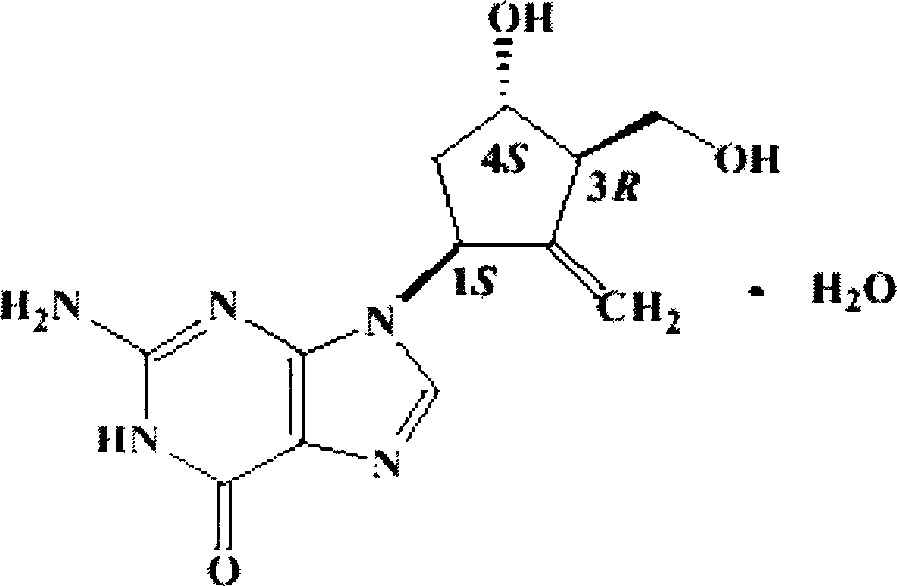
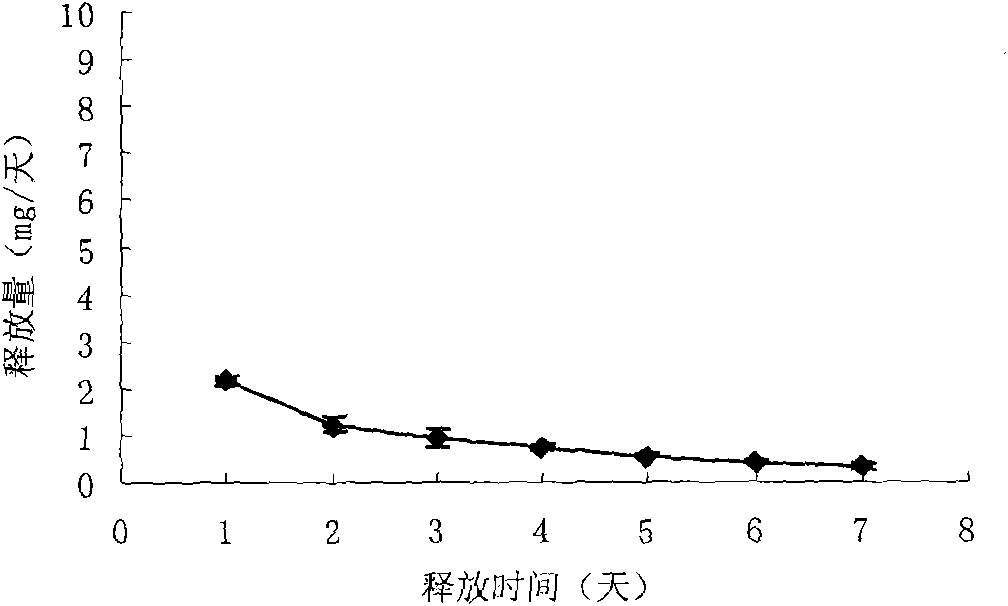
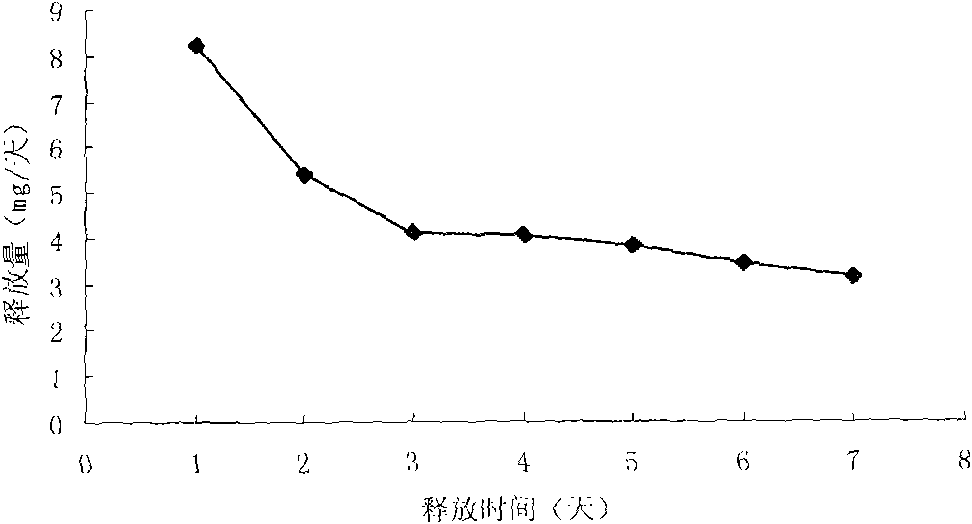
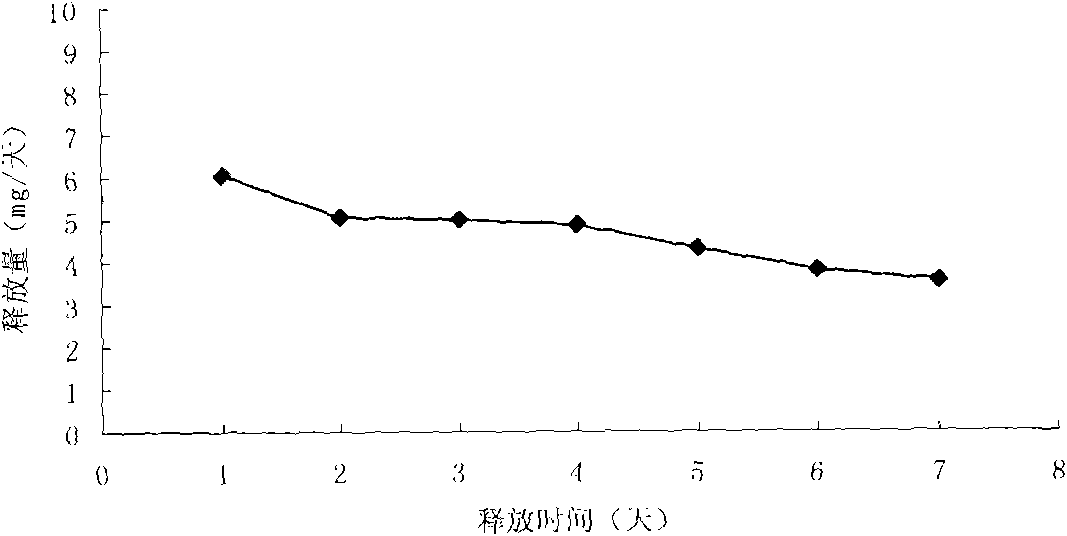
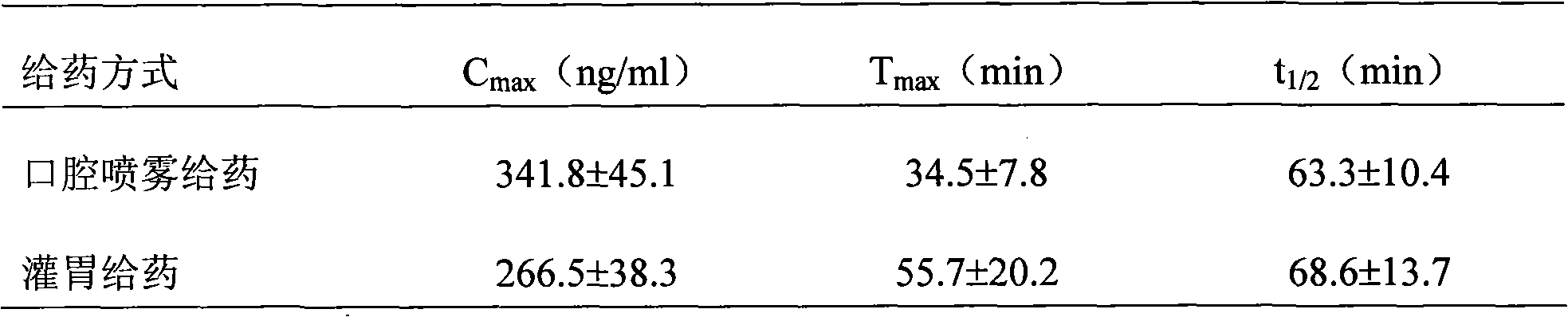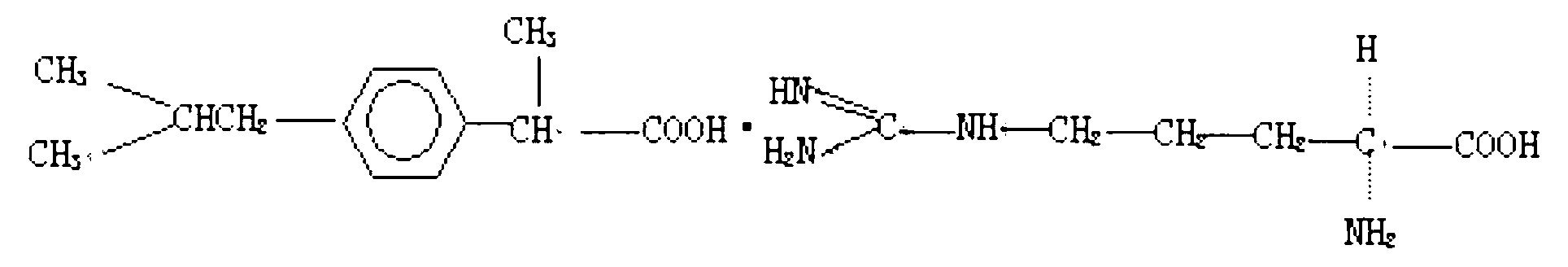Patents
Literature
461 results about "Hepatic first pass effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Illustration showing the hepatic portal vein system. The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug is greatly reduced before it reaches the systemic circulation.
