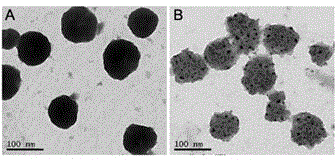
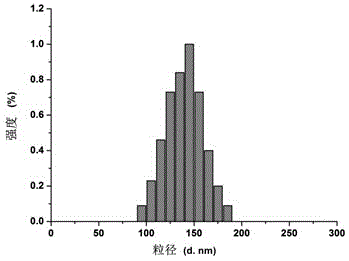
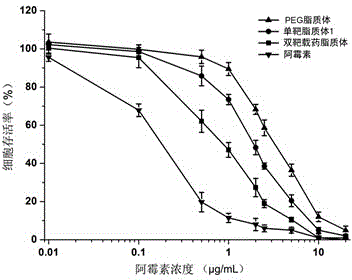
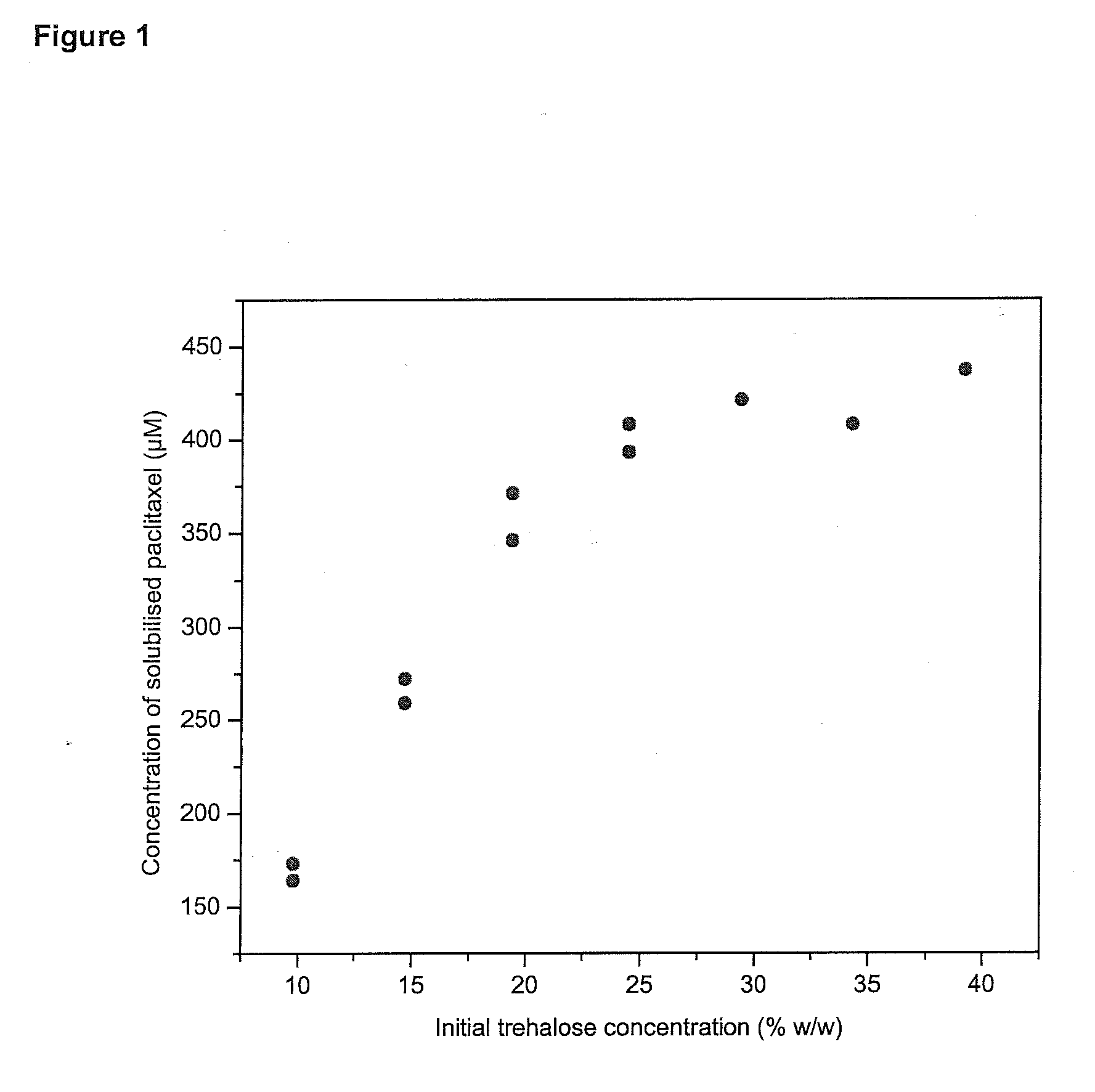
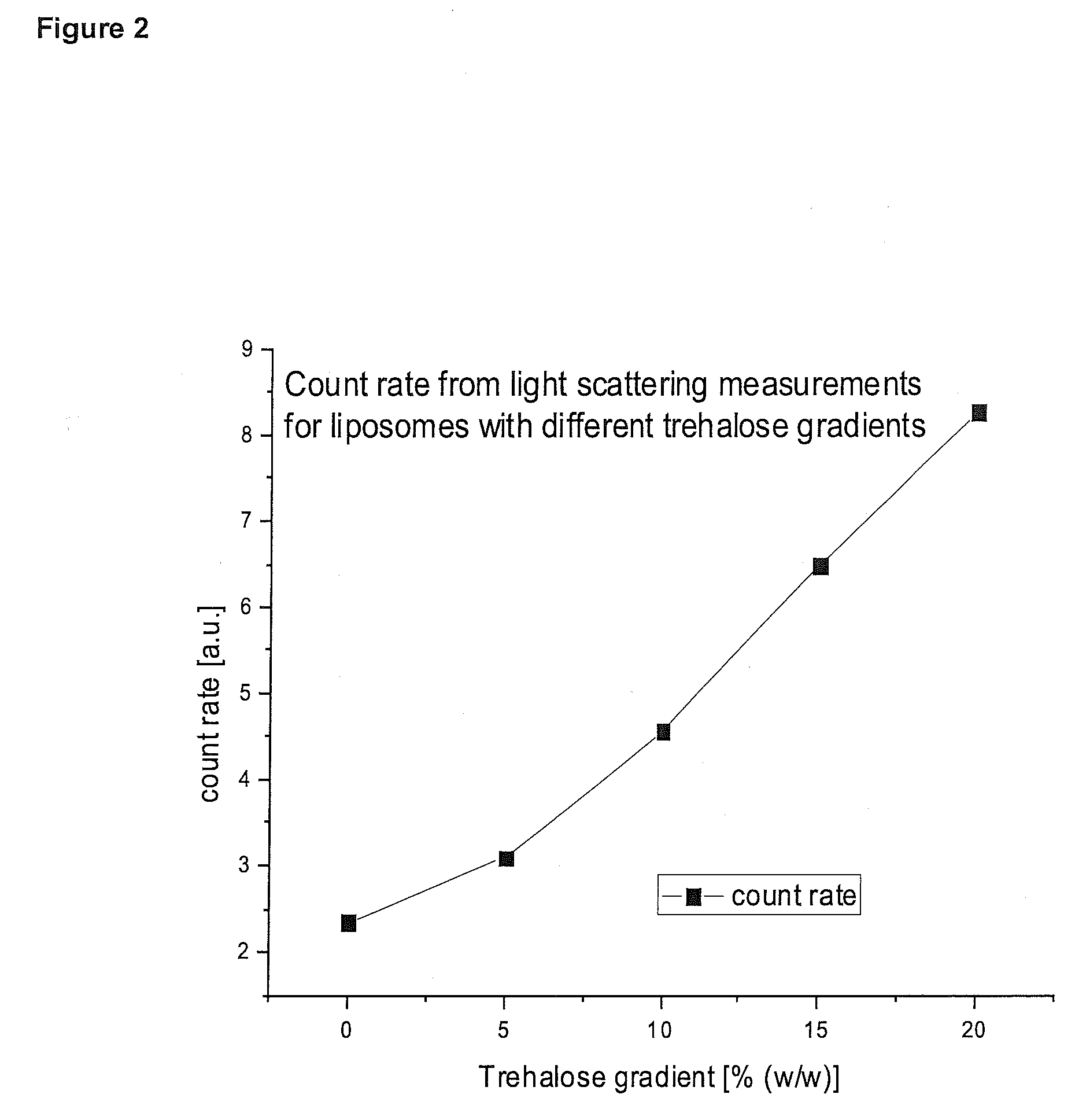
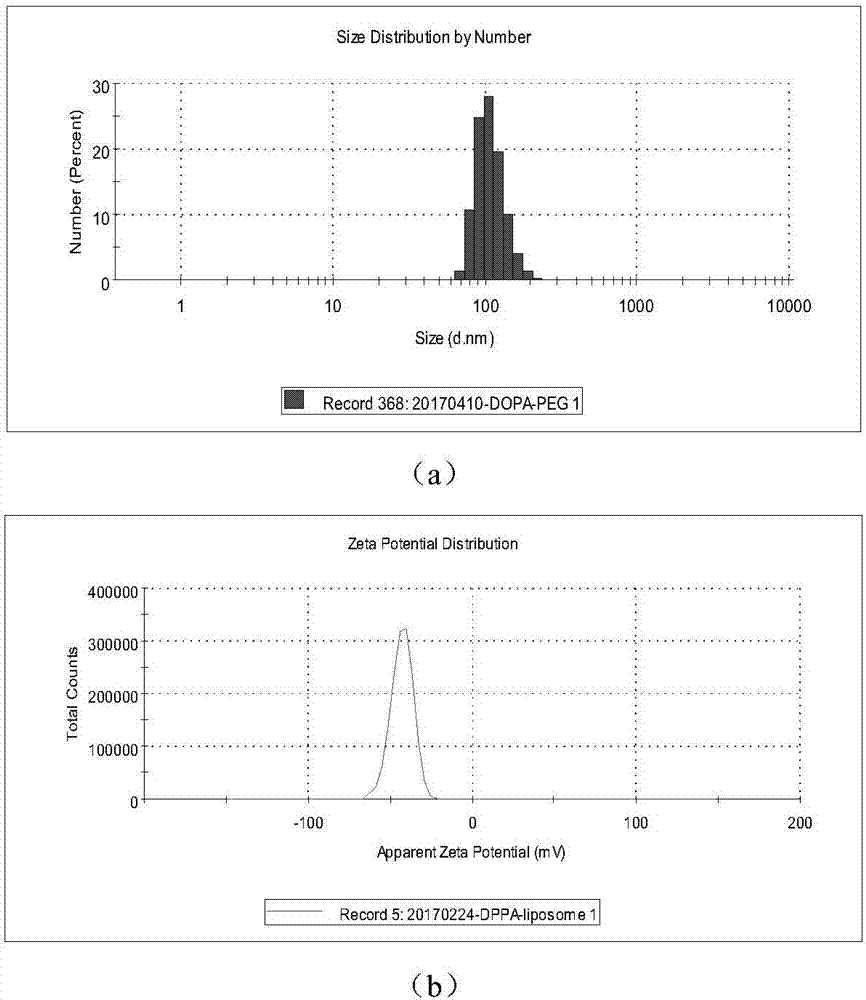
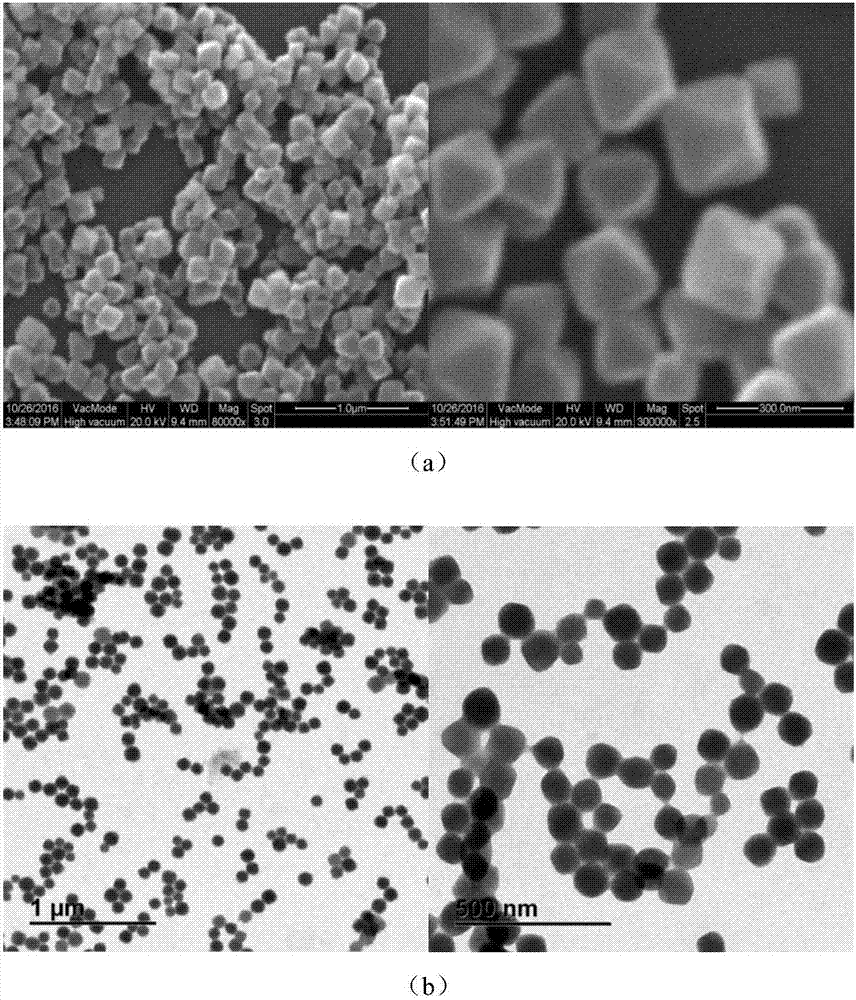
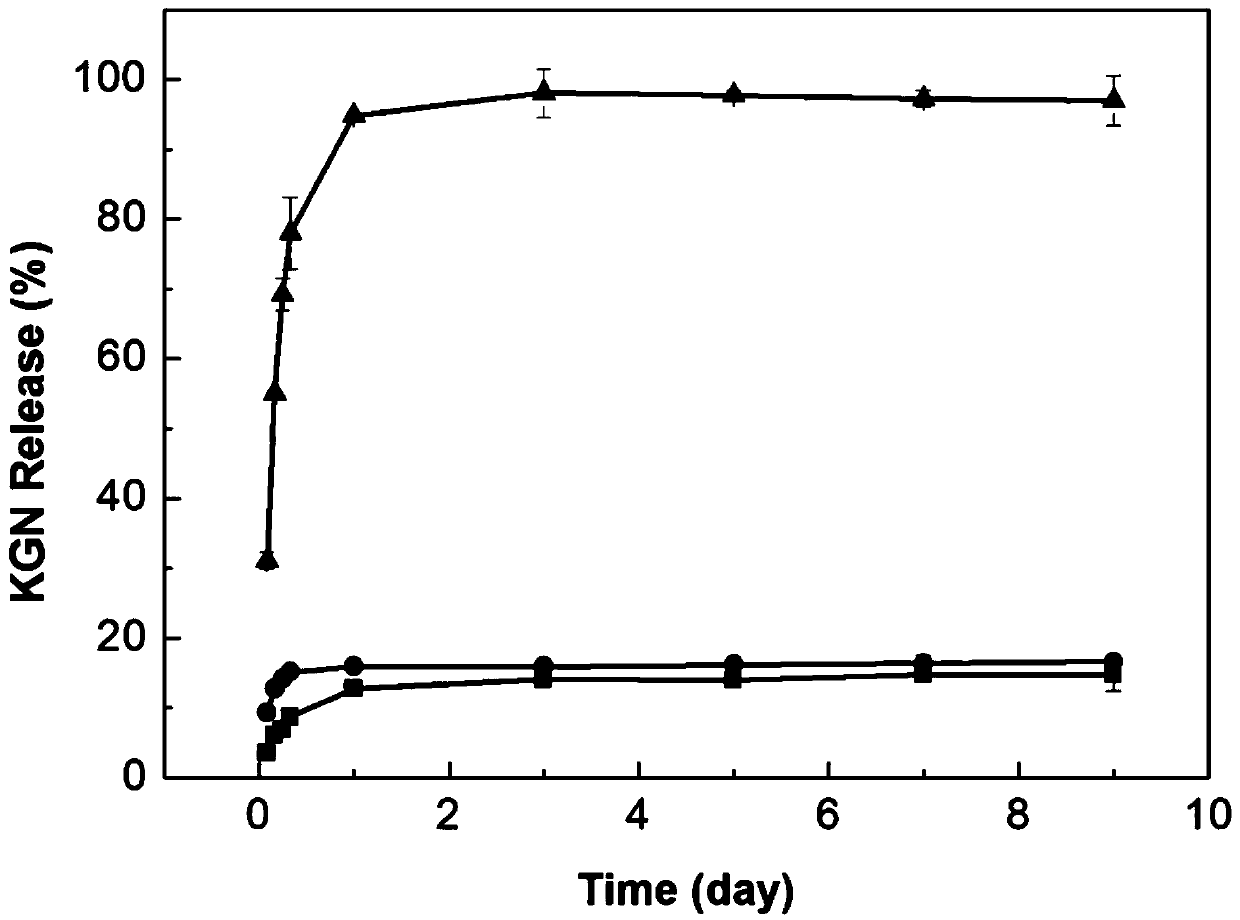
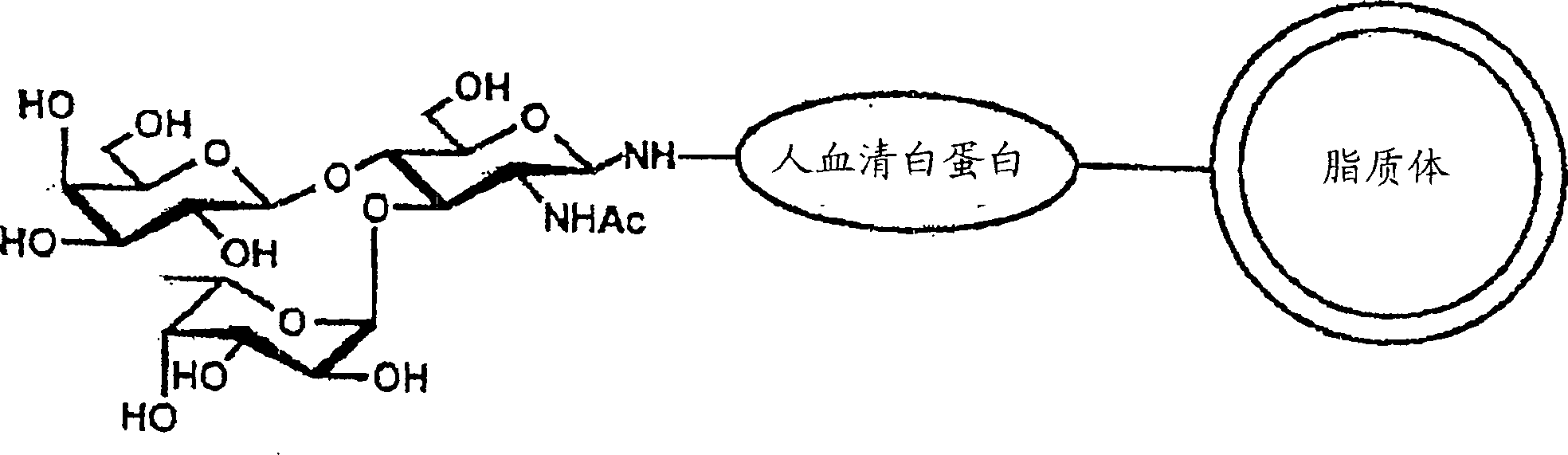
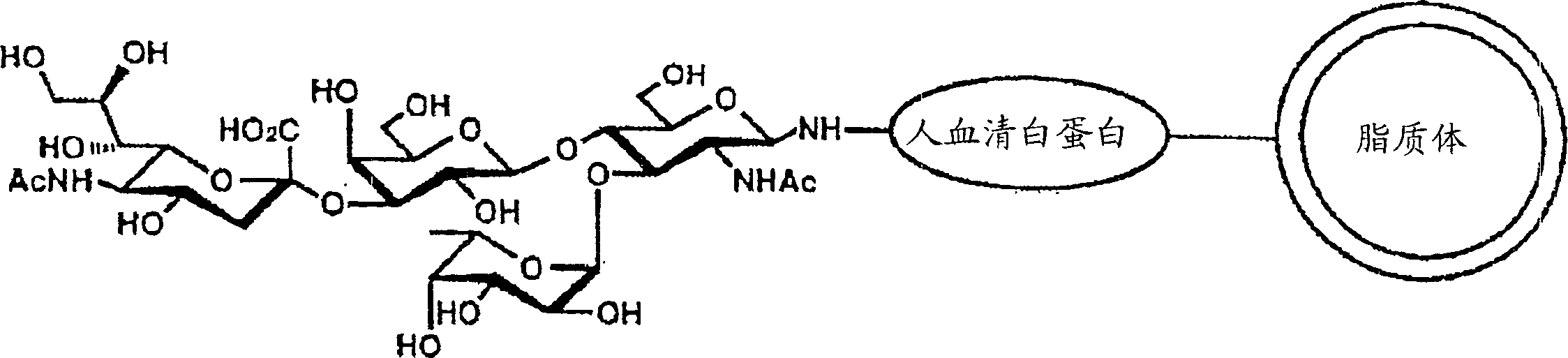
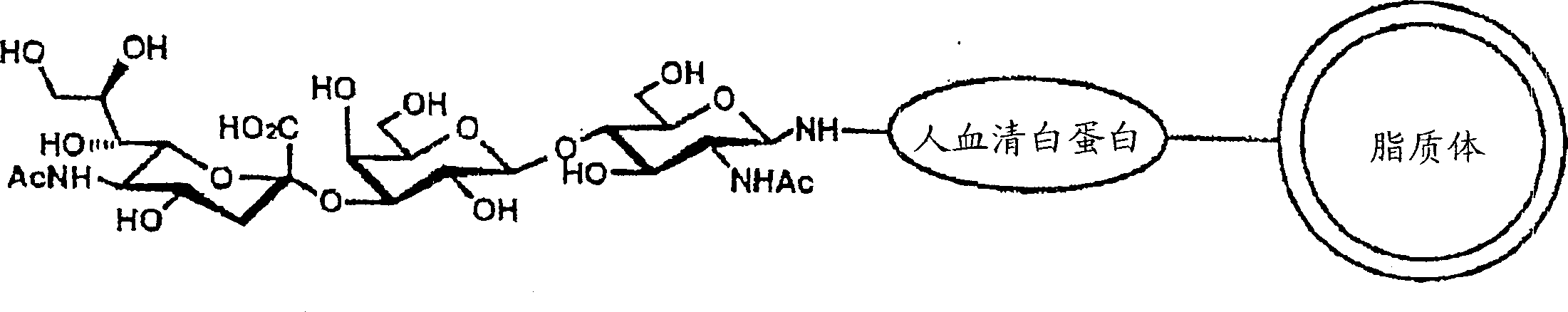
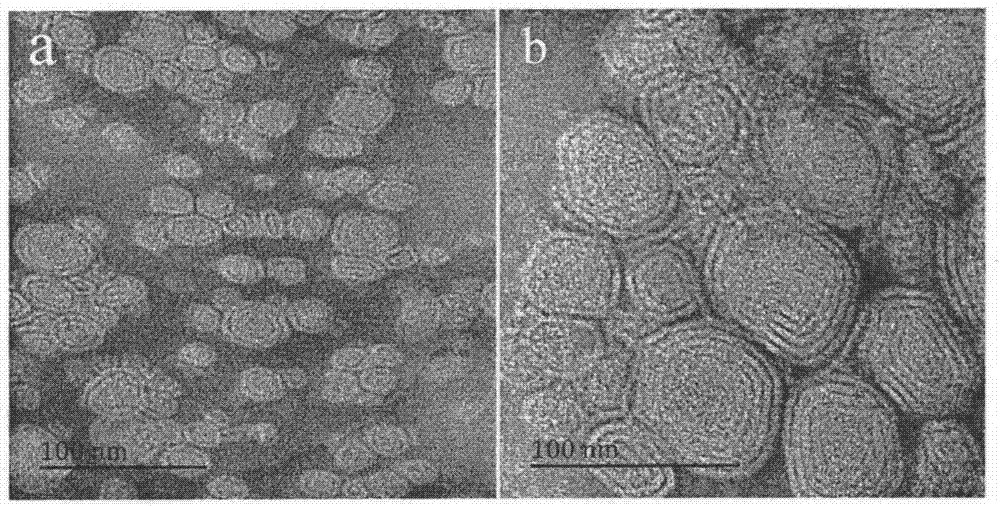
