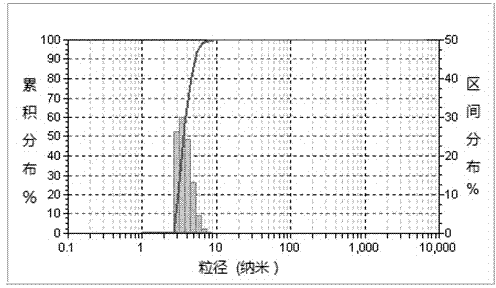
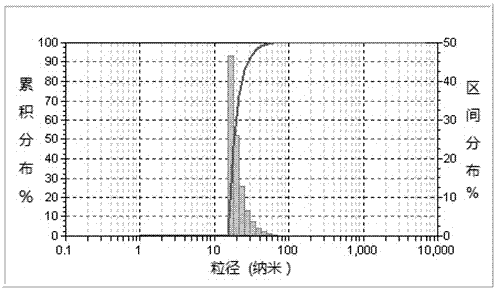
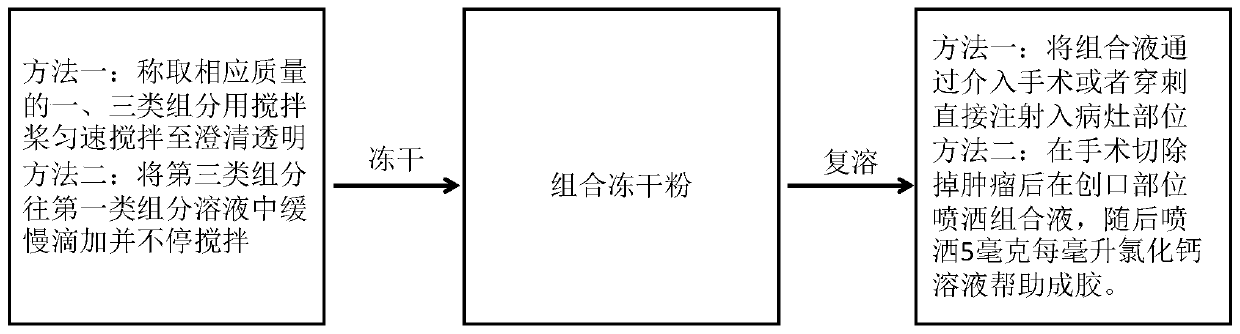
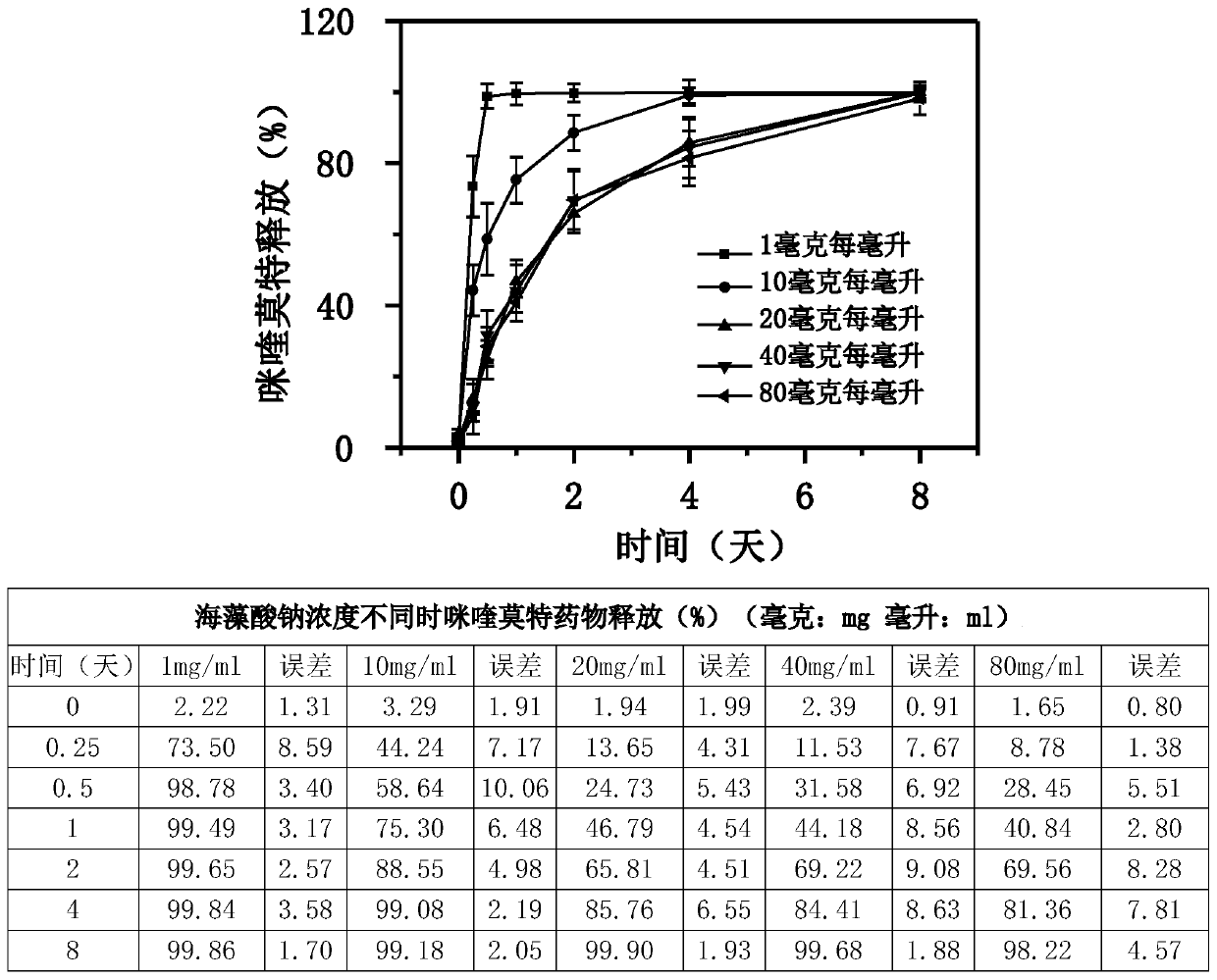
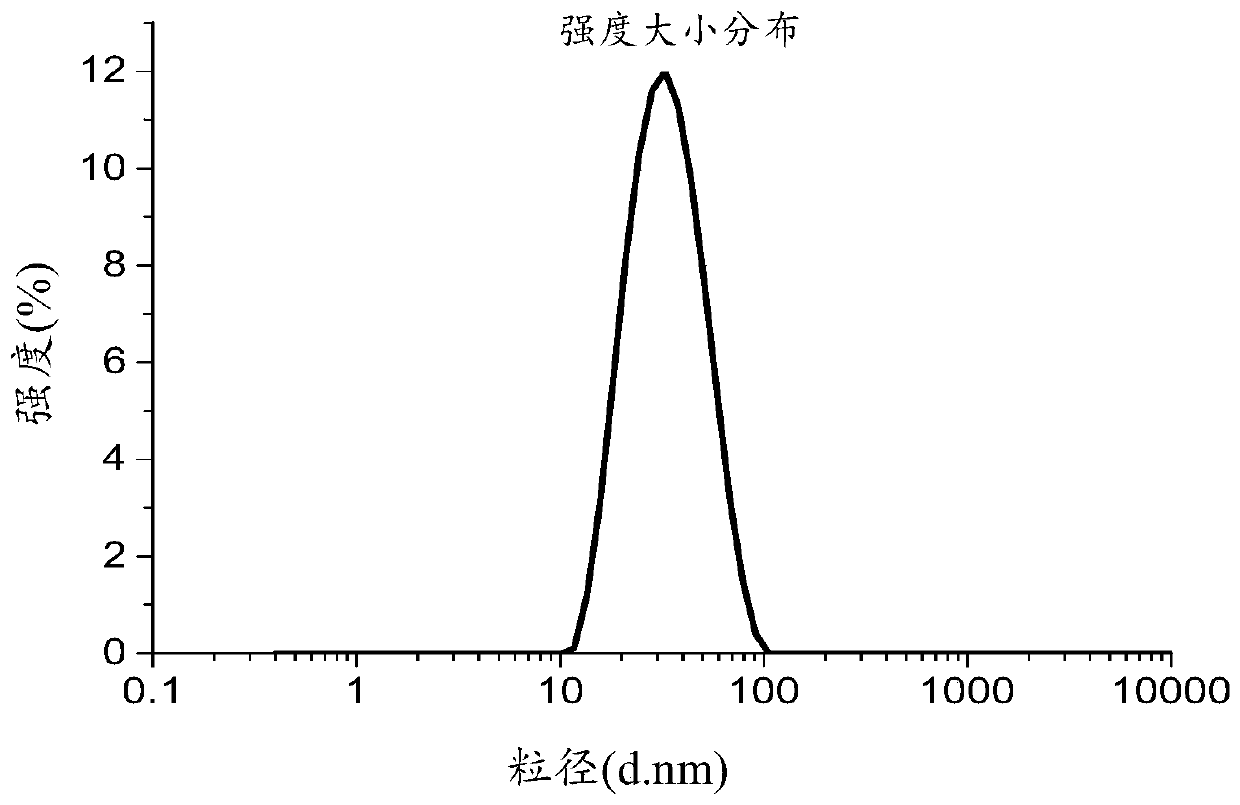
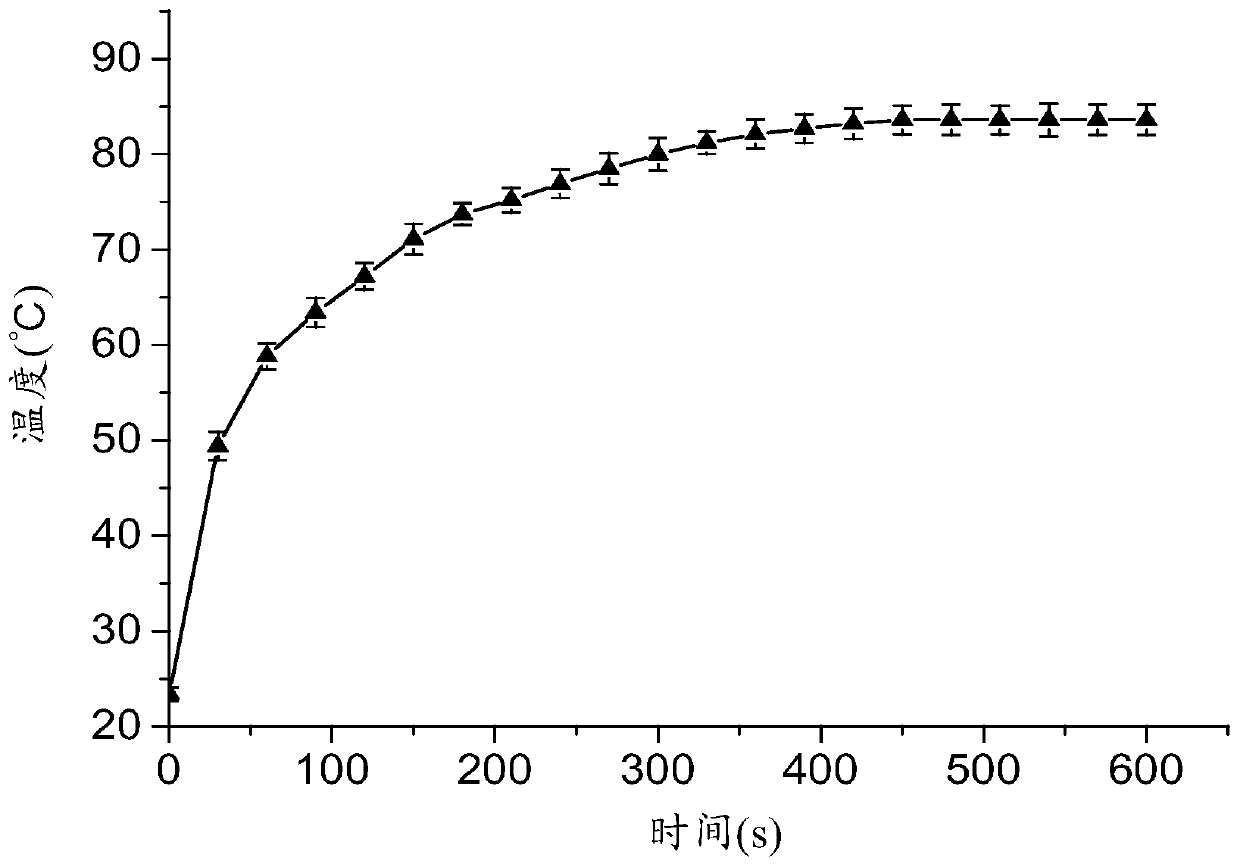
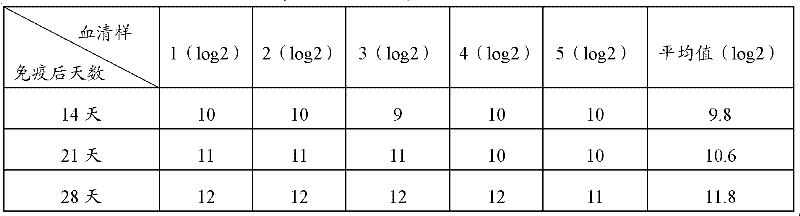
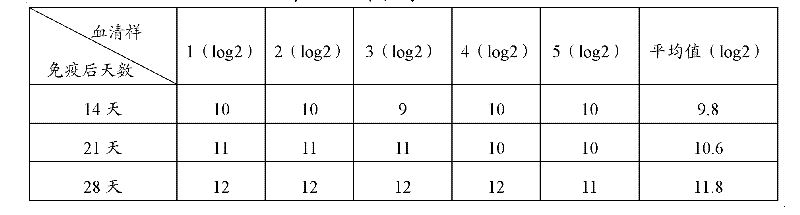
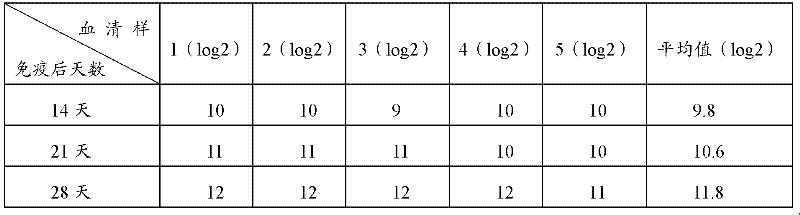
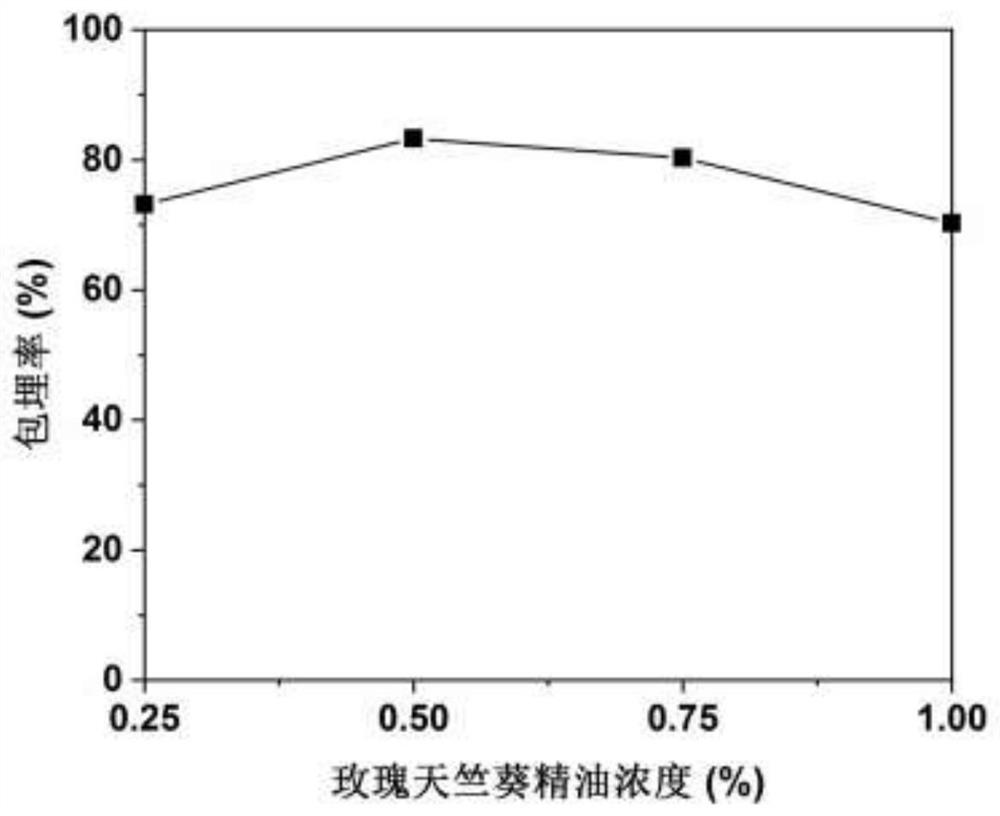
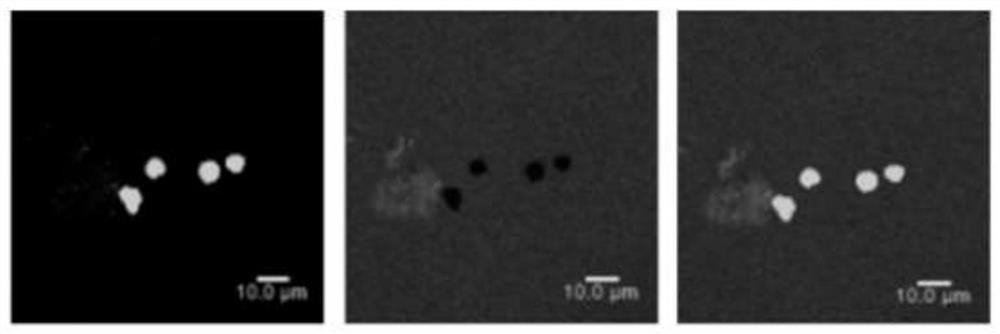
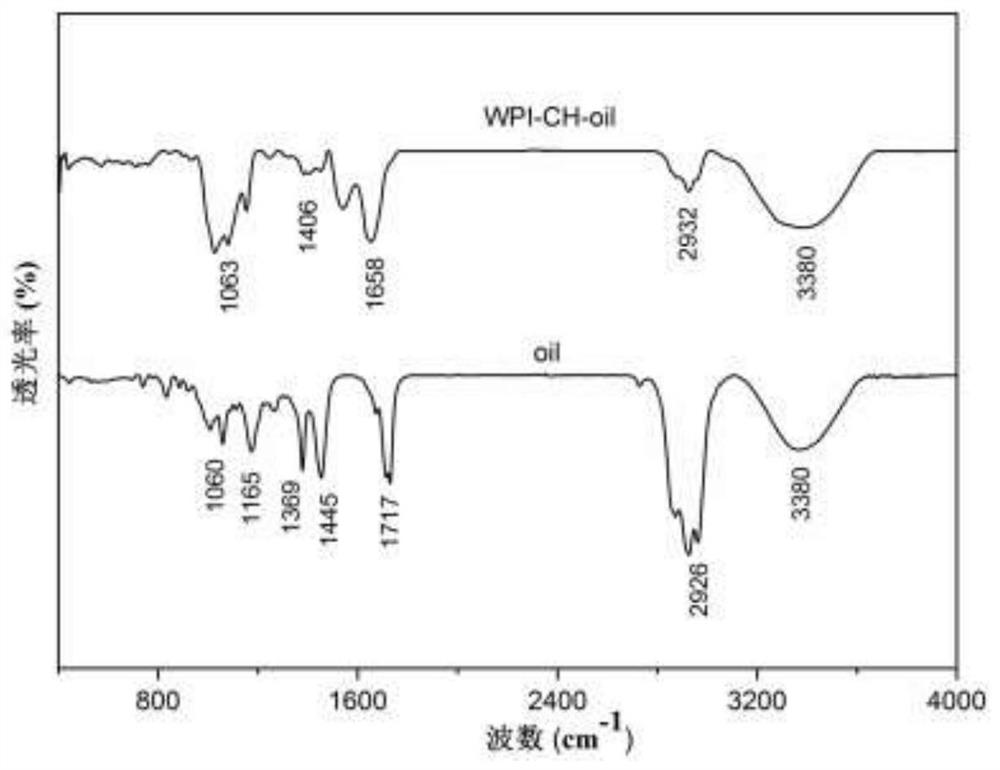
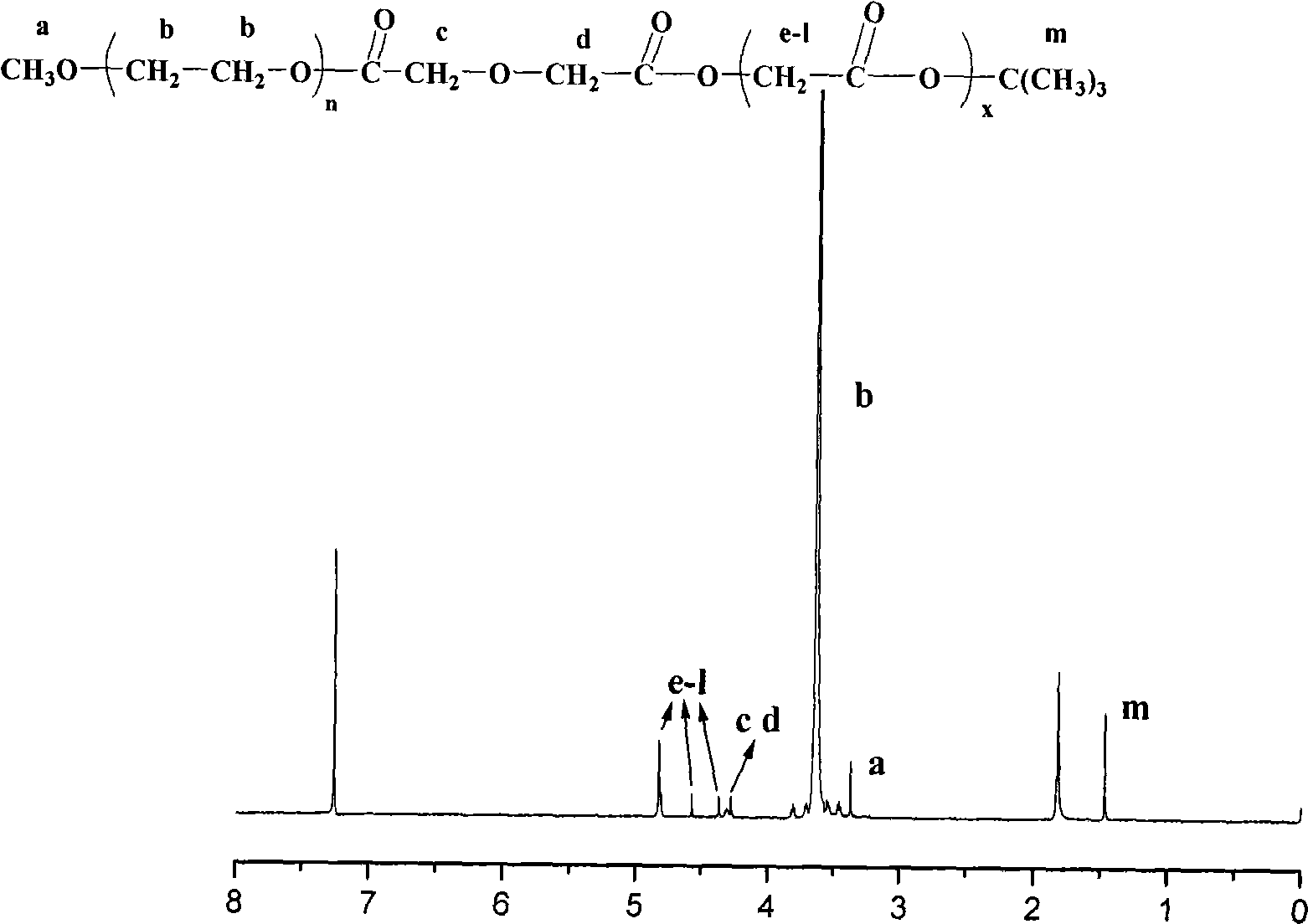Patents
Literature
109results about How to "Solve the problem of insoluble in water" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sterilization soil-improvement slow-release composite fertilizer and preparation method thereof
InactiveCN105347916ASolve the problem of insoluble in waterHas a bactericidal effectFertilizer mixturesSlagCopper carbonate
The invention discloses sterilization soil-improvement slow-release composite fertilizer. The sterilization soil-improvement slow-release composite fertilizer comprises, by weight, 20-25 parts of urea, 10-15 parts of ammonium biphosphate, 10-20 parts of potassium phosphate, 5-10 parts of potassium sulfate, 8-12 parts of sodium silicate, 4-6 parts of magnesium phosphate, 7-9 parts of calcium chloride, 0.2-0.4 parts of zinc ammonium phosphate, 0.1-0.3 parts of copper carbonate, 0.1-0.2 parts of manganese ammonium phosphate, 0.4-0.6 parts of potassium borate, 0.3-0.5 parts