Patents
Literature
72 results about "Ondansetron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or with other medications to prevent nausea and vomiting caused by cancer drug treatment (chemotherapy) and radiation therapy. It is also used to prevent and treat nausea and vomiting after surgery.
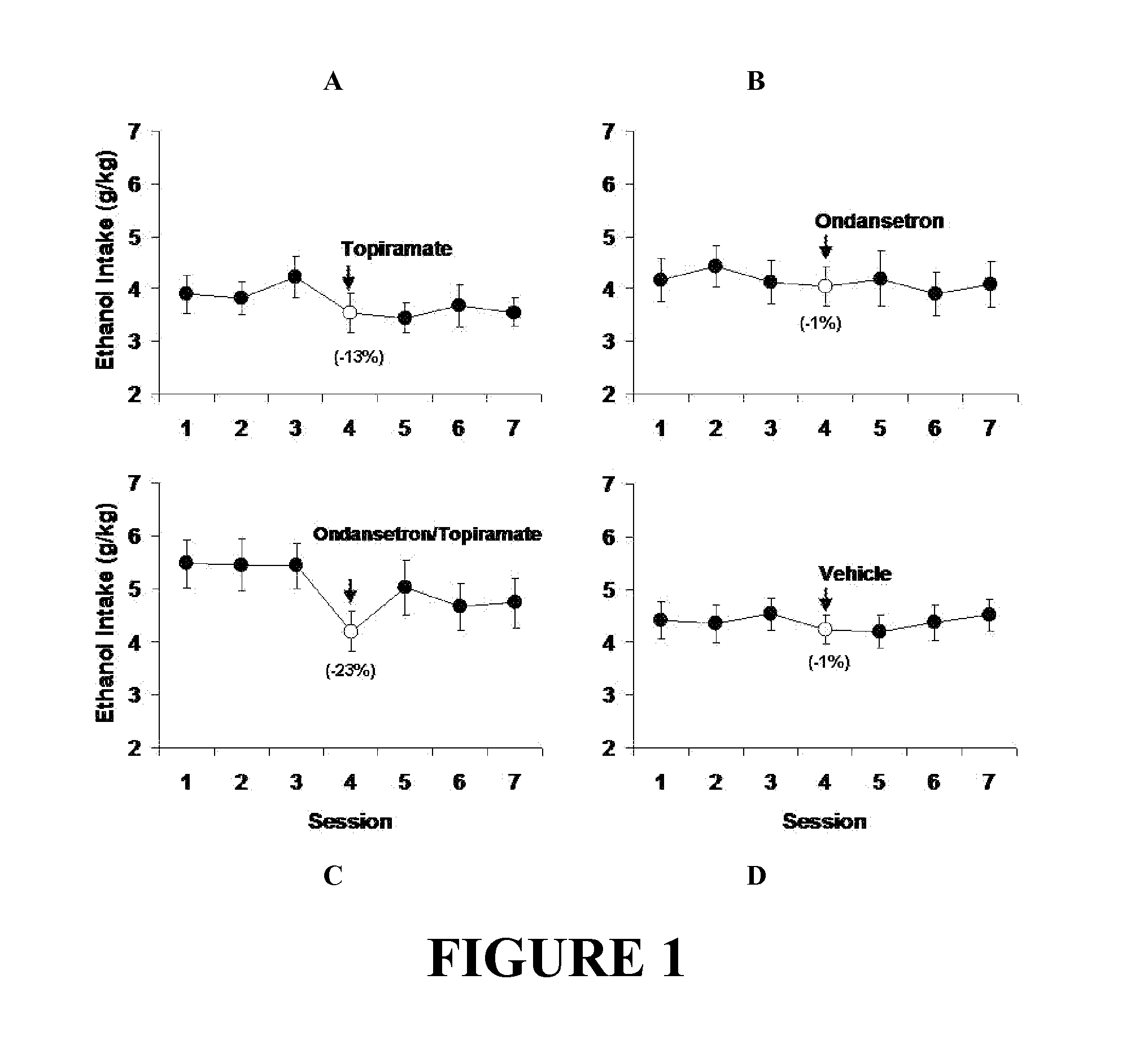
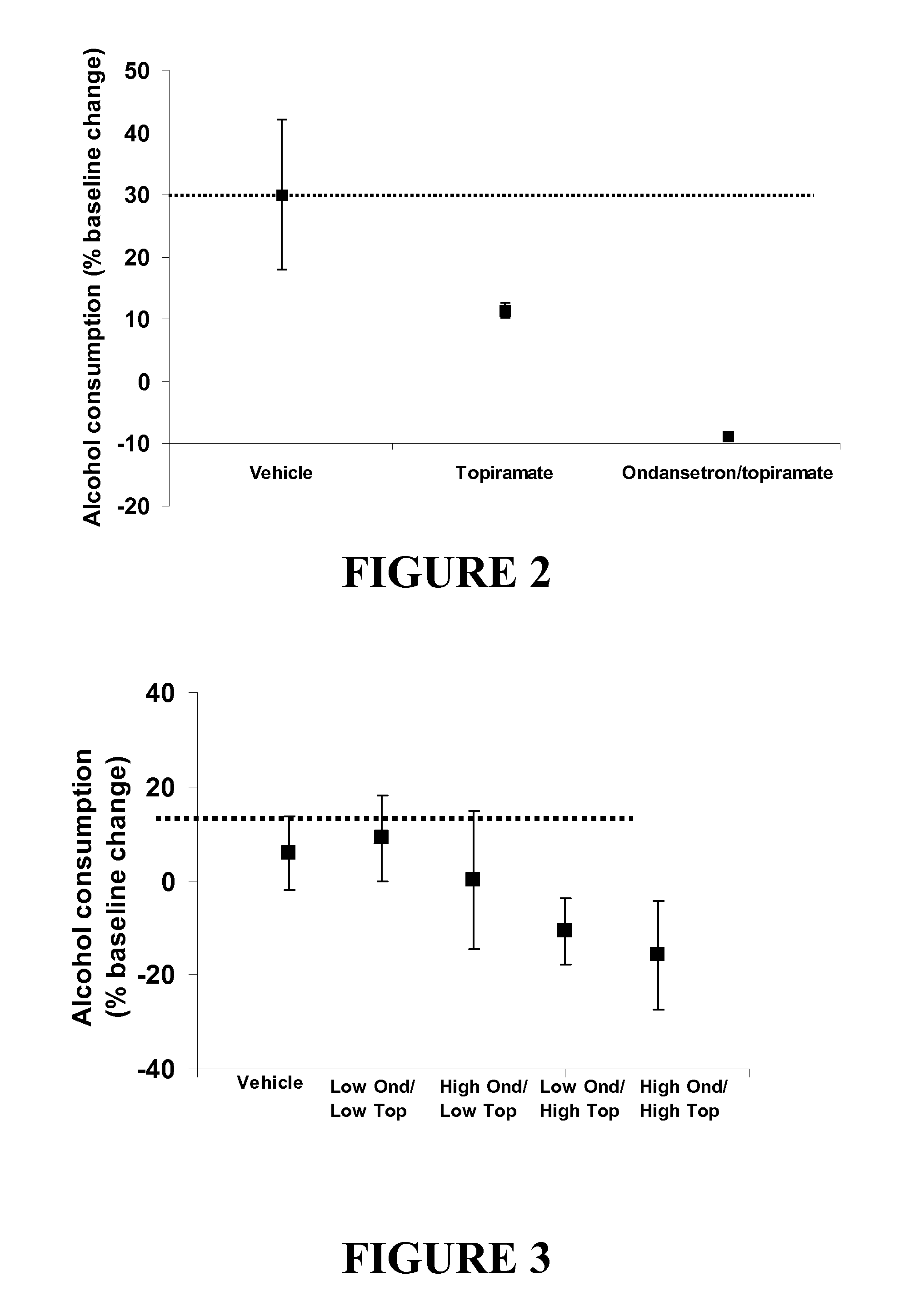
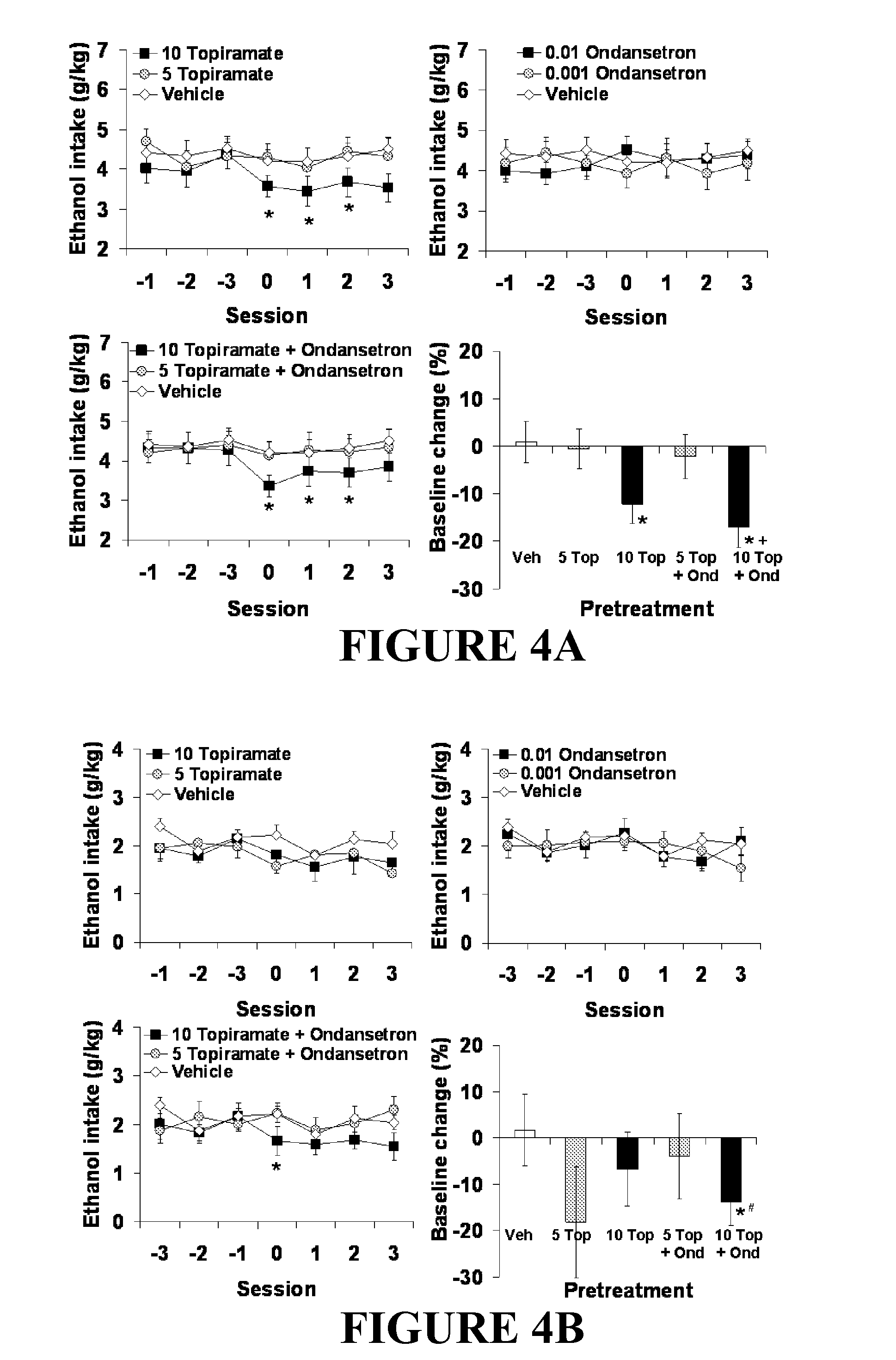
Medication Combinations for the Treatment of Alcoholism and Drug Addiction
