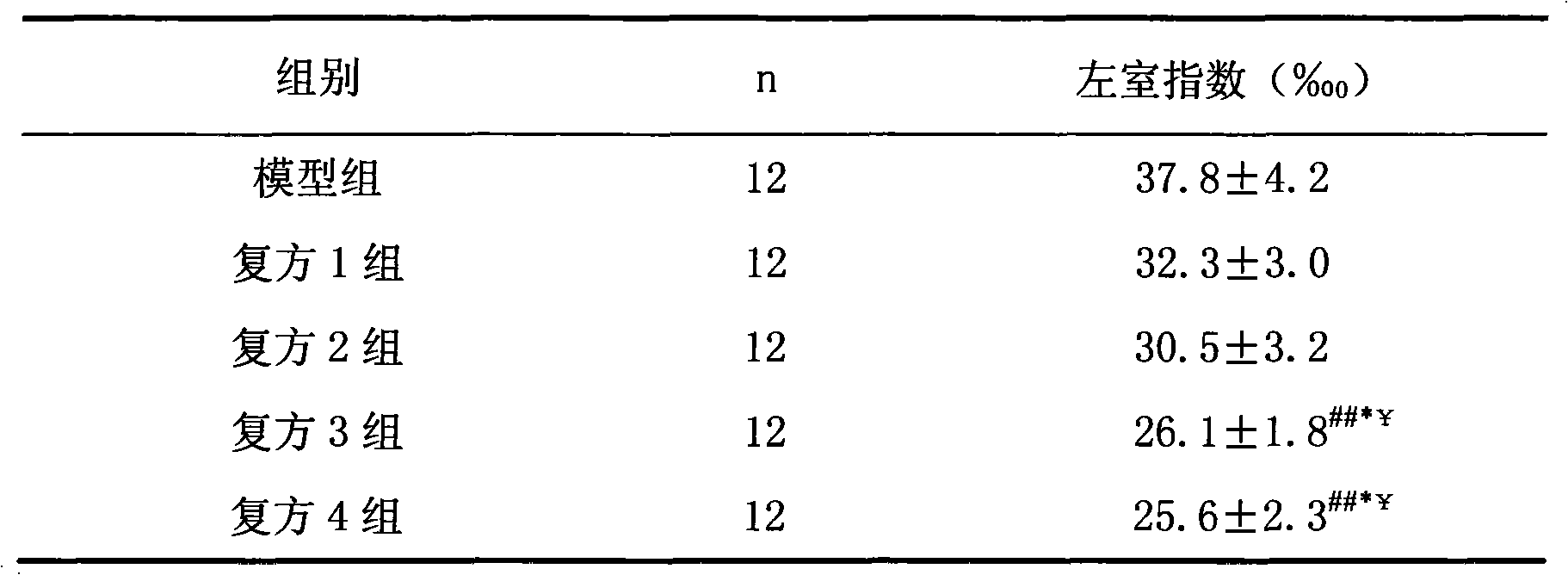
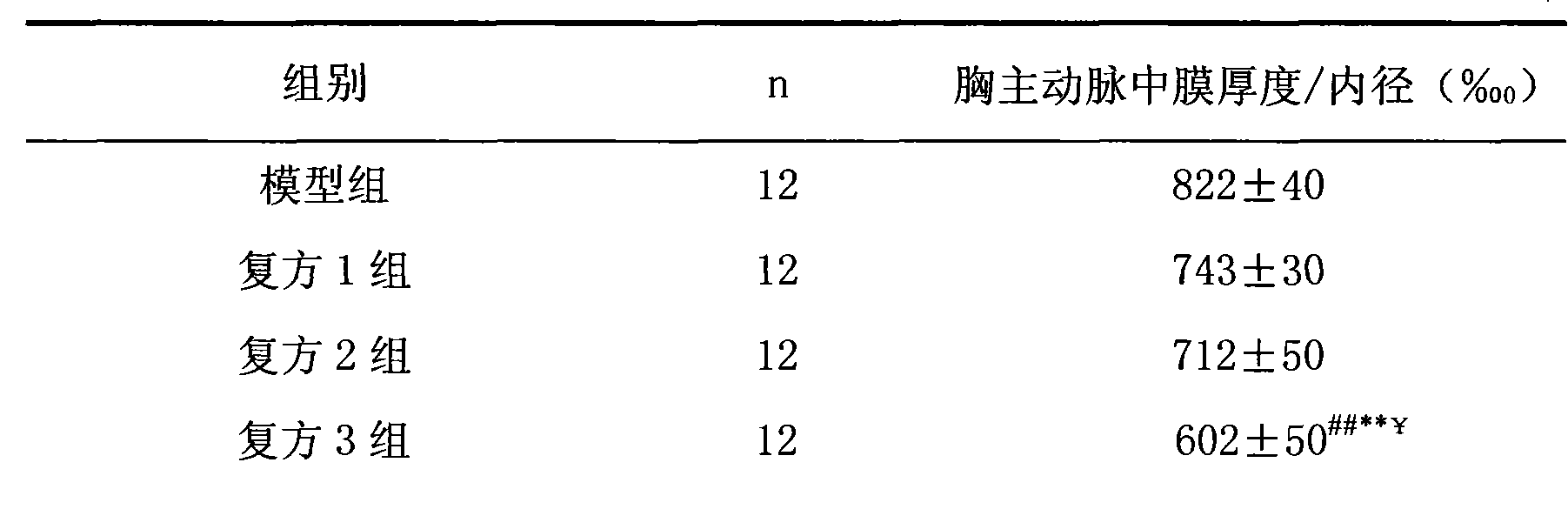
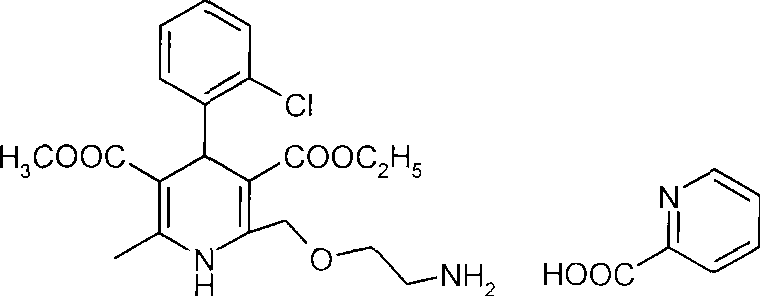
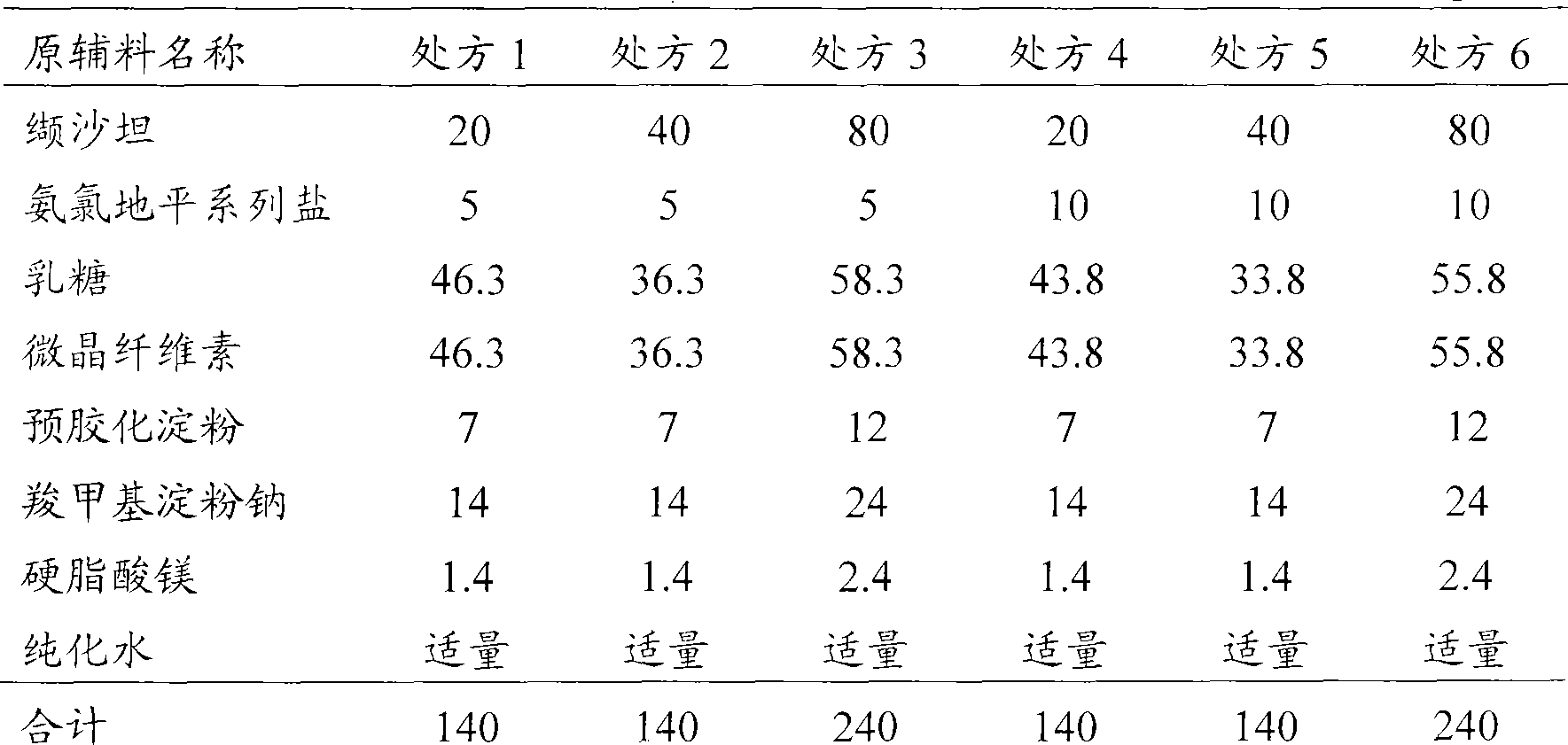
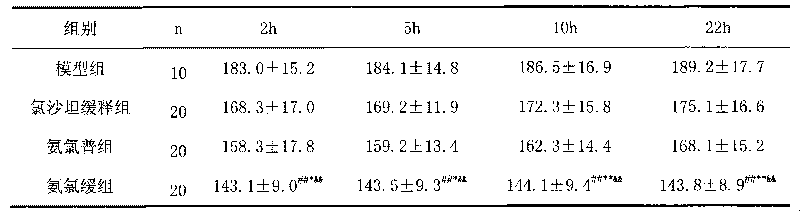
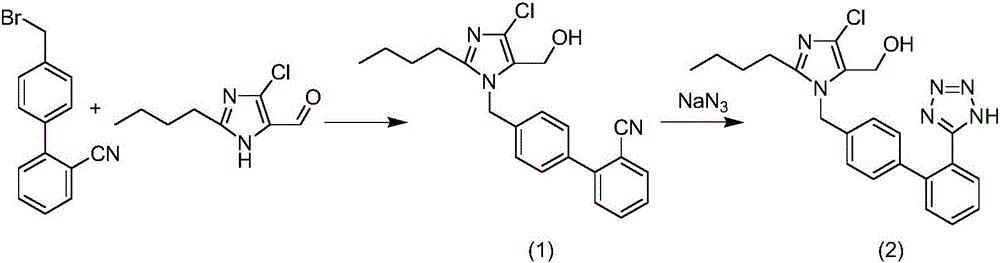
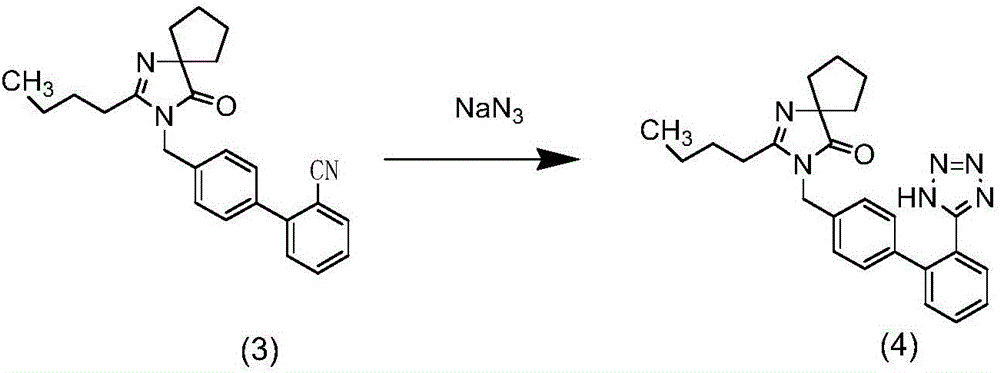
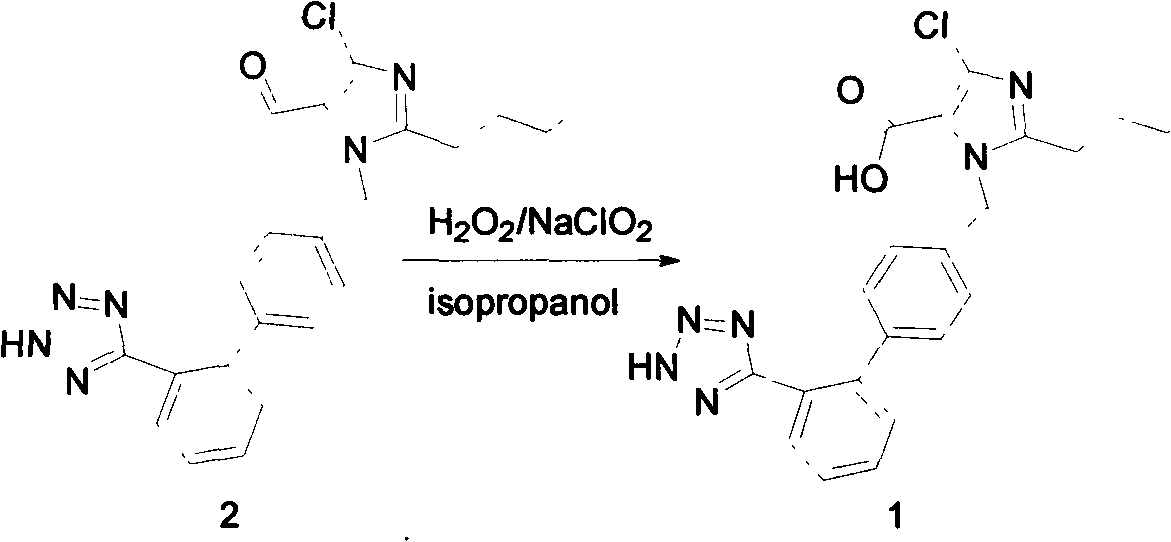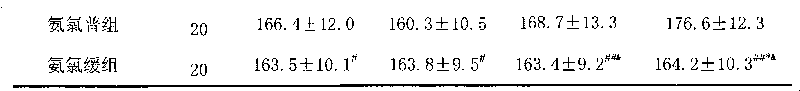Patents
Literature
81 results about "Losartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Losartan is used to treat high blood pressure (hypertension) and to help protect the kidneys from damage due to diabetes. It is also used to lower the risk of strokes in patients with high blood pressure and an enlarged heart.
