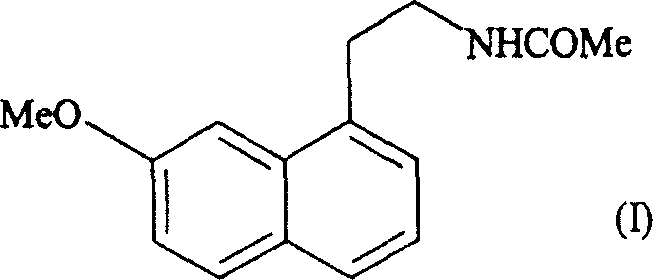
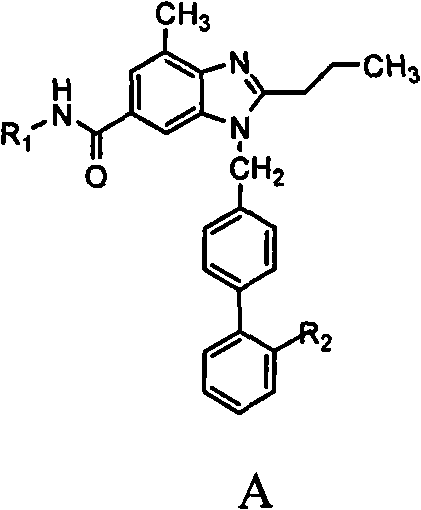
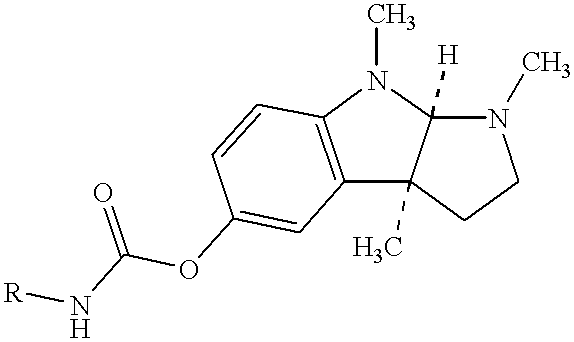
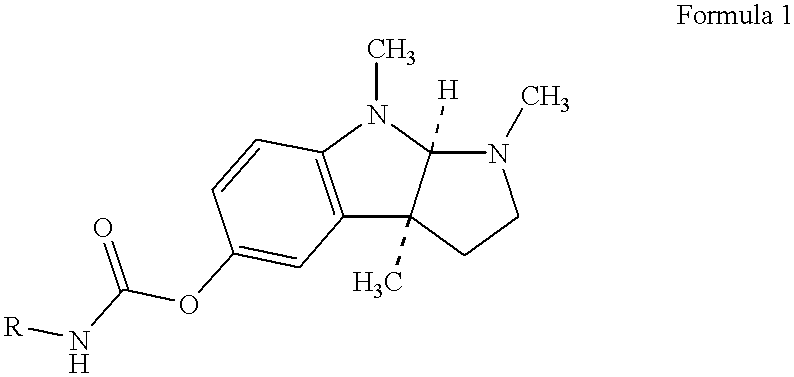
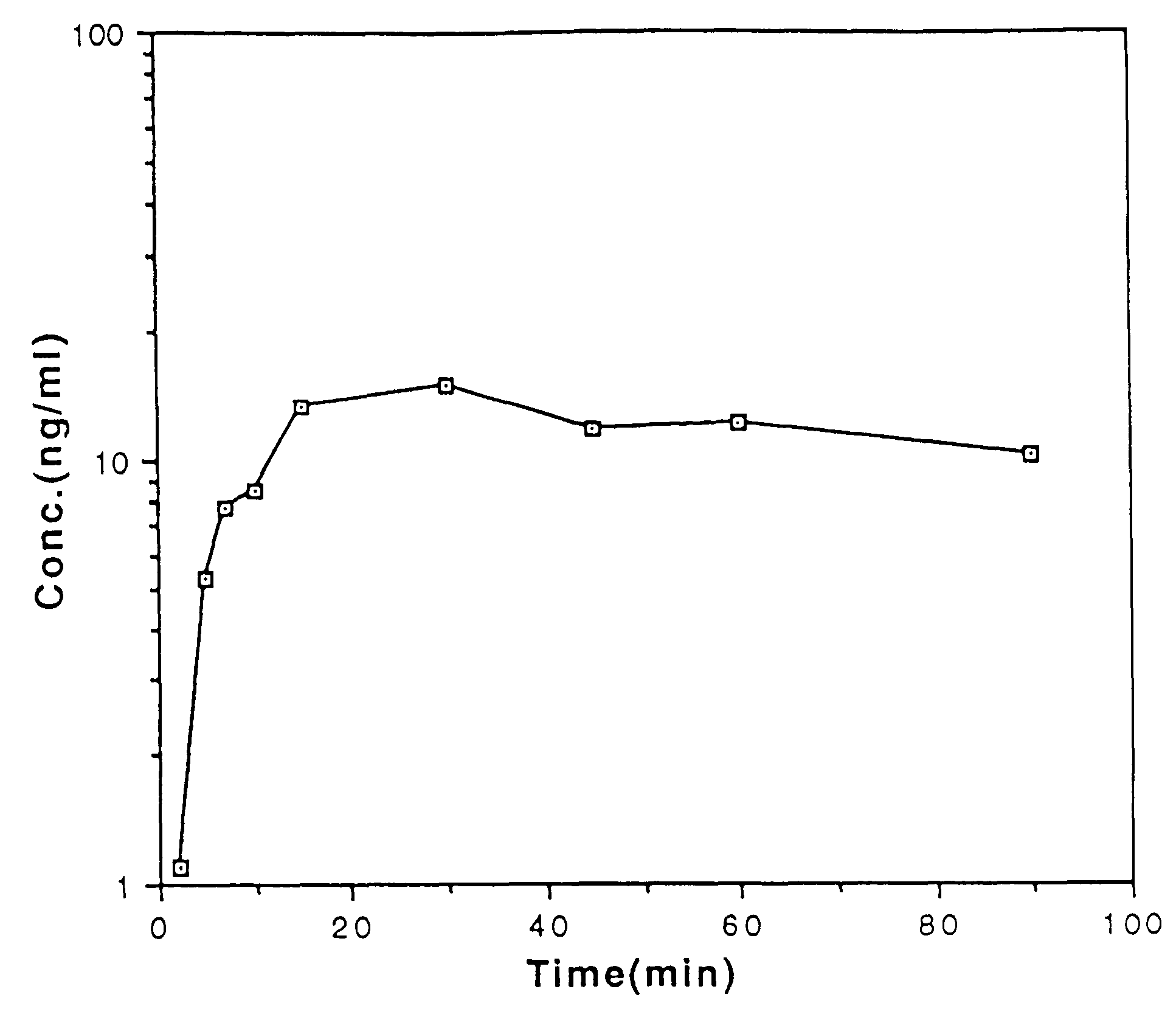
Patents
Literature
118 results about "Sublingual administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sublingual (abbreviated SL), from the Latin for "under the tongue", refers to the pharmacological route of administration by which substances diffuse into the blood through tissues under the tongue. Many drugs are designed for sublingual administration, including cardiovascular drugs, steroids, barbiturates, benzodiazepines, opioid analgesics, enzymes and, increasingly, vitamins and minerals.
Fentanyl composition for the treatment of acute pain
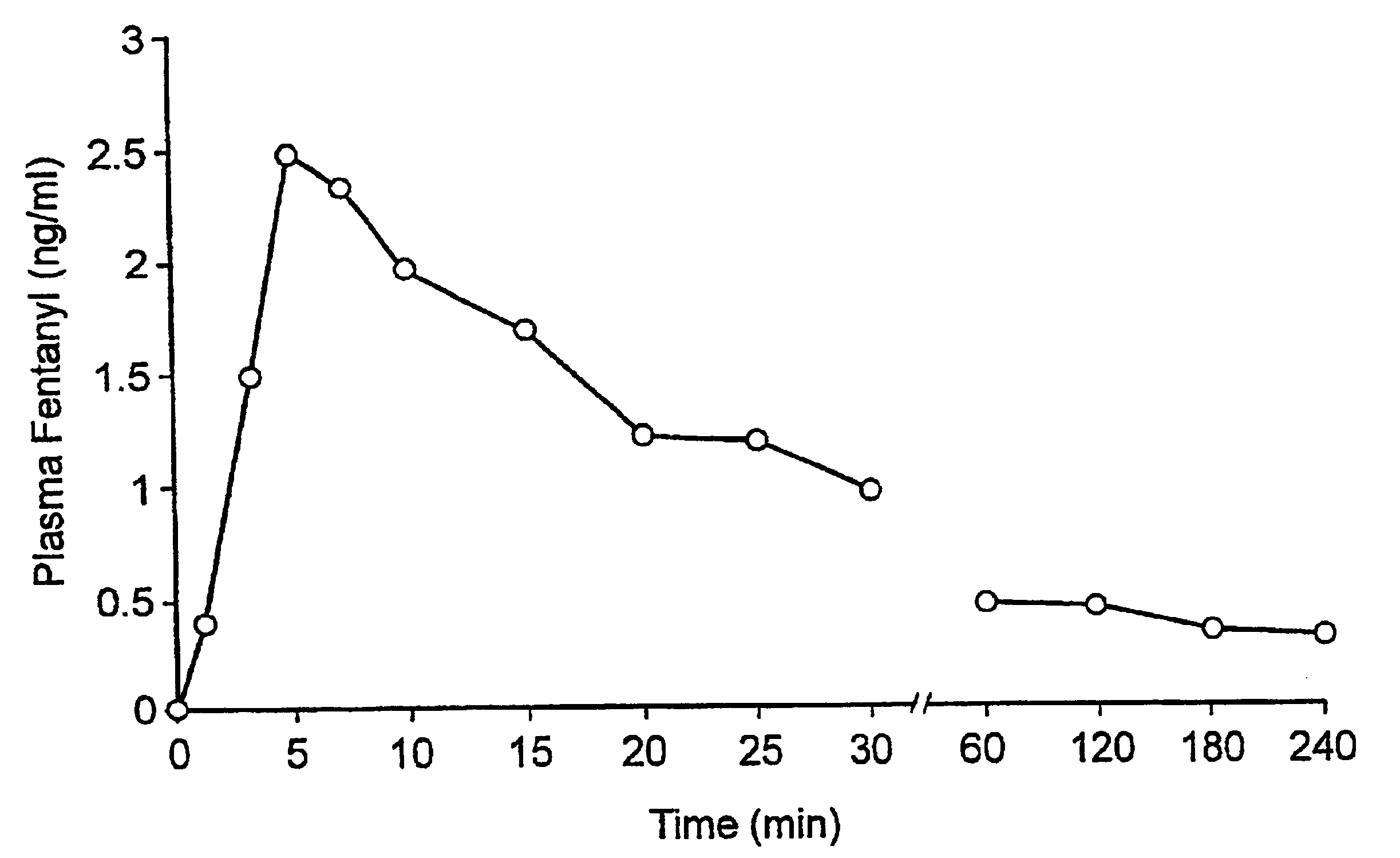
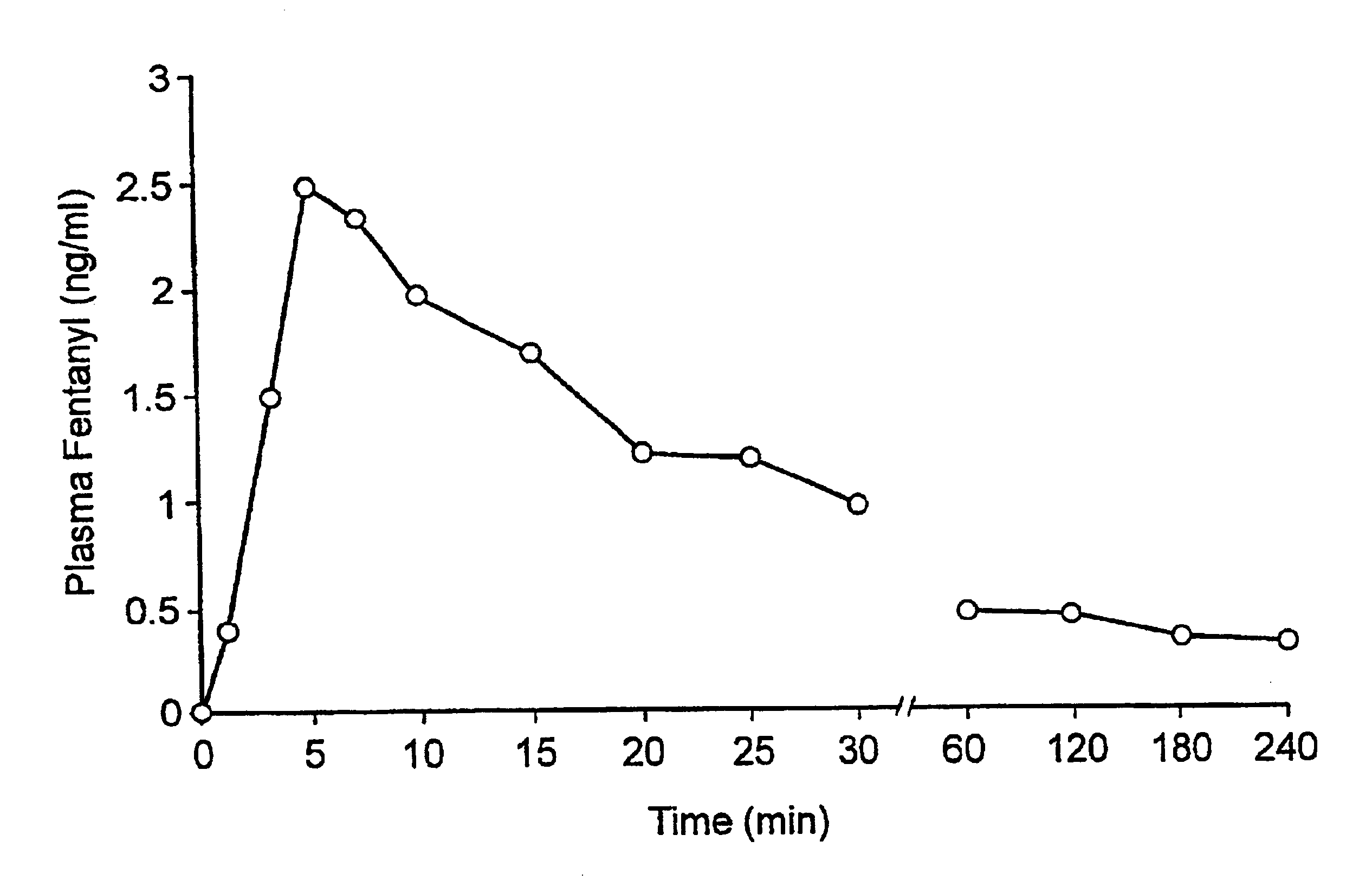