InactiveUS20110065628A1Decrease and cessationReverses effectBiocideNervous disorderAlcoholismsOndansetron
The present invention provides for the use of combinations of drugs to treat addictive disorders. More specifically, the present invention provides compositions and methods for treating disorders using combinations of drugs such as topiramate, ondansetron, and naltrexone.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
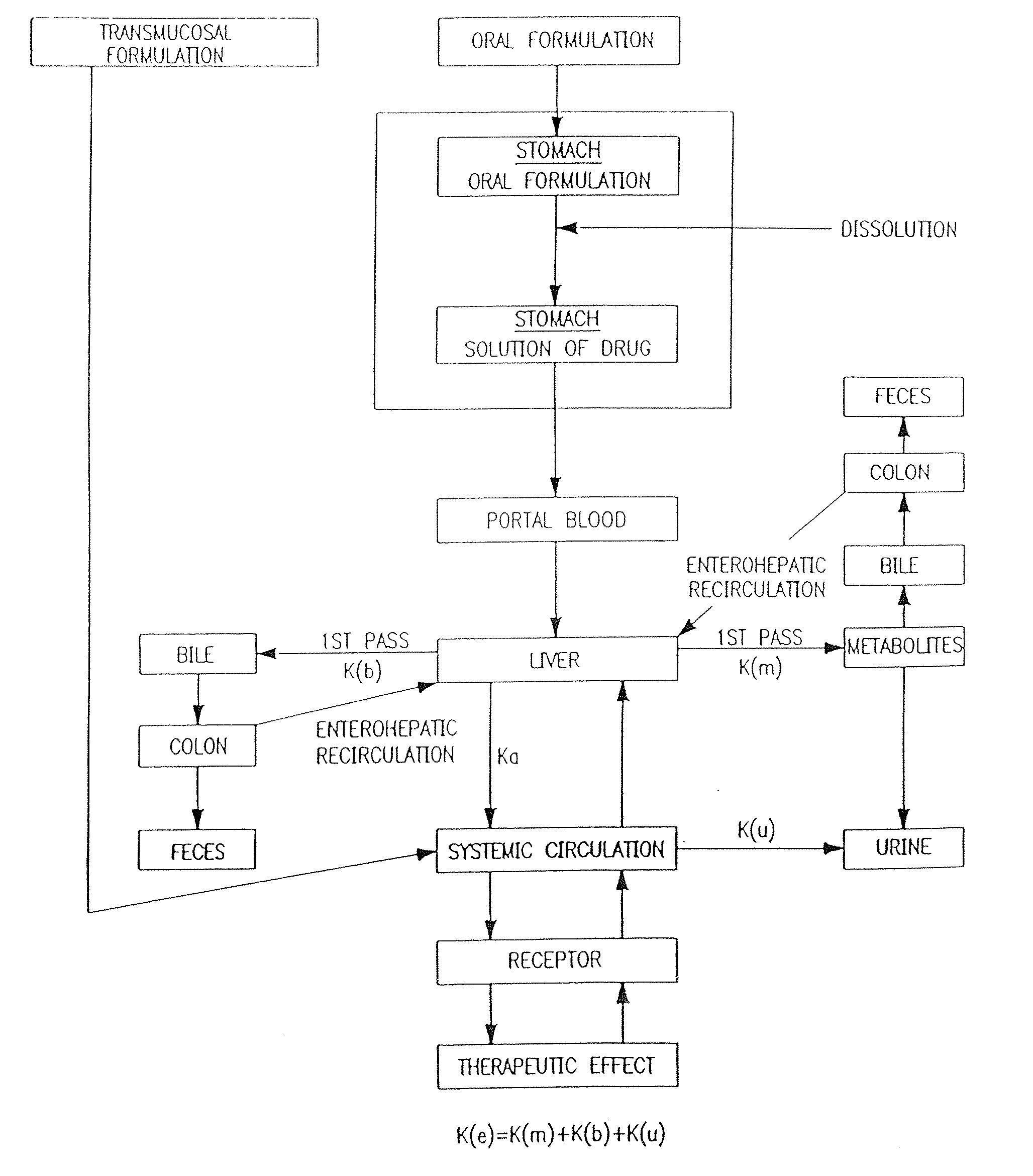
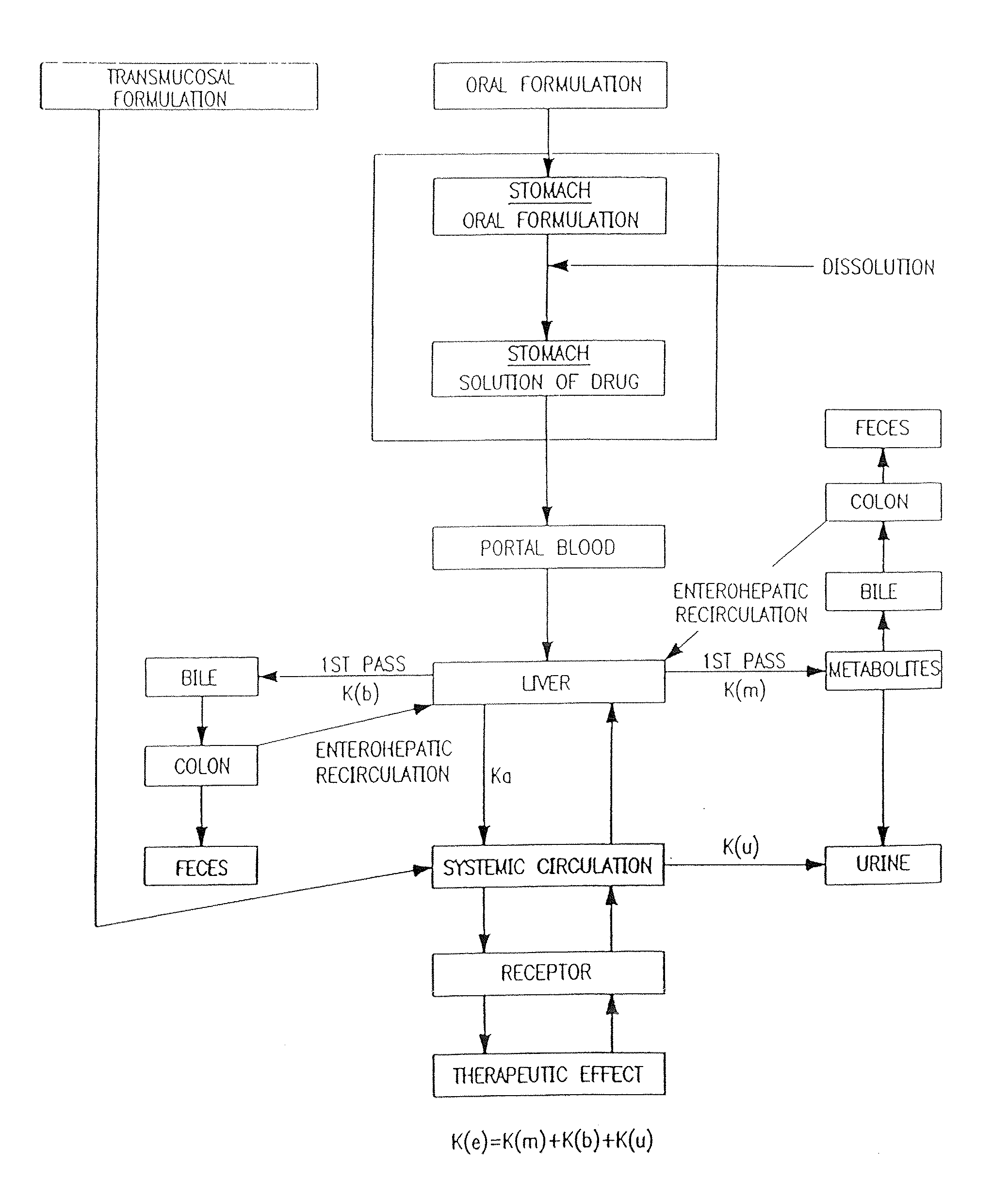
Transmucosal administration of drug compositions for treating and preventing disorders in animals
