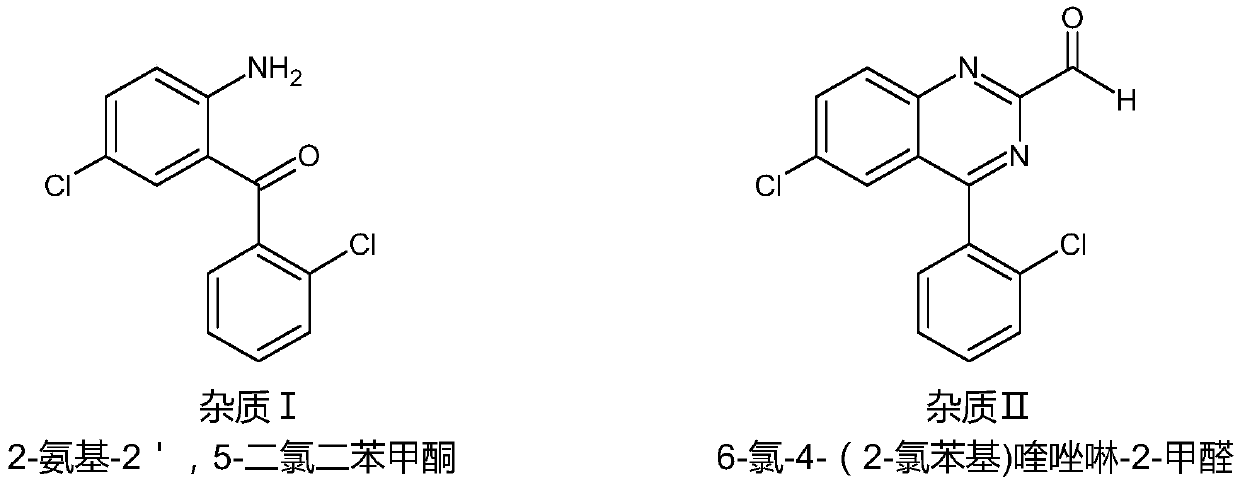
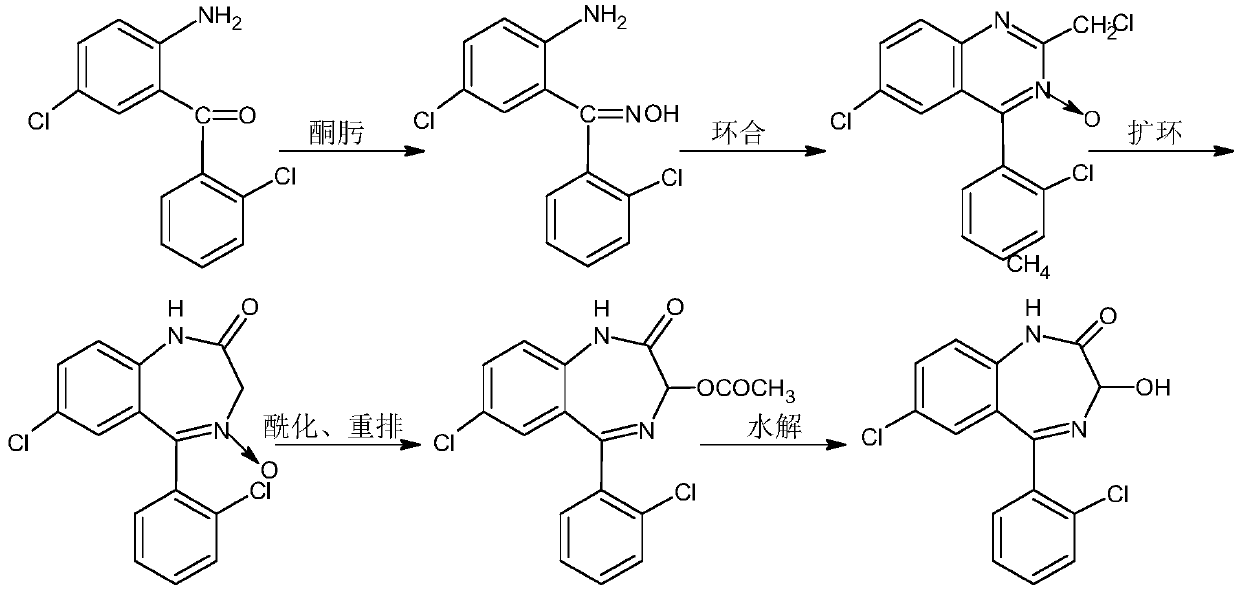
Purification method of lorazepam
A purification method, the technology of lorazepam, applied in the field of medicine and chemical industry, can solve the problems such as difficult to meet the quality standards, and achieve the effect of reducing content, simple operation and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The present invention proposes a method for purifying lorazepam, comprising the steps of:
[0023] Add 100ml of tetrahydrofuran into the reaction flask, raise the temperature to reflux, and maintain the reflux for degassing for 30 minutes. Under the protection of nitrogen, lower the temperature to 55°C-60°C, add 20g of lorazepam crude product under stirring, keep it at 55°C-60°C for 2 hours after feeding, add 1g of activated carbon and keep it at 55°C-60°C for 1 hour. Filtrate while it is hot, heat up the filtrate to 55°C-60°C, add 300ml of petroleum ether, control the speed of adding petroleum ether, the system temperature is 50°C-60°C during the process of adding petroleum ether, and keep stirring at 55°C-60°C for 30 Minutes, then first lower the temperature to 40°C-45°C and keep for 1 hour; then cool down to 20°C-25°C and keep for 1 hour; continue to cool down to -5°C-0°C to crystallize, crystallize for 2 hours, filter and dry 16.3 g of lorazepam product was obtaine...
Embodiment 2
[0025] The present invention proposes a method for purifying lorazepam, comprising the steps of:
