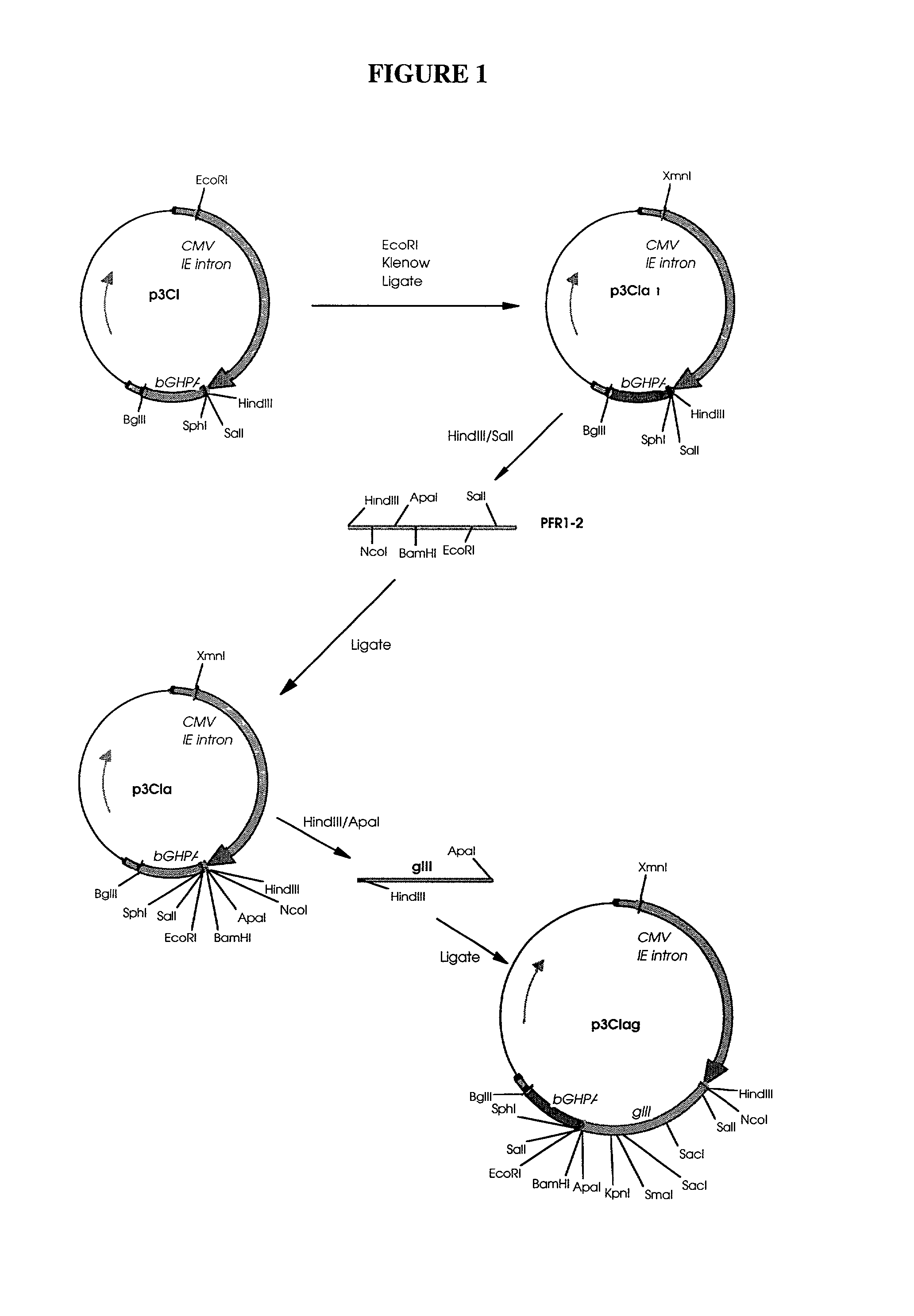
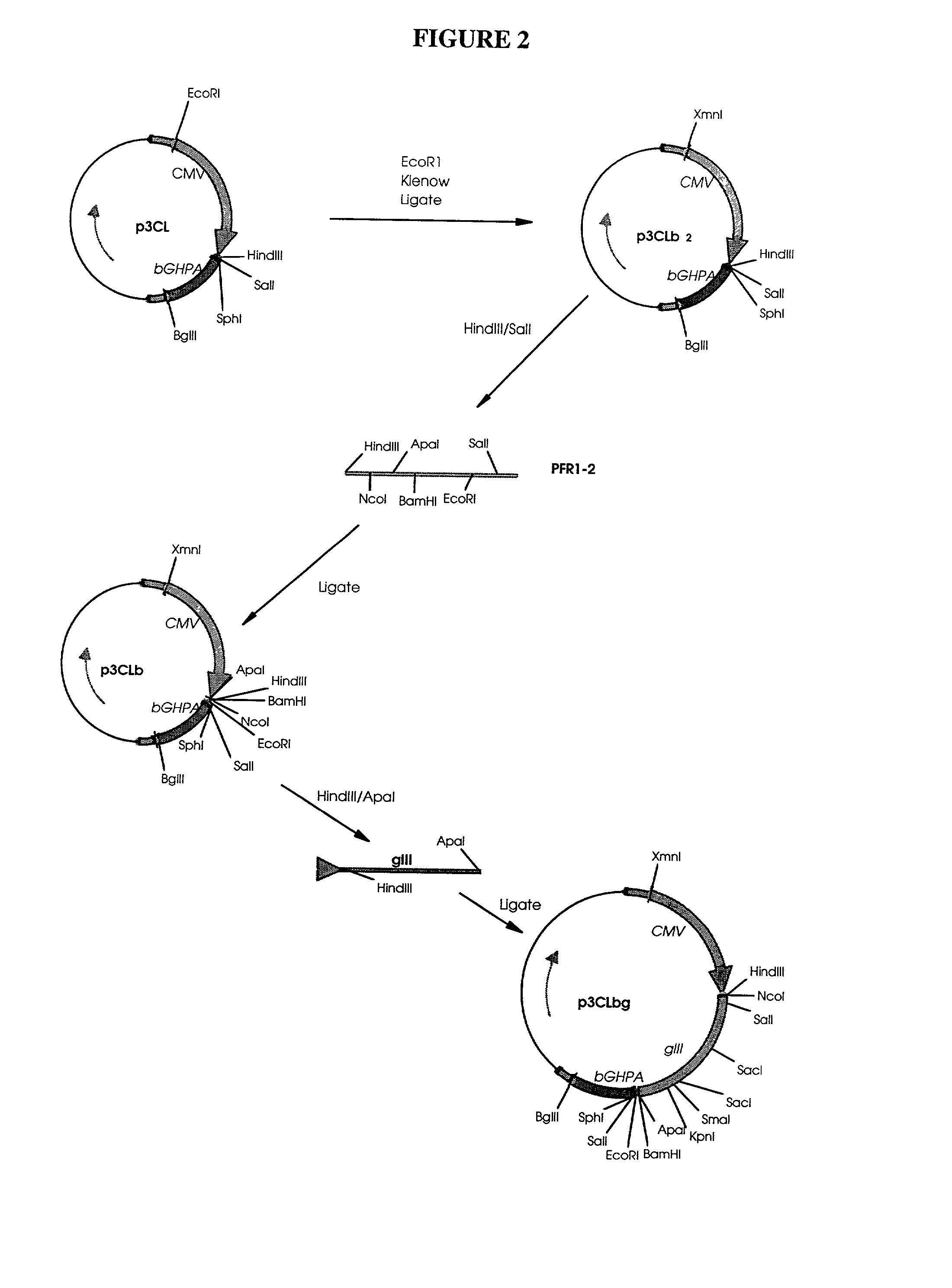
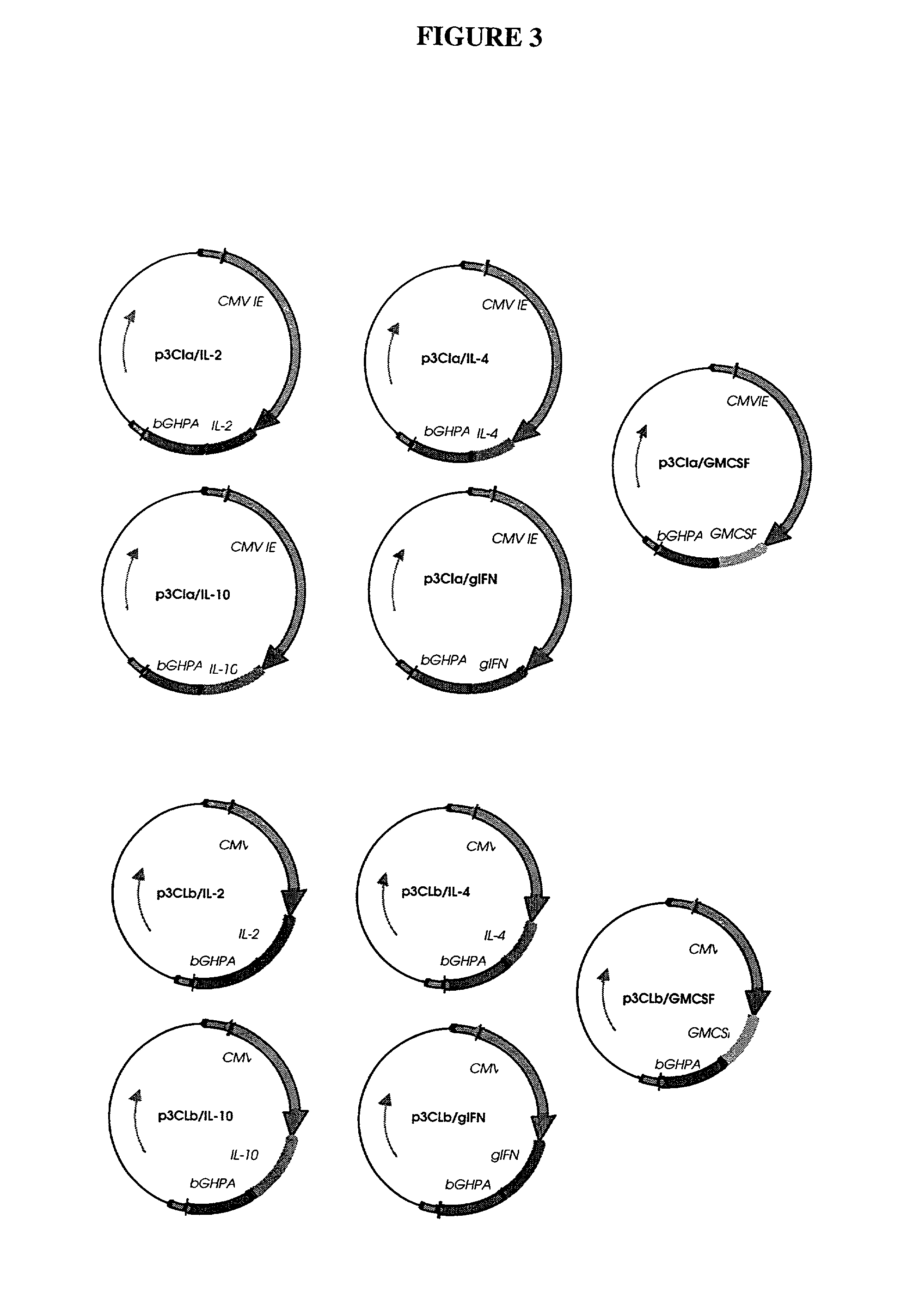
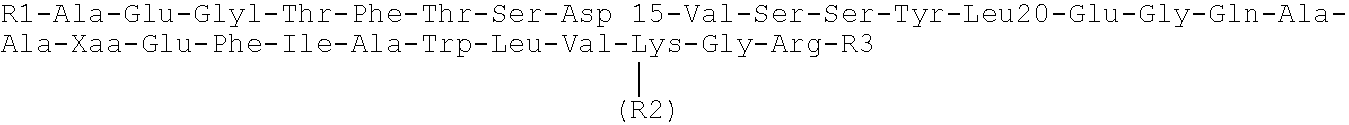Patents
Literature
68 results about "Peptide hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptide hormones or protein hormones are hormones whose molecules are peptides or proteins, respectively. The latter have longer amino acid chain lengths than the former. These hormones have an effect on the endocrine system of animals, including humans. Most hormones can be classified as either amino acid–based hormones (amine, peptide, or protein) or steroid hormones. The former are water-soluble and act on the surface of target cells via second messengers; the latter, being lipid-soluble, move through the plasma membranes of target cells (both cytoplasmic and nuclear) to act within their nuclei.