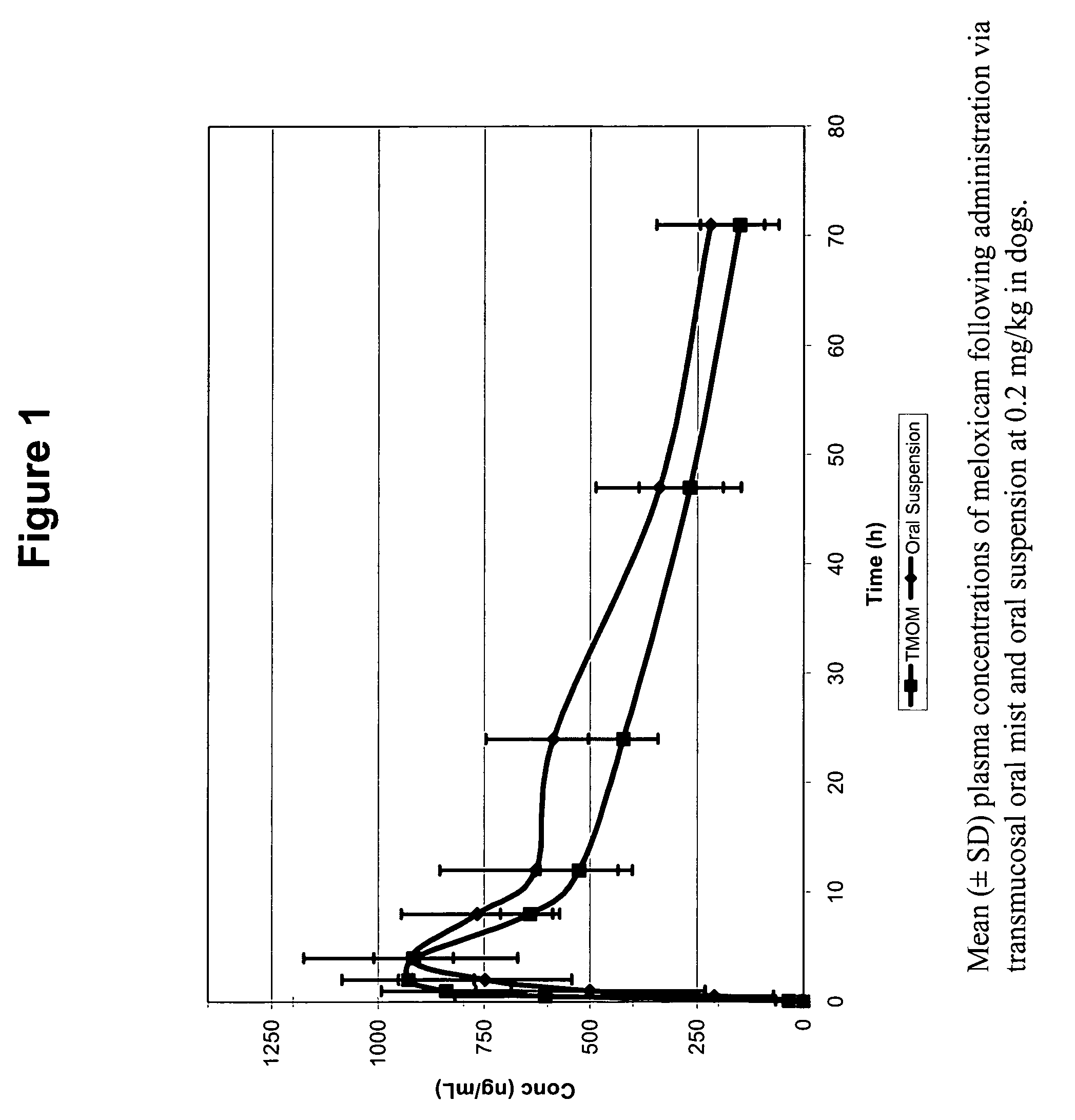
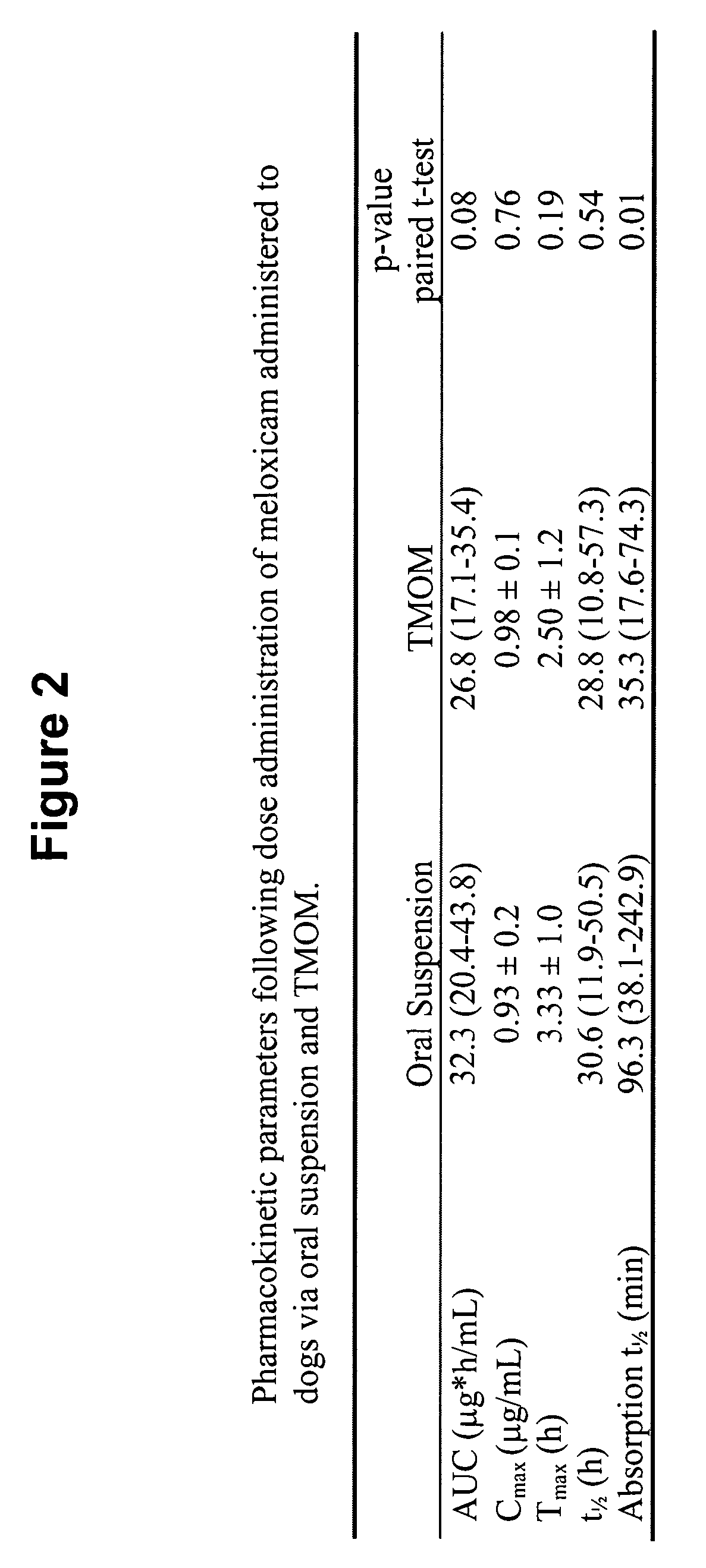
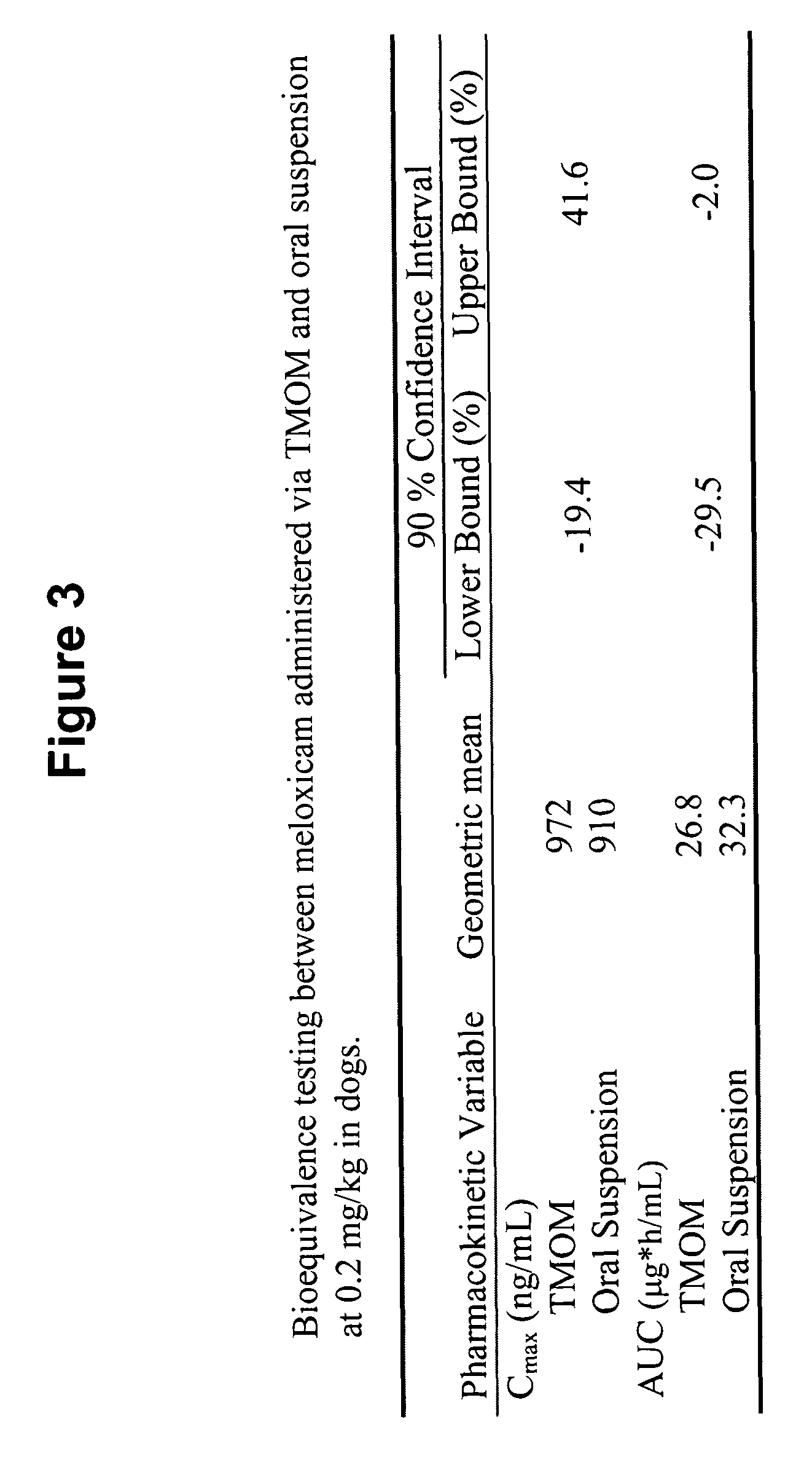
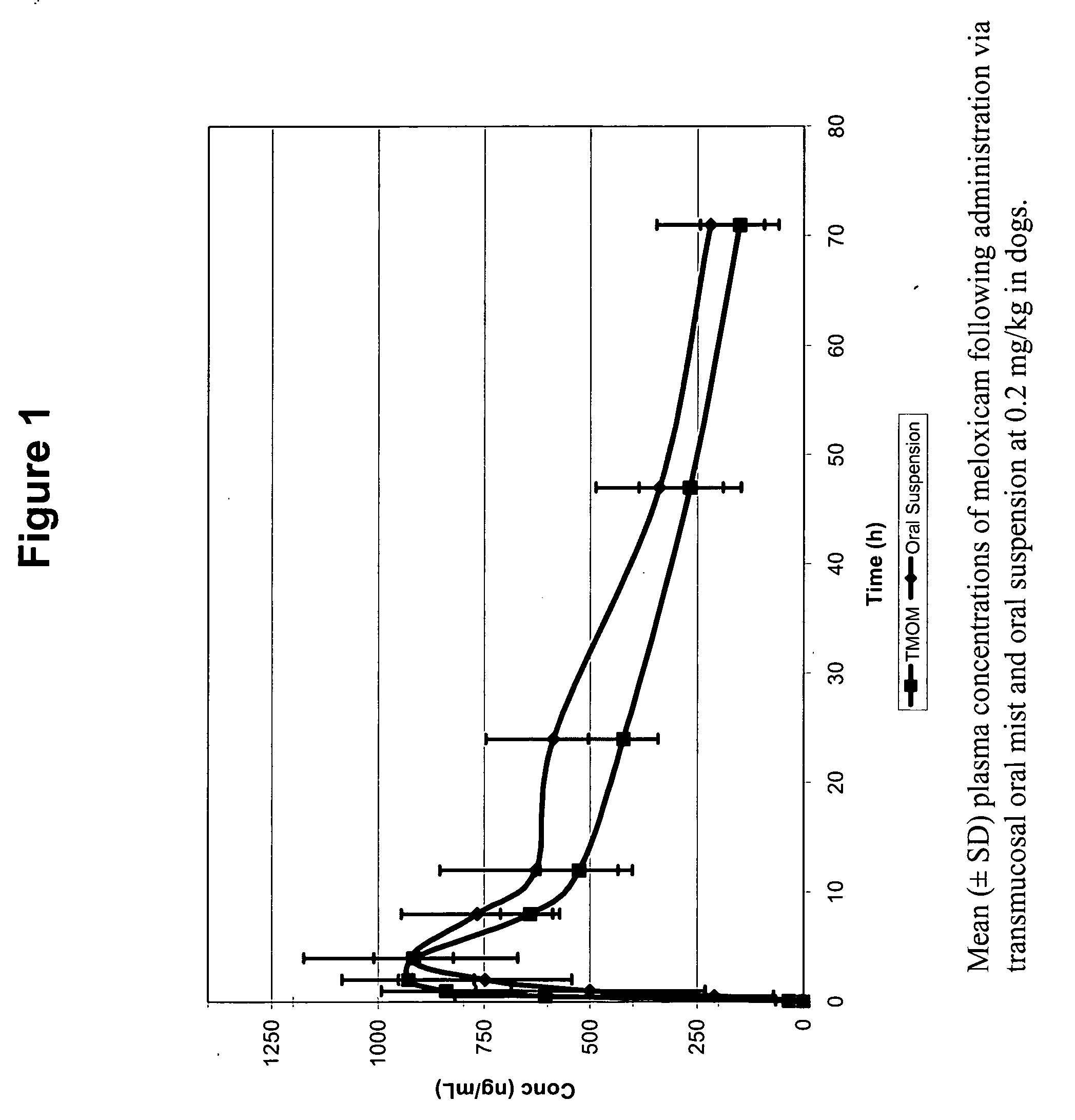
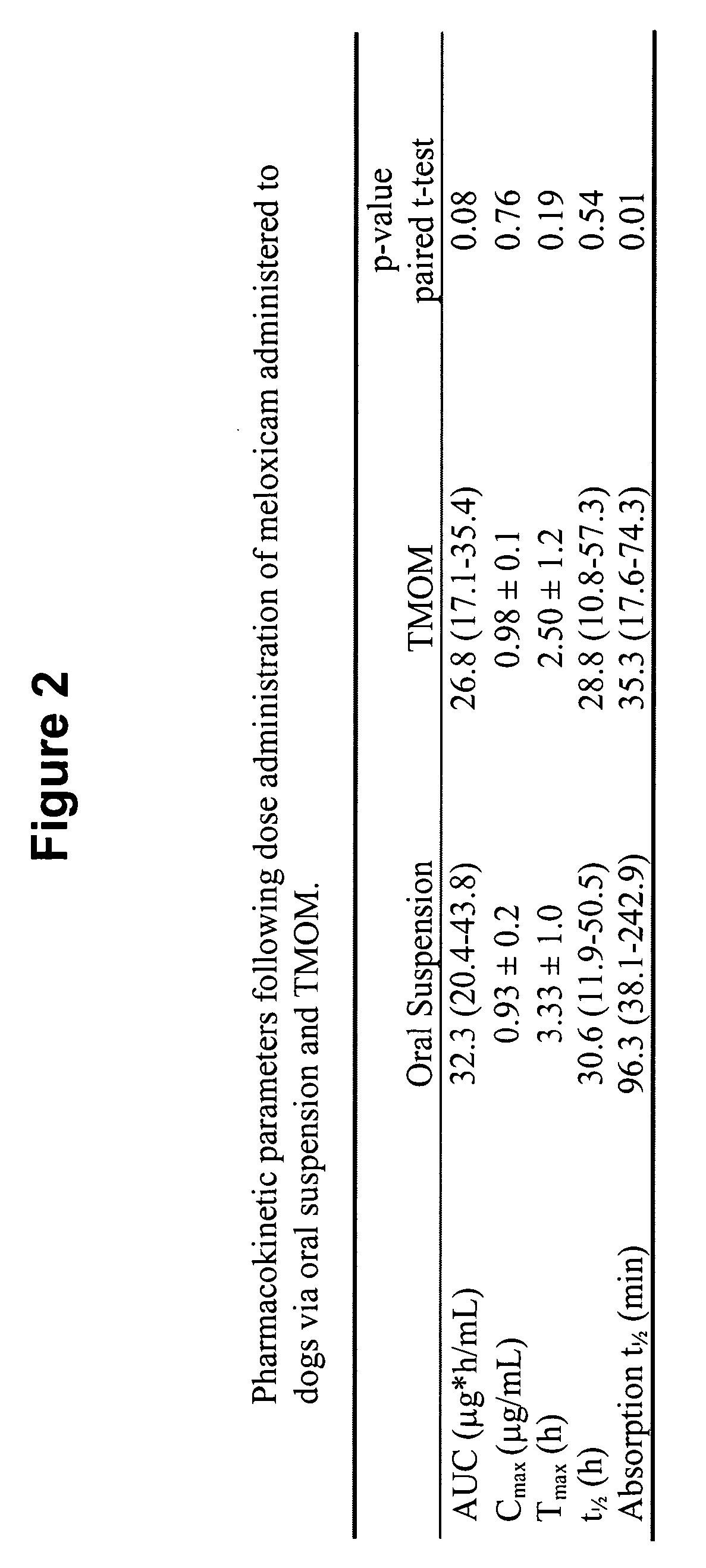
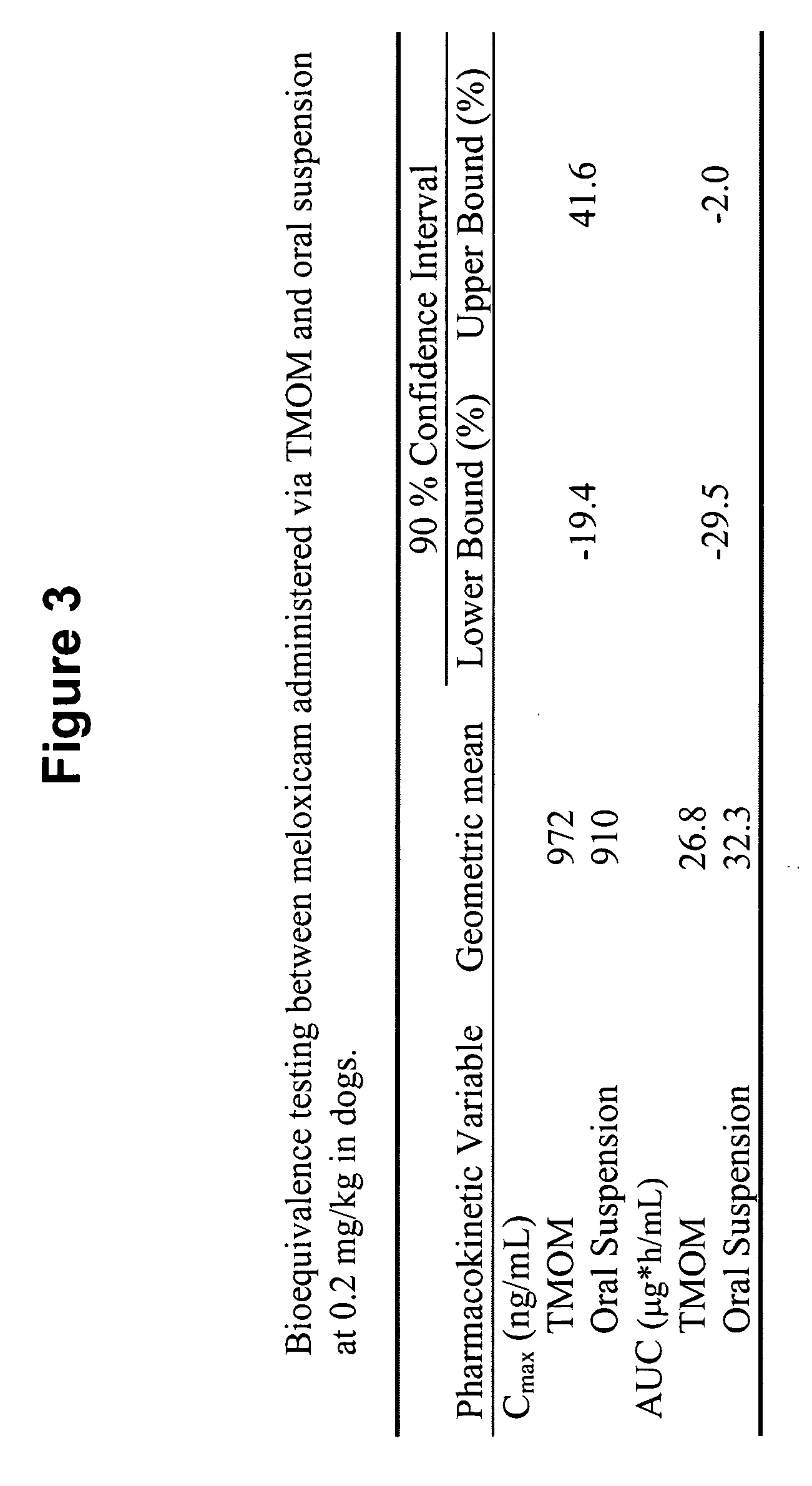
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
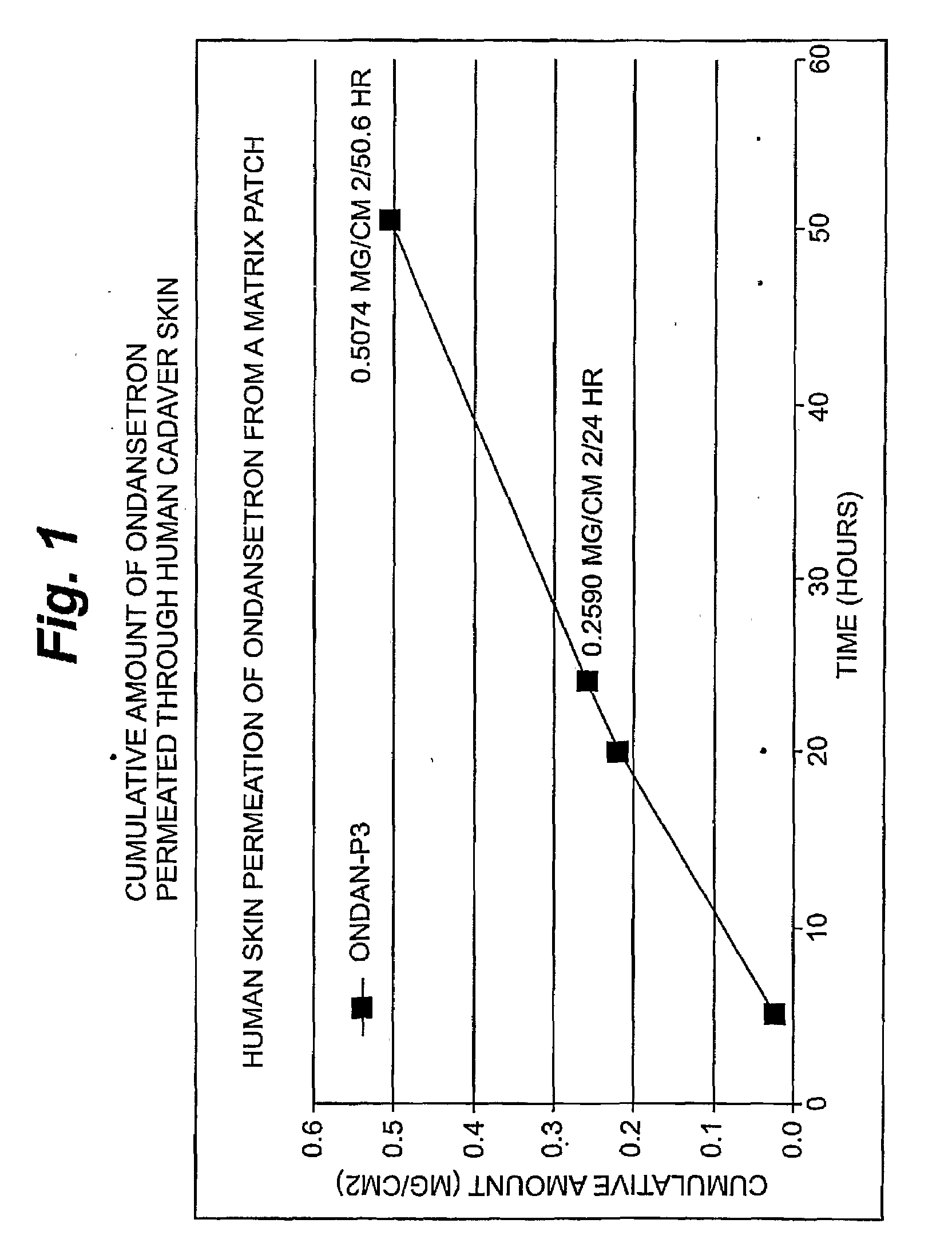
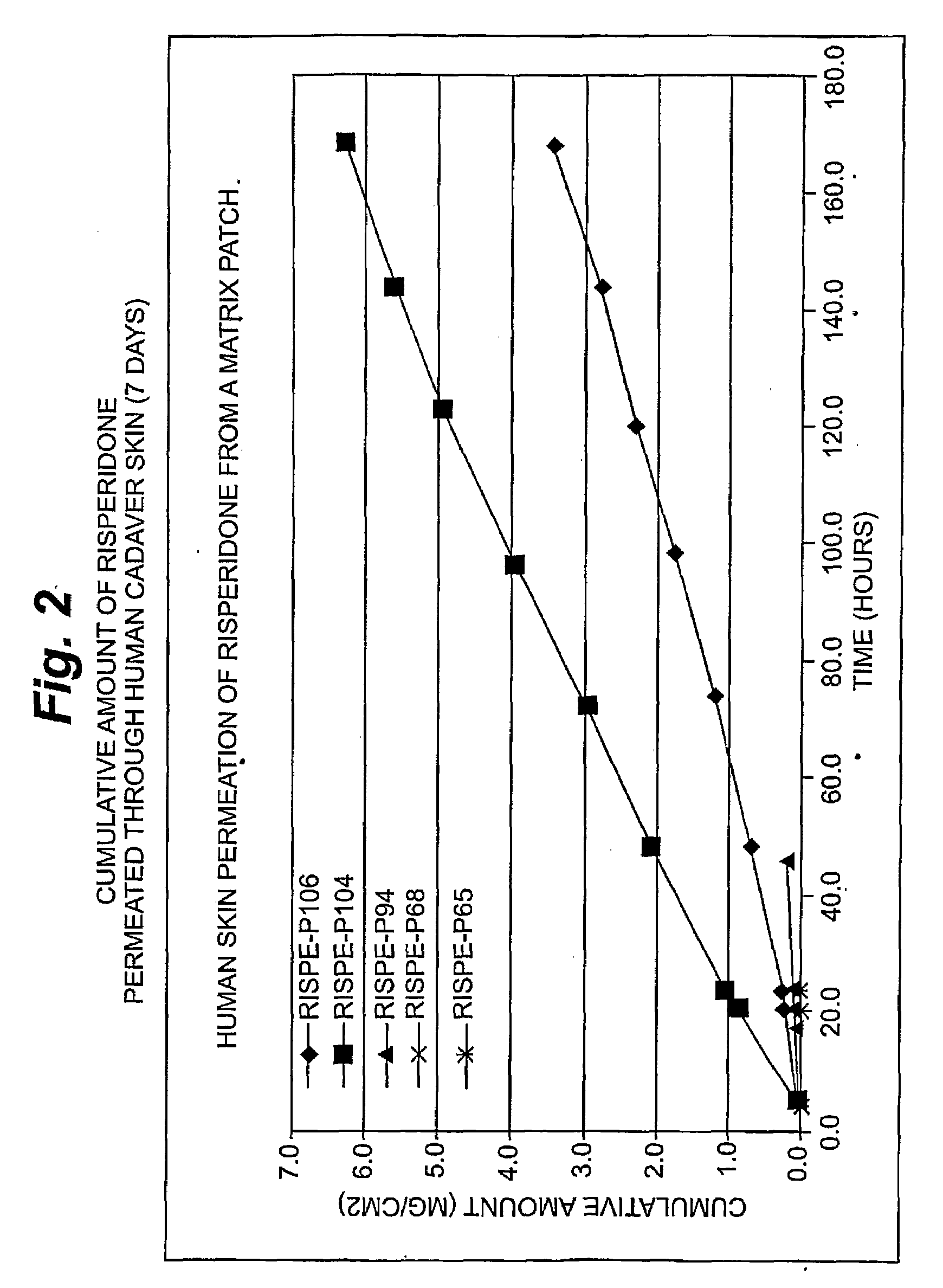
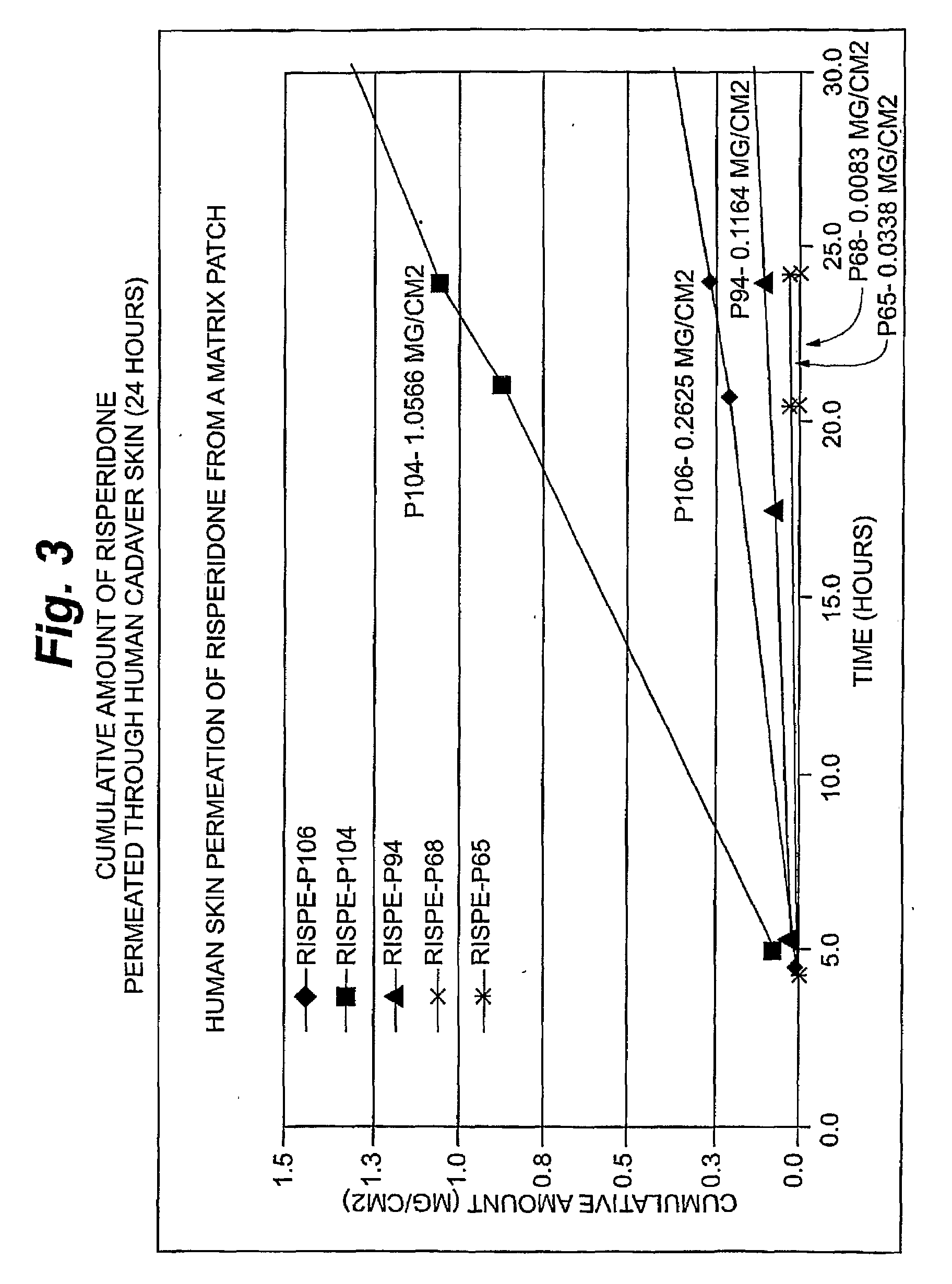
Transdermal Delivery of Hydrophobic Bioactive Agents
InactiveUS20080262445A1Improve solubilityImprove permeabilityBiocideAdhesive dressingsSerotoninFlumazenil
A method and related compositions, including the use of N-acyl derivatives of sarcosine, provide for the delivery of bioactive agents through tissue surfaces such as the skin. The method and composition are particularly well suited for hydrophobic active agents such as serotonin (5HT3) receptor antagonists (e.g., ondansetron), antipsychotic agents (e.g., risperidone), benzodiazepines (e.g., flumazenil), and progestins (e.g., levonorgestrel).