of ferric sulfate, 0.2-0.4 parts of sodium molybdate, 20-25 parts of sodium alginate, 35-45 parts of a coating material, 5-10 parts of cassava slag, 5-8 parts of furfural slag, 10-20 parts of chicken manure, 5-10 parts of carbonized rice husk, 10-15 parts of humic acid, 10-15 parts of straws, 5-8 parts of plant ash, 0.2-0.5 parts of biological fungi, 4-6 parts of thermopsine and 3-5 parts of Artemisia argyi powder. The invention also discloses a preparation method of the sterilization soil-improvement slow-release composite fertilizer.
Owner:全椒县琪悦家庭农场
Fat-soluble vitamin freeze-dried prepn. and its prepn. method
InactiveCN1903207ASolve the problem of insoluble in waterIncrease fat solubilityPowder deliveryHydroxy compound active ingredientsSolubilityFreeze-drying
A freeze-dried liposoluble vitamin with high water solubility and stability and its preparing process is also disclosed.
Owner:青岛众智医药科技有限公司
Anti-corrosive protective film agent for marine boiler
ActiveCN101498001AMaintain propertiesSolve the problem of insoluble in waterMarine engineeringAntiseptic
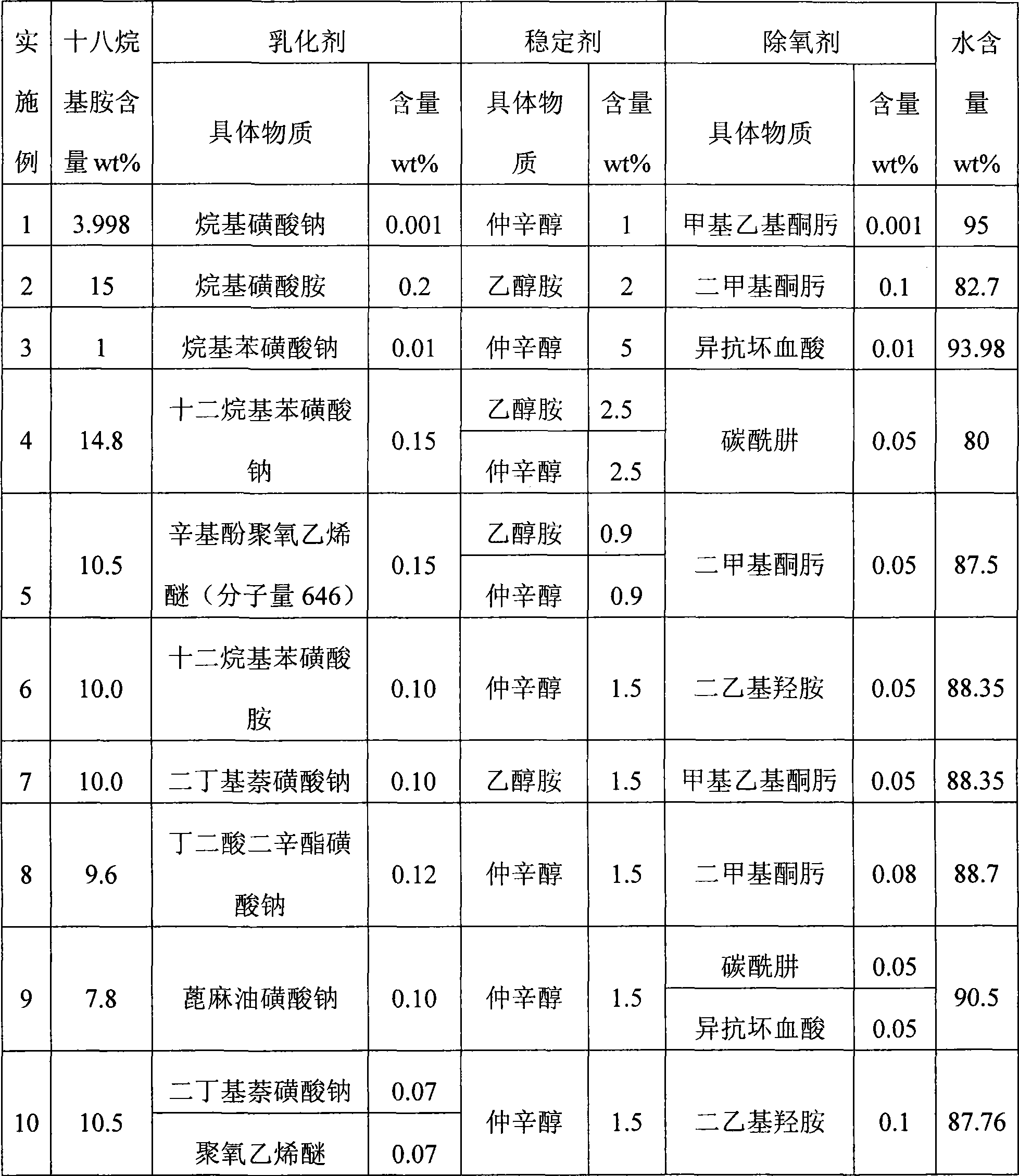
The invention discloses an antiseptic protective film used in a ship boiler, which comprises octadecylamine, emulsifier, stabilizer, deoxidant and water. The antiseptic protective film used in the ship boiler has good antiseptic effect, high stability, long preservation time, safety and environmental protection and can be used in both an operation condition and an idle condition when used in the ship boiler.
Owner:SHANGHAI EMPEROR OF CLEANING HI TECH
Wood protection processing method
ActiveCN101195228ASolve the problem of insoluble in waterSolve the anti-churn problemWood impregnation detailsTarSlurry
A processing method for protecting wood belongs to the technical field of antiseptic, mould proofing and insect prevention processing method, which is characterized in that the processing method comprises that adding NaOH or KOH in biomass gasification tars, mixing and stirring the mixture to react for 1-2 hours, then obtaining liquid A, crashing leaves and fruits of camptotheca acuminata decne to 50-200 courses to be mixed with aqueous solution of NaOH or KOH with weight of 5-20 times of camptotheca acuminata decne materials and consistency of 0.1-0.4%, after being stirred homogeneously, the mixture employs ultrasonic cell-break to react for 4-8 hours, after the slurry is filtered and concentrated, obtaining liquid B, the wood for treatment goes through dipping treatment by liquid A or liquid B which are diluted 20-100 times, then the wood after the dipping treatment goes through acidification dipping treatment. The processing method for protecting wood has antiseptic, mould proofing and insect prevention effects, includes no heavy metal which produces toxins to human beings and domestic animals, is safe for human being and causes no environmental pollution, which further is low in cost.