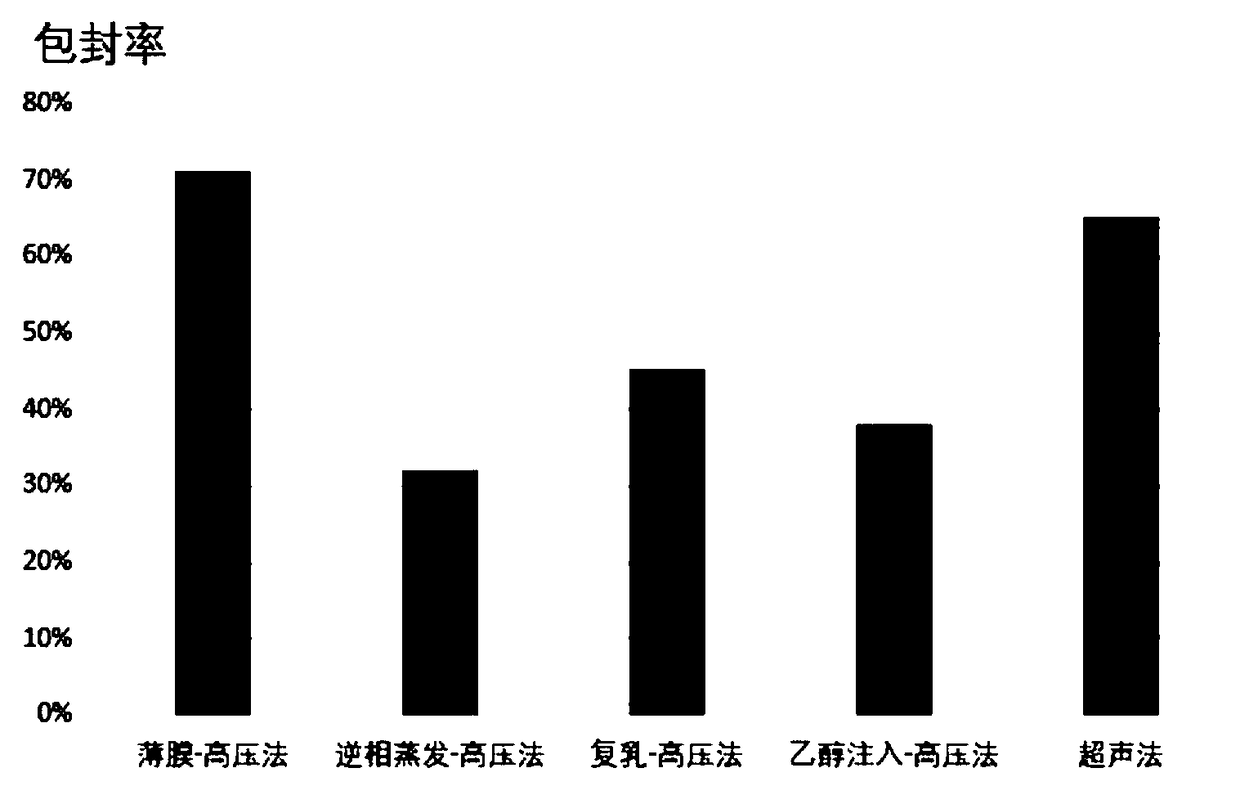
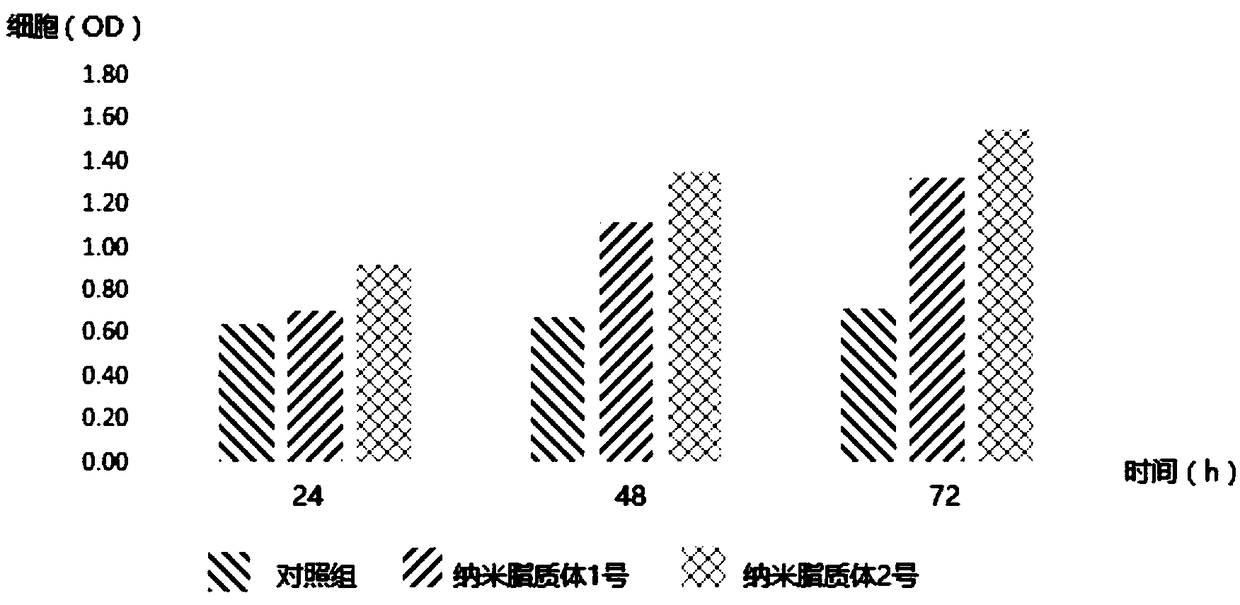
Patents
Literature
126 results about "Liposome membrane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A liposome is a spherical-shaped vesicle that is composed of one or more phospholipid bilayers, which closely resembles the structure of cell membranes. The ability of liposomes to encapsulate hydrophilic or lipophilic drugs have allowed these vesicles to become useful drug delivery systems.
