Silicone-containing acrylic polymers for transdermal drug delivery compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0093]Silicone-containing acrylic copolymers are synthesized by copolymerization using methodologies known in the art. For example, acrylic monomers are copolymerized with silicone-containing acrylic monomers in an appropriate solvent, such as ethyl acetate, with an appropriate initiator, such as 2,2′-azobisisobutyronitrile (AIBN), at an appropriate temperature.
[0094]Silicone-containing acrylic copolymer A is prepared as follows: An initial charge containing 9.7 g methyl acrylate (MA), 20.9 g 2-ethylhexyl acrylate (2-EHA), 95.1 g 3-(tris(trimethylsilyloxy)silyl)propyl methacrylate (TRIS), 0.074 g AIBN, and 125.5 g ethyl acetate (solvent) is mixed in a 2-L round bottom flask, which is installed with a thermometer, condenser, stainless steel stirrer, water bath and dropping funnel. Under stirring, the initial charge is heated to 80° C. and allowed to reflux for 15 minutes. Then a mixture of 29.0 g MA, 62.5 g 2-EHA, 285.3 g TRIS, 0.22 g AIBN and 377.0 g ethyl acetate is uniformly added...
example 2
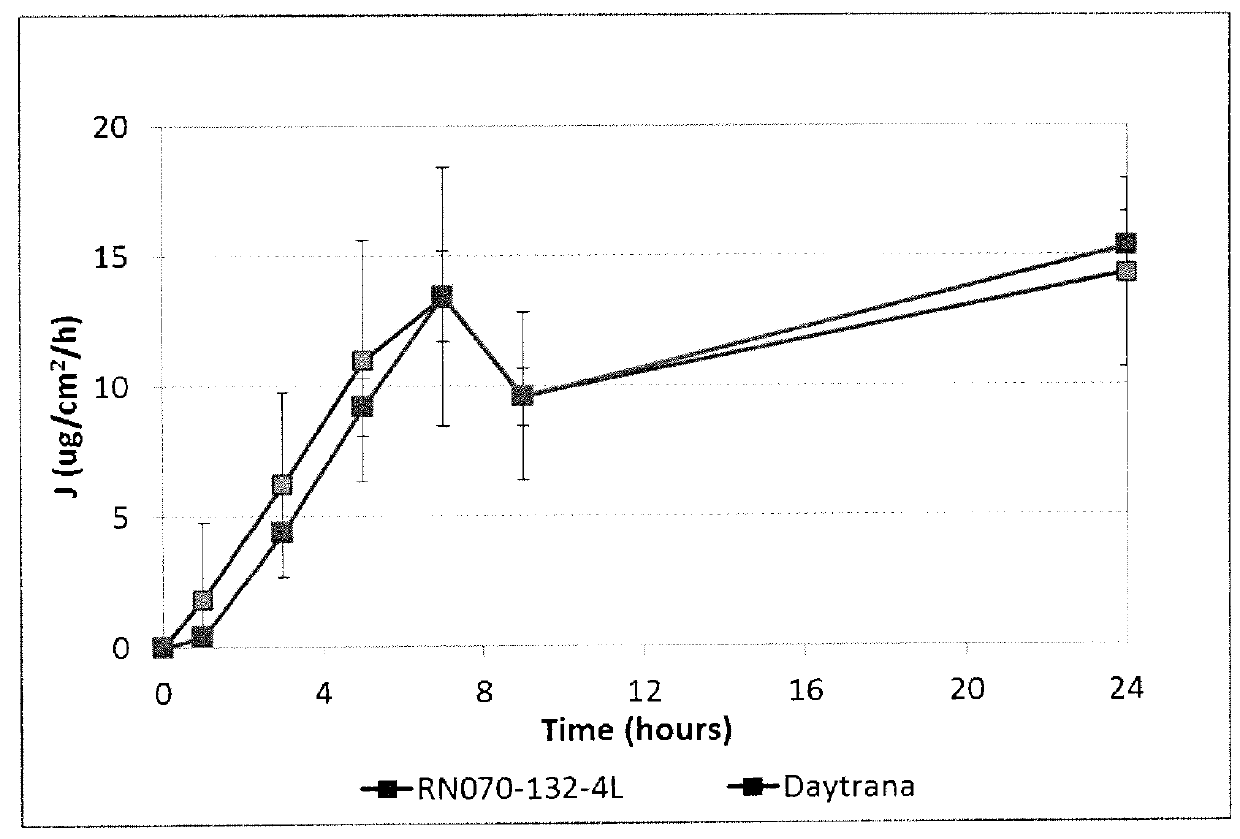
[0097]Methylphenidate was formulated in silicone-containing acrylic polymer B (described above) to prepare a composition comprising 20% methylphenidate and 80% polymer B. Drug flux through human cadaver skin over 24 hours was assessed in vitro. Results as compared to results achieved with the polymer matrix of the commercial Daytrana® product (20% methylphenidate, 80% blend of acrylic pressure-sensitive adhesive polymer and silicone pressure-sensitive adhesive polymer) are shown in FIG. 1 and summarized below.
