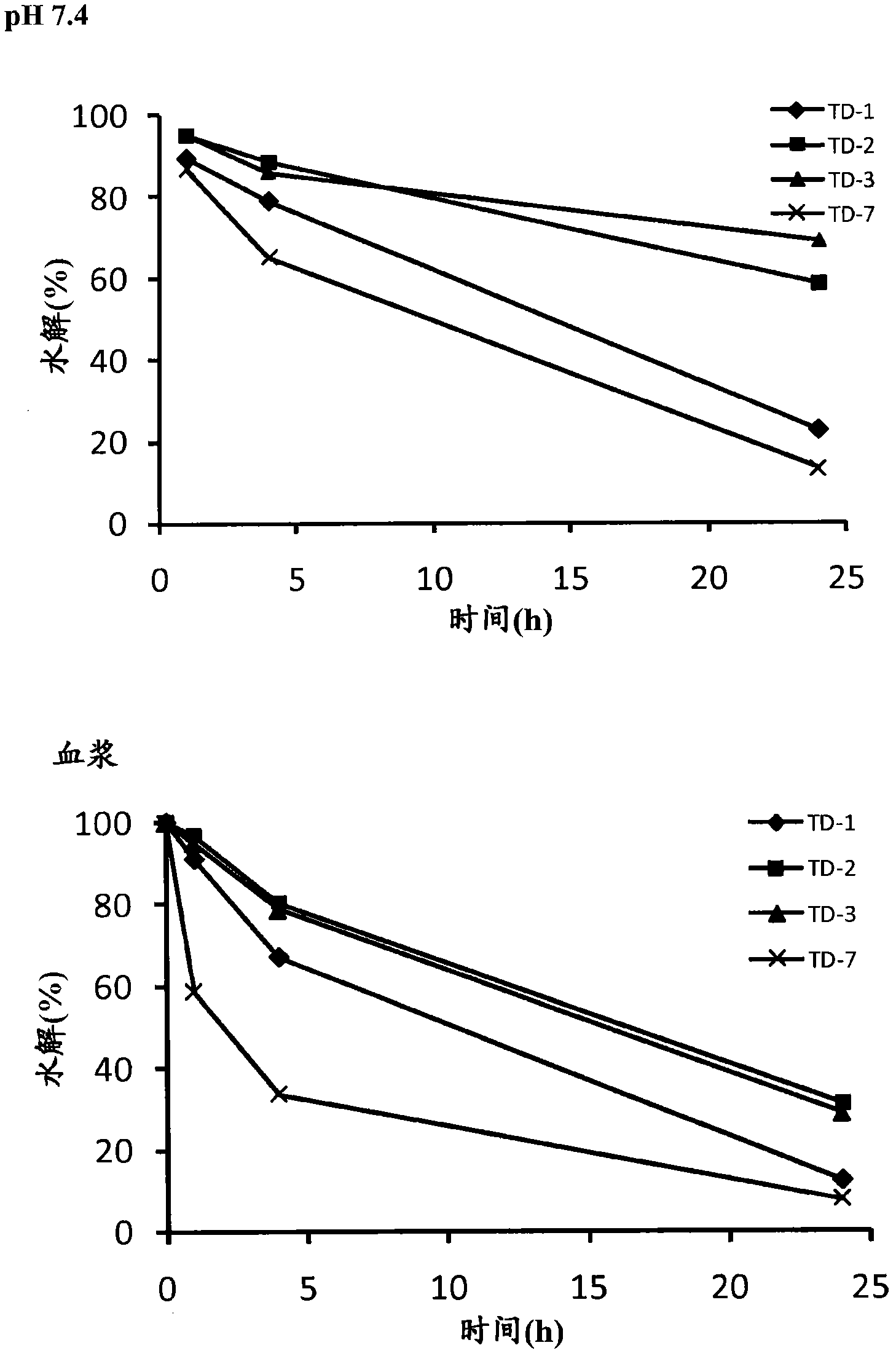
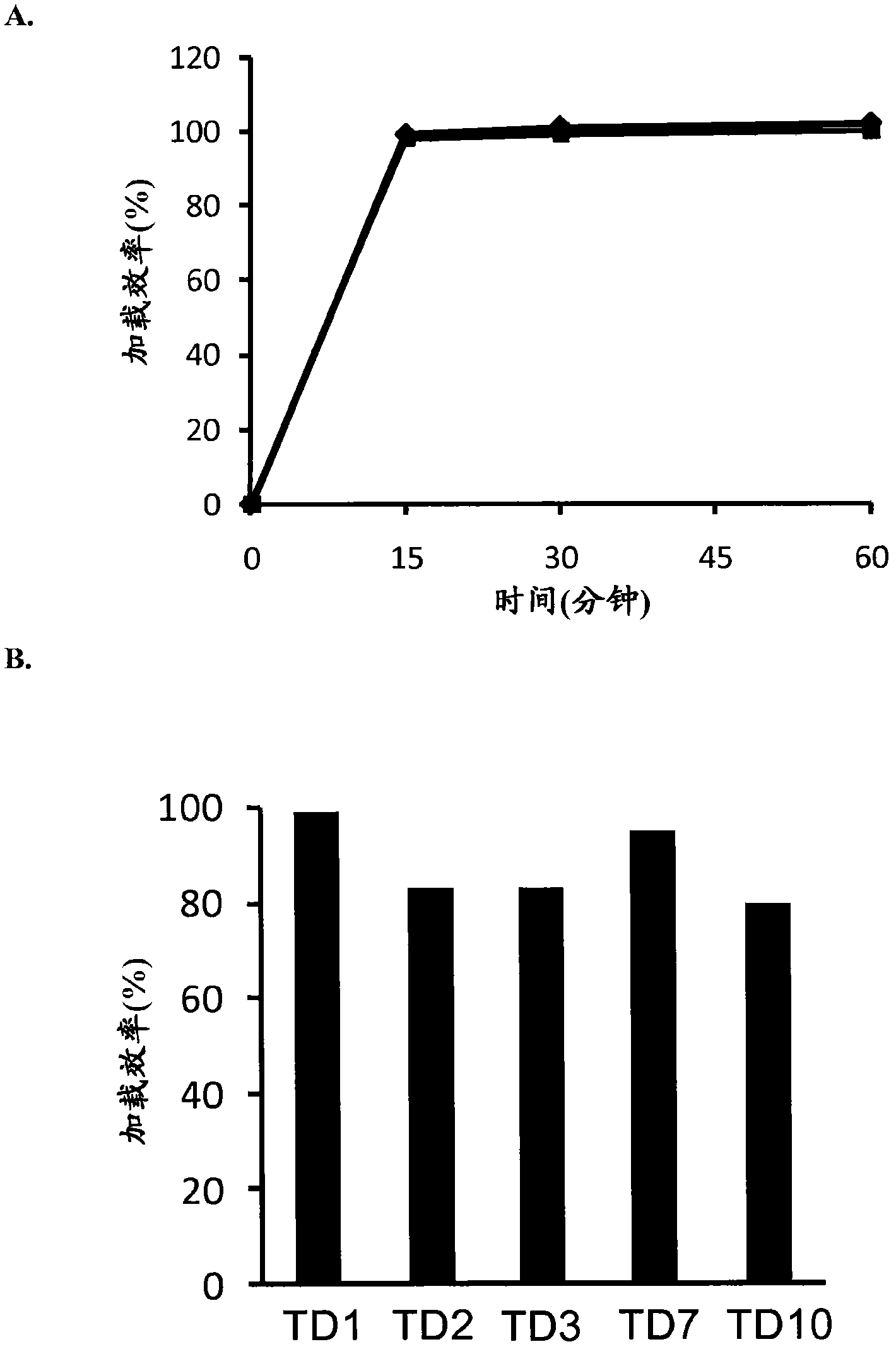
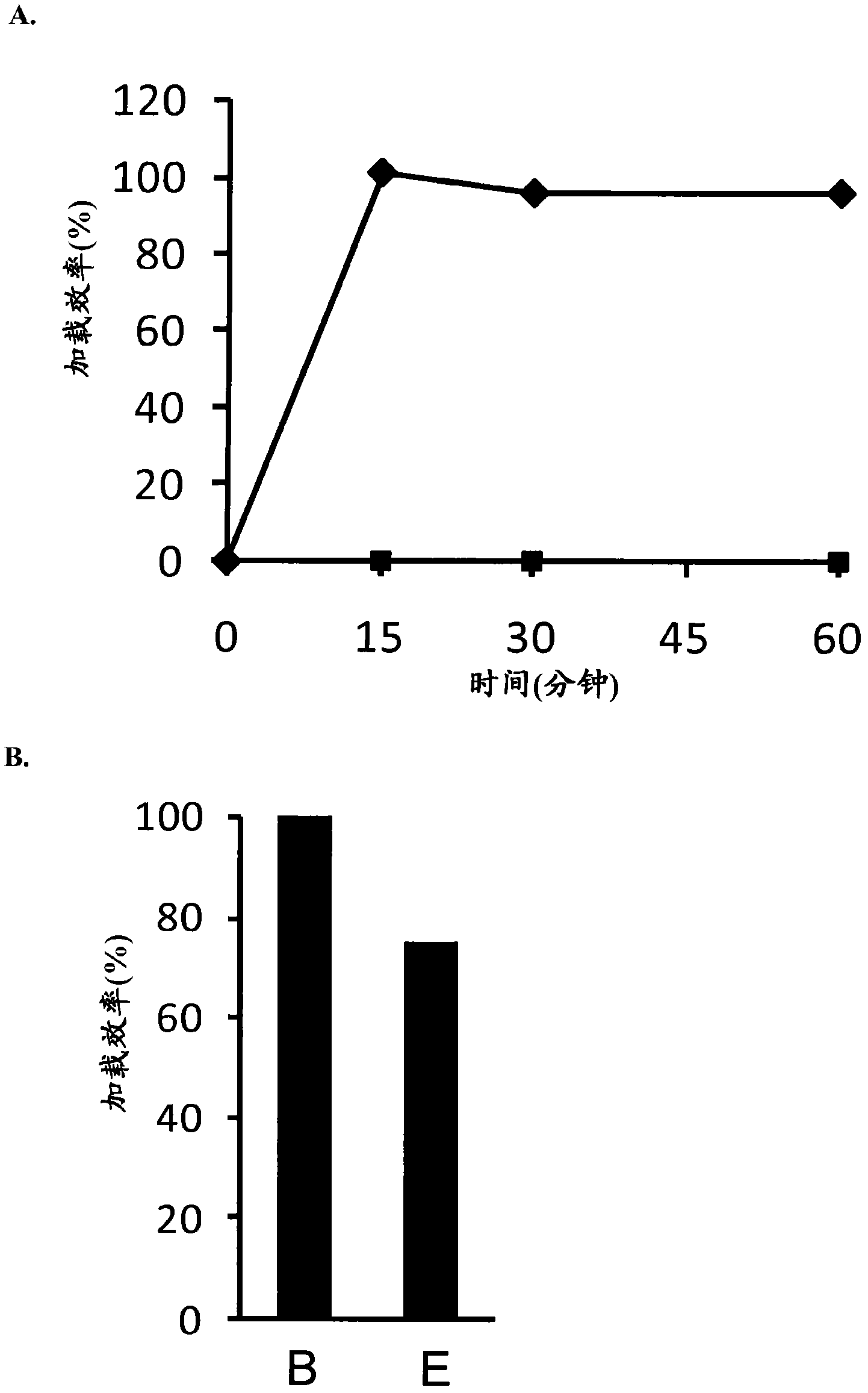
Modified drugs for use in liposomal nanoparticles
A nanoparticle and liposome technology, applied in the field of drugs for the modification of liposome nanoparticles, can solve problems such as limiting therapeutic potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0313] Various methods available for preparing liposomes are described, for example, in: Szoka et al., Ann. Rev. Biophys. Bioeng. 9:467 (1980); U.S. Pat. , ed., Marcel Dekker, Inc., New York, 1983, Chapter 1; and Hope et al. Chem. Phys. Lip. 40:89 (1986), the entire contents of which are incorporated herein by reference. In some preferred aspects, liposomes are small liposomes with a diameter of about 100 nm formed by extrusion of a hydrated lipid dispersion through a filter membrane with 100 nm pores, generally as described in Hope et al., Biochim. Biophys. Acta, 812:55-65 (1985), which is incorporated herein by reference.