Owner:DERMATRENDS INC
Methods for treating apnea and apnea disorders using optically pure R(+) ondansetron
Methods for the treatment, management, or prevention of apnea and apnea disorders, or symptoms thereof, using a therapeutically effective amount of substantially optically pure R(+) ondansetron, or a pharmaceutically acceptable salt thereof, substantially free of its S(-) stereoisomer.
Owner:SEPACOR INC
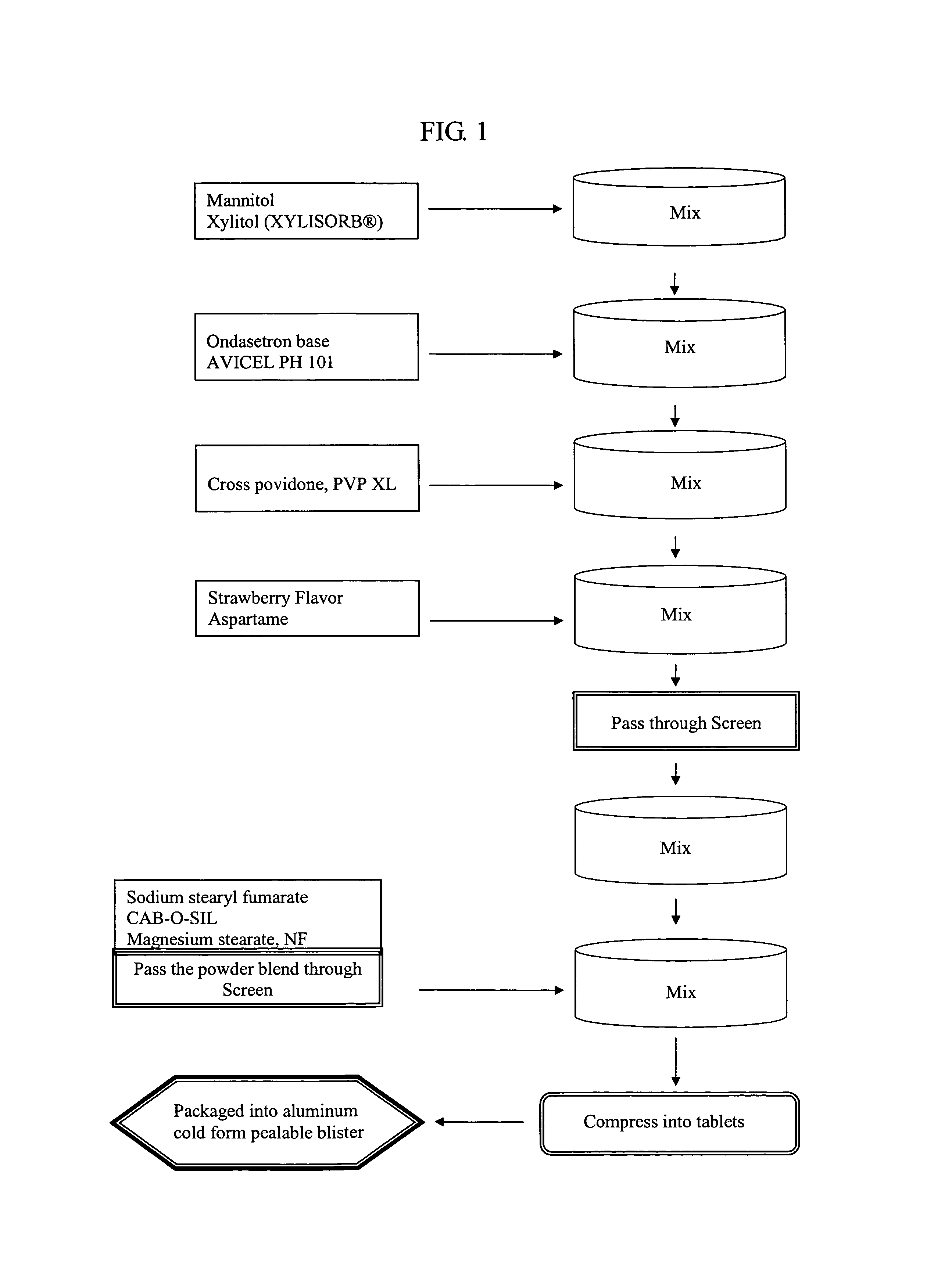
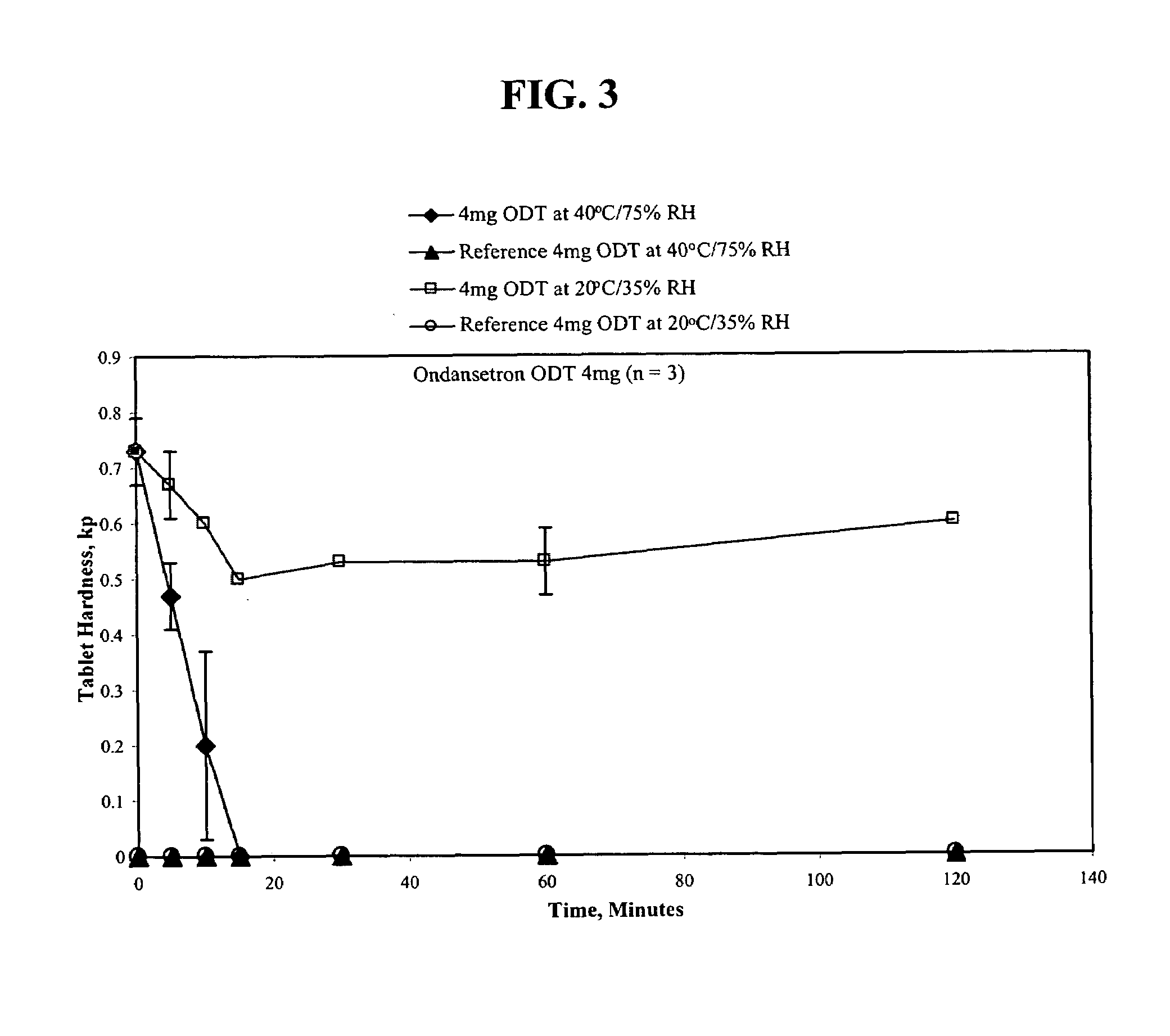
Ondansetron orally disintegrating tablets
InactiveUS7390503B1Safe and effective absorptionImprove bioavailabilityPowder deliveryPill deliveryWater dispersibleOrally disintegrating tablet
An ondansetron solid orally disintegrating dosage form for oral administration having at least one first water-dispersible component or water-insoluble cellulose derivative, a component having a —CHOH functional group, a disintegrating agent and at least one lubricant is provided. The dosage form can comprise ondansetron, a hydrophilic polymer such as microcrystalline cellulose, a component having a —CHOH functional group such as mannitol or xylitol and a disintegrating agent such as crospovidone. The lubricant may be a mixture of magnesium stearate, sodium stearyl fumarate and colloidal silicon dioxide. The present invention provides a non-effervescent tablet comprising the ondansetron dosage form. Another aspect of the invention is the treatment of emesis such as nausea and vomiting caused by cancer chemotherapy and radiation by the administration of the ondansetron formulation of the present composition. Finally, a process of forming an ondansetron disintegrating tablet using the ondansetron dosage form is disclosed.
