Patents
Literature
700results about How to "Patient compliance is good" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
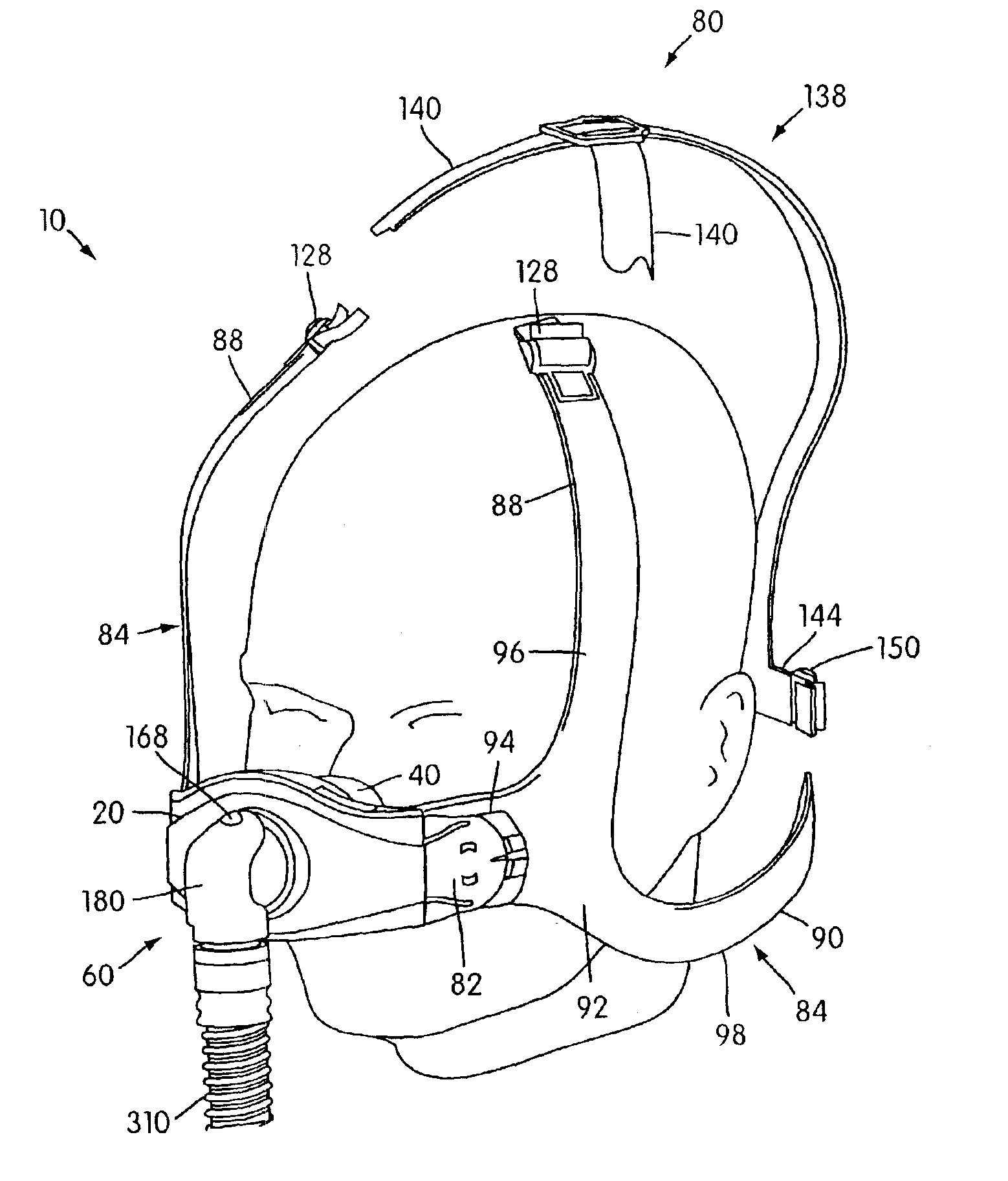
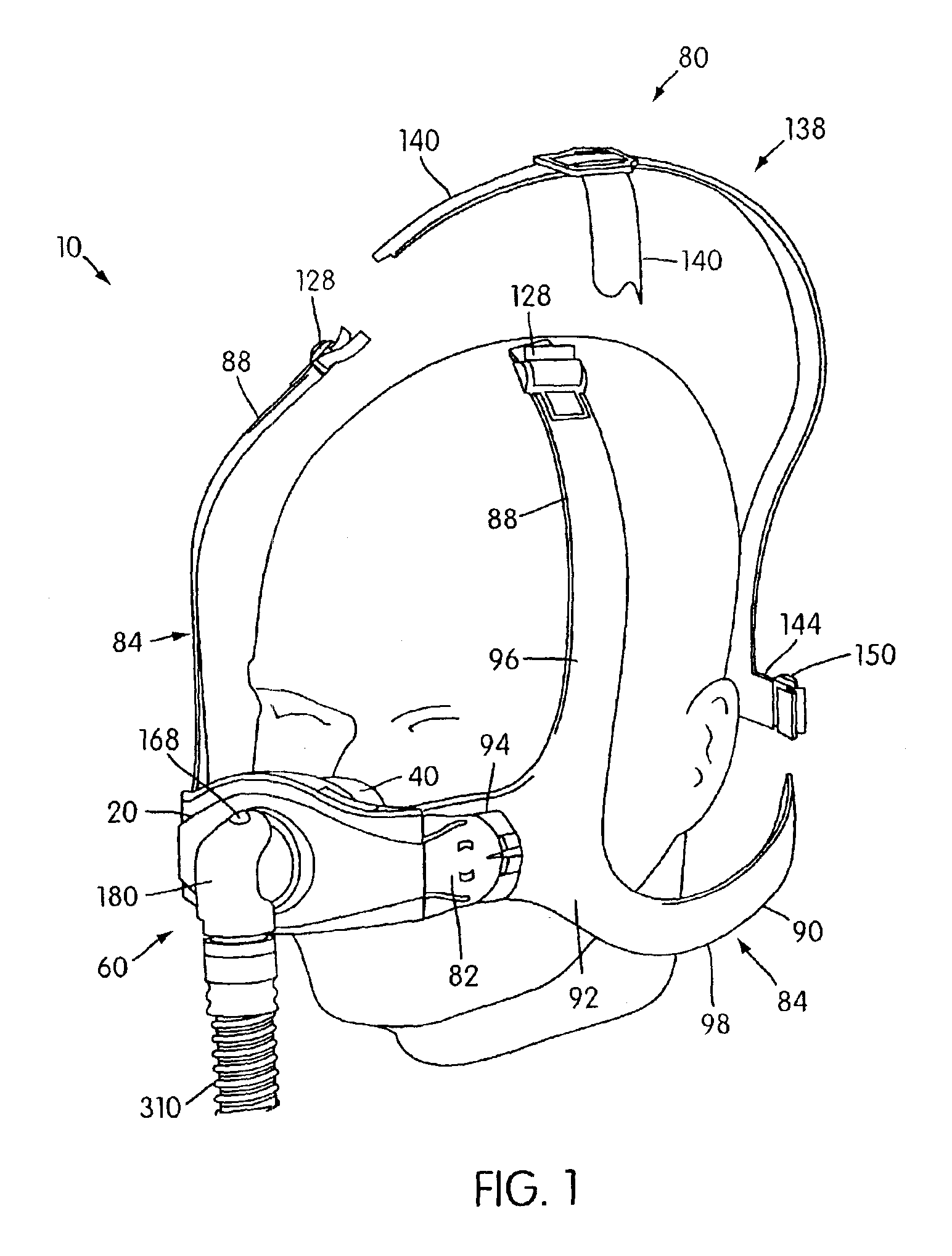
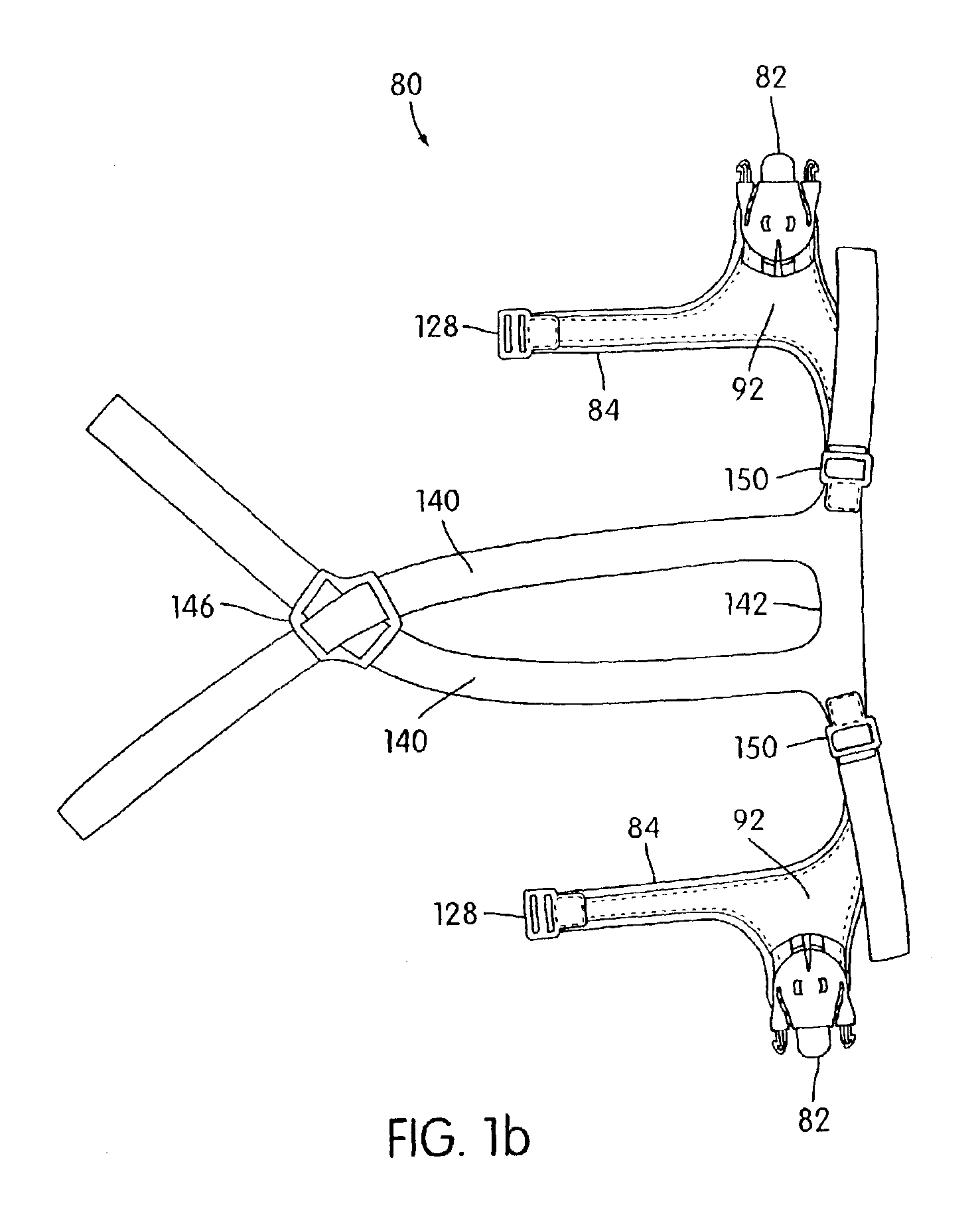
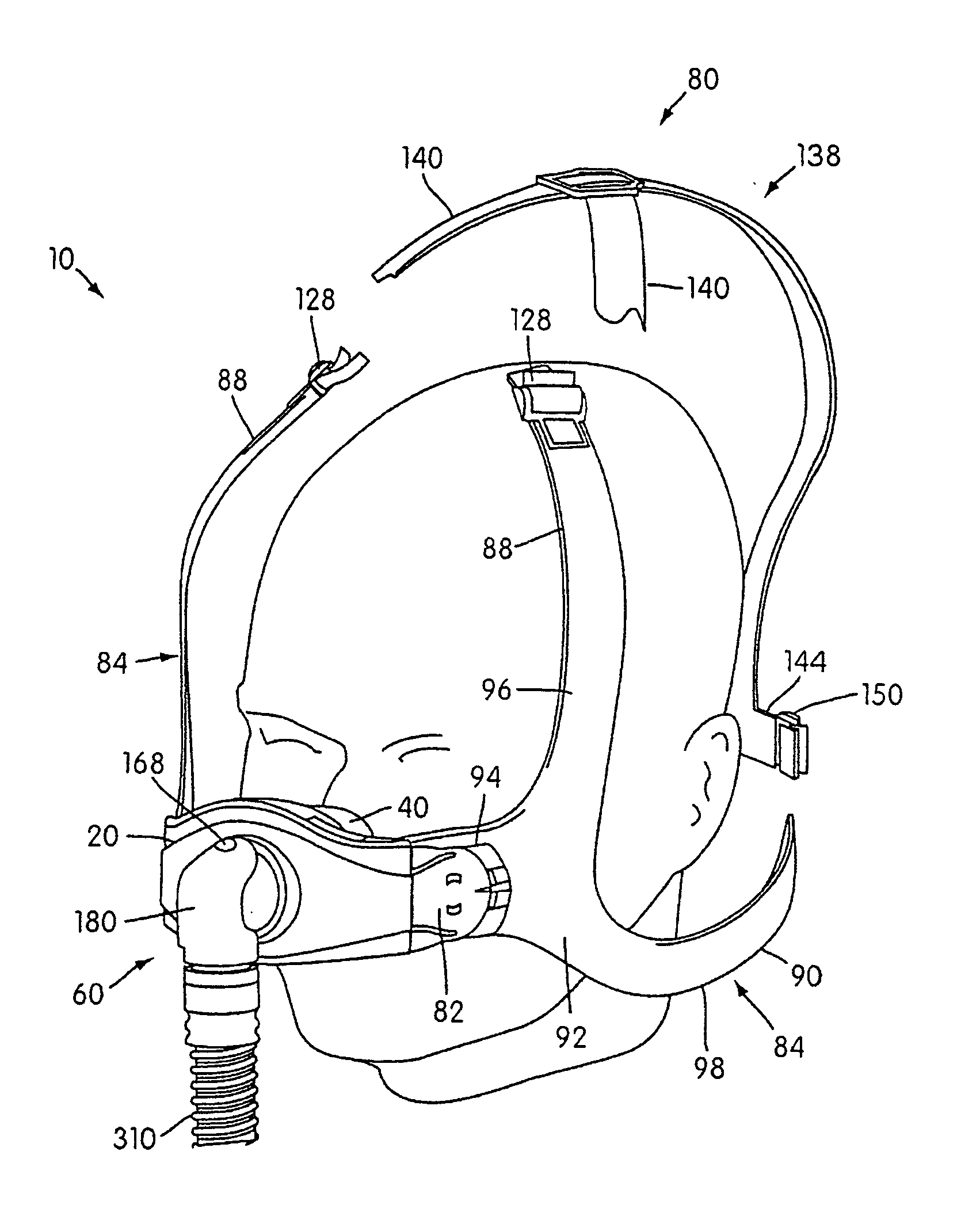
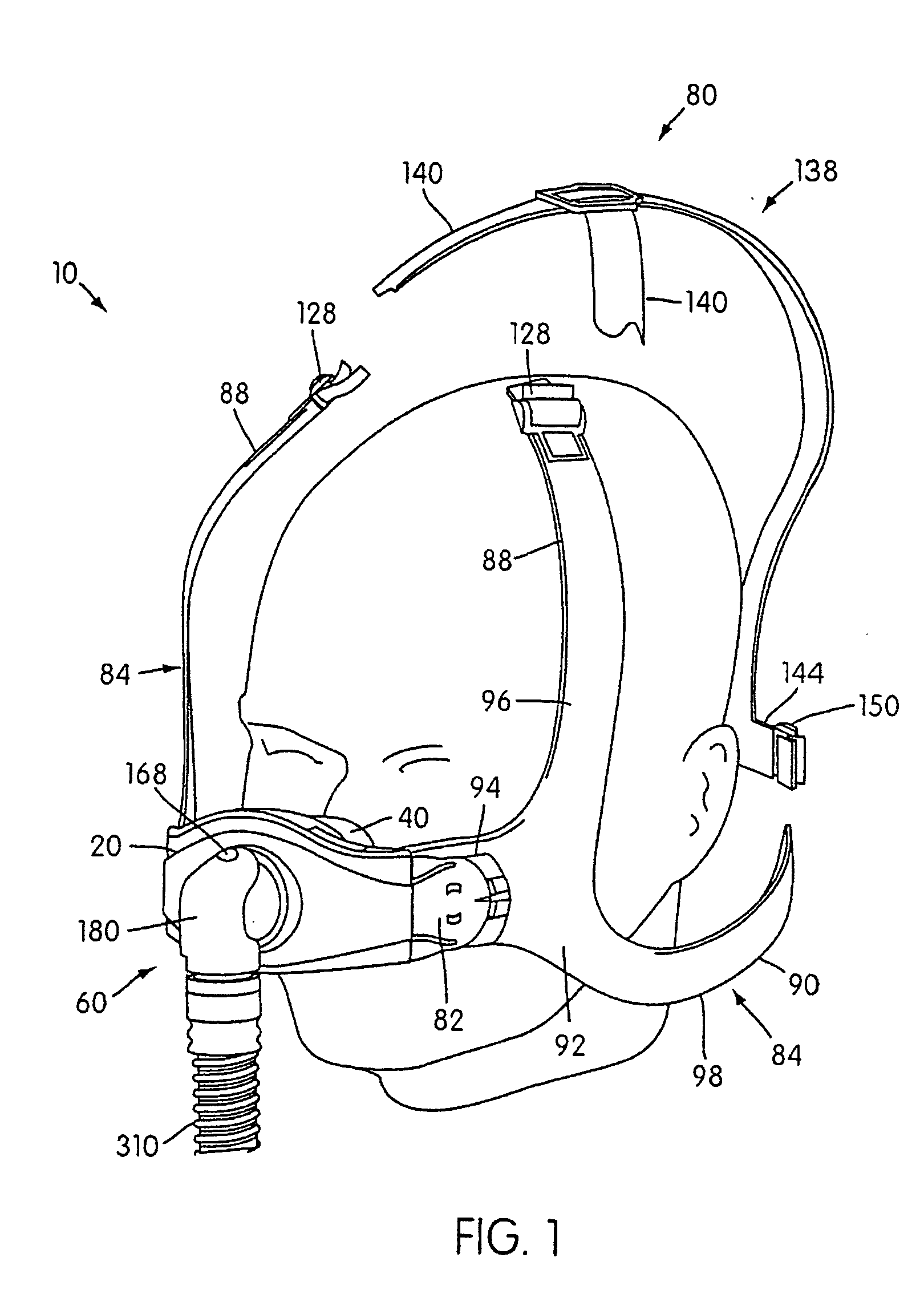
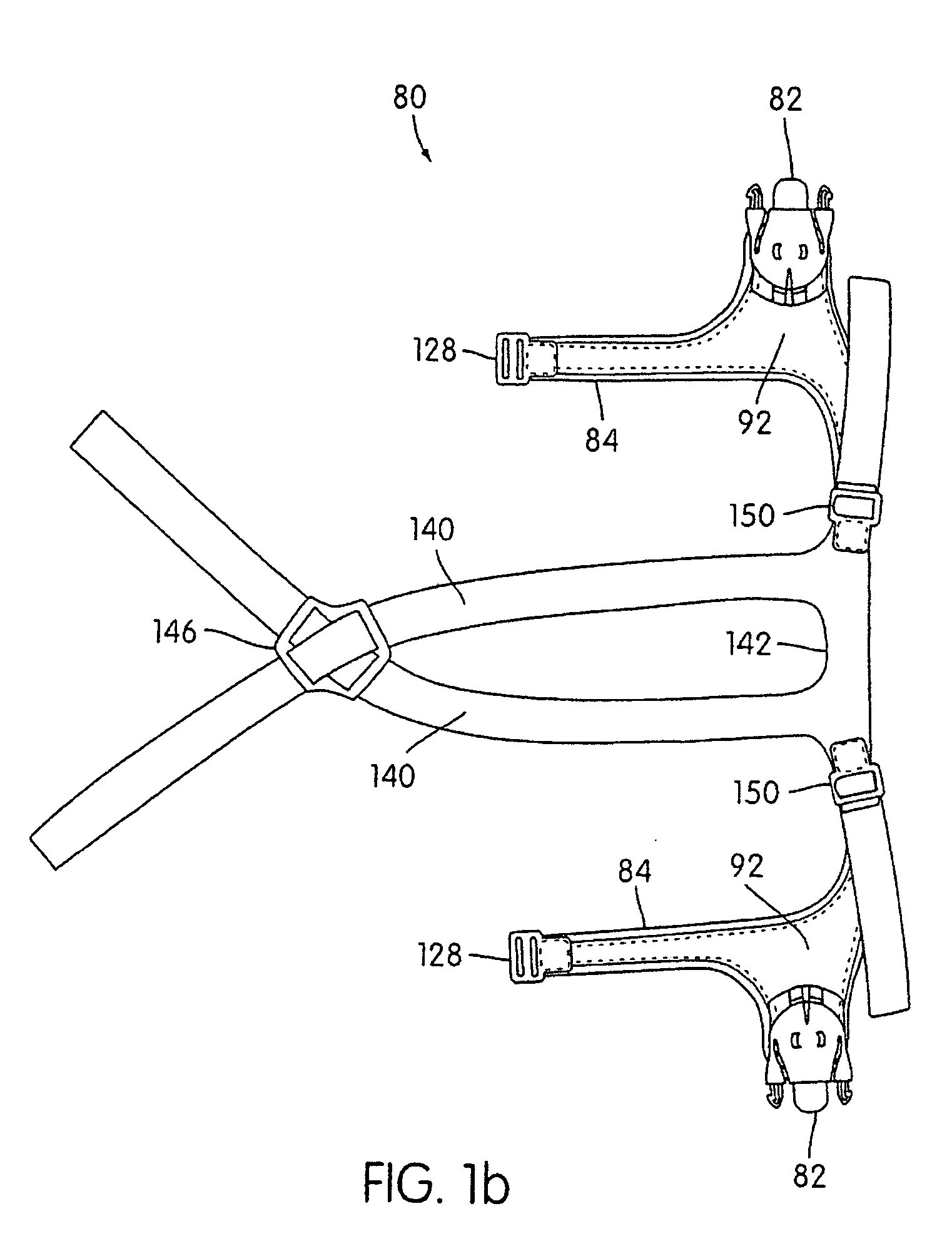
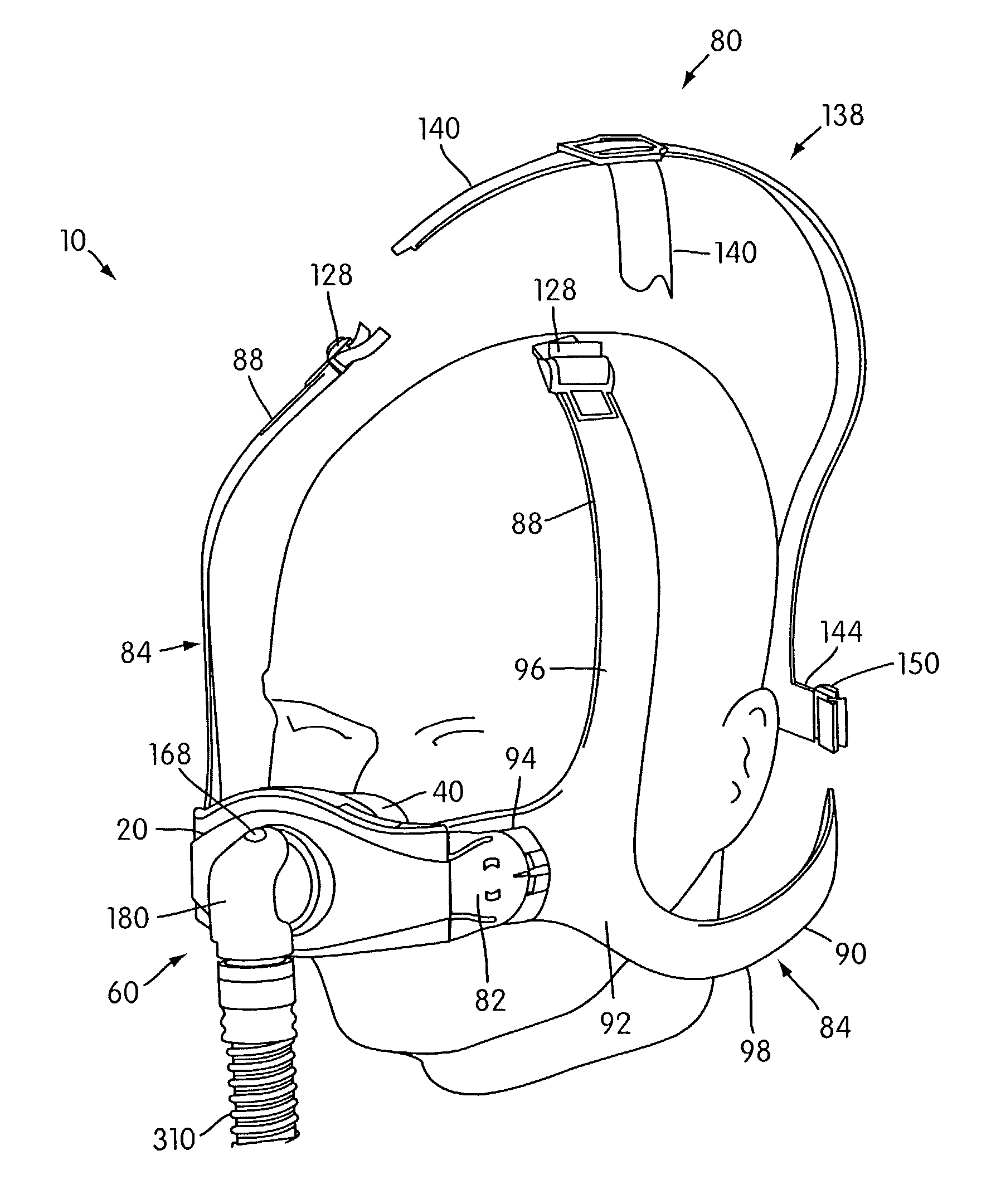
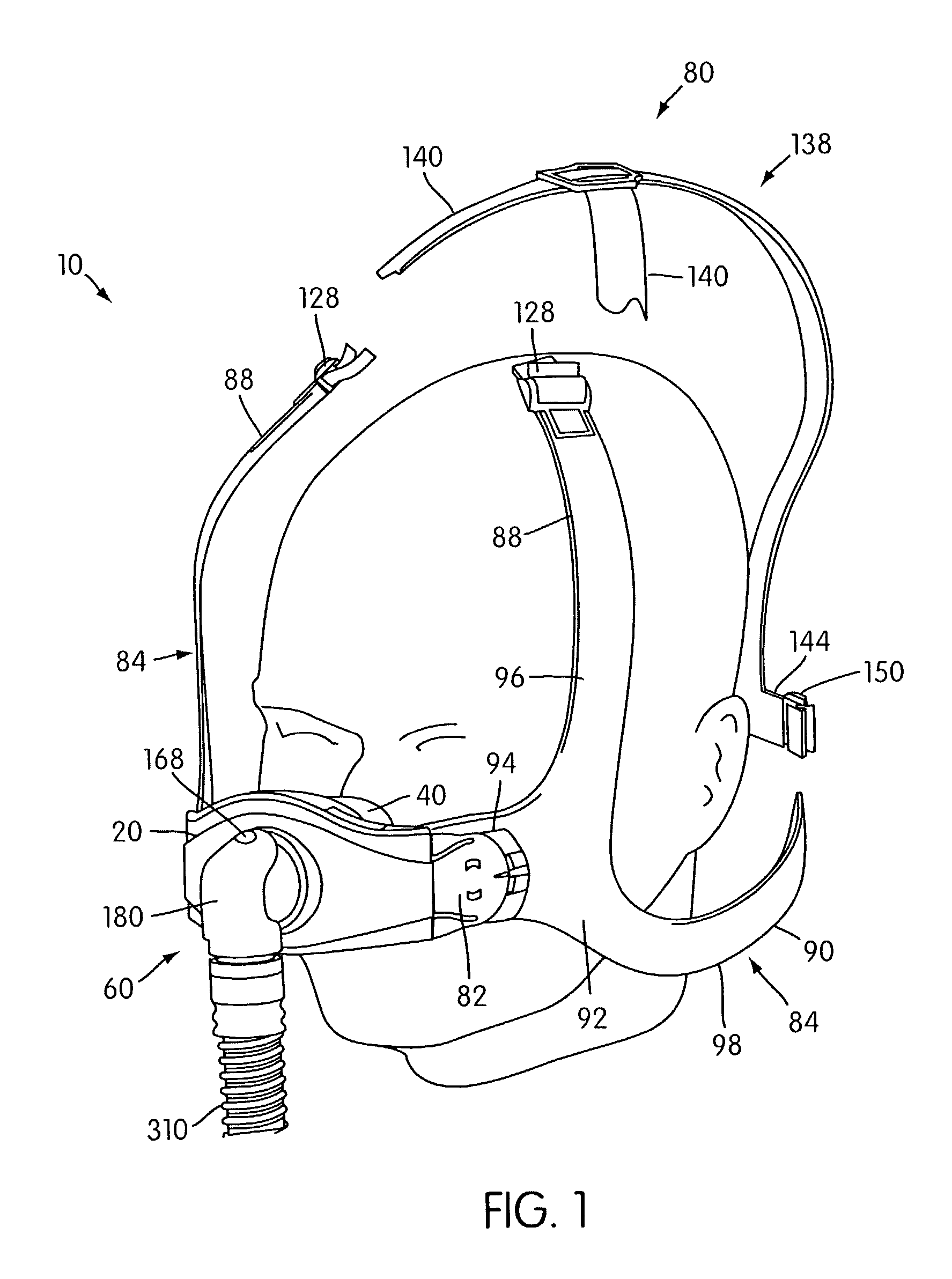
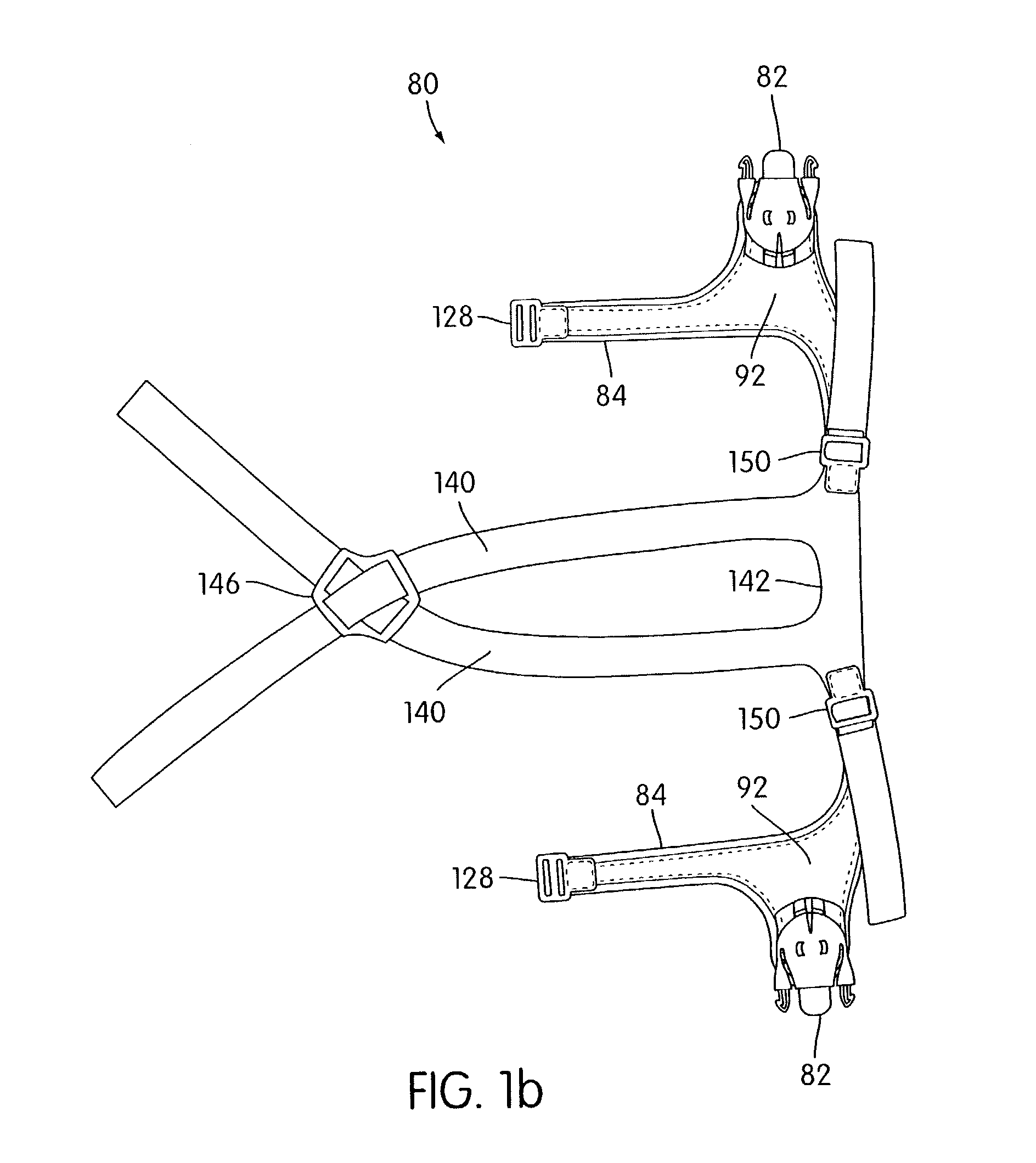
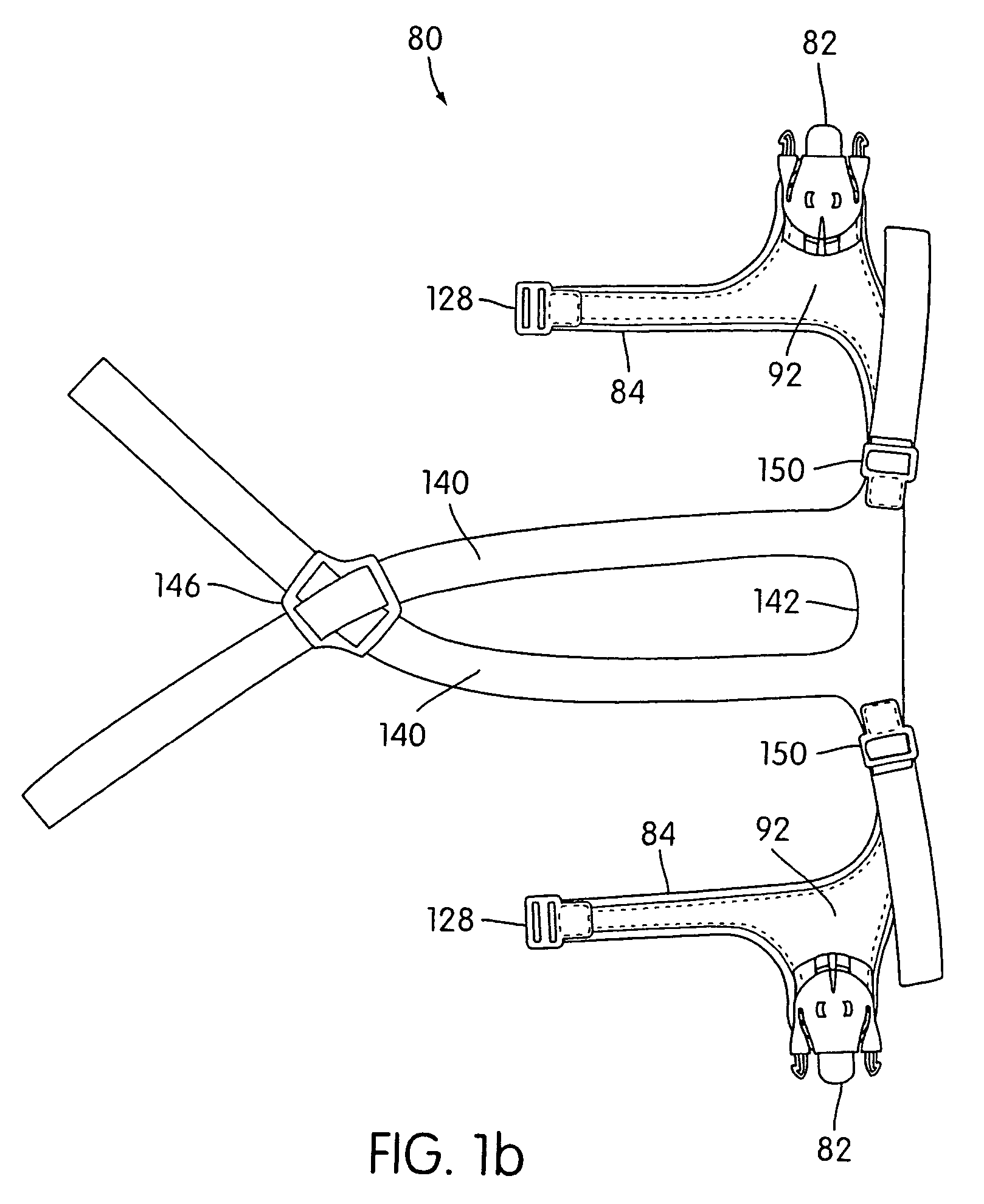
Ergonomic and adjustable respiratory mask assembly with headgear assembly
ActiveUS6907882B2Comfortable patient interfacePatient compliance is goodChemical protectionHeat protectionRespiratory maskEngineering
A respiratory mask assembly for delivering breathable gas to a patient includes a frame having a main body and a side frame member provided on each lateral side of the main body. Each side frame member includes an integrally formed first connector portion. A headgear assembly