Compositions for Drug Administration
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
Fiber capable of slow releasing health Chinese herbal medicines and face material thereof
InactiveCN101067228AImprove securityImprove effectivenessMedical devicesArtifical filament manufactureFiberHepatic first pass effect
The present invention is health fiber and fabric capable of releasing health Chinese medicine components. The health fiber is spun with melt containing micron level fine powder of cassia, rehmannia root, tuckahoe and other six kinds of Chinese medicinal materials. When the underwear of the health fiber is worn, the medicine components are released constantly from the health fiber and absorbed by the body through the skin effectively without first pass effect of liver.
Owner:JIANGSU SHENGHONG CHEM FIBRE CO LTD
Creamier formulation containing turmeric and extractive of flax seed and method of producing the same
InactiveCN101129311AImprove skin appearanceAvoid first pass effectCosmetic preparationsToilet preparationsFlaxseed extractAdemetionine
The invention relates to a cosmetic especially for a cream with 0. 65%-1. 8% turmeric extract and 8%-10% flax seed extract and making method, which is characterized by the following: extracting form natural plants; utilizing the extract of the turmeric and flax seed to be human body's forerunner substance effectively; improving autoregulation hormone secretion of human body; having much security in treating menopause symptom; avoiding the risk of inducing canceration to galactophore and endometrium through supplementing estrogen; improving appearance surface skin of human and adjusting and balancing internal secretion for women in menopausal period. The invention can improve chronic fatigue syndrome to improve metabolism and quality of life, which can treat acne and avoid liver primacy effect of oral medication with a simple making method.
Owner:陈苏莉
Nanometer antifungal econazole nitrate emulsion medicine and its prepn process
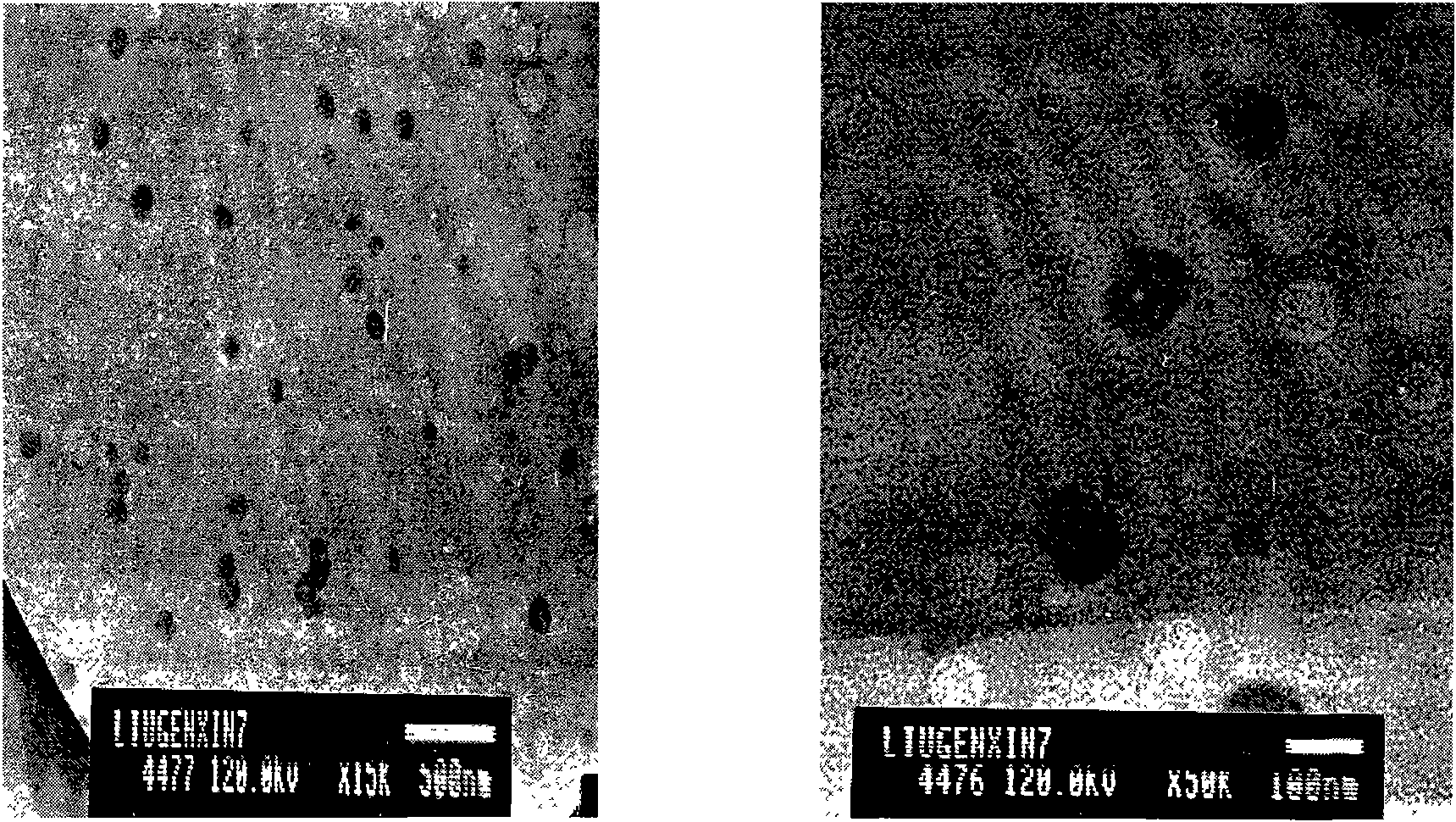
InactiveCN100448443CTransparent appearanceReduce surface tensionOrganic active ingredientsAntimycoticsAntifungalHepatic first pass effect
The nanometer antifungal econazole nitrate emulsion medicine consists of econazole nitrate, surfactant, oil and distilled water. Its preparation process includes the following steps: weighing the materials; mixing the surfactant and oil and dissolving econazole nitrate in dimethyl sulphoxide; mixing the solution of econazole nitrate with the surfactant and oil; and final adding distilled water slowly while stirring to form stable homogeneous transparent nanometer antifungal econazole nitrate emulsion medicine. The medicine is used in treating candidal tinea corporis, tinea cruris and tinea pedis, aural mycosis, seborrheic dermatitis, etc. The medicine is absorbed through skin to enter into blood circulation, and this avoids the first pass effect of liver, prolongs the medicine release and prevents stimulation to gastrointestinal tract. The preparation process is simple and low in power consumption.
Owner:NORTHWEST A & F UNIV
Oseltamivir lyophilized orally-disintegrating tablets and preparation method thereof
InactiveCN104367558AOral convenienceEasy to takeOrganic active ingredientsAntiviralsFreeze-dryingOrally disintegrating tablet
The present invention provides oseltamivir lyophilized orally-disintegrating tablets and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The oseltamivir lyophilized orally-disintegrating tablets comprise oseltamivir phosphate or oseltamivir and a matrix, wherein the oseltamivir lyophilized orally-disintegrating tablets contain (calculated as the oseltamivir) 10-75 parts by weight of the effective component, and the matrix contains 1-60 parts by weight of a framework support agent, 1-50 parts by weight of a binder, 0-10 parts by weight of a lyoprotectant, and 0-10 parts by weight of a flavoring agent, and can further contains 0-10 parts by weight of a flavoring agent and 0-69 parts by weight of an inorganic alkali. The oseltamivir lyophilized orally-disintegrating tablet preparation method comprises dissolving, mold injection, rapid freezing, freeze-drying and product packaging. The oseltamivir lyophilized orally-disintegrating tablets of the present invention have characteristics of convenient taking, taking without water, rapid absorption, and first pass effect avoiding.
Owner:BEIJING SUNHO PHARMA
Esomeprazole magnesium injection liquid
InactiveCN101513387AConvenient for clinical operationImprove bioavailabilityOrganic active ingredientsDigestive systemPatient needReflux
The invention discloses an esomeprazole magnesium injection liquid capable of treating gastroesophageal reflux disease. At present, esomeprazole magnesium in the market is only tablets, and the patient needs to take the esomeprazole magnesium for 1 to 3 times per day; and because the dosage taken by the patient per day is large and the frequency is high, side effects generated by the medicine are large, too. In order to solve the problems, the invention aims to provides the esomeprazole magnesium injection liquid with convenient clinical use, high bioavailability and low price, which can make the medicine quickly reach effective treatment concentration in vivo through direct intravenous injection or intramuscular injection, reduce first-pass effect of the medicament in liver and improve the bioavailability of the medicament in vivo. The esomeprazole magnesium injection liquid provided by the invention mainly comprises the esomeprazole magnesium or pharmaceutically acceptable salts thereof and solvent for injection; and 1 ml of the injection liquid comprises 10 to 200 mg of the esomeprazole magnesium or the pharmaceutically acceptable salts thereof, and the pH of the injection liquid is between 3.0 and 8.0.
Owner:李铁军
Voriconazole slow-release suppository and preparation method thereof
ActiveCN102058519AReasonable workmanshipSmall toxicityOrganic active ingredientsAntimycoticsMicrosphereWhole body
The invention discloses a voriconazole slow-release suppository and a preparation method thereof. The voriconazole slow-release suppository disclosed by the invention is prepared from voriconazole, a solubilizer, a slow-release material, an emulsifier, a dispersing medium and an excipient; and the preparation method disclosed by the invention comprises the following steps of: preparing slow-release microspheres by solubilizing the extremely indissolvable voriconazole, carrying out cast pouring and cooling to obtain the voriconazole slow-release suppository. The preparation method disclosed bythe invention is simple, the prepared suppository has a slow-release characteristic, the first pass effect generated by oral administration and the toxic action of intravenous administration for the organs of a whole body are avoided, and particularly for the patients of intensive care units, the patients with serious fungous infection and child and senile patients, the pain brought to the patients by frequent administration is reduced, the administration compliance is improved, and the curative effect of medicaments is improved.
