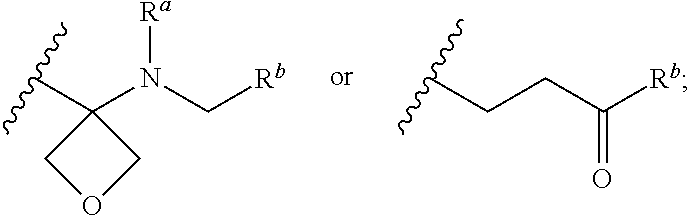Patents
Literature
666 results about "Active metabolite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An active metabolite is an active form of a drug after it has been processed by the body.
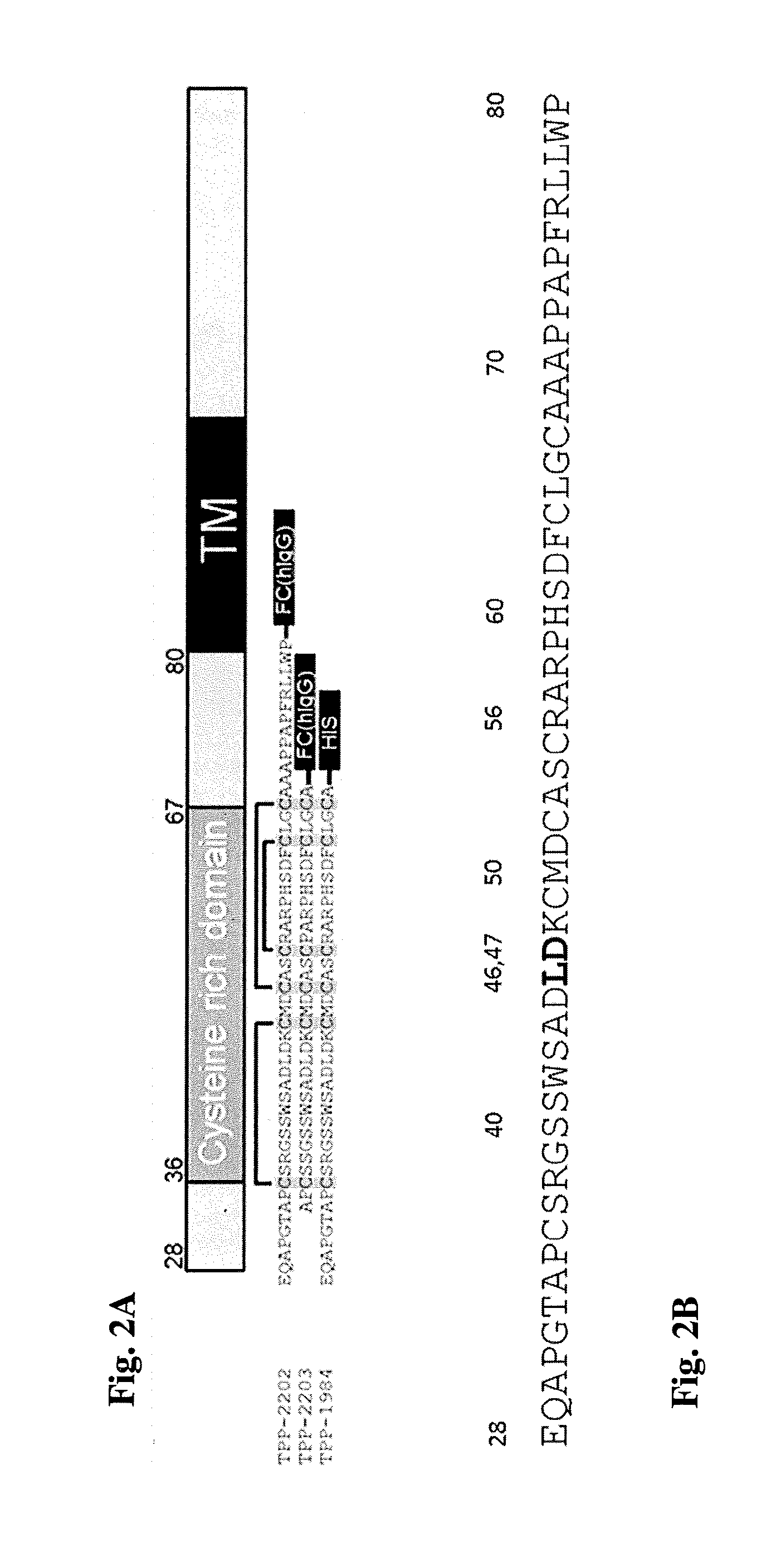
Method for production of antibodies to specific sites of rapamycin
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
Novel compound useful for the treatment of degenerative and inflammatory diseases
ActiveUS20120277247A1Modify activityModulate activityOrganic active ingredientsOrganic chemistryAutoimmune conditionAutoimmune disease
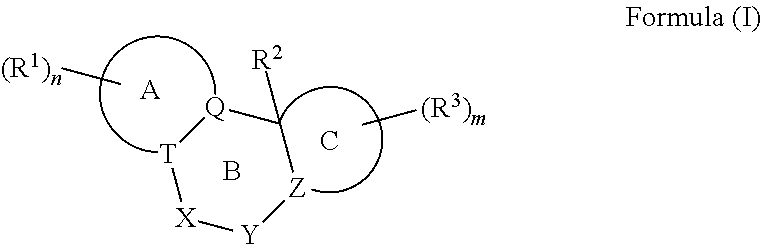
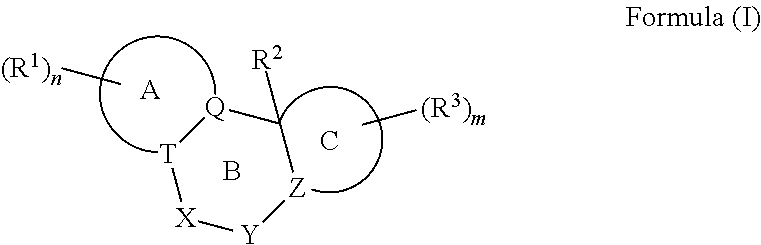
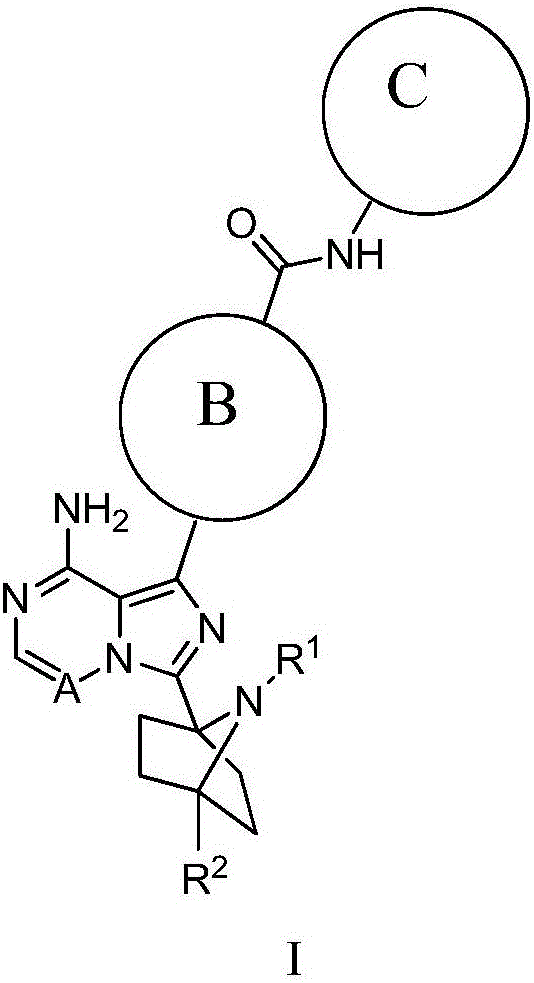
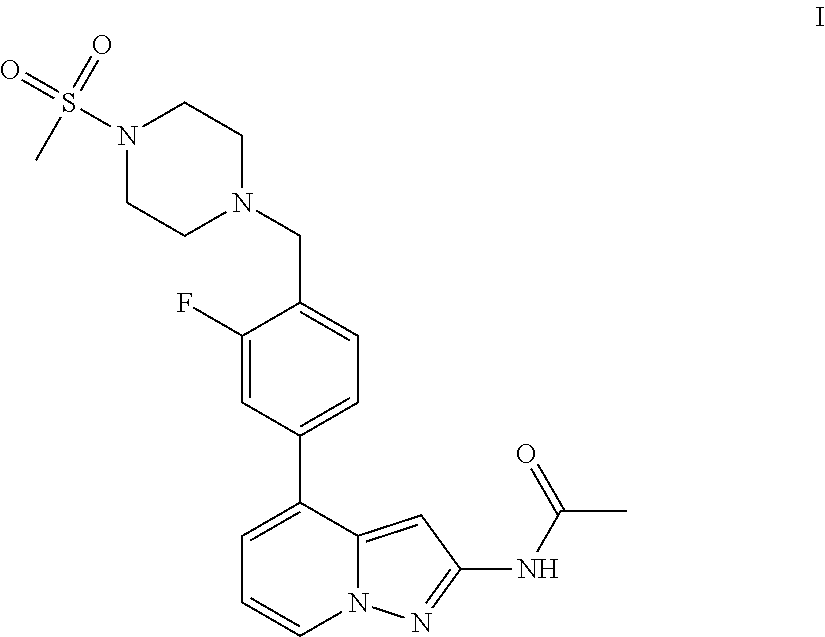
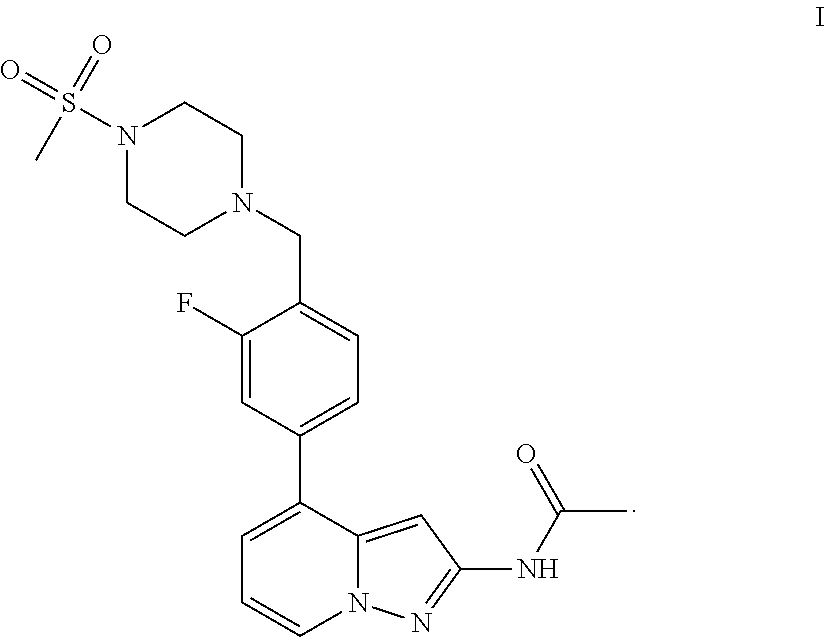
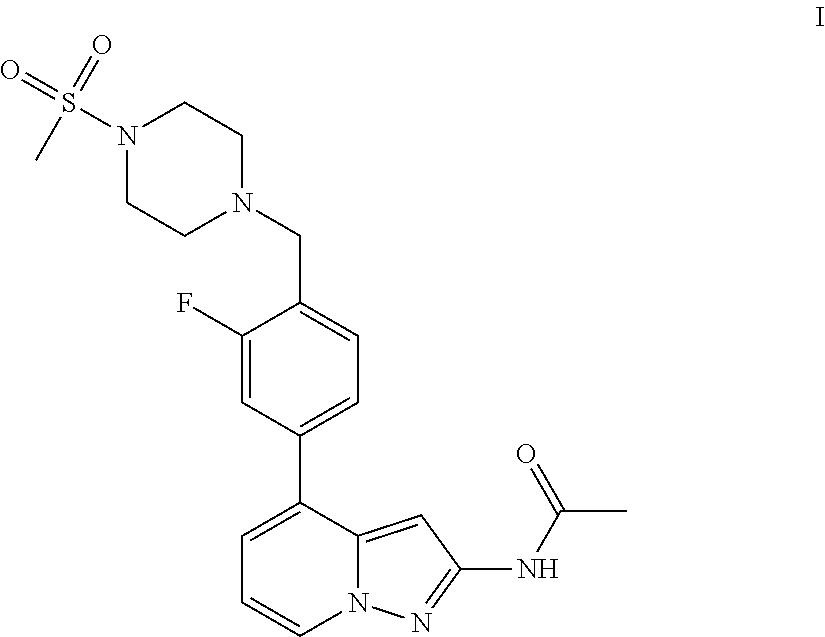
A pyrazolopyridine compound according to Formula I, able to inhibit JAK is disclosed, as well as pharmaceutically acceptable salts, a solvate thereof, solvates of the pharmaceutically acceptable salts and biologically active metabolites thereof. The compound may be prepared as a pharmaceutical composition, and may be used for the treatment or prophylaxis of a variety of conditions in mammals including humans, and particularly, such conditions as may be associated with aberrant JAK activity, including by way of non-limiting example, allergy, inflammatory conditions, autoimmune diseases, proliferative diseases, transplant rejection, diseases involving impairment of cartilage turnover, congenital cartilage malformations, and / or diseases associated with hypersecretion of IL6.
Owner:GALAPAGOS NV
Optically active isomers of ketotifen and therapeutically active metabolites thereof
Racemic norketotifen, racemic 10-hydroxy-ketotifen, racemic 10-hydroxy-nor-ketotifen and optically active isomers of ketotifen, norketotifen, 10-hydroxy-ketotifen and 10-hydroxy-norketotifen were found to have antiallergic and anti-inflammatory effects while being devoid of the severe dose-limiting sedative side effects of ketotifen.
