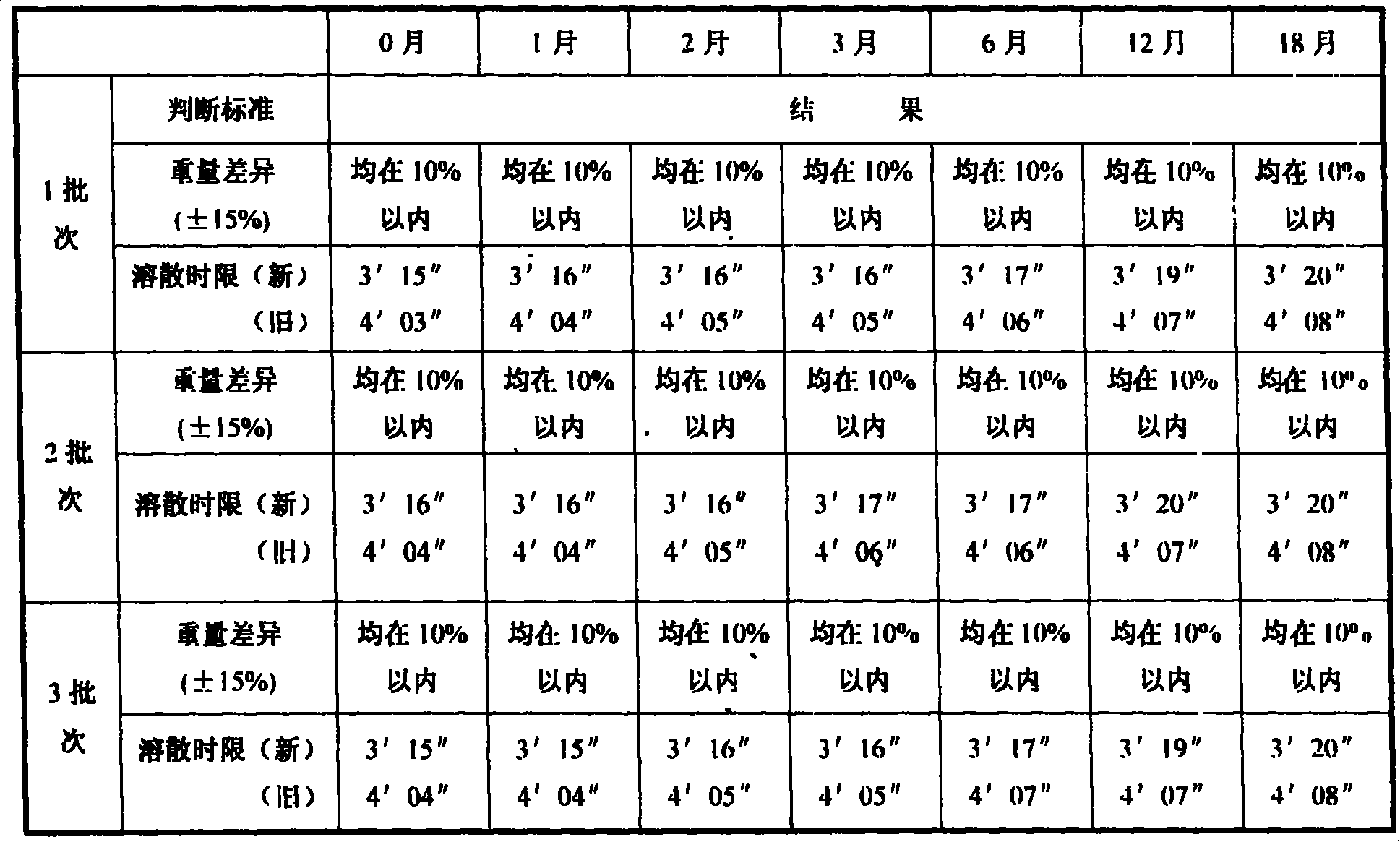
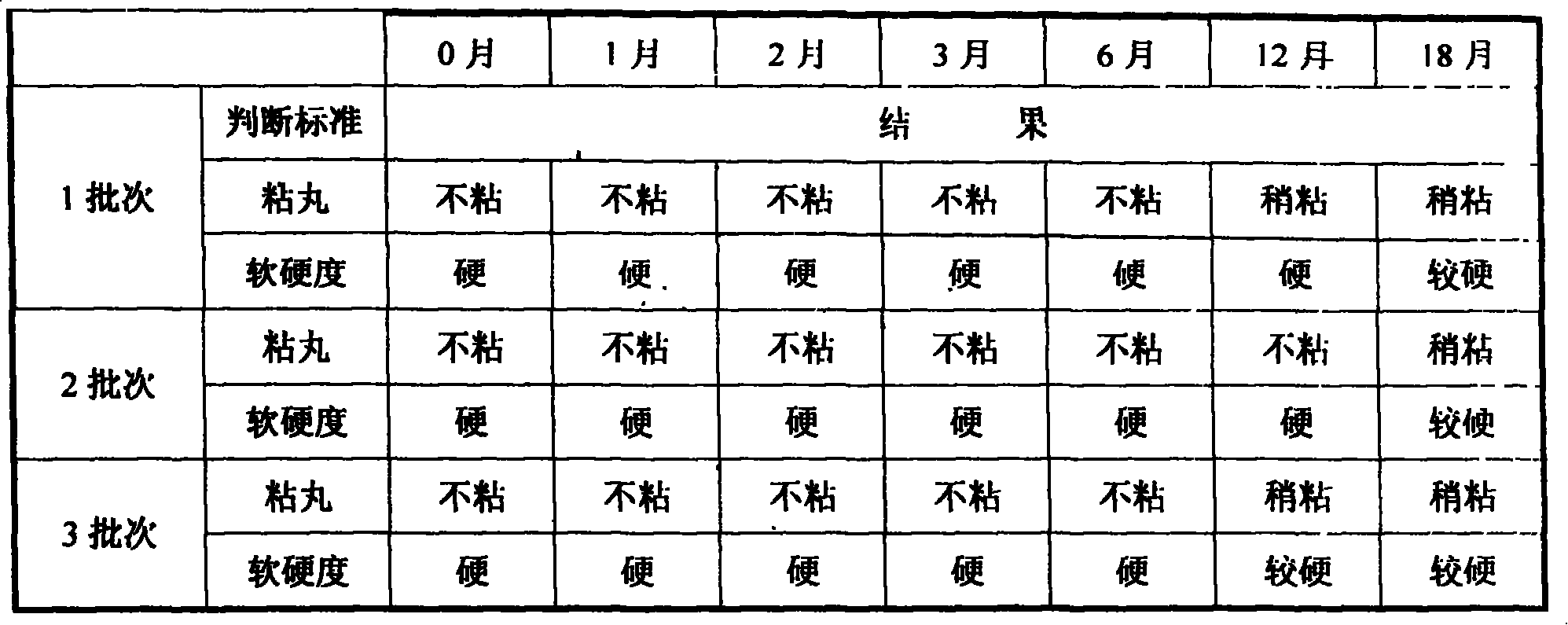
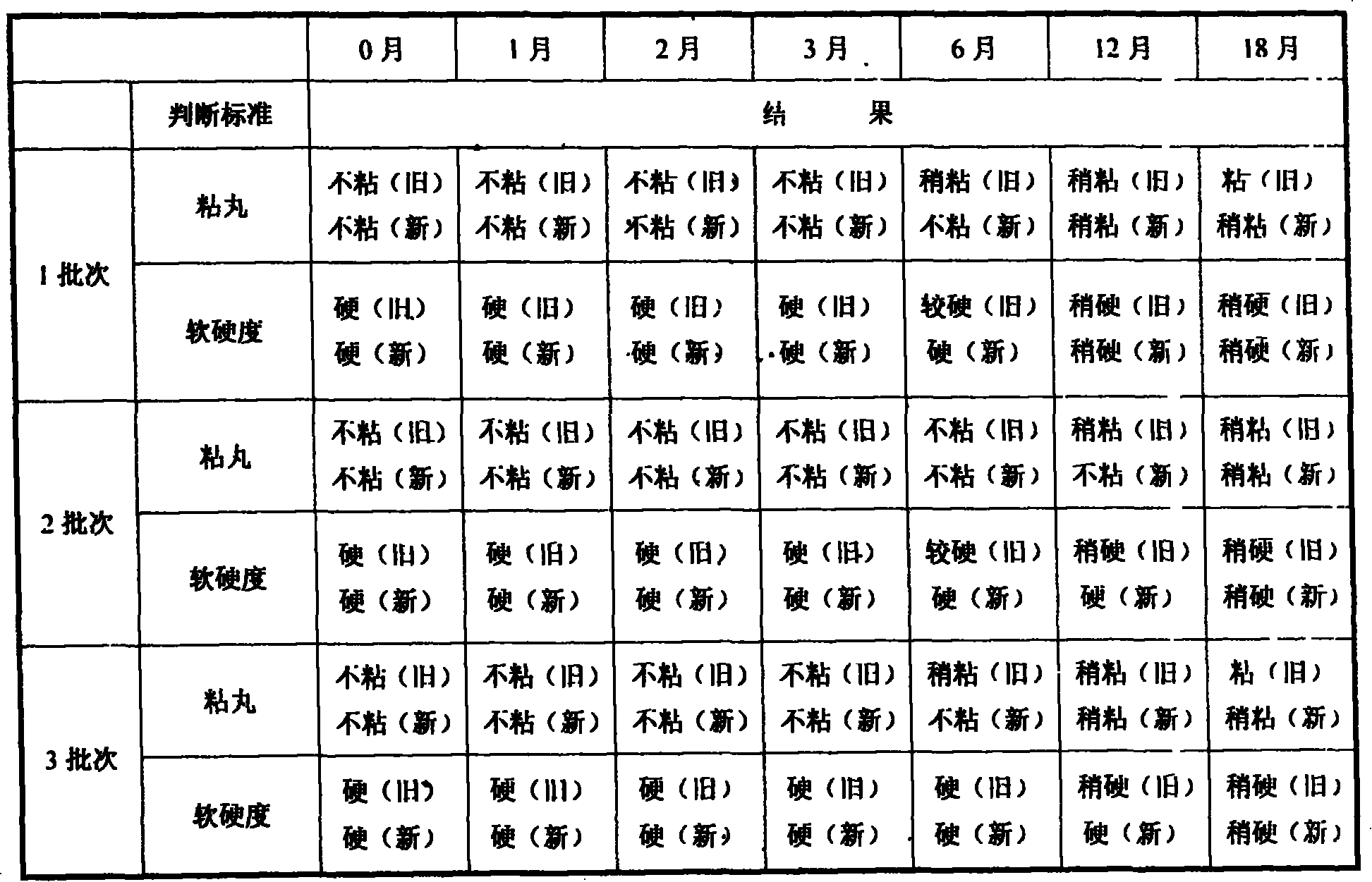
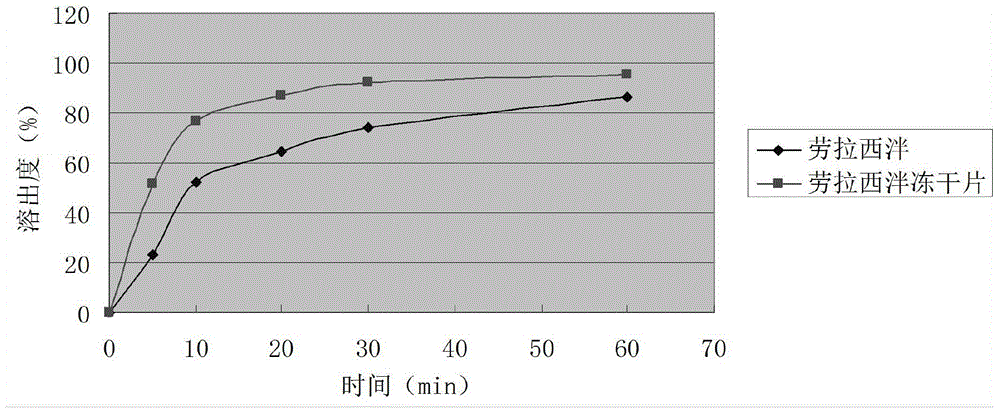
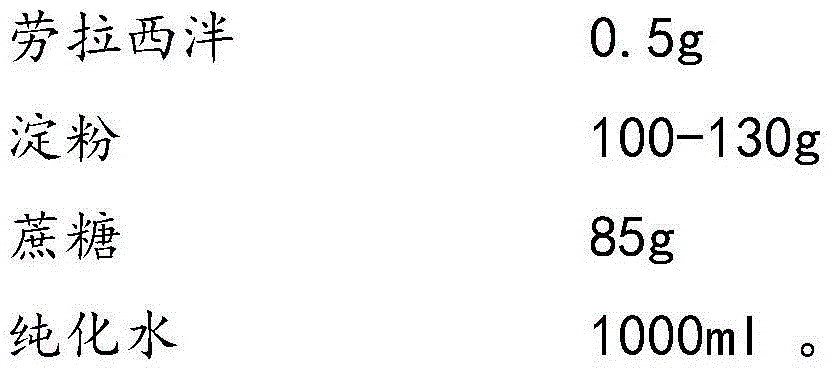Patents
Literature
37 results about "Lorazepam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
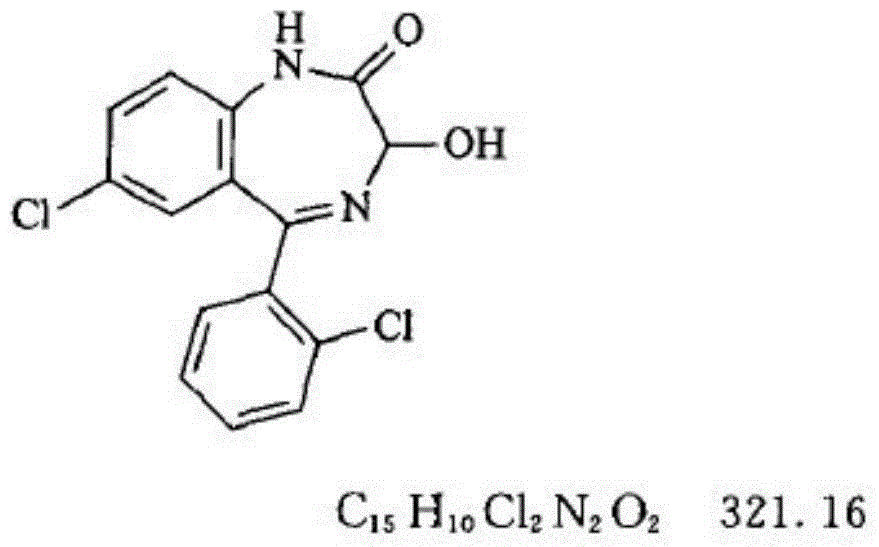
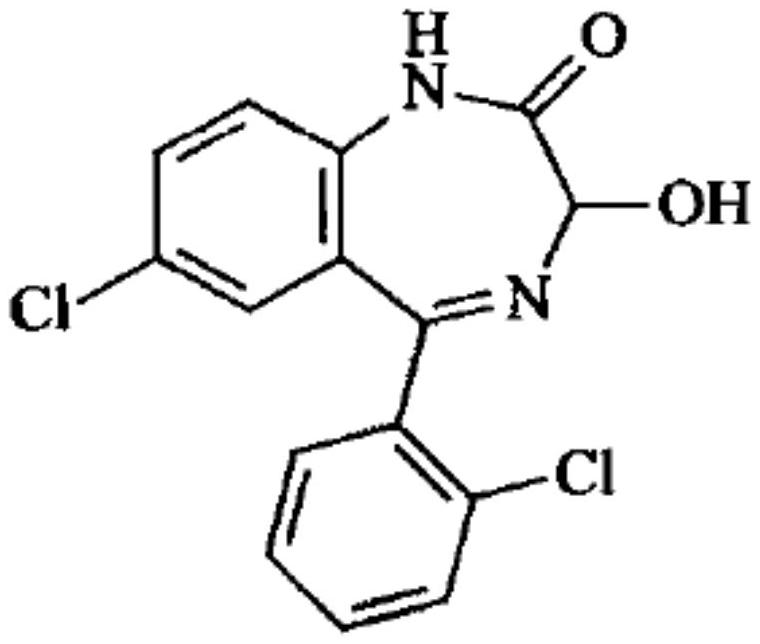
This medication is used to treat anxiety.