A pharmaceutical composition for the treatment of acute pain by sublingual administration is described. The composition comprises an essentially water-free, ordered mixture of fentanyl or a pharmaceutically acceptable salt thereof in the form of microparticles which are adhered to the surface of carrier particles which are substantially larger than the particles of fentanyl, and are essentially water-soluble. In a preferred embodiment, the composition also contains a bioadhesion and / or mucoadhesion promoting agent. The invention also relates to the preparation of the composition, and to the use of the composition for the treatment of acute pain.
Owner:DIABAKT AB
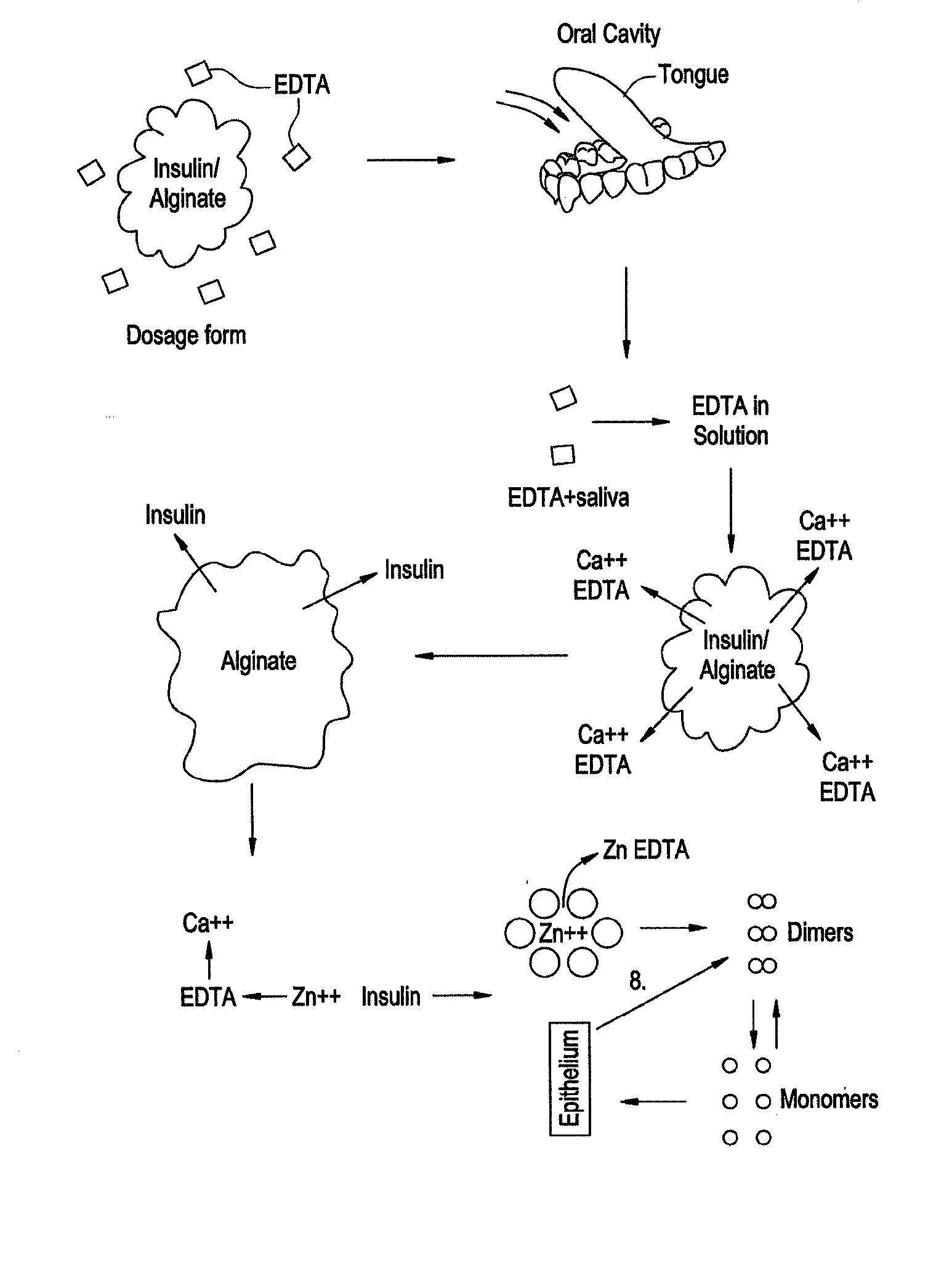
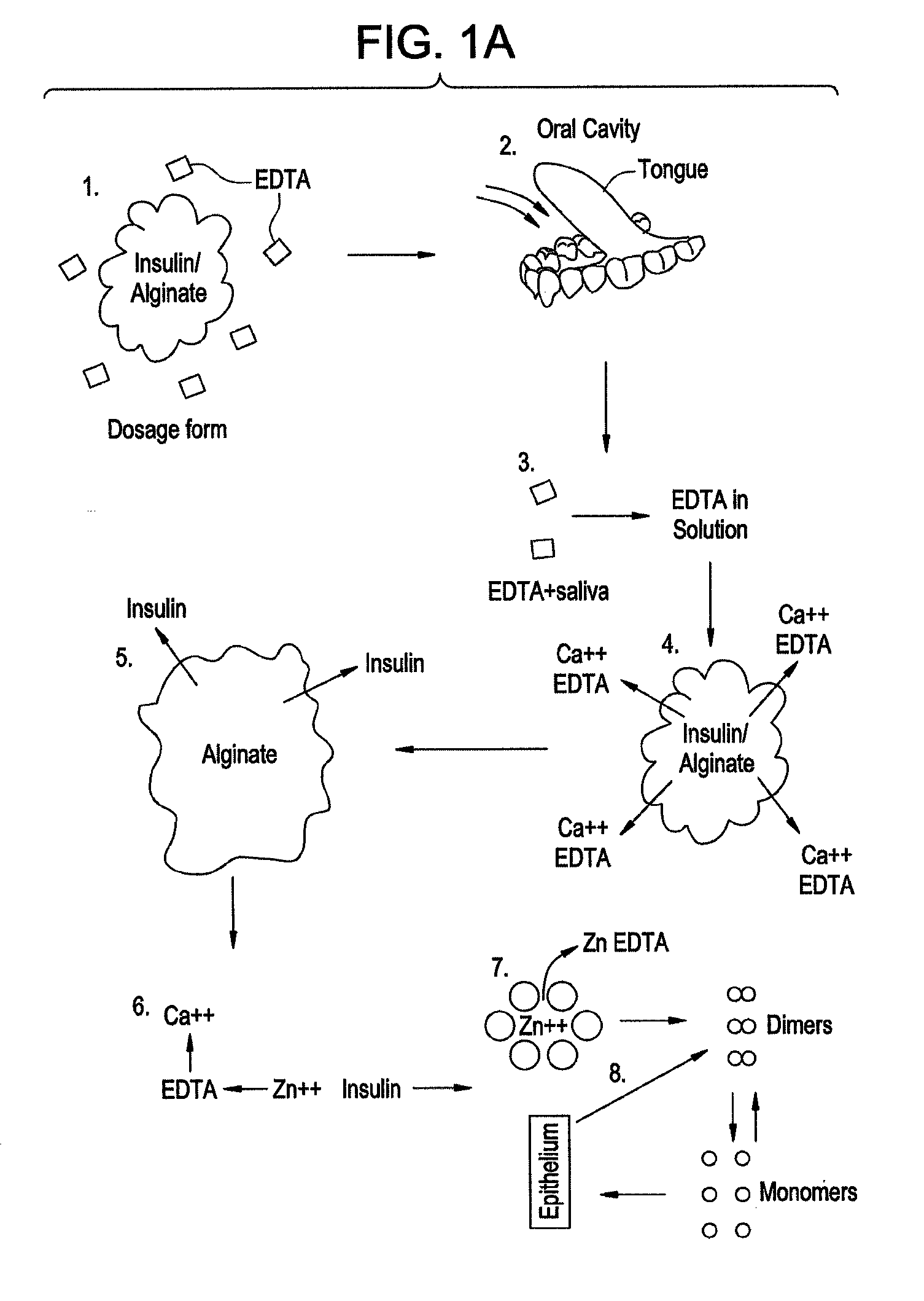
Rapid acting drug delivery compositions
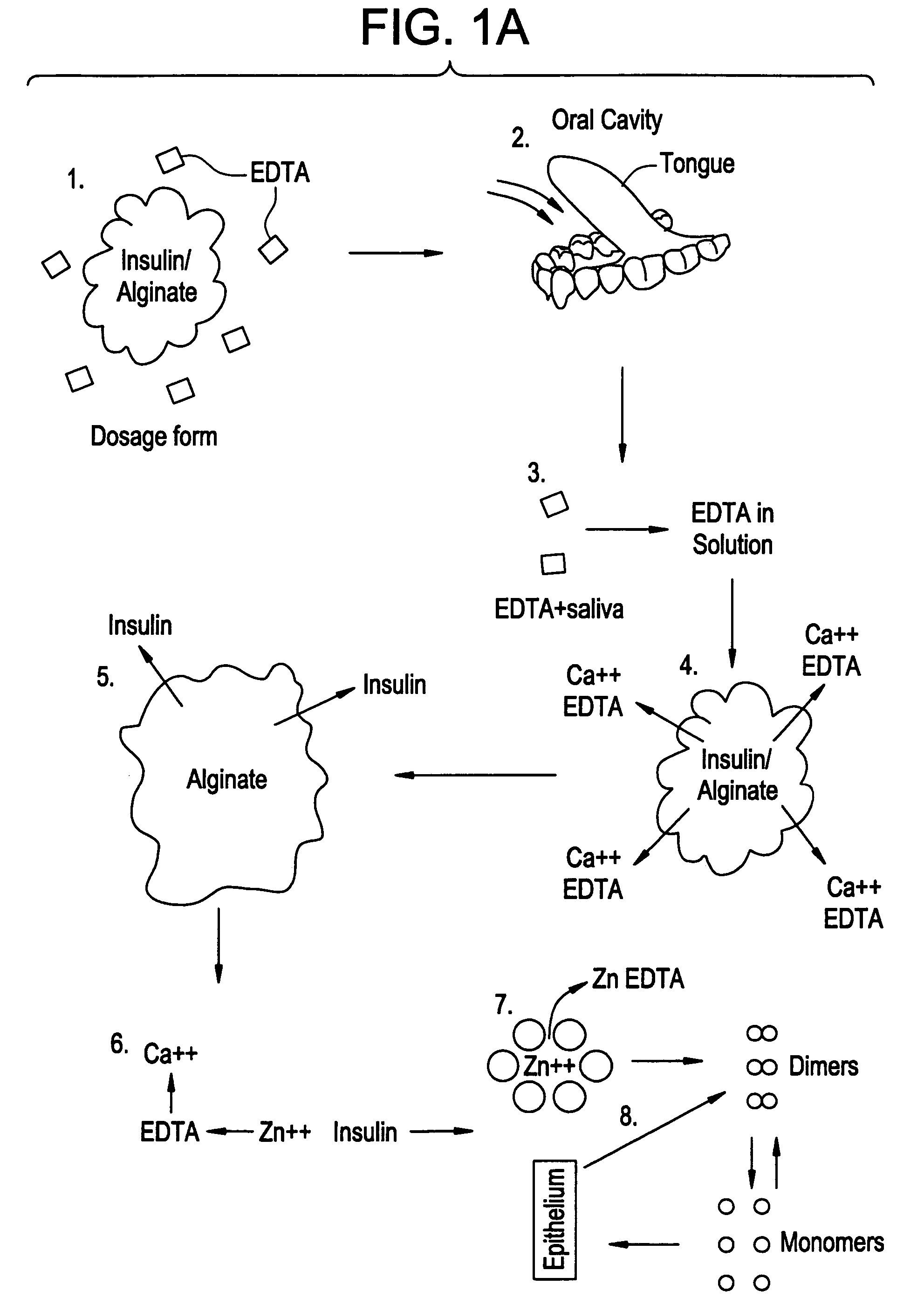
