Patents
Literature
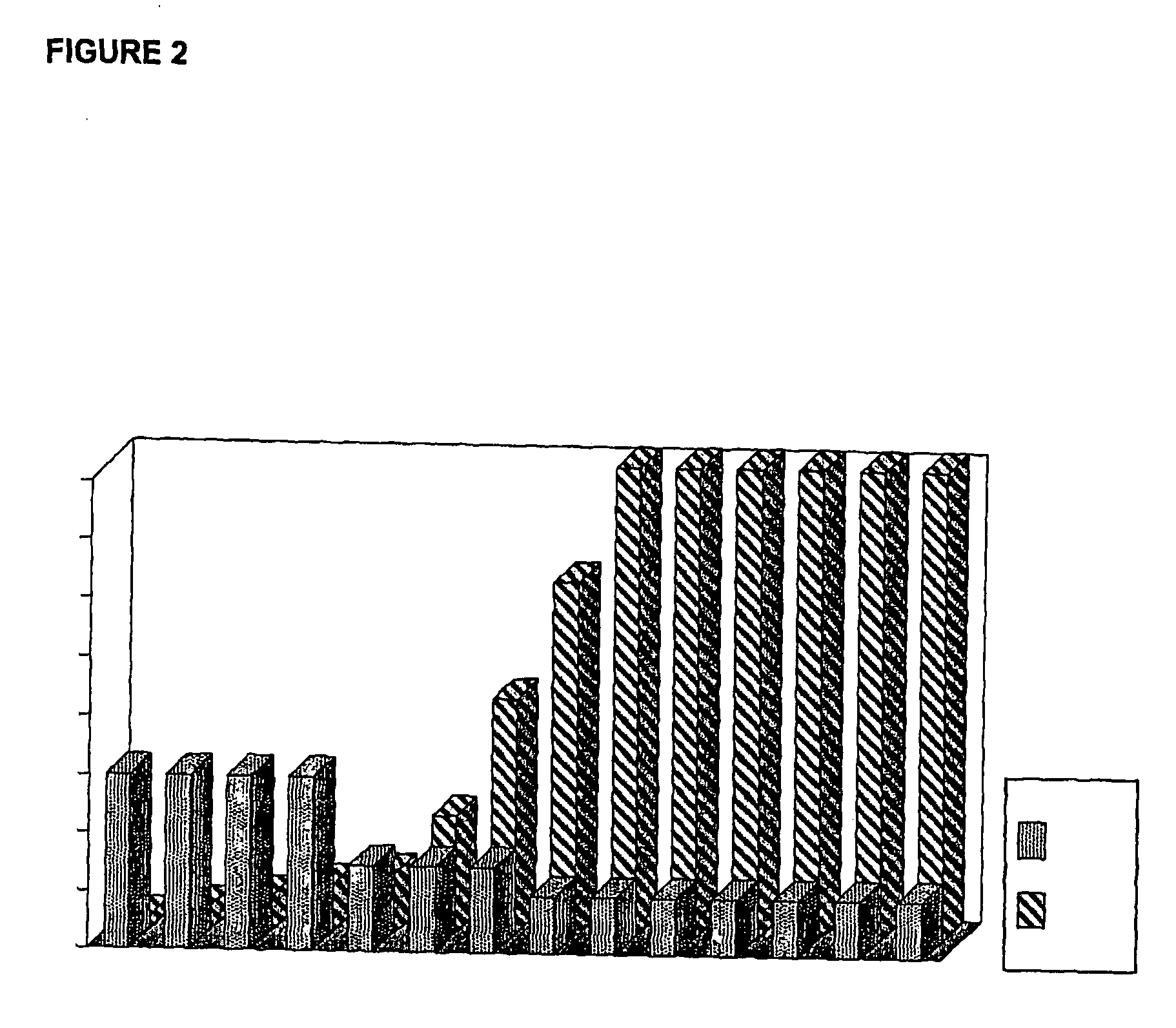
270 results about "Gonadotropin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gonadotropins are glycoprotein polypeptide hormones secreted by gonadotrope cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH), luteinizing hormone (LH), and placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant humans and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.
Enhancement of endogenous gonadotropin production
Provided herein is a method of enhancing endogenous gonadotropin and androgen production comprising administering a therapeutically effective amount of at least one GnRH agonist to a patient in need of such treatment.
Owner:TAP PHARM PROD INC
Ligand/lytic peptide compositions and methods of use
InactiveUS6635740B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideAbnormal tissue growth
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Non-peptide GNRH agents, methods and intermediates for their preparation
InactiveUS7101878B1Modulate activityGood biodistributionBiocideOrganic chemistrySteroidal hormonesGonadotropin-releasing hormone
Non-peptide GnRH agents capable of inhibiting the effect of gonadotropin-releasing hormone are described. Such compounds and their pharmaceutically acceptable salts, multimers, prodrugs, and active metabolites are suitable for treating mammalian reproductive disorders and steroid hormone-dependent tumors as well as for regulating fertility, where suppression of gonadotropin release is indicated. Methods for synthesizing the compounds and intermediates useful in their preparation are also described.
Owner:AGOURON PHARMA INC
Enhancement of Endogenous Gonadotropin Production
InactiveUS20070232548A1Enhancing and increasing productionImprove the level ofHormone peptidesPeptide/protein ingredientsAndrogenAgonist
