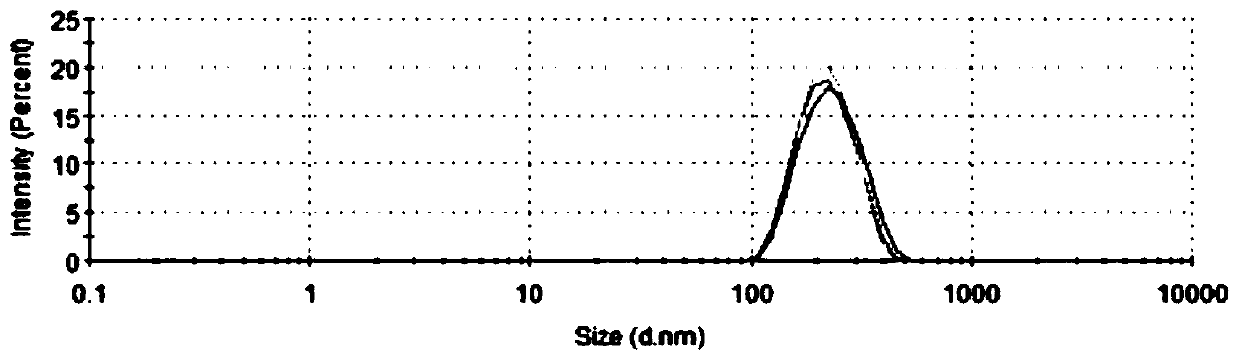
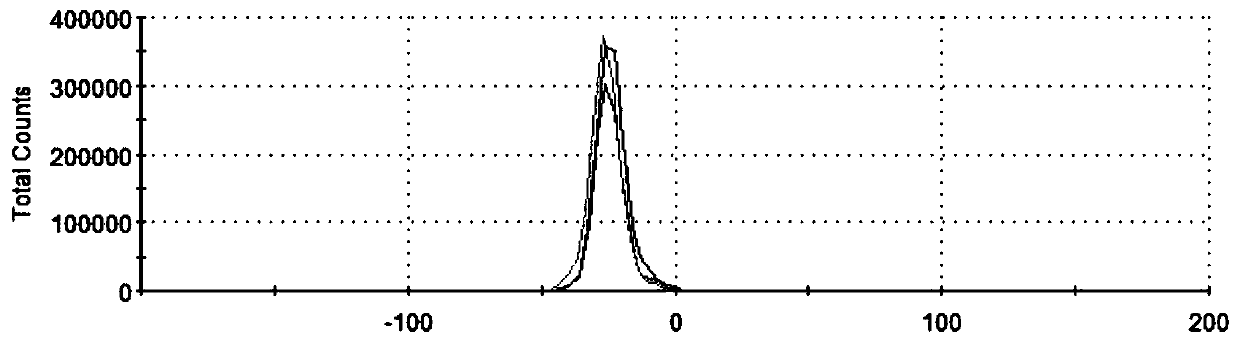
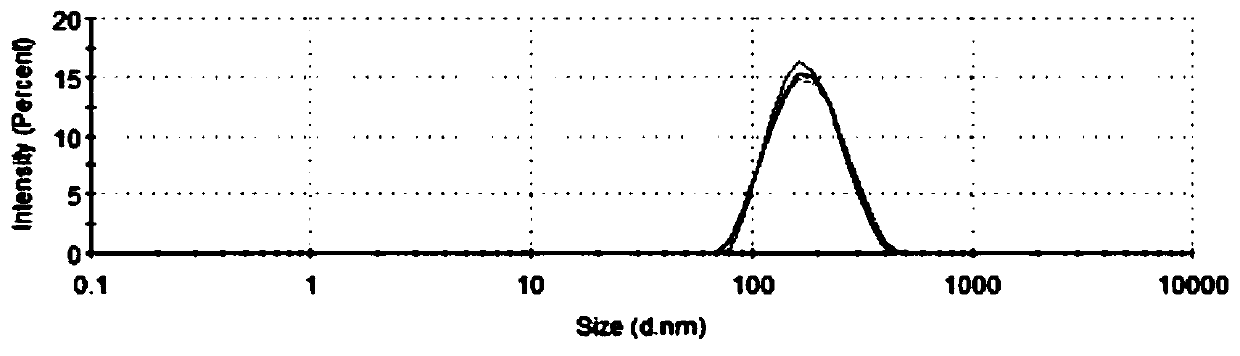
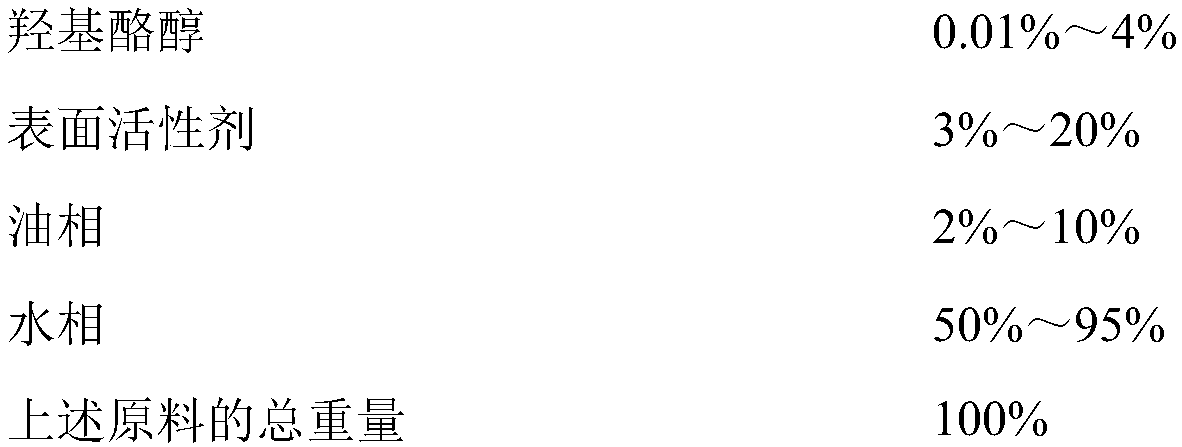
Patents
Literature
57 results about "Opalescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Opalescence refers to the optical phenomena displayed by the mineraloid gemstone opal (hydrated silicon dioxide). However, there are three notable types of opal (precious, common, and fire), each with different optical effects, so the intended meaning varies depending on context. The optical effects seen in various types of opal are a result of refraction (precious and fire) or reflection (common) due to the layering, spacing, and size of the myriad microscopic silicon dioxide spheres and included water (or air) in its physical structure. When the size and spacing of the silica spheres are relatively small, refracted blue-green colors are prevalent; when relatively larger, refracted yellow-orange-red colors are seen; and when larger yet, reflection yields a milky-hazy sheen.
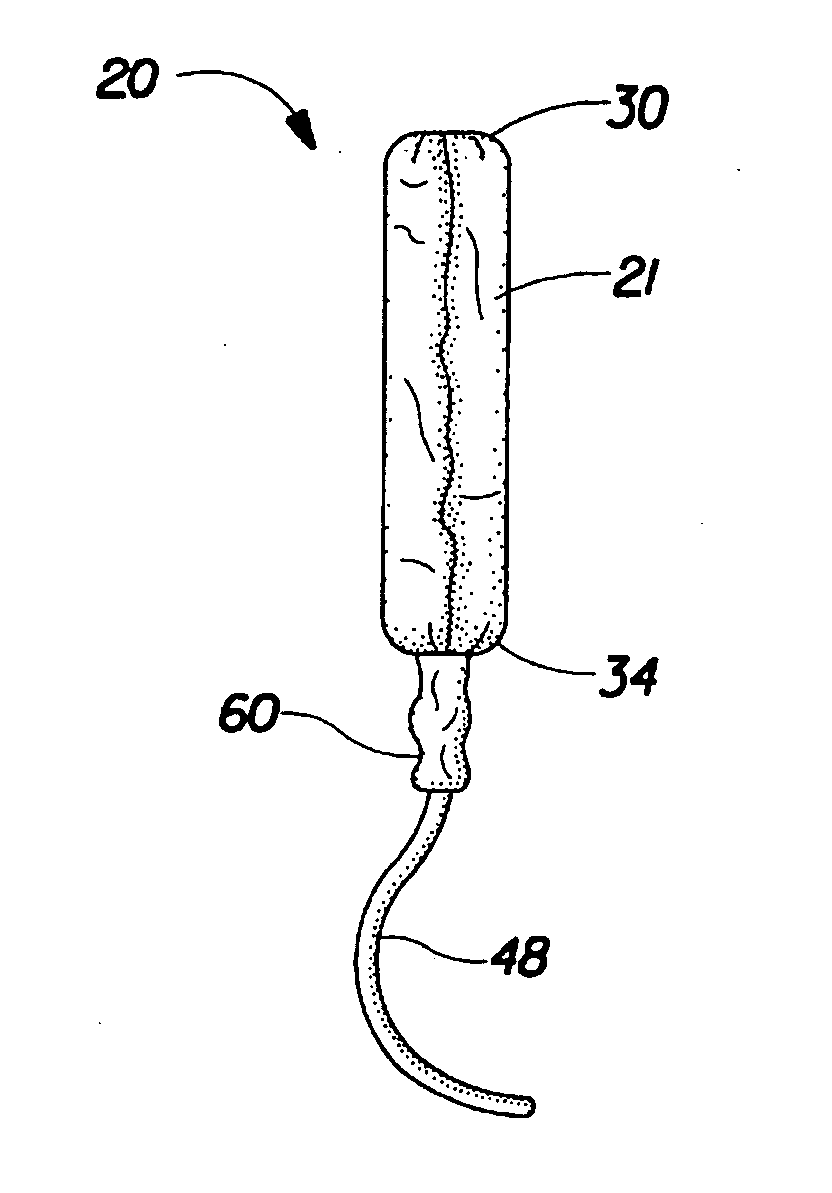
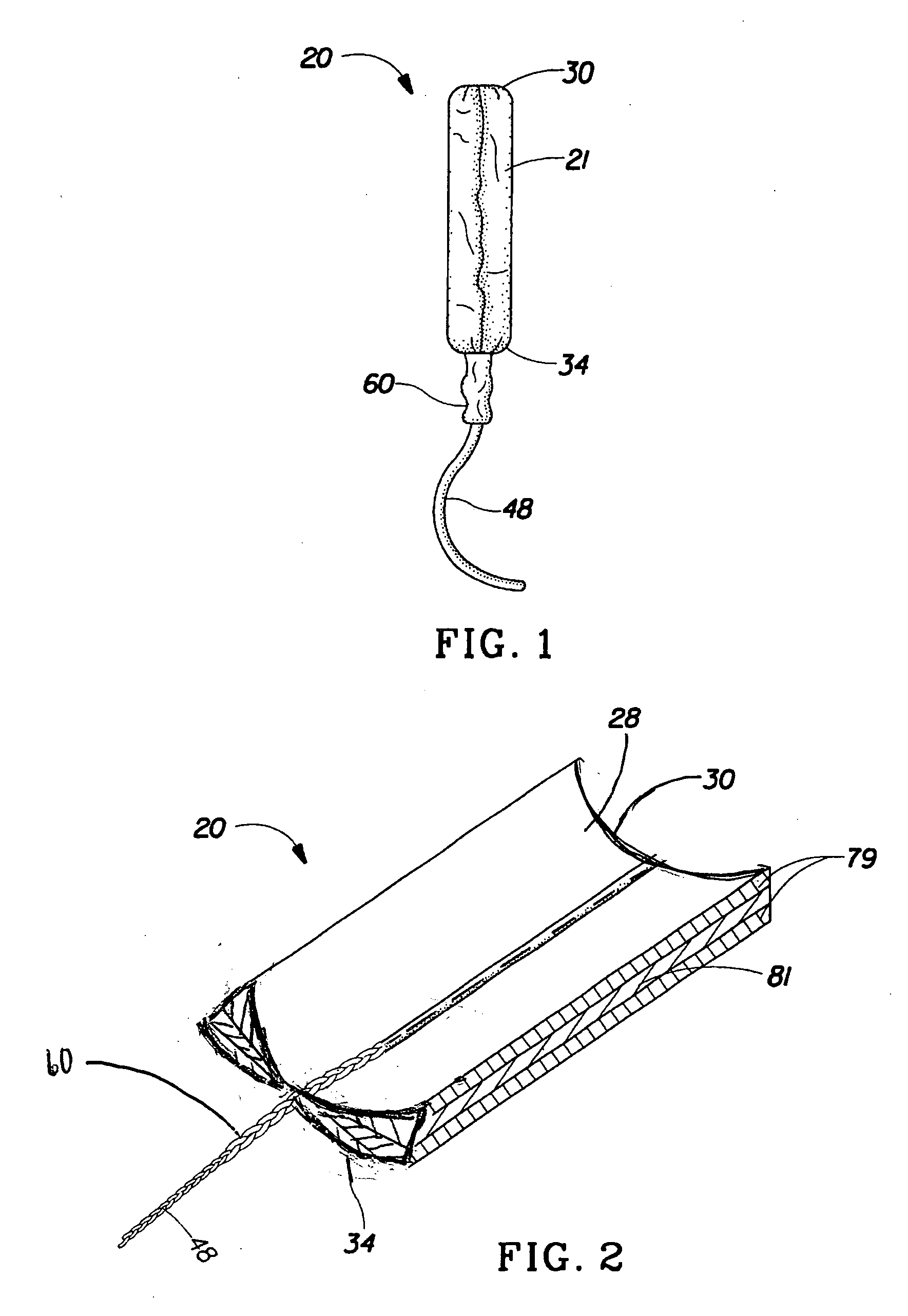
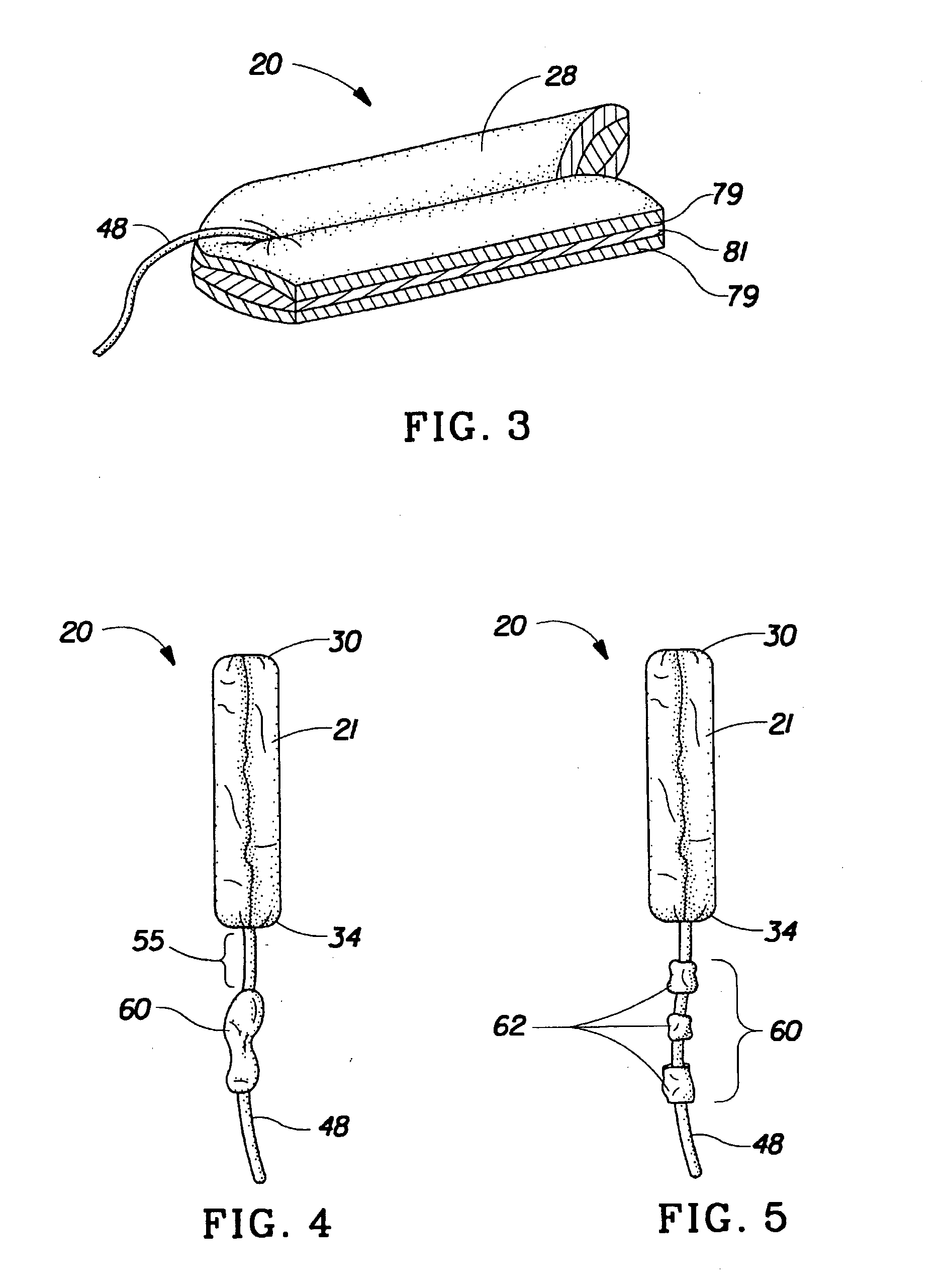
Absorbent tampon comprising a visually distinct withdrawal member
A tampon that includes a primary absorbent member, secondary absorbent member and a withdrawal member that is visually distinct from the secondary absorbent member. The visual distinctiveness of the withdrawal member with respect to the secondary absorbent member is due to a difference in color, shade, pattern, reflectance, opacity, translucence, opalescence, fluorescence, luminescence, phosphorescence, chemiluminescence, whiteness and the mixtures thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Synthesis, uses and compositions of crystal hydrogels
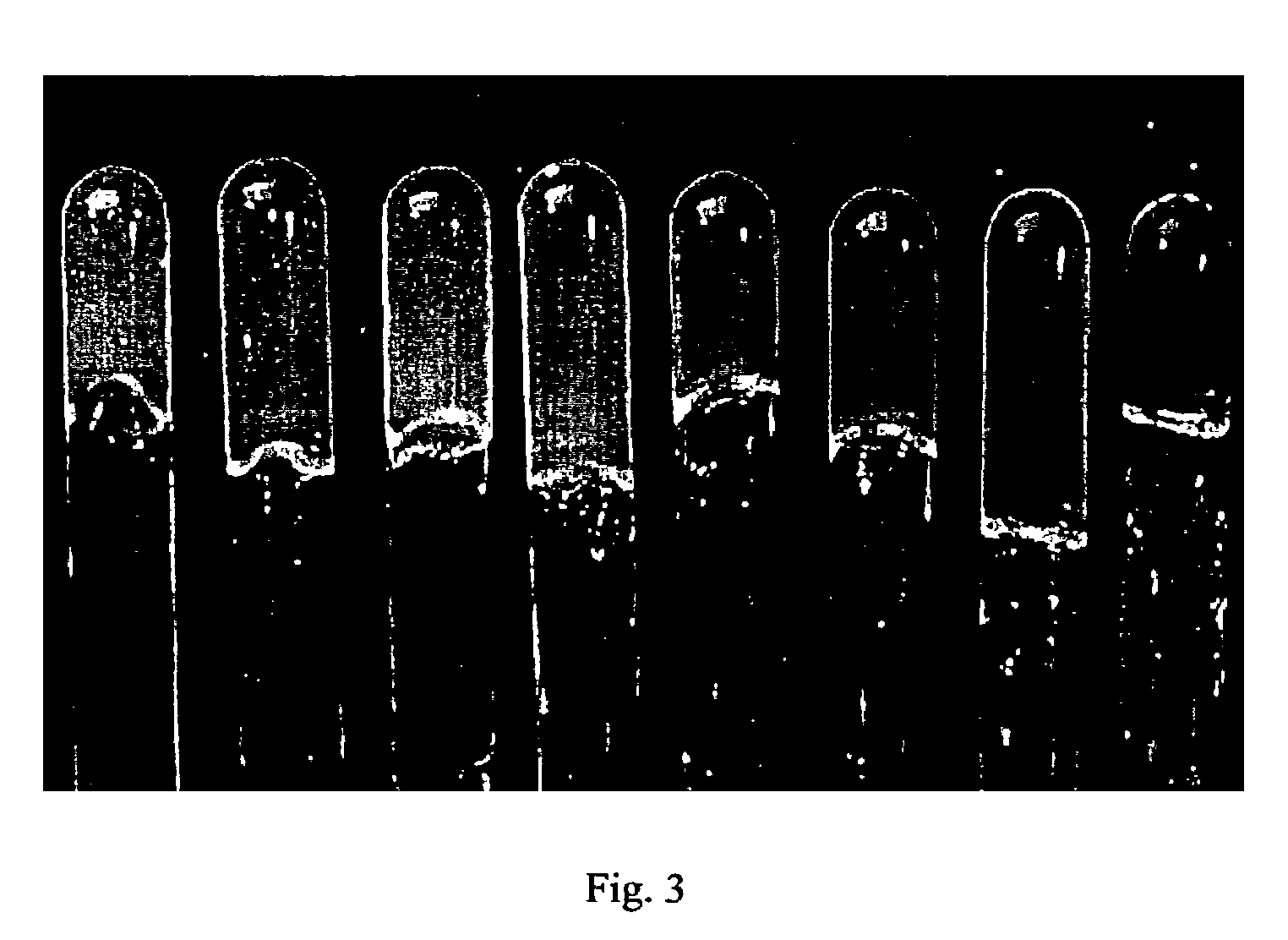
A method is disclosed for creating hydrogels with ordered crystalline structures that exhibit a characteristic colored opalescence. In addition to the unique optical properties, these materials contain a large amount of water in their crosslinked networks. The manufacturing processes include synthesizing monodispersed hydrogel nanoparticles containing specific reactive functional groups, self-assembly of these particles to form a crystalline structure, and subsequent crosslinking neighboring spheres to stabilize the entire network. Polymerizing a hydrogel monomeric composition around the crystalline structure can enhance the mechanical strength. The resulting network is dimensionally and thermodynamically stabile under various pH and temperature conditions. The color and volume of these crystalline hydrogel networks can reversibly change in response to external stimuli such as temperature, pH and other environmental conditions. These new materials may lead to a variety of technological and artistic applications, ranging from sensors, displays, controlled drug delivery devices, jewelry and decorative consumer products.