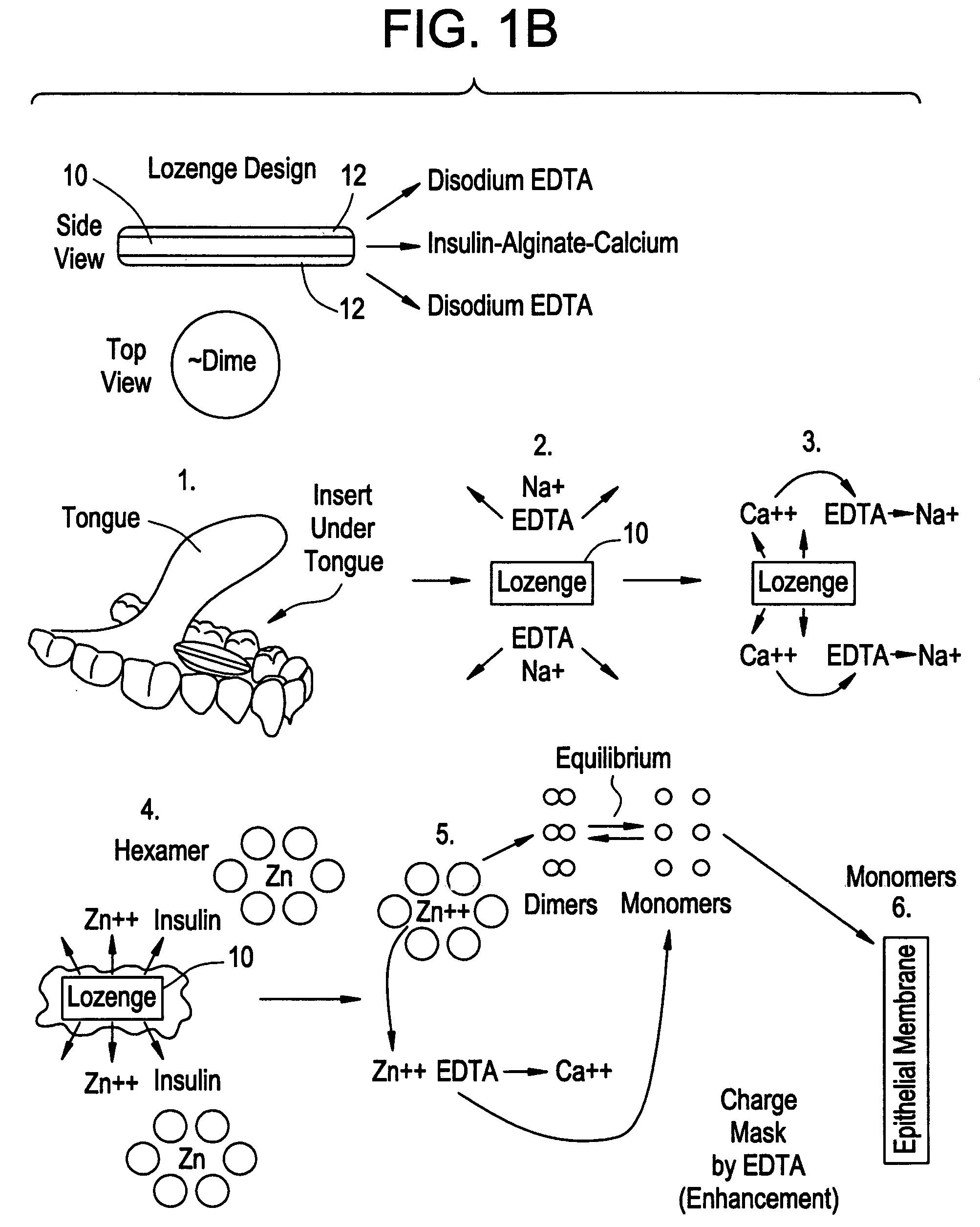
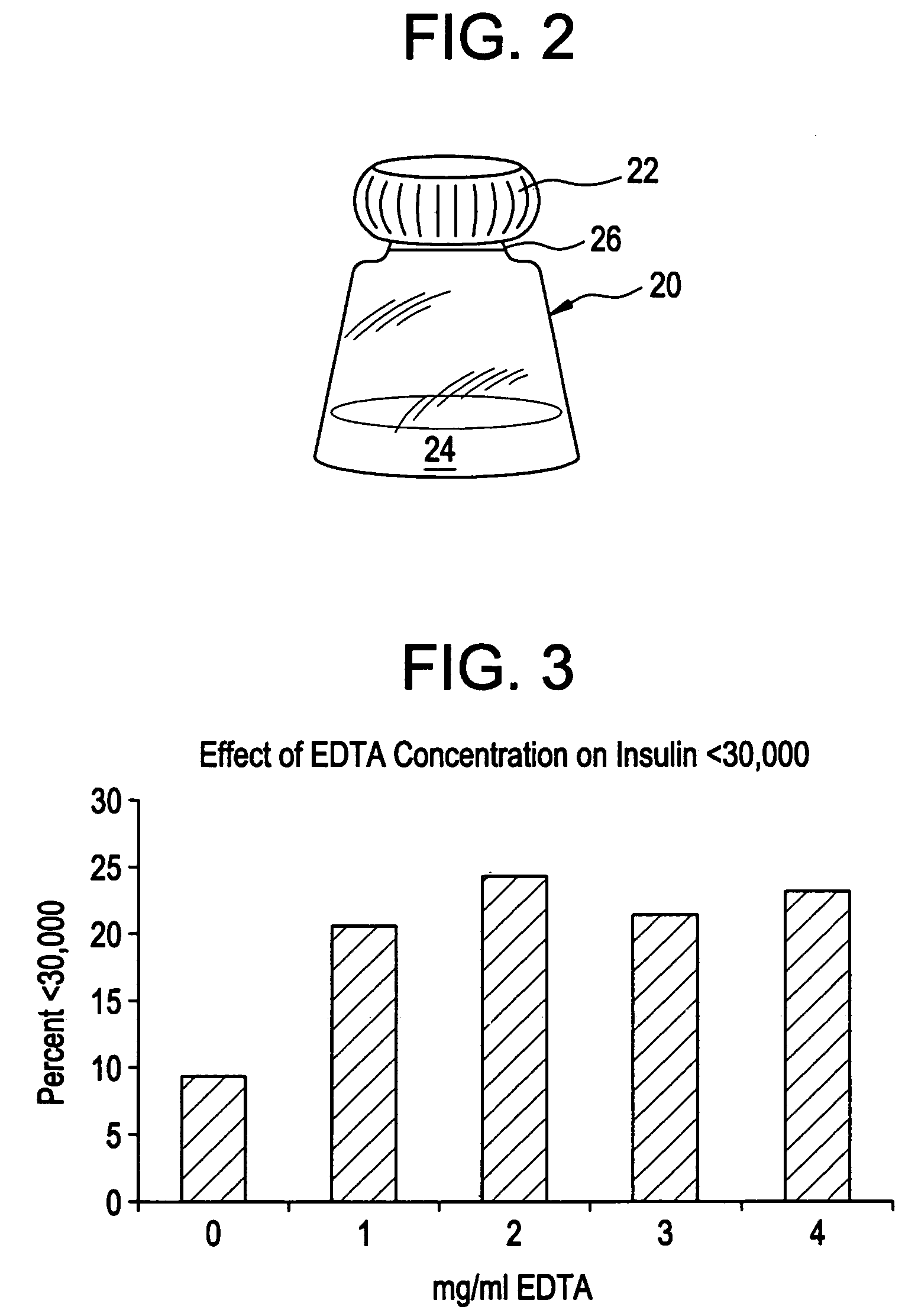
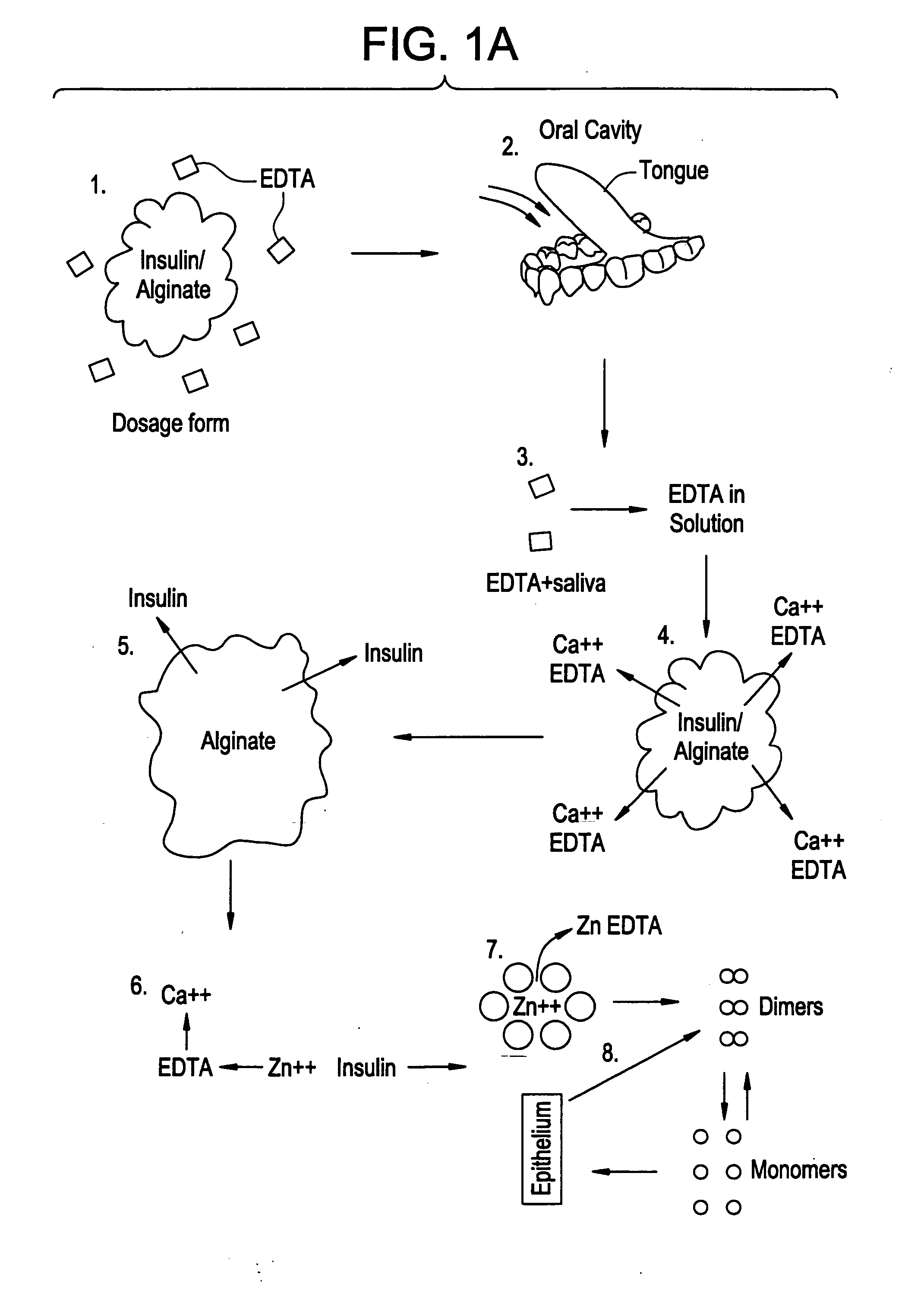
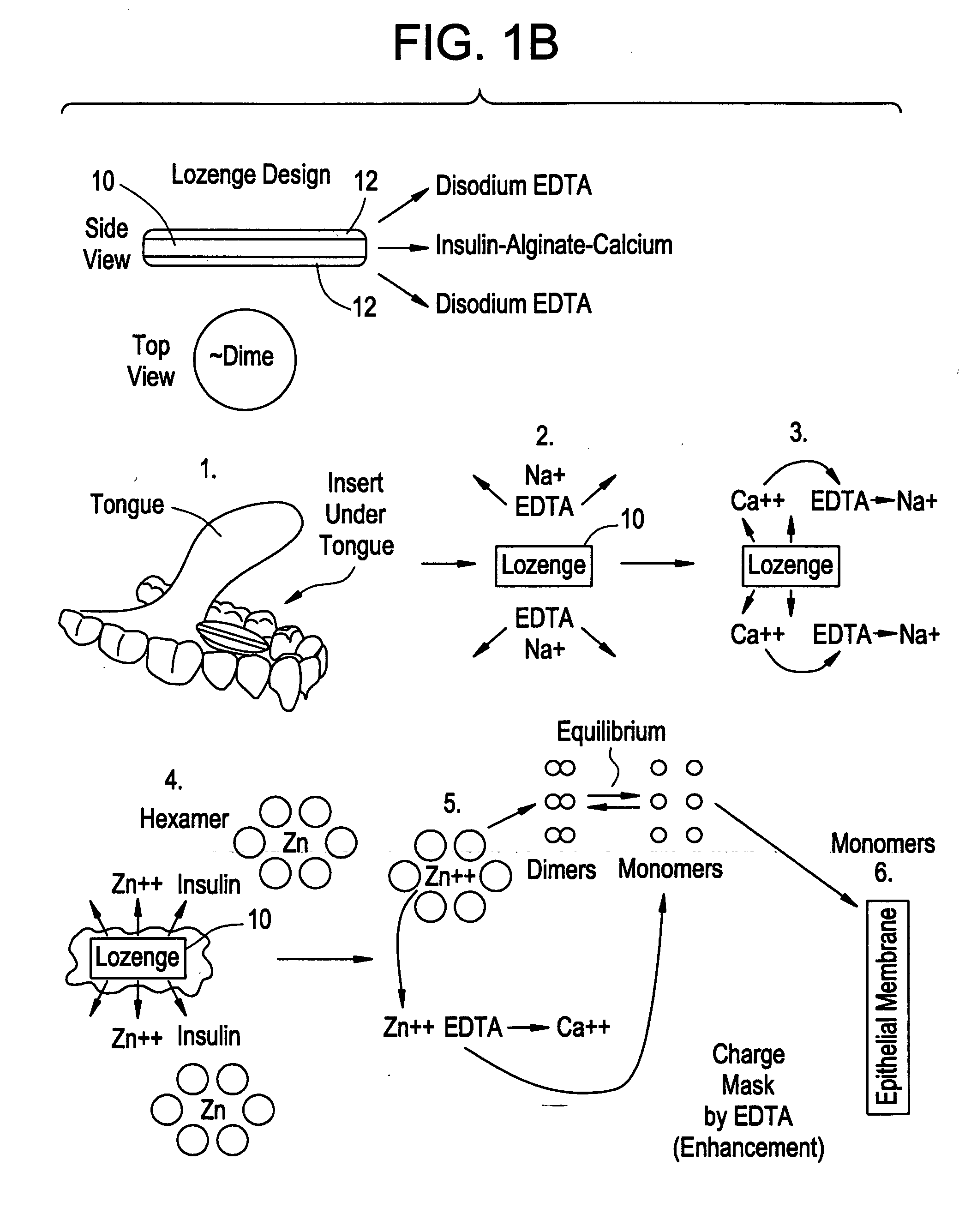
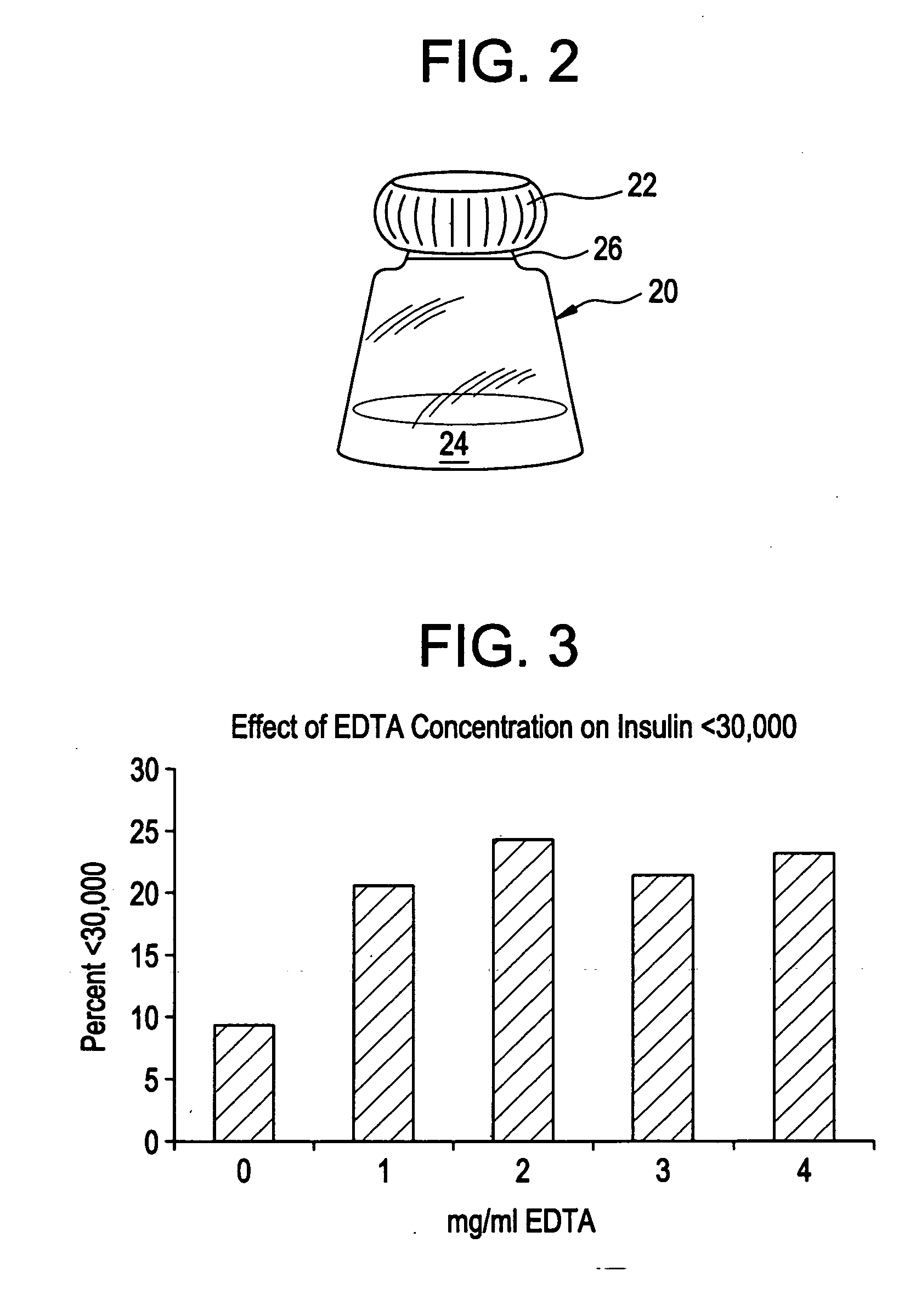
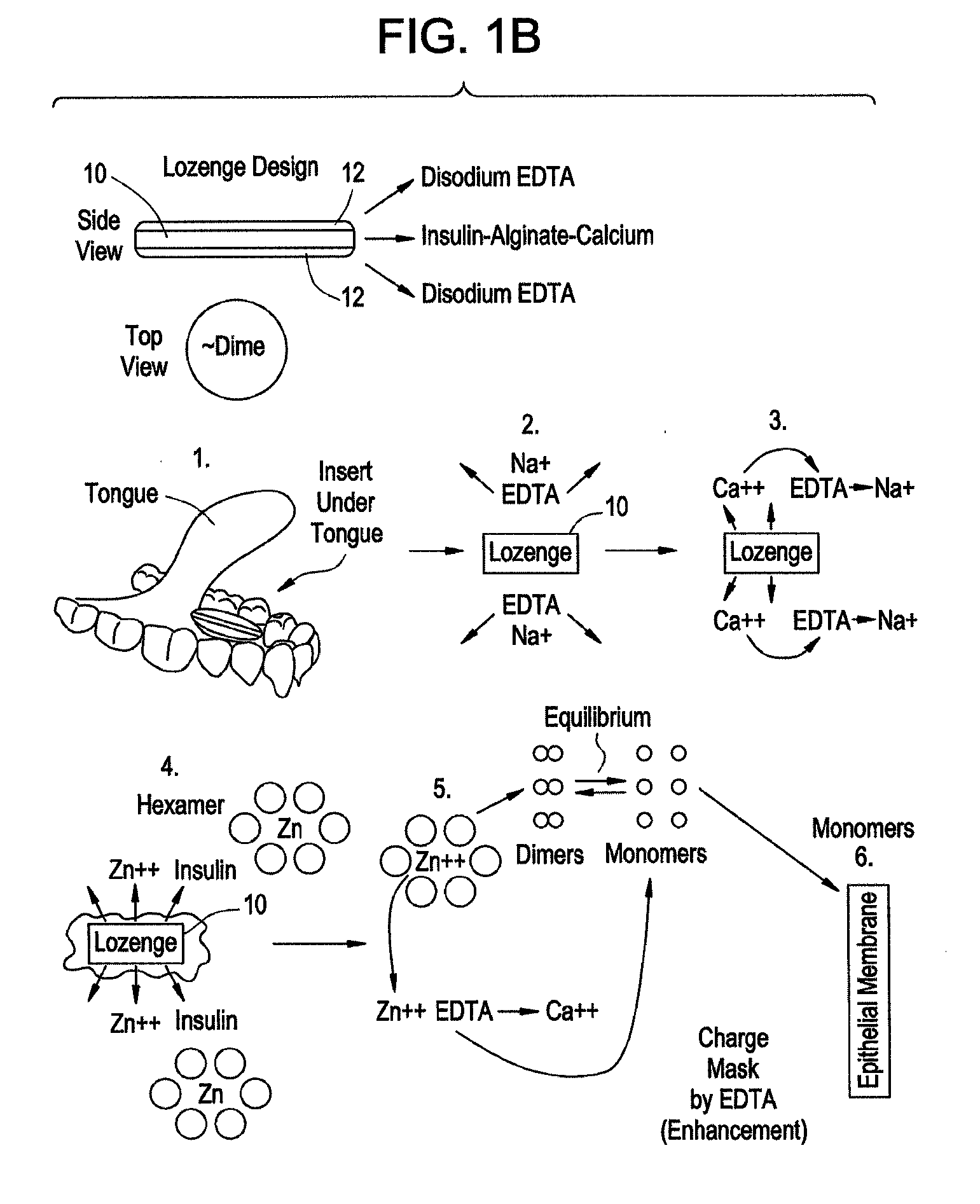
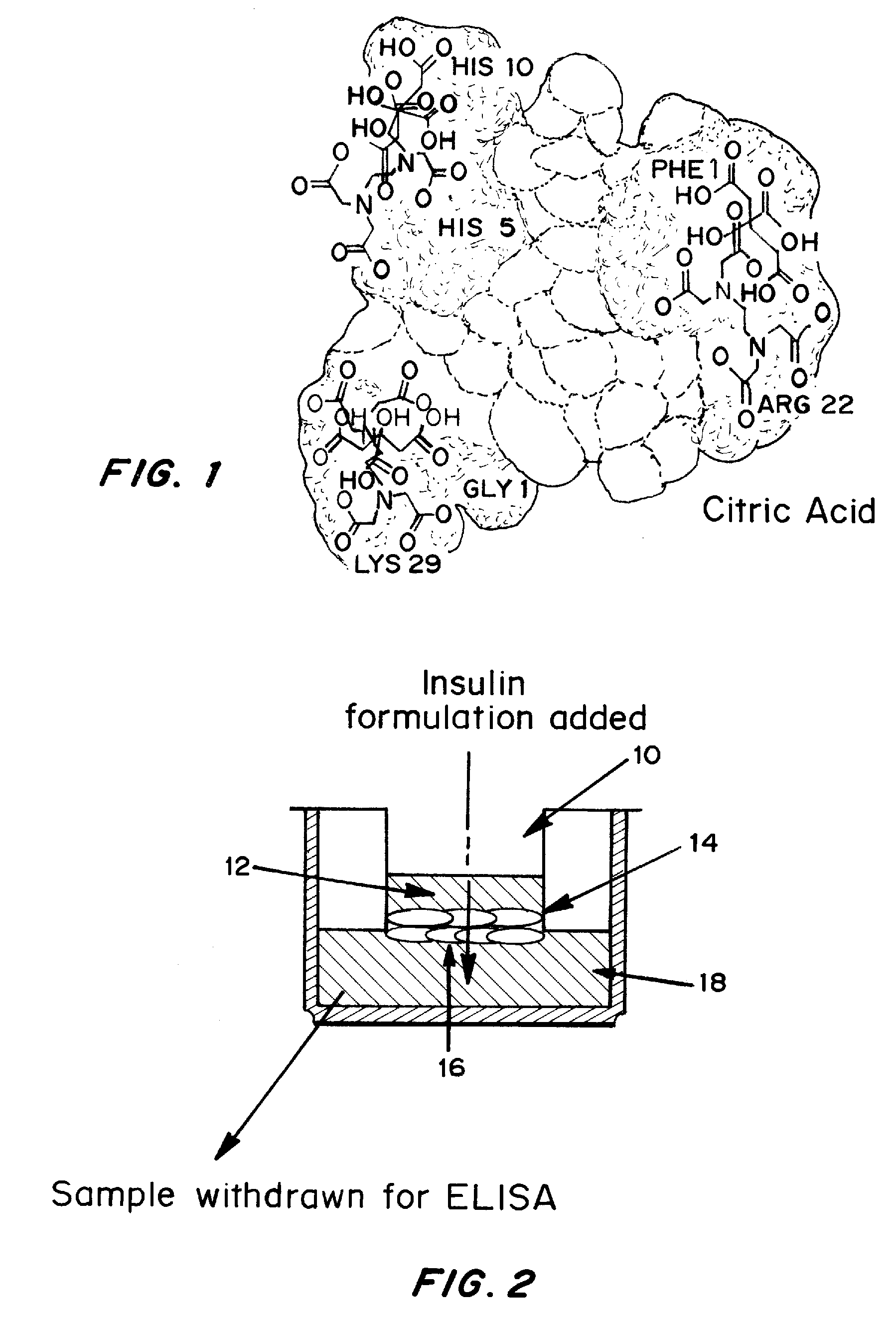
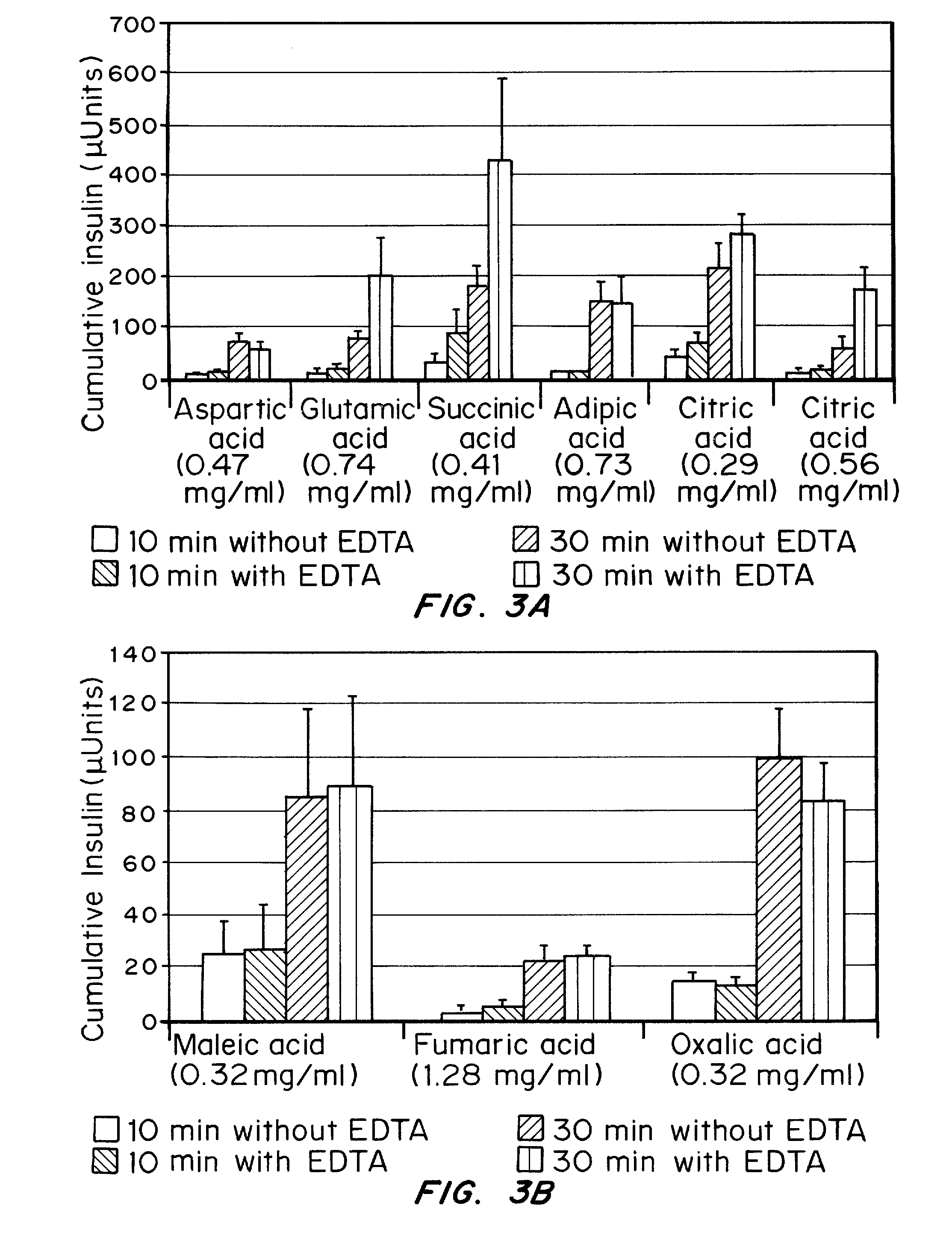
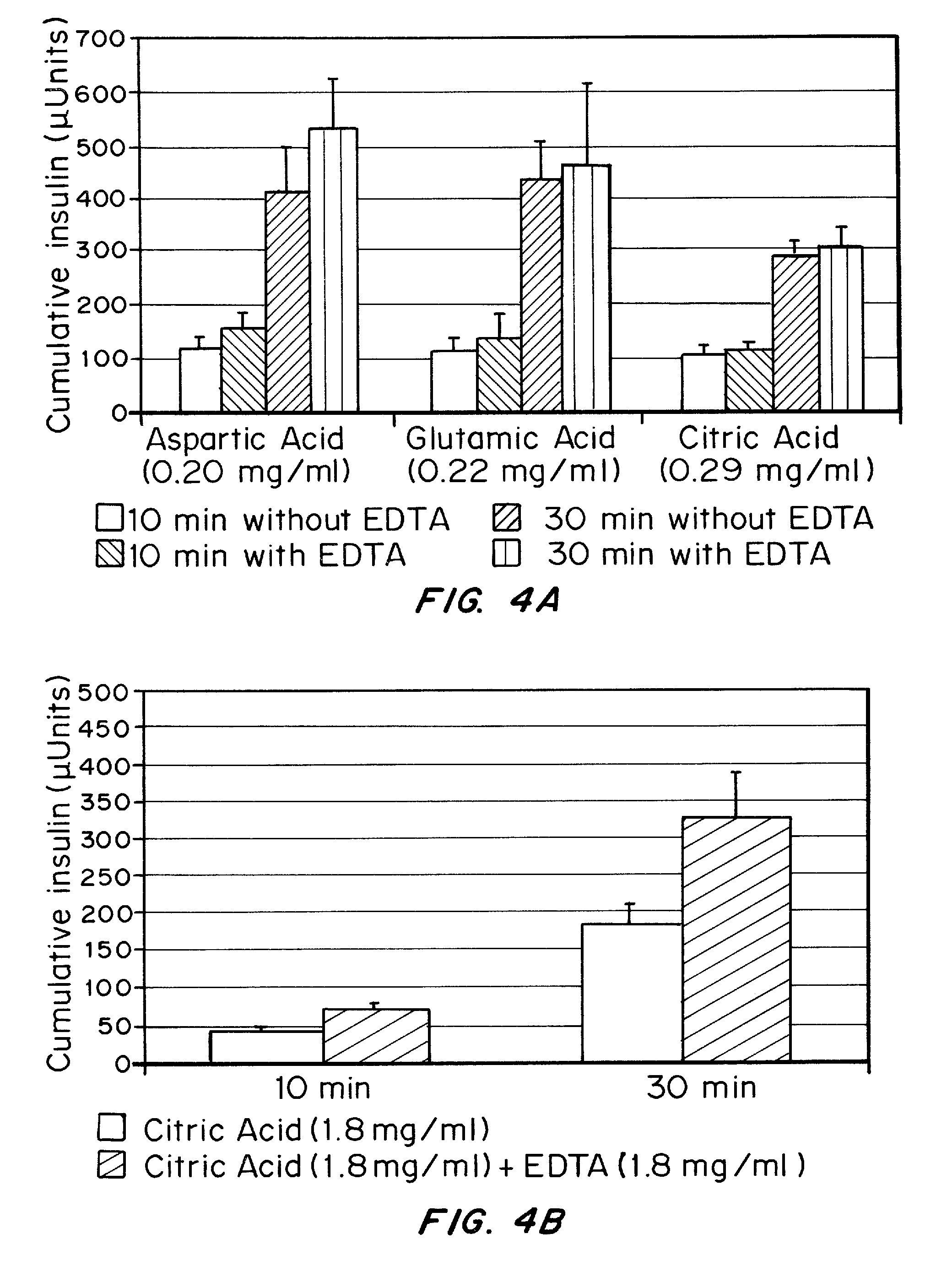
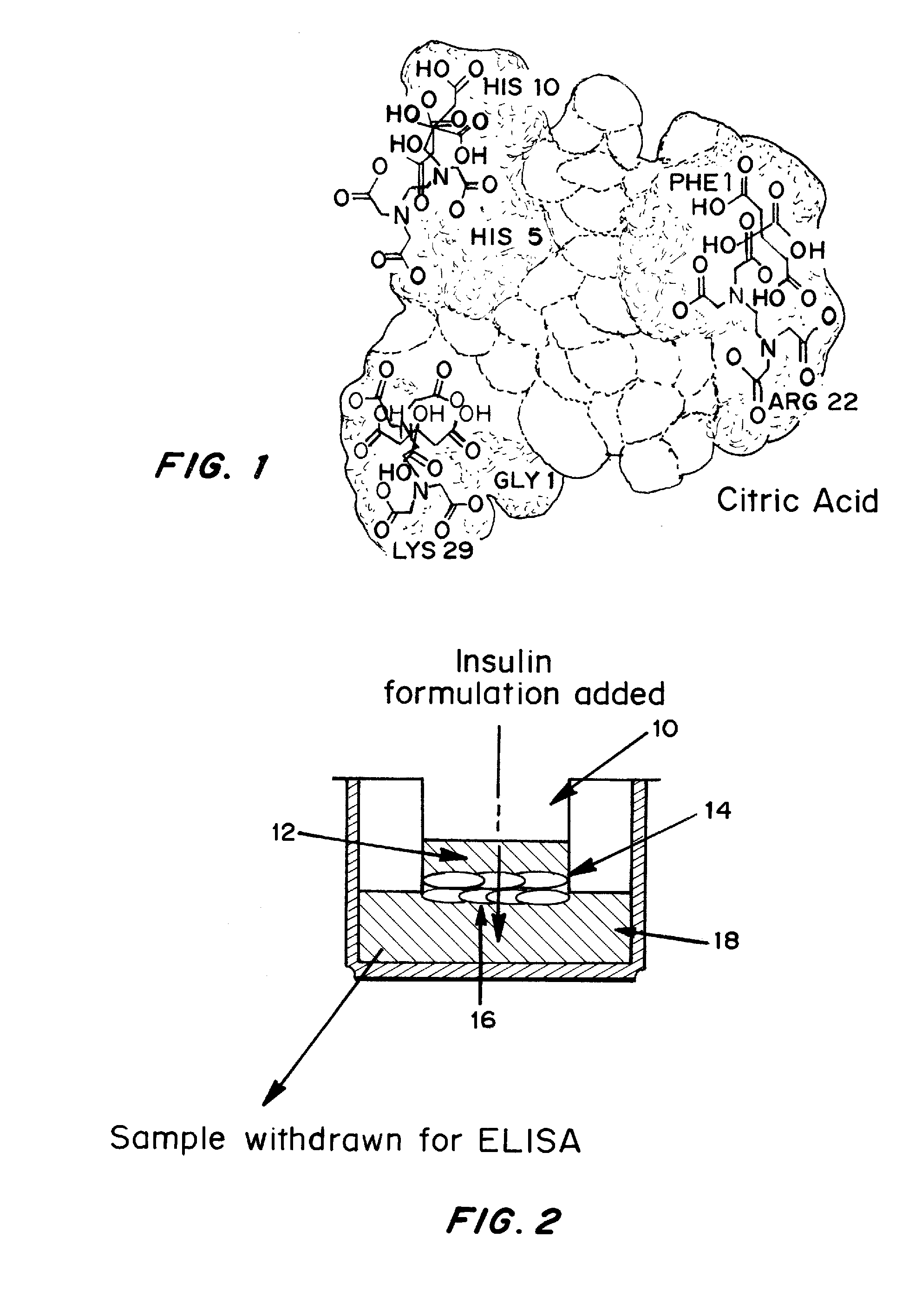
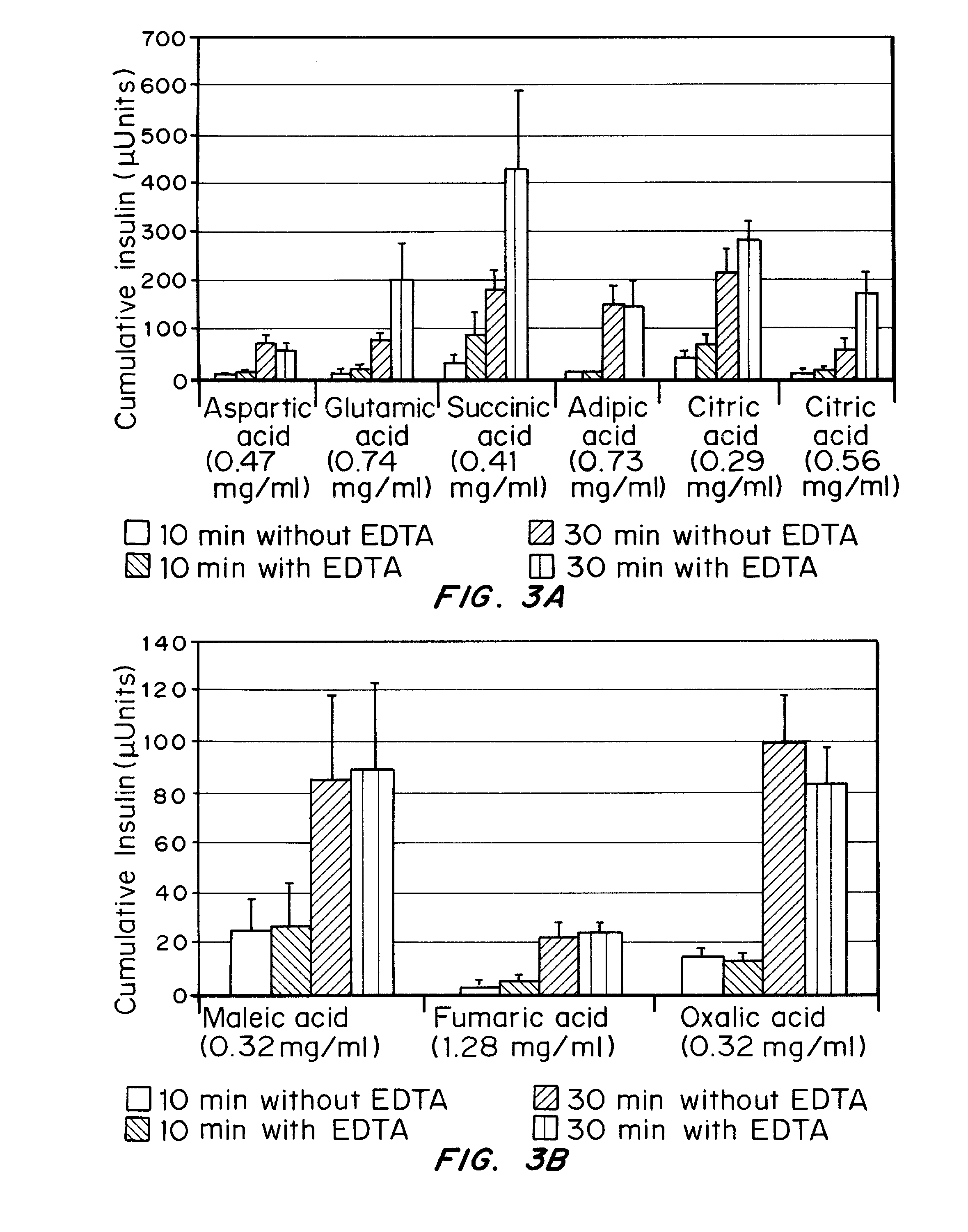
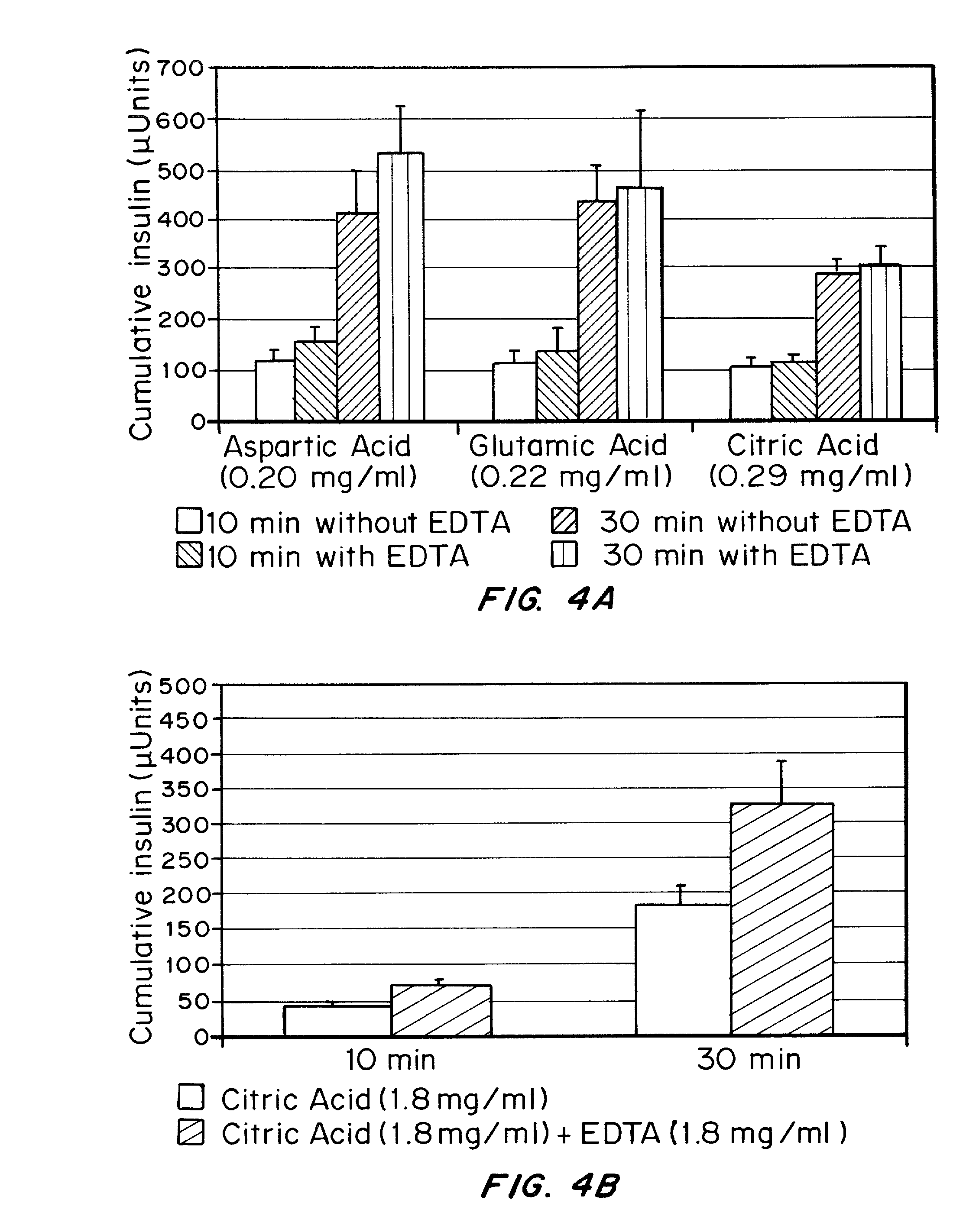
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
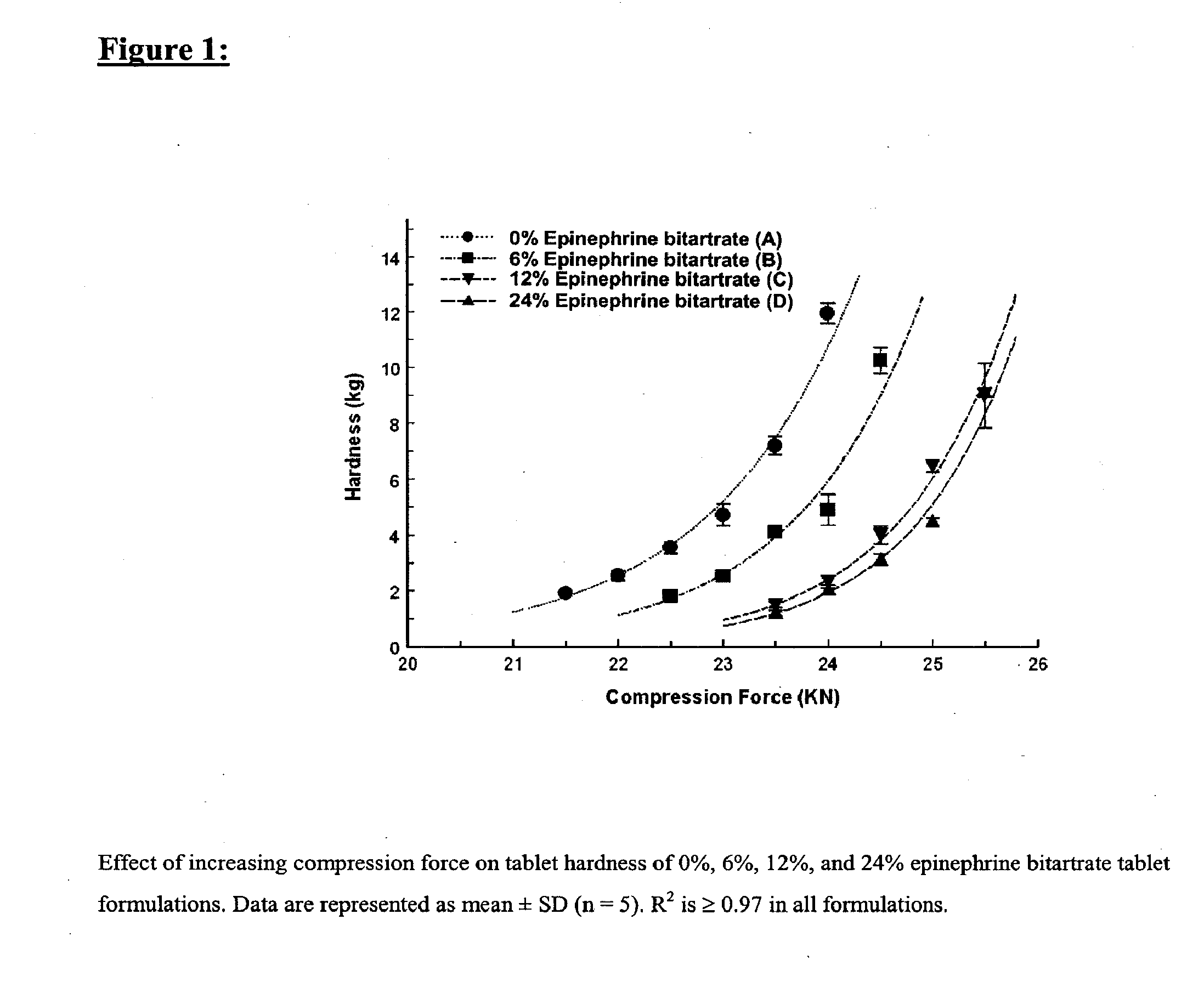
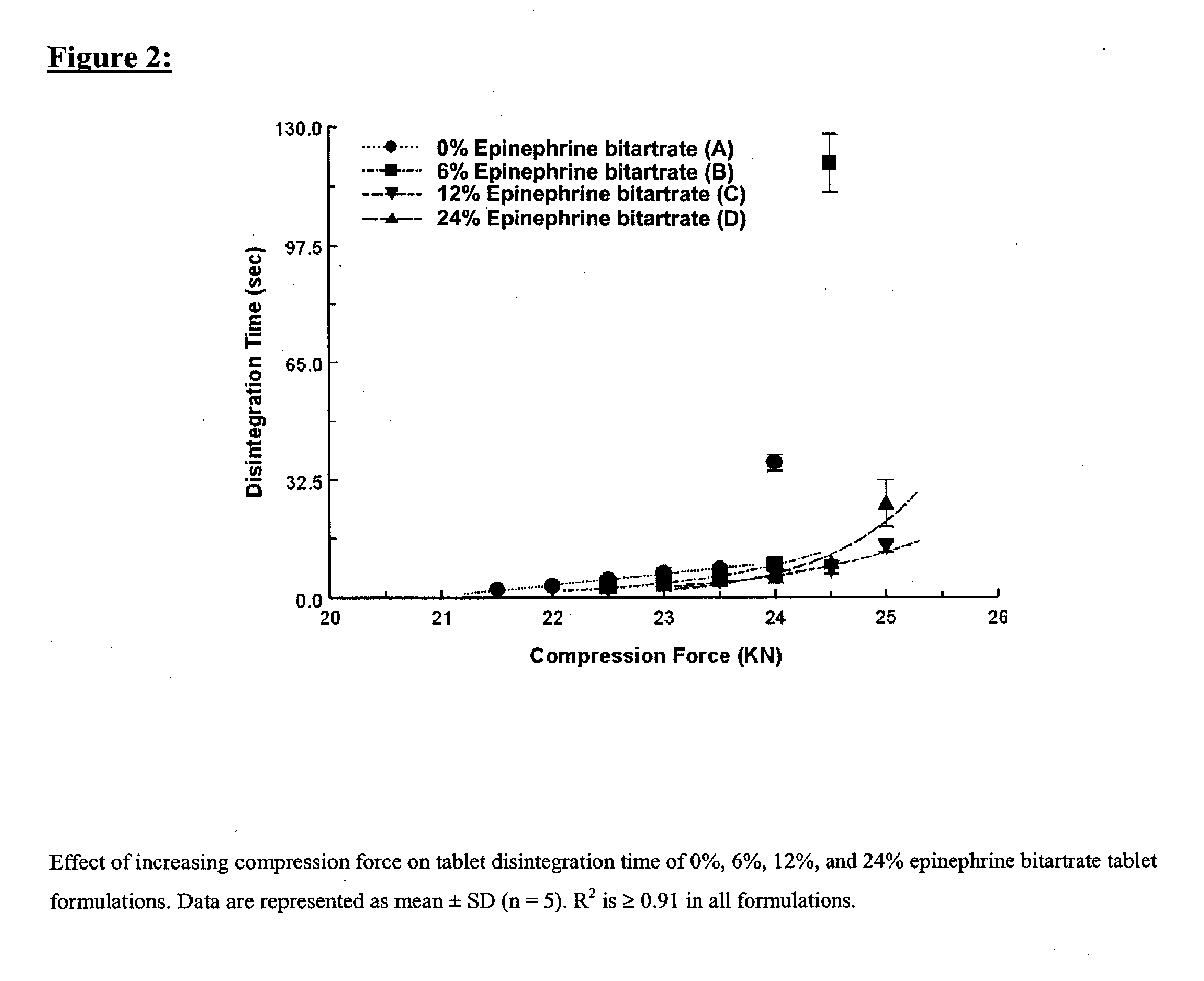
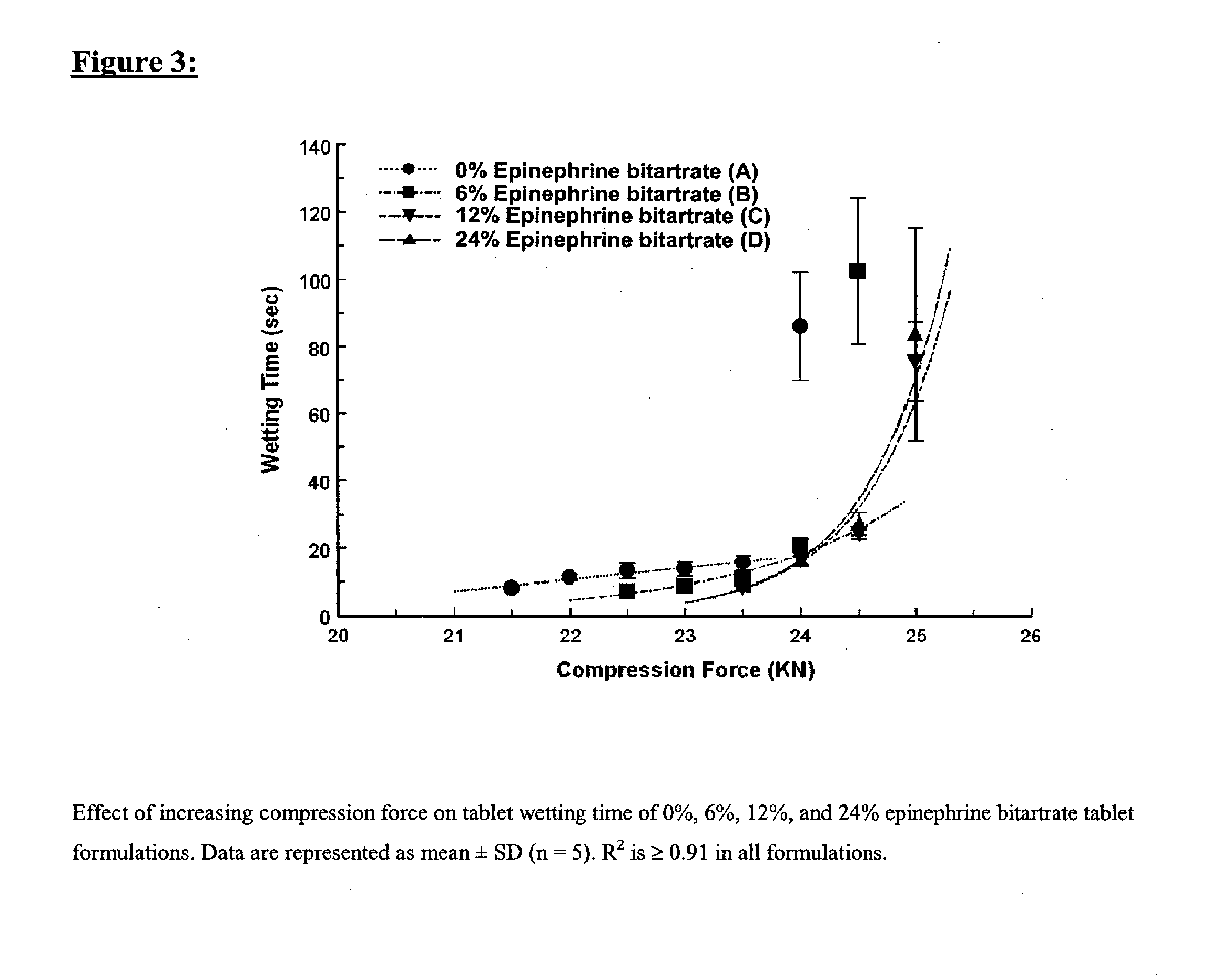
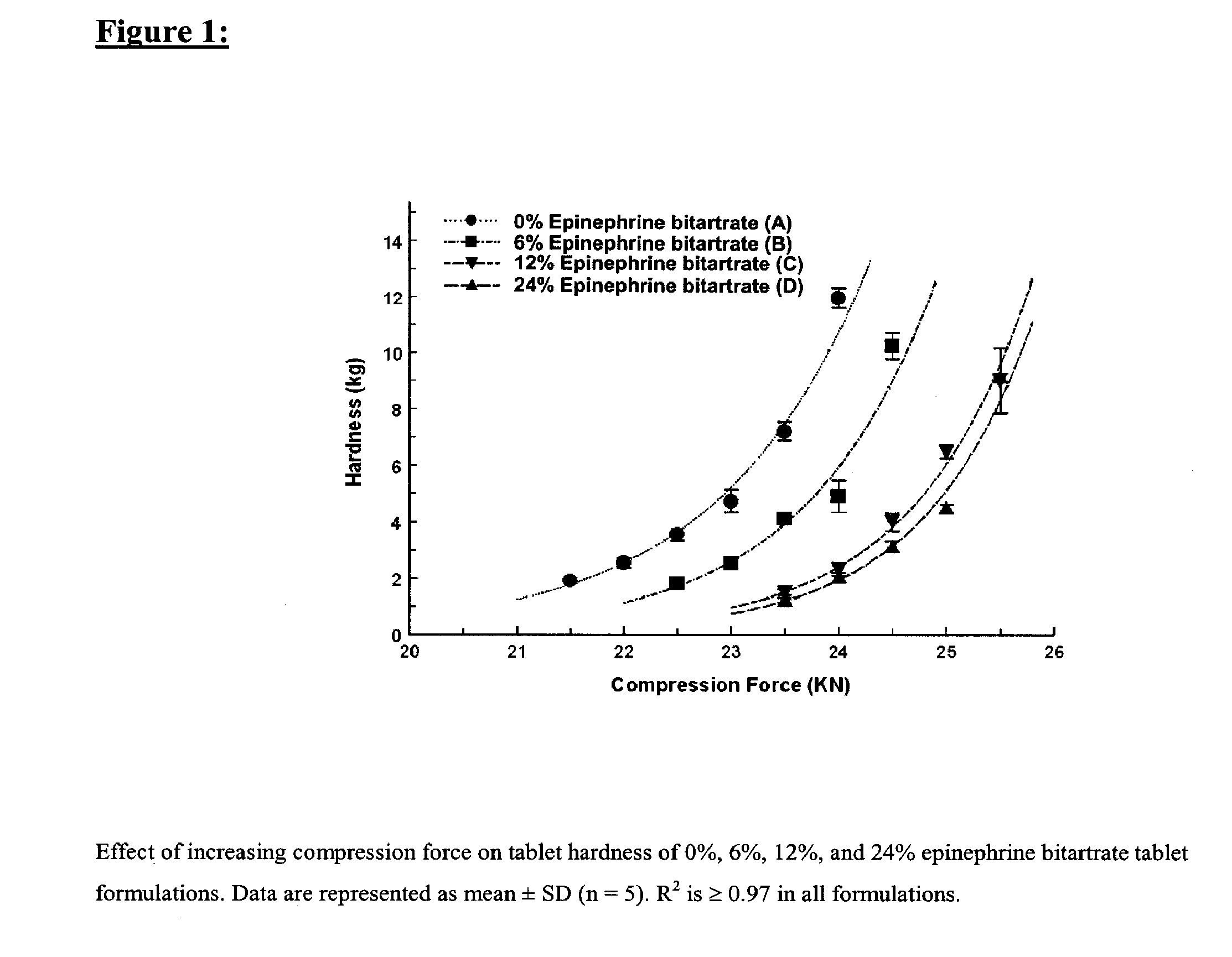
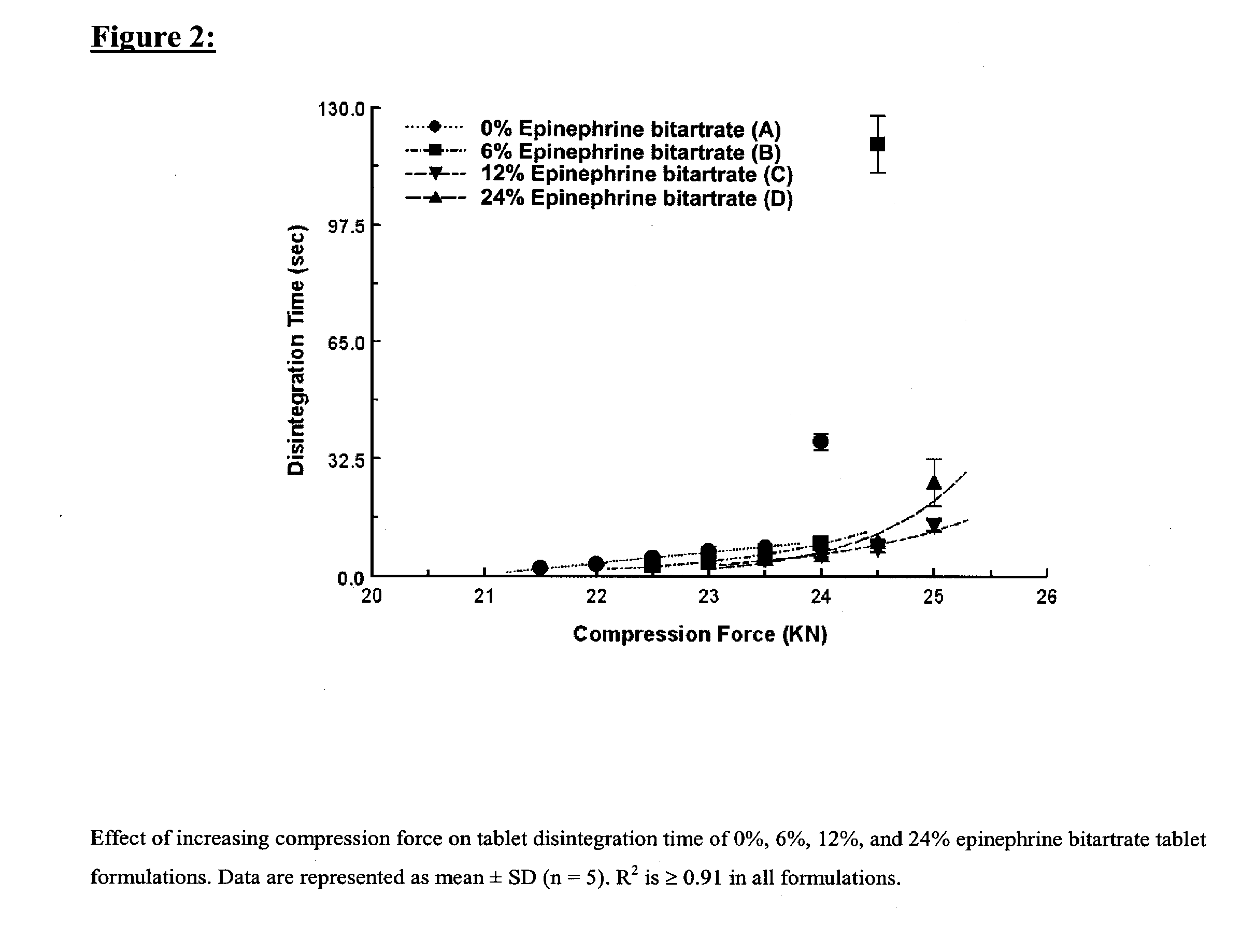
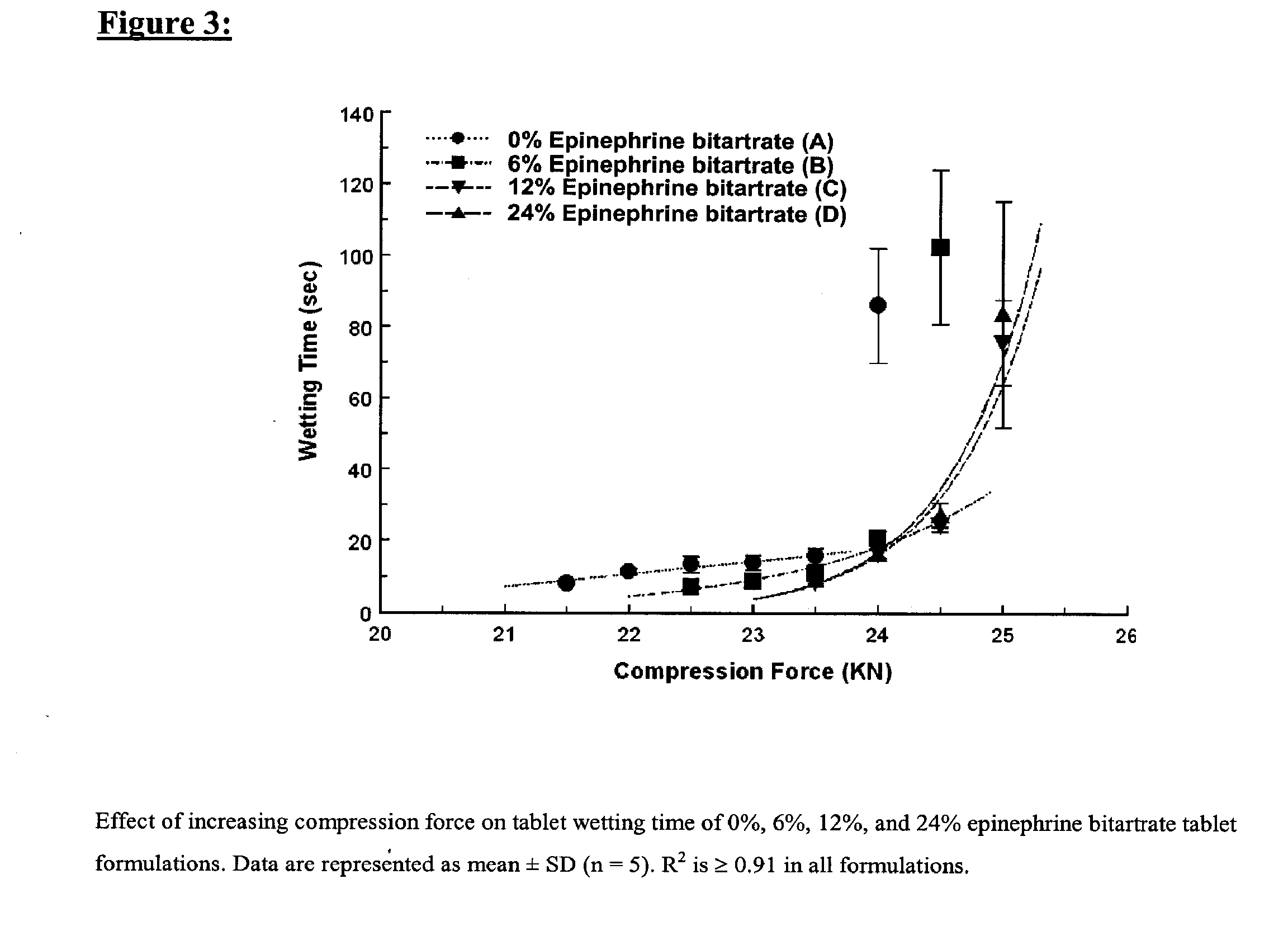
Fast-disintegrating epinephrine tablets for buccal or sublingual administration
Described herein are formulations for fast-disintegrating epinephrine tablets which can be prepared for buccal or sublingual administration, wherein the fast-disintegrating epinephrine tablets can produce plasma epinephrine concentrations substantially equivalent to those achieved by traditional injectable dosage forms comprising epinephrine injected either subcutaneously or intramuscularly.
Owner:UNIVERSITY OF MANITOBA
Fast-disintegrating epinephrine tablets for buccal or sublingual administration
Described herein are formulations for fast-disintegrating epinephrine tablets which can be prepared for buccal or sublingual administration, wherein the fast-disintegrating epinephrine tablets can produce plasma epinephrine concentrations similar to those achieved by an approximately 0.3 mg epinephrine dose in the thigh (Epi-Pen).
