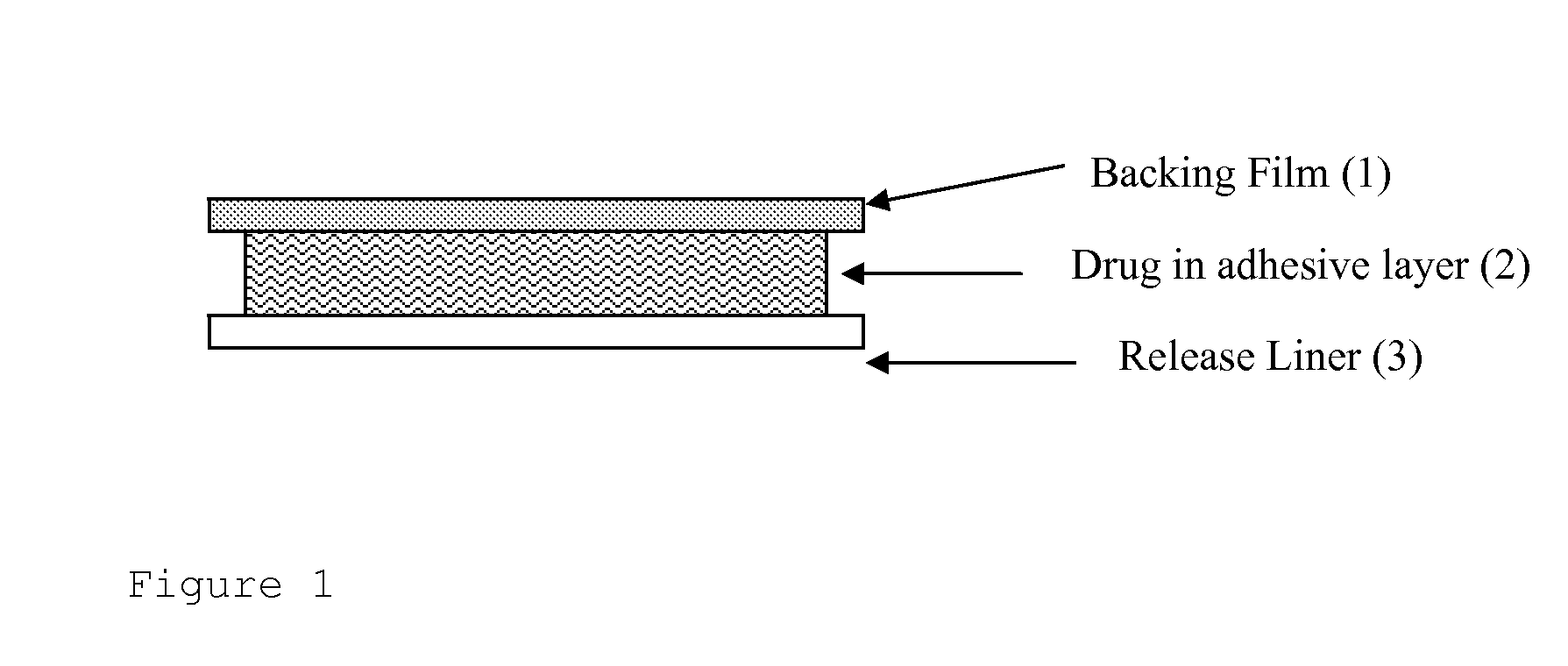
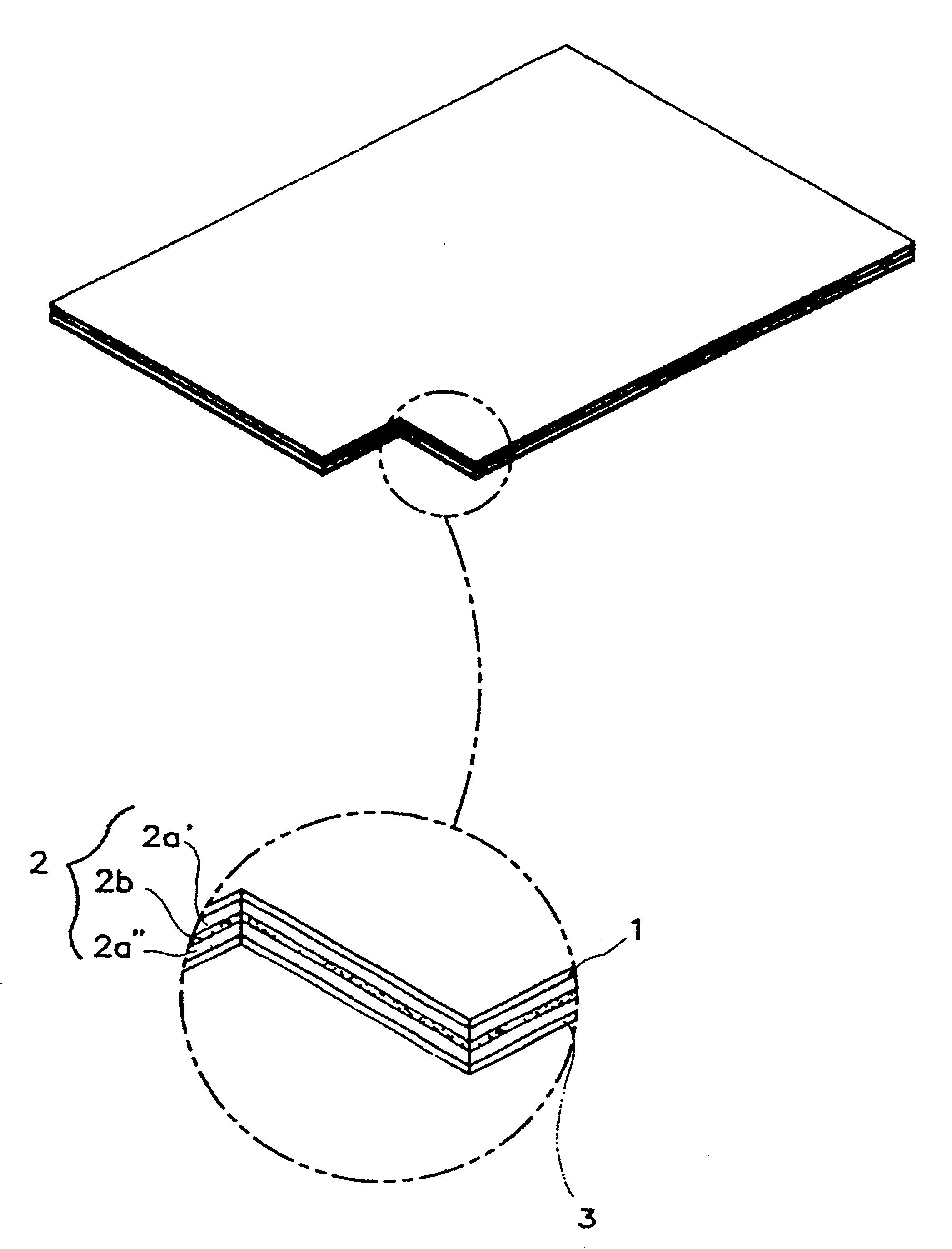
InactiveUS6723337B1Improve solubilityPromote absorptionPowder deliveryPeptide/protein ingredientsAdditive ingredientDissolution
The invention herein relates to a transdermal drug delivery systern for anti-Inflammatory analgesic. agent comprising diclofenac diethylammonium salt, wherein a backing film (1), a matrix layer (2) containing active ingredients, a release liner (3) which is removed before application onto the skin are laminated therein. Mome particularly, the invention herein relates to a transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonum salt, wherein the transdermal penetration and adhesion of the patch to the body are enhanced by means of a matrix layer which comprises a diclofenac diethylammonum salt as active ingredient in addition to acrylic polymer as adhesive constituent, non-ionic surfacant as absorption enhancer, terpene and dissolution assistant, and the volatile and non-volatile constituents of the composition arc separately applied therein for significantly reducing the manufacturing time thereof.