is removably attachable to the frame. The headgear assembly has a second connector portion adapted to be removably coupled with the first connector portion provided on the frame. The second connector portion is manually movable to a releasing position to detach the headgear assembly from the frame. The headgear assembly is rotationally adjustable with respect to the frame.
Owner:RESMED LTD
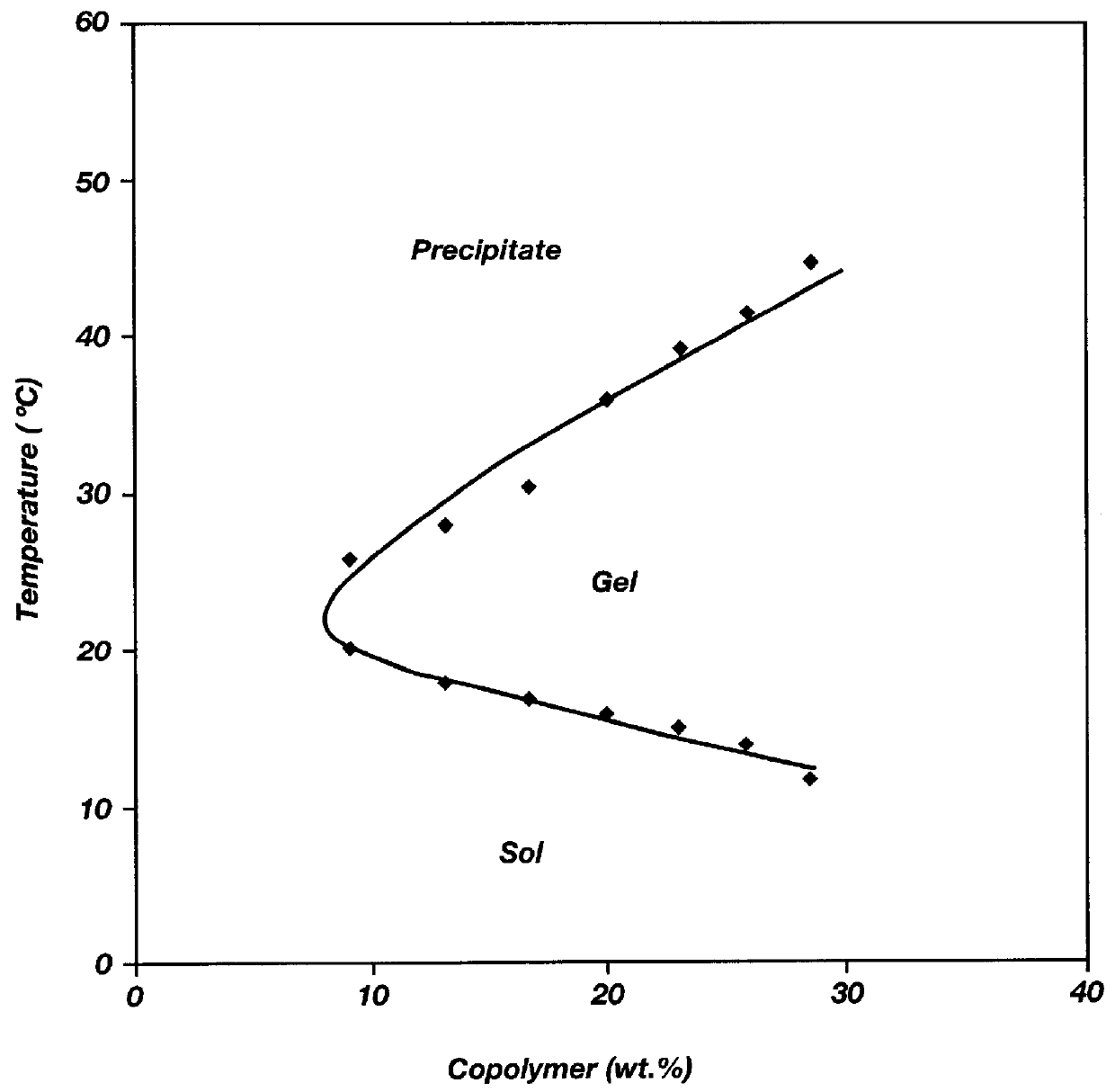
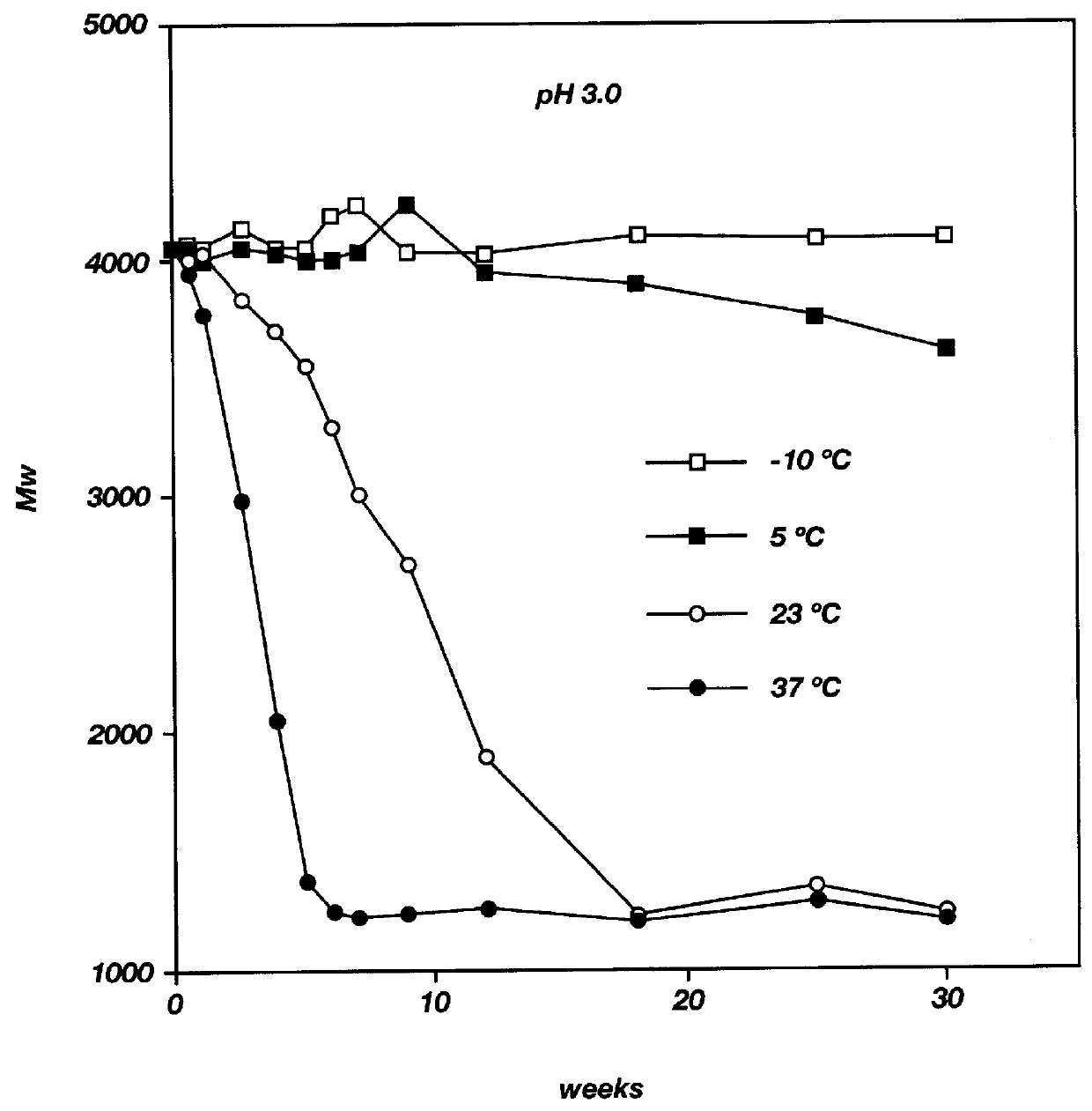
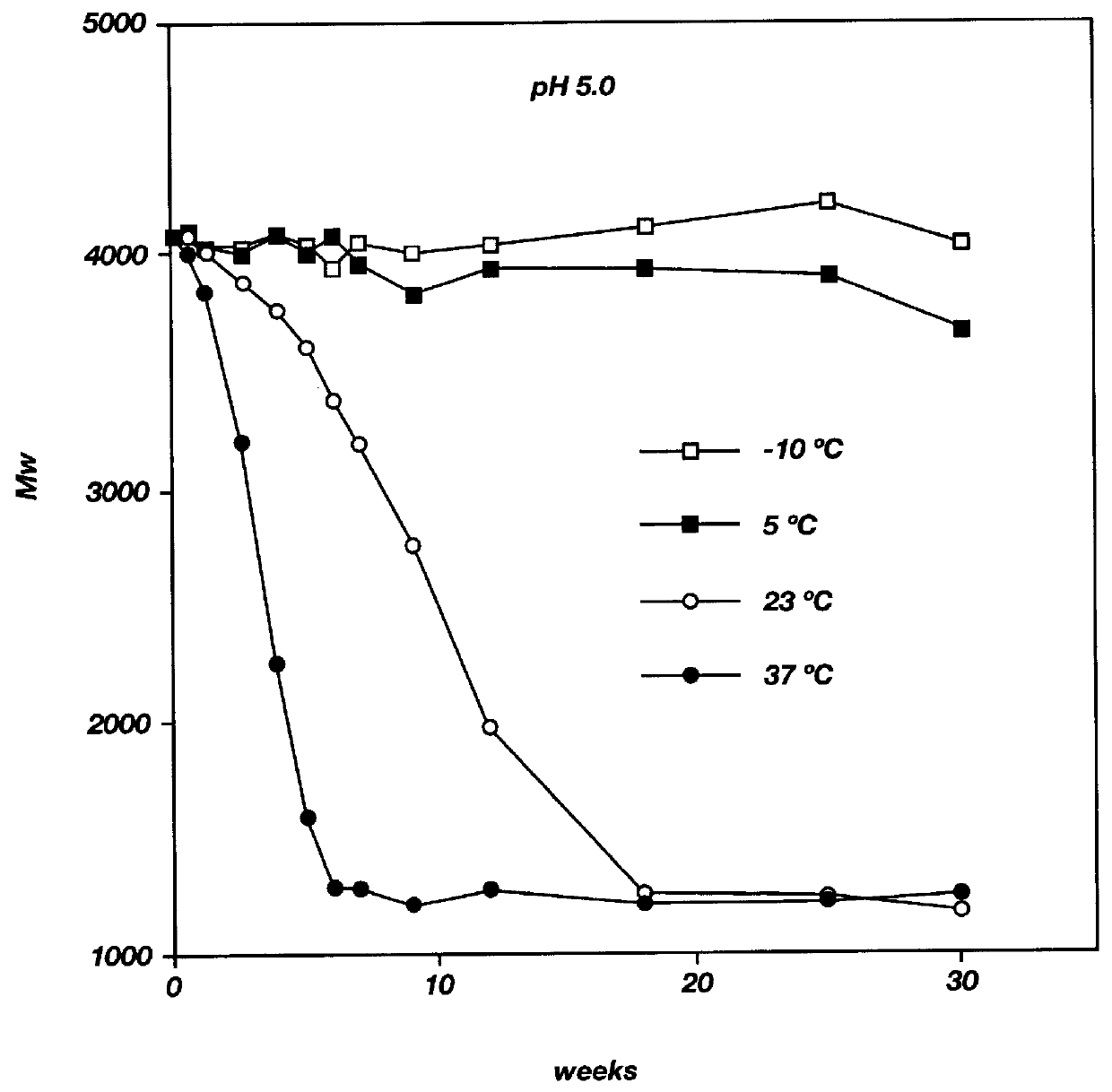
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Respiratory mask assembly with magnetic coupling to headgear assembly
ActiveUS20050155604A1Decrease and minimize and numberDecrease and minimize requirementRespiratory masksBreathing masksCouplingBreathing gas
A respiratory mask assembly for delivering breathable gas to a patient includes a frame and a headgear assembly which are adapted to being removeably magnetically coupled to one another at a desired angular orientation therebetween.