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveUS20100047341A1Improve Medication AdherenceConstant controlBiocideAnimal repellantsCo administrationSide effect
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:HANALL PHARMA CO LTD
Oral liquid losartan compositions
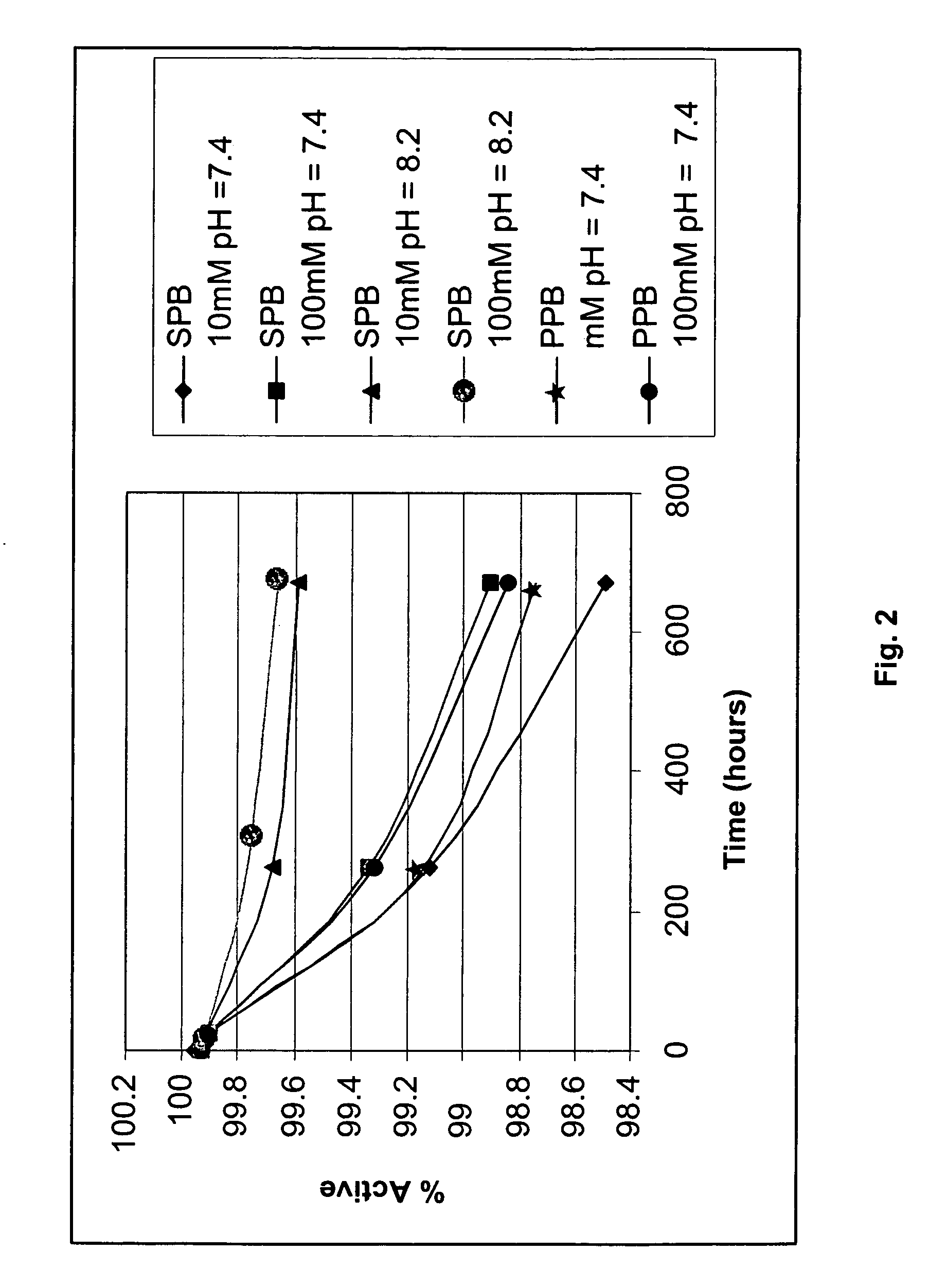
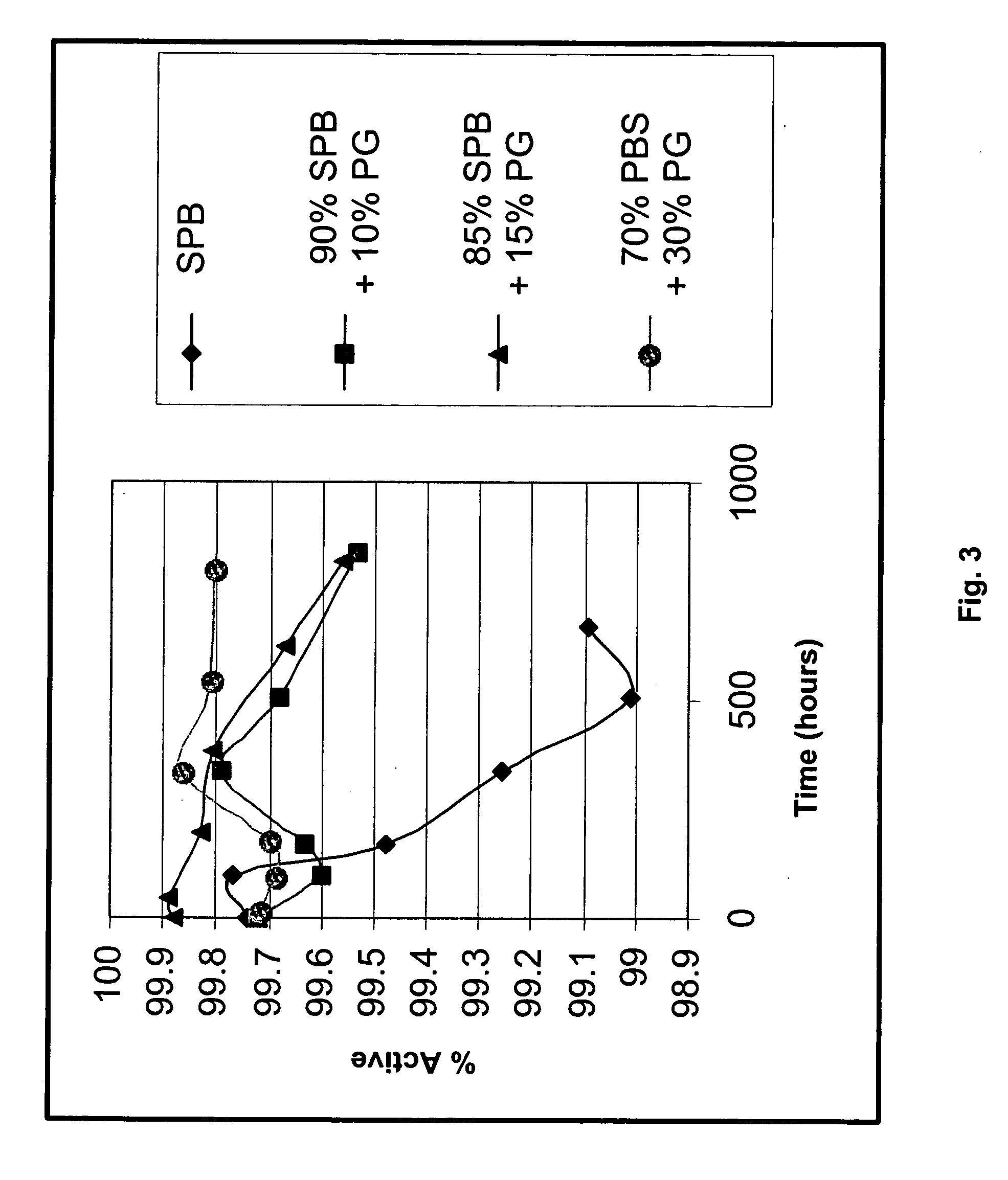
The present invention is directed to improved oral liquid compositions that include losartan, or a pharmaceutically acceptable salt or metabolite thereof, and at least one pharmaceutically acceptable carrier in an amount sufficient to provide a pH of about 6 or higher. Processes of preparing such compositions and methods of administering such compositions are also included.
Owner:WOCKHARDT EU OPERATIONS SWISS
Compound of losartan compound or its medical salt and calcium channel blocker or its medical salt
InactiveCN101347427ALittle side effectsGood curative effectOrganic active ingredientsCardiovascular disorderCarboxylic acidLosartan
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Stable non-crystalline formulation comprising losartan
InactiveUS20060160871A1Handling and advantageWell formedBiocidePowder deliveryLosartanTreatment hypertension
One or more embodiments of the invention provide various novel formulations, and tablet dosage forms, comprising losartan that are non-crystalline, stable, and / or otherwise improvements over known losartan formulations. One or more embodiments of the invention further provide methods for preparing the formulation, methods for preparing the tablet dosage form, and to methods of administering the tablet dosage and / or formulation comprising losartan. The losartan-containing formulations may be administered to a user to treat hypertension, and related conditions.
Owner:NEKTAR THERAPEUTICS INC
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveCN101528203ASignificant blood pressure lowering effectExcellent suppression of side effectsOrganic active ingredientsAerosol deliverySide effectCo administration
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:韩诺生物制约株式会社
Method for preparing high-purity losartan