Owner:UNIVERSITY OF NORTH TEXAS
Agate-containing dark green glaze for ru-porcelain, processing method of glaze as well as ru-porcelain and firing method thereof
The invention relates to agate-containing dark green glaze for ru-porcelain, a processing method of the glaze as well as the ru-porcelain and a firing method thereof and relates to the technical field of silicate ceramics. The agate-containing dark green glaze for the ru-porcelain is prepared from components in parts by weight as follows: 6-20 parts of ru jade, 1-15 parts of agate, 10-70 parts of feldspar, 2-8 parts of calcite, 1-20 parts of wollastonite, 0-2 parts of talc, 0-15 parts of chest wood ash, 1-8 parts of ammolite and 1-20 parts of black bluestone. The enamel of the ru-porcelain fired with the adoption of the agate-containing dark green glaze for the ru-porcelain takes dark green as a base, can present the deep and full sky blue, azure, light greenish blue, bluish white, pale blue and bright green and can present bright and transparent pea green and onion green color. The ru-porcelain adopting the agate-containing dark green glaze for the ru-porcelain has good jade sense and opalescence effects and has a smoother glaze surface, higher mechanical strength, lower water absorption rate and high firing qualification rate.
Owner:郭秀贞
Preparation method of long-circulating nanoparticle
InactiveCN102451160AEasy to operateImprove targetingPowder deliveryOrganic active ingredientsFreeze-dryingPolyethylene glycol
The invention which belongs to the medicine processing field concretely relates to a preparation method of a long-circulating nanoparticle. The preparation method of the long-circulating nanoparticle provided in the invention has the advantages of simple operation, and good targeting and good in vitro release of products. The method comprises the following steps: 1, dissolving 5-Fu (5-fluorouracil), PEG-PHDCA (polyethylene glycol-poly(hexadecyl cyanoacrylate)) and a phosphatide in a mixed organic solvent of tetrahydrofuran and ethanol to form an organic phase; 2, slowly adding the organic phase to a water phase of a surfactant in a dropwise manner under magnetic stirring, and fully diffusing the organic phase by continuously stirring for 1h after finishing the dropwise addition; 3, carrying out reduced pressure evaporation to remove the organic solvent to obtain a nanoparticle colloidal suspension with a blue opalescence; 4, carrying out ultracentrifugation separation deposition on the nanoparticle, and washing; and 5, carrying out ultrasonic dispersion with a 4% mannitol solution, and freeze-drying.