Owner:BRIDGE PHARMA INC
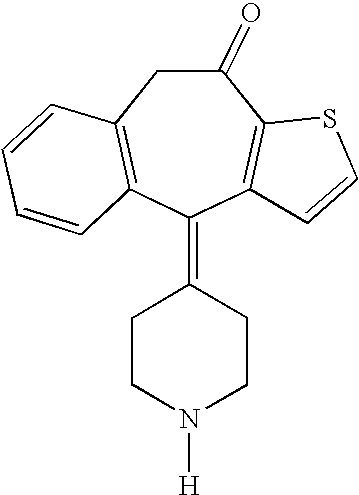
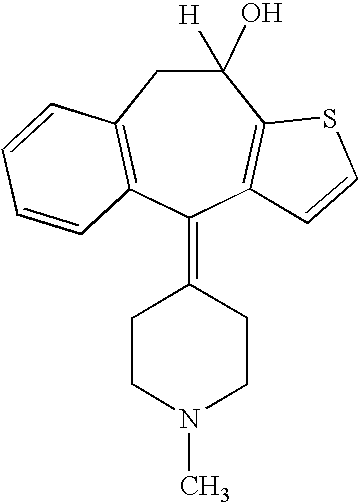
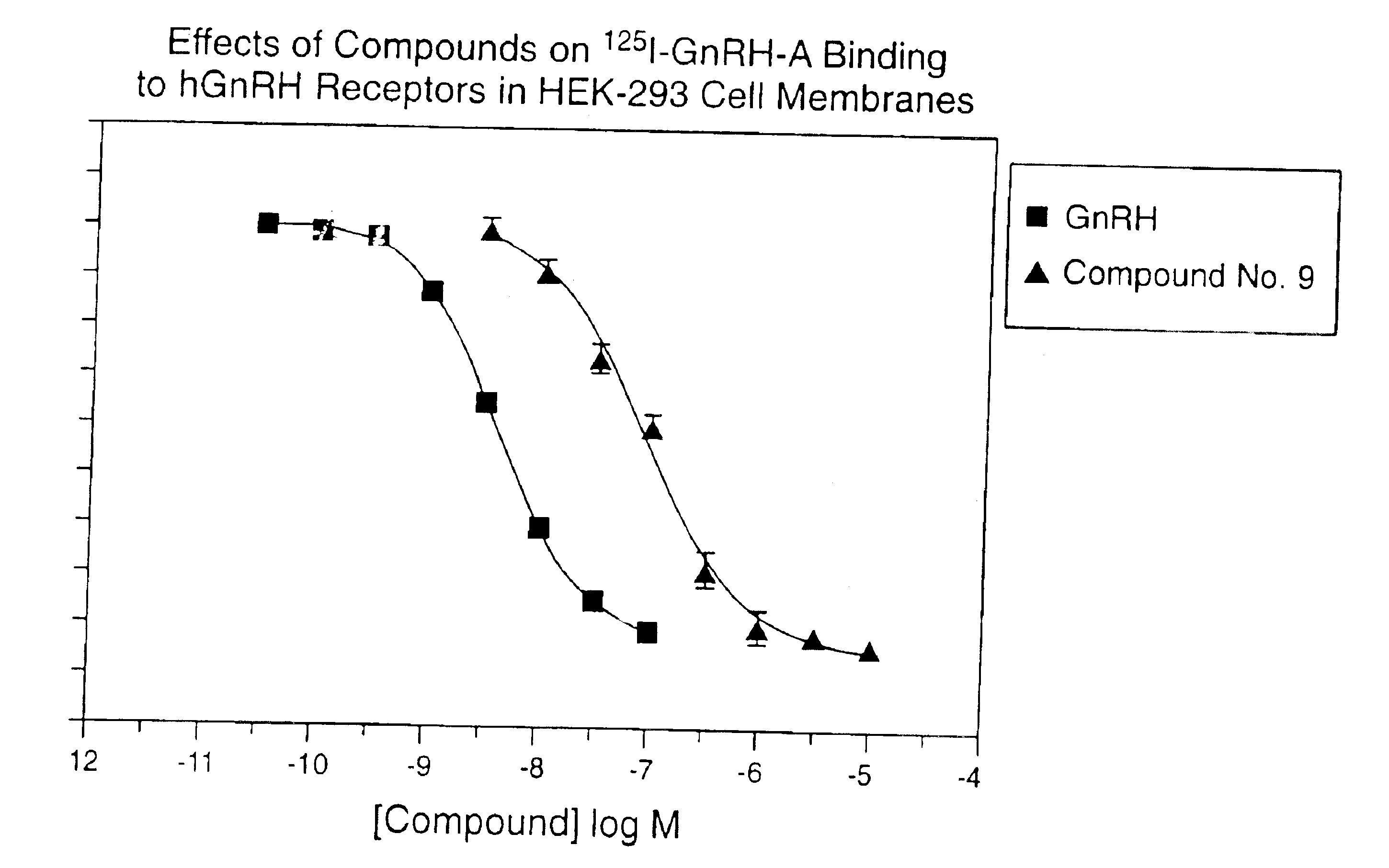
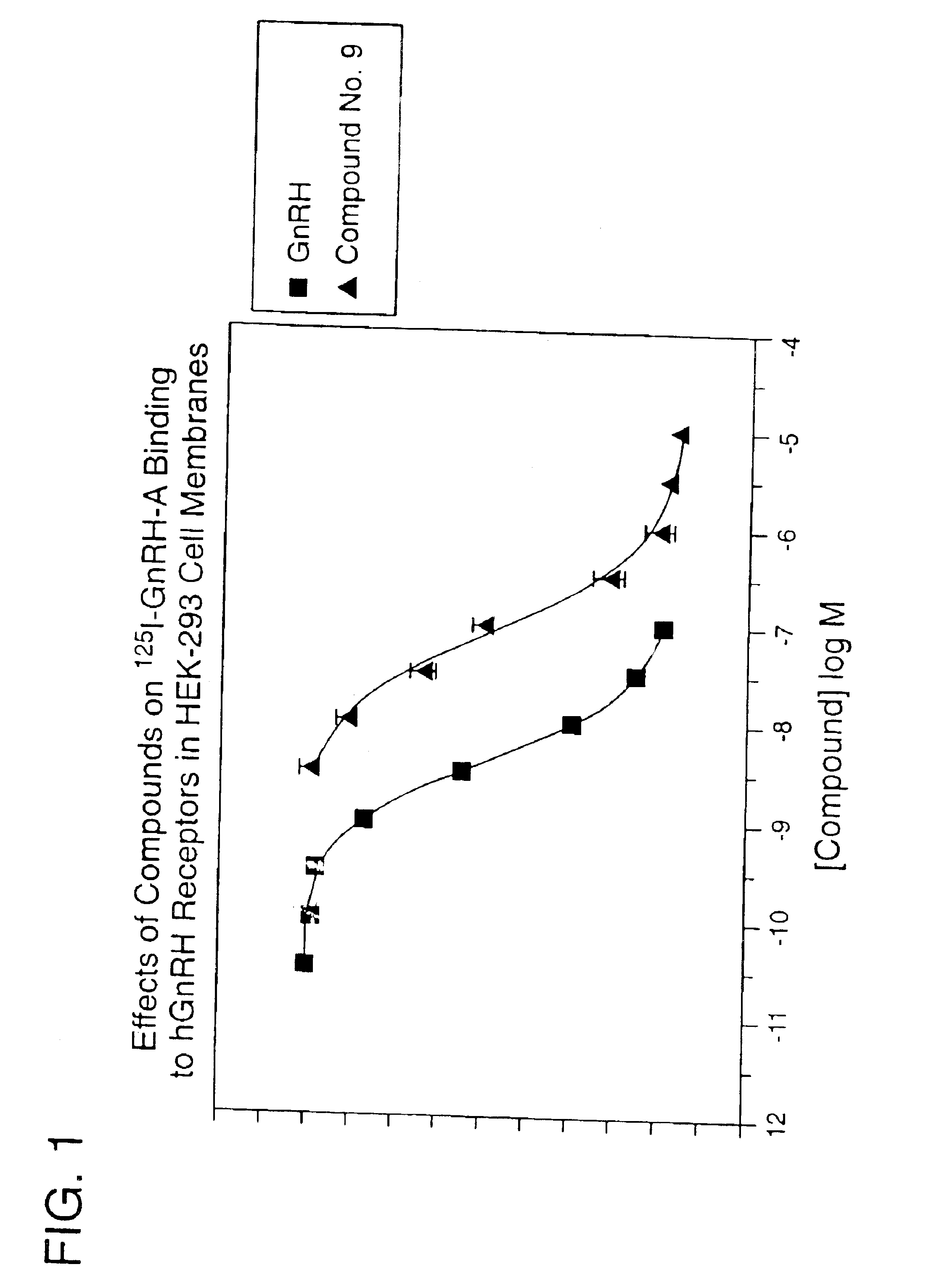
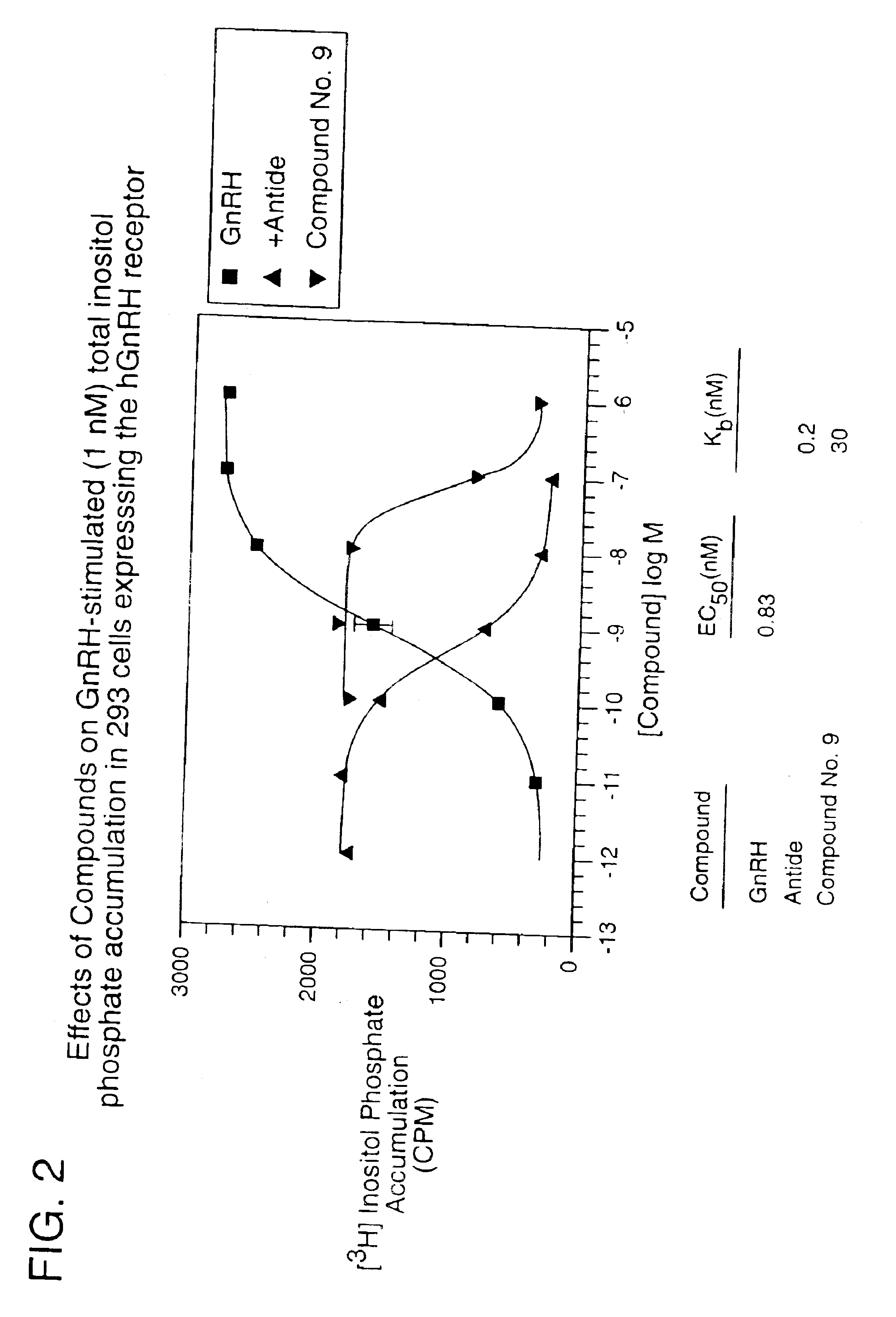
Non-peptide GNRH agents, methods and intermediates for their preparation
InactiveUS7101878B1Modulate activityGood biodistributionBiocideOrganic chemistrySteroidal hormonesGonadotropin-releasing hormone
Non-peptide GnRH agents capable of inhibiting the effect of gonadotropin-releasing hormone are described. Such compounds and their pharmaceutically acceptable salts, multimers, prodrugs, and active metabolites are suitable for treating mammalian reproductive disorders and steroid hormone-dependent tumors as well as for regulating fertility, where suppression of gonadotropin release is indicated. Methods for synthesizing the compounds and intermediates useful in their preparation are also described.
