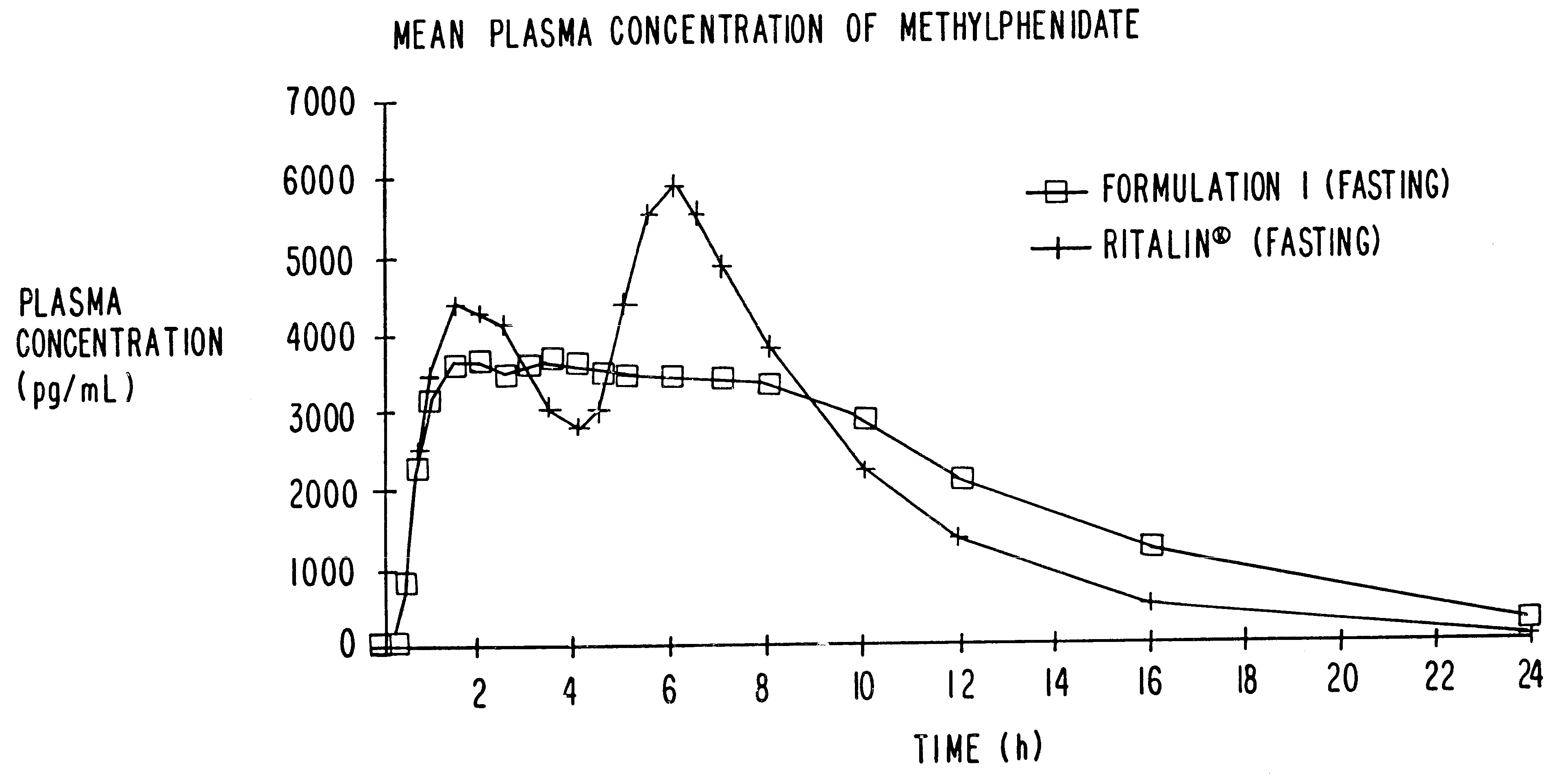
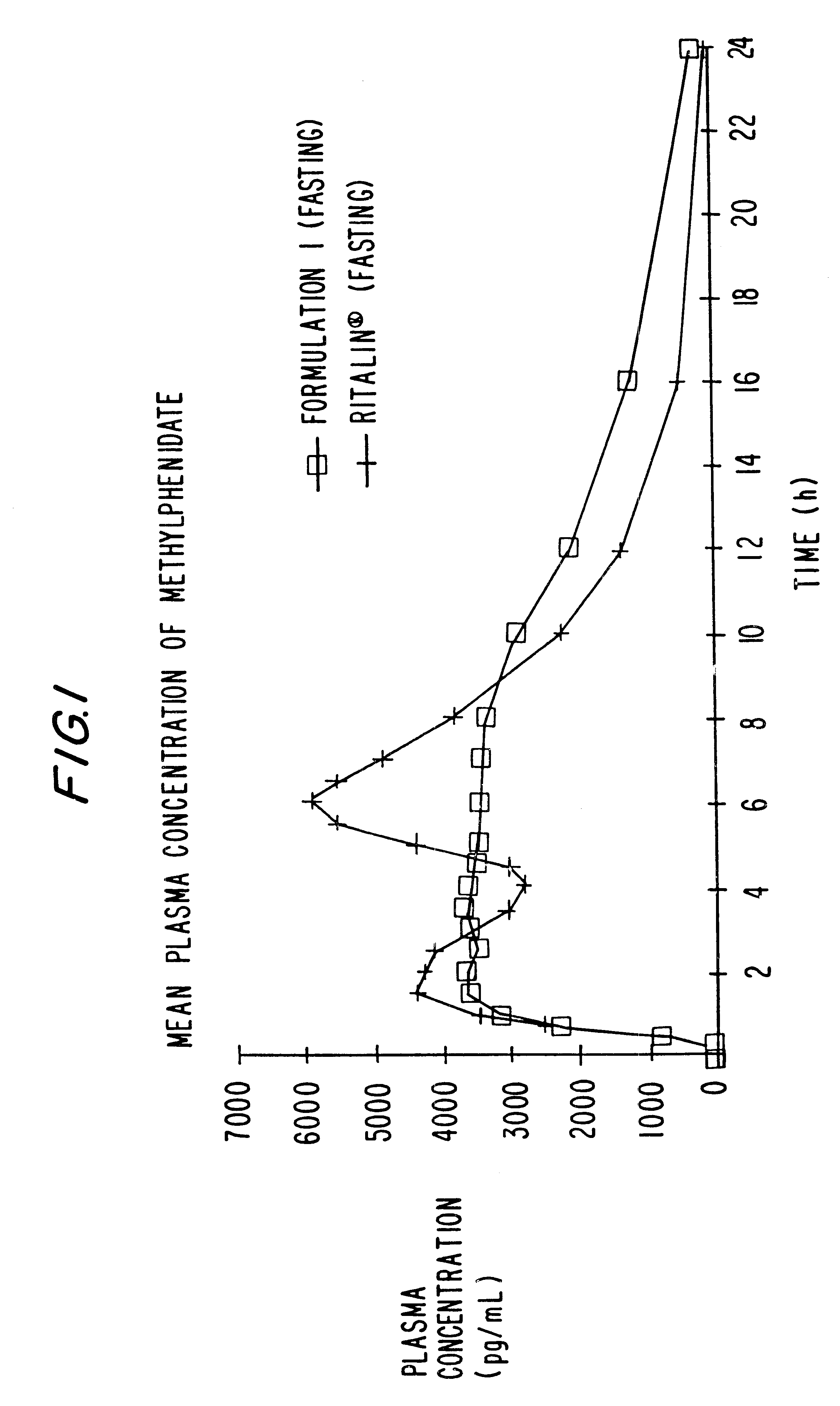
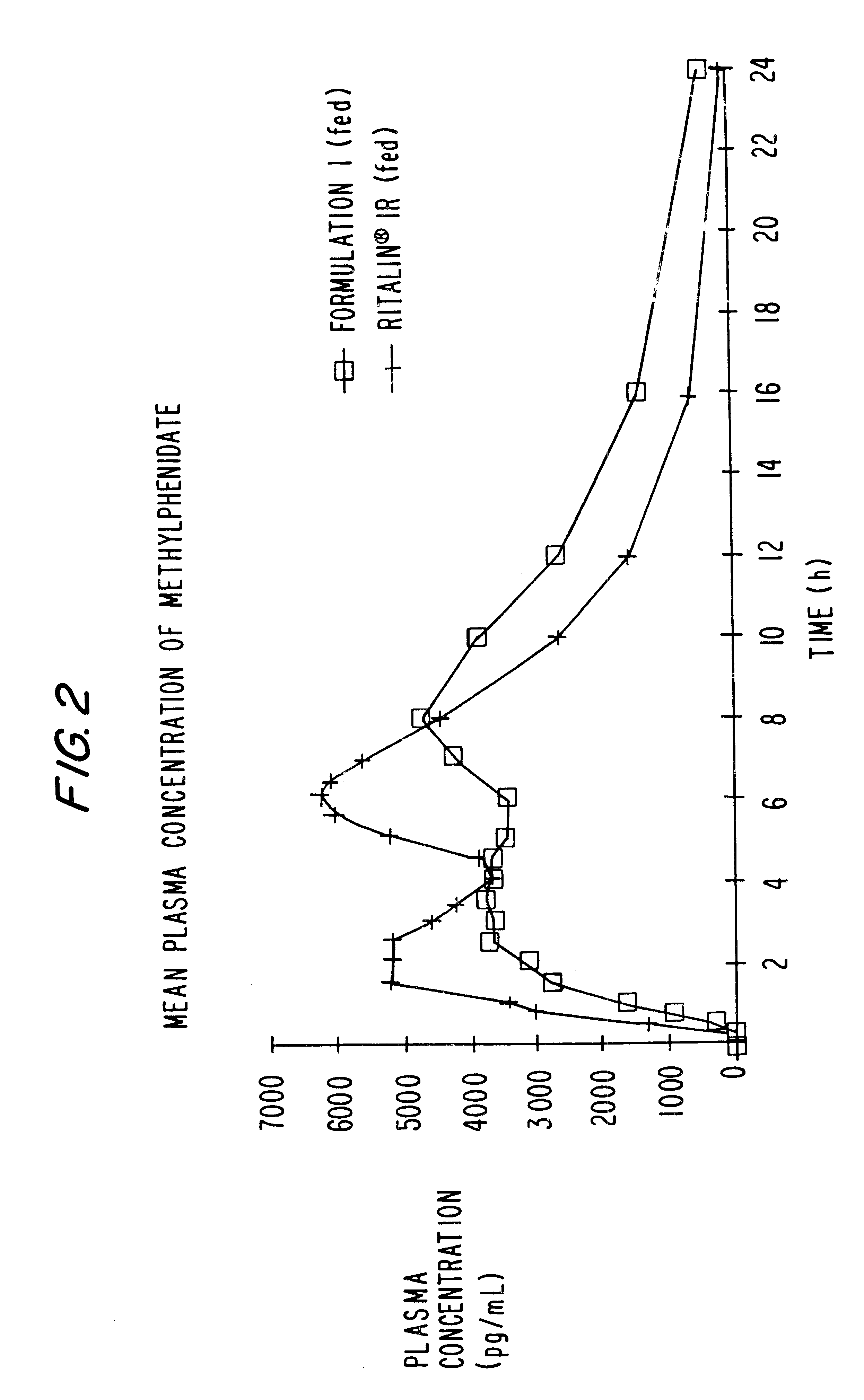
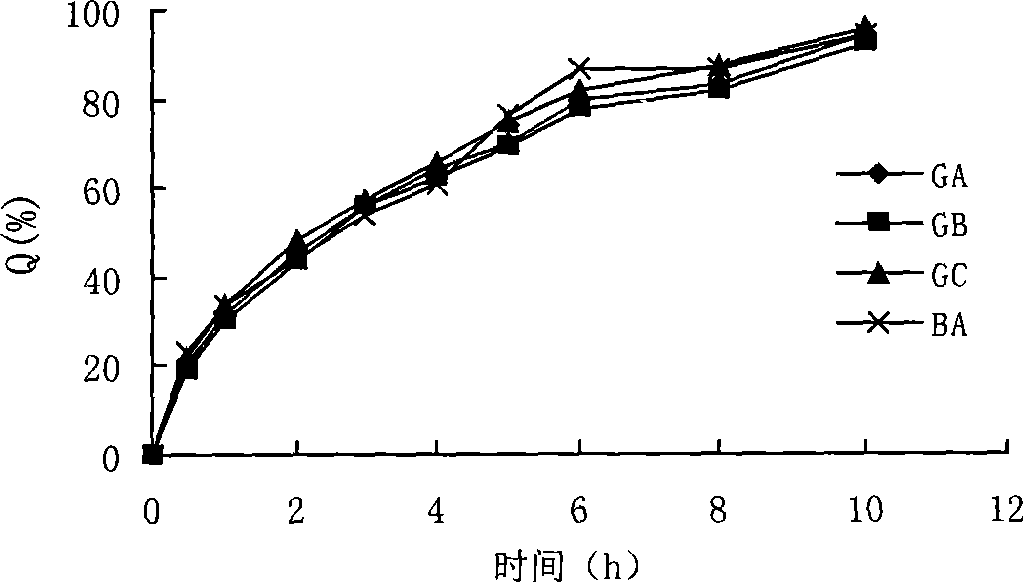
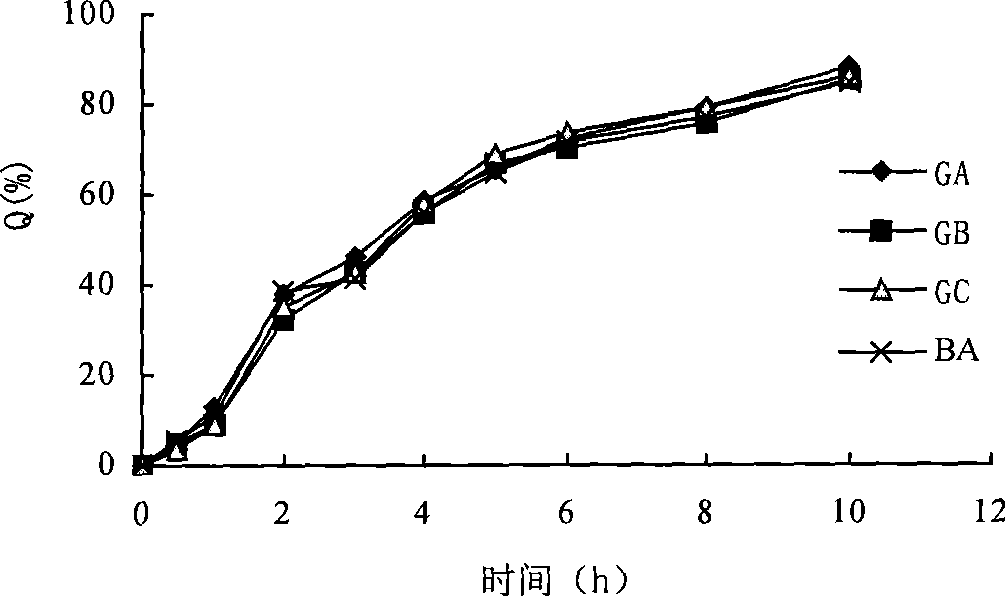
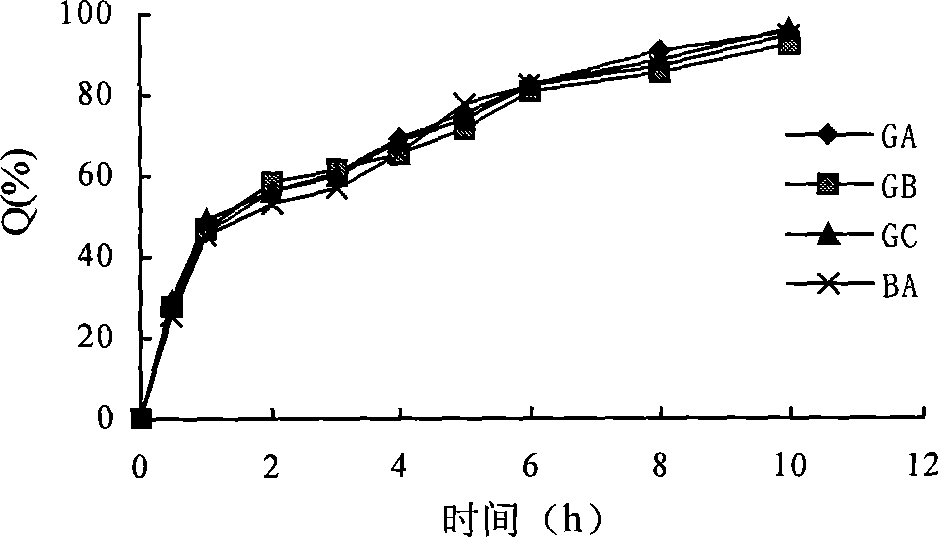
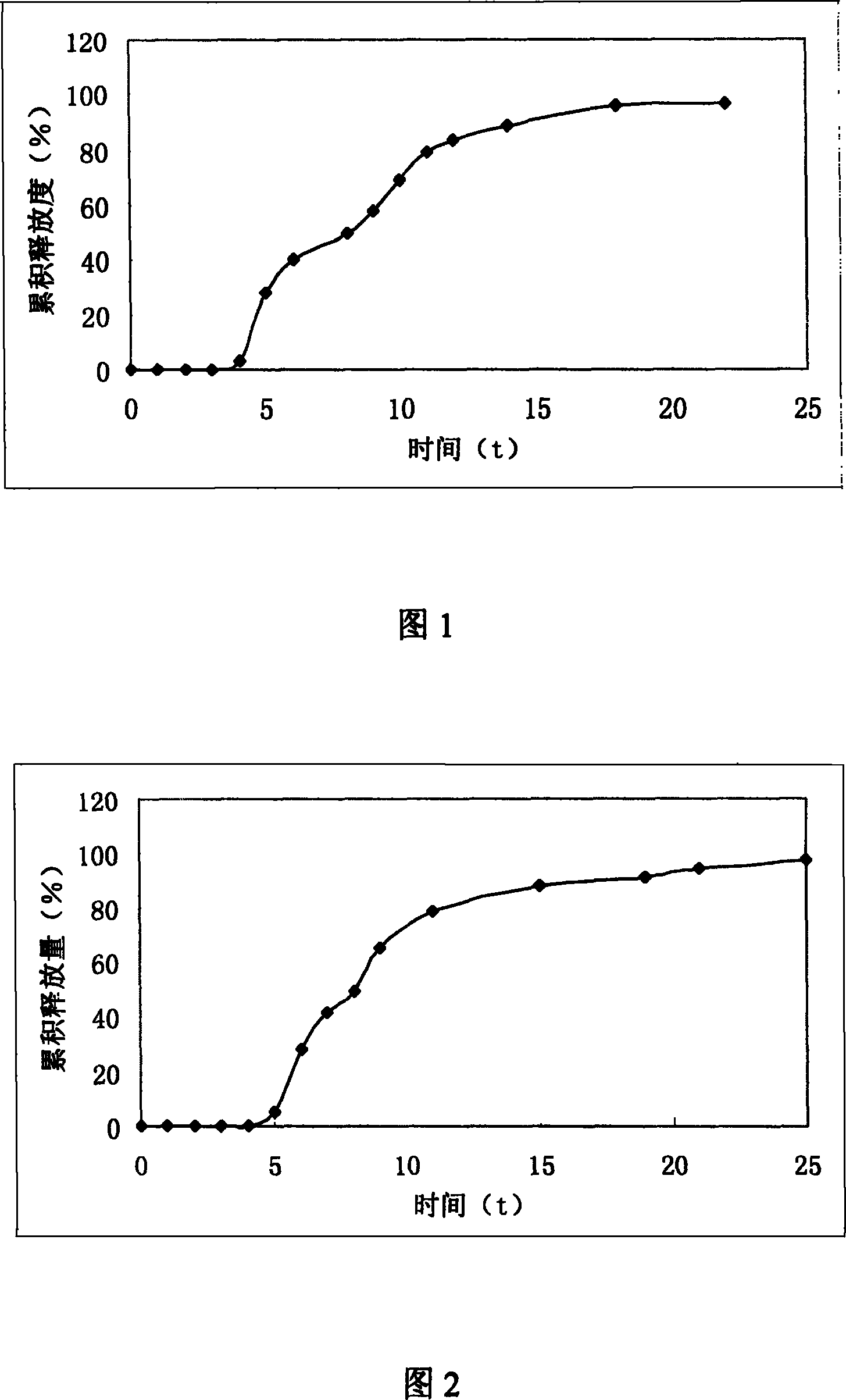
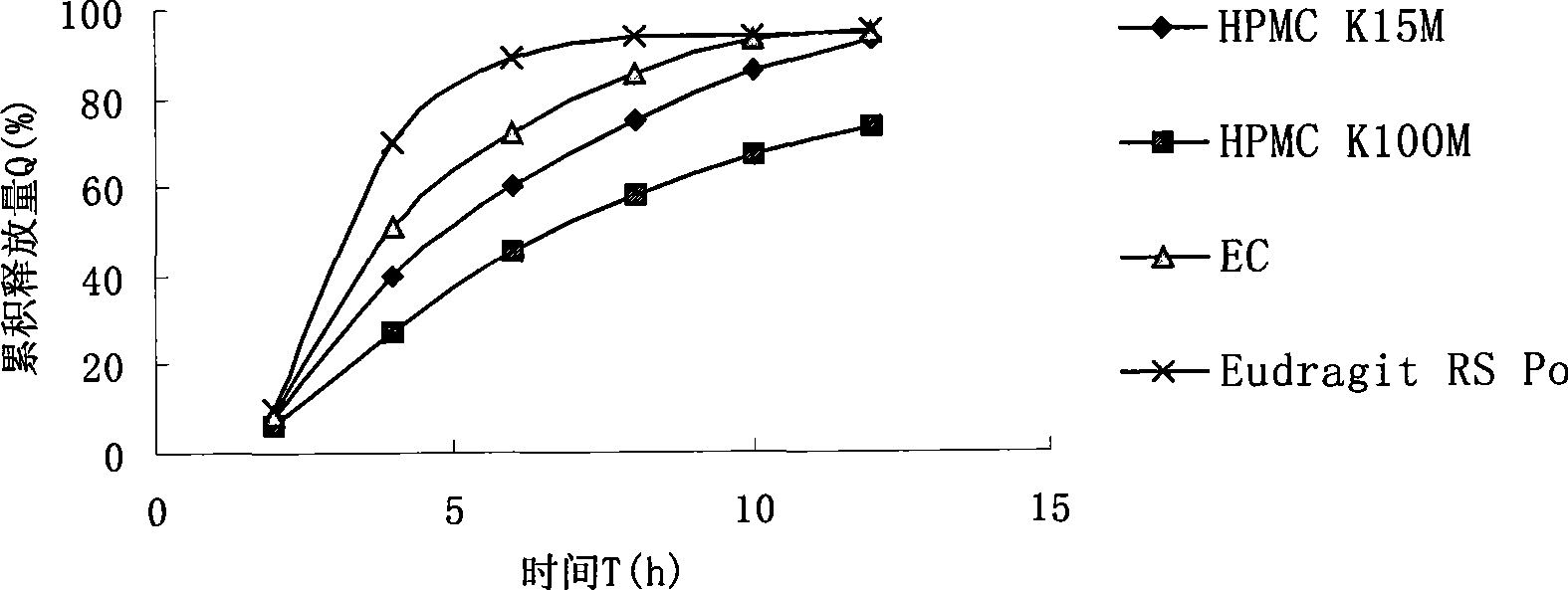