Owner:夏落
Glass ceramics
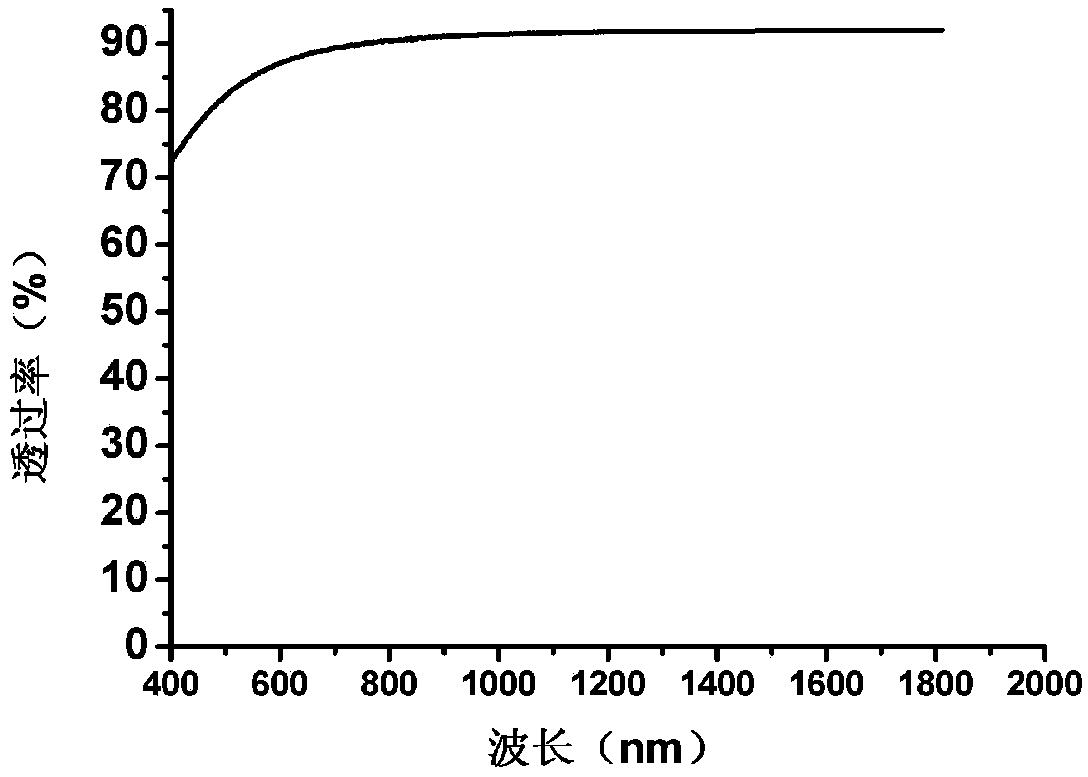
The invention discloses glass ceramics. The glass ceramics is prepared from the following components, in percentage by weight: 65-80% of SiO2, 0-3.5% of Al2O3, 7-12.5% of Li2O, 0.5-3% of K2O, greaterthan or equal to 3% and less than or equal to 10% of Y2O3, 1.5-4% of P2O5, 1-5% of ZrO2, 0.5-2% of MgO and 0.5-2% of ZnO. The glass ceramics solve problem of molding and opalescence presenting of theglass ceramics which take a quartz solid solution and lithium disilicate as main crystal phases by adjusting the components of the glass ceramics, the internal quality of the glass ceramics is uniform, the glass ceramics can be efficiently and stably produced, and the glass ceramics have the high hardness, the high Young modulus and the higher transmittance, and can be widely applied.
Owner:CDGM GLASS LLC
Auto air duct plate open-type foaming device and its air duct plate production method
The invention discloses an open foaming device for car air channel board, comprising foaming units, forming mold and mold platform. The forming mold is set on mold platform, and it concludes bottom and top mold, said bottom mold is an integral, said top mold comprises multi-section top mold in relative to bottom mold. The multi-section top mold is clamped section by section according to the sequence of polyurethane material casting. The clamping and forming of polyurethane in front section are finished before coagulation and solidification, and clamping and forming of polyurethane cast in next section is finished gradually. The invention is characterized by no filling of catalyst for prolonging opalescence time, high producing efficiency, and good quality of air channel board.
Owner:董升顺
3D printing false tooth material
InactiveCN105832563AImprove bindingGood roasting stabilityImpression capsDentistry preparationsLithiumSilicate glass
The invention relates to the technical field of false teeth, in particular to a 3D printing false tooth material .The 3D printing false tooth material comprises zirconia enhanced lithium silicate glass ceramic used as a substrate material, the zirconia enhanced lithium silicate glass ceramic is matched with a facing material for carrying out personalized decoration on the zirconia enhanced lithium silicate glass ceramic .The facing material is the low-melting-point fine particle feldspar material .By means of perfect matching of coefficients of thermal expansion of the substrate material and the facing material, good bonding of the two materials and long-term stability of the facing material are ensured; the low-melting-point fine particle feldspar material is uniform, compact in surface and good in roasting stability and has excellent grinding and polishing performance, and therefore the surfaces of false teeth can be smooth and compact; the low-melting-point fine particle feldspar material has the high permeability and warn tone, meanwhile, the zirconia enhanced lithium silicate glass ceramic has an opalescence effect, and therefore the surface color can be fresh and attractive .
Owner:AI JIA DENTAL LAB
Porcelain white glass formula
ActiveCN103482869AGood lookingMeets requirements for reflective opacificationAluminium hydroxideCalcite
The invention relates to a porcelain white glass formula. The porcelain white glass formula is characterized by being prepared from the following raw materials by weight percent: 60-90% of batch, and 10-40% of cullet, wherein the batch comprises the following components in parts by weight: 50-60 parts of quartz sand, 13.5-16 parts of sodium carbonate, 10-12 parts of aluminium hydroxide, 9-11 parts of sodium fluosilicate, 5-7 parts of fluorite, 0-1.5 parts of barium sulfate, 0.5-2 parts of sodium nitrate, 0-3 parts of calcite and 0.3-0.7 parts of compound clarifier. The formula gives consideration to demands of each manufacturing link on properties of glass and demands of users on physical and chemical properties of the glass in the glass production process; control of grain sizes is facilitated; reflected opalescence is generated, so that the glass product produced according to the formula disclosed by the invention obtains the appearance effect of high white porcelain; the product quality is greatly improved; the formula disclosed by the invention is especially suitable for manufacturing bottles for cosmetics, wine bottles and wine bowls.
Owner:吴江光华玻璃厂
Emerald green glaze
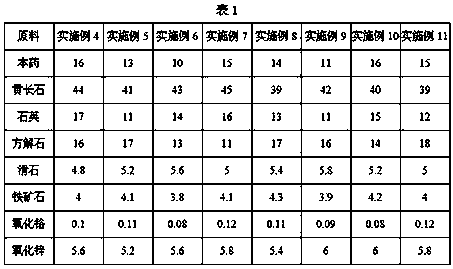
The invention provides emerald green glaze which is prepared from, by weight, 10-16 parts of primary medicines, 39-45 parts of melilite, 11-17 parts of quartz, 13-19 parts of calcite, 4-6 parts of talcum, 3.5-4.3 parts of iron ores, 0.08-0.12 part of chromium oxide and 5-7 parts of zinc oxide. Compared with the prior art, the emerald green glaze has the advantages that iron and chromium serve as coloring agents and are fired in reducing atmosphere to form the emerald green glaze, and the emerald green glaze with pure natural colors, strong jade texture and opalescence and high yield can be prepared by firing without removing iron in raw materials.
