Agomelatine-containing medicinal composition for oral mucosa or sublingual administration
A technology for sublingual administration of agomelatine, which is applied to drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as high cost and complicated preparation process, and achieve low cost and excellent preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 7
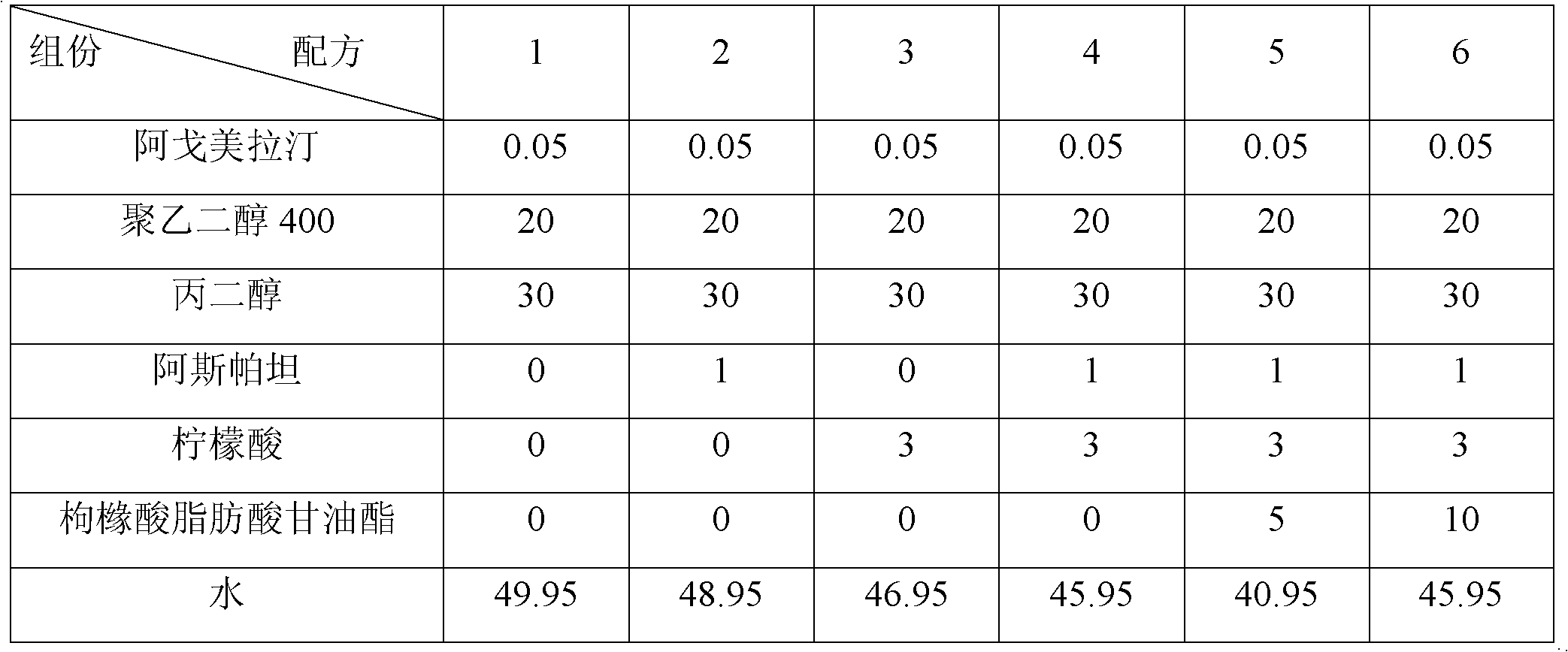
[0084] The pharmaceutical composition was prepared by adopting agomelatine with different particle sizes, and the formula is shown in Table 7. Preparation method (wet granulation and tabletting): mix agomelatine with mannitol and cross-linked polyvinylpyrrolidone evenly, add an appropriate amount of 5% hypromellose for wet granulation, and mix the obtained dry granules with magnesium stearate, Mix micronized silica gel evenly, and press into tablets.
