Simvastatin tablet and preparation method thereof
A technology for simvastatin and tablets, which is applied in the field of simvastatin tablets and its preparation, can solve the problems that the dissolution behavior cannot be simulated, the rationality of the prescription process needs to be studied, and the quality evaluation method needs to be improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
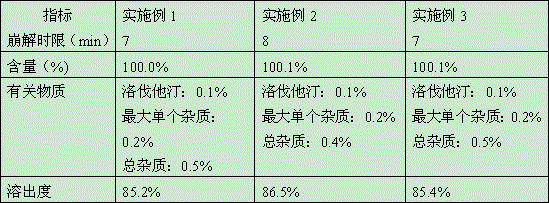
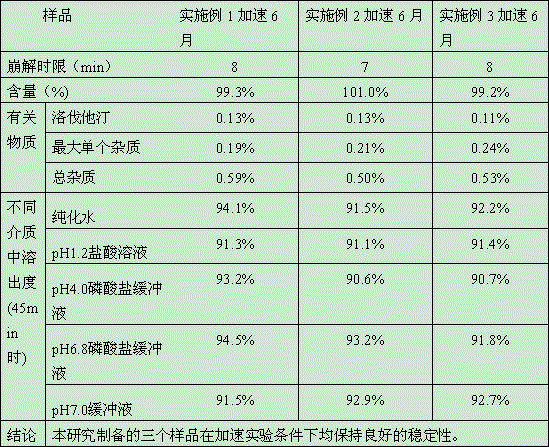
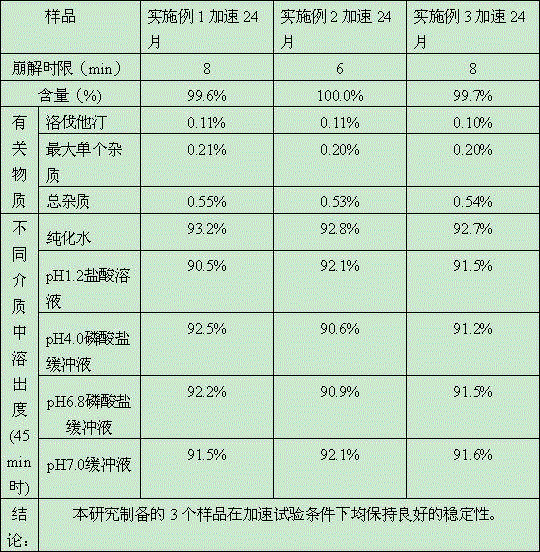
Embodiment 1
[0024] Simvastatin 100g
[0025] Butylated hydroxyanisole 0.05g
[0026] Hypromellose 5.0g
[0027] Proper amount of ethanol
[0028] Appropriate amount of purified water
[0029] Spherical lactose 1000g
[0030] Croscarmellose Sodium 30g
[0031] Silica 8.0g
[0032] Magnesium Stearate 3.0g
[0033] Film coating powder 30.0g
[0034] Preparation:
[0035] Dissolving butylated hydroxyanisole in an appropriate amount of ethanol solution, adding hypromellose to disperse evenly, and adding an appropriate amount of water to prepare a gel-like adhesive. Pour the adhesive into the raw material of simvastatin, stir it into loose and uniform small particles by stirring and granulating, transfer the material to the boiling tower for drying at a temperature lower than 50°C, stop drying when the moisture content of the particles is lower than 2%, and press into tablet. After uniformly suspending the film coating premix with 60% ethanol solution, spray coating evenly until the ...
Embodiment 2
[0037] Simvastatin 100g
[0038] Butylated hydroxyanisole 0.07 g
[0039] Hypromellose 5.0 g
[0040] Proper amount of ethanol
[0041] Appropriate amount of water
[0042] Spherical lactose 2000 g
[0043] Croscarmellose Sodium 50 g
[0044] Silica 15 g
[0045] Magnesium stearate 10 g
[0046] Film Coating Powder 50 g
[0047] Preparation:
[0048] Dissolving butylated hydroxyanisole in an appropriate amount of ethanol solution, adding hypromellose to disperse evenly, and adding an appropriate amount of water to prepare a gel-like adhesive. Pour the adhesive into the raw material of simvastatin, stir it into loose and uniform small particles by stirring and granulating, transfer the material to the boiling tower for drying at a temperature lower than 50°C, stop drying when the moisture content of the particles is lower than 2%, and press into tablet. After uniformly suspending the film coating premix with 60% ethanol solution, spray coating evenly until the tablet...
Embodiment 3
[0050] Simvastatin 100.0 g
[0051] BHA 0.1 g
[0052] Hypromellose 10.0 g
[0053] Proper amount of ethanol
[0054] Appropriate amount of purified water
[0055] Spherical lactose 2000.0 g
[0056] Croscarmellose Sodium 40.0 g
[0057]Silica 10.0 g
[0058] Magnesium stearate 10.0 g
[0059] Film coating powder 50.0 g
[0060] Dissolving butylated hydroxyanisole in an appropriate amount of ethanol solution, adding hypromellose to disperse evenly, and adding an appropriate amount of water to prepare a gel-like adhesive. Pour the adhesive into the raw material of simvastatin, stir and granulate into loose and uniform small particles, transfer the material to the boiling tower for drying at a temperature lower than 50°C, stop drying when the moisture content of the particles is lower than 2%, and press into tablet. After uniformly suspending the film coating premix with 60% ethanol solution, spray coating evenly until the tablet core is evenly covered with a layer of f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com