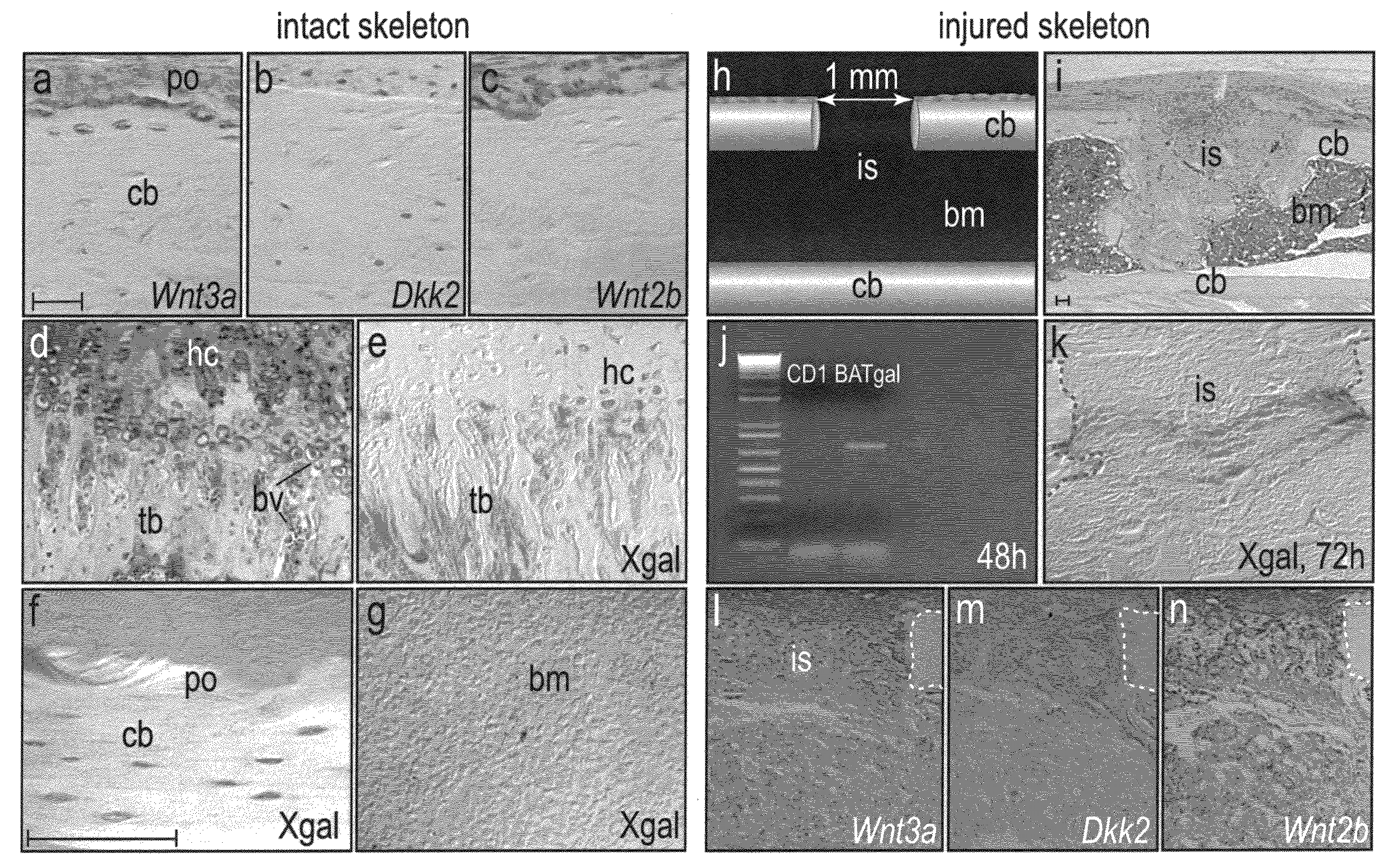
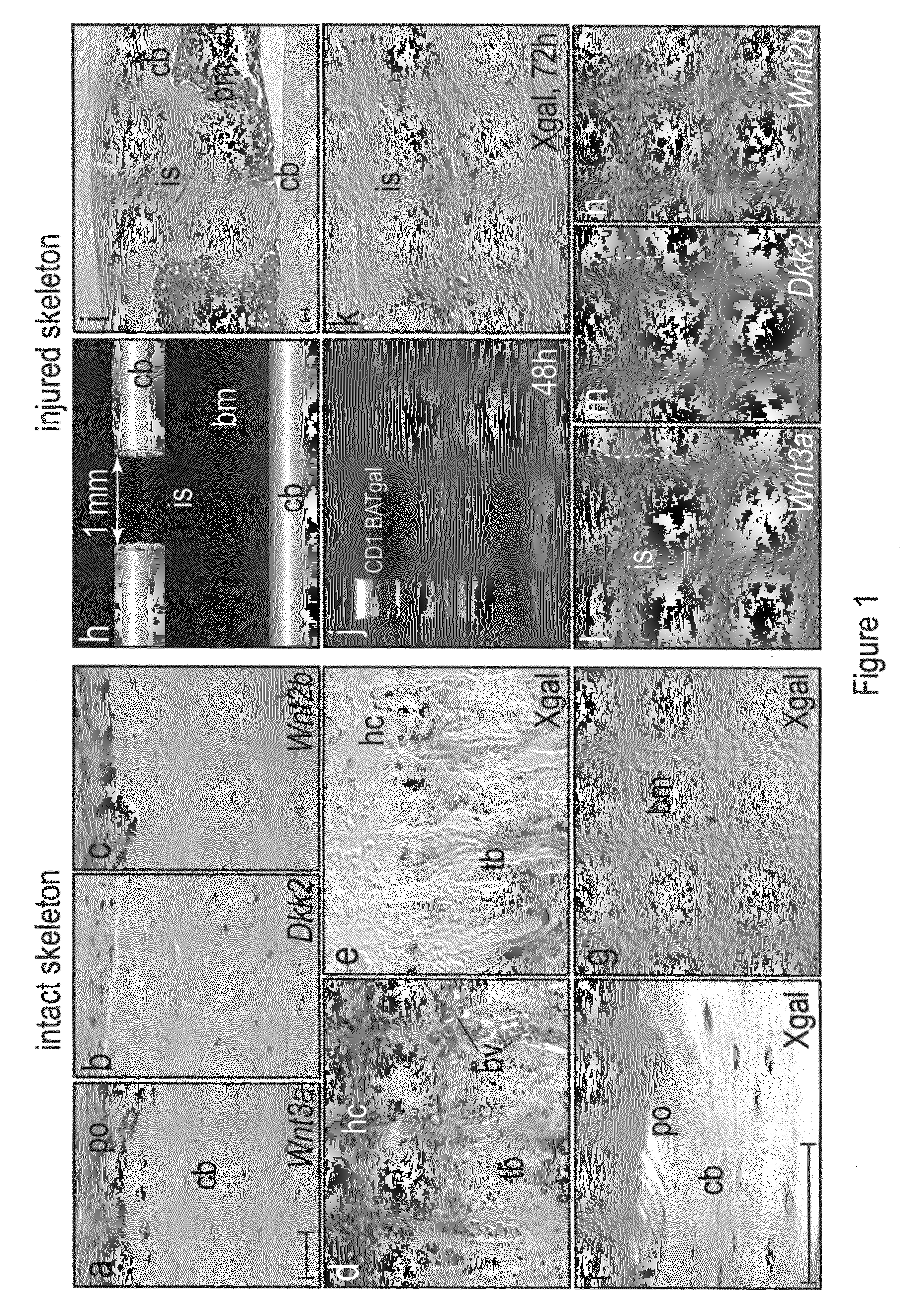
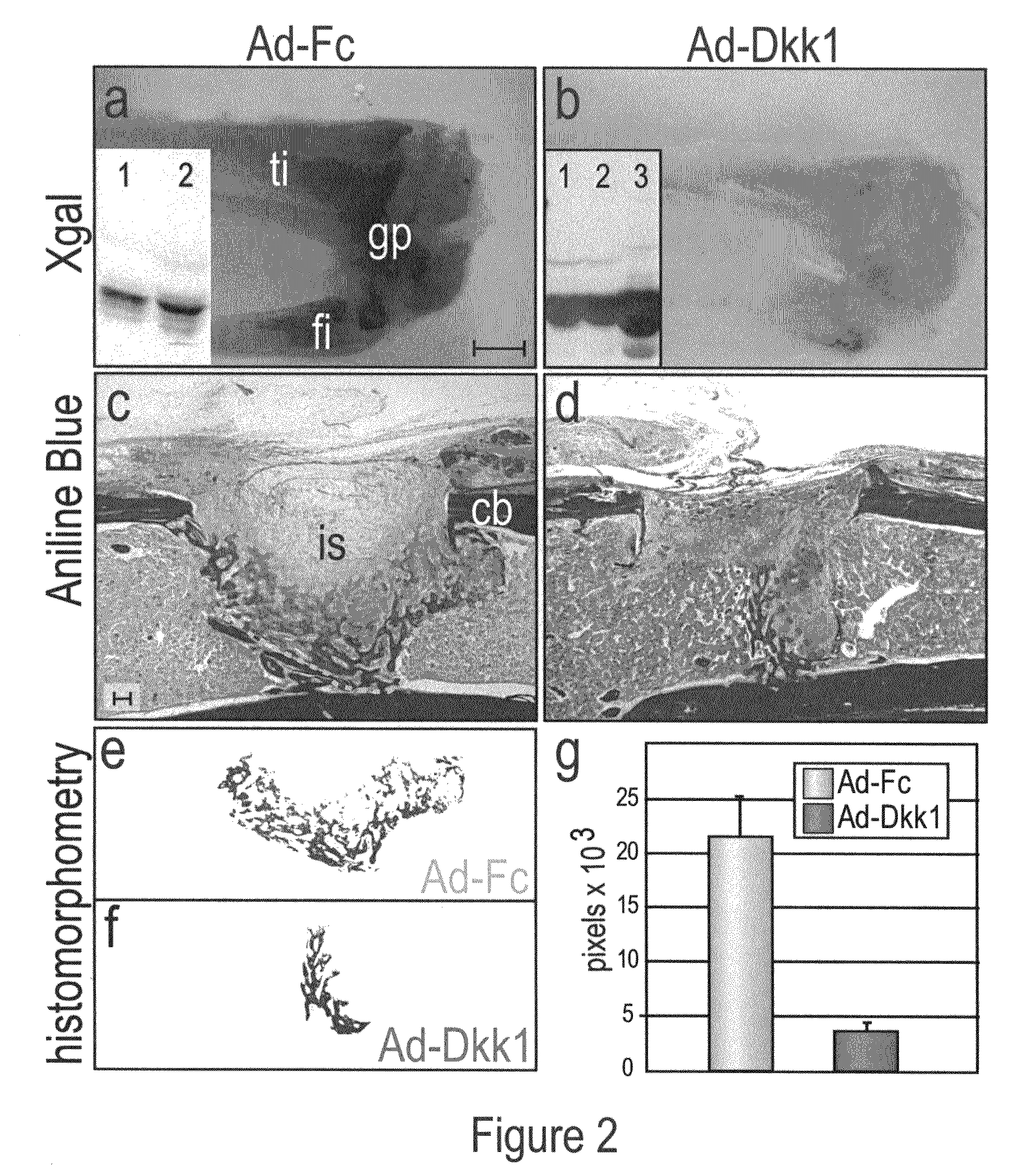
WNT compositions and methods of use thereof
InactiveUS20080226707A1Accelerate bone repairAccelerate bone regenerationPeptide/protein ingredientsSkeletal disorderLiposome membraneTherapeutic intent
Methods and compositions are provided for the therapeutic use of Wnt proteins, where the Wnt protein is inserted in the non-aqueous phase of a lipid structure. In some embodiments the Wnt protein is presented in its active conformation on an outer liposome membrane or micelle. Pharmaceutical compositions of the present invention can be administered to an animal for therapeutic purposes. In some embodiments of the invention, the compositions are administered locally, e.g. by injection at the site of an injury. For certain conditions it is desirable to provide Wnt activity for short periods of time, and an effective dose will be administered over a defined, short period of time.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for preparing natural material-liposome composite nanofiber based on electrostatic spinning technology
InactiveCN102797074ALow priceEasy to operateMonocomponent protein artificial filamentFilament/thread formingFiberCross-link
The invention relates to a method for preparing a natural material-liposome composite nanofiber based on electrostatic spinning technology. The method comprises the following steps of: (1) adding lecithin, cholesterol and octadecylamine into a reaction vessel, then adding anhydrous alcohol, stirring for dissolving, and finally depressurizing to remove alcohol so as to obtain a liposome membrane; (2) adding deionized water into the reaction vessel containing the liposome membrane, stirring at room temperature, and then carrying out ultrasonic treatment so as to obtain a liposome suspension with uniform particle sizes; (3) preparing a spinning solution containing natural materials by taking the liposome suspension as a solvent, and then carrying out electrostatic spinning so as to obtain a nanofiber; and (4) fumigating and cross-linking the nanofiber in a genipin solution or alcohol steam. The method provided by the invention has the advantages of simplicity in operation, low cost of raw materials, mild reaction conditions and good biocompatibility; and a composite nanofiber bracket prepared by the method provided by the invention can control the release of genes, growth factors and various medicaments, has stable performance, is easy to preserve, and has wide application prospects.