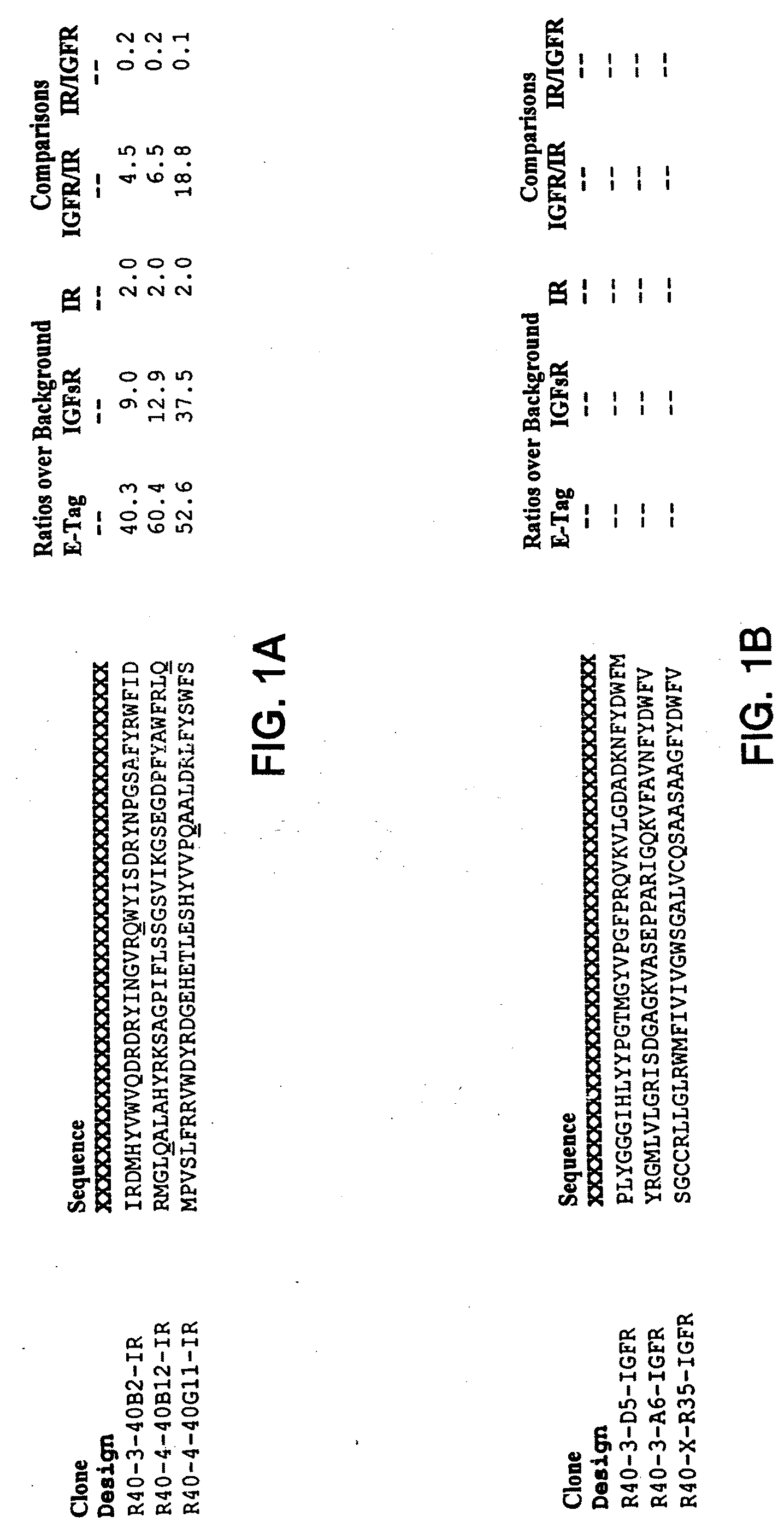
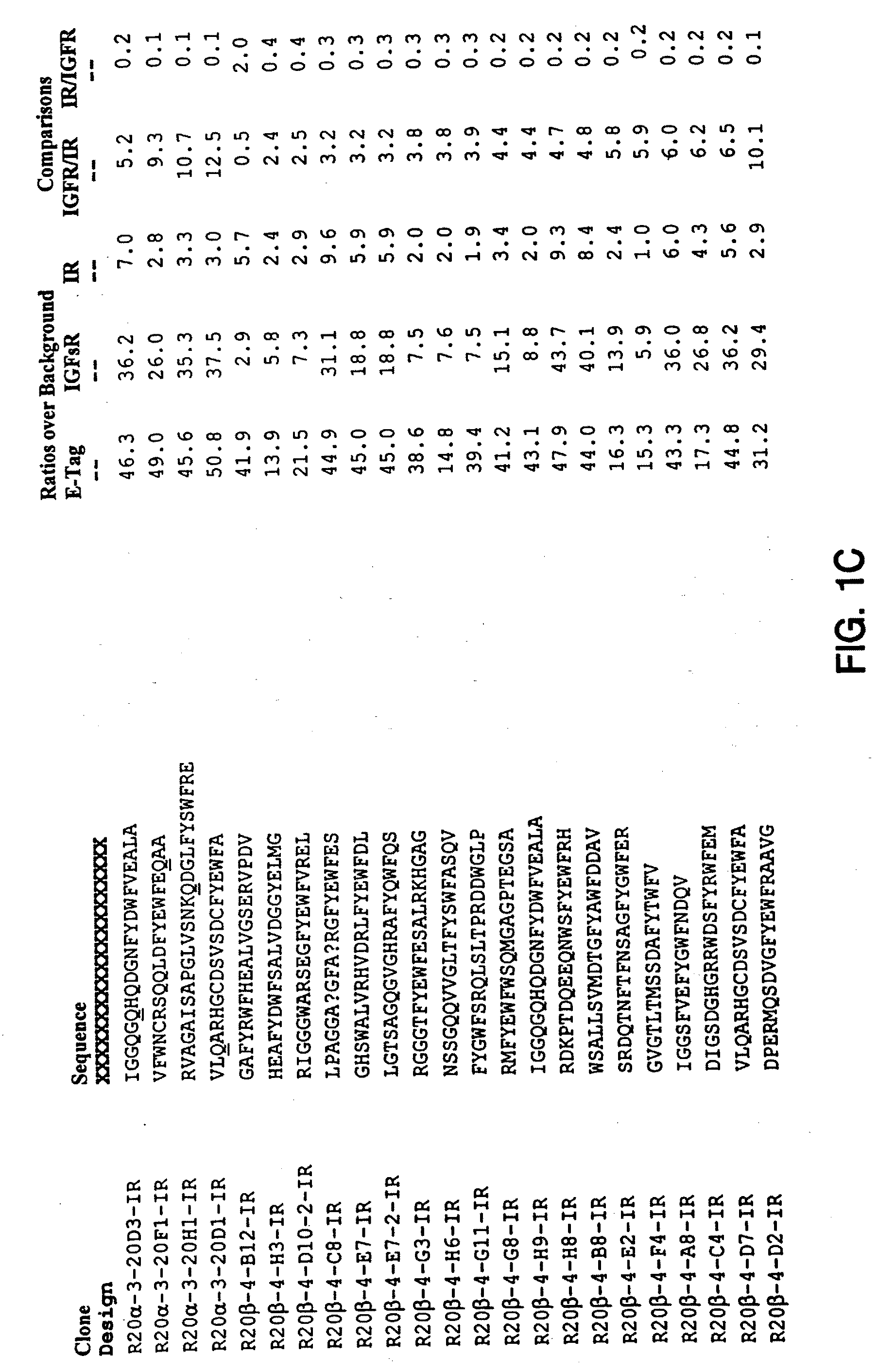
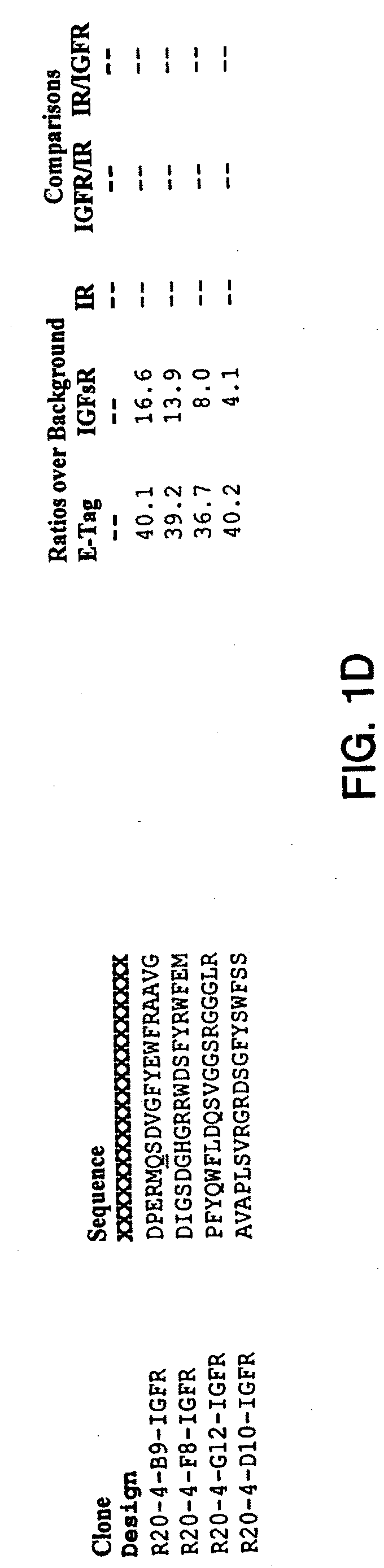
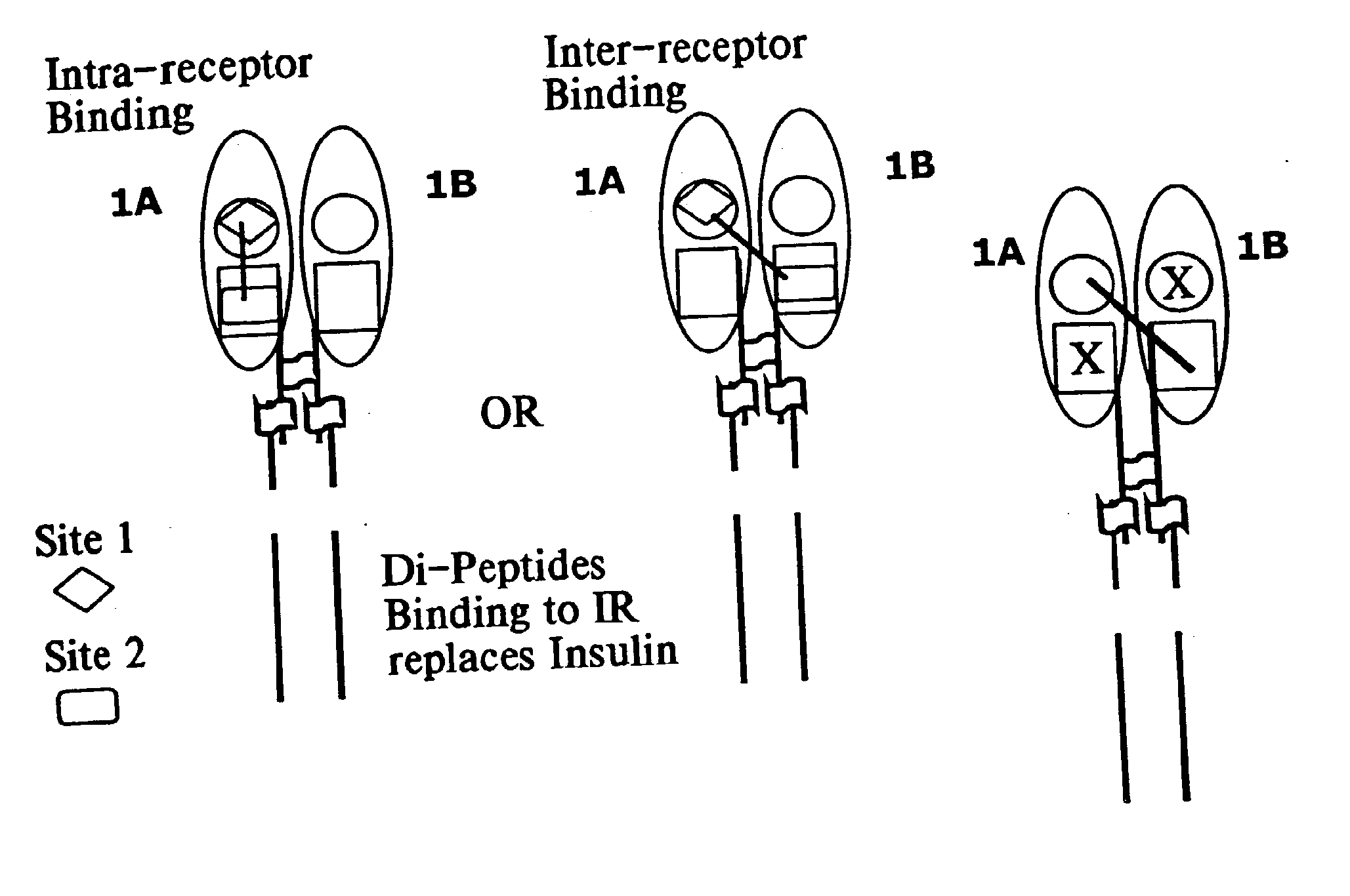
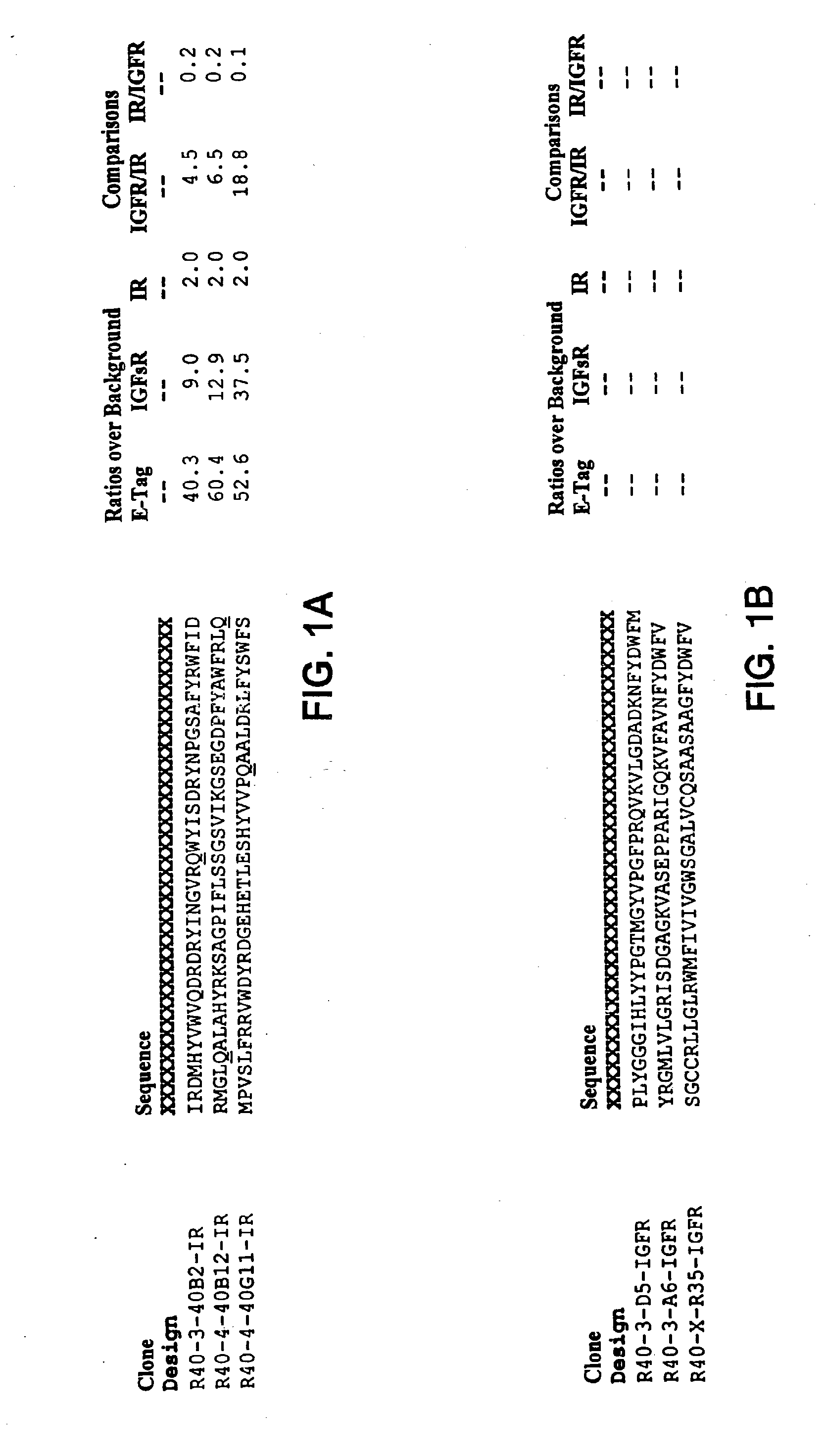
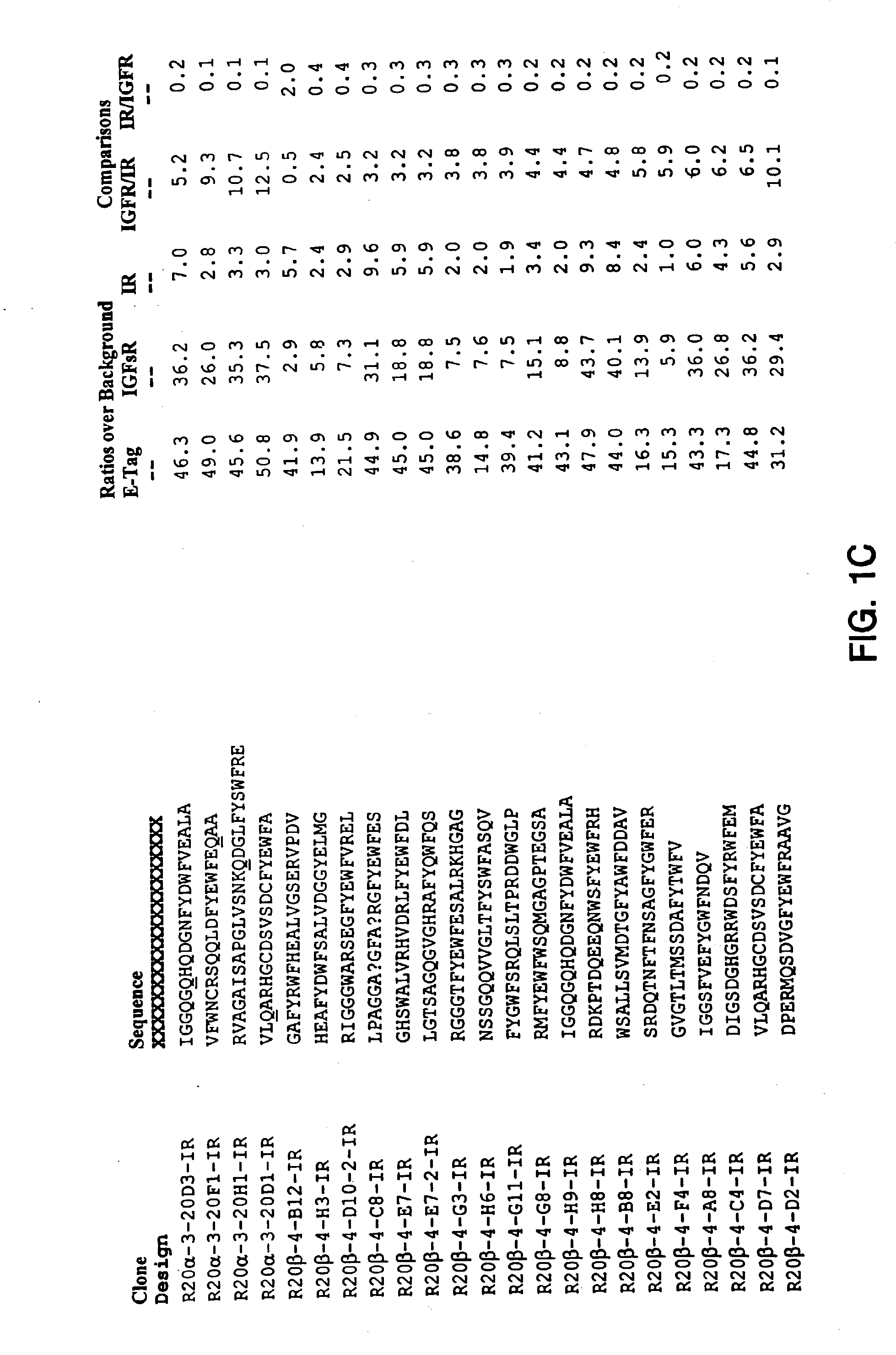
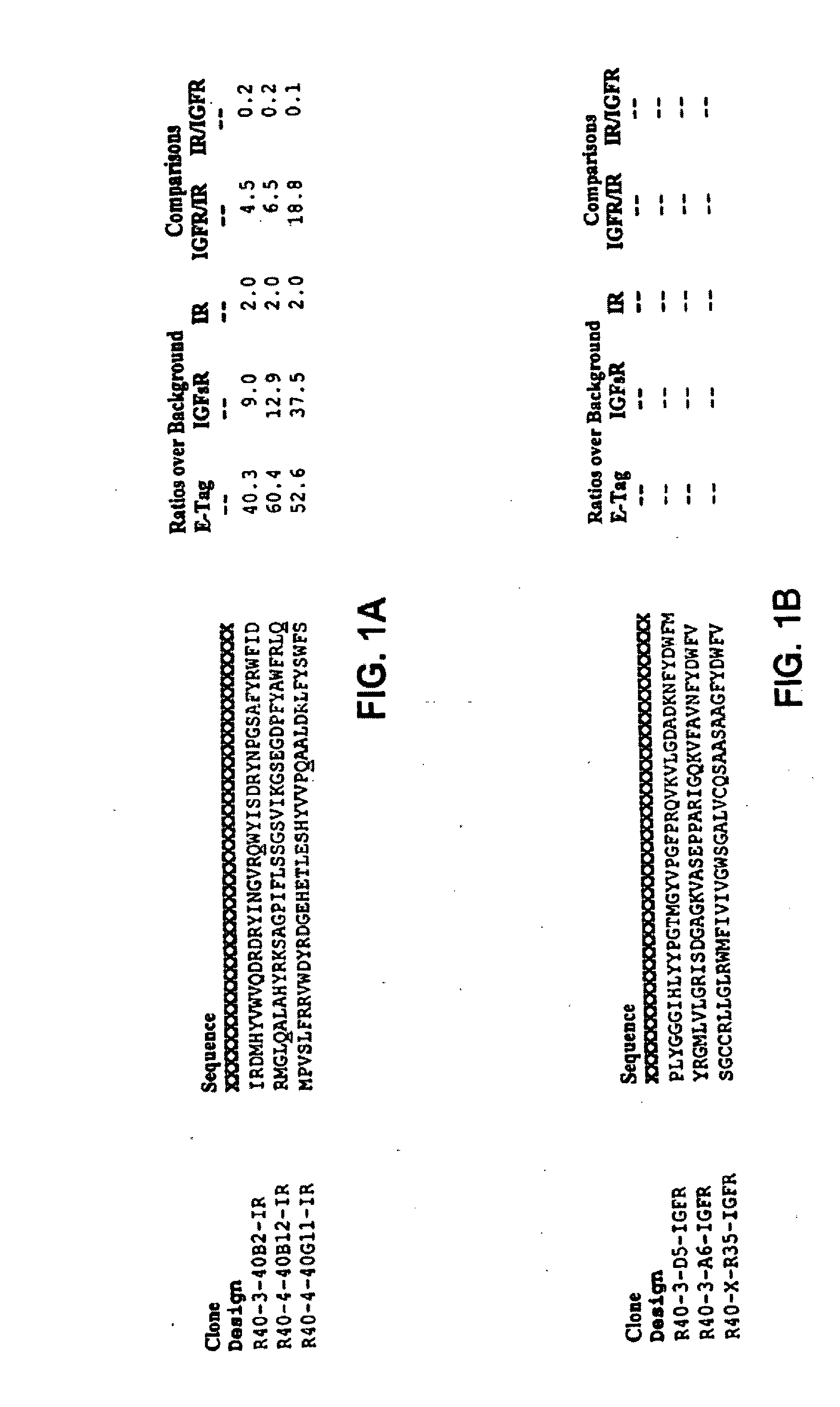
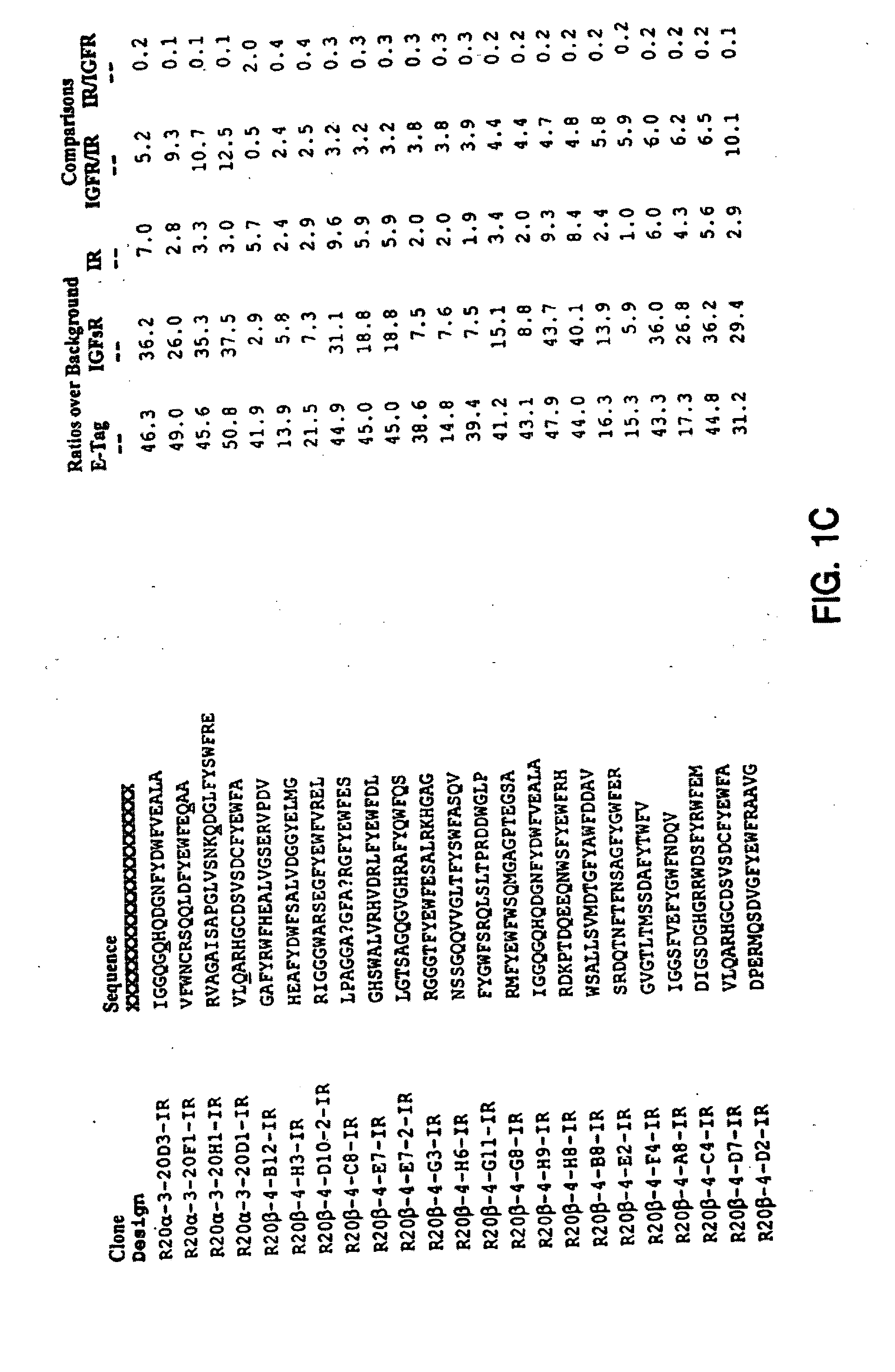
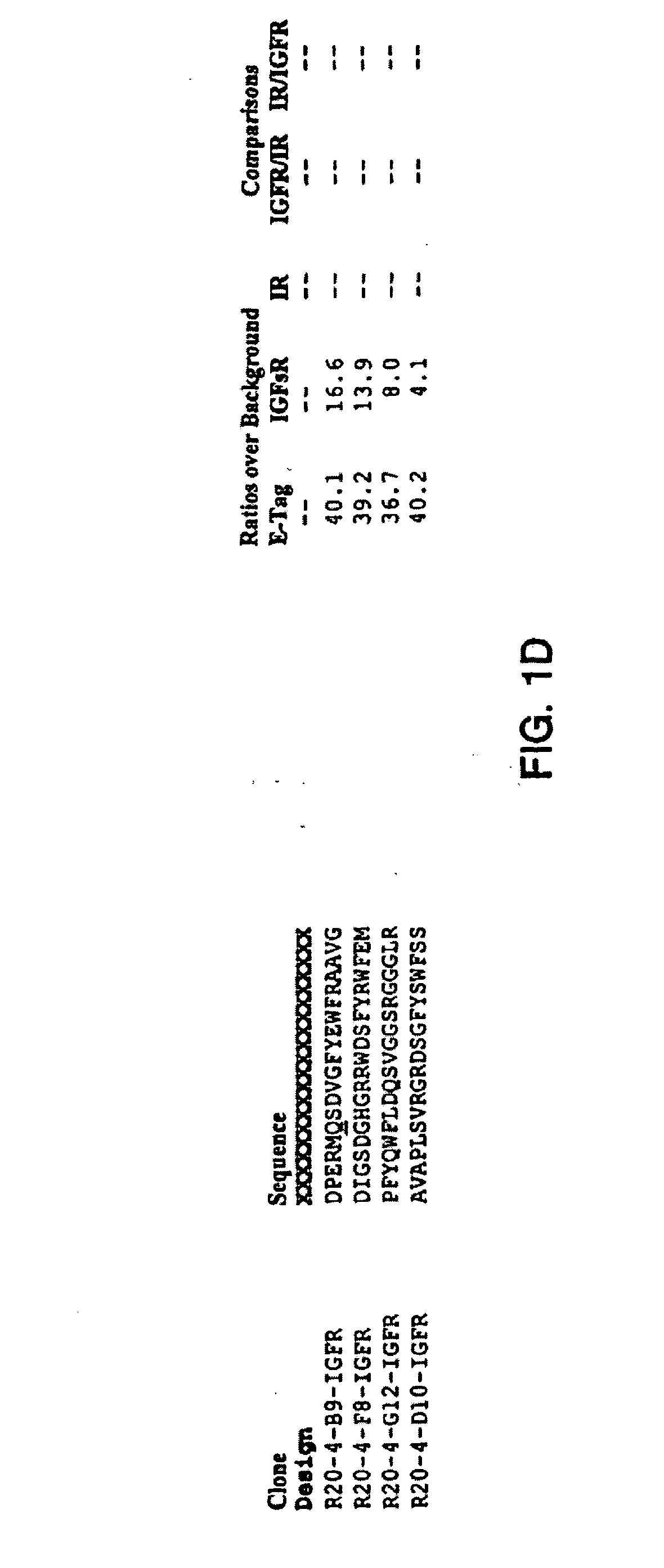
Insulin and IGF-1 receptor agonists and antagonists
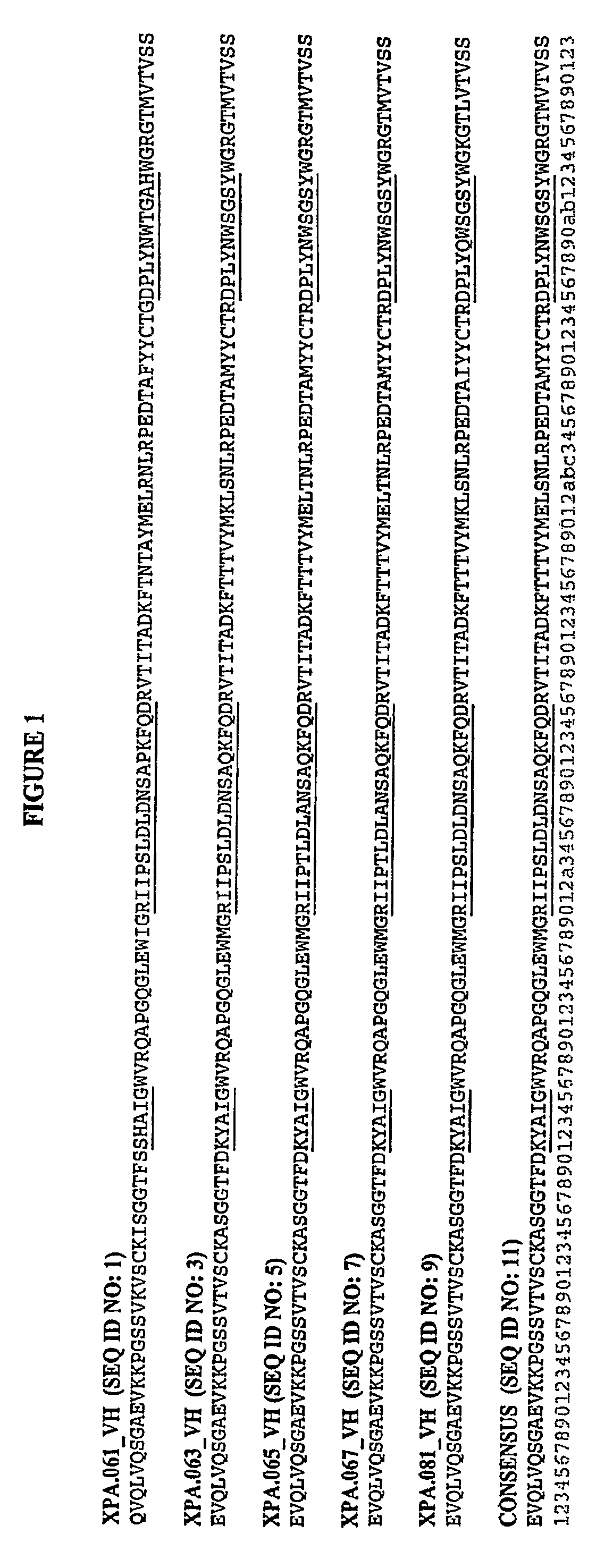
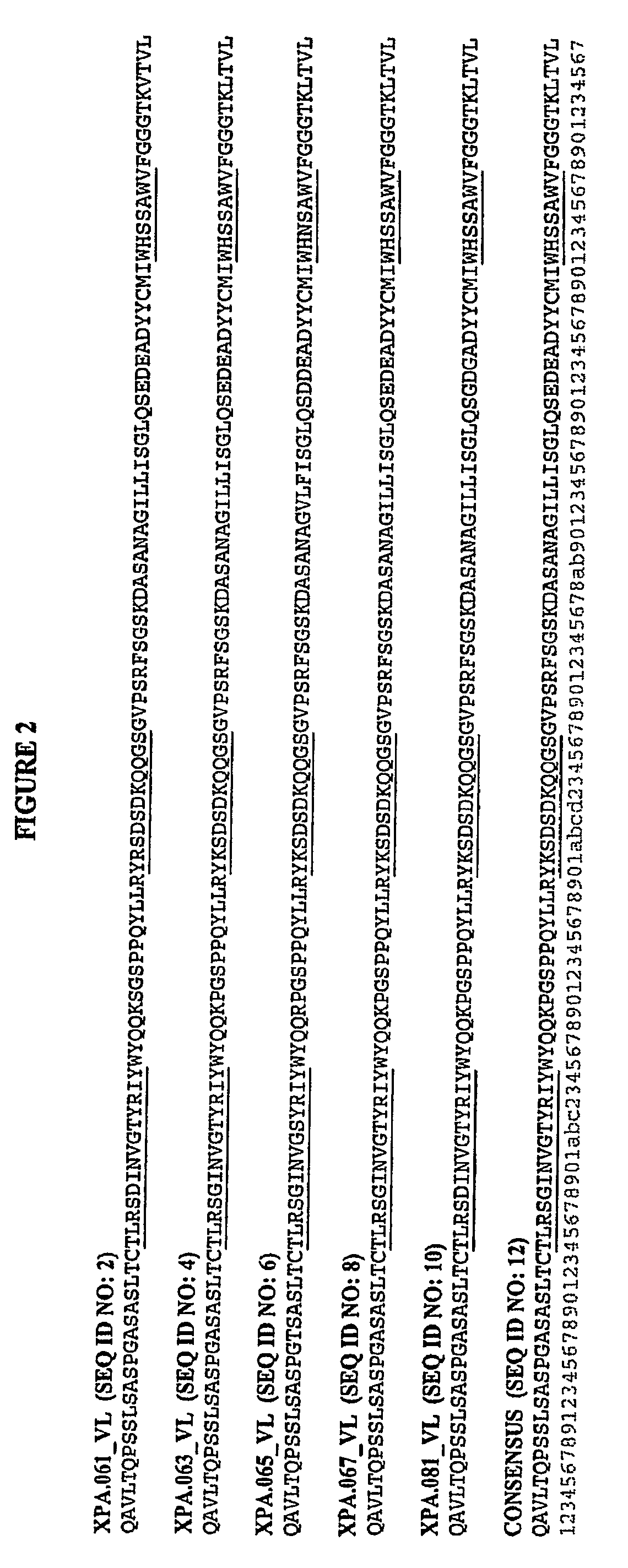
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonist peptides may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Isulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Insulin and IGF-1 receptor agonists and antagonists
InactiveUS6875741B2Peptide/protein ingredientsGenetic material ingredientsBinding sitePancreatic hormone
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
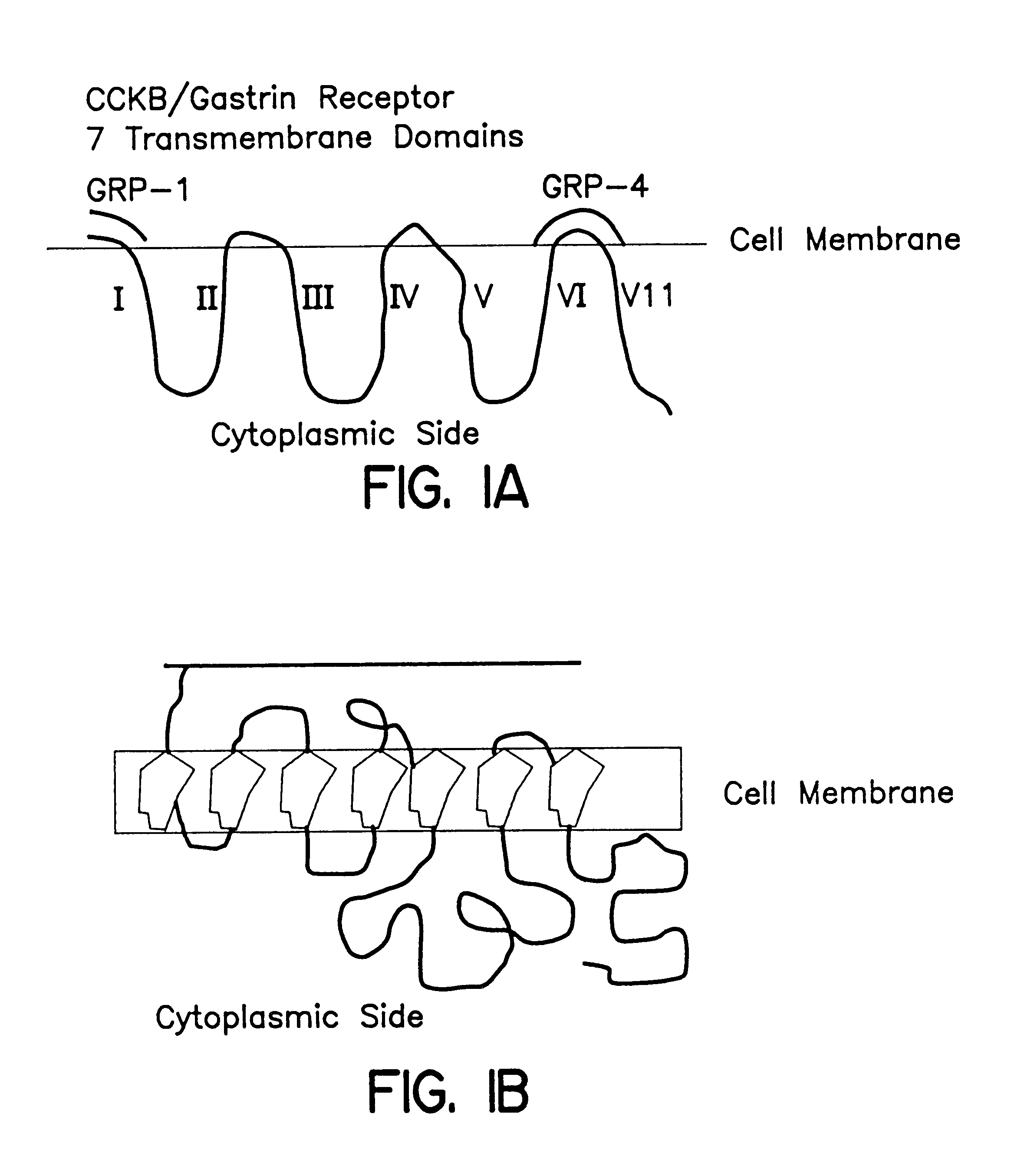
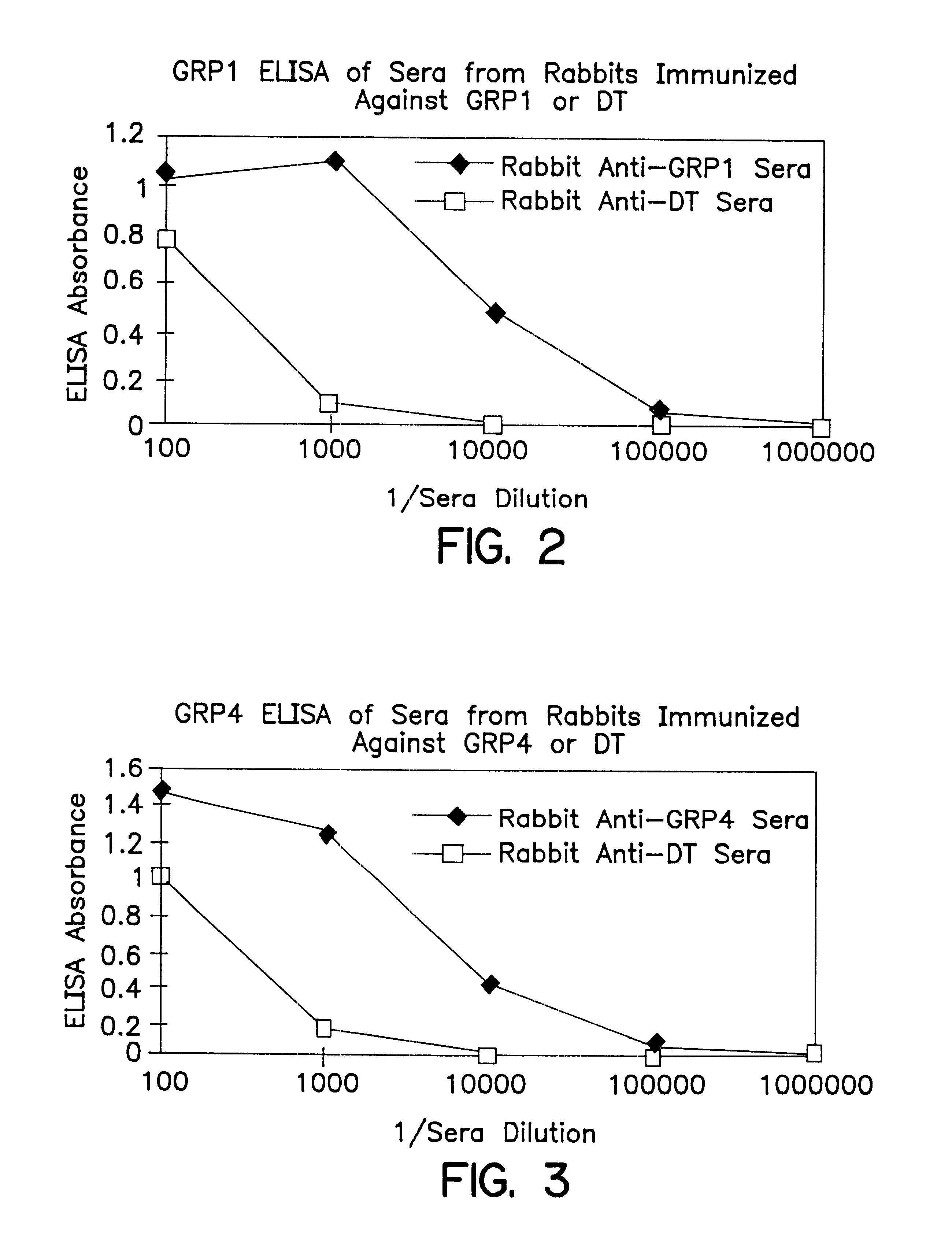
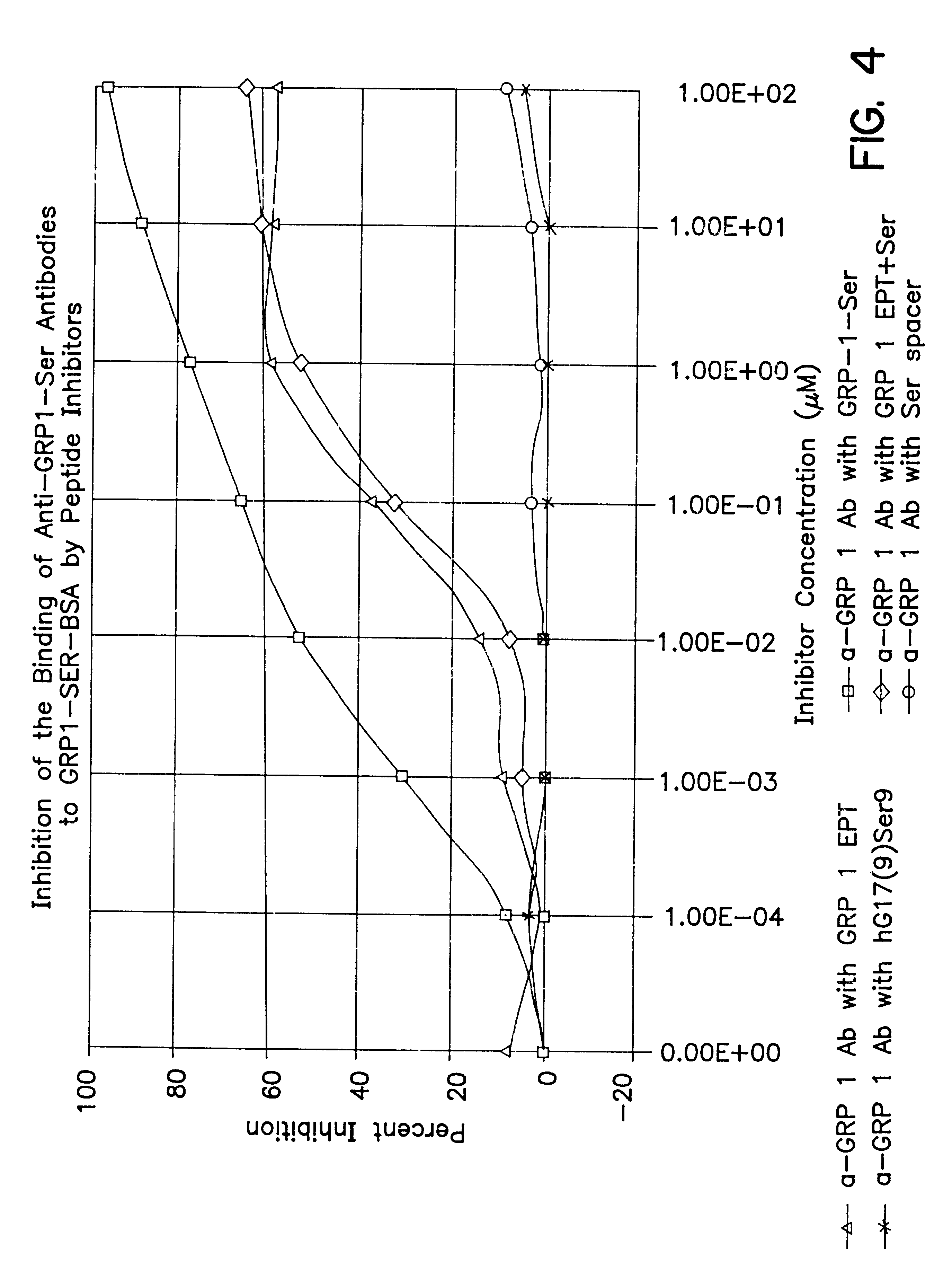
Immunogenic compositions to the CCK-B/gastrin receptor and methods for the treatment of tumors
InactiveUS6548066B1Inhibiting autocrine growth-stimulatory pathwayEffectively prevent the binding of the peptide hormones to the receptorsPeptide/protein ingredientsReceptors for hormonesTissue biopsyPassive Immunizations
The invention concerns immunogens, immunogenic compositions and method for the treatment of gastrin-dependent tumors. The immunogens comprise a peptide from the CCK-B / gastrin-receptor conjugated to a spacer and to an immunogenic carrier. The immunogens are capable of inducing