Owner:SAMYANG BIOPHARMLS CORP
Transdermal drug patch
InactiveUS20010033858A1Sufficient transdermal permeabilityConstant concentrationNervous disorderSheet deliverySolubilityTransdermal patch
The present invention is directed toward a formulation for supplying additional drug for delivery in a transdermal drug delivery device. The invention comprises a drug, such as fentanyl that is capable of transdermal delivery, and a solution having a pre-designed solubility for the drug. The solution dissolves only a portion of said drug and allows a significant portion of the drug to remain undissolved in solution, thus providing extra drug to be delivered at a consistent, controlled delivery rate. The invention may used in conjunction with controlled heat.
Owner:ZARS INC
Transdermal drug delivery devices comprising a polyurethane drug reservoir
InactiveUS20050048104A1Easy to processImprove versatilityAntibacterial agentsOrganic active ingredientsDrug reservoirMedicine
The present invention relates to the field of transdermal drug delivery. More specifically, the present invention relates to drug reservoir materials for use in transdermal drug delivery devices. The drug reservoirs of the present invention comprise a polyurethane polymer which can be processed at temperatures below those which cause degradation of temperature sensitive drugs and / or excipients. The present invention is also directed to tailoring the release characteristics of the polyurethane material to accommodate a range of suitable drugs to be delivered from the transdermal drug delivery device and / or provide a range of delivery rates for a particular drug.
Owner:VENKASTREETCARAN SUBRAMANIAN S +3
Compositions and methods for delivering estradiol in transdermal drug delivery systems
ActiveUS20060078601A1Simple and inexpensive to manufactureLoss in componentAdhesive dressingsAbsorbent padsHuman skinPharmaceutical drug
A blend of at least two polymers in combination with a drug provides a pressure-sensitive adhesive composition for a transdermal drug delivery system in which the drug is delivered from the pressure-sensitive adhesive composition and through dermis when the pressure-sensitive adhesive composition is in contact with human skin.