Owner:UNIVERSITY OF MANITOBA
Rapid Acting Drug Delivery Compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:BIODEL INC
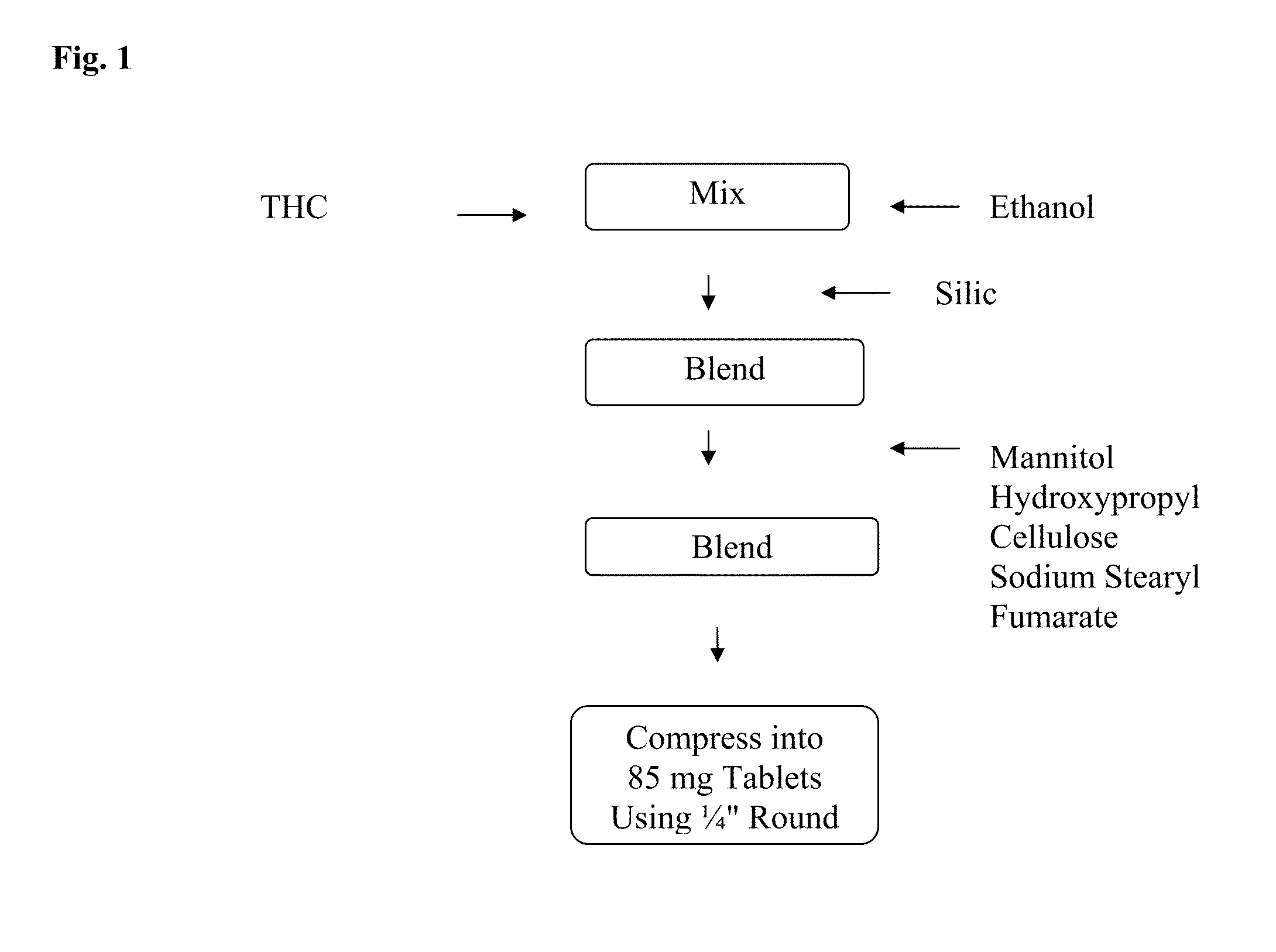
Solid dosage form composition for buccal or sublingual administration of cannabinoids
ActiveUS20160015683A1Rapid onsetImprove oral bioavailabilitySmall article dispensingBiocideCannabisCannabinoid
The present invention relates to solid dosage forms of cannabinoid pharmaceutical formulations comprising a solvated cannabinoid for buccal or sublingual administration, and methods of making and using the same.
Owner:AVENTIS PHARMACETICAL PRODUCTS INC
Rapid mucosal gel or film insulin compositions
InactiveUS20080096800A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsWhole bodyDissolution
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL INC
Sublingual administration of dihydroergotamine for the treatment of migraine
InactiveUS20030008005A1Prevent movementProlong lifeBiocidePharmaceutical delivery mechanismDihydroergotamineSide effect
Owner:ALAMO PHARMA
Pharmaceutical composition based on agonist of benzodiazepine
The present invention describes the use of pharmaceutical compounds in pharmaceutical compositions for sublingual administration, including as active ingredient thereof, an agonist of the central receptor of benzodiazepinics chosen among diazepam, lorazepam, bromazepam, triazolam, alprazolam, flunitrazepam, nitrazepam and midazolam maleate, in a mixture with a pharmaceutical excipient consisting of, at least, 70% of the weight of the final formulation containing 40-45% by weight of lactose, 15-27% by weight of sorbitol and 12-16% by weight of cellulose.
Owner:SIGMA PHARMA LTDA
Methods for Buccal, Lingual or Sublingual Dosing Regimens of Epinephrine for the Treatment of Allergic Emergencies
InactiveUS20070293581A1Patient compliance is goodReduce worriesBiocideOrganic active ingredientsDosing regimenDosage form
The present invention relates to methods of administering dosage forms which comprise epinephrine, including buccal, lingual, sublingual or transmucosal dosage forms comprising epinephrine for treatment of allergic emergencies, including anaphylaxis. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
Rapid Mucosal Gel or Film Insulin Compositions
InactiveUS20080085298A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsNasal cavityWhole body
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL
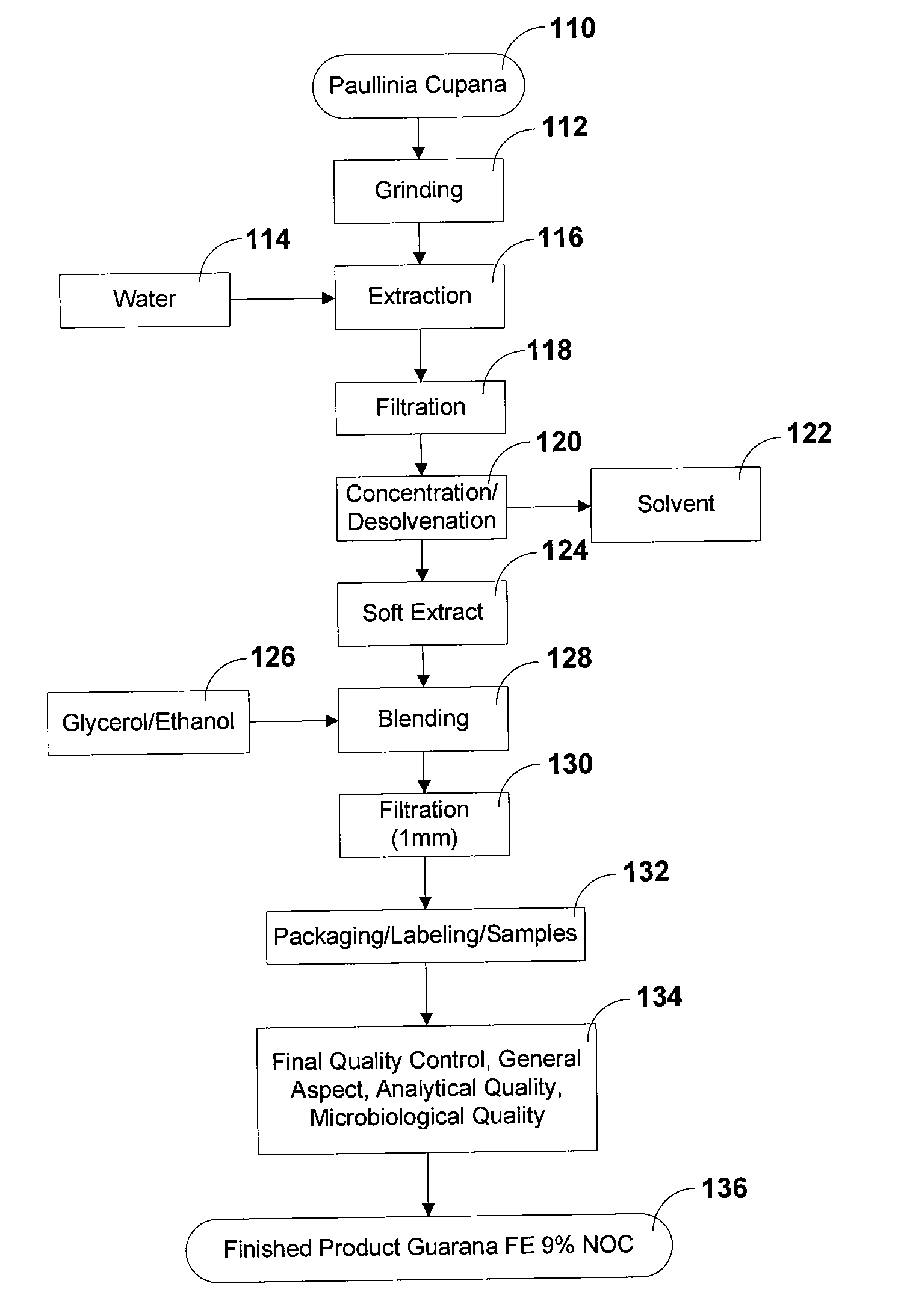
Methods and Systems for Sublingual Guarana Administration
InactiveUS20090155392A1Low variabilityImprove bioavailabilityBiocideNervous disorderBuccal useHerbal supplement
Embodiments of the present invention relate generally to methods and systems for the sublingual and buccal administration of herbal supplements, and more particularly, to the sublingual and buccal administration of Guaraná, which allows for considerably reducing the therapeutic dose, with the additional advantage of increasing the quickness of the beneficial effects.
Owner:NELSON BRET D
Sublingual spray for the treatment of pain
This invention relates to a spray for sublingual administration, used in the treatment and management of pain, especially acute pain. Also provided are methods of treatment and management of pain, a metered dosage system for administration of the spray, and combination therapies.
Owner:UNIV OF KENTUCKY RES FOUND
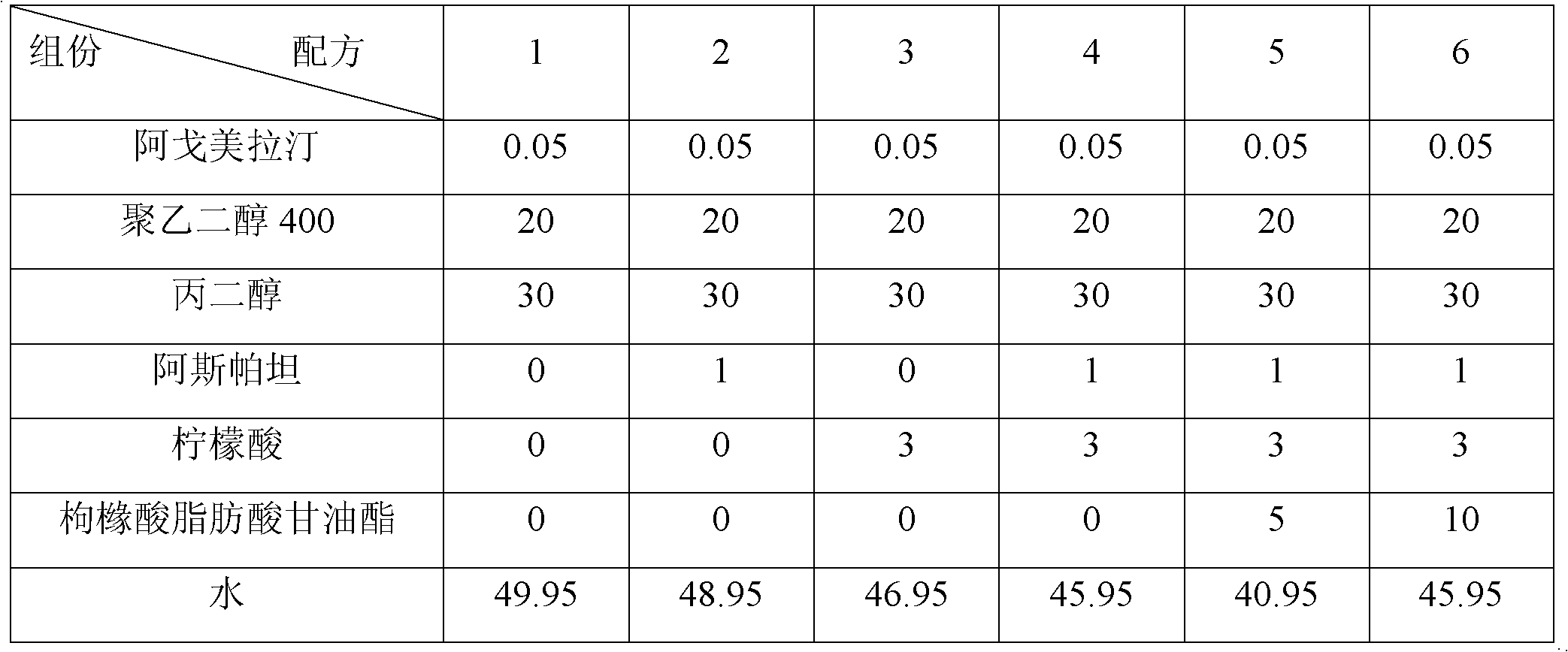
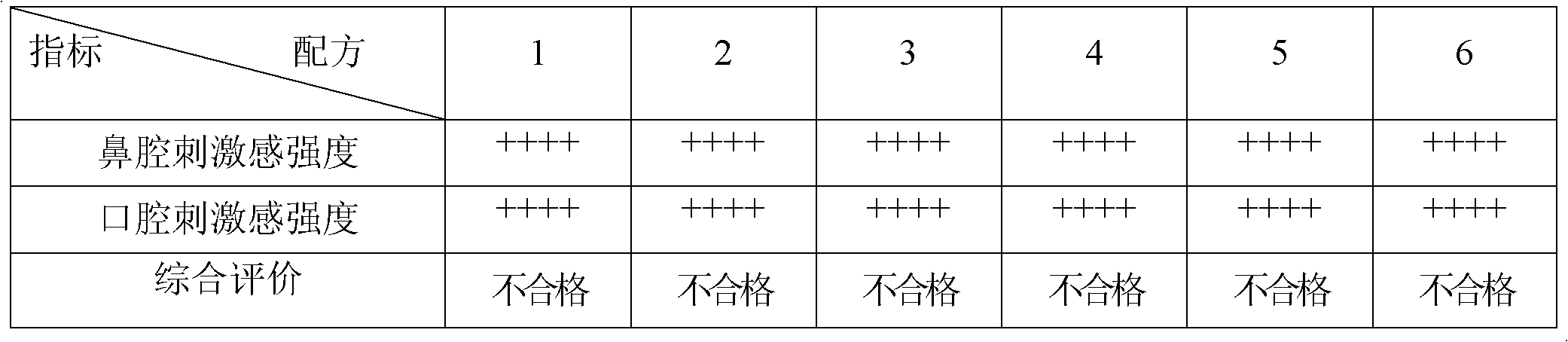
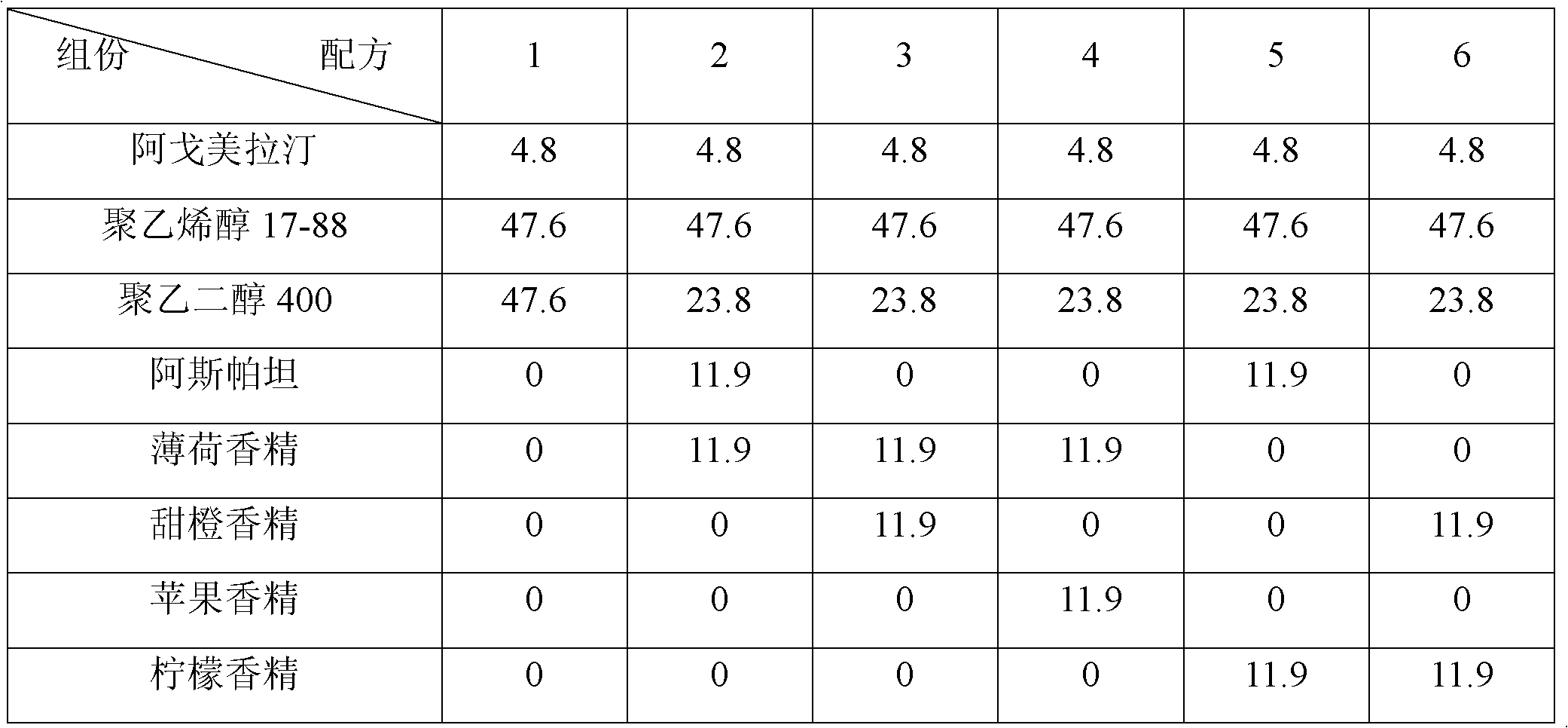
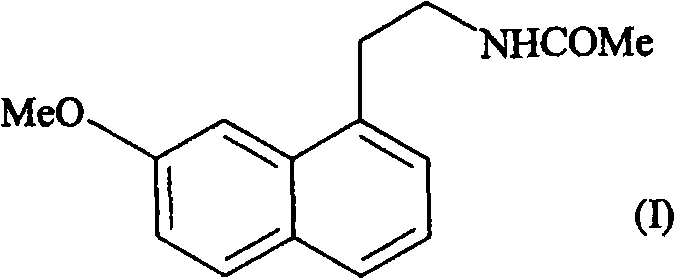
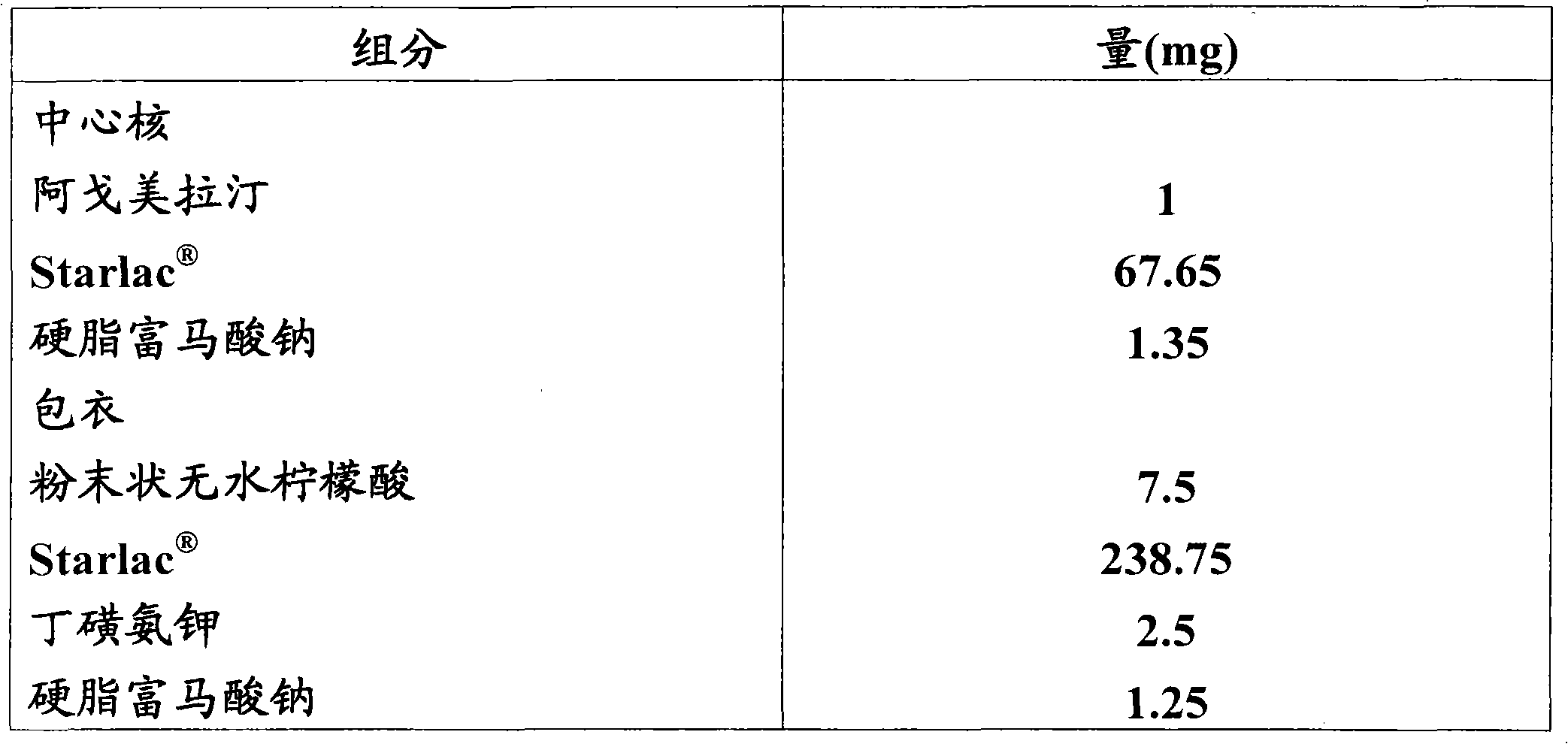
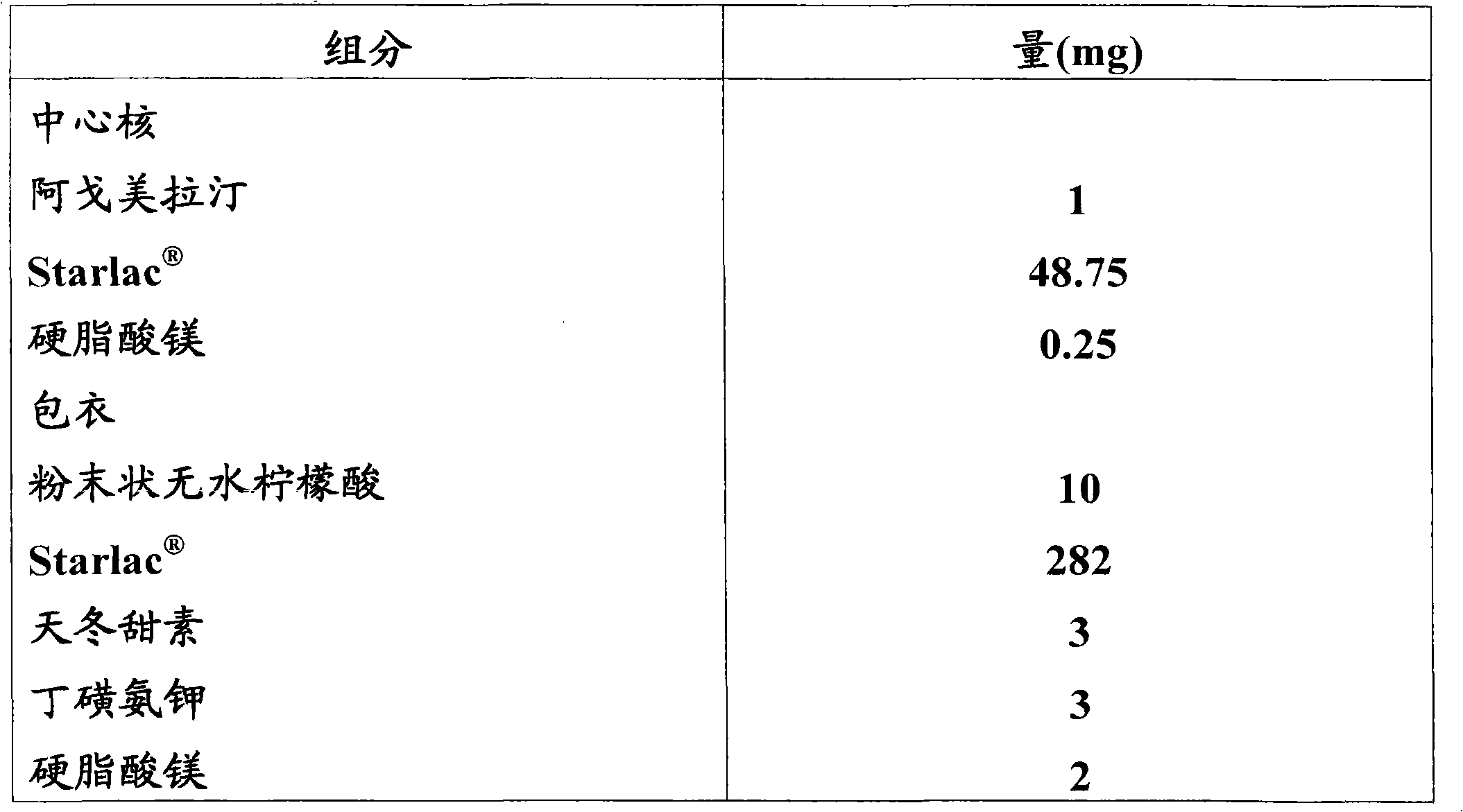
Orodispersible pharmaceutical composition for oromucosal or sublingual administration of agomelatine
InactiveCN1981752AShort disintegration timeSpeed up entryOrganic active ingredientsNervous disorderSublingual administrationOral cavity
The invention relates to a coated solid orodispersible pharmaceutical composition for oral, oromucosal or sublingual administration of agomelatine.