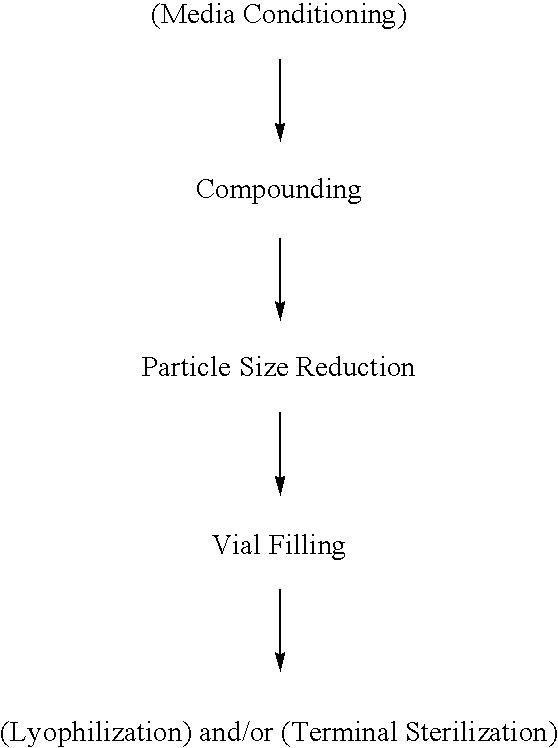
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20060198896A1Easy doseReduce injection volumeBiocidePowder deliveryBenzodiazepinePolyethylene glycol
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
Pharmaceutical composition based on agonist of benzodiazepine
The present invention describes the use of pharmaceutical compounds in pharmaceutical compositions for sublingual administration, including as active ingredient thereof, an agonist of the central receptor of benzodiazepinics chosen among diazepam, lorazepam, bromazepam, triazolam, alprazolam, flunitrazepam, nitrazepam and midazolam maleate, in a mixture with a pharmaceutical excipient consisting of, at least, 70% of the weight of the final formulation containing 40-45% by weight of lactose, 15-27% by weight of sorbitol and 12-16% by weight of cellulose.
Owner:SIGMA PHARMA LTDA
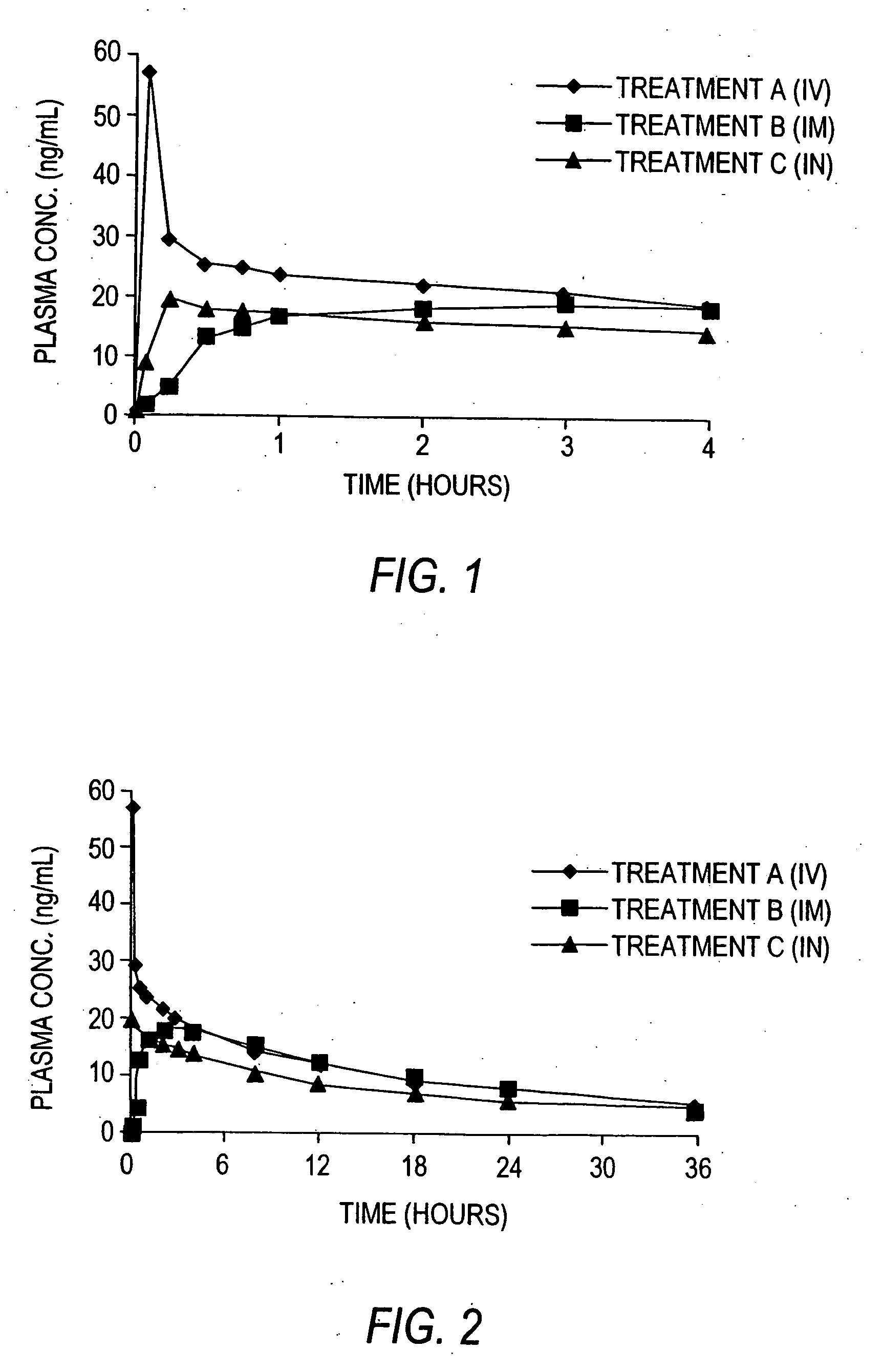
System and method for intranasal administration of lorazepam
InactiveUS20060147386A1Avoid small quantitiesModerate durationNervous disorderAerosol deliveryLorazepamMedicine
A therapeutic composition of lorazepam and its pharmaceutically acceptable derivatives are provided for intranasal administration of at least one predetermined volumetric unit dose in the form of a spray by means that delivers one or more therapeutically prescribed unit doses that are highly accurate as to the volume discharged and which leave no significant quantity of the composition in the delivery means.
Owner:UNIV OF KENTUCKY RES FOUND
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20090304801A1Easy to atomizeFacilitate depositionPowder deliveryBiocideBenzodiazepineNanoparticle
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
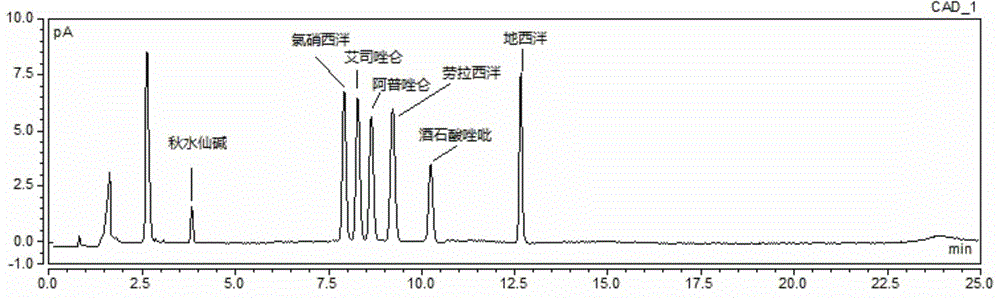
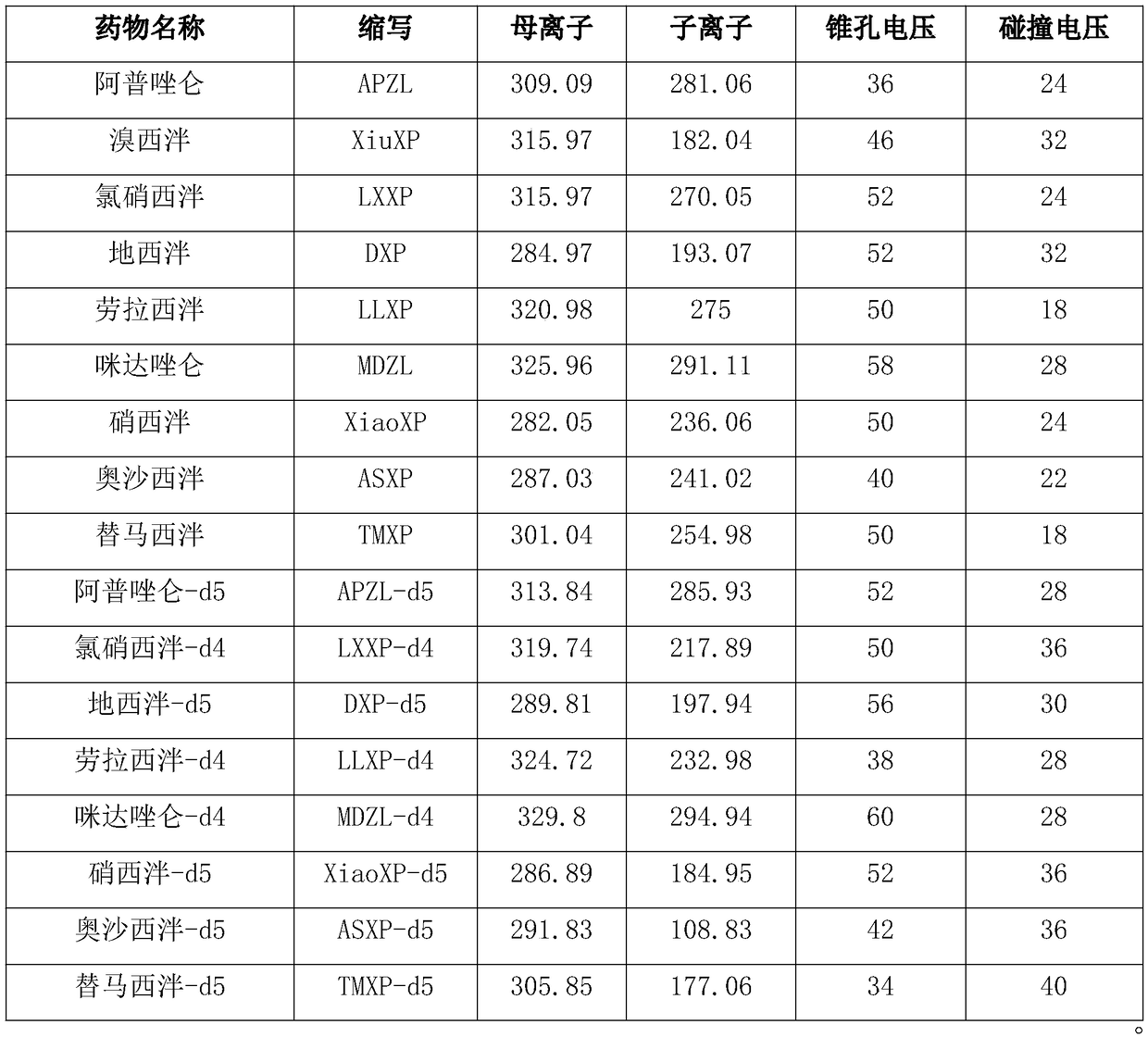
Method for simultaneous determination of contents of 6 kinds of sedative-hypnotic drugs in serum by UHPLC-CAD technology
ActiveCN104133030ASimultaneous measurementShort analysis timeComponent separationSide effectSedative/hypnotic
The invention relates to a method for simultaneous determination of the contents of 6 kinds of sedative-hypnotic drugs in serum by a UHPLC-CAD combined technology, wherein the 6 kinds of sedative-hypnotic drugs are diazepam, lorazepam, alprazolam, estazolam, clonazepam and zolpidem tartrate. The method is characterized by including the following steps: 1) reference substance preparation, 2) internal standard solution preparation, 3) sample solution preparation, 4) mobile phase solution preparation, 5) chromatographic condition setting, 6) electrospray detector condition optimization, 7) sample determination, 8) gradient-concentration reference substance solution preparation, 9) standard curve preparation, and 10) data calculation and analysis. The method is advanced, has good clinical application prospects and effects, can rationally use drugs for clinic, increases drug curative effects, lowers toxic and side effects and drug costs, and plays a positive role.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Kit for determining antianxiety/hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085265AReduce matrix effectThe test result is accurateComponent separationBromazepamTandem mass spectrometry
The invention provides a kit for determining antianxiety / hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry. The kit comprises the following constituents: drug standards comprising bromazepam, clonazepam, diazepam, lorazepam, midazolam, nitrazepam, oxazepam and Temazepam; drug internal standard compounds comprising alprazolam-d5, clonazepam-d4, diazepam-d5, lorazepam-d4, midazolam-d4, nitrazepam-d5, oxazepam-d5 and Temazepam-d5; drug extraction compositions comprising, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropanol solution and 10% of purified water; negative plasma; and diluent: 50% of carbinol water solution. The kit can be used for simultaneously determining antianxiety / hypnotic type drugs and active metabolites thereof, and has the advantages of short determination time and high flux.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Stabilization of lorazepam
InactiveUS20080031944A1Good storage stabilityImproved disintegration timeBiocidePowder deliveryGlass transitionLorazepam
This invention relates to orally disintegrable, lorazepam-containing dosage forms which are storage stable and disintegrable within about 90 seconds or less. In one embodiment, there is provided a storage stable, orally disintegrable dosage form comprising: protected lorazepam particles comprising lorazepam and polymeric material having a glass transition temperature of about 65° C. or above. Also disclosed is a method of producing a storage stable lorazepam containing tablet.
Owner:CEPHALON INC +1
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveCN101189001ASolve the problem of insoluble in waterSmall injection volumeOrganic active ingredientsPowder deliveryBenzodiazepinePolyethylene glycol
Described herein are nanoparticulate formulations of benzodiazepines, such as lorazepam, which do not require the presence of polyethylene glycol and propylene glycol as stabilizers; and methods of making and using such formulations. The formulation is especially useful in aerosol and injectable dosage forms and contains nanoparticulate benzodiazepines such as lorazepam and at least one surface stabilizer. The preparation is useful in the treatment of epileptic states, in the treatment of irritable bowel syndrome, sleep induction, acute psychosis and as a pre-anesthesia drug.