Owner:BARR LAB
Ondansetron film compositions
The present invention relates to products and methods of making products having a taste masked active component. In particular, the present invention relates to taste-masked film dosage forms including at least one active component and a slow dissolving basic composition.
Owner:MONOSOL RX
Buccal, polar and non-polar spray containing ondansetron
InactiveUS20090162297A1Rapid onsetFast absorptionBiocideOrganic active ingredientsOndansetronSolvent
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide ondansetron for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, ondansetron, and optional flavoring agent; formulation II: aqueous polar solvent, ondansetron, optionally flavoring agent, and propellant; formulation III: non-polar solvent, ondansetron, and optional flavoring agent; formulation IV: non-polar solvent, ondansetron, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, ondansetron, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, ondansetron, optional flavoring agent, and propellant.
Owner:DUGGER III HARRY A +1
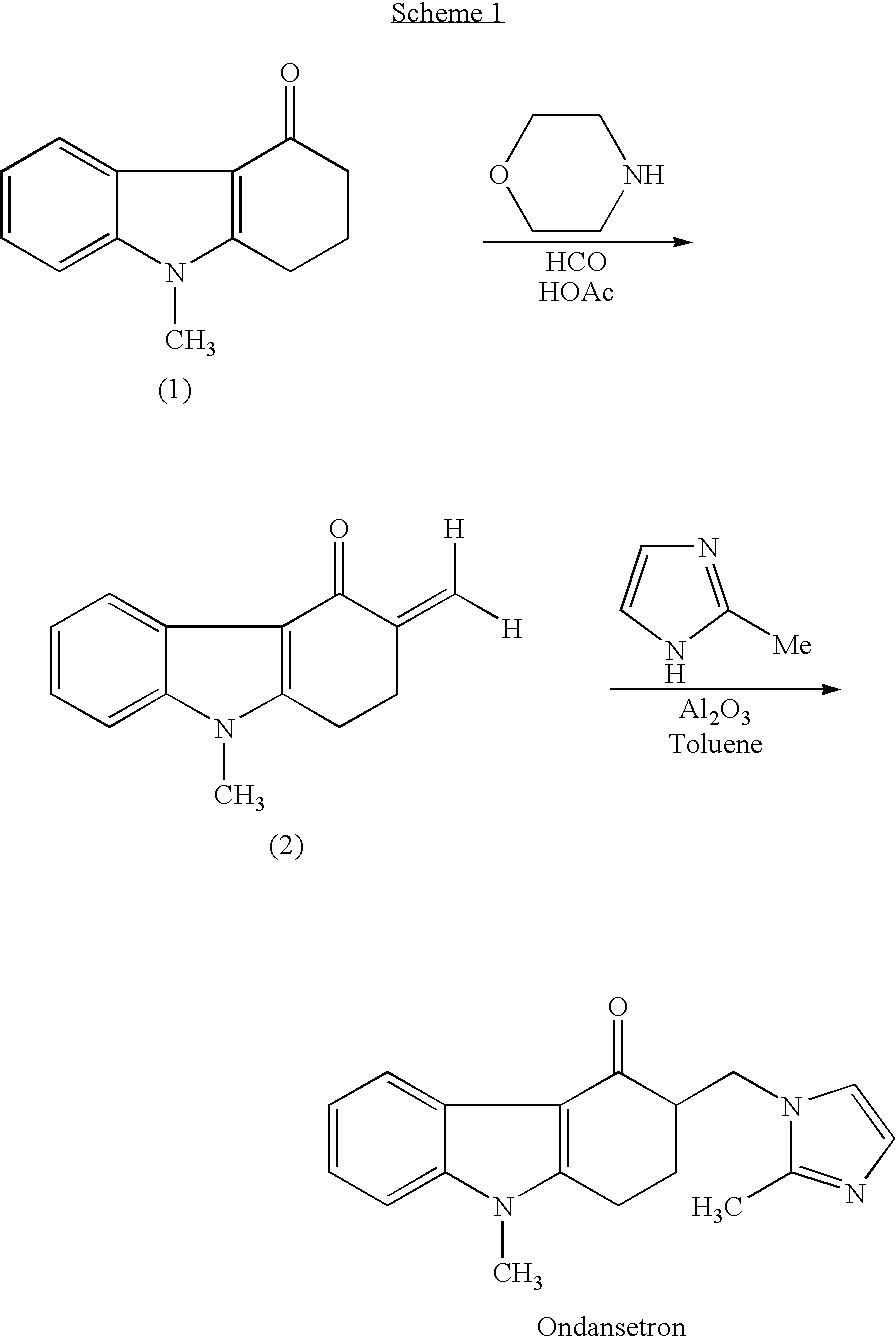
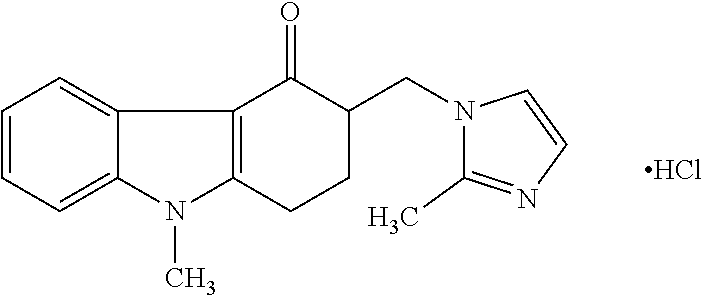
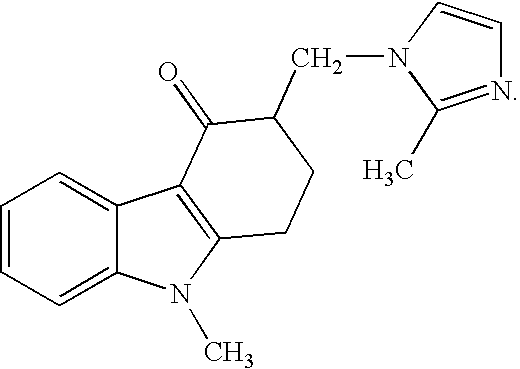
One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O
InactiveUS20080009635A1Improve reaction speedReduce yieldOrganic chemistryHydrocarbon solventsAcetic acid
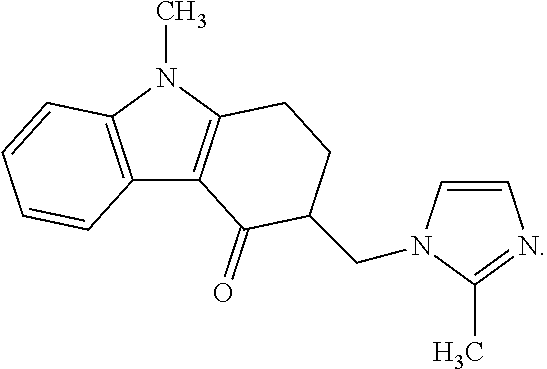
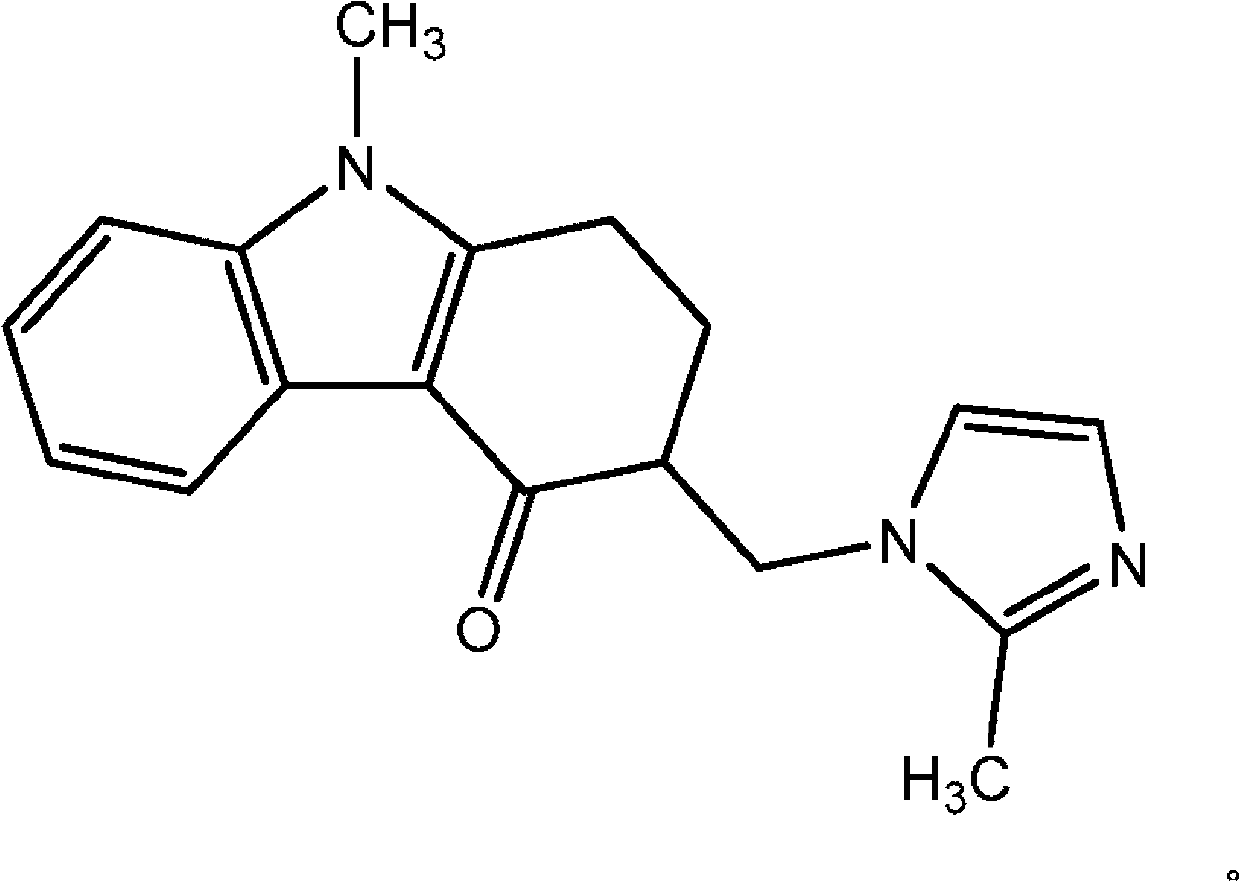
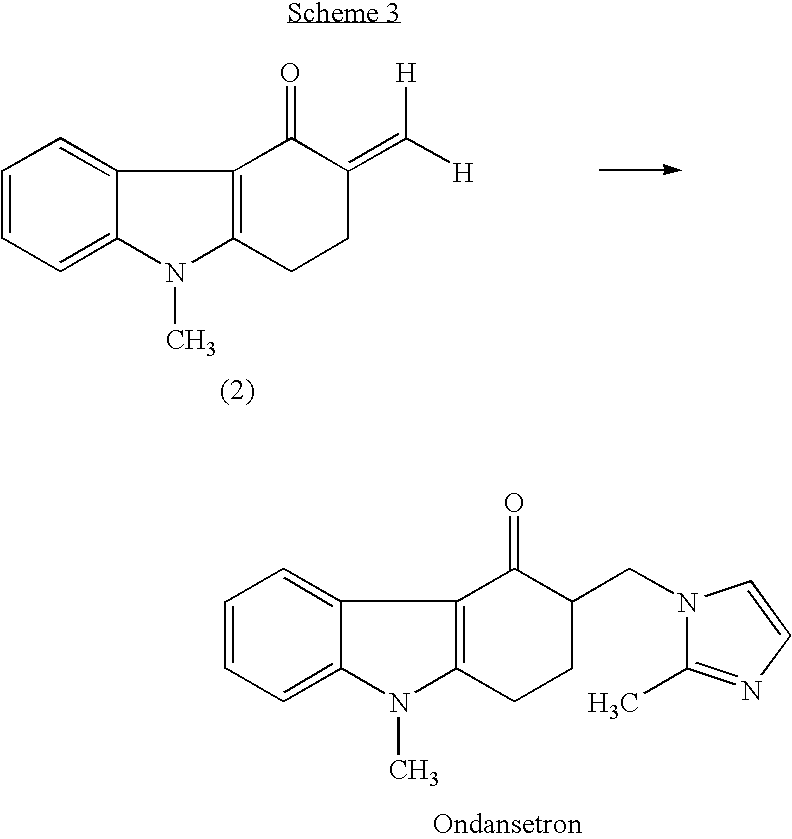
A one-pot industrial process for preparing 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazole-1-yl)methyl]-4H-carbazol-4-one of Formula-(I) from 1,2,3,9-tetrahydro-9-methyl-4H-carbazol-4-one of Formula-(IV) involves reaction of Formula (IV) with HNR1R2 salt and paraformaldehyde, where R1,R2 are independently alkyl groups or together forms a cyclic alkyl group, in a solvent system of acetic acid and hydrocarbon solvent to form a crude mixture of intermediate compounds of Formula (III) and (VIII), which is converted to ondansetron (Formula (I)) without isolation by reaction with 2methyimidazole in a suitable solvent system in the same pot.
