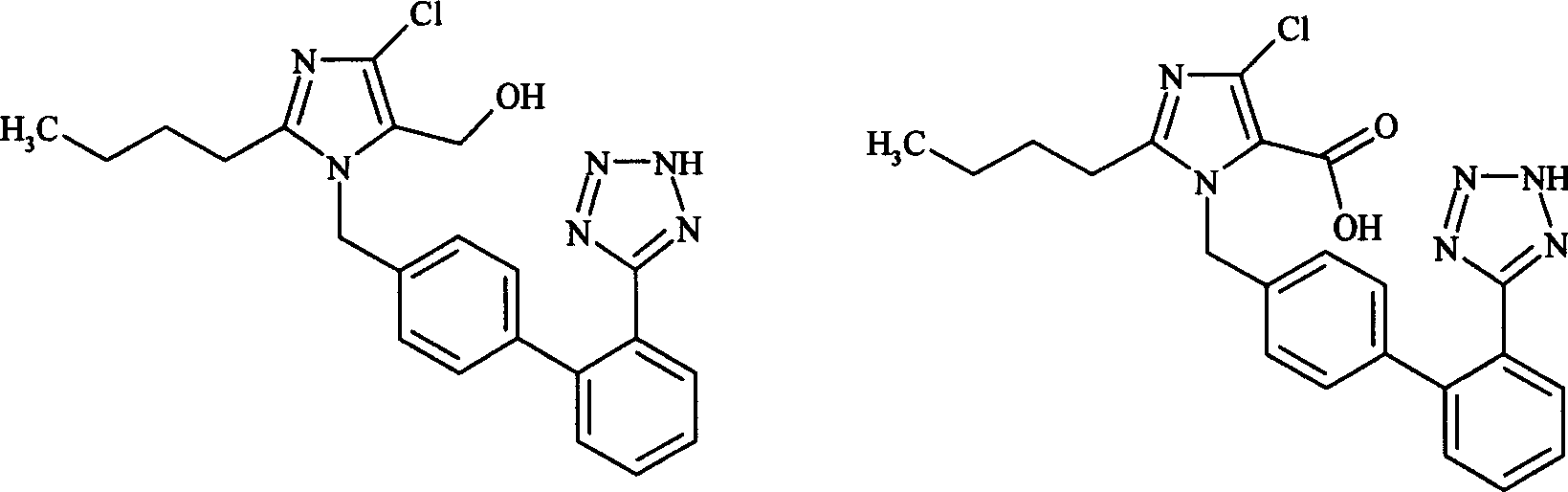
Prepn process of 5-losartan carboxylate
The technology of losartan carboxylate and formic acid is applied in the field of preparation of 5-carboxylate losartan, which can solve the problems of containing many impurities, being difficult to industrialize production, and failing to meet product purity requirements well, and achieving a simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: 500 ml of distilled water and 0.25 mol of potassium permanganate were added to a 2000 ml three-neck flask protected by nitrogen and mechanically stirred. 0.30 mol of tetrabutylamine chloride was divided into four equal portions and added over 20 minutes. After stirring for 30 minutes, 1000 ml of pyridine was added. After stirring for 15 minutes, the insoluble matter was filtered off. The filtrate was put back into the original three-neck flask with nitrogen protection and mechanical stirring. Heat to forty degrees. 0.1 mol of the compound of formula (I) was added in portions within 30 minutes. Keep the reaction temperature below fifty degrees. After stirring for one hour, 30% aqueous formaldehyde (200ml) was added. Stir until the red color of the reaction subsides. The reaction formed a large brown precipitate which was filtered. The solid was washed three times with 100 ml of 1M NaOH solution. The almost colorless filtrate was concentrated to one thi...
Embodiment 2
[0012] Example 2: 250 ml of distilled water and 0.25 mol of potassium permanganate were added to a 2000 ml three-neck flask protected by nitrogen and mechanically stirred. 0.30 mol of tetrabutylamine chloride was divided into four equal portions and added over 20 minutes. After stirring for 30 minutes, 1000 ml of pyridine was added. After stirring for 15 minutes, the insoluble matter was filtered off. The filtrate was put back into the original three-neck flask with nitrogen protection and mechanical stirring. Heat to seventy degrees. 0.1 mol of the potassium salt of the compound of formula (I) was added in portions within 30 minutes. Keep the reaction temperature below eighty degrees. After stirring for one hour, 30% aqueous formaldehyde (200ml) was added. Stir until the red color of the reaction subsides. The reaction formed a large brown precipitate which was filtered. The solid was washed three times with 100 ml of 1M NaOH solution. The almost colorless filtrate wa...
Embodiment 3
[0013] Example 3: 500 ml of distilled water and 0.25 mol of potassium permanganate were added to a 2000 ml three-neck flask protected by nitrogen and mechanically stirred. 0.30 mol of tetrabutylamine chloride was divided into four equal portions and added over 20 minutes. After stirring for 30 minutes, 1000 ml of dimethylformamide were added. After stirring for 15 minutes, the insoluble matter was filtered off. The filtrate was put back into the original three-neck flask with nitrogen protection and mechanical stirring. 0.1 mol of the potassium salt of the compound of formula (I) was added in portions within 30 minutes. Keep the reaction temperature below twenty degrees. After stirring for ten hours, 30% aqueous formaldehyde (200 ml) was added. Stir until the red color of the reaction subsides. The reaction formed a large brown precipitate which was filtered. The solid was washed three times with 100 ml of 1M NaOH solution. The almost colorless filtrate was concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com