Owner:ZHEJIANG ANJI FENGHUI BAMBOO PROD & GARMENTS
Amphiphilic tri-block copolymer taxol bonding medicament and synthesis method thereof
InactiveCN1961962ASolve the problem of insoluble in waterGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterSynthesis methods
The invention relates to an amphipathy block copolymer-Paclitaxel compound and relative preparation, wherein said invention is formed by bonded aliphatic polyester-carbowax-aliphatic polyester block copolymer and Paclitaxel; with hydroxyl carbowax (PEG), solvent and catalyst, it processes the ring-opening polymerization of aliphatic ester to obtain the liphatic polyester-carbowax-aliphatic polyester block copolymer, then converting the hydroxyl grouyp into end carboxyl; with condensating agent, processing genate reaction with Paclitaxel, to obtain the inventive drug. The invention has amphipathy property, to be made into liquid agent or freeze dried. And its block structure can improve the Paclitaxel content, adjusted between 10-40%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Application of linker of polyethylene glycol and fat-soluble compounds in biological catalysis
InactiveCN102174191ASolve the problem of insoluble in waterThe separation method is simpleMicroorganism based processesFermentationTriterpenePolyethylene glycol
The invention relates to application of a linker of polyethylene glycol and fat-soluble compounds in biological catalysis. In the invention, mPEG (methoxy polyethylene glycol) is linked with various fat-soluble compounds such as sterides, triterpene and the like by linking bridges to synthesize mPEG-modified water-soluble derivatives: OS-Z-mPEG. Via the derivatives, the original fat-soluble organic compounds can be completely dissolved in water without adding any cosolvents to perform homogeneous biological conversion or catalysis; and after reaction, by the solvent precipitation characteristic of the mPEG polymer, the converted products are selectively precipitated, filtered and collected from the fermentation liquid, thereby achieving the aims of dissolving the fat-soluble compounds and simplifying separation of the converted products.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Sustained-release mixed fertilizer and preparation method thereof
InactiveCN105294257AImprove the bactericidal effectPromote degradationFertilizer mixturesCopper nitrateMonopotassium phosphate
The invention discloses a sustained-release mixed fertilizer. The sustained-release mixed fertilizer comprises the following raw materials in parts by weight: 30 to 40 parts of urea, 15 to 25 parts of monopotassium phosphate, 10 to 20 parts of potassium silicate, 3 to 5 parts of magnesium sulfate, 8 to 10 parts of calcium chloride, 0.2 to 0.4 part of zinc sulfate, 0.3 to 0.5 part of copper nitrate, 0.5 to 1.5 parts of sodium borate, 0.1 to 0.3 part of ferric chloride, 0.1 to 0.3 part of manganese sulfate, 0.3 to 0.5 part of ammonium molybdate, 22 to 29 parts of sodium alginate, 40 to 50 parts of coating material, 5 to 10 parts of biochar, 10 to 15 parts of soybean meal, 10 to 15 parts of humic acid, 10 to 15 parts of straw, 8 to 12 parts of plant ash, and 5 to 7 parts of Gaotouwu powder. The invention also discloses a preparation method of the sustained-release mixed fertilizer. The sustained-release mixed fertilizer can control various elements to be released slowly, can improve the soil and can be used for sterilization and weed control, the coating material can be biologically degraded, and the environment can be protected.
Owner:全椒县琪悦家庭农场
Anti-tumor prodrug having accurate structure and taking novel amphipathic polymer as carrier and synthetic method thereof
InactiveCN101543632ASolve the problem of insoluble in waterFacilitate dissociationOrganic active ingredientsPharmaceutical non-active ingredientsSide effectAdditive ingredient
The invention discloses a prodrug system prepared by connecting a series of oligopolymers synthesized by a group protection and deprotection method and polyethyleneglycol to be used as a drug carrier, belonging to the technical field of chemical drugs. Laden drugs are water-insoluble anti-tumor drugs such as taxol, or other cancer therapy drugs such as doxorubicine and camptothecin. A research discovers that drug compositions prepared by the method greatly improve the hydrophobic nature of the anti-tumor drugs and can accurately control the gather time of the oligopolymers on focus parts and the hydrolyzation rupture time of ester bonds by accurately controlling the lengths of segmers of the oligopolymers so as to control the drug release time so that drugs are stably released, thereby the method prevents burst release causing toxic side effect on normal cells, reduces the drug dosage to enhance the curative effect and sufficiently plays a sustained and controlled release role; and in addition, the structure of the oligopolymers is accurate, and the molecular weight of synthesized amphipathic block polymers which are uniformly distributed is accurate, thereby the method can ensure synthesized drug ingredients to be definite and stable and the pharmacokinetics and the treatment effect (activity and toxicity) of the synthesized drugs to have good reproduction quality and be accurately defined.
Owner:XIANGTAN UNIV
Method for preparing water-soluble plant melanin
ActiveCN102747105AGood water solubilitySolve the problem of insoluble in waterFermentationSolubilityCatalytic oxidation
The invention discloses a method for preparing a water-soluble plant melanin. The method comprises the following steps of preparing a melanin precursor from blueberry leaves as raw materials and carrying out enzyme catalytic oxidation, macroporous resin purification and spray drying to obtain the water-soluble plant melanin. The water-soluble plant melanin obtained by the method has good water-solubility and solves the problem that a natural melanin cannot be dissolved in water.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Liquidambaric acid or ursolic acid nano-granule and preparation method thereof
ActiveCN104721144AImprove water solubilitySmall particle sizePowder deliveryOrganic active ingredientsNanocapsulesHuman prostate
The invention discloses a liquidambaric acid or ursolic acid nano-granule, a preparation method and an application thereof. The liquidambaric acid or ursolic acid nano-granule is prepared by the following steps: taking the liquidambaric acid or ursolic acid as a core material, taking one or two mixtures of the gelatin, B-cyclodextrin, rubusoside, soluble starch, stevioside and thea saponin as a wall material, wherein the mass ratio of the core material to the wall material is 1:(5-100). The preparation method is an ultrasonic high-speed refiner method or a high energy ball milling method. By adopting the liquidambaric acid or ursolic acid nano-granule, the cell permeability can be improved and the growths of the human colon carcinoma cell HT-29, the human prostate cancer cell DU145 or the human breast cancer cell MDA-MB-231 can be inhibited.
Owner:SHANGHAI APPLIED TECHNOLOGIES COLLEGE
Immunochemotherapy pharmaceutical composition and preparation method therefor
ActiveCN110917345AGood biocompatibilityGood sustained release effectOrganic active ingredientsAntibody ingredientsChemotherapeutic drugsTumor recurrence
The invention discloses an immunochemotherapy pharmaceutical composition. The immunochemotherapy pharmaceutical composition is formed by mixing a first mixture with a second mixture; the first mixturecontains immunoadjuvant imiquimod R837; the second mixture contains chemotherapeutic drug oxaliplatin which can cause immunogenicity death, and further contains poloxamer 188 and sodium alginate (ALG); the first mixture is an imiquimod emulsion obtained by mixing the imiquimod R837 with the poloxamer 188; imiquimod particles each have a particle size of 0.5-3 mum; the imiquimod emulsion undergoesmoist heat sterilization; and the second mixture is a mixture prepared in a manner that the sodium alginate (ALG) and the oxaliplatin are stirred and mixed with water, and filtration sterilization isperformed with a micrometer filter membrane. The new anti-cancer pharmaceutical composition can exert the synergistic anticancer effect, reduces the side effect, the cancer metastasis probability andthe cancer recurrence probability, and can inhibit the growth of a distant metastatic tumor and reduce the tumor recurrence probability through the immunoreaction while effectively killing a tumor insitu; and meanwhile, the production process is relatively optimal, and the product stability is high.
Owner:SUZHOU INNOVATIVE BIOMATERIALS & PHARM CO LTD
Photo-thermal sensitive carboxymethyl chitosan nano drug-loaded microsphere and preparation method thereof
InactiveCN110859823ASolve the problem of insoluble in waterPrecision releaseOrganic active ingredientsEnergy modified materialsHydrazoneIsopropyl
The invention belongs to the technical field of biomedical materials, and particularly relates to a photo-thermal sensitive carboxymethyl chitosan nano drug-loaded microsphere, a nano microsphere is formed by taking a polymer carboxymethyl chitosan / N-isopropylacrylamide as a skeleton and loading hydrazone bond bonded nanogold-doxorubicin hydrochloride on the surface of the skeleton. The inventionalso provides a preparation method of the nano drug-loaded microsphere. The invention provides the photo-thermal sensitive carboxymethyl chitosan nano drug-loaded microsphere, the problem that chitosan is difficult to dissolve in water is solved, meanwhile, by changing the conditions of near-infrared illumination and temperature, the nano microsphere material is subjected to phase change, hydrophilic chain collapse and solvation layer damage, a medicine is slowly released, the medicine carrying rate of the medicine is increased, and the medicine is controlled to be accurately released.
Owner:AGRI PRODS PROCESSING RES INST CHINESE ACAD OF TROPICAL AGRI SCI +1
Preparation method of chicken infectious bronchitis virus HA antigen
InactiveCN102391366ASolve the problem of reducing potencySolve inaccurateDepsipeptidesWater bathsAntigen
The invention discloses a preparation method of a chicken infectious bronchitis virus HA antigen. In the preparation method, an A-type clostridium perfringen is adopted to treat a virus solution, so that the problem that in the conventional method, the preparation cost of the antigen is high is solved; an adopted stabilizing agent is N-2-hydroxypropyl trimethyl ammonia chloride chitosan and is water-soluble, so that the problem that the conventional stabilizing agent chitosan is water-insoluble is solved; an adopted deactivating method is deactivation for 30 minutes in water bath of 56 DEG C, so that the problem that the titer is reduced after a deactivating agent is added is solved; an adopted chicken red blood cell suspension has the concentration of 0.5 percent, so that the judgment result is more stable and the problem that the judgment result is not accurate is solved; due to adoption of dialysis bag for concentration, the problem that the titer of the antigen is low is solved; therefore, the invention provides the preparation method of the chicken infectious bronchitis virus HA antigen, which is low in cost, high in titer, and high in stability and safety, and the prepared IBV (infectious bronchitis virus) HA antigen can be successfully applied to detection on the titer of a serum HI antibody after chickens are immunized by an IBV vaccine.
Owner:哈药集团生物疫苗有限公司
Repaglinide-metformin hydrochloride tablet and preparing method thereof
InactiveCN104337811AUse less excipientsSolve the problem of insoluble in waterOrganic active ingredientsMetabolism disorderMetformin HydrochlorideSolvent
The invention discloses a prescription of a repaglinide-metformin hydrochloride tablet and a preparation technology thereof. In particular, a solid dispersion water solution technology is used; the problem that repaglinide is insoluble in water can be solved only through common wet granulations; dissolved objects and related matter are both superior to those of foreign objects. Microcrystalline celluloses and sorbitol serve as fillers; polyvinylpolypyrrolidone serves as disintegrating agents; PVPk30 serves as bonding agents; silicon dioxide serves as lubricating agents; meglumine serves as saltiness agents; and poloxamer 188 serves as solubilizers. The repaglinide-metformin hydrochloride tablet prepared with the technology is fewer in use auxiliary material; the high dissolving performance and the high stability can be kept; the preparation technology is simple; the technology cost is lower; and the repaglinide-metformin hydrochloride tablet is suitable for industrial production.