The invention relates to a method for preparing high-purity losartan. The method comprises steps as follows: (1), a losartan crude product is added to an organic solvent or a mixed solvent of an organic solvent and water, and the mixture is heated to reach 20-80 DEG C and stirred; (2), the system is cooled directly or cooled after water is added or cooled to 0-5 DEG C after a part of solvent is evaporated, a material is precipitated, filtered and dried, and losartan is obtained, wherein the organic solvent used in the step (1) is any one of tetrahydrofuran, butanone, acetone and methyl alcohol or is a mixed solvent of any one of the four solvents and water. The losartan obtained with the method has high purity, any individual impurity can be reduced to 0.2% or even under 0.1%, the purity of the losartan can reach 99.5%, the cost of the method is lower, the refining yield is high, and the method is very simple in operation, environment-friendly and suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Preparation of losartan
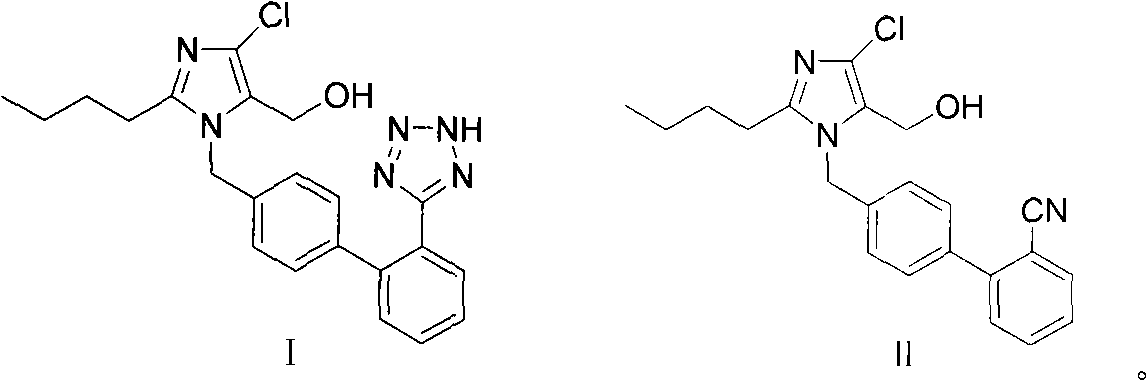
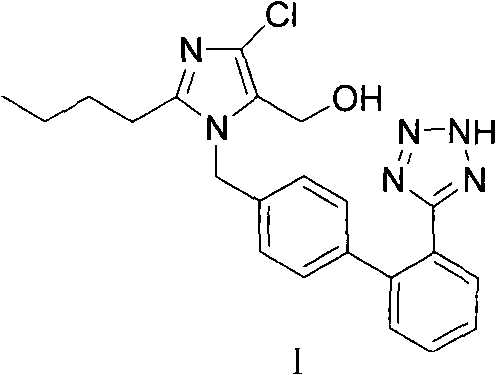
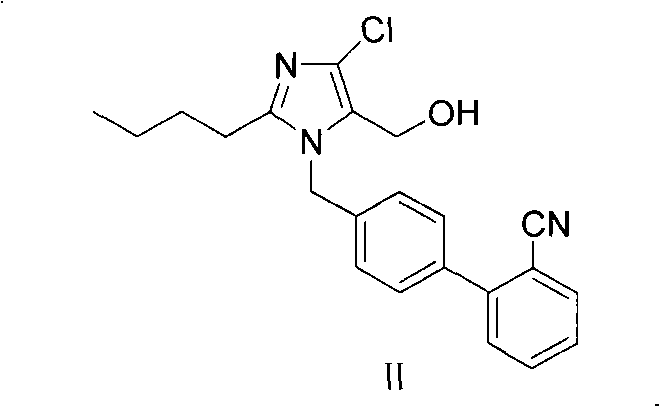
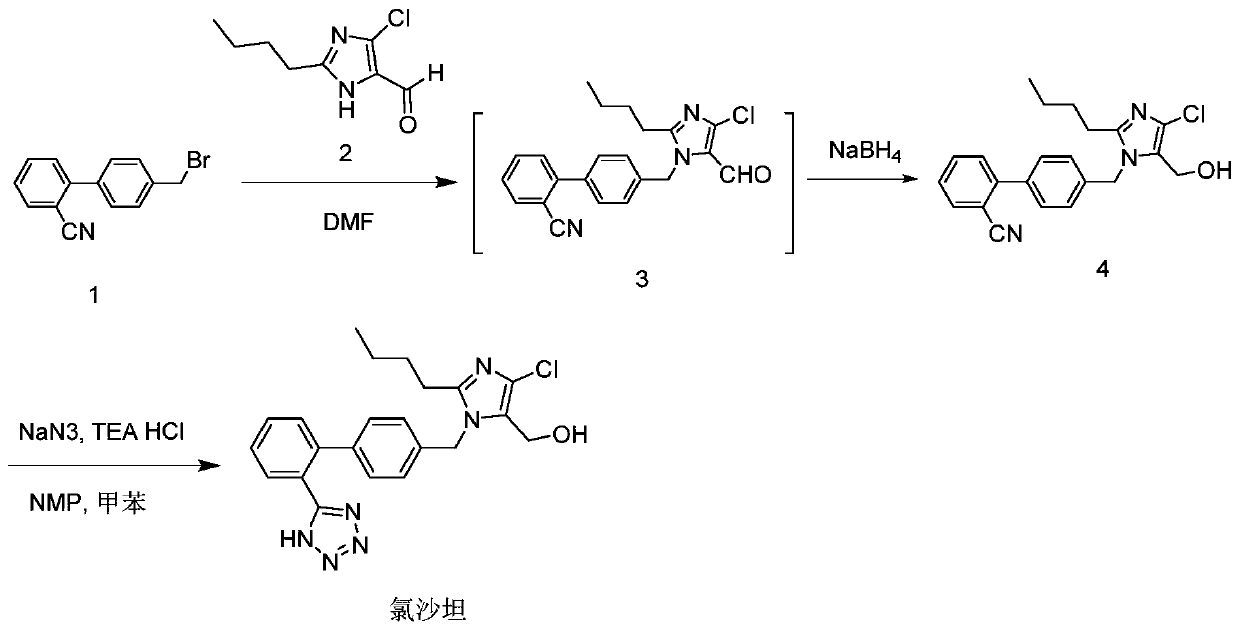
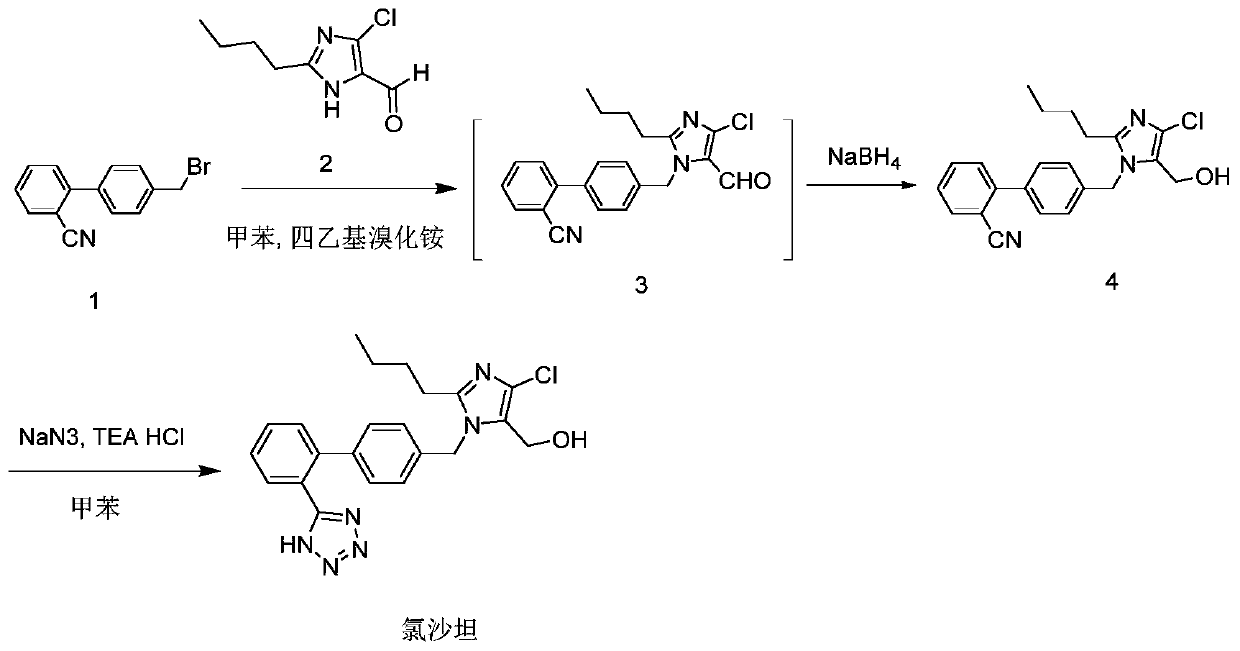
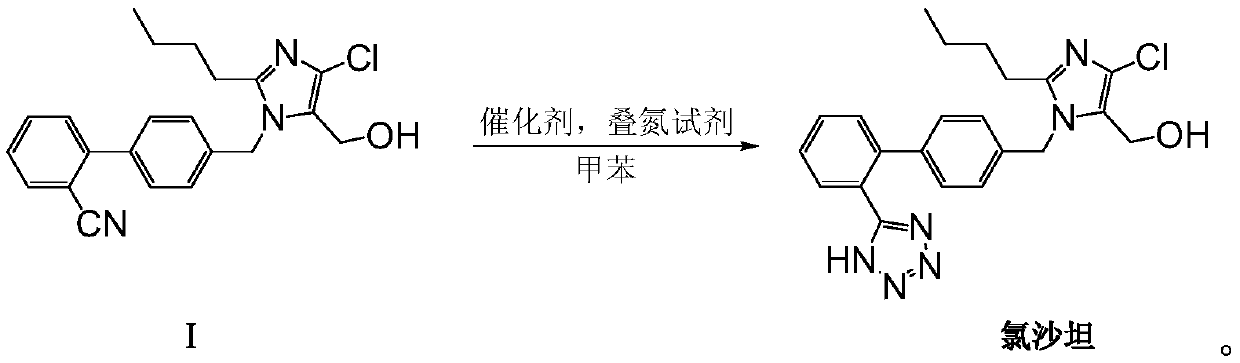
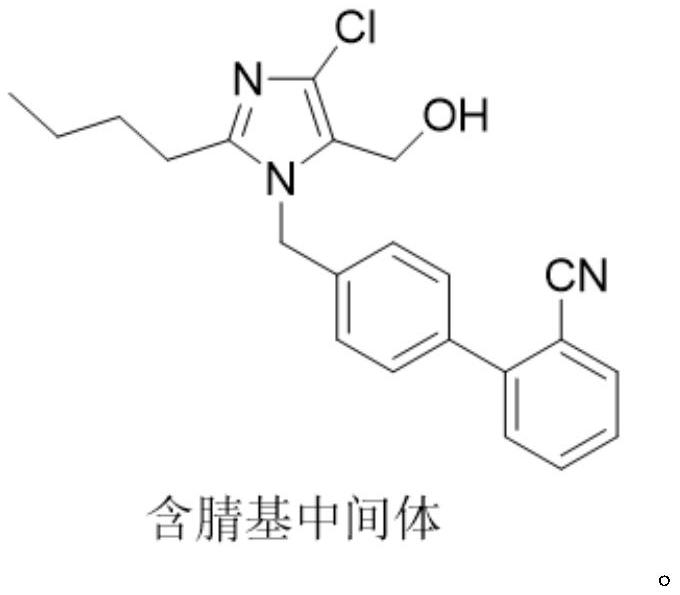
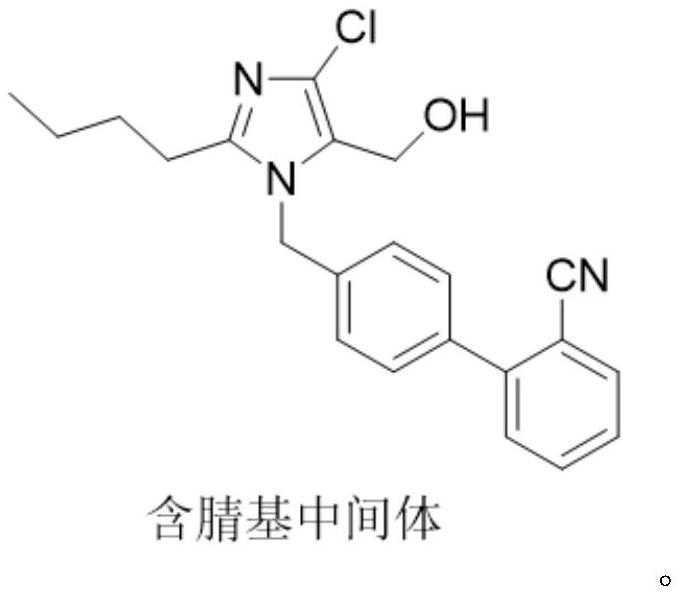
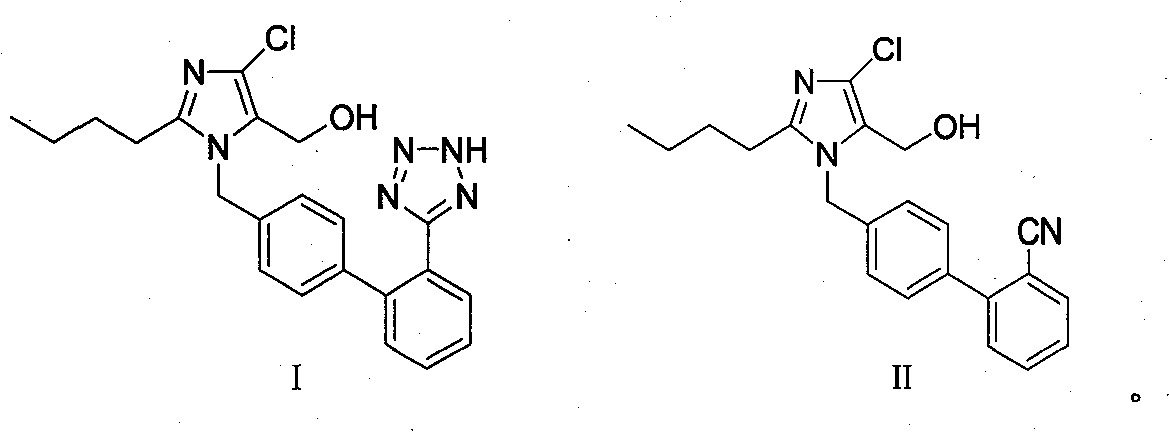
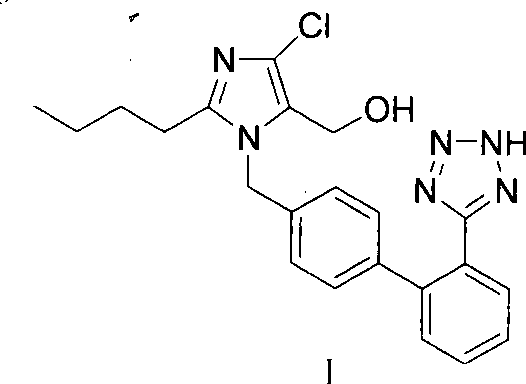
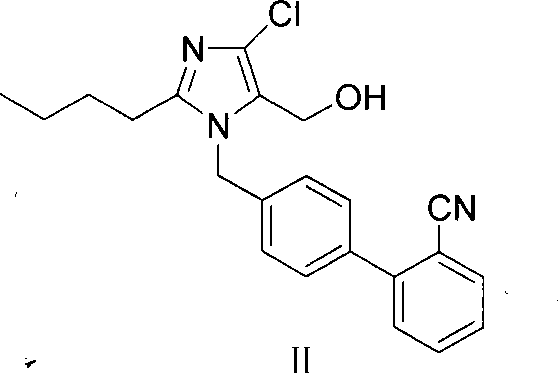
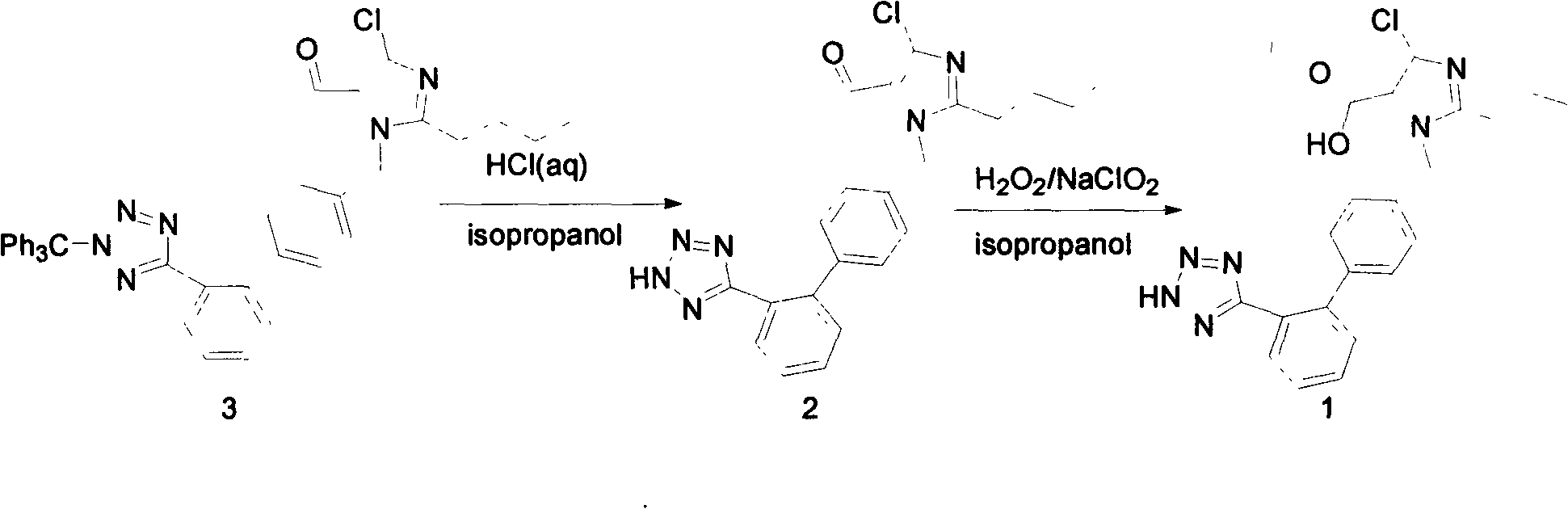
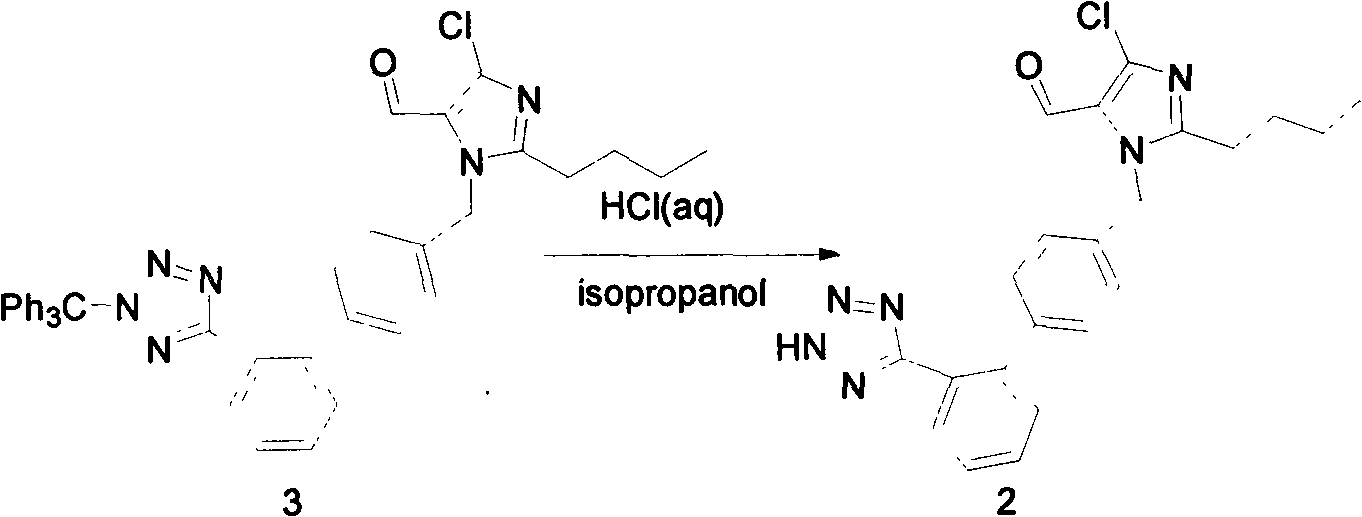
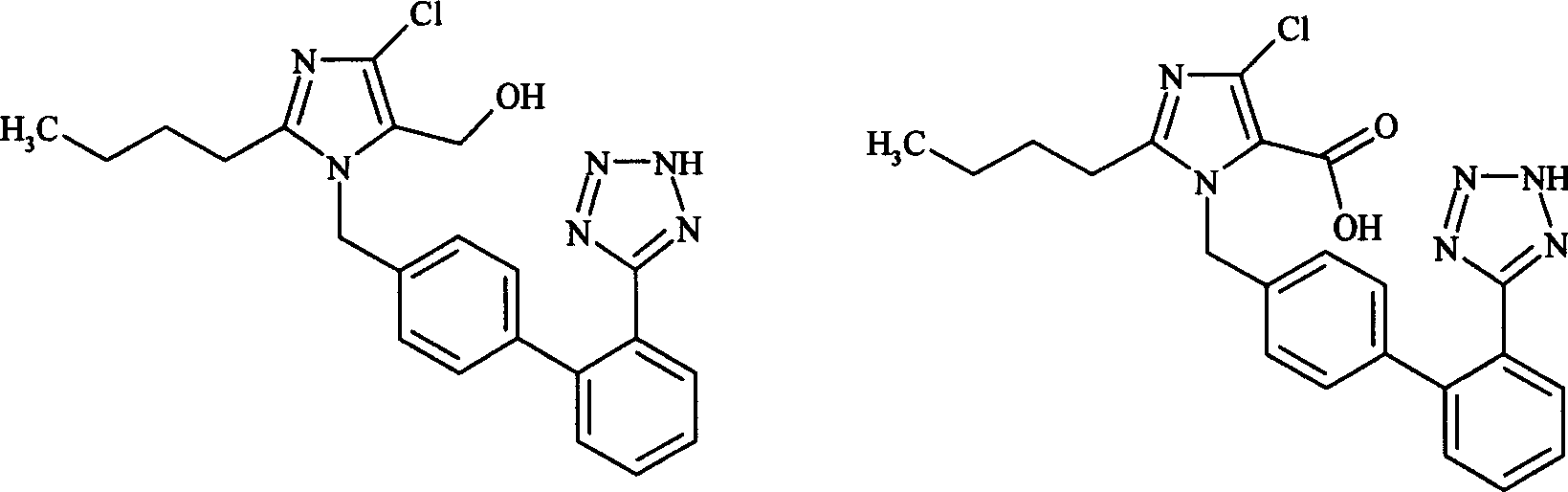
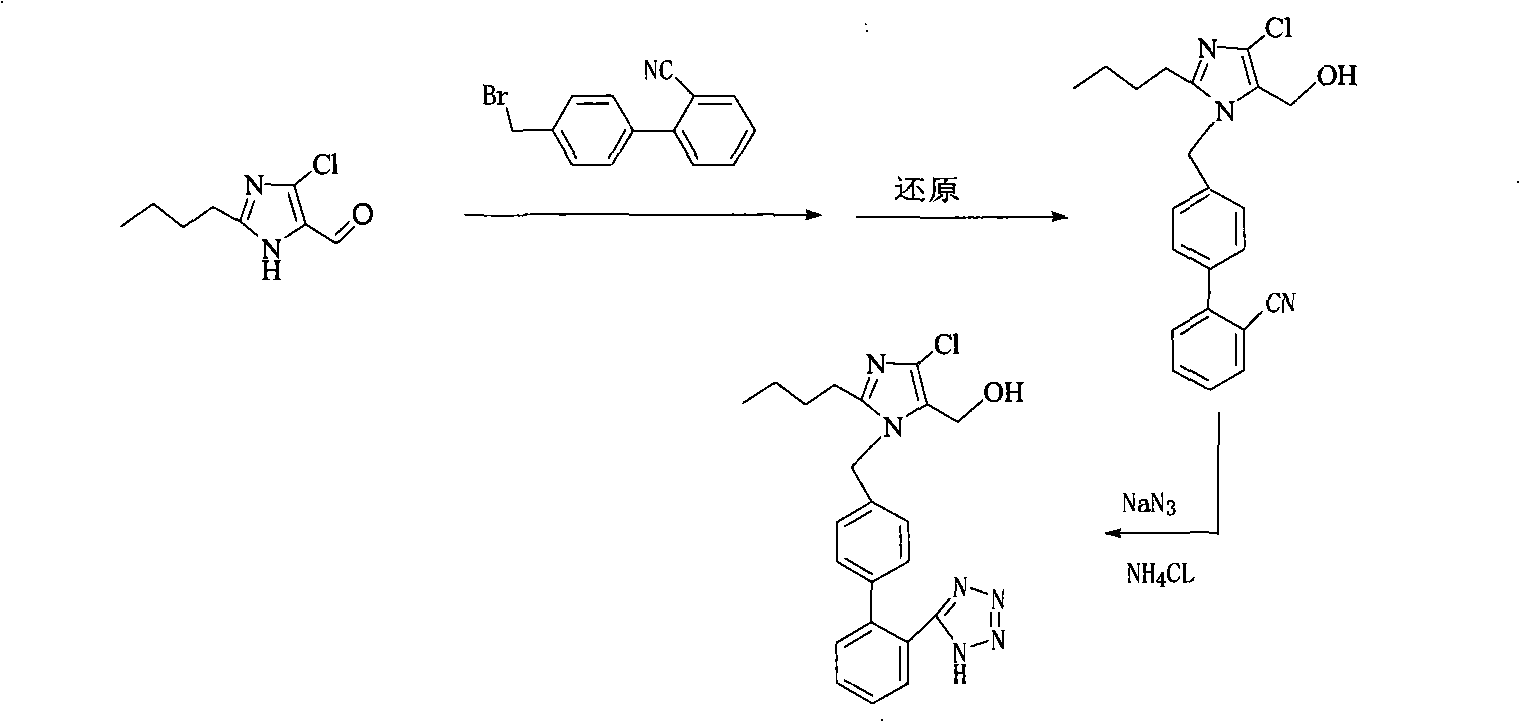
The invention provides a method for preparing losartan shown in a formula (I). The method comprises the following steps: reacting 2-butyl-4-chlorine-5-(hydroxymethyl)-1-{[(2'- cyano-group) xenyl-4-group]methyl} imidazole and natrium azide and triethylamine hydrochloride shown in a formula (II) in toluol; regulating the obtained reaction liquid by alkali to separate organic layers; adding a reducing agent into the collected liquid; regulating the obtained reaction liquid by acid to obtain losartan solid; and if required, refining the losartan solid by isopropyl alcohol. The method has the advantages of high yield, high purity of the losartan, low cost, little environmental pollution and suitability for industrialized production.
Owner:ZHEJIANG MENOVO PHARMA
Medicinal composition for treating hypertension
ActiveCN101849942AImprove protectionReduce incidenceOrganic active ingredientsCardiovascular disorderTreatment effectPotassium