Owner:DONGHUA UNIV
Process for preparing Paclitaxel liposome preparation
InactiveCN101011357ASlow down removalExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsSucroseLiposome membrane
The invention relates to a method for preparing drug rehabilitation liposome agent, which comprises that using film disperse method or atomizing drying method to prepare long-circulation rehabilitation liposome, using cholesterol, distearin acyl phosphatidyl choline and myristate as stabilizers, using sucrose as freezing preservative, using chloroform and chloroform as organic solvents; and using amphipathic carbowax derivative to decorate the liposome membrane; and using compression or high-pressure homogeneity method to make the diameter of liposome smaller than 100nm and the package rate higher than 85%. The invention has less toxicity and high stability, as one novel drug slow-release target agent.
Owner:XIAN LIBANG PHARMA TECH
Drug-carrying liposome co-modified by folic acid and TAT peptide and preparation method thereof
InactiveCN103976954AImprove transport efficiencyEasy to manufactureMacromolecular non-active ingredientsAntineoplastic agentsTat peptideLiposome membrane
The invention discloses drug-carrying liposome co-modified by folic acid and TAT peptide and a preparation method thereof. The drug-carrying liposome comprises liposome, a long chain targeted membrane material, a short chain targeted membrane material and a drug. Meanwhile, the invention provides the preparation method of the drug-carrying liposome co-modified by folic acid and TAT peptide. The method comprises the following steps: weighing phospholipid, cholesterol, the long chain targeted membrane material, the short chain targeted membrane material and the drug, dissolving the components in an organic solvent, and then carrying out rotary evaporation at 50 DEG C under reduced pressure to remove the organic solvent so as to obtain a medicated liposome membrane; adding a phosphate buffer solution into the medicated liposome membrane for dissolving, carrying out ultrasonic treatment for 2 minutes, and filtering for 10 times by using a 0.22mu m membrane to obtain double-target drug-carrying liposome. According to the drug-carrying liposome disclosed by the invention, the TAT peptide is connected with specific ligands by means of PEG with different weight-average molecular weights to establish a nanometer carrier modified by double ligands, and the prepared drug-carrying liposome can be used for efficiently conveying the drug in tumor cells.
Owner:SUZHOU UNIV
Liposomal formulations of lipophilic compounds
ActiveUS20130259922A1Good conditionMaintaining polydispersityBiocideOrganic active ingredientsLiposome membraneMedicine
The present invention relates to the preparation of liposomes with enhanced loading capacity for pharmaceutically and / or diagnostically active agents and / or cosmetic agents which are substantially solubilized by the liposomal membranes, to liposome dispersions with enhanced stability with respect to release of the active agent and / or cosmetic agent from the liposomes obtainable by the process, and to pharmaceutical or cosmetic compositions comprising said stabilized liposome dispersions. The preparation may involve dehydration and rehydration steps of liposome dispersions which may be carried out by spray drying.
Owner:SYNCORE BIOTECH
Method of manufacturing pharmaceutical preparation containing liposomes
InactiveUS20060239925A1Raise the ratioEfficient transportX-ray constrast preparationsLiposomal deliveryLiposome membraneCompound (substance)
A method of manufacturing a liposome-containing preparation is disclosed, comprising (a) mixing one or more constituents of a liposome membrane, an aqueous solution of a water-soluble chemical and supercritical carbon dioxide at a temperature of 32 to 65° C. in a pressure vessel, and (b) evacuate the carbon dioxide to form an aqueous dispersion of liposomes enclosing an aqueous solution of a water-soluble chemical, wherein the constituents include at least one phospholipid exhibiting a transition temperature.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
Surface functionalization modification method for metal organic framework (MOF) material based on liposome membrane
ActiveCN107189074AImprove biostabilityRealization of independent integrationLiposome membraneMetal-organic framework
The invention belongs to the technical field of preparation of nanomaterials and discloses a surface functionalization modification method for a metal organic framework (MOF) material based on a liposome membrane. The surface functionalization modification method comprises the following steps: selecting MOFs materials and liposome which are mutually matched, and enabling an electrostatic adsorption or covalent linkage effect to exist between the liposome membrane and exposed active sites of the MOFs materials; directly mixing corresponding MOFs materials and a liposome solution, and carrying out fusion coating and centrifugal washing to obtain a final product; carrying out quality evaluation and performance evaluation on the final product, coating the MOFs materials with the liposome by electrostatic adsorption or covalent linkage, and finishing surface functionalization modification of the MOFs materials. Compared with a traditional polyethylene glycol modification method, the surface functionalization modification method has the advantages that higher biological stability can be provided, simplicity and convenience in operation are realized, and modification efficiency is relatively high; no organic solvents or toxic solvents are needed; the MOFs materials are expected to be endowed with novel functions when the surface functionalization modification method is applied to the aspects of drug delivery, molecular image and the like.
Owner:XIDIAN UNIV
Irinotecan liposome and preparation method thereof
InactiveCN102485213AOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholEthanol Injection
The invention belongs to the technical field of medicine and relates to irinotecan liposome and a preparation method thereof. The method can control ethanol content in liposome during a preparation process and ensure no substantial influence of residual ethanol on liposome property in the preparation process. The invention employs improved ethanol injection method to prepare irinotecan liposome, and a membrane material comprises phospholipid or / and other components. The method comprises steps of: preparing a hydrating medium at 45-75 DEG C and insulating for standby; dissolving lipid phase with ethanol, including absolute ethyl alcohol, in a volume less than 10% of a final volume of a preparation at 45-75 DEG C; adding the hydrating medium into the lipid phase at a certain speed; stirring and dispersing to reduce grain size and obtain a blank liposome; and loading according to a gradient method. The invention can effectively control residual ethanol amount during the preparation process, and physical and chemical properties of the liposome have no significant change.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of composite drug-loaded delivery material based on hydrogel and liposome
The invention relates to a preparation method of a composite drug-loaded delivery material based on a hydrogel and liposome, which belongs to the technical field of biomaterials, and aims to solve thetechnical problem of fast release of the hydrogel as a scaffold material for repairing cartilage defects. The preparation method comprises the following steps: 1. dissolving the lipid and a drug in acontainer in an organic solvent, after fully dissolving, removing the organic solvent by rotary evaporation, forming a liposome film on the surface of the container, adding a buffer solution, and fully shaking the material to make the liposome film for hydration shedding to obtain a liposome solution; and 2. thoroughly mixing the liposome solution and a polyethylene glycol solution, adding a chitosan solution or a chitosan derivative solution, and obtaining the composite drug-loaded delivery material based on the hydrogel and the liposome after standing. The liposome of the present inventioncan be loaded with the drug to achieve long-term slow release, and can be used as a carrier delivery system for the drug; and the drug and the liposome have synergism to achieve long-term slow releaseof the drug loaded on a stent material.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Preparation method of polycationic liposome/calcium phosphate nanoparticle drug delivery vector
InactiveCN103071161AImprove stabilityOvercomes the tendency to aggregate and precipitateGenetic material ingredientsInorganic non-active ingredientsCalcium biphosphateLiposome membrane
The invention discloses a preparation method of a gene drug delivery vector with polycationic liposome coating calcium phosphate nanoparticles. The calcium phosphate nanoparticles are prepared with an improved coprecipitation method or a reverse microemulsion method, and the stability of the calcium phosphate nanoparticles is improved; and an amphiphilic compound polyethyleneimine-cholesterol, phosphatide and / or cholesterin are / is taken as liposome membrane material(s), and incubation is performed by adopting both the lipidosome and the calcium phosphate nanoparticles, so that the gene drug delivery vector is obtained. According to the preparation method, the stability of the calcium phosphate nanoparticles is improved through improvements of a calcium phosphate nanoparticle prescription and a preparation process; the trend that the calcium phosphate nanoparticles can gather and precipitate easily is further overcome by a lipidosome coating technology; and polycations on the surface of the lipidosome increase the uptake of a cell for the vector and improve the endosomal escape capability. The prepared compound gene drug delivery vector improves the cellular uptake and transfection efficiencies on the basis of safely and economically reservation of the calcium phosphate vector, and has a broad application prospect.