Owner:ELAN PHRMA INT LTD
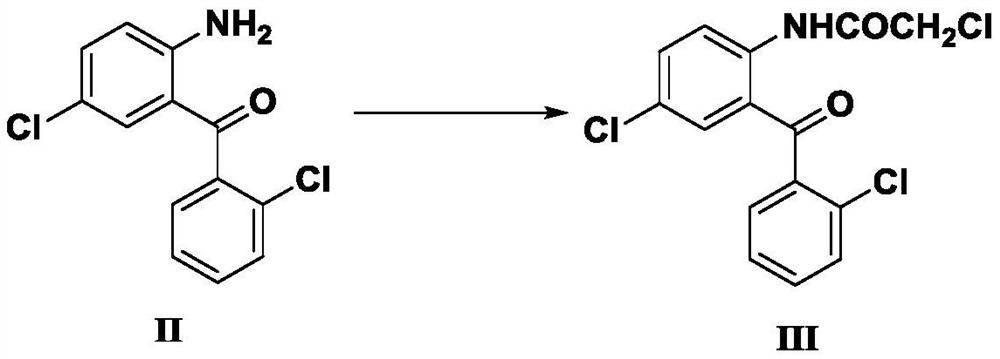
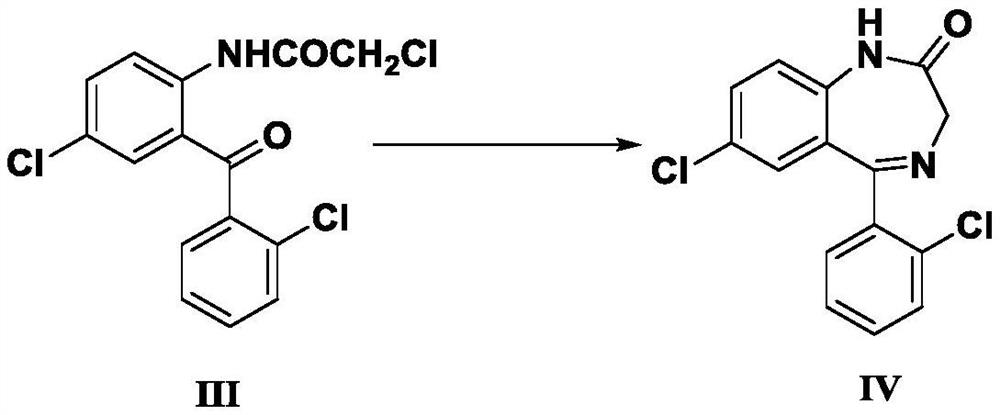
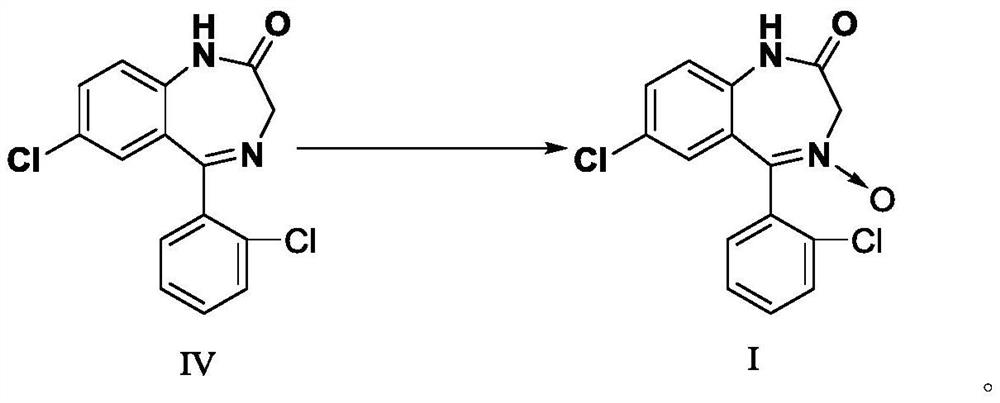
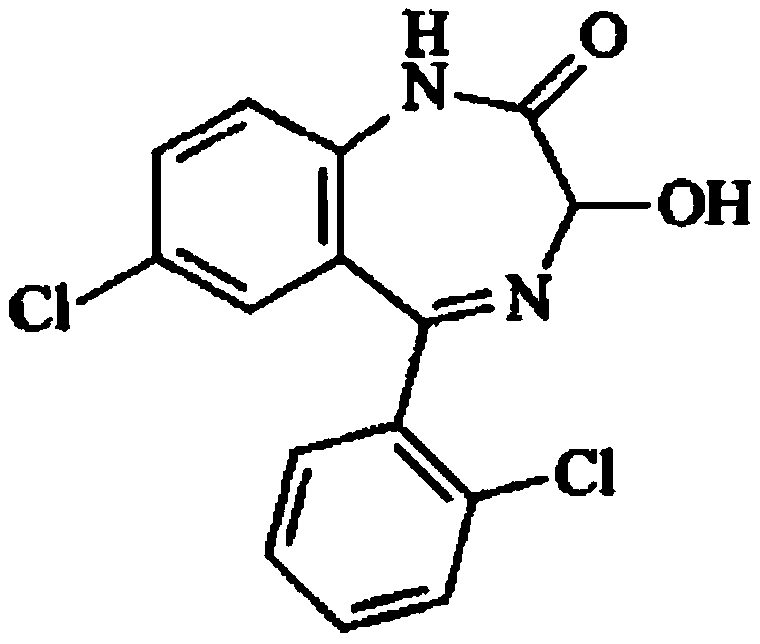
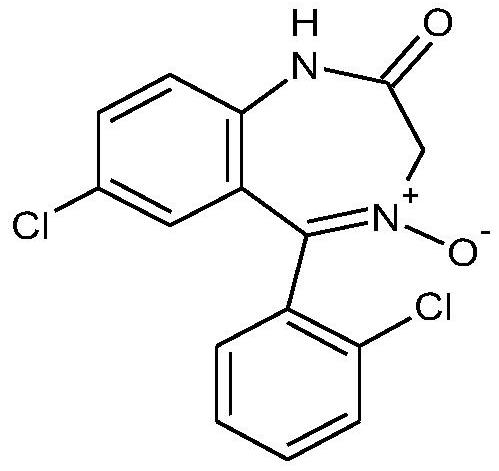
Preparation method of lorazepam intermediate
InactiveCN112028844ASimple and fast operationMild reaction conditionsOrganic chemistryChlorobenzeneAcetyl chloride
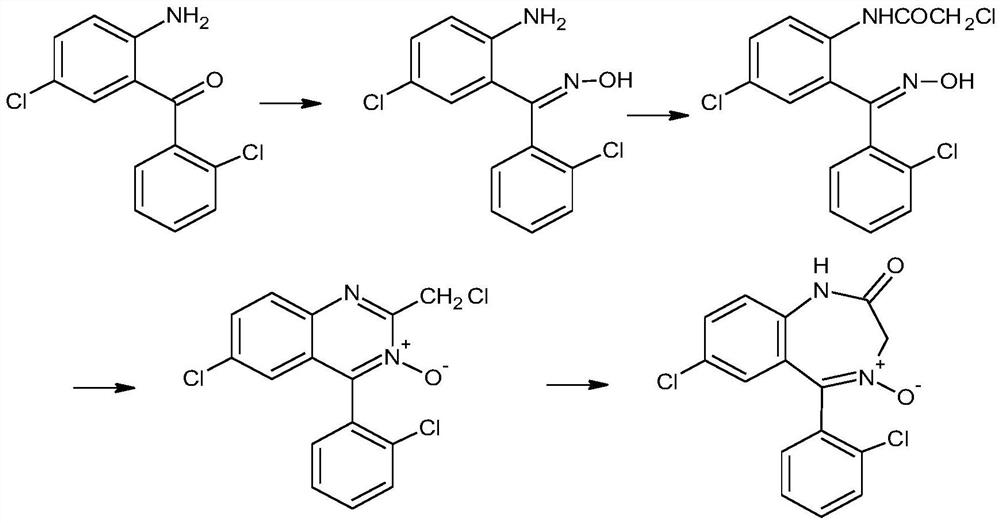
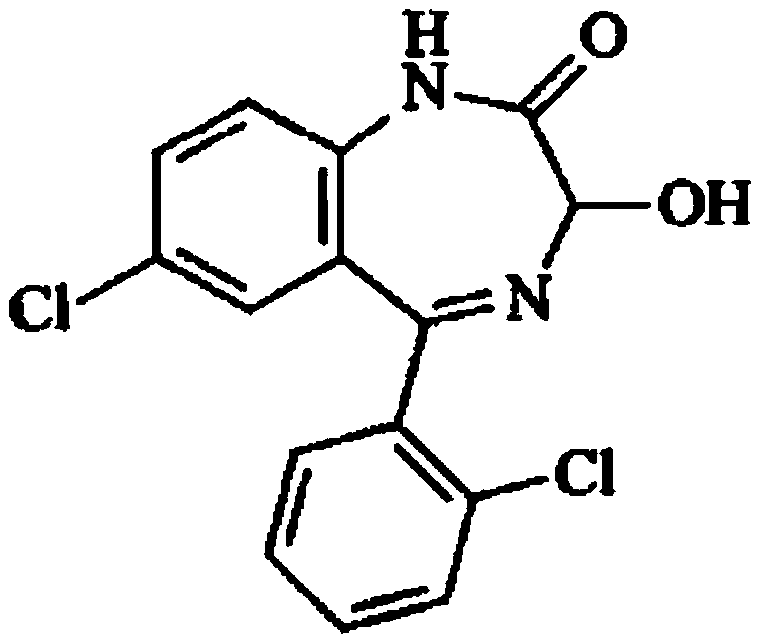
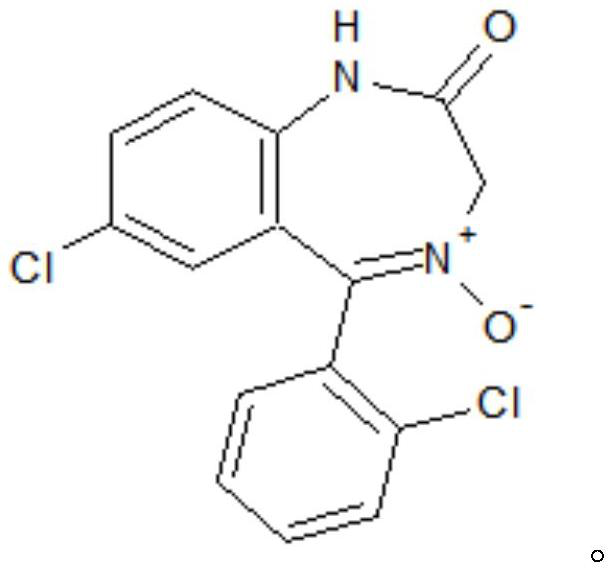
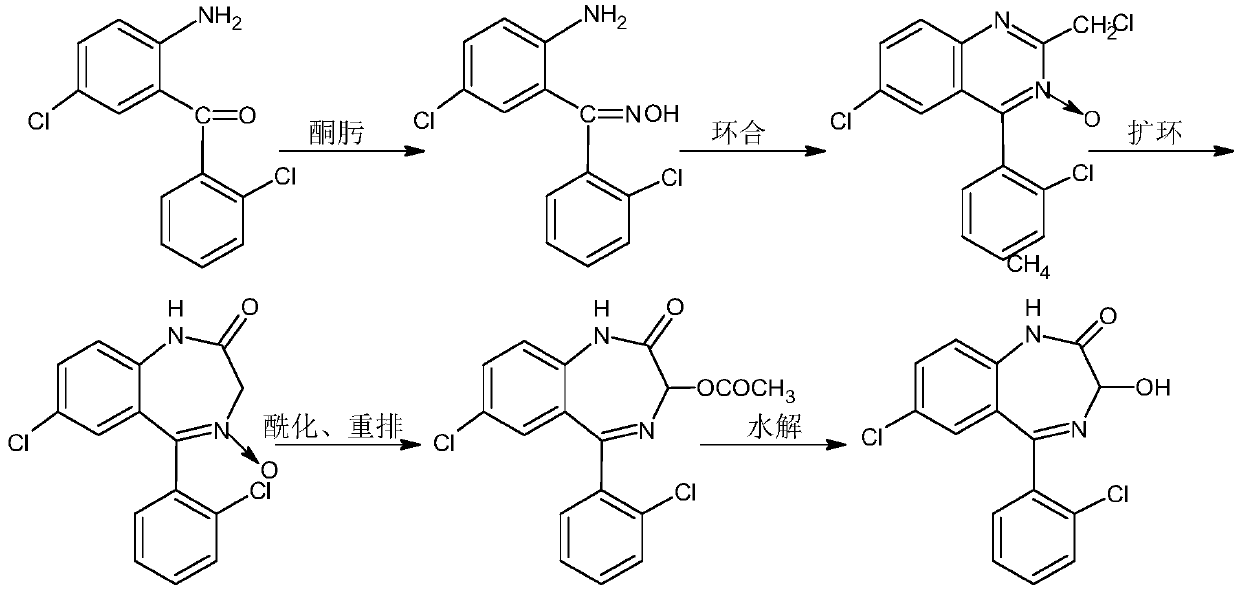
The invention provides a preparation method of a lorazepam intermediate, the lorazepam intermediate is 7-chloro-5-(2-chlorphenyl)-1, 3-dihydro-2H-1, 4-benzodiazepin-2-one-4-oxide, and the preparationmethod comprises the following steps: (1) carrying out acylation reaction on 2-amino-2', 5-dichlorobenzophenone and chloroacetyl chloride to prepare 2-chloroacetamido-2', 5-dichlorobenzophenone, (2) reacting 2-chloroacetamido-2', 5-dichlorobenzophenone with urotropine and ammonium acetate to prepare 7-chloro-5-(2-chlorphenyl)-1, 3-dihydro-2H-1, 4-benzodiazepin-2-one, and (3) carrying out a reaction on the 7-chloro-5-(2-chlorphenyl)-1, 3-dihydro-2H-1, 4-benzodiazepin-2-one and ammonium acetate to obtain 7-chloro-5-(2-chlorphenyl)-1, 3-dihydro-2H-1, 4-benzodiazepin-2-one-4-oxide. The preparationmethod disclosed by the invention is simple and convenient, mild in reaction condition, high in yield, good in product quality and low in cost.
Owner:BEIJING YIMIN PHARMA
Dropping pills containing lorazepam and method for preparing the same
InactiveCN101194908ASmall molecular weightFast dissolutionOrganic active ingredientsNervous disorderChemical synthesisFood additive
The invention provides a dripping pill which contains lorazepam and a process for preparation. The invention is a medicament which has the function of anti anxiety disorder, insomnia, and epilepsy. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
Pharmaceutical composition based on agonist of benzodiazepine
Owner:SIGMA PHARMA LTDA
Lorazepam composition freeze-dried tablet and preparation method thereof
InactiveCN104546749AReduce typesReduce dosageOrganic active ingredientsNervous disorderFreeze-dryingTwo temperature