Owner:LES LAB SERVIER
Compound of losartan compound or its medical salt and calcium channel blocker or its medical salt
InactiveCN101347427ALittle side effectsGood curative effectOrganic active ingredientsCardiovascular disorderCarboxylic acidLosartan
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Buccal and sublingual administration of physostigmine
Physostigmine, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b] indol-5ol methylcarbamate, administered buccally or sublingually in non-sustained release dosage form provides extremely prolonged blood levels. This active agent is physically compounded with materials of some or all of classes of ingredients that function as pH controls, preservative agents, viscosity control agents, absorption enhancers, stabilizing agents, solvents, and carrier vehicles. This compounding will produce a pharmaceutical composition in the form of a liquid, tablet, gel, patch or lozenge for administration of the active agent, Physostigmine, by absorption through the buccal or sublingual mucosa of the patient. This method of delivery of Physostigmine and similar compounds is useful for treatment of cognitive deficiencies and / or neurological function deficits, mood and / or mental disturbances in mammals including human beings.
Owner:MADHAT MAHER N
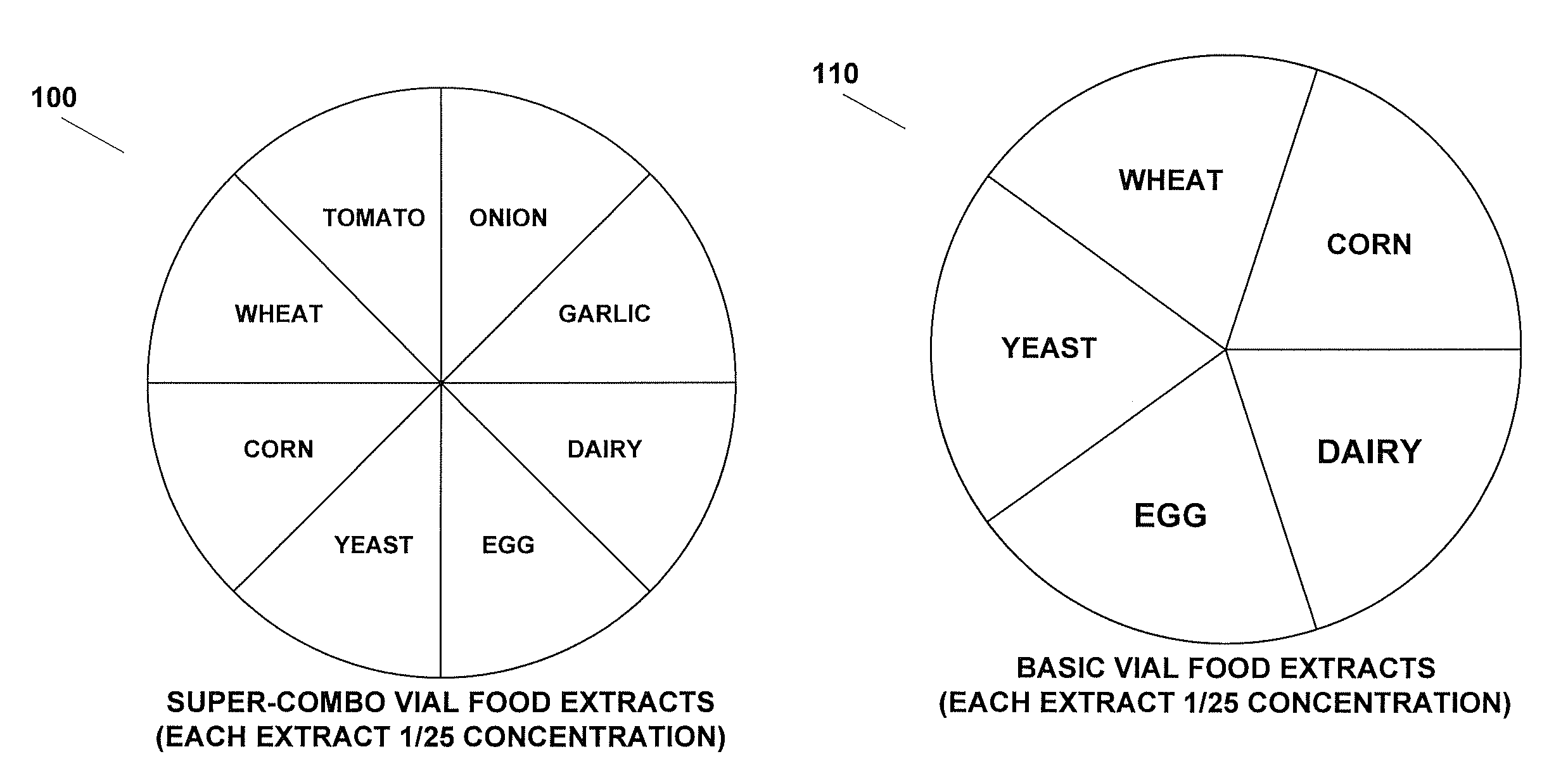
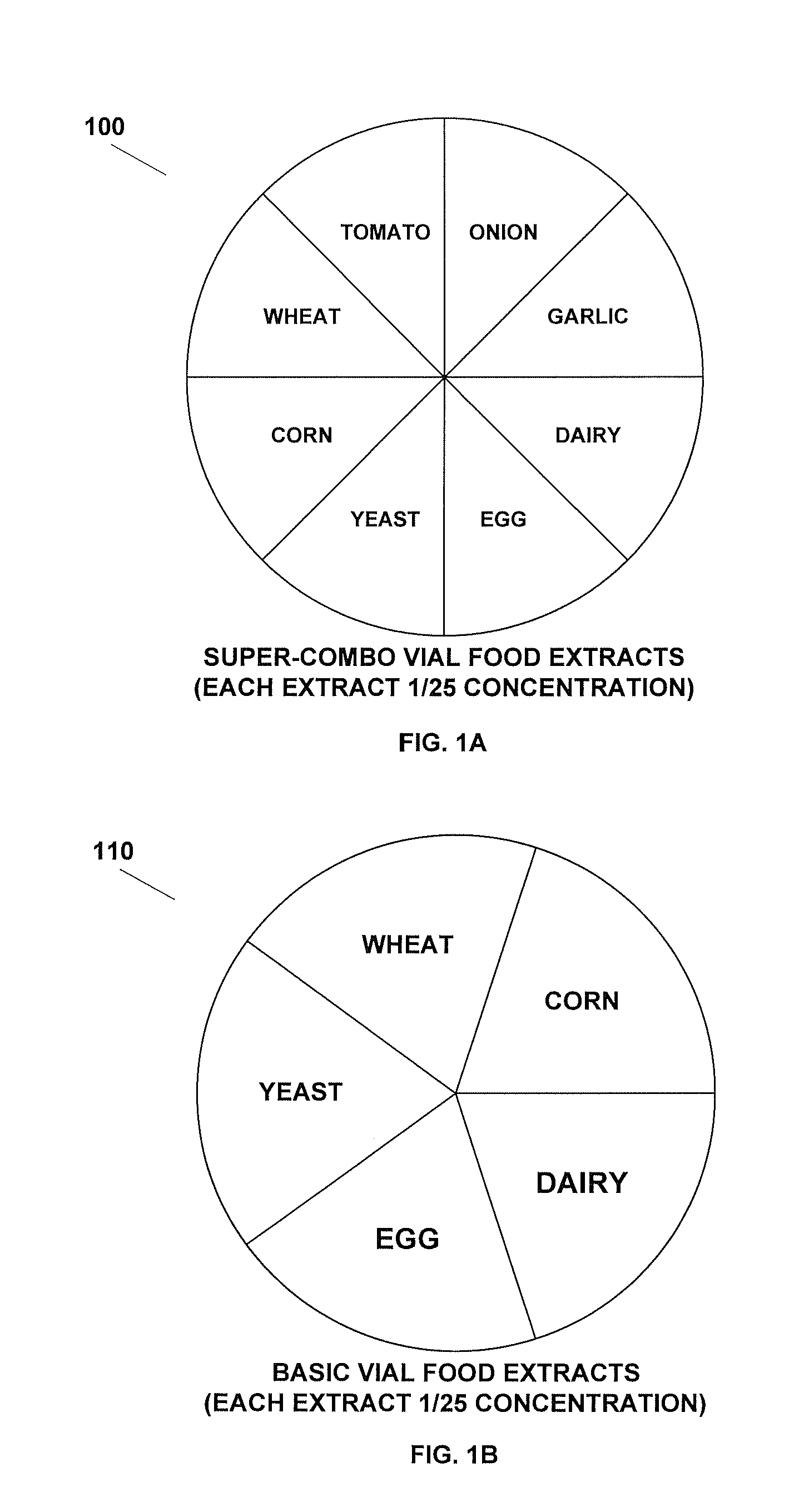
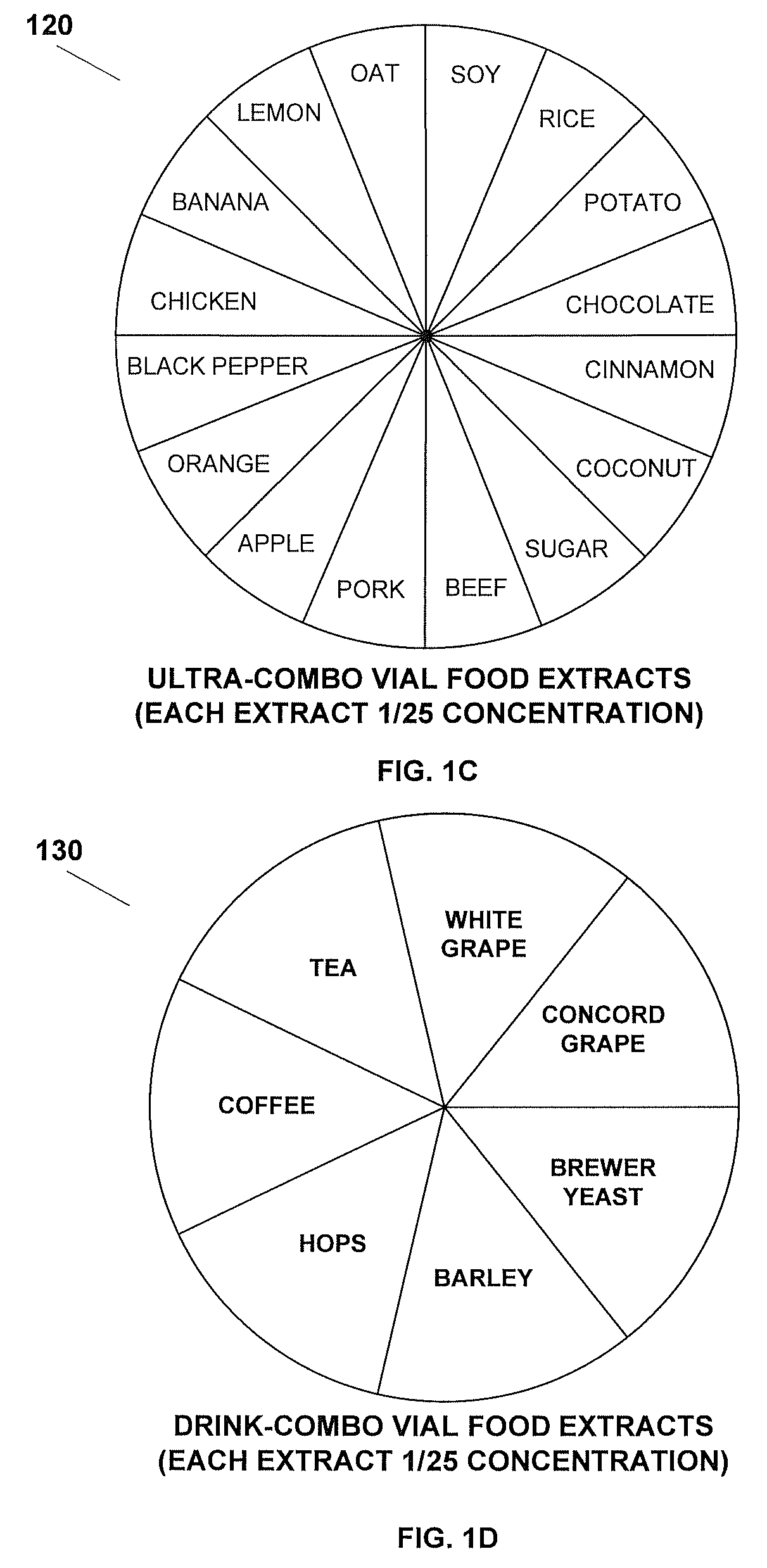
Method for testing and treating delayed food allergies
A method for testing, treating, and preventing delayed food allergies includes: receiving detailed symptom, medical, and dietary histories from a patient; formulating a combination of one or more food extracts at selected concentration for sublingual administration over a trial period; determining whether the patient's symptoms have improved, worsened, or had no change, in response to the administration of the combination; and altering the combination in response to whether the patient's symptoms have improved, worsened, or not changed, so as to induce immune system food tolerance.
Owner:SHEA JOHN
Verifiable absorption drug delivery form based on cyclodextrins
Solid dosage forms are produced by absorbing solutions of drug:cyclodextrin inclusion complexes on absorbing matrices and then drying. The matrices may, but need not, disintegrate in water. The resulting forms are suitable for oral or sublingual administration, and also can be used in topical administrations to mucosa covered tissues. They are particularly suitable for sublingual administration because the eluted, nondisintegrated matrix (e.g., absorbing paper) can be recovered and checked for completion of elution. This enables documentation that effective absorption occurred from the mouth cavity.
Owner:PITHA JOSEF
Agomelatine-containing medicinal composition for oral mucosa or sublingual administration
ActiveCN102579415AEasy to prepareLow costOrganic active ingredientsNervous disorderHepatic first pass effectSublingual administration
The invention discloses an agomelatine-containing medicinal composition for oral mucosa or sublingual administration and a preparation method thereof. The medicinal composition consists of a main medicament of which the particle diameter is 48-250 mum and is preferably 48-150 mum, and medicinal auxiliary materials. The medicinal composition can be prepared through a simple preparation process without special production equipment, has low manufacturing cost, and is suitable industrial production; moreover, the use compliance of a patient is improved through the excitement of the taken medicament in an oral cavity; and the medicinal composition is absorbed directly through oral mucosa, so that the first pass effect of the conventional oral medicament is avoided, and the bioavailability is high.
Owner:CHENGDU KANGHONG PHARMA GRP
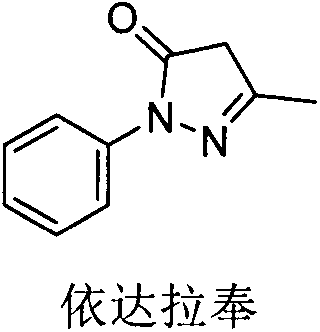
Edaravone and (+)-2-camphol sublingual medicine composition
InactiveCN107773545AHydroxy compound active ingredientsPill deliveryPharmaceutical drugSublingual administration
The invention discloses an edaravone and (+)-2-camphol composition sublingual medication preparation and a preparation method thereof, wherein the sublingual medication preparation is prepared from edaravone, (+)-2-camphol, excipients, filling agents, bonding agents, disintegrating agents, lubricating agents and the like. The sublingual medication preparation has the advantages that the first passeffect of the liver can be avoided; the sample stability is high; the transportation is convenient; the use is convenient, and the like.