Owner:NOVEN PHARMA
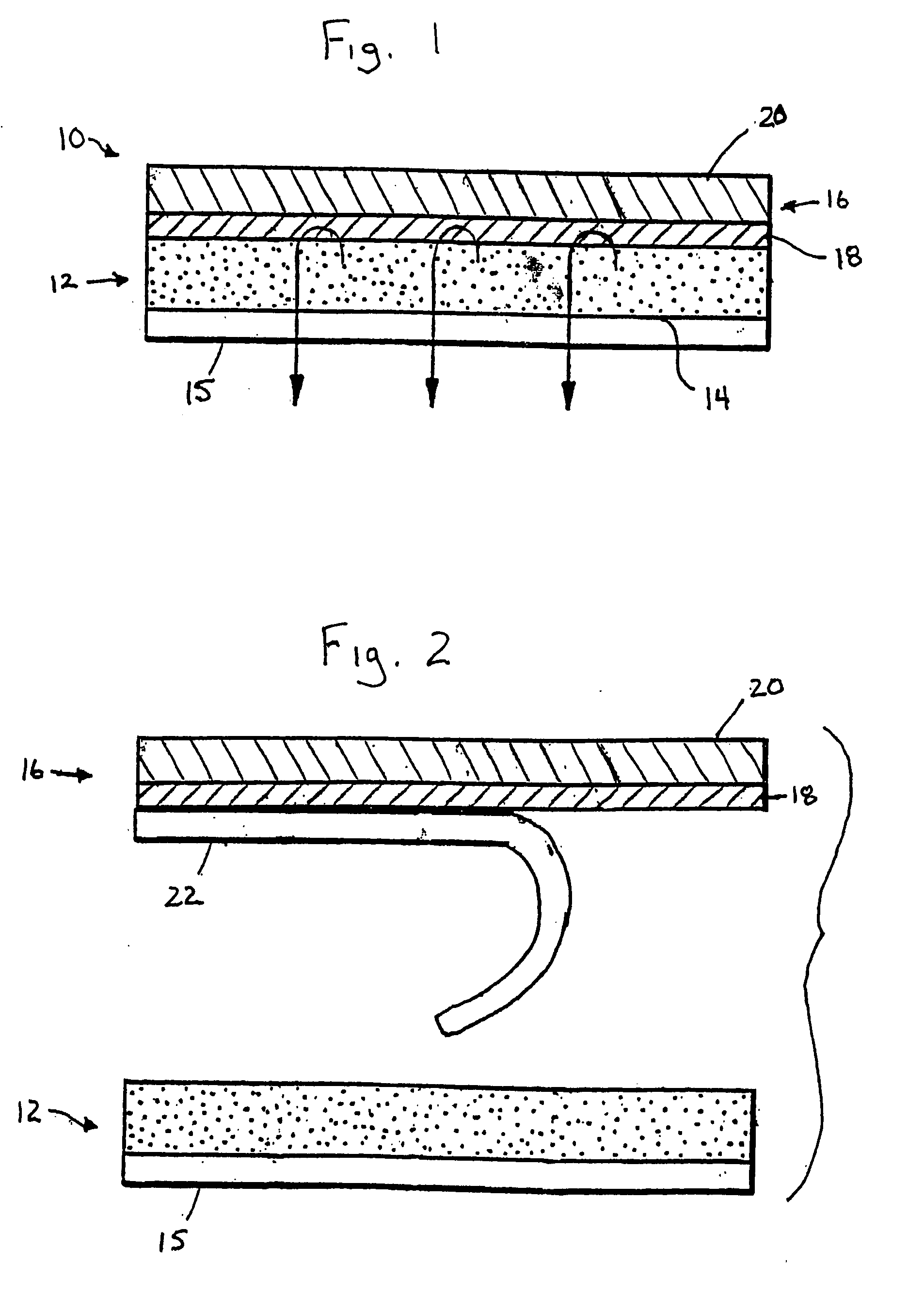
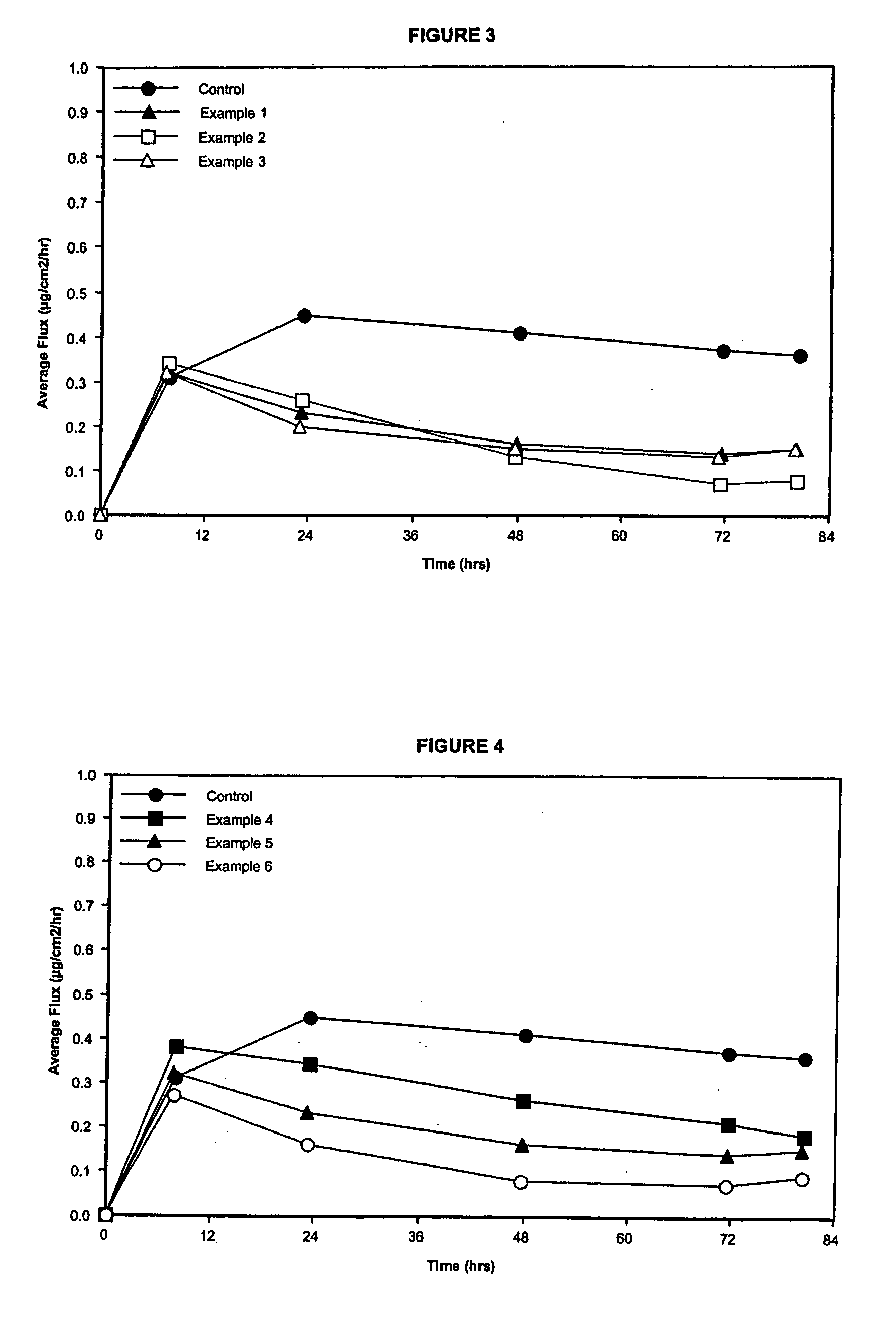
Transdermal Drug Delivery using an Osmolyte and Vasoactive Agent
ActiveUS20100076035A1Increase blood flowImprove permeabilityBiocideHydroxy compound active ingredientsOtic AgentsActive agent
Owner:BIOCHEMICS +1
Skin permeation device for analyte sensing or transdermal drug delivery
Devices, systems, kits and methods for increasing the skin's permeability controlled by measured skin electrical parameter are described herein. They may be used for transdermal drug delivery and / or analyte extraction or measurement. The controlled abrasion device contains (i) a hand piece, (ii) an abrasive tip, (iii) a feedback control mechanism, (iv) two or more electrodes, and (v) an electrical motor. The feedback control mechanism may be an internal feedback control mechanism or an external feedback control. The kit contains the controlled abrasion-device, one or more abrasive tips, optionally with a wetting fluid. The method for increasing the skin's permeability requires applying the controlled abrasion device to a portion of the skin's surface for a short period of time, until the desired level of permeability is reached. Then the abrasion device is removed, and a drug delivery composition or device or an analyte sensor is applied to the treated site.
Owner:ECHO THERAPEUTICS INC
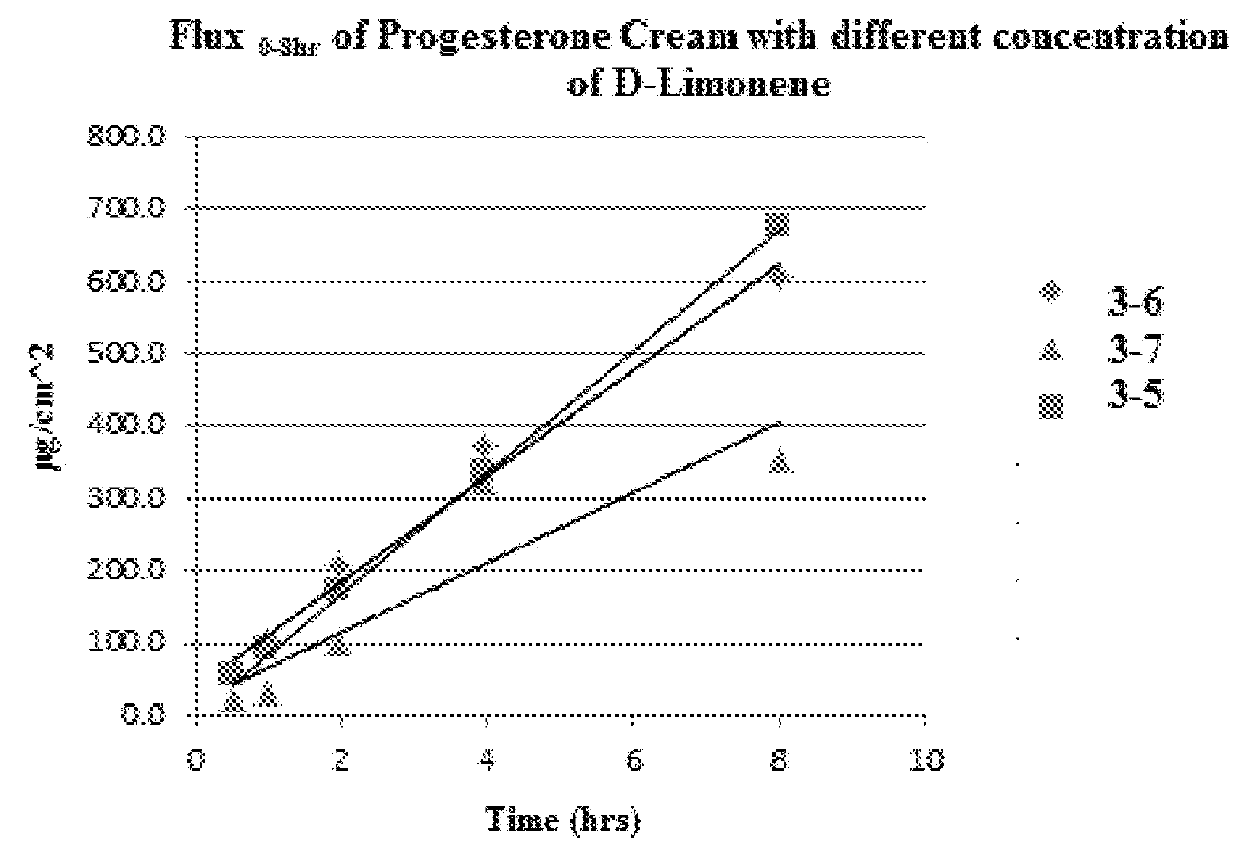
Transdermal cream
This disclosure relates to transdermal pharmaceutical compositions containing progesterone in combination with one or more solubilizing agents and penetration enhancers, wherein the pharmaceutical compositions are formulated as creams for topical administration. In some embodiments, the transdermal pharmaceutical compositions contain progesterone, a medium-chain oil, and d-limonene. In some embodiments, the transdermal pharmaceutical compositions contain progesterone, a medium-chain oil, a penetration enhancer (e.g., propylene glycol, a fatty acid ester of propylene glycol, a glycol ether), and optionally d-limonene. In certain embodiments, the pharmaceutical compositions further include estradiol. Methods for treating conditions associated with hormone deficiency in a subject are also described.