Owner:RESMED LTD
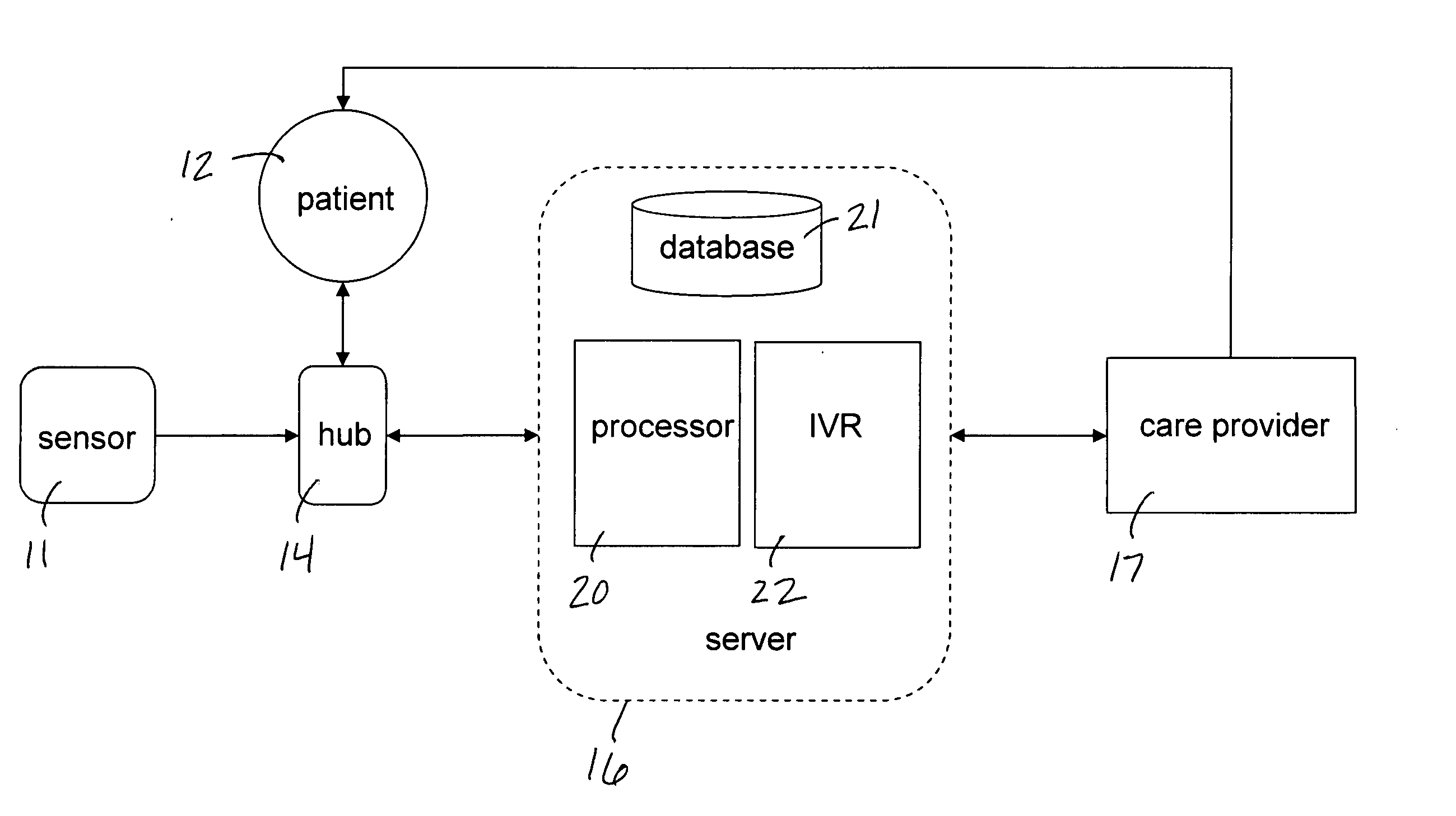
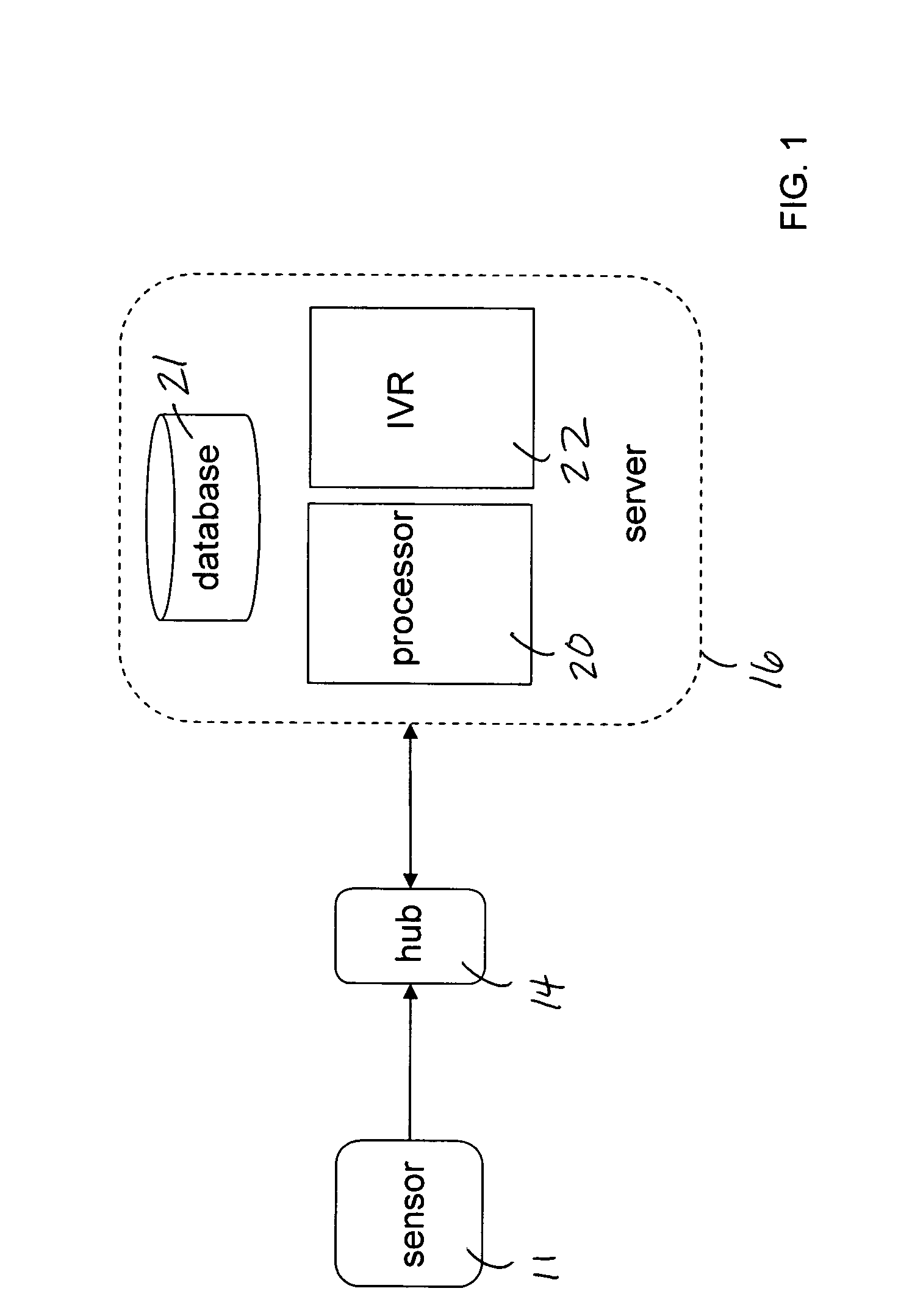
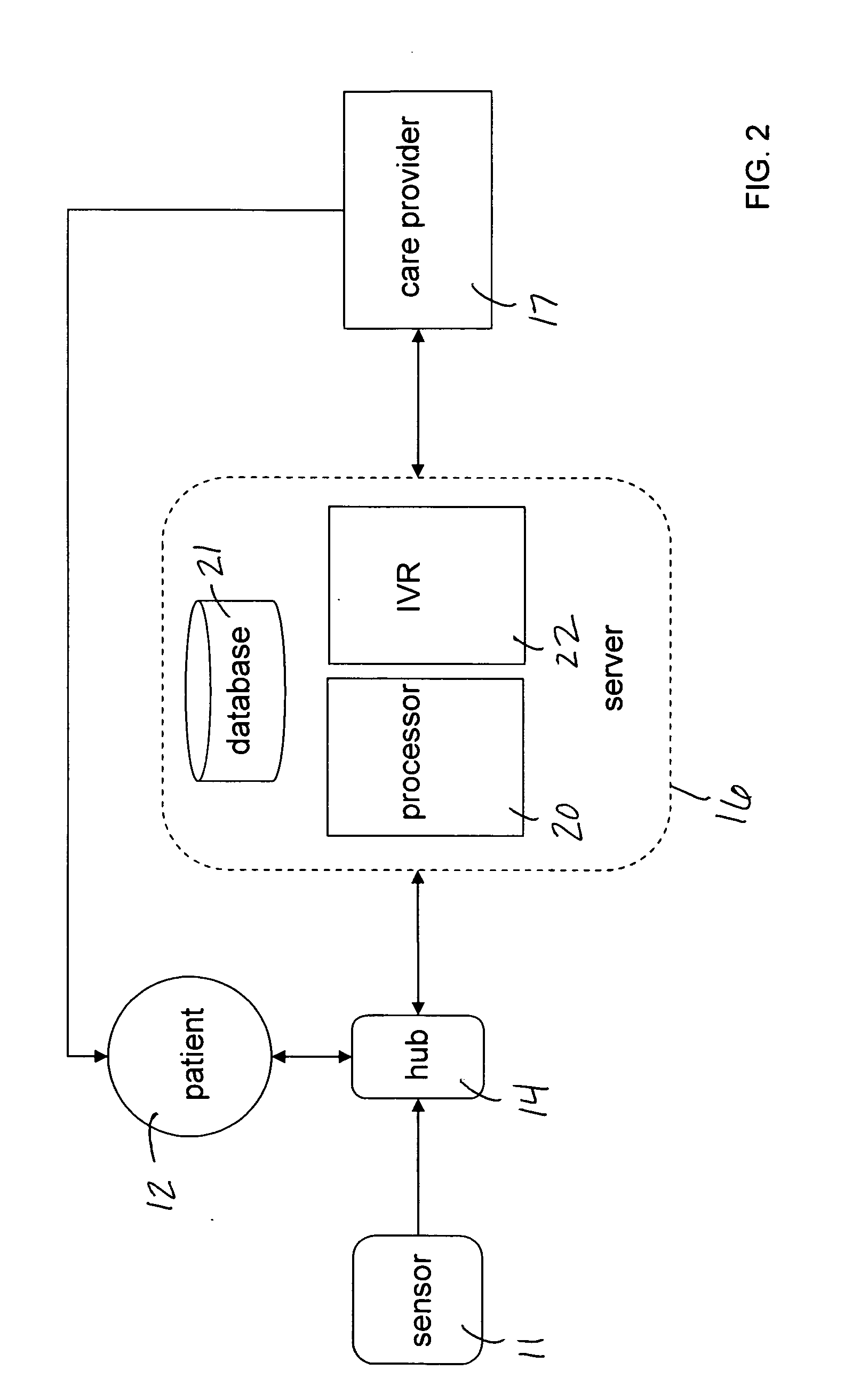
Mobile patient monitoring system with automatic data alerts
InactiveUS20060089542A1Improve complianceLower cost of careMedical communicationSurgeryPatient complianceEmergency medicine
A system to increase compliance with patient monitoring protocols for patients with chronic disease. The system uses a wireless telecommunication device as the hub of the system. The hub is configured to increase patient compliance with a monitoring protocol by being integrated with a mobile device, such as a cellular phone or PDA, that the patient normally carries or wears. The hub is further configured to increase compliance by displaying games that incorporate monitored conditions and providing rewards to the patient when he complies with the monitoring protocol. The hub receives physiological data about the patient from a medical sensor then collates the sensed data with certain data input by the patient. The reading is transmitted to a server that uses a software application to automatically examine and interpret the data. Alerts are sent to the health care provider only when the reading is outside specified parameters. The health care provider may contact the patient about the outlying event via the network.
Owner:SAFE N SOUND SOLUTIONS
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20070292461A1Pleasant and easy to spreadPatient compliance is goodCosmetic preparationsMetabolism disorderActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Ergonomic and adjustable respiratory mask assembly with frame
ActiveUS7743767B2Comfortable patient interfacePatient compliance is goodChemical protectionHeat protectionEngineeringRespiratory mask
A respiratory mask assembly for delivering breathable gas to a patient includes a frame having a front surface and a rear surface, opposite the front surface, and adapted in use to face the patient. The frame defines an inner wall and an outer wall extending from the rear surface, the inner and outer walls being spaced to define a channel therebetween. A cushion is removably attachable to the frame such that the cushion and frame are repeatably engagable with and disengagable from one another. The cushion includes a side wall to be inserted into the channel of the frame, the side wall having a first interlocking surface that engages a second interlocking surface provided in the channel when the cushion and frame are engaged with one another. The first and second interlocking surfaces interlock with one another to removably attach the cushion to the frame.
Owner:RESMED LTD
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS20020010127A1Increase efficacyPromote patient complianceBiocideNervous disorderOpioid antagonistSide effect
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of said opioid antagonist effective to attenuate a side effect of said opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
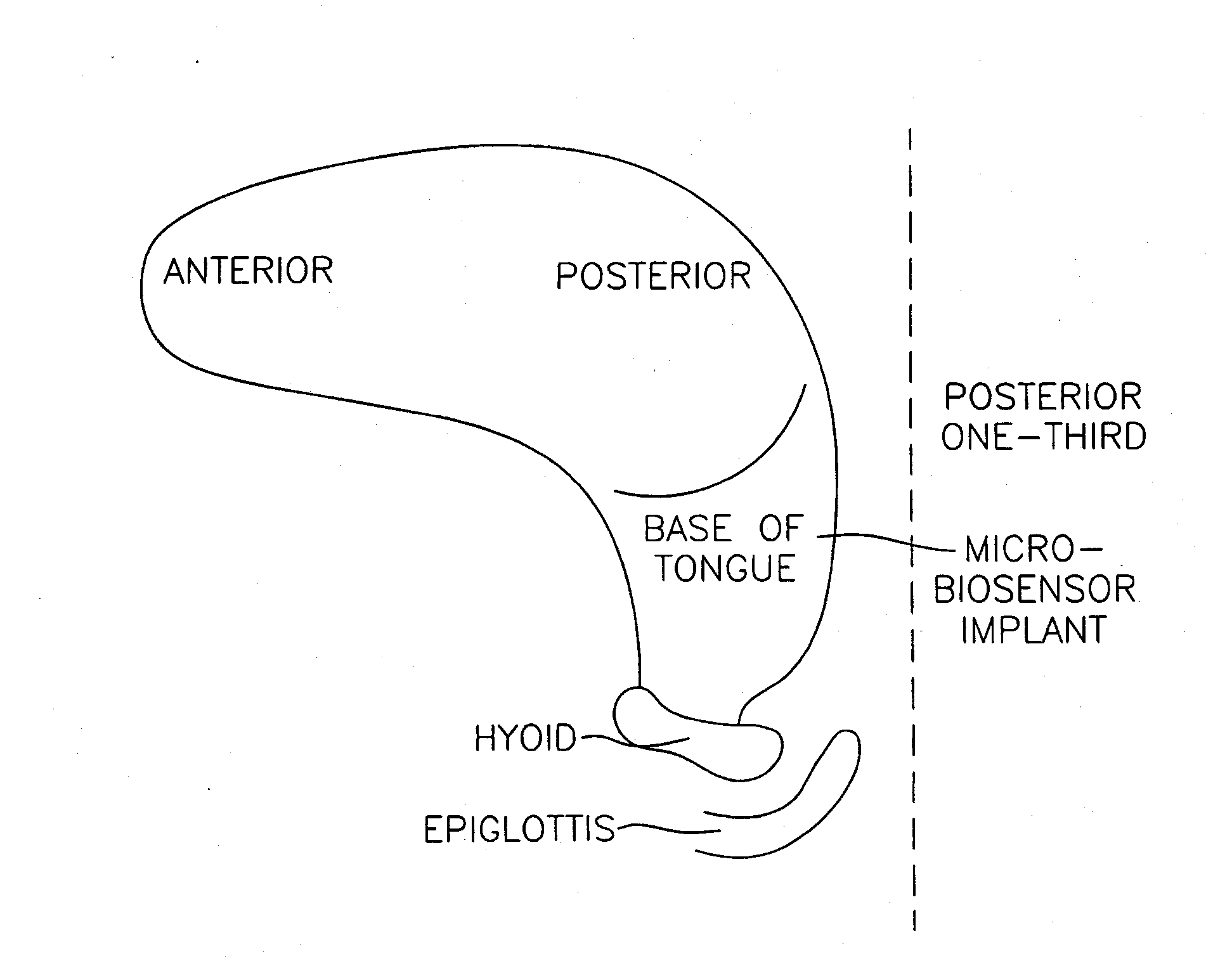
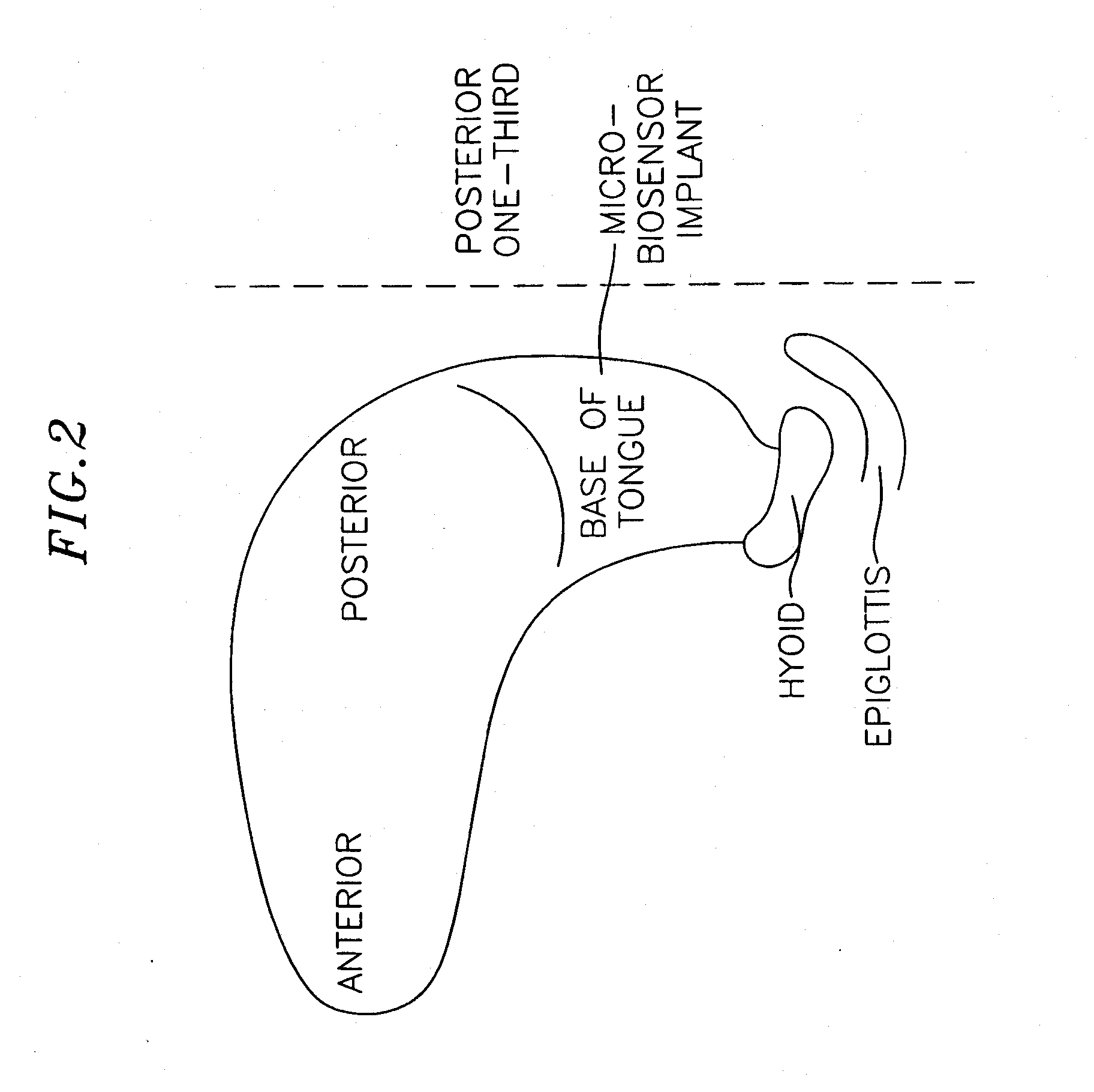
Method and apparatus for preventing obstructive sleep apnea
InactiveUS20070173893A1Patient compliance is goodRelieve symptomsElectrotherapyArtificial respirationMuscle toneGenioglossus muscle
A method and device for creating an afferent stimulus for preventing obstructive sleep apnea are disclosed. The device includes at least one electrode and a stimulator, of which at least one electrode stimulates the genioglossus muscle of a patient having obstructive sleep apnea. The electrode is capable of conducting selected electrical stimulation generated by the stimulator, and the system is capable of delivering the selected electrical stimulation during a selected time of day. The electrical stimulation is selected to maintain sufficient muscle tone of the genioglossus muscle to prevent it from obstructing the airway during sleep, preferably at a stimulus intensity low enough to avoid awakening the patient during sleep. A removable mouthpiece having a battery, at least one electrode, and a controller, and optionally a sensor, can be used for providing the stimulation.