Owner:YANTAI YENEPHARMA BIOMEDICAL
Methods for Buccal, Lingual or Sublingual Dosing Regimens of Epinephrine for the Treatment of Allergic Emergencies
InactiveUS20070293580A1Patient compliance is goodReduce worriesBiocideOrganic active ingredientsDosing regimenMedicine
The present invention relates to methods of administering dosage forms which comprise epinephrine, including buccal, lingual, sublingual or transmucosal dosage forms comprising epinephrine for treatment of allergic emergencies, including anaphylaxis. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
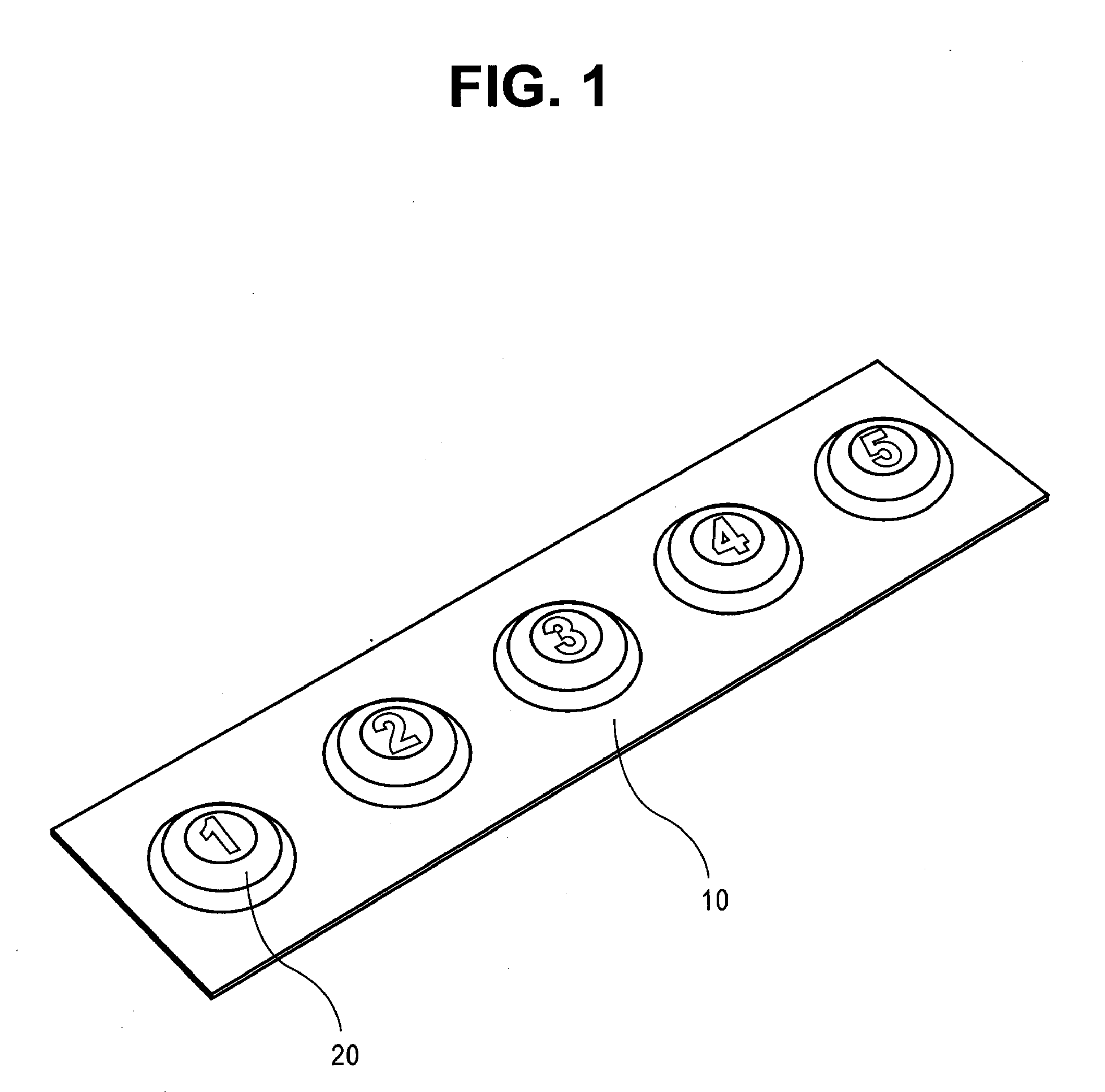
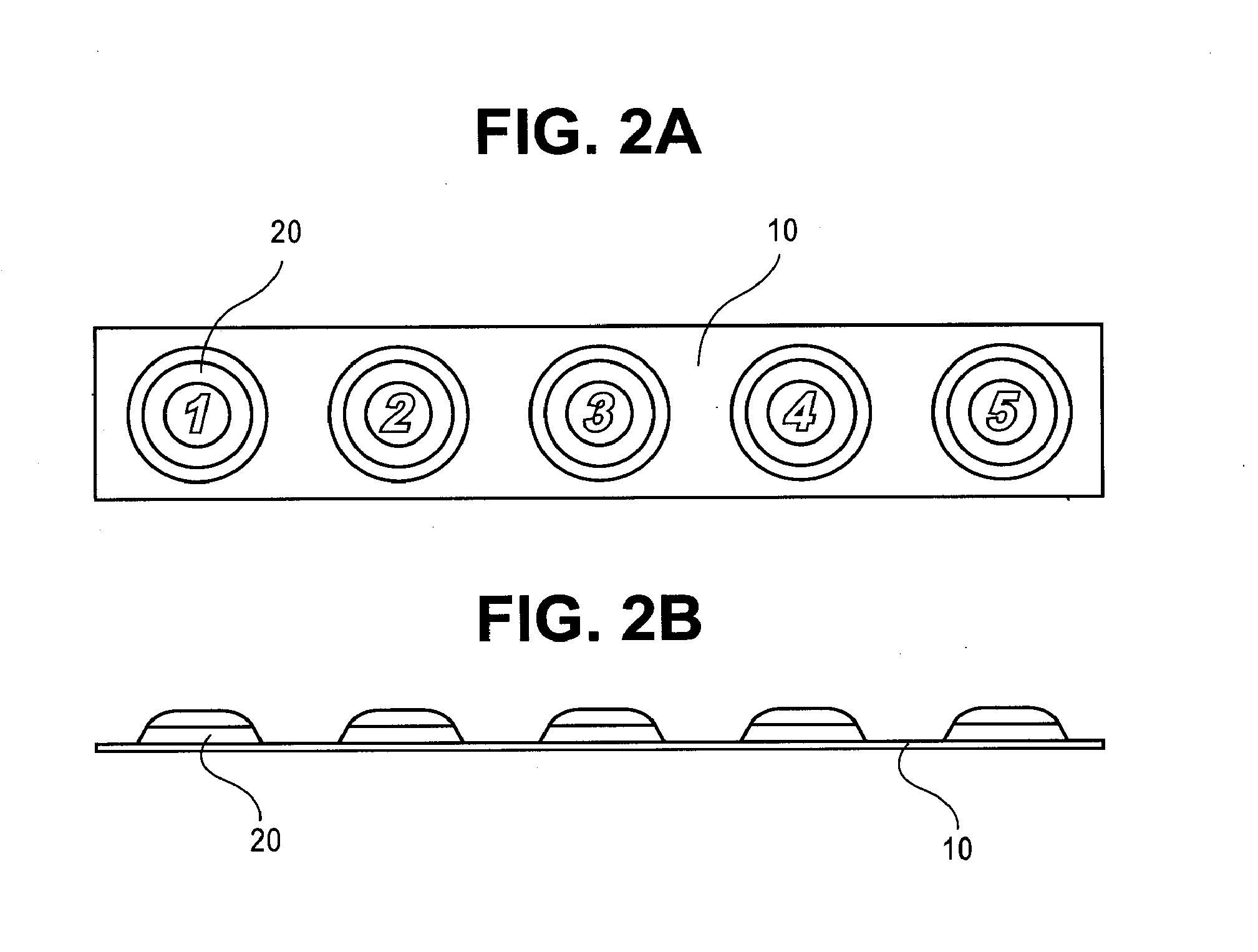
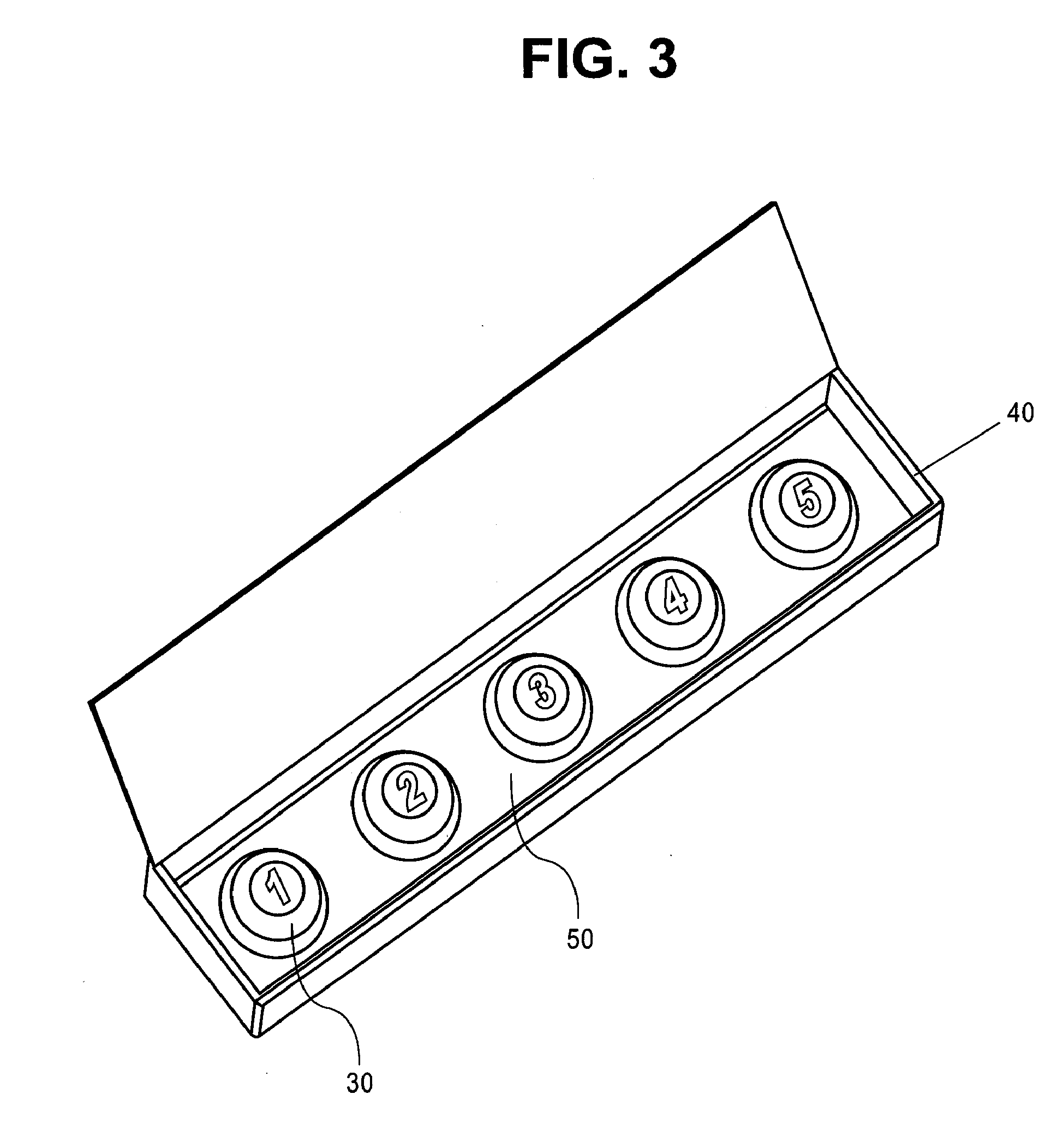
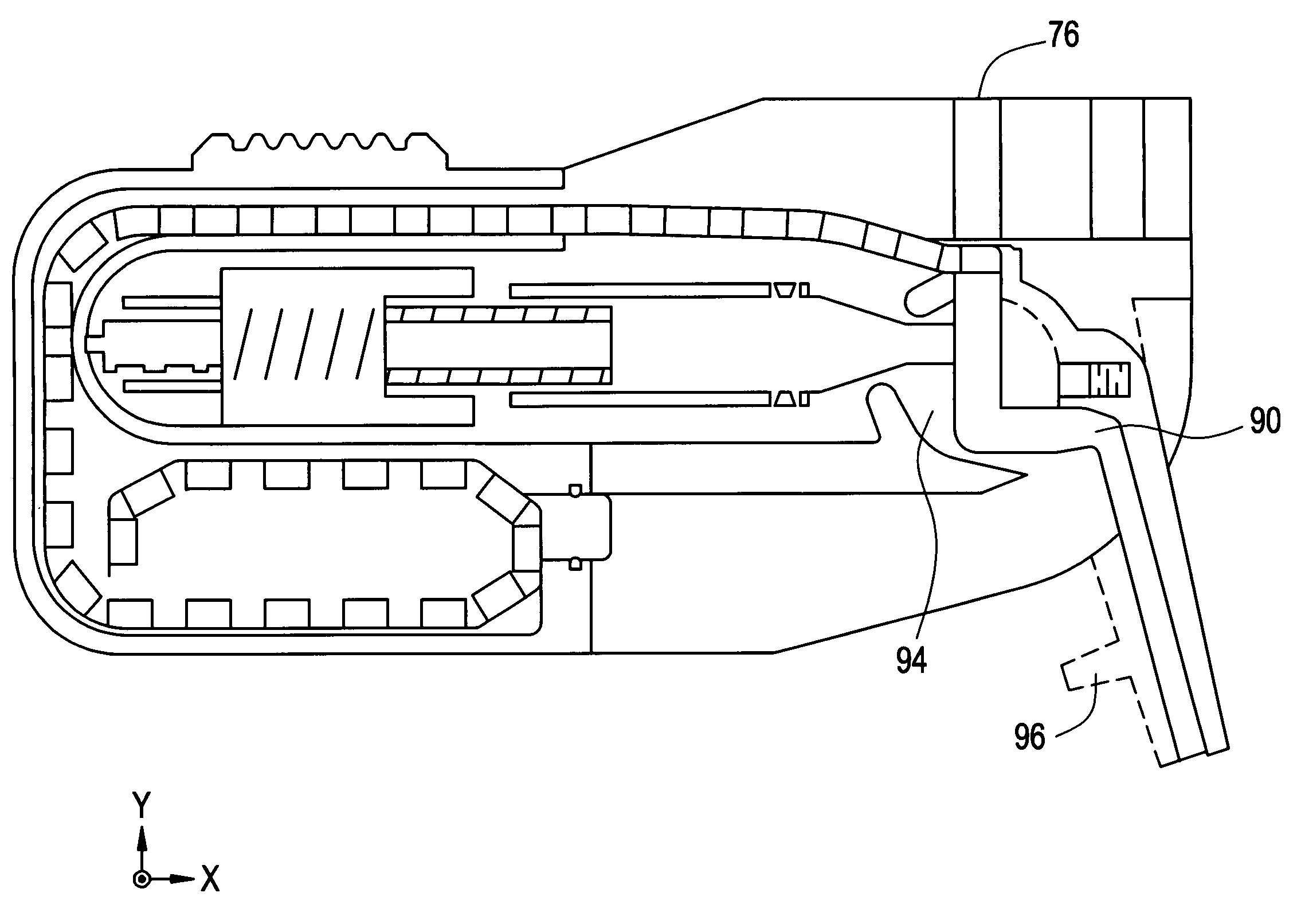
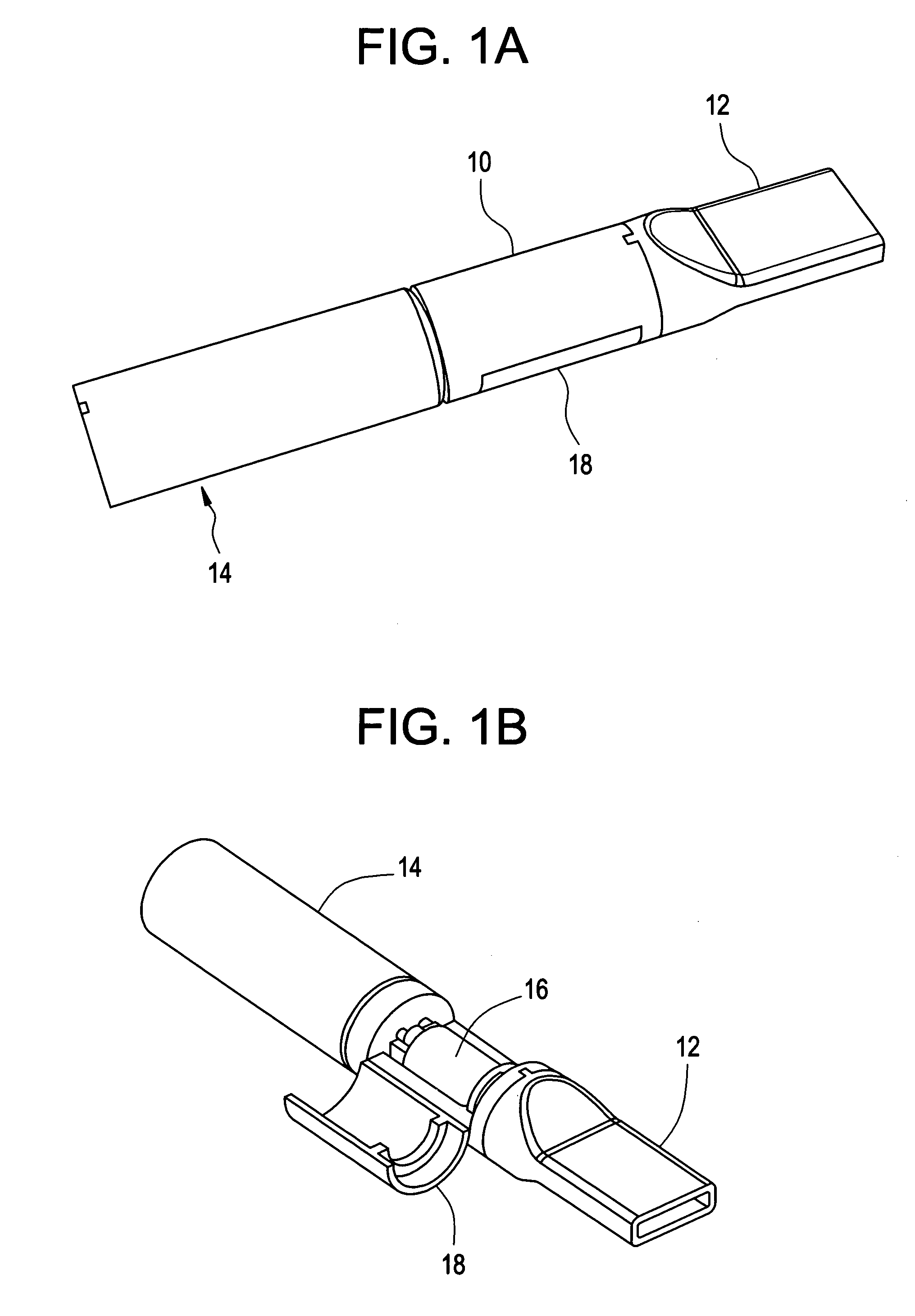
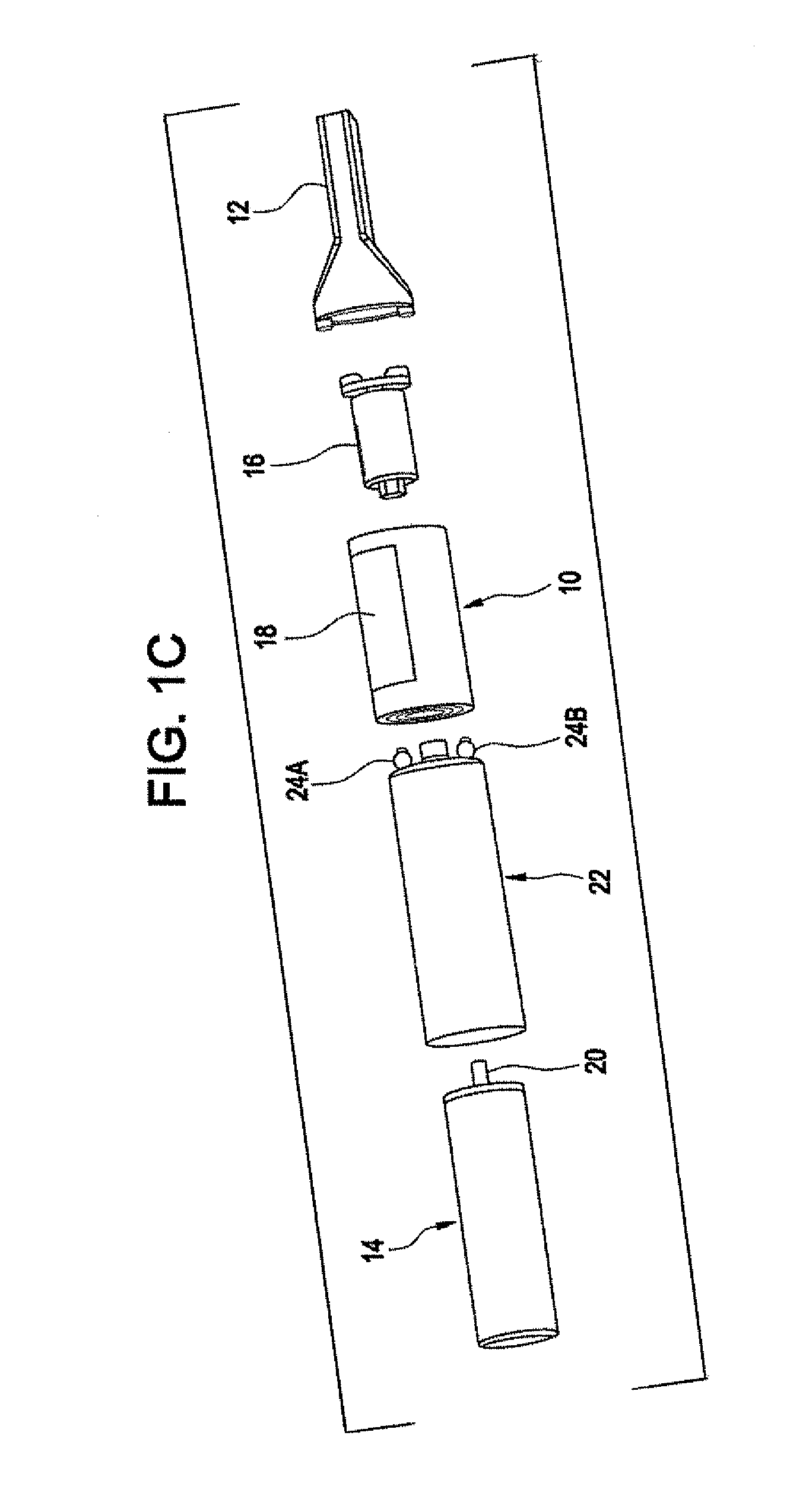
Sublingual drug delivery device
A drug delivery device that aerosolizes a dry powder formulation so that it forms a fine coating in the oral cavity and, more specifically in the sublingual region of the oral cavity is described herein. In the preferred embodiment, the device contains five main parts: (i) a compressed gas canister, (ii) a dispenser body (also referred to herein as the main housing ), (iii) a means for storing one or more doses of a drug formulation, (iv) a means for releasing a dose of the drug formulation such as a gas canister or spring piston and (v) a mouthpiece. Preferred configurations include circular, tubular, and rectangular. The means for storing the drug formulation may be configured to separately store one or more materials. In one embodiment, the means for storing the active agent is in the form of one or more drug discs, where the drug discs contain a plurality of blister packs, each storing one dose of the drug formulation. In another embodiment, the means for storing the active agent is a dosage cartridge containing a single dose of the drug formulation. In yet another embodiment, the drug formulation is stored on a ribbon containing a plurality of blister packs, each storing one dose of the drug formulation.
Owner:BIODEL INC
Orodispersible pharmaceutical composition for oral, oromucosal or sublingual administration of agomelatine
The invention relates to a coated solid orodispersible pharmaceutical composition for oral, oromucosal or sublingual administration of agomelatine.
Owner:LES LAB SERVIER
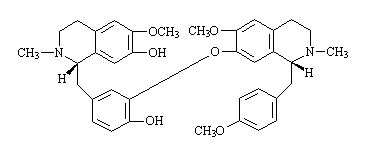
New usages of lotus plumule and alkaloid thereof and derivative thereof
The invention provides new usages of lotus plumule and alkaloid thereof and derivative thereof, such as lotus total alkaloids, liensinine, isoliensinine and methyl liensinine, in particular to the usages of lotus plumule and extract thereof, lotus plumule total alkaoids, bis-benzyl-isoquinoline alkaloid derivative or analogue in preparing drugs for curing senile dementia or healthcare food or food additives. The new usages are characterized in that the lotus plumule and extract thereof, the lotus plumule total alkaoids, the bis-benzyl-isoquinoline alkaloid derivative or the analogue are adopted as main active ingredients, and other compatible active ingredients are combined to form a new formula to prevent and treat dementia (including senile dementia and vascular dementia), improve learning and memory and the like. The dosage forms can be solid forms, liquid forms and the like used for different administrations such as oral taking, sublingual administration, nasal mucosa administration, percutaneous administration, muscle administration, vein administration and rectum administration.
Owner:李宏
Fentanyl composition for treatment of acute pain
Pharmaceutical compositions for the treatment of acute pain via sublingual administration are disclosed. The composition contains an ordered mixture of fentanyl or a pharmaceutically acceptable salt thereof in the form of substantially anhydrous, microparticles adhered to substantially water-soluble particles that are much larger than the fentanyl particles. The surface of the carrier particles. In a preferred embodiment, the composition also contains a bioadhesion and / or mucoadhesion promoter. The invention also relates to a method for the preparation of the composition and the use of the composition for the treatment of acute pain.
Owner:欧莱克斯欧公司
Methods and compositions for treating neoplasms
InactiveUS20060034821A1Reduce the amount requiredPeptide/protein ingredientsAntineoplastic agentsNasal cavityVein
The present invention comprises the periodic administration of a solution of neuraminidase to a patient with a neoplasm until the neoplasm has receded. The administration of the solution of neuraminidase can be administered by subcutaneous injection, intramuscular injection, intravenous injection, nasal administration, sublingual administration or transdermal administration. The present invention is effective in treating a wide variety of neoplasms.
Owner:SIGNAL COORDINATING THERAPY
Ginkgo flavone and lactone dispersion tablet and preparation method thereof
The invention relates to a gingko ketonic ester dispersible tablet and its preparation method, and belongs to the field of Chinese traditional medicine. The tablet mainly comprises gingko ketonic ester and proper adjuvant. The inventive preparation has effects in activating blood circulation, dispelling blood stasis, and dredging meridians. The inventive dispersible tablet has improved water solubility and can rapidly disinregrate in cold water and form uniform suspensoid liquid. It has various administration manner including direct drink, direct deglutition, sublingual administration. It has convenient administration, high bioavailability, good taste, easy acceptability, rational and feasible technology, and stable and controllable quality.