Owner:THERAPEUTICSMD INC
Adhesively applied external nasal strips and dilators containing medications and fragrances
InactiveUS7013889B2Extending olfactory effectivenessEfficient deliveryRespiratorsBreathing filtersTransdermal medicationBreathing process
Owner:WINTER BREATH
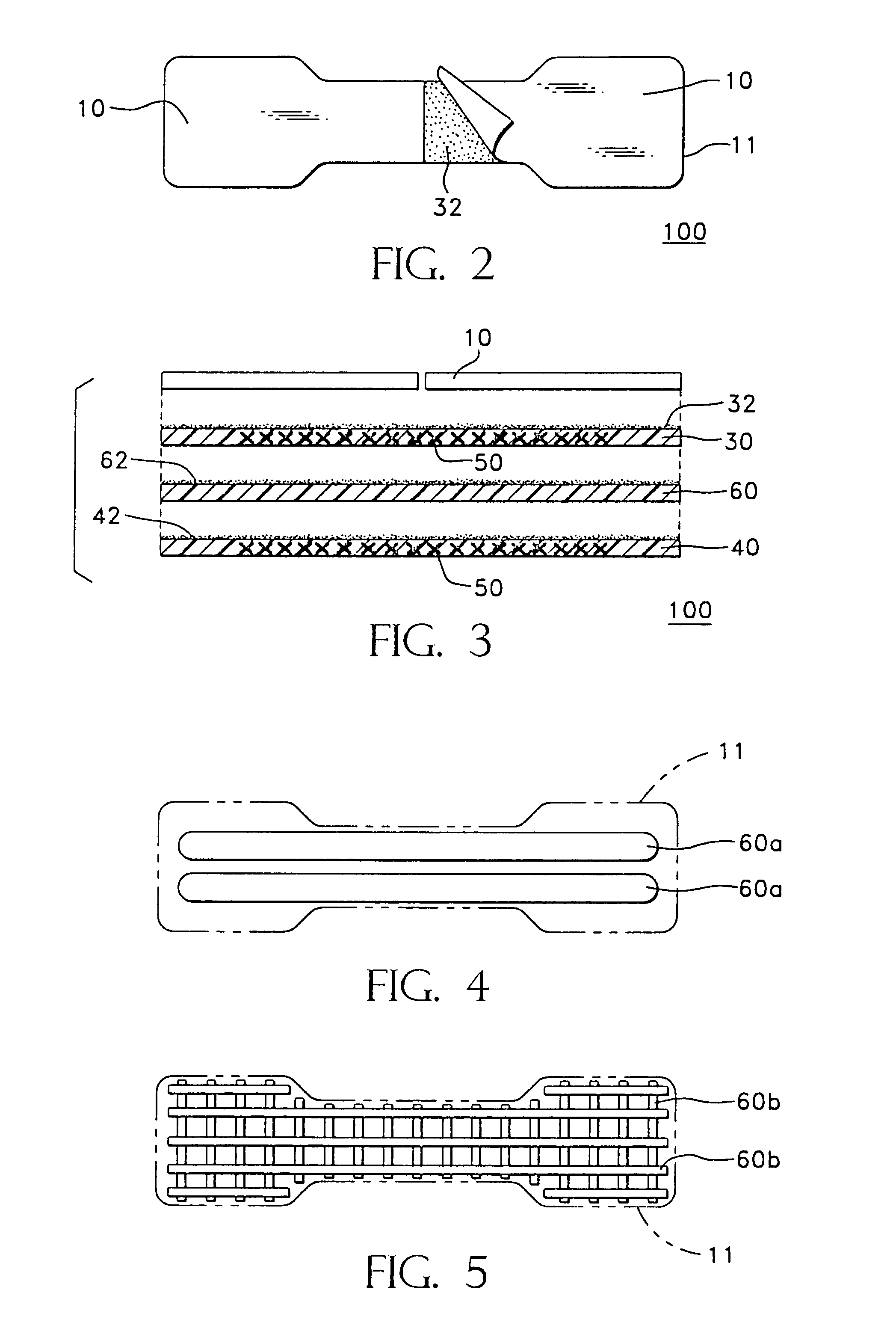
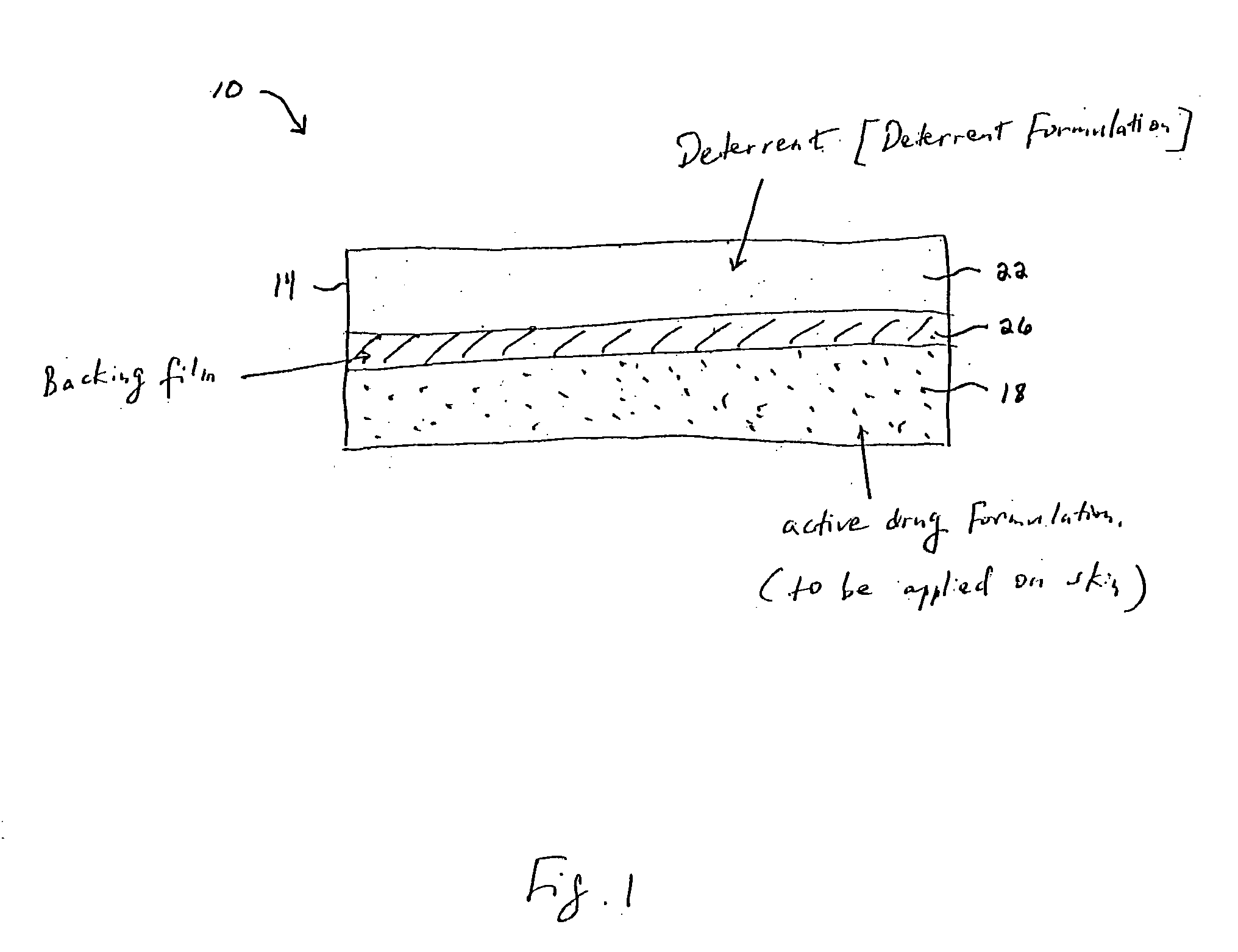
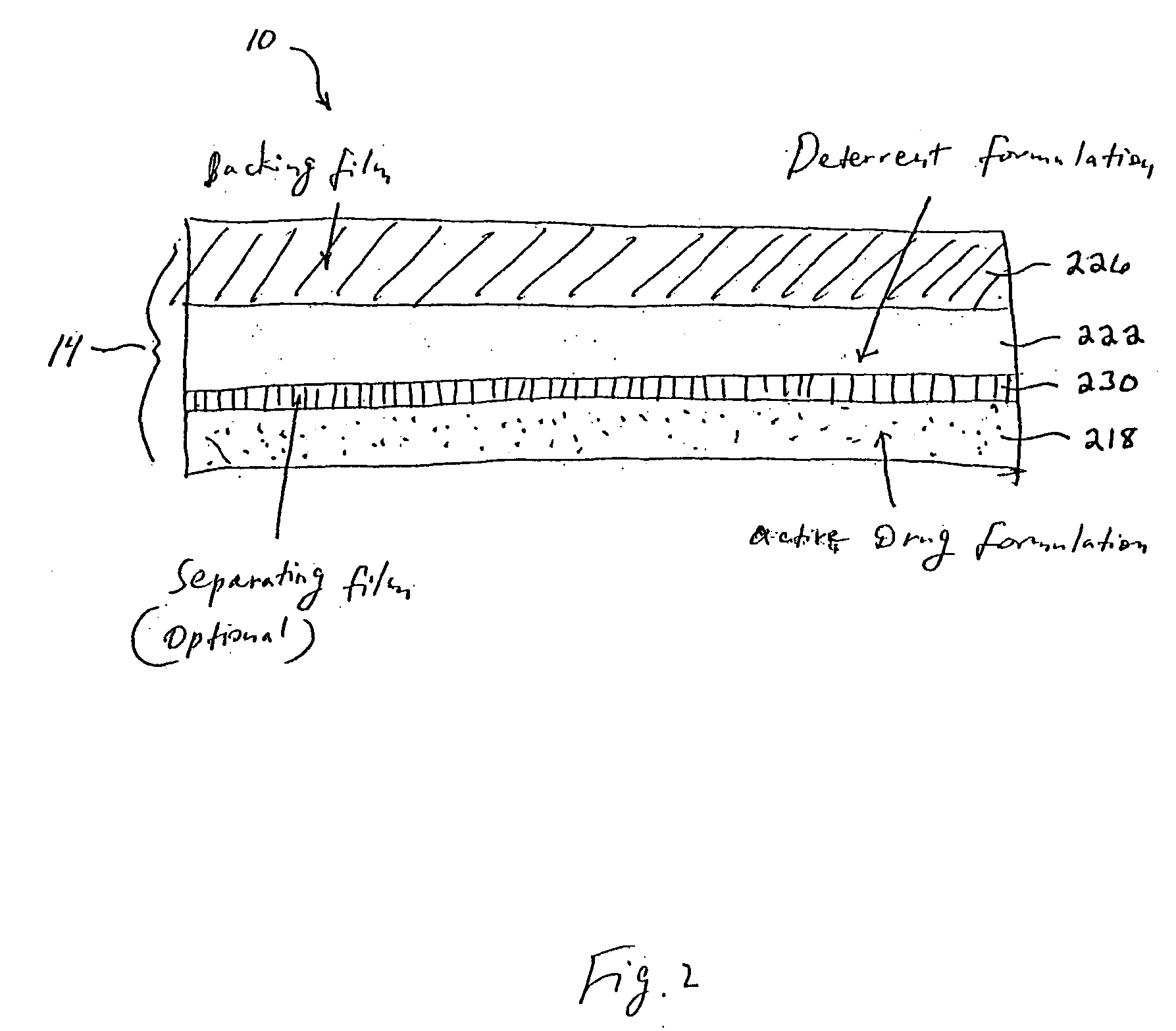
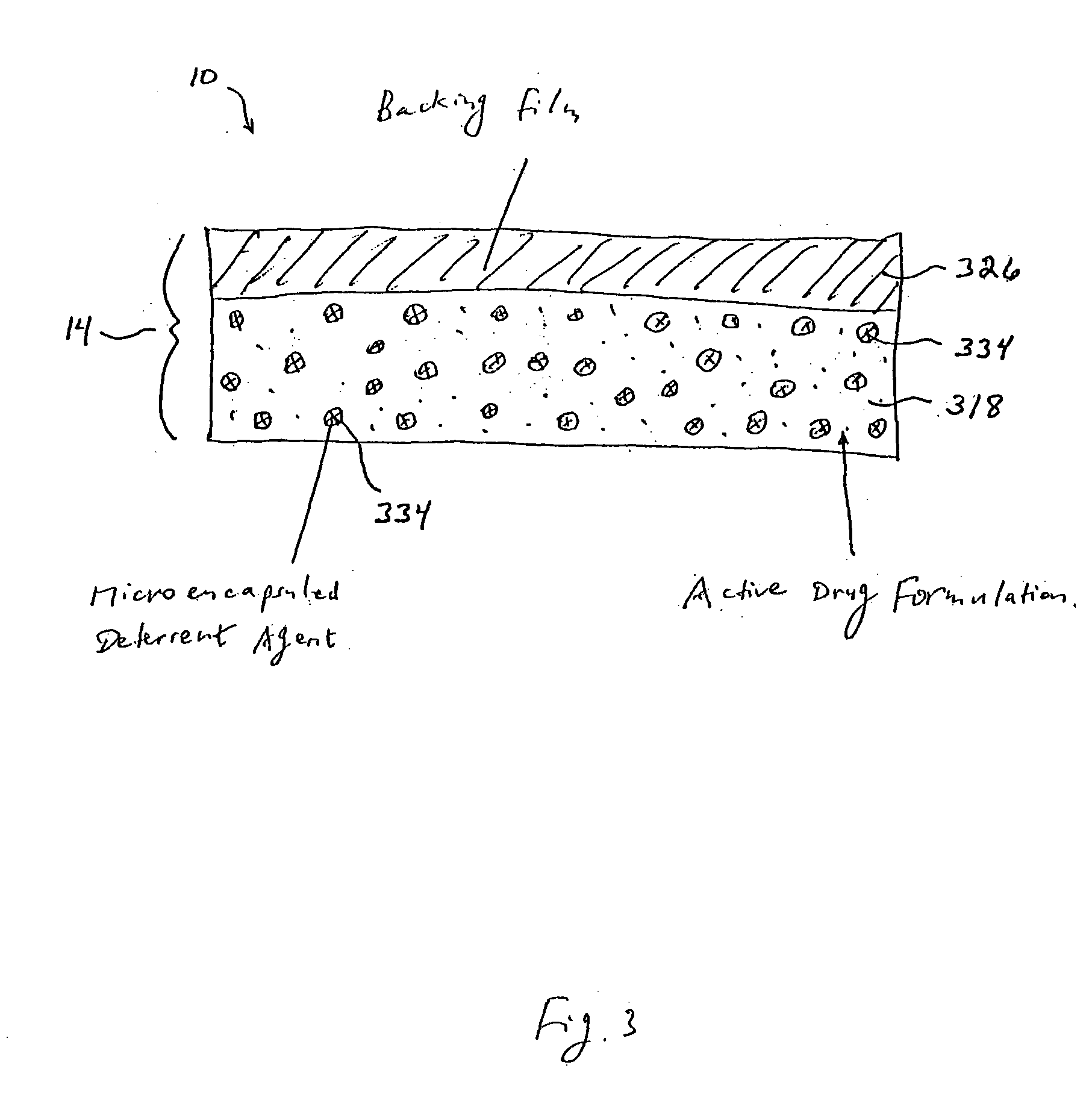
Methods and apparatus for transdermal delivery of abusable drugs with a deterrent agent
InactiveUS20040109886A1Reduced potential for abuseEfficient managementBiocideAnimal repellantsAnesthetic AgentTransdermal medication
The present invention describes a system and method for reducing the abuse potential of drugs, particularly or especially narcotic agents, in transdermal drug delivery systems. This is achieved by designing the transdermal drug delivery system such that when the active drug is extracted out of the transdermal delivery system, a deterrent agent is also timely co-extracted upon introduction of the system to an extraction solution where abuse may normally be allowed take