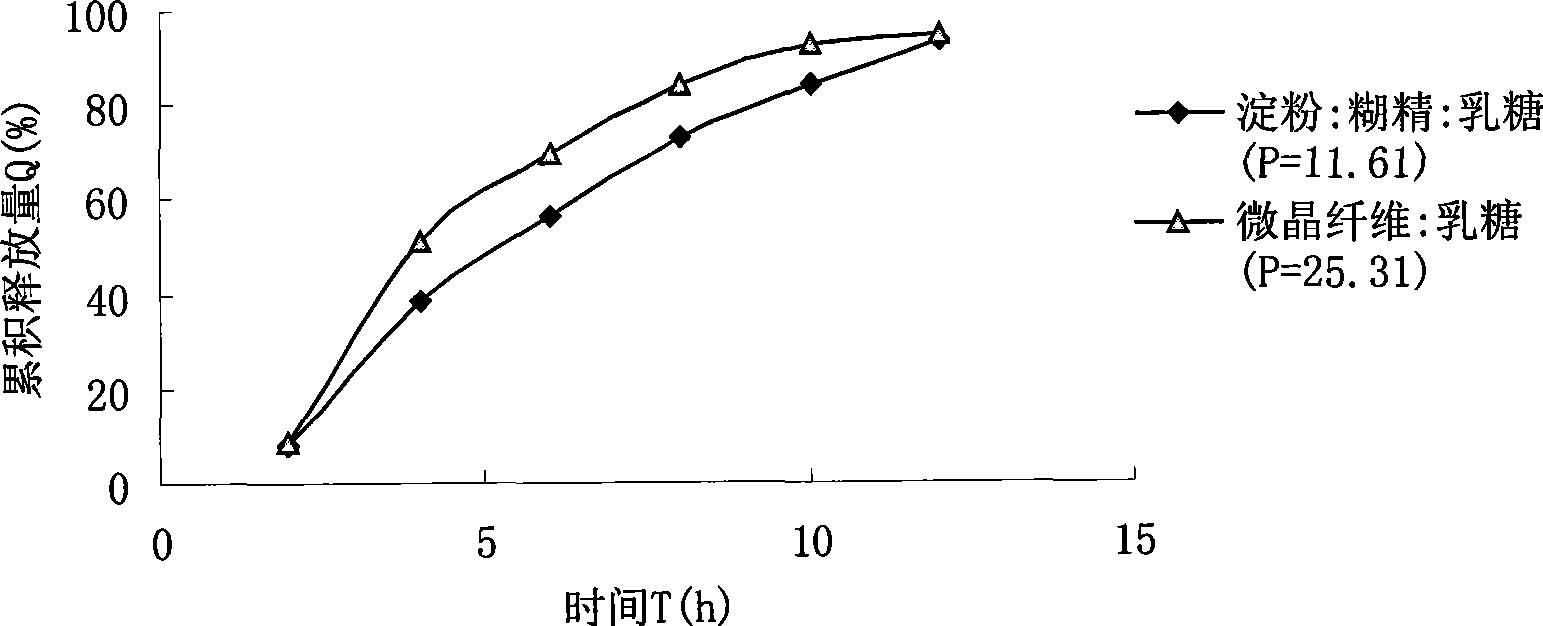
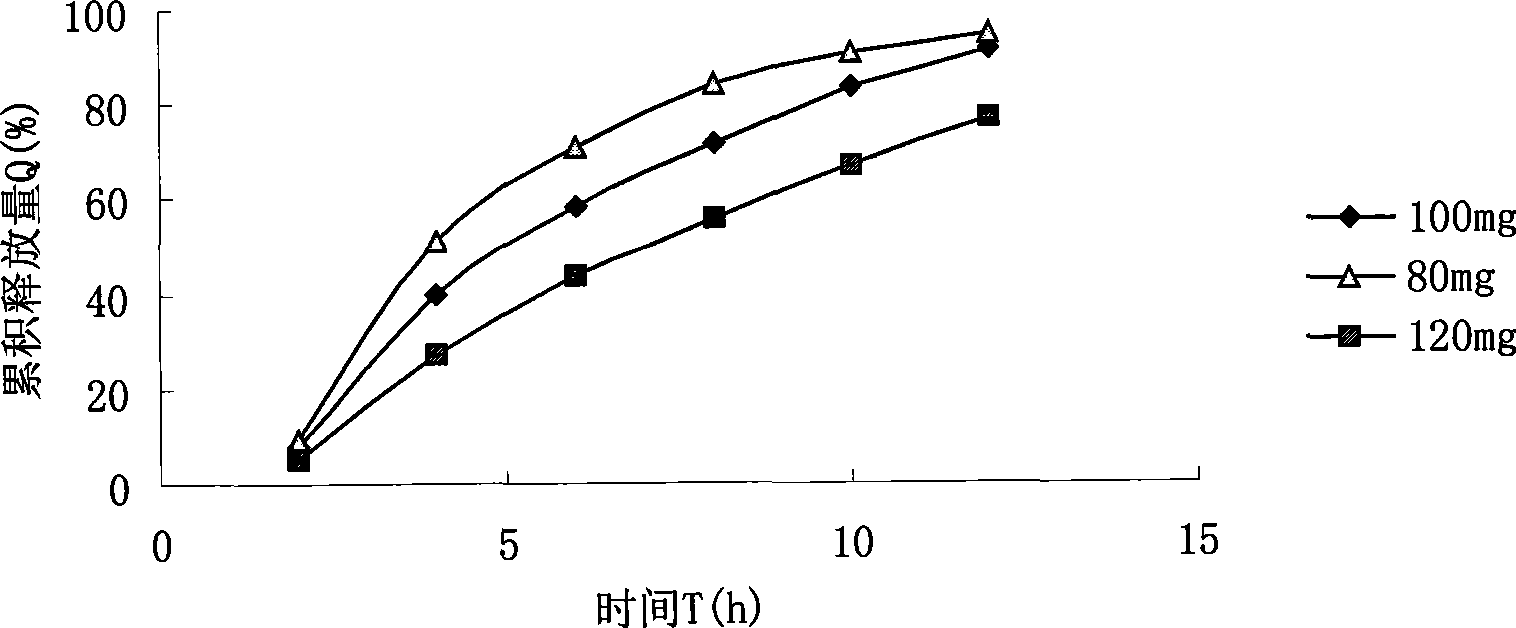
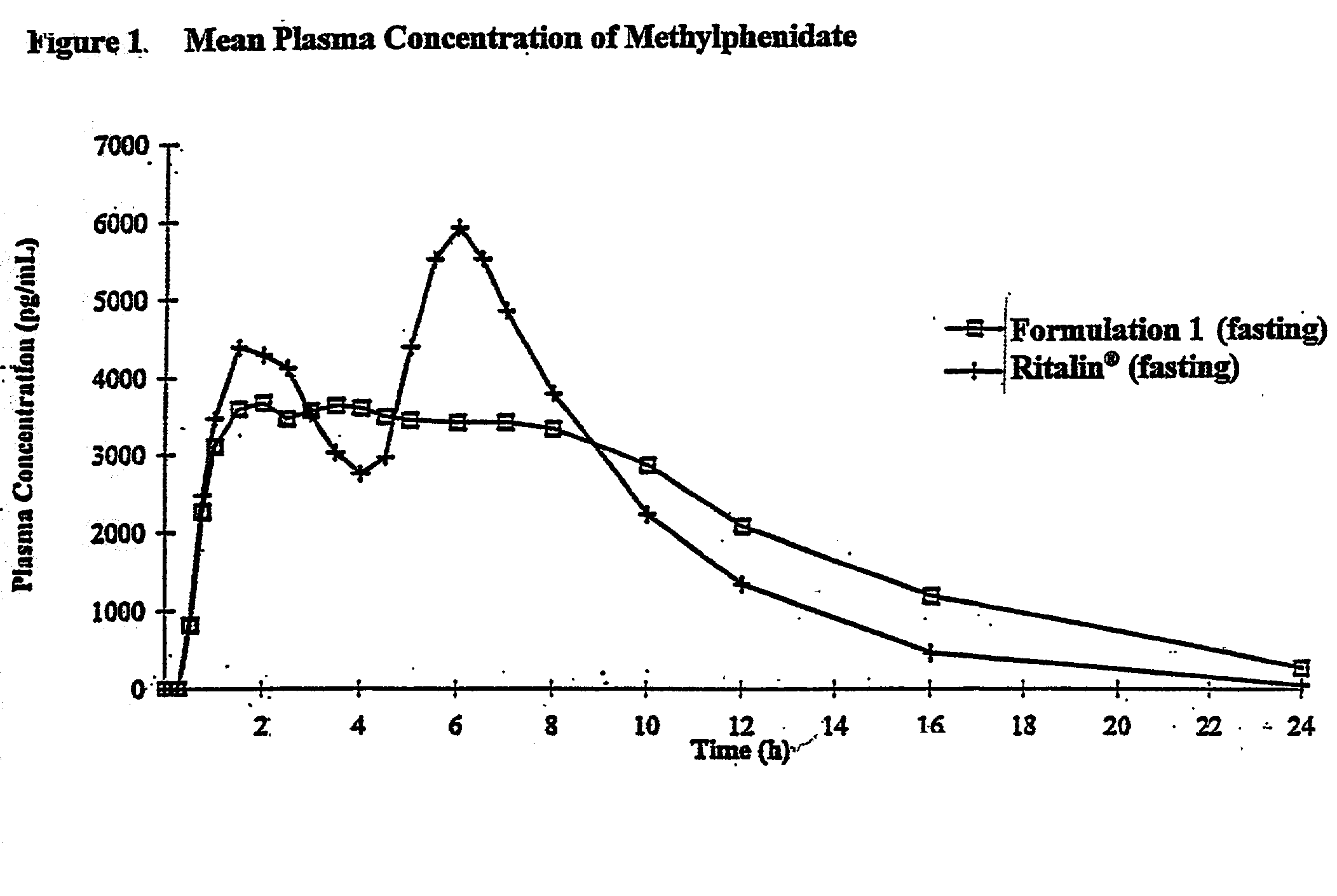
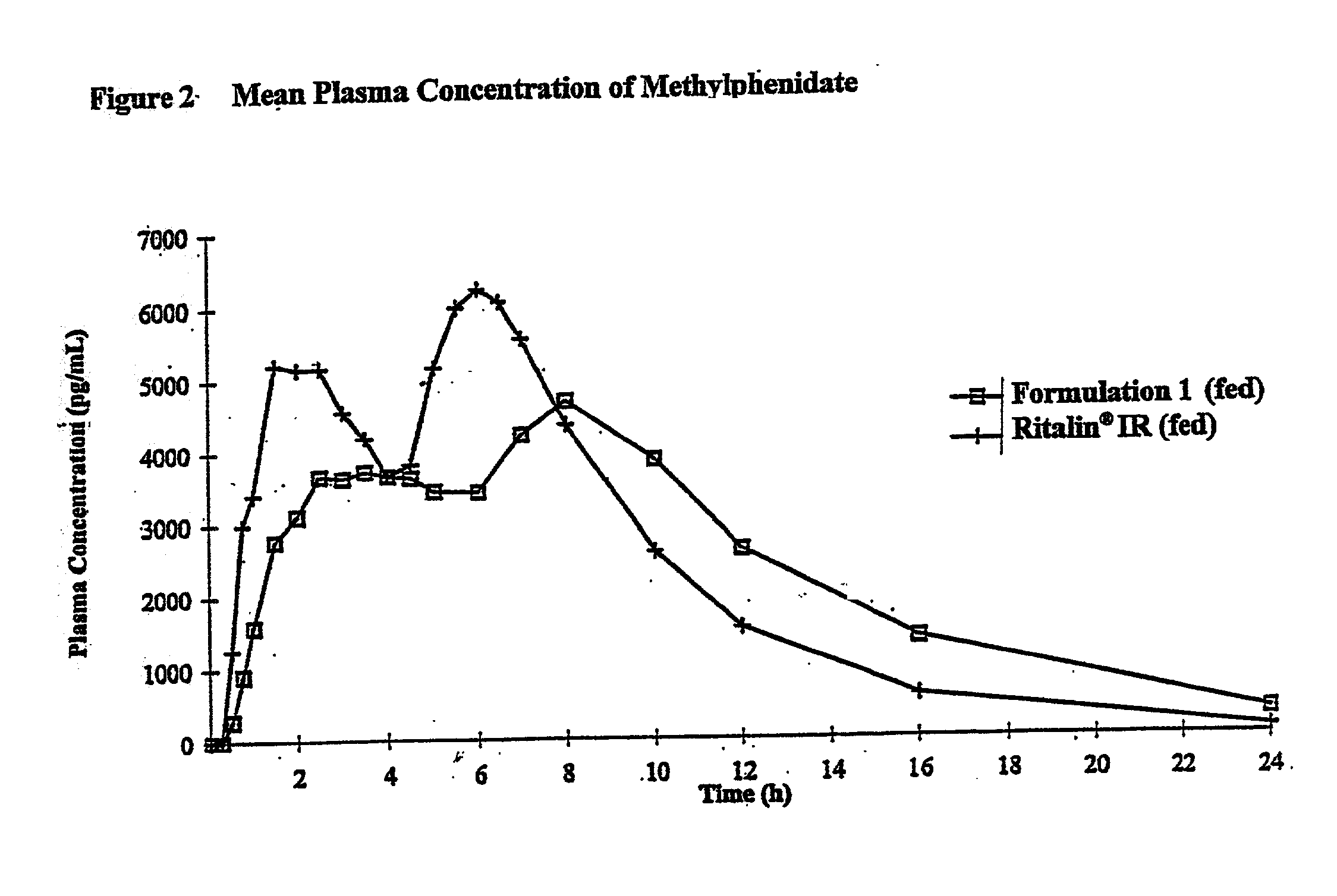
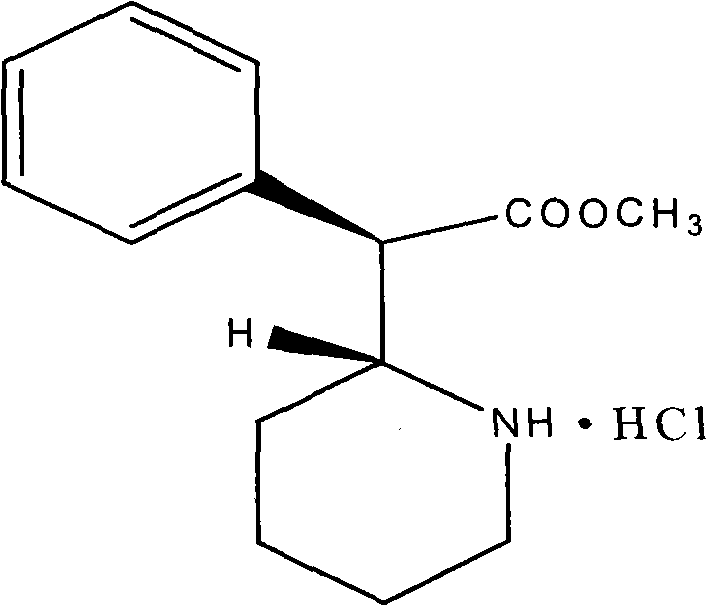
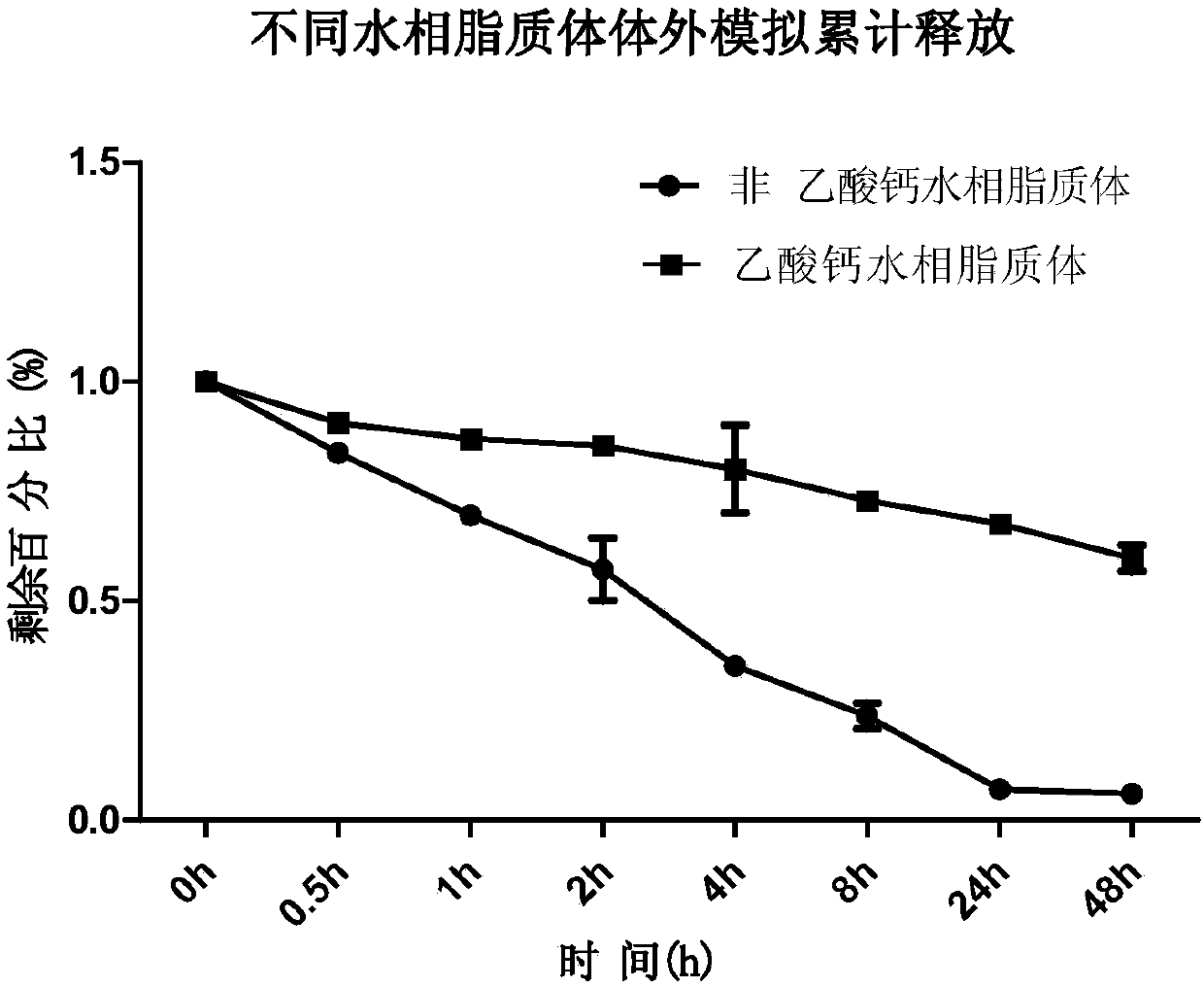
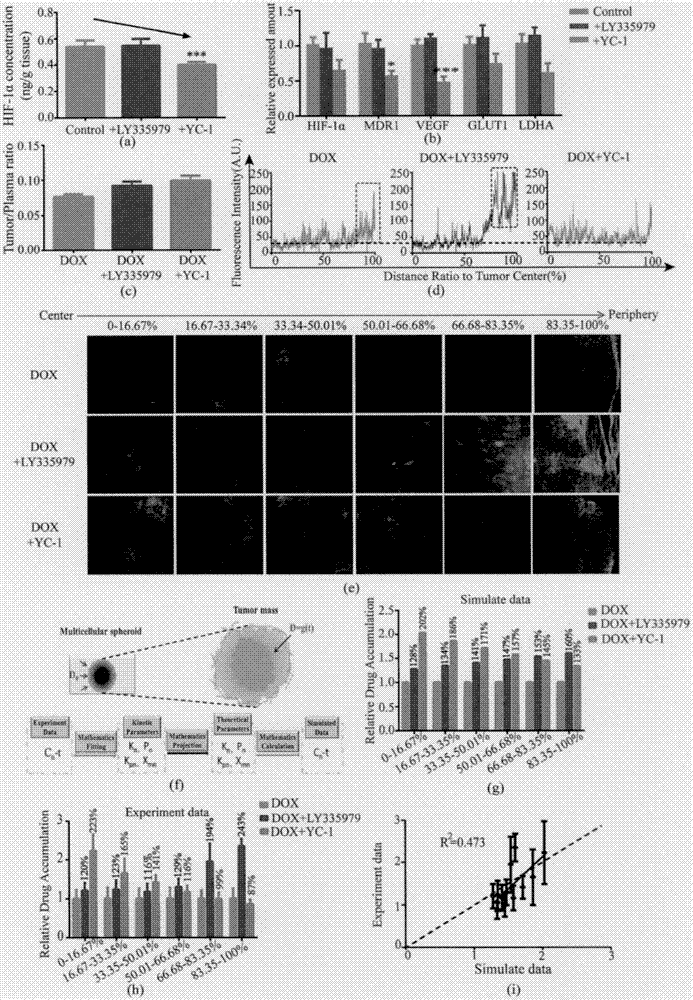
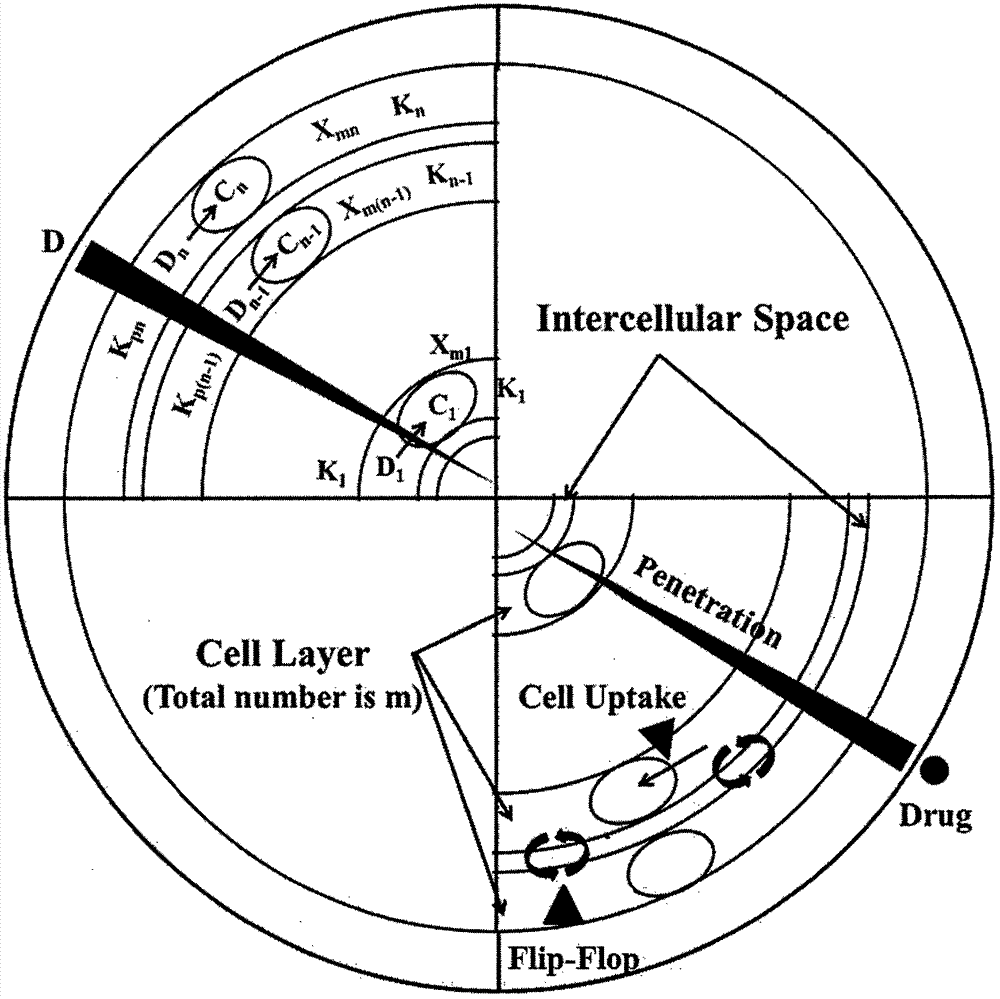