The invention provides a lorazepam composition freeze-dried tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The lorazepam composition freeze-dried tablet is prepared from lorazepam, starch and cane sugar, and prepared by the following steps: using starch and cane sugar as auxiliary materials, performing heating process treatment on the ordinary corn starch to improve the bonding and disintegration functions of starch in the tablet and the formability of the tablet, wherein the lorazepam composition freeze-dried tablet only needs the two auxiliary materials including starch and cane sugar. The lorazepam composition freeze-dried tablet is prepared by the two-temperature-rising two-temperature-decrease freeze-drying technology; the two-temperature-rising process and the two-temperature-decrease process can facilitate better formability and improve the dissolution rate of the tablet, so as to improve the bioavailability of the tablet; the tablet overcomes the defect of the common lorazepam, is high in dissolution rate and high in bioavailability, and ensures the curative effect and the safety of the clinical medication; the preparation method can reduce the varieties and the dosage of the auxiliary materials in lorazepam.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
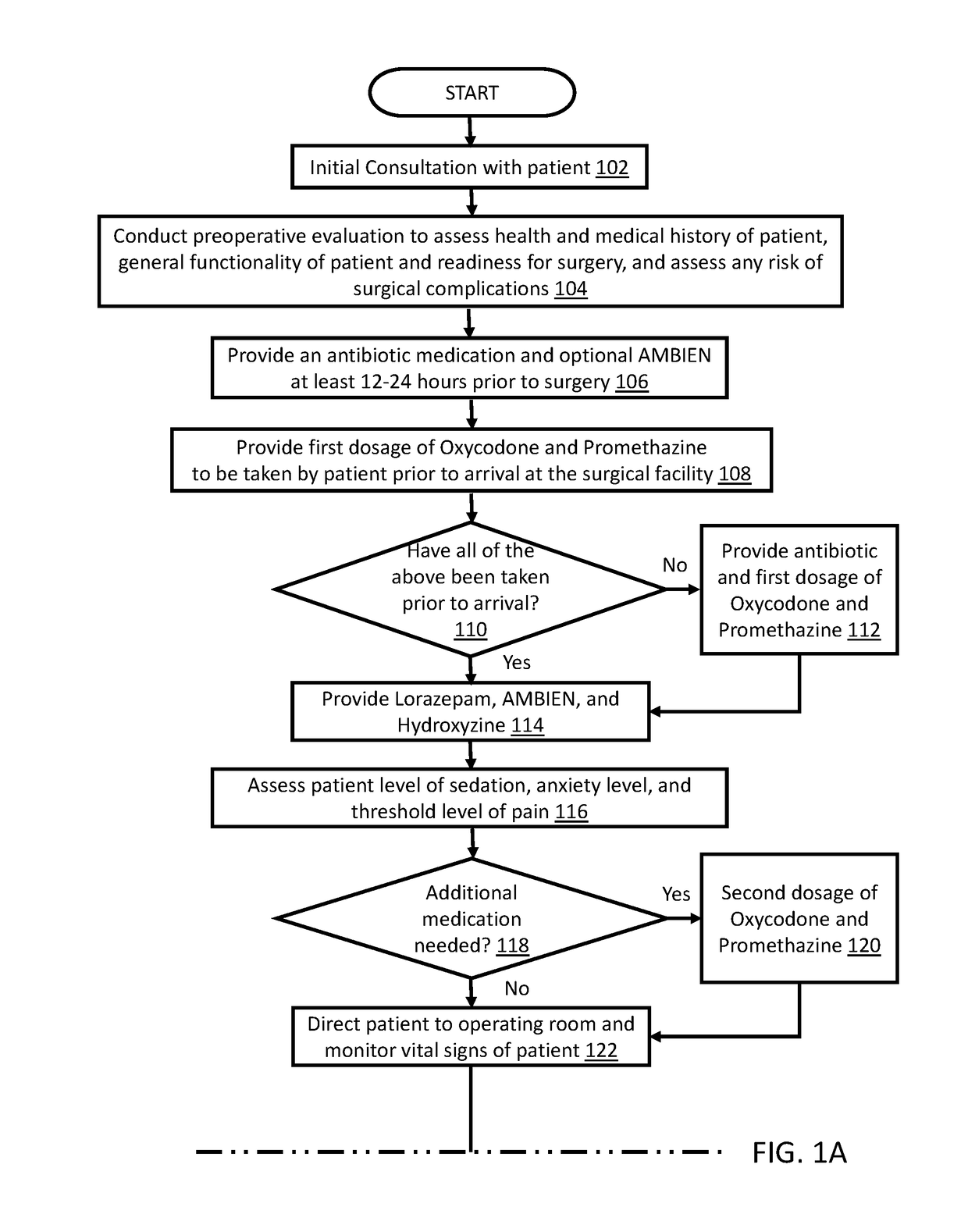
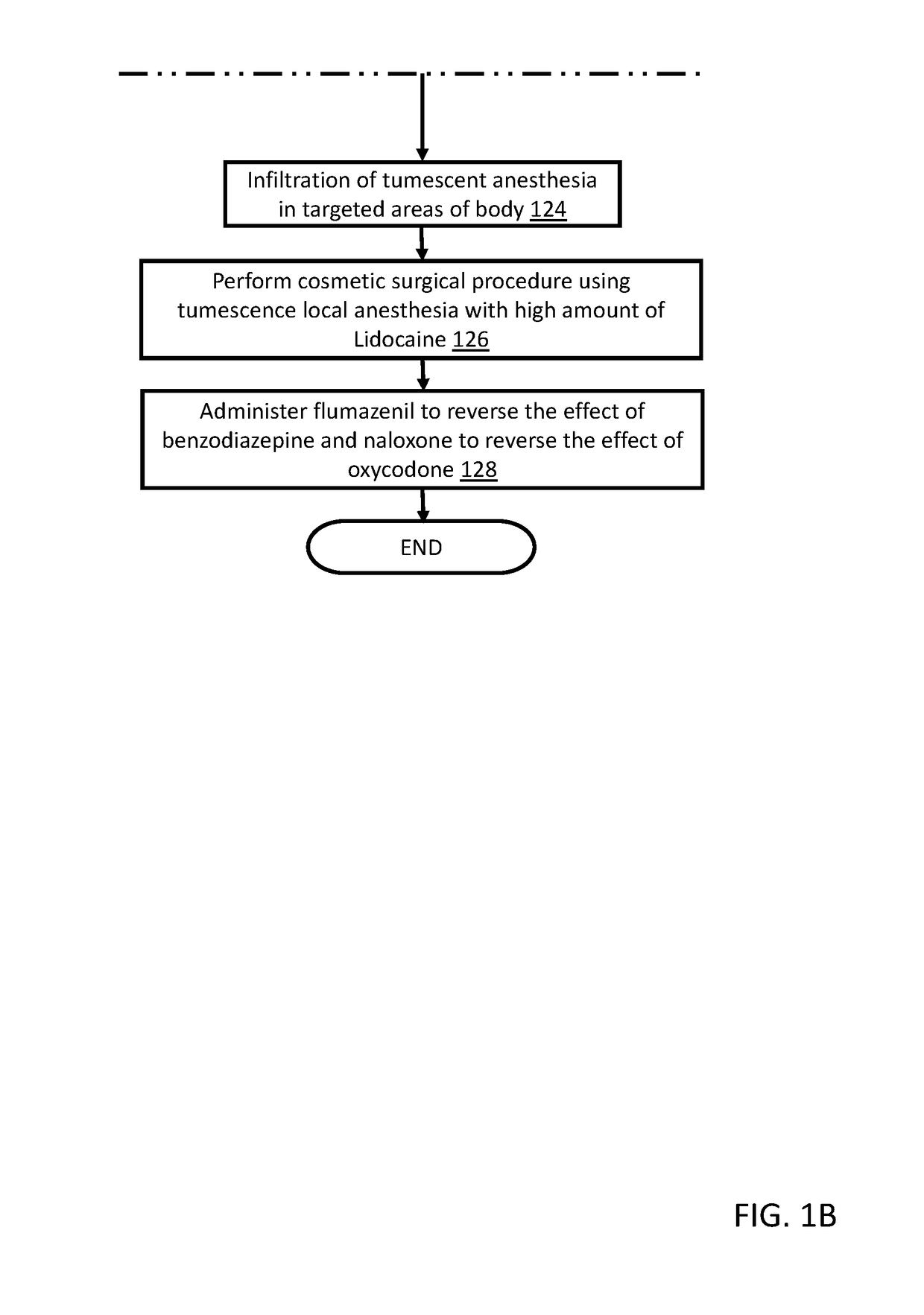
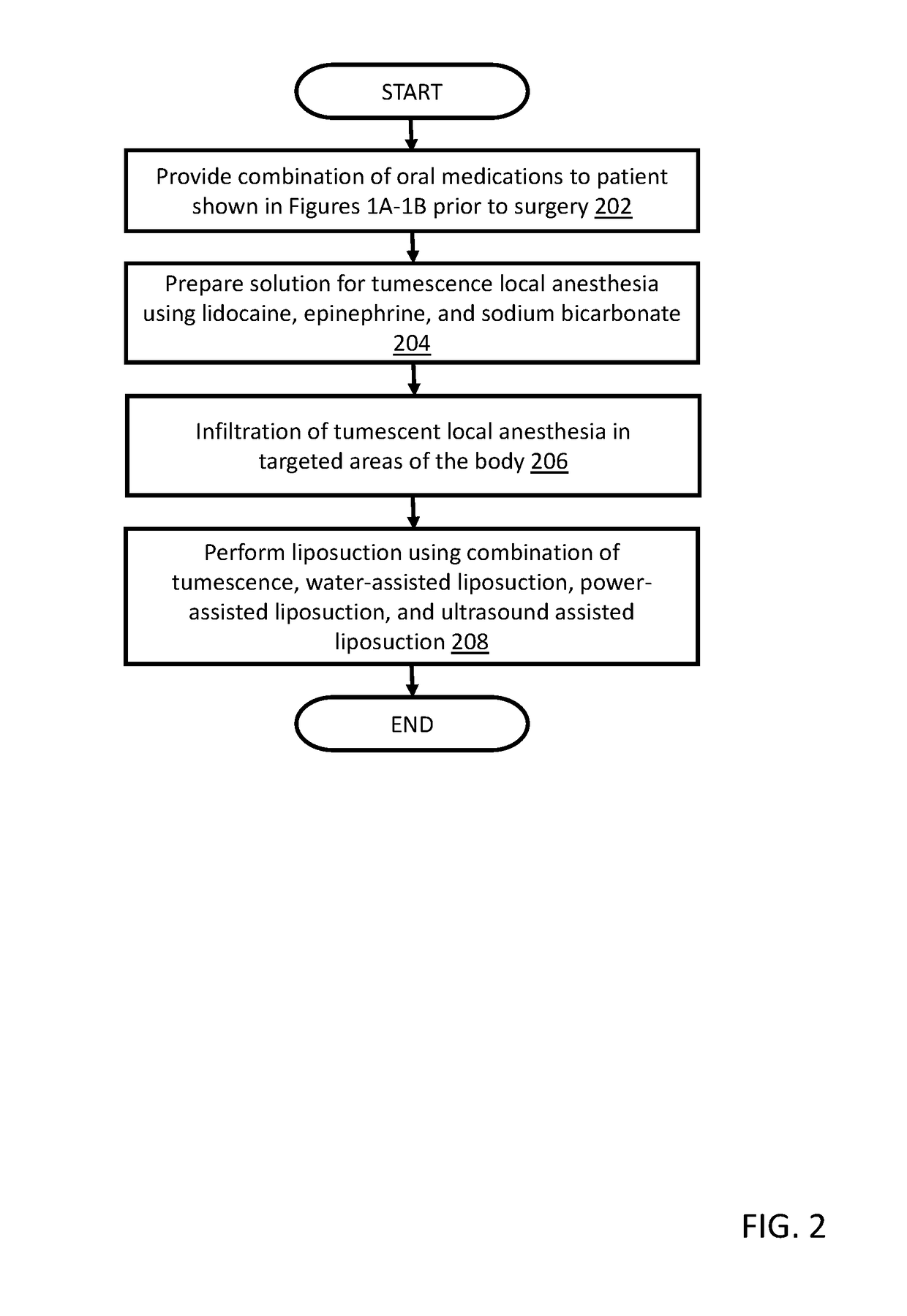
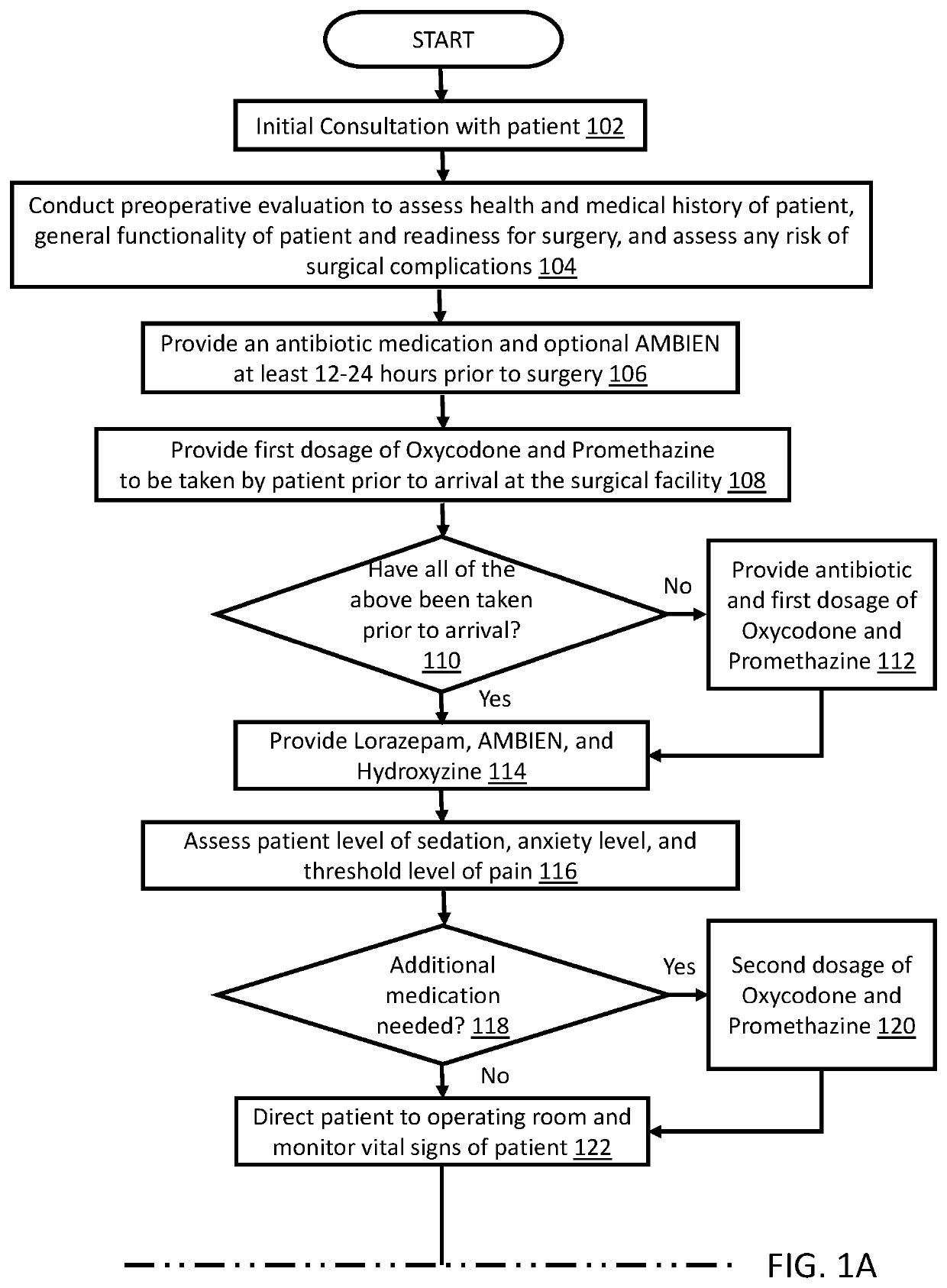
Method for performing cosmetic surgical procedures using tumescent anesthesia and oral sedation
ActiveUS20190038549A1Fast recovery timeGreat patient comfortCosmetic preparationsOrganic active ingredientsSodium bicarbonateHydroxyzine
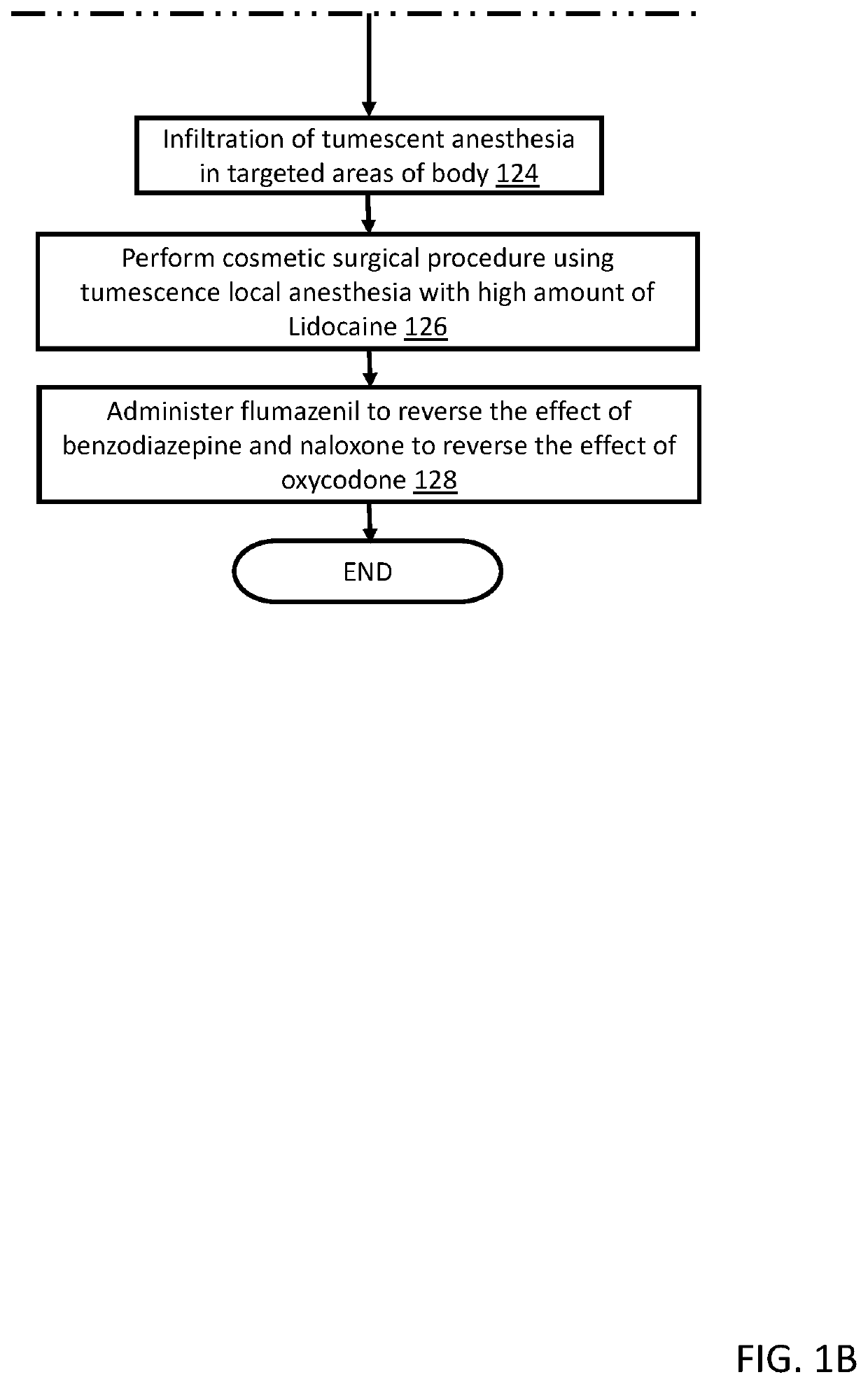
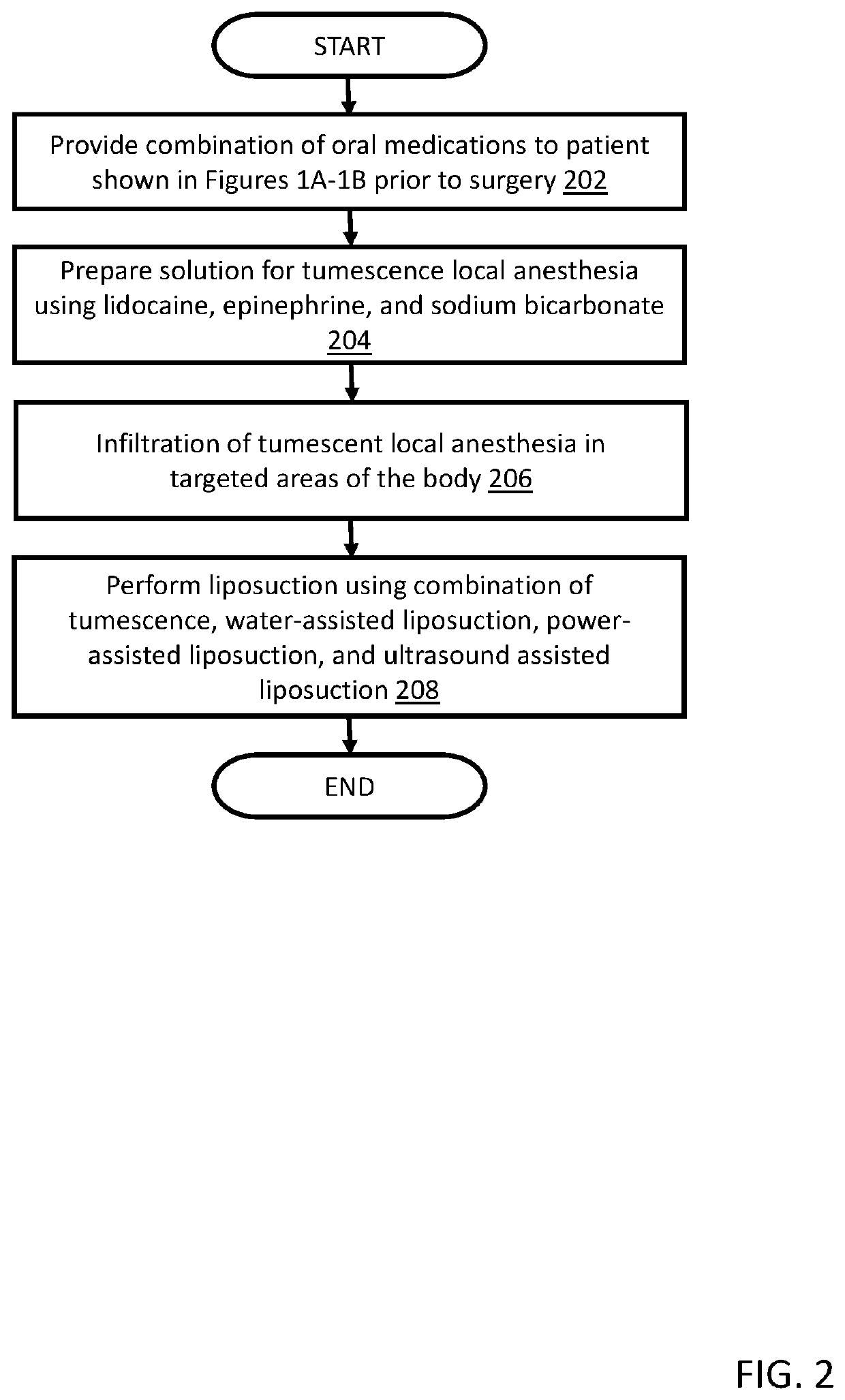
A method for anesthetizing a patient undergoing a cosmetic surgical procedure comprising: providing an antibiotic medication at least from about 12 hours to about 24 hours prior to the cosmetic surgical procedure; providing a first dosage of oxycodone and a first dosage of promethazine to be taken by the patient prior to arrival at a surgical facility where the cosmetic surgical procedure is to occur; and providing a dosage of lorazepam, a dosage of zolpidem, and a dosage of hydroxyzine just prior to the cosmetic surgical procedure, wherein the zolpidem comprises a dosage of AMBIEN. In one embodiment, the method further comprises: preparing an anesthetizing solution comprising lidocaine, epinephrine, and sodium bicarbonate; and infiltrating the anesthetizing solution in a targeted area of the patient. In another embodiment, the method further comprises: providing one or more reversing agents to the patient after the cosmetic surgical procedure.