Owner:ZHEJIANG UNIV
Therapeutic or Diagnostic Drug for Inflammatory Disease Comprising Targeting Liposome
InactiveUS20070286896A1Improve efficiencyLess side effectsBiocideSenses disorderLiposome membraneTarget tissue
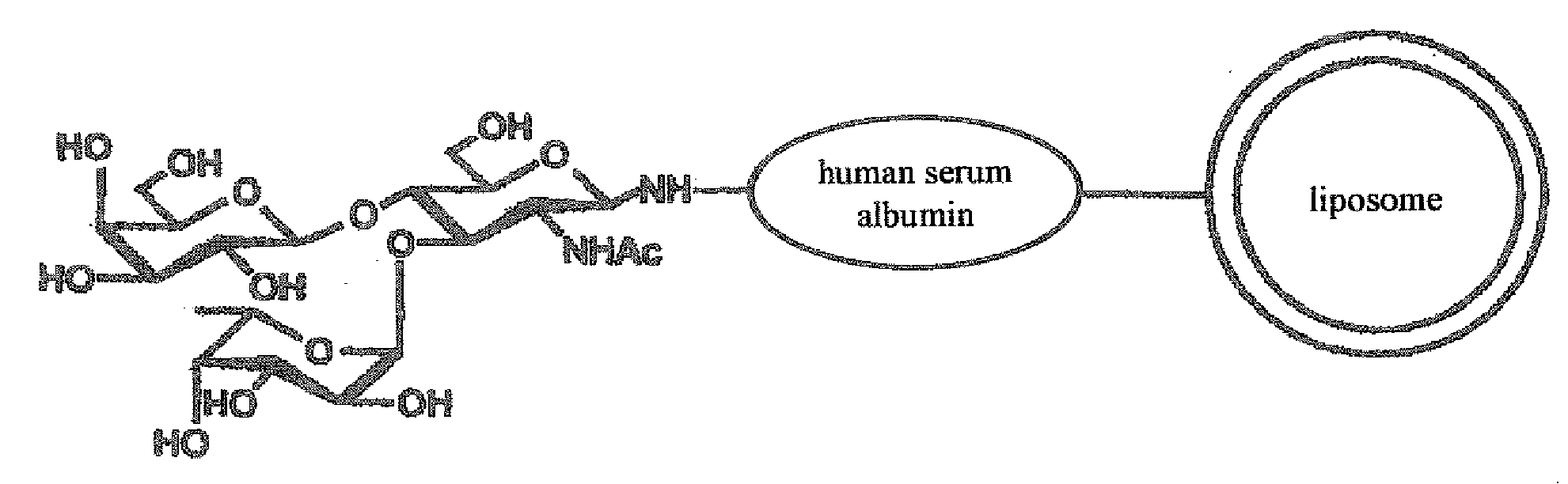
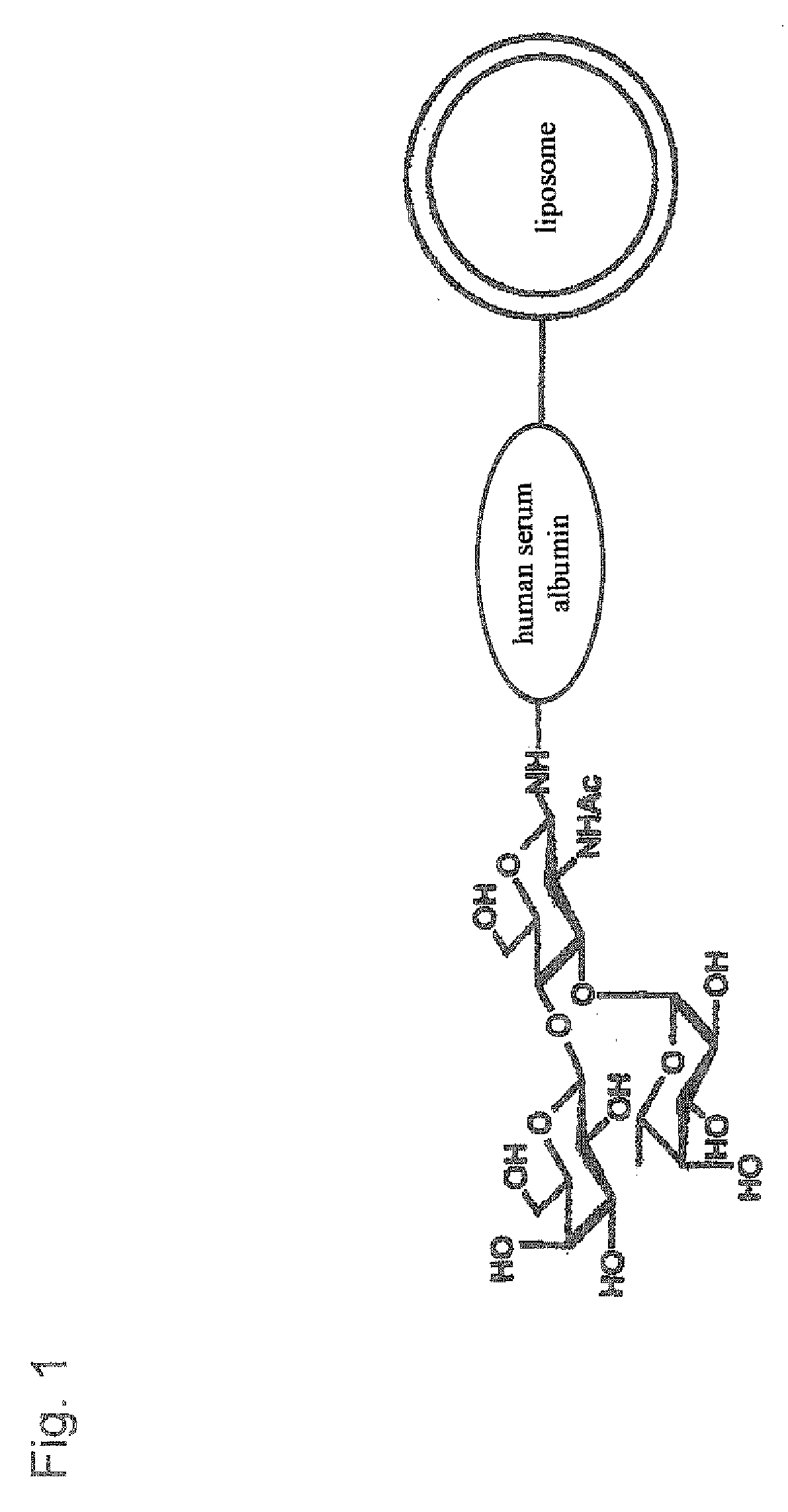
There is provided targeting drug delivery system (DDS) nanoparticles that can be accumulated in target tissues such as inflammatory sites of inflammatory diseases and can thus be utilized in a therapeutic or diagnostic DDS for locally delivering drugs or genes to the affected parts. The present invention provides a pharmaceutical composition for the medical treatment or diagnosis of an inflammatory disease comprising a sugar-modified liposome having a sugar chain bound to the membrane of the liposome.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Novel blank liposomes taking ginsenoside derivatives as membrane materials, and preparation method and application thereof
ActiveCN109833298AHigh activityLow hemolyticOrganic active ingredientsCosmetic preparationsLiposome membraneNiosome
The invention discloses novel blank liposomes taking ginsenoside derivatives as membrane materials as well as a preparation method and application thereof. The ginsenoside derivatives used in the novel blank liposomes taking the ginsenoside derivatives as the membrane materials are high in activity and low in hemolytic activity, and can be used as liposome membrane materials for preparing blank liposomes; moreover, structures of some of the compounds are novel. In addition, the novel blank liposomes prepared by using the ginsenoside derivatives meet the requirements for hemolytic activity; andthus, the liposomes are higher in safety, better in film-forming properties and more excellent in stability. Therefore, the liposomes are of important application values.
Owner:XIAMEN GINPOSOME PHARM CO LTD
Method of manufacturing pharmaceutical preparations containing liposomes
InactiveUS20060034907A1Increased formationPromote formationUltrasonic/sonic/infrasonic diagnosticsAerosol deliveryLiposome membraneWater soluble
A method of manufacturing liposome-containing preparations which contain liposomes exhibiting superior stability in vivo and high enclosure rate of a drug is disclosed, comprising mixing a supercritical or subcritical carbon dioxide, one or more liposome membrane constituents including a phospholipid exhibiting a phase transition temperature and a water-soluble chemical.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
Modified drugs for use in liposomal nanoparticles
Drag derivatives are provided herein which are suitable for loading into liposomal nanoparticle carriers. In some preferred aspects, the derivatives comprise a poorly water-soluble drag derivatized with a weak-base moiety that facilitates active loading of the drag through a LN transmembrane pH or ion gradient into the aqueous interior of the LN. The weak-base moiety can optionally comprise a lipophilic domain that facilitates active loading of the drag to the inner monolayer of the liposomal membrane. Advantageously, LN formulations of the drag derivatives exhibit improved solubility, reduced toxicity, enhanced efficacy, and / or other benefits relative to the corresponding free drags.
Owner:THE UNIV OF BRITISH COLUMBIA
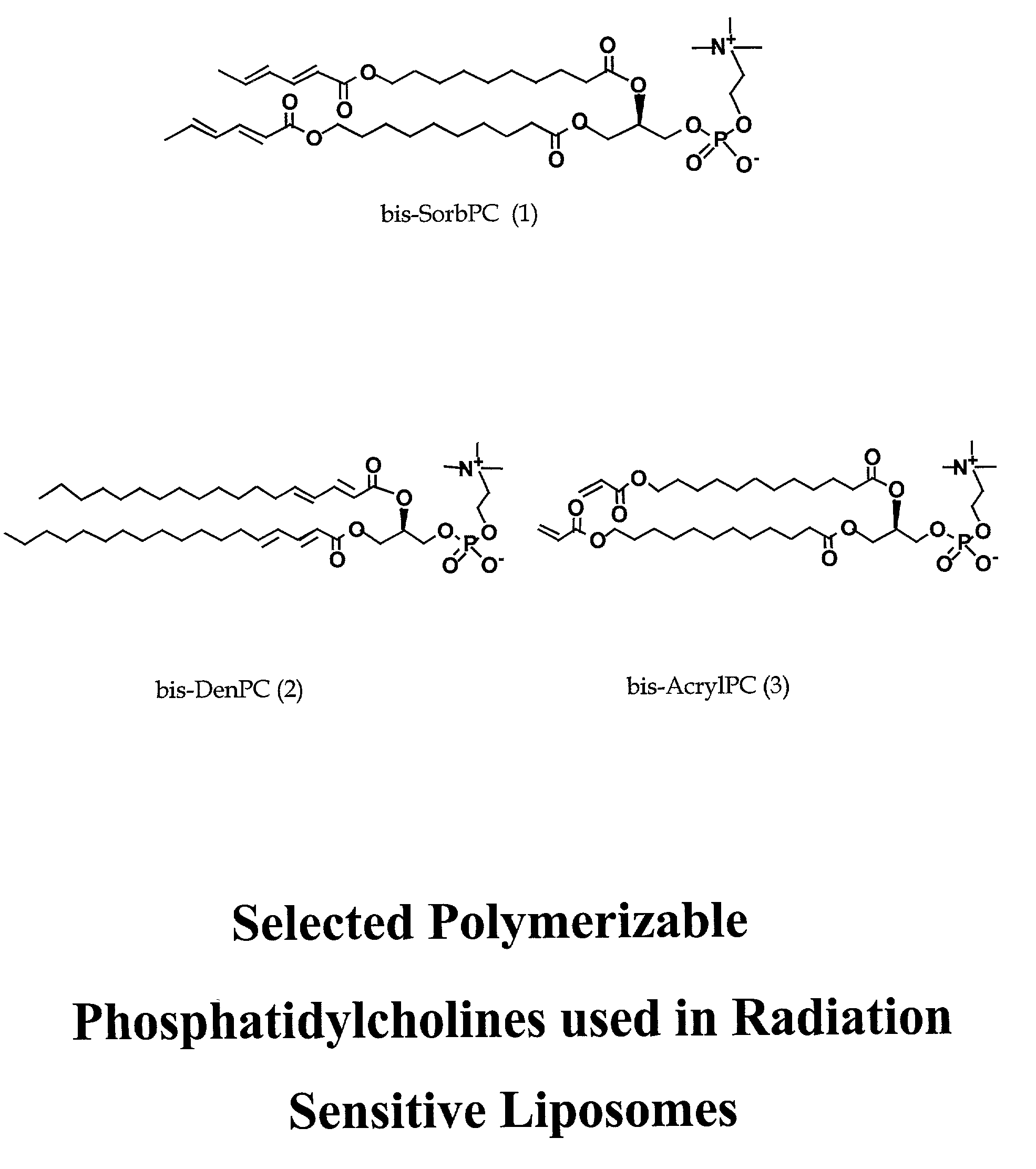
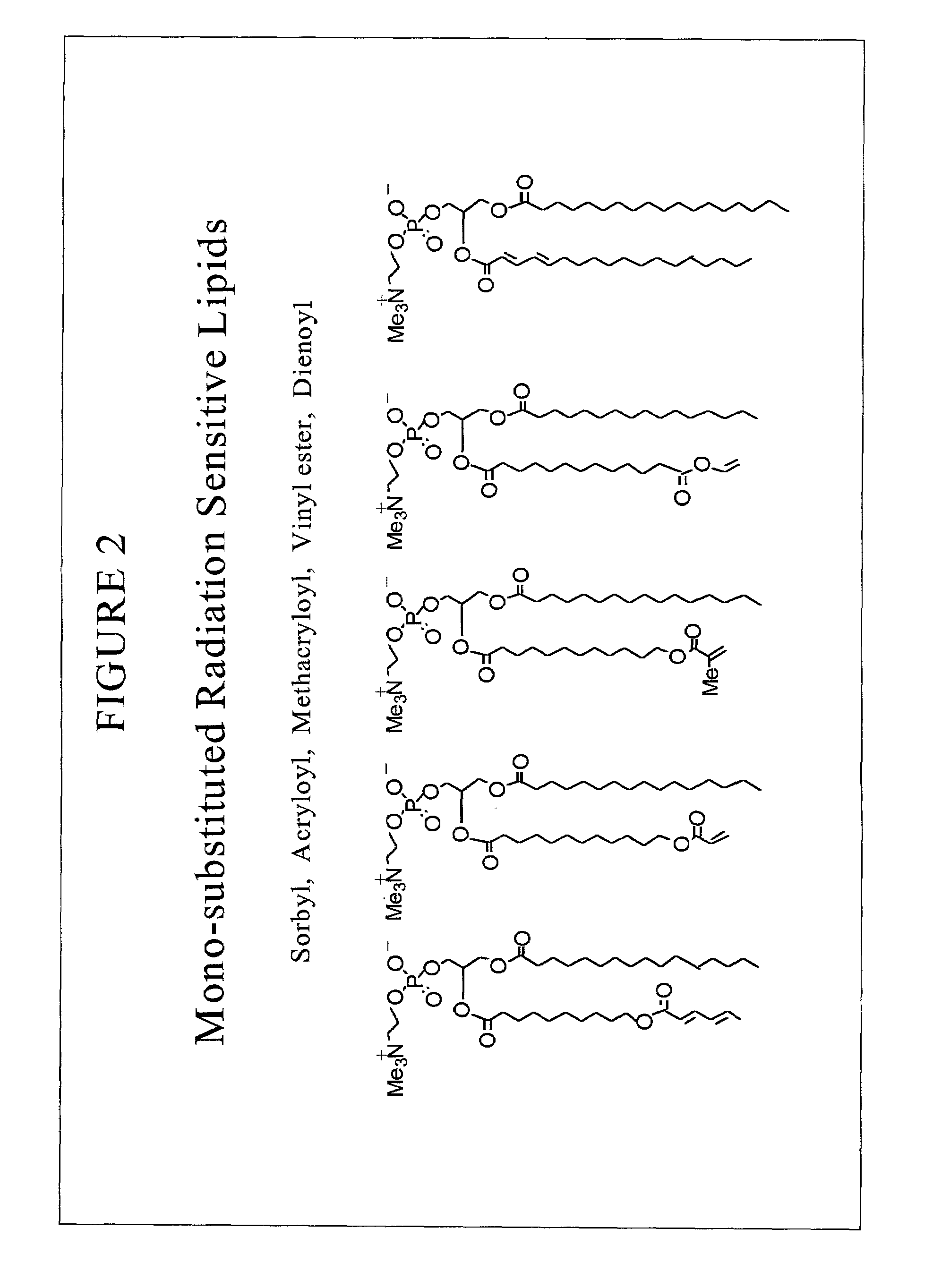
Radiation sensitive liposomes
InactiveUS6989153B2Facilitated releaseEfficient convenient meanUltrasonic/sonic/infrasonic diagnosticsBiocideLipid formationDisease
The present invention relates to a radiation sensitive liposome, and the use of this liposome as carrier for therapeutic and diagnostic agent(s). In particular, the invention encompasses a liposomal delivery system, comprising a stable liposome-forming lipid and a polymerizable colipid, a fraction of which polymerizable colipid polymerizes upon exposure to ionizing radiation, thereby destabilizing the liposomal membrane. Destabilization of liposomes allows for leakage of liposomal contents. The present invention further contemplates methods of diagnosing and treating conditions and diseases that are responsive to liposome-encapsulated or associated agents.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Method of manufacturing pharmaceutical preparations containing liposomes
InactiveCN101001609AX-ray constrast preparationsPharmaceutical non-active ingredientsBiological bodyLiposome membrane
It is intended to provide a method of producing a liposome-containing preparation which is excellent in the stability in vivo and contains liposomes having a drug entrapped therein at an elevated ratio, and a liposome-containing preparation obtained by this method. The method of producing a liposome-containing preparation as described above is characterized by comprising mixing carbon dioxide in the supercritical or subcritical state, a liposome membrane-constituting substance at least containing a phospholipid having a transition temperature and a water-soluble drug in a pressure container and discharging the carbon dioxide by depressurizing the inside of the container to thereby prepare an aqueous dispersion of liposomes having the water-soluble drug entrapped therein.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
Liposome injection of pharmaceutical composition comprising piperacillin sodium and tazobactam sodium
InactiveCN101890015ASimple preparation processFacilitated releaseAntibacterial agentsLiposomal deliveryPiperacillin Sodium/ Tazobactam SodiumLiposome membrane
The invention discloses a liposome injection of a pharmaceutical composition comprising piperacillin sodium and tazobactam sodium. The liposome injection mainly comprises the following components in part by weight: 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4.5 to 13.5 parts of liposome membrane material and membrane material additive, 0.9 to 1.8 parts of frozen dry excipient and 0.45 to 0.9 part of antioxidant.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of galactosylated chitosan modified immune ethosome