Owner:PITTS WALTER C
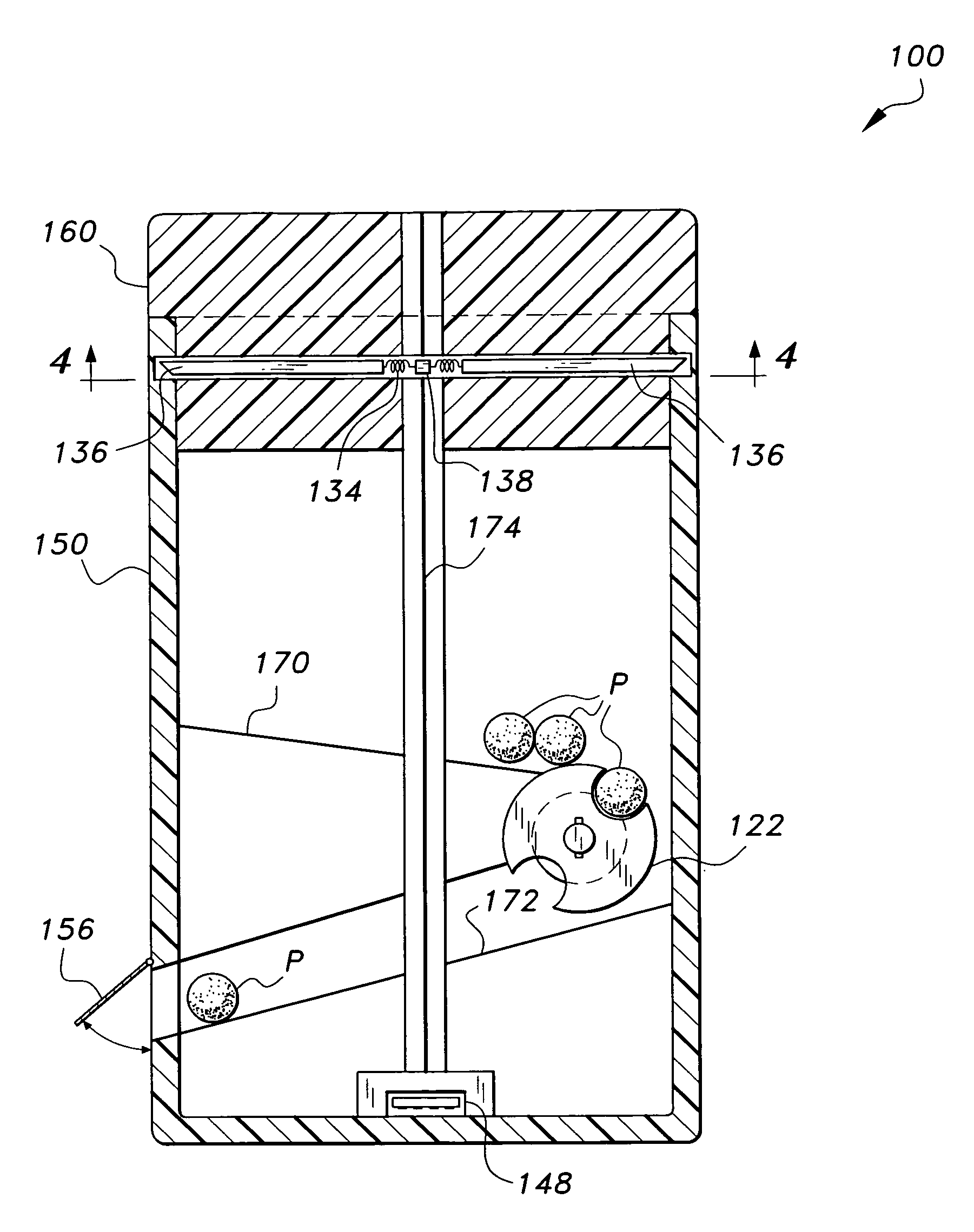
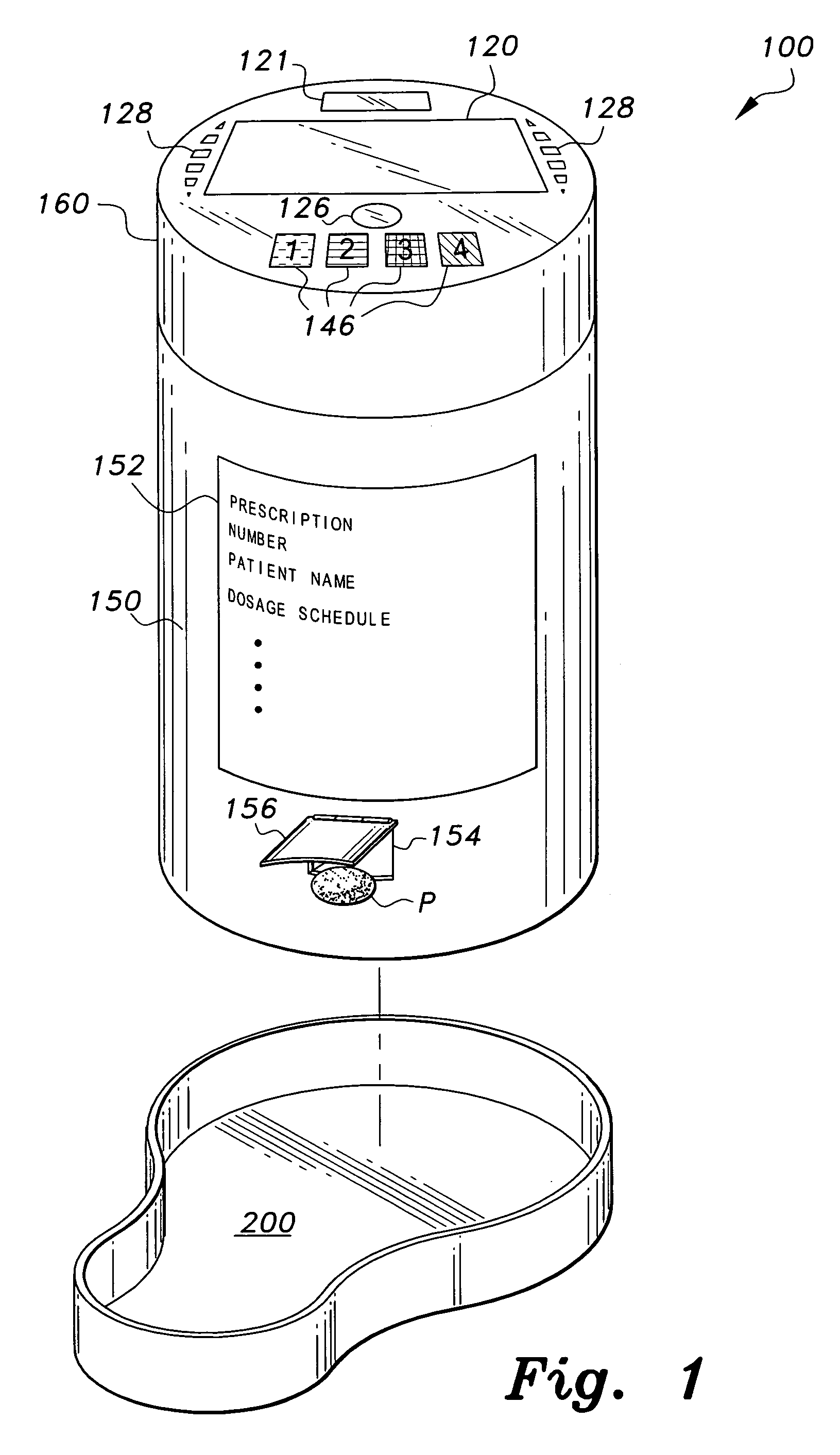
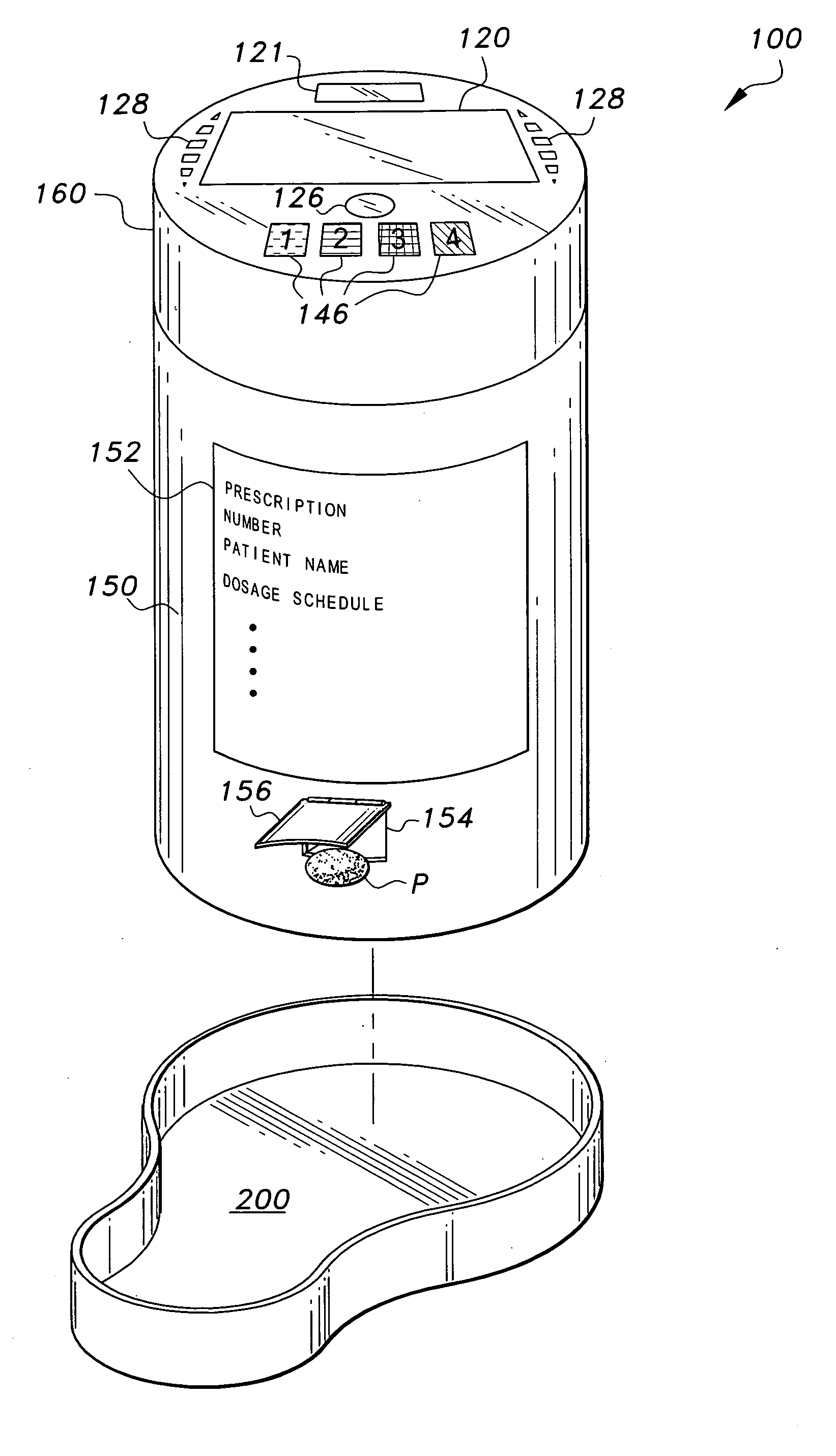
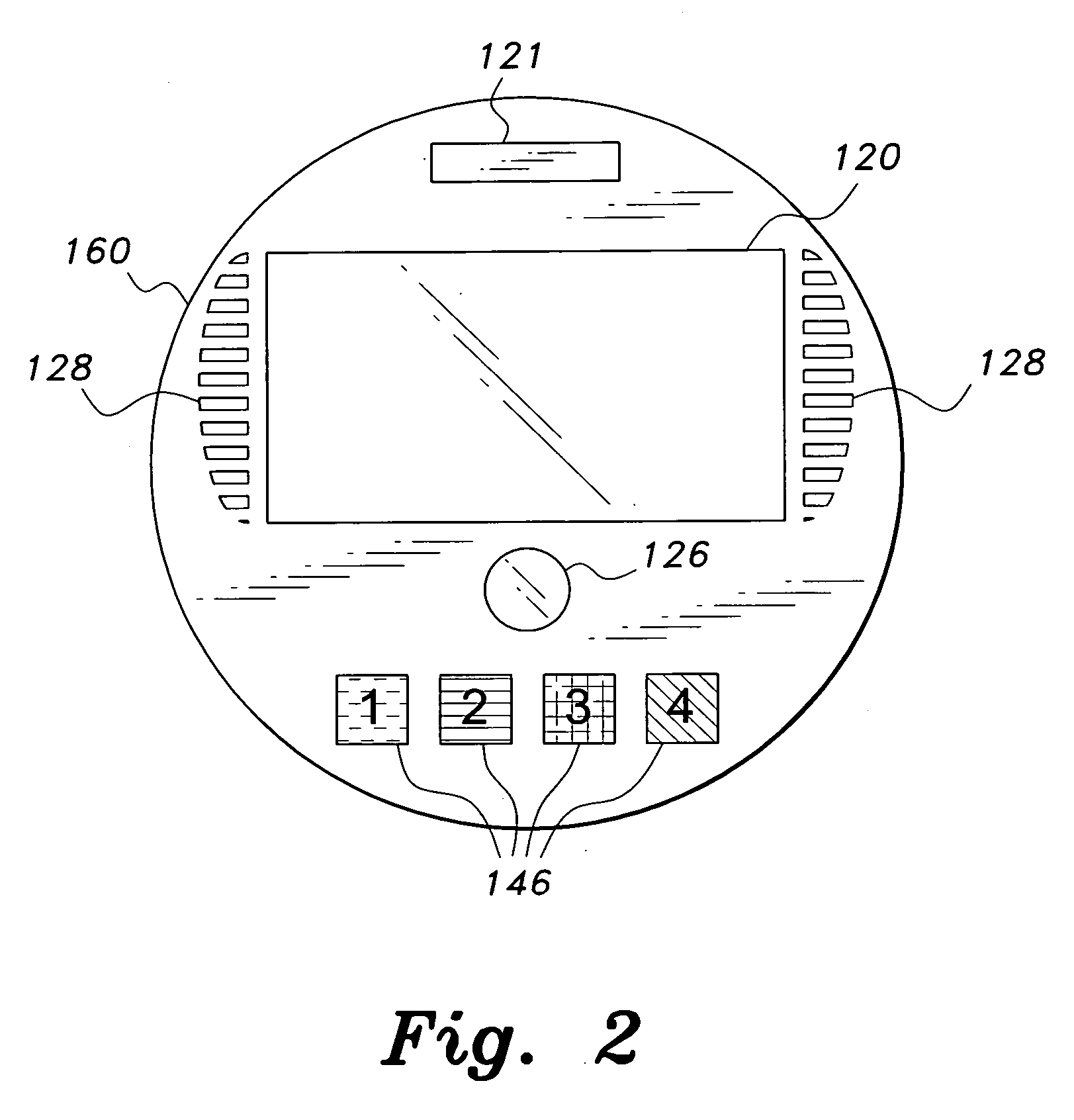
Interactive medication container
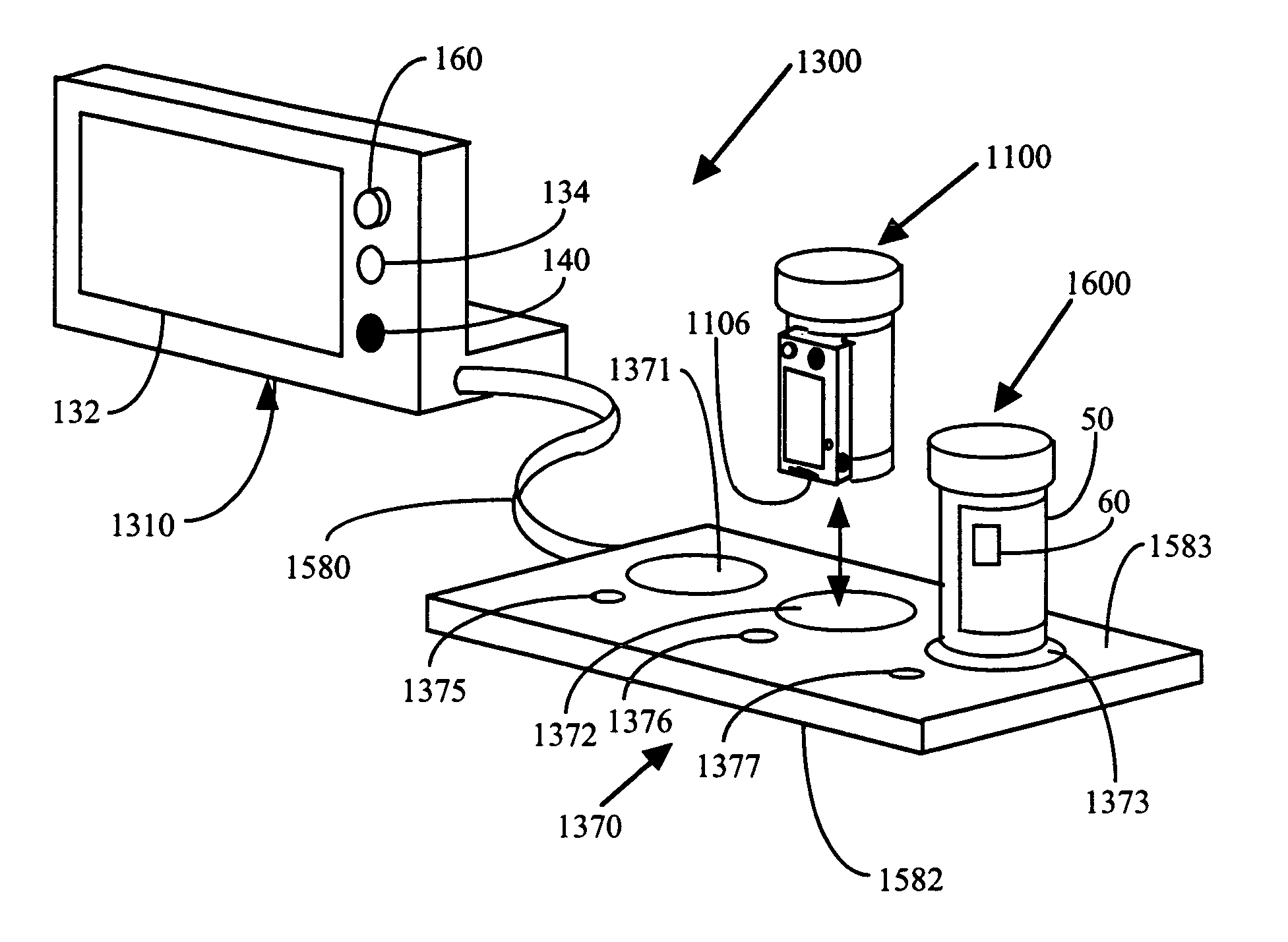
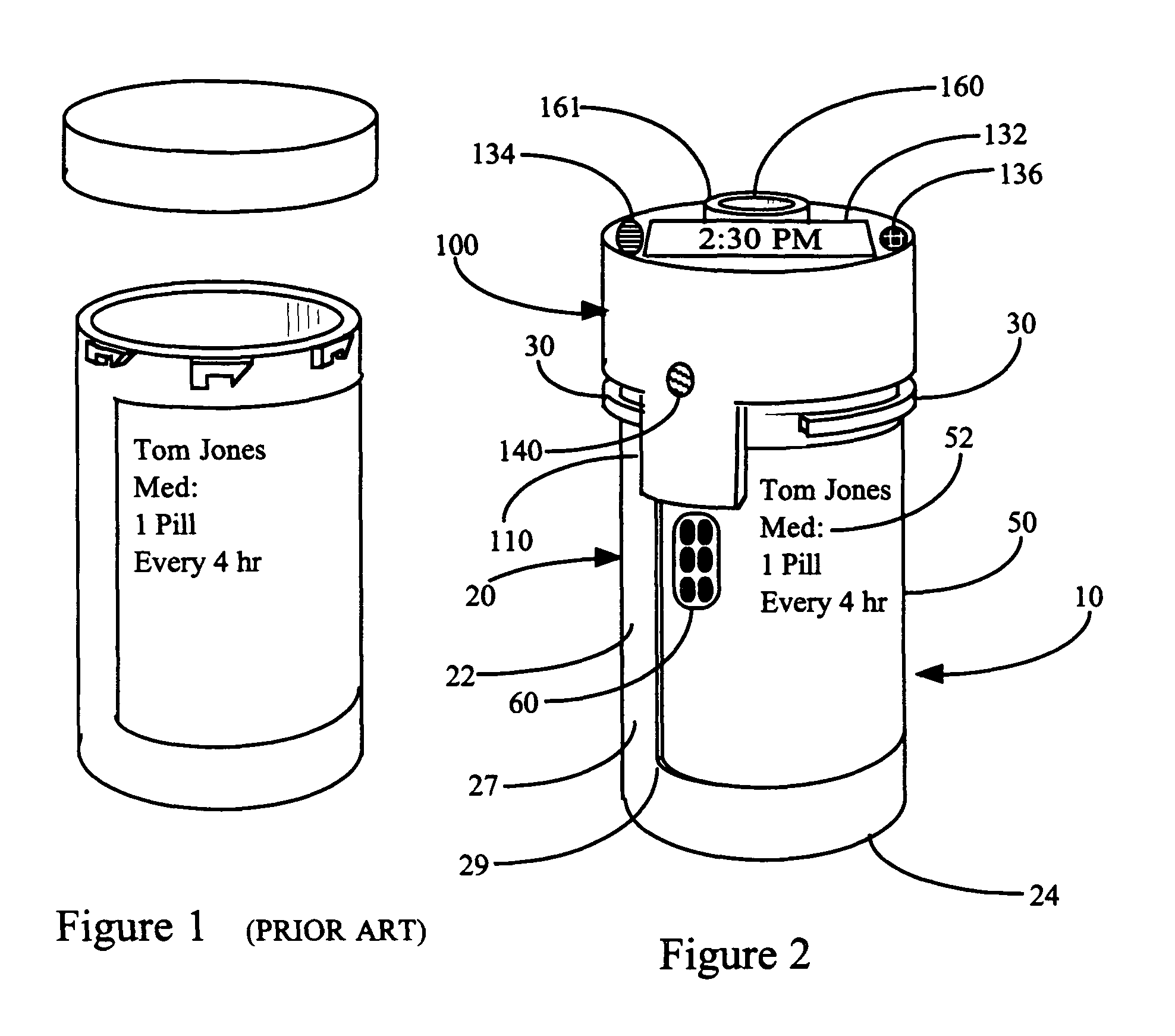
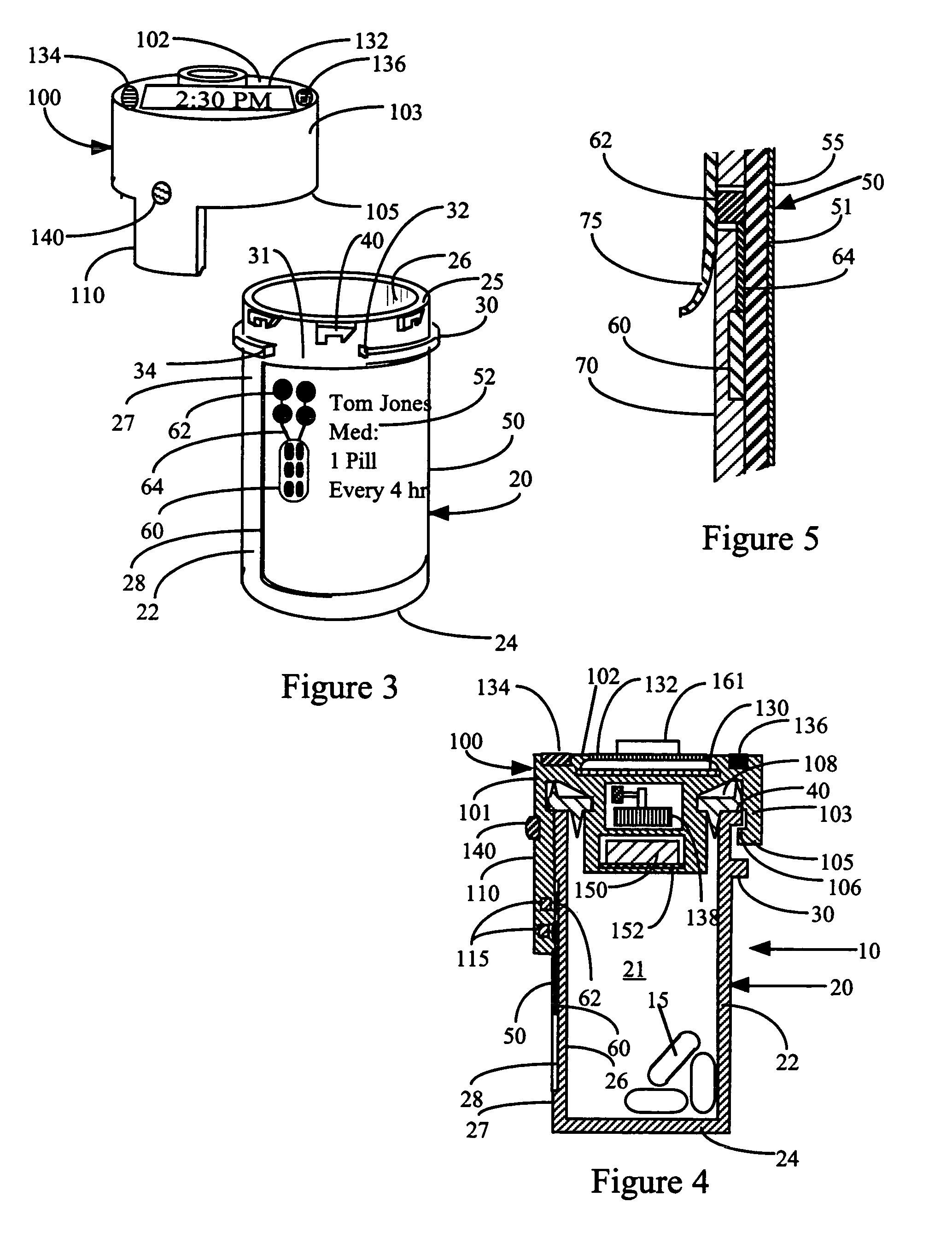
InactiveUS7978564B2Patient compliance is goodLow costMechanical clocksCoin-freed apparatus detailsPatient inputDosing regimen
This invention relates to an interactive medication container or console that hold or otherwise organizes one or more medication vials or containers. Each vial has a memory strip containing medication and prescription information. Each vial can also include a reminder unit that is attached to and portable with the individual vials. The console or reminder unit reads the information strip of the vial and communicates this information to or interacts with a patient to remind them to take the medication. The medication container or reminder unit also gathers or tracks information such as consumption time, quantity remaining, patient feedback, and contraindication information. The medication container or reminder unit interacts with the patient by displaying questions or receiving and recording input from the patient before, during or after a dose of medication is taken. The patient input can be used to modify the dosing regimen for future doses of medication. The medication container reorders medication when the quantity remaining reaches a threshold level. Contraindication information in the memory strip is downloaded to a personal home computer or a hospital or nursing home computer.