Owner:BEIJING YINKERUISI BIOLOGICAL PODUCTS RES INST
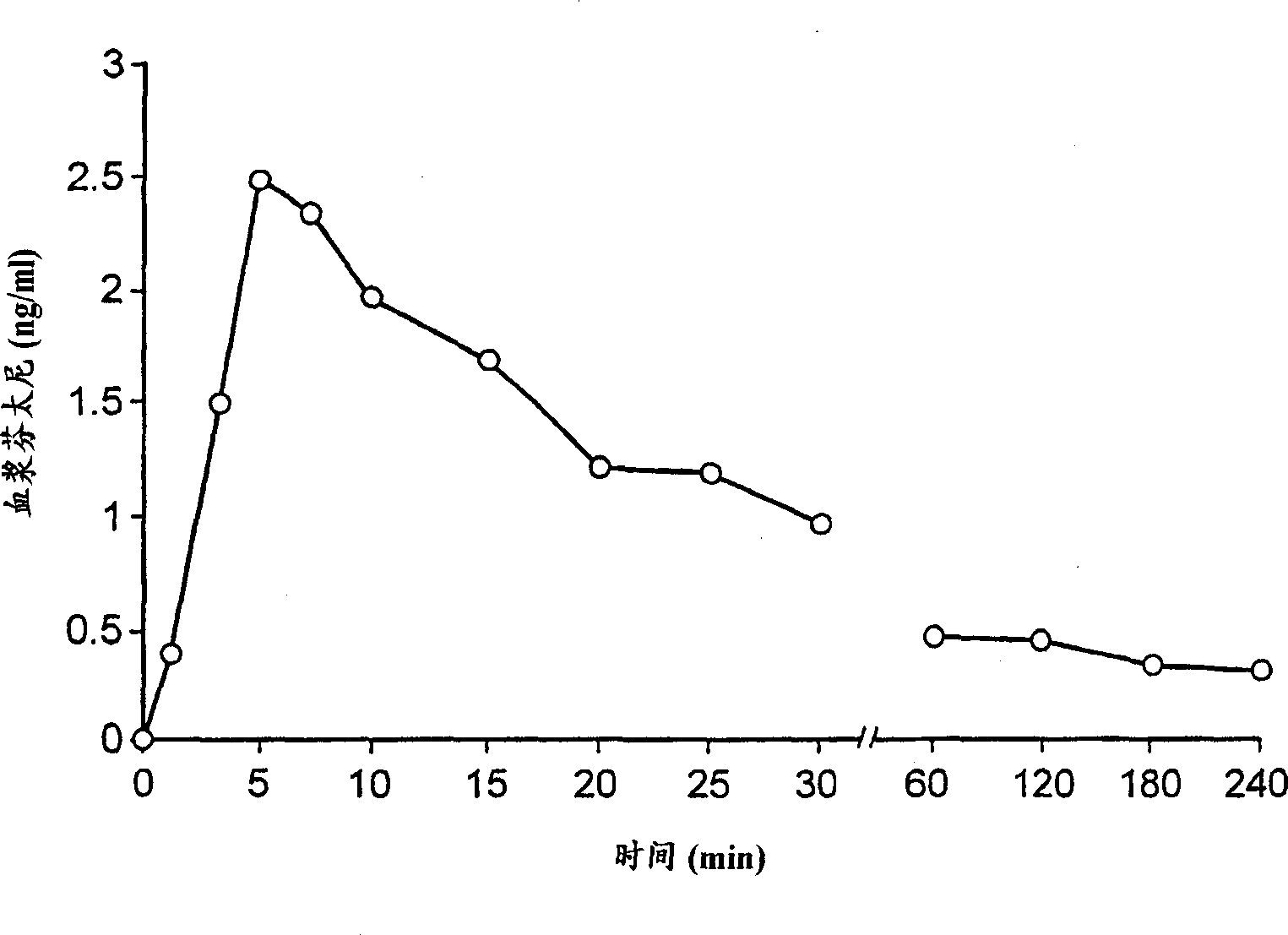
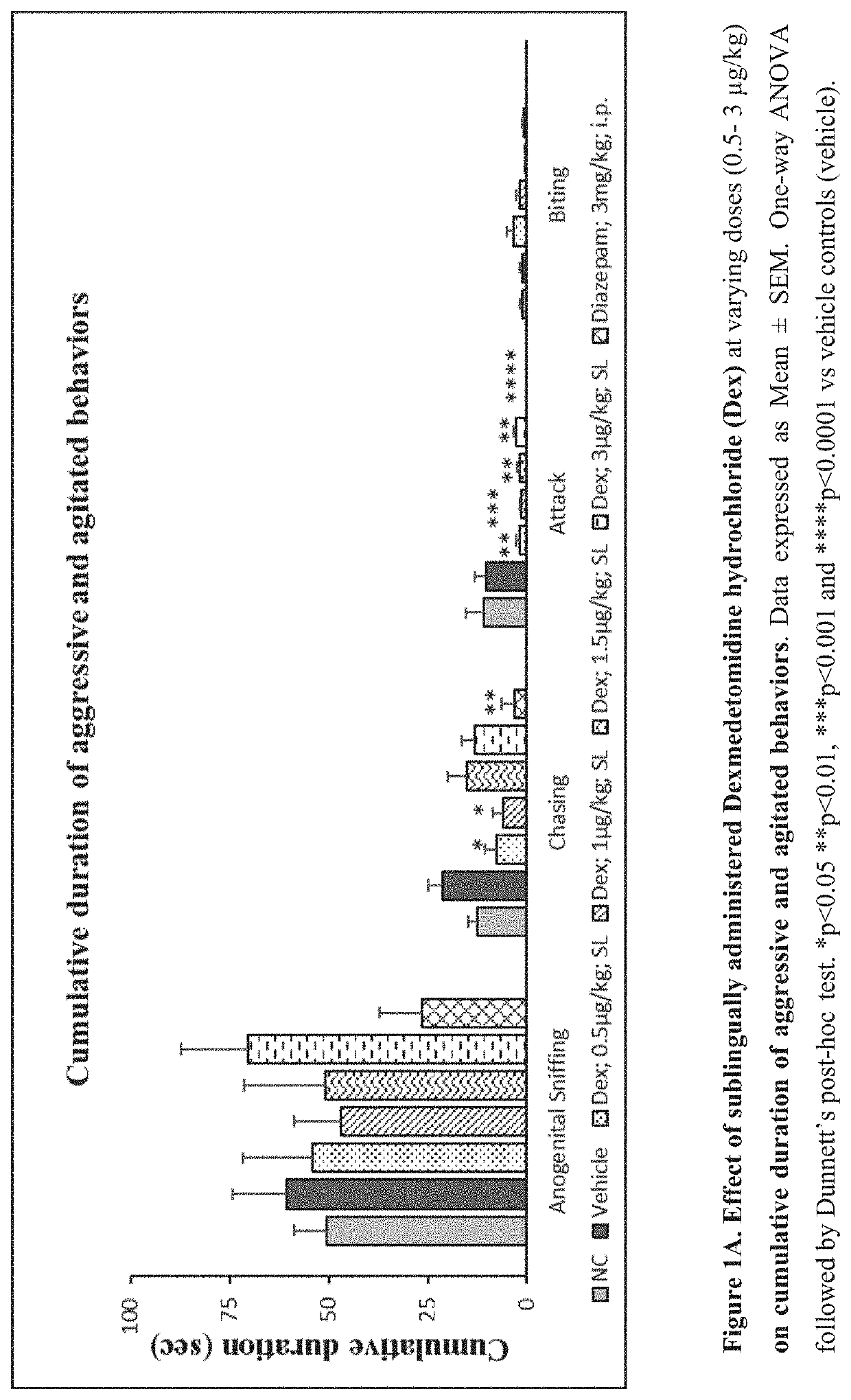
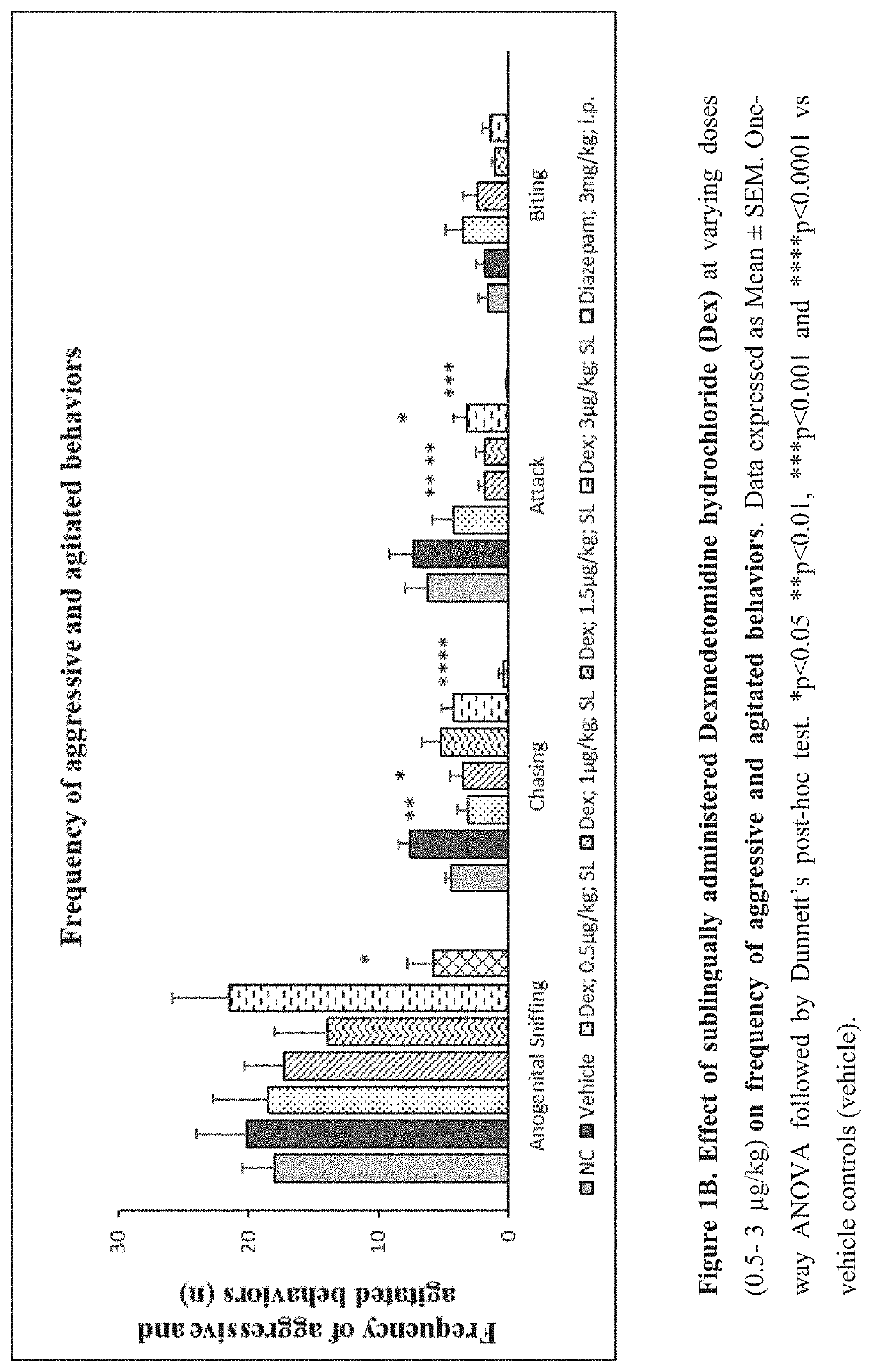
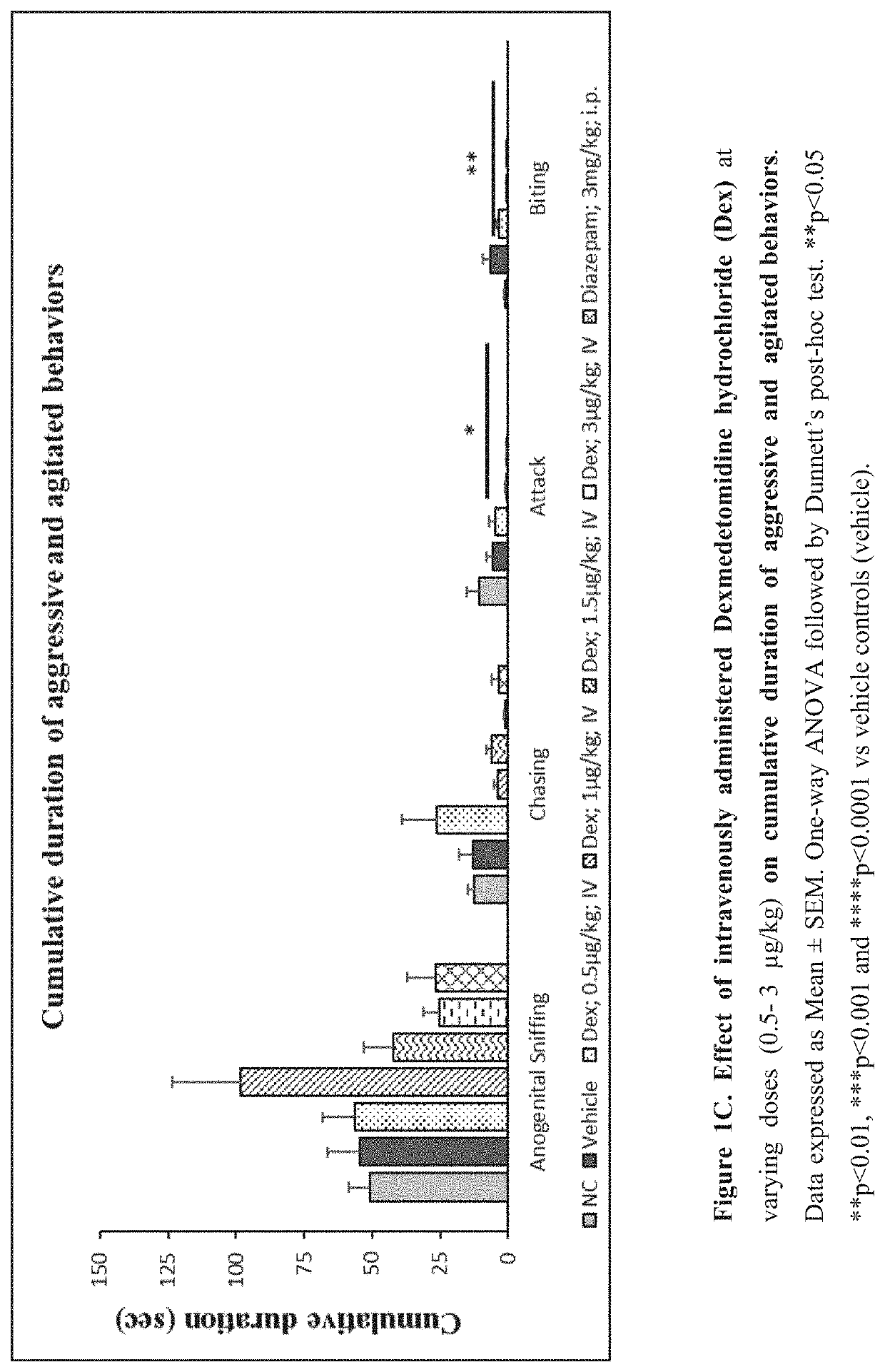
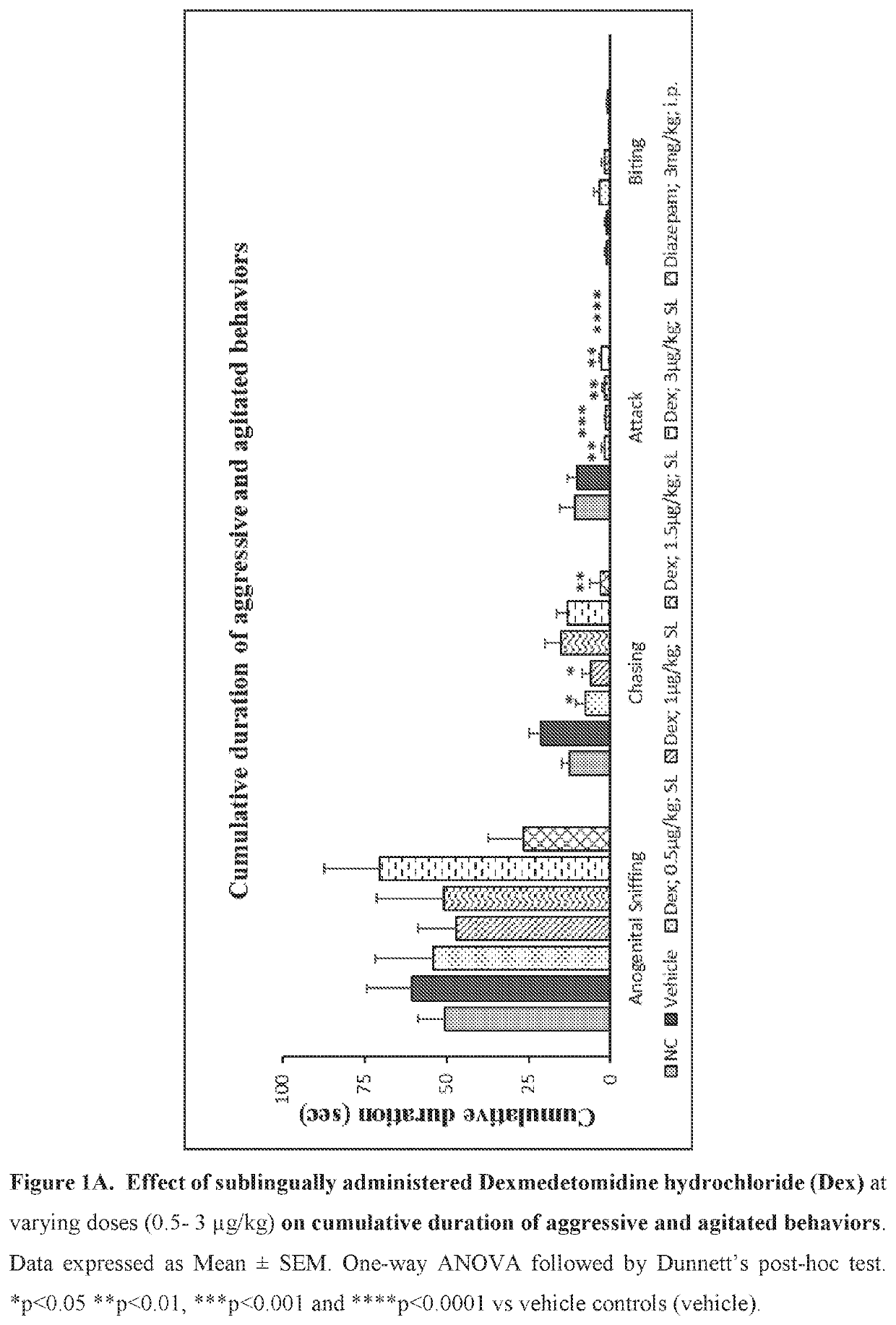
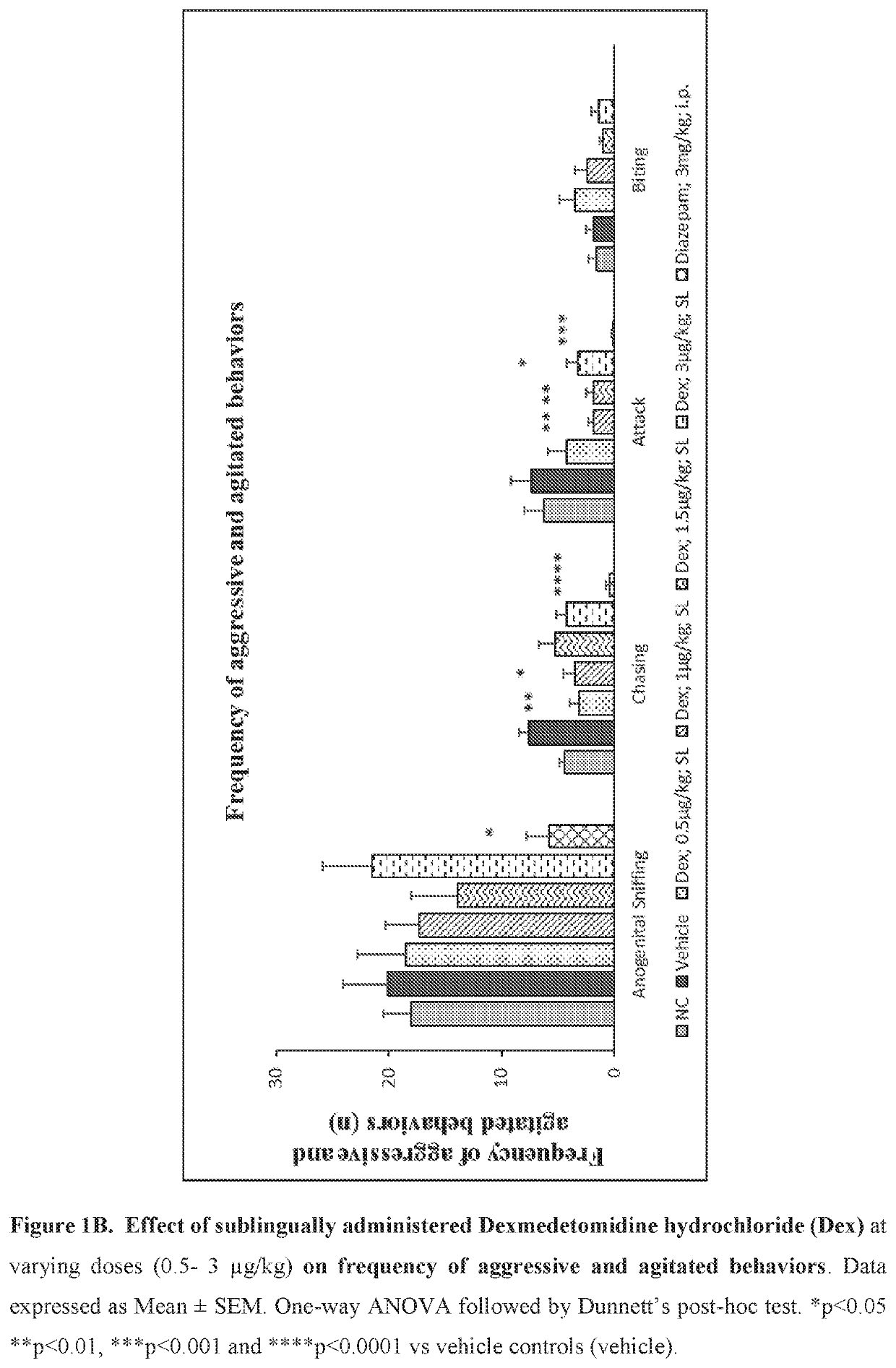
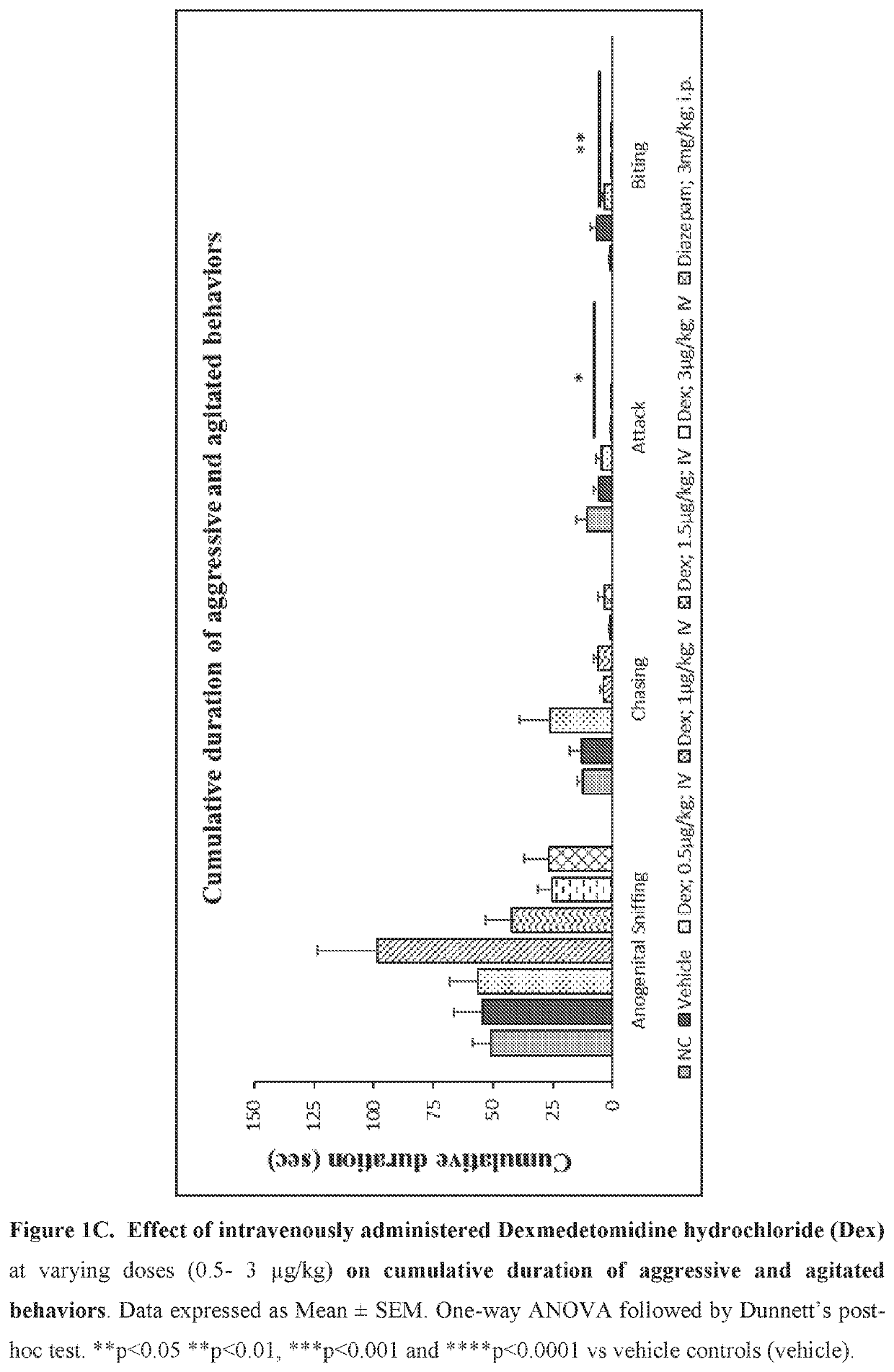
Use of sublingual dexmedetomidine for the treatment of agitation
PendingUS20220031663A1Preventing and reducing signOrganic active ingredientsNervous disorderAdrenergic receptor agonistsNeuro-degenerative disease
The present invention discloses a method of treating agitation or the signs of agitation in a subject comprising the sublingual administration of an effective amount of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine, or a pharmaceutically acceptable salt thereof. The method is particularly suitable for the treatment of agitation associated with neurodegenerative and / or neuropsychiatric diseases. The present invention also discloses the sublingual administration of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine or a pharmaceutically acceptable salt thereof at a dose that is effective to treat agitation or the signs of agitation in a subject, but does not cause significant sedation.
Owner:BIOXCEL THERAPEUTICS INC +1
Use of sublingual dexmedetomidine for the treatment of agitation
PendingUS20190365715A1Preventing and reducing signOrganic active ingredientsNervous disorderNeuropsychiatric diseaseSublingual administration
The present invention discloses a method of treating agitation or the signs of agitation in a subject comprising the sublingual administration of an effective amount of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine, or a pharmaceutically acceptable salt thereof. The method is particularly suitable for the treatment of agitation associated with neurodegenerative and / or neuropsychiatric diseases. The present invention also discloses the sublingual administration of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine or a pharmaceutically acceptable salt thereof at a dose that is effective to treat agitation or the signs of agitation in a subject, but does not cause significant sedation.
Owner:BIOXCEL THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com