Provided herein is a method of enhancing endogenous gonadotropin and androgen production comprising administering a therapeutically effective amount of at least one GnRH agonist to a patient in need of such treatment.
Owner:TANEJA RAJNEESH
Ligand/lytic peptide compositions and methods of use
InactiveUS20040018967A1Inhibition of maturationLysis of tumor cells is rapidAntibacterial agentsOrganic active ingredientsLytic peptideAutoimmune condition
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Quinoline derivatives, their production and use
The present compounds are intermediates for the preparation of quinoline derivatives and compositions having gonadotropin-releasing hormone antagonistic activity useful as propylactics or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, uterine or cervical cancer, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; are effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a male or female contraceptive, as an ovulation-inducing agent; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; and are useful for modulating estrous cycles in animals in the field of animal husbandry, as agents for improving the quality of edible meat or promoting the growth of animals, and as agents for promoting spawning in fish.
Owner:TAKEDA PHARMA CO LTD
Unitary combinations of FSH and hCG
InactiveUS20080108571A1Increase stimulationSafer and more successful ovulatory stimulationHormone peptidesPeptide/protein ingredientsOvulation inductionTreatment goals
A novel ovulatory induction paradigm entails administration of hCG in combination with FSH during all stages of treatment, where the ratio of FSH to hCG is adjusted to optimize ovulatory stimulation and minimize complications. The use of compositions characterized by various FSH:hCG ratios enables the practitioner readily to tailor the treatment regimen and accommodate different therapeutic goals as well as individual patient responses to gonadotropin administration.