Owner:JIANGSU CAREFREE PHARM CO LTD
Preparation method for water soluble compound cooling agent
InactiveCN105166835ACool and mildLong-lasting coolnessFood preparationPhysical chemistryCyclodextrin
The present invention relates to a preparation method for a water soluble compound cooling agent. The preparation method comprises the following steps: step (1) uniformly mixing Tween-80 and poloxamer to obtain solution A; (2) adding poloxamer into the solution A and oscillating the solution A with ultrasound wave to obtain solution B; step (3) dissolving a compound cooling agent in dipropylene glycol, adding the obtained solution into solution B, and uniformly mixing the solution by stirring to obtain the water soluble compound cooling agent. According to the present invention, a plurality of cooling agents are compounded according to a certain ratio, the compounded cooling agent has a gentle and lasting cooling flavor, and has no bitterness. Meanwhile, the problem that the cooling agent is insoluble in water is solved, and the water soluble compound cooling agent can be widely used in foods, daily products, beverages and other products.
Owner:ANHUI AIDI SPICE CO LTD
Vitamin A nano emulsion and preparation method thereof
InactiveCN106727310AImprove stabilitySolve the problem of insoluble in waterCosmetic preparationsSenses disorderVitamin A AlcoholVitamin a1
The invention discloses a vitamin A nano emulsion and a preparation method thereof. The preparation method comprises the following steps: uniformly mixing an emulsifier with vitamin A oil, slowly dropping water, and preparing the vitamin A nano emulsion by using a phase transformation method, wherein the particle size of the vitamin A nano emulsion is within 1-100nm. Due to preparation of the vitamin A nano emulsion, the difficulty that vitamin A is not dissolved in water can be solved, and convenience is brought to production and application of the vitamin A nano emulsion. Due to preparation of the vitamin A nano emulsion, the stability of vitamin A is also improved, and the vitamin A nano emulsion has relatively great application values in fields such as food, healthcare products and cosmetics.
Owner:SHAANXI UNIV OF SCI & TECH
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveCN101189001ASolve the problem of insoluble in waterSmall injection volumeOrganic active ingredientsPowder deliveryBenzodiazepinePolyethylene glycol
Described herein are nanoparticulate formulations of benzodiazepines, such as lorazepam, which do not require the presence of polyethylene glycol and propylene glycol as stabilizers; and methods of making and using such formulations. The formulation is especially useful in aerosol and injectable dosage forms and contains nanoparticulate benzodiazepines such as lorazepam and at least one surface stabilizer. The preparation is useful in the treatment of epileptic states, in the treatment of irritable bowel syndrome, sleep induction, acute psychosis and as a pre-anesthesia drug.
Owner:ELAN PHRMA INT LTD
Preparation method of rose geranium essential oil microcapsules with good oxidation resistance and antibacterial properties
PendingCN113712144ASolve the problem of insoluble in waterNo chemical changes involvedFood ingredient as antioxidantFood preservationBiotechnologyPelargonium roseum
The invention discloses a preparation method of rose geranium essential oil microcapsules with good oxidation resistance and antibacterial properties, and belongs to the technical field of food processing. The preparation method comprises the following steps of using protein and polysaccharide as wall materials, and using rose geranium essential oil as a core material; performing high-speed shearing and high-pressure homogenizing so that the wall materials and the core material are jointly emulsified, adjusting pH, adding a curing agent, and performing a complex coacervation method so as to obtain turbid liquid of the rose geranium essential oil microcapsules; and then performing freeze drying so as to obtain the rose geranium essential oil microcapsules. The microcapsules prepared by the preparation method disclosed by the invention can load and protect the rose geranium essential oil, the stability of the rose geranium essential oil is improved, the oxidation resistance of the rose geranium essential oil is well exerted, and the rose geranium essential oil microcapsules have the effect of restraining bacteria; and besides, the preparation method disclosed by the invention is simple in technology, mild in conditions, low in cost, safe and harmless, and is suitable for various fields of foods, health-care products, cosmetics and the like.
Owner:NORTHWEST A & F UNIV
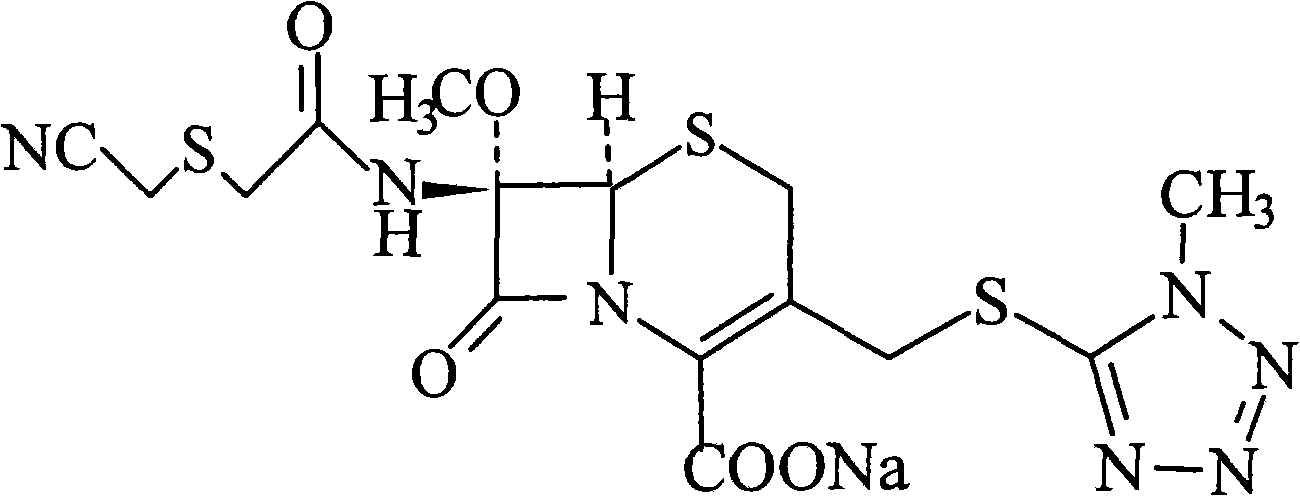
Composition of cefmetazole acid
InactiveCN101548977AEasy to purifyImprove product qualityPowder deliveryOrganic active ingredientsSide effectArginine
The invention relates to a composition of a cefmetazole acid, which is characterized in that the mix of cefmetazole acid and arginine aseptic powders with the weight ratio of 1: 0.35 to 1:0.70 is dissolved in water. The invention includes the detail steps of weighting 50kg material drug of cefmetazole acid and arginine aseptic powders by the weight percentage of 1: 0.35 to 1:0.70; pouring the material drug in a three-dimensional motion mixer and setting the rotating speed of the three-dimensional motion into 5 rotation per minute; well mixing the material drug for 60 minutes to 90 minutes; discharging the material and sub-packaging the material. The invention has the advantages that the composition improves the quality of products and reduces impurities, the composition markedly improves the stability of product, effectively reduces the content of impurities and solves the problem of water insolvability, and cefmetazole acid is more stable than cefmetazole sodium and can be purified easily; if cefmetazole acid is used to obtain preparation together with arginine proportionally, the all relative material can be stabilized in 2 percent for a long time, thereby reducing the incidence rate of side effects and allergy and achieving actual meaning and value.
Owner:国药集团致君(苏州)制药有限公司
Preparation method of self-emulsifying glycerol monolaurate
PendingCN110169496ASolve the problem of insoluble in waterImprove solubilityAccessory food factorsFood ingredient as emulsifierGlycerolRoom temperature
The invention discloses a preparation method of self-emulsifying glycerol monolaurate, comprising the following steps of a, respectively weighing glycerol monolaurate, an emulgator, a solubilizer, anemulsion stabilizer and a carrier; b, heating the glycerol monolaurate to 40 to 60 DEG C, then adding the emulgator and dissolving to obtain an oil phase; c, adding the emulsion stabilizer into water,heating to 40 to 60 DEG C for dissolving to obtain a water phase for later use; d, adding the oil phase solution obtained in the step b into the water phase solution obtained in the step c, stirringand homogenizing to ontain a glycerol monolaurate solution; e, cooling the solution obtained in the step d to room temperature, adding the solubilizer, performing secondary homogenization to obtain asemitransparent or transparent solution; f, mixing the solution obtained in the step e with the carrier, and performing spray drying to prepare solid particles. The self-emulsifying glycerol monolaurate prepared by the invention has the advantages that a self-emulsifying solution is adsorbed by the carrier, the fluidity is good, moisture absorption is not easy to occur, the use is convenient, andthe uniform mixing of a feed is facilitated.