Owner:CINDERELLA ANESTHESIA LLC
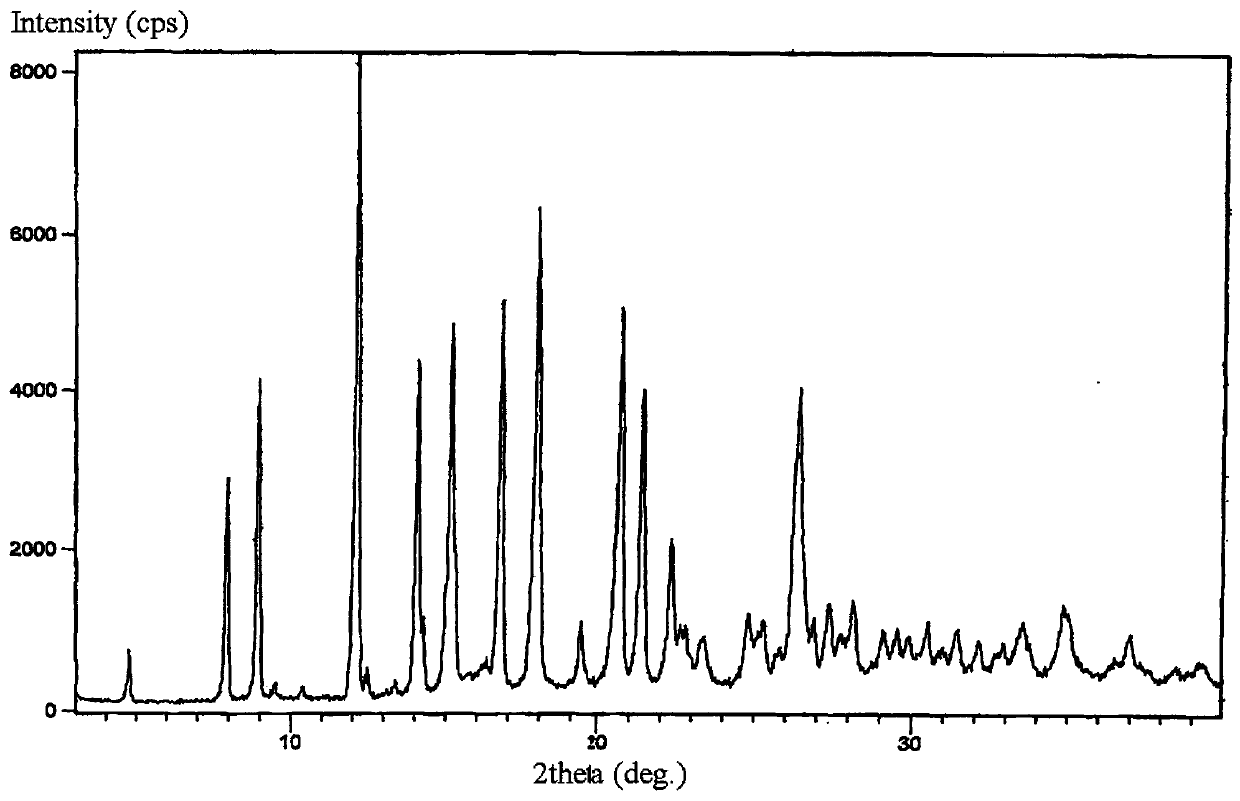
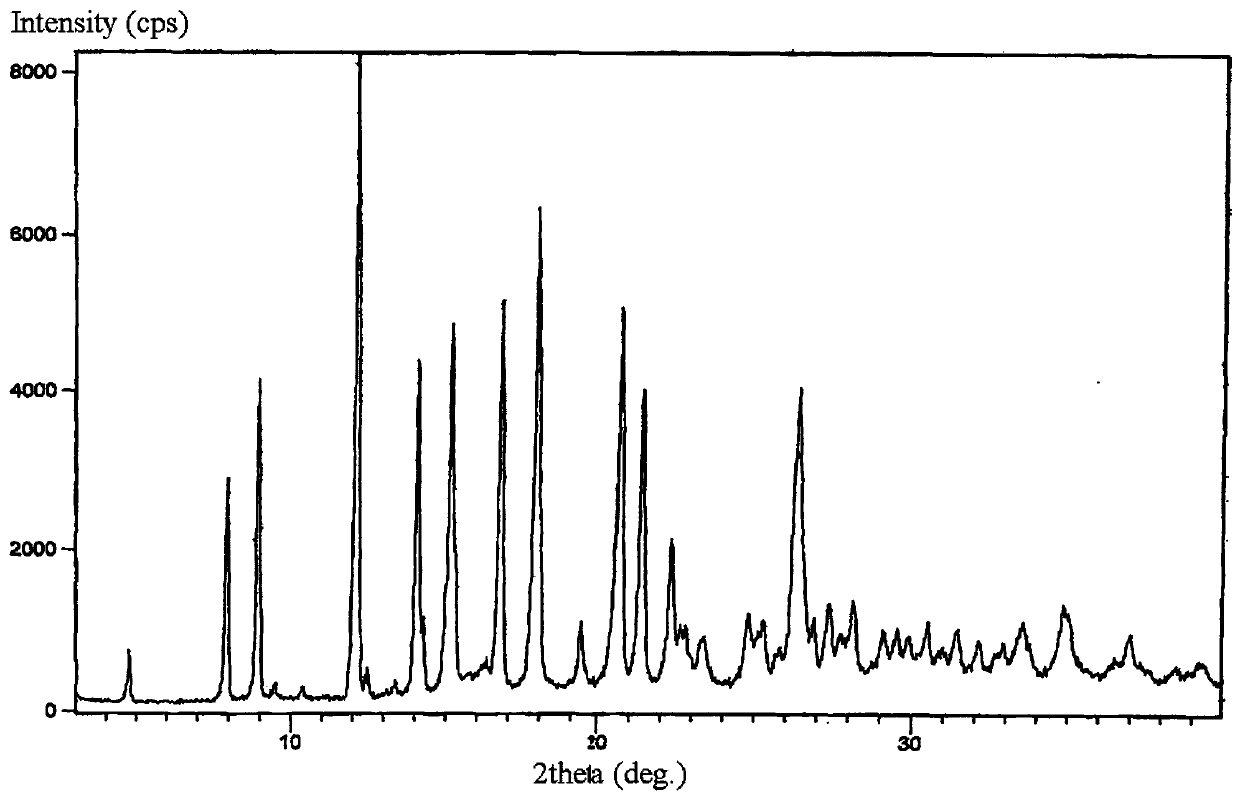
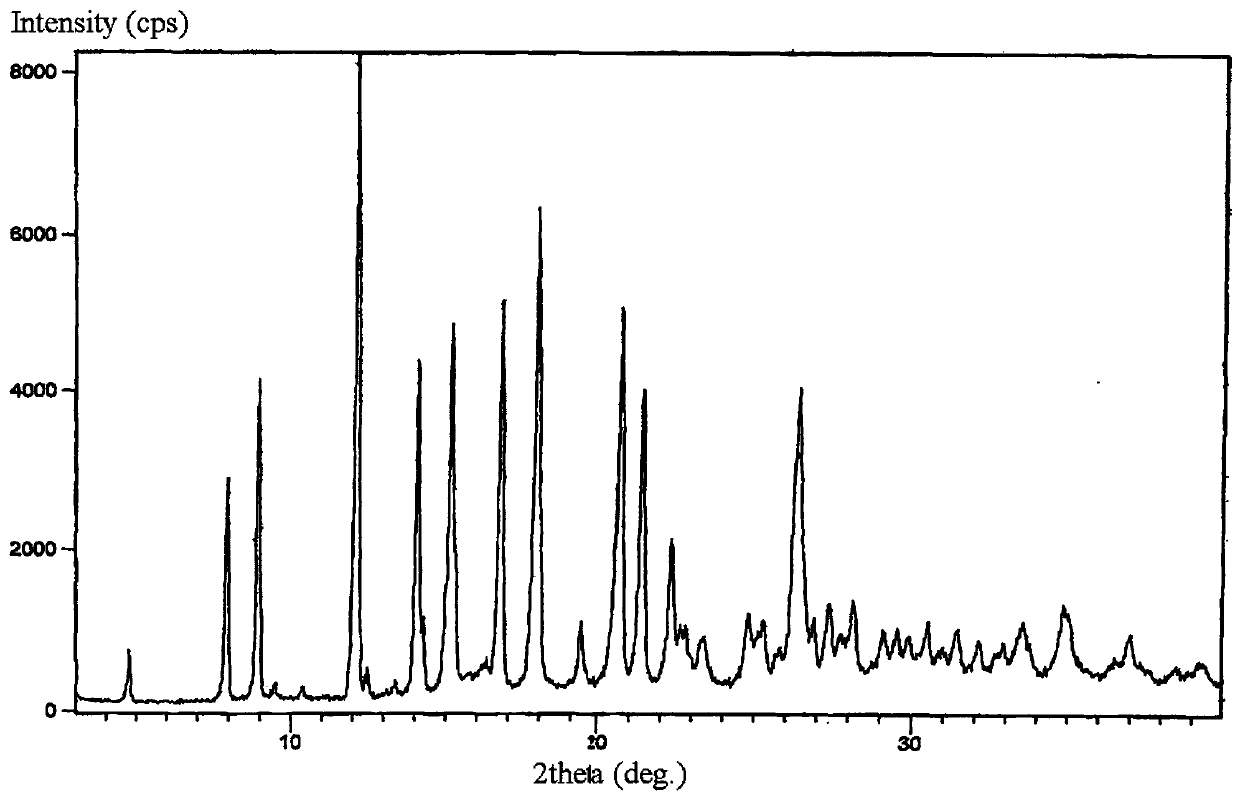
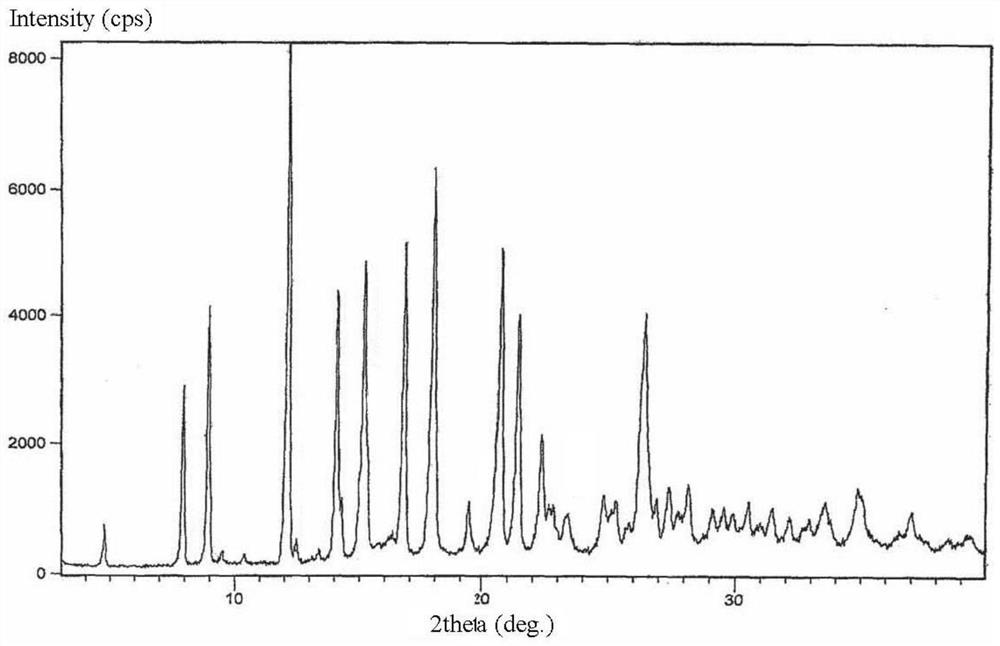
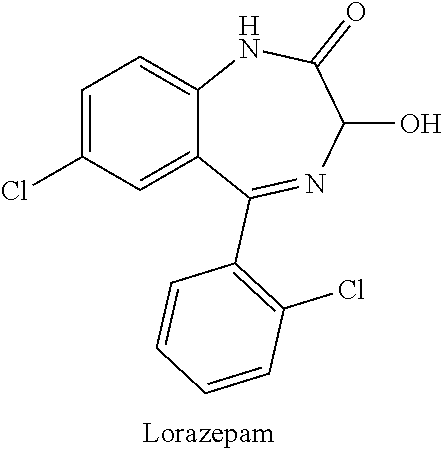
New crystal form of lorazepam, preparation method and pharmaceutical applications thereof
The invention relates to a new crystal form for preparing lorazepam, a preparation method and pharmaceutical applications thereof, wherein specifically the lorazepam crystal has diffraction peaks at about 12.17 DEG, about 14.15 DEG, about 15.27 DEG, about 16.84 DEG, about 17.91 DEG and about 20.81 DEG in a powder X-ray diffraction pattern represented by a 2[theta] angle by using Cu-Kalpha radiation, for example, the crystal has diffraction peaks at about 7.93 DEG, about 9.04 DEG, about 12.17 DEG, about 14.15 DEG, about 15.27 DEG, about 16.84 DEG, about 17.91 DEG, about 20.81 DEG, about 21.44 DEG and about 26.38 DEG. The invention further provides a preparation method of the new crystal of larazepam, and pharmaceutical applications of the new crystal form. According to the invention, the prepared new crystal form for preparing lorazepam shows excellent properties defined in the specification.
Owner:HUNAN DONGTING PHARMA
Lorazepam dripping pills and its preparation process
InactiveCN1546035ADisintegration and dissolution fastQuality improvementOrganic active ingredientsNervous disorderMedicineDysphagia
The invention relates to a Lorazepum drop pill prepared by utilizing ultramicro disintegration and drop pill manufacturing process, which has the advantages of improving collapse and dissolving speed, quick effect, increased medicament stability, reduced adjuvant consumption, lowered production costs, and easiness in carrying and use. It has good compliance, thus is especially suitable for children, the elderly, bedridden patients and dysphagia patients.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Method for performing cosmetic surgical procedures using tumescent anesthesia and oral sedation
ActiveUS10624840B2Avoid painFast recovery timeOrganic active ingredientsCosmetic preparationsSodium bicarbonateHydroxyzine
A method for anesthetizing a patient undergoing a cosmetic surgical procedure comprising: providing an antibiotic medication at least from about 12 hours to about 24 hours prior to the cosmetic surgical procedure; providing a first dosage of oxycodone and a first dosage of promethazine to be taken by the patient prior to arrival at a surgical facility where the cosmetic surgical procedure is to occur; and providing a dosage of lorazepam, a dosage of zolpidem, and a dosage of hydroxyzine just prior to the cosmetic surgical procedure, wherein the zolpidem comprises a dosage of AMBIEN. In one embodiment, the method further comprises: preparing an anesthetizing solution comprising lidocaine, epinephrine, and sodium bicarbonate; and infiltrating the anesthetizing solution in a targeted area of the patient. In another embodiment, the method further comprises: providing one or more reversing agents to the patient after the cosmetic surgical procedure.
