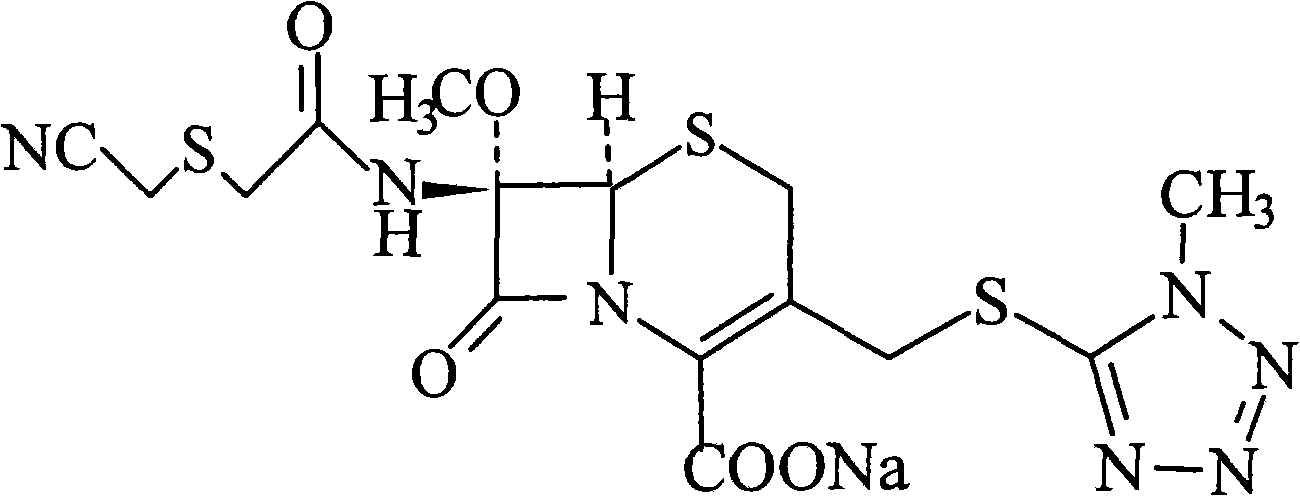
Composition of cefmetazole acid
A technology of cefmetazole acid and its composition, which is applied in the field of drug preparation, can solve the problems of high side effects and allergy rate, easy increase of related substances, high content of impurities, etc., achieve easy purification, reduce the incidence of allergic reactions, and reduce impurities The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0010] Cefmetazole acid sterile powder is used, the particle size is adjusted, mixed with arginine sterile powder at a weight ratio of 1:0.35 to 1:0.70, and dissolved in water. The specific steps are as follows: in the aseptic batching workshop, weigh 50 kg of cefmetazole acid and arginine aseptic raw materials with a ratio of 1:0.35 to 1:0.70, and pour them into a high-efficiency three-dimensional motion mixer. Set the speed of the three-dimensional powder mixer to 5 rpm, and start the powder mixing operation according to the existing powder mixing packaging standard operating procedures. After mixing for about 60min to 90min, the material is discharged and packaged separately.
[0011] The optimum weight ratio is 1:0.40. Because its molar ratio is about 1:1, its pH is about 7±1, which is close to neutral, which is beneficial for injection. Otherwise, if the pH is too high or too low, it will stimulate the intravenous injection.
example 2
[0013] Cefmetazole acid sterile powder is used, the weight ratio of particle size and sodium carbonate sterile powder is adjusted to be 1:0.13-1:0.20, mixed and dissolved in water. The specific steps are as follows: in the aseptic batching workshop, weigh 50 kg of cefmetazole acid and sodium carbonate aseptic raw materials in a ratio of 1:0.13 to 1:0.20, and pour them into a high-efficiency three-dimensional motion mixer. Set the speed of the three-dimensional powder mixer to 5 rpm, and start the powder mixing operation according to the powder mixing packaging standard operating procedures. After mixing for about 60min to 90min, the material is discharged and packaged separately.
[0014] The optimum weight ratio is 1:0.14. Because its molar ratio is about 1:1, its pH is about 7±1, which is close to neutral, which is beneficial for injection. Otherwise, if the pH is too high or too low, it will stimulate the intravenous injection.
example 3
[0016] Cefmetazole acid sterile powder is used, the weight ratio of particle size and sodium bicarbonate sterile powder is adjusted to be 1:0.18-1:0.25, and the mixture is dissolved in water. The specific steps are as follows: In the aseptic batching workshop, weigh 50 kg of cefmetazole acid and sodium bicarbonate sterile raw materials with a ratio of 1:0.18 to 1:0.25, and pour them into a high-efficiency three-dimensional motion mixer. Set the speed of the three-dimensional powder mixer to 5 rpm, and start the powder mixing operation according to the standard operating procedures for powder mixing packaging. Mix until about 60min to 90min and mix evenly, then discharge and pack separately.
[0017] The optimum weight ratio is 1:0.19. Because its molar ratio is about 1:1, its pH is about 7±1, which is close to neutral, which is beneficial for injection. Otherwise, if the pH is too high or too low, it will stimulate the intravenous injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com