Owner:ZHEJIANG WANFANG BIO TECH CO LTD
Anti-blue-light-pollution skin care composition capable of resisting blue light pollution and application thereof
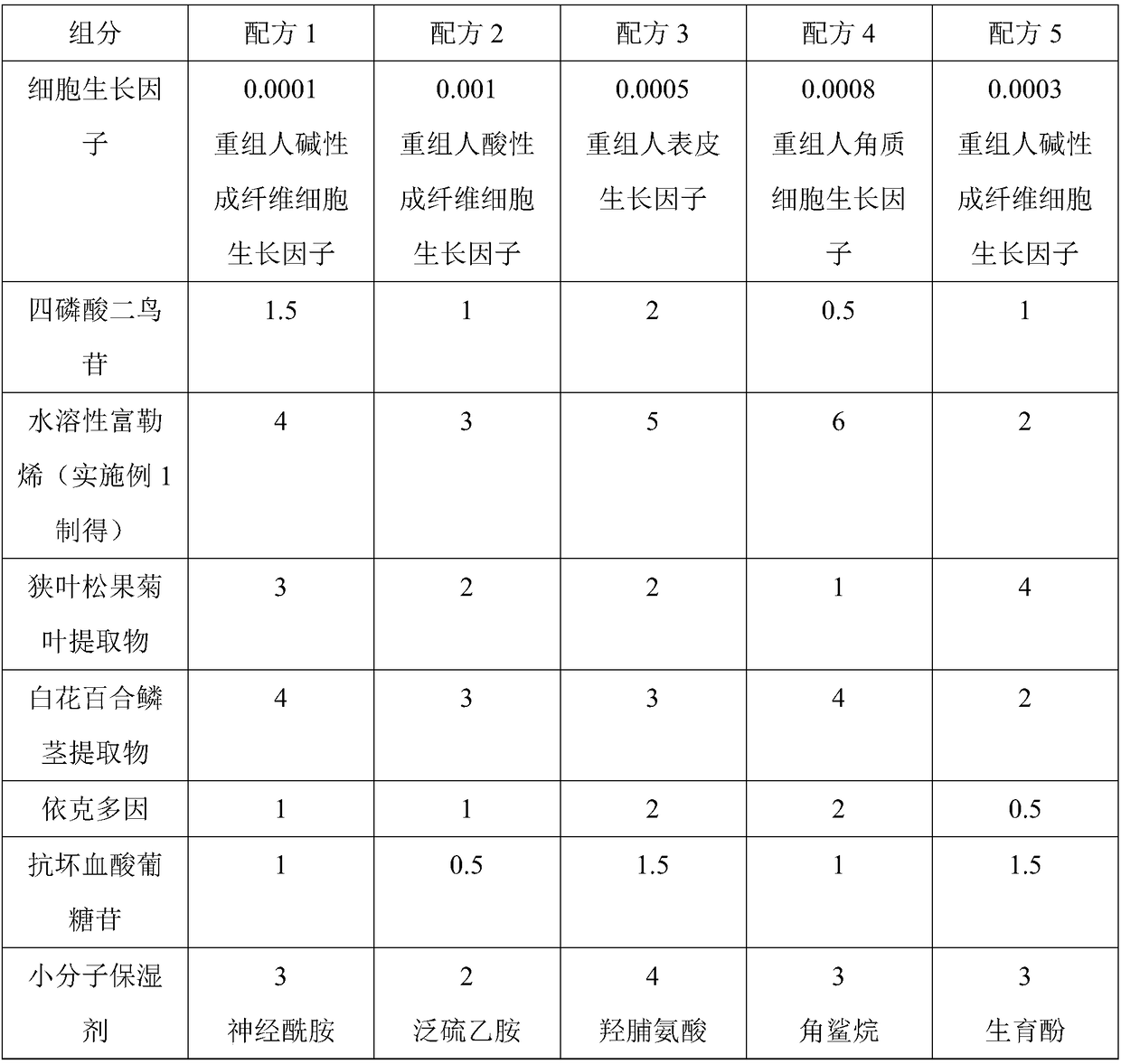
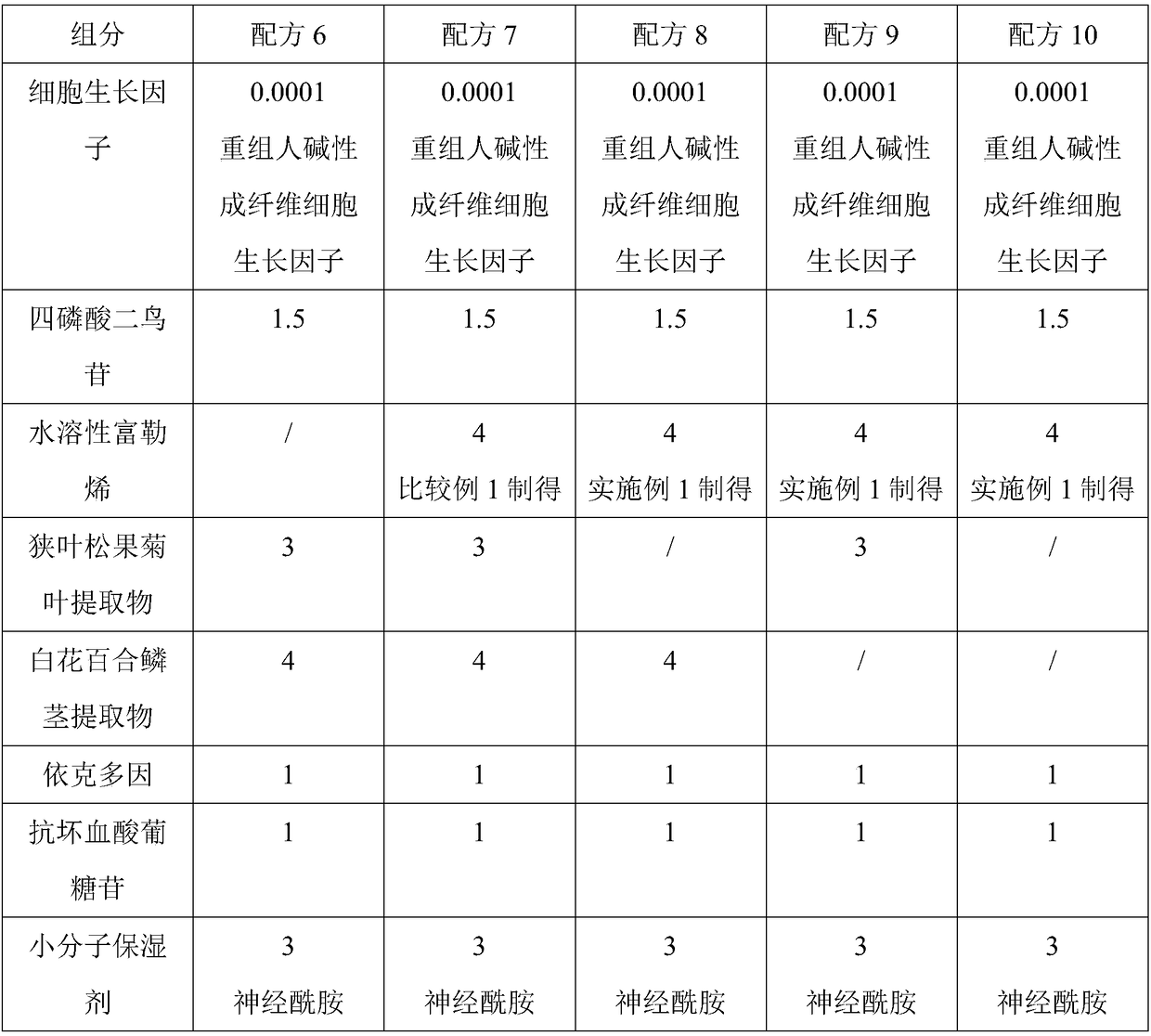
ActiveCN108852869ASignificant free radical scavenging effectSolve the problem of insoluble in waterCosmetic preparationsToilet preparationsEchinacea angustifoliaHyperpigmentation
The invention discloses an anti-blue-light-pollution skin care composition and application thereof. The anti-blue-light-pollution skin care composition is prepared from the following components: a cell growth factor, diguanosine tetraphosphate, water-soluble fullerene, an echinacea angustifolia leaf extract, a lilium candidum bulb extract, ectoin, ascorbyl glucoside and a -molecule wetting agent.The skin care composition is designed to solve various skin problems induced by blue light pollution, the components are reasonably collocated and scientifically proportioned, so that the phenomena ofthe loss of collagen, the pigmentation and the skin sensitivity caused under the blue light pressure can be effectively avoided, and a better anti-blue-light protecting and repairing effect is achieved.
Owner:GUANGDONG COOWAY BIOTECH CO LTD +1
Water soluble ethopabate composition and compound composition thereof
ActiveCN103622944ASolve the problem of insoluble in waterImprove solubilityOrganic active ingredientsAntiparasitic agentsDrugChemistry
The invention provides a water soluble ethopabate composition and a compound composition thereof. The composition and the compound composition thereof can be used to manufacture drugs for preventing and treating coccidiosis. The drugs can be taken through drinking water, so the problem that a premix is hard to mix uniformly in the feed and the curative effect is affected is effectively solved.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Carbon nano tube compounded hydrogel for tritium prevention and filtration and preparation method thereof
InactiveCN105418859ASolve the problem of insoluble in waterHigh mechanical strengthFiltrationCarbon nanotube
The invention discloses carbon nano tube compounded hydrogel for tritium prevention and filtration and a preparation method thereof. The carbon nano tube compounded hydrogel can keep air permeability under the condition of water adsorption and meanwhile has a certain mechanical strength. The preparation method adopts a two-step irradiation method, acrylic acid is successfully grafted to the walls of carbon nano tubes through first-step irradiation, and the problem that the carbon tubes are insoluble in water is solved; gel and the carbon nano tubes are successfully and uniformly compounded together through second-step irradiation, the mechanical strength of the carbon nano tube compounded hydrogel is also accordingly improved, and the air moisture adsorption efficiency is also improved. The method is simple and easy to operate, the doping of other reagents is not needed, and the obtained hydrogel material is purer.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV
Tree insect pest preventing and controlling process method
InactiveCN101194631ASolve the problem of insoluble in waterGood control effectBiocideAnimal repellantsTree rootTree trunk
A method for preventing and curing pests of trees belongs to the technical field of a method for processing trees, which comprises the following preparations and the process for preventing and curing trees, firstly, the process for preparation is that biomass gasification oil tar is reacted with NaOH or KOH for 30-80 minutes and is added with H2O to be diluted to make trees preventing and curing process preparation, and the weight ratio of biomass gasification oil tar: NaOH:H2O is 1:10.-0.4:0.5-2.0, and the weight ratio of biomass gasification oil tar:KOH:H2O is 1:0.1-0.5:0.1-2.0, and the biomass gasification oil tar is bamboo oil tar or wooden oil tar or grass oil tar, secondly, the trees preventing and curing process is that the preparations prevent and cure pests of trees by adopting ways of tree trunk injection or tree root irrigation or stems and leaves spray or essential positions coating, the method for preventing and curing pests of trees has the advantages of low manufacturing cost, excellent disinfecting effect, and little environmental affect.
Owner:杭州天香园林有限公司
Water-dispersible cannabidiol product and preparation method thereof
InactiveCN110693027ASolve the problem of insoluble in waterImprove bioavailabilityDispersion deliveryHydroxy compound active ingredientsBiotechnologyOrganosolv
The present invention relates to the field of food processing and particularly relates to a water-dispersible cannabidiol product and a preparation method thereof. The water-dispersible cannabidiol product comprises an obtained product prepared by the following method: adding an organic phase containing cannabidiol into a water phase for emulsification, adding glycosylase for a glycosylation treatment after removing organic solvent in an emulsion, and conducting drying to prepare the water-dispersible cannabidiol product. The prepared water-dispersible cannabidiol product can be quickly dispersed and dissolved in an aqueous solution, the solution is clear, and no precipitate is produced after long-term placing. The product after the glycosylation treatment is a glucoside mixture with the cannabidiol as a parent nucleus structure and introducing different sugar chain structures to phenolic hydroxyl groups. The product solves a problem that the cannabidiol is insoluble in water and can be widely applied to the food and beverage industry.