Owner:CINDERELLA ANESTHESIA LLC
A compound anesthetic for ferrets and its preparation method and application
InactiveCN104873518BGood anesthesiaSlow activityOrganic active ingredientsAnaestheticsMuscle relaxationSide effect
The invention discloses compound anesthetic for minks and a preparation method and application of the compound anesthetic and belongs to the fields of preparation and application of the compound anesthetic for minks. The compound anesthetic for minks comprises detomidine, lorazepam and nalbuphine. The preparation method incudes: (1) mixing the detomidine, lorazepam and nalbuphine; (2) adding water of injection with uniform mixing to obtain the compound anesthetic. The compound anesthetic is quick in anesthesia induction on minks, long in anesthesia duration, good in analgesia, sedation and muscle relaxation effects, smooth and steady in analepsia and simple to operate, has slight impact on all clinical conventional indexes and special indexes, almost has no side effect and can be used in operations such as long-distance mink transportation, electro-ejaculation, cesarean section, trauma and fracture needing chemical restraints or sedation.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
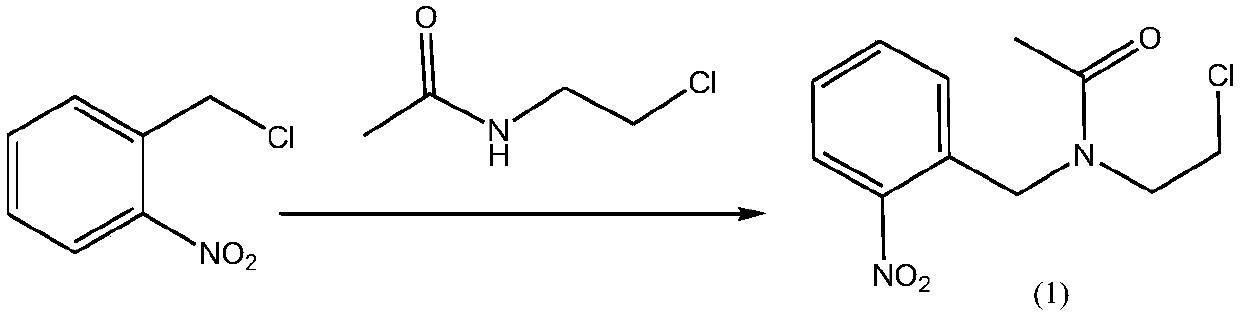
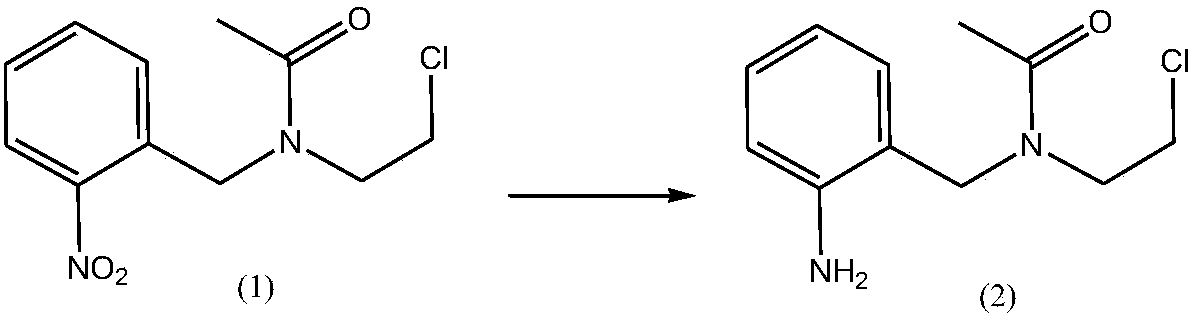
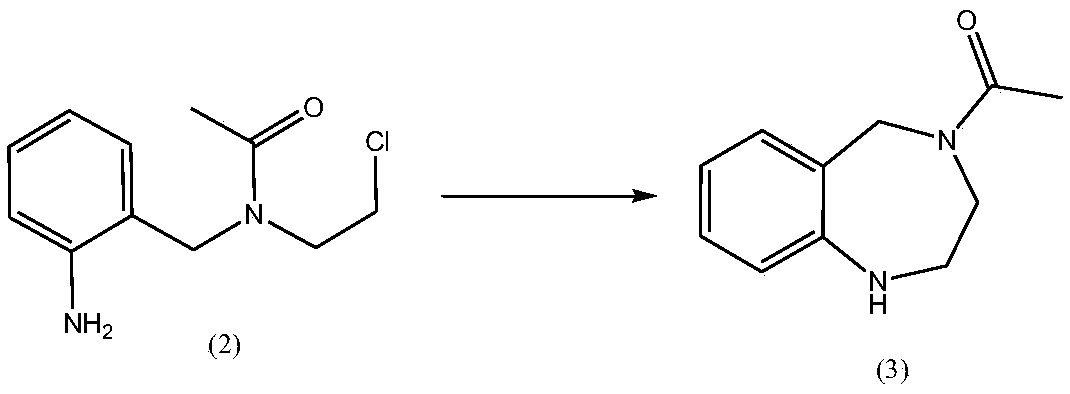
Simple synthesis process of anxiolytic drug lorazepam intermediate
InactiveCN109678746ARaw materials are easy to obtainSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationIce waterBottle
The invention discloses a simple synthesis process of an anxiolytic drug lorazepam intermediate. The simple synthesis process comprises the steps of putting N-(2-chloroethyl)acetamide, triethylamine and dichloromethane into a reaction bottle; stirring the mixture in an ice water bath; dropwise adding an o-nitrobenzyl chloride DCM (Dichloromethane) solution to the ice water bath; removing the ice water bath; separating the liquid; extracting the liquid with dichloromethane, and combining organic phases; drying the combined organic phase; concentrating the organic phase to dryness under reducedpressure to obtain a compound (1); putting the compound (1), acetic acid and ethanol into the reaction bottle for stirring; adding iron powder to the mixture to perform a temperature rise reaction; extracting the mixture twice with toluene, and combining the organic phases; drying the combined organic phase; filtering the organic phase; and collecting a filtrate to obtain a toluene solution of a compound (2); stirring the toluene solution of the compound (2) and potassium iodide, preserving heat until the raw materials are completely reacted; extracting an aqueous phase once with toluene, andcombining the organic phases; drying the combined organic phase; filtering the organic phase; collecting a filtrate; concentrating the filtrate to dryness under reduced pressure; adding methyl tert-butyl ether, refluxing and pulping, cooling and separating the material; and filtering the material to obtain a final product. The yield of the two steps is 84.2% and the purity is 99%. According to thesimple synthesis process of the anxiolytic drug lorazepam intermediate disclosed by the invention, the raw materials are simple and easy to obtain, the operation is simple and convenient, the yield is high, the three wastes are less, and the synthesis process is suitable for industrial production.
Owner:南京诺希生物科技有限公司
Preparation method of lorazepam intermediate
ActiveCN112500359AOxidation reaction goes wellShorten the processing cycleOrganic chemistryBenzodiazepineChlorobenzene
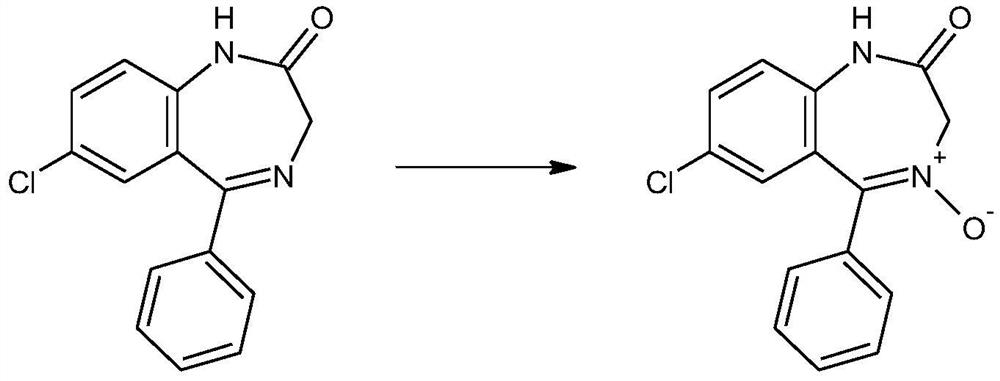
The invention discloses a preparation method of a lorazepam intermediate. The preparation method comprises the following steps: taking 7-chloro-5-(-2-chlorphenyl)-1, 3-dihydro-2H-1, 4-benzodiazepine-2-ketone as a raw material, carrying out oxidation reaction with hydrogen peroxide in an aprotic polar solvent without an acidic solvent, and after the reaction is finished, carrying out aftertreatmentto obtain the 7-chloro- dioxo-5-(2chlorphenyl) 1, 3-dihydro-2H-1, 4-benzodiazepine. The invention relates to a 4-benzodiazepine-4-oxide. According to the preparation method, expensive raw materials do not need to be used for catalysis in the reaction process, operations such as reaction quenching, solvent extraction and concentration distillation do not need to be carried out in the post-treatment process, operation steps are reduced, the production period is shortened, the production cost is reduced, and the preparation method is suitable for industrial production.
Owner:HUAZHONG PHARMA
A kind of electrochemical chiral recognition detection method of lorazepam
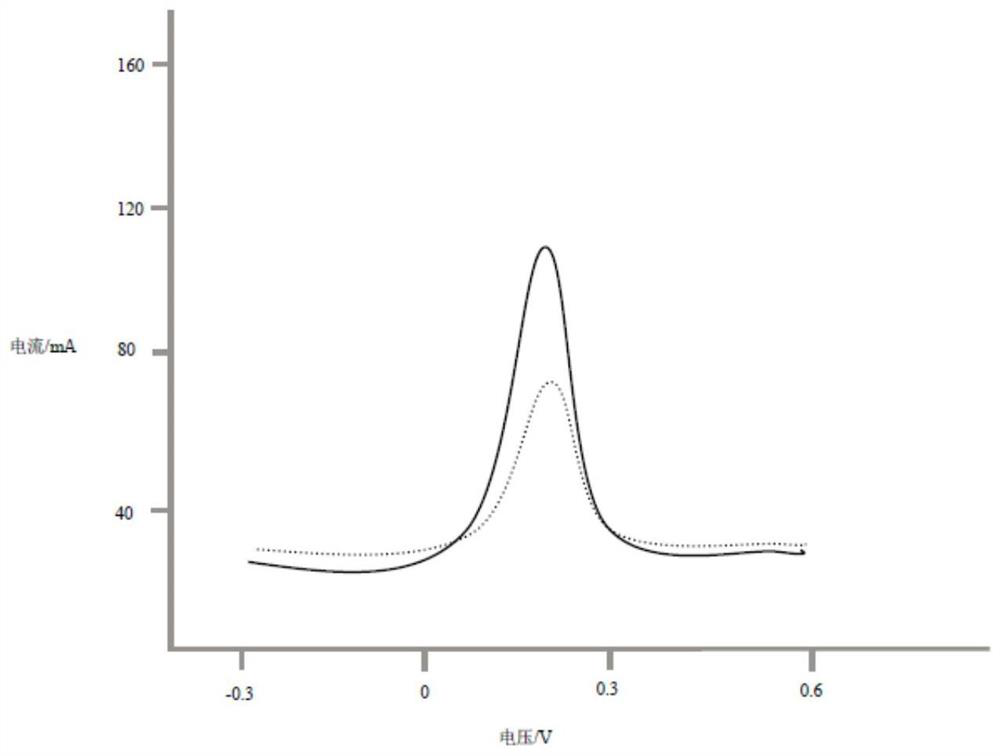
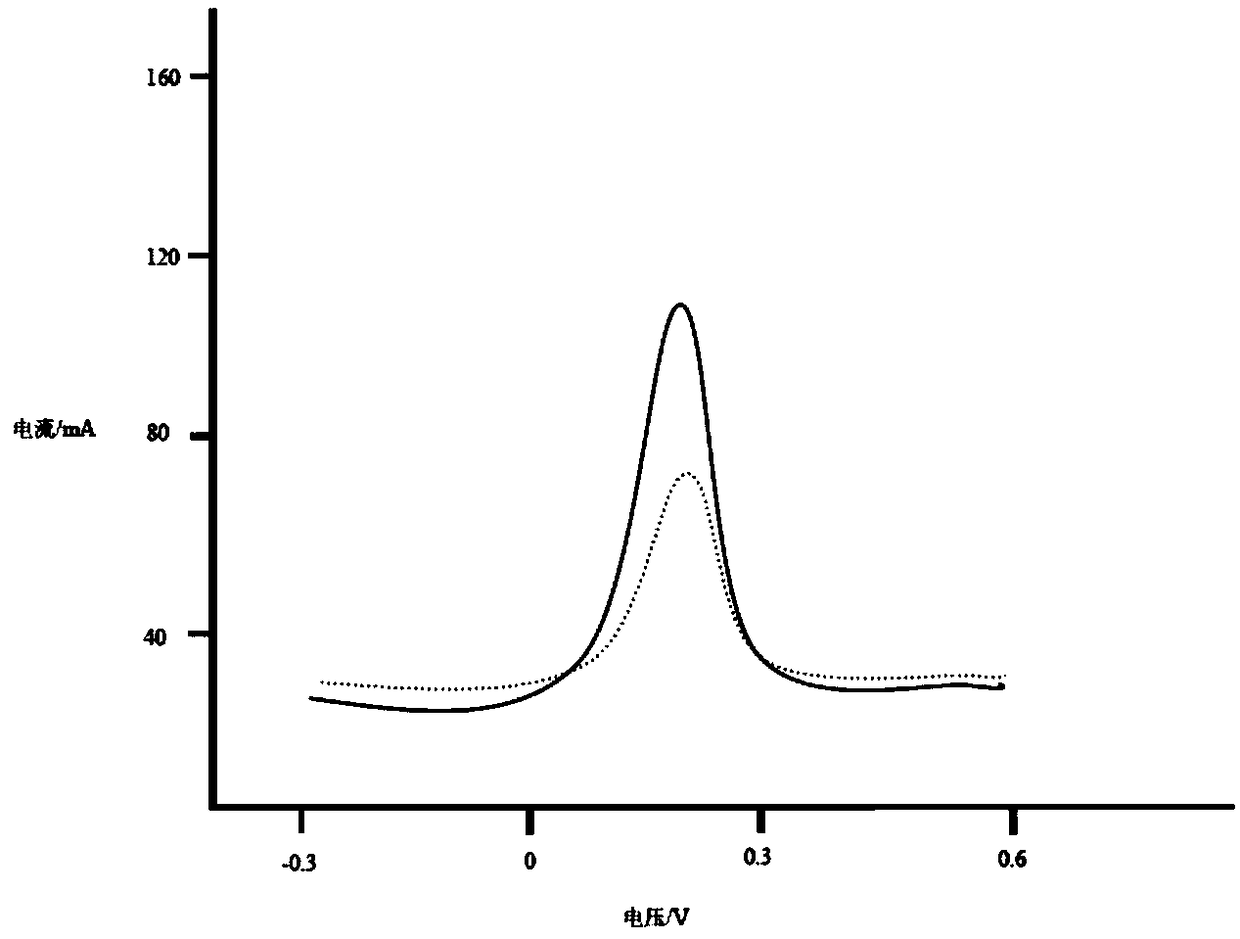
ActiveCN109239172BEasy to operateQuick identificationGrapheneMaterial electrochemical variablesPeak currentElectrochemistry
The invention discloses a chiral detection method of lorazepam, which is realized by adopting the following steps: preparing graphene, carboxylating the graphene and modifying the graphene on a glassy carbon electrode, and then electrochemically modifying poly-L-leucine, using Differential pulse voltammetry was used to detect the isomers of lorazepam, and the scanning potential was from ‑0.3 to 0.6V. The results showed that R‑lorazepam had a higher peak current and S‑lorazepam had a lower peak current. peak current. The detection method of the present invention can realize rapid identification of R-lorazepam and S-lorazepam according to the difference in peak current, and the detection method is simple and the detection efficiency is high.