Owner:AGOURON PHARMA INC
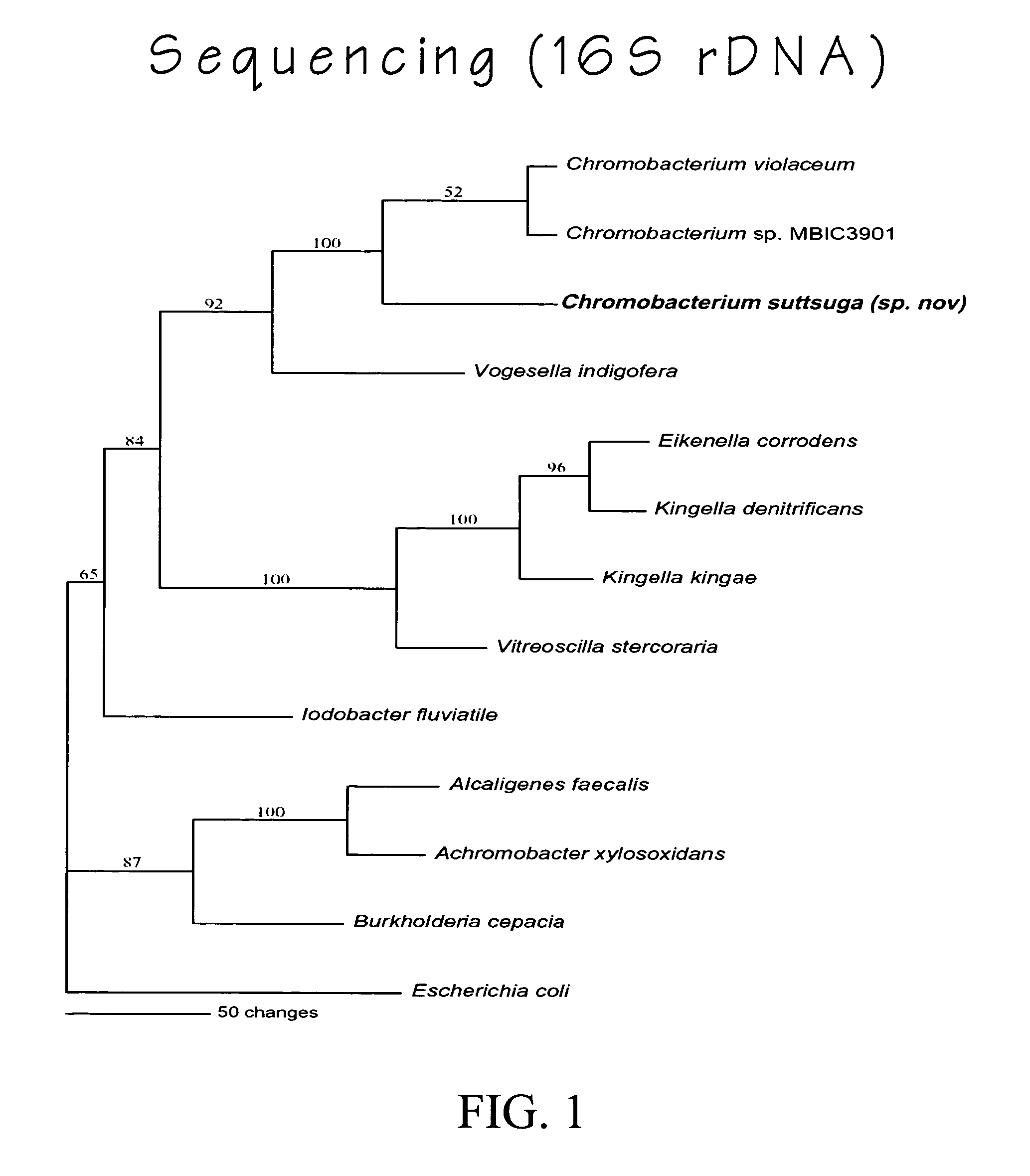
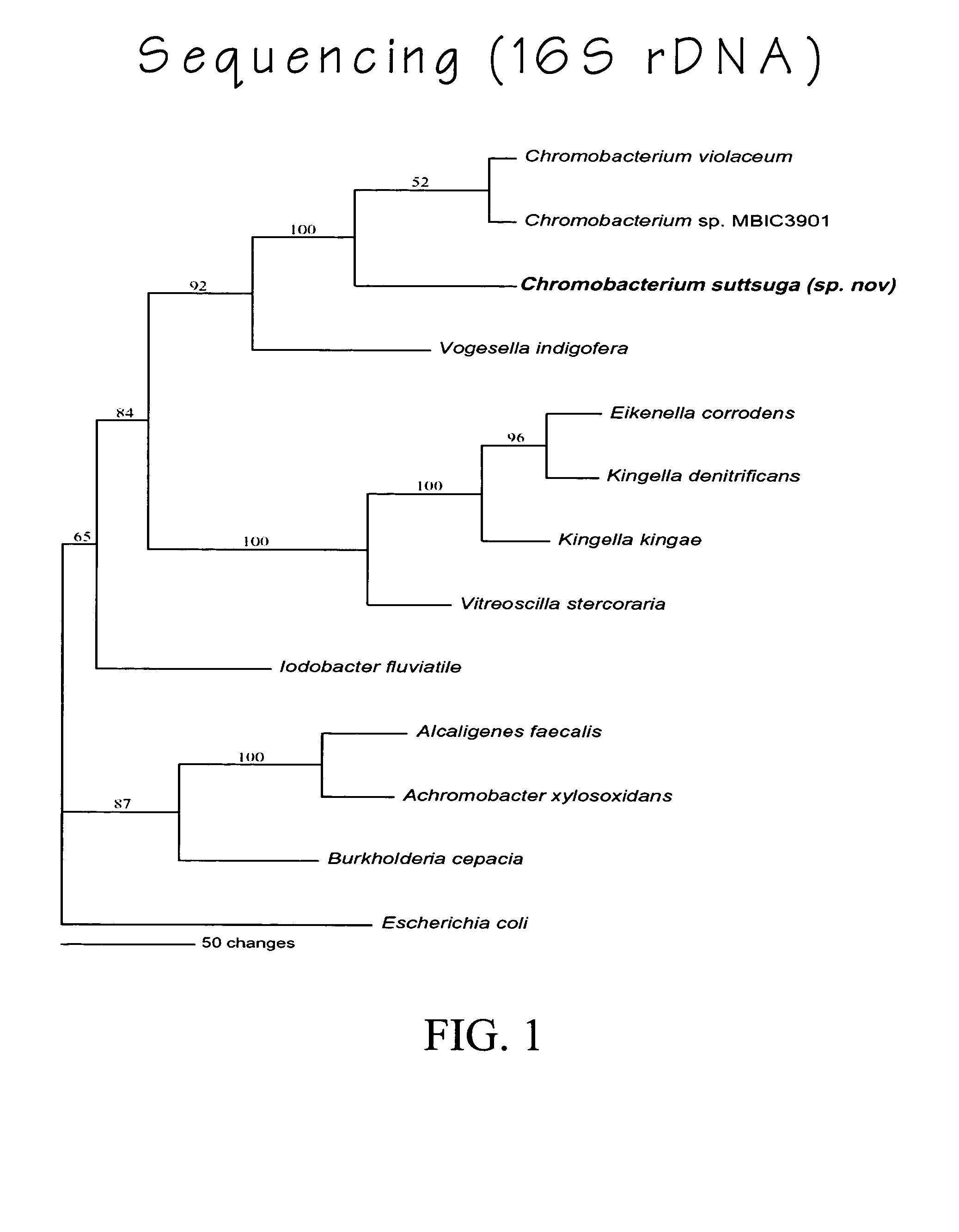
Chromobacterium subtsugae sp. nov. for control of insect pests
Chromobacterium suttsuga sp. nov., a new species of the genus Chromobacterium which possesses insecticidal activity, is described. The invention also relates to insecticidally-active metabolites obtained from the strain and to insecticidal compositions comprising cultures of the strain and / or supernatants, filtrates, and extracts obtained from the strain, and use thereof to control insect pests.
Owner:US SEC AGRI +1
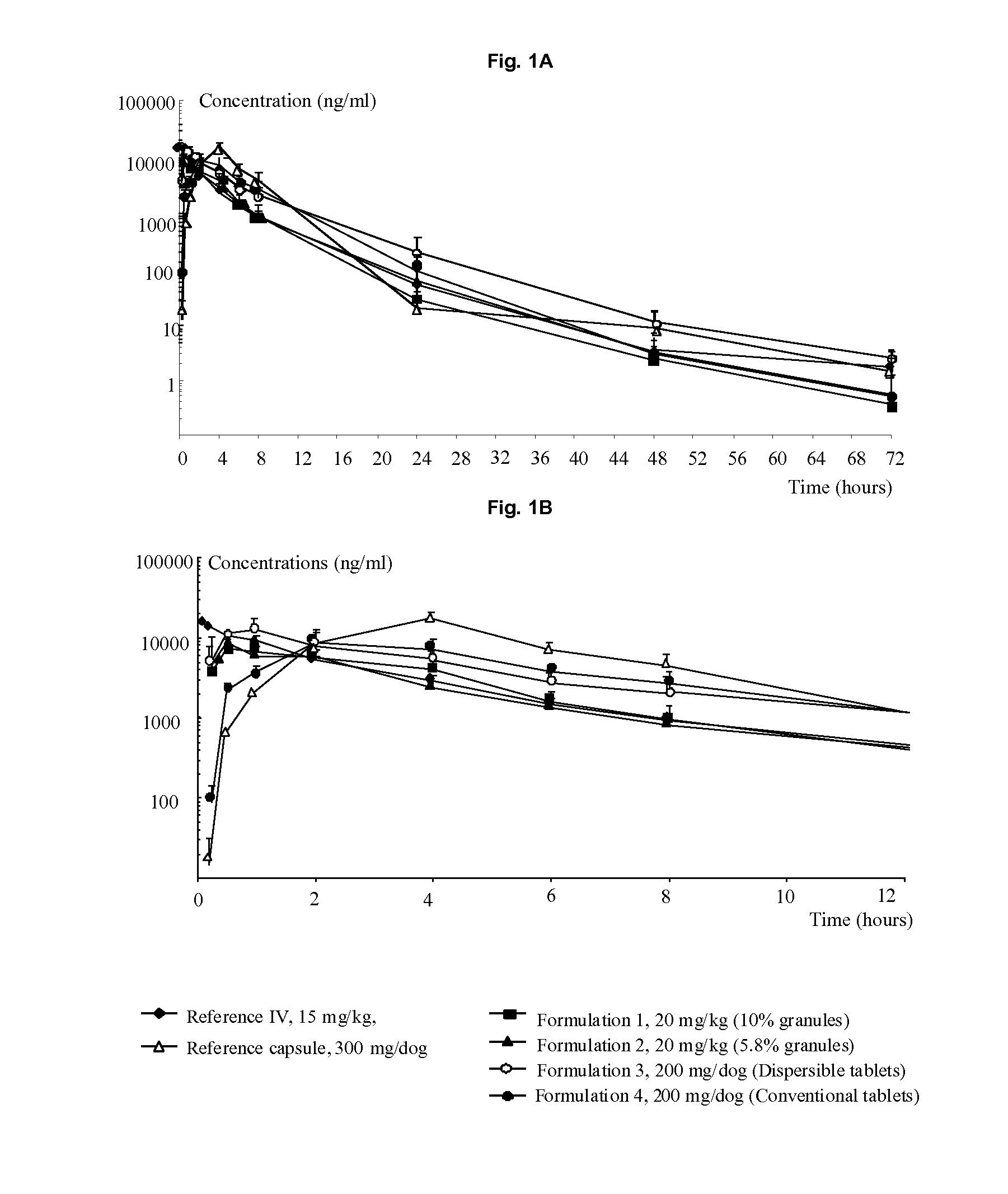
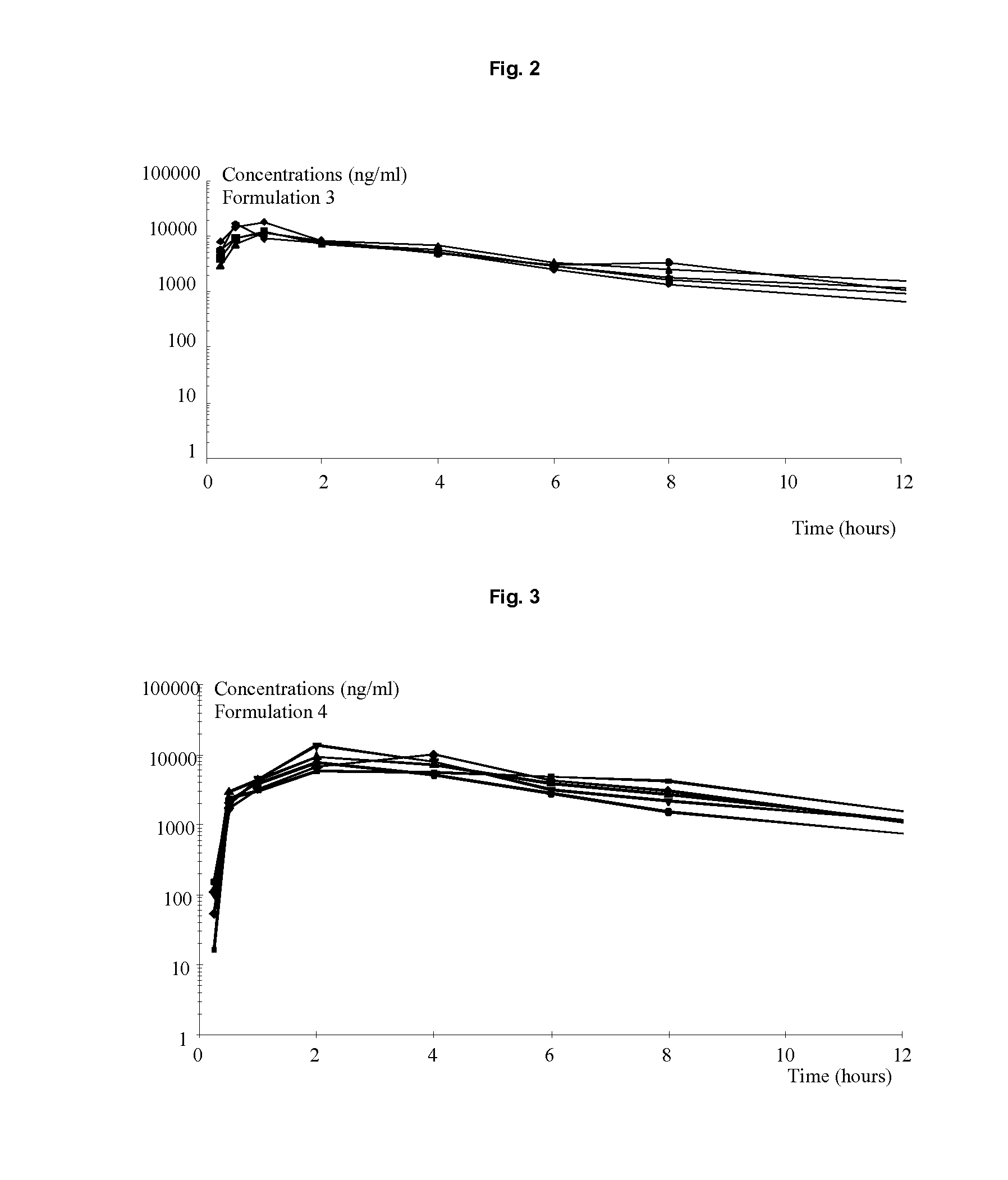
Oral formulations of pyrrolidine derivatives
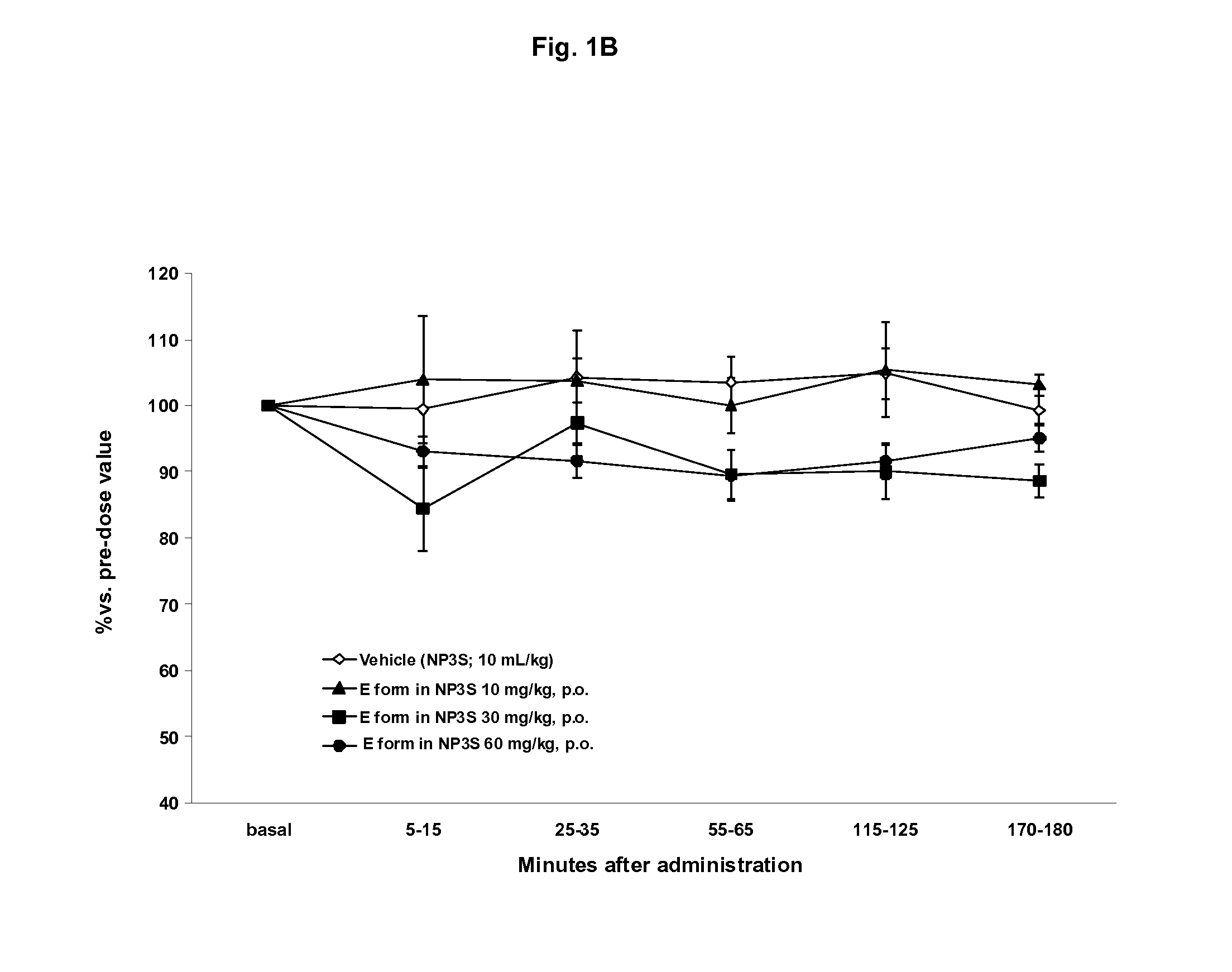
The present invention relates to solid oral formulations comprising a compound of formula (3Z,5S)-5-(hydroxymethyl)-1-[(2′-methyl-1,1′-biphenyl-4-yl)carbonyl]pyrrolidin-3-one-O-methyloxime, and / or an active metabolite thereof, and the use of said formulations in the treatment and / or prevention of preterm labor, premature birth, dysmenorrhea and embryo implantation failure due to uterine contractions. The present invention is furthermore related to processes for their preparation.