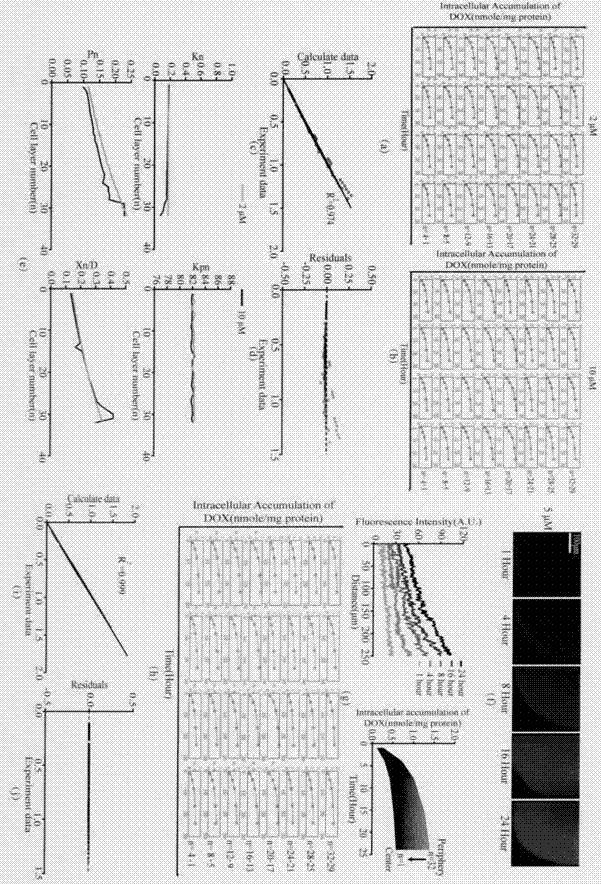
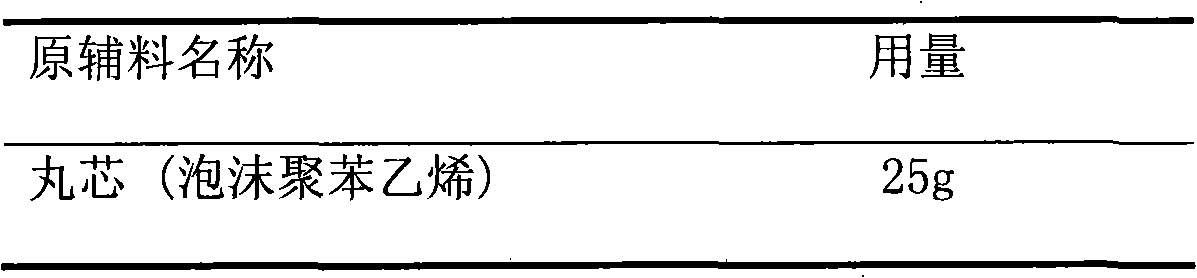
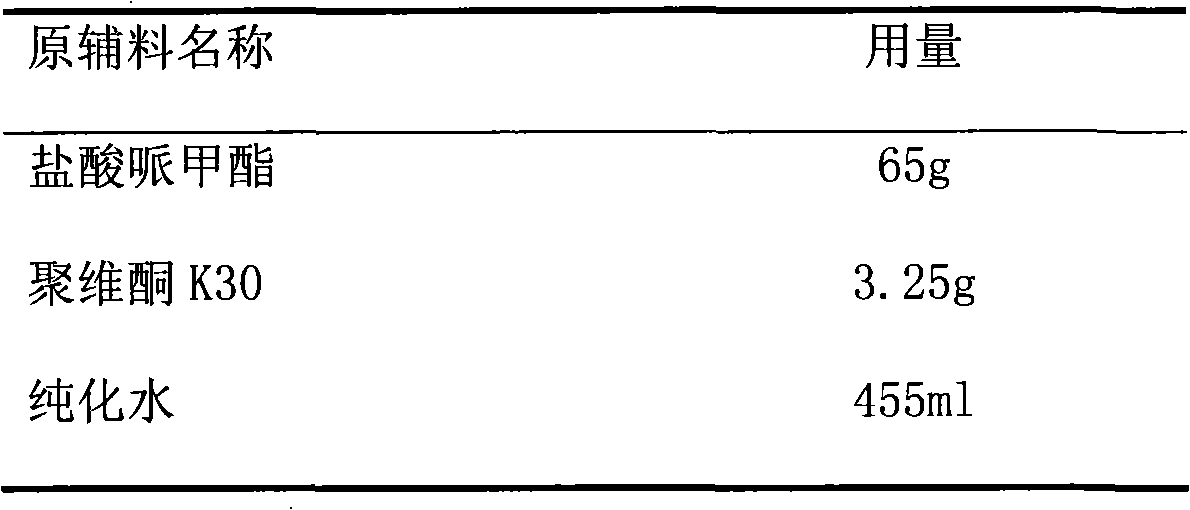
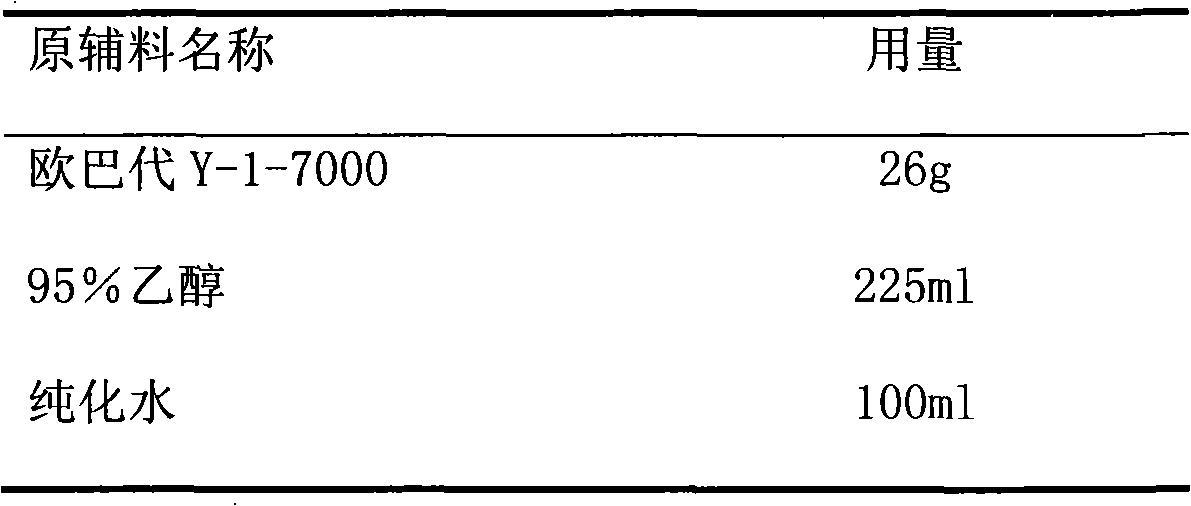
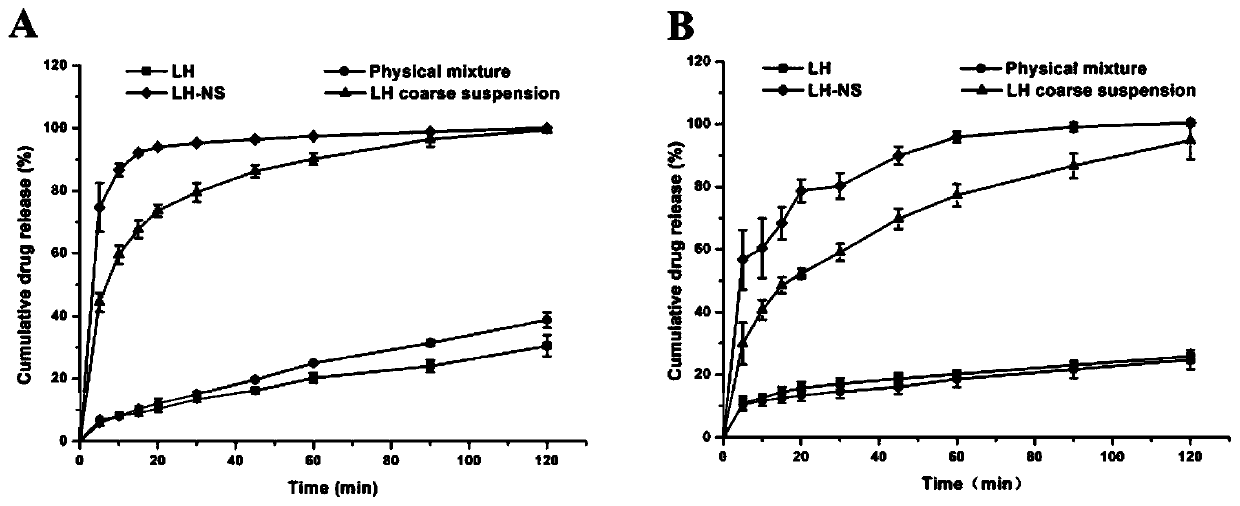
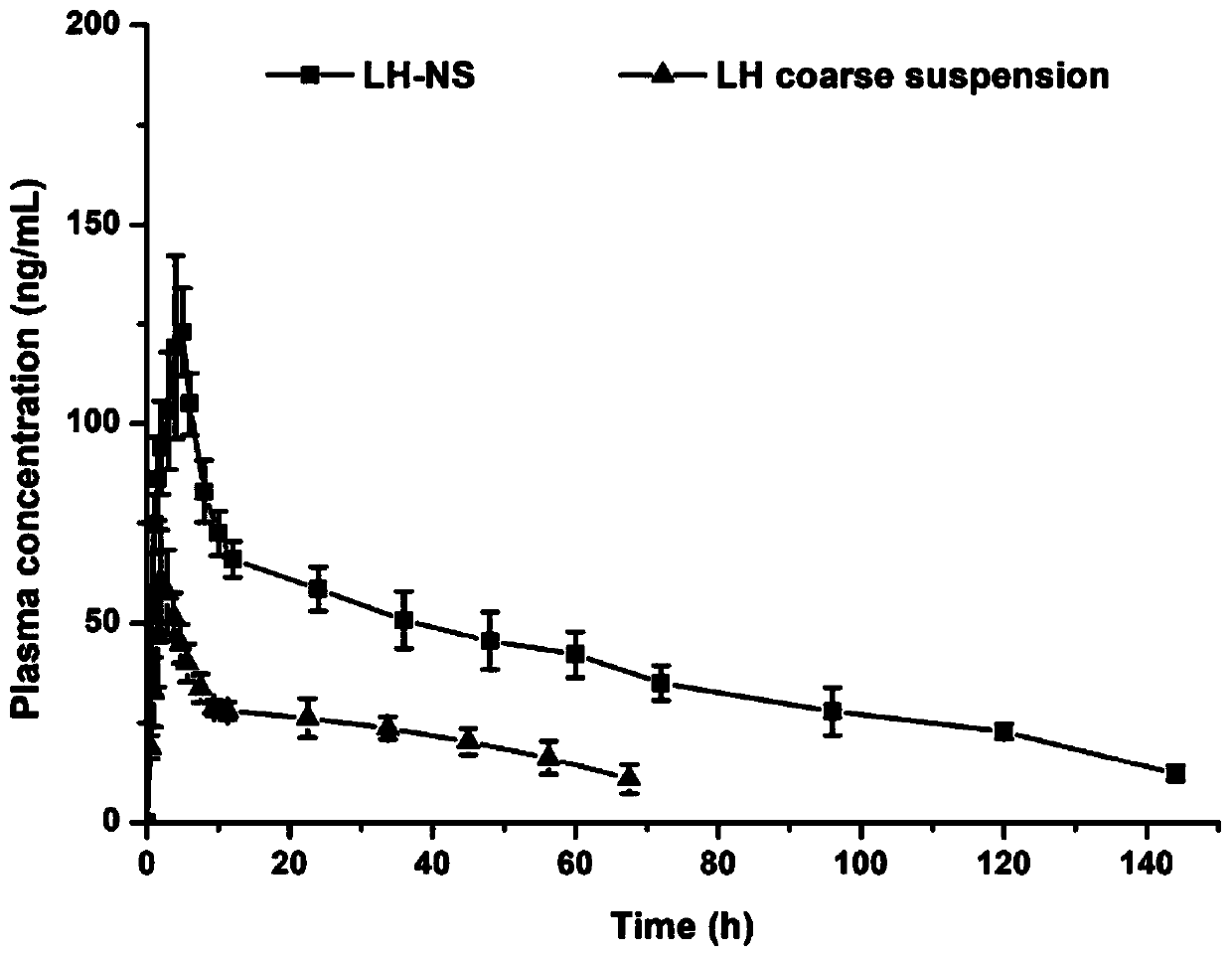
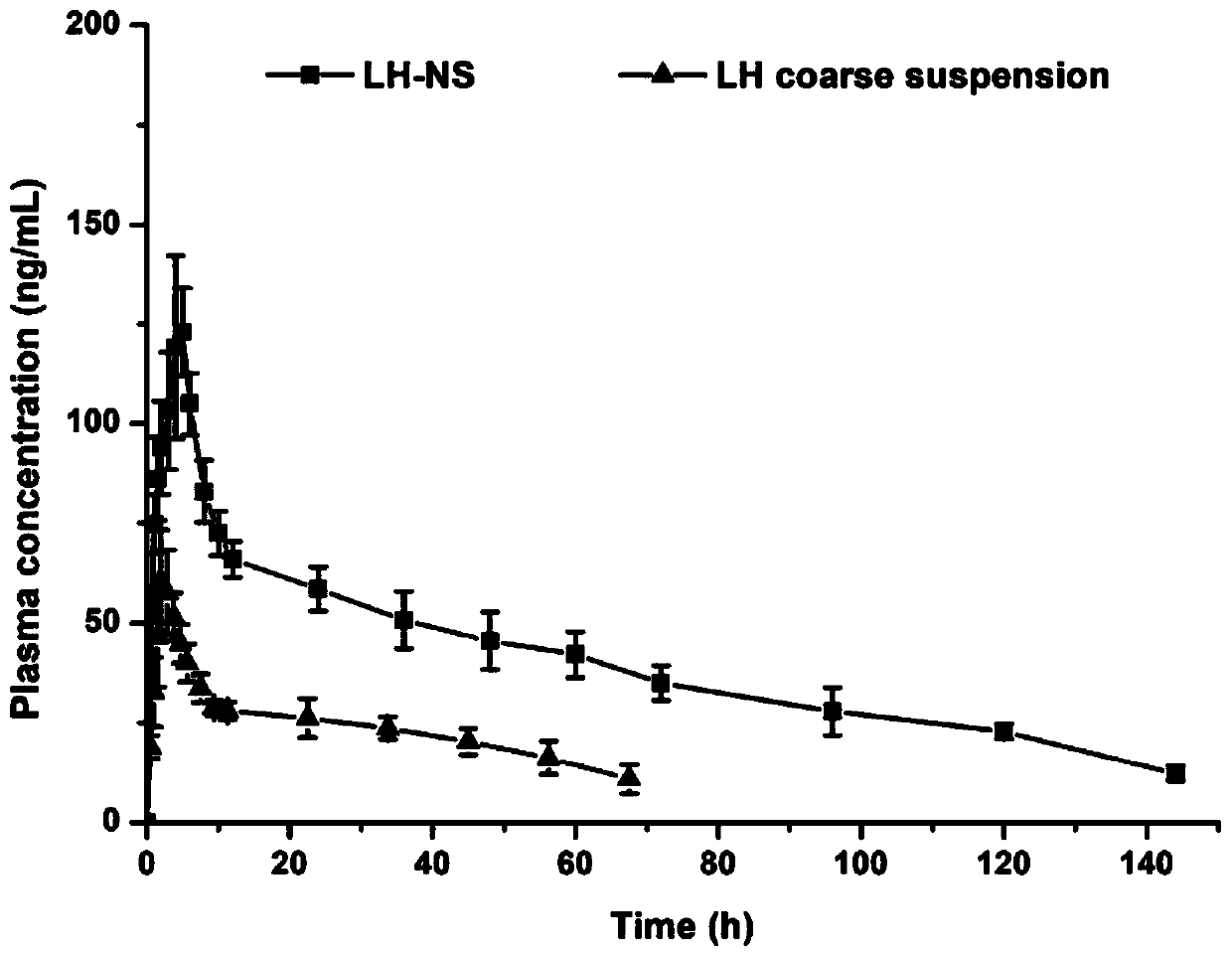
Patents
Literature
80 results about "Plasma drug concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In first order kinetics, fixed fraction of drug is eliminated in unit time. If plasma concentration of a drug is 100 mg and fixed fraction is 10%, after first hour 10 mg will be eliminated, after second hour 9 mg will be eliminated, and so on.