Owner:苏州特瑞药业股份有限公司
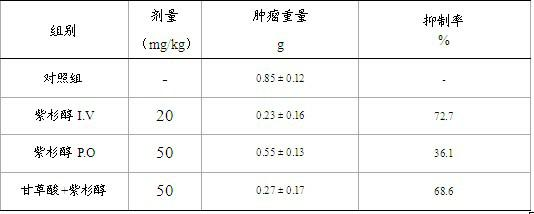
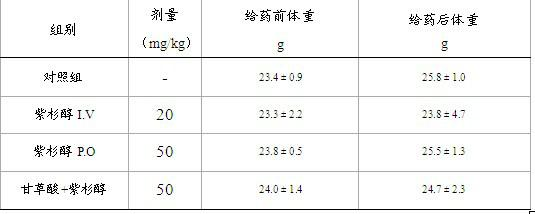
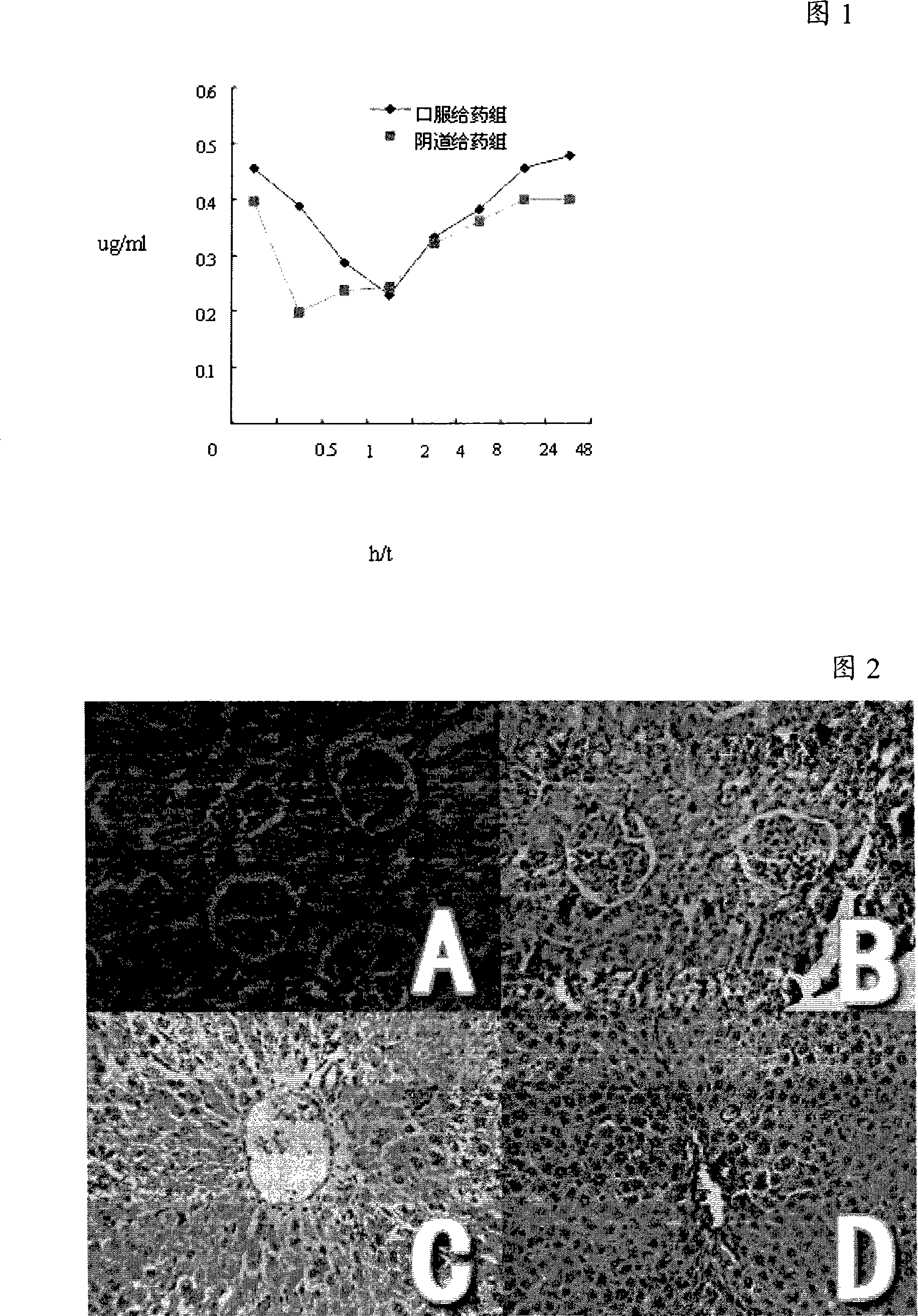
Compound pharmaceutical composition for improving oral bioavailability of taxol and application thereof
InactiveCN102091085AImprove oral bioavailabilityImprove anti-tumor activityEther/acetal active ingredientsKetone active ingredientsBlood concentrationOral medication
The invention discloses a compound pharmaceutical composition for improving oral bioavailability of taxol and application thereof. The invention also discloses a pharmaceutical composition containing the compound pharmaceutical composition and a taxol monomeric compound. The compound pharmaceutical composition provided by the invention can be used for improving the oral bioavailability of the taxol and can improve the anticancer effect of the taxol when used for preparing anticancer drugs. In the compound pharmaceutical composition provided by the invention, the taxol is the main anticancer component, and the traditional Chinese medicine extract monomeric compound improves the oral bioavailability and the anticancer effect of the taxol by inhibiting the first-pass effect of the taxol. Shown by experimental study, the compound pharmaceutical composition provided by the invention can be prepared into an oral medicament to improve the blood concentration and the anticancer effect of the taxol after oral administration.
Owner:SUN YAT SEN UNIV
Female surface coating contraceptive
InactiveCN101181638AImprove permeabilityAvoid damageOrganic active ingredientsSexual disorderAnti-ProgestinOral medicine
The invention relates to a surface coating adopting contraceptive for women, which consists of one or more than one active components such as compound, infiltrating accelerant, and humectant in anti-progestin medicine or progestational hormone medicine and estrogen medicine as well as normal raw materials such as oily component, aqueous component, macromolecule material, surface active agent, bacteriostat, chemical inhibitor and spice and so on. The invention can be made into electuary, emulsion, cream, jellies, spray and liquid cream so as to be used in contraception, urgent contraception, lactation contraception and reproductive health. The contraceptive adopts a method of being coated on the skin or the mucosa surface to reach the aim so as to prevent the first-pass effect and the gastrointestinal tract effect of the oral medicine, thereby reducing the injure to the liver and the kidney, reducing toxic and side effect, providing new preparation, method and route of administration for enlarging the range of the medicine-user, and making the contraception become more simple, convenient, easier and more willing to be accepted.
Owner:程定超
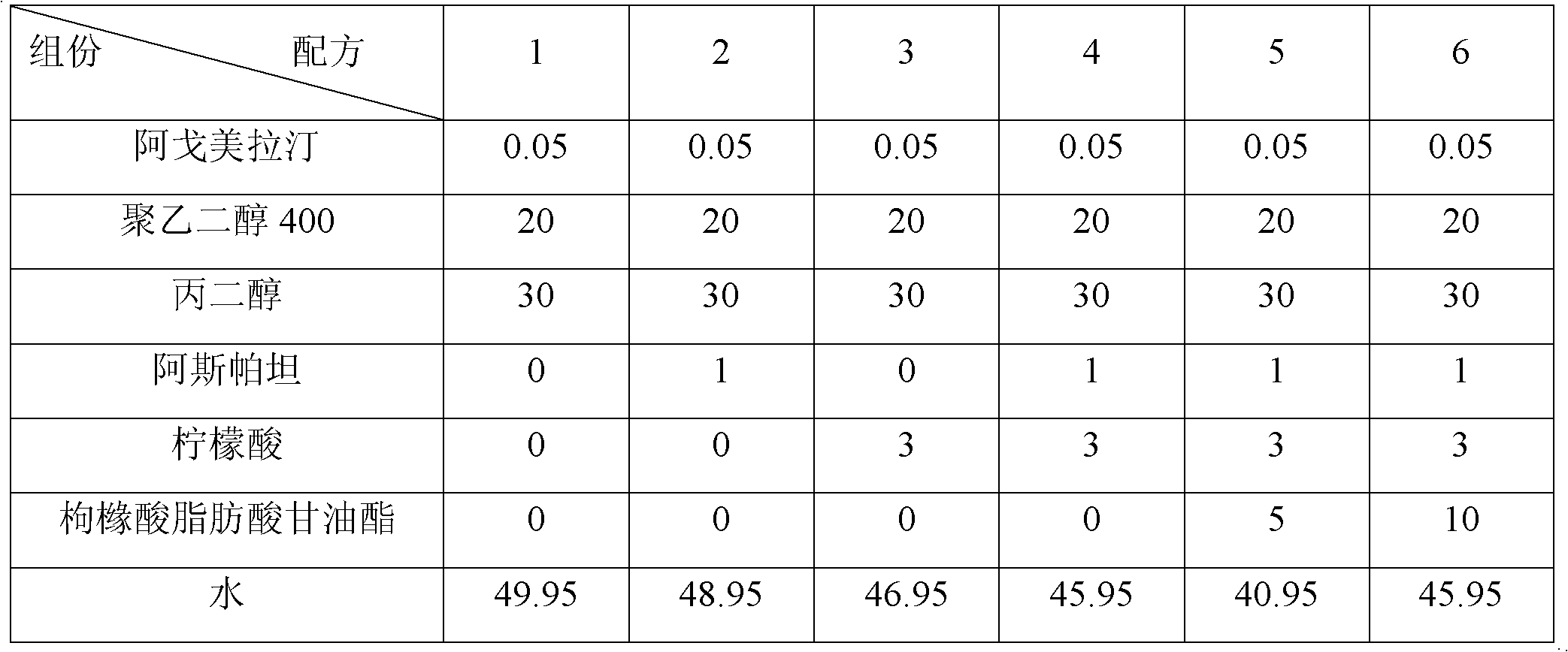
Agomelatine-containing medicinal composition for oral mucosa or sublingual administration
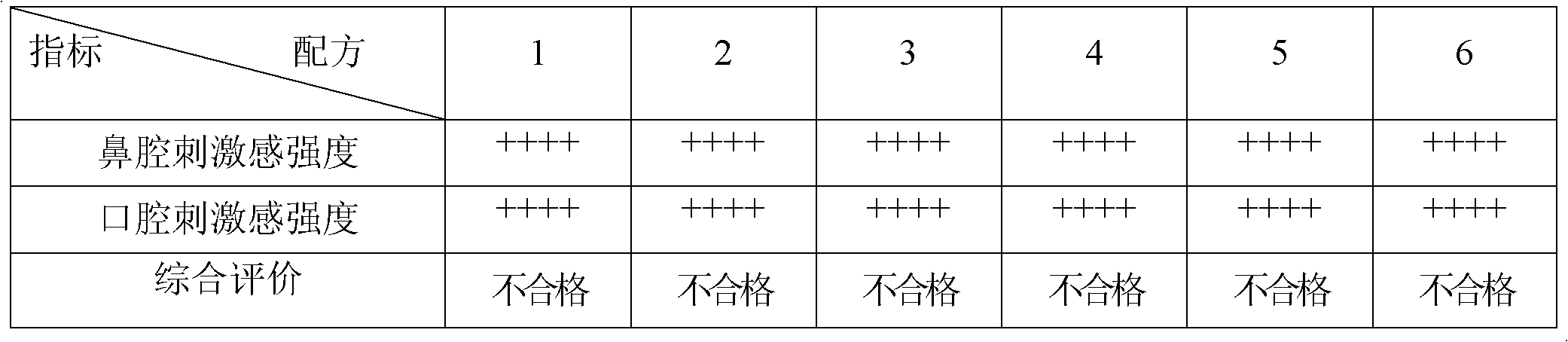
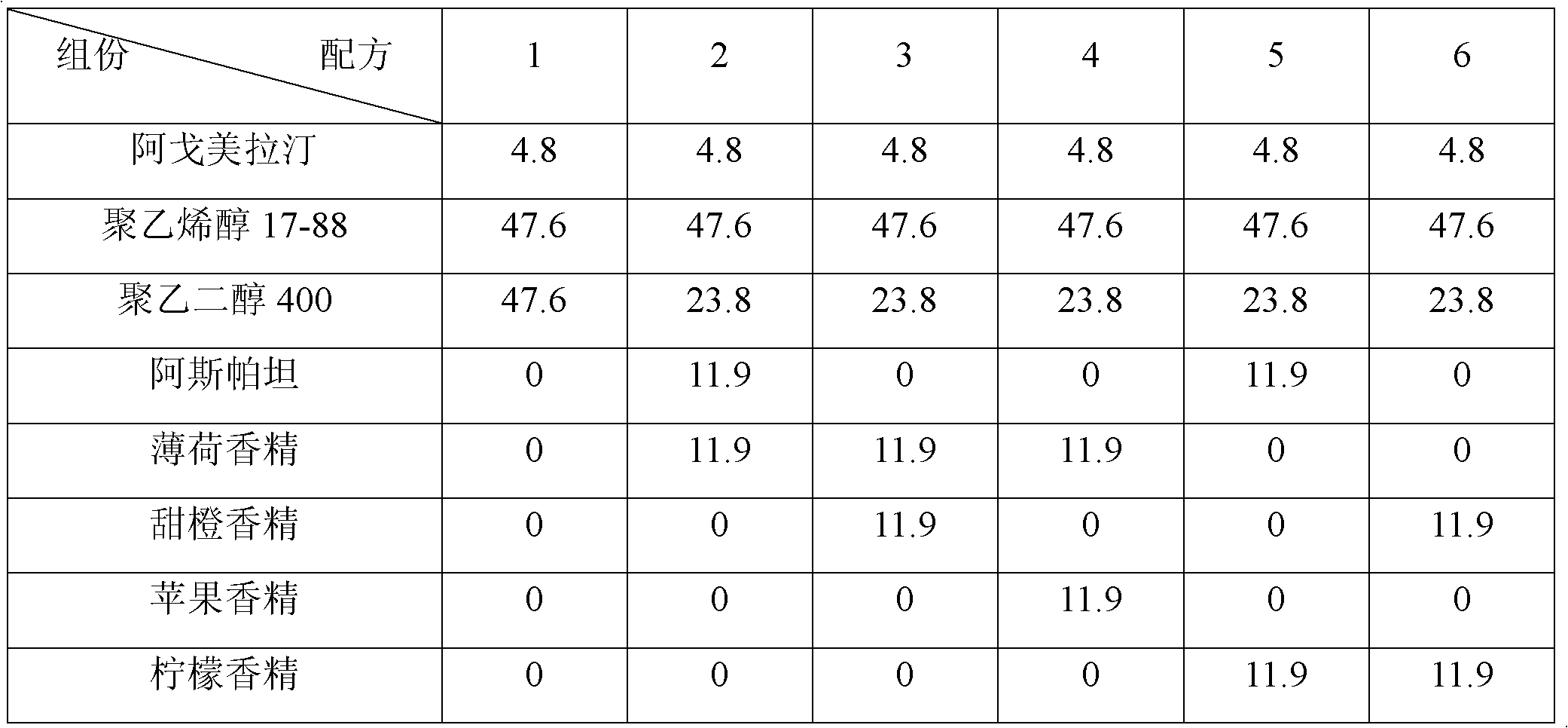
ActiveCN102579415AEasy to prepareLow costOrganic active ingredientsNervous disorderHepatic first pass effectSublingual administration