place. Preferably, the deterrent agent of the present invention is capable of inducing or causing one or more intensely repugnant effects within the abusing person, thus reducing the potential for abuse of the active drug formulation.
Owner:ZARS INC
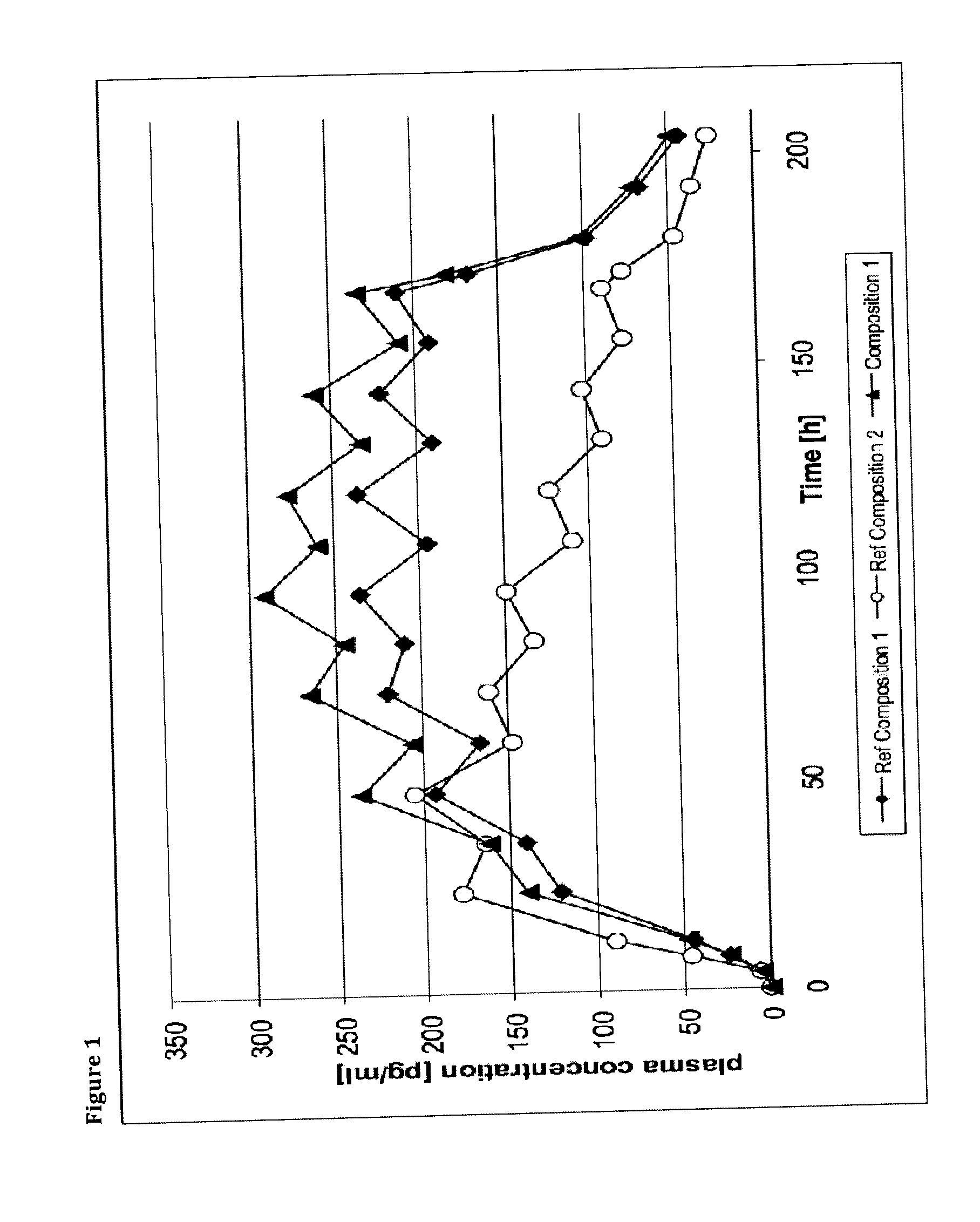
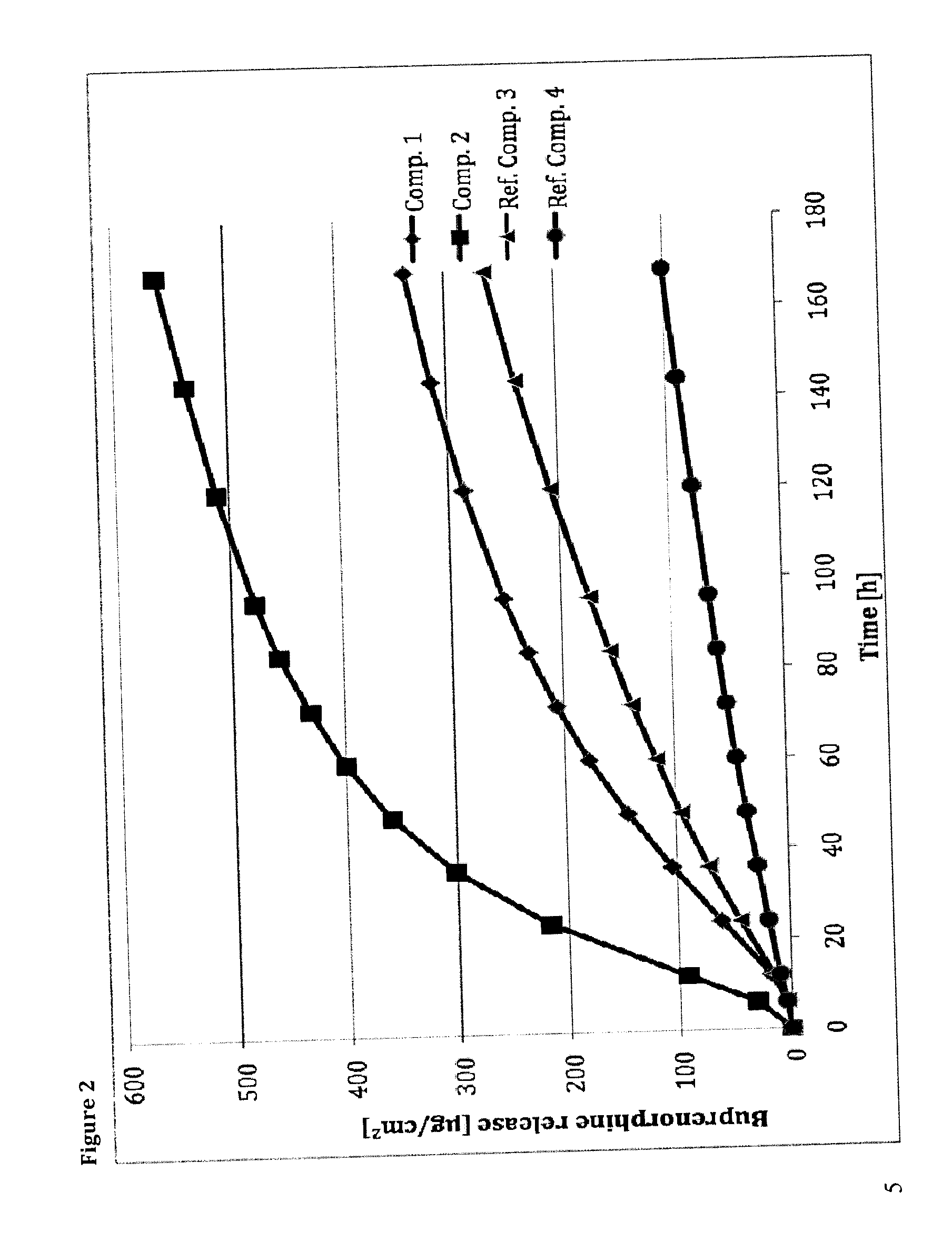
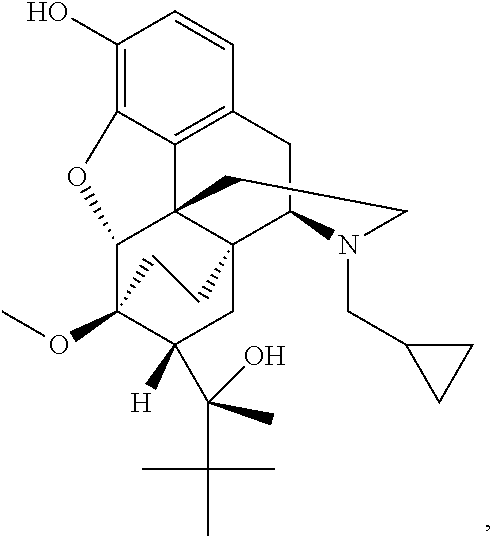
Transdermal therapeutic system comprising buprenorphine
ActiveUS20130331803A1Improve comfortBiocideAdhesive dressingsTransdermal medicationPenetration enhancer
The invention is concerned with a transdermal therapeutic system (TTS) comprising buprenorphine and a method of manufacturing such a TTS. The transdermal therapeutic system is used for the transdermal administration of buprenorphine and analogues thereof. In particular, the invention relates to the use of a transdermal therapeutic system (TTS) for analgesic purposes. The TTS according to the invention comprises a transdermal drug delivery composition comprising buprenorphine and an adhesive component, which is a mixture of a crosslinked and a non-crosslinked acrylic polymer and a penetration enhancer comprising a keto acid.