antibodies in vivo which bind to the CCK-B / gastrin-receptor in tumor cells, thereby preventing growth stimulating peptide hormones from binding to the receptors, and inhibiting tumor cell growth. The immunogens also comprise antibodies against the CCK-B / gastrin-receptor for passive immunization. The invention also concerns diagnostic methods for detecting gastrin-dependent tumors in vivo or from a tissue biopsy using the antibodies of the invention.
Owner:CANCER ADVANCES INC
Ligand/lytic peptide compositions and methods of use
InactiveUS6635740B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideAbnormal tissue growth
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Medical prosthetic devices and implants having improved biocompatibility
Disclosed are medical prosthetic devices or medical implants which exhibit improved biocompatitibly. The devices or implants include a metal material, e.g. titanium, in which the metal surface parts are coated with a corresponding hydride material that contains one or more biomolecule substance. This biomolecule substance may contain one or more biologically active molecules, e.g. bio-adhesives, biopolymers, blood proteins, enzymes, extra cellular matrix proteins, extra cellular matrix biomolecules, growth factors and hormones, peptide hormones, deoxyribonucleic acids, ribonucleic acids, receptors, enzyme inhibitors, drugs, biologically active anions and cations, vitamins, adenosine monophosphate (AMP), adenosine diphosphate (ADP), adenosine triphosphate (ATP), marker biomuolecules, amino acids, fatty acids, nucleotides (RNA and DNA bases), or sugars.