Owner:IPCA LAB LTD
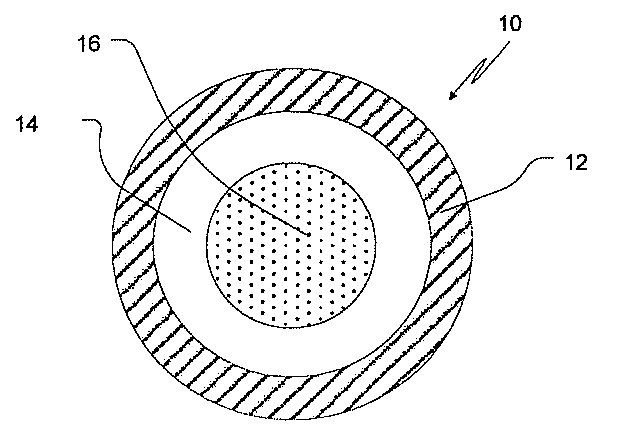
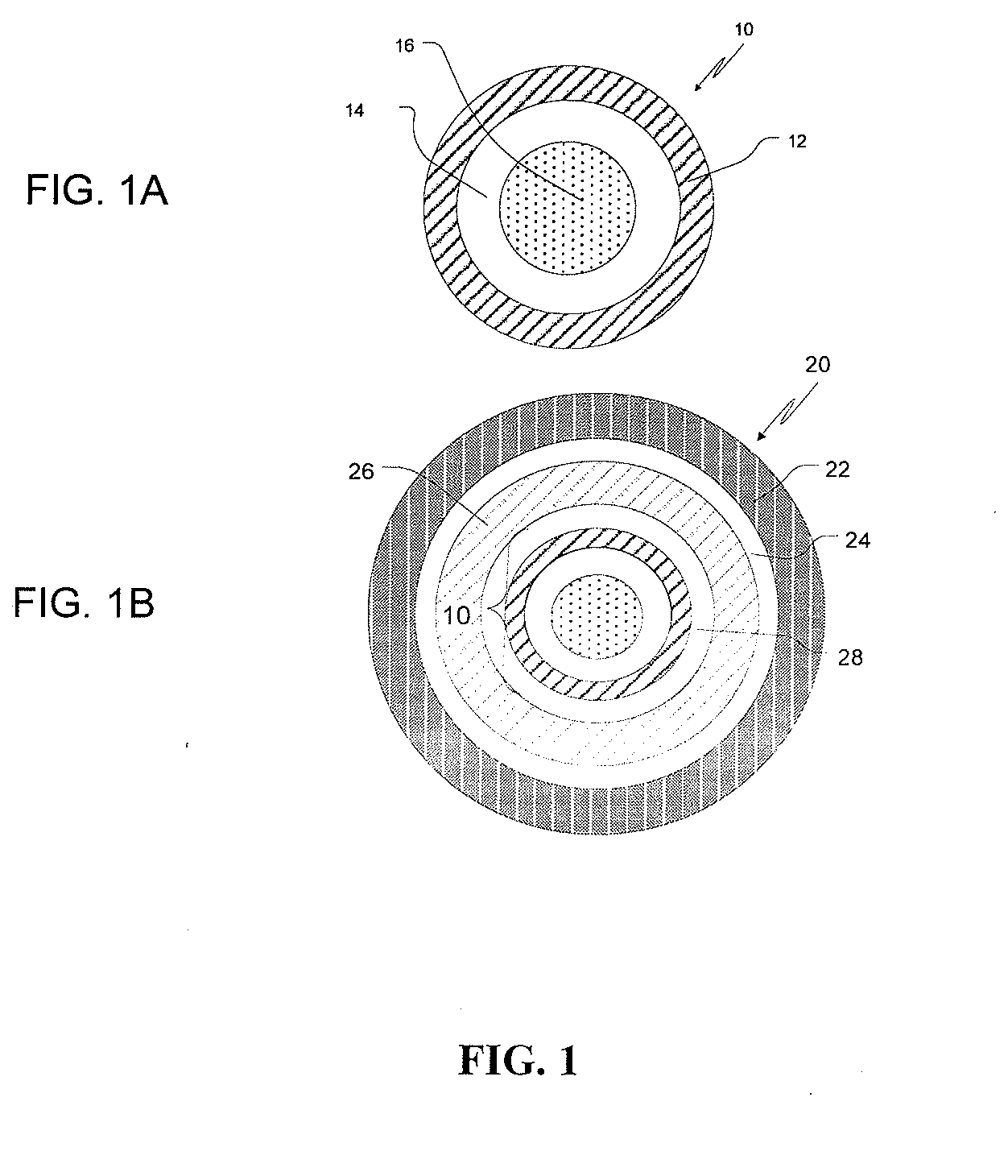
Methods of Treating PDNV and PONV with Extended Release Ondansetron Compositions
InactiveUS20110003005A1Organic active ingredientsBiocideOndansetronPostoperative nausea and vomiting
Extended release ondansetron compositions of the present invention are useful for treating postoperative nausea and vomiting (PONV) and / or postdischarge nausea and vomiting (PDNV).
Owner:APTALIS PHARMATECH
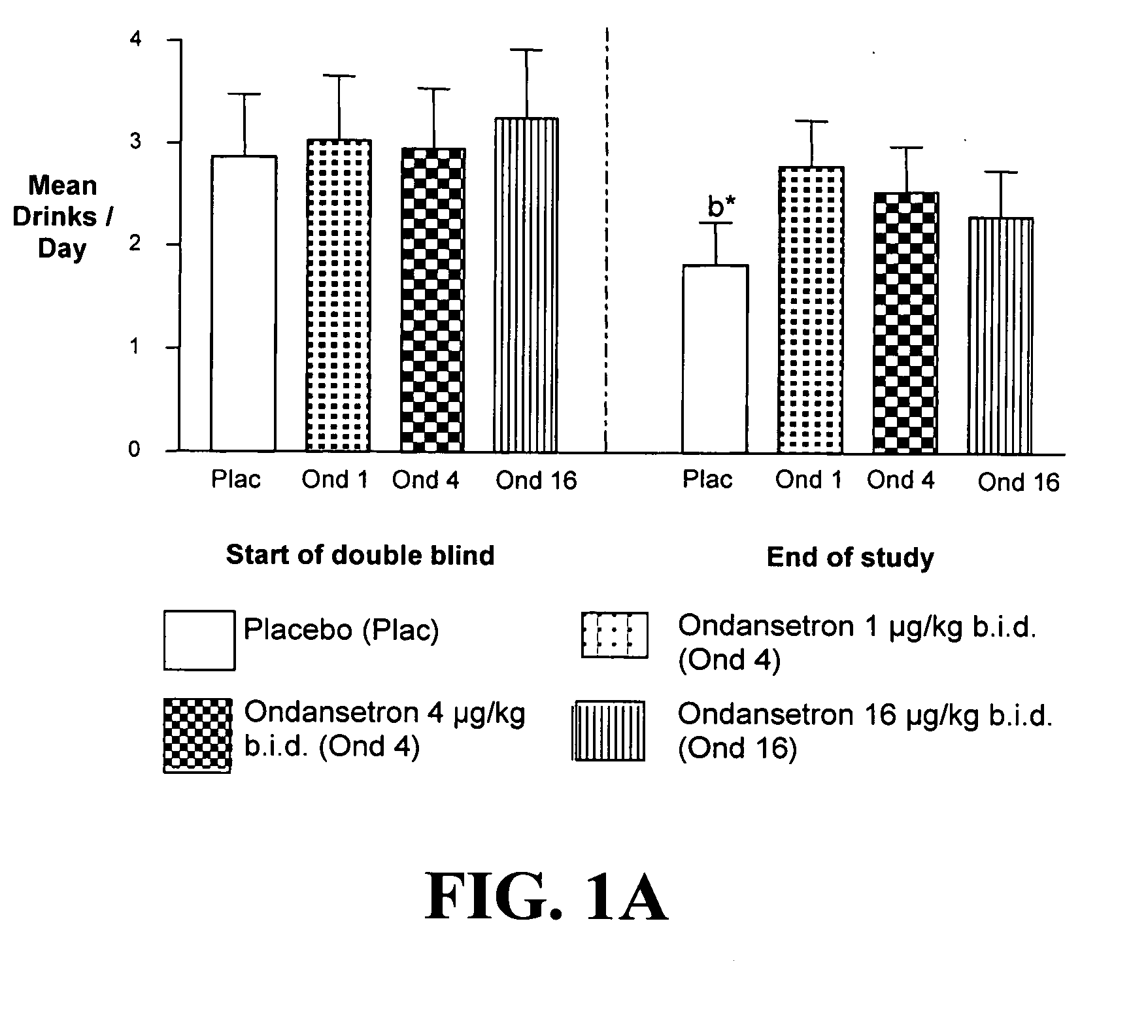
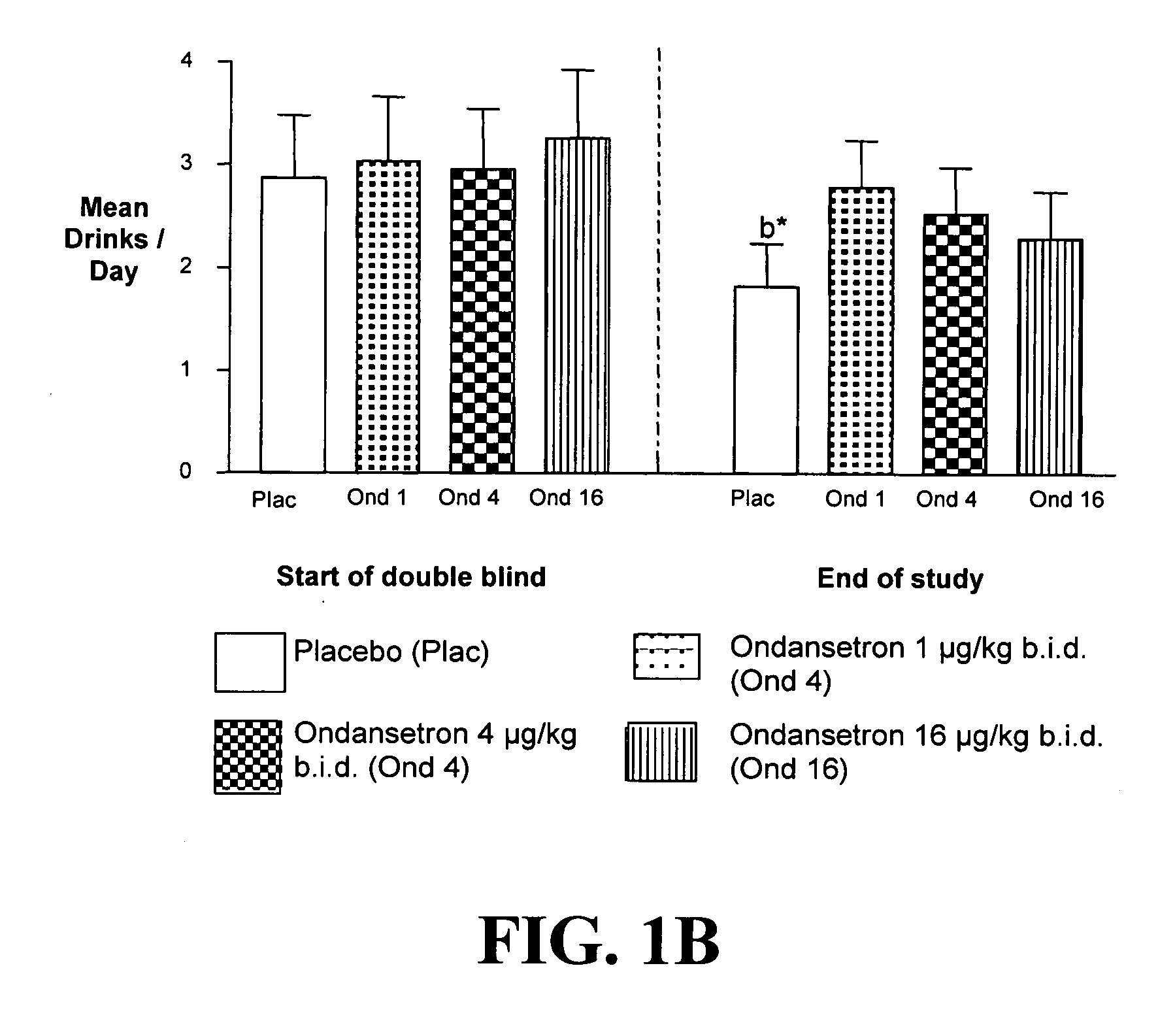
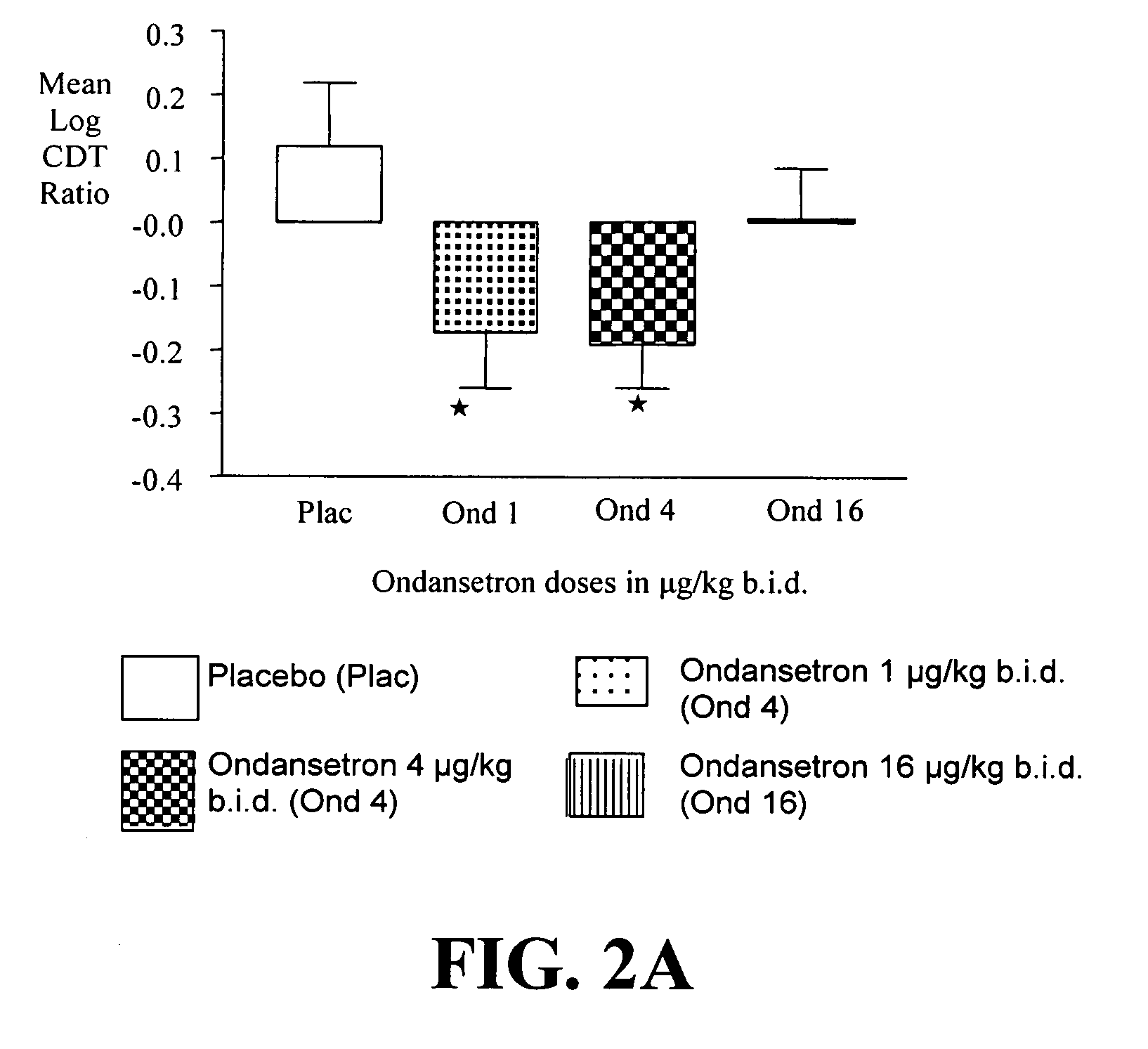
Combined Effects of Topiramate and Ondansetron on Alcohol Consumption