Owner:FERRING BV
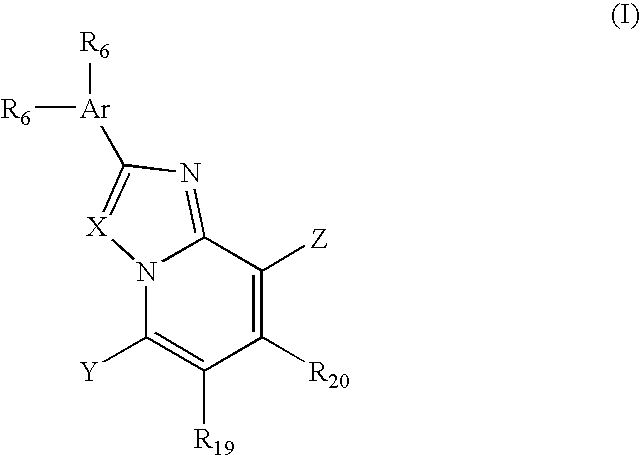
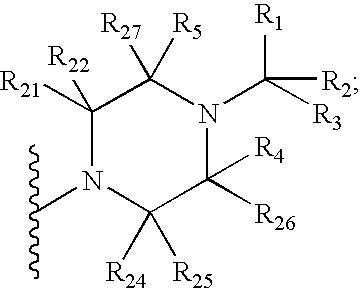
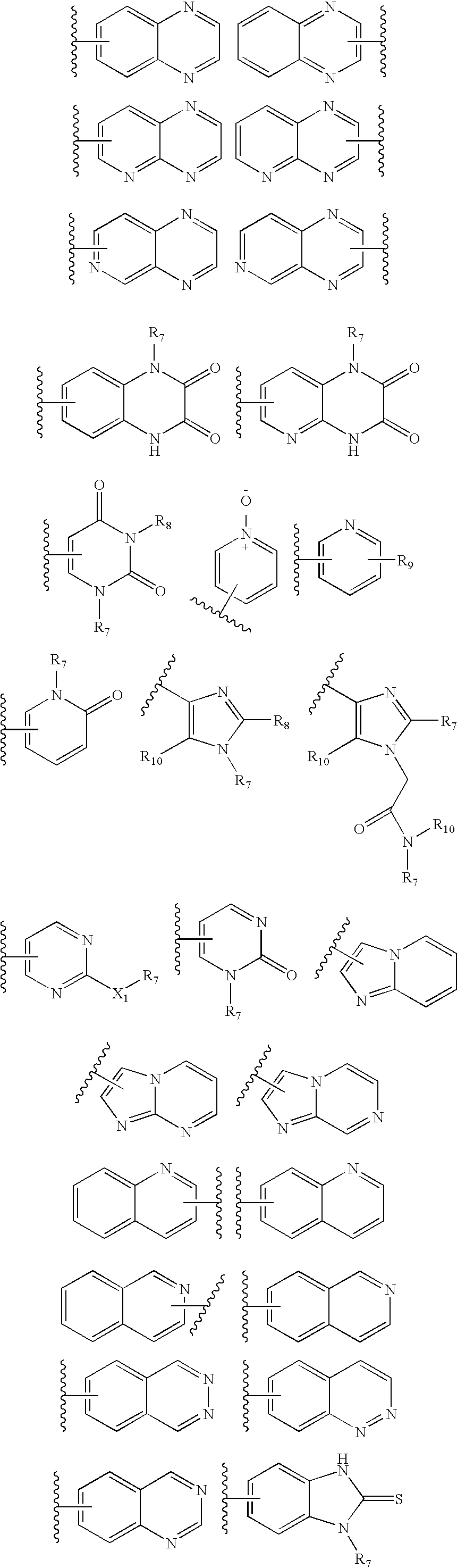
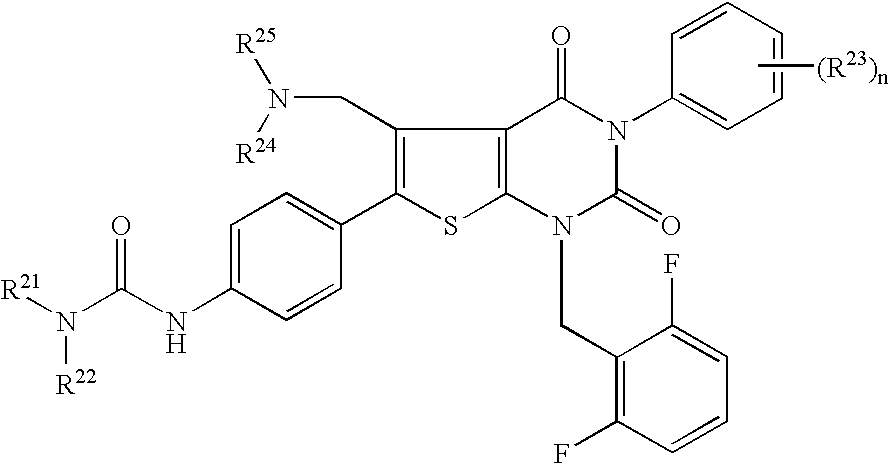
7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060189616A1BiocideOrganic chemistryGonadotropin-releasing hormone receptorHormones regulation
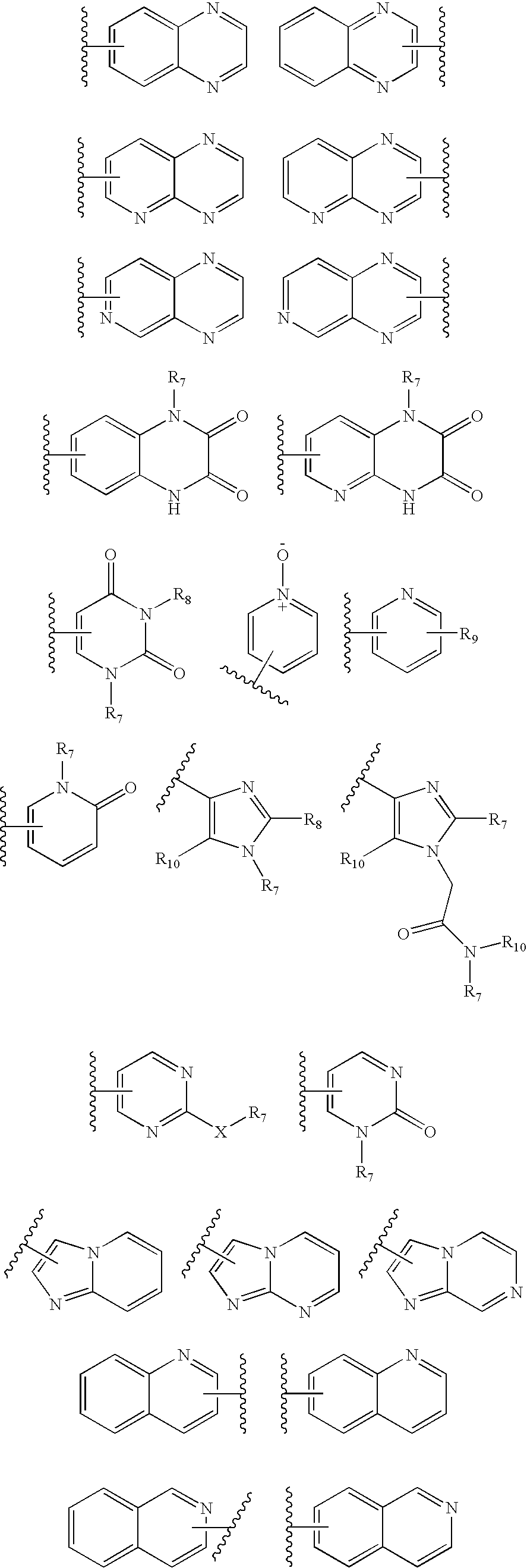
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
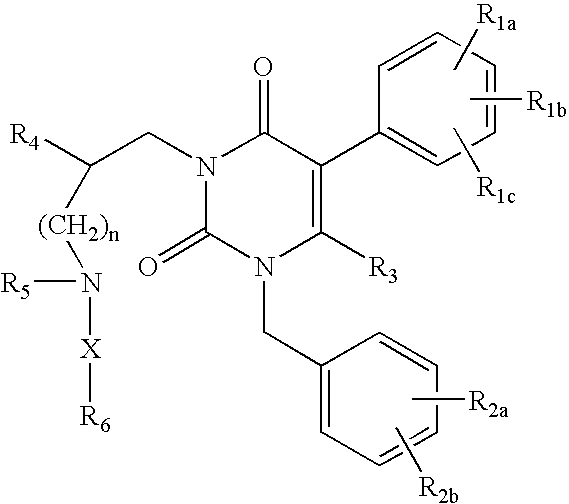
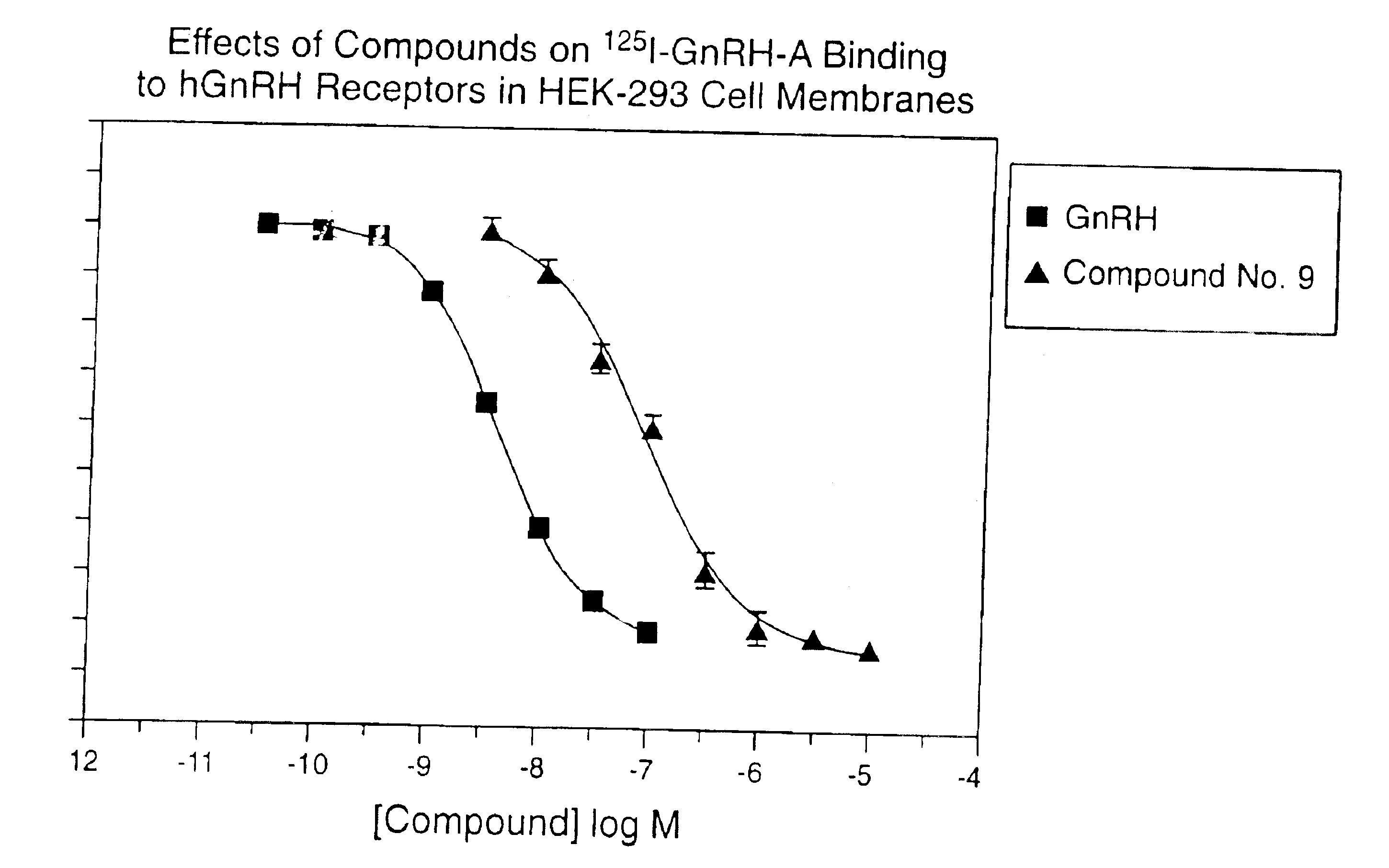
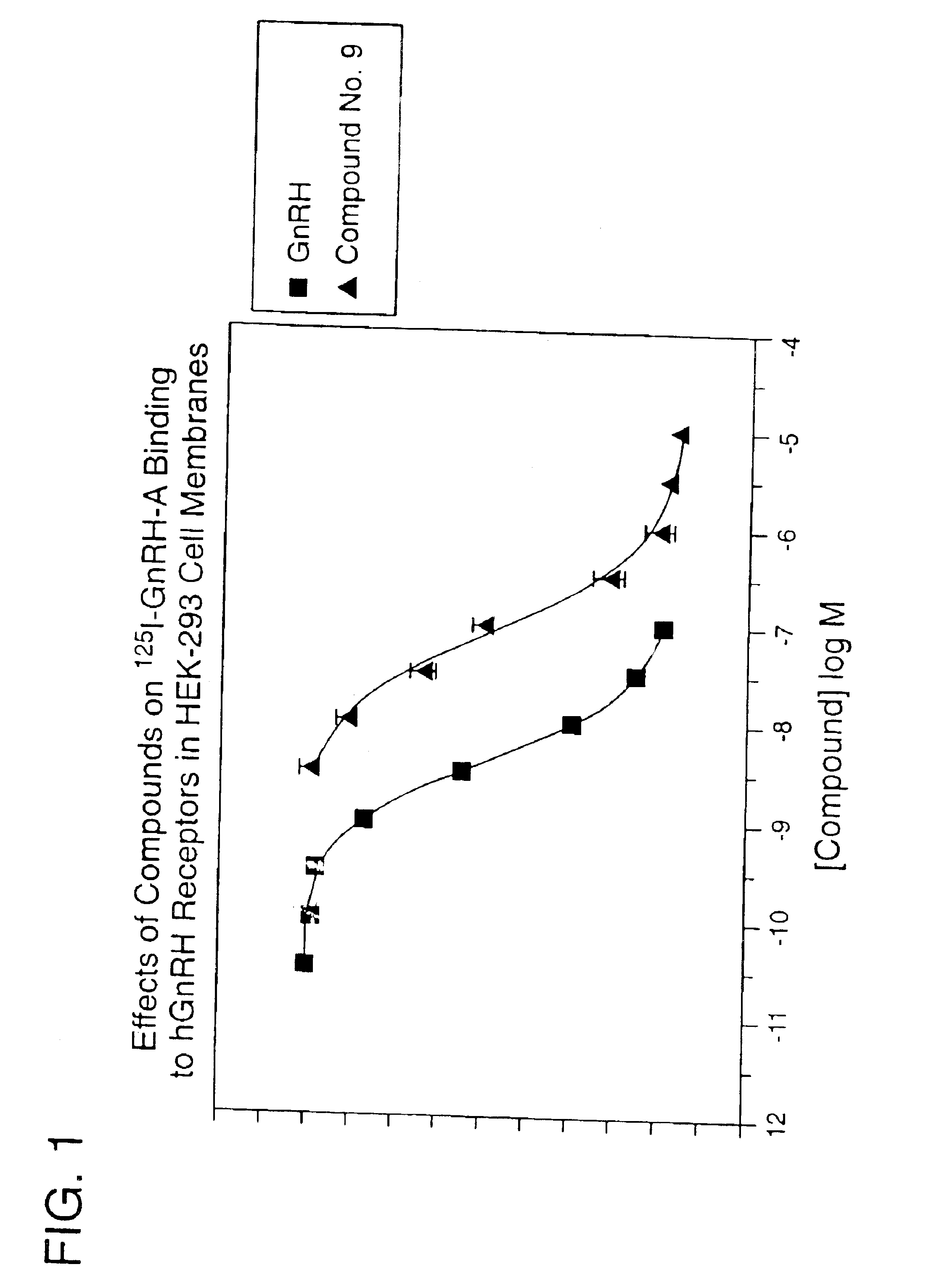
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
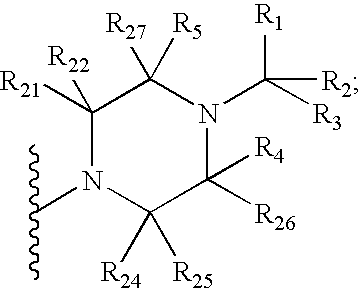
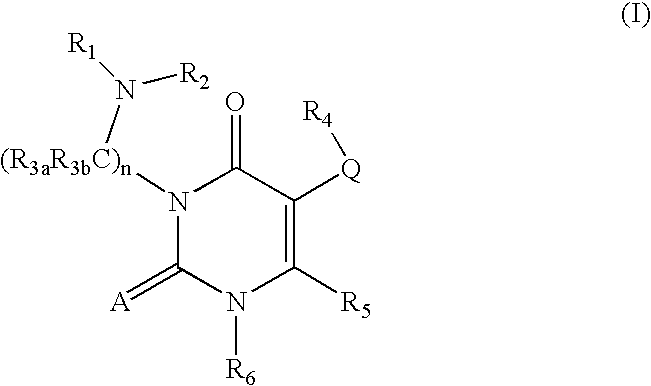
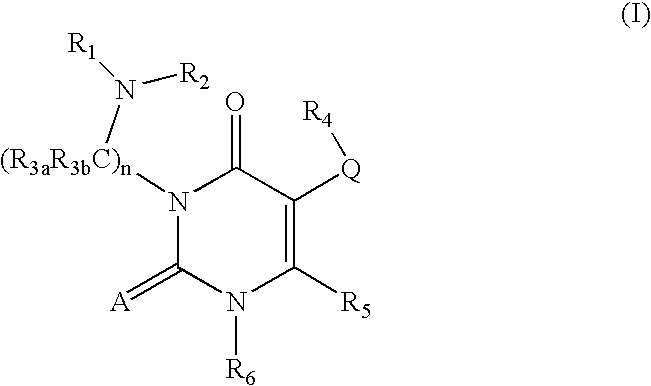
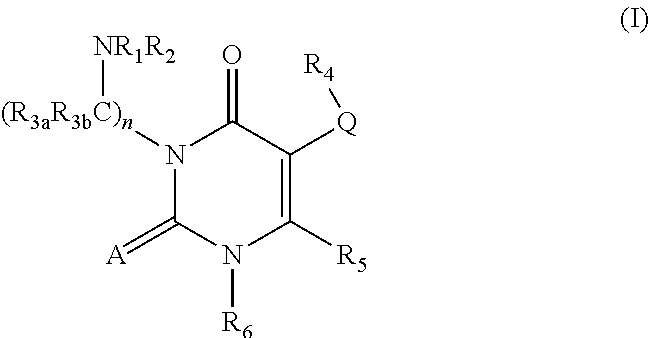
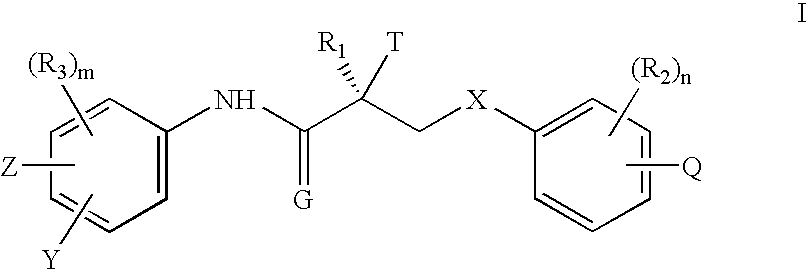
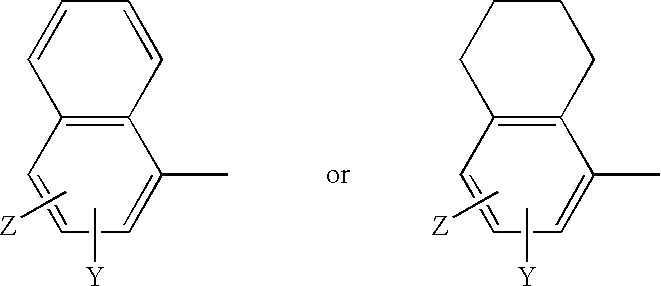
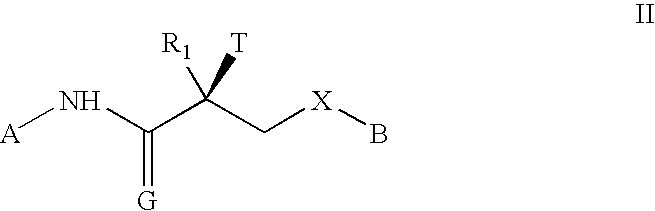
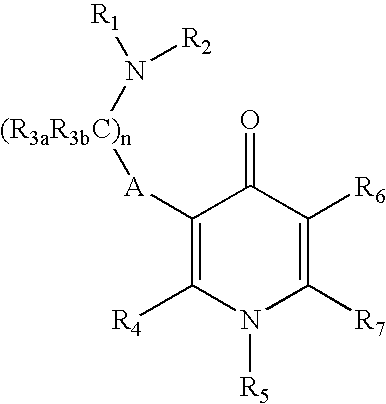
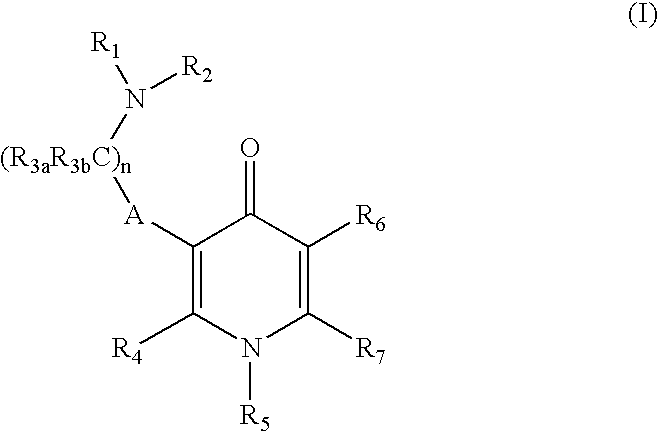
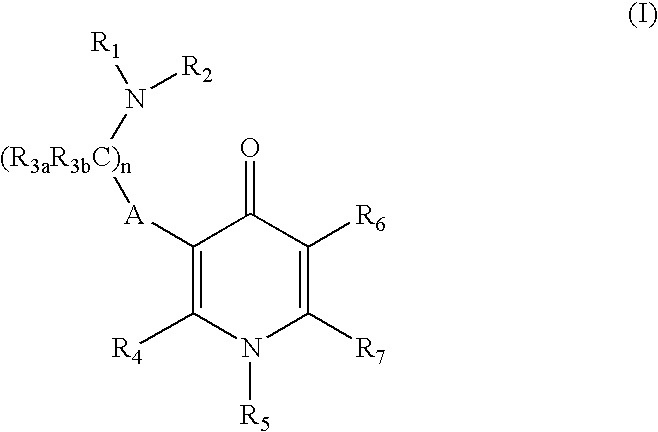
GnPRH receptor antagonists are disclosed which have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein A, R1, R2, R3a, R3b, R4, R5, R6 R7 and n are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
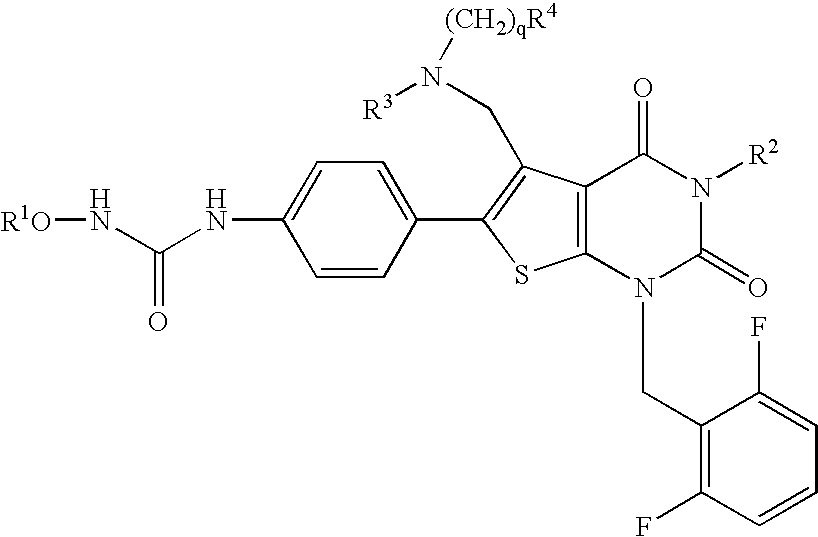
4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060189618A1BiocideOrganic chemistryGonadotropin-releasing hormone receptorLutenizing hormone
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
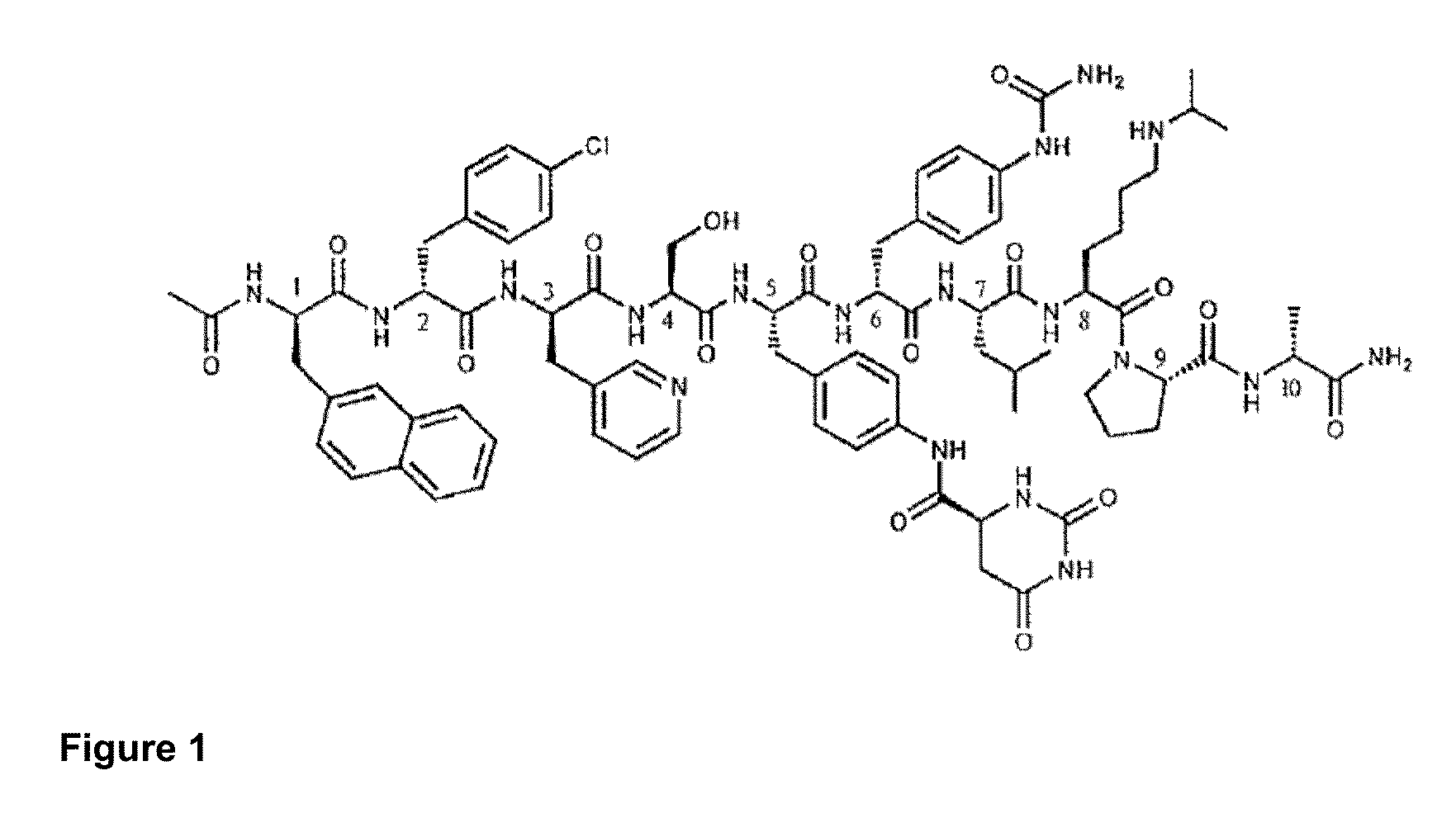
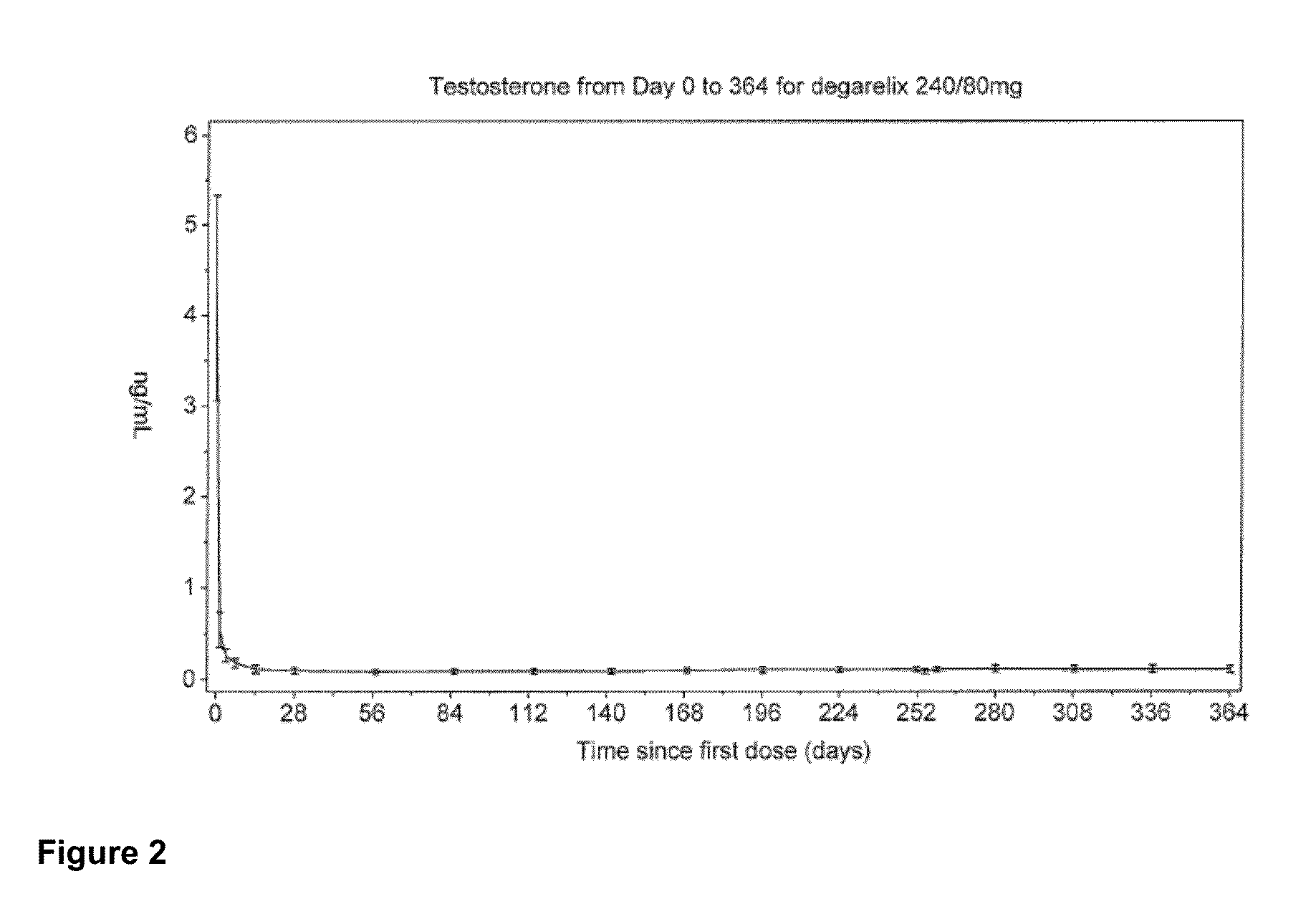
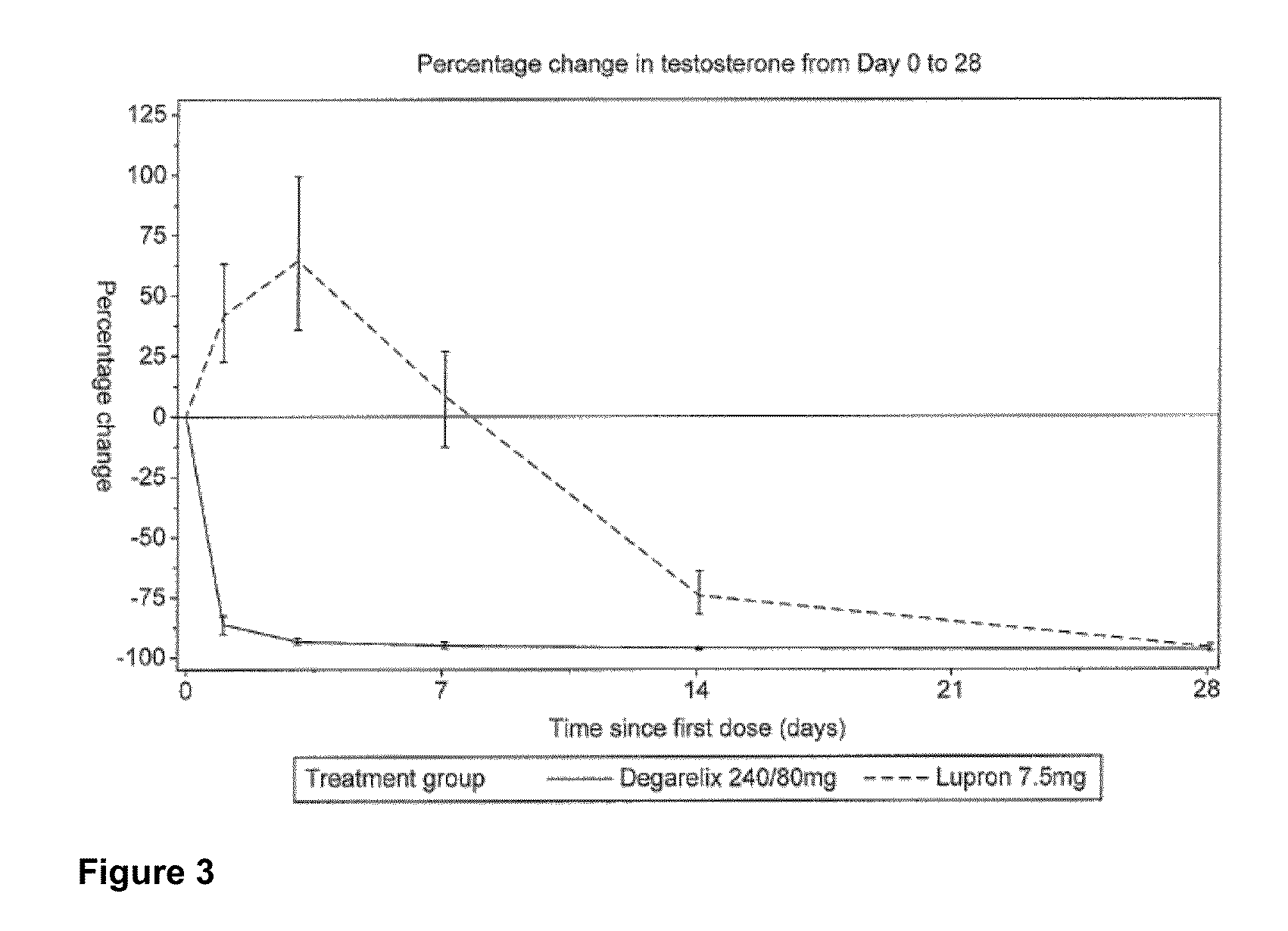
METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST
The invention provides methods and dosing regimens for safely and effectively treating androgen-dependent prostate cancer with a gonadotrophin releasing hormone (GnRH) antagonist without causing a testosterone spike and / or other side effect of GnRH agonist therapy such as a urinary tract infection, or an arthralgia-related or cardiovascular side effect. The present disclosure also provides for methods for treating prostate cancer in a patient with a history of at least one cardiovascular event, wherein administration of degarelix to the subject decreases the likelihood of developing or experiencing an additional cardiovascular event compared to treatment with a gonadotrophin releasing hormone (GnRH) agonist.
Owner:FERRING BV
Liposomal vaccine
InactiveUS20070082043A1High weight ratioHigh encapsulation efficiencyPeptide/protein ingredientsMicroencapsulation basedWater solubleLiposome
The invention provides liposomal vehicles for encapsulating relatively high levels of water-soluble substances including immunogens directed against gastrin and gonadotropin releasing hormone. The liposome encapsulating large amounts of immunogens can be injected parentally to induce effective immune responses without exhibiting significant adverse tissue reactogenicity.
Owner:RECEPTOR BIOLOGIX
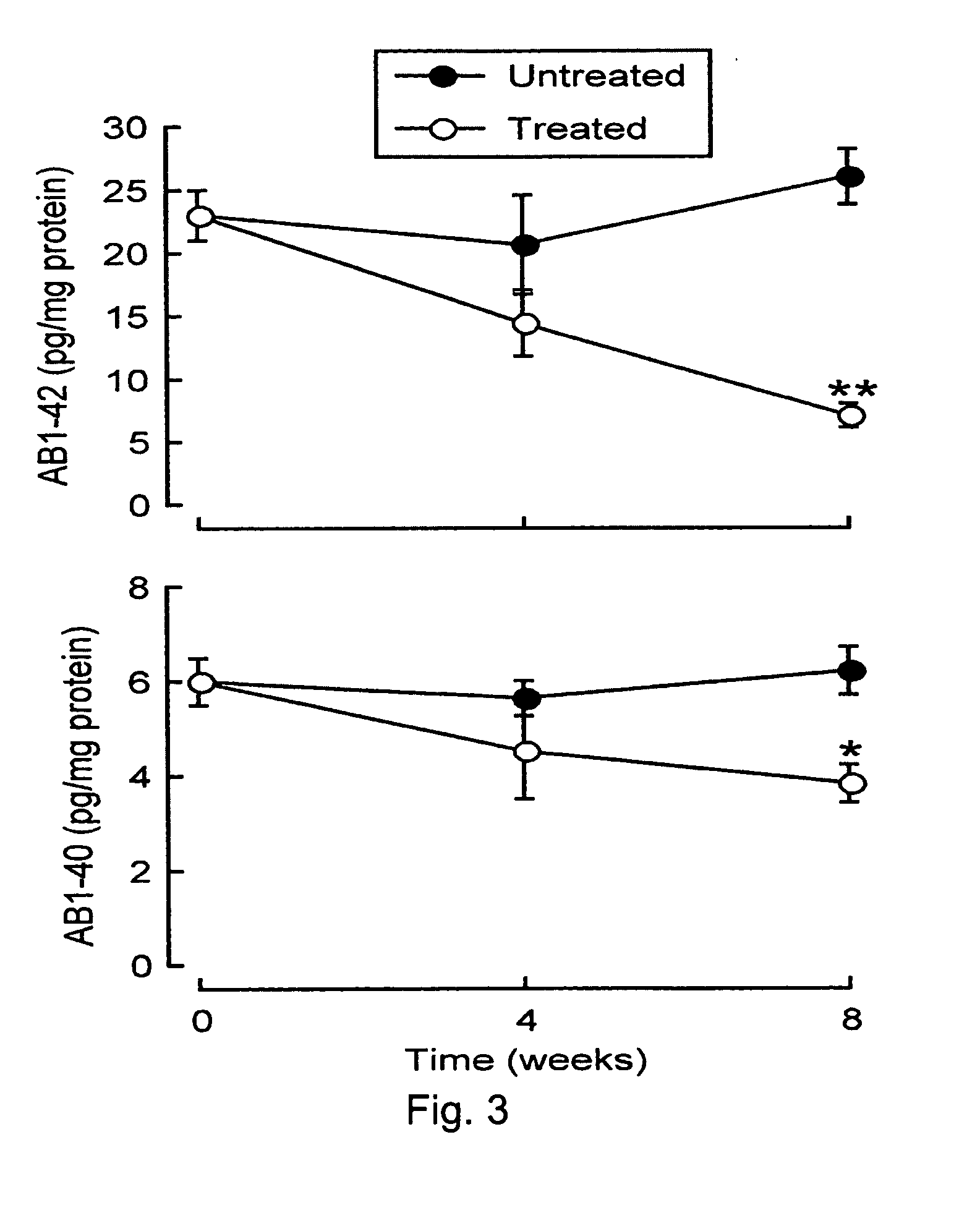
Brain-derived gonadotropins and cognition
InactiveUS20100028361A1Reduce and eliminate and and functionReduce and eliminate levelBiocideOrganic active ingredientsDiseasePhysiology
A method of treating or preventing neurodegenerative disease in a subject, the method includes administering to the subject a therapeutically effective amount of at least one physiologically acceptable agent that modulates levels, production, and / or function of brain-derived hormones of the hypothalamic-pituitary-gonadal (HPG) axis or their receptors.