Owner:SOUTHWEST TECH INNOVATIONS +2
System and Methods for Improved Diabetes Data Management and Use Employing Wireless Connectivity Between Patients and Healthcare Providers and Repository of Diabetes Management Information
ActiveUS20100069730A1Shorten development timeExtended service lifePhysical therapies and activitiesDrug and medicationsDiseaseInformation repository
Methods, devices and a system for disease management are provided that employ diagnostic testing devices (e.g., blood glucose meters) and medication delivery devices (e.g., insulin delivery devices) for providing data to a repository in real-time and automatically. Repository data can be analyzed to determine such information as actual test strip use, patient health parameters to outside prescribed ranges, testing and medication delivery compliance, patient profiles or stakeholders to receive promotional items or incentives, and so on. Connected meters and medication delivery devices and repository data analysis are also employed to associate a diagnostic test to a mealtime based on timing of a therapeutic intervention performed by an individual.
Owner:EMBECTA CORP
Ergonomic and adjustable respiratory mask assembly with headgear assembly
InactiveUS7047972B2Patient compliance is goodEasy to separateRespiratory masksBreathing masksEngineeringRespiratory mask
Owner:RESMED LTD
Solid form
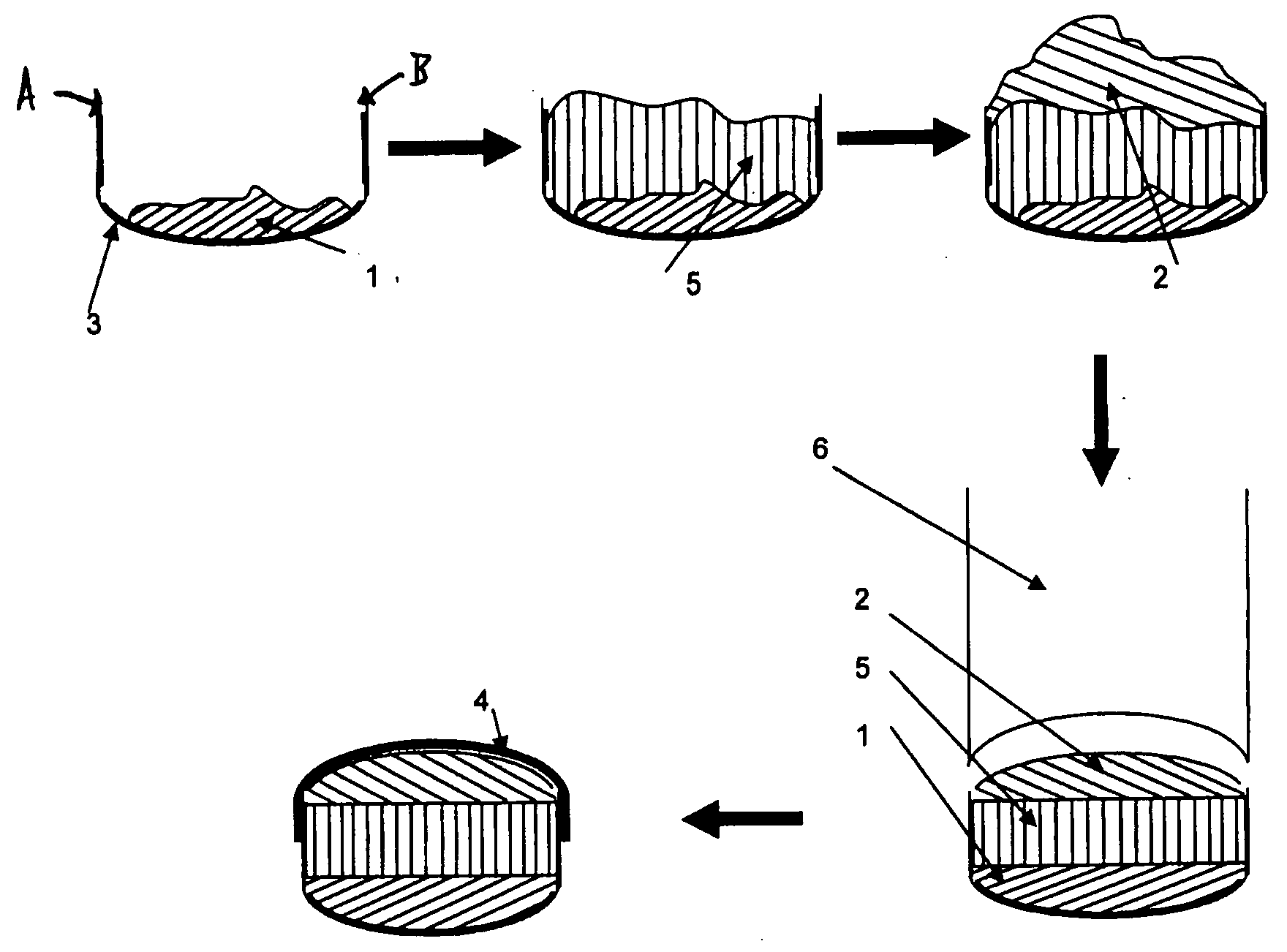
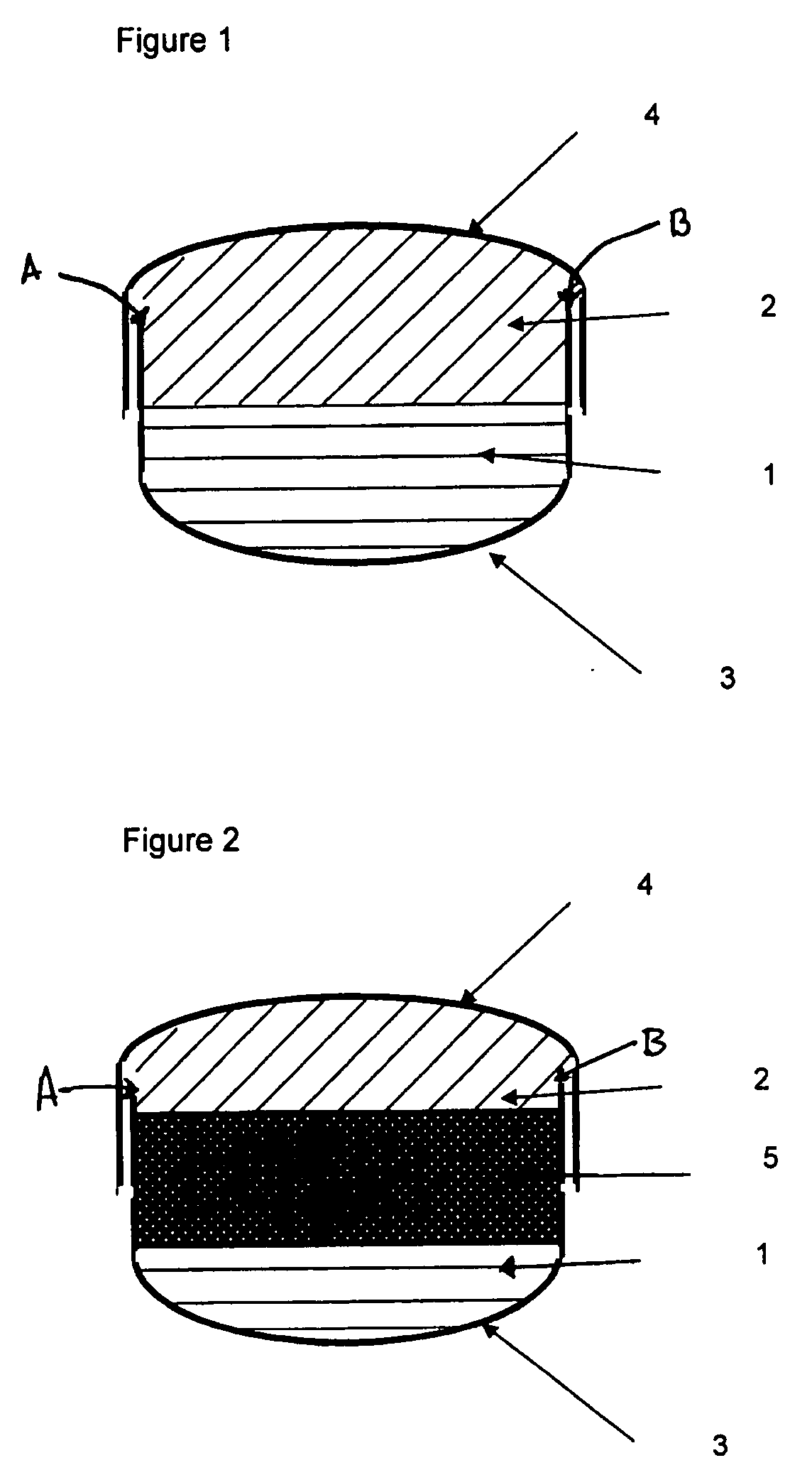
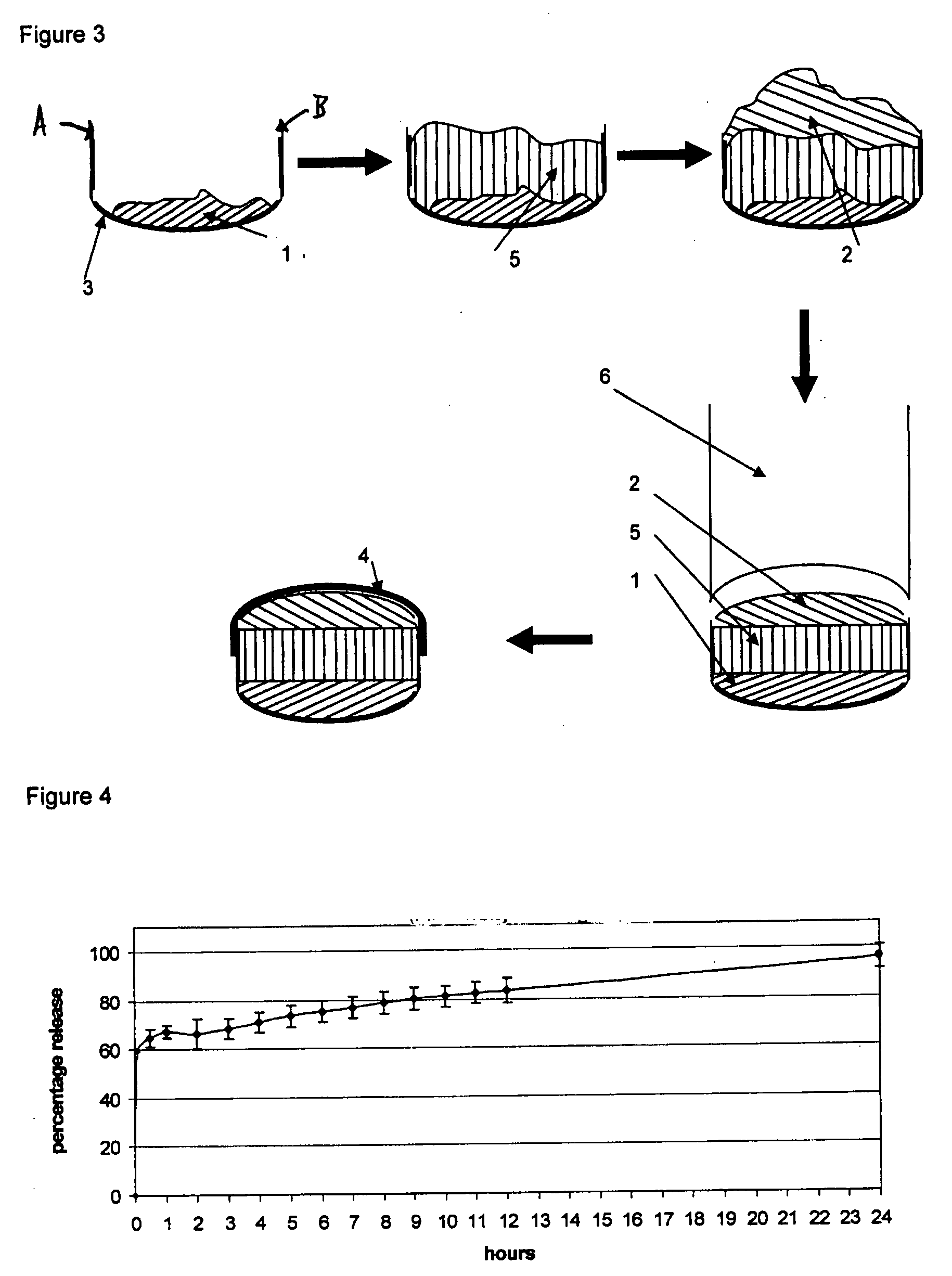
InactiveUS20080286344A1Strong enoughAvoids and reduces processing and product drawbackPowder deliveryNervous disorderFilling materialsVolumetric Mass Density
A solid form comprising at least one film enrobing a compacted fill material wherein:i) the compacted fill material comprises at least one active material;ii) the solid form shows a weight loss that is less than 1% during a 30 minutes USP friability test United States Pharmacopeia (USP) 29 Test Number 1216 (page 3046);iii) the compacted fill material has a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength of less than 0.9 MPa; andiv) the compacted fill material is present in the solid form in at least a first zone and a second zone and the active material is present in at least one of the zones.
Owner:FMC CORP
Topical foam/mousse compositions for treating psoriasis
InactiveUS20050281755A1Stable compositionPatient compliance is goodOrganic active ingredientsCosmetic preparationsPsoriasisVitamin D Analogue
Topically applicable, pharmaceutical foam / mousse compositions, well suited for the treatment of psoriasis, include a hydrophobic phase, at least one surfactant, a therapeutically effective amount of a vitamin D analogue and a therapeutically effective amount of a corticosteroid.
Owner:GALDERMA SA
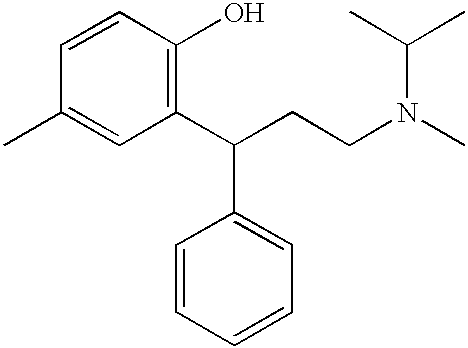
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS6517864B1Achieve effectClinical efficacyOrganic active ingredientsAerosol deliveryMuscarinic antagonistMetabolite
Device for transdermal administration of tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, optionally together with pharmaceutically acceptable carrier(s) to a human being or an animal in order to achieve an effect against overactive bladder. Use of a compound having an effect against overactive bladder comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s), for the manufacture of a composition to be administered transdermally for achieving an effect against overactive bladder. Method for achieving an effect against overactive bladder in a living body by transdermal administration of a compound comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s).
Owner:MCNEIL AB +1
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20080063607A1Pleasant and easy to spreadPatient compliance is goodBiocideCosmetic preparationsActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Electronic pill dispenser
InactiveUS7359765B2Patient compliance is goodEffects of lossCoin-freed apparatus detailsOral administration deviceUltrasound attenuationModem device
Owner:VARVARELIS NICHOLAS M +1
Controlled release pharmaceutical compositions with improved bioavailability
PendingUS20070196396A1Efficient retentionImprove bioavailabilityHeavy metal active ingredientsBiocideControlled releaseActive agent
The present invention provides a controlled release oral pharmaceutical composition having a therapeutically effective amount of one or more pharmacologically active agent having low bioavailability; one or more solubilizers; one or more biocompatible swelling agents; and a swelling enhancer. The swelling agent, in combination with swelling enhancer, swells in the presence of water in gastric fluid such that the size of the dosage form is sufficiently increased to provide retention of the dosage form in the stomach of a patient, which gradually erodes within the gastrointestinal tract over a prolonged time period.
Owner:RUBICON RES PTY LTD
Electronic pill dispenser
InactiveUS20060071011A1Patient compliance is goodEffects of lossCoin-freed apparatus detailsOral administration devicePatient complianceTransceiver
An electronic pill dispenser includes a container and a cap removably attached to the container. Components of the pill dispenser include a power source, pill dispenser circuitry, a real time clock, a counter, a display, a dispensing mechanism, a sensor, a visual indicator, an audible indicator, an input / output interface, an input output port, and a communication bus electrically interconnecting the components. The pill dispenser may also include a physical indicator, a locking mechanism, a transceiver, an antenna, and a modem. The pill dispenser enhances patient compliance for following through a particular drug regimen by offsetting negative effects of memory loss and other cognitive dysfunctions, attenuation of special senses, poor eyesight, lack of patient education, etc. The pill dispenser also helps the mentally unstable. The pill dispenser reminds users and dispenses pills to authorized individuals at appropriate times, and is economical and convenient.