ActiveCN105726485AEffective transdermal deliveryEasy to operatePharmaceutical non-active ingredientsAntibody medical ingredientsAntigenProtein solution
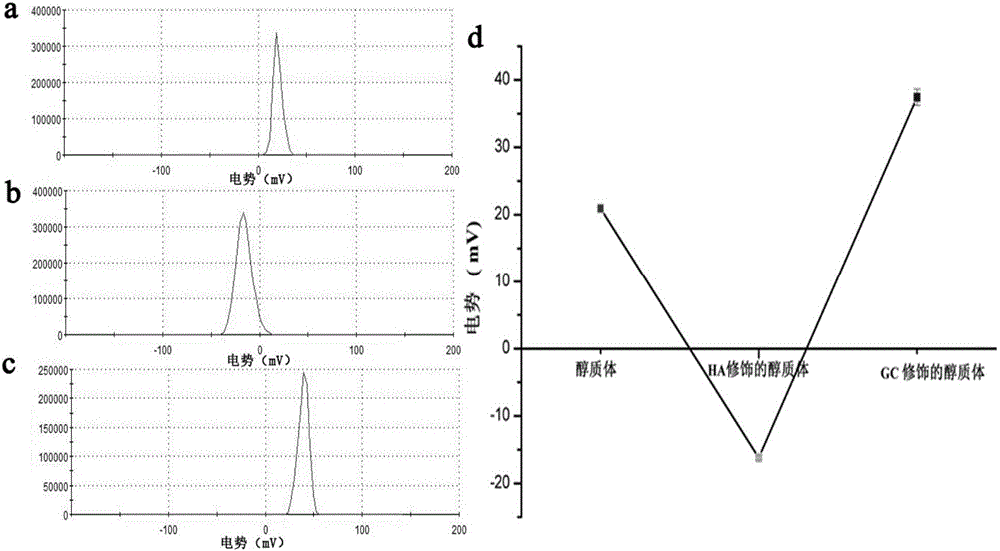
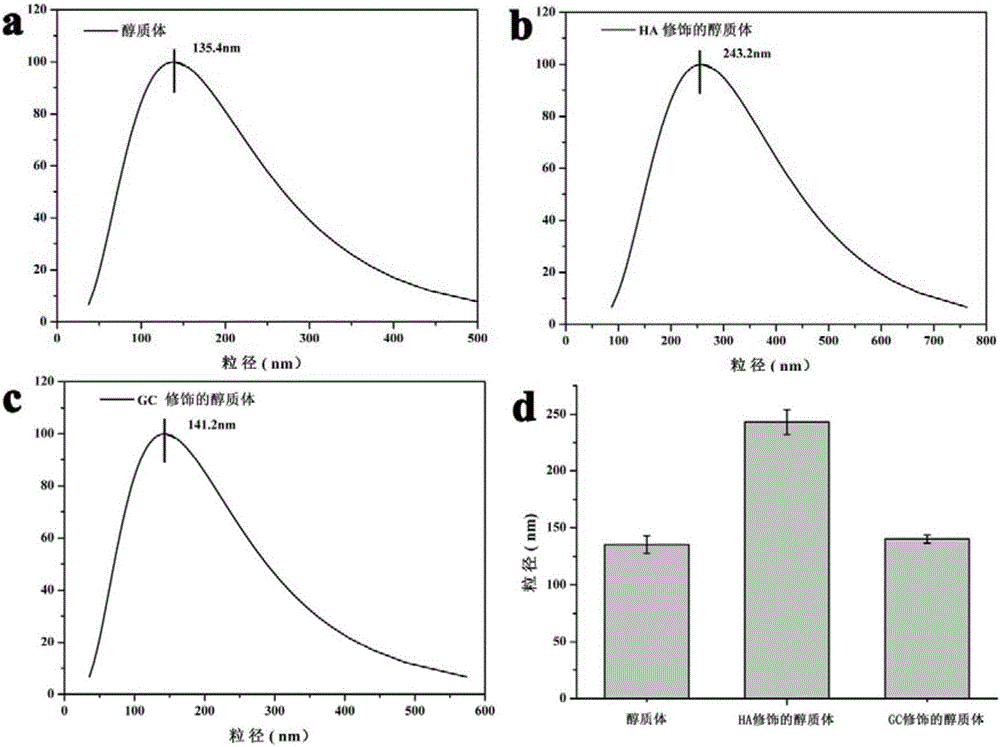
The invention relates to a preparation method of a galactosylated chitosan modified immune ethosome. The preparation method comprises the following steps: mixing lecithin, cholesterol and octadecylamine, then dissolving in ethanol, carrying out rotary evaporation to obtain a liposome membrane, then adding antigen protein solution, and carrying out ultrasonic dispersion to obtain an immune ethosome loaded with antigen protein; by utilizing a layer-by-layer self-assembly technology, dropwise adding the immune ethosome loaded with the antigen protein into hyaluronic acid (HA) solution, stirring to obtain mixed solution, then dropwise adding into galactosylated chitosan (GC) solution, and stirring, so that the galactosylated chitosan modified immune ethosome is obtained. The prepared GC modified immune ethosome has the advantages of high deformability, high encapsulation efficiency, cutin softening and complete skin permeation (so that an organism is immunized and obtains corresponding resistance), no stimulation to skin, stable properties and simple preparation method and is an ideal carrier for constructing a transcutaneous immune patch.
Owner:DONGHUA UNIV
Protein or nucleic acid drug liposome preparation and preparation method thereof based on tetraether lipid
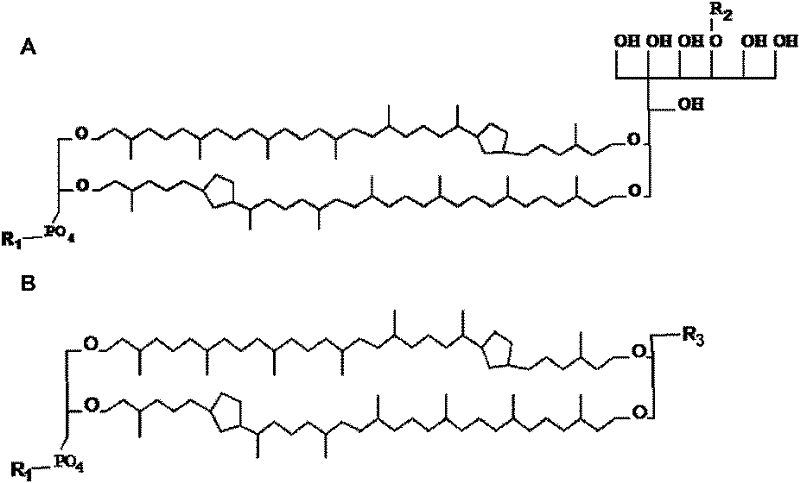
InactiveCN101744768AProtection stabilityImprove bioavailabilityPeptide/protein ingredientsGenetic material ingredientsLiposome membraneVaccine antigen
Disclosed are a protein or nucleic acid drug liposome preparation and a preparation method thereof based on tetraether lipid in large biological molecular drug technical field; the liposome membrane comprises the monomolecular layer of the tetraether lipid; wherein, the tetraether lipid accounts 50-100% of the total lipid mass, the protein drug or nucleic acid drug are dissolved in the water solution phase of the liposome membrane interior. The invention can be used as the oral carrier for polypeptide, protein, oligonucleotide and genetic drugs, guarantees the stability of the drugs in the gastrointestinal tract and improves the bioavailability and efficacy, wherein the polypeptide and protein drugs include insulin, human leukocyte colony growth factor, erythropoietin, human growth factor, calcitonin, exenatide, intestinal peptide hormone, thymosin, etc.; the vaccine antigen includes the antigens of various hepatitis virus, aids virus, HPV virus and herpes virus; the nucleic drugs include the plasmids of various genes, oligonucleotide molecules with various gene sequences and chemical modification structures thereof.
Owner:SHANGHAI JIAO TONG UNIV
Liposome composite phospholipid capable of adjusting phase-transition temperature and application thereof
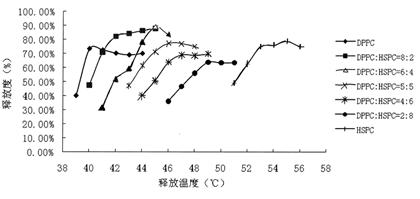
ActiveCN102210870AOvercome the shortcomings of two phase transition temperaturesThe effect of phase transition temperature is eliminatedOrganic active ingredientsPowder deliveryLiposome membranePhosphorylcholine
The invention discloses liposome composite phospholipid capable of adjusting phase-transition temperature and application thereof. The liposome composite phospholipid is prepared from 1-10 molar parts of dipalmitoyl phosphorylcholine (DPPC) and 1-10 molar parts of hydrogenated soya phosphatidylcholine (HSPC), and can be used for preparing thermosensitive target liposome preparation. According to the invention, different phospholipid materials are screened through a large number of experiments, and the experimental result indicates that the liposome composite phospholipid capable of adjusting phase-transition temperature can be obtained by scientifically combining DPPC and HSPC; the composite phospholipid can form a new phase when being prepared into a liposome membrane, avoid phase separation, has a single phase-transition temperature and can overcome the shortcoming of the prior art that two different phospholipid materials have two phase-transition temperatures; and moreover, the composite phospholipid has better stability and target release effect compared with the single-DPPC liposome, has phase-transition temperature with better tolerance to human body compared with the single-HSPC liposome, and has wide application range.
Owner:上海洁士宝日化集团有限公司
Docetaxel liposome and preparation method thereof
ActiveCN103622924ASimple preparation processSmall particle sizeOrganic active ingredientsPowder deliveryLipid formationLiposome membrane
The invention belongs to the technical field of medicine and provides a docetaxel liposome suitable for industrial production and a preparation method thereof. The preparation method provided by the invention comprises the following steps: an organic solvent dissolved in a liposome membrane material of main medicine is dispersed into superfine water-soluble proppant micro powder; the organic solvent is recovered under the decompression condition; lipid is adsorbed to a water-soluble carrier to obtain a powder liposome. When the powder liposome is in contact with a hydration medium, the lipid swells and the water-soluble vector is rapidly dissolved so as to form a multilayer liposome in a water phase; a clarified liposome with blue opalescence is obtained by externally applying acting force to reduce the particle size; liposome powder is obtained by adding a freeze-drying protective additive to carry out freezing and drying; liposome solution is obtained by redissolving the liposome powder before use. According to the invention, the liposome is prepared by utilizing a decompression carrier freeze-drying technology; stability of the liposome can be greatly improved; the preparation method is easy for production scale-up and provides a novel thinking for industrialization of the liposome.