The invention discloses an agomelatine-containing medicinal composition for oral mucosa or sublingual administration and a preparation method thereof. The medicinal composition consists of a main medicament of which the particle diameter is 48-250 mum and is preferably 48-150 mum, and medicinal auxiliary materials. The medicinal composition can be prepared through a simple preparation process without special production equipment, has low manufacturing cost, and is suitable industrial production; moreover, the use compliance of a patient is improved through the excitement of the taken medicament in an oral cavity; and the medicinal composition is absorbed directly through oral mucosa, so that the first pass effect of the conventional oral medicament is avoided, and the bioavailability is high.
Owner:CHENGDU KANGHONG PHARMA GRP
Transdermal medicament delivery system containing donepezil compound, preparation and preparation method
ActiveCN102188363AImprove complianceAvoid first pass effectNervous disorderMacromolecular non-active ingredientsDonepezilCross linker
The invention discloses a transdermal medicament delivery system containing a donepezil compound, a transdermal preparation and a preparation method. The transdermal medicament delivery system comprises the following components in percentage by weight: 0.1 to 50 percent of donepezil or acid radical salt thereof, 1 to 95 percent of skeleton polymer, 0.1 to 60 percent of transdermal penetration enhancer, 0 to 10 percent of cross linker, 0.5 to 60 percent of humectant, 0.02 to 10 percent of bacteriostatic agent, 0.02 to 30 percent of pH regulator and 0 to 90 percent of solvent. The system is used for treating light, medium and severe senile dementia, can maintain long-time stable medicament delivery of at least 3 days, has better performance, is convenient for medicament delivery, and can reduce the administration frequency and increase the compliance of patients; and meanwhile, the transdermal path avoids first-pass effect on gastrointestinal tracts and liver due to oral administration of medicaments, and the system has higher bioavailability and obvious advantages in medicinal application.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Nanometer antifungal econazole nitrate emulsion medicine and its prepn process
InactiveCN1931165ATransparent appearanceReduce surface tensionOrganic active ingredientsAntimycoticsAntifungalHepatic first pass effect
The nanometer antifungal econazole nitrate emulsion medicine consists of econazole nitrate, surfactant, oil and distilled water. Its preparation process includes the following steps: weighing the materials; mixing the surfactant and oil and dissolving econazole nitrate in dimethyl sulphoxide; mixing the solution of econazole nitrate with the surfactant and oil; and final adding distilled water slowly while stirring to form stable homogeneous transparent nanometer antifungal econazole nitrate emulsion medicine. The medicine is used in treating candidal tinea corporis, tinea cruris and tinea pedis, aural mycosis, seborrheic dermatitis, etc. The medicine is absorbed through skin to enter into blood circulation, and this avoids the first pass effect of liver, prolongs the medicine release and prevents stimulation to gastrointestinal tract. The preparation process is simple and low in power consumption.