Owner:STRAUMANN HLDG AG
Insulin and IGF-1 Receptor Agonists and Antagonists
Owner:NOVO NORDISK AS +1
Methods for improved targeting of antibody, antibody fragments, hormones and other targeting agents, and conjugates thereof
InactiveUSRE38008E1Improve localizationReduce productionIn-vivo radioactive preparationsPeptide/protein ingredientsAntibody fragmentsEphA Receptors
Methods for improved targeting of antibody, antibody fragments, peptides hormones, steroid hormones and conjugates thereof are disclosed. Enhanced delivery to target cells of antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, specific for a population of cells of a mammal comprises steps of administering to said mammal an adequate dosage of blocking antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, and administering to said mammal an effective dosage of said antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, specific for said population of cells. In the preferred embodiment, the specific antibodies are monoclonal antibodies directed toward tumor-associated antigen in man.
Owner:IDEC PHARM CORP
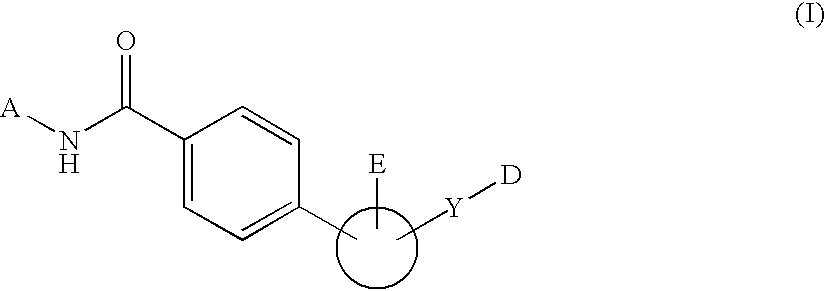
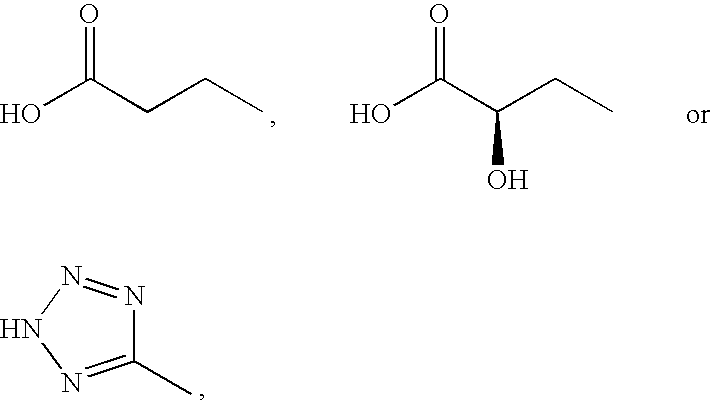
Peptide derivatives
A pharmacologically active peptide hormone derivative in which the parent peptide hormone has been modified by introducing either a lipophilic substituent, W, in the N-terminal amino acid or a lipophilic substituent, Z, in the C-terminal amino acid of the parent peptide hormone or an analogue thereof, said lipophilic substituent having from 8 to 40 carbon atoms, has a protracted profile of action.