Owner:山东信开源科技创新发展有限责任公司
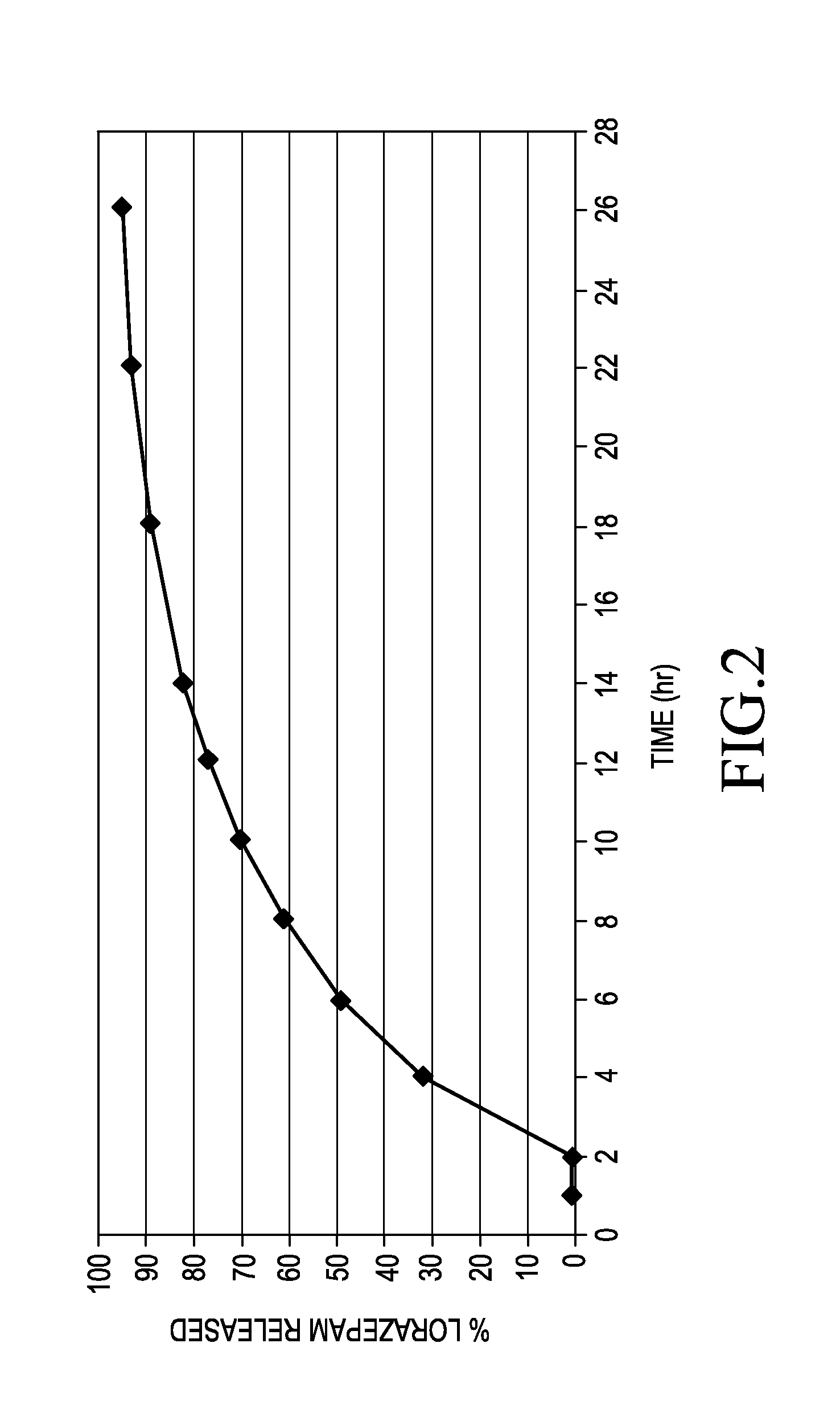
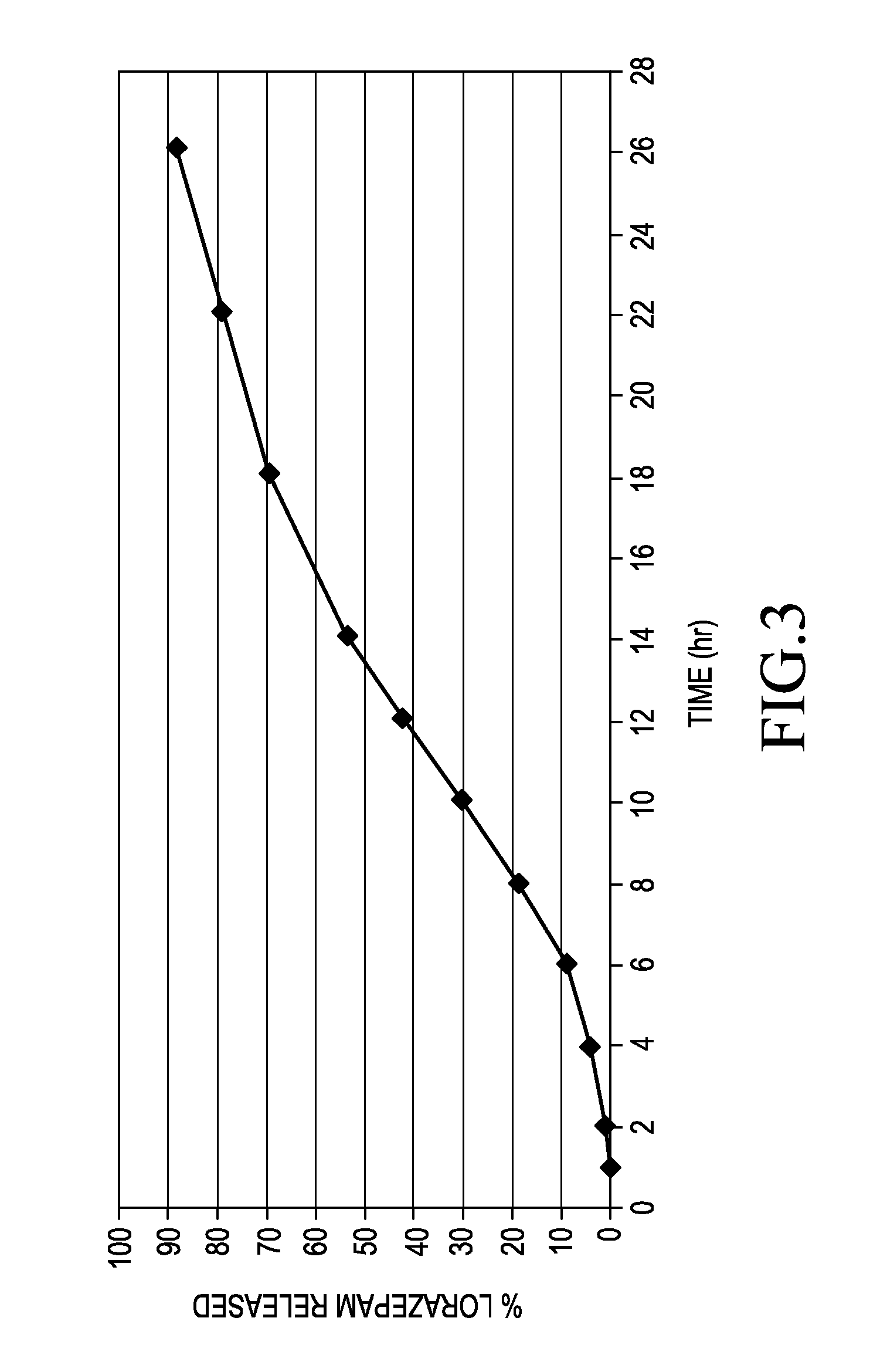
Sustained release formulations of lorazepam
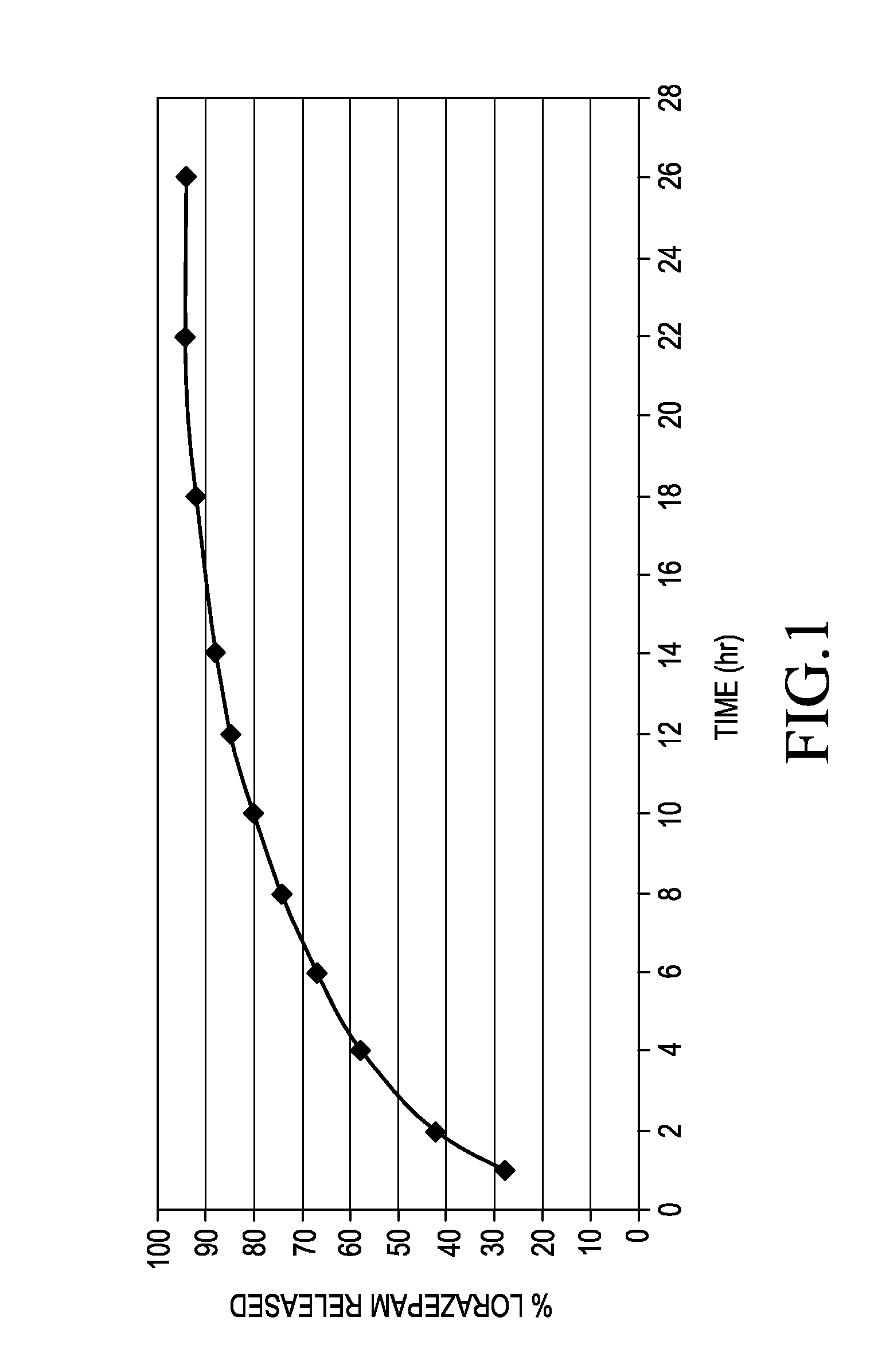
ActiveUS20150110874A1Promote absorptionFavorable steady state pharmacokinetic profilesBiocidePowder deliveryTherapeutic effectProlonged release
A pharmaceutical composition for delivering lorazepam in a prolonged fashion is achieved with prolonged release lorazepam pharmaceutical beads. The composition typically contains sustained release lorazepam beads and delayed sustained release lorazepam beads. The composition can provide once daily dosing that maintains 24 hour therapeutic effect under steady state conditions.
Owner:EDGEMONT PHARMA LLC LIQUIDATING TRUST
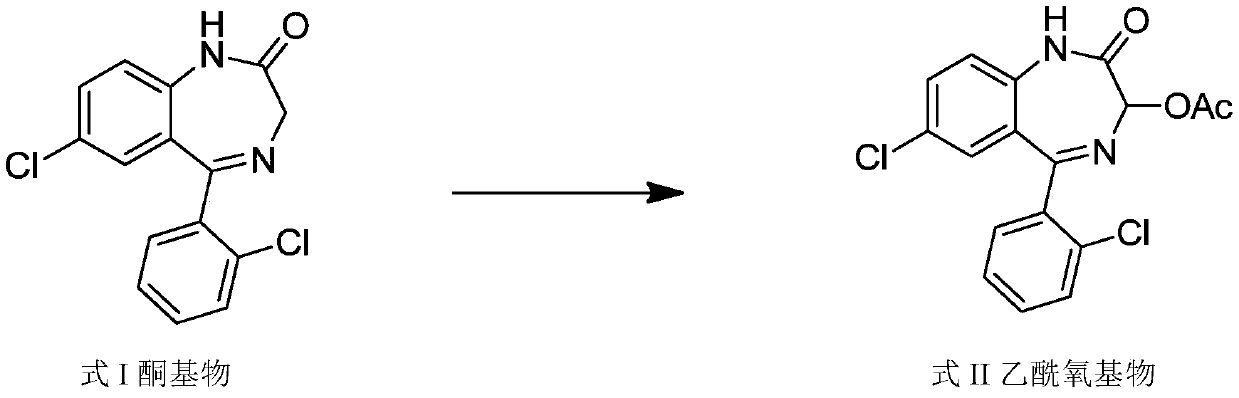
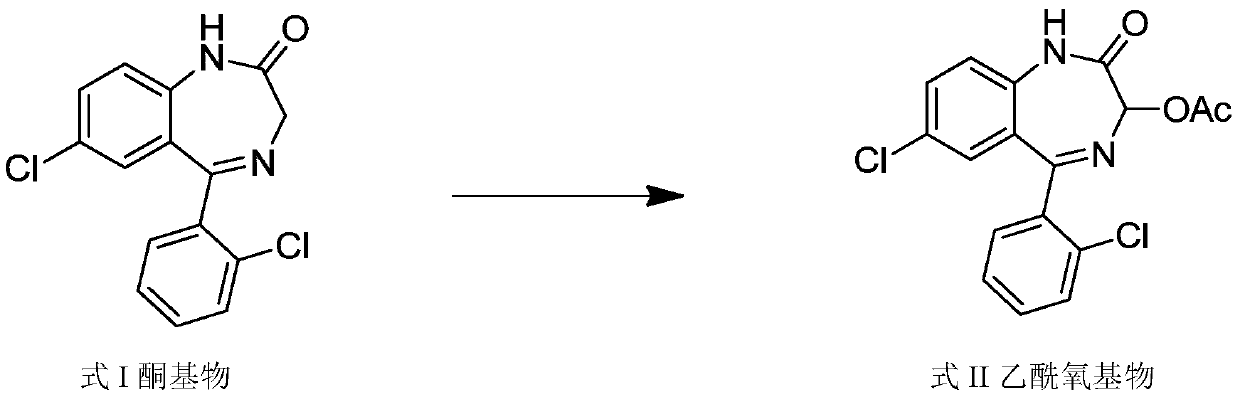
Method for preparing lorazepam
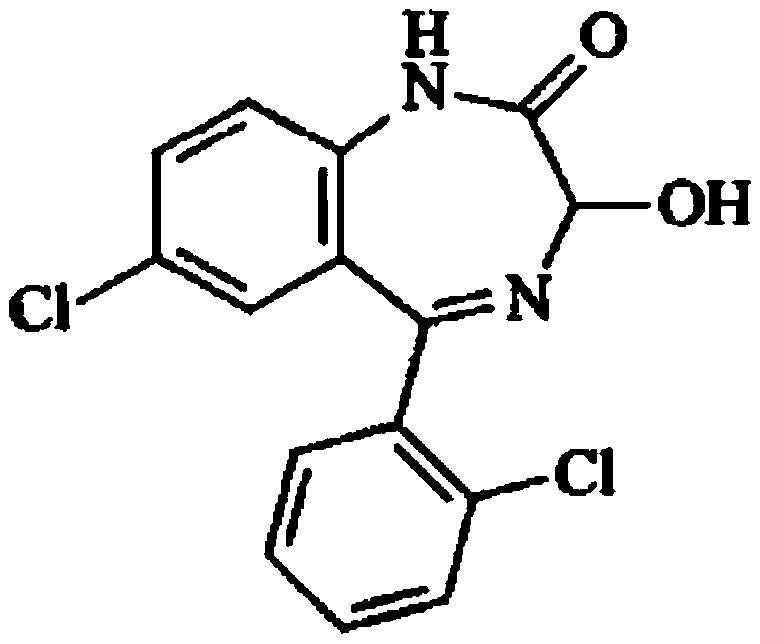
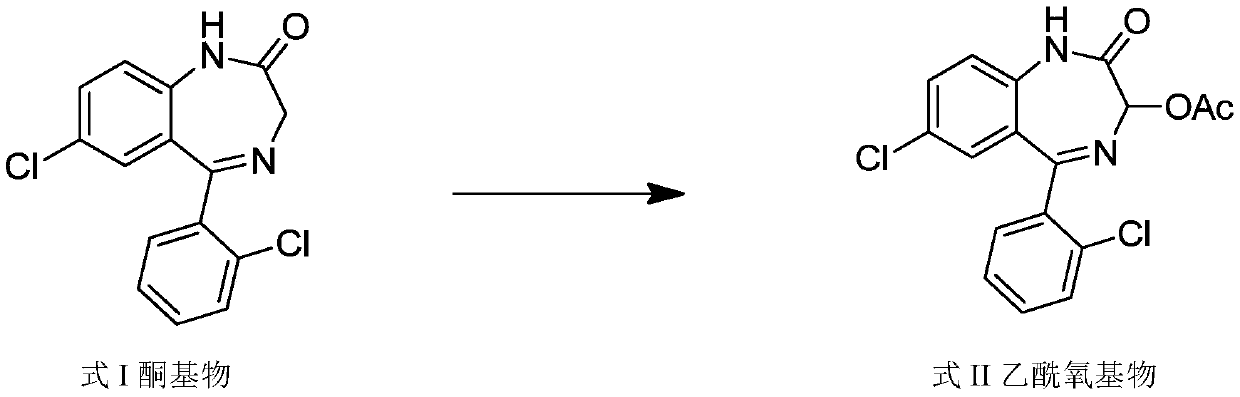
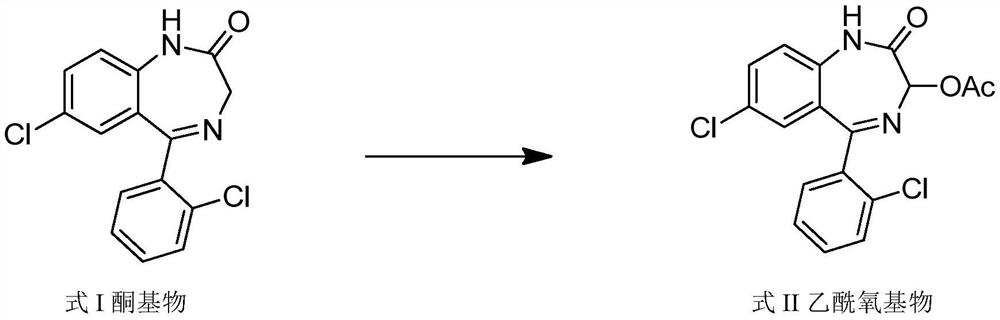
The invention relates to a method for preparing lorazepam. The method comprises the following steps: subjecting a ketone substrate as shown in a formula I, glacial acetic acid, potassium acetate, potassium persulfate and iodine to a reaction under heating and stirring conditions to obtain an acetoxy substrate as shown in a formula II; dropwise adding a sodium hydroxide solution into a mixture of ethanol and the acetoxy substance as shown in the formula II, carrying out stirring to realize a complete reaction, performing filtering to obtain a filter cake, and reacting the filter cake with ethylacetate and a citric acid solution to obtain a crude lorazepam product; and crystallizing the crude lorazepam product by using ethanol and ethyl acetate in sequence. The invention further relates tothe pharmaceutical application of larazepam prepared by using the method. The larazepam is particularly used for treating or preventing anxiety, epilepsy, convulsion, sedation and hypnosis. The methoddisclosed by the invention has excellent technical effects as described in the specification.