Owner:NORTHWEST A & F UNIV
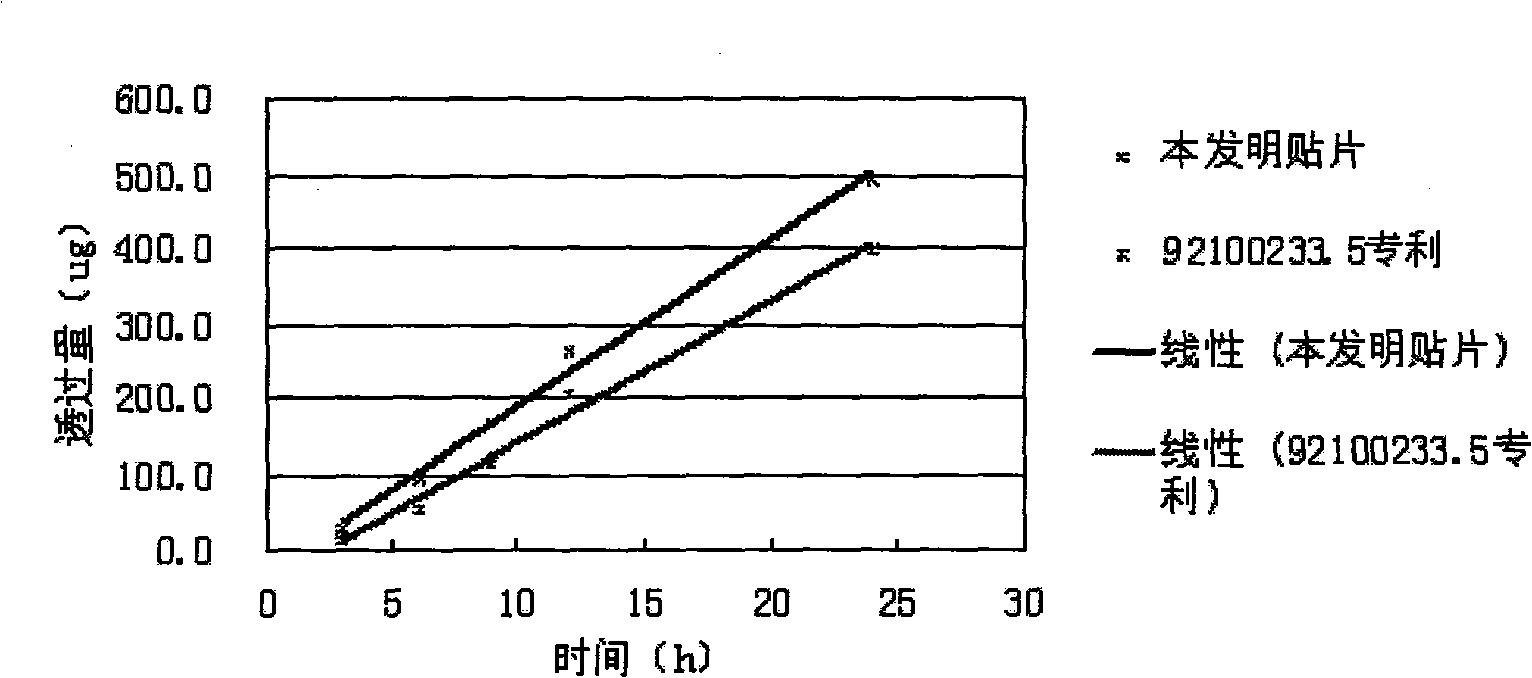
Slow-release patch with daphne giraldii bark extract, and preparation method thereof
InactiveCN101518599AImprove adhesionImprove flexibilityAntipyreticAnalgesicsAntioxidantTherapeutic effect
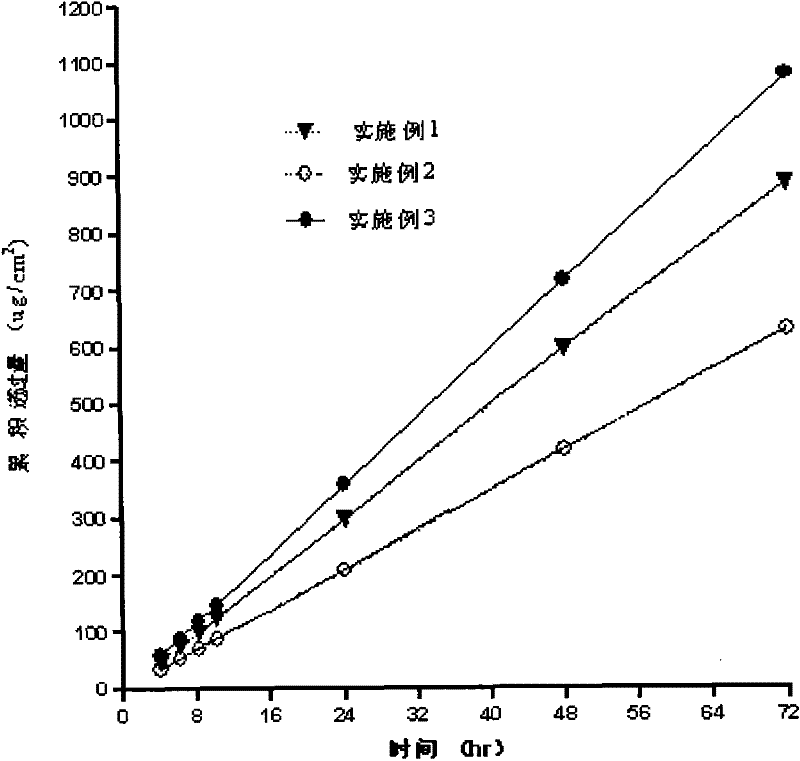
The invention belongs to the technical field of medicament, and discloses a percutaneous slow-release patch with a daphne giraldii bark extract, and a reparation method thereof. The patch with the daphne giraldii bark extract comprises a back lining layer, a drug reservoir and an antisticking layer, wherein the drug reservoir consists of the daphne giraldii bark extract, pressure sensitive adhesive and a percutaneous absorption accelerator; plasticizer, tackifier and antioxidant and the like can be added if needed; the selected pressure sensitive adhesive is silicone pressure sensitive adhesive, isobutylene pressure sensitive adhesive or acrylate pressure sensitive adhesive, and can select one or more compounds therein. The percutaneous patch has the advantages that the percutaneous patch can prevent oral administration from causing liver first-pass effect and irritating gastrointestinal tracts, overcomes deficiency that ointment, plaster, liniment, cream and other preparations are uncertain in dosage, easy to pollute clothes and the like, is constant in drug-releasing speed, ensures lasting steady therapeutic effect, and is convenient to use, good in flexibility and suitable in adhesion. The 24-hour accumulated permeation amount of daphnetin in a daphne giraldii bark patch prepared by the method is obviously higher than that of the commercially available daphne giraldii bark plaster.
Owner:SHENYANG PHARMA UNIVERSITY
Cetirizine hydrochloride gel
InactiveCN1457765AImprove complianceAvoid gastrointestinal irritationOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideOral medication
The present invention relates to the field of medicine technology and is one externally applied form of Cetirizine hydrochloride gel as one histamine resisting medicine and its preparation. The present invention prepares the gel with Cetirizine hydrochloride as main medicine component and different supplementary material, and the gel is administrated via skin or mucous membrane. The present invention can avoid liver reaction and stimulation to gstrointestinal tract of the medicine, reduce medicine concentration in blood, reduce central nerve suppressing effect, raise the medicine concentration in local affected part, decrease the administration times and raise patient's compatibility.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Zolpidem tartrate oral spraying agent and preparation method thereof
InactiveCN101780038AQuick effectSmall individual differencesOrganic active ingredientsNervous disorderZolpidem TartrateOral medication
The invention discloses a zolpidem tartrate oral spraying agent and a preparation method thereof. The oral spraying agent uses water as a solvent and 100ml of oral spraying agent comprises 1 to 10g of zolpidem tartrate, 0.1 to 50g of solubilizer, 0.1 to 5g of sorbefacient, 0.01 to 5g of flavoring agent and 0.1 to 3g of antioxidant. After the zolpidem tartrate oral spraying agent is sprayed and taken by oral cavity or hypoglottis, the medicament is mainly absorbed through the oral cavity or sublingual mucosa to avoid first pass effect of liver and food influence; therefore, the medicament is absorbed more rapidly, takes effect more quickly, and has smaller individual difference; and the medicament has convenient use, is taken without water before bedtime, and is particularly suitable for patients who are incompliant to oral administration and injection. Due to wide distribution range of the medicament in oral mucosa after spraying and administration and the effect of the sorbefacient, the medicament is rapidly absorbed from the oral mucosa and has quick-acting effect.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Traditional Chinese medicine preparation for curing gout and method of preparing the same
InactiveCN101249173APrevent relapseRelieve symptomsHydroxy compound active ingredientsSkeletal disorderSide effectAdditive ingredient
The invention discloses a traditional Chinese medicine preparation for treating gout and a preparation method thereof. The traditional Chinese medicine preparation is mainly made from the raw ingredient medicines by weight parts as follows: radix rehmanniae, pawpaw, Radix Aucklandiae, angelica dahurica, Wu Chia Pee, achyranthes and radix aconiti feri are respectively 100 to 250 parts; panax notoginseng and fennel are respectively 10 to 40 parts; camphor is 25 to 75 parts; borneol is 10 to 30 parts; mush / muskone is 0.1 to 0.5 parts. Compared with the prior art, the traditional Chinese medicine preparation has specific antiphlogistic effect to non-steroid inflammation, and has the functions of clearing heat as well as removing dampness, activating blood circulation and dredging collaterals, and promoting vascular regeneration; by adopting the modern extraction technology, the invention fully extracts the active ingredients in the traditional Chinese medicine to make a external preparation which can be applied on affected parts directly; through percutaneous absorption, no liver first pass effect occurs, the traditional Chinese medicine preparation has no damage to the liver, stomach, kidney, etc., without toxic and side effects, the curative effect is good and thorough, and the relapse is avoided.
Owner:杨漓 +2
Capparis spinosa fruit gel ointment, production method thereof, and application thereof to antirheumatic medicaments
ActiveCN102068474AEvenly dispersedAffect comfortAntipyreticAerosol deliveryPolyesterTectorial membrane
The invention relates to capparis spinosa fruit gel ointment, a production method thereof, and application thereof to antirheumatic medicaments. The capparis spinosa fruit gel ointment comprises a backing layer, a medicament-containing ointment body and a covering layer protective film, wherein the medicament-containing ointment body is prepared from the following raw materials: a medicinal part of the capparis spinosa and a medicinal excipient; a covering material of the covering layer protective film is one of release paper, a plastic film, a polyester, an aluminum foil-polyethylene composite film and hard gauze; and a backing material of the backing layer is one of cotton cloth, non-woven fabric and chemical fiber cloth. The apparis spinosa fruit gel ointment provided by the invention substitutes the apparis spinosa fruit serving as a medicament for the traditional root bark, so the contradiction between the resource utilization of the apparis spinosa medicinal plants and ecological protection is solved. More importantly, the apparis spinosa fruit gel ointment provided by the invention solves the problems of irritability and hypersensitivity caused by the clinical application of the traditional formulation and administration way of the apparis spinosa, and has the characteristics of long action time, high moisture retention, high air permeability, avoidance of the first pass effect of livers, using comfortableness and high compliance of patients.