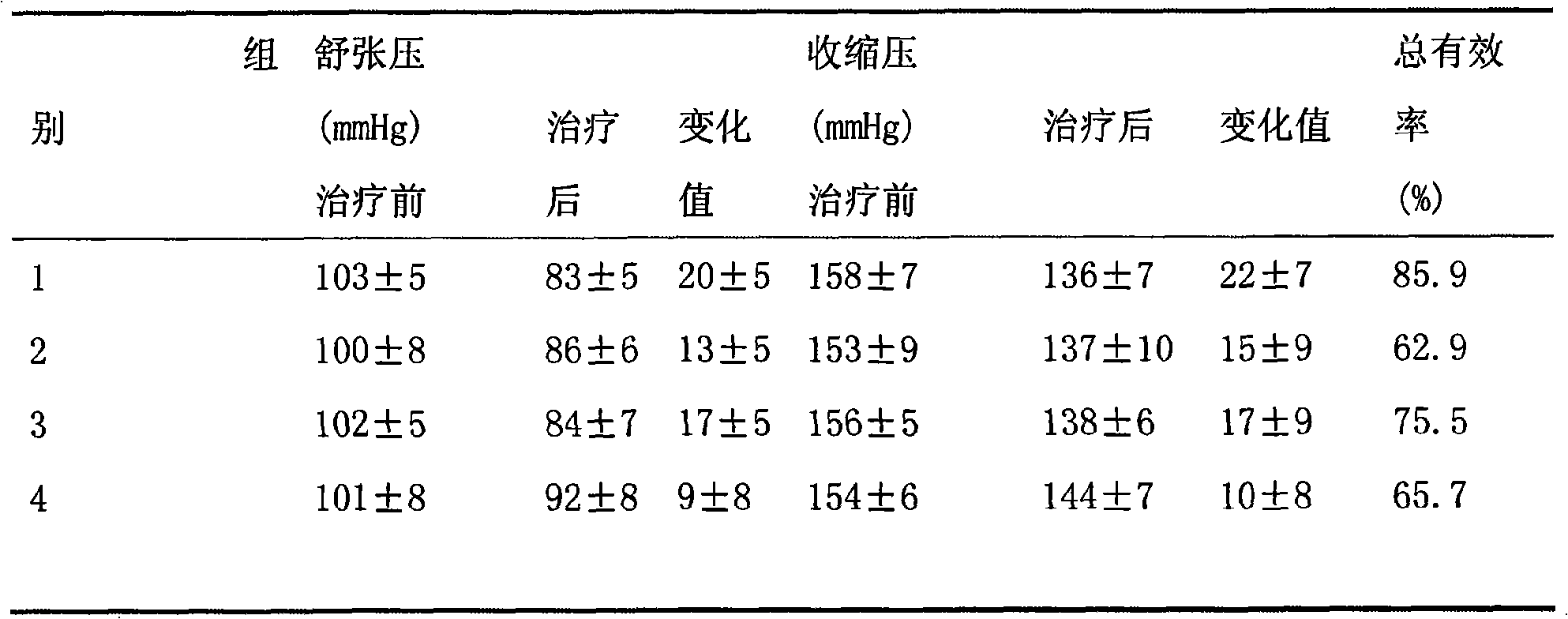
The invention discloses a medicinal composition for treating hypertension, and belongs to the field of medicaments. The antihypertensive medicinal composition comprises a pharmaceutically effective dose of levoamlodipine or a pharmaceutically acceptable salt thereof and losartanpotassium, wherein the levoamlodipine is weighed in free alkali; the losartan is weighed in free acid; and the weight ratio of the levoamlodipine to the losartan is 1 to 11-19. The medicinal composition does not damage the target organ while reducing the blood pressure so as to enhance the therapeutic effect and reduce the risk for therapy of a patient.
Owner:LUNAN PHARMA GROUP CORPORATION
Therapeutic composition containing amlodipine salt and losartan medicine
The invention relates to amlodipine series salt, generally comprising camphor sulfonic acid salt, L-aminobutanedioic acid salt, maleic acid salt and methanesulfonic acid salt and sartan compound or pharmaceutical composition of pharmaceutically acceptable thereof and preparation method of same, and kit for drug combination containing amlodipine serious salt and sartan compound composition. The composition or kit can be applied to treat patient suffering angina pectoris, atherosclerosis, and / or hypertension complicated with hyperlipemia and patient having risk of heart diseases (including human).
Owner:BEIJING ROCK PHARMA
Method of removing the triphenylmethane protecting group
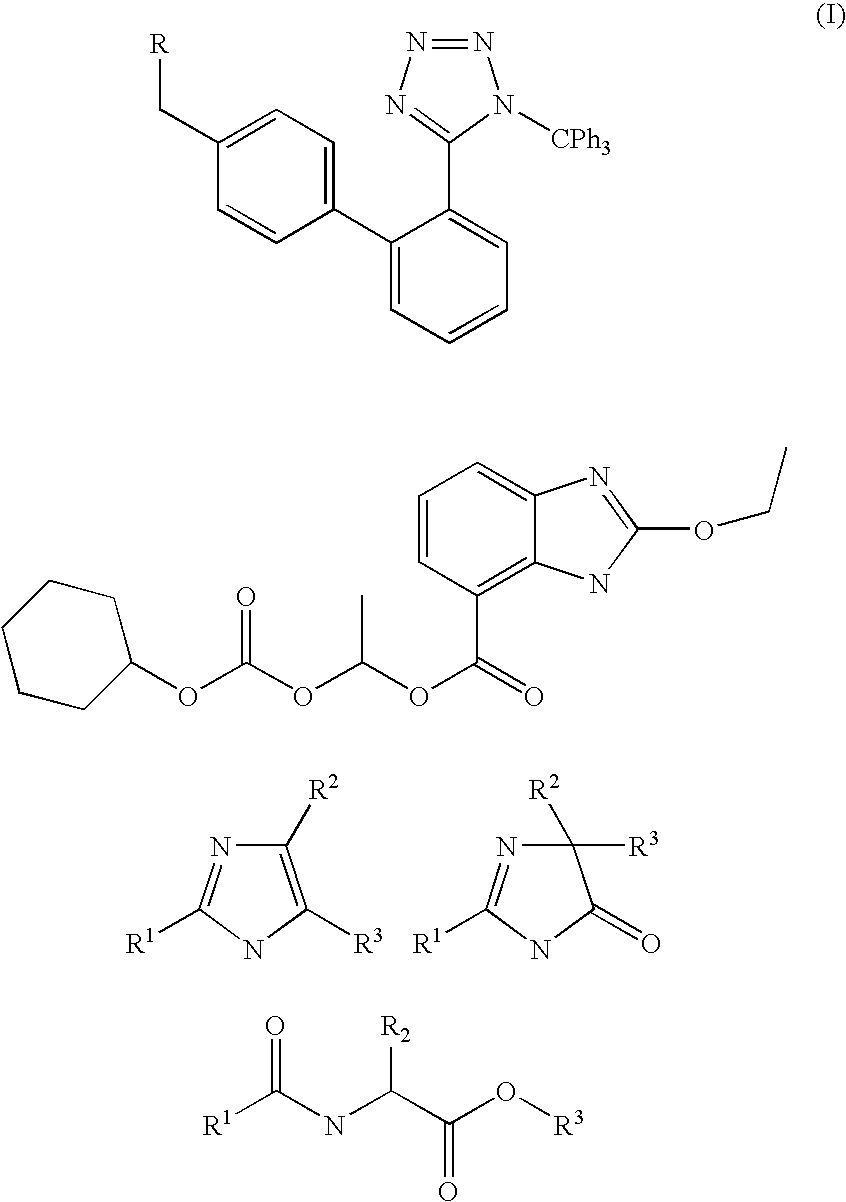
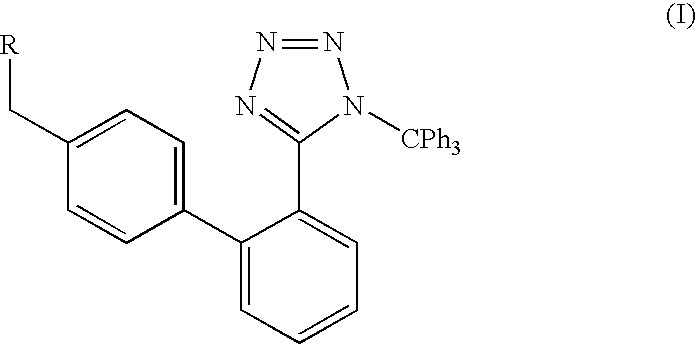
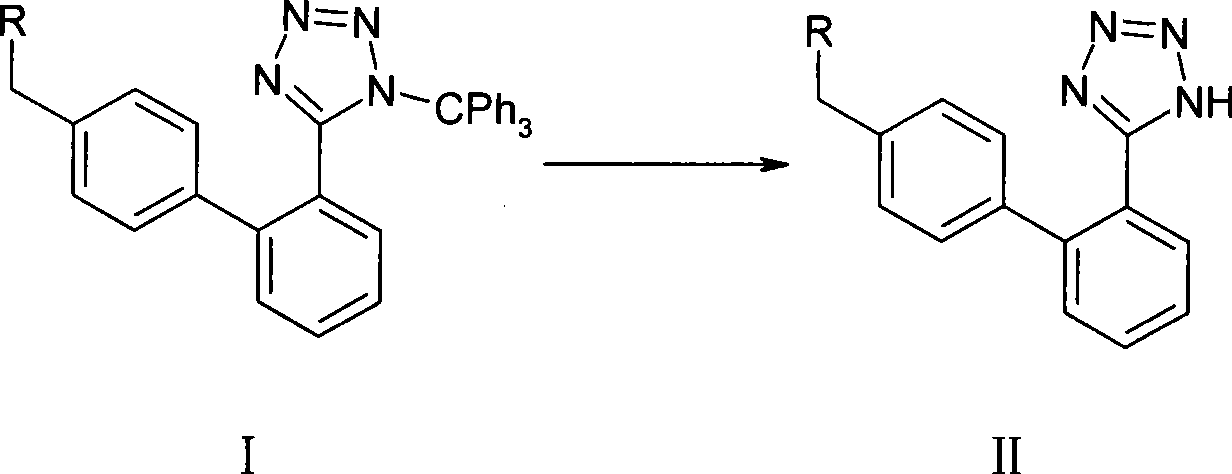
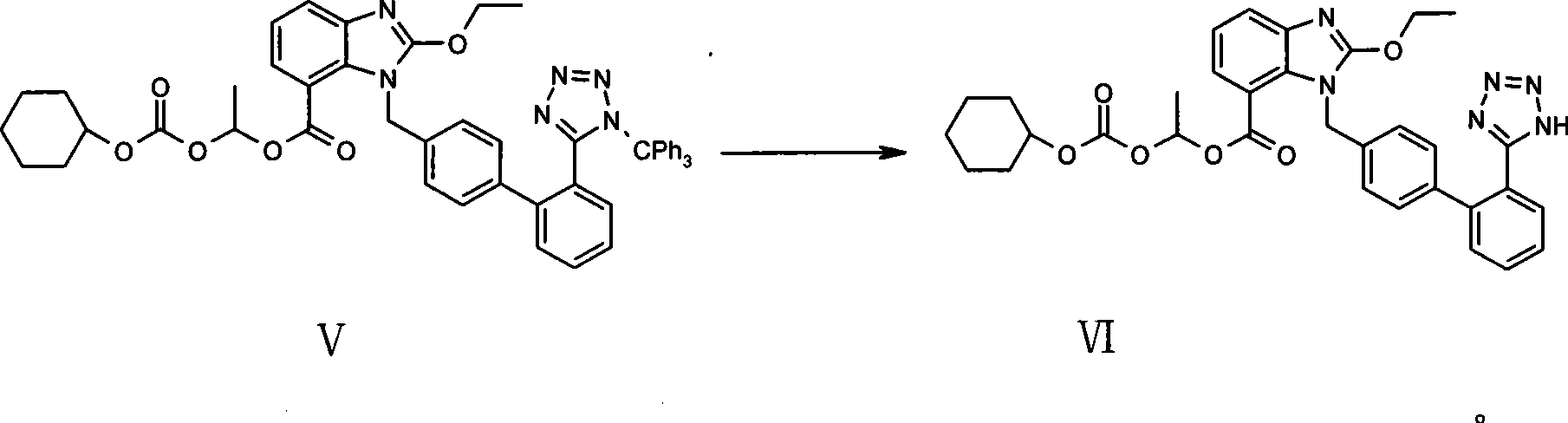
A method of removing the triphenylmethane protecting group from 1-triphenylmethy1-5-(4′-subst. methyl-1,1′-biphenyl-2-yl)-1H-tetrazoles of general formula I wherein R represents the groups of formulae and where R1, R2 and R3 can be H, a halogen, an unbranched or branched C1-C5 alkyl, C1-C5 hydroxyalkyl, C1-C5 alkoxy, C1-C5 alkoxymethyl or benzyl, or wherein R2 and R3 can form together a saturated or unsaturated C5-C7 ring, optionally an unsubstituted or substituted aromatic ring, is carried out by solvolysis in a simple anhydrous C1 to C5 alcohol in a neutral or slightly basic medium. The method is suitable for the preparation of drugs, such as the potassium salts of losartan, irbesartan or valsartan or candesartan cilexetil.