[0085] Table 7 Embodiment 1-7 (calculated by weight percentage)
[0086]
[0087]
[0088] Remarks: Add about 8g (0.4g) of 5% hypromellose solution per 100g of solid material, and the amount of hypromellose is not included in the formula.
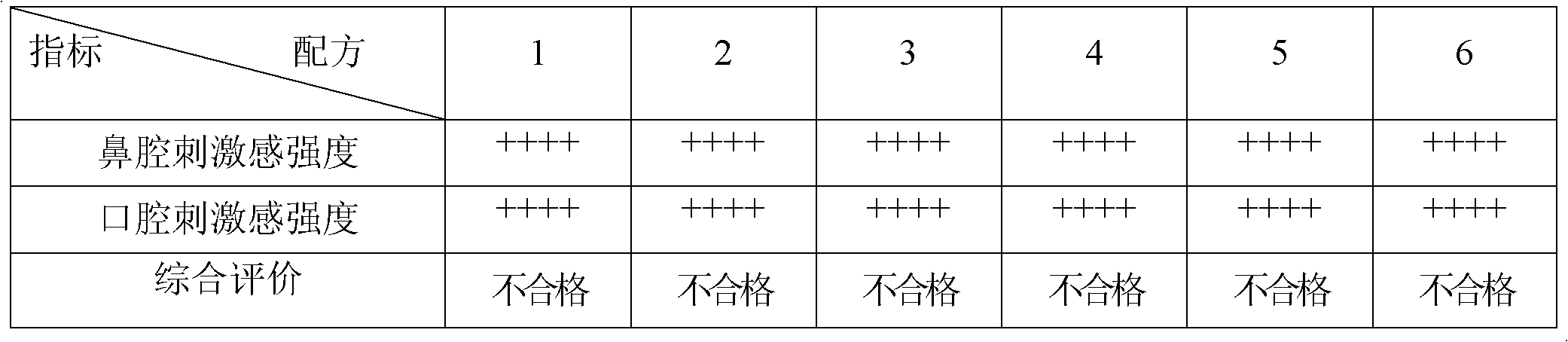
[0089] Find 6 healthy volunteers to use, through oral mucosa and sublingual administration, evaluate its stimulation intensity and detect its dissolution rate. The results are shown in Table 8.
[0090] Table 8 embodiment 1-7 evaluation result
[0091]
[0092] Summary: It can be...
Embodiment 8 to 18
[0094] The choice of diluent has a certain influence on the irritation and dissolution rate of this product. It is known to those skilled in the art that soluble excipients such as mannitol, sorbitol, lactitol, sucrose, lactose, glucose, or insoluble excipients such as starch, dextrin, calcium sulfate and microcrystalline cellulose can be used as the ingredients of tablets. thinner. The inventor studies the situation of different adjuvants as the diluent of the present invention through sufficient experiments, and the results show that the diluent suitable for the pharmaceutical composition of agomelatine of the present invention can be any excipient commonly used for dilution in the pharmaceutical field. Excipients, including but not limited to one or more of the above-mentioned diluents.