Owner:SMITH MARK A +1
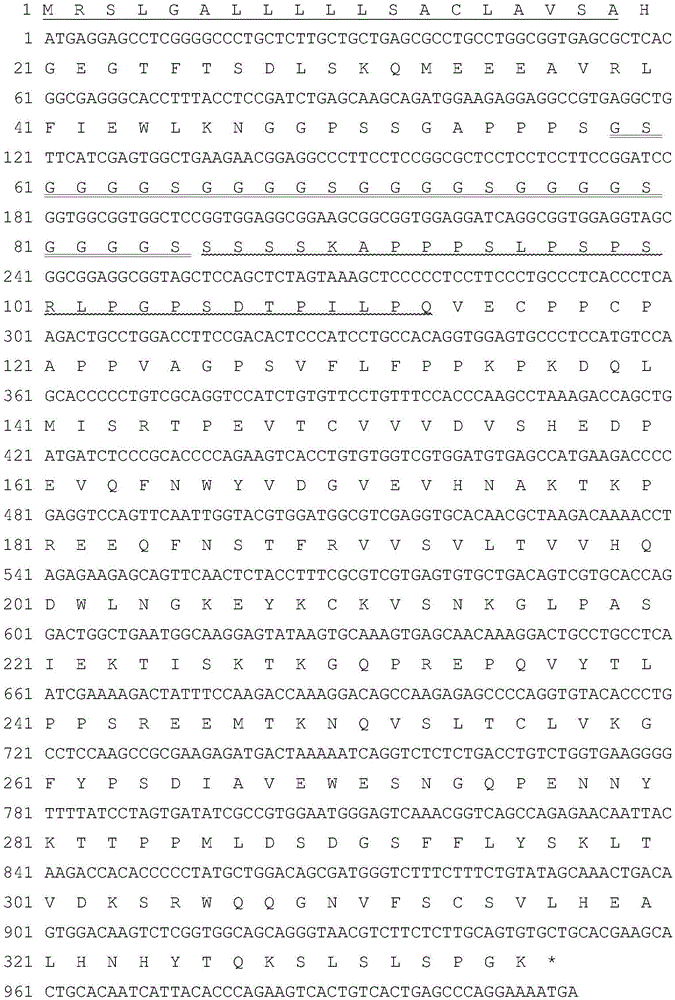
Hyperglycosylated Extendin-4, fusion protein of analogue thereof, and preparation method and application of fusion protein
ActiveCN106117370AReduce fluctuations in drug concentrationReduce generation riskPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseHuman Chorionic Gonadotropin Beta Subunit
The invention discloses hyperglycosylated Extendin-4, fusion protein of analogue thereof, and a preparation method and the application of fusion protein. The fusion protein comprises Extendin-4, the analogue of the Extendin-4, a flexible peptide joint, at least one human chorionic gonadotropin beta carboxyl terminal peptide rigid unit and a human immunoglobulin Fc fragment. The invention also discloses the preparation method and the application of the fusion protein. The fusion protein has optimal biological activity, obviously prolonged circulation half-time, lowered immunogenicity and improved bioavailability. The fusion protein can be used for treating diabetes, obesity and other diseases benefited by lowering fasting plasma glucose, inhibiting stomach and / or bowel movement and inhibiting and / or bowel evacuation or inhibiting food intake.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Health-care product for preventing and treating premature ovarian failure and preparation method thereof
InactiveCN101961419APrevent premature agingImproves symptoms of dullness and lack of elasticityAerosol deliveryOintment deliveryReflux extractionEpimedium
The invention discloses a health-care product for preventing and treating premature ovarian failure and a preparation method thereof. The health-care product comprises medlar, glossy privet fruit, prepared rehmannia, epimedium, malaytea scurfpea fruit, Chinese dodder seeds, raspberry, peanut, Chinese date and soy. The preparation method comprises the following steps of: weighing raw materials according to a weight proportion; soaking with ethanol; performing reflux extraction; filtering, and keeping the filtrate for later use; performing reflux extraction on the residue twice with ethanol; re-filtering, and keeping the filtrate for later use; combining the filtrate, and concentrating the filtrate until ethanol odor does not exist so as to obtain ethanol extract; and decocting the residue twice, filtering to obtain water extract, and combining the ethanol extract and the water extract to obtain the health-care product. The health-care product has excellent effect of improving the ovary function, and has the effects of improving the ovary function, achieving the effect of estrogen, improving the symptoms of weight loss, desudation and the like caused by ovarian failure, regulating the response of the ovary to gonadotropin, promoting follicular development and the like for 40-year-old women particularly.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Compositions and methods for contraception in or sterilization of mammals
InactiveUS6680058B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideMammal
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to induce sterility or long-term contraception in mammals. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in mammals in vivo. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Compositions and methods for treating precocious puberty
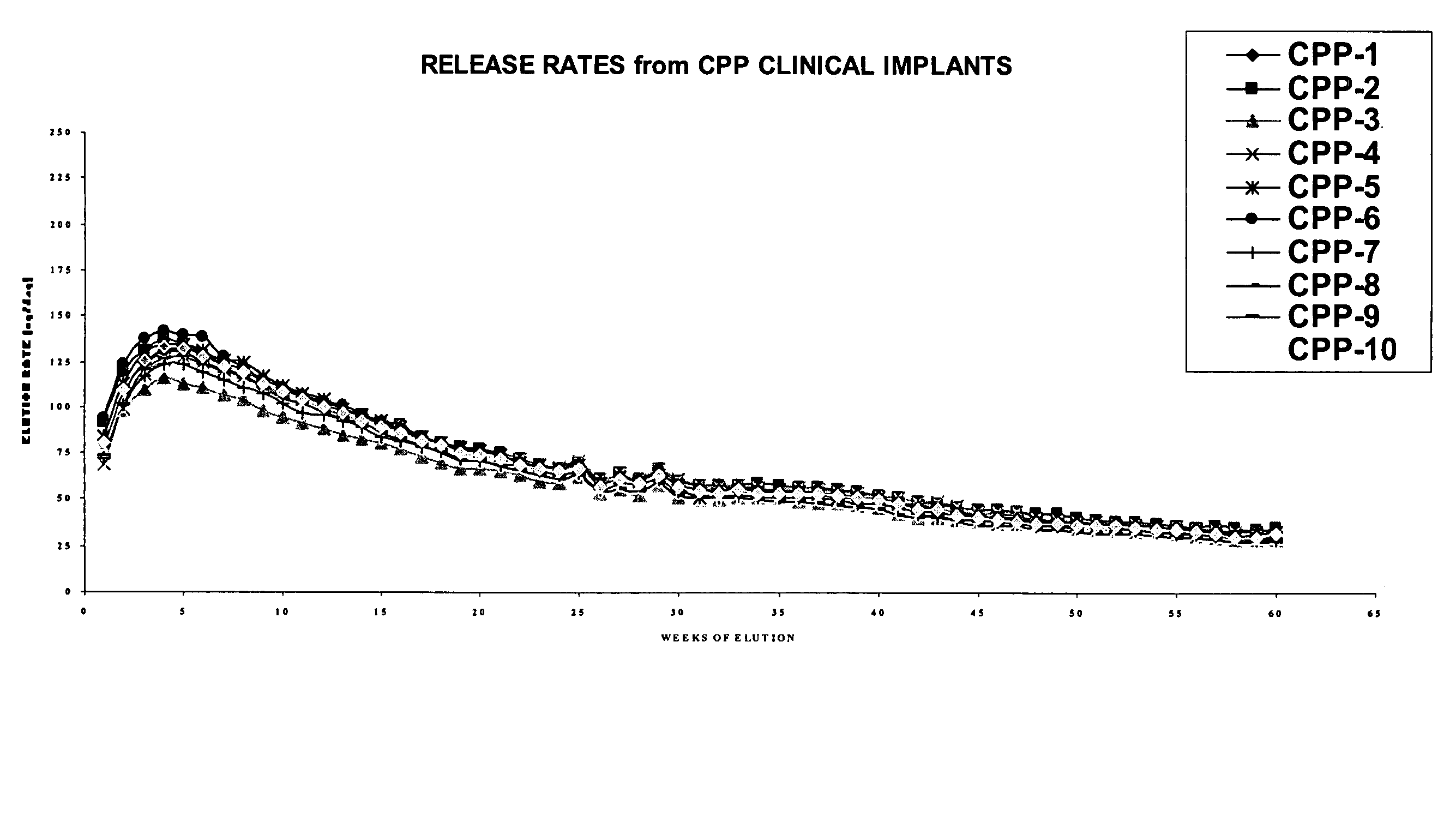
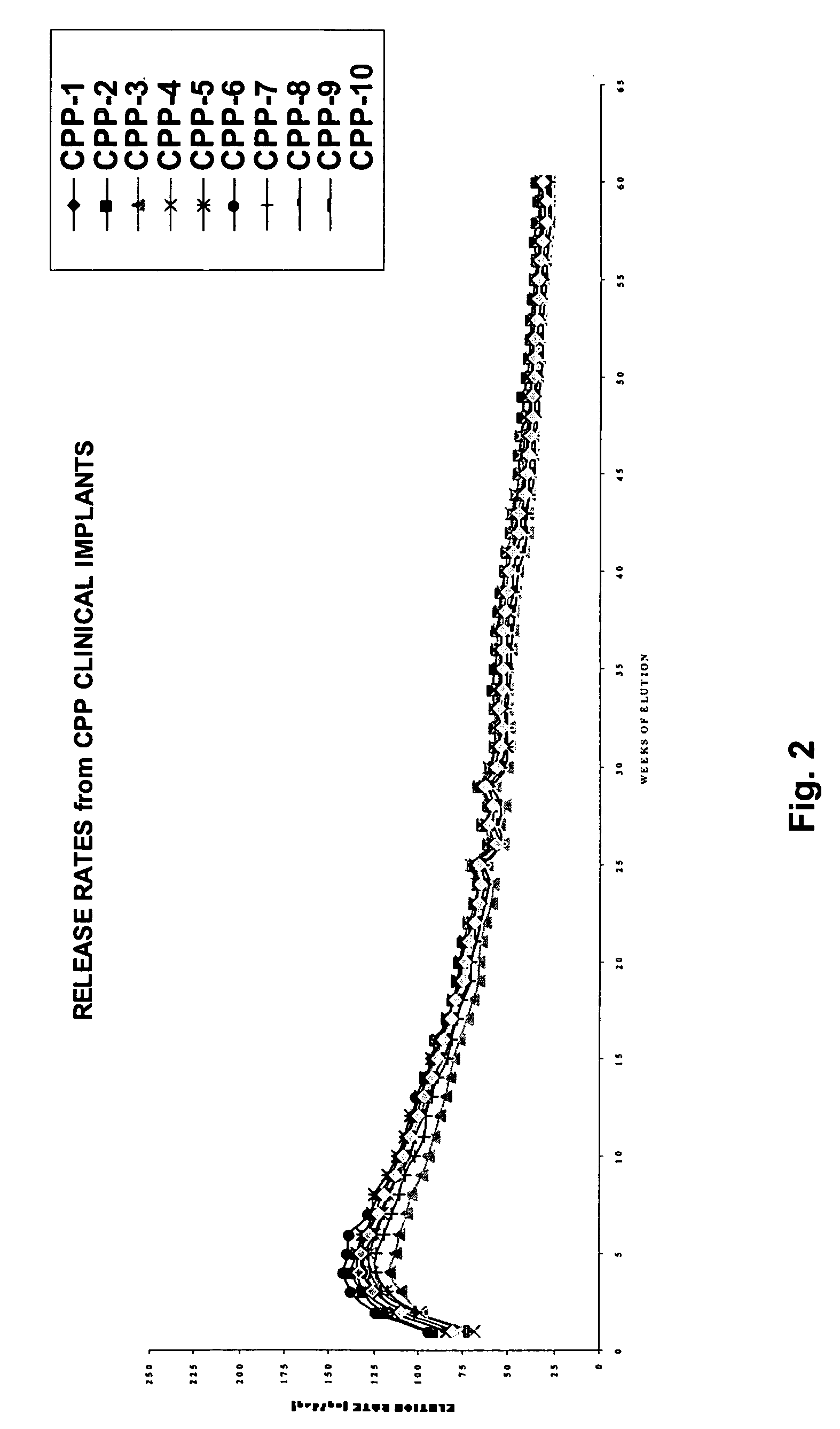
ActiveUS20060019903A1Avoid lostPeptide/protein ingredientsPharmaceutical delivery mechanismHistrelinPhysiology
The present invention is directed to the controlled delivery of gonadotropin-releasing hormone (GnRH) agonists, preferably from a polymeric material that is implanted in the body. More specifically, the present invention relates to compositions comprised of a GnRH agonist, preferably histrelin, in a polymeric material that results in a desired and controlled delivery of a therapeutically effective amount of GnRH agonist over an extended period of time in order to treat central precocious puberty (CPP).
Owner:ENDO PHARMA SOLUTIONS
Condensed-ring thiophene derivatives, their production and use
A gonadotropin-releasing hormone antagonistic composition, which comprises an optionally substituted condensed-bicyclic compound consisting of a homo or hetero 5 to 7 membered ring and a homo or hetero 5 to 7 membered ring is effective as a propylactic or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, cancer of uterine cervix, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; is effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a contraceptive of male or female, as an ovulation-inducing agent of female; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; is useful as modulating estrous cycles in animals in the field of animal husbandry, as an agent fro improving the quality of edible meat or promoting the growth of animals; is useful as an agent of spawning promotion in fish.