Owner:SHENYANG PHARMA UNIVERSITY +1
Remedy or diagnostic for inflammatory disease containing target-directing liposome
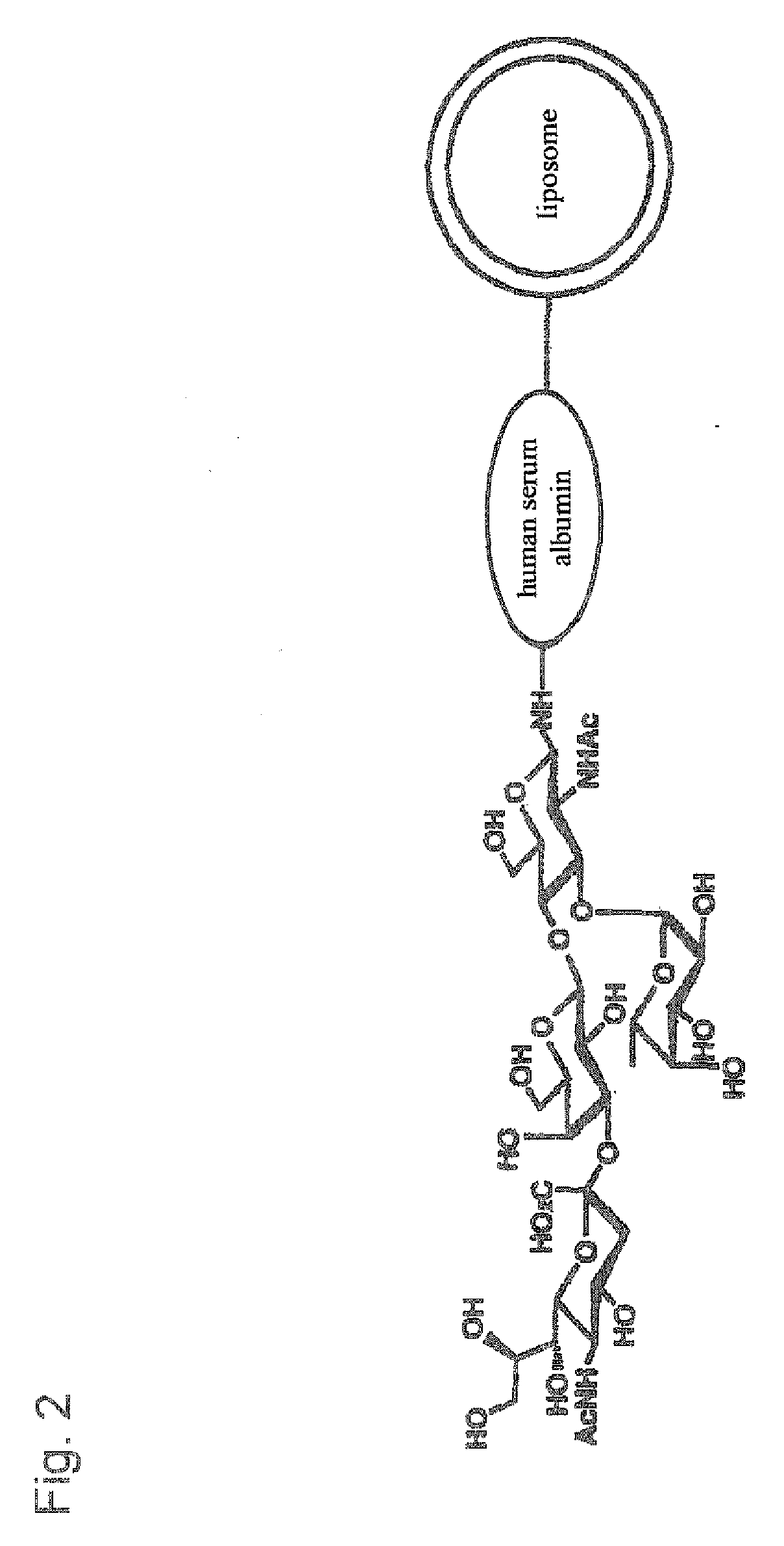
There is provided targeting drug delivery system (DDS) nanoparticles that can be accumulated in target tissues such as inflammatory sites of inflammatory diseases and can thus be utilized in a therapeutic or diagnostic DDS for locally delivering drugs or genes to the affected parts. The present invention provides a pharmaceutical composition for the medical treatment or diagnosis of an inflammatory disease comprising a sugar-modified liposome having a sugar chain bound to the membrane of the liposome.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Preparation method of medicine-carrying ethosome modified by galactosed polyethyleneimine
InactiveCN107184552AEasy to operateShort preparation timeOrganic active ingredientsGenetic material ingredientsLiposome membraneCholesterol
The invention discloses a preparation method of a medicine-carrying ethosome modified by galactosed polyethyleneimine. The method is characterized by mixing lecithin, cholesterol and octadecylamine, then dissolving the mixture in alcohol, carrying out rotary evaporation to obtain a liposome membrane, and then adding the liposome membrane into a medicine-containing solution to obtain medicine-carrying ethosome; dropwise adding the medicine-carrying ethosome into a galactosed polyethyleneimine solution by using a layer-by-layer self-assembly technique, simultaneously stirring and carrying out ultrasonic dispersion, then adding nucleic acid medicines to obtain the medicine-carrying ethosome modified by galactosed polyethyleneimine. Through the preparation method of the medicine-carrying ethosome modified by galactosed polyethyleneimine, galactosed polyethyleneimine and nucleic acid medicines are successively assembled outside the medicine-carrying ethosome through electrostatic adsorption by mainly using an ethosome preparation technique and the layer-by-layer self-assembly technique; a plurality of medicines (including gene medicines) are effectively loaded and delivered; and the therapy targeted to liver tumor cells of the medicines can be improved.
Owner:DONGHUA UNIV
Biological adhesive liposome preparation for eyes and preparation method thereof
InactiveCN101669909AIncrease contact timeImproves ocular bioavailabilitySenses disorderPharmaceutical non-active ingredientsLiposome membranePoor compliance
The invention belongs to the field of medicinal preparations, and relates to liposome for eyes and a preparation method thereof. In order to overcome the defects that common eye drops have short residence time in the conjunctival sac to cause low bioavailability at the eyes and the semi-solid dosage form has poor compliance and is not easy to be accepted by patients and the like in the prior art,the invention provides a biological adhesive liposome preparation for eyes. The biological adhesive liposome preparation for the eyes consists of the liposome, a liposome membrane modification material and a medicament wrapped in the liposome, wherein the surface of the liposome is modified with free mercapto, and a covalent binding disulfide bond can be formed by the free mercapto and a mucoprotein subdomain rich in cysteine on the surface of the eye to anchor the liposome on the surface of a mucous membrane and serve as a medicament store to slowly release the medicament in the conjunctivalsac and provide permanent driving force for the absorption of the medicament. The preparation is helpful for promoting the absorption of the medicament at the eyes, and can improve the bioavailabilityof the medicament.
Owner:FUDAN UNIV
Liposome-based immunotherapy
PendingUS20160338953A1Highly effective in treatmentEffective treatmentNervous disorderAntipyreticAntigenDisease
The present invention provides a liposome encapsulating an autoantigen, wherein the liposome has a size comprised from 500 to 15000 nm and the liposome membrane comprises phosphatydilserine (PS) in an amount comprised from 10 to 40% by weight with respect to the total membrane liposomal composition. Pharmaceutical or veterinary compositions comprising a therapeutically effective amount of said liposome are also provided. Further, the invention provides liposomes and pharmaceutical or veterinary compositions as defined above for use as a medicament, particularly for the treatment of autoimmune diseases. Finally the present invention provides liposomes and pharmaceutical or veterinary compositions as defined above for use in the restoration of tolerance to self in a patient suffering from an autoimmune disease.
Owner:FUNDACIO INST DINVESTIGACIO & CIENCIES DE LA SALUT GERMANS TRIAS I PUJOL +2
Tumor-targeting nanoparticles, preparation method and application thereof
InactiveCN109172830AGood dispersionGood biocompatibilityPowder deliveryEnergy modified materialsTumor targetLiposome membrane
Owner:CHONGQING MEDICAL UNIVERSITY
Liposome composite body
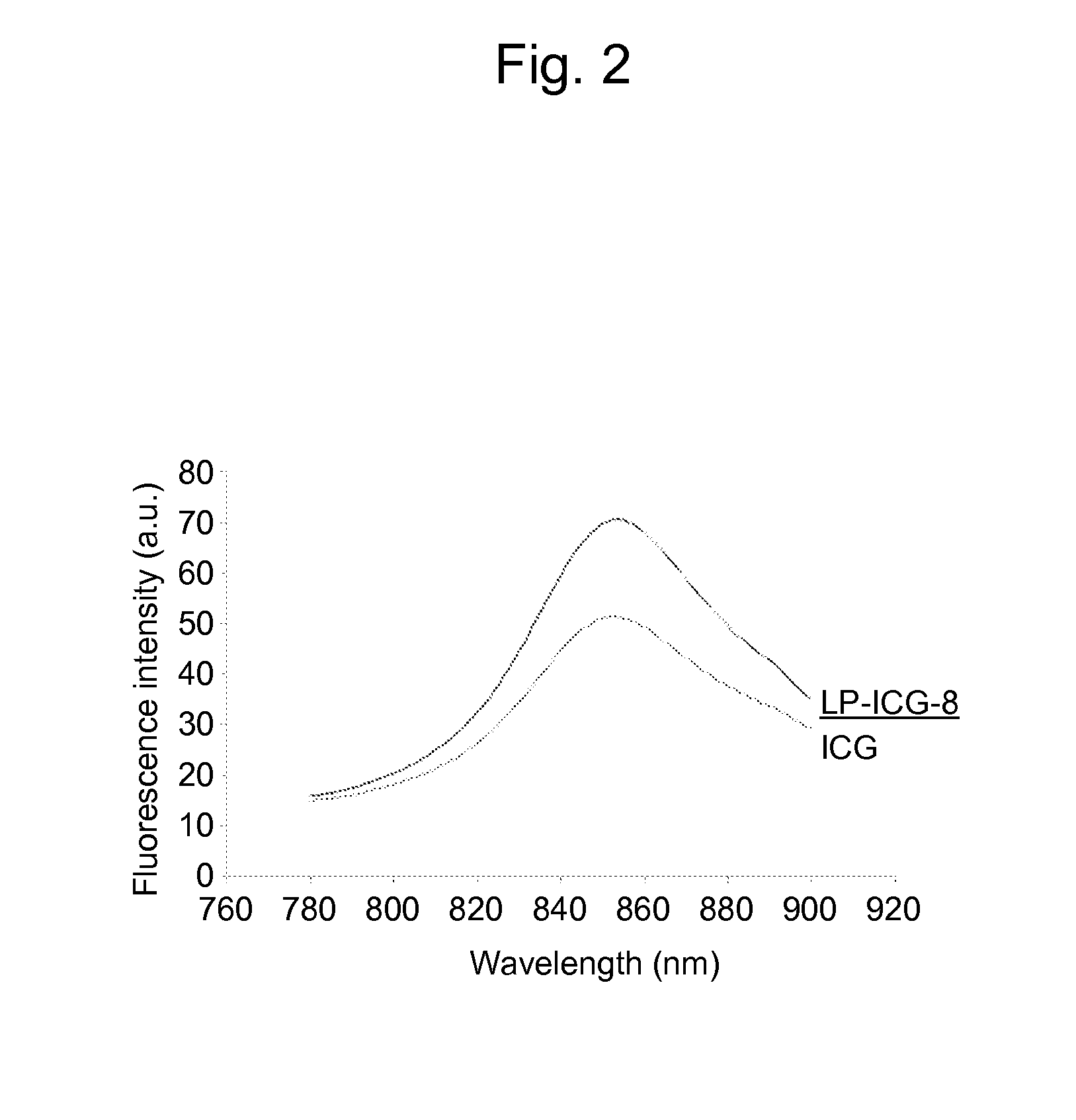
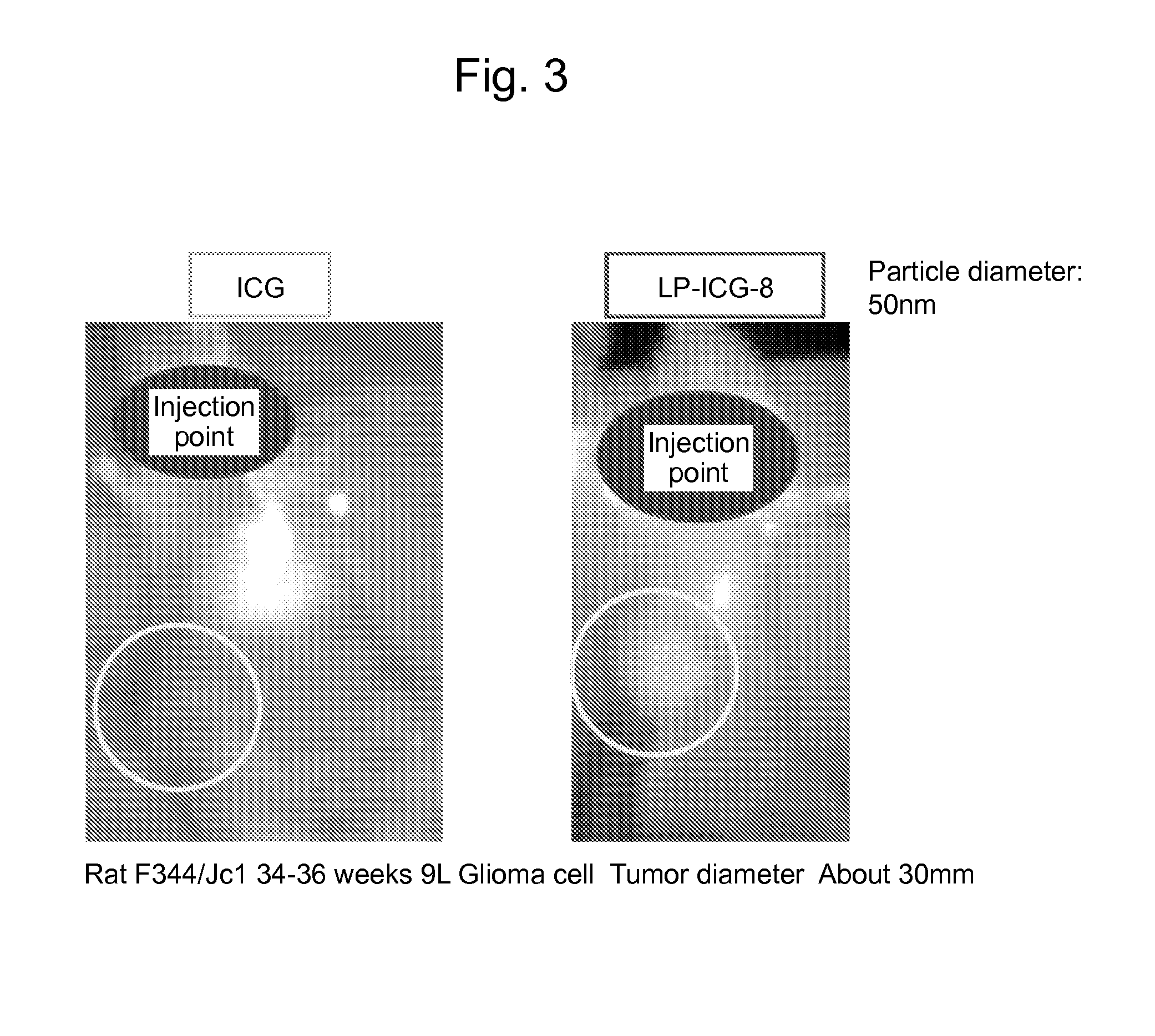
An object of the present invention is to provide a drug delivery system capable of sustainedly releasing a drug noninvasively at any given point in time. The present invention relates to a liposome complex comprising a liposome membrane-constituting substance bonded to a light-absorbing compound having an absorption wavelength in the near-infrared region, selected from the group consisting of indocyanine green dyes, phthalocyanine dyes, squarylium dyes, croconium dyes, and diimmonium dyes.