Owner:OBSEVA
Chromobacterium suttsuga sp. nov. and use for control of insect pests
ActiveUS20050074431A1Improve resistance managementReduce the use of pesticidesBiocideBacteriaInsect pestMicrobiology
Chromobacterium suttsuga sp. nov., a new species of the genus Chromobacterium which possesses insecticidal activity, is described. The invention also relates to insecticidally-active metabolites obtained from the strain and to insecticidal compositions comprising cultures of the strain and / or supernatants, filtrates, and extracts obtained from the strain, and use thereof to control insect pests.
Owner:US SEC AGRI +1
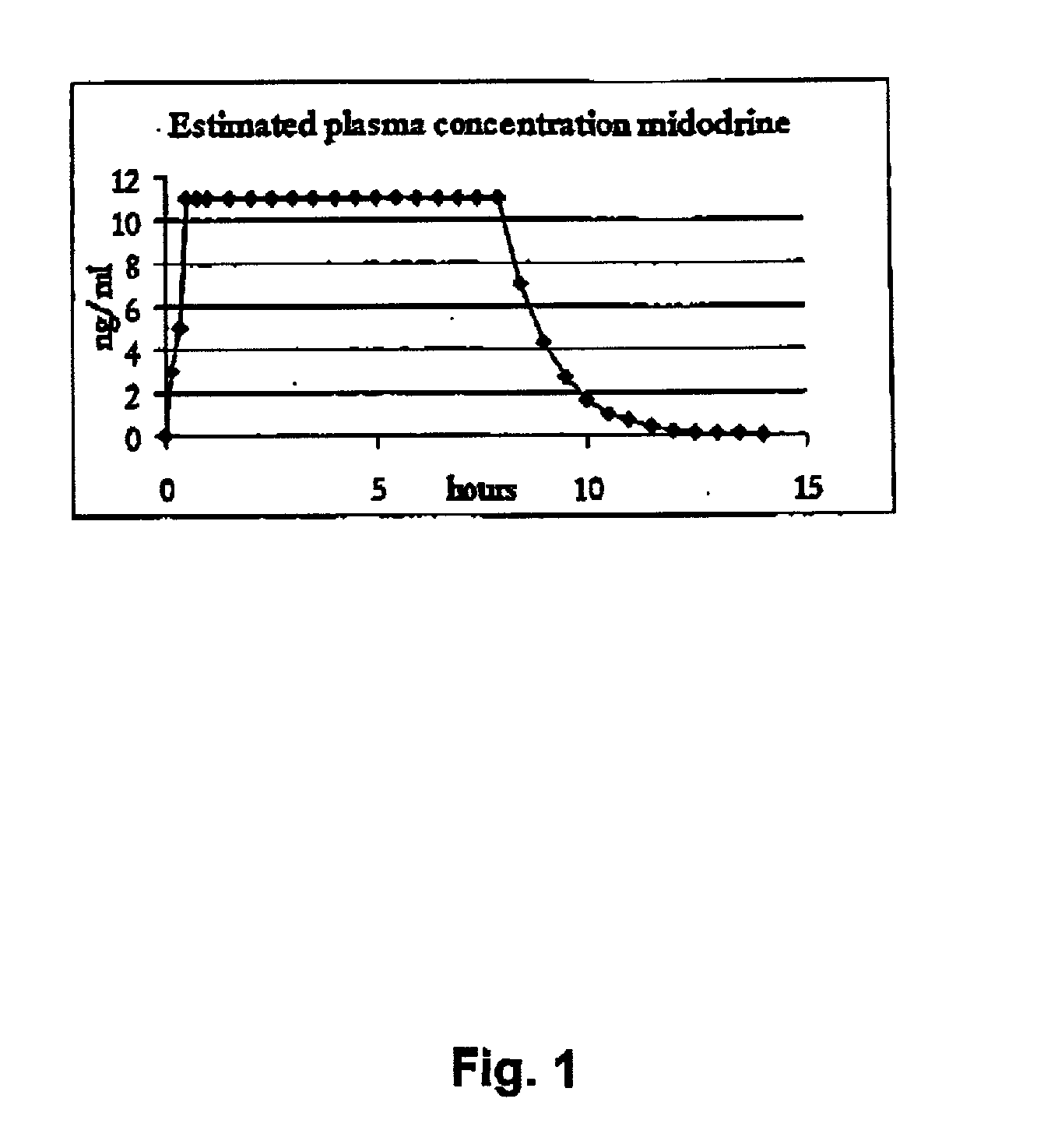
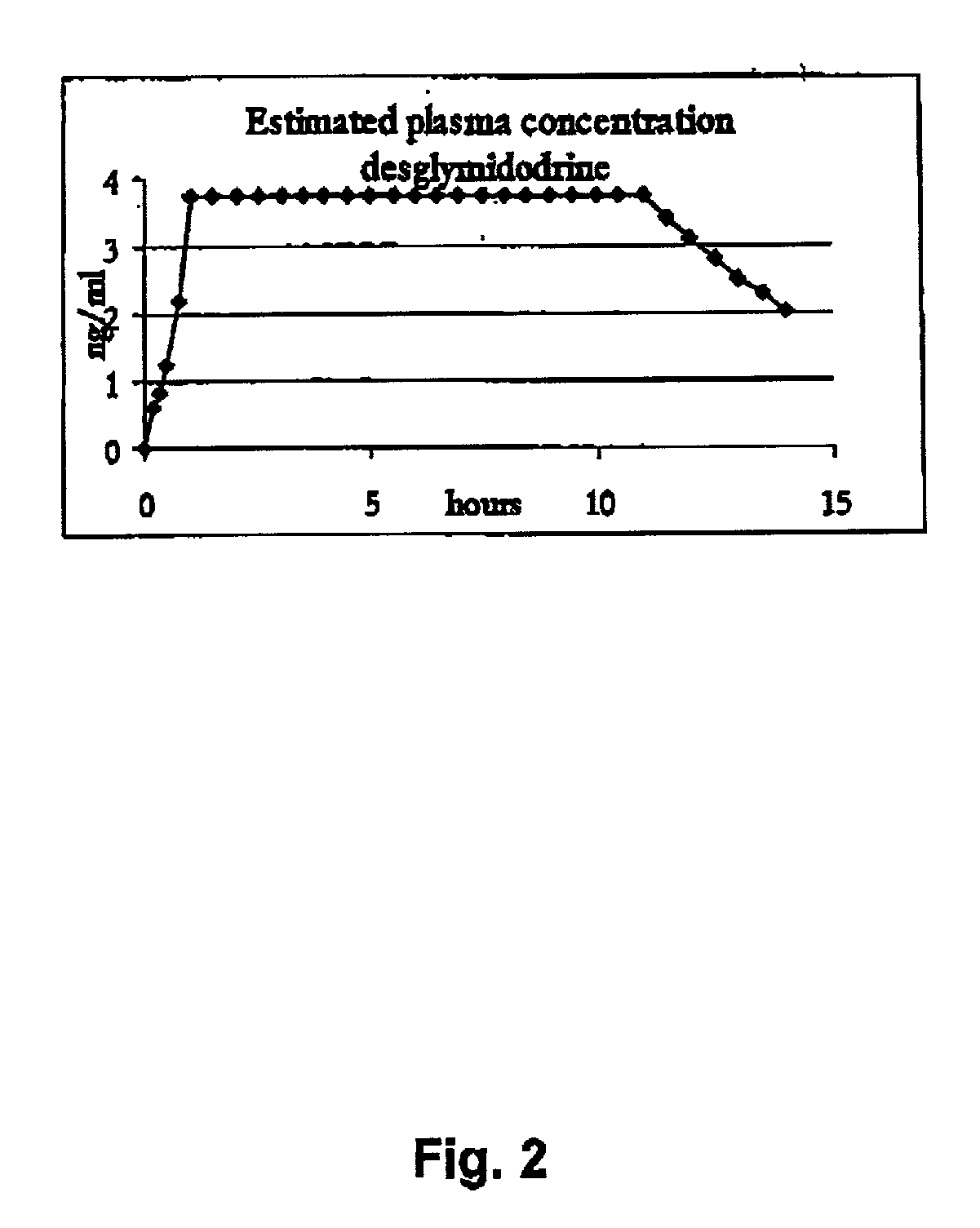
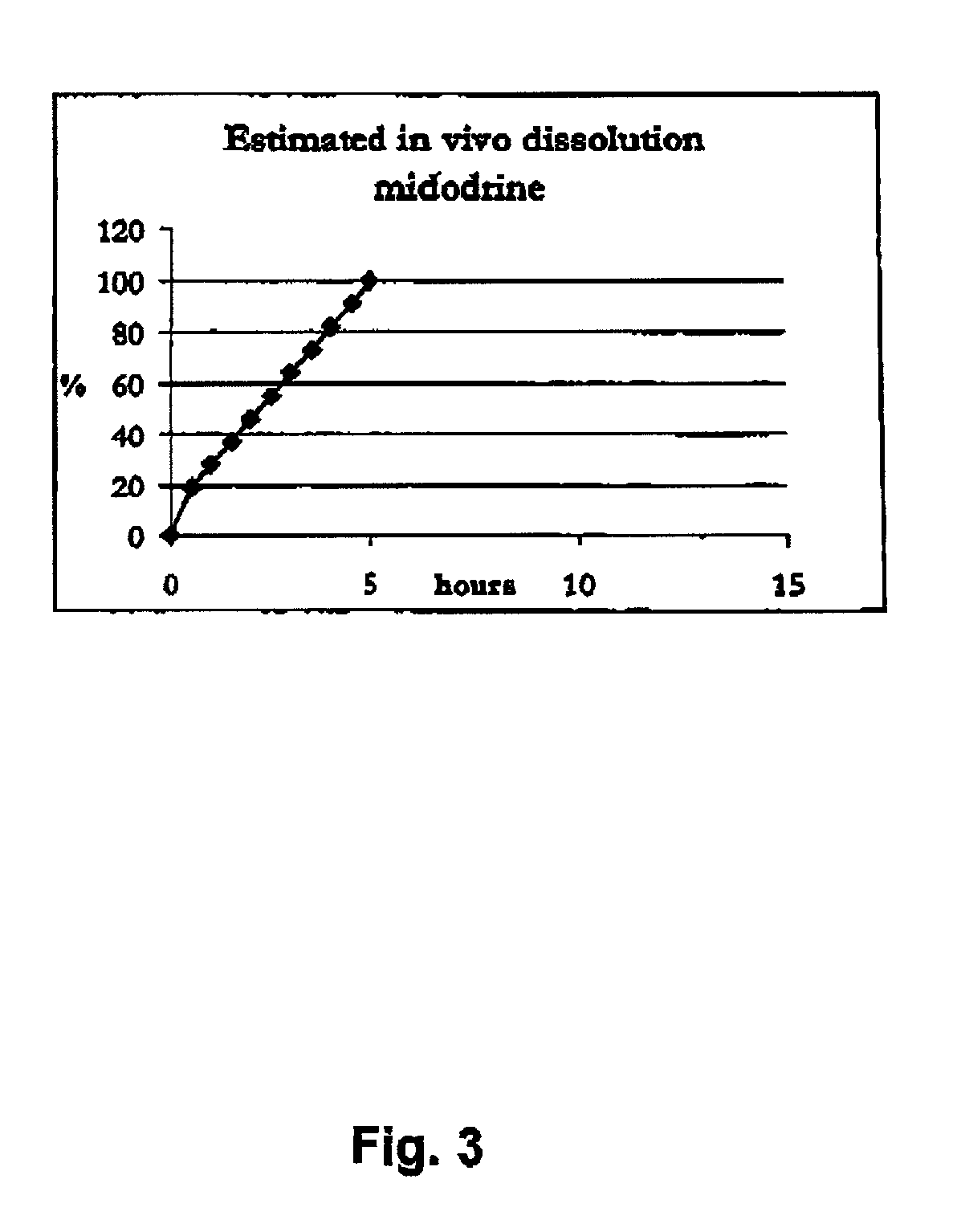
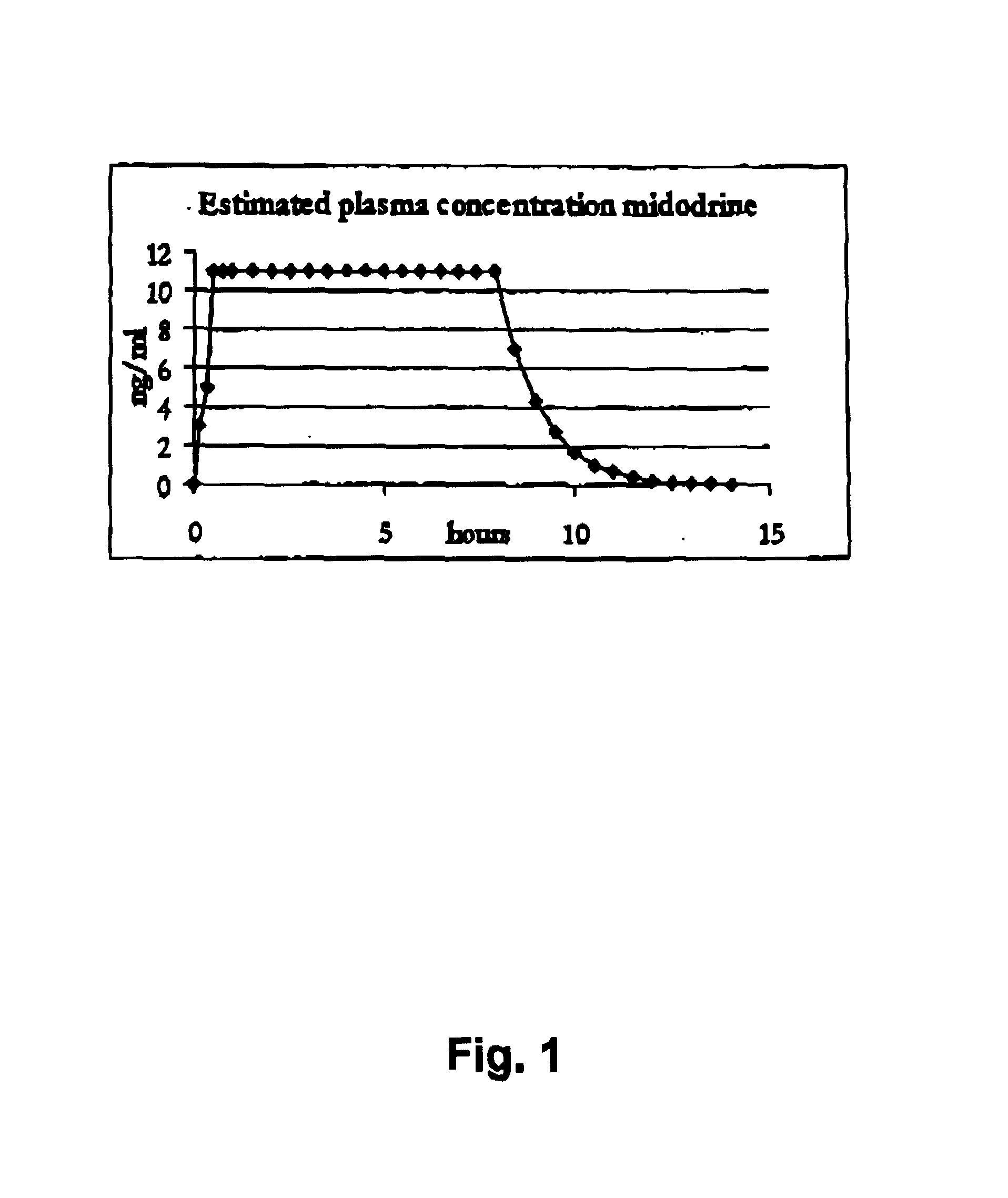
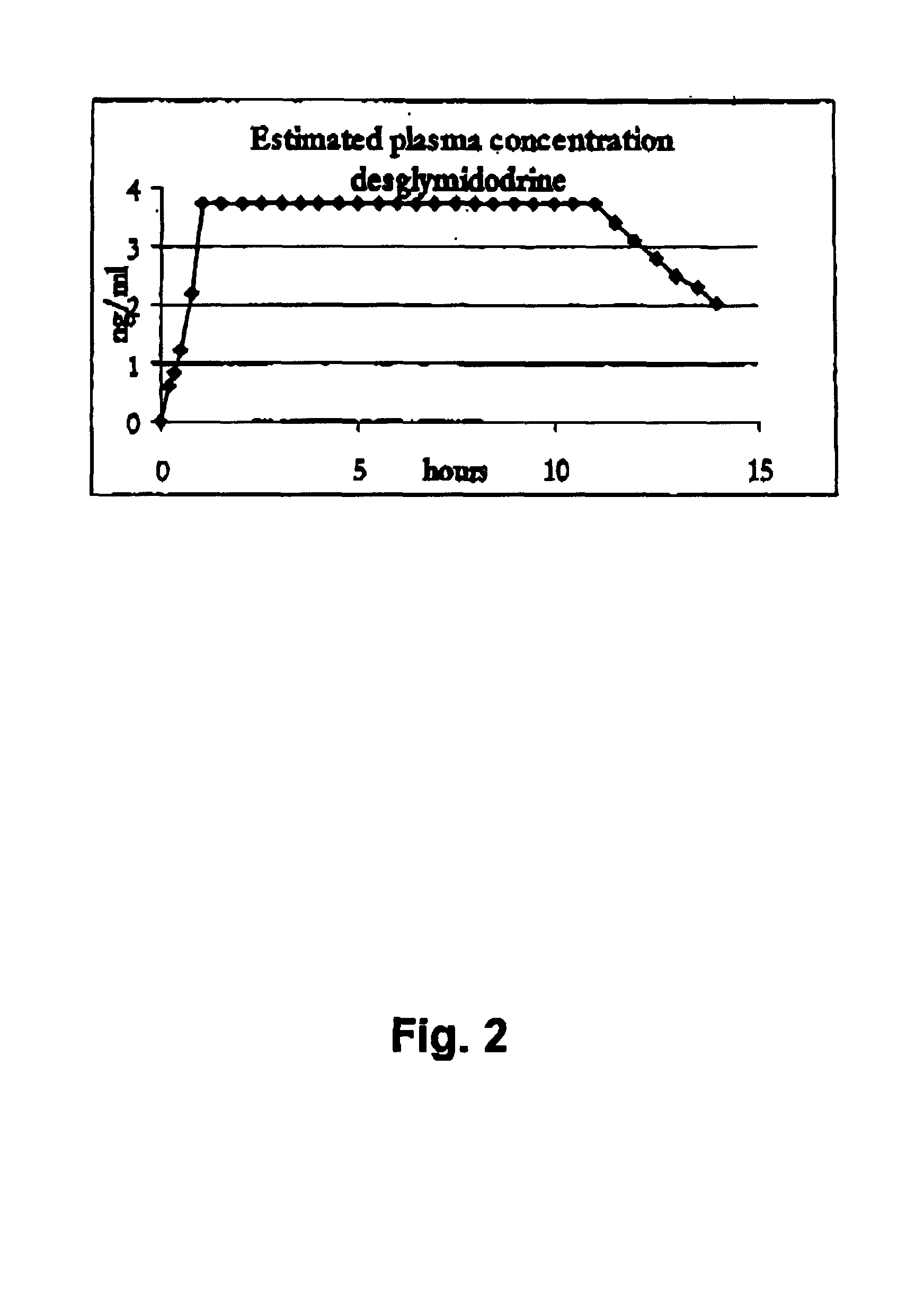
Controlled release pharmaceutical composition for oral use containing midodrine and/or active metabolite, desglymidodrine
InactiveUS7070803B2Increase surface areaEfficient processOrganic active ingredientsNanomedicineSide effectDesglymidodrine
Novel controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine. The novel compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine.The novel compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps: i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desglymidodrine have been released from the composition.Also disclosed is a method for treating orthostatic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a composition according to the invention.
Owner:NYCOMED AUSTRIA
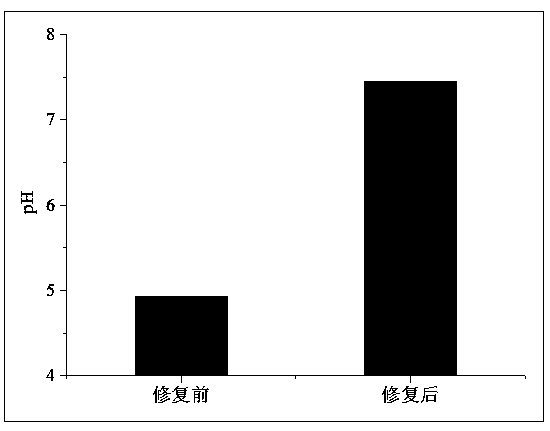
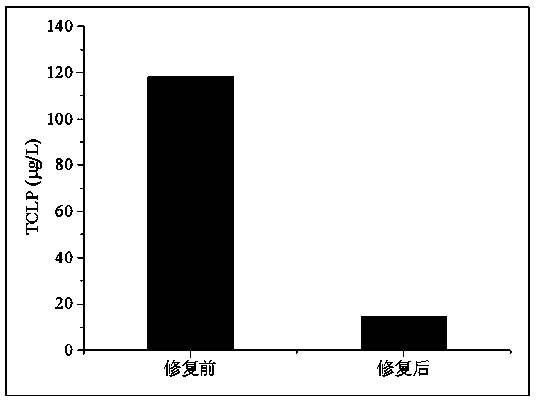
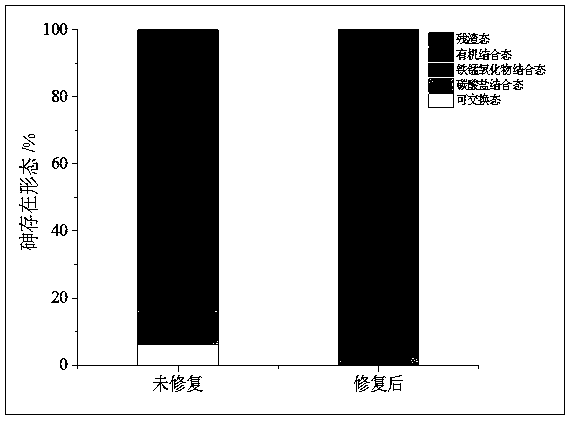
Method for remedying acidified arsenic contaminated soil by biochar-loaded nano-scale zero-valent iron cooperated with bacteria
ActiveCN109570227AReduce dosageAvoid destructionContaminated soil reclamationChemical reactionPseudomonas putida
The invention relates to a method for remedying acidified arsenic contaminated soil by biochar-loaded nano-scale zero-valent iron cooperated with bacteria. The method includes selecting Pseudomonas putida strain MnB1 (ATCC23483); carrying out enrichment culture on the Pseudomonas putida strain in enrichment culture media; inoculating strains in culture media with divalent manganese and carrying out culture on the strains to obtain active metabolites; adding the active metabolites and the green synthetic biochar-loaded nano-scale zero-valent iron