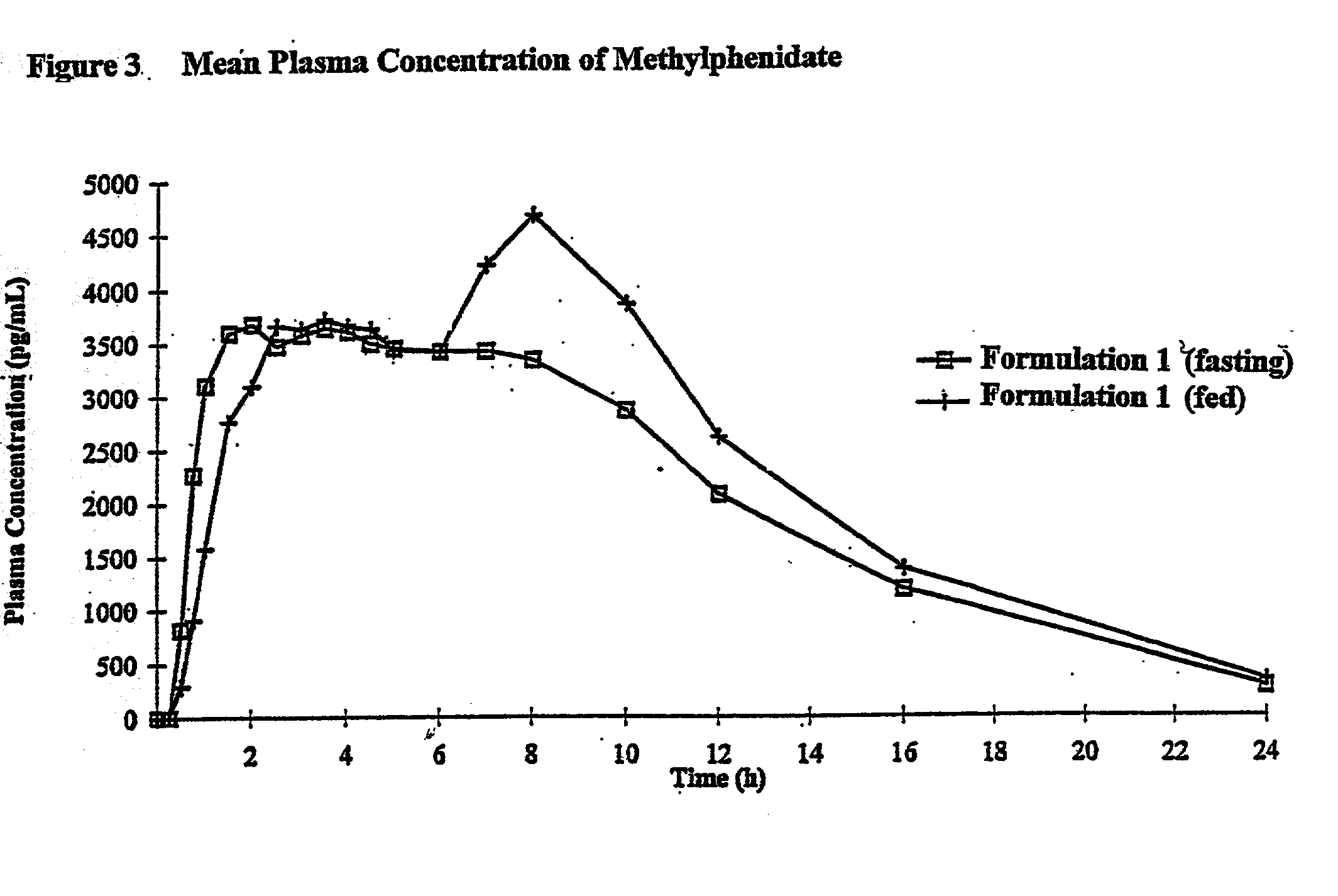
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Isosorbide mononitrate timely quick-release and slow-release preparation
ActiveCN101143140AImprove stabilityEffective plasma concentration stablePharmaceutical non-active ingredientsGranular deliveryControl layerMedicine
The invention discloses a time quick-release and slow-release preparation of isosorbide mononitrate. The technical problem for the invention to solve is to provide a time quick-release and slow-release preparation, which is provided with long time of retaining the effective plasma-drug concentration and a low interval of 5-ISMN plasma-drug concentration. Each structure layer of the invention is orderly arranged from the inside to the outside as a parent nuclear of a pill core, a drug layer of a second dosage, an insulating layer, a slow-release coating layer, a drug layer of a first dosage, an expanding layer and a release controlling layer. The weight percentages of each layer are 15 to 40 percent of the parent nuclear of the pill core, 0.3 to 5.7 percent of the drug layer of the second dosage, 0 to 20 percent of the insulating layer, 5 to 40 percent of the slow-release coating layer, 0.7 to 13.4 percent of the drug layer of the first dosage, 5 to 30 percent of the expanding layer and 5 to 40 percent of the release controlling layer. The invention combines the characteristics of a time quick-release and slow-release medication system to avoid the drug resistance as well as accurately meet the clinical requirement of the angina treatment of the patient of the coronary heart disease.
Owner:SHANGAI PHARMA GRP CO LTD +1
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
The invention discloses a sinomenine or an enteric-coated controlled-release tablet of hydrochloride thereof. The prepared enteric-coated controlled-release tablet hardly releases the drug in artificial simulated gastric juice, but can slowly and smoothly release the drug in artificial simulated intestinal juice; the sustained release time of the drug can achieve more than 12 hours or even 24 hours; the enteric-coated controlled-release tablet is taken once or twice daily, the plasma drug concentration in vivo is smooth, and the peak-valley phenomenon of the plasma drug concentration is reduced; as the prepared enteric-coated controlled-release tablet hardly releases the drug in stomach, the contacted concentration of the drug with the gastric mucosa is small, the stimulation of the stomach caused by the drug is alleviated. As the prepared enteric-coated controlled-release tablet sustainedly slowly releases the drug in intestinal tract, the times of the drug administration are reduced, and the patient compliance is improved, thereby being applicable to the needs of the clinical development.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS20030054033A1Patient compliance is goodOrganic active ingredientsNervous disorderImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Hydrochloric tamsulosin sustained-release capsule and its preparation method
ActiveCN101125134APrecise Controlled ReleaseControl releaseOrganic active ingredientsPharmaceutical delivery mechanismSide effectOral medication
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
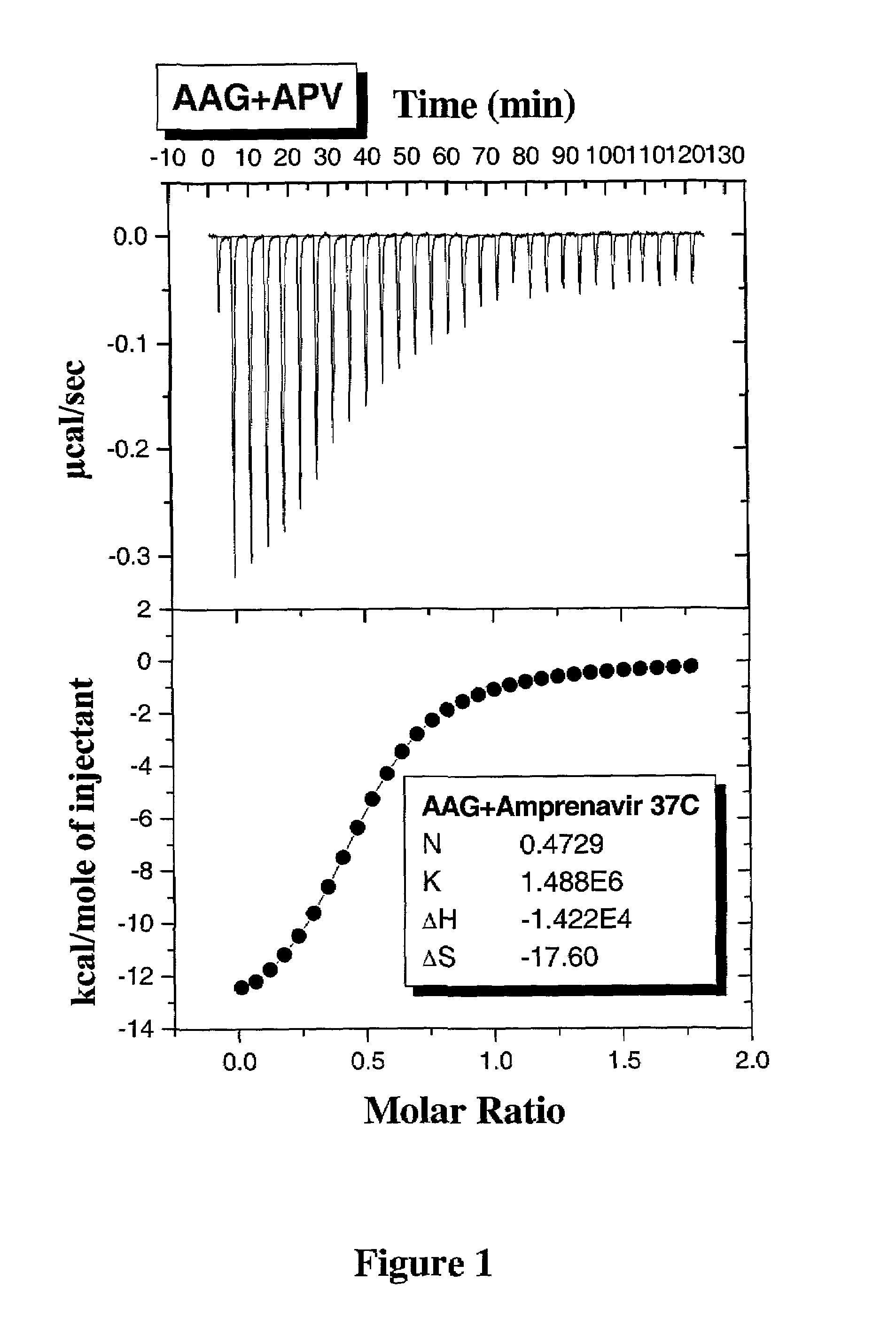
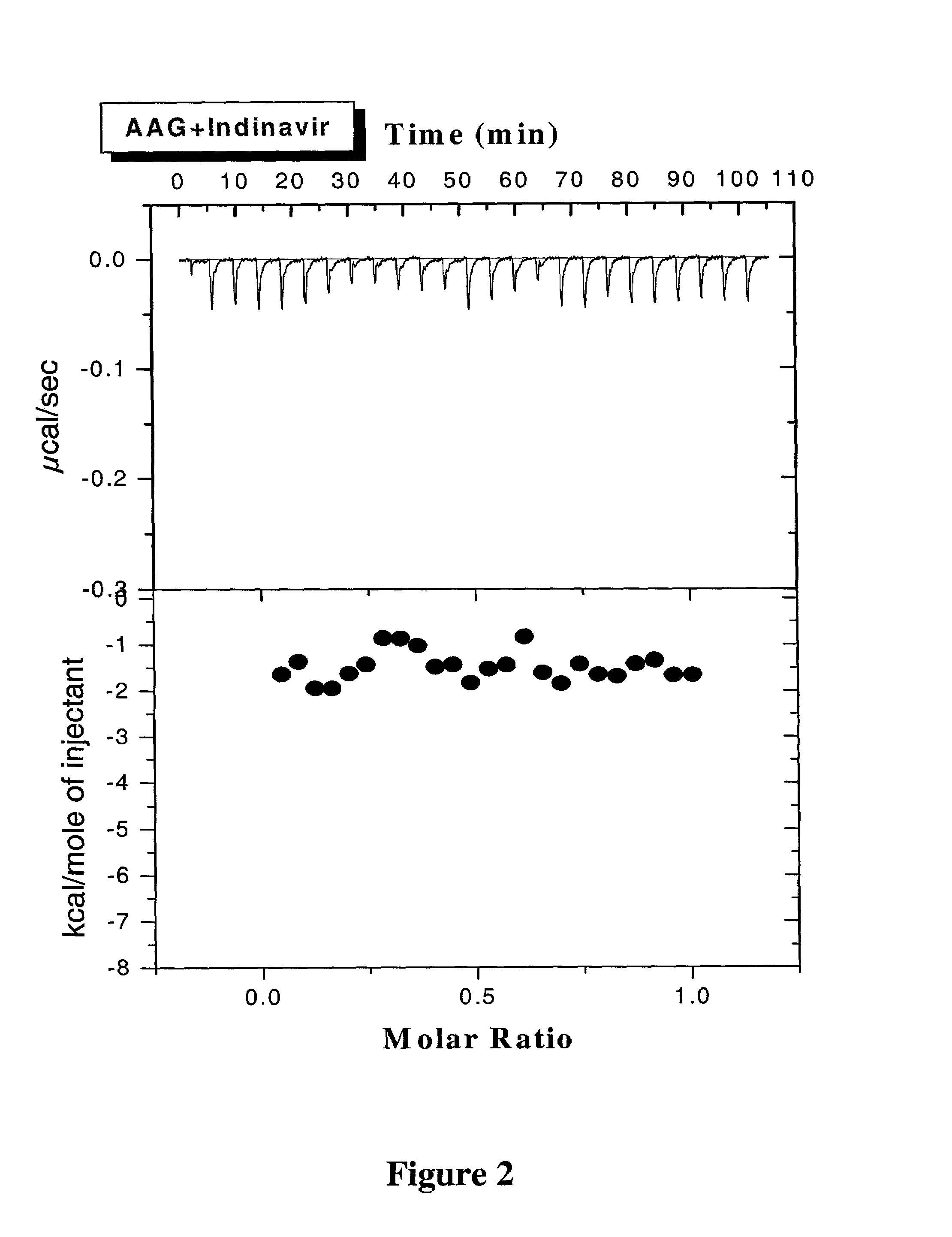
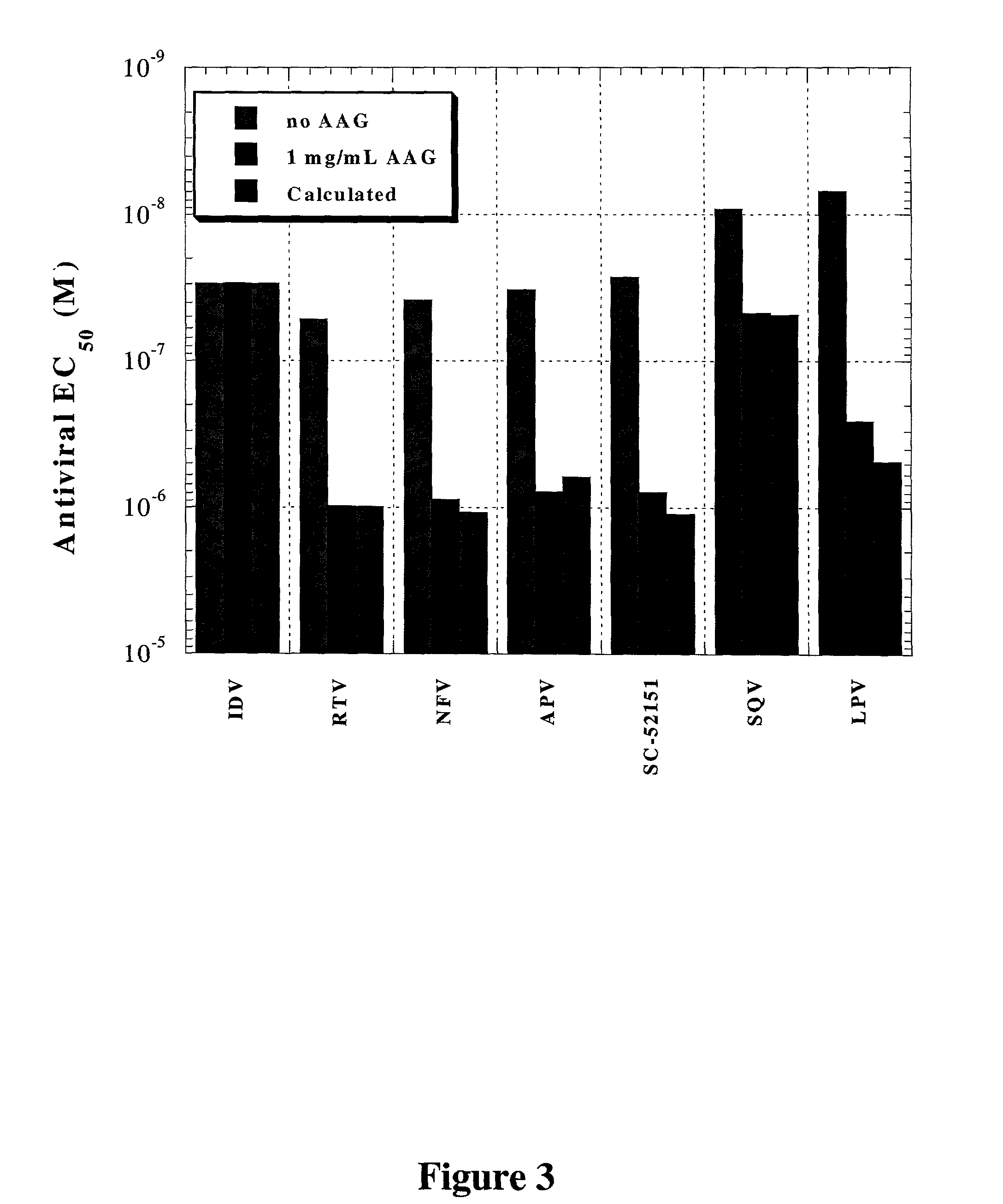
