Lurasidone hydrochloride nano mixed suspension solution for durable intramuscular injection and preparation method of mixed suspension solution
A technology of lurasidone hydrochloride and nanosuspension is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas and the like, can solve problems such as low solubility, and achieves simple preparation process, Ease of industrialization, effect of increasing solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
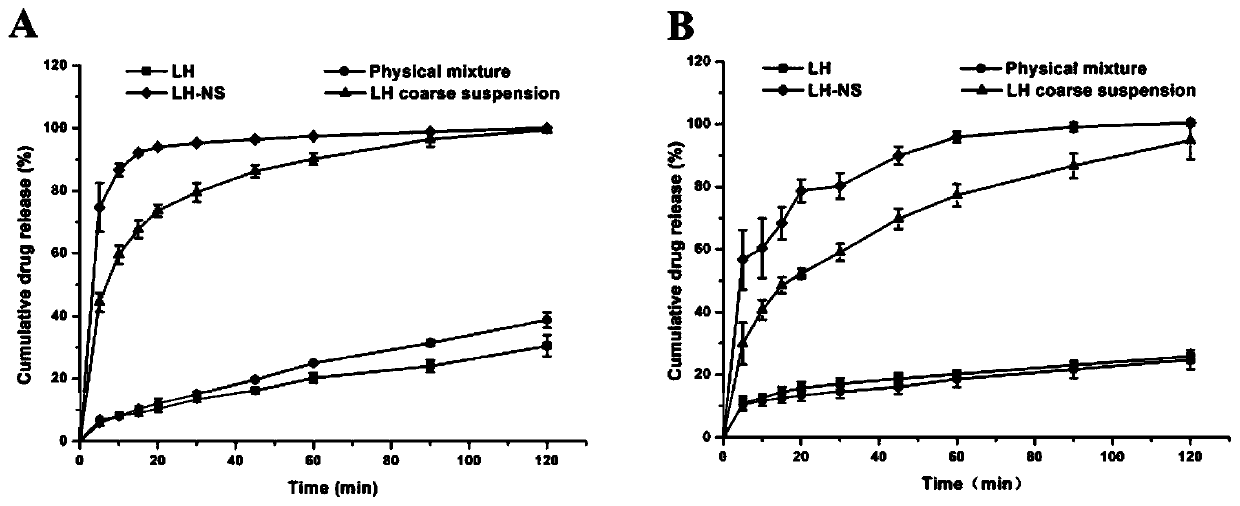
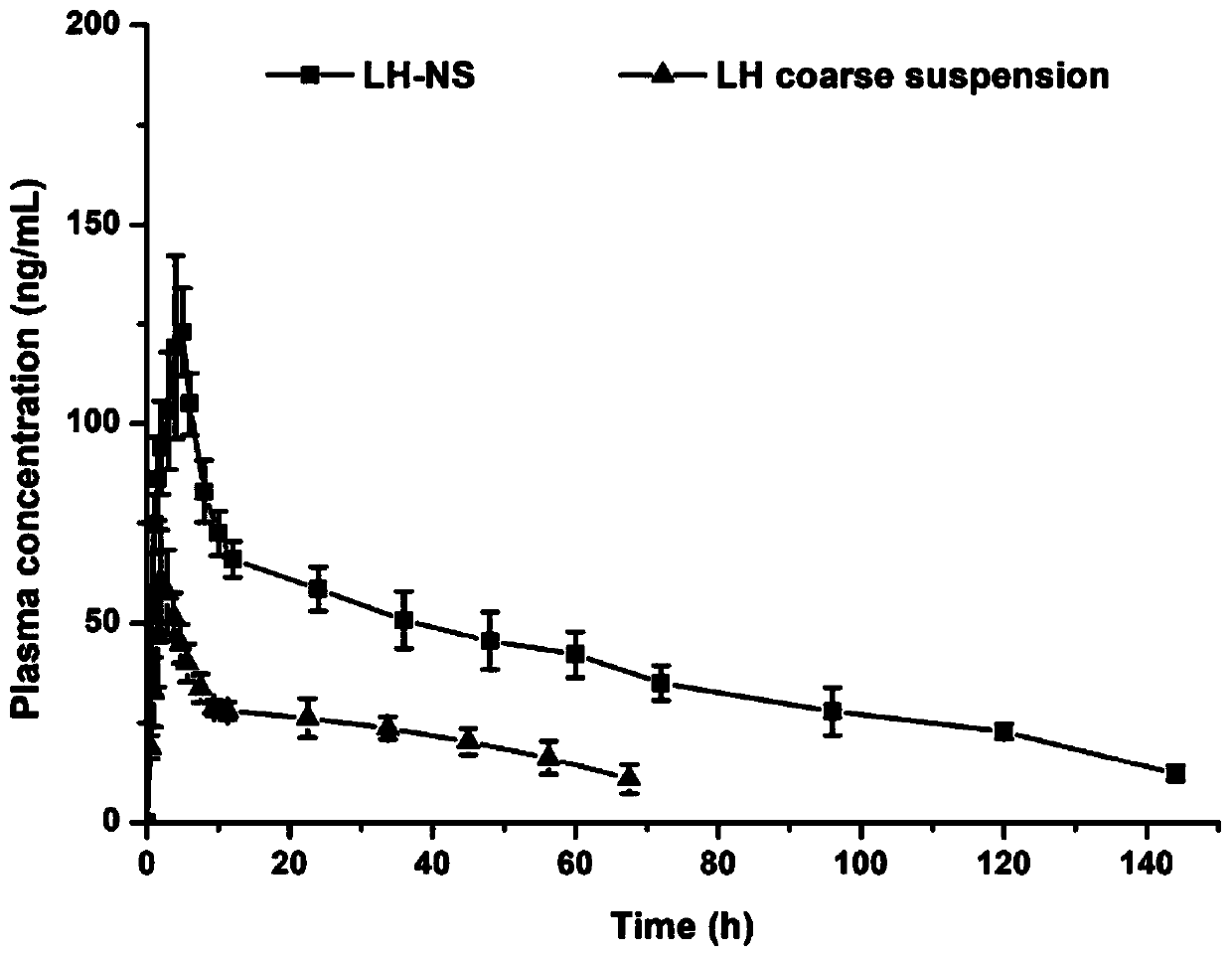
[0040] Weigh 0.1% (w / v) Tween 20 and place it in isotonic water for injection with pH 7.0, dissolve it ultrasonically to obtain a stabilizer solution, then weigh 0.1% (w / v) lurasidone hydrochloride and disperse it in the above stabilizer In the solution, disperse at a high speed of 9000 rpm for 5 minutes to obtain a coarse suspension of lurasidone hydrochloride. Then, the lurasidone hydrochloride nanosuspension can be prepared by stirring for 6 hours under the condition of rotating speed of 1200 r / min by wet media grinding method. Detected with a laser particle size analyzer, the average particle diameter is 460.6nm, and the polydispersity index is 0.365.
Embodiment 2