into the acidified arsenic contaminated soil anduniformly stirring the active metabolites, the biochar-loaded nano-scale zero-valent iron and the acidified arsenic contaminated soil; carrying out a series of physical-chemical reaction on active manganese oxide, zero-valent iron, biochar and trivalent arsenic or pentavalent arsenic in the soil; converting the arsenic in exchangeable forms into arsenic in residual forms. The method has the advantages that the arsenic in the soil can be effectively immobilized, the pH (potential of hydrogen) of the soil can be increased, and the double purposes of remedying soil acidification and arsenic contamination can be simultaneously achieved; the method is short in remediation time, high in efficiency, wide in treatment range and free of secondary pollution, and stable effects can be realized.
Owner:QINGDAO TECHNOLOGICAL UNIVERSITY
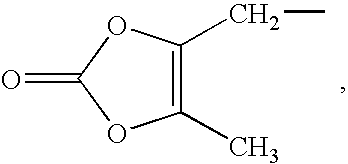
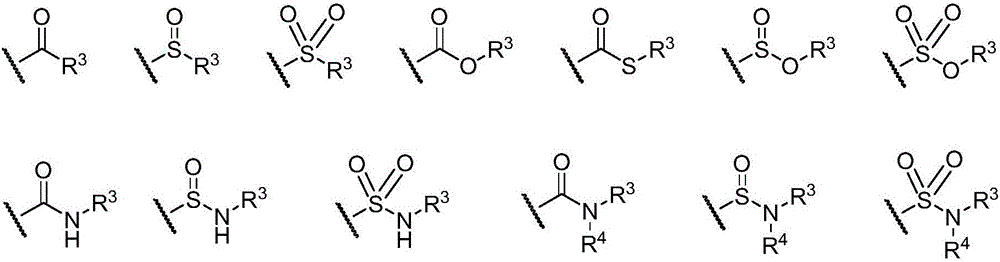
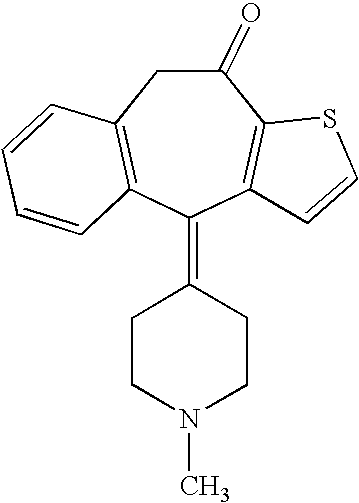
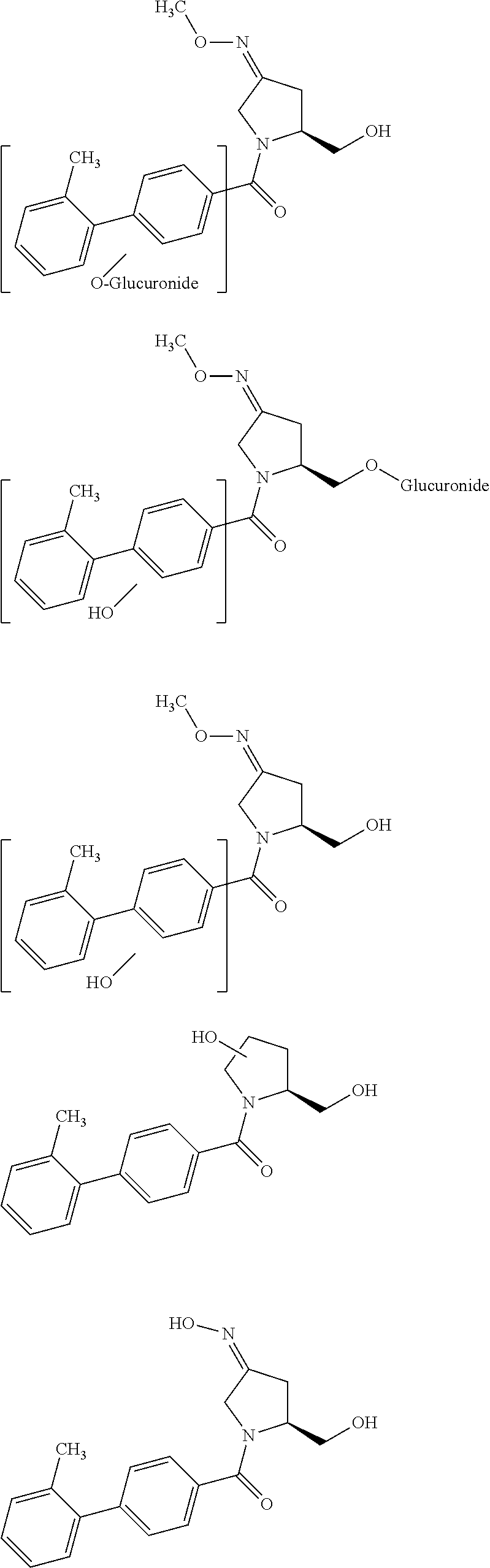
Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof
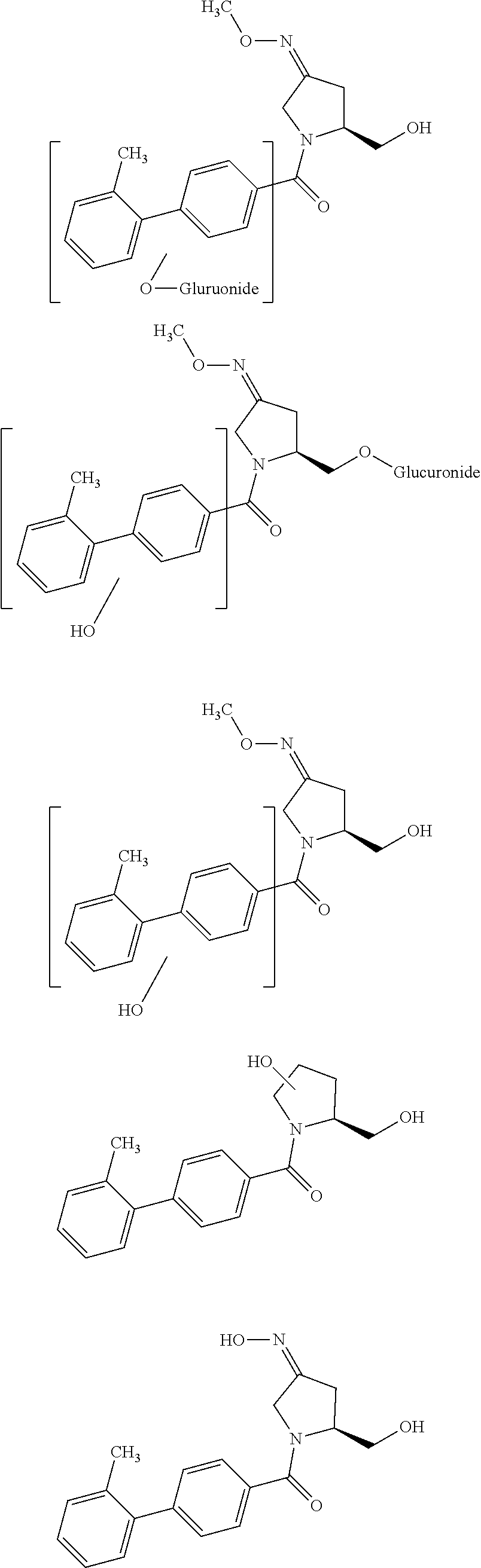
The invention relates to tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and a preparation method and medicament applications thereof, belonging to the field of medicinal chemistry. The derivatives contain salt of a compound in the formula I, and enantiomer and racemate of the compound in the formula I. The in-vitro whole-blood hydrolysis experiment results show that the stability of the compound in the formula I on esterase is good, and the hydrolysis rate of methyl carboxylate is obviously slower than that of non-deuterated methyl ester. The compound in the formula I can be effectively converted into pharmacological active metabolite in a human body to achieve the effect of inhibiting the platelet aggregation, and the concentration of the active metabolite is obviously higher than that of a non-deuterated compound of clopidogrel or a corresponding structure. The pharmacodynamic experiment results show that the compound in the formula I has the effect of obviously inhibiting the platelet aggregation, and the effect of inhibiting the platelet aggregation is obviously better than that of the non-deuterated compound of clopidogrel and the corresponding structure. Therefore, the compound in the formula I can be used for preparing medicaments for preventing or treating related thrombus and embolism diseases.