Owner:禹州市土魂钧瓷有限公司
Method for preparing hydroxypropyl chitose nano particle
The present invention relates to a hydroxypropyl chitosan nanometer particle and the preparation method for the hydroxypropyl chitosan nanometer particle. The nanometer particle is obtained through ion gel method, including: chitosan, sodium hydroxidand and isopropyl alcohol are mixed for stirring at room temperature; excessive propylene oxide is added in to the mixture for sufficient reaction at 60 DEG C; After the reaction, the Ph value is adjusted to be neutral; the reaction product is deposited by acetone and is cleaned for several times; then the product is dried in vacuum to get hydroxypropyl chitosan; with constant magnetic-force stirring, the water solution of hydroxypropyl chitosan is slowly added with sodium polyphosphate solution; when the solution is of opalescence, hydroxypropyl chitosan nanometer particles are obtained. The diameter of the hydroxypropyl chitosan nanometer particles obtained by the present invention can be controlled. The hydroxypropyl chitosan nanometer particles can be biologically degraded with in a non-toxic and harmless way. The preparation method is moderate. The hydroxypropyl chitosan nanometer particles can be used as the carriers of protein drugs.
Owner:STATE GRID CORP OF CHINA +1
Ropivacaine nano particle, preparation method thereof and optimizing experimental method of effect of the ropivacaine nano particle
ActiveCN104146972AAvoid damageHigh encapsulation efficiencyPowder deliveryAnaestheticsFreeze-dryingPolyvinyl alcohol
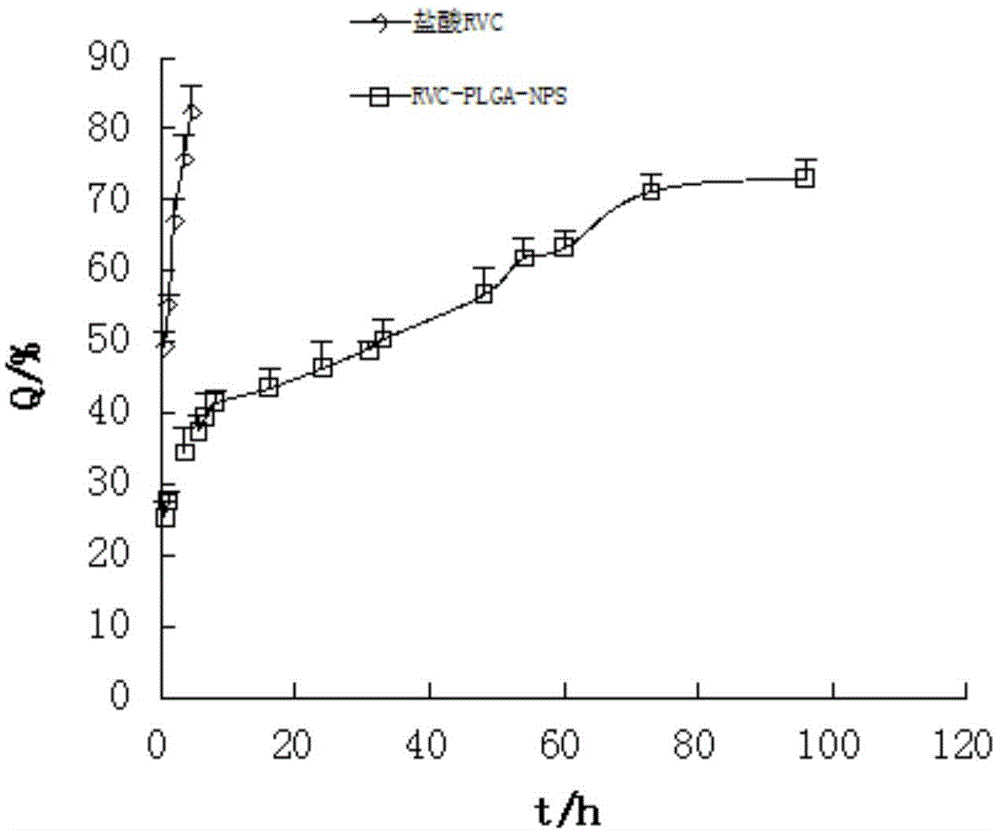
The invention relates to the field of preparation of medicines and particularly relates to ropivacaine nano particles, a preparation method thereof and an optimizing experimental method of effects of the ropivacaine nano particles. The preparation method comprises following steps: (A) dissolving ropivacaine free alkali and polylactic acid-polyglycollic acid segmented copolymer in dichloromethane to form an organic phase while a polyvinyl alcohol solution is employed as an aqueous phase; (B) performing evaporation to a white emulsion at 30-40 DEG C to remove the organic phase to obtain a pale blue opalescence suspension liquid; and (C) performing centrifugal separation to the pale blue opalescence suspension liquid to obtain a precipitate, washing the precipitate, and performing ultrasonic dispersion and a vacuum freeze-drying process to obtain the ropivacaine nano particles. By means of the prepration method of the ropivacaine nano particles, an in-vitro releasing research proves that the ropivacaine nano particles has a releasing rate being about 73% in 96 h, a slow-releasing effect is quite good and a pain-relieving requirement on acute pains, such as post-operation pain and the like, can be satisfied just through one-time dosing.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
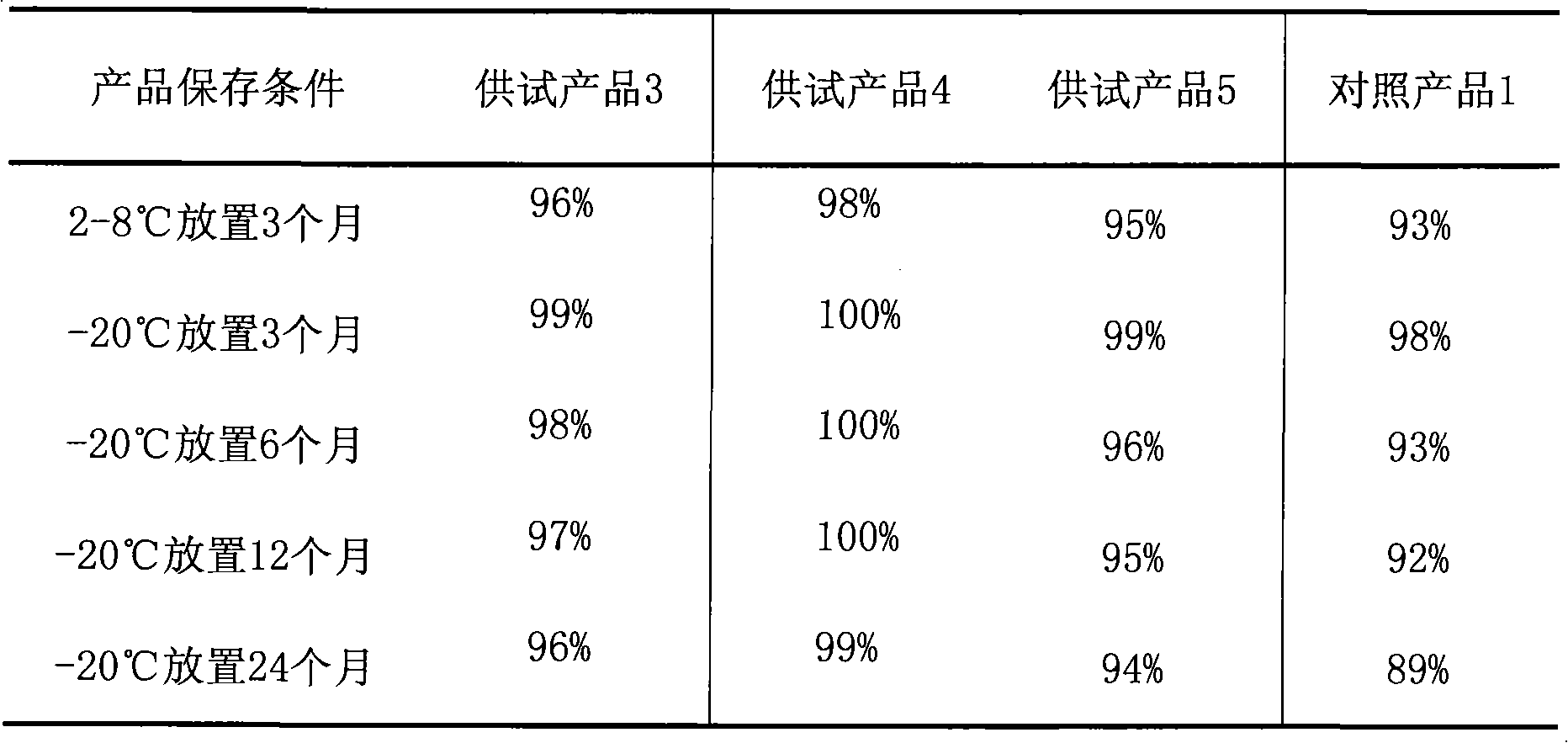
Stable ibuprofen arginine injection and preparation method thereof
InactiveCN102335114AImprove white opalescenceImprove the problem of turbidityOrganic active ingredientsPowder deliveryIbuprofen arginineIothalamate Meglumine
The invention relates to a stable ibuprofen arginine injection and a preparation method thereof. The injection comprises ibuprofen, arginine, and meglumine. Also, sodium dihydrogen phosphate and sodium thiosulfate can be contained in the injection. The injection can be an injection liquid, a transfusion liquid or a lyophilized powder injection. The ibuprofen arginine injection provided by the invention brings no problem in respects of haemolyticus, vascular stimulation or allergy. When a prior product is diluted by using physiological saline or a 5% glucose injection, a white opalescence or turbid phenomenon is occurred. With the injection and the preparation provided by the invention, the problem is solved, the physical stability problem of the product is solved, and the quality stability and safety of the product are ensured.
Owner:CHONGQING PHARMA RES INST
Intravenous anesthetics 2,6-diisopropyl phenol microemulsion composition and method of making the same
ActiveCN101411685AImprove securitySmall particle sizeHydroxy compound active ingredientsAnaestheticsHemolysisAdditive ingredient
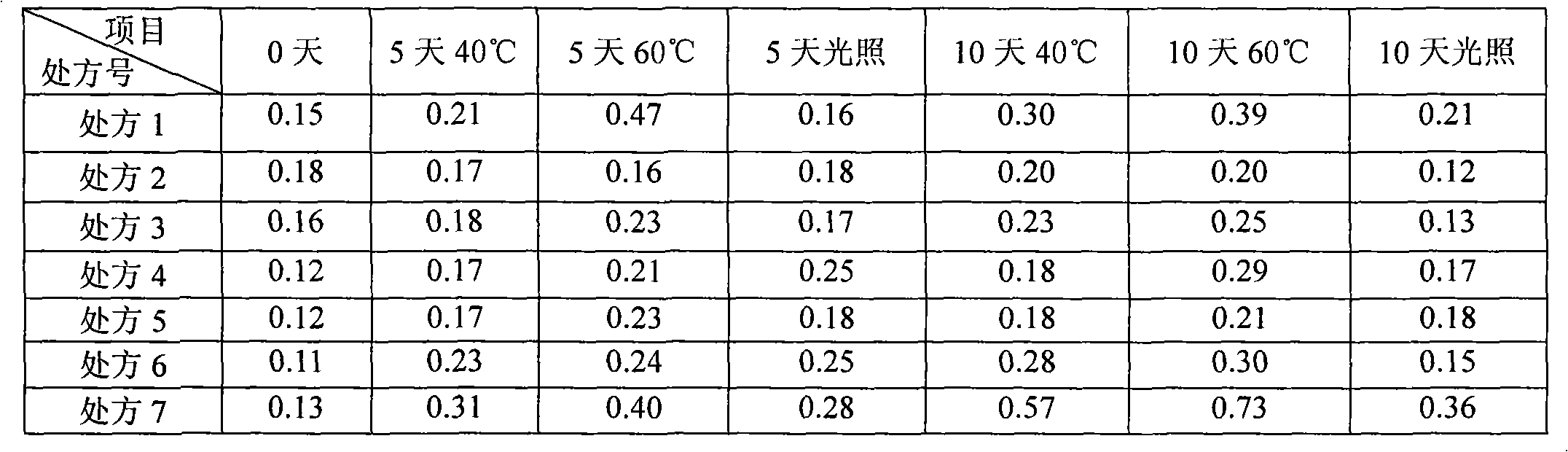
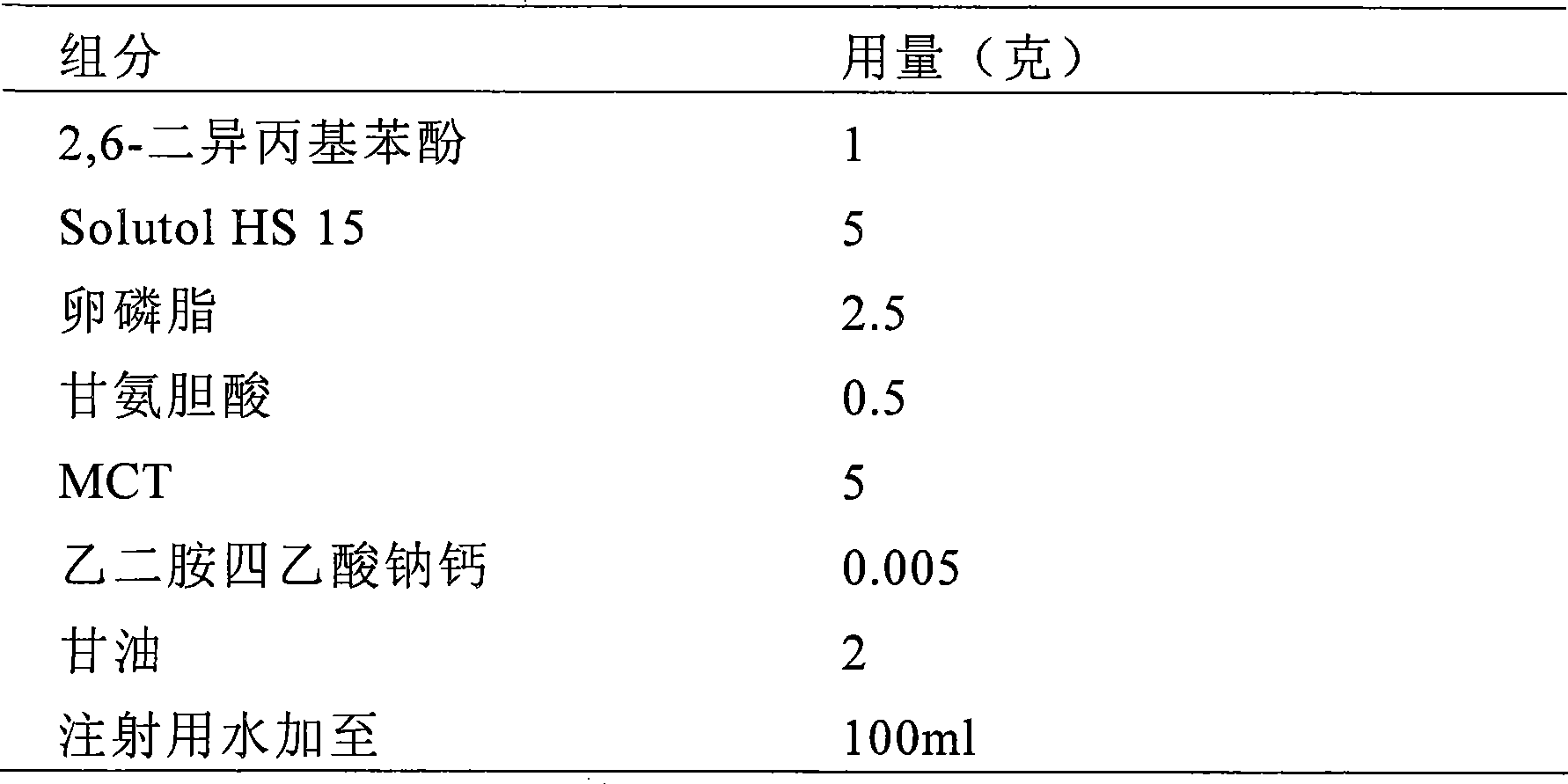
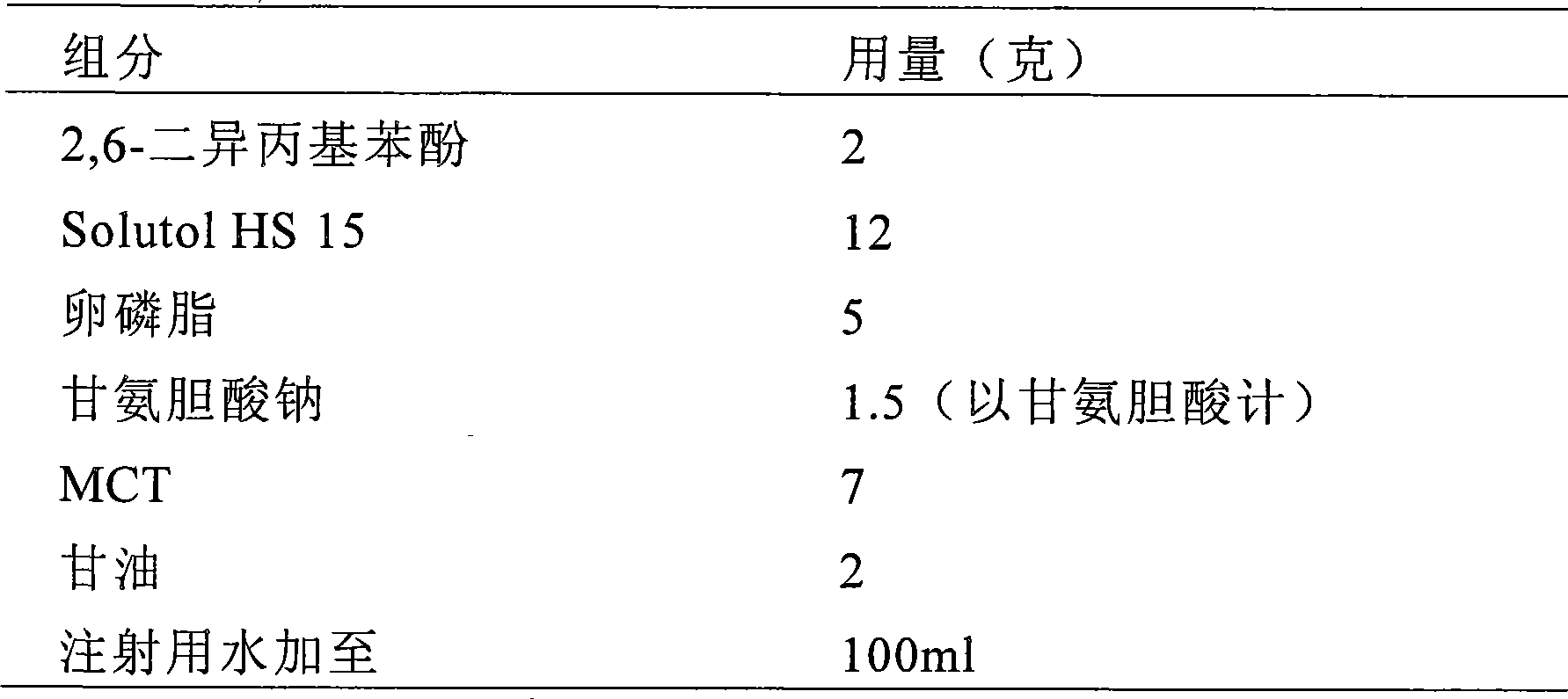
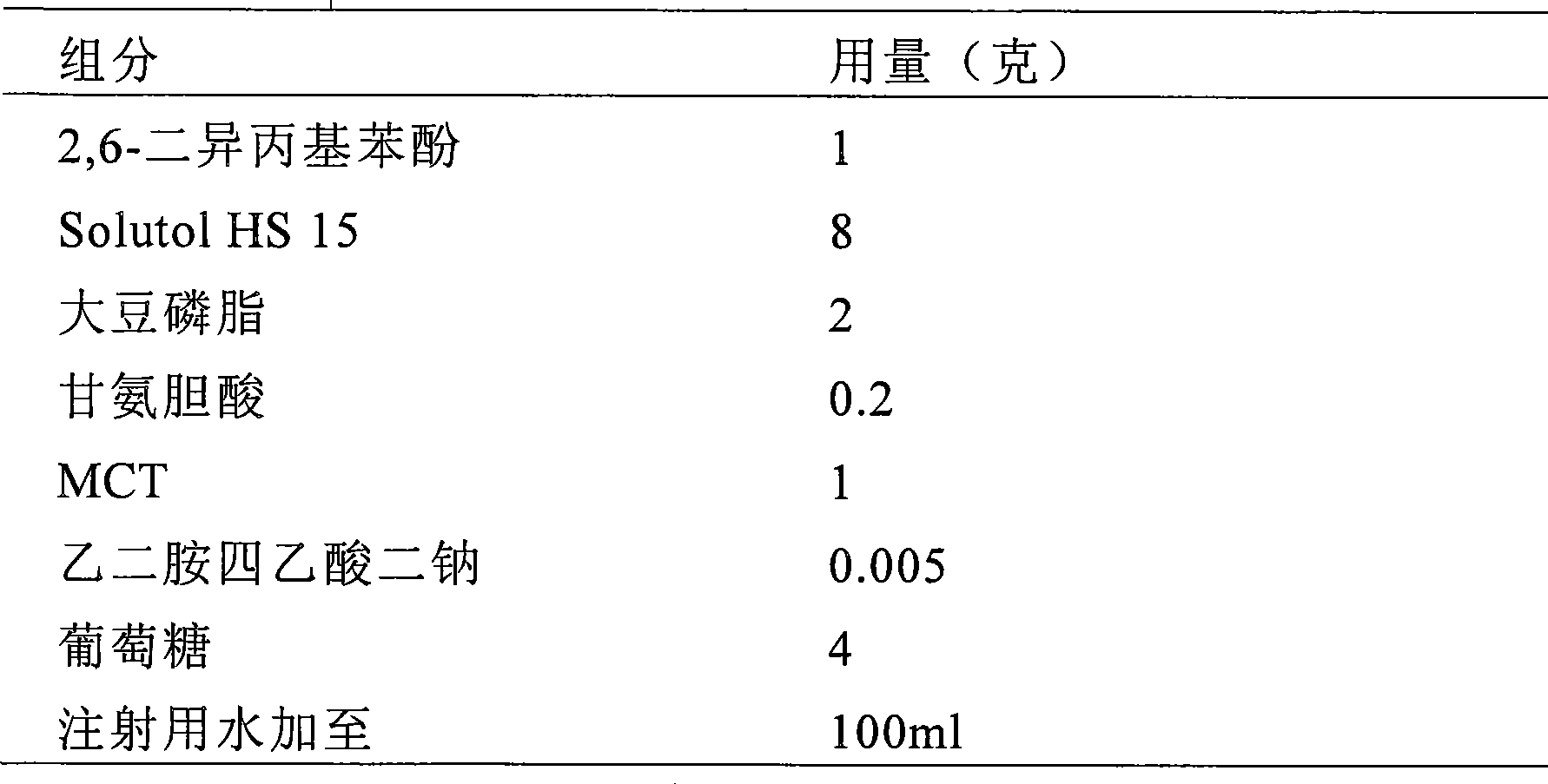