Owner:ZENTIVA AS
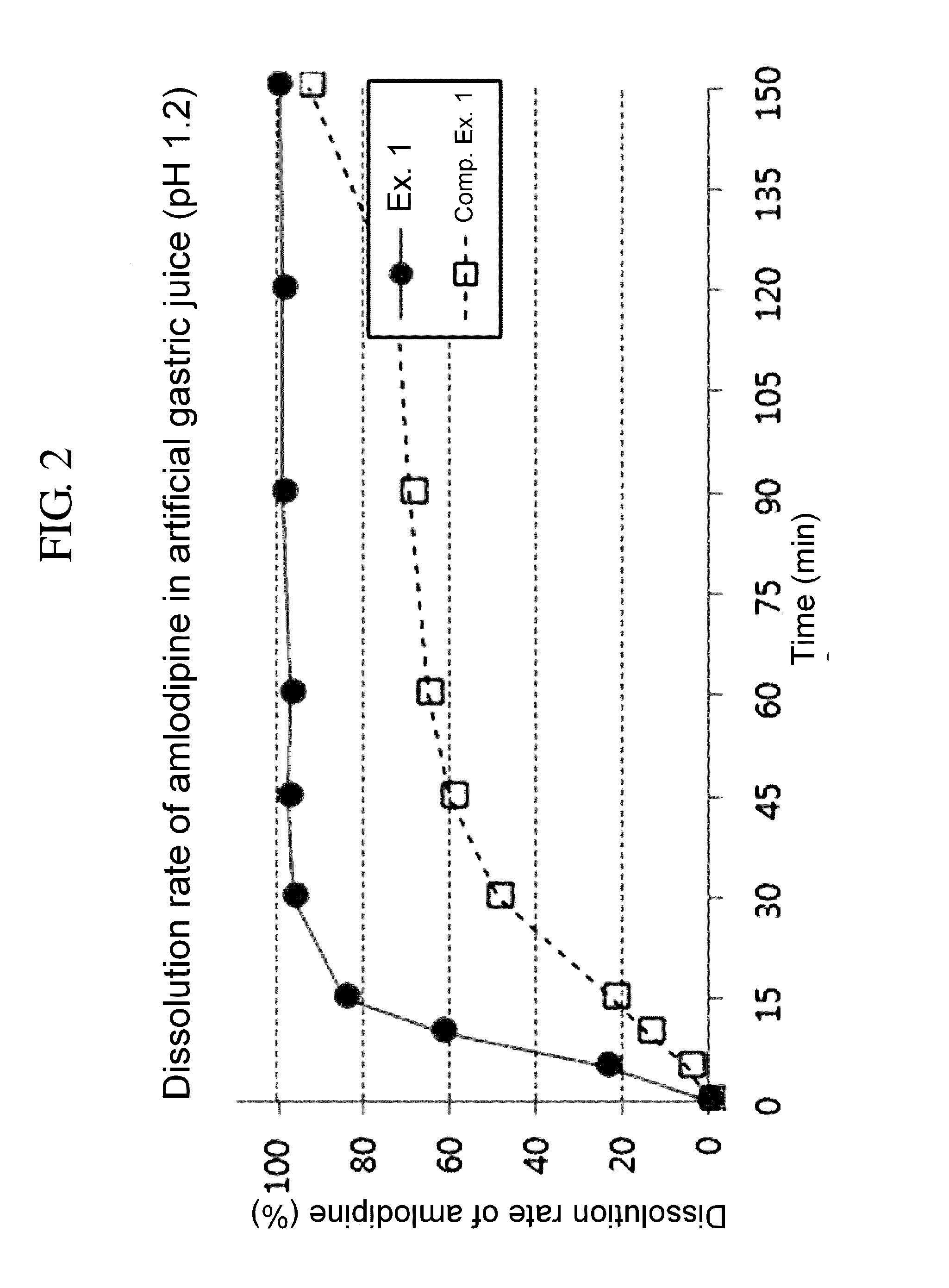
Pharmaceutical combination formulation comprising amlodipine, losartan and rosuvastatin
ActiveUS20170027871A1Excellent dissolution and stability propertyMinimize interactionOrganic active ingredientsMetabolism disorderPharmaceutical industryMechanism of action
Disclosed is a pharmaceutical combination formulation comprising a first discrete part containing amlodipine and rosuvastatin and a second discrete part containing losartan, which exhibits improved dissolution rate and stability. The inventive combination formulation comprising amlodipine, losartan and rosuvastatin having different action mechanisms from one another can be effectively used to prevent or treat a cardiovascular disorder. Designed to minimize an interaction among active ingredients, the pharmaceutical combination formulation exhibits excellent storage stability and dissolution rates of amlodipine, losartan and rosuvastatin, and thus can be useful in pharmaceutical industries.
Owner:HANMI PHARMA
Preparation method of losartan
The invention provides a preparation method of losartan. The losartan is prepared by reacting a cyano-containing intermediate as shown in a formula (I), which is described in the specification, with an azide reagent in toluene in the presence of a catalyst. After the reaction is finished, azide ions are removed through the following procedures: adding water to divide a reaction system into three layers, separating out a middle layer, adding n-butyl alcohol into the middle layer for dilution, and adding triphenylphosphine into the obtained diluted solution to remove the residual azide ions in the diluted solution. According to the preparation method, sodium nitrite is not needed, so formation of the genotoxic impurity nitrosamine is fundamentally eradicated; the obtained target losartan isgood in purity and high in yield; and the method is simple and convenient in preparation process, mild and easily controllable in operation conditions, good in safety, and suitable for large-scale industrial production.
Owner:ZHEJIANG TIANYU PHARMA +1
Method for recovering valsartan racemate
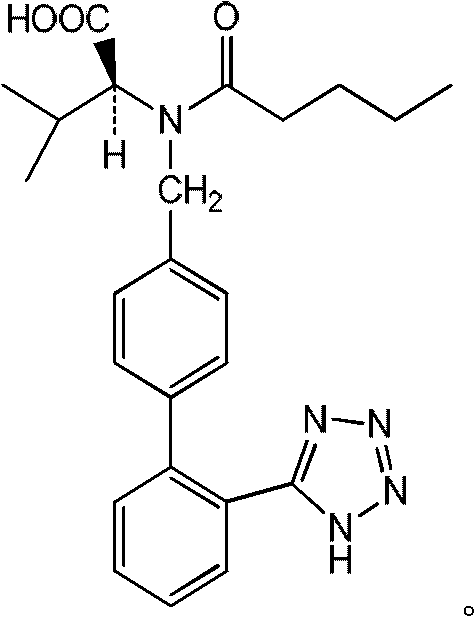
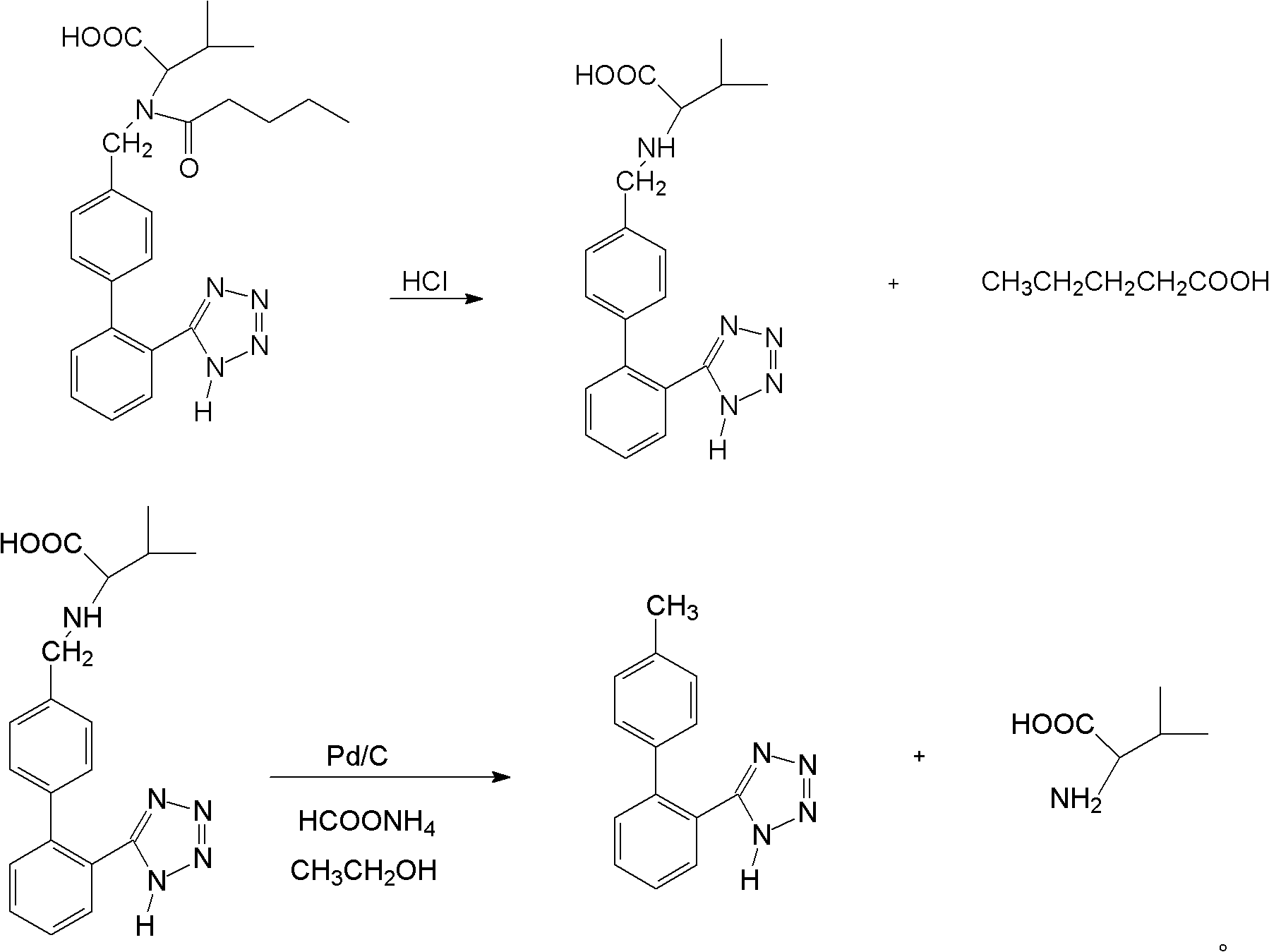
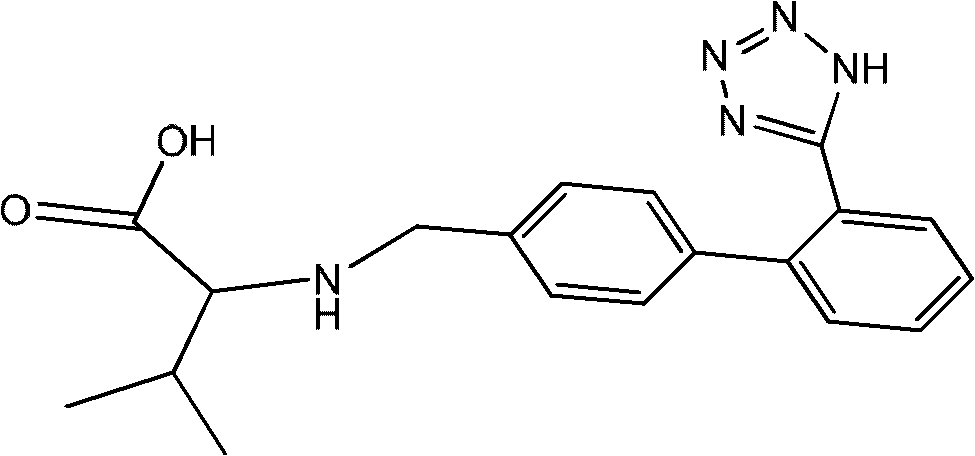
ActiveCN102351804AEasy to purifyHigh selectivityOrganic compound preparationPreparation from carboxylic acid amidesAlcoholLosartan
The invention discloses a method for recovering valsartan racemate, which comprises the following steps that: (1) pentanoic acid and valsartan depentanized acyl products are obtained through hydrolysis reaction of valsartan racemate in acid aqueous solution; and (2) in alcohol solvents, the valsartan depentanized acyl products take reduction reaction under the effect of reducing agents and catalysts to obtain valine and 4'-methyl phenylbenzene-2-hydroxyphenyl tetrazole. The method has the advantages that the steps are simple and convenient, the method is applicable to industrial production, and the 4'-methyl phenylbenzene-2- hydroxyphenyl tetrazole, the valine and the pentanoic acid can be recovered, wherein the 4'-methyl phenylbenzene-2-tetrazole is an important raw material for synthesizing losartan important intermediate N-(trityl)-5-(4'-bromine methyl phenylbenzene)-2-hydroxyphenyl) tetrazole.