Owner:吉林敖东创新医药科技有限公司
Fermentation material for composite microorganism and preparation method thereof
InactiveCN101186896AImprove palatabilityIncrease profitFungiBacteriaCell culture mediaEnzyme inducer
The invention relates to a composite microorganism ferment material which is characterized in that production strains select production strains of which the growth rate is quick and the enzyme production quantity is large by combining with through ray inducement and production performance detection. The processing technique employs innovative symbiosis compatibility design of probiotics and multi-compound fermentation technology, the number of the live probiotics in the products achieves five billion per gram, and the products simultaneously contain polyoses, polypeptide, organized enzyme, organic acid, phytosterin, triterpennoids saponin and the like active metabolite. Specific enzyme producing inducer is added to growth medium, the latest single creature venting valve is employed on the package, which not only can be added to complete feed of livestock or poultry but also can be directly added into feedstuff, thereby effectively promoting breakdown and absorption of giant molecule nutrient substance in the intestinal canal of livestock or poultry, thereby eliminating the action of anti-nutritional factors in the feedstuff, remarkably increasing utilization ratio of the feedstuff, and reducing the feeding cost.
Owner:SHANGHAI CHUANGBO ECOLOGICAL ENG
Nuclear Hormone Receptor Modulators
Owner:ABBVIE INC
Antibody drug conjugates (ADCS) with kinesin spindel protein (KSP)
ActiveUS20160346402A1Improved profileImmunoglobulins against cell receptors/antigens/surface-determinantsUnknown materialsDiseaseAntiendomysial antibodies
The present application relates to novel antibody drug conjugates (ADCs), to active metabolites of these ADCs, to processes for preparing these ADCs, to the use of these ADCs for the treatment and / or prophylaxis of diseases and to the use of these ADCs for preparing medicaments for treatment and / or prevention of diseases, in particular hyperproliferative and / or angiogenic disorders such as, for example, cancer diseases. Such treatments can be carried out as monotherapy or else in combination with other medicaments or further therapeutic measures.
Owner:BAYER PHARMA AG
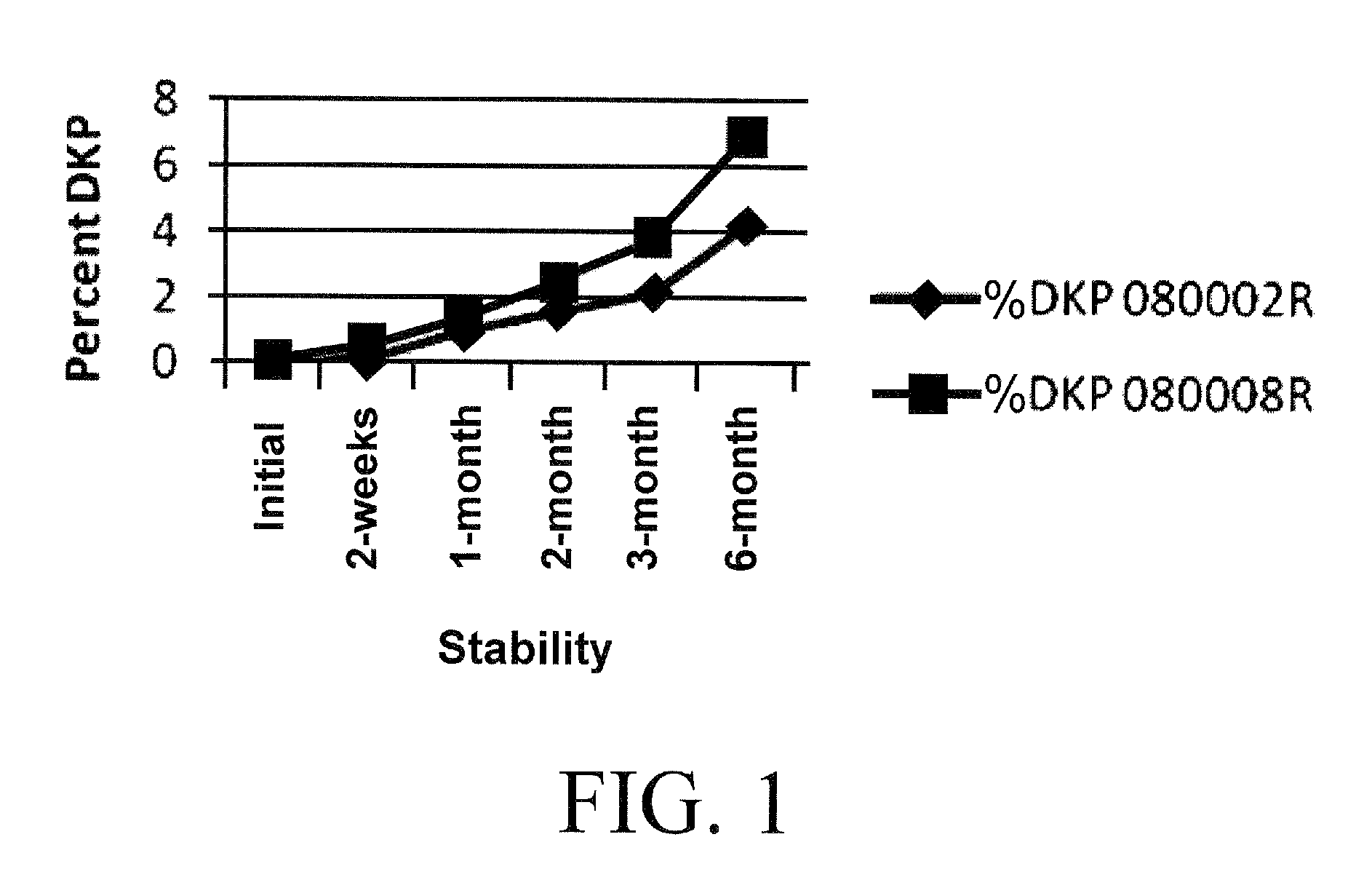
Stabilized Coating for Pharmaceutical Formulations
InactiveUS20100062062A1Improve stabilityEfficiently coat and stabilizeBiocidePharmaceutical non-active ingredientsPolyvinyl alcoholDiketopiperazines
A process is described for preparing stabilized tablet formulations for temperature and moisture sensitive active drugs. Water soluble polyvinyl alcohol is processed with drugs such as angiotensin converting enzyme (ACE) inhibitors and compressed into solid form once excess water is removed. Low dose polyvinyl alcohol ramipril tablets prepared by this process are stable under conditions of high humidity and heat for periods of at least up to six months with less than 8% hydrolysis of the prodrug to the active metabolite diketopiperazine (DKP).
Owner:AETHOS PHARMA
Inhibition of abnormal cell proliferation with camptothecin and combinations including the same
InactiveUS20050118180A1Enhance immune responseReduce inhibitionBiocideGenetic material ingredientsLeukemiaAbnormal cells
A method for treating diseases associated with abnormal cell proliferation comprises delivering to a patient in need of treatment a compound selected from the group consisting of 20(S)-camptothecin, analog of 20(S)-camptothecin, derivative of 20(S)-camptothecin, prodrug of 20(S)-camptothecin, and pharmaceutically active metabolite of 20(S)-camptothecin, in combination with an effective amount of one or more agents selected from the group consisting of alkylating agent, antibiotic agent, an alkylating agent, antibiotic agent, antimetabolic agent, hormonal agent, plant-derived agent, anti-angiogenesis agent and biologic agent. The method can be used to treat benign tumors, malignant or metastatic tumors, leukemia and diseases associated with abnormal angiogenesis.
Owner:SUPERGEN
Pharmaceutical kit comprising midodrine as active drug substance
InactiveUS20020193445A1Fast curative effectRapid onsetBiocidePowder deliveryHigh concentrationSide effect
Novel phannaceutcal kit comprising a controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine and a relatively fast onset composition. The controlled release compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine. The controlled release compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps; i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desgtymidodrine have been released from the composition. One of the advantages of the invention is that the controlled release composition provides a base line plasma concentration, which during most of the day is therapeutically effective. When a higher concentration is needed, only a minor supply of active drug substance is necessary to obtain a very fast relief from symptoms. If the constant base line plasma concentration was absent, it would be necessary to use a relative higher fast onset dose to reach the high therapeutically effective level. The kit according to the present invention is a superior tool for obtaining an optimal treatment with a minimum of active drug substance. Also disclosed is a method for treating orthostaic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a kit according to the invention.
Owner:NYCOMED AUSTRIA
Microorganism formulation for eliminating nutrilit-resistance function and preparation method
InactiveCN101371682AElimination of anti-nutritional factor effectsImprove immunityAnimal feeding stuffBiotechnologyNutrition
The invention relates to a microorganism preparation used for eliminating the effect of anti-nutritional factors and is characterized by consisting of the following materials by weight percentage: 28 to 35 percent of bacillus subtilis, 45 to 53 percent of lactobacillus plantarum and 12 to 27 percent of yeast of brewer; a preparation method of the microorganism preparation comprises the following steps: the production of the strain is produced by combining the ray induction and production performance test, the production strain with high yield and large enzyme production are screened, the production technique adopts the technology of liquid single culture and solid composite culture, product accelerators are added into the culture medium so as to lead the product to be rich in active metabolites such as beneficial living bacterium, enzyme, peptide, polysaccharide and amino acids, and the like; after the feed material and complete feed are added with the microorganism preparation, the effect of anti-nutritional factors to the nutrient components in the feed can be eliminated greatly, the palatability of the feed is improved, feed intake is improved, stress resistance and immunity of the cultured animals is enhanced, group uniformity is improved, the growing speed and feed utilization can be improved and the culture cost can be reduced.
Owner:SHANGHAI CHUANGBO ECOLOGICAL ENG
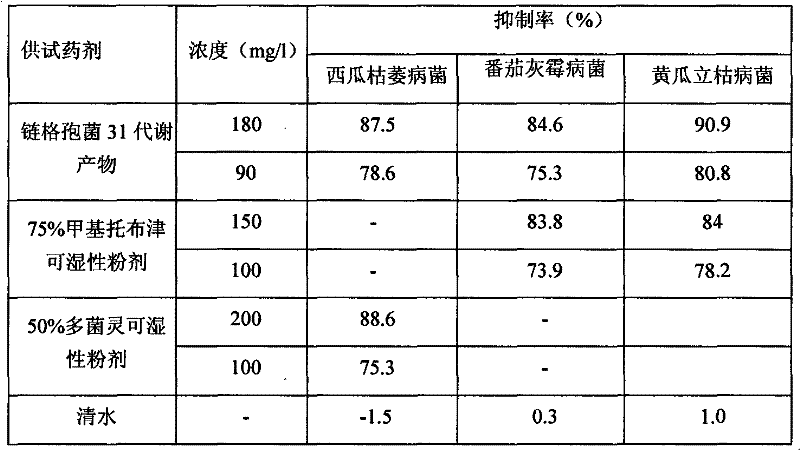
Application of alternaria alternate metabolic products in cucumber rhizoctonia solani prevention and control
The invention relates to the field of biotechnology, in particular to application of alternaria alternate metabolic products in cucumber rhizoctonia solani prevention and control. In the invention, endophytic fungus of camphor trees is separated from camphor tree living bodies collected from Tianmu mountain in Hangzhou, Zhejiang province by adopting endophytic fungus separation technology, and isnamed alternaria alternata 31 through microbial taxonomy identification, and the strain is stored in China General Microbiological Culture Collector Center (CGMCCC) on December 31, 2010, and the collection register number is CGMCC No.4508. The invention has a most obvious characteristic that the strain liquid can be fermented to produce active metabolic products which have inhibiting effect on pathogenic bacteria of plant fungus diseases such as Rhizoctonia solani, Fusarium oxysporium, Botrytis cinerea and the like, thus alternaria alternate is an important microbial resource in agriculture.
Owner:CHINA JILIANG UNIV
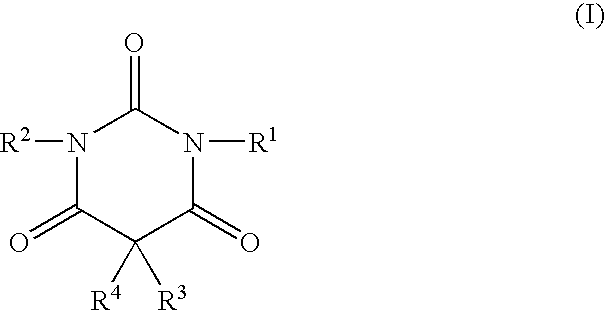
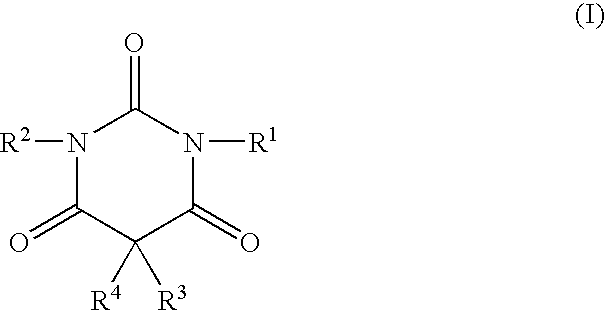
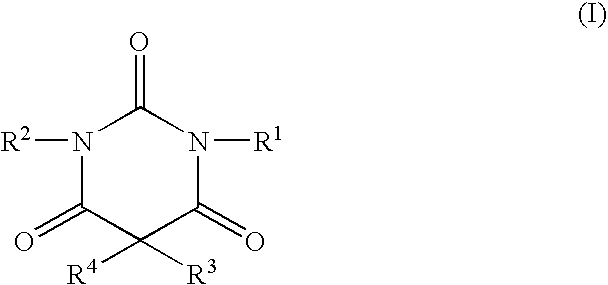
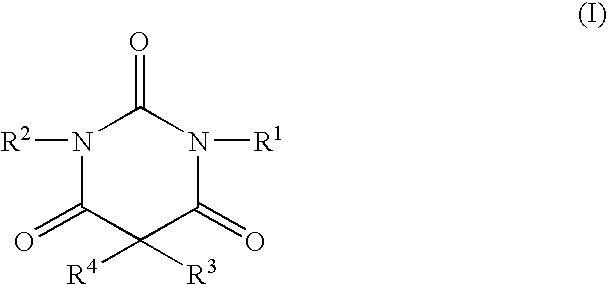
Non-sedating barbituric acid derivatives
The present invention relates to novel non-sedating barbituric acid derivatives, pharmaceutical compositions containing them and methods of neuroprotection in cases of cerebral ischemia, head trauma and other acute neurologic injuries, and prevention of resulting neuronal damage. The invention also relates to the use of non-sedating barbituric acid derivatives given in a manner and dosage effective to produce blood levels and brain levels of these drugs and / or their active metabolites sufficient to provide a therapeutic effect.