The invention provides intravenous anesthetics 2, 6-diisopropyl phenol microemulsion composition, which is characterized by containing 2, 6-diisopropyl phenol, Solutol HS15, oil for injection, phospholipids or other aqueous components. The invention discloses a preparation method for the microemulsion composition at the same time. The grain diameter of the microemulsion composition is less than 100 nanometers, and the microemulsion composition is transparent in appearance, has slight opalescence, does not cause the occurrence of hemolysis, and can be used for intravenous injection and administration.
Owner:BEIJING SHIQIAO BIOPHAM +1
Bioactive peptide milk substrate liposome essence water having blue opalescence and Tyndall phenomenon and preparation method of bioactive peptide milk substrate liposome essence water
ActiveCN110522655APenetrate fastFull penetrationCosmetic preparationsToilet preparationsPolyphenolLiposome
The invention relates to bioactive peptide milk substrate liposome essence water having blue opalescence and a Tyndall phenomenon. The bioactive peptide milk substrate liposome essence water consistsof the following components in parts by weight of 10-60 parts of milk substrate liposome, 0.1-1 part of bioactive peptide, 0.1-1 part of ginsenoside, 0.002-0.01 part of yeast essence, 0.001-0.01 partof alpha-bisabolol, 1-3 parts of tea polyphenols, 0.01-1 part of hyaluronic acid, 0.5-5 parts of trehalose, 0.01-3 parts of epsilon-polylysine and the balance distilled water, wherein all of the components are 100 parts. The essence water firstly adopts naturally-extracted bisabolol and polylysine united preservative, is free from chemical additives, has the effects of moistening, resisting wrinkles and whitening skin, is quick to seep, good in absorption and rich in nutrients, and can solve the problems that at current, essence water is difficult to deliver to a skin base layer or only a fewof active components can be delivered to the skin base layer for development of effects.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of long-circulating nanoparticles
InactiveCN103006563AEasy to operateImprove targetingOrganic active ingredientsPowder deliveryFreeze-dryingEvaporation
The invention relates to a preparation method of long-circulating nanoparticles and belongs to the field of drug processing. The preparation method can be operated simply and can produce the long-circulating nanoparticles having good targeting performances and external release properties. The preparation method comprises the following steps of dissolving 5-Fu, PEG-PHDCA and phospholipid in a mixed organic solvent of tetrahydrofuran and ethanol to obtain an organic phase, slowly and dropwisely adding the organic phase into a water phase of a surfactant with magnetic stirring, then persistently stirring for 1 hour so that the organic phase is dispersed fully, carrying out reduced pressure evaporation to remove the mixed organic solvent and to obtain a nanoparticle colloidal suspension liquid having blue opalescence, carrying out overspeed centrifugal separation precipitation and washing of nanoparticles, carrying out ultrasonic dispersion by a mannitol solution having the content of 4%, and carrying out freeze drying.
Owner:魏奇
Hydroxytyrosol containing nanoemulsion preparation and preparation method of lyophilized agent thereof
InactiveCN108888595AImprove stabilitySolve the shortcomings of poor stabilityCosmetic preparationsPowder deliveryWater bathsHydroxytyrosol
The invention discloses a hydroxytyrosol containing nanoemulsion preparation and a lyophilized agent thereof. The hydroxytyrosol containing nanoemulsion preparation is prepared from hydroxytyrosol, asurface active agent, an oil phase and a water phase. A preparation method of the hydroxytyrosol containing nanoemulsion preparation comprises the steps that 1, the surface active agent and hydroxytyrosol are weighed and added into oil to serve as the oil phase, and the materials are stirred and mixed in a water bath; 2, purified water is weighed and added into a beaker, the materials are placed in the water bath, the oil phase is slowly and dropwise added into the water bath under the water bath condition, and the materials are fully stirred to be completely emulsified until and a clear transparent solution with bluish opalescence and with the viscosity similar to an aqueous solution is formed; 3, the nanoemulsion preparation is frozen and dried, water is removed, and the hydroxytyrosol nanoemulsion freeze-dried preparation is prepared. The hydroxytyrosol nanoemulsion prepared through the method has the advantages of being good in stability, low in viscosity, small in grain size and capable of being diffused and permeating into the skin more easily, the lyophilized preparation is conveneitn to store and transport, and can be added into cosmetics, medicine and healthcare food moreflexibly and conveniently.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
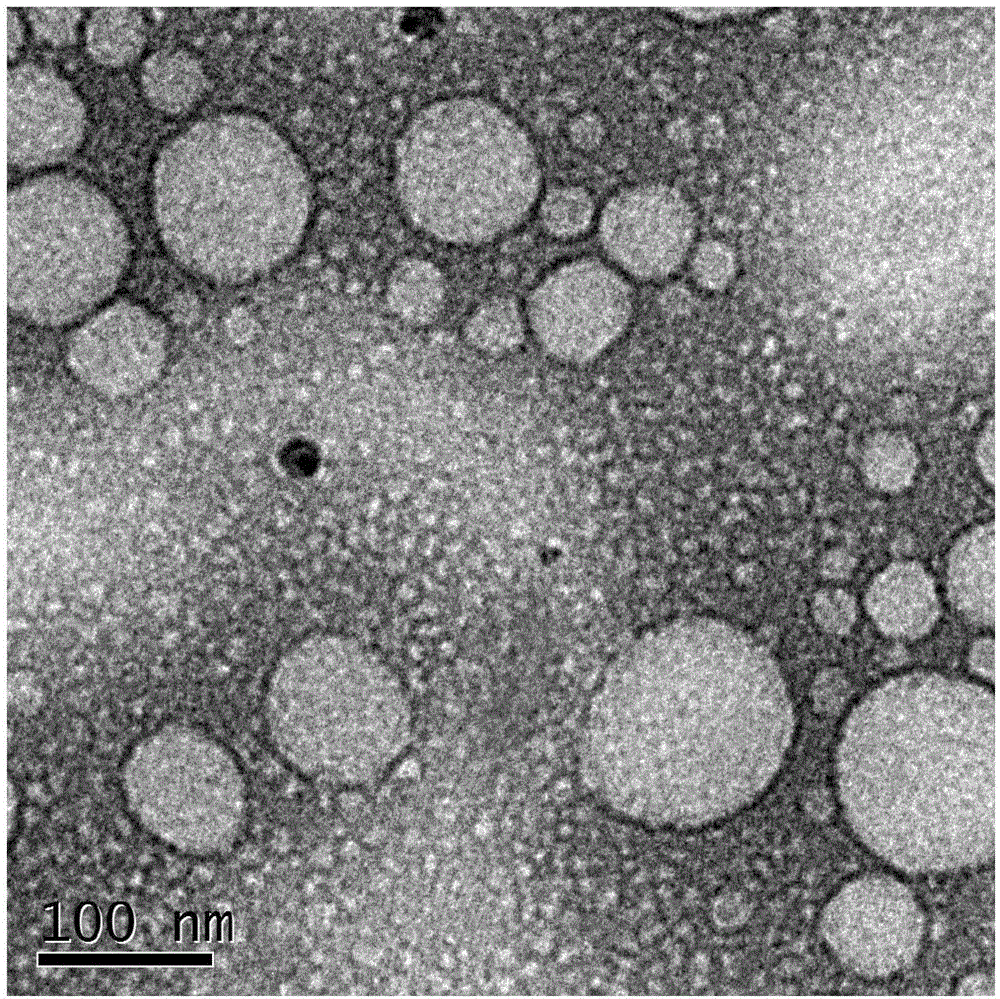
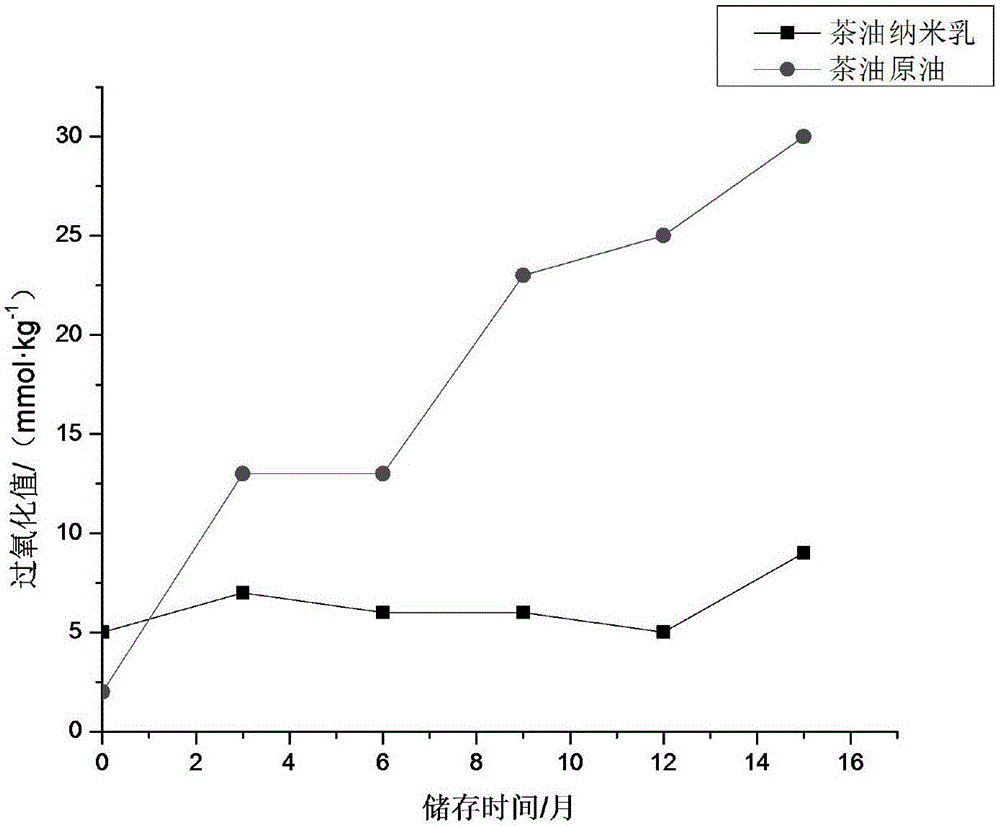