Owner:XINJIANG INST OF MATERIA MEDICA
Sublingual tablet for anaesthesia and preparation method thereof
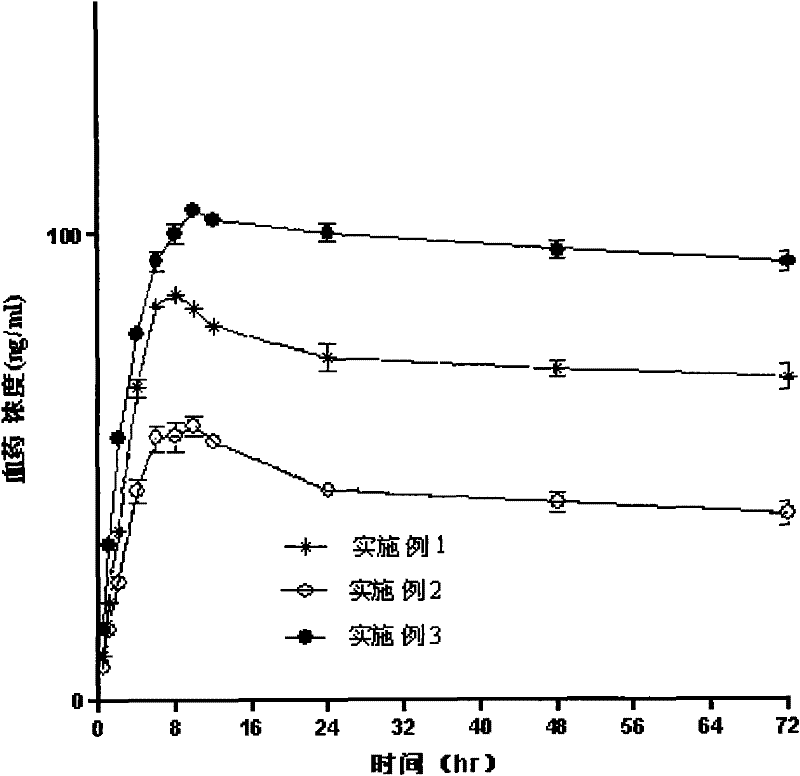
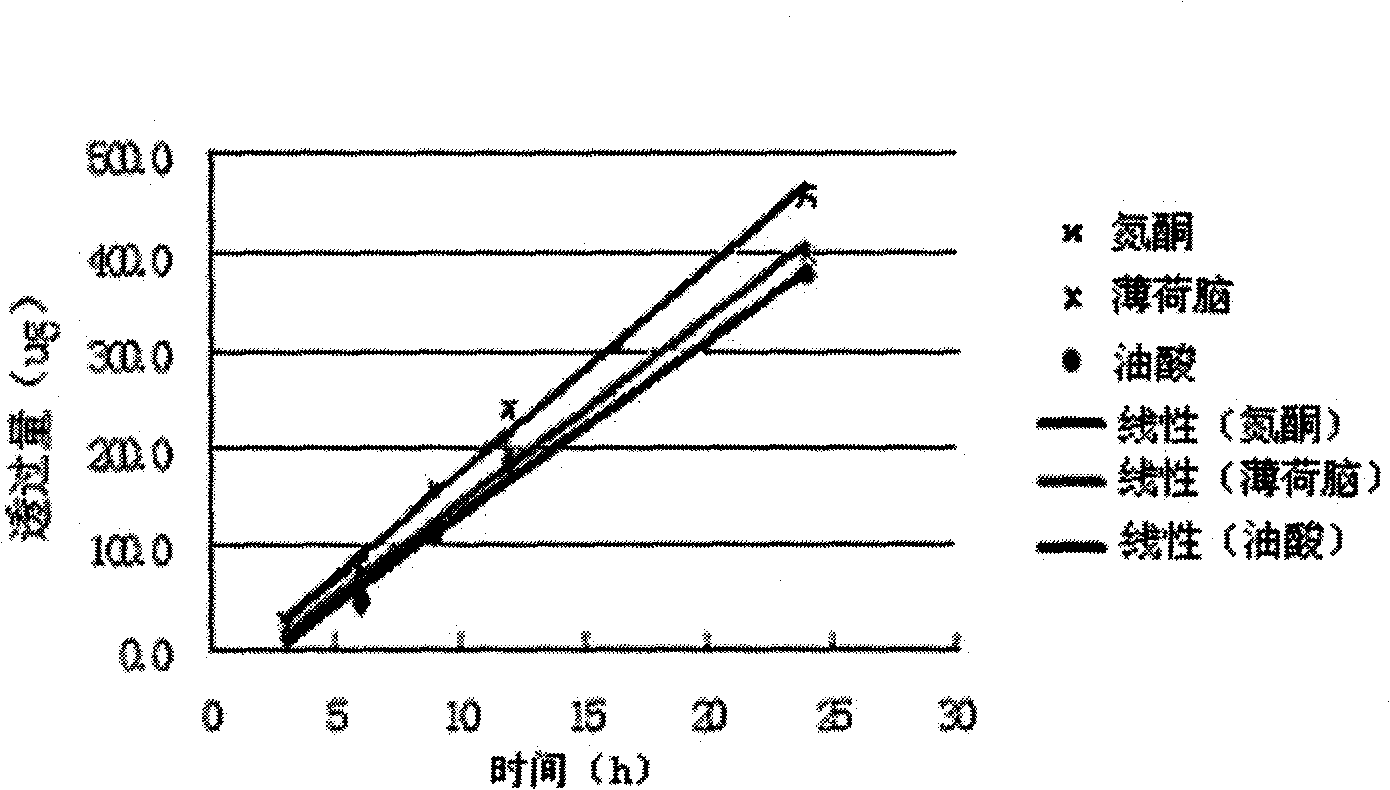
InactiveCN107137399AAvoid first pass effectDisintegrates quicklyOrganic active ingredientsAnaestheticsAdhesiveHepatic first pass effect
The invention discloses a sublingual tablet for anaesthesia. The preparation method of the sublingual tablet comprises the following steps: taking dexmedetomidinehydrochloride and ketamine hydrochloride as raw materials, adding a certain amount of a filling agent, a disintegrating agent, a corrigent, an adhesive and a lubricant, and performing pretreatment, mixing, granulatinganddrying, total blending, aluminum plastic inner packaging, outer packaging and the likeseparately. The sublingual tablet does not require building a special administrationchannel, is directly dosed through sublingual veins, avoids the liver first pass effect, takes effect quickly and is good in effect; particle angles of the sublingual tablet are all smaller than 35 DEG C duringpreparation, so that the particle mobility of the sublingual tablet is good, finished products is rapid to disintegrate and can be completelydisintegrated within 3 minutes, the weight difference of finished products is small, the increment of impurities is small during storage, the stability is good, the shelf life reaches 24 months, the preparation technology is simple and practical, and the sublingual tablet is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
External medicine combination for treating skin allergic disease
ActiveCN101417131AGood effectGood synergySalicyclic acid active ingredientsInorganic boron active ingredientsDrug synergismTopical medication
Anaphylactic diseases comprising urticaria, papilla, subacute dermatitis, eczema, and the like, are skin diseases which seriously puzzle the human beings. In the invention, antihistamine, zinc salt and weak acid are prepared into a topical compound preparation, which not only reduces the untoward effect of drugs, avoids the first pass effect of the liver when feeding the drugs by mouth, avoids the blood concentration peak valley phenomenon caused by oral feeding, but also can improve the curative effect and is convenient for clinic application. Simultaneously pharmacological tests show that the topical compound preparation obtains excellent drug synergism to the anaphylactic skin diseases and has excellent effects on curing the anaphylactic diseases.
Owner:LUNAN PHARMA GROUP CORPORATION
Improved entecavir oral disintegrating tablet and its preparing method
InactiveCN1781485AImprove bioavailabilityIncrease blood concentrationDigestive systemAntiviralsSide effectEntecavir
The present invention relates to entecavir soft capsule for treating hepatitis B and its preparation process, and aims at providing oral disintegrating entecavir tablet with fast absorption, high bioavailability, convenient taking, less intestinal residue, less side and no first pass effect of liver and intestine. The present invention is prepared with entecavir as main material and certain amount of supplementary material.
Owner:FUKANGREN BIO PHARMA
Slow-release mifepristone vaginal ring preparation and application thereof
InactiveCN102600001APromote dissolutionImprove bioavailabilityOrganic active ingredientsFemale contraceptivesPharmacyHepatic first pass effect
The invention relates to a preparation method for two types of slow-release mifepristone vaginal ring preparations. The first type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant and from 62 to 97.45wt% of medical high polymer release control materials. The second type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant, from 62 to 97.45wt% of medical high polymer release control materials, and a thin release control membrane covered on the outside of each slow-release mifepristone vaginal ring preparation. Due to the preparation method, a slow-release mifepristone vaginal ring can slowly release the mifepristone within seven days, a first pass effect and gastrointestinal reaction of mifepristone orally taken drug are avoided, bioavailability of the drug and pharmacy compliance of a patient are improved.