Owner:TARO PHARMA INDS
Compositions containing opioid antagonists
ActiveUS7700626B2Improve bioavailabilityImprove solubilityBiocideNervous disorderOpioid antagonistOpioid Agonist
Compositions containing opioid antagonists, particularly alvimopan and its active metabolite, with improved solubility and bioavailability for oral or parenteral administration, injectable dosage formulations, kits, and methods of making and using same are disclosed. In preferred embodiments, invention provides injectable formulations containing opioid antagonists, particularly alvimopan and its active metabolite, having low solubility that may be readily prepared, are stable during storage, and provide maximum levels of opioid antagonists when administered parenterally, particularly via injection. The results are achieved by a combination of processing techniques and component selection.
Owner:MERCK SHARP & DOHME LLC
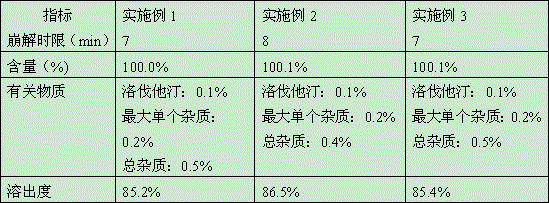
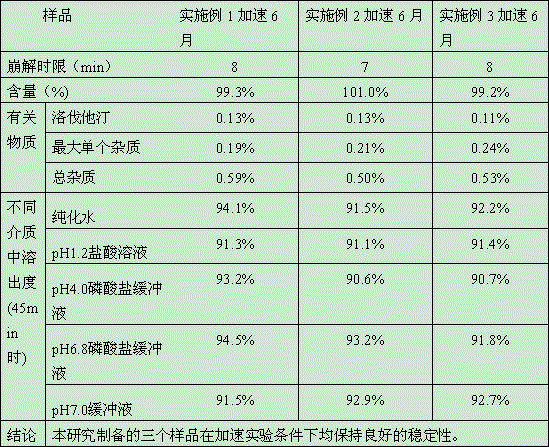
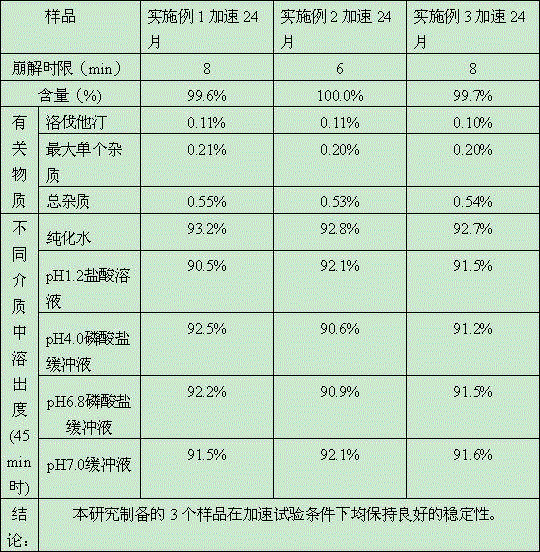
Simvastatin tablet and preparation method thereof
The invention discloses a simvastatin tablet and a preparation method thereof. The simvastatin tablet comprises an active ingredient simvastatin and pharmaceutical excipients, wherein the pharmaceutical excipients are spherical lactose, cross-linked sodium carboxymethyl cellulose, butylhydroxyanisole, hydroxypropyl methyl cellulose, silicon dioxide, magnesium stearate and film coating premix, which are added according to a specific mass ratio and process. The simvastatin and the pharmaceutical excipients are incompatible, and are prone to hydrolysis and oxidation, lactone bonds break to open loop to generate an active metabolite simvastatin hydroxy acid under the high-humidity condition, the intramolecular diene bond is subjected to a slow oxidative copolymerization reaction to generate a dimer or polymer under the high-temperature condition, and the preparation of a stable preparation is greatly difficult. In recent years, with the continuous disclosure of information, the difference between the quality standards of simvastatin tablets produced by different manufacturers is great, wherein the dissolution behavior difference is more significant, so that the situation that the simvastatin tablets have the same name but have different quality is very obvious. The prescription process determined by the study can continuously produce the simvastatin tablet at large scale, and the prepared simvastatin tablet has a good dissolution performance in various PH-value dissolution media, and keeps good stability in the long-term storage process.
Owner:DIAO GRP CHENGDU PHARMA
Modified-release compositions of at least one form of venlafaxine
InactiveUS20050244498A1Reduce morbidityReduce releaseBiocideAnimal repellantsControlled releaseOral medication
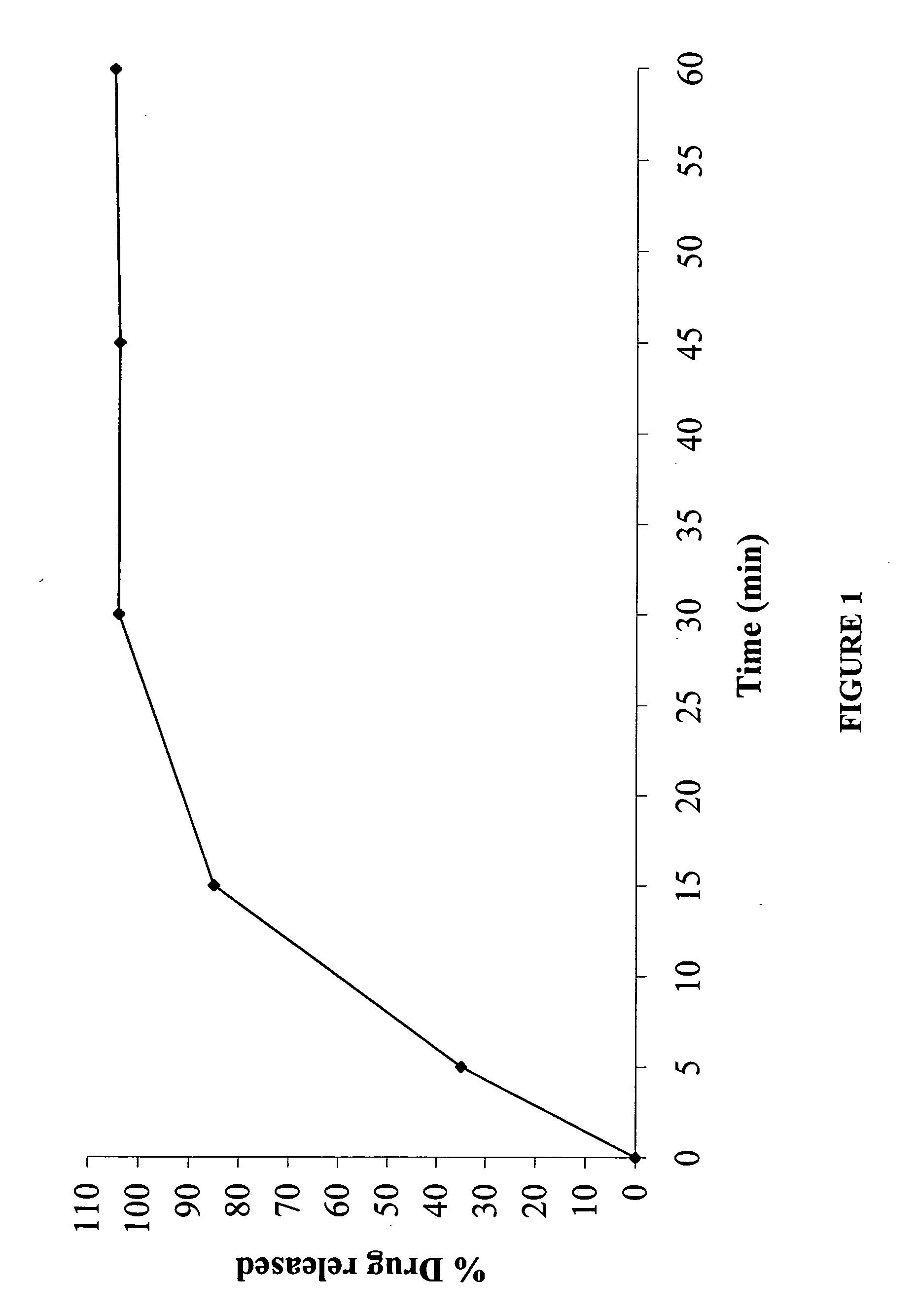
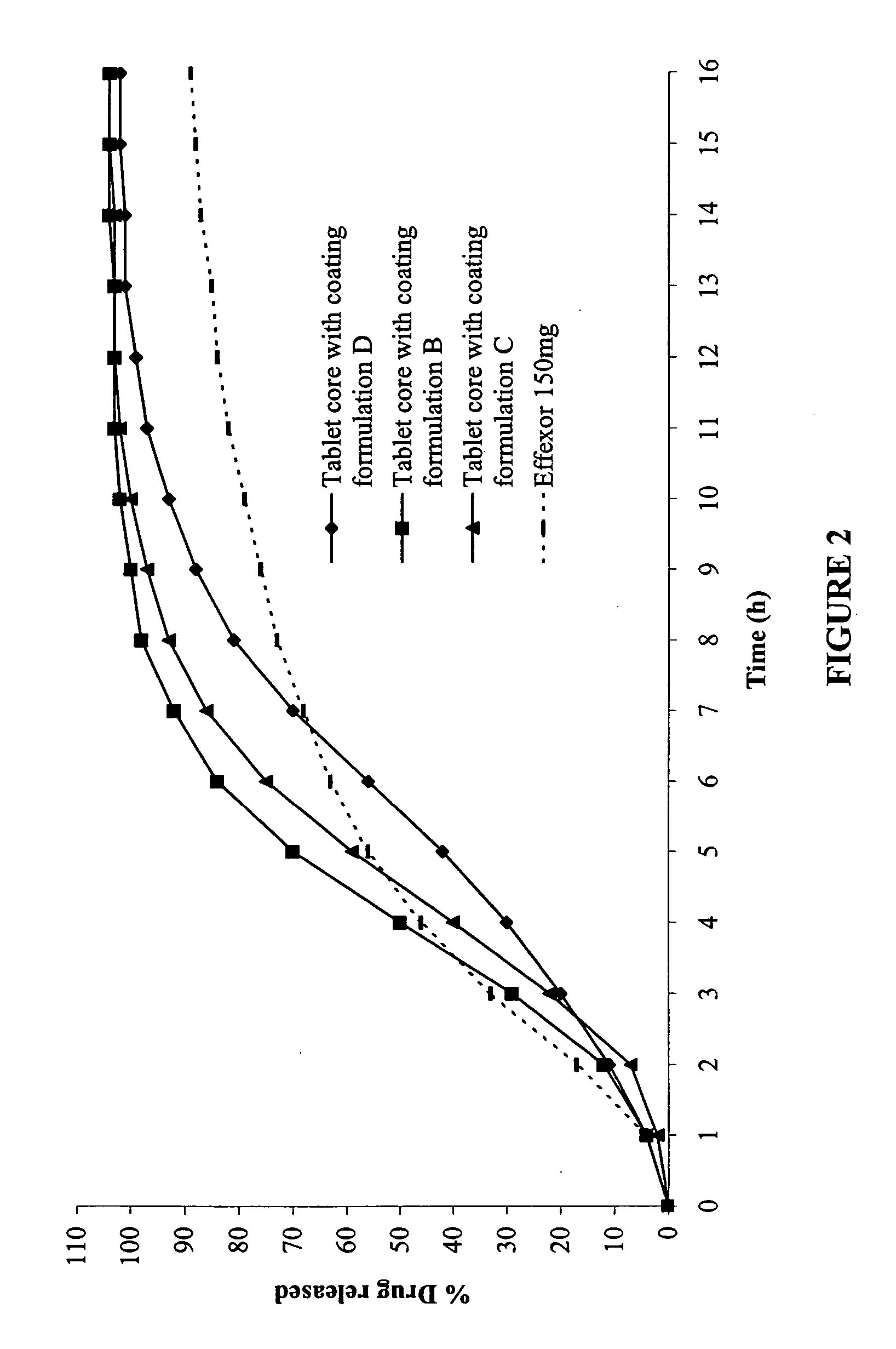
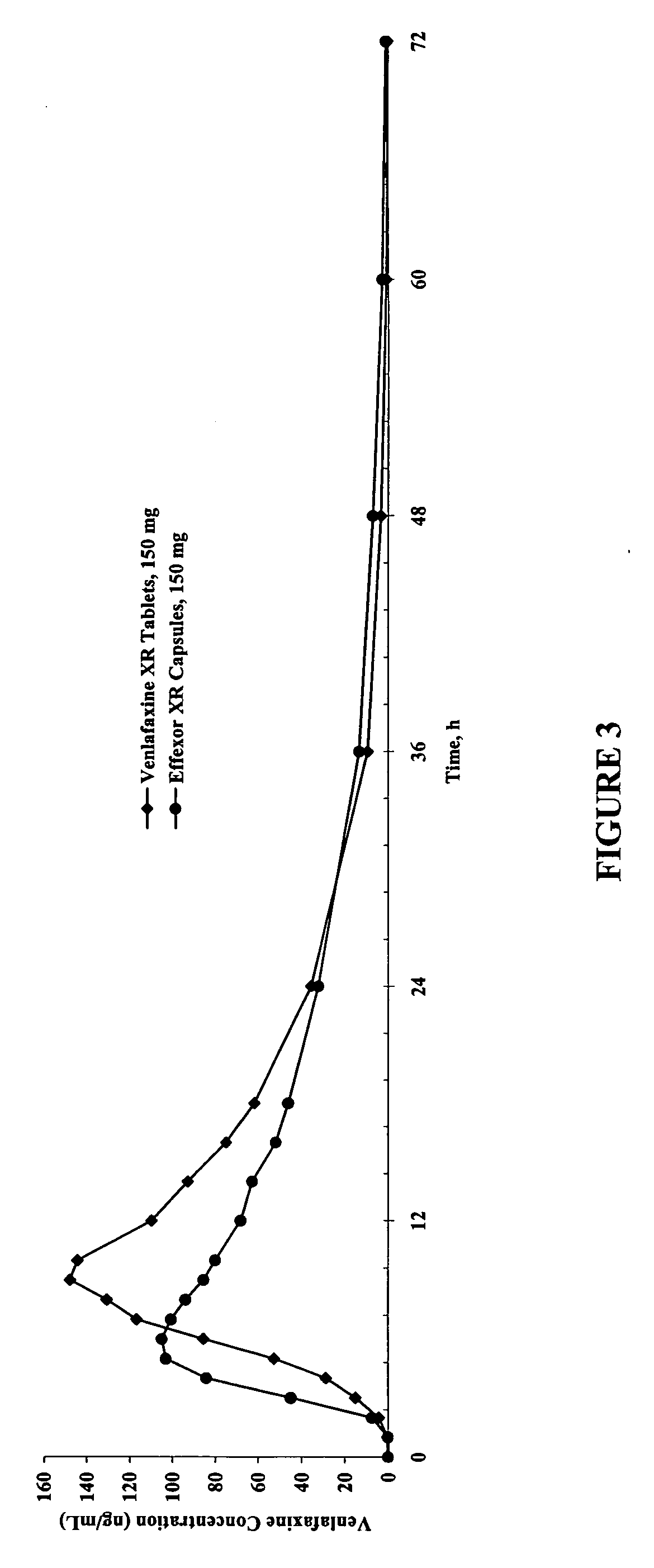
The present invention relates to a modified release composition of at least one form of venlafaxine, which is an enhanced absorption delayed controlled release composition for oral administration suitable for once daily dosing. The composition comprises a core comprising at least one form of venlafaxine selected from the group consisting of venlafaxine, an active metabolite of venlafaxine, a pharmaceutically acceptable salt of venlafaxine, a pharmaceutically acceptable salt of an active metabolite of venlafaxine, and combinations thereof, and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core. The compositions of the invention provide enhanced absorption delayed controlled release of the at least one form of venlafaxine such that the combined geometric mean ratio of the composition of the invention to the reference product for the AUC0-t or the Cmax for venlafaxine and its active metabolite O-desmethylvenlafaxine is greater than 2 after first administration of the composition under fed or fasting conditions.