Owner:NOVO NORDISK AS
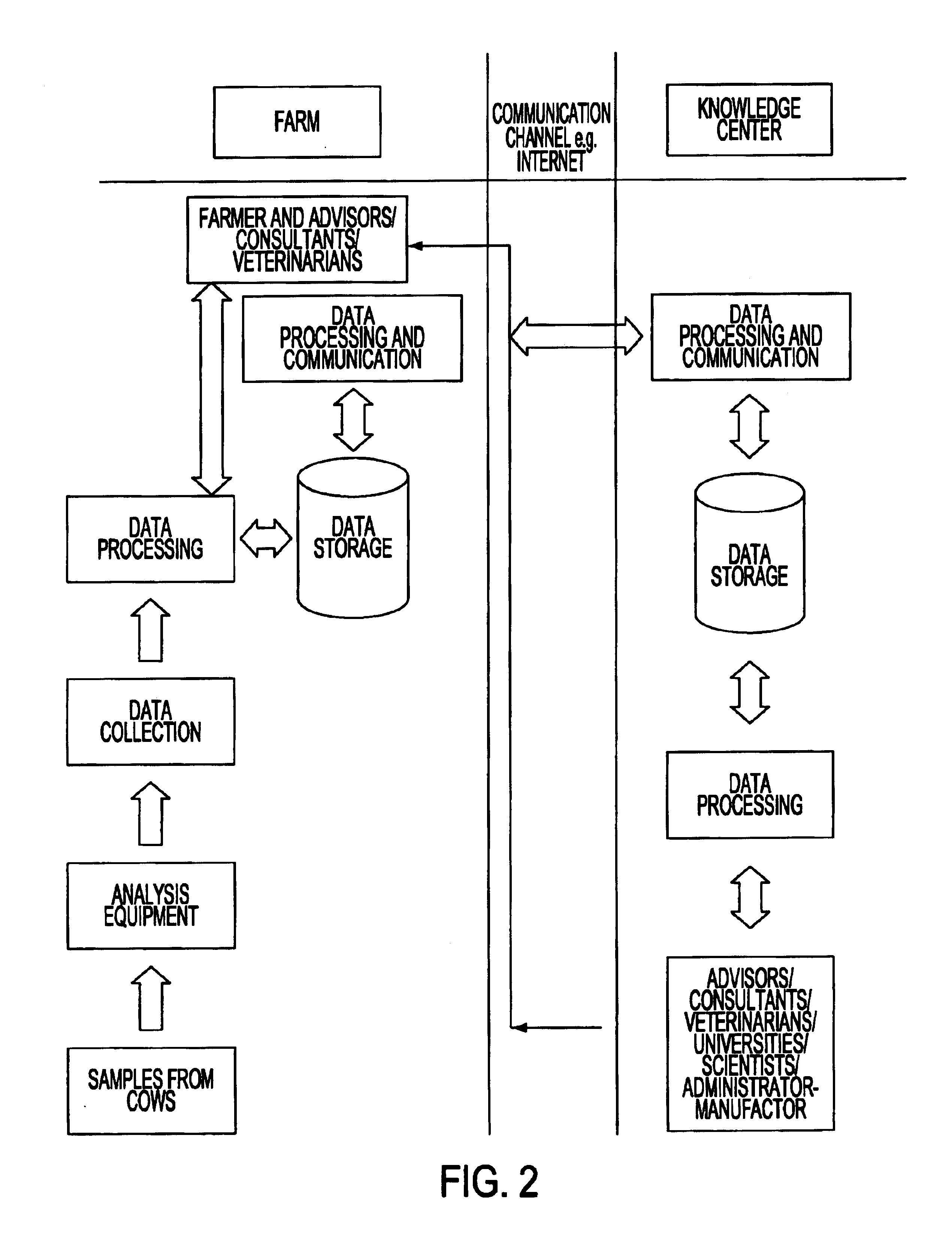
System for optimizing the production performance of a milk producing animal herd
InactiveUS6814025B2Increase productivityIncrease profitabilitySamplingCathetersLactate dehydrogenaseAgricultural science
Owner:LATTEC
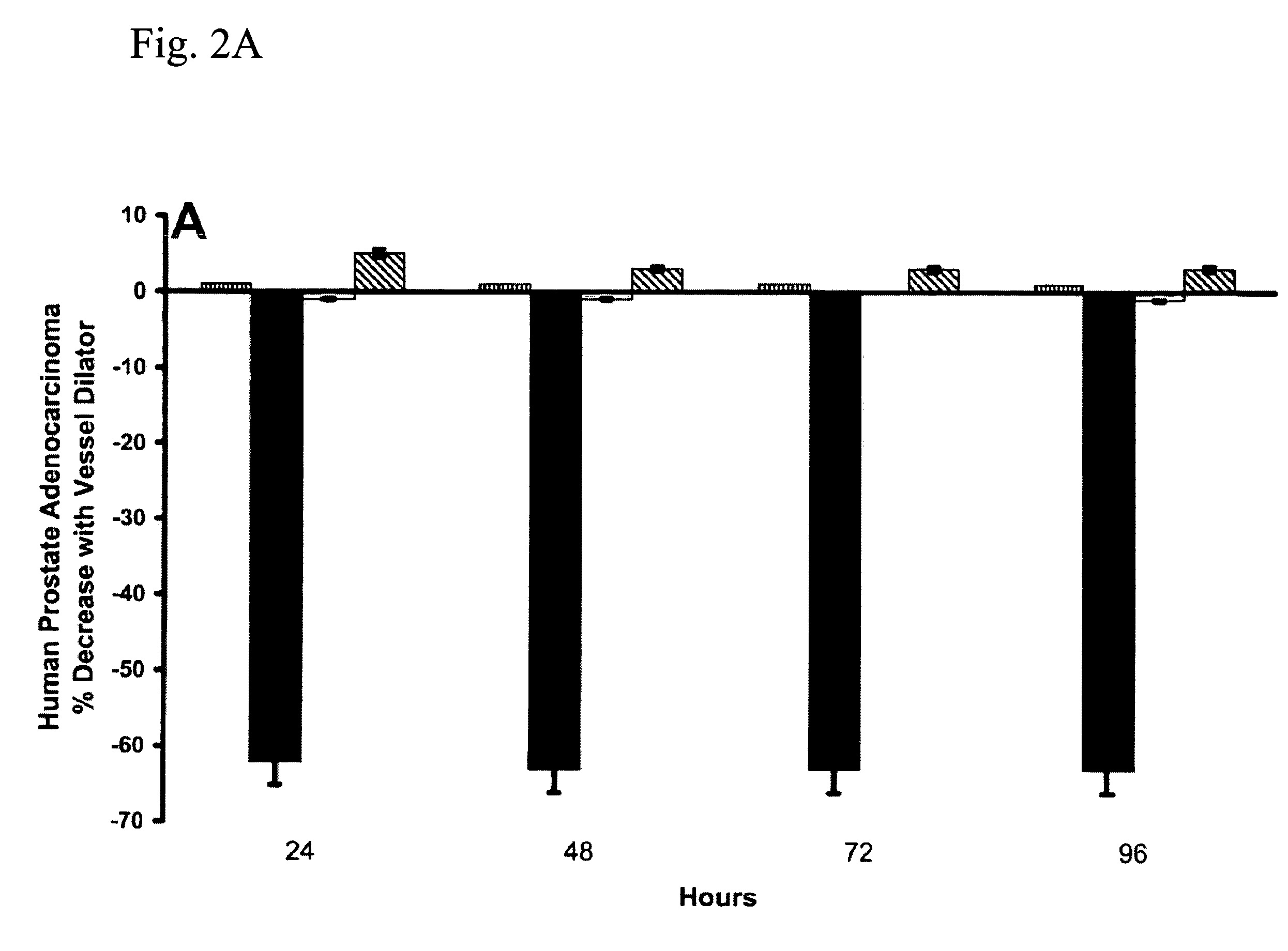
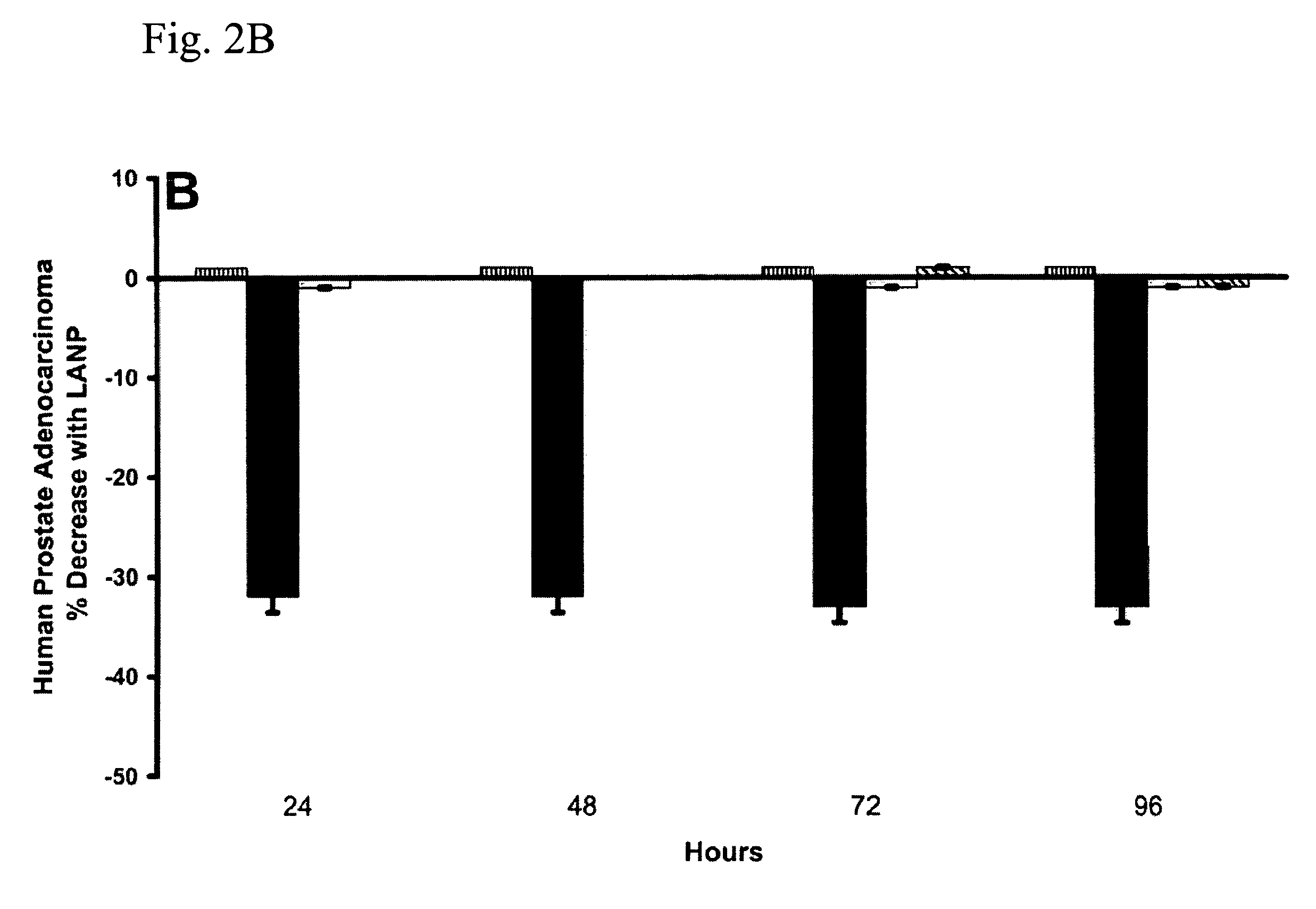
Cancer Treatment Using C-Type Natriuretic Peptides
InactiveUS20050209139A1Reduce in quantityComplete remission ratePeptide/protein ingredientsDepsipeptidesC-type natriuretic peptideCancer type
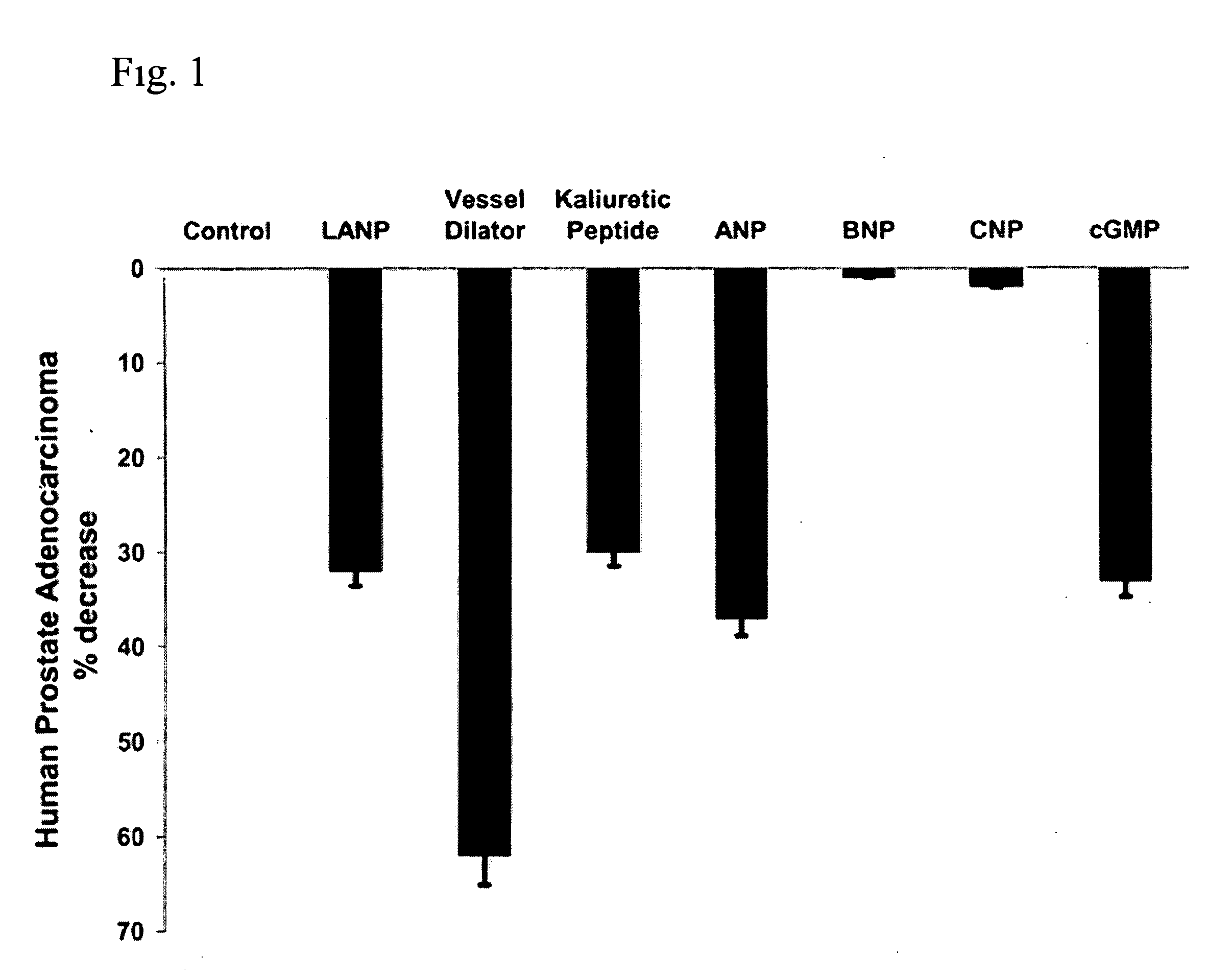
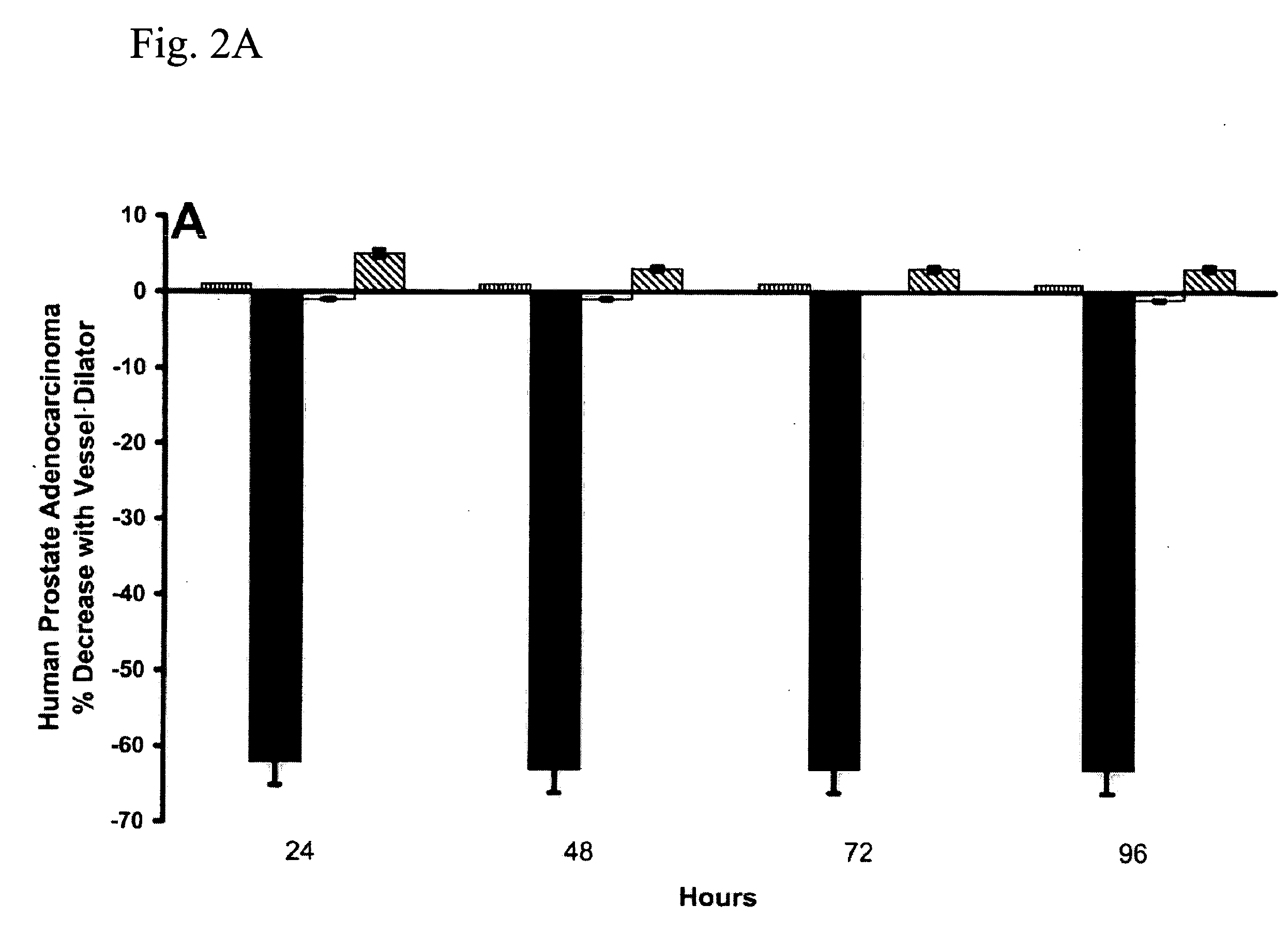
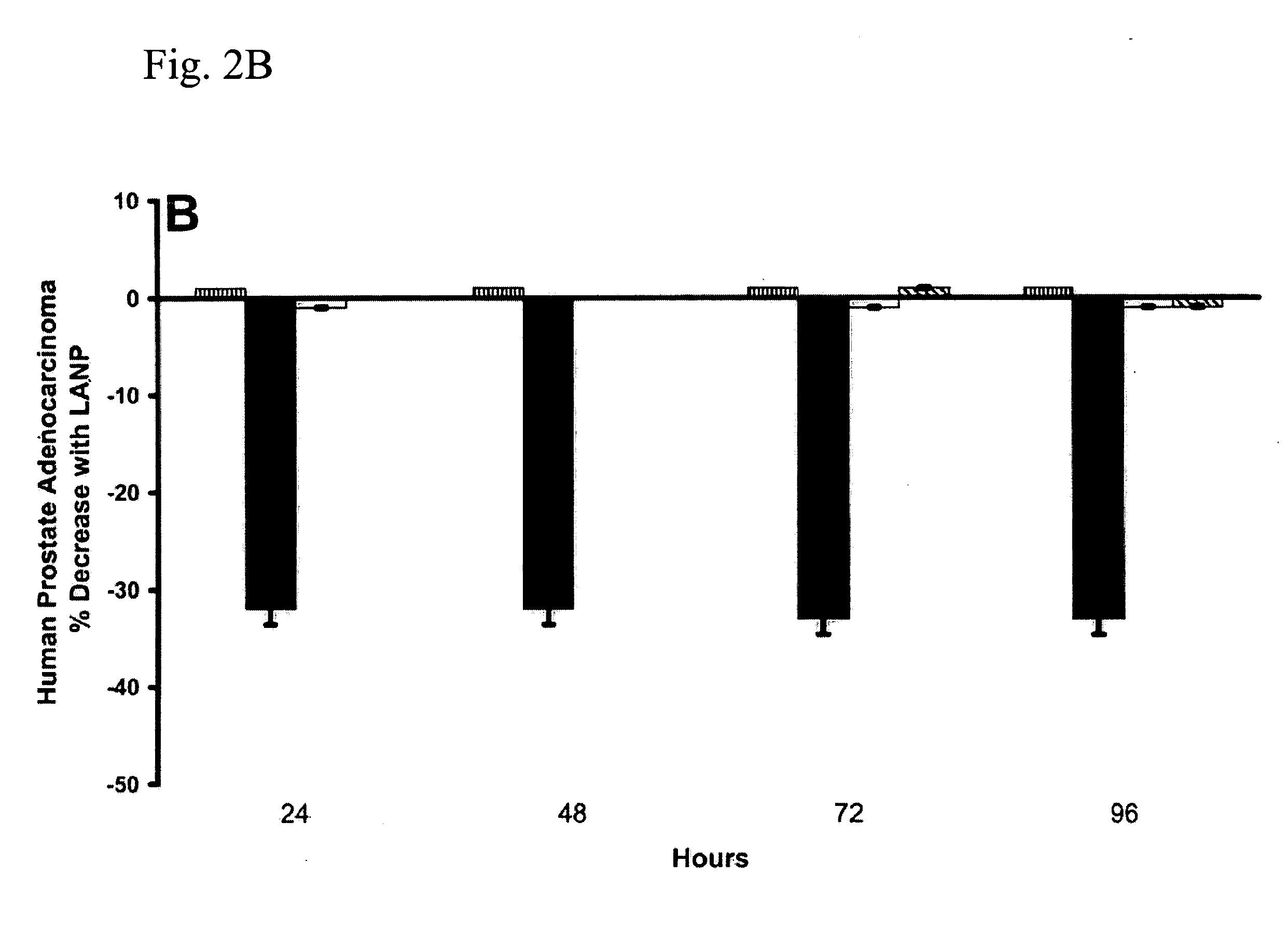
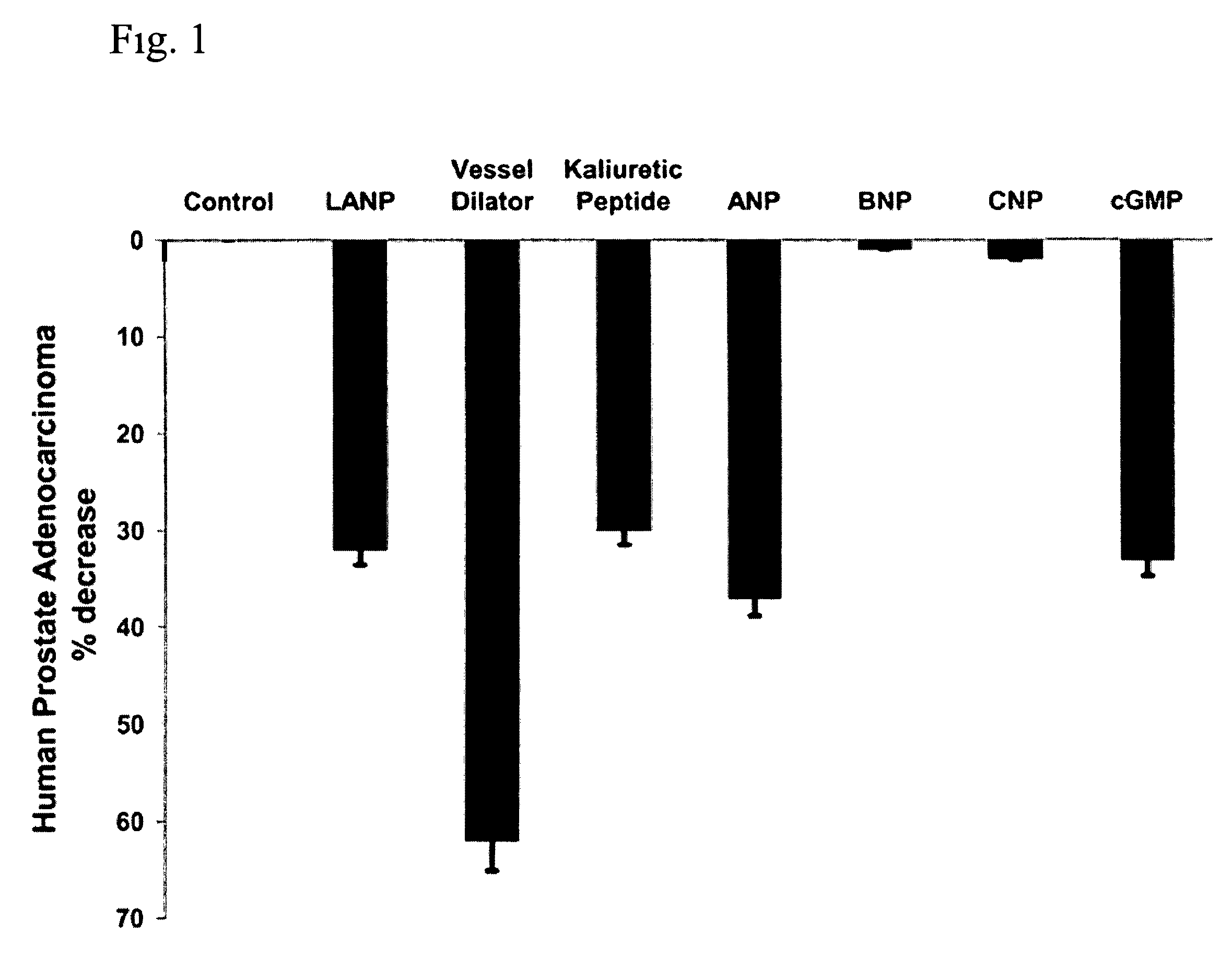
The present invention includes a method of utilizing four peptide hormones to inhibit the growth of cancer(s). A dramatic decrease in the number of human pancreatic adenocarcinoma cells (i.e., the type of cancer with the highest mortality, with patients only surviving four months) was observed responsive to treatment. The application of the invention would be to utilize one or more of these peptide hormones alone and / or in combination to treat cancer. The ability of these peptide hormones to decrease the number of adenocarcinoma cells has implications for adenocarcinomas at other sites in the body with the majority of cancers of the breast, colon and prostate also being adenocarcinomas. Adenocarcinomas also occur in the lung and other tissues. Treatment of a wide variety of cancers in addition to adenocarcinomas is anticipated by the present invention.
Owner:UNIV OF SOUTH FLORIDA
Ligand/lytic peptide compositions and methods of use
InactiveUS20040018967A1Inhibition of maturationLysis of tumor cells is rapidAntibacterial agentsOrganic active ingredientsLytic peptideAutoimmune condition
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Cancer treatment using C-type natriuretic peptides
InactiveUS7488713B2Reduce in quantityComplete remission ratePeptide/protein ingredientsDepsipeptidesC-type natriuretic peptideProstate cancer
The present invention includes a method of utilizing four peptide hormones to inhibit the growth of cancer(s). A dramatic decrease in the number of human pancreatic adenocarcinoma cells (i.e., the type of cancer with the highest mortality, with patients only surviving four months) was observed responsive to treatment. The application of the invention would be to utilize one or more of these peptide hormones alone and / or in combination to treat cancer. The ability of these peptide hormones to decrease the number of adenocarcinoma cells has implications for adenocarcinomas at other sites in the body with the majority of cancers of the breast, colon and prostate also being adenocarcinomas. Adenocarcinomas also occur in the lung and other tissues. Treatment of a wide variety of cancers in addition to adenocarcinomas is anticipated by the present invention.
Owner:UNIV OF SOUTH FLORIDA
Introduction of naked DNA or RNA encoding non-human vertebrate peptide hormones or cytokines into a non-human vertebrate
The present invention relates to the introduction of naked DNA or RNA molecules encoding non-human vertebrate peptide hormones or cytokines into a non-human vertebrate to achieve delivery of the non-human vertebrate peptide hormone or cytokine. The invention thus provides an alternative to directly administering the polypeptide of interest.
Owner:MARTIN STEPHEN +1
Hydrolyzation of pig blood with complex enzyme method for producing blood peptide native
The production of serum peptide hormone by the hydrolysis of pig blood with a compound enzyme method belongs to the biochemical technology field. The invention relates to the expansion of the space structure of globulin and the breaking and hydrolysis of a molecular chain, the main processes are as follows: red blood cells are extracted from fresh blood in a centrifugal way and then swelled and mixed with plasma; later pH2-3 is adjusted, and 95% of ethanol and 2.8% of H2O2 with equivalent weight are added; next, sodium hydroxide is added with the heat preserved for 2h at 80 DEG C; pH8 is adjusted, and papain, trypsin, alkaline protease and the like are added with 4h of enzymolysis at 37 DEG C and then pH3.0 is adjusted; after pepsin is added with 4h of enzymolysis at 37 DEG C, a serum peptide hormone product is obtained by enzyme destroying and drying. The technology comprises the expansion of internal gene of the globulin, the oxidation of heme, the hydrolysis of compound enzyme and the like, has over 90% of hydrolysis rate, and solves the problems of difficult hydrolysis of blood globulin and complex process in the conventional process; the serum peptide hormone product which is produced by the invention has over 60% of peptide content, simultaneously preserves the AgI immune function factors and the like in blood, enhances the organism immunity function, and is suitable for blood comprehensively processing such as slaughterhouses, etc.
Owner:SICHUAN ACAD OF FOOD & FERMENTATION INDS +1
Medical prosthetic devices and implants having improved biocompatibility