[0314] In one method, heterogeneously sized multilamellar vesicles are prepared by dissolving vesicle-forming lipids in a suitable organic solvent or solvent system, and drying the mixture under vacuum (or under inert gas conditions) to form thin lipids. plasma membrane. Alternatively, lipids can be dissolved in a suitable solvent such as tert-butanol a...
Embodiment 1
[0329] Embodiment 1-chemical synthesis method
[0330] Quantification of weakly basic derivatives and unmodified drugs using ultra-performance liquid chromatography (UPLC). Use an instrument consisting of: Waters Acquity TM UPLC system with photodiode array detector (PDA) and triple-quadrupole (TQ) MS detector; Empower TM Data acquisition software version 2.0 (Waters, USA). Separation was performed as follows: using Waters Acquity TM BEH C18 column (1.7 μm, 2.1×100 mm), flow rate 0.25 mL / min, mobile phase A and B consisted of water containing 0.1% trifluoroacetic acid (TFA) and acetonitrile containing 0.1% TFA, respectively. For prednisone and etoposide derivatives and unmodified drugs, the mobile phase consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The mobile phase was delivered under a programmed linear gradient at a column temperature of 23°C.
[0331] For the docetaxel derivative and docetaxel, the separation was initiat...
Embodiment 2
[0342] Example 2 - Taxane Derivatives
[0343] At the hydroxyl group at the C-2' position, docetaxel was derivatized with N-methyl-piperazinylbutanoic acid to form the amino ester prodrug (TD1), as described below.
[0344] 2′-O-(N-methyl-piperazinylbutyrate) derivative of docetaxel (TD1)
[0345] Linker Synthesis: 4-(4-Methylpiperazin-1-yl)butyrate hydrochloride
[0346]
[0347] 1-Methylpiperazine (7.68 mL, 70 mmol, 4 equiv) was added to a stirred solution of ethyl 4-bromobutyrate (2.5 mL, 17.3 mmol) in ethyl acetate (50 mL) at room temperature. The solution was stirred at 25°C for 1 hour, a white precipitate formed and then heated to 70°C on an oil bath for 1 hour. TLC analysis (20% ethyl acetate (EtOAc) / hexanes, Rf = 0.9 (starting material), 0.1 (product), visualized with iodine (I2)) indicated complete consumption of the bromide reagent. The reaction was diluted with EtOAc (100mL), transferred to a separatory funnel, water (100mL), sodium bicarbonate (NaHCO 3 , s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com