InactiveUS20100041689A1Avoid controlReduce impulseBiocideNervous disorderDiseaseBehavioral interventions
The present invention provides for the use of combinations of drugs to treat addictive disorders. More specifically, the present invention relates the use of drugs in conjunction with behavioral intervention to treat alcohol-related diseases and disorders as well as treatment of obesity and regulating weight.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
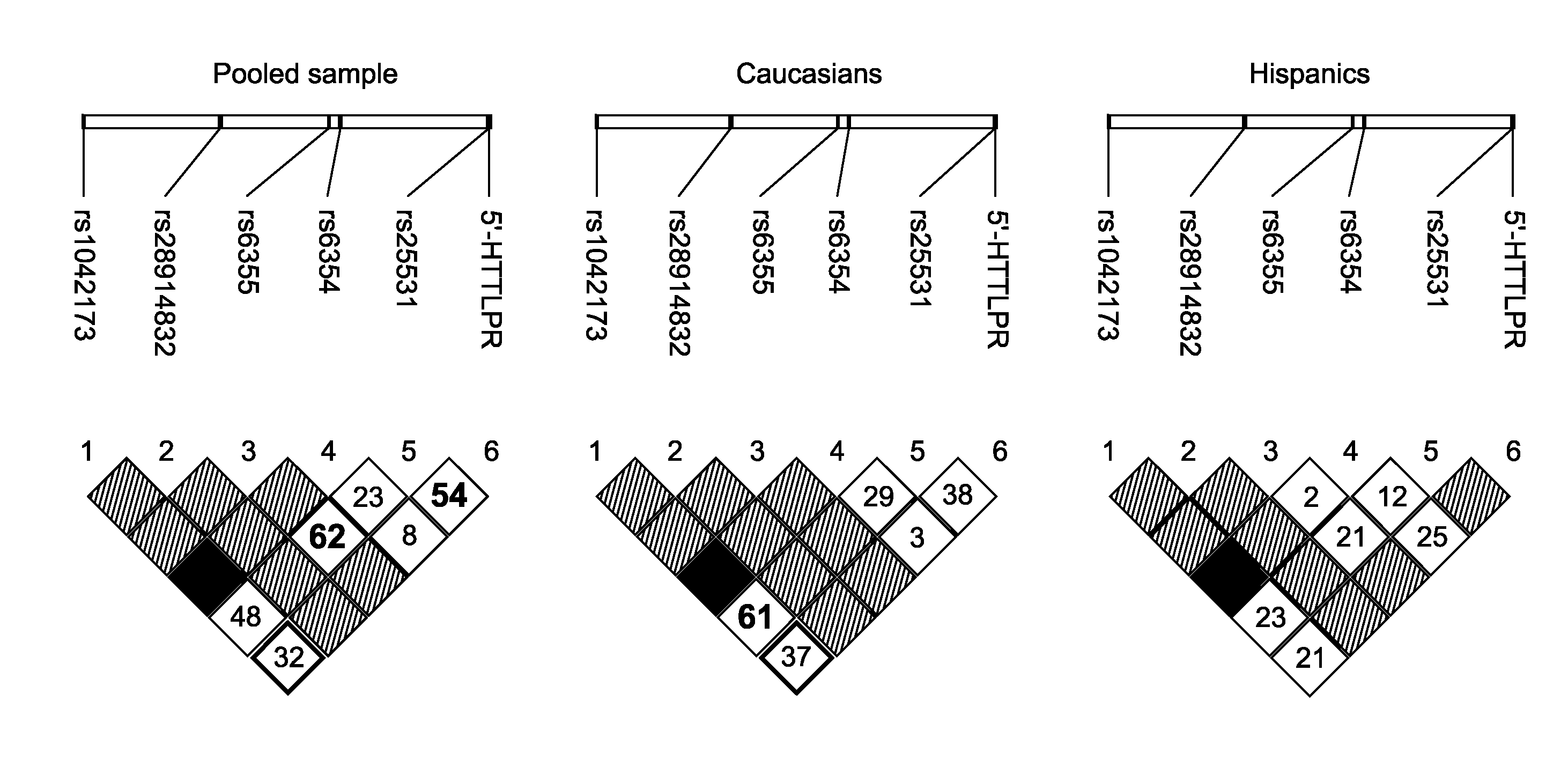
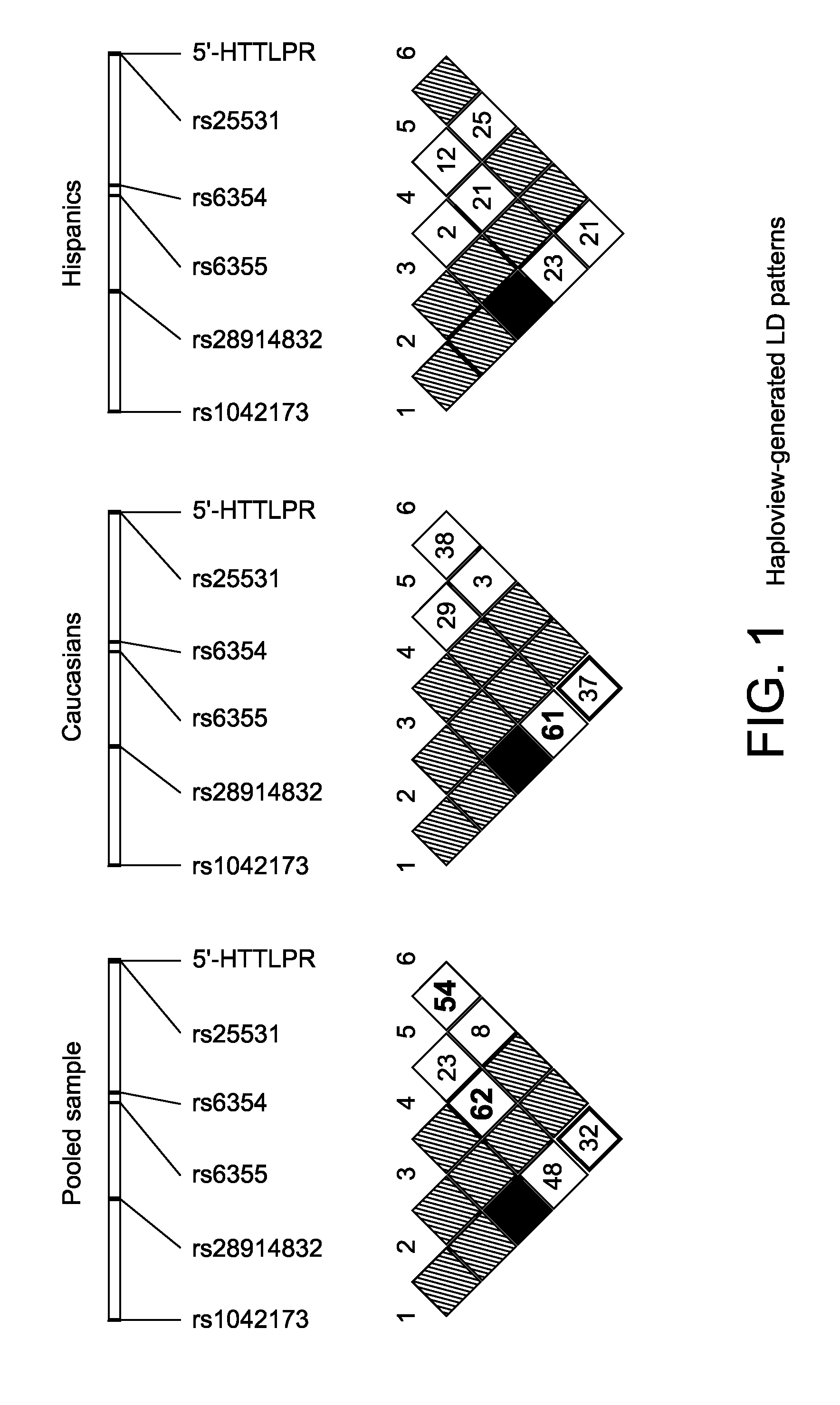
Serotonin transporter gene and treament of alcoholism
ActiveUS20110112159A1Physical improvementImproving psychological sequelaOrganic active ingredientsBiocideDiseaseAlcoholisms