InactiveUS20060155384A1Electrolytic inorganic material coatingBone implantZirconium hydrideEnzyme Inhibitor Drugs
A medical prosthetic device or medical implant containing a metal material (A) selected from the group consisting of titanium or an alloy thereof, zirconium or an alloy thereof, tantalum or an alloy thereof, hafnium or an alloy thereof, niobium or an alloy thereof and a chromium-vanadium alloy, wherein surface parts of the metal material (A) are coated with a layer of a corresponding hydride material (B) selected from titanium hydride, zirconium hydride, tantalum hydride, hafnium hydride, niobium hydride and chromium and / or vanadium hydride, respectively, said device or implant being characterised in that the layer of hydride material (B) comprises one or more biomolecule substances (C) associated therewith. The device or implant exhibits improved biocompatibility. The metal material (A) is preferably titanium. The biomolecule substance (C) may be selected from the following types of substances: Natural or recombinant bio-adhesives; natural or recombinant cell attachment factors; natural, recombinant or synthetic biopolymers; natural or recombinant blood proteins; natural or recombinant enzymes; natural or recombinant extracellular matrix proteins; natural or synthetic extracellular matrix biomolecules; natural or recombinant growth factors and hormones; natural, recombinant or synthetic peptide hormones; natural, recombinant or synthetic deoxyribonucleic acids; natural, recombinant or synthetic ribonucleic acids; natural or recombinant receptors; enzyme inhibitors; drugs; biologically active anions and cations; vitamins; adenosine monophosphate (AMP), adenosine diphosphate (ADP) or adenosine triphosphate (A TP); marker biomolecules; amino acids; fatty acids; nucleotides (RNA and DNA bases); and sugars.
Owner:NUMERICAL TECH INC
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
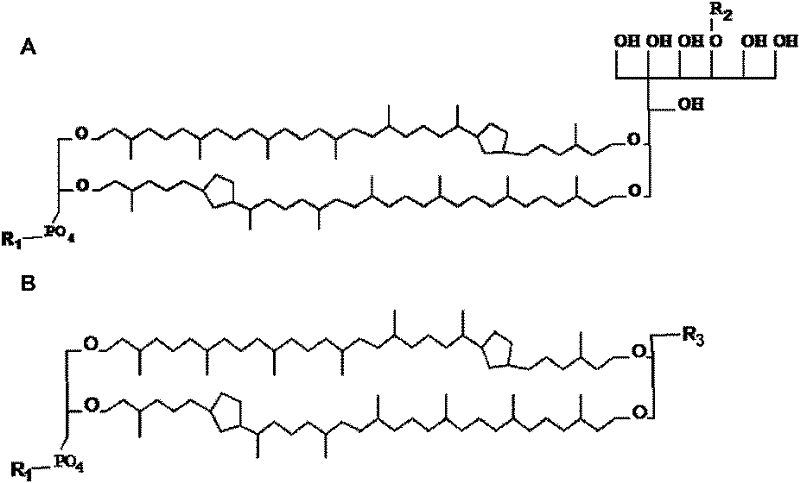
Protein or nucleic acid drug liposome preparation and preparation method thereof based on tetraether lipid
InactiveCN101744768AProtection stabilityImprove bioavailabilityPeptide/protein ingredientsGenetic material ingredientsLiposome membraneVaccine antigen
Disclosed are a protein or nucleic acid drug liposome preparation and a preparation method thereof based on tetraether lipid in large biological molecular drug technical field; the liposome membrane comprises the monomolecular layer of the tetraether lipid; wherein, the tetraether lipid accounts 50-100% of the total lipid mass, the protein drug or nucleic acid drug are dissolved in the water solution phase of the liposome membrane interior. The invention can be used as the oral carrier for polypeptide, protein, oligonucleotide and genetic drugs, guarantees the stability of the drugs in the gastrointestinal tract and improves the bioavailability and efficacy, wherein the polypeptide and protein drugs include insulin, human leukocyte colony growth factor, erythropoietin, human growth factor, calcitonin, exenatide, intestinal peptide hormone, thymosin, etc.; the vaccine antigen includes the antigens of various hepatitis virus, aids virus, HPV virus and herpes virus; the nucleic drugs include the plasmids of various genes, oligonucleotide molecules with various gene sequences and chemical modification structures thereof.
Owner:SHANGHAI JIAO TONG UNIV
Treatment and prevention of cancerous and pre-cancerous conditions of the liver, lung and esophagus
InactiveUS20110117108A1Inhibit transferInhibition transitionHeavy metal active ingredientsSnake antigen ingredientsEsophageal cancerAdjuvant therapy
The invention relates to the treatment and / or prevention of cancerous and / or, precancerous conditions of the liver, lung and esophagus by actively and / or passively immunizing a patient against the peptide hormone gastrin and / or a gastrin receptor, e.g., the CCK-B / gastrin receptor. The immunizations of the invention may be employed as a monotherapy, an adjunctive therapy, or as part of a combination therapy.
Owner:CANCER ADVANCES INC
Use of a glp-1 molecule for treatment of biliary dyskinesia and/or biliary pain/discomfort
The present invention relates to molecules, compositions and methods suitable for the treatment or prevention of biliary dyskinesia and / or pain and / or discomfort originating from the biliary tree. The peptide hormone glucagon-like peptide-1 (GLP-1) has both anti-secretory effects and smooth muscle relaxatory properties in the gastrointestinal tract. GLP-1 exists in several forms, where the natural occurring GLP-1 molecules are GLP-1 (1-37), GLP-1 (7-37), and the amidated versions GLP-1 (1-36)amide, GLP-1 (7-36)amide. Other molecules capable of binding to and activating the GLP-1 receptor is herein included in the tern GLP-1 molecules. GLP-1 molecules may be naturally occurring or homologues of GLP-1 having one or more amino acid substitutions or modifications. The GLP-1 molecules are used for the manufacture of a medicament for the treatment or prevention of biliary dyskinesia and / or pain and / or discomfort originating from the biliary tree.