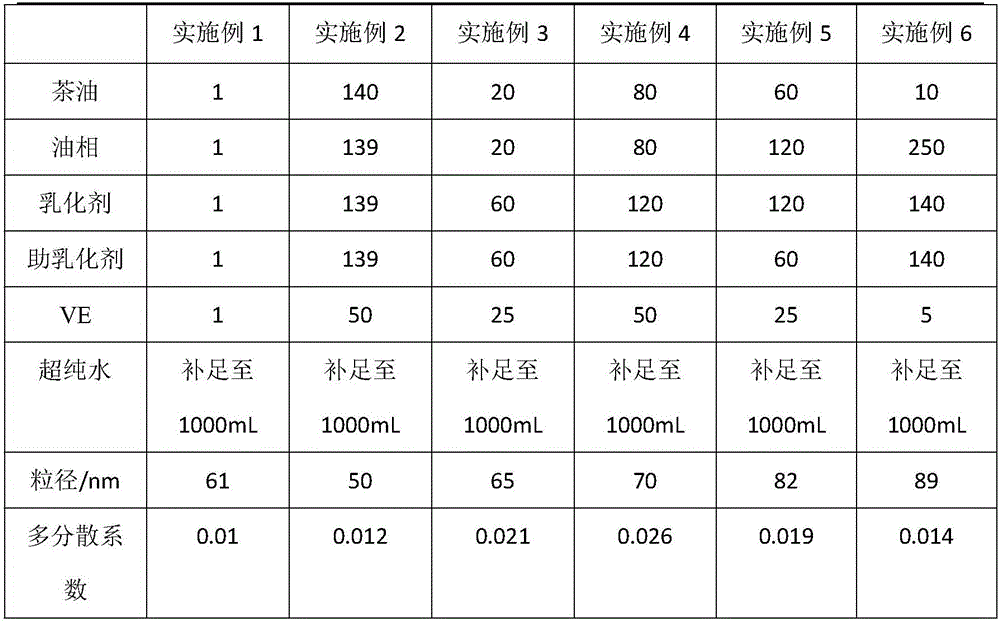
Camellia oil nano-emulsion and preparation method thereof
InactiveCN106821977AImprove bioavailabilityReduce incidenceEmulsion deliveryDermatological disorderAntioxidantCurative effect
The invention relates to camellia oil nano-emulsion and a preparation method thereof. Each 1000 mL of camellia oil nano-emulsion comprises the following components: 1 to 140 g of camellia oil, 1 to 250 g of oil phase, 1 to 140 g of an emulsifier, 1 to 140 g of a co-emulsifier, 1 to 50 g of an antioxidant and the balance of ultrapure water, wherein the total volume is 1000 mL and the average grain size is less than 100 nanometers. The preparation method comprises the following steps: (1) preparation of the oil phase: weighing the camellia oil, the emulsifier, the oil phase and the antioxidant, mixing, and heating and stirring at 37+ / -3 DEG C to obtain the oil phase; and (2) preparation of the camellia oil nano-emulsion: under the condition of continuously heating, dropwise adding the ultrapure water into the oil phase while stirring until the oil phase is clear and transparent or blue opalescence occurs to obtain nano-emulsion. After the camellia oil nano-emulsionis prepared, the mechanical stability of the camellia oil is improved, the bioavailability of the camellia oil is improved and the curative effect is enhanced.
Owner:FUJIAN MEDICAL UNIV
Color-unchangeable lead-containing opalescent frit
The invention discloses color-unchangeable lead-containing opalescent frit which is prepared from the following raw materials in part by weight: 40-50 parts of SiO2, 5-10 parts of Al2O3, 1-5 parts of CaO, 30-40 parts of PbO, 1-5 parts of ZrO2 and 1-5 parts of alkali metal. The color-unchangeable lead-containing opalescent frit disclosed by the invention has the beneficial effects that the composition proportion is determined through a lot of experimental screening, experimental results show that all the components are more scientifically and reasonably proportioned, ceramics prepared by the frit disclosed by the invention has good glazed smoothness, good enamel, uniform opalescence and wide application scope.
Owner:CARLOBBIA GLAZE KUNSHAN
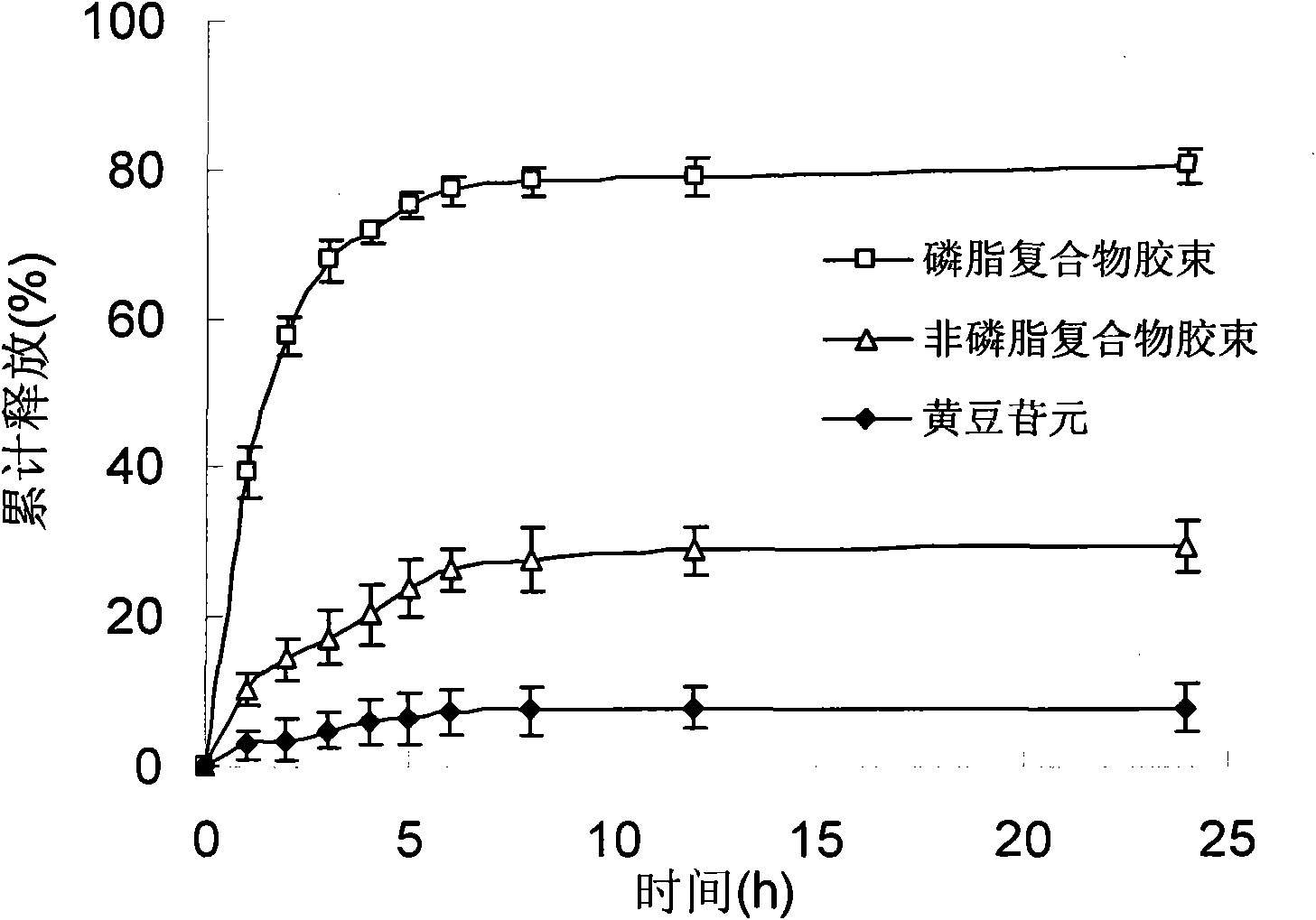
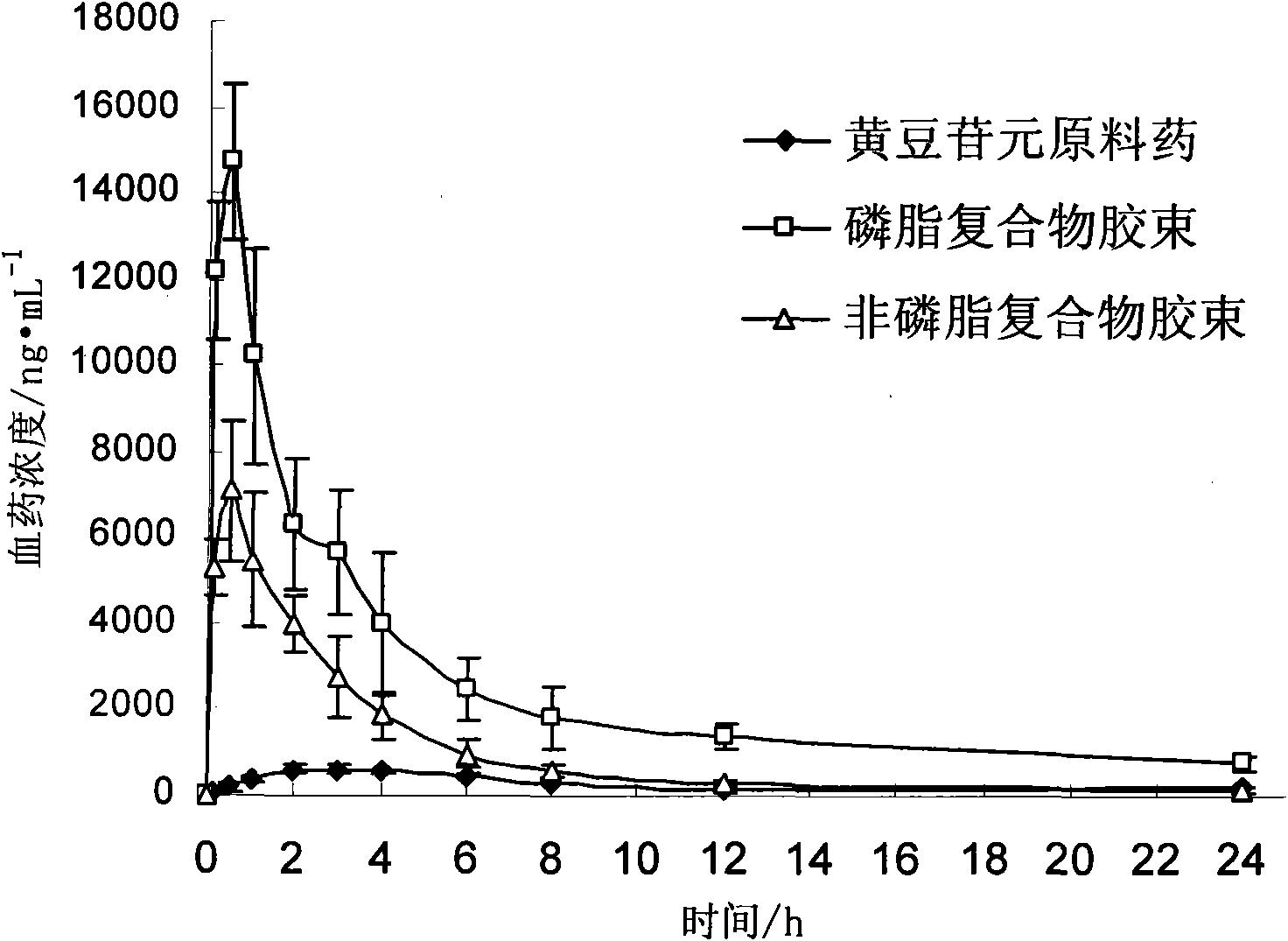
Daidzein micelles and preparation method thereof
InactiveCN102058528AImprove bioavailabilityIncrease route of administrationOrganic active ingredientsPowder deliveryFreeze-dryingDaidzein
The invention relates to daidzein micelles and a preparation method thereof. The daidzein micelles contain 1 part of daidzein, 12 to 30 parts of phospholipid and 1 to 25 parts of additive. The preparation method comprises: preparing a daidzein and phospholipid composite, namely, adding daidzein and 50 to 95 percent of phospholipid into an organic solvent, heating the solution to 40 to 60 DEG C, refluxing under reduced pressure, keeping temperature and stirring for 2 to 10 hours, recovering the organic solvent, drying and crushing to obtain the daidzein and phospholipid composite; and preparing daidzein micelles from the phospholipid composite, namely, dissolving the daidzein and phospholipid composite, the rest phospholipid and the additive in an organic solvent, subjecting the solution to rotary evaporation to form a film, and hydrating at 40 to 60 DEG C to obtain daidzein micelle suspension with opalescence. The average particle size of the daidzein micelles is less than 50 nanometers. The daidzein micelles can be further prepared into oral or injection preparations including capsules, oral mixed suspension, oral liquid, injection, injection freeze-dried powder injection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Eye drop for treating xerophthalmus and preparation method of eye drop
ActiveCN105012404AAvoid damageIncrease irritationSenses disorderInorganic boron active ingredientsEye dropBorax
The invention discloses an eye drop for treating xerophthalmus and a preparation method. The eye drop is prepared from the following raw materials and auxiliary materials in parts by weight: 130-170 parts of an extract containing bear gall powder, 1.5-3.3 parts of borneol, 1-2 parts of a bacteriostatic agent, 6-10 parts of a solubilizer, 40 parts of sodium borate and 10 parts of boric acid. According to the physical and chemical properties of chemical components contained in the medicinal material in the formula, use of volatile oil is removed; the dosage of the borneol and the bear gall powder is adjusted; the borneol and the bear gall powder are added in different manners; muddy opalescence and the like caused by a conventional bear gall powder adding method are reduced; the medicinal composition is ensured; meanwhile, the preparation directly acts on the medication part; the dosage is greatly reduced; the onset time of the medicine is quickened; and the curative effect is improved.