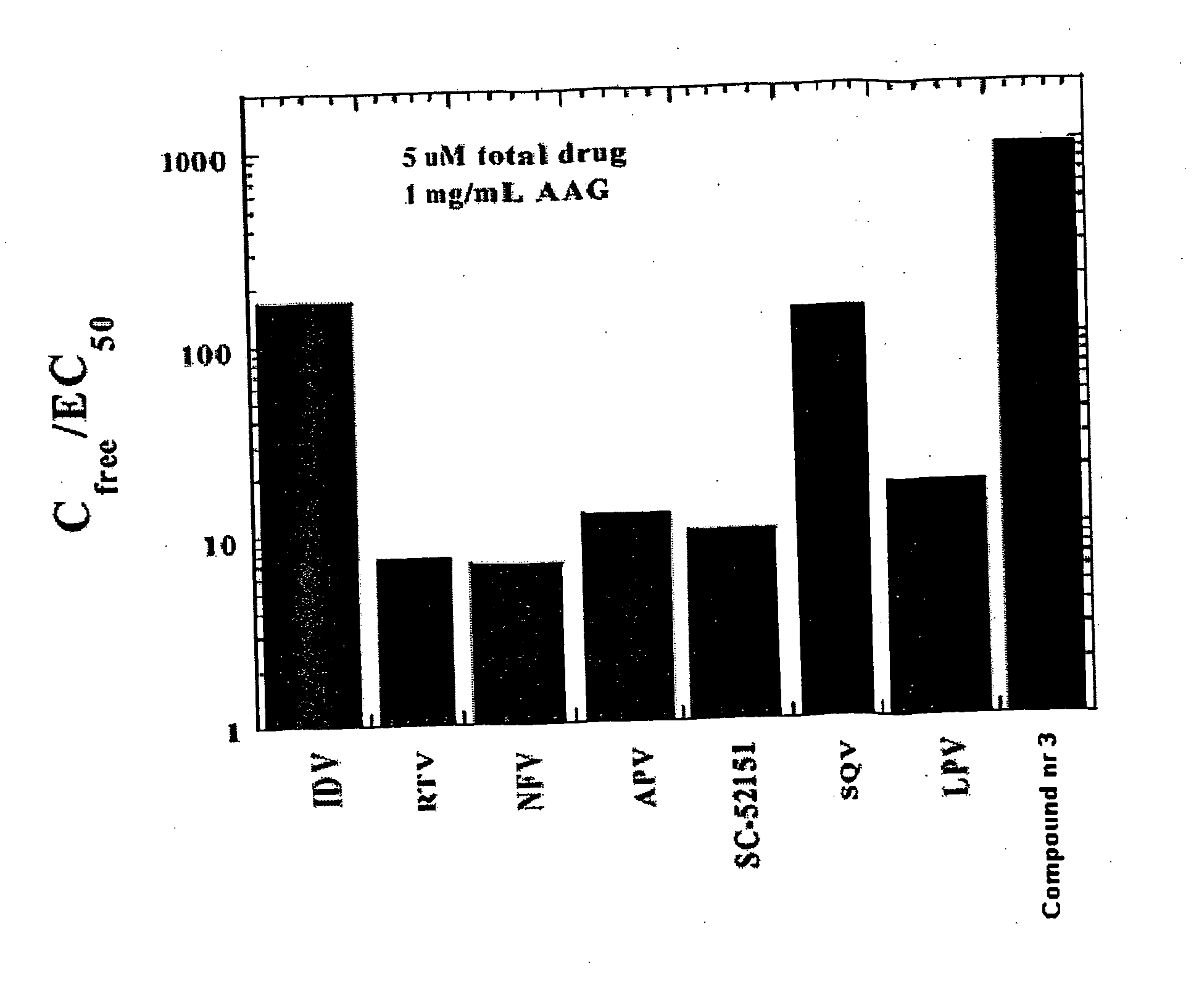
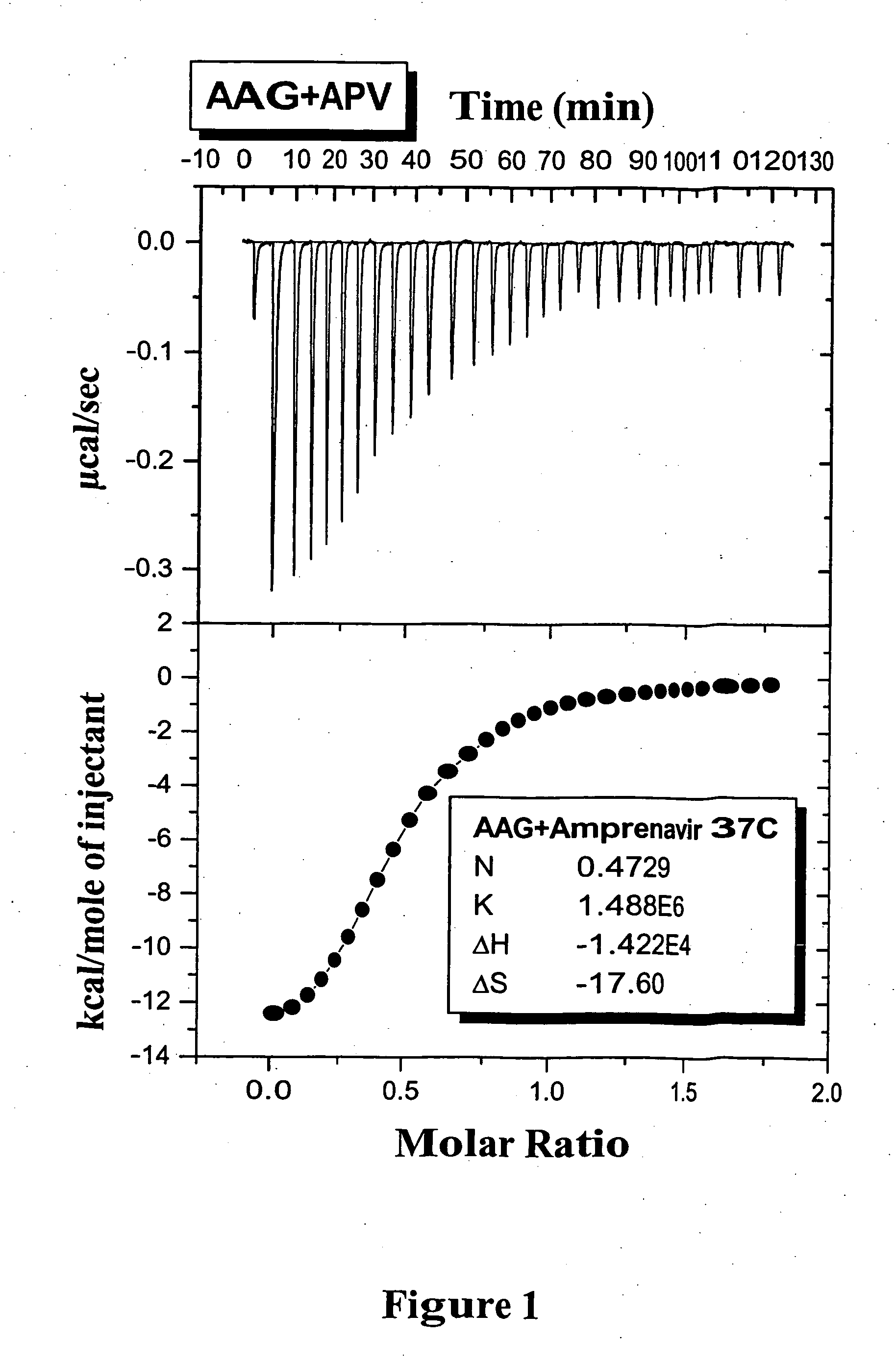
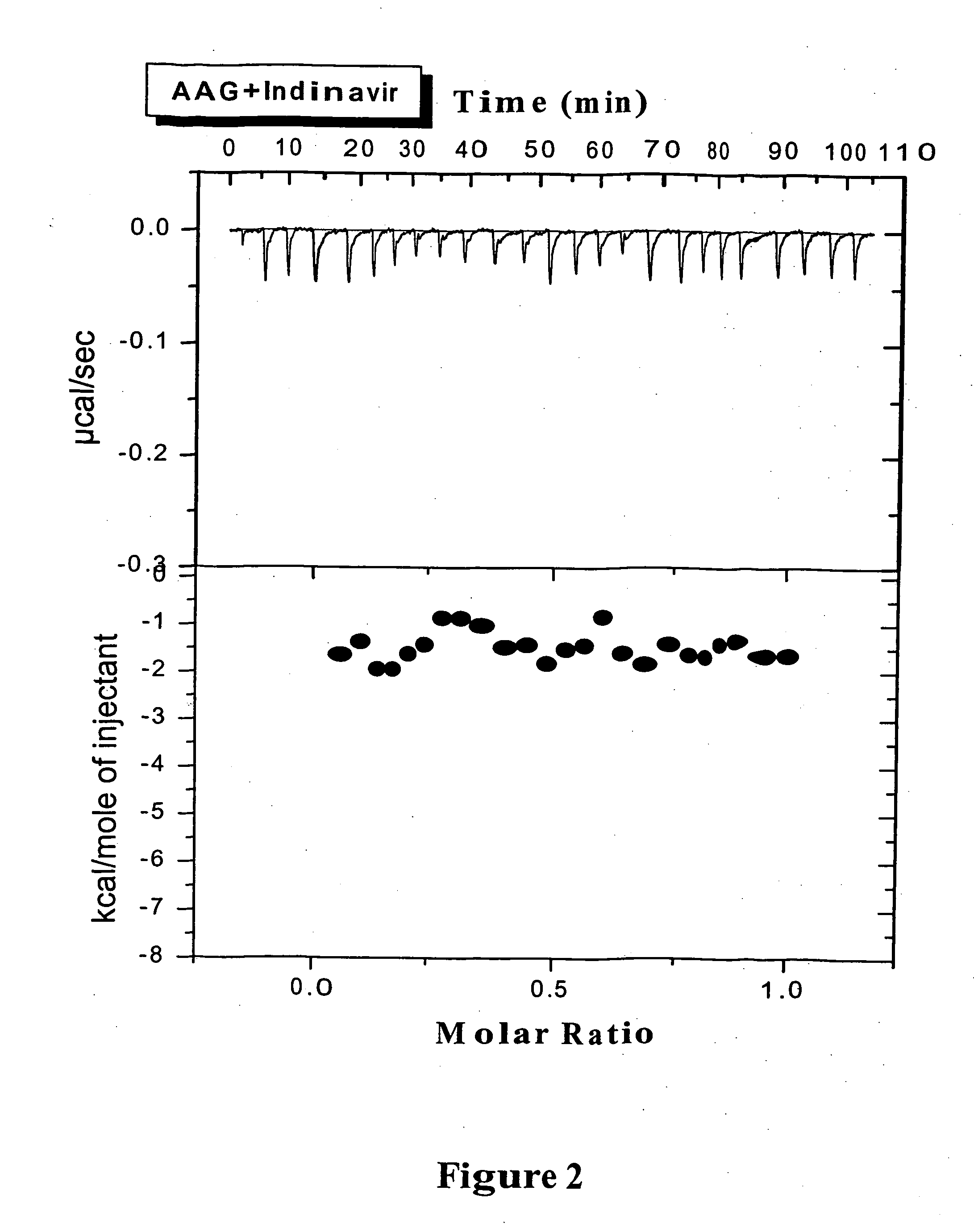
Methods for determining plasma free drug concentration by direct measurement of binding affinity of protease inhibitors to plasma proteins
InactiveUS7087373B2Evaluate the in vivo anti-HIV efficacy of PIsCompound screeningApoptosis detectionRegimenIn vivo
Methods for isothermal titration calorimetry analysis of the binding affinity of protease inhibitors to plasma proteins. A method that can quantitatively calculate free drug concentrations of protease inhibitors in human plasma, as well as a method to calculate therapeutic amounts and dosage regimens. Furthermore, the present invention provides a method that can calculate the effect of plasma proteins on the antiviral activity (EC50 values) of protease inhibitors from their binding affinities to plasma proteins. The present invention provides as well a method that can evaluate the in vivo anti-HIV efficacy of PIs in human plasma.
Owner:TIBOTEC PHARMA
Dexmethylphenidate hydrochloride dual-release preparation and preparation method thereof
InactiveCN101933913ASimple processIncrease productivityOrganic active ingredientsNervous disorderDual releaseSide effect
The invention belongs to the field of medicine, and in particular relates to a dexmethylphenidate hydrochloride dual-release preparation and a preparation method thereof. The preparation of the invention comprises pellets with different release performance, wherein the pellets comprise 10 to 70 percent of quick-response pellets and 20 to 60 percent of enteric-coated pellets based on the total weight of the pellets. The release mode in the preparation can achieve an ideal treatment effect and ensure that the medicament concentration in blood plasma in vivo is maintained as long as 16 to 24 hours. Compared with a dexmethylphenidate hydrochloride common preparation which is taken twice per day, the dual-release preparation can reduce a peak value of the medicament concentration in the blood plasma in vivo after the medicament is taken for the second time, and simultaneously the bioavailability of the medicament is not influenced. The dexmethylphenidate hydrochloride adopted by the invention can effectively reduce toxic and side effects brought by a racemic compound and improve a treatment effect and the medication compliance of a patient.
Owner:孙卫东
All-trans retinoic acid liposome preparation and preparation and application thereof
ActiveCN107753427AHigh drug loadingIn vivo stabilityHydroxy compound active ingredientsMacromolecular non-active ingredientsHalf-lifePlasma drug concentration
The invention belongs to the technical field of biological pharmacy, and particularly relates to an all-trans retinoic acid liposome preparation and a preparation technology thereof. The all-trans retinoic acid liposome preparation comprises all-trans retinoic acid and a liposome vector. According to the method, the all-trans retinoic acid liposome preparation is prepared through an active drug loading method, and an all-trans retinoic acid preparation which is high in drug loading capacity and stable in liposome is formed. The all-trans retinoic acid liposome preparation prepared through themethod greatly improves plasma drug concentration in all-trans retinoic acid liposome, and prolongs the half life.