Owner:浙江新赛科药业有限公司
Method for the production of losartan
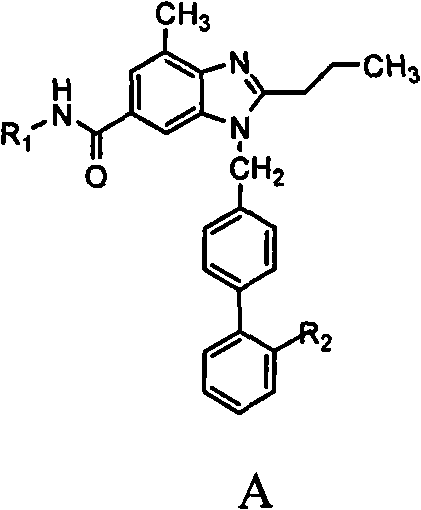
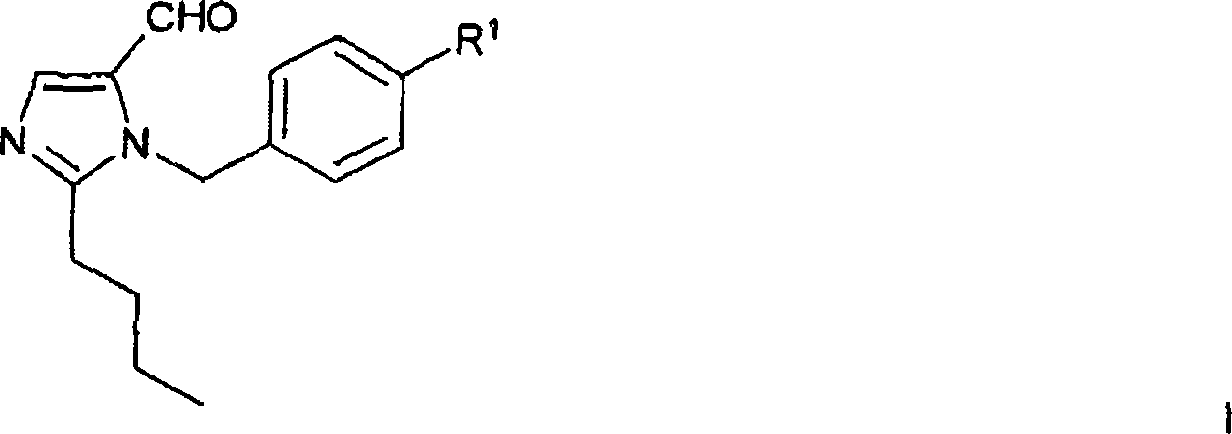
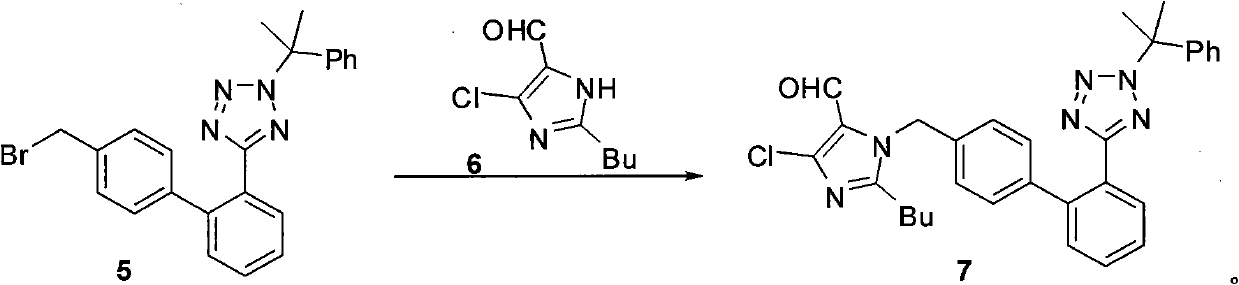
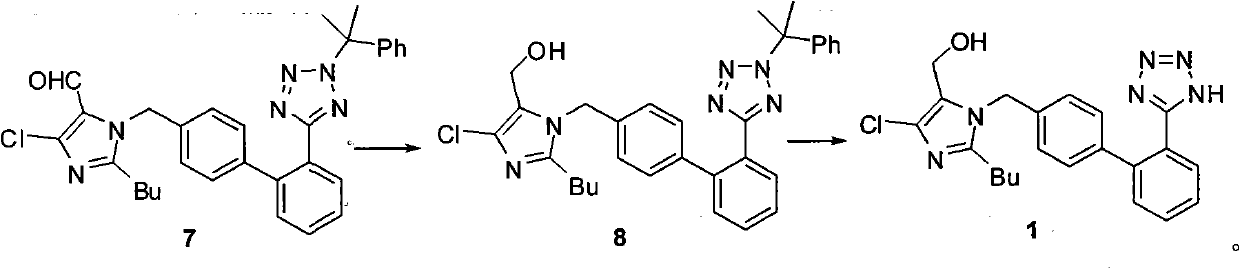
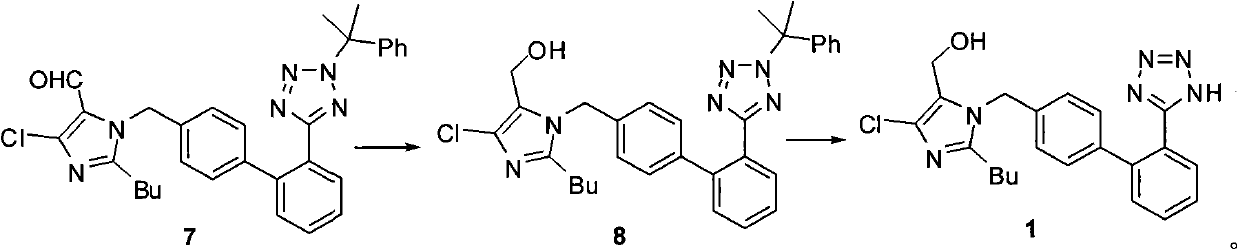
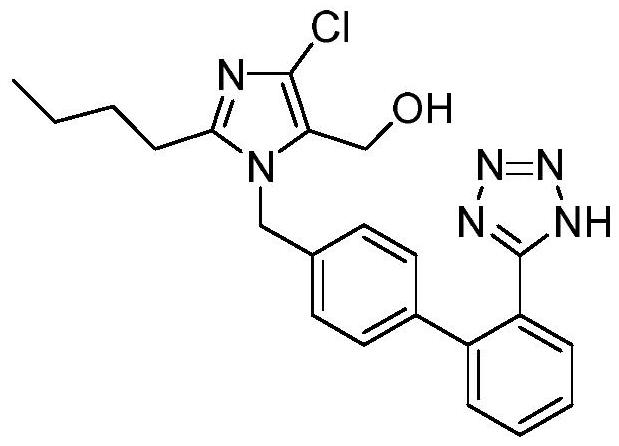
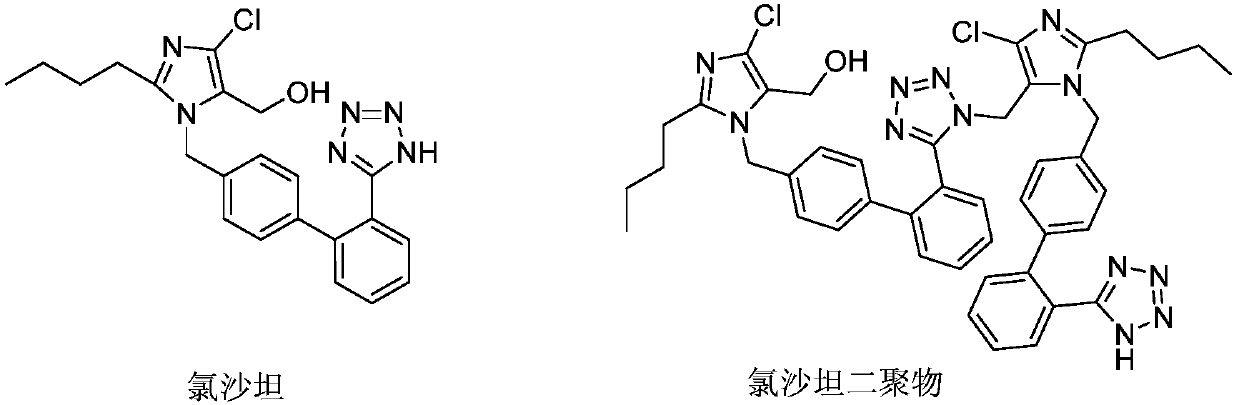
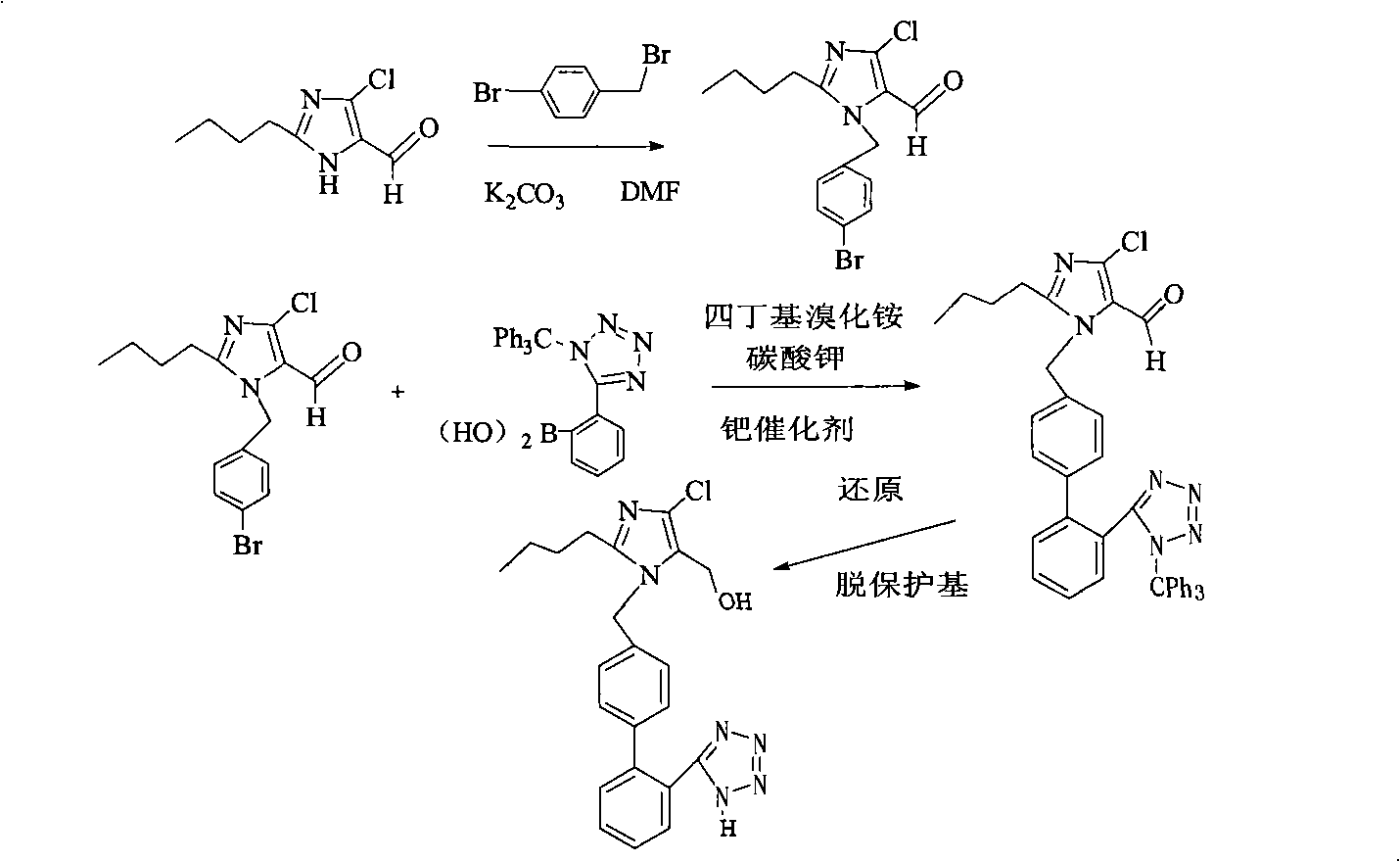
The invention relates to a novel method for the production of losartan, an imidazol derivative with the chemical name 2-n-butyl-4-chloro-5-hydroxymethyl-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-]methyl}imidazol and the pharmacologically active salts thereof. The invention also relates to novel intermediate products which are suitable for the production of losartan, and to novel methods for the production of intermediate compounds which are suitable for the production of losartan. One aspect of the invention is a method for the production of a compound of general formula (I), which can arise as an intermediate step in the inventive representation of losartan.
Owner:RATIOPHARM GHBH (DE)
Method of synthesizing losartan and losartan intermediates
InactiveCN102675294APracticalSuitable for industrial productionOrganic chemistrySynthesis methodsDecomposition
The invention relates to a method for synthesizing losartan and losartan intermediates and belongs to the technical field of medicine and medicine intermediates. 5-(4'-bromomethyl biphenyl-2-radical)-2-(1- methyl-1- phenylethyl) tetrazole and 2-butyl-4-chlorine-5-formyl radical imidazole are subjected to condensation, reduction and decomposition protection processes, and the losartan is obtained. The method has the advantages that the synthesis process is suitable for industrial production, in addition, the economic value can be generated, the synthesis process is safe, the raw material cost is saved, subsequent products are easy to treat, the reaction raw materials are single, and the synthesis method is convenient.