Owner:SHANDONG JINHE DRUG RES DEV
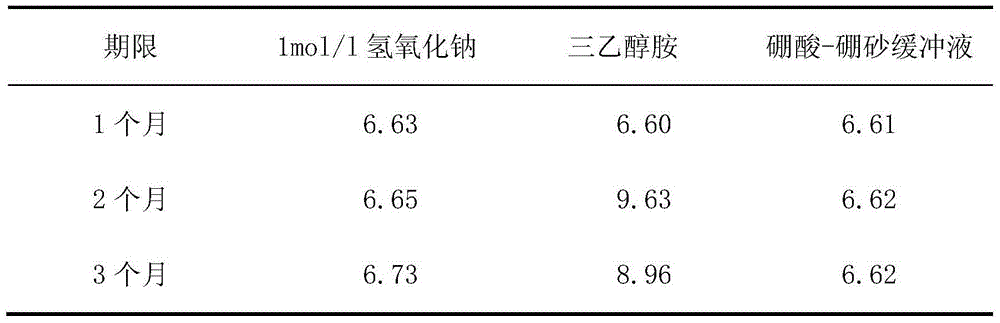
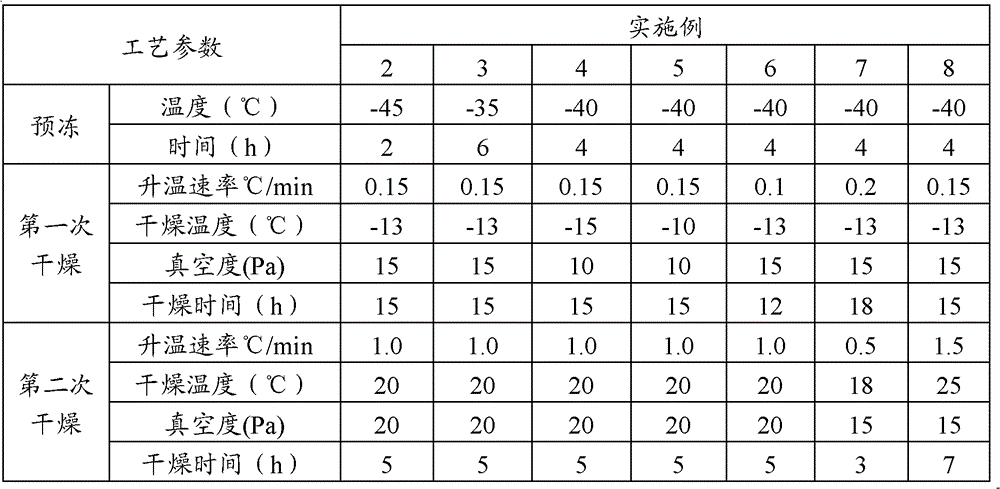
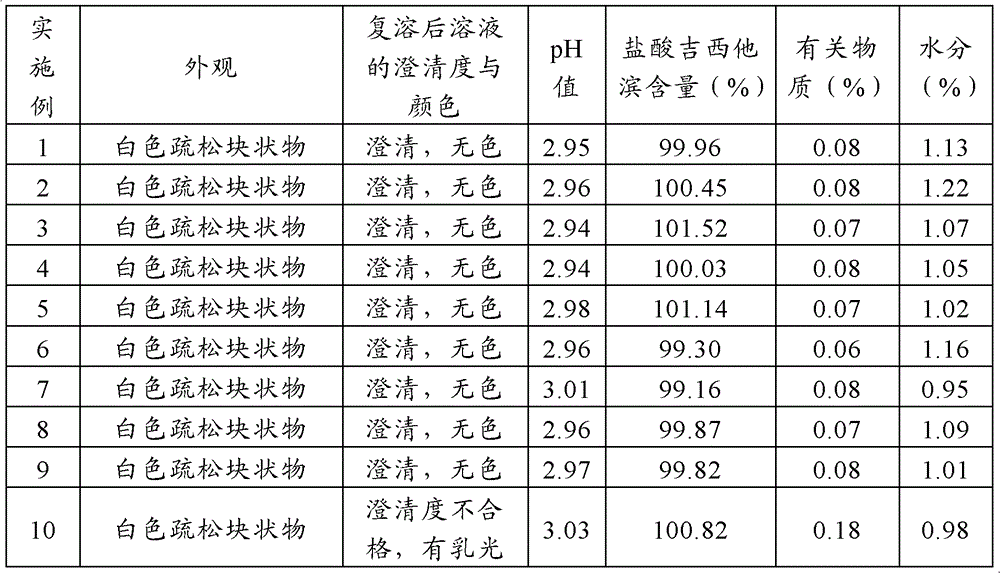
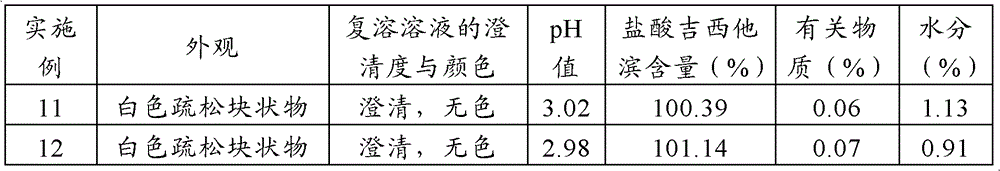
Method for preparing gemcitabine hydrochloride lyophilized powder
ActiveCN102793677AAvoid dehydrochlorinatioAvoid efficiencyOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideLow vacuum
The invention relates to the field of pharmaceutic preparations and discloses a method for preparing gemcitabine hydrochloride lyophilized powder. According to the method for preparing the gemcitabine hydrochloride lyophilized powder, a primary pre-freezing process and a stage drying process are used for lyophilization; appropriate vacuum degrees and temperature are matched at all drying stages to avoid the problem that hydrochloric acid is removed from gemcitabine hydrochloride under high vacuum degree, and drying efficiency is low due to low vacuum degree; meanwhile, temperature is slowly raised at different speed at different drying stages, and the problem that the gemcitabine hydrochloride is deacidified due to too high heating speed can be solved. Compared with the prior art, the method for preparing the gemcitabine hydrochloride lyophilized powder has the advantages that the method is easy to operate, lyophilization time is short, and drying temperature is low; the prepared gemcitabine hydrochloride lyophilized powder product is high in outer structural quality and redissolution performance; a redissolution solution is clear and transparent, and opalescence is avoided; relative substances are low in content, and the prepared gemcitabine hydrochloride lyophilized powder is safe and reliable in quality; and the method is suitable for preparing the gemcitabine hydrochloridelyophilized powder and can be widely applied to large-scale production of the gemcitabine hydrochloride lyophilized powder.
Owner:CHONGQING LUMMY PHARMA
Method of heat-treating packaged product and heat-treated packaged product
InactiveUS20050106346A1Suppressing opalescence of packaging materialPoor appearanceWrappers shrinkageFlexible coversMedical productWater soluble
A packaged product formed by enclosing a content material, such as food or beverage, sanitary products or medical products, within a packaging material including at least a layer of hydrophilic resin, is heat-treated with hot water for, e.g., boil sterilization or retort sterilization. The hot water is caused to contain a water-soluble compound in an amount substantially exceeding a level contained in tap water, whereby it becomes possible to suppress the opalescence of the packaging material causing inferior appearance or transparency and leading to a lowering in gas-barrier property, which has been problematic in the conventional boil or retort hot water treatment.
Owner:KUREHA KAGAKU KOGYO KK
Camptothecinum derivative emulsion agent and preparation thereof
InactiveCN101259103AImprove stabilitySolve the problem of insoluble in waterOrganic active ingredientsEmulsion deliveryEmulsionDrug content
The invention provides an emulsion of camptothecin derivative which comprises the components by weight: 0.01 to 0.1 percent of 10-methyl-9 nitryl camptothecin, 5 to 20 percent of oil for injection, 1-4 percent of emulsifier, 1-4 percent of co-emulsifier, 0 to 60 percent of solubilizer, 2 to 3 percent of glycerin of isotonizing agent and Vitamin E and the rest is water for injection; the sum of the percentages of all components is 100 percent. The emulsifier and a co-surfactant are dissolved in the oil for injection, heated and added with the solubilizer and 10-methyl-9 nitryl camptothecin to be dissolved, ant then added with the glycerin of isotonizing agent and the water for injection and made into primary emulsion by means of high speed homogenization or ultrasound, then further made into the emulsion by means of high pressure homogenization or micro jet. The prepared emulsion of the invention has uniform particle diameter and high content of drug and the appearance of the emulsion is a milky white or blue opalescence homogeneous system; meanwhile, compared with other preparation methods, the micro jet technique is adopted by the invention to prepare the emulsion, so as to ensure less loss of drug and better stability of the emulsion. The emulsion of camptothecin derivative of the invention has reasonable design and easy operation, which is suitable for practical use.