Owner:TAKEDA PHARMA CO LTD
Inducing sterility in fish by disrupting the development of the GnRH system
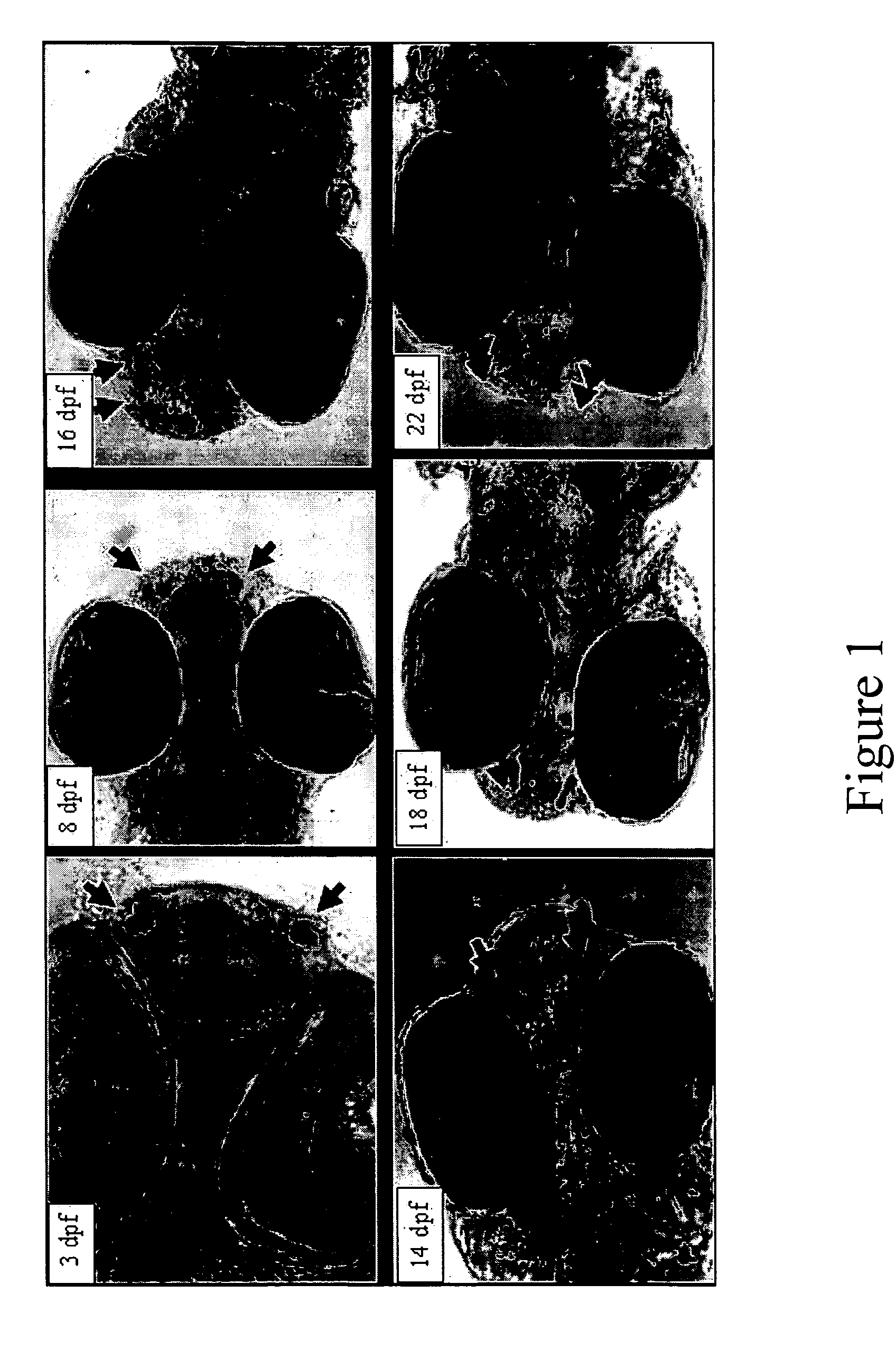
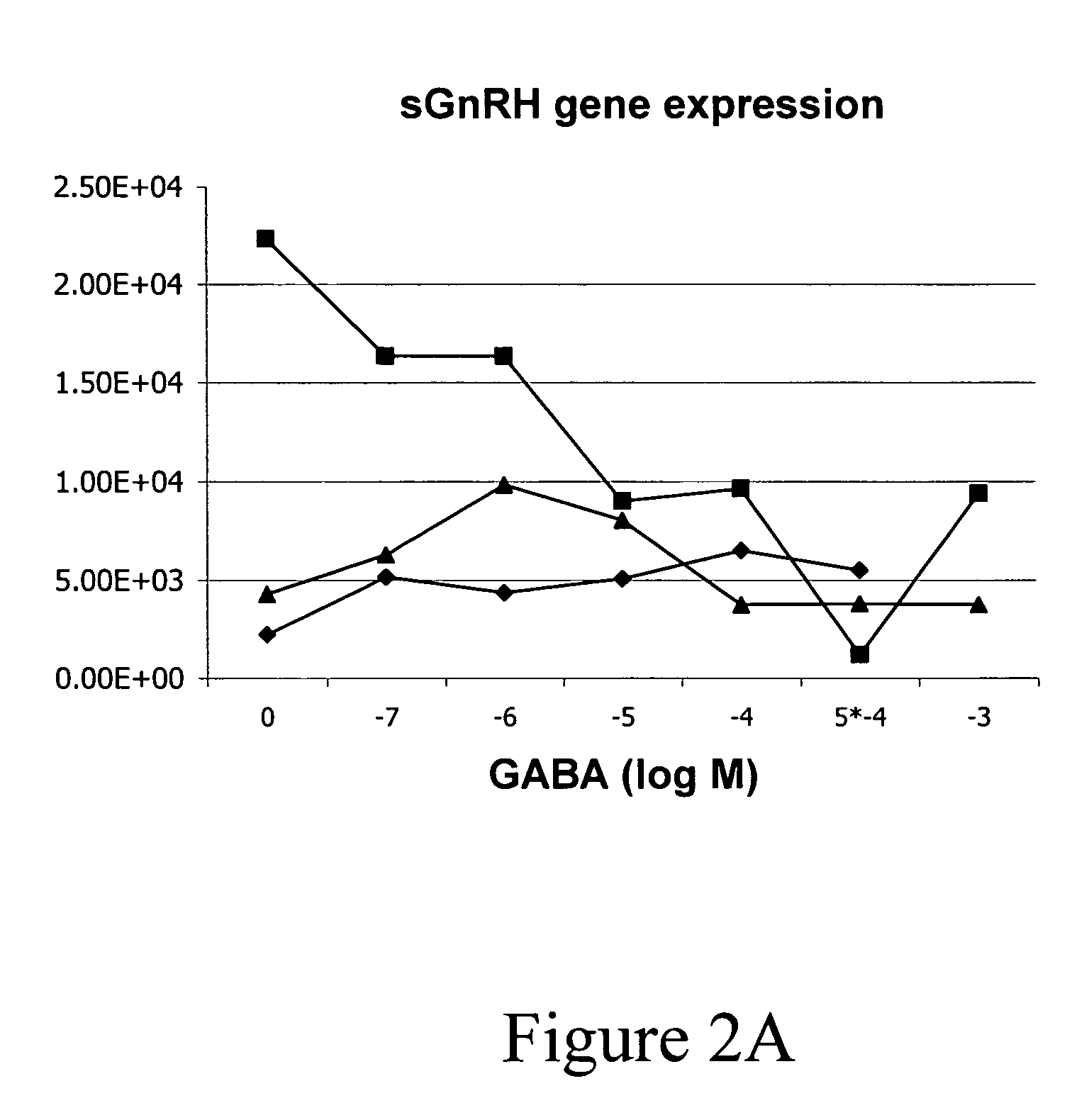
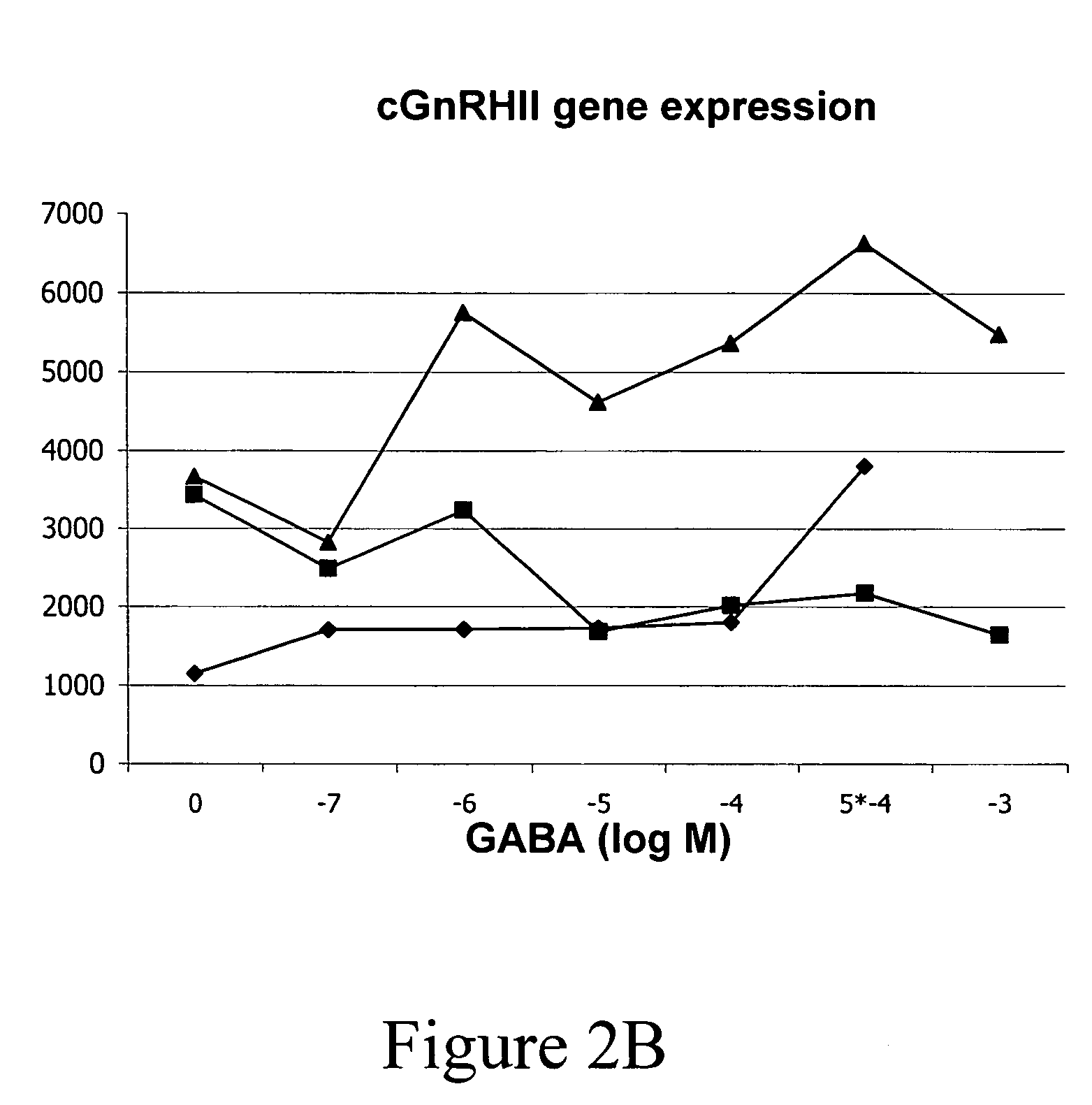
InactiveUS20050132969A1Less gonadMore muscleHormone peptidesPisciculture and aquariaGABA receptor agonistGABA receptor antagonist
The present invention provides for a method for inducing sterility in fish by administering at least one compound that disrupts the establishment of the gonadotropin-releasing hormone system during early development thereby inhibiting sexual maturity in the treated fish. Effective compounds include GABA, GABA receptor agonists, or GABA receptor antagonists.
Owner:RAMOT AT TEL AVIV UNIV LTD +2
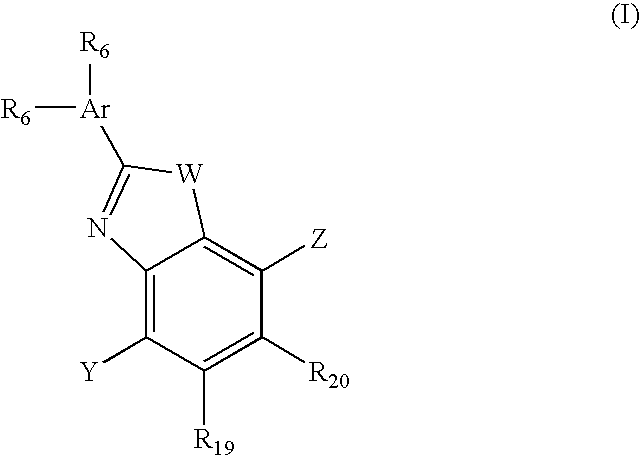
Benzooxazole and benzothiazole antagonists of gonadotropin releasing hormone receptor
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
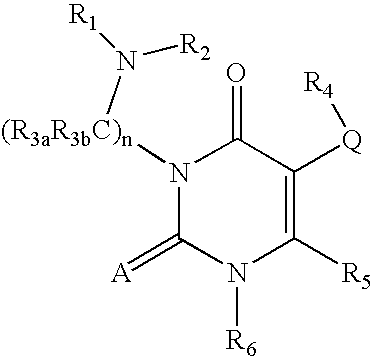
GnRH receptor antagonists are disclosed which have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein A, Q, R1, R2, R3a, R3b, R4, R5, R6 and n are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Processes for the preparation of uracil derivatives
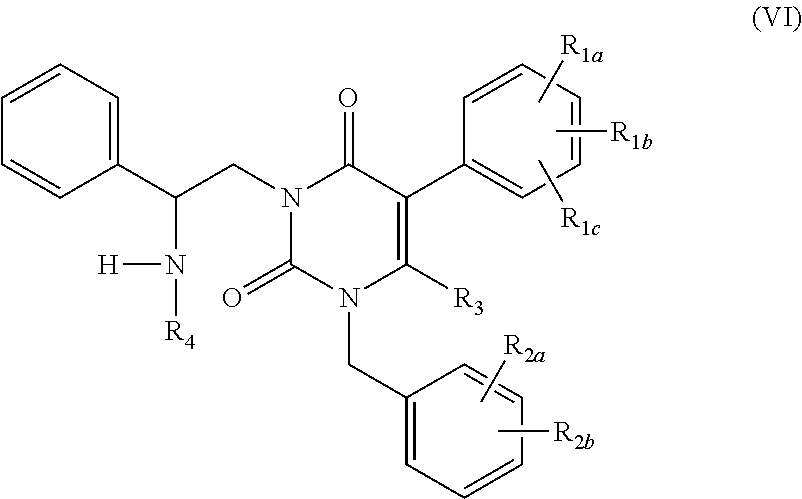
The present invention relates to processes and intermediates for preparing Gonadotropin-Releasing Hormone (GnRH) receptor antagonists of structure (VI); and stereoisomers and pharmaceutically acceptable salts thereof.