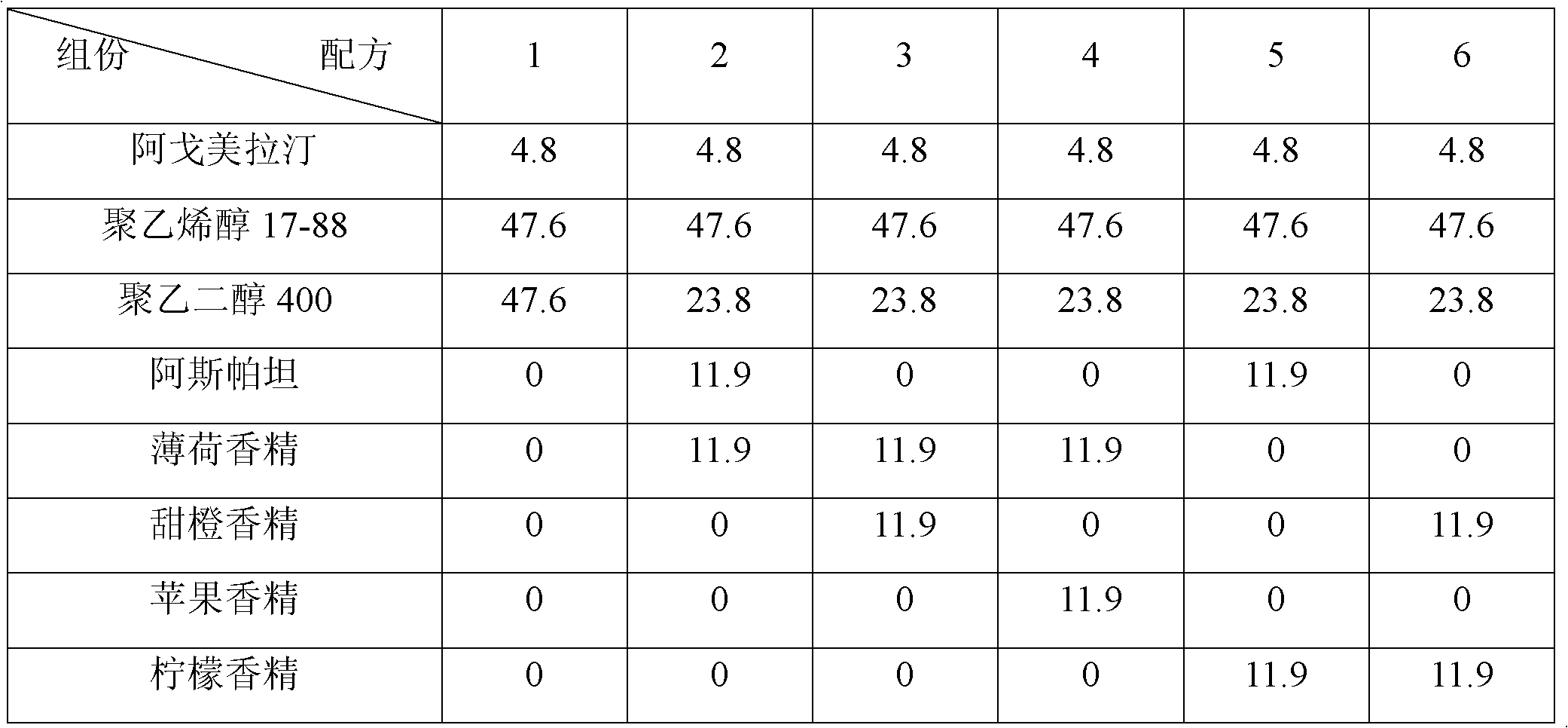
[0095] The present invention is further illustrated by the following specific experiments: using the above composition formulations of different diluents (see Table 9 and Table 10), the p...
Embodiment 19-38
[0112] In addition to diluents, disintegrants, and lubricants, the pharmaceutical composition of the present invention may also contain other pharmaceutical excipients. The specific formulations are shown in Table 13 and Table 14. The particle size range of agomelatine used is 48 μm- 150 μm. The preparation method is as follows:
[0113] Step 1: crush the main drug, diluent, lubricant, disintegrant, flavoring agent, antioxidant, opacifier, penetration enhancer, and sieve for pretreatment.
[0114] Step 2: Mix the pretreated main drug, diluent, lubricant, disintegrant, flavoring agent, antioxidant, opacifier and penetration enhancer evenly;
[0115] Step 3: compress the uniformly mixed above-mentioned materials into tablets.
[0116] Table 13 Embodiment 19-29 (calculated in weight percentage)
[0117]
[0118] Note: the lactose used in Examples 22 and 24 is ground lactose; the lactose used in Example 29 is spray-dried lactose.
[0119] Table 14 Embodiment 30-38 (calculat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com