Owner:NAT RES INST FOR FAMILY PLANNING
Nebulizer for treating respiratory system diseases
ActiveCN103893165AQuick effectAvoid the first pass effectAntibacterial agentsAntimycoticsDiseaseNebulizer
The invention provides a nebulizer for treating respiratory system diseases. The nebulizer contains (A) 0.1-20 g / L of terpenoid, (B) 0.1-20 g / L of a non-ionic surfactant, and (C) 0.1-20 g / L of an osmotic pressure regulator. The nebulizer can form medicinal steam, mist or aerosol through a sprayer or an atomizer and the like, and is inhaled by the respiratory tract or locally sprayed. The nebulizer has the following advantages that 1, the target is directly arrived, the response is quick, and local action or general action can be exerted; 2, the first-pass effect of the liver and the destroying and degradation of gastrointestinal tracts can be avoided, and the bioavailability is high; 3, the nebulizer has good compliance.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Arginine ibuprofen composition for injection
InactiveCN103301118AGood treatment effectAvoid instabilityOrganic active ingredientsPowder deliveryTreatment effectHalf-life
The invention provides an arginine ibuprofen composition for injection, and relates to the technical field of medicine manufacturing. The main medicine of the composition comprises arginine ibuprofen and melatonin, wherein the melatonin comprises a quick release part and a cyclodextrin-included slow release part. According to the arginine ibuprofen composition for injection provided by the invention, the therapeutic effect of the arginine ibuprofen is improved, instability caused by oral administration of MT (Melatonin) is avoided and MT is quick to distribute and eliminate and the like, and the first pass effect of MT is reduced. The dosage of MT is reduced. The design of dosage combining quick release and slow release is in accordance with secretion characteristic of MT, so that the problem of half-life period of MT is solved and the bioavailability of a product is improved. Melatonin combined with arginine ibuprofen has a good synergistic effect for treating arthritis.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
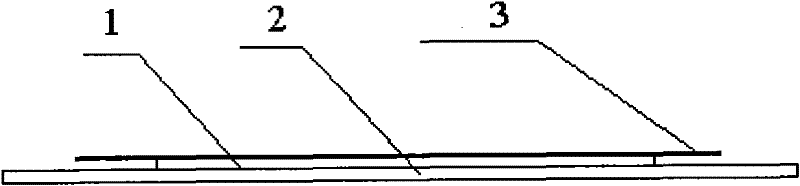
Desloratadine patch and preparation method thereof
InactiveCN101933914AImprove solubilityGood curative effectOrganic active ingredientsImmunological disordersCross-linkSolubility
The invention relates to a desloratadine patch and a preparation method thereof. The desloratadine patch comprises a patch film and medicament components on the patch film, wherein the medicament components comprise main component desloratadine and auxiliary materials; and the auxiliary materials comprise a solubilizing agent, a substrate, a cross-linking agent, an excipient and an osmosis reinforcing agent. The invention overcomes the bias that in the prior art, the desloratadine is considered to be difficult to externally penetrate through the skin cuticle and not to reach effective tissue concentration in the skin tissue so as not to exert the curative effects of allergic resistance and inflammation resistance, solves the problem of poor solubility of the desloratadine, improves the permeability of the desloratadine on the skin cuticle, ensures that the desloratadine rapidly arrives at the affected part and takes the effect rapidly and solves the problems of inconspicuous effect-taking and obvious first pass effect on gastrointestinal tract of the traditional patch. The desloratadine patch has the advantages of good curative effect, rapid action, less toxic or side effect and convenient use.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
Oral loratadine disintegrating tablet and its prepn
ActiveCN1739513AReduce stimulationQuick effectPill deliveryImmunological disordersDiseaseOrally disintegrating tablet
The present invention belongs to the field of medicine technology, and is especially oral loratadine disintegrating tablet for treating allergic diseases and its preparation process. The oral loratadine disintegrating tablet includes loratadine as effective medicine component, and excipient mixture comprising disintegrating agent, stuffing, soluble polyol and penetrant. The oral loratadine disintegrating tablet consists of loratadine 20-50 wt%, disintegrating agent 5-15 wt%, stuffing 10-30 wt%, soluble polyol 30-60 wt%, and penetrant 1-5 wt%. The oral loratadine disintegrating tablet, after being disintegrated fast, can cover gastrointestinal mucous membrane widely, and has fast acting, no first pass effect, high bioavailability, and convenient taking.
Owner:HAINAN PULIN PHARMA +1
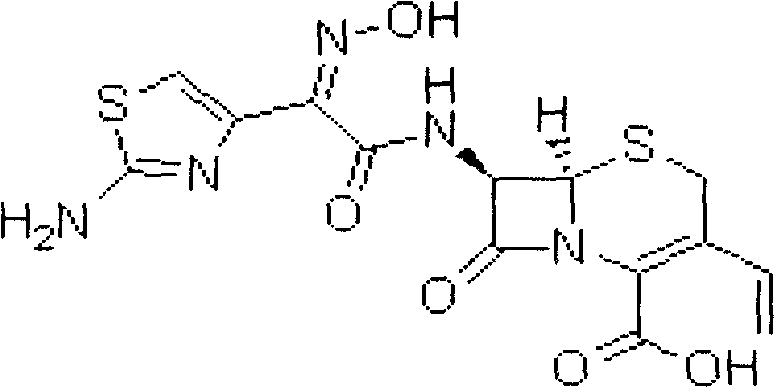
Child cefdinir composition freeze-dried oral disintegrating tablet and preparation method thereof
ActiveCN102579378ADisintegrates quicklyQuick effectAntibacterial agentsOrganic active ingredientsSide effectMedicine
A child cefdinir composition freeze-dried oral disintegrating tablet and a preparation method thereof relate to the technical field of medicine and preparation methods for medicine. The child cefdinir composition comprises, by weight, 24% to 49% of cefdinir, 49% to 72% of mannitol, 2% to 4% of gelatin and 0.1% to o.15% of sucralose. A tert butyl alcohol- water cosolvent is used as a menstruum. The freeze-dried oral disintegrating tablet has the advantages that components are simple, water is not required when a user takes the freeze-dried oral disintegrating tablet, chewing is not required, disintegrating time in human mouths is no more than two seconds, absorption is quick, bioavailability is high, intestinal canal residue is few, side effect is low, hepatic first pass effect is small, and the freeze-dried oral disintegrating tablet tastes good and is particularly suitable for infant patients.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
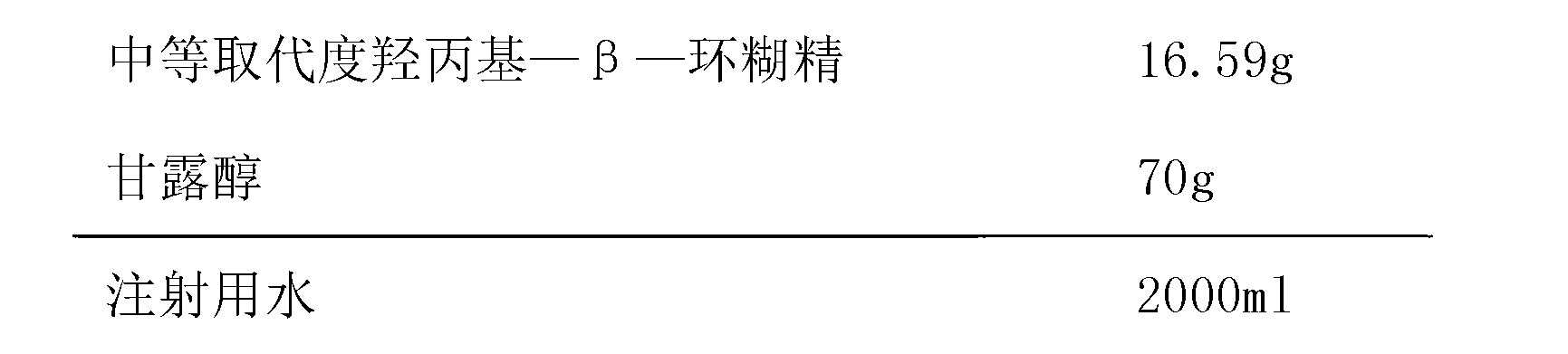
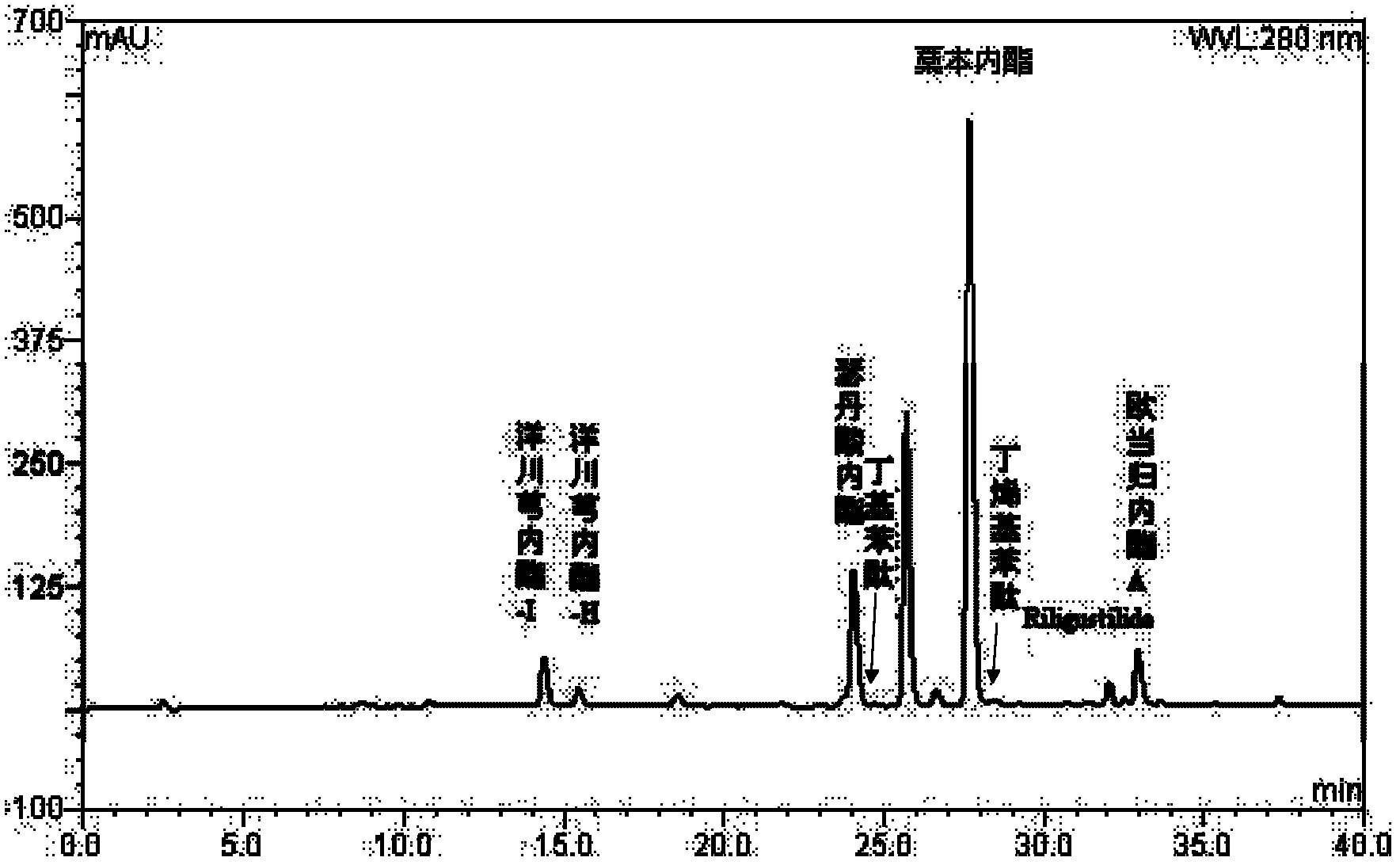
Preparation containing ligustilide type component for treating cardio-cerebrovascular disease and preparation method thereof
InactiveCN102631387APrevent escapeImprove bioavailabilityCardiovascular disorderPlant ingredientsDiseaseWater vapor
The invention discloses a preparation containing a ligustilide type component and a preparation method thereof. The ligustilide type component comprises the following components by weight percent: ligustilide 14%-42%, sedanolide 3%-20%, butylidenephthalide 0.1%-5%, butylphthalide 0.1%-3%, senkyunolide-H 0.2%-3%, senkyunolide-I 0.4%-5%, levistilide A 0.5%-1.5%, and riligustilide 0.4%-1.2%. The preparation method prevents the ligustilide type component from being destroyed by the wet distillation method and the solvent extraction method, and the quality of the ligustilide type component is more ensured without solvent residue. The preparation containing the ligustilide type component is safe and controllable on the effective part. Drop pills and sublingual tablets containing the ligustilide type component can avoid the defects of the oral preparation after administration such as liver first pass effect and gastrointestinal reaction, and an injection containing the ligustilide type component can also avoid the possible situations during using the injections such as acute poisoning reaction and allergic reaction. The preparation containing the ligustilide type component is safer and more effective, and has good economic benefit and social benefit.