Owner:HUNAN DONGTING PHARMA
Compound anesthetic for minks and preparation method and application of compound anesthetic
InactiveCN104873518AGood anesthesiaMarked respiratory depressionOrganic active ingredientsAnaestheticsMuscle relaxationSide effect
The invention discloses compound anesthetic for minks and a preparation method and application of the compound anesthetic and belongs to the fields of preparation and application of the compound anesthetic for minks. The compound anesthetic for minks comprises detomidine, lorazepam and nalbuphine. The preparation method incudes: (1) mixing the detomidine, lorazepam and nalbuphine; (2) adding water of injection with uniform mixing to obtain the compound anesthetic. The compound anesthetic is quick in anesthesia induction on minks, long in anesthesia duration, good in analgesia, sedation and muscle relaxation effects, smooth and steady in analepsia and simple to operate, has slight impact on all clinical conventional indexes and special indexes, almost has no side effect and can be used in operations such as long-distance mink transportation, electro-ejaculation, cesarean section, trauma and fracture needing chemical restraints or sedation.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Preparation method of lorazepam intermediate
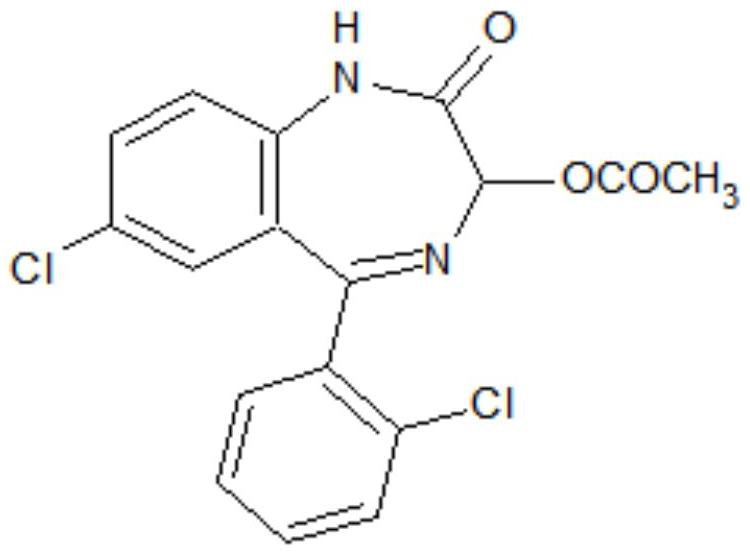
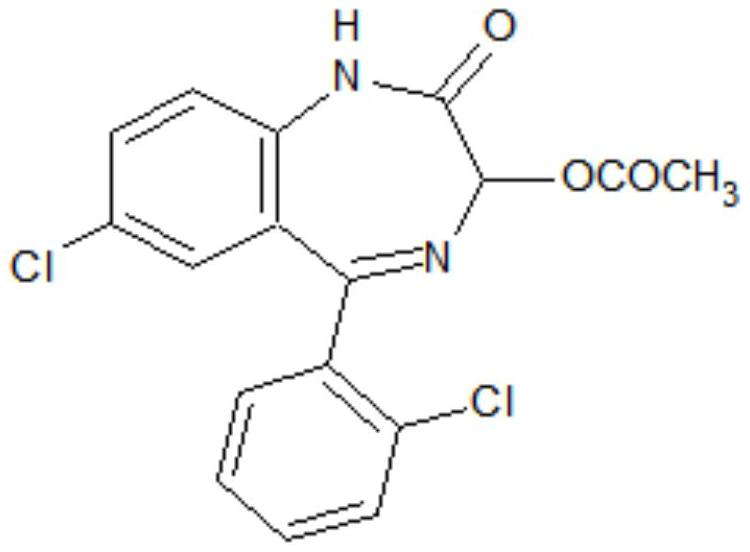
The invention discloses a preparation method of a lorazepam intermediate, which comprises the following steps: adding 4-dimethylaminopyridine into a mixed solution of 7-chloro-2-oxo-5-(2-chlorphenyl)-1, 4-benzodiazepin-4-oxide, acetic anhydride and an aprotic polar solvent, stirring, heating, carrying out heat preservation reaction, cooling after the reaction is finished, adding water to separate out materials, filtering, washing with water, refining to obtain a target product. According to the invention, under the condition of greatly reducing the dosage of acetic anhydride, the yield of acylation and rearrangement reactions of lorazepam is ensured, and the discharge of acid-containing wastewater is greatly reduced; in addition, in the reaction process, the temperature is stable, the phenomenon of violent rising does not occur, and the production safety is greatly improved.
Owner:HUAZHONG PHARMA
Light-stabilized pharmaceutical composition, and preparation method and pharmaceutical application thereof
The invention relates to a light-stabilized pharmaceutical composition, and a preparation method and pharmaceutical application thereof. Specifically, the pharmaceutical composition comprises a lorazepam crystal and pharmaceutical adjuvants. The lorazepam crystal is radiated by Cu-K alpha. In a powder X-ray diffraction pattern expressed by an angle of 2 theta, there are diffraction peaks at approximately 12.17 degrees, approximately 14.15 degrees, approximately 15.27 degrees, approximately 16.84 degrees, approximately 17.91 degrees and approximately 20.81 degrees. For example, the crystal hasdiffraction peaks at approximately 7.93 degrees, approximately 9.04 degrees, approximately 12.17 degrees, approximately 14.15 degrees, approximately 15.27 degrees, approximately 16.84 degrees, approximately 17.91 degrees, approximately 20.81 degrees, approximately 21.44 degrees and approximately 26.38 degrees. A new crystal form and the pharmaceutical composition, which are used for preparing lorazepam and prepared by the method, exhibit excellent properties as described in the description of the invention.
Owner:HUNAN DONGTING PHARMA
Light-stabilized pharmaceutical composition and its preparation method and pharmaceutical use
ActiveCN110840898BOrganic active ingredientsNervous disorderPharmaceutical AidsPharmaceutical Substances
The invention relates to a light-stabilized pharmaceutical composition, a preparation method and a pharmaceutical use thereof. Specifically, the pharmaceutical composition provided by the present invention comprises lorazepam crystals and pharmaceutical excipients; said lorazepam crystals use Cu-Kα radiation, and in the powder X-ray diffraction spectrum represented by 2θ angle, in There are diffraction peaks at about 12.17°, about 14.15°, about 15.27°, about 16.84°, about 17.91°, and about 20.81°; There are diffraction peaks at 15.27°, about 16.84°, about 17.91°, about 20.81°, about 21.44°, and about 26.38°. The invention also provides a preparation method of the pharmaceutical composition. Pharmaceutical uses of the pharmaceutical composition are also provided. The new crystal form of lorazepam and the pharmaceutical composition prepared by the method of the present invention exhibit such excellent properties as described in the description of the present invention.
Owner:HUNAN DONGTING PHARMA
Purification method of lorazepam
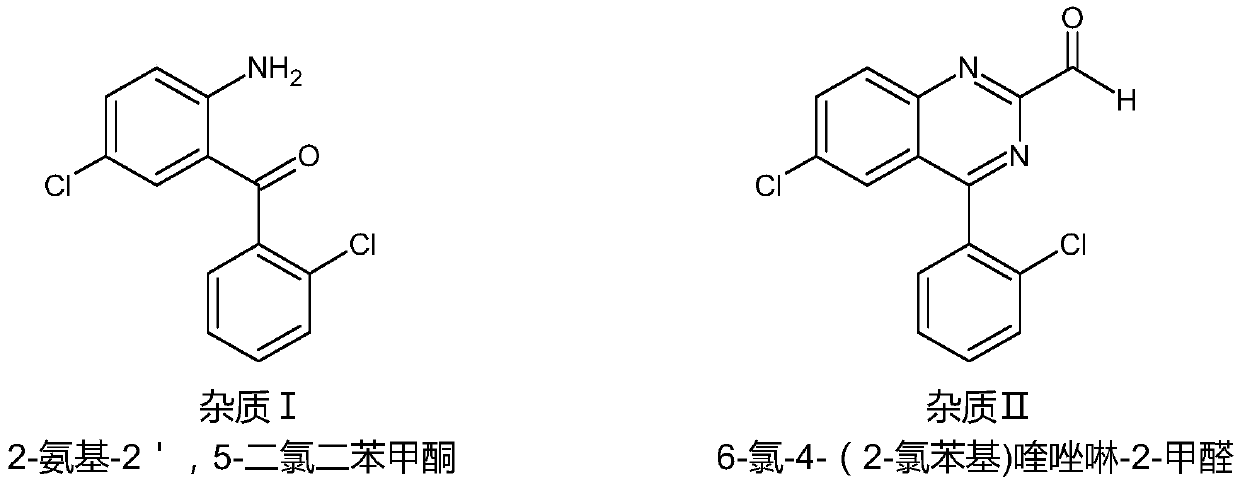
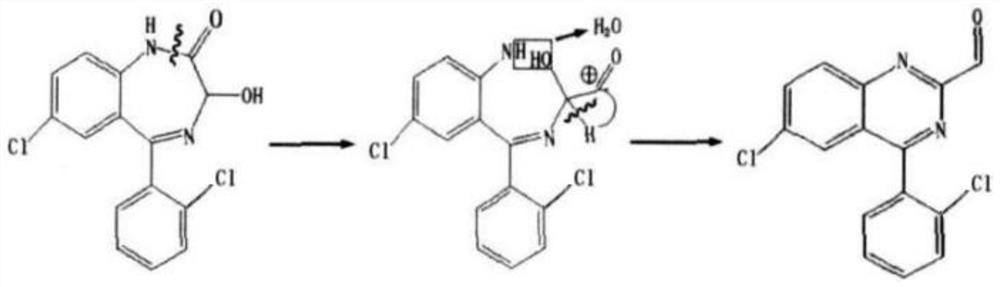
The invention discloses a purification method of lorazepam. The purification method specifically comprises the following steps: heating for refluxing a good solvent, cooling the refluxed solvent underthe protection of an inert gas, adding crude lorazepam, stirring for dissolving, adding activated carbon to decolorize, performing filtration, adding an inert solvent into the obtained filtrate, carrying out cooling crystallization, and performing filtration to obtain the lorazepam product. The purity of the lorazepam product is not less than 99.85%, and the content of an impurity 6-chloro-4-(2-chlorphenyl)quinazoline-2-carboxaldehyde in the lorazepam product does not exceed 0.05%. The method has the advantages of simplicity in operation, controllable quality, and suitability for industrial production.
Owner:HUAZHONG PHARMA
Preparation process of lorazepam impurity C
InactiveCN111732549AImprove production efficiencyThe reaction process is simpleOrganic chemistryFluid phasePtru catalyst
The invention provides a preparation process of a lorazepam impurity C. According to the process, lorazepam is taken as a raw material, a catalyst and a buffer reagent are added into an aprotic solvent, a heat preservation reaction is performed, and after the reaction is finished, the lorazepam impurity C is obtained through post-treatment. The method has the following advantages: the reaction andpost-treatment process is simple; separation and purification are performed without using a thin-layer chromatography (PTLC) technology or a liquid-phase preparative column technology; and the prepared lorazepam impurity C has higher purity and the like, and can be used for qualitative and quantitative analysis of impurities in lorazepam production, so that the quality standard of lorazepam is improved, and important guiding significance is provided for safe medication.
Owner:HUAZHONG PHARMA
Electrochemical chiral recognition detection method for lorazepam
ActiveCN109239172AEasy to operateQuick identificationGrapheneMaterial electrochemical variablesPower flowPeak current
The invention discloses a chiral detection method for lorazepam, realized by adopting the following steps: preparing graphene, performing carboxylation on the graphene and modifying on a glassy carbonelectrode, then electrochemically modifying poly-L-leucine, detecting a lorazepam isomer by adopting a differential pulse voltammetry procedure, and scanning a potential from -0.3V to 0.6V, wherein results show that R-lorazepam has a relatively high peak current and S-lorazepam has a relatively low peak current. The detection method disclosed by the invention can realize fast recognition of R-lorazepam and S-lorazepam, and the detection method is simple and high in detection efficiency.
Owner:山东信开源科技创新发展有限责任公司
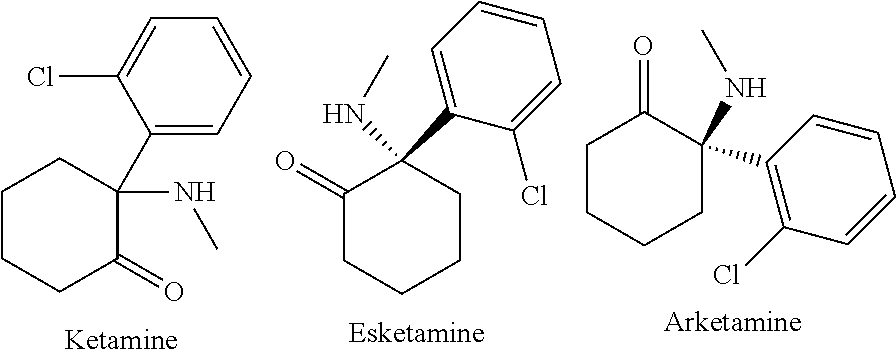
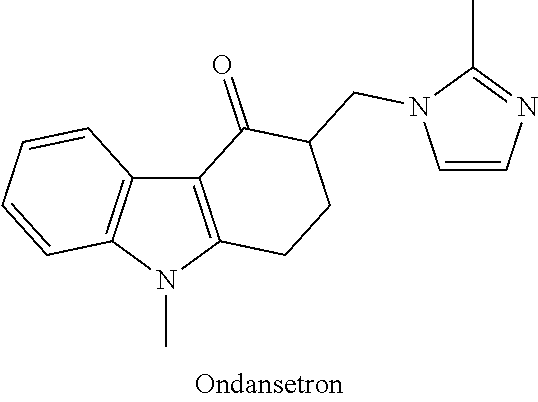
Method of treatment for ketamine infusion
Methods for treatment for patients having depression and / or pain are contemplated as including an administration of first preparation comprising an antiemetic agent, preferably ondansetron, followed by a second preparation of a synergistic combination of ketamine and a benzodiazepine, preferably lorazepam, administered via a continuous intravenous infusion. Such methods may be seen to better alleviate depression and pain symptoms, and may result in reduced need for other medications.
Owner:ELEUSIS THERAPEUTICS US INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com