Owner:ZHEJIANG TIANYU PHARMA
1,3 and 1,3,5 substituted imidazoles as antihypertensives
InactiveUS20100216854A1High affinityEnhanced inhibitory effectBiocideOrganic chemistryLosartanLipophilicity
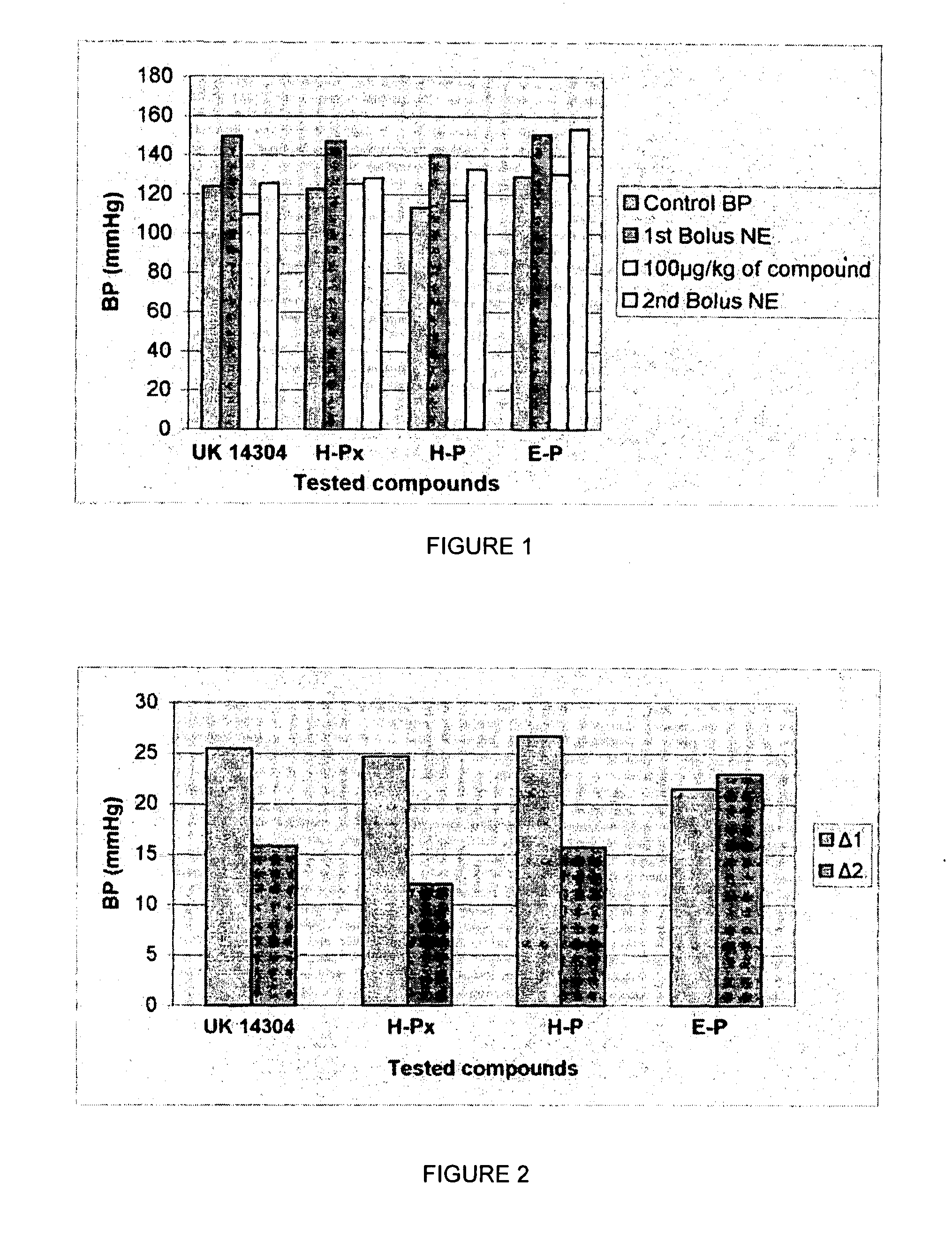
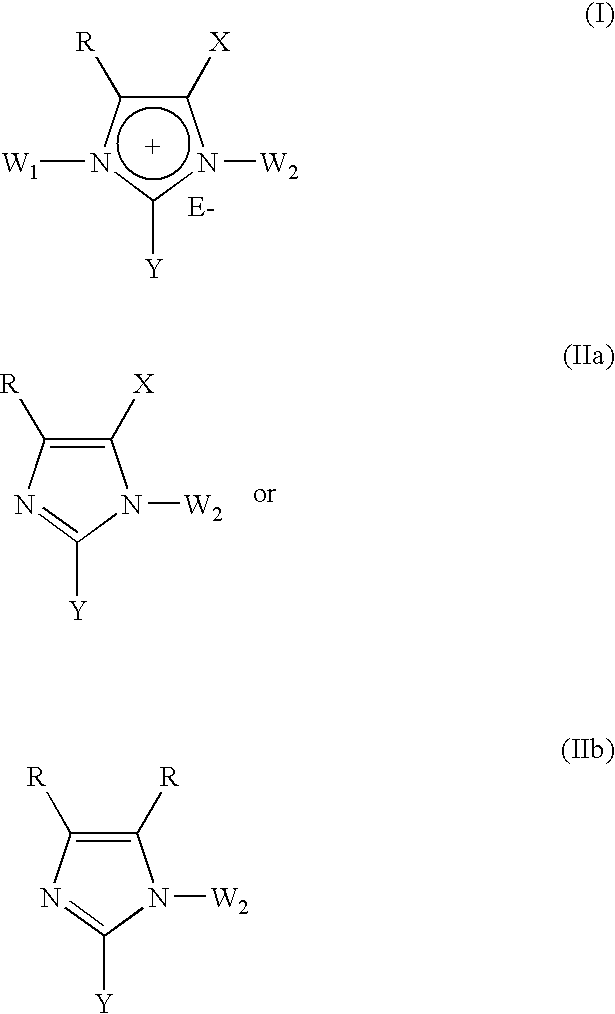
The present invention provides novel 1,5 and 1,3,5-substituted imidazole compounds of formulas (I), (IIa), (IIIb) in hydrophilic or lipophilic form, which are useful as angiotensin II AT1 receptor antagonists with sympathetic suppressant properties. In particular, the invention provides pharmaceutical compositions containing the pharmacophoric groups of Losartan and Clonidine as well compounds, processes and intermediates for preparing compounds and their use in methods of treating hypertension and cardiovascular diseases through Renin Angiotensin System (RAS) and Sympathetic System (SS). Alkylated histamine based double action Saltans are lipophilic and can act transdermally.
Owner:ELDRUG SA
Preparation method of losartan
The invention provides a preparation method of losartan, wherein the preparation method comprises the steps: reacting a nitrile intermediate shown in the specification with an azide reagent in a solvent, adding an inorganic alkali aqueous solution selected from carbonate or bicarbonate, washing, and separating out an intermediate material layer; and further separating the intermediate material layer to obtain losartan. The azide ions after the reaction can be basically and completely removed by using the conventional inorganic alkali solution, the removal effect is good, the preparation process is simple and convenient, the operation conditions are mild and easy to control, and the method is suitable for large-scale industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Amlodipine and losartan-containing compound preparation for treating hypertension
InactiveCN102038687AQuick resultsHigh blood pressureOrganic active ingredientsCardiovascular disorderSide effectAdditive ingredient
The invention relates to an amlodipine and losartan-containing compound preparation for treating hypertension. The compound preparation comprises amlodipine or pharmaceutically-acceptable salts thereof, and hydrochlorothiazide and losartan or pharmaceutically-acceptable salts thereof which are used as main ingredients and pharmaceutically acceptable carriers. The compound preparation has the advantages that: the curative effect is improved according to the action mechanism that the medicaments are combined to ensure that the medicaments are complementary to each other to reach the standard quickly, so that the standard-reaching rate of blood pressure can reach 80 percent, the adverse effect related to increasing of a certain dosage can be conveniently reduced, and longer action time is kept. The compound preparation has the characteristics of quick response, high standard-reaching rate, small side effect, and low cost.
Owner:邬林祥 +1
Preparation of losartan
The invention provides a method for preparing losartan shown in a formula (I). The method comprises the following steps: reacting 2-butyl-4-chlorine-5-(hydroxymethyl)-1-{[(2'- cyano-group) xenyl-4-group]methyl} imidazole and natrium azide and triethylamine hydrochloride shown in a formula (II) in toluol; regulating the obtained reaction liquid by alkali to separate organic layers; adding a reducing agent into the collected liquid; regulating the obtained reaction liquid by acid to obtain losartan solid; and if required, refining the losartan solid by isopropyl alcohol. The method has the advantages of high yield, high purity of the losartan, low cost, little environmental pollution and suitability for industrialized production.
Owner:ZHEJIANG MENOVO PHARMA
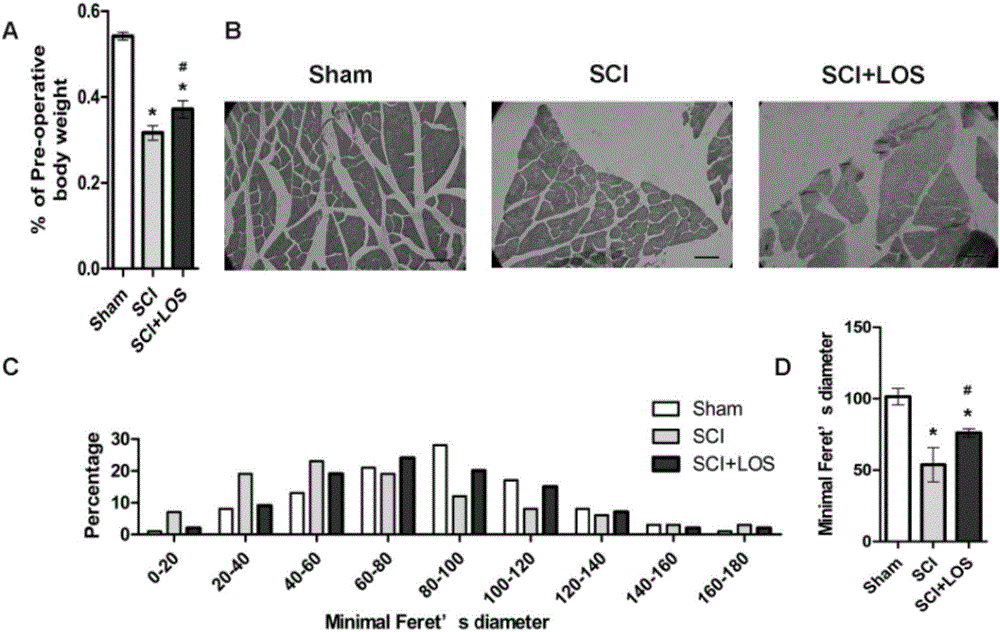
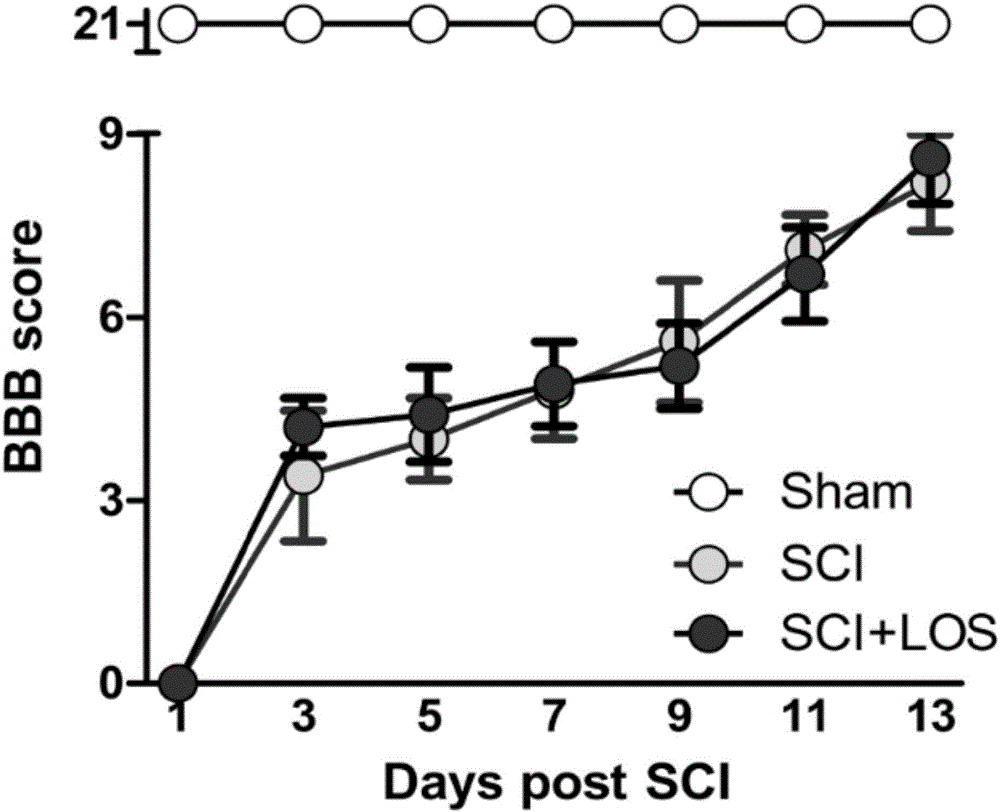
Medicine that cures muscle atrophy after spinal cord injury and its application method
InactiveCN106474119ARelieve atrophyPrevent atrophyOrganic active ingredientsNervous disorderSkeletal muscle atrophyPharmacon
The invention discloses a medicine that cures muscle atrophy after spinal cord injury and its application method: the medicine that cures muscle atrophy after spinal cord injury is losartan. The application method is that losartan activates a signal pathway of IGF1 / Akt / mTOR and inhibits an expression of MuRF-1; A receptor blocking pharmacon of angiotensin II takes effect through activating the pathway of IGF-1 / Art / mTOR and inhibiting a ubiquotin-proteasomes system. The receptor blocking pharmacon of angiotensin II can improve the skeletal muscle atrophy after spinal cord injury of rats and may take effect through activating the pathway of IGF-1 / Akt / mTOR and inhibiting the ubiquitin-proteasomes system, which provides a part of basis for later clinical application and combined therapy.