Owner:NEUROCRINE BIOSCI INC
Piperazinylimidazopyridine and piperazinyltriazolopyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060270848A1Organic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorLutenizing hormone
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
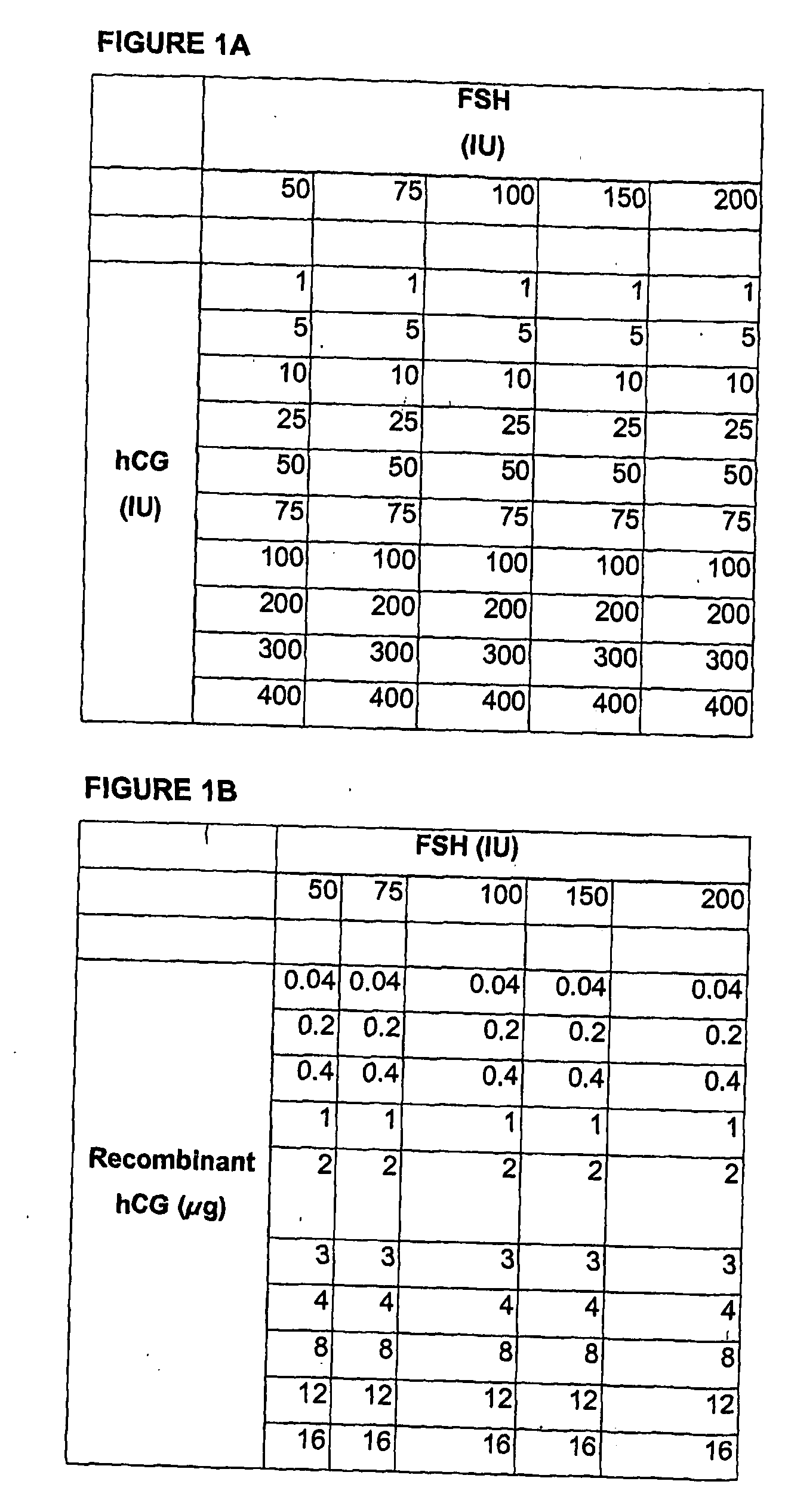
Method of controlled ovarian hyperstimulation and pharmaceutical kit for use in such method
InactiveUS20050235374A1Easy to solveImprove developmentPeptide/protein ingredientsPeptide preparation methodsGanirelixCo administration
One aspect of the present invention is concerned with a method of controlled ovarian hyperstimulation in a mammalian female, said method comprising the co-administration to said female of a substance having follicle stimulating hormone activity (FSH substance) in an amount effective to stimulate multiple follicular development;—gonadotropin releasing hormone (GnRH) antagonist in an amount equivalent to a daily subcutaneous dose of at least 0.5 mg ganirelix to prevent a premature LH-surge; and—a LH substance in an amount effective to prevent or suppress symptoms of luteinising hormone (LH) deficiency resulting from the administration of the GnRH antagonist; followed by administering a meiosis and luteinisation inducing substance (ML substance) in an amount effective to stimulate resumption of meiosis and luteinisation, and wherein the LH substance is not obtained from the urine of human females. Another aspect of the to invention relates to a pharmaceutical kit for use in a method of controlled hyperstimulation, which kit comprises:—at least one parenteral or oral dosage unit containing one or more FSH substances in an amount equivalent to a subcutaneous dose of 50-1500 I.U. FSH;—at least one parenteral dosage unit containing one or more GnRH antagonists in an amount equivalent to a subcutaneous dose of 0.5-25 mg ganirelix;—at least one parenteral dosage unit containing one or more LH substances in an amount equivalent to a subcutaneous dose of 50-3000 I.U. recombinant LH; wherein the LH substance is not obtained from the urine of human females.
Owner:ZONE IND DE IOURIETTAZ
Ovarian large cortex piece vitrified cryopreservation protection liquid and cryopreservation method thereof
InactiveCN102771471APromote perfusion timeImprove biological activityDead animal preservationOvarian transplantationIn vivo
The invention relates to an ovarian large cortex piece vitrified cryopreservation protection liquid and a cryopreservation method thereof. Although the vitrified cryopreservation method is widely applied to experimental animals, a universal cryopreservation method and a standard cryopreservation liquid preparation scheme are absent at present, so that the sized transplantation subjected to vitrified cryopreservation is at a small-size stage, an ovarian small cortex piece has limited follicles, and the service life of the transplanted follicles is influenced. The invention provides vitrified cryopreservation protection liquid suitable for an ovarian large cortex piece, and gonadotropin is added on the basis of the common vitrified liquid. According to the vitrified cryopreservation protection liquid suitable for the ovarian large cortex piece, especially the ovaries of experimental animals, improved variety livestock and wild endangered species in sudden death are conveniently cryopreserved at the most proper osmotic equilibrium time of performing vitrified cryopreservation on the ovarian large cortex pieces of different sizes; and moreover, according to the method, the survival rate of the follicles can be improved, the blood reperfusion time of the transplant is shortened, and the survival rate of the cryopreserved ovarian transplantation in vivo is improved.
Owner:NINGXIA MEDICAL UNIV
Protein derivatives of human granzyme B, and use thereof in targeted therapy on adenocarcinoma
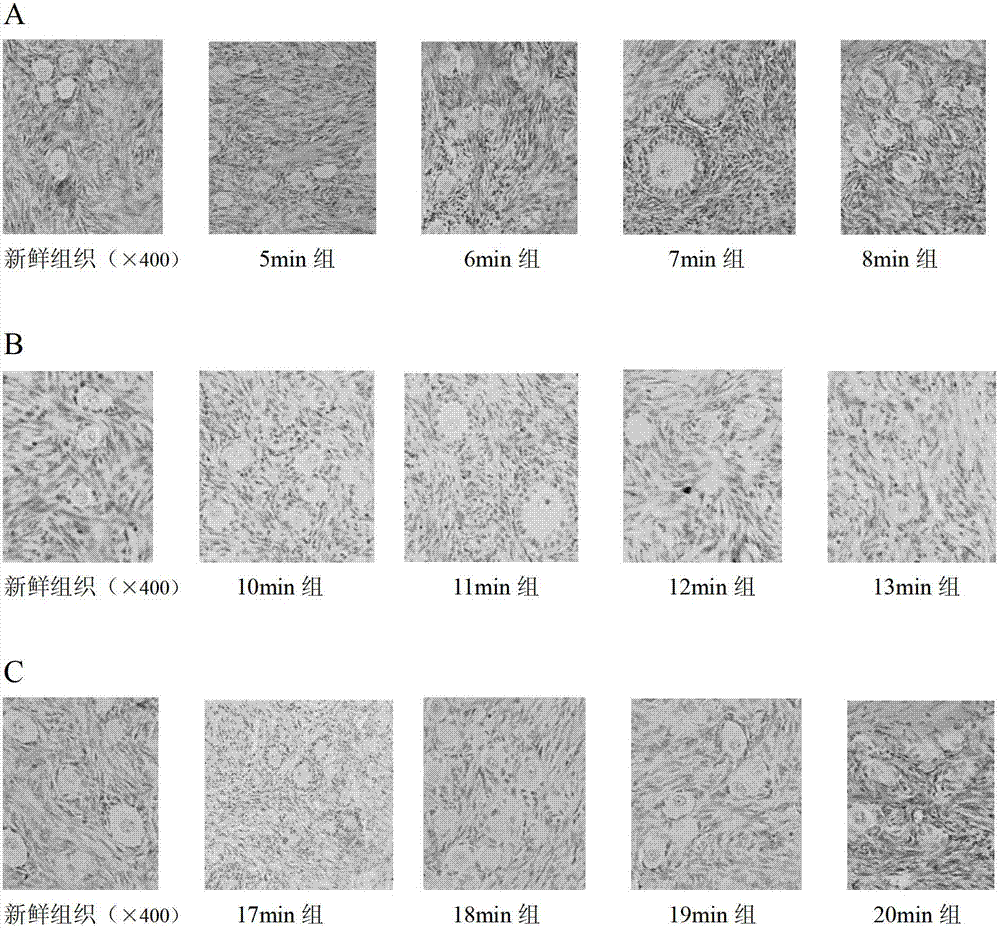
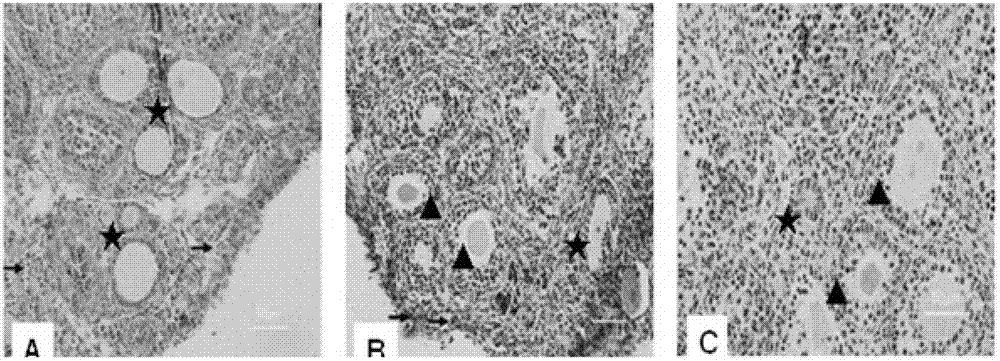
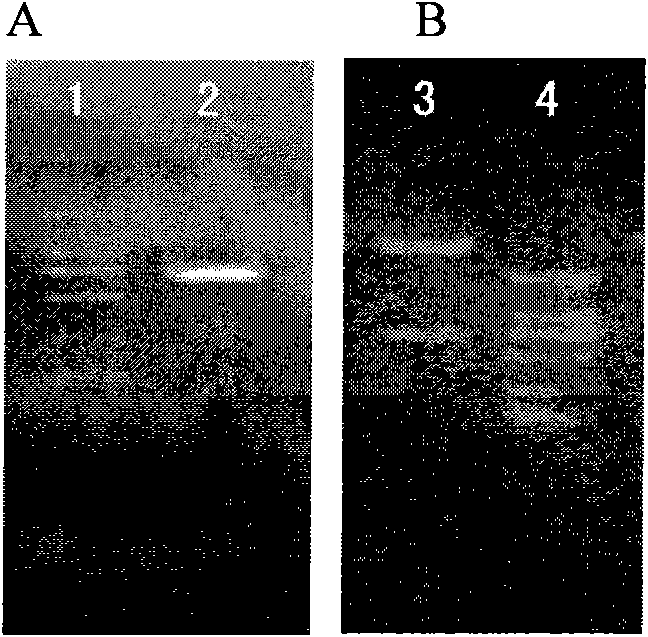
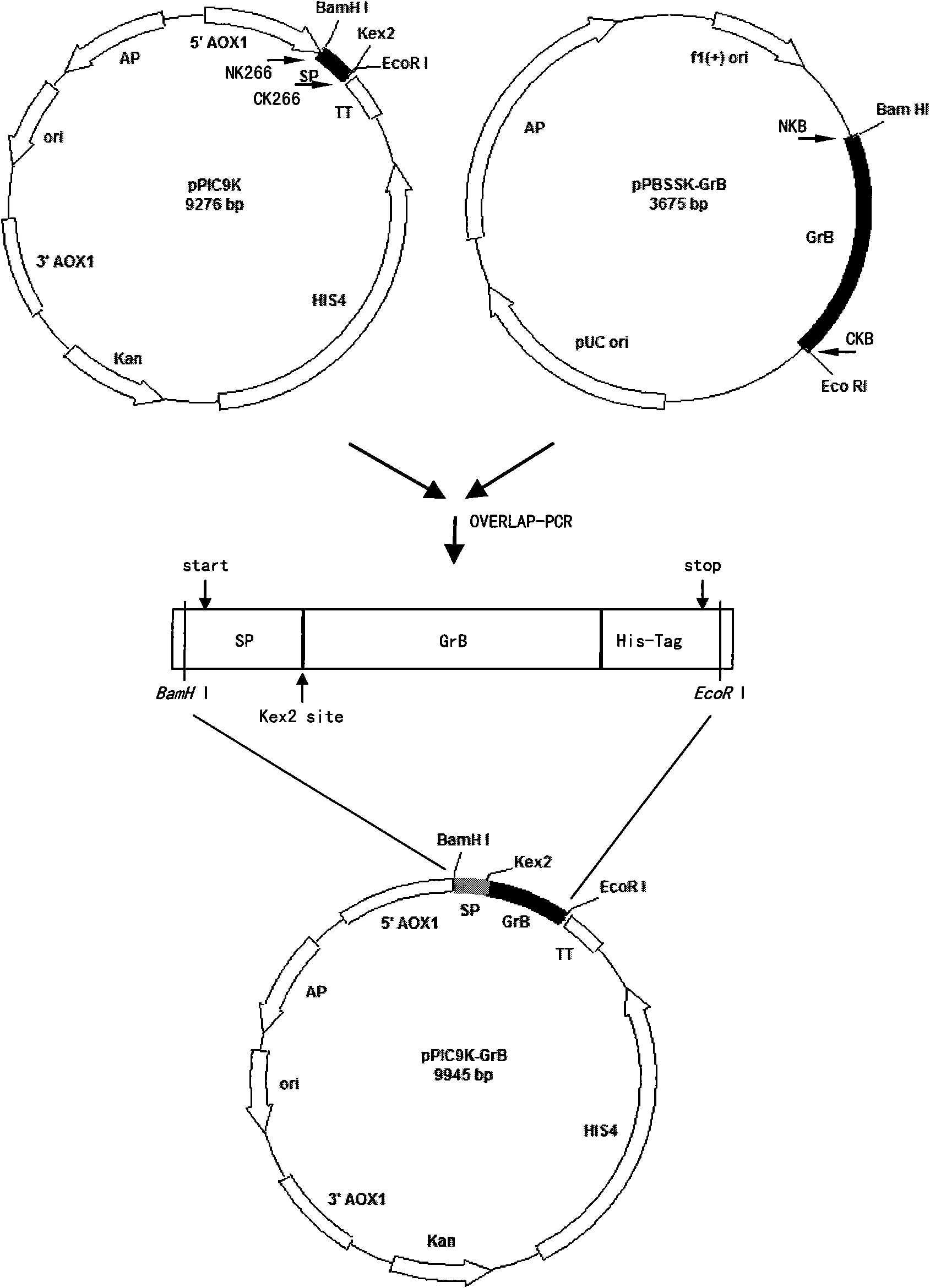