[0042] Weigh 0.1% (w / v) polyethylene glycol 4000 and place it in isotonic water for injection with pH 7.0, dissolve it ultrasonically to obtain a stabilizer solution, then weigh 0.15% (w / v) lurasidone hydrochloride and disperse it in the above In the stabilizer solution, disperse at a high speed of 9000 rpm for 5 minutes to obtain a coarse suspension of lurasidone hydrochloride. Then, the lurasidone hydrochloride nanosuspension can be prepared by stirring for 8 hours under the condition of rotating speed of 900 r / min by wet media grinding method. Detected with a laser particle size analyzer, the average particle diameter is 384.9nm, and the polydispersity index is 0.274.
Embodiment 3
[0044] Weigh 0.07% (w / v) polyethylene glycol 4000 and 0.04% poloxamer 188 in the isotonic water for injection of pH 7.0, ultrasonically dissolve to obtain a stabilizer solution, and then weigh 0.15% (w / v ) Lurasidone hydrochloride is dispersed in the above stabilizer solution, and dispersed at a high speed of 9000rpm for 5 minutes to obtain a coarse suspension of lurasidone hydrochloride. Then adopt the wet media grinding method and stir at 900r / min for 6h to prepare the lurasidone hydrochloride nanosuspension. Detected with a laser particle size analyzer, the average particle diameter is 351.5nm, and the polydispersity index is 0.237.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com