Owner:SHANGHAI JIAO TONG UNIV
Construction of drug penetration dynamic model based on three-dimensional cell model and application of drug penetration dynamic model to drug evaluation
The invention creatively discloses a drug penetration dynamic evaluation method based on a three-dimensional cell model. The method comprises the steps that (1) a three-dimensional somatic cell model and a "penetration dynamics" mathematic model are established, the penetration behavior of an antitumor drug in somatic cells and an accumulation process in all layers of cells are described semi-quantitatively, and fitting is performed to obtain corresponding dynamic parameters; (2) drug concentrations in all layers of cells at different time points after the drug is given according to different concentrations can be subjected to backward prediction based on the obtained dynamic parameters, and the prediction result is highly consistent with an experience result; (3) biological factors influencing characteristic dynamic parameters are analyzed, the penetration behavior of the drug in tumor tissue is speculated according to a plasma drug concentration time course, and the speculation result is highly consistent with an experiment result; and (4) the model and the evaluation method are applied to a second-phase clinical drug INNO-206, and reasons for superiority of the clinical effect of the drug to that of doxorubicin are expounded from the aspect of in-tumor penetration, so that the universality of the method is further verified.
Owner:CHINA PHARM UNIV
Positively charged water-soluble prodrugs of oxicams and related compounds with very high skin penetration rate
InactiveCN101522692AAvoid side effectsImprove absorption rateSenses disorderNervous disorderSolubilitySide effect
The novel positively charged pro-drugs of oxicams and related compounds in the general formula (1) 'Structure 1' were designed and synthesized. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin approximately 100 times faster than do oxicams and related compounds. It takes 1-2 hours for oxicams and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about approximately 50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the parent drugs in a few minutes. The prodrugs can be used medicinally in treating any oxicams-treatable conditions in humans or animals. Second, the prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of oxicams. Controlled transdermal administration systems of the prodrugs enable oxicams and related compounds to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of oxicams and related compounds. Another great benefit of the transdermal administration of these pro-drugs is that administering medication, especially to children, will be much easier.
Owner:于崇曦 +1
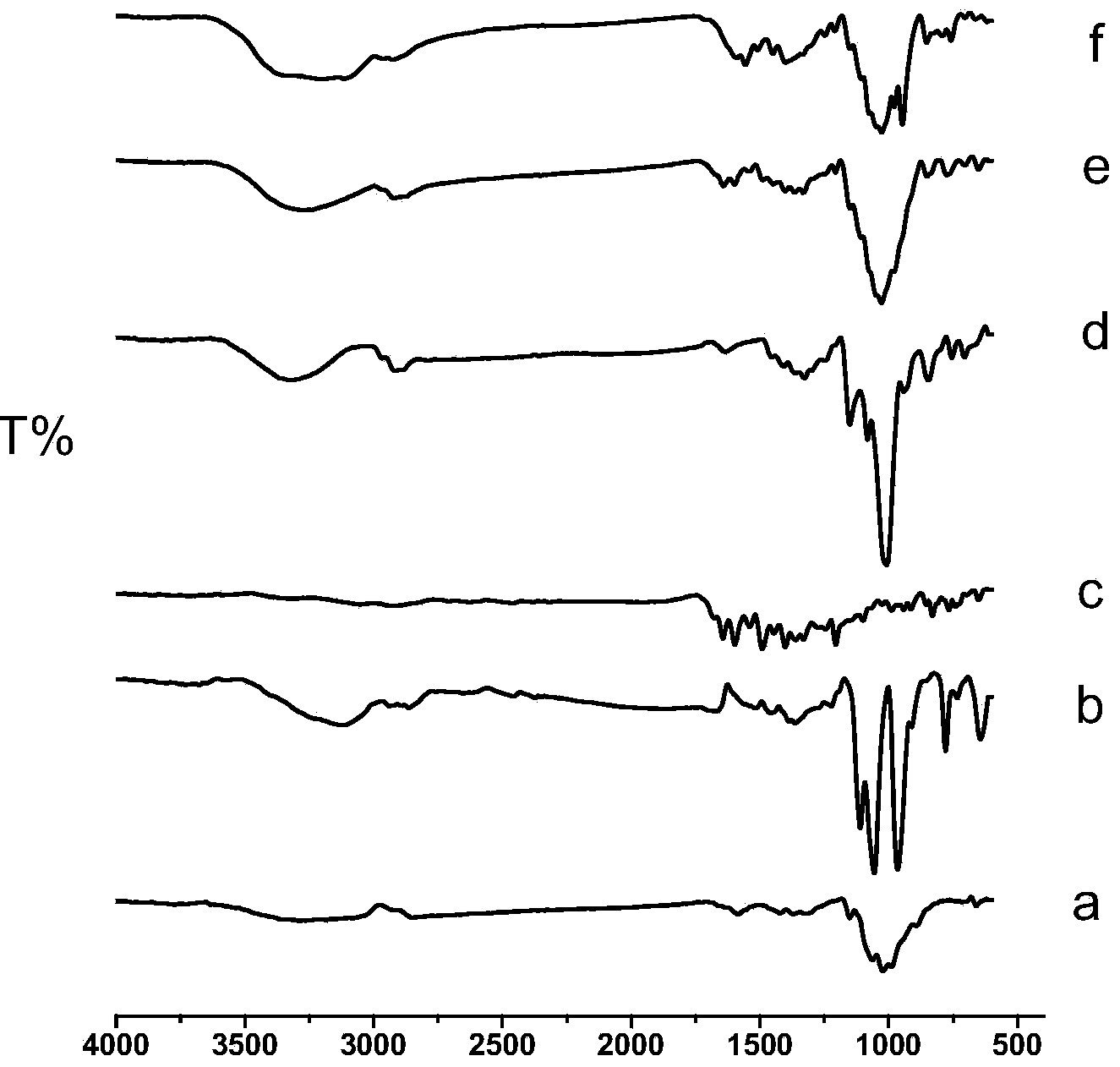
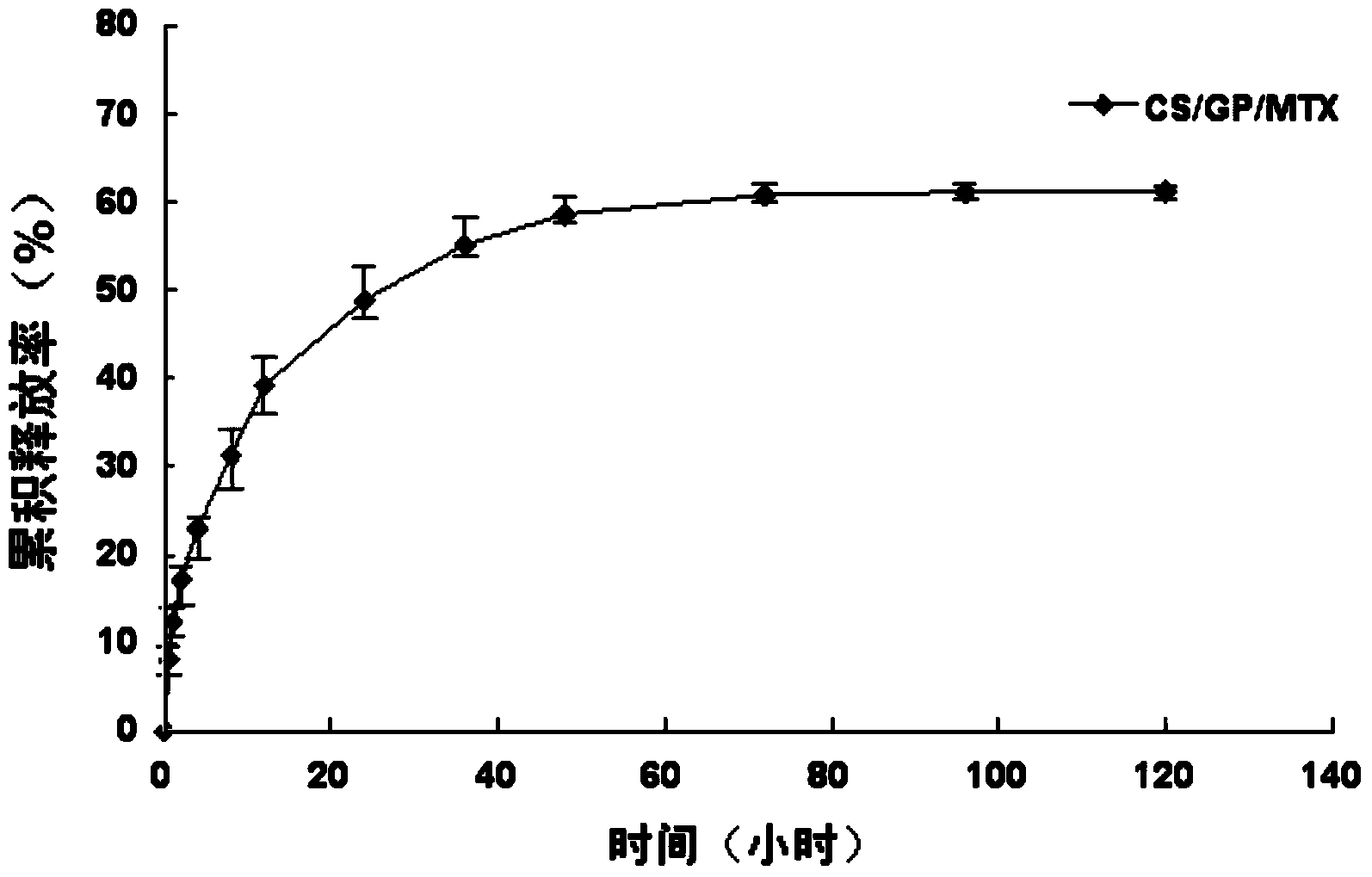
CS/GP/MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel) and application thereof
ActiveCN103735501AStable in natureQuality is easy to controlOrganic active ingredientsAntipyreticPhosphateBeta-glycerophosphate
The invention provides MTX subcutaneous sustained-release injection CS / GP / MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel). Every liter of aqueous solution of the CS / GP / MAX contains 16 to 22 grams of chitosan, 0.05 to 0.1 mole of acetic acid, 70 to 150 grams of beta-glycerophosphate, 0.1 to 0.12 mole of methotrexate, 0.1 to 0.12 mole of a solubilizer and 5 to 50 grams of an isoosmotic adjusting agent and pH modifier. The CS / GP / MAX has stable properties and controllable quality, and the external properties, tgel (the time of gelation), eta, pH value, content and preliminary stability of the CS / GP / MAX meet requirements on thermosensitive hydrogel; 70 percent of drugs of the CS / GP / MAX in a PBS (Phosphate Buffer Solution) are released within 5 days under the condition of 37 DEG C; compared with those of MTX injection, the Cmax (Maximum Plasma Drug Concentration) is reduced by 82 percent, the AUC0-t (area under the plasma concentration time curve from time 0 to t hours) is enlarged by 2.37 times, and the t0.02muM (the length of time the MTX concentration-time curve remained above 0.02 muM in one week) is prolonged by 12.93 times after the CS / GP / MAX is subcutaneously injected to the back of a rabbit; by the CS / GP / MAX, adverse effects can be reduced, and curative effects can be improved.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
Novel isosorbide mononitrate micro-porous osmotic pump controlled-release preparation and preparation method thereof
InactiveCN104644599AReduces carryoverImprove compliancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsTime lagCellulose acetate
The invention relates to a novel isosorbide mononitrate micro-porous osmotic pump controlled-release preparation and a preparation method thereof. The preparation comprises a medicated tablet core, and a semipermeable controlled-release membrane coating the tablet core. The tablet core contains isosorbide mononitrate, general osmotic pressure active substance, delayed-release micro pills permeating the active substance and filler; the semipermeable membrane comprises cellulose acetate and a pore-foaming agent. The invention further relates to a preparation method of the controlled-release preparation. The most important characteristic of the invention is that the delayed-release micro pill having certain time lag and permeating the active substance is added into the table core, so as to supplement osmotic pressure in a pump chamber at late period of release and promote further release of the drug, thus, the pharmaceutics requirement that end release residue in the pump chamber is not more than 5% is fulfilled, meanwhile, zero-order release of drug is guaranteed, plasma drug concentration is steady, frequency of use is reduced for the patient, and safety of medication, effectiveness and patient compliance are all improved.
Owner:CHINA PHARM UNIV
Quantitative analysis method of plasma-drug concentration of novel compound WSJ-557 in SD rat plasma
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Riboflavin gastric-floating tablet and preparation method thereof
ActiveCN104473891AReduce toxic and side effectsImprove medication complianceOrganic active ingredientsMetabolism disorderSodium bicarbonateSide effect
The invention provides a riboflavin gastric-floating tablet and a preparation method thereof. The riboflavin gastric-floating tablet prepared by the method comprises the following raw materials in percentage by weight: 1%-5% of riboflavin, 10%-50% of hydroxypropyl methyl cellulose, 5%-25% of ethylcellulose, 2%-40% of hexadecanol, 5%-40% of lactose, 1%-10% of sodium bicarbonate, 1%-10% of light pharmaceutical calcium carbonate and 1%-10% of magnesium stearate. The riboflavin gastric-floating tablet is low in toxic and side effects of medication, has the functions of prolonging the action time of medicines, and maintaining stable plasma drug concentration; and the bioavailability of the medicine is improved.
Owner:CHANGSHA BAISHUN BIOTECH
Simvastatin apellagrin sustained-release capsule and method of producing the same
InactiveCN101428008ASmall toxicityReduce the number of dosesOrganic active ingredientsMetabolism disorderDyslipidemiaSide effect
The invention provides a compound simvastatin niacin sustained-release capsule and a preparation method thereof. In the compound sustained-release capsule preparation, each capsule comprises 200 to 500 mg of niacin and 20 mg of simvastatin. The compound sustained-release capsule is prepared by the following steps: preparing blank pills, preparing niacin sustained-release pills; preparing simvastatin sustained-release pills, and preparing capsules. The product is suitable for treating primary hypercholesterolemia and mixed dyslipidemia; according to the characteristics, the niacin part is used as the sustained-release part and the simvastatin is used as the immediate-release part to produce the compound preparation, so that all the components exert synergism in the body, exert the efficacy to the largest extent, reduce the toxic and side effects of the drug, reduce the fluctuation of the plasma drug concentration in the body and reduce the drug taking times of the patient; the efficacy on relieving hypercholesterolemia of the product is obviously superior to that of any single prescription preparation.
Owner:BEIJING HONGYANG HENGFENG MEDICINE TECH
Vortioxetine sustained-release capsules and preparation method of vortioxetine sustained-release capsules
InactiveCN106727438AGood compatibilityPrevent infiltrationOrganic active ingredientsNervous disorderSide effectSucrose
The invention discloses vortioxetine sustained-release capsules as well as a preparation method and application of the vortioxetine sustained-release capsules. The vortioxetine sustained-release capsules are prepared by loading vortioxetine sustained-release micro-pills into hollow capsules. Each vortioxetine sustained-release micro-pill is composed of a medicine-carrying pill core, an isolation type coating layer, a sustained-release coating layer and a protective coating layer from inside to outside, wherein the medicine-carrying pill core is composed of vortioxetine, an adhesive and a sucrose hollow pill core, and the specific weight percent is as follows: 0.1 percent to 10 percent of the vortioxetine, 10 percent to 30 percent of the adhesive and 10 percent to 60 percent of the sucrose hollow pill core. A sustained-release preparation can be used for prolonging the holding time of treatment concentration of the vortioxetine in a body to 12h and reducing the side effect caused by the fact that the fluctuation of plasma drug concentration is too great; meanwhile, the number of times of orally taking drug is reduced, and the vortioxetine sustained-release capsules have remarkable effect on patients with depressive disorder, who need to be treated, and are convenient to use.
