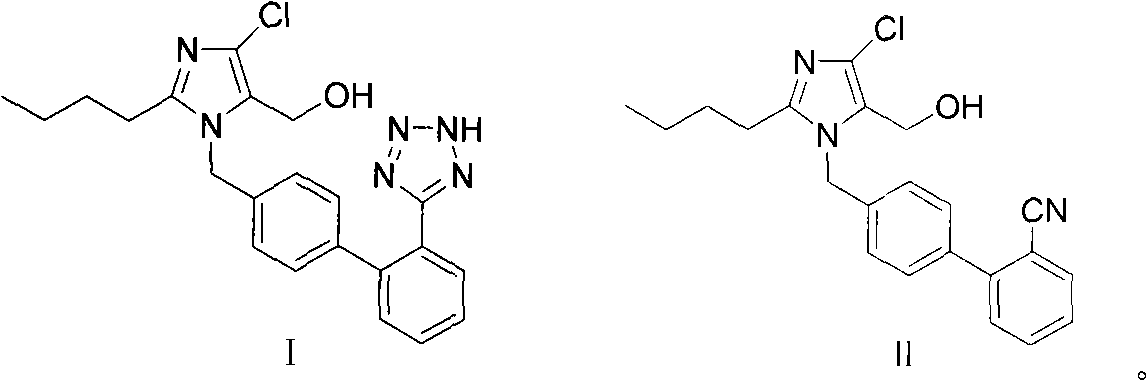
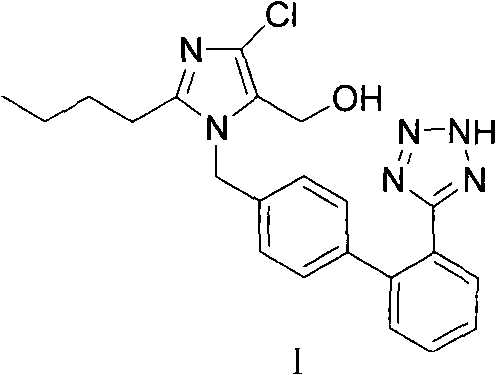
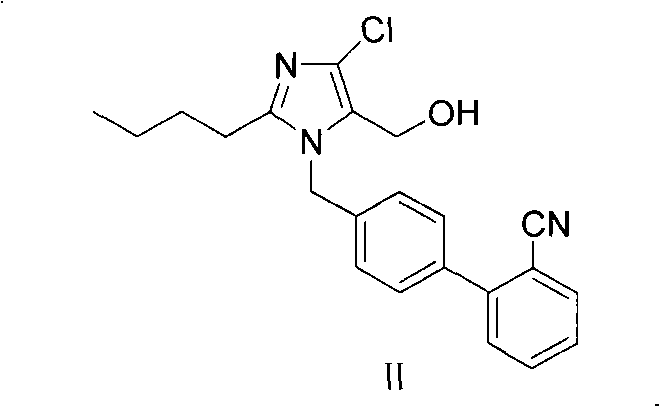
Preparation of losartan
A reaction solution and the obtained technology are applied in the field of preparation of the hypertensive drug losartan, can solve problems such as affecting product yield, environmental impact, and difficulty in solvent recovery, and achieve the effects of less damage, less solvent, and lower costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of losartan
[0029] To 2-butyl-4-chloro-5-(hydroxymethyl)-1-{[(2'-cyano)biphenyl-4-yl]methyl}imidazole (200g, 0.53mol), azide Add 400ml of toluene to sodium (100g, 1.54mol), triethylamine hydrochloride (215g, 1.54mol), react at 100°C for 40 hours, cool down to 20-30°C, adjust the pH of the reaction solution with alkaline water 9 to 10, separate the toluene layer, add sodium metabisulfite (50 g, 0.26 mol) to the resulting reaction solution, stir for 30 minutes, add 200 ml of ethyl acetate, adjust the pH of the reaction solution to 3.5 with dilute hydrochloric acid, and stir for 6 hours to obtain a solid wet product, the wet product was dissolved in 400ml of isopropanol, heated to reflux and cooled to 10-20°C, filtered and dried to obtain losartan (201g, molar yield 90%).
Embodiment 2
[0030] Embodiment 2: the preparation of losartan
[0031] To 2-butyl-4-chloro-5-(hydroxymethyl)-1-{[(2'-cyano)biphenyl-4-yl]methyl}imidazole (200g, 0.53mol), azide Add 400ml of toluene to sodium (100g, 1.54mol), triethylamine hydrochloride (215g, 1.54mol), react at 100°C for 40 hours, cool down to 20-30°C, adjust the pH of the reaction solution with alkaline water 9 to 10, divided into three layers, take the middle layer and add sodium metabisulfite (50g, 0.26mol), stir for 30 minutes, add 200ml of ethyl acetate, adjust the pH of the reaction solution to 3.5 with dilute hydrochloric acid, stir for 6 hours to obtain a solid wet product, The wet product was dissolved in 400ml of isopropanol, heated to reflux and cooled to 10-20°C, filtered and dried to obtain losartan (203g, molar yield 91%).
Embodiment 3
[0032] Embodiment 3: the preparation of losartan
[0033] To 2-butyl-4-chloro-5-(hydroxymethyl)-1-{[(2'-cyano)biphenyl-4-yl]methyl}imidazole (200g, 0.53mol), azide Add sodium (100g, 1.54mol), triethylamine hydrochloride (215g, 1.54mol) to 400ml toluene, react at 120°C for 40 hours, cool down to 20-30°C, adjust the pH of the reaction solution to 9 with alkaline water ~10, separate the toluene layer, add sodium nitrite (36.6g, 0.53mol) to the resulting reaction solution, stir for 30 minutes, add 200ml of ethyl acetate, adjust the pH of the reaction solution to 3.0 with dilute hydrochloric acid, stir for 8 hours, filter and dry Losartan (215 g, 96% molar yield) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com