Owner:VARVARELIS NICHOLAS M +1
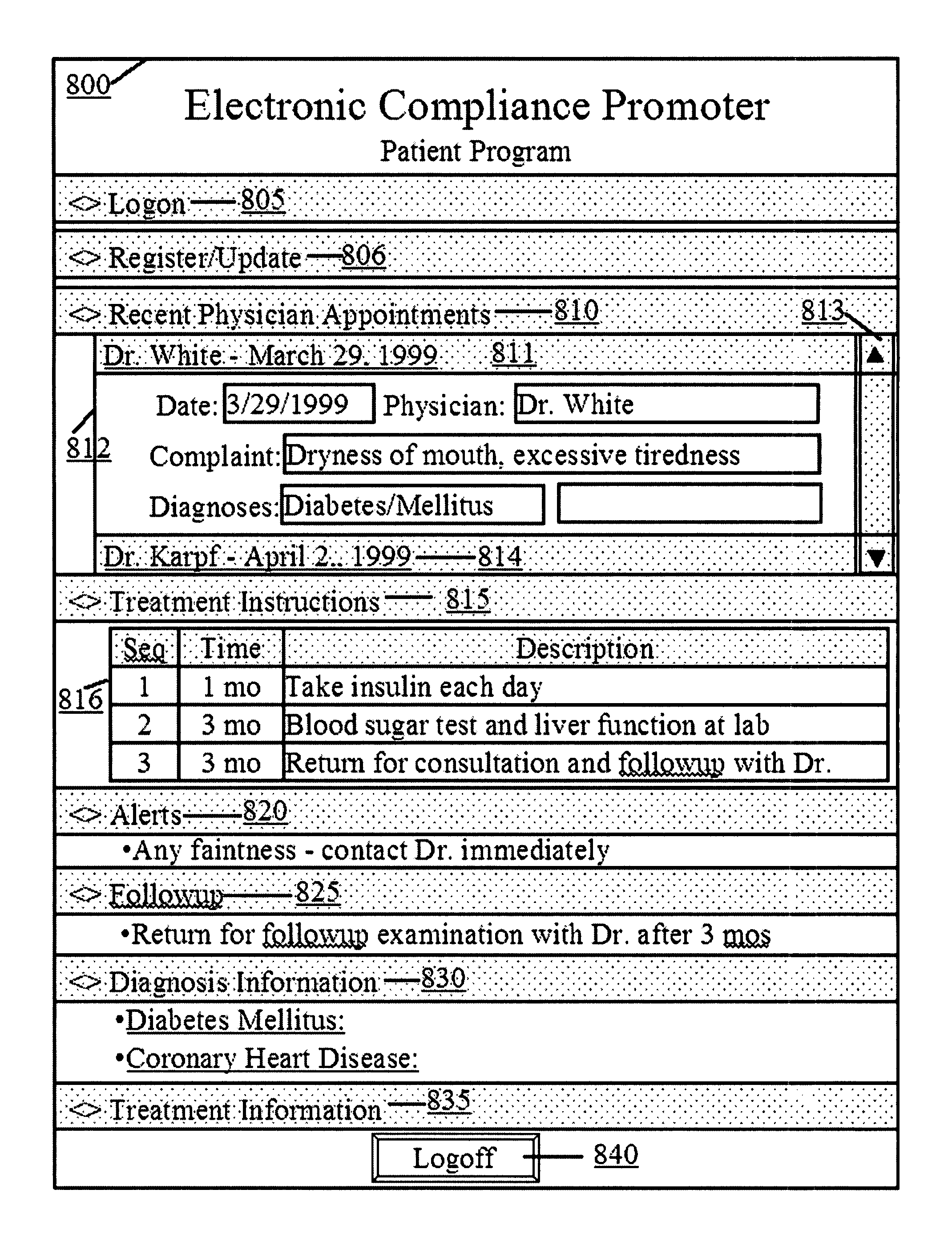
Computer system and method for increasing patients compliance to medical care instructions
InactiveUS7287031B1Maximize availabilityEasy accessLocal control/monitoringDiagnostic recording/measuringGuidelinePatient compliance
Owner:KARPF RONALD STEVEN +1
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
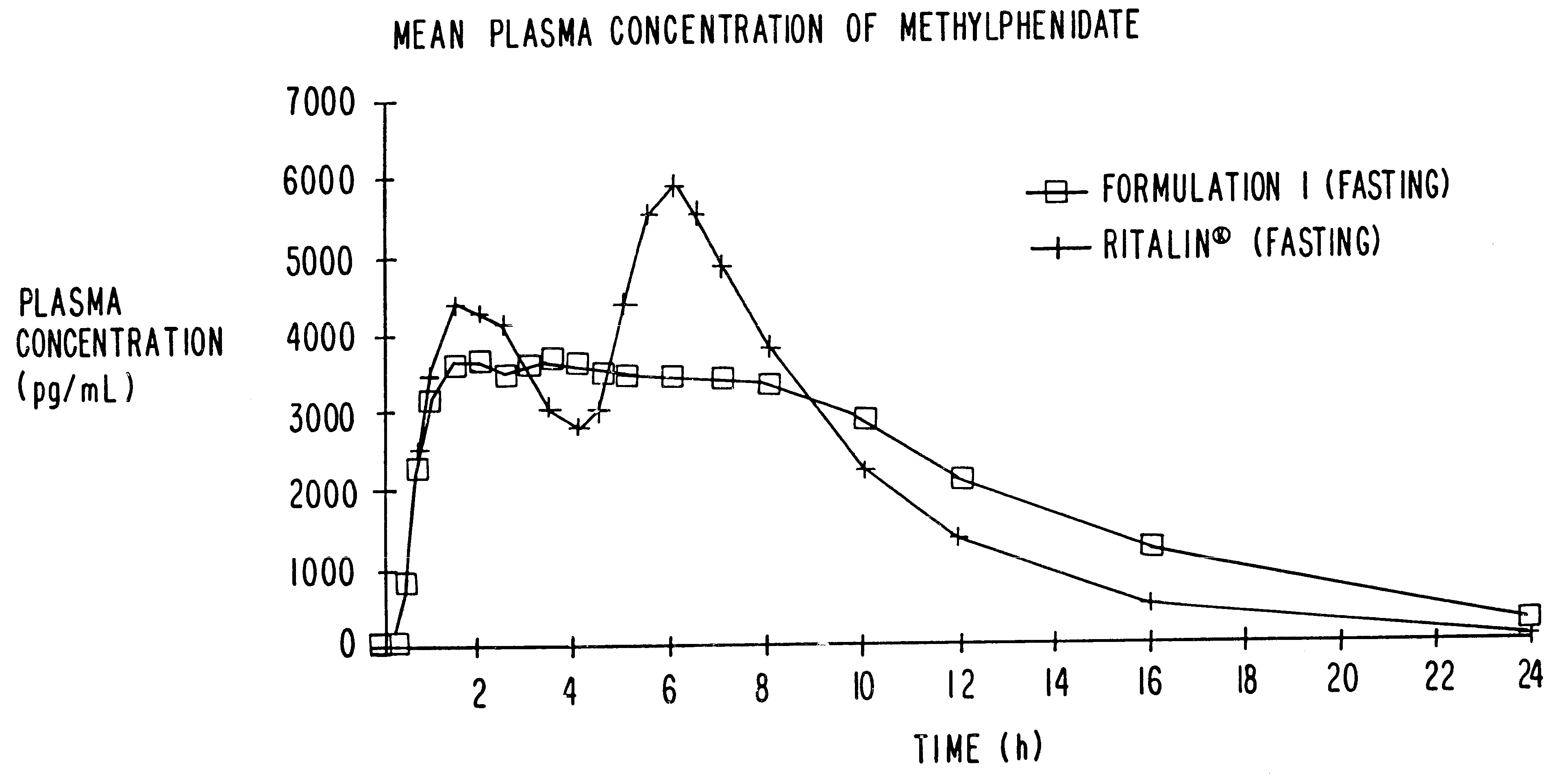
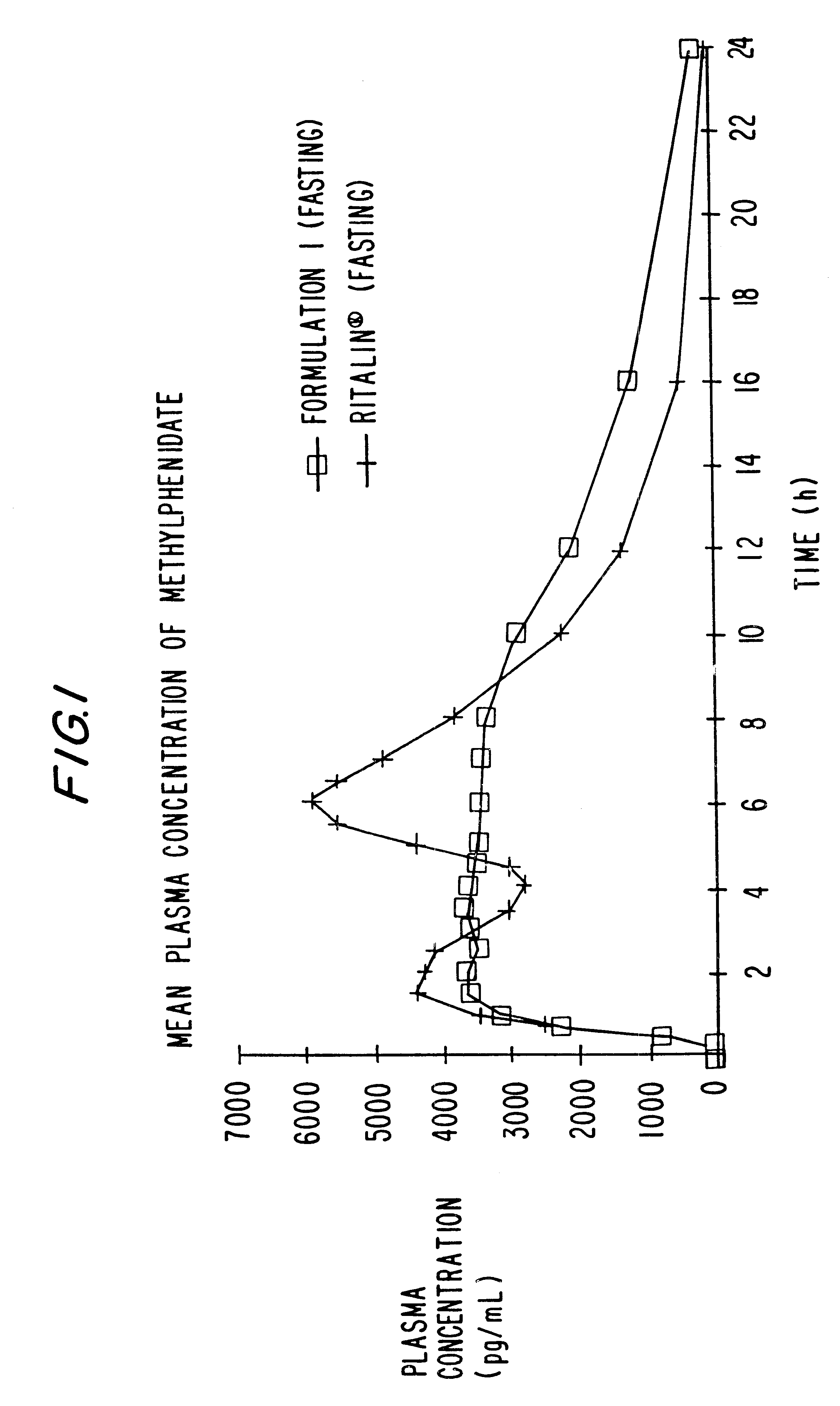
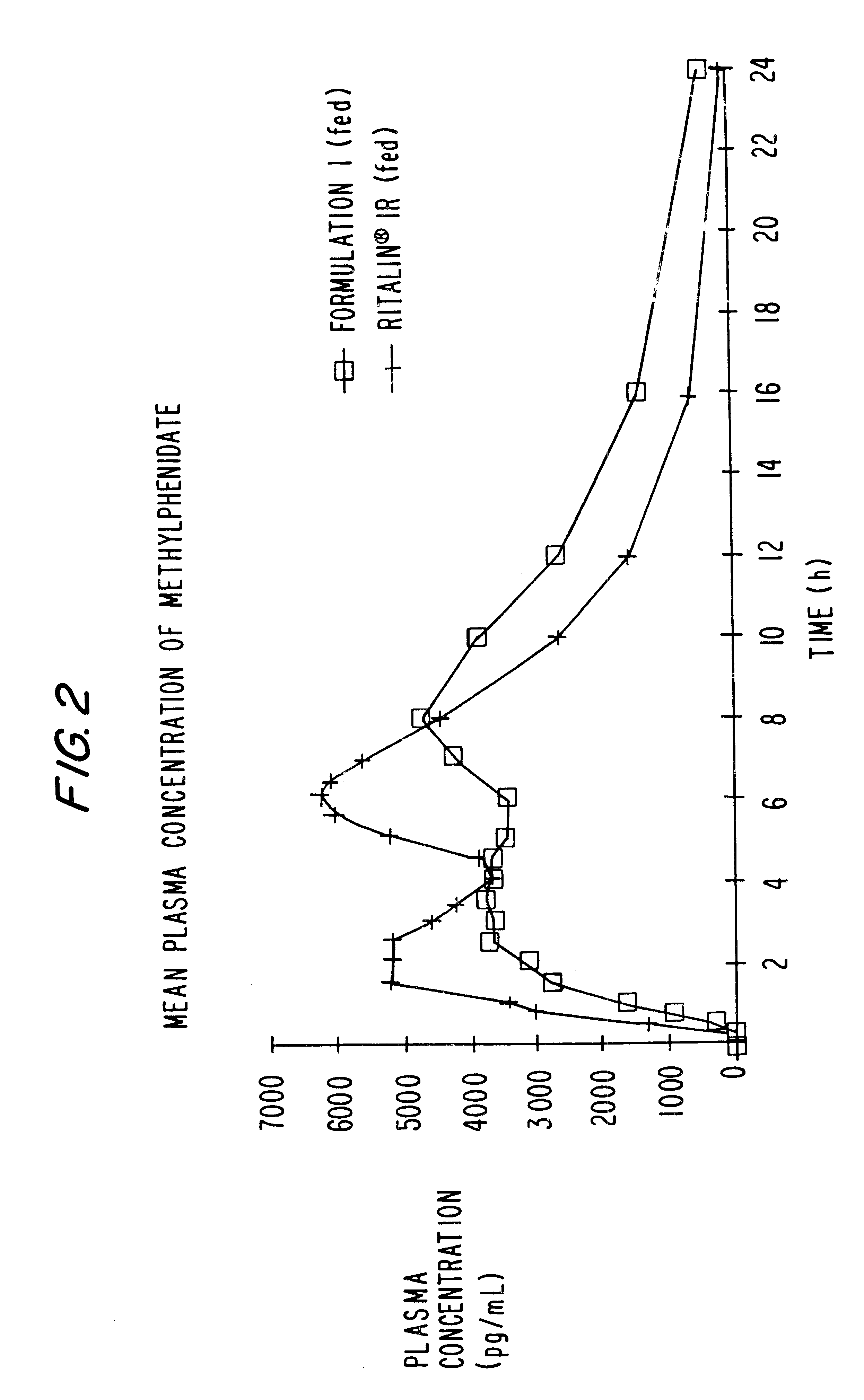
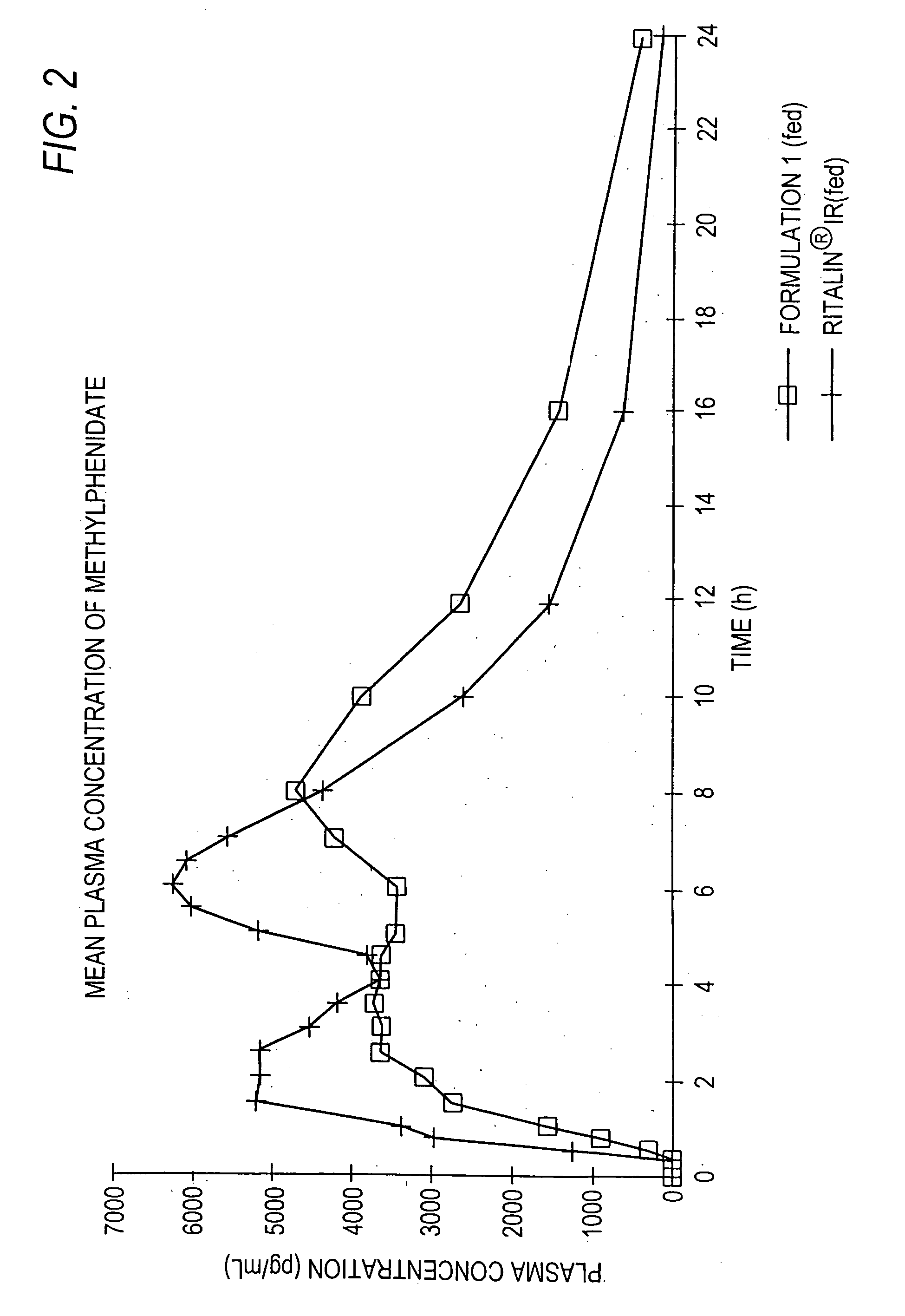
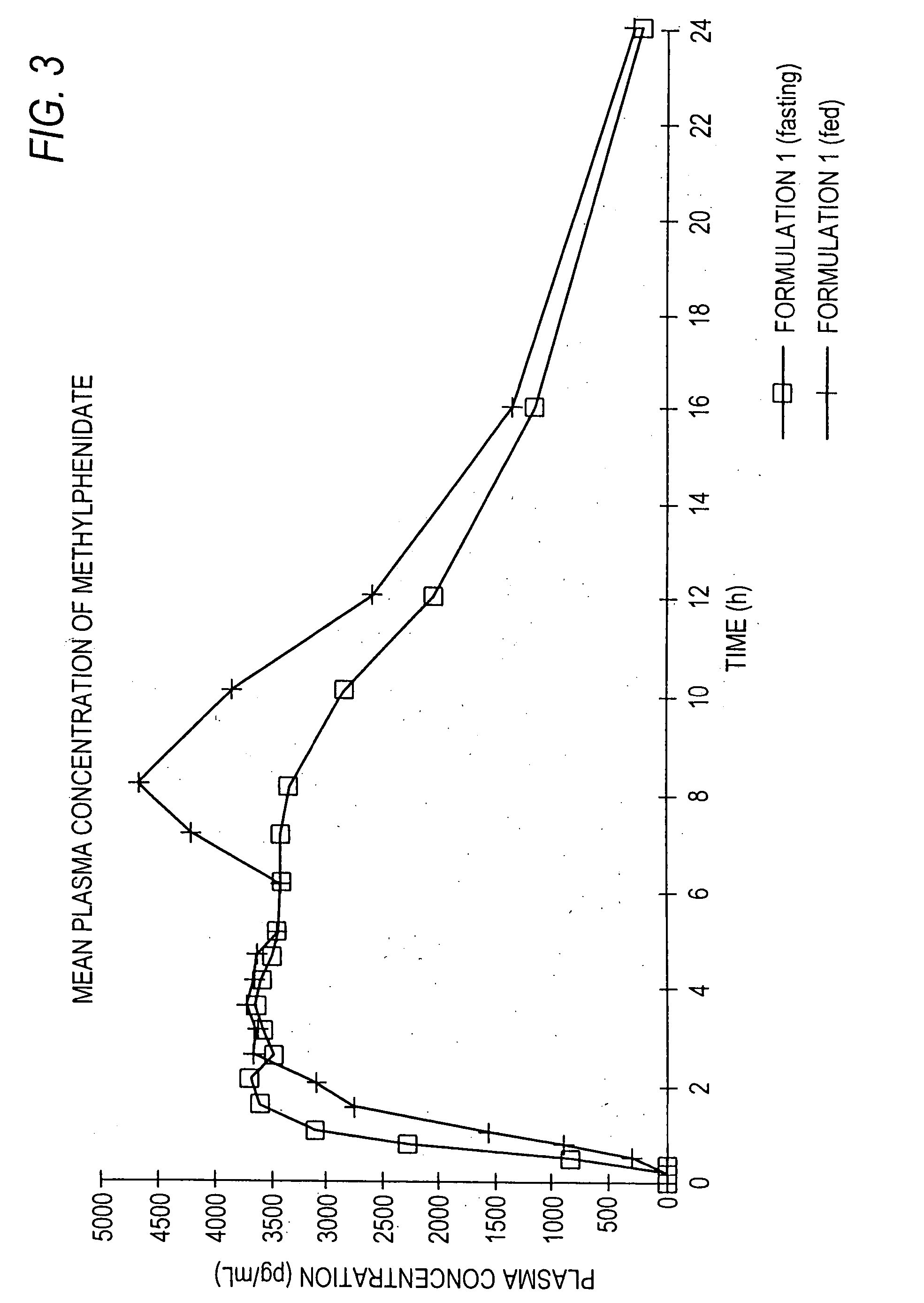
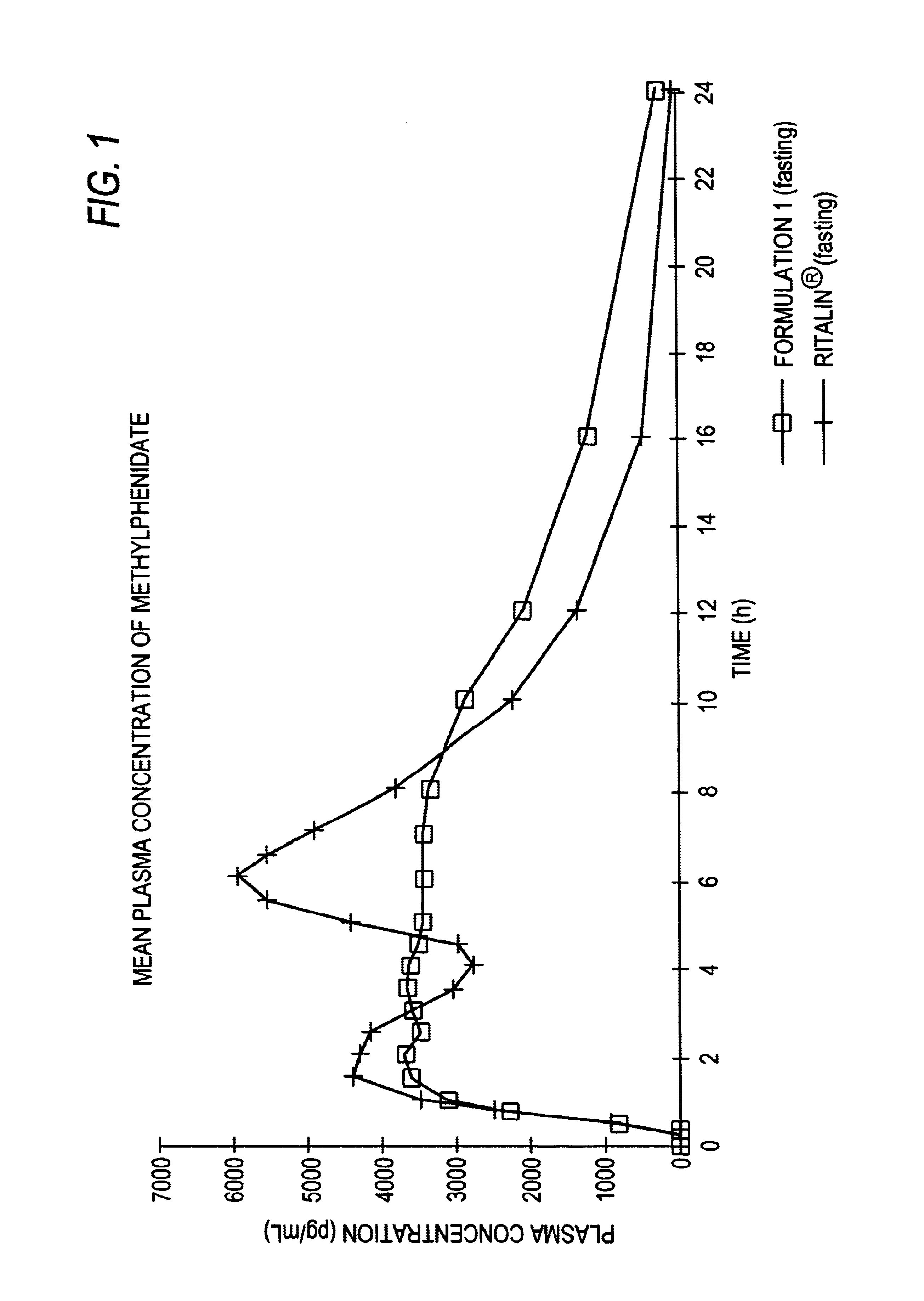
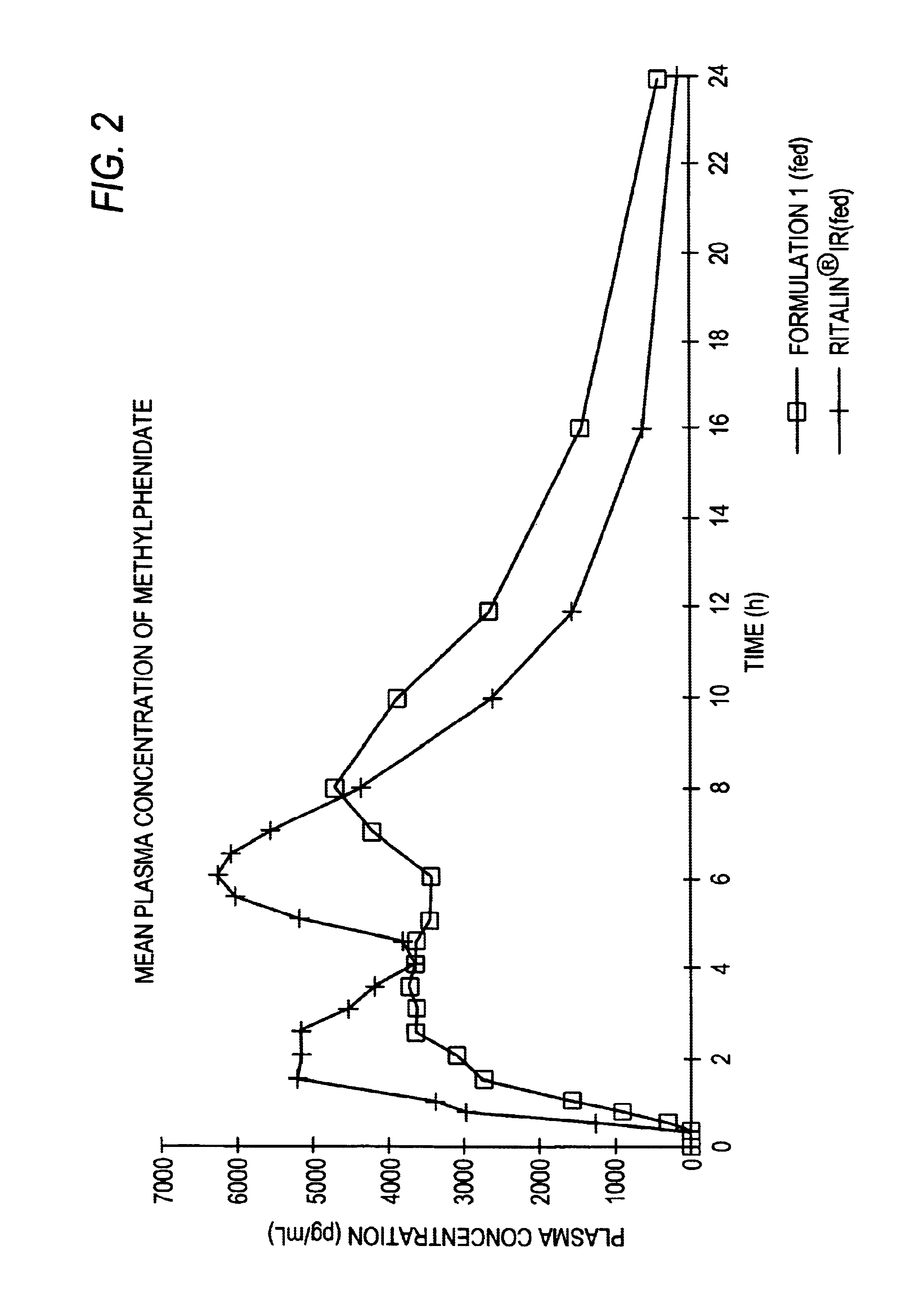
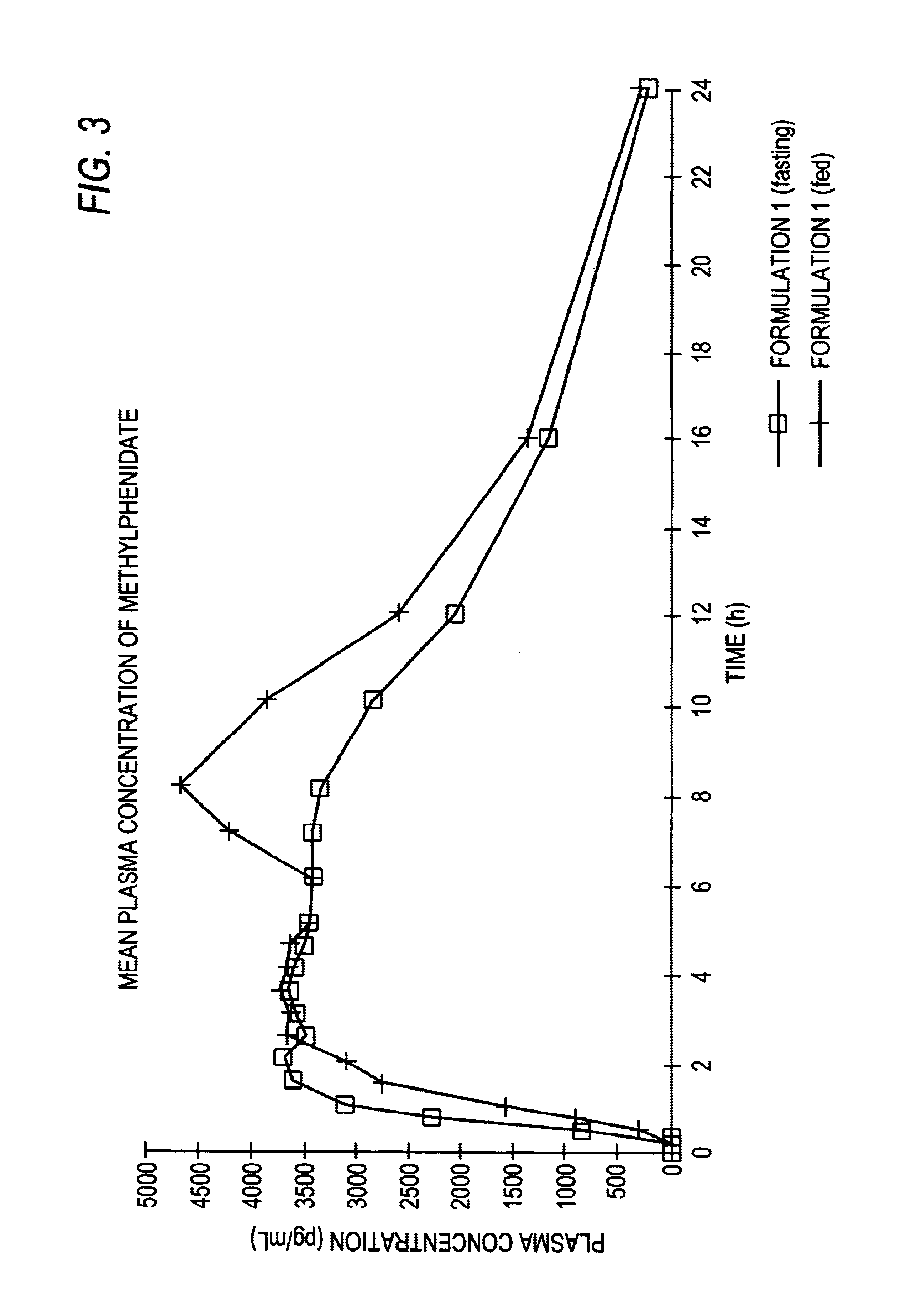
Controlled/modified release oral methylphenidate formulations
InactiveUS20040131680A1Patient compliance is goodPowder deliveryOrganic active ingredientsDuration of effectMethylphenidate
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
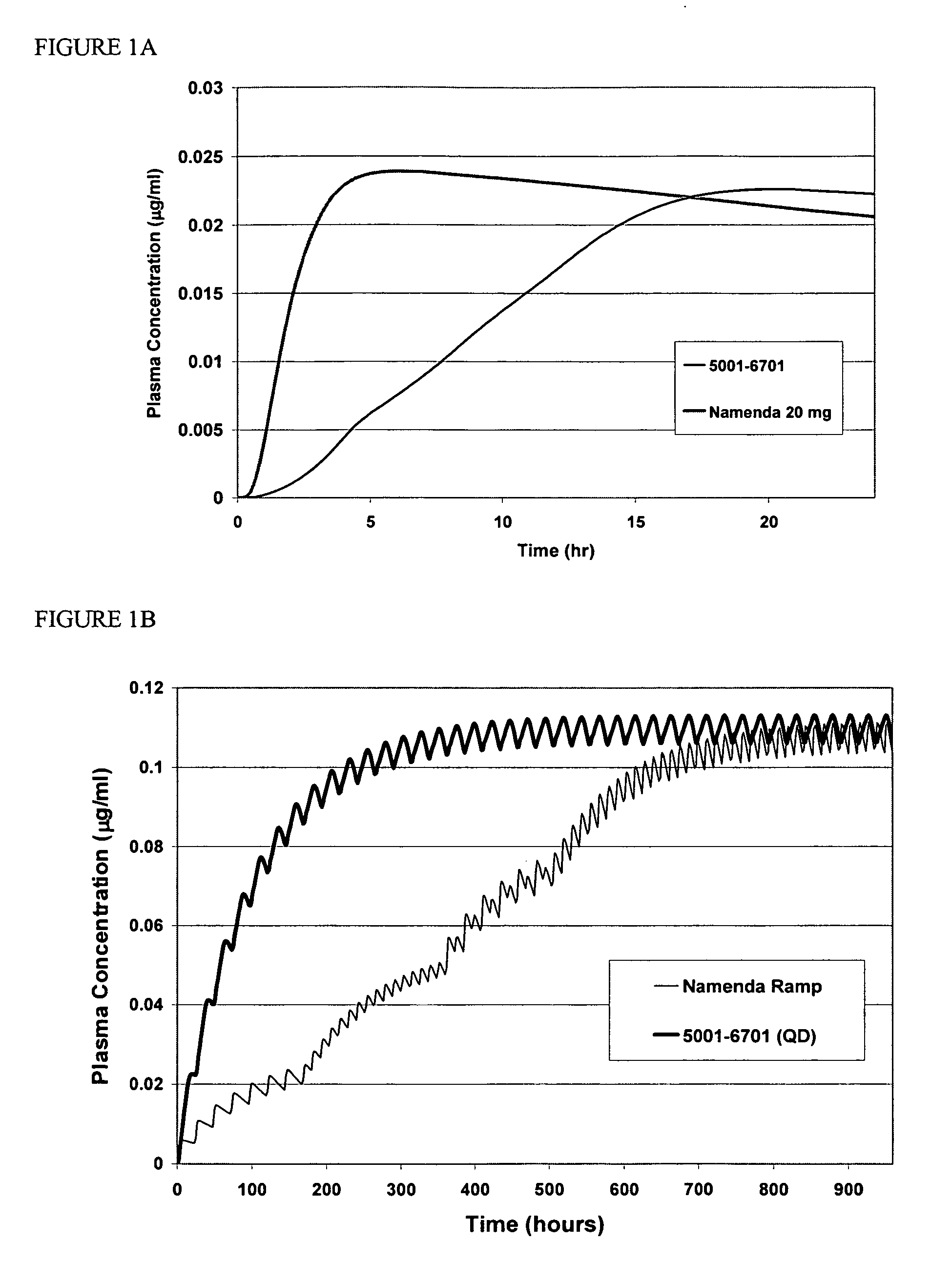
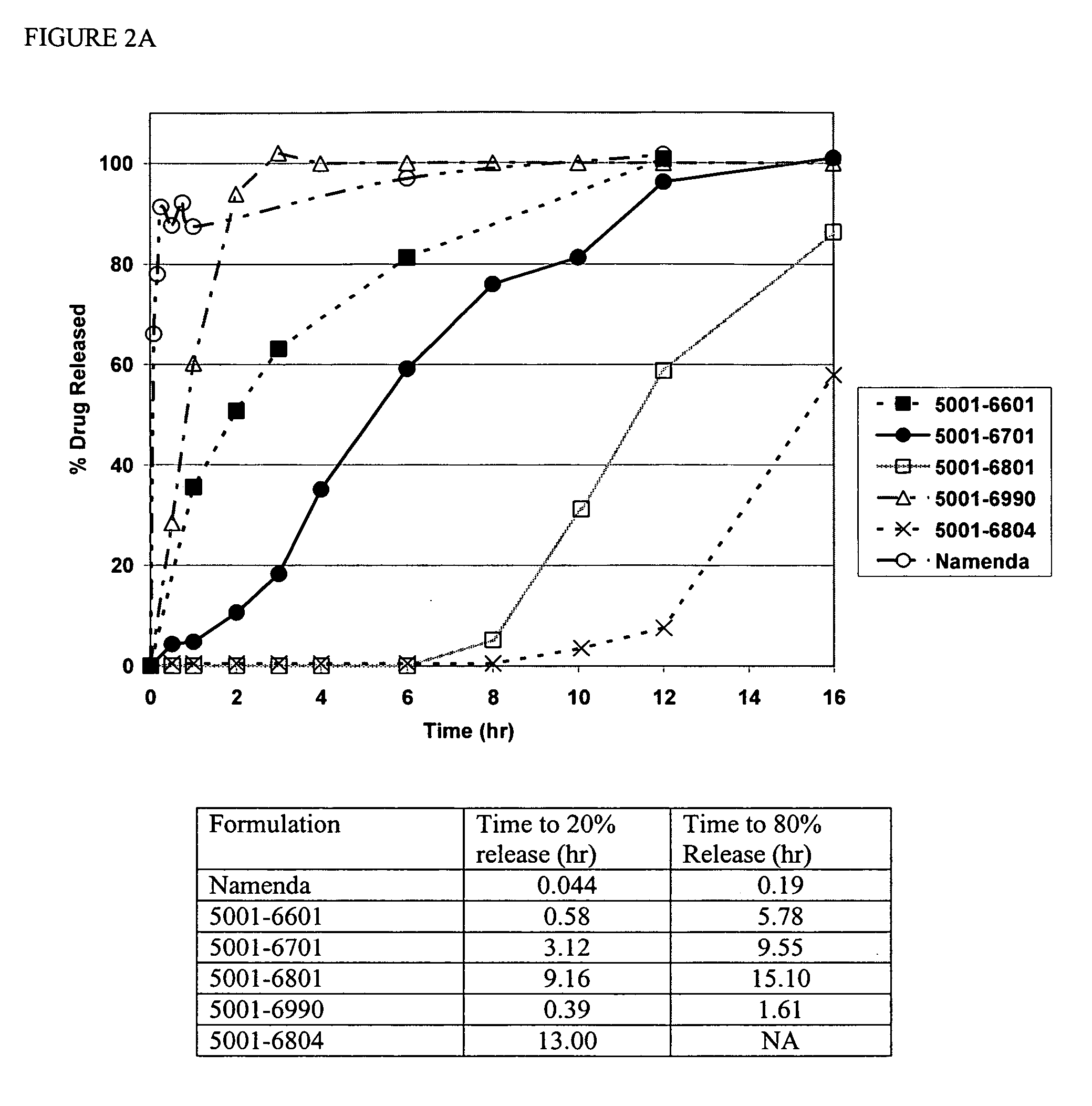
Method and composition for adminstering an NMDA receptor antagonist to a subject
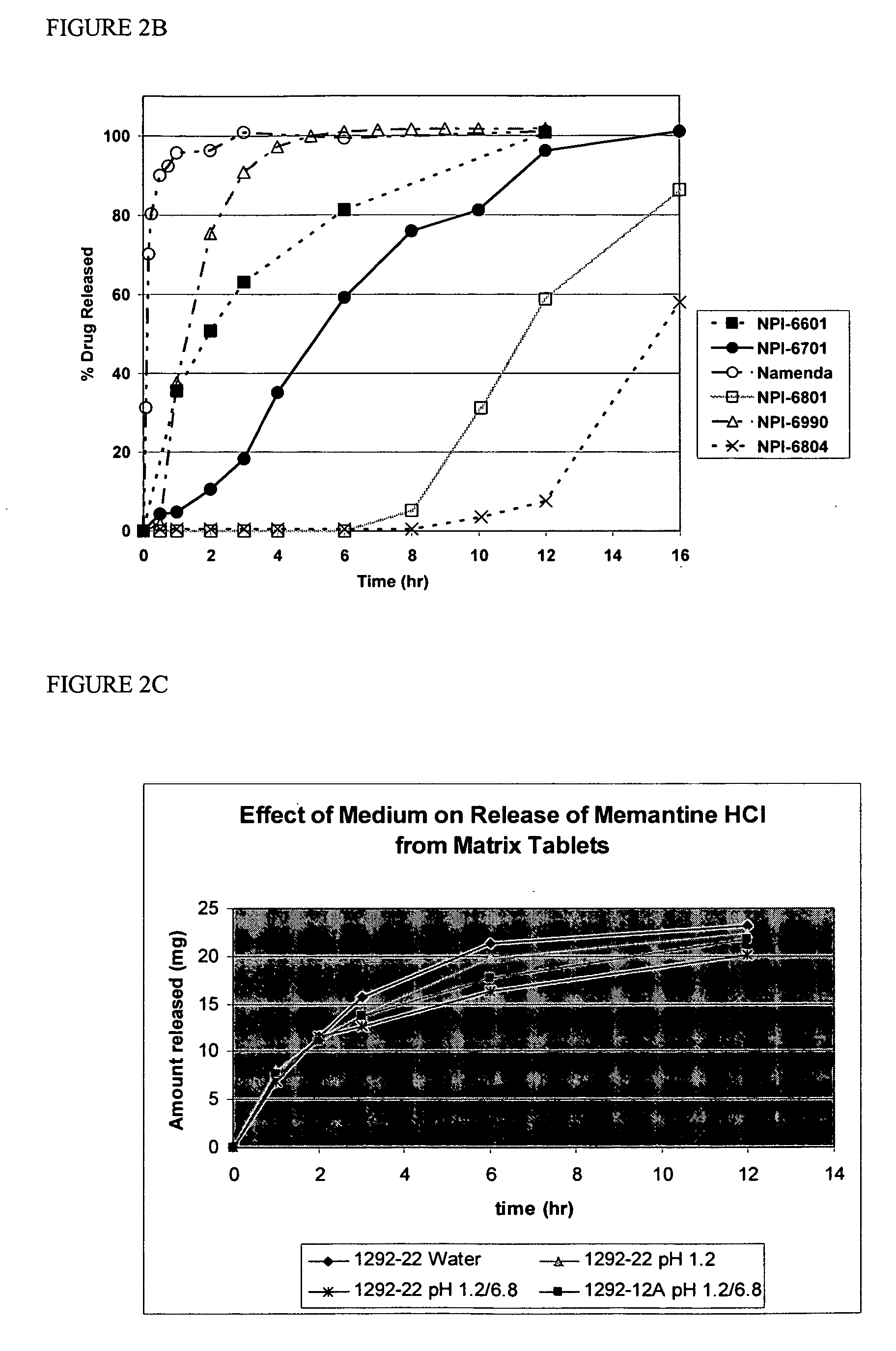
ActiveUS20060142398A1Prevent adverse side effectsPatient compliance is goodBiocideNervous disorderNR1 NMDA receptorPharmacology
The invention provides methods and compositions for administering an NMDA receptor antagonist (e.g., memantine) to a subject.
Owner:ADAMAS PHARMA LLC
Apparatus and method for establishing and/or improving binocular vision
ActiveUS20120307203A1Reliable performanceImprove visionEye exercisersEye diagnosticsVisual systemVisual presentation
An apparatus and method are disclosed that provide for establishing and / or improving binocular vision by adaptation of perceptual motor learning for the visual system of a patient suffering of a binocular vision disorder. The apparatus has a set of two units, one for respectively eye, for manipulation of a vision parameter of the visual presentation, e.g. picture, movie, image. To control the visual presentation on respectively unit a processing unit is connected. The processing unit is adapted to determine a boundary value of a first and / or second vision parameter for the first and / or second manipulation of the first and second unit, where binocular vision disappears for the patient. Furthermore, the processing unit controls at least one vision parameter by manipulating, including oscillating or fluctuating, it within a first range, the first range having a maximum value that is less than the boundary value, for the perceptual motor learning.