Owner:佛山市弘泰药物研发有限公司
Piribedil sustained-release tablet and preparation method thereof
ActiveCN103006606AFacilitated releaseImprove stabilityOrganic active ingredientsNervous disorderSustained Release TabletClinical efficacy
The invention provides a piribedil sustained-release tablet and a preparation method thereof. The piribedil sustained-release tablet comprises the following raw material components in percent by weight: 10-80% of piribedil, 1-70% of framework material, 10-80% of filler, 0.1-5% of lubricating agent and an appropriate amount of adhesive. The piribedil sustained-release tablet provided by the invention has the advantages that releasing rate of piribedil is increased, drug stability is improved, the defects that a drug therapeutic effect is reduced owning to fluctuation of plasma drug concentration is overcome, stable plasma drug concentration is maintained for a long time, motion complication caused by a dopamine receptor stimulated by fluctuation of drug concentration is avoided, the requirement of a continuous activated dopamine receptor is met, compliance of a patient is improved and a clinical effect of the piribedil sustained-release tablet is guaranteed.
Owner:KANGYA OF NINGXIA PHARMA
Ticagrelor slow-release preparation as well as preparation and application thereof
InactiveCN109806261AGood treatment effectImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPatient compliance
The invention belongs to the technical field of ticagrelor and in particular relates to a ticagrelor slow-release preparation as well as preparation and application thereof. The ticagrelor slow-release preparation provided by the invention comprises at least one rapid release layer and at least one slow release layer, wherein each of the rapid release layer and the slow release layer contains theticagrelor; the mass ratio of the content of the ticagrelor in the rapid release layer to the content of the ticagrelor in the slow release layer is 1 to (3 to 7); the ticagrelor slow-release preparation is used as a medicine for anti-platelet treatment; on one hand, the preparation has reasonable plasma drug peak concentration; on the other hand, the residual ticagrelor is slowly released and stable plasma drug concentration is maintained; the anti-platelet treatment effect is good and the bleeding tendency is reduced; the preparation is orally taken once each day and has good patient compliance and high utilization safety; the preparation method of the ticagrelor slow-release preparation has the advantages of simple technology, relatively low cost and good practicability.
Owner:梁江丽
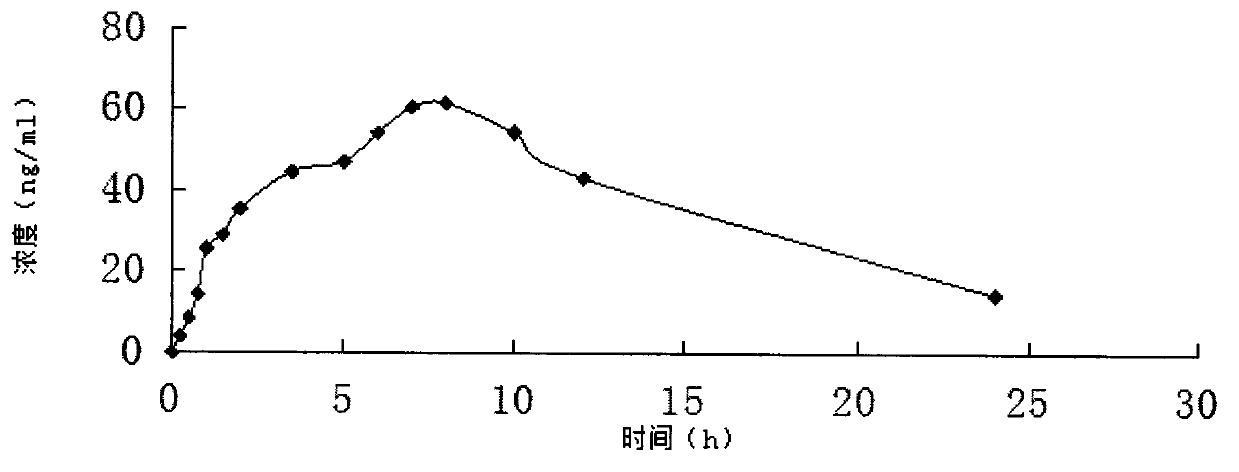
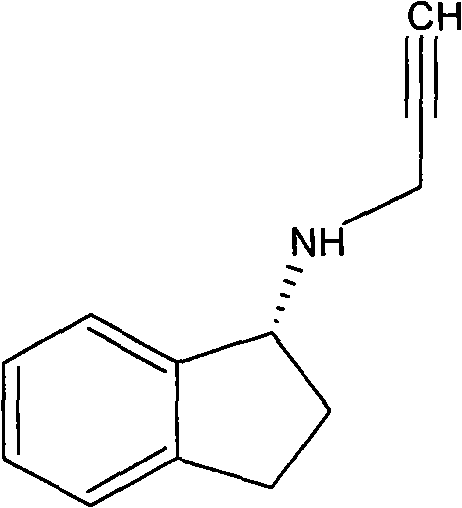
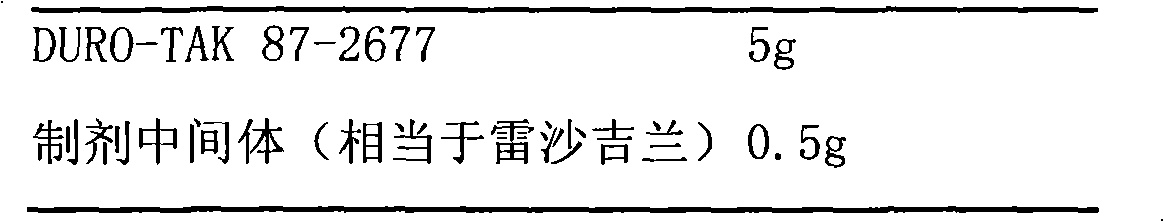
Slow release long-acting rasagiline transdermal patch with high bioavailability and preparation method thereof
InactiveCN102100682AStable absorptionAvoid the first pass effectOrganic active ingredientsNervous disorderTransdermal patchAcrylic polymer
The invention relates to a slow release long-acting rasagiline transdermal patch with high bioavailability and a preparation method thereof. The patch is characterized in that effective amount of rasagiline and a substrate are contained, wherein the concentration of rasagiline is in the range of 0.1 mg / cm2 - 2 mg / cm2, every patch contains 1 mg - 10 mg of rasagiline, and the substrate contains one or more than two kinds of the following substances: acrylic polymers containing no carboxylic group and silicone polymers with silanol groups capped by alkyls. The time of maximum plasma drug concentration of the patch is prolonged to be 5 times or more that of oral tablets, and the bioavailability of the patch is 130 percent or more of that of oral tablets. The patch in the invention can be applied to effectively inhibit monoamine oxidase B (MAOB) for at least 3 days, in addition, the preparation method is simple and suitable for industrial production.
Owner:CHONGQING PHARMA RES INST
Lithium carbonate sustained release tablet
InactiveCN108721238ALow therapeutic indexReduce adverse reactionsNervous disorderInorganic active ingredientsSustained Release TabletFoaming agent
The invention discloses a lithium carbonate sustained release tablet. The sustained release tablet is prepared from lithium carbonate, a sustained-release material and other ingredients. The sustained-release material is a hydrophilic gel skeleton material or an erodible matrix material, wherein the other ingredients comprise an adhesive, a pore-foaming agent, a surfactant, a lubricant, a flow aid, filler, a disintegrant and a wetting agent. The lithium carbonate sustained release tablet has the advantages that administration times are reduced, the peak and valley phenomenon of plasma-drug concentration is avoided or reduced, a stable and lasting effective plasma-drug concentration is provided and the safety and the effectiveness of a drug are improved.
Owner:上海葆隆生物科技有限公司
Hemsleyadin sustained-release preparation
ActiveCN101244068AReduce releaseReduce peak and valley absorptionAntibacterial agentsOrganic active ingredientsWater insolubleAntibiotic Y
The invention relates to a special sustained-release preparation of natural antibiotics curcurbitacin, in particular to a membrane controlled or / and matrix release oral preparation with the composition as follows: curcurbitacin 5 to 20%, water insoluble medical macromolecular material 30 to 65%, water soluble medical macromolecular material 5 to 25%, and conventional auxiliary material 15 to 45%. The special sustained-release preparation of natural antibiotics curcurbitacin has the advantages of retarding the release of curcurbitacin from drug preparation, reducing the peak and peak valley for drug absorption, facilitating stable plasma drug concentration, and especially facilitating the disease treatment when the sustained release is combined with rapid release since the rapid release part can be rapidly released to produce the plasma drug concentration required for treatment as soon as possible, while the sustained release part can subsequently provide stable plasma drug concentration and reduce the fluctuation of plasma drug concentration.
Owner:云南蓝绿康药业有限公司
Sustained-release agent of nitric acid dinitrate and preparation method thereof
ActiveCN101181259AReduce the frequency of takingEasy to takePharmaceutical delivery mechanismPharmaceutical non-active ingredientsDiseaseCarmellose Sodium
An isosorbide dinitrate sustained-release preparation is a tablet which consists of isosorbide dinitrate, sustained-release materials and other pharmaceutical excipients, wherein, the sustained-release materials are selected from hypromellose (E4M) and carmellose sodium. As the drug is released in digestive tract according to the procedure after the oral administration, the effective plasma drug concentration in human body can be maintained stably and sustainedly, therefore the invention has the advantages of good healing efficacy, long drug effect, less side effects, small number of the times of the administration of the patients, convenient usage and so on; the invention not only has the function of treating and alleviating angina symptoms, but also has the special advantages of preventing sudden onset and protecting the daily lives of the patients and safely passing through the incidence peak of the diseases, therefore, the invention provides reliable means and great convenience for the treatment of the patients and the self-prevention of sudden onset; the price of the drug is cheap, which is conductive to the administration of ordinary patients.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Solvent of artesunate for intramuscular injection
InactiveCN108309932AEasy to operateImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityDrug administration
The invention discloses a solvent of artesunate for intramuscular injection, and particularly relates to a solvent of artesunate for intramuscular injection, which has a slow release function and is used by animals. The solvent is prepared from the following components in percentage by volume: 20% of 1,2-propanediol, 15% of ethanolamine, 15% of polyvinylpyrrolidone-K15, and the balance of sterilewater for injection. The solvent can realize high solubility and safety of artesunate well, has the advantage of slow release at the same time, can maintain effective plasma drug concentration of a drug for a long time in an organism, and reduces the times of drug administration; and compared with oral administration and intravenous injection, the intramuscular injection is easier to operate in veterinary clinical practice.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Positively charged water-soluble prodrugs of n-arylanthranilic acids with very fast skin penetration rate
ActiveCN101506168AGood absorption rateGood curative effectOrganic compound preparationAntipyreticSolubilityFlufenamic acid
The novel positively charged pro-drugs of arylanthranilic acids in the general formula (1) 'Structure 1' were designed and synthesized. The compounds of the general formula (1) 'Structure 1' indicated above can be prepared from mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds, by reaction with suitable alcohols, thiols, or amines and coupling reagents, such as N, N'-Dicyclohexylcarbodiimide, N, N'-Diisopropylcarbodiimide, O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate, O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate, Benzotriazol-1-yl-oxy-tris (dimethylamino)phosphonium hexafluorophosphate, et al. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin ~200 times faster than does mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds. It takes 2-4 hours for mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about ~50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the parent drugs in a few minutes. The prodrugs can be used medicinally in treating any NSAIAs-treatable conditions in humans or animals. The prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and thus avoid most of the side effects of NSAIAs, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis.
Owner:TECHFIELDS BIOCHEM CO LTD
Methods for determining plasma free drug concentration by direct measurement of binding affinity of protease inhibitors to plasma proteins
InactiveUS20060205002A1Evaluate the in vivo anti-HIV efficacy of PIsWell formedCompound screeningApoptosis detectionRegimenIn vivo
Owner:TIBOTEC PHARMA
Uncaria slow-release pill preparation and preparation method thereof
InactiveCN101884683AIncrease profitReduce fluctuations in concentrationPill deliveryMacromolecular non-active ingredientsSide effectMedical expenses
The invention discloses an uncaria slow-release pill preparation and a preparation method thereof. The uncaria slow-release pill preparation is prepared from 10-40 parts by weight of uncarine or rhomotoxine and 50-90 parts by weight of substrates, wherein the substrates comprise 40-60 parts by weight of hydrophilic substrates and 10-30 parts by weight of hydrophobic substrates. In the invention, the uncaria slow-release pill preparation is slowly released by a slow-release technique so that the fluctuation of plasma drug concentration can be effectively reduced, the phenomenon of 'peak valley' generated from frequent administration of the common preparation can be avoided, the utilization rate of the drug is improved, and medical expenses can be reduced; the uncaria slow-release pill preparation reduces the direct stimulation of the drug to gastrointestinal tracts to reduce the generation of side effects of the drugs and be easier for patients to tolerate, is beneficial to long-term treatment and is more beneficial to the treatment of hypertension. In addition, the process is simplified so that the uncaria slow-release pill preparation process is easy to operate, the uncaria slow-release pill preparation is easy to carry and take, the problems existing in the prior art are solved and the purpose of the invention is achieved.
Owner:GUIZHOU BAIHUA PHARMA
ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting methotrexate and application thereof
InactiveCN107219357ASmall standard deviationChromatography mass spectrometry is easyMaterial analysisSerum igeElisa kit
The invention provides an ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting methotrexate and application thereof. The kit is prepared from the following reagents: a methotrexate coated 96-pore microporous plate, a methotrexate monoclonal antibody, an antibody diluting solution, an HRP (Hypothalamic Regulatory Peptide)-labeled goat anti-mouse secondary antibody, a methotrexate standard product, a washing liquid concentrated solution, a sample diluting solution, a TMB (Tetramethylbenzidine) color-developing solution and a stopping solution. The stable, rapid and simple methotrexate detection kit is obtained through improving the combination of all reagents and improvement on parameters of the reagents; when the ELISA kit is applied, the standard deviation value of the kit is relatively small. The ELISA kit can be used for detecting the plasma drug concentration of patients who utilize the methotrexate with a large dosage and detecting the plasma drug concentration of patients who utilize the methotrexate with a small dosage.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Methylphenidate hydrochloride sustained-release floating micropill in stomach and its preparation method
InactiveCN101288653AReduce entryAvoid damageNervous disorderCapsule deliveryMethylphenidate HydrochlorideSustained release pellets
The invention relates to the technical field of medicine, which discloses an anti-ADHD drug methylphenidate hydrochloride gastric floating sustained-release pellet and a preparation method thereof. The gastric floating sustained-release pellet is composed of a pellet core, a drug layer, an isolation layer and a sustained-release layer, the density thereof is less than 1g / cm<3>, which can be floated in the stomach for the sustained-release of methylphenidate hydrochloride, the pellet can be prepared into a gastric floating sustained-release capsule by being filled in a capsule; compared with the ordinary preparation, the gastric floating sustained-release pellet or the gastric floating sustained-release capsule reduces the times of drug administration, leads the methylphenidate hydrochloride to maintain the comparatively stable plasma-drug concentration in vivo, ensures the long effectiveness of the methylphenidate hydrochloride, relatively reduces the side effects of the methylphenidate hydrochloride compared with the conventional sustained-release preparation, improve the bioavailability and reduces the dosage. The gastric floating sustained-release pellet adopts the conventional process for preparation, which is easy to realize the industrial production.
Owner:石家庄蒎格医药科技有限公司
Lurasidone hydrochloride nano mixed suspension solution for durable intramuscular injection and preparation method of mixed suspension solution
InactiveCN109998991AImprove solubilityImprove dissolution rateOrganic active ingredientsNervous disorderLurasidone HydrochlorideIntramuscular injection
The invention discloses a lurasidone hydrochloride nano mixed suspension solution for durable intramuscular injection and a preparation method of the nano mixed suspension solution. The raw materialsof the nano mixed suspension solution comprise 0.1-0.5 wt.% of lurasidone hydrochloride and 0.1-0.3 wt.% of a stabilizer, and a solvent is water for injection. Through high-speed dispersion, in combination with a wet-method medium grinding method, the lurasidone hydrochloride is prepared into the nano mixed suspension solution for intramuscular injection, the first-pass effect is avoided, and thesolubility and dissolution rate of the medicine after muscle injection are improved. After muscle injection, the effect can be achieved continuously for 3-7 days, the drug administration frequency isreduced, fluctuation of the plasma drug concentration is reduced, and the compliance and safety of a patient during medicine taking are improved.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com