[0026] Add 120ml of tetrahydrofuran into the reaction flask, raise the temperature to reflux, and maintain reflux for 30 minutes for degassing. Under the protection of argon, lower the temperature to 50°C-55°C, add 20g of lorazepam crude product under stirring, keep it at 50°C-55°C for 2 hours after feeding, add 1g of activated carbon and keep it at 50°C-55°C for 1 hour. Filtrate while it is hot, raise the temperature of the filtrate to 55°C-60°C, add 360ml of n-hexane, control the speed of adding n-hexane, the system temperature is 50°C-60°C during the process of adding n-hexane, and keep stirring at 55°C-60°C for 30 Minutes, then first lower the temperature to 40°C-45°C and keep for 1 hour; then cool down to 20°C-25°C and keep for 1 hour; continue to cool down to -5°C-0°C to crystallize, crystallize for 2 hours, filter and dry 15.7 g of lorazepam product was obtained, the HPLC...
Embodiment 3
[0028] The present invention proposes a method for purifying lorazepam, comprising the steps of:
[0029] Add 100ml of tetrahydrofuran into the reaction flask, raise the temperature to reflux, and maintain the reflux for degassing for 30 minutes. Under the protection of argon, lower the temperature to 55°C-60°C, add 20g of crude lorazepam under stirring, keep it at 55°C-60°C for 2 hours after feeding, add 1g of activated carbon and keep it at 55°C-60°C for 1 hour. Filtrate while it is hot, heat the filtrate to 55°C-60°C, add 250ml of n-hexane, control the speed of adding n-hexane, the system temperature is 50°C-60°C during the addition of n-hexane, and keep stirring at 55°C-60°C for 30 minutes, then lower the temperature to 40°C-45°C and keep for 1 hour; then lower the temperature to 20°C-25°C and keep for 1 hour; continue to cool down to 5°C-10°C to crystallize, crystallize for 2 hours, filter and dry to obtain 15g of lorazepam product, HPLC purity 99.93%, impurity 6-chloro-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com