Owner:GASTROTECH PHARMA AS
Non Peptide Agonists and Antagonists of Adrenomedullin and Gastric Releasing Peptide
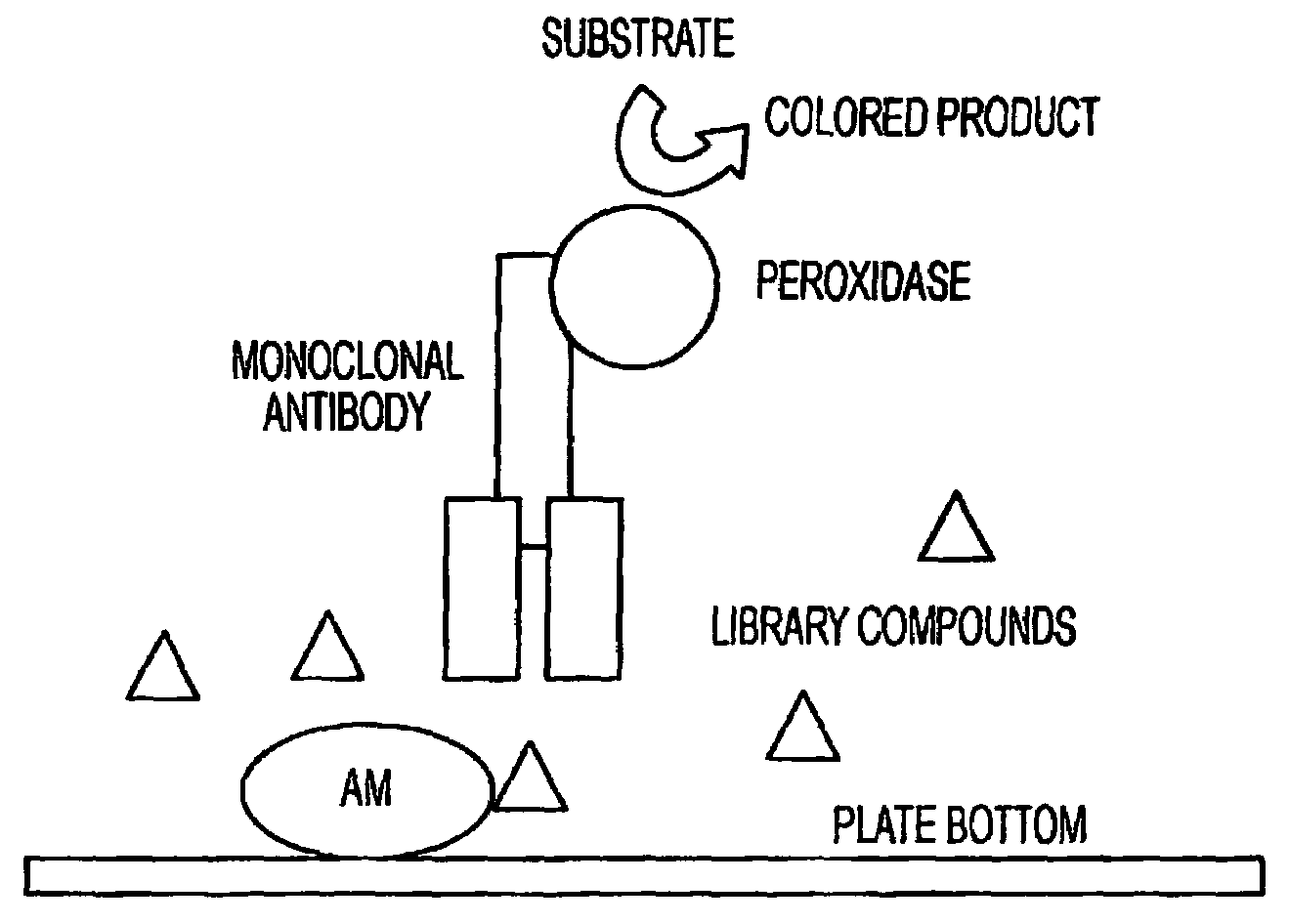
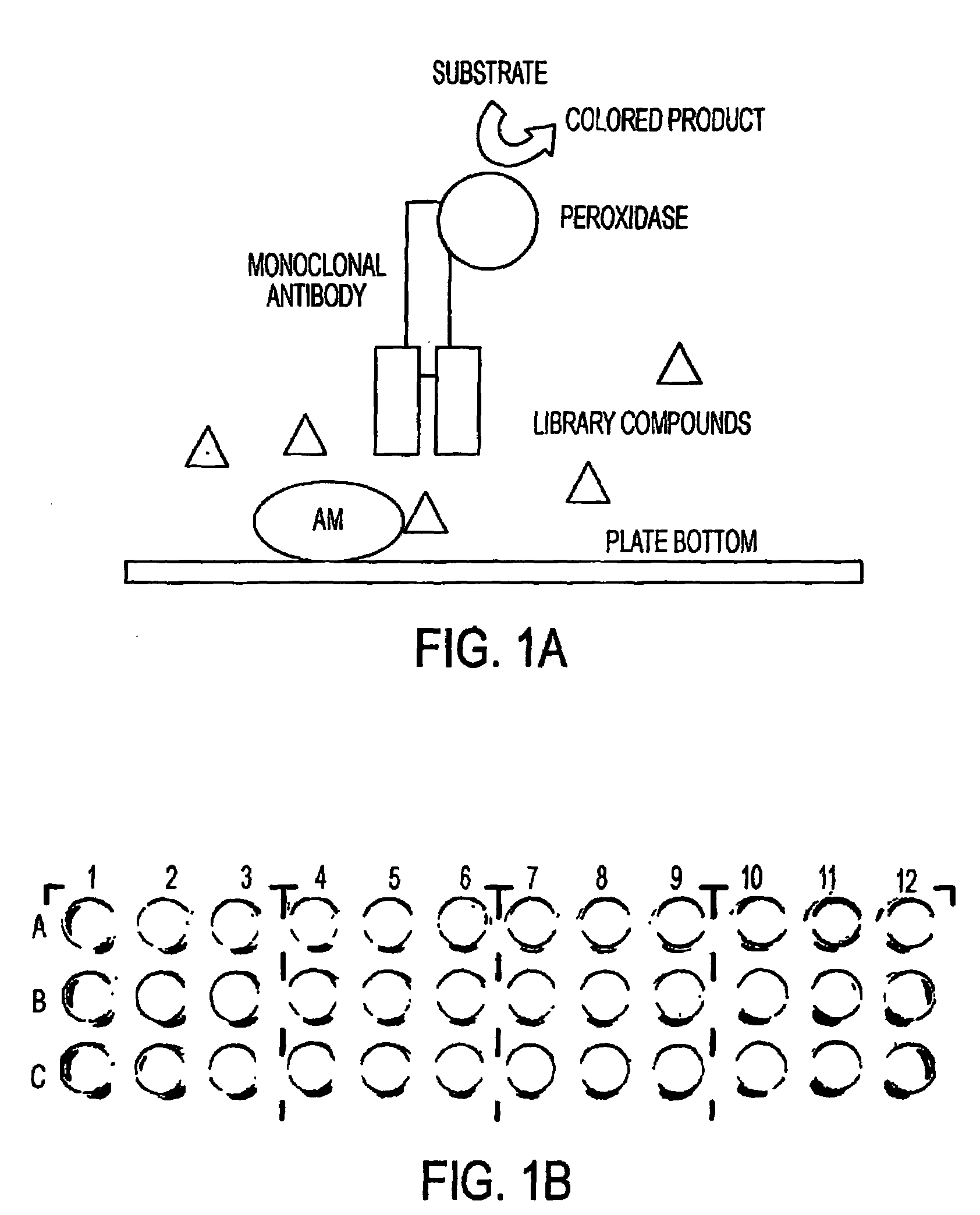
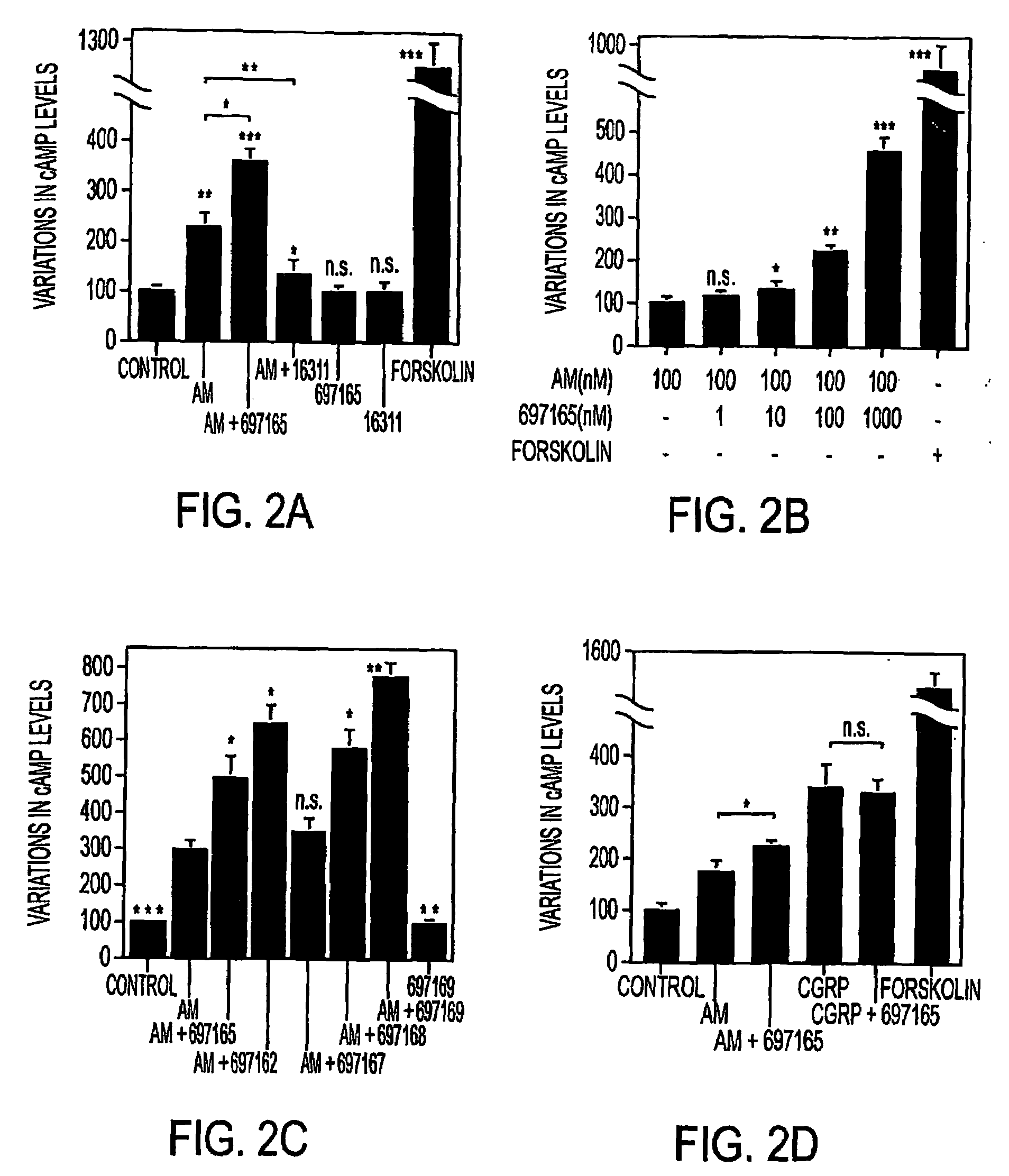
This invention relates, e.g., to methods for inhibiting or stimulating an activity of an adrenomedullin (AM) or gastrin releasing peptide (GRP) peptide hormone, comprising contacting the peptide with a small molecule, non-peptide, modulatory agent of the invention. Complexes of these modulatory agents with other components, such as the peptides or blocking antibodies specific for the peptides, are also described, as are pharmaceutical compositions comprising the modulatory agents, and methods for using the modulatory agents to diagnose or treat patients.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonist peptides may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
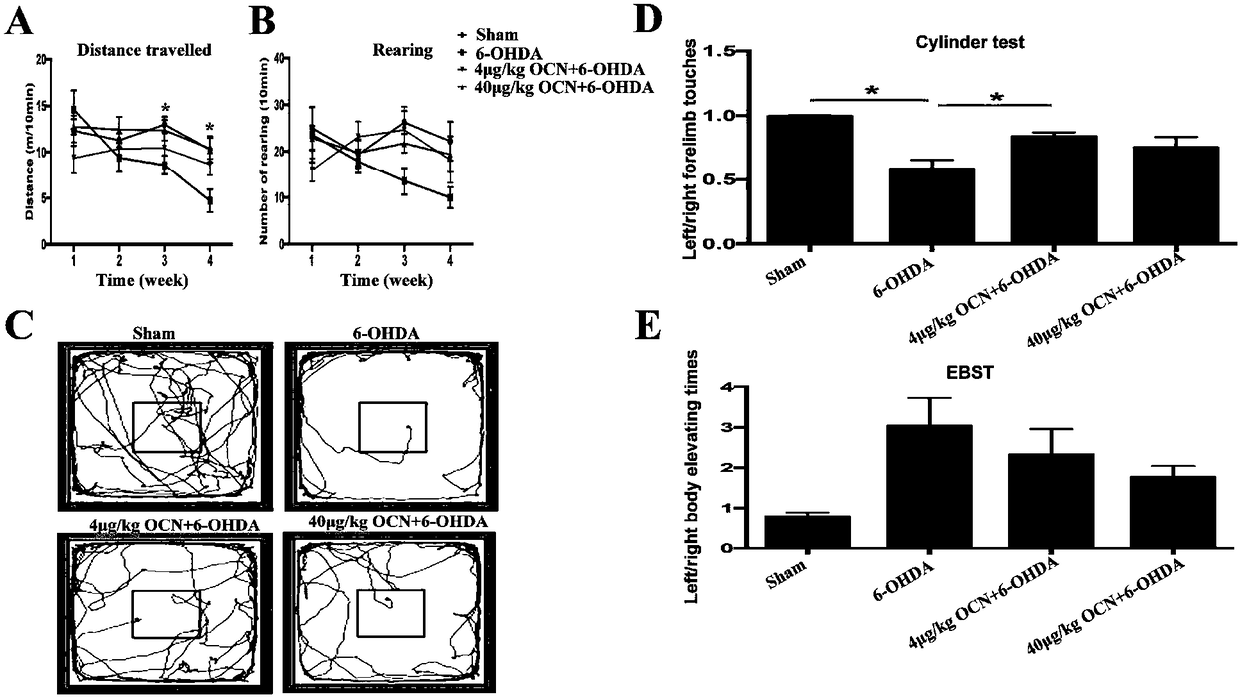
Use of osteocalcin for preparing medicine for treating Parkinson's diseases
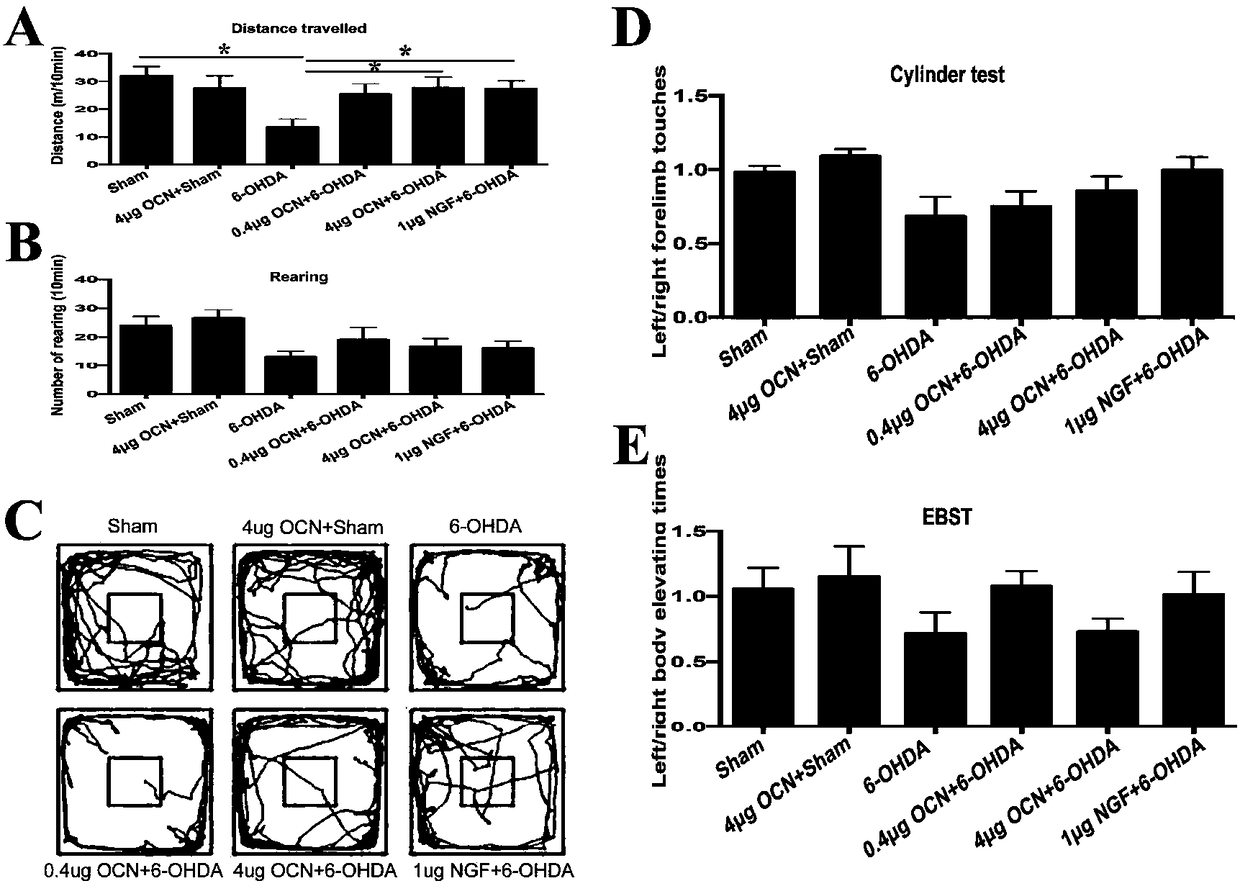
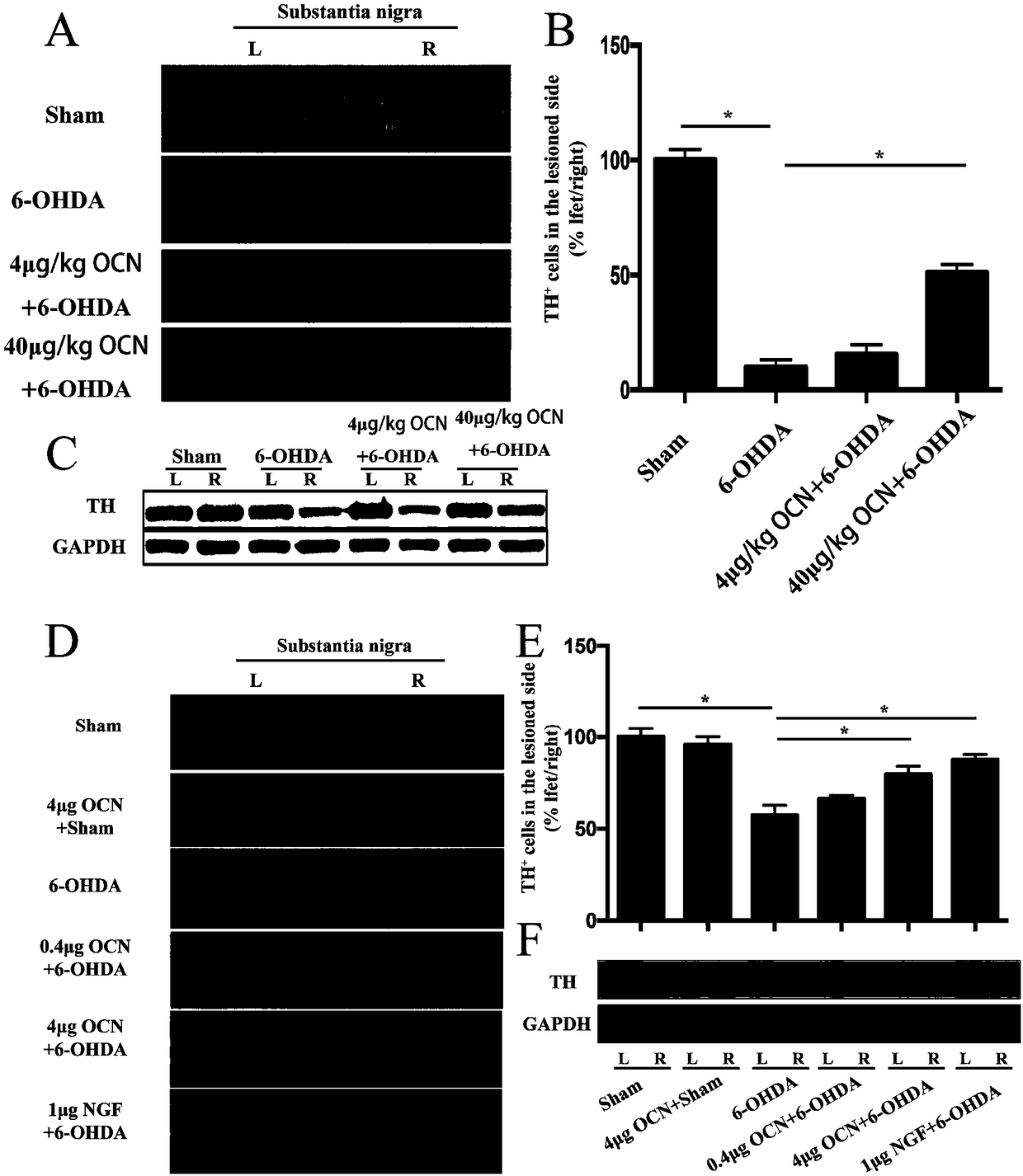
The invention discloses a use of osteocalcin for preparing medicine for treating Parkinson's diseases. The effects that exogenous application of the osteocalcin can relieve the dyskinesia of a rat model with the Parkinson's diseases and can reduce the substantia nigra dopaminergic neuron loss, and the PC12 cell apoptosis caused by 6-OHDA can be reduced through AKT / GSK path in vitro. Therefore theosteocalcin as a small molecule polypeptide hormone capable of passing through blood brain barrier can become fire-new medicine for preventing and treating the occurrence and the development of the Parkinson's diseases.