Owner:CHIBA UNIVERSITY
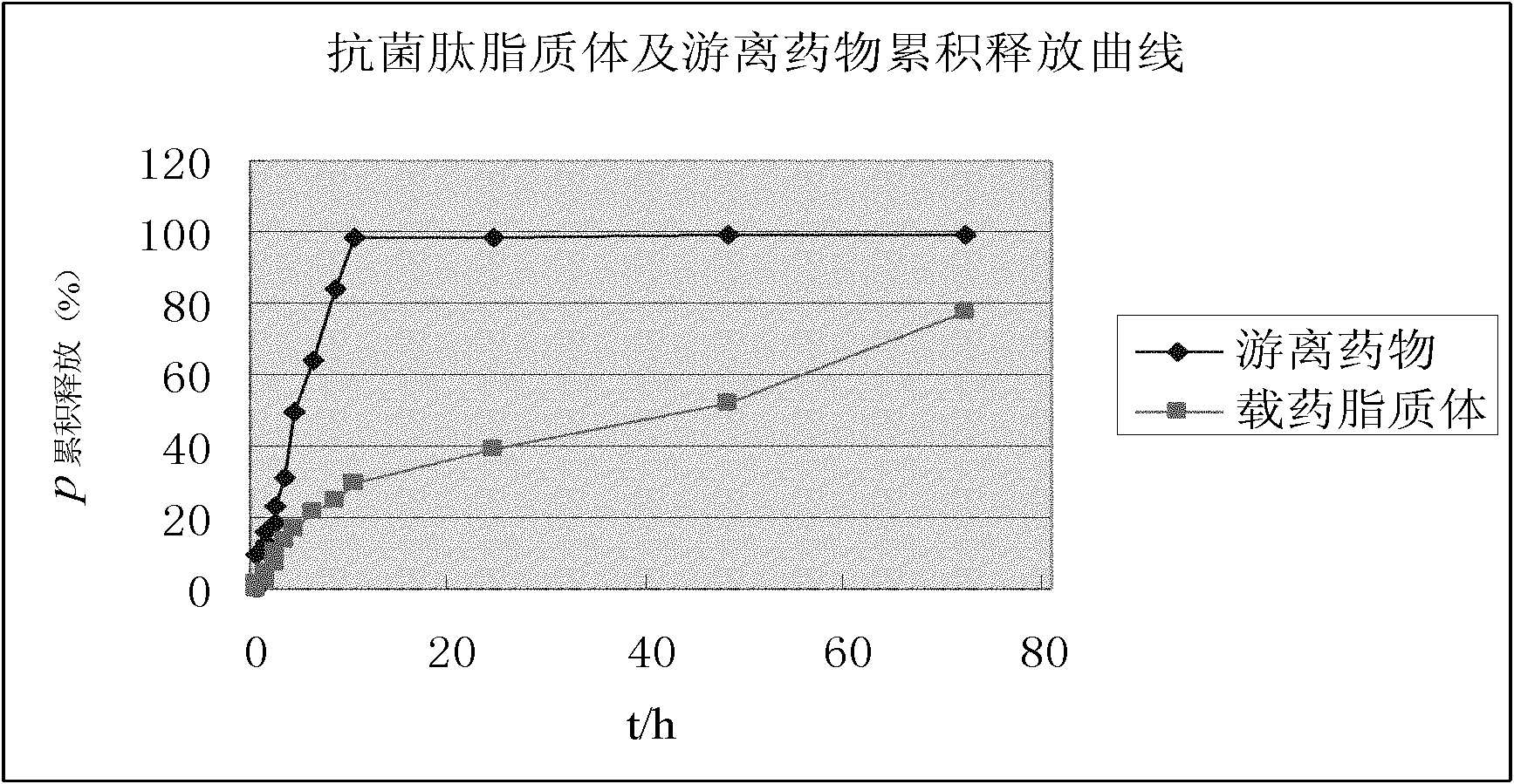
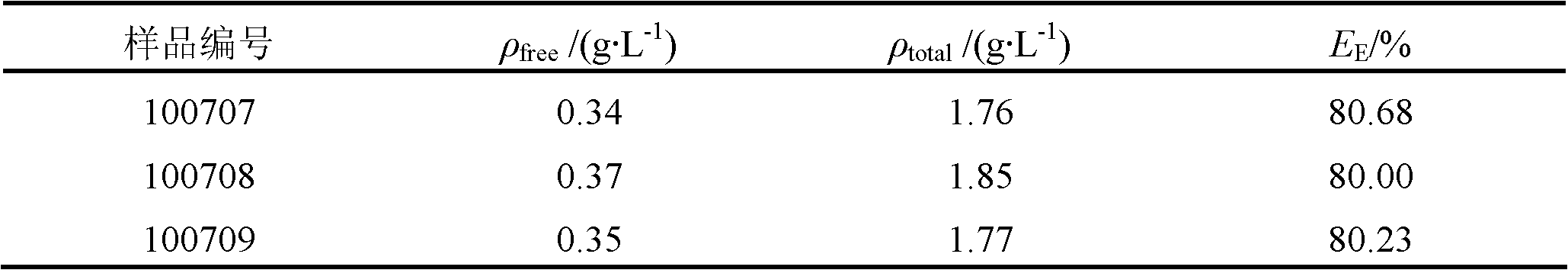
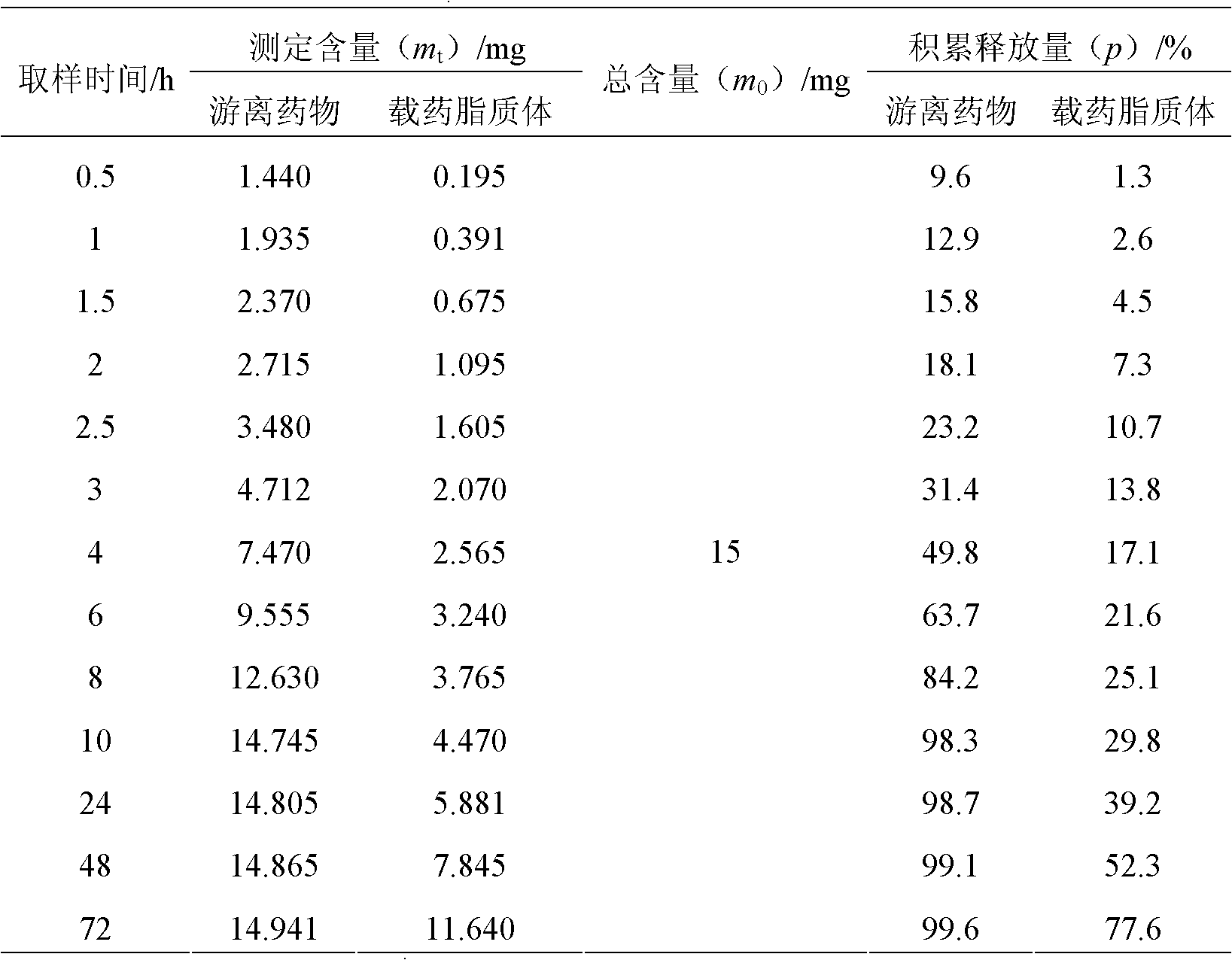
Preparation method for antibacterial peptide liposome
InactiveCN102462661AImprove stabilityProlonged resistance activityAntibacterial agentsPeptide/protein ingredientsEmbedding rateLiposome membrane
The invention provides a preparation method for an antibacterial peptide liposome. Medicinal active ingredients include an antibacterial peptide, a liposome film additive, a preservative and the like. The preparation method has the advantages of high embedding rate, stable preparation process, stable medicament effect, low leak rate and the like. The antibacterial peptide is prepared into the active antibacterial peptide liposome, so that the embedding rate of the antibacterial peptide can be increased, and the active period of the anti-bacteria peptide is prolonged.
Owner:TIANJIN RINGPU BIO TECH
Polyglutamic acid and human epidermal growth factor nano-liposome and preparation method thereof
ActiveCN109316446AAvoid influenceImprove placement stabilityOrganic active ingredientsCosmetic preparationsLiposome membraneCuticle
The invention belongs to the technical field of bioengineering and discloses a polyglutamic acid and human epidermal growth factor nano-liposome and a preparation method thereof. The nano-liposome comprises high molecular weight polyglutamic acid, low molecular weight polyglutamic acid, a human epidermal growth factor and a liposome membrane material. According to the polyglutamic acid and human epidermal growth factor nano-liposome, the high molecular weight polyglutamic acid and the low molecular weight polyglutamic acid are compounded with the human epidermal growth factor and the preservative-free nano-liposome is prepared by utilizing a bacterium-inhibition effect of epsilon-polylysine; the nano-liposome has an addition and multiplying effect on skin repairing; the transdermal performance and skin absorption rate of active components are greatly improved; the nano-liposome has stable performance, good utilization compatibility and relatively high encapsulation rate.
Owner:NANJING SHINEKING BIOTECH CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com