Owner:ZHEJIANG UNIV
Method for preparing folic acid coupling hydroxypropyl chitosan nano particles
InactiveCN101235102AStrong targetingParticle size controllablePharmaceutical non-active ingredientsAntineoplastic agentsNon toxicityMicroparticle
The invention relates to a folic acid coupling hydroxypropyl chitose nanometer particle and a corresponding preparation method, wherein the preparation method comprises mixing hydroxypropyl chitose water solution with folic acid in same volume, reacting completely at 70 DEG C without light, dialyzing for 24h, freezing and drying dialyzed product to obtain folic acid coupling hydroxypropyl chitose light yellow solid, adjusting the pH value of folic acid coupling hydroxypropyl chitose water solution to 5-6, adding sodium polyphosphate solution until the light yellow fades and appears opalescence, to obtain nanometer particle. The prepared folic acid coupling hydroxypropyl chitose nanometer particle has significant targeting effect on tumor cell, controllable particle diameter, biological degradation, non toxicity and mild process, thus is suitable for target slow-release carrier of protein drug.
Owner:STATE GRID CORP OF CHINA +1
Polyethylene glycol-integrated interferon variant lyophilized preparation
ActiveCN102327242AReduce the amount of dissolutionHigh retention rate of interferon activityPowder deliveryPeptide/protein ingredientsAlferon N InjectionFreeze-drying
The invention relates to polyethylene glycol-integrated interferon variant injection. Each 1ml of injection comprises 50 to 500 micrograms of polyethylene glycol-integrated interferon variant protein, a buffer system with the pH value of between 4.5 and 7.5 and a freeze-dried protective agent formed by a mannitol-alkaline amino acid mixed system. The polyethylene glycol-integrated interferon variant injection is stable and good in redissolution effect, and does not have the phenomenon of opalescence.
Owner:BEIJING KAWIN TECH SHARE HLDG
A preparing process for instant precipitate-free freeze-dried human fibrinogen
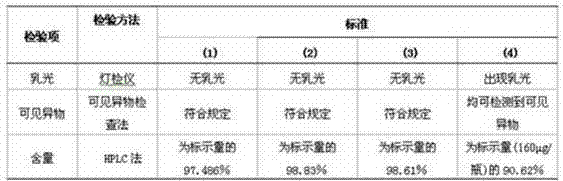
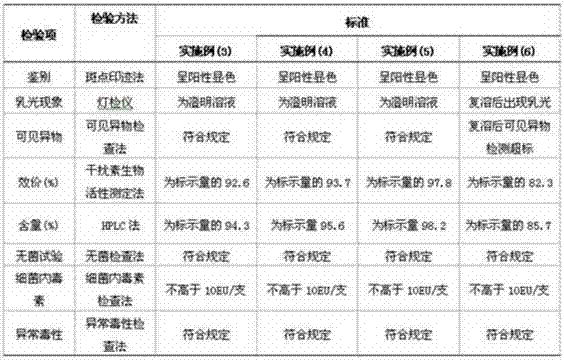
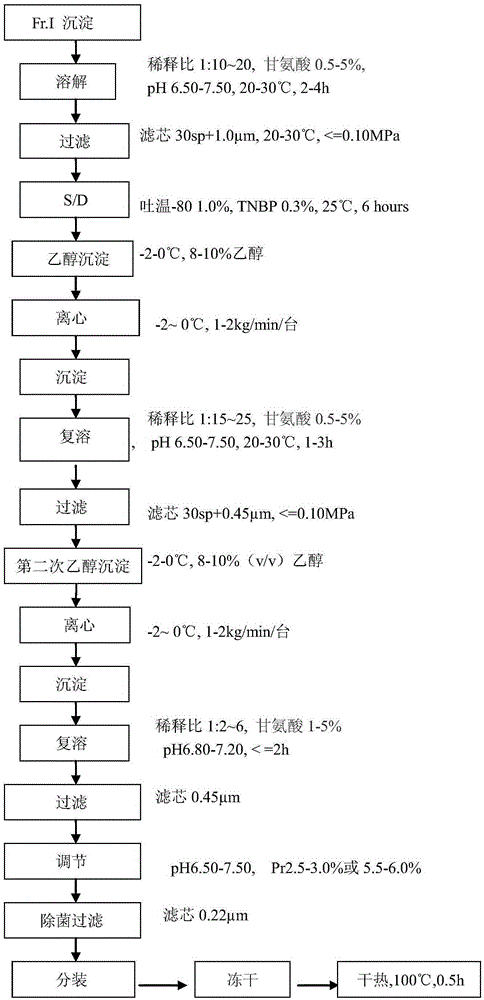
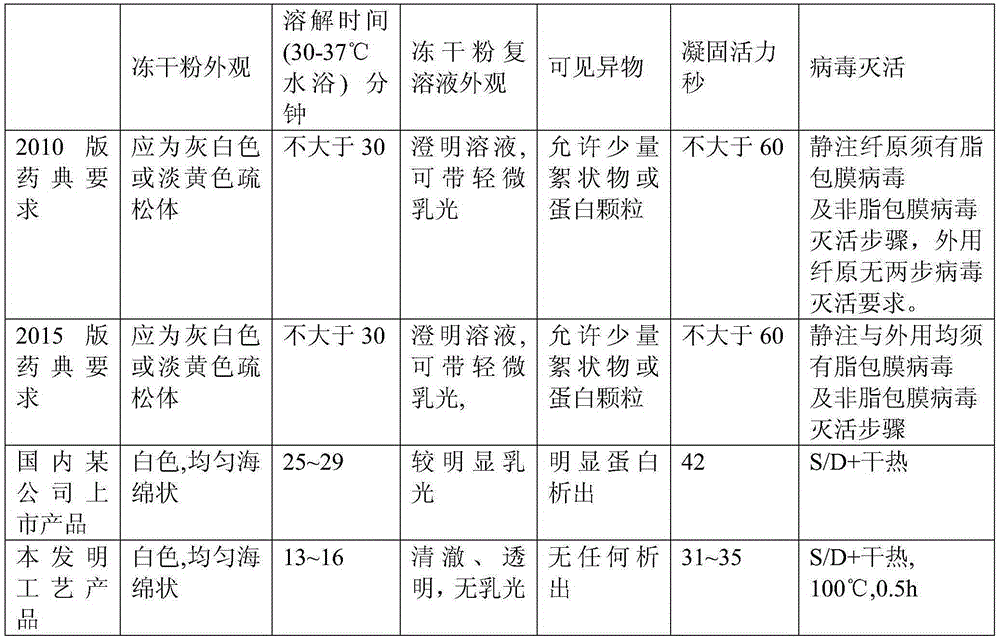
PendingCN106800583AEasy to produceShorten the production cyclePeptide preparation methodsFiberHigh concentration
The invention relates to the field of preparing processes for human fibrinogen, and particularly relates to a preparing process for instant precipitate-free freeze-dried human fibrinogen. The process includes five steps, namely the method includes a first step of component I precipitate dissolving and filtering; a second step of S / D virus inactivation; a third step of twice low-temperature ethanol precipitation rectification; a fourth step of freeze-drying; and a fifth step of virus inactivation in a dry heat manner. Through the improved fibrinogen production process, the fibrinogen which is instant, precipitate-free, opalescence-free and free of protein particles can be prepared. The process can prepare the human fibrinogen used for traditional intravenous injection, and can prepare high-concentration human fibrinogen compatible in human fibrous protein adhesives.
Owner:上海洲跃生物科技有限公司
Sevoflurane venous microemulsion and preparation method thereof
InactiveCN101791291AIncrease relative volatilityReduce volatilityEther/acetal active ingredientsAnaestheticsSide effectIrritation
The invention relates to a medicinal preparation and a preparation method thereof, in particular to a sevoflurane venous microemulsion and a preparation method thereof, and belongs to the technical field of medicaments. The sevoflurane venous microemulsion is prepared from sevoflurane, oil for injection and auxiliary materials such as emulsifying agent and the like by using the microemulsion preparation technology. The sevoflurane venous microemulsion is clear and transparent liquid, has light blue opalescence, and has Tyndall phenomenon; and the medicament-loading rate is 0.01 to 2.00 percent, the average grain diameter is 10 to 100 nanometers, and the pH is 4.5 to 9.5. On the one hand, the sevoflurane venous microemulsion improves the bioavailability and treatment effect of the sevoflurane by changing an administration route, and on the other hand, the venous microemulsion has targeting and can reduce the toxic or side effect of the medicament; and meanwhile, the volatility of the sevoflurane is reduced to be minimum due to the package of the microemulsion so as to reduce the acrimony of the sevoflurane, alleviate the pain of a patient during injection, make the patient accept easily and improve the administration compliance of the patient.
Owner:XUZHOU MEDICAL COLLEGE
Method for preparing interleukin-11 semi-finished product solution, and products thereof
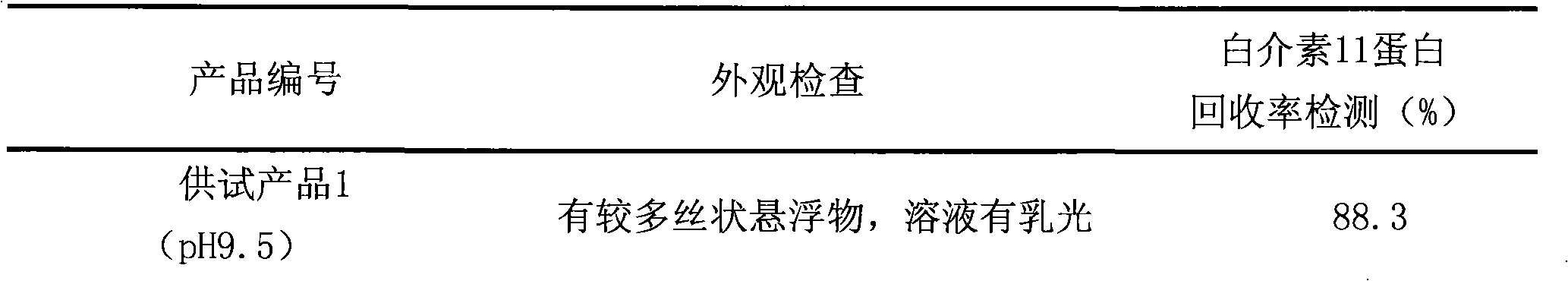
ActiveCN101837119AQuantitatively accurateHigh clarityPowder deliveryPeptide/protein ingredientsGlycinInterleukin 11
The invention discloses a method for preparing an interleukin-11 semi-finished product solution, and products thereof. The method comprises the following steps of: dissolving interleukin-11 into a phosphate buffer, adding glycin, and stirring to obtain the mixed solution; adjusting the pH value of the mixed solution; supplementing injection water to fix volume, and stirring to obtain the interleukin-11 semi-finished product solution, wherein the pH value of the mixed solution is adjusted to 8.0-9.5 by using diluted alkaline or is adjusted to 1.0-6.0 by using diluted acid. The method better solves the problems of accumulation and settlement of the interleukin-11 in the preparing, sterile filtering and packaging processes of the semi-finished product. The prepared semi-finished product solution is clear and transparent, and has no opalescence, and the volume of the protein of the main drug is accurately fixed. The sterile filtration of the prepared semi-finished product solution semi-finished product solution is easy to implement, the filter membrane can not be blocked, and the accumulation and settlement can not be caused during the packaging process of the semi-finished product. The clearness of the finished product is good after redissolution, no flocky precipitate or opalescence can be produced, the stable activity is maintained, and the use quantity is reduced in clinic.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Lujun Jun porcelain prepared from nano copper ion and preparation method of Lujun Jun porcelain
The invention discloses Lujun Jun porcelain prepared from nano copper ions and a preparation method of the Lujun Jun porcelain. A glaze of the Lujun Jun porcelain consists of the following raw materials in percentage by weight: 35-45 parts of melilite, 10-20 parts of calcite, 10-20 parts of quartz, 10-16 parts of traditional Chinese medicines, 5-10 parts of frit, 5-10 parts of plant ash, 1-3 partsof copper ore, 3-8 parts of Qingdao soda ash, 6-8 parts of soft soil and 2-5 parts of an adhesive. The preparation method of the glaze comprises the following steps: (1) treating the raw materials ina ball mill; (2) preparing the adhesive; (3) preparing glaze slurry; (4) carrying out primary glazing, and drying; (5) carrying out secondary glazing, and drying; (6) sintering in a furnace. Due to reasonable proportioning and temperature control, preparation procedures are simple and convenient, and the sintered Lujin glaze is rich in glaze color and natural in glaze surface and has a blue opalescence effect.
Owner:杨鹏飞
Stable ibuprofen arginine injection and preparation method thereof
InactiveCN102335114BAddress physical stabilityChemical stability preventsOrganic active ingredientsPowder deliveryIbuprofen arginineSodium thiosulfate
Owner:CHONGQING PHARMA RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com