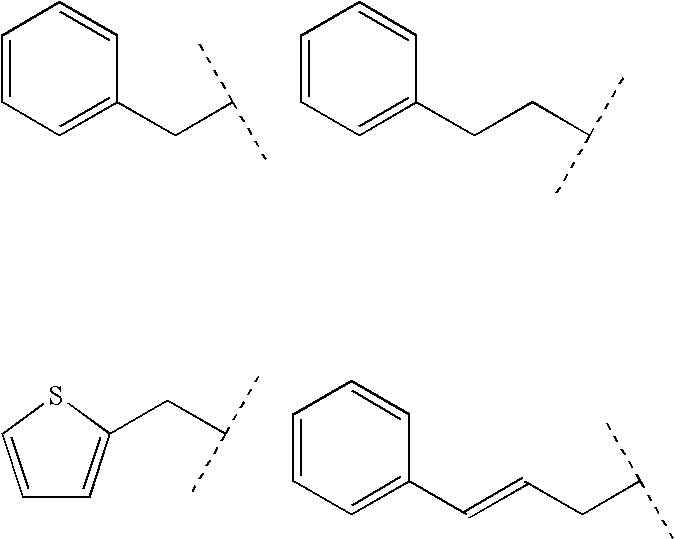
Novel glucagon antagonists/inverse agonists
a technology of glucagon antagonists and inverse agonists, which is applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problem of general knowledge that is not orally availabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
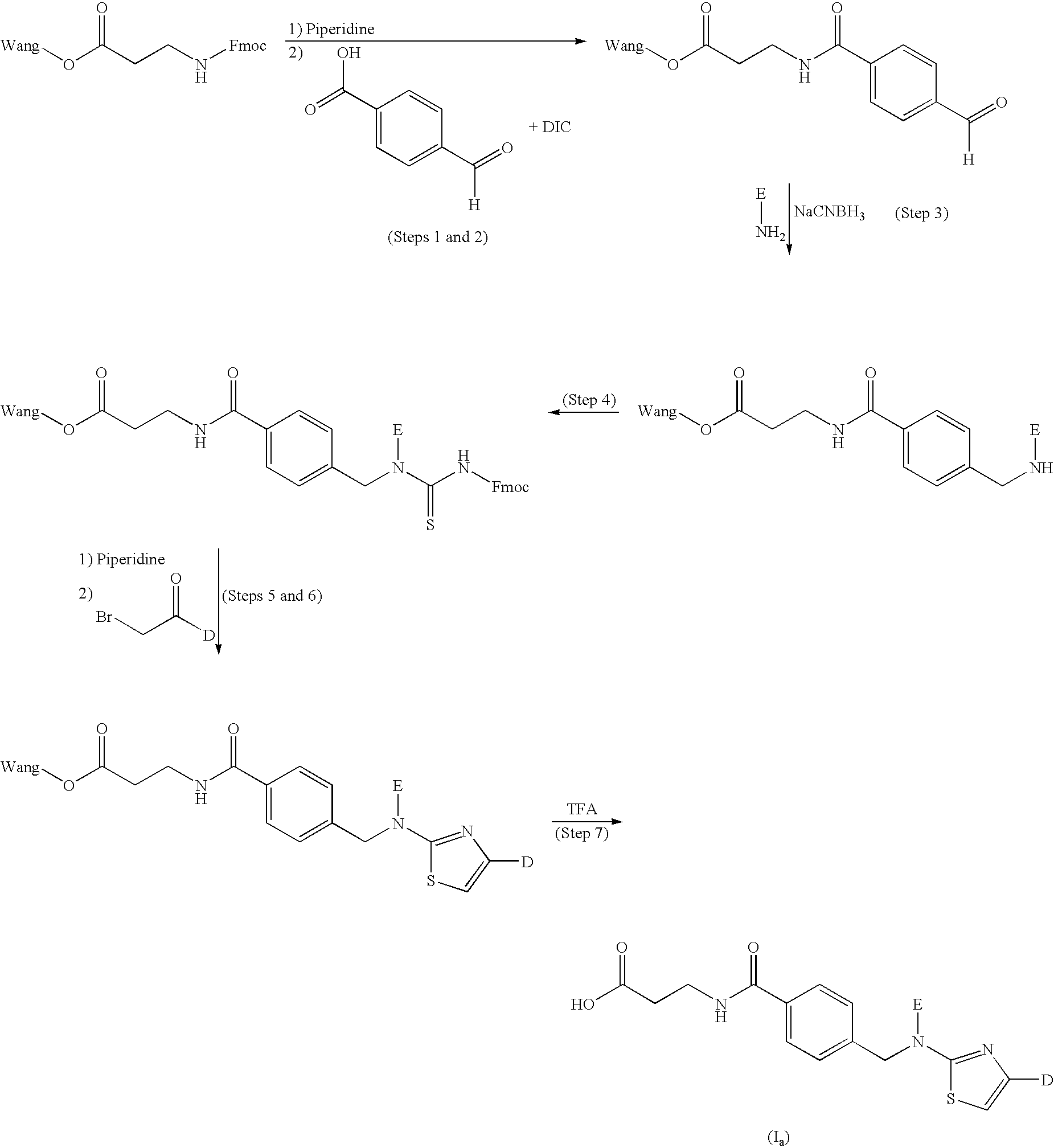
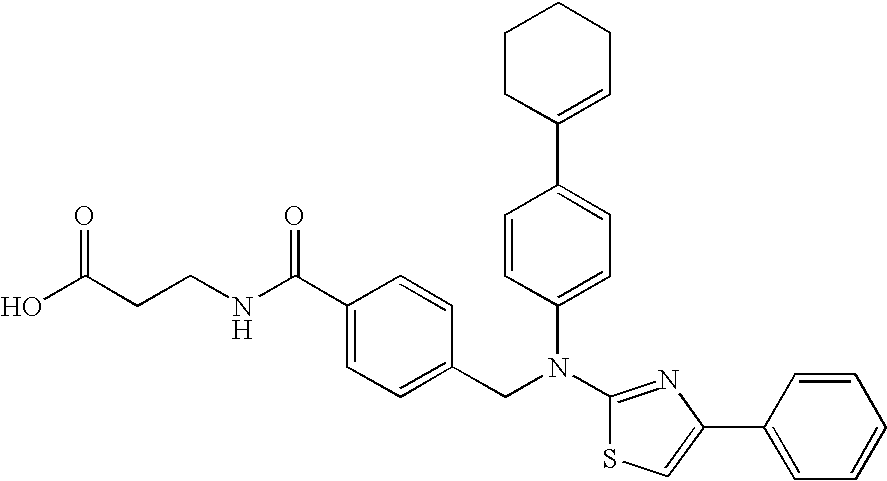
example 1
General Procedure (A)
3-(4-[(4-Cyclohex-1-enylphenyl)-(4-phenylthiazol-2-yl)amino]methyl}benzoylamino)propionic acid
[0154]
[0155] Fmoc-β-Ala-Wang resin (0.57 mmol / g, 50 μmol) was treated with piperidine (20% in NMP, 1000 μL) for 10 min and the resin was drained. This was repeated once. The resin was washed with NMP (6×1000 μL). 4-Carboxybenzaldehyde (0.5 M in NMP, 500 μL), HOBt (0.5 M in NMP, 500 μL) and DIC (0.5 M in toluene, 500 μL) were added and the resulting mixture was shaken for 15 hours at room temperature. The resin was drained and washed with DMF (3×1000 μL). The resulting resin-bound aldehyde was added the appropriate amine, in this case 4-(cyclohex-1-enyl)aniline, prepared as described in WO 00 / 69810 (1.0 M in NMP, 600 μL), NaCNBH3 (1.0 M in NMP:methanol (7:3), 600 μL) and acetic acid (140 μL). The resulting mixture was shaken for 9 hours at room temperature, drained and washed with MeOH (1000 μL) for 1 hour, followed by washing with with 5% DIPEA in methanol (1×) and NM...
example 2
General Procedure (A)
3-(4-{[(4-Cyclohexylphenyl)-(4-phenylthiazol-2-yl)amino]methyl}benzoylamino)propionic acid
[0158]
example 3
General Procedure (A)
3-(4{[(4-Cyclohexylphenyl)-(5-methyl-4-phenylthiazol-2-yl)amino]methyl}benzoylamino)propionic acid
[0159]
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com