Owner:GUILIN NATURAL INGREDIENTS CORP
Beta-cyclodextrin derivative grafted hydroxypropyl chitosan hydrogel and preparation method thereof
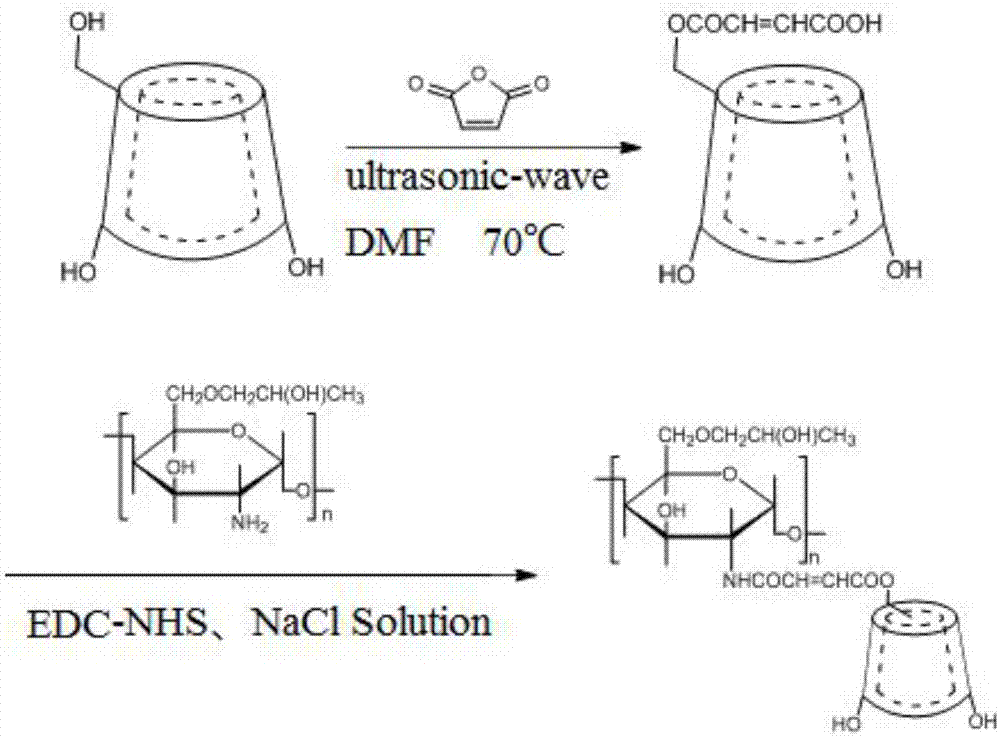
ActiveCN107383393ASolve the problem of insoluble in waterGood biocompatibilityMaleic anhydrideChemistry
The invention provides beta-cyclodextrin derivative grafted hydroxypropyl chitosan hydrogel and a preparation method thereof. The invention provides novel maleic anhydride modified beta-cyclodextrin grafted hydroxypropyl chitosan hydrogel and a preparation method thereof. Firstly, beta-cyclodextrin and maleic anhydride are used as raw materials; a multifunctional ultrasonic extractor is used for preparing the maleic anhydride modified beta-cyclodextrin; then, the maleic anhydride modified beta-cyclodextrin is used for reacting with hydroxypropyl chitosan through carboxyls and aminos; amido bonds are generated. The preparation method has the advantages that the cost is lower; the yield is high; the reaction conditions can be easily controlled. The prepared hydrogel is uniform and stable and has good biocompatibility.
Owner:QILU UNIV OF TECH
Yiqing granule preparing method
ActiveCN103977097AProblems Affecting SolubilityImprove efficacyDigestive systemPharmaceutical non-active ingredientsAqueous sodium hydroxideScutellariae radix
The invention discloses a Yiqing granule preparing method. The method comprises the following steps: respectively extracting dry extract powder from rhizoma coptidis, rheum officinale and radix scutellaria; adding a pharmaceutically-acceptable auxiliary material in the dry extract powder of rhizoma coptidis, evenly mixing, and adding an ethanol-water solution or water so as to prepare rhizoma coptidis granules; adding a pharmaceutically-acceptable auxiliary material in the dry extract powder of rheum officinale, evenly mixing, and adding a sodium hydroxide water solution or an ethanol-water solution containing sodium hydroxide so as to prepare rheum officinale granules; adding a pharmaceutically-acceptable auxiliary material in the dry extract powder of radix scutellariae, evenly mixing, and adding a sodium hydroxide water solution or an ethanol-water solution containing sodium hydroxide so as to prepare radix scutellaria granules; finally mixing rhizoma coptidis granules, rheum officinale granules and radix scutellaria granules to obtain mixed granules, and sub-packaging the mixed granules in bags, thereby obtaining Yiqing granules. The Yiqing granules prepared by virtue of the method have qualified dissolubility and a strong curative effect and are safe and effective to take; besides, the technology is simple; the application prospect is broad.
Owner:SICHUAN FENGCHUN PHARMA
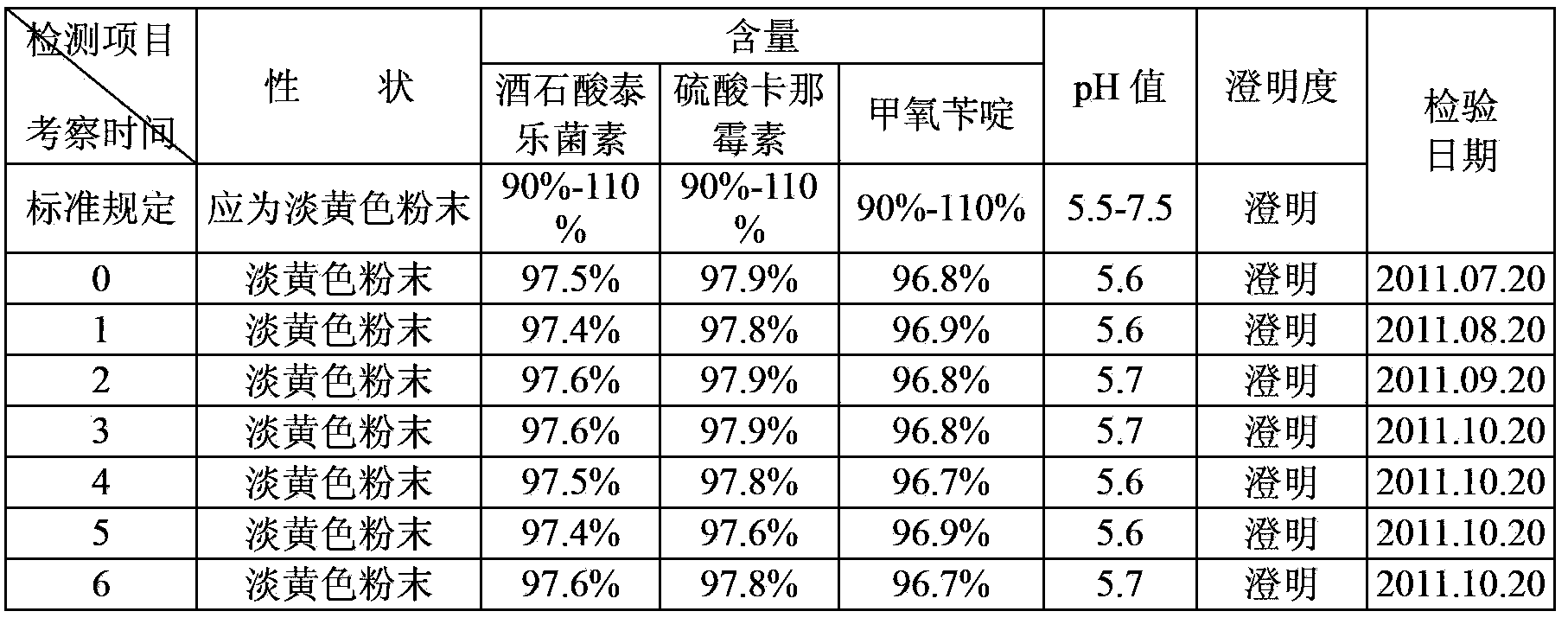
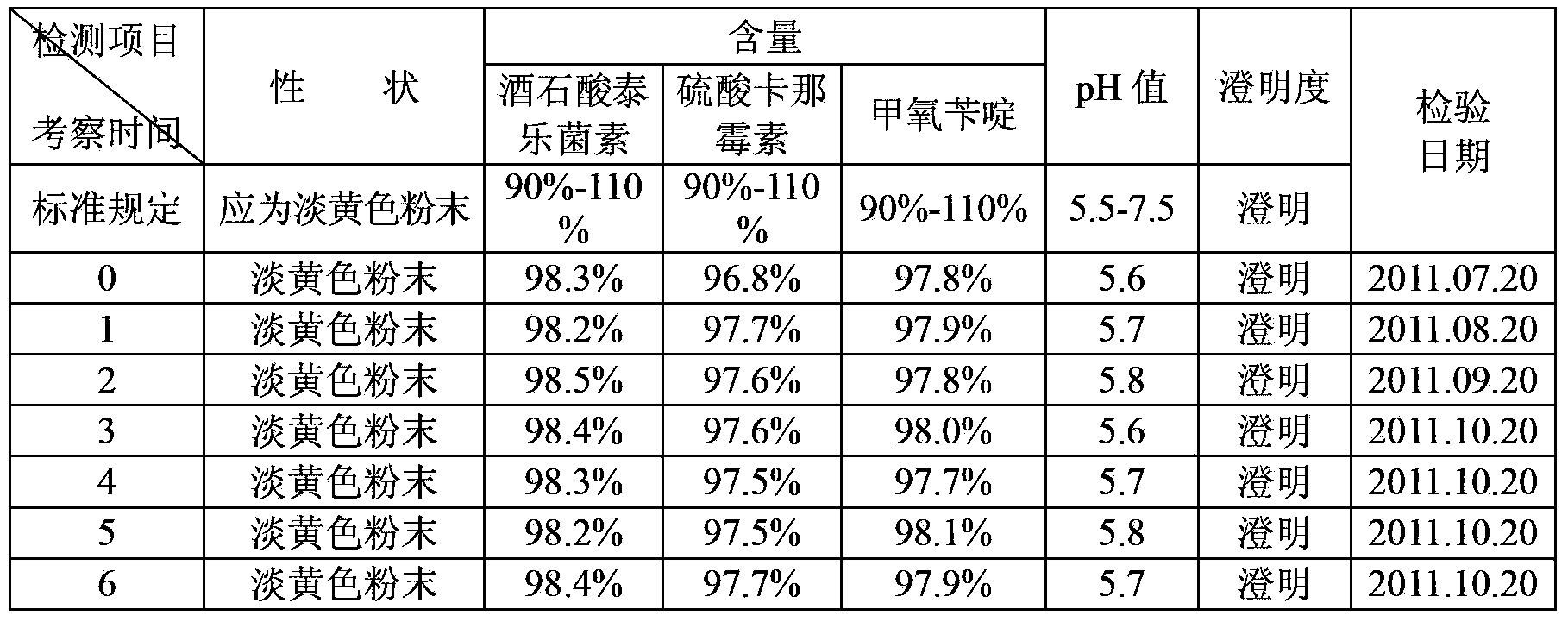
Preparation method of synergistic tylosin tartrate soluble powder compound medicine
InactiveCN103405463AWide range of indicationsGood curative effectAntibacterial agentsOrganic active ingredientsTrimethoprimSecondary Infections
The invention aims at providing a preparation method of a synergistic tylosin tartrate soluble powder compound medicine. The synergistic tylosin tartrate soluble powder compound medicine has a rapid and definite curative effect on respiratory system infections and complications of livestock and poultry. The preparation method comprises the following steps of: mixing 0.2 part of trimethoprim and 0.2 part of cosolvent, then crushing, and sieving by using a 120 meshes sieve or a finer sieve; and then uniformly mixing with 1 part of tylosin tartrate, 1 part of kanamycin sulfate and 0.05-0.1 part of pain alleviant by an equivalently successive increase method, thus obtaining the synergistic tylosin tartrate soluble powder compound medicine. The cosolvent is citric acid or succinic acid or the mixture of citric acid and succinic acid and the pain alleviant is procaine hydrochloride or lidocaine hydrochloride or the mixture of procaine hydrochloride and lidocaine hydrochloride. The animal synergistic tylosin tartrate soluble powder compound medicine for injection contains three medical components which are tylosin tartrate, kanamycin sulfate and trimethoprim, can be used for solving the problems that the secondary infection cannot be controlled very well during the treatment of mycoplasma infection due to the narrow antibacterial spectrum of the tylosin tartrate, and the trimethoprim is insoluble in water, has a synergistic effect on tylosin tartrate and kanamycin sulfate and has an obvious medicine curative effect.