Owner:TIANJIN UNIV
Artesunate nanoemulsion drug composition and preparation method thereof
InactiveCN101623255AHigh thermodynamic stabilityGood storage stabilityElcosanoid active ingredientsPharmaceutical non-active ingredientsSide effectAcute toxicity testing
The invention discloses an Artesunate nanoemulsion drug that is an O / W (oil-in-water type) nanoemulsion system consisting of ethyl oleate, Tween-80, normal butanol, ultrapure water and Artesunate. The nanoemulsion drug effectively overcomes the first-pass effect of traditional tablets in liver, causes no sense of pain in injection and greatly improves the insecticidal and helminthic effect of original Artesunate. Meanwhile, the nanoemulsion system has high drug-loading rate, is stable when stored, has certain slow release function compared with merchant Artesunate injection, and further is convenient for administration, thus greatly improving the bioavailability. Besides, shown by acute toxicity tests and clinical drug effect tests on mice, the nanoemulsion has no obvious toxic or side effect and is a safe, reliable and high-efficiency nano level antiparasitic drug.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
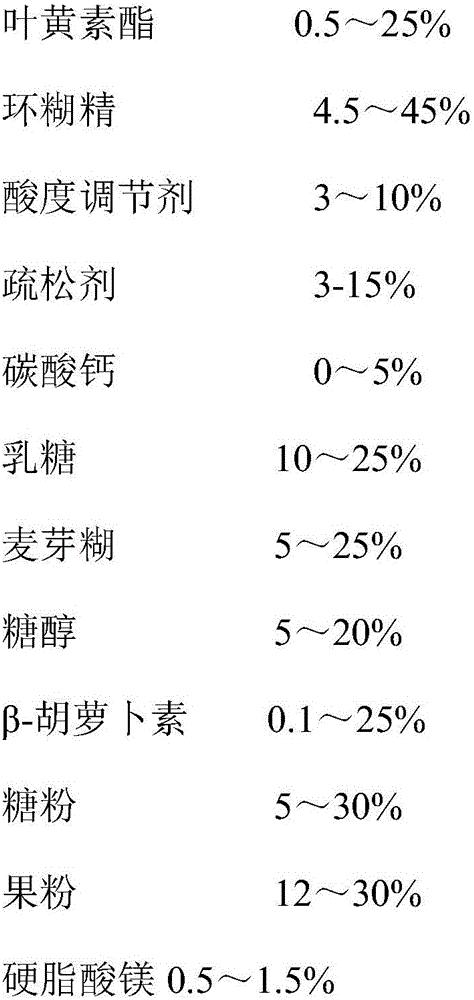
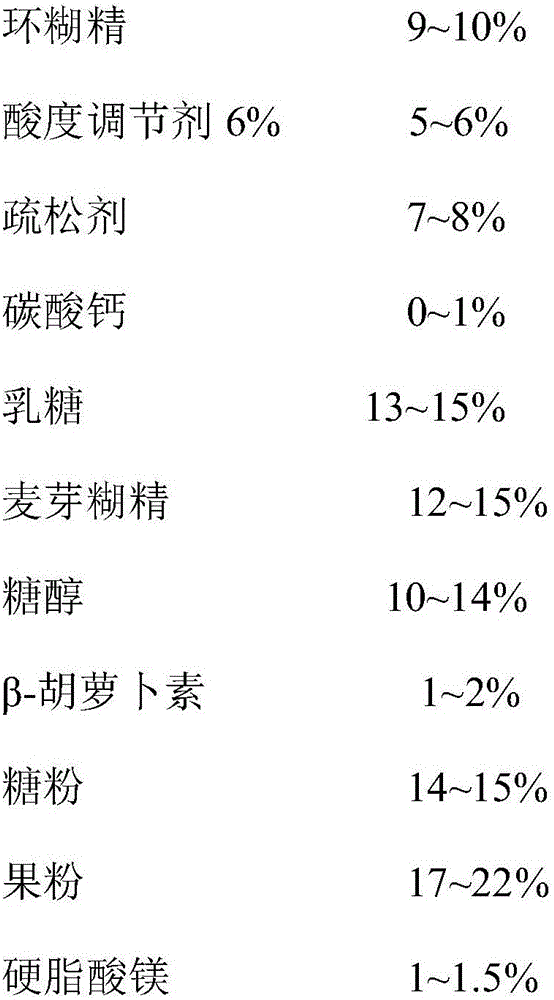
Composition containing xanthophylls/lutein ester and application thereof
ActiveCN106236739APrevent morbiditySignificant health careSenses disorderOrganic chemistryBeta-CaroteneOrally disintegrating tablet
The invention discloses a composition containing xanthophylls / lutein ester and application thereof. The composition containing xanthophylls / lutein ester contains xanthophylls / lutein ester and beta-carotene and a pharmaceutical acceptable carrier, wherein weight ratio of xanthophylls / lutein ester to beta-carotene is 1: 0.1-100. By coordinated use of xanthophylls / lutein ester and beta-carotene and with assistance of natural healthy ingredients, the dosage form of an orally disintegrating tablet is prepared. The orally disintegrating tablet is absorbed through oral cavity, has fast effectiveness, has small first-pass effect, reduces damage of gastric acid to xanthophylls / lutein ester, has high bioavailability, and has characteristics of nutritional and health-care effects, good mouthfeel and fast absorption. By the use of the product, visual power can be obviously enhanced, ocular blood flow is increased, and eye muscle fatigue is improved. Therefore, morbidity of cataract, senile retina macular degeneration and juvenile myopia is reduced.
Owner:SINO NUTRACEUTICAL CO LTD
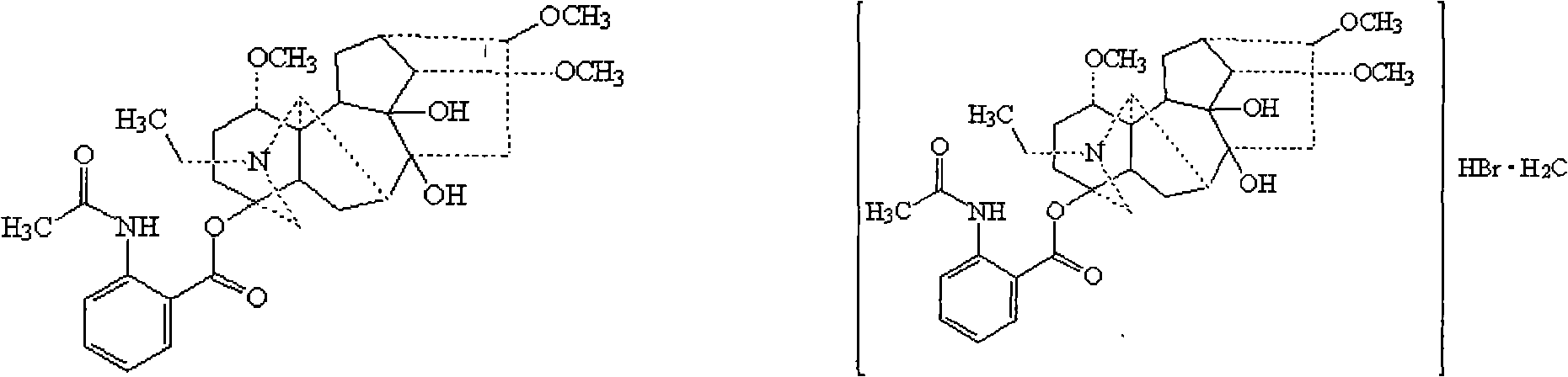
Lappaconitine transdermal patch and preparation method thereof
InactiveCN101574331AEasy to useImprove bioavailabilityOrganic active ingredientsAntipyreticTransdermal patchSide effect
The invention discloses a lappaconitine transdermal patch and preparation method thereof. The patch comprises a back sheet, a drug storing layer and a protection layer, wherein, the drug storing layer is composed of the raw materials of lappaconitine and pressure sensitive adhesive, or a right amount of transdermal accelerant is added to finally prepare lappaconitine transdermal patch. The lappaconitine transdermal patch of the invention has the advantages of convenient use, high bioavailability and lasting action, avoids first-pass effect of liver as well as damage to gastrointestinal tract, reduces drug toxicity and side effect, improves bioavailability of drug and safety of treatment, and has obvious effect on anti-inflammatory and analgesia.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com