Owner:天津运三泽生物医药科技有限公司 +1
Preparation method of 5-losartan carbonate
InactiveCN102558156AHigh yieldHigh purityOrganic chemistryCardiovascular disorderLosartanEthyl Chloride
The invention provides a method for preparing a losartan derivative 5-losartan carbonate from 2-butyl-4-chloro-5-formoxyl-1-{[2'-2(1-H-tetrazole-5-yl)-biphenyl-4]-methyl} imidazole. According to the invention, the total yield of 5-losartan carbonate is 75.6%; and on the existing technology basis, the yield is further improved, cost is reduced, product purity is improved, maneuverability is increased. Thus, the method provided by the invention is suitable for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Prepn process of 5-losartan carboxylate
The present invention discloses preparation process of 5-losartan carboxylate. In polar solvent, such as DMF, pyridine, acetone, the mixed solvent with water, etc, losartan or its ammonium salt, alkali metal salt, hydrochloride, sulfate, formate, etc and the organic salt or inorganic salt of permanganic acid react to produce 5-losartan carboxylate or its ammonium salt, alkali metal salt, hydrochloride, sulfate, formate, etc. The preparation process is direct, economic, efficient and suitable for industrial production.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Method for reducing bipolymer impurities in losartan
ActiveCN108047208ALow costImprove conversion rateOrganic chemistryTemperature controlOrganic solvent
The invention discloses a method for reducing bipolymer impurities in losartan. The method comprises the following steps: firstly, adding losartan condensate into an organic solvent, adding acid, adjusting the pH value to be 1 to 5, and carrying out stirring reaction for 8 to 20 DEG C at a temperature controlled to be 10 to 40 DEG C; secondly, cooling down a system, adding alkali to adjust the pHto be alkaline, completely evaporating an organic layer, adding water, filtering, adjusting the acidity of filtrate, and performing crystallization, so as to obtain losartan. The content of the bipolymer impurities in the formed losartan is smaller than 0.2 percent and even smaller than 0.1 percent, the method is low in cost, the yield is high, and the method is extremely simple to operate, environmentally friendly and suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Losartan preparation method
ActiveCN101362750AGuaranteed qualityOrganic chemistryBulk chemical productionAlcoholRoom temperature
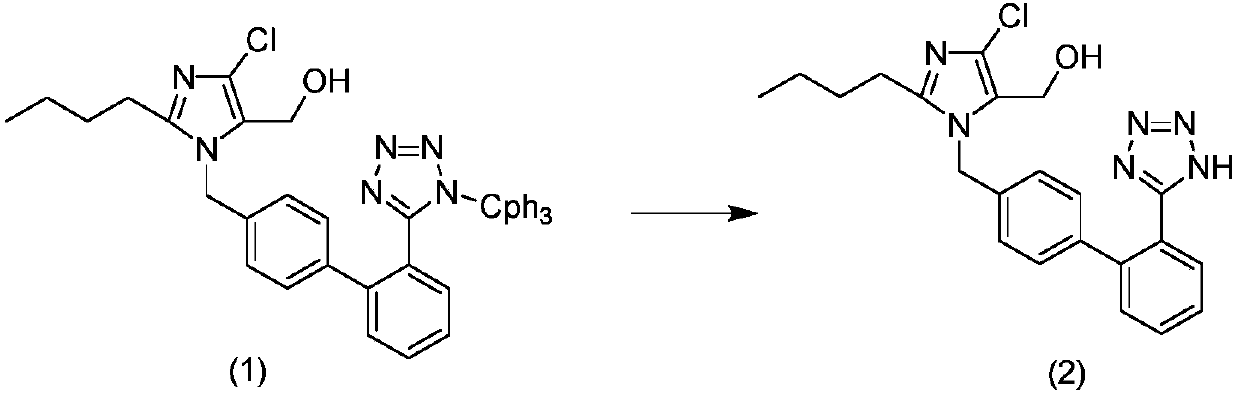
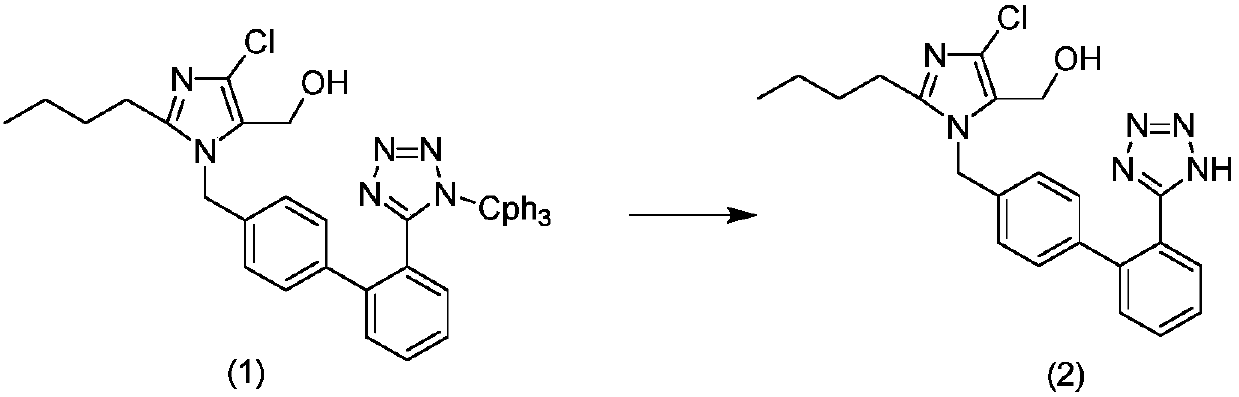
The invention relates to a preparation method of losartan, which comprises the following steps: triphenylmethyl losartan (structure III) is dissolved in a long chain liquid alcohol over C12, inorganic acid (hydrochloride, sulfate, phosphate) aqueous solution is added as a catalyst and the losartan (structure II) is finally obtained after 24 hours -36 hours of reaction at room temperature. By the improvement of the reaction conditions, no side reaction occurs during the reaction process, thereby ensuring the quality of the final products.
Owner:CHINA RESOURCES SAIKE PHARMA
Glioma-targeting composite nanometer preparation, preparation method and applications thereof
ActiveCN107890570AHigh transfection efficiencyEffective gene silencing effectOrganic active ingredientsPeptide/protein ingredientsLosartanCopolymer
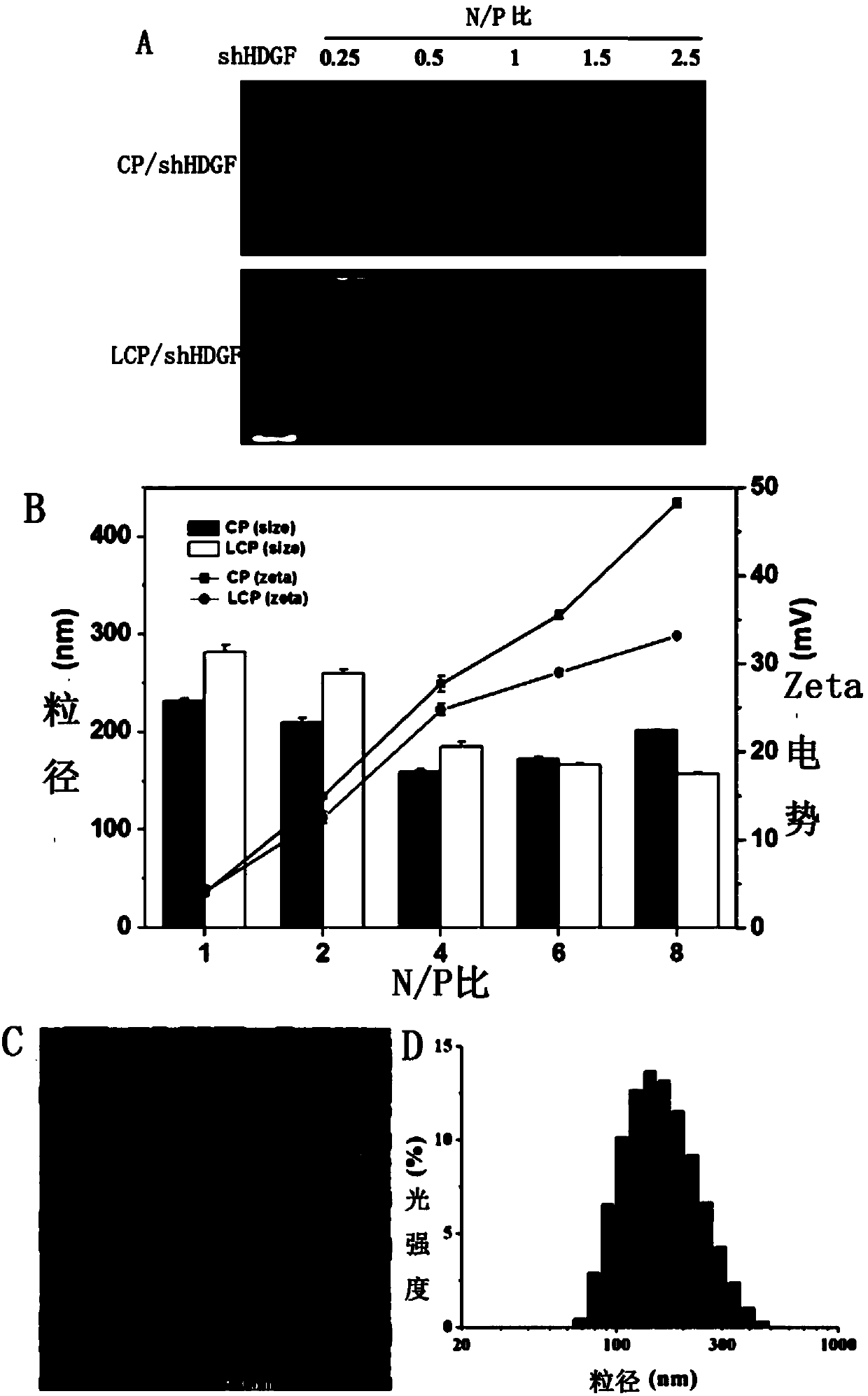
The invention provides a glioma-targeting composite nanometer preparation, a preparation method and applications thereof, wherein the glioma-targeting composite nanometer preparation is a nasal nanometer complex assembled by coating a coated material hepatoma-derived growth factor delivery shRNA with a coating material losartan-chitosan-polyethyleneimine vector copolymer, and the particle size is50-250 nm. The preparation method comprises: carrying out an oxidation reaction on chitosan and potassium periodate to obtain oxidized chitosan; carrying out a reaction on the oxidized chitosan and polyethyleneimine to obtain a chitosan-polyethyleneimine copolymer; carrying out a Schiff base reaction on the chitosan-polyethyleneimine copolymer and carboxylated losartan to obtain a losartan-chitosan-polyethyleneimine vector copolymer; and assembling the losartan-chitosan-polyethyleneimine vector copolymer and shHDGF to obtain the nasal nanometer complex. According to the present invention, after the nasal cavity product is prepared from the glioma-targeting composite nanometer preparation, the good brain glioma targeting property can be provided.
Owner:NANJING MEDICAL UNIV
Preparation of losartan
ActiveCN101328167AEasy to separateEasy to operateOrganic chemistryBulk chemical productionLosartanMethyl group
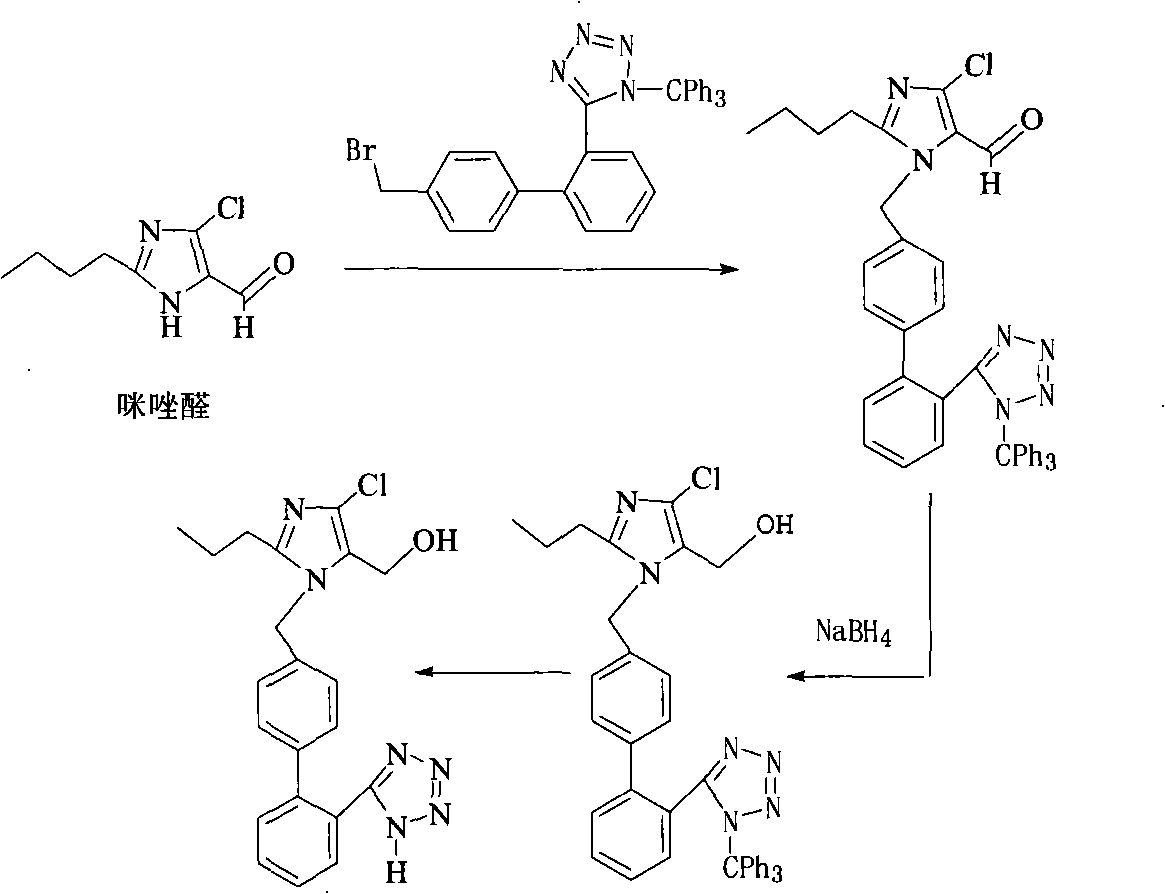
The invention relates to a method for preparing losartan. The method comprises the following steps of: removing a protective group of triphenylmethyl from 2-butyl-4-chloro-5-formyl-l-[(2'-(1-triphenylmethyl-tetrazole-5-yl)-biphenyl-4-)methyl] imidazole in the presence of a strong acid to give an intermediate of 2-butyl-4-chloro-5-formyl-1-[(2'-(1-H-tetrazole-5-)-biphenyl-4-)methyl] imidazole; producing 2-butyl-4-chloro-5-(hydroxymethyl)-1[(2'-(1-H-tetrazole-5-yl)-biphenyl-4-) methyl] imidazole in the presence of a reducer.
Owner:CHINA RESOURCES SAIKE PHARMA
Medicine composition for treating hypertension
InactiveCN101711762AImprove protectionReduce incidenceOrganic active ingredientsPharmaceutical non-active ingredientsWhite blood cellLevamlodipine
The invention belongs to the field of medicine preparations, which relates to a medicine composition containing the active components of levorotatory amlodipine / amlodipine and losartan. Concretely, the medicine composition is an osmotic pump preparation. The osmotic pump preparation contains main medicines, high molecular substances, a surfactant, an osmotic pressure promoter, an osmosis promotion polymer and auxiliary materials of other conventional preparation tablets and a coating. The medicine composition represents an obvious advantage in the indexes of the pressure relief, the sugar tolerance, the urine content of urine trace albumin, blood leucocyte after stress and the like.
Owner:LUNAN PHARMA GROUP CORPORATION
Double-skin milk and losartan mixed nutrient solution and preparation method thereof
The invention discloses a double-skin milk and losartan mixed nutrient solution and a preparation method thereof and relates to the technical field of food processing. The double-skin milk and losartan mixed nutrient solution mainly contains 3,000g of water, 50-60g of cream, 30-40g of chocolate, 50-60g of red beans, 20-30g of fruits, 200-250g of white sugar, 30-40g of pudding, 40-50g of dried rose petals, 5-10g of ginger juice, 5-10g of acanthopanax bark, 5-10g of shell powder of areca nut, 10-20g of Chinese waxgourd peel, 10-20g of chive seed, 20-25g of peach kernel, 20g of black beans, 20g of cola and 20g of losartan. The method comprises the steps of selecting materials, mincing, melting, cooling, mixing and stirring, cupping, and charging, thereby completing preparation. The double-skin milk and losartan mixed nutrient solution is diverse in flavor and unique in taste, so that the requirements of people on good food are met; the preparation method is simple and is low in cost, so that the increase of the economic benefit is facilitated; and the dried rose petals can be used for treating gynecological diseases such as infrequent menstruation, abdominal pain due to blood stasis, irregular menstruation, amenorrhea, dysmenorrhea and the like.
Owner:安徽省鸿运生物医药股份有限公司
Sartan compound discoloration method
The invention relates to a sartan compound discoloration method. The method comprises the following steps: adding irbesartan or losartan crude products containing pigment impurities into solvent, and dissolving; and adding hydroboration reagent, stirring for discoloration, and crystallizing through dissociation, cooling, distillation and other means to obtain white losartan or irbesartan. The method has the advantages of mild reaction conditions, short operating cycle, high discoloration efficiency and environment friendliness, and is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for removing trityl group of 1-trityl-5-(1,1'-biphenylyl-2 yl)-1H-tetrazole compound
The invention discloses a method for removing trityl from 1-trityl-5-(1,1'-biphenyl-2yl)-1H-tetrazole compound (I). The method is characterized by adding acyl halide in low-grade alcohol at low moisture condition to remove the trityl. The method has the advantages of rapid reaction speed, greatly shortened reaction time, higher product purity, and being fit for industrialized production. The method can be used for preparing related candesartan (VIII), losartan (X), irbesartan (XII), valsartan (XIV), olmesartan medoxomil (XVI) or olmesartan (XVIII).
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com