94 hr FluxFlux Lot #Formulation (SP 9732 Backing)(mcg / cm2 / h)Ratio65654Daytrana9.101.0RN070-132-4L20% MPH + 80% Copolymer B10.481.15
[0098]The results show that the composition based on polymer B achieved a drug flux comparable to that of Daytrana®.
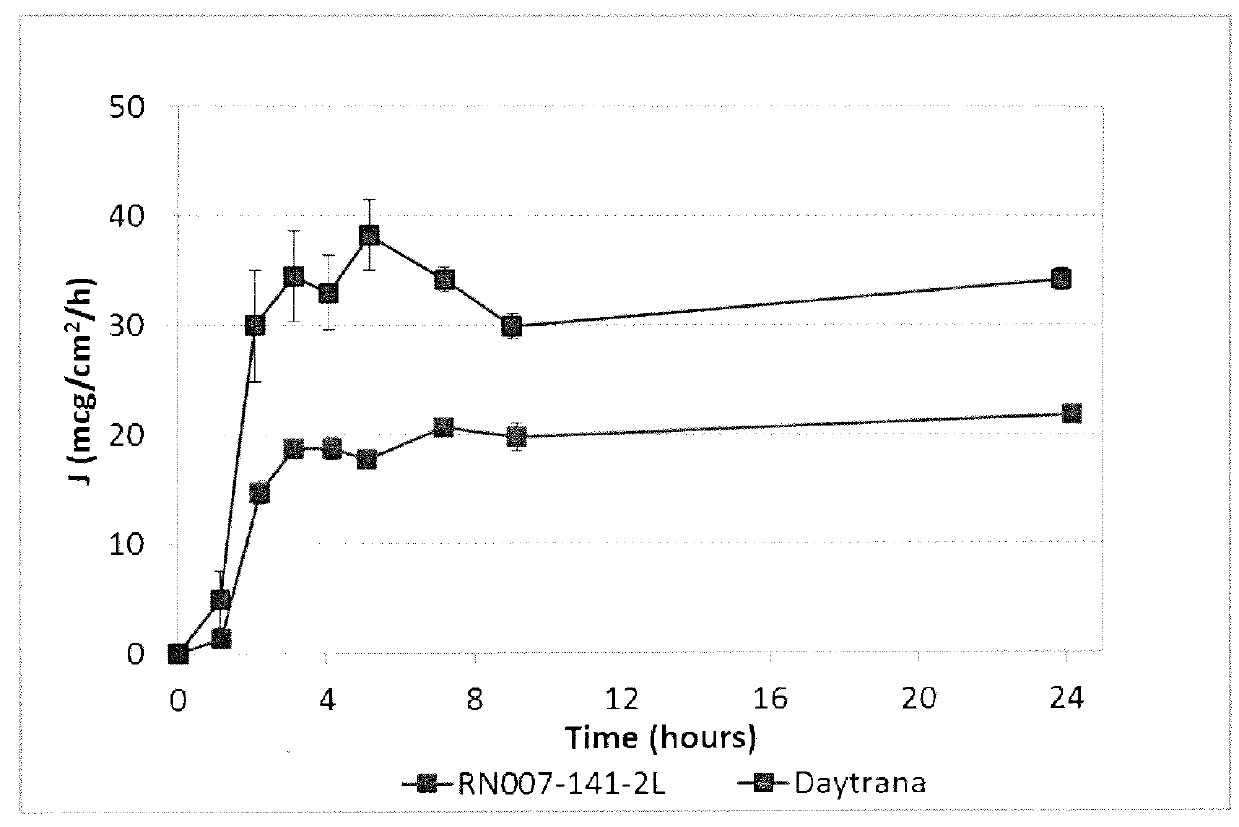
[0099]For comparison, the in vitro flux from a composition prepared with 20% methylphenidate in 80% acrylic pressure-sensitive adhesive (Gelva® 3087) and from Daytrana® are plotted in FIG. 2 and summarized below.
94 hr FluxLot #Formulat...
example 3
[0101]The methylphenidate / polymer B composition described above was applied to a backing and a release liner, and peel force from the release liner was assessed over 4 months. Results as compared to results achieved with the commercial Daytrana® product are shown below.
Peel from Release Liner (g / 0.5″, n = 3)Lot #Formulation (w / w)1M2M3M4MRN056-Freshly made 9.222.439.980.632-4LDaytranaRN070-20% MPH + 80% 23.325.022.418.7132-4LCopolymer B
[0102]The results show that the peel force for the polymer B composition remained stable and low while that of the Daytrana® product increased over time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com