The invention belongs to the pharmacological field of gene engineering, and provides a group of protein derivatives of human granzyme B by adopting gene engineering technology. The group of protein derivatives of the human granzyme B comprises GrB-G4S-GnRH (GrBLG) and mGrB-C4S-GnRH (mGrBLG) which are targeted fusion proteins formed by connecting human matured granzyme B (GrB) and mutagenic human matured granzyme B (mGrB) with human gonadotropin-releasing hormone (GnRH) through flexible connecting short peptide GlyGlyGlyGlySer (G4S) respectively and which can be combined with human GnRH receptors (GnRHR) on the surfaces of cells through ligand human GnRH. The mGrB eliminates the functions of the combination through a personal 'electrostatic exchange-absorption mode' and the entering into target cells of the prior GrB, and simultaneously reserves the enzymatic activity and the cytocidal function of the GrB. The invention provides experimental evidences for peculiarly targeted-killing GnRHR positive cells and minimizing toxic side effect by the fusion proteins, and shows the use of the group of protein derivatives of the human granzyme B in the targeted therapy on the positive adenocarcinoma of a type of gonadotropin-releasing hormone receptors.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Premature ovulation preventive agent
InactiveUS20090048273A1Low toxicIncrease resistanceBiocideOrganic active ingredientsBuccal administrationBiology
The present invention provides a premature ovulation inhibitor for use in in vitro fertilization or embryo transfer process, which contains a nonpeptidic compound having a gonadotropin releasing hormone antagonistic action. The premature ovulation inhibitor for use in in vitro fertilization or embryo transfer process of the present invention is low toxic, permits oral administration, and has a superior inhibitory effect on premature ovulation in in vitro fertilization or embryo transfer process.
Owner:TAKEDA PHARMA CO LTD
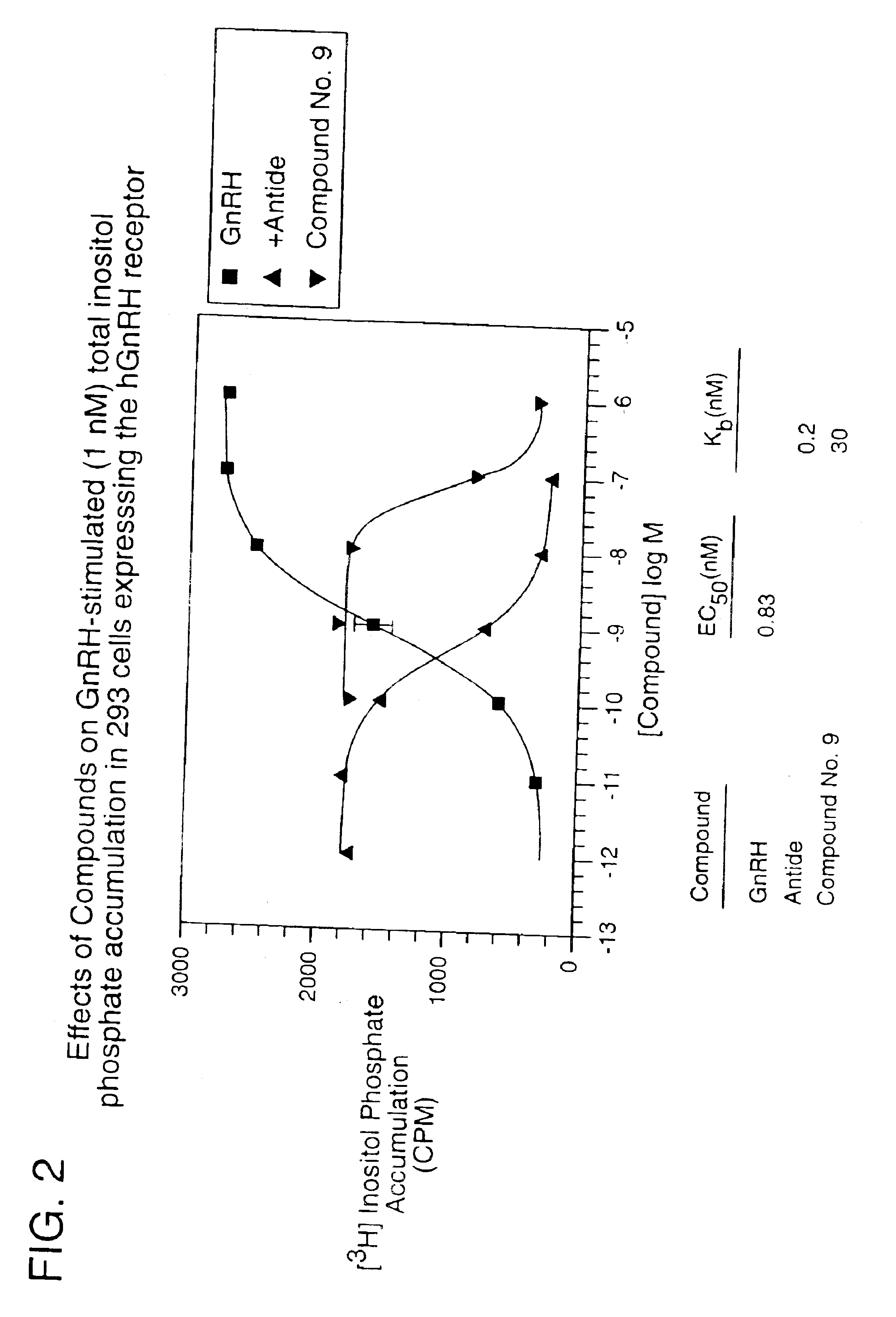
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
ActiveUS7015226B2Organic active ingredientsBiocideGonadotropin-releasing hormone receptorNK1 receptor antagonist
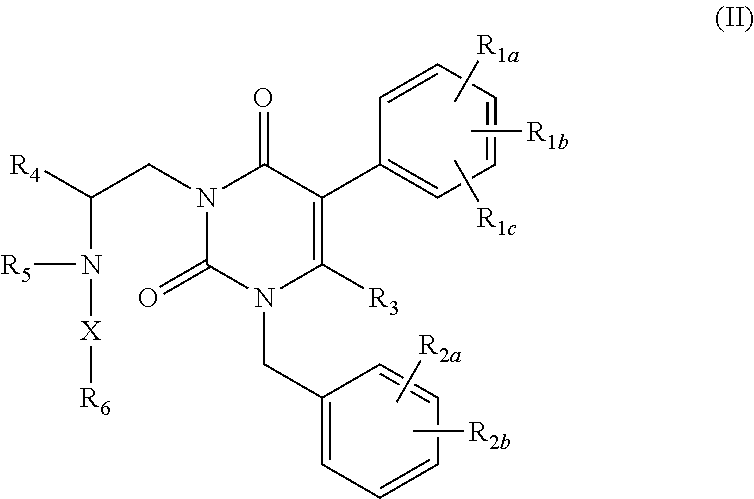
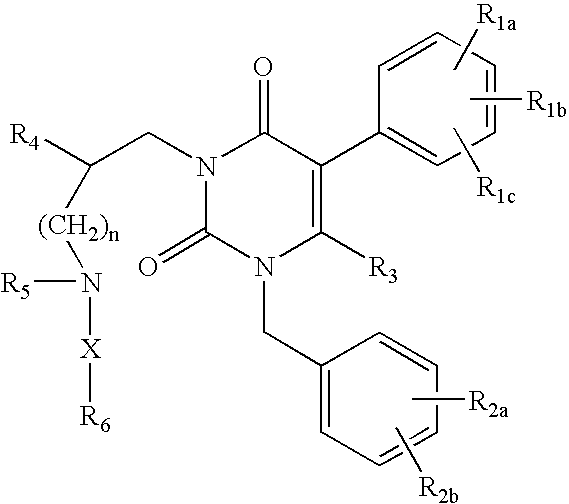
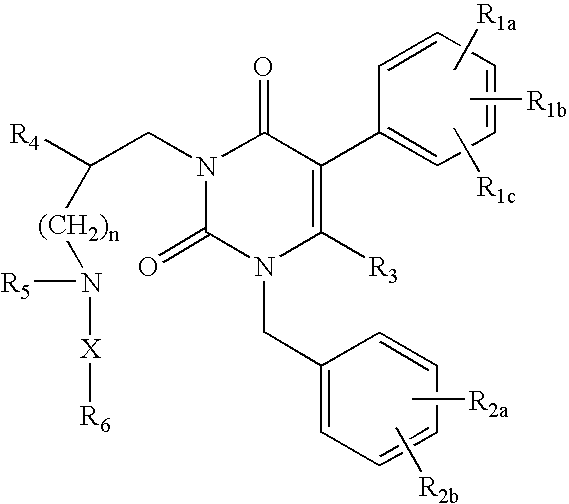
GnRH receptor antagonists are disclosed that have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein n, R1a, R1b, R1c, R2a, R2b, R3, R4, R5, R6 and X are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Gonadotrophins for folliculogenesis
InactiveUS20050085412A1High Z-numberSaccharide peptide ingredientsDepsipeptidesSialic acid aldolaseFSH preparation
The invention provides an FSH preparation having a high degree of sialylation, and showing increased efficacy.
Owner:MERCK SERONO SA
Compositions comprising a SARM ad GnRH agonist or a GnRH antagonist, and methods of use thereof
InactiveUS20060287282A1Reduce morbidityDecreased sexual libidoBiocidePhosphorous compound active ingredientsSelective androgen receptor modulatorAndrogen Receptor Gene
The present invention relates to compositions comprising a selective androgen receptor modulators (SARM) and a gonadotropin releasing hormone (GnRH) agonist or a GnRH antagonist, and their use, inter-alia for treating hormone-associated conditions in males and females, which arise as a result of androgen decline, suppression or abrogation, or in treating, suppressing, inhibiting or preventing prostate cancer.
Owner:GTX INCORPORATED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/7d0575ed-1c35-439a-aa29-5f182c626cec/US20060189616A1-20060824-C00001.png)
![7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/7d0575ed-1c35-439a-aa29-5f182c626cec/US20060189616A1-20060824-C00002.png)
![7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 7-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/7d0575ed-1c35-439a-aa29-5f182c626cec/US20060189616A1-20060824-C00003.png)
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00001.png)
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00002.png)
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00003.png)