Owner:SHANGHAI INST FOR ENDOCRINE & METABOLIC DISEASES +1
Human antibodies specific for gastrin materials and methods
InactiveUS8278421B2Reduce formationEnhance and decrease propertySugar derivativesDigestive systemAntibodyPeptide hormone
Owner:XOMA TECH LTD
Peptide antagonists of the calcitonin cgrp family of peptide hormones and their use
The embodiments provide a modified calcitonin gene-related peptide antagonist including an N-terminal fragment of modified calcitonin gene-related peptide or related protein family member where at least two residues of the N-terminal fragment are cysteine (Cys) and at least one amino acid comprises a non-threonine substitution of a threonine (Thr) residue; a central core where the central core comprises an oc-helix; and a C-terminal fragment of modified calcitonin gene-related peptide or related protein family member comprising a C-terminal amide and where at least one amino acid of the C-terminal fragment is phenylalanine (Phe), proline (Pro), tyrosine (Tyr) or hydroxyproline (Hyp) or pharmaceutically acceptable salt thereof, as well as compositions, including pharmaceutical compositions, comprising a subject peptide. The embodiments further provide treatment methods, including methods of treating a migraine, the methods generally involving administering to an individual in need thereof an effective amount of a subject peptide or composition.
Owner:SOARES CHRISTOPHER J
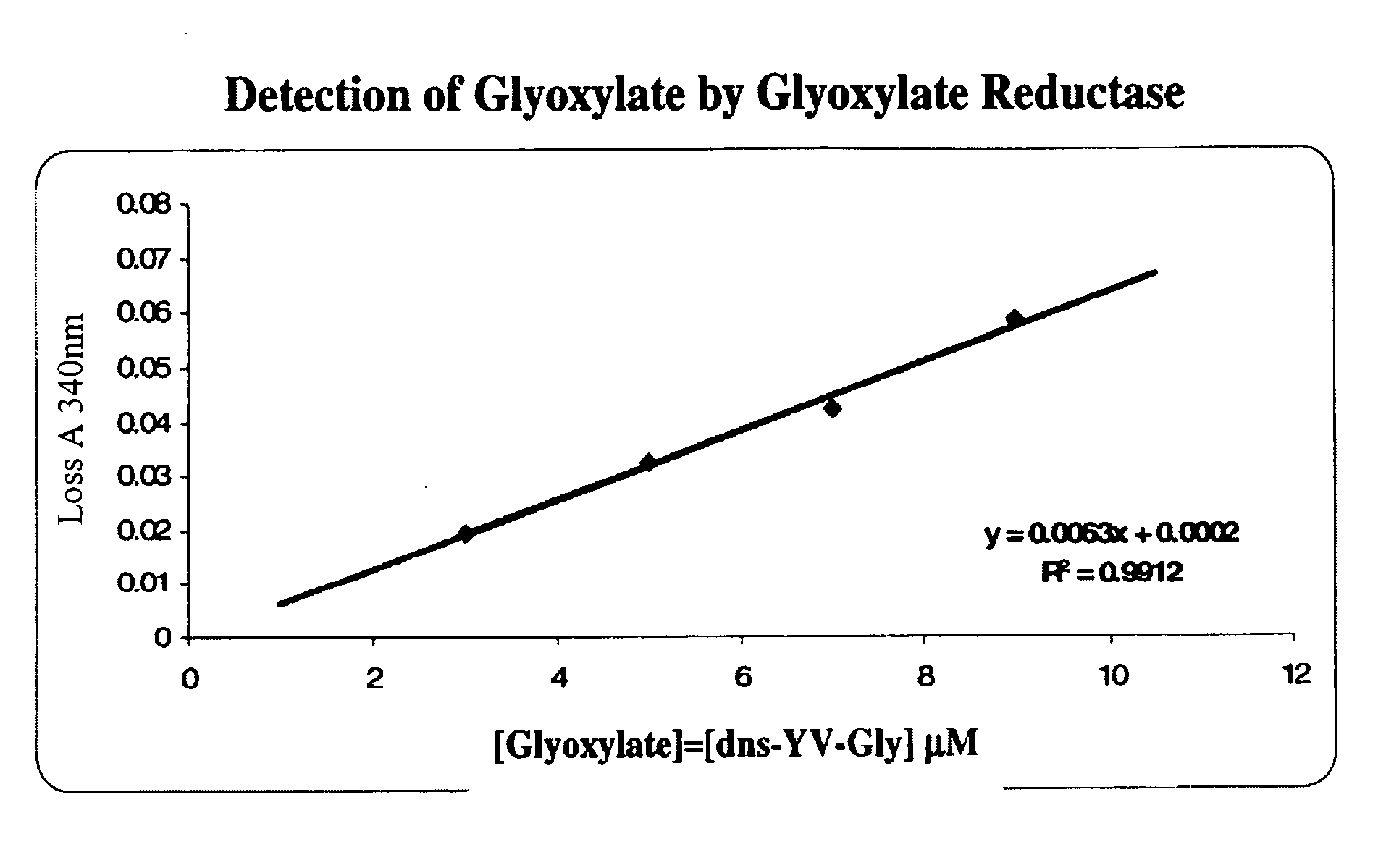
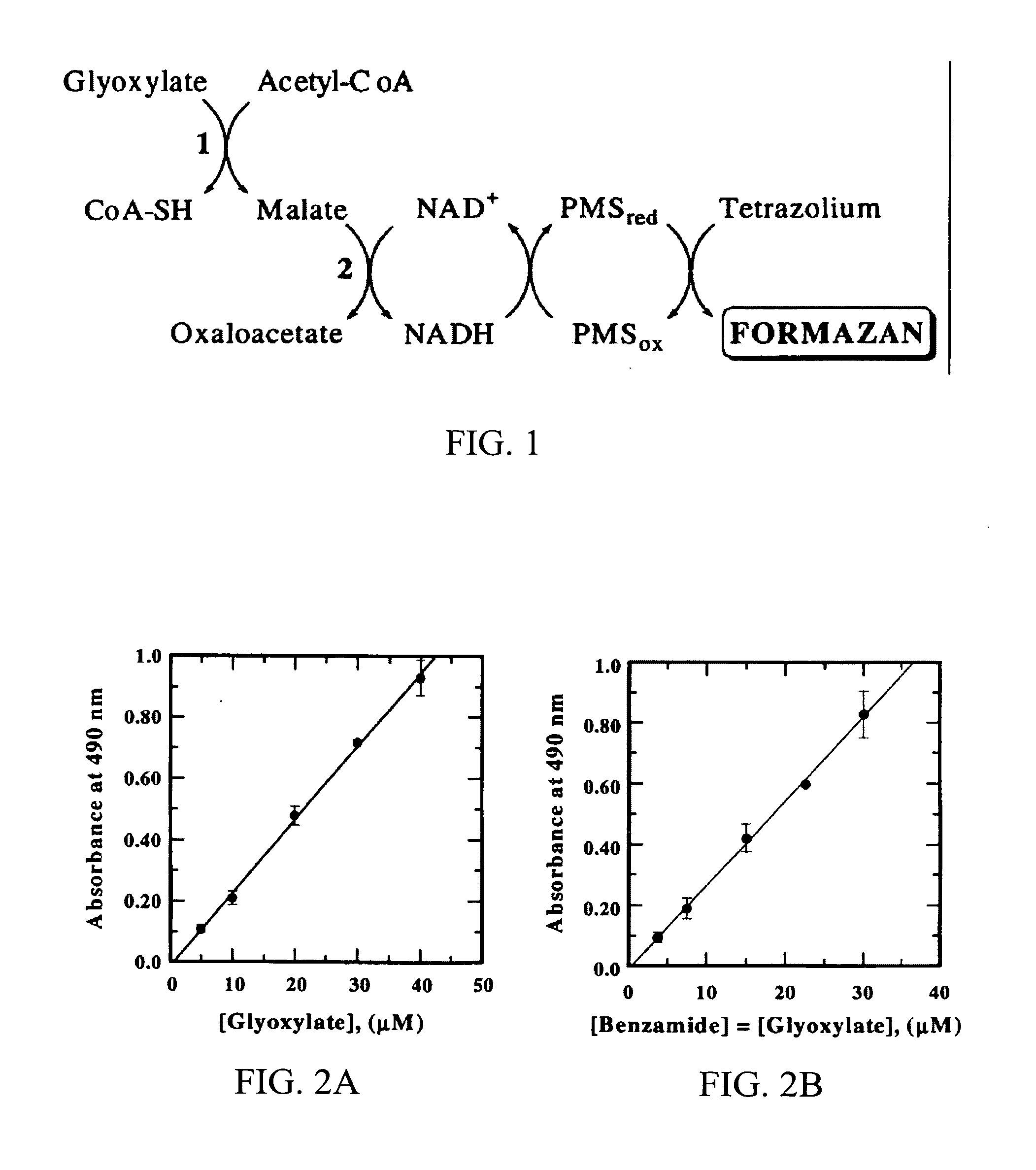
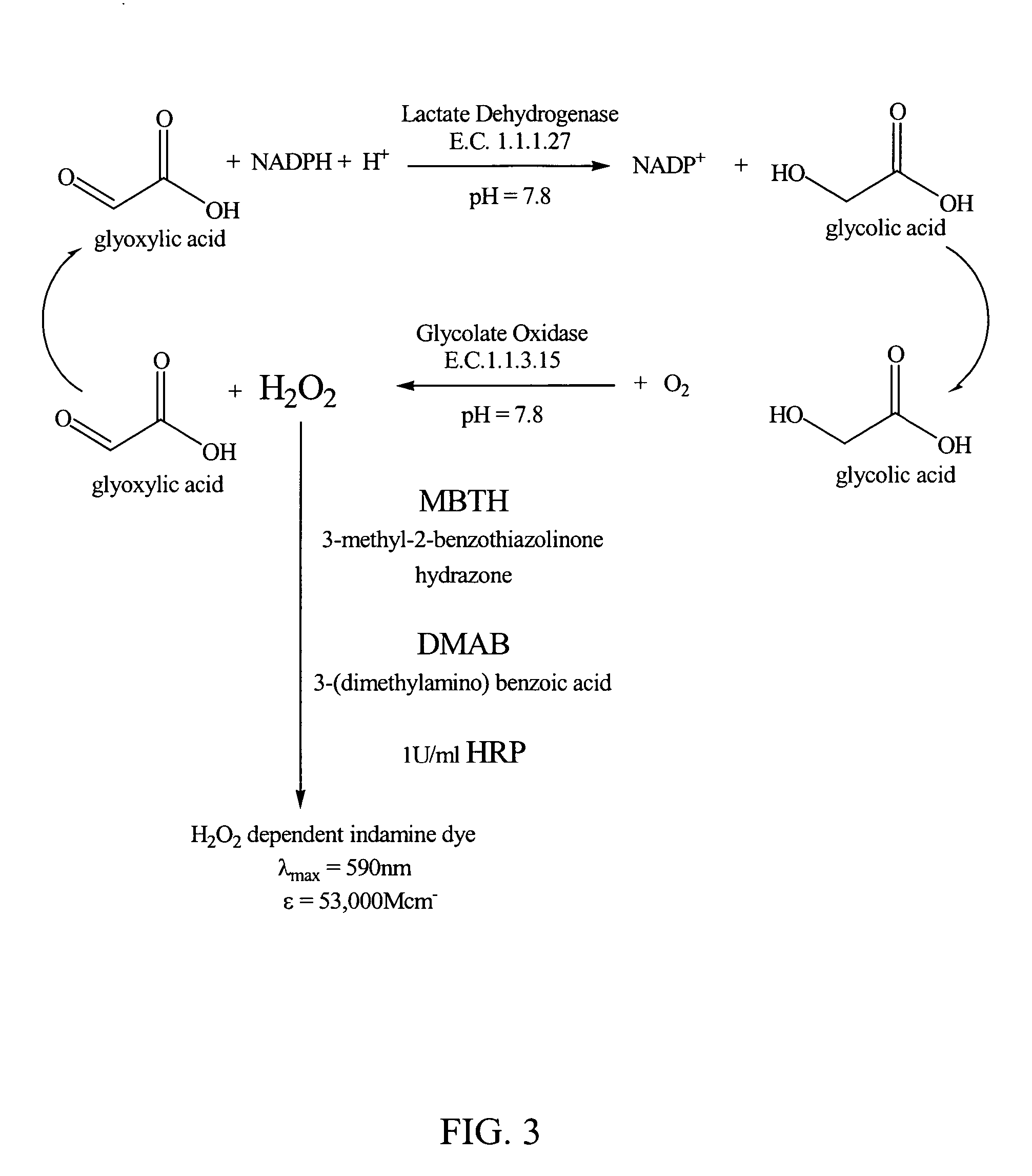
Glyoxylate assays and their use of inden tifying natural amidated compounds
Methods for detecting and assaying for glyoxylate, include enzyme-based assays and / or assays for hydrogen peroxide following liberation of hydrogen peroxide from glyoxylate, are disclosed. In some embodiments, the invention is directed to methods for assaying for glyoxylate produced by the reaction of peptidylglycine alpha-amidating monooxygenase (PAM). The subject invention also concerns methods for assaying for the enzyme peptidylglycine alpha-amidating monooxygenase and / or its substrates. The detection of glyoxylate is indicative of the presence of PAM and / or its substrates. The subject invention also concerns methods for screening for peptide hormones, amidated fatty acids, any N-acyl-glycine or N-aryl-glycine conjugated molecule, and generally compounds having a glycine reside in free acid form and attached to a carbonyl group.
Owner:UNIGENE LABORATORIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com