Owner:BIOVAIL LAB INT SRL
Bruton's tyrosine kinase inhibitor
The invention relates to a compound with a formula I and a pharmaceutically acceptable salt, a solvent compound, an active metabolite, a polycrystalline compound, an ester, an isomer or a prodrug thereof, a pharmaceutical composition containing the compound in the formula I, and application using the same as a selective Bruton's tyrosine kinase inhibitor to prepare a medicine for preventing or treating heterologous immunity diseases and self immunity diseases or cancers. The formula is shown in the specification.
Owner:NANJING GENTAI PHARMA TECH CO LTD
Pharmaceutical composition containing GKAs (glucokinase activators) and B vitamins and application of pharmaceutical composition
InactiveCN106474480AReduce complicationsImprove high blood sugarMetabolism disorderUrinary disorderB Vitamin FamilyVitamin
The invention relates to pharmaceutical composition containing GKAs (glucokinase activators) and B vitamins. The pharmaceutical composition is prepared from a therapeutic effective dose of one of GKAs as well as medicinal precursors, active metabolites or GKA salts, one or more of a therapeutic effective dose of the B vitamins and pharmaceutically acceptable carriers, wherein the GKAs mainly comprise TTP399, HMS5552, PF-04937319, LY2608204, PF-04991532 and GKM-001; the content of the B vitamins is 0.01-50 mg and adopt folic acid compounds as the first choice. The invention further relates to an application of the composition in preparation of drugs for treating diabetes mellitus and preventing or delaying complications of diabetic angiopathy.
Owner:深圳泰乐德医疗有限公司 +1
Medicinal composition containing calcium passage paralysor and B family vitamin and its use
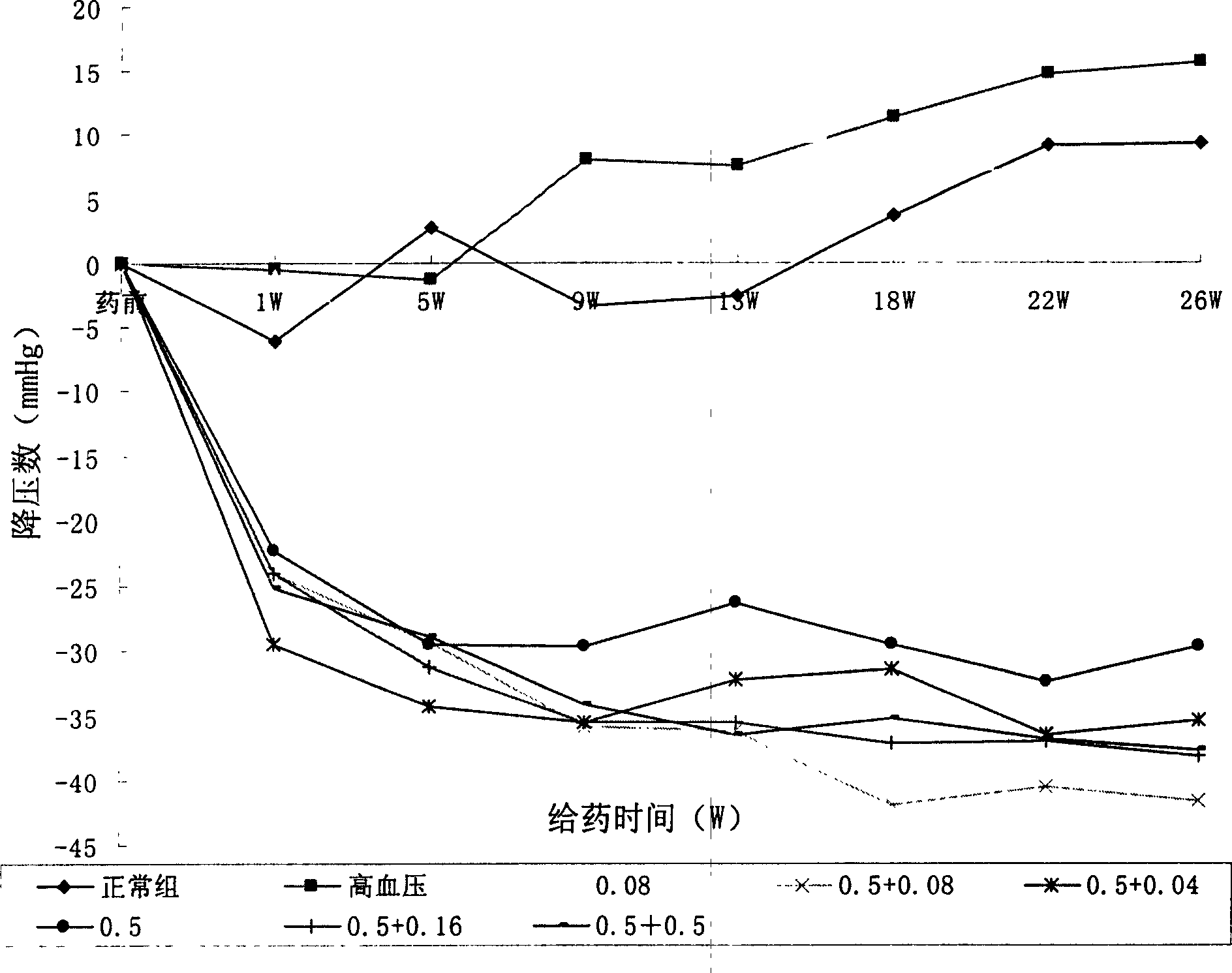
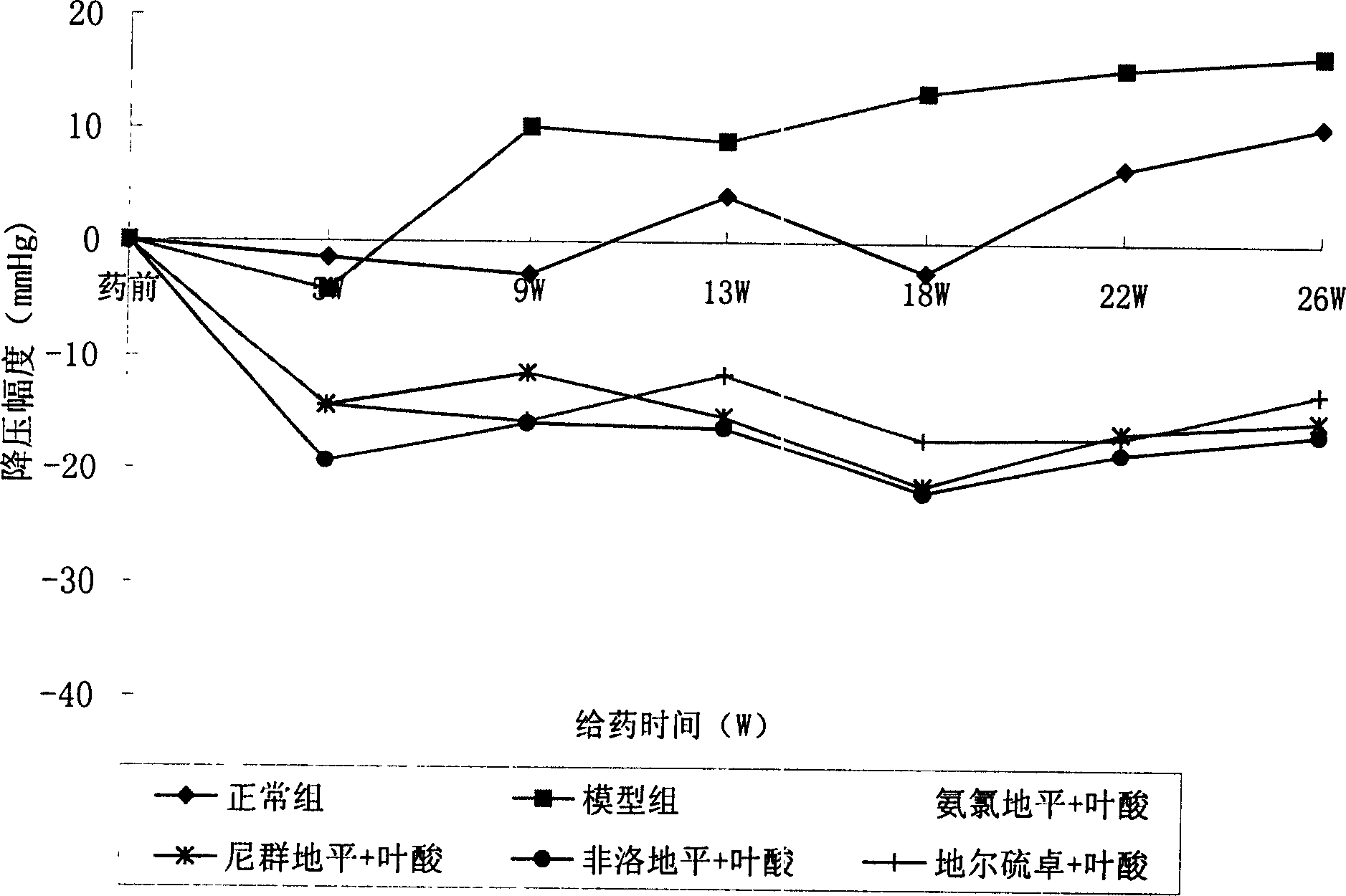
ActiveCN1824320AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderPatient complianceAngina
A composite medicine for improving the curative effect of the antihypertensive medicine containing Ca channel retardant and preventing the complications such as eyeground hemorrhage, angina pectoris, myocardial infarction, cerebral apoplexy, heart failure and kidney failure is composed of Ca channel vetardant or its precursor or its active metabolite or its salt, one or more of vitamins in B family and the pharmacologically acceptable carrier.
Owner:SHENZHEN AUSA PHARMA +1
Anti-enterovirus 71 (EV 71) caprolactam aldehyde compound and preparation method and purpose thereof
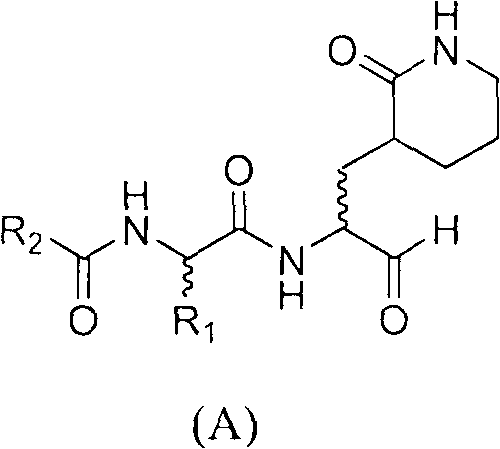
A structure general formula of a caprolactam aldehyde enterovirus 71 (EV 71) 3C protease inhibitor is as shown in chemical combination A, all variables in the structure are defined in an instruction book, and the compound effectively restrains or blocks the copy of the enterovirus 71. The invention relates to a compound with the structure as shown in formula (A), various types of optical isomers of the compound with the structure as shown in formula (A), drug active metabolites, officinal salt, solvate and discovery and application when the compound with the structure as shown in formula (A), the various kinds of optical isomers of the compound with the structure as shown in formula (A), the drug active metabolites and the officinal salt are used for preparing antiviral drugs for curing hand-foot-mouth virus infection diseases. The invention further relates to an intermediate and a synthetic method of preparing the compound with the structure as shown in formula (A).
Owner:NANKAI UNIV +2
Pyrrolidine derivatives as oxytocin/vasopressin v1a receptors antagonists
The present invention relates to a compound of formula (3Z,5S)-5-(hydroxymethyl)-1-[(2′-methyl-1,1′-biphenyl-4-yl)carbonyl]pyrrolidin-3-one O-methy19243-yloxime, and / or an active metabolite thereof having antagonist action at the oxytocin receptor and / or vasopressin V1a receptor, to processes for their preparation, pharmaceutical compositions containing them and their use.
Owner:OBSEVA
Non-sedating barbituric acid derivatives
The present invention relates to novel non-sedating barbituric acid derivatives, pharmaceutical compositions containing them and methods of neuroprotection in cases of cerebral ischemia, head trauma and other acute neurologic injuries, and prevention of resulting neuronal damage. The invention also relates to the use of non-sedating barbituric acid derivatives given in a manner and dosage effective to produce blood levels and brain levels of these drugs and / or their active metabolites sufficient to provide a therapeutic effect.
Owner:TARO PHARMA INDS
Ophthalmic pharmaceutical compositions for the treatment of neoangiogenic pathologies of the eye
The present invention relates to ophthalmic pharmaceutical compositions comprising Sorafenib, its derivatives or active metabolites, for the treatment and prevention of ocular neoangiogenic pathologies.
Owner:S I F I SPA
Endogenetic streptomycete for preventing kiwi berry bacterial canker and preparation thereof
InactiveCN101486982AInhibit synthesisGood water solubilityBiocideBacteriaStreptomyces glaucusKiwi fruit
The invention belongs to the technical field of biological pesticides and discloses a method for identifying a Streptomyces glaucus sp.nov yangling TIASA5 strain and a method for preparing an active substance of the strain and applications of the method. The strain is preserved in China General Microbiological Culture Center of Microbial Culture Collection Management Committee in Microbiology Institute, Chinese Academy of Science on August 16th, 2007 with the preservation number of CGMCC No.2132. The strain has better preventive and therapeutic effects on bacterial canker of kiwi fruits. The strain is separated from plant tissues, generates active metabolite that does not have toxic action to the plant tissues, and has stability, convenient spraying and convenient popularization and application.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof](https://images-eureka.patsnap.com/patent_img/76bf92ed-2246-4456-a2b2-070a9f9b2232/FDA0000406631610000014.png)
![Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof](https://images-eureka.patsnap.com/patent_img/76bf92ed-2246-4456-a2b2-070a9f9b2232/FDA0000406631610000015.png)
![Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof Tetrahydrothieno-[2,3-c]pyridine deuterated derivatives and preparation method and medicament applications thereof](https://images-eureka.patsnap.com/patent_img/76bf92ed-2246-4456-a2b2-070a9f9b2232/FDA0000406631610000021.png)