Owner:IMVI LABS AB
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
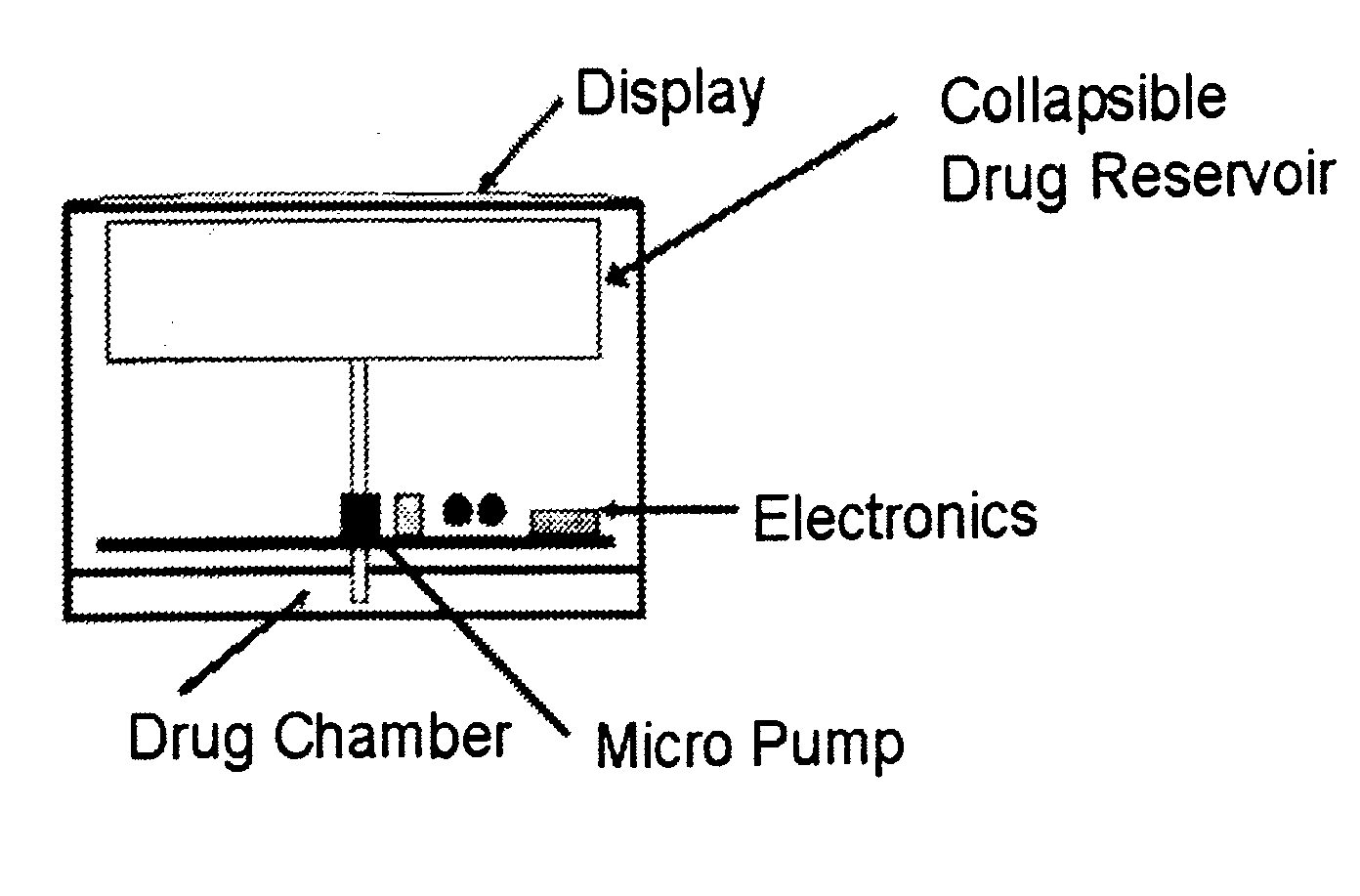
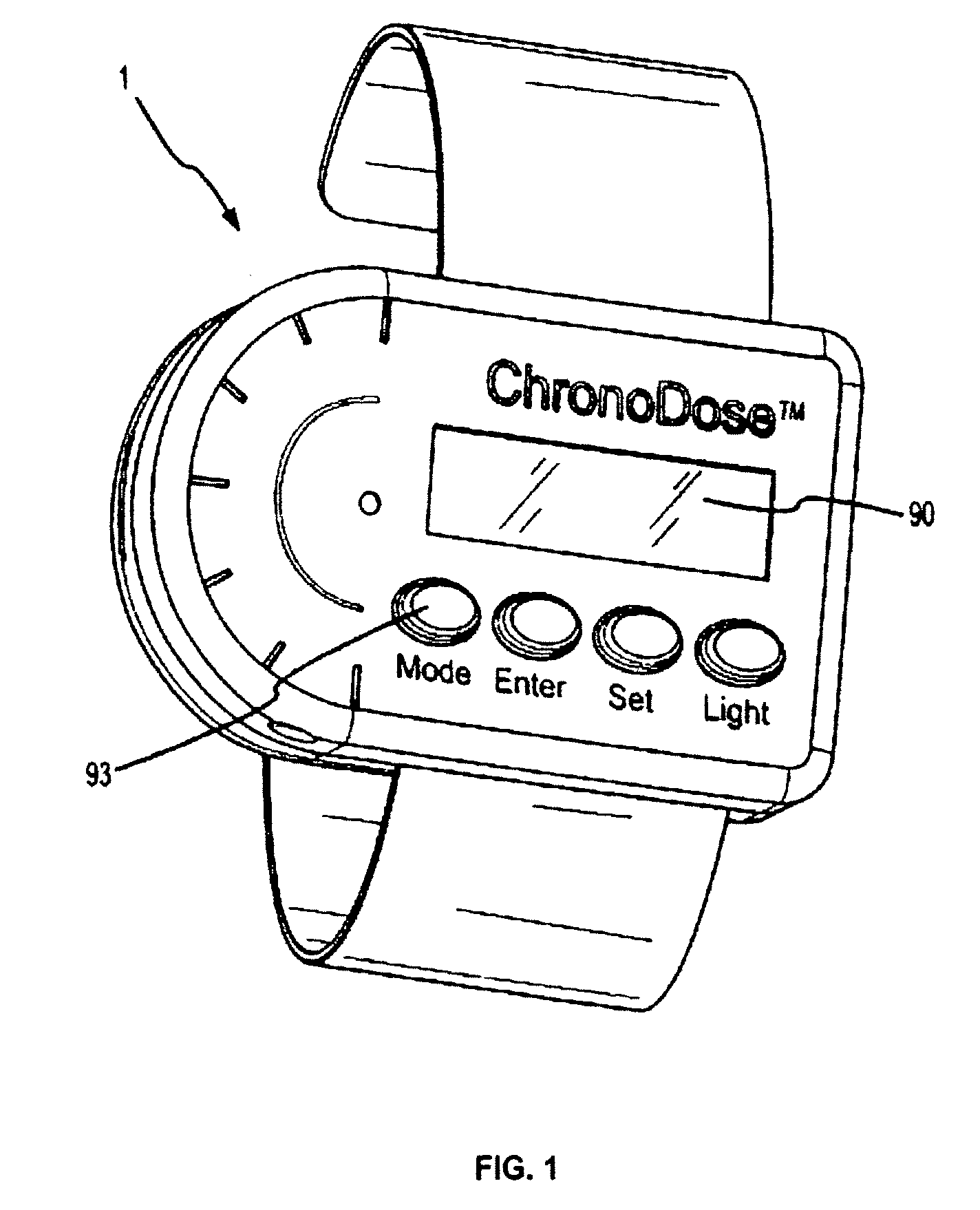
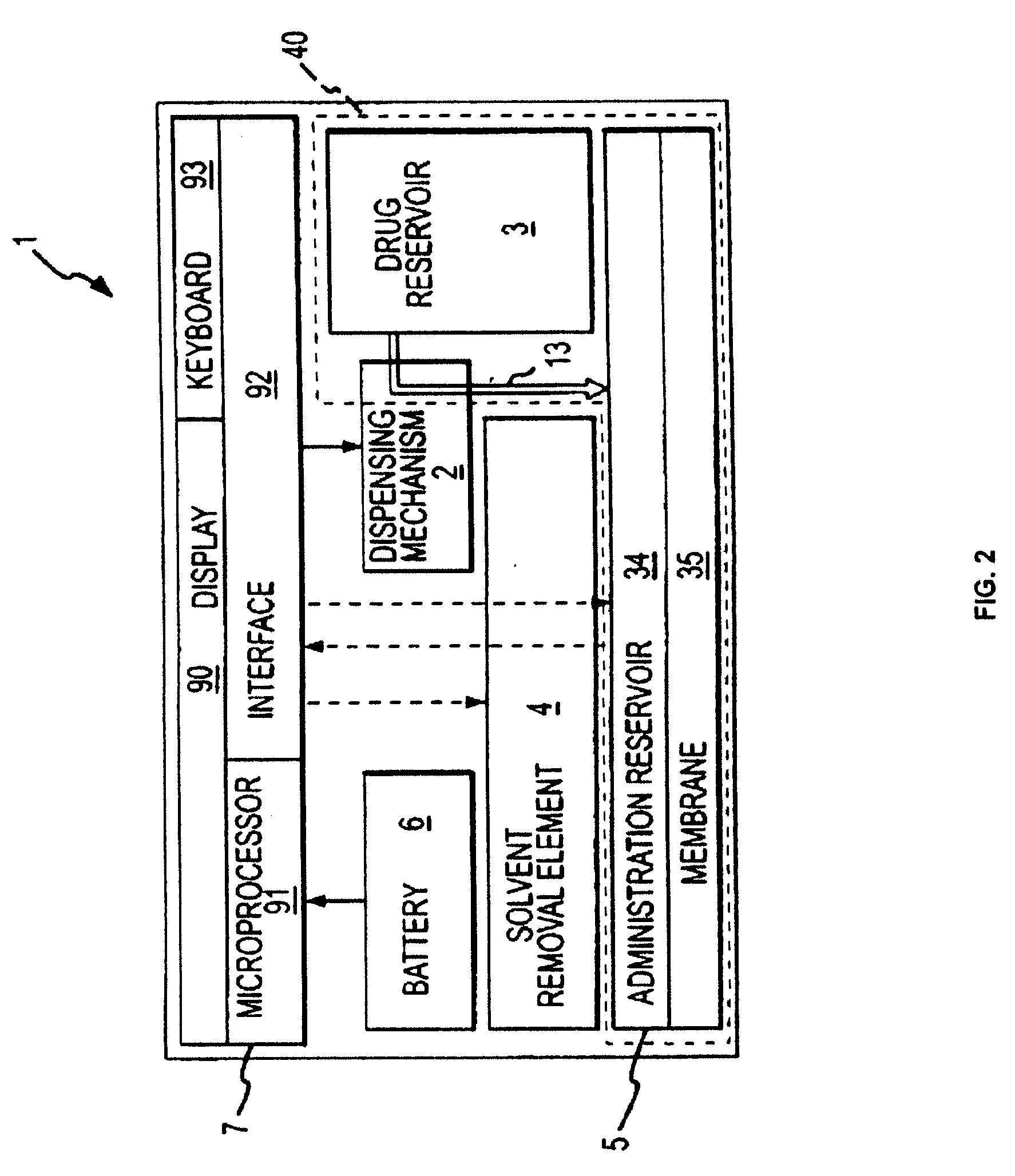
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
Controlled/modified release oral methylphenidate formulations
InactiveUS6673367B1Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
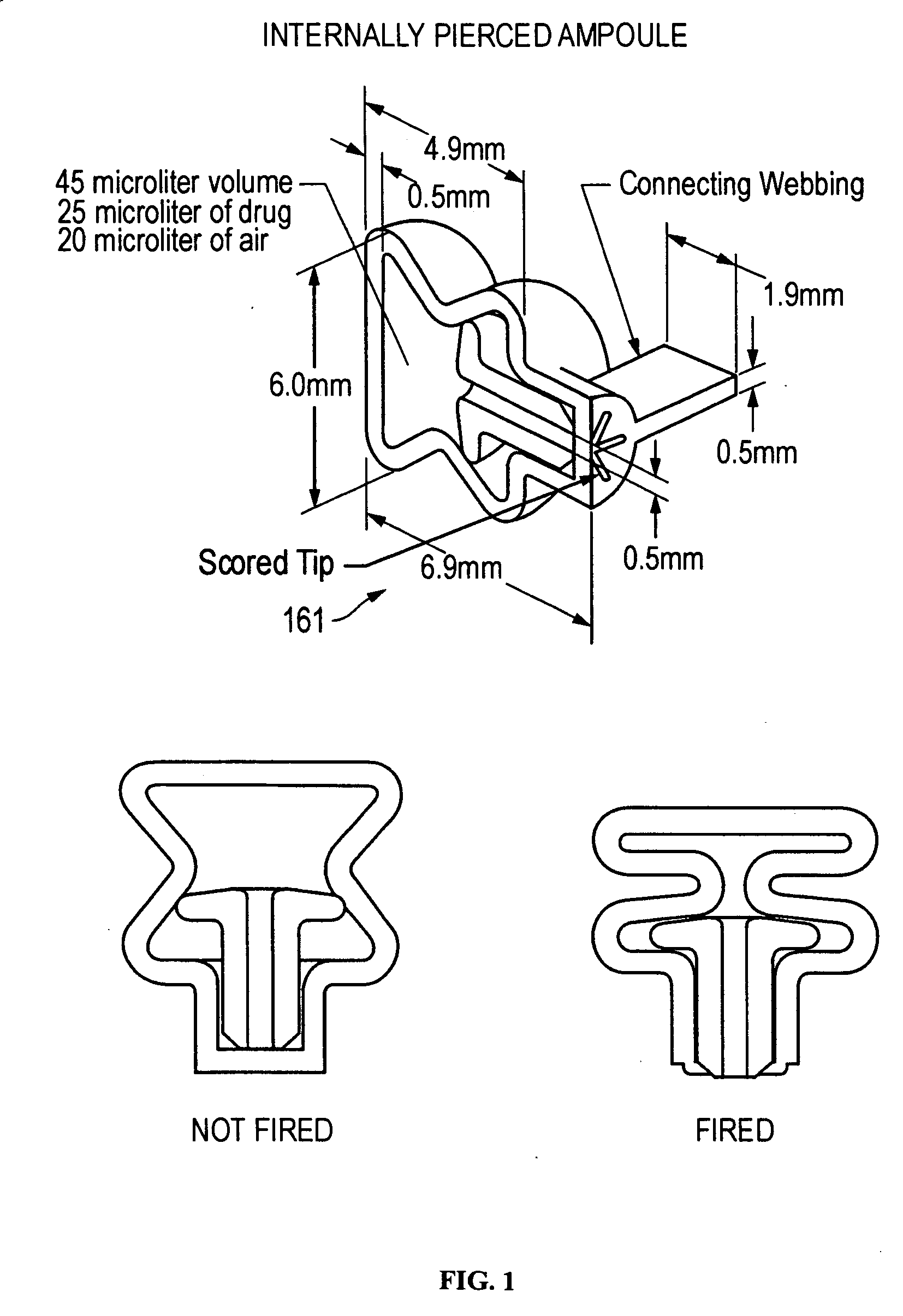
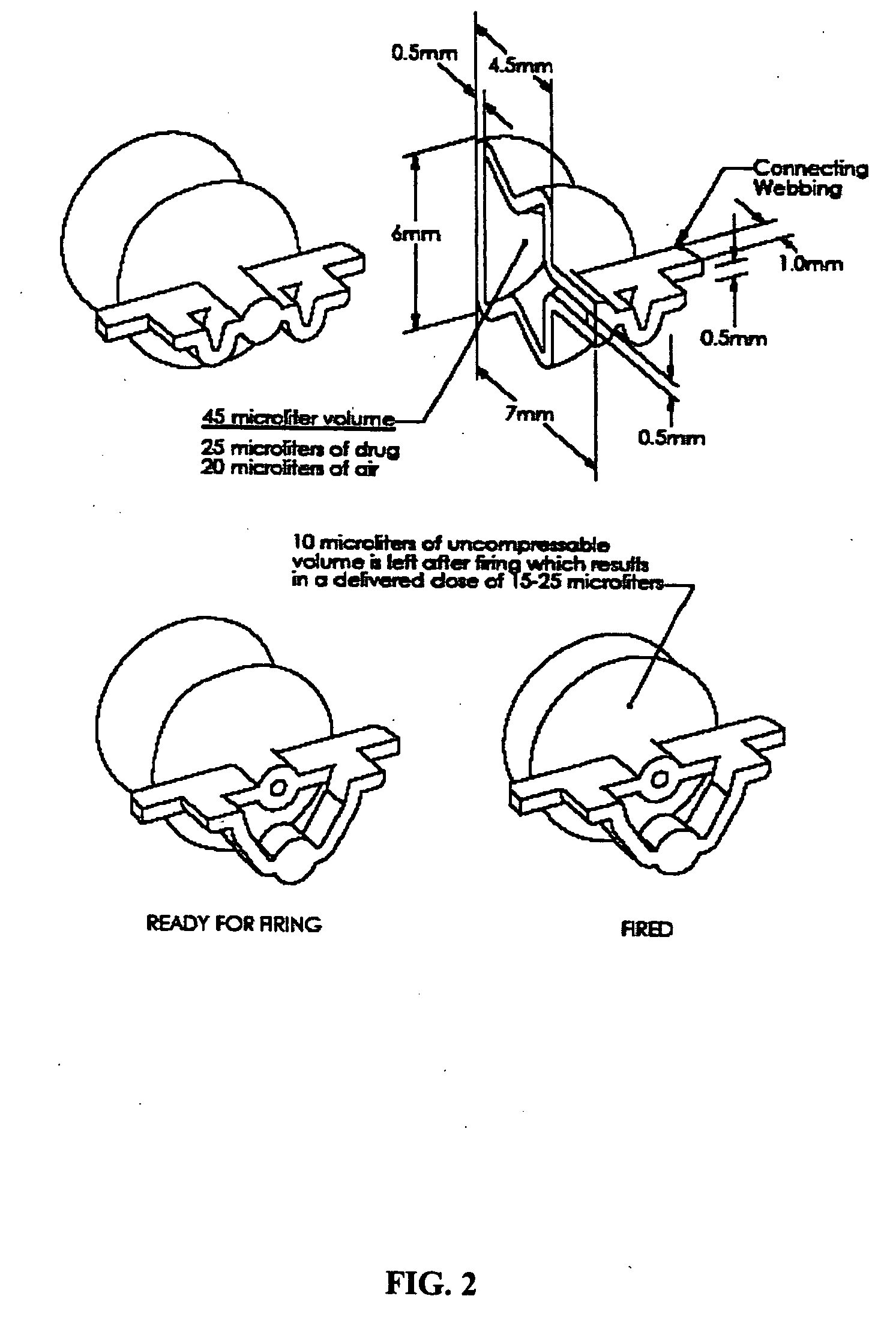
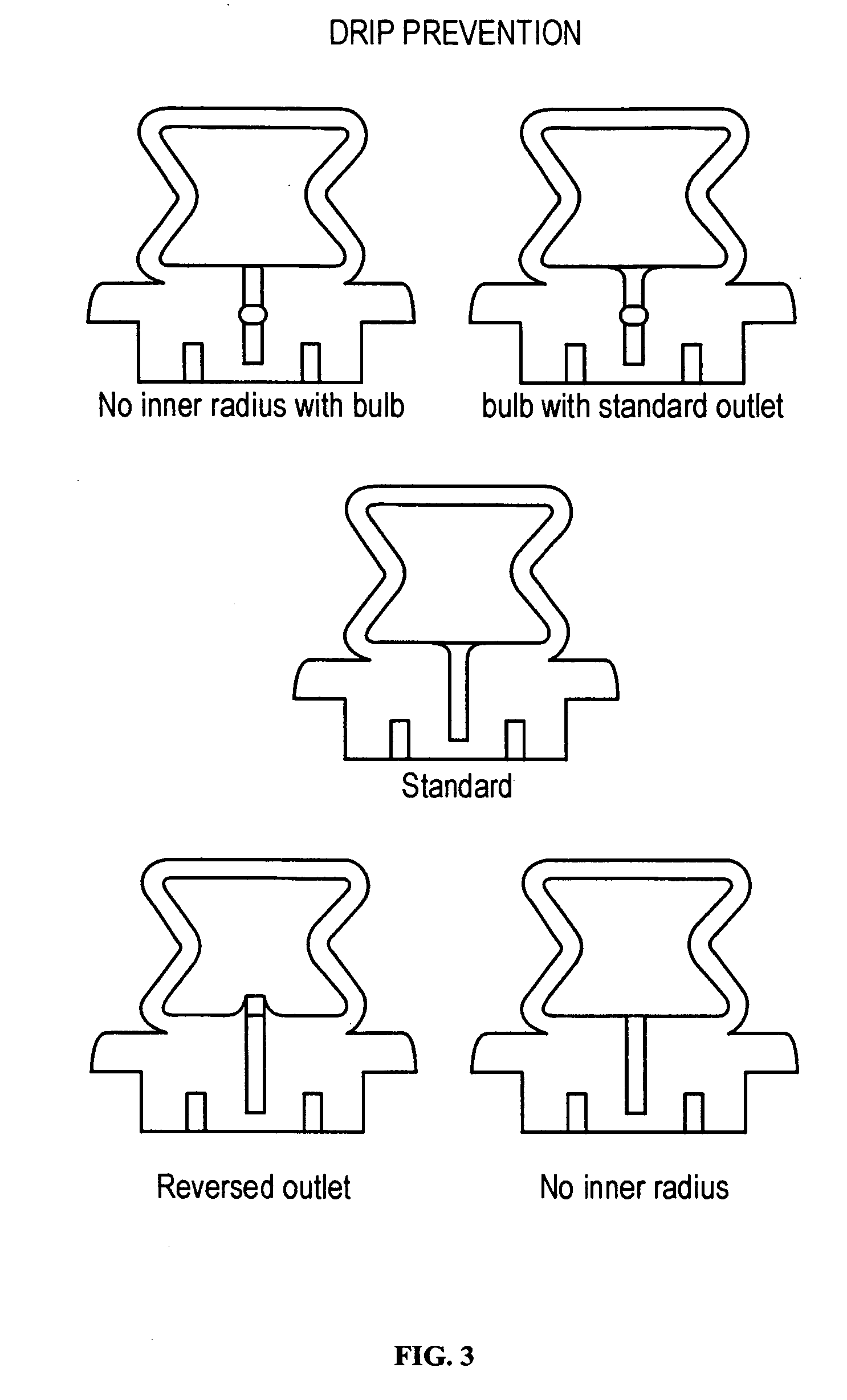
Multiple unit dose drug delivery system
InactiveUS20070051362A1Reduces potentially unpleasant side effectConvenient, fast and safeBiocideMedical devicesOphthalmologyNose
The present disclosure is directed to devices that administer single or multiple doses of one or more substances to the eye, nose, or ear of a user. The precise and repeatable dosing features of the presently disclosed devices overcome many of the disadvantages associated with known methods for dispensing substances to, for example, the eye of a user. The devices administer precise doses of a substance to a precise location from ampoules that may be single-dose or two-dose ampoules, which may be externally or internally pierced.
Owner:MYSTIC PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com