Owner:四川联美生物药业有限公司
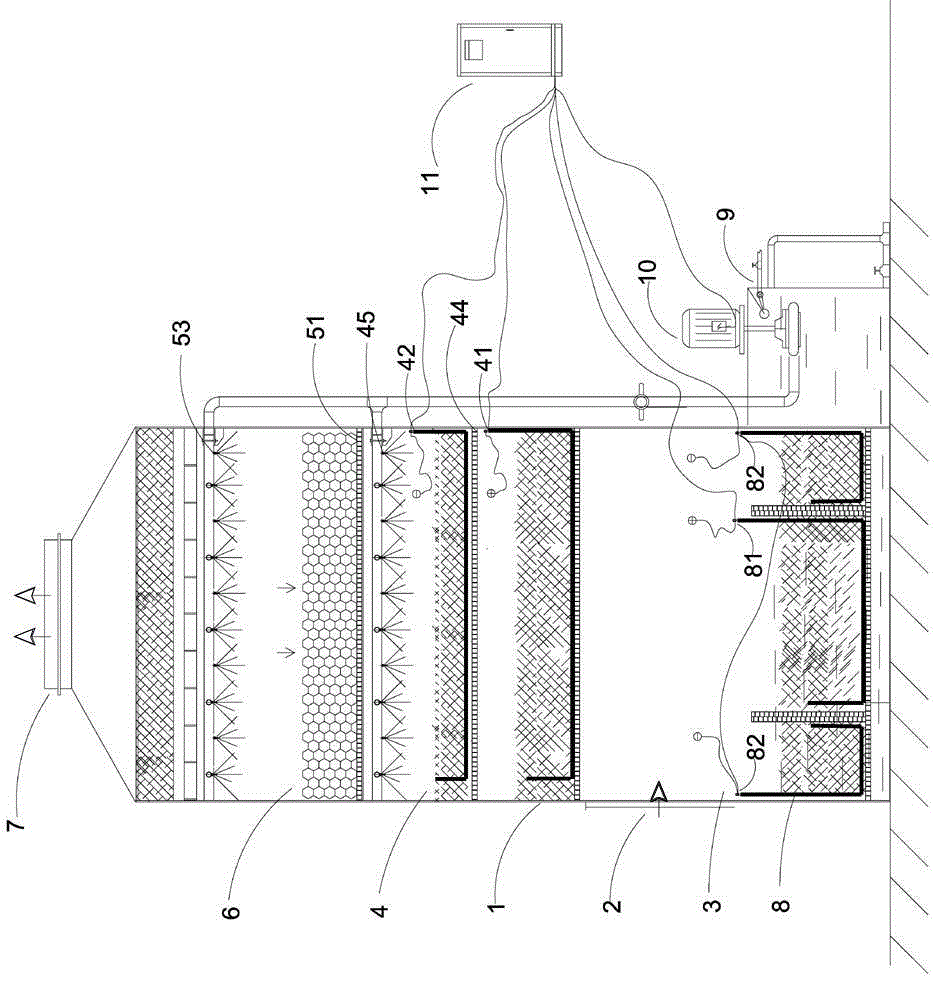
Micro-charge water spray waste gas treatment method
InactiveCN105413374AEffective interceptionEfficient captureCombination devicesElectricityWater storage
The invention particularly relates to a micro-charge water spray waste gas treatment method. The micro-charge water spray waste gas treatment method comprises the following steps: step 1, waste gas enters from the gas inlet of a reaction tower body; step 2, the waste gas continually rises under the action of a suction force and passes through a micro-charge field to form waste gas with polarity particles, and the waste gas with the polarity particles is intercepted and captured; step 3, the spray flushing procedure is carried out to enable the waste gas with the polarity particles to be adhered to a liquid, and the waste gas with the polarity particles falls down together with the liquid to a water storage micro-charge reaction chamber for advanced electrochemical oxidation and flocculent sedimentation treatment; step 4, the waste gas subjected to purification treatment is discharged out of the reaction tower body after being converted into a pollutant-free gas. The micro-charge water spray waste gas treatment method has the following benefits: waste gas enters the micro-charge field to form waste gas with polarity particles to be adhered onto the liquid, and the waste gas with the polarity particles is effectively intercepted and captured and carried away by the running liquid.
Owner:陈西安
Polyvinyl alcohol with advantages of defoaming function and low alcoholysis degree, and preparation method thereof
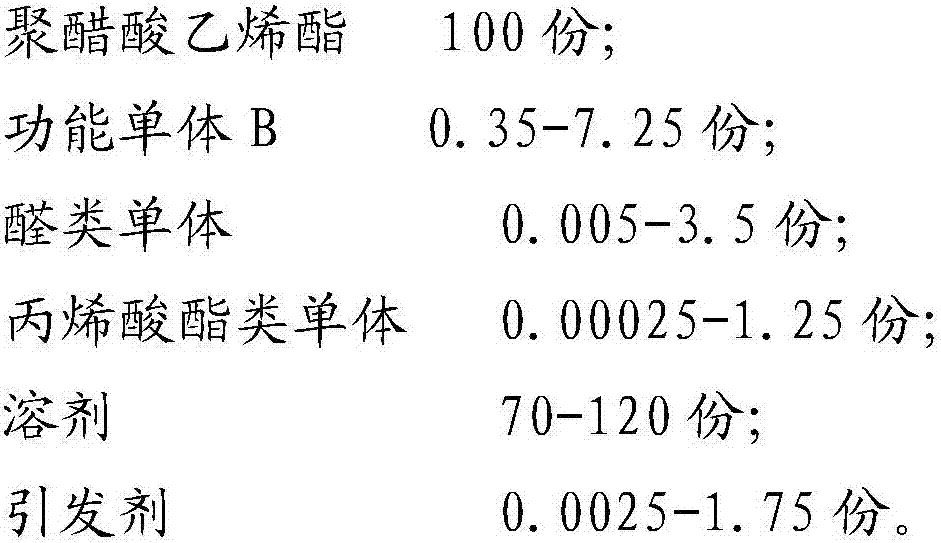
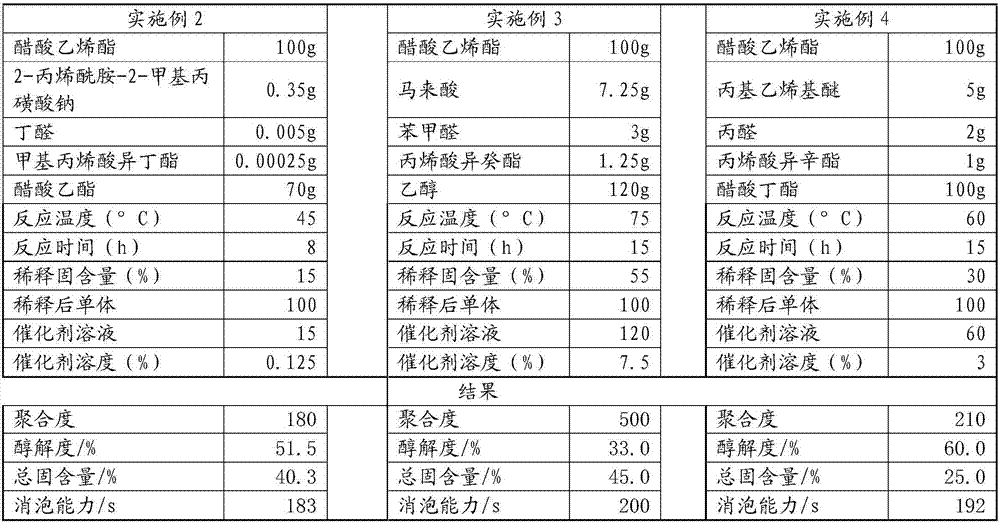
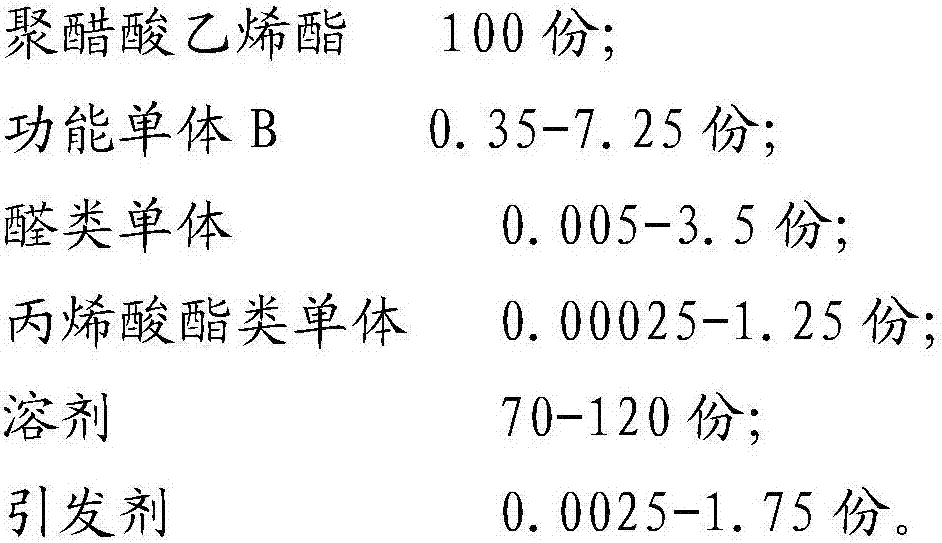
ActiveCN107189003ASolve the problem of insoluble in waterSolve the problem of fragmentationPolyvinyl acetateAlcohol
The present invention belongs to the field of preparation of polyvinyl alcohol, and particularly relates to polyvinyl alcohol with advantages of defoaming function and low alcoholysis degree, and a preparation method thereof. The preparation method comprises: a) polyvinyl acetate polynary monomer copolymerization, and b) catalytic alcoholysis, wherein the copolymerization monomer used in the step a) comprise, by weight, 100 parts of polyvinyl acetate, 0.35-7.25 parts of a functional momomer B, 0.005-3.5 parts of an aldehyde monomer, 0.00025-1.25 parts of an acrylate monomer, 70-120 parts of a solvent, and 0.0025-1.75 parts of an initiator. According to the present invention, the problem that the polyvinyl alcohol with the alcoholysis degree of less than 56 mol% is insoluble in water is solved so as to make the auxiliary dispersant become the environmentally friendly product, the problem of the water phase dispersing during vinyl chloride suspension polymerization is solved, and the auxiliary dispersant has the defoaming function.
Owner:TIANJIN SUNNYMER +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com