Owner:HEXAL AG
Silicone-containing acrylic polymers for transdermal drug delivery compositions
Described herein are silicone-containing acrylic polymers useful, for example, in transdermal drug delivery compositions, to methods of making and using them, to transdermal drug delivery compositions comprising them, and to methods of making and using such transdermal drug delivery compositions. The polymers are particular suitable for formulating amine drugs, such as amphetamine, methylphenidate, rivastigmine, paroxetine and clonidine.
Owner:NOVEN PHARMA
Transdermal drug delivery patch and method of controlling drug release of the same by near-ir
ActiveUS20120283695A1Control releaseEfficient deliveryBiocideOrganic active ingredientsNanoparticlePhotothermal conversion
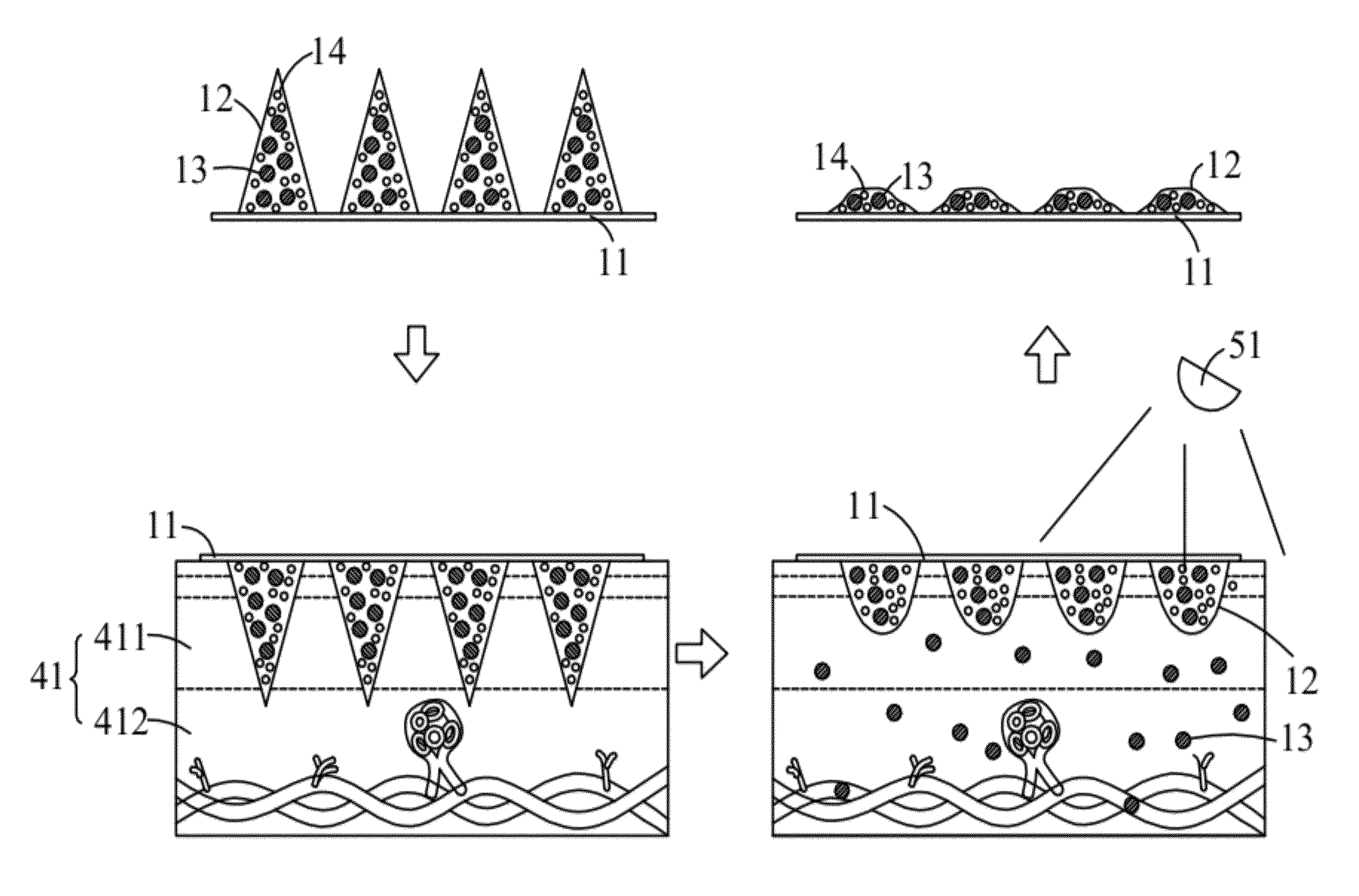
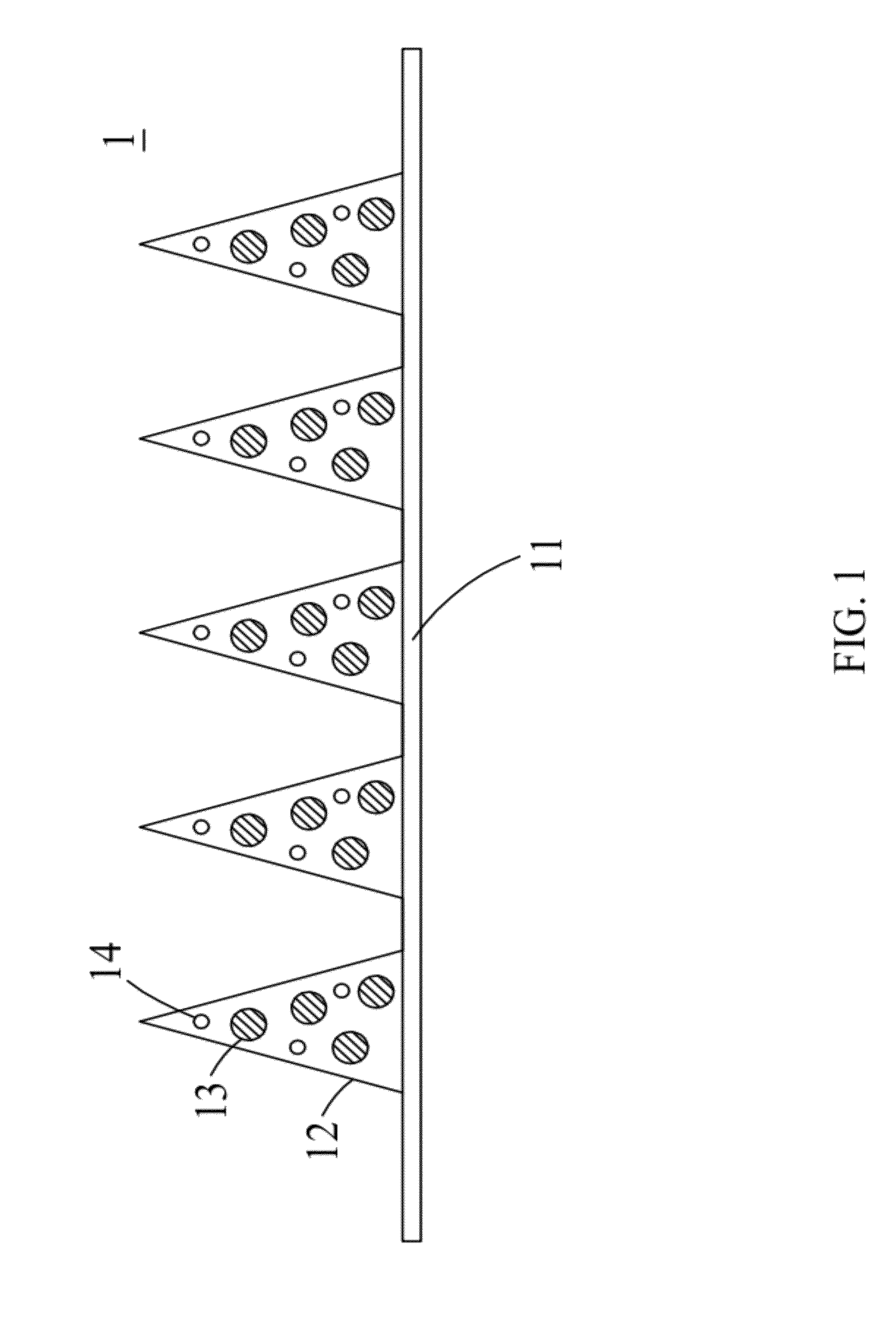
A transdermal drug delivery patch and a method of controlling the drug release of the transdermal drug delivery patch by near-IR are disclosed. The transdermal drug delivery patch comprises a substrate, carriers and drugs. The drugs are encapsulated in the carriers, and the carriers having the drugs are disposed on a surface of the substrate. The carriers are formed of biodegradable polymers, and nano-particles with a photothermal conversion effect are loaded in the carrier. When the carriers are punctured into the skin and the nano-particles in the carrier absorb the near-IR, the near-IR is converted into heat by the nano-particles to melt the carrier and thus releasing the drugs encapsulated in the carrier into the skin. Accordingly, the speed of releasing the drugs encapsulated in the carrier can be controlled accurately by the near-IR.
Owner:NAT CHENG KUNG UNIV
Prophylactic and therapeutic treatment of topical and transdermal drug-induced skin reactions
Botanically derived anti-irritants for prophylactic and therapeutic treatment of adverse skin reactions from application of transdermal or topical drug delivery system, permits the effective administration of a drug from a delivery system in which the drug, of a component of the delivery system comprises a skin irritant; and the delivery systems formed thereby.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Microencapsulated fragrances and methods of coating microcapsules
InactiveUS7011093B2Extending olfactory effectivenessMinimizing adhesive residueOrganic active ingredientsBreathing filtersTransdermal medicationPharmaceutical Substances
Nasal dilators and strips, methods of their manufacture, and methods for improving the breathing of individuals are provided. The strips and dilators include an elongated substrate, with or without a dilating component or portion, having top and bottom surfaces and a pressure-sensitive adhesive disposed on the bottom surface. The dilator is designed to provide a gentle expanding force to the nasal wall tissue when the dilator is adhesively attached to the nose. This invention further includes a cosmetic fragrance, an aromatic medication and / or transdermal medication disposed on the strips or dilators. In order to improve the shelf-life and in-use olfactory effectiveness of such products, fragrance delivery mechanisms are used. Separation of volatile oils and adhesives are also provided to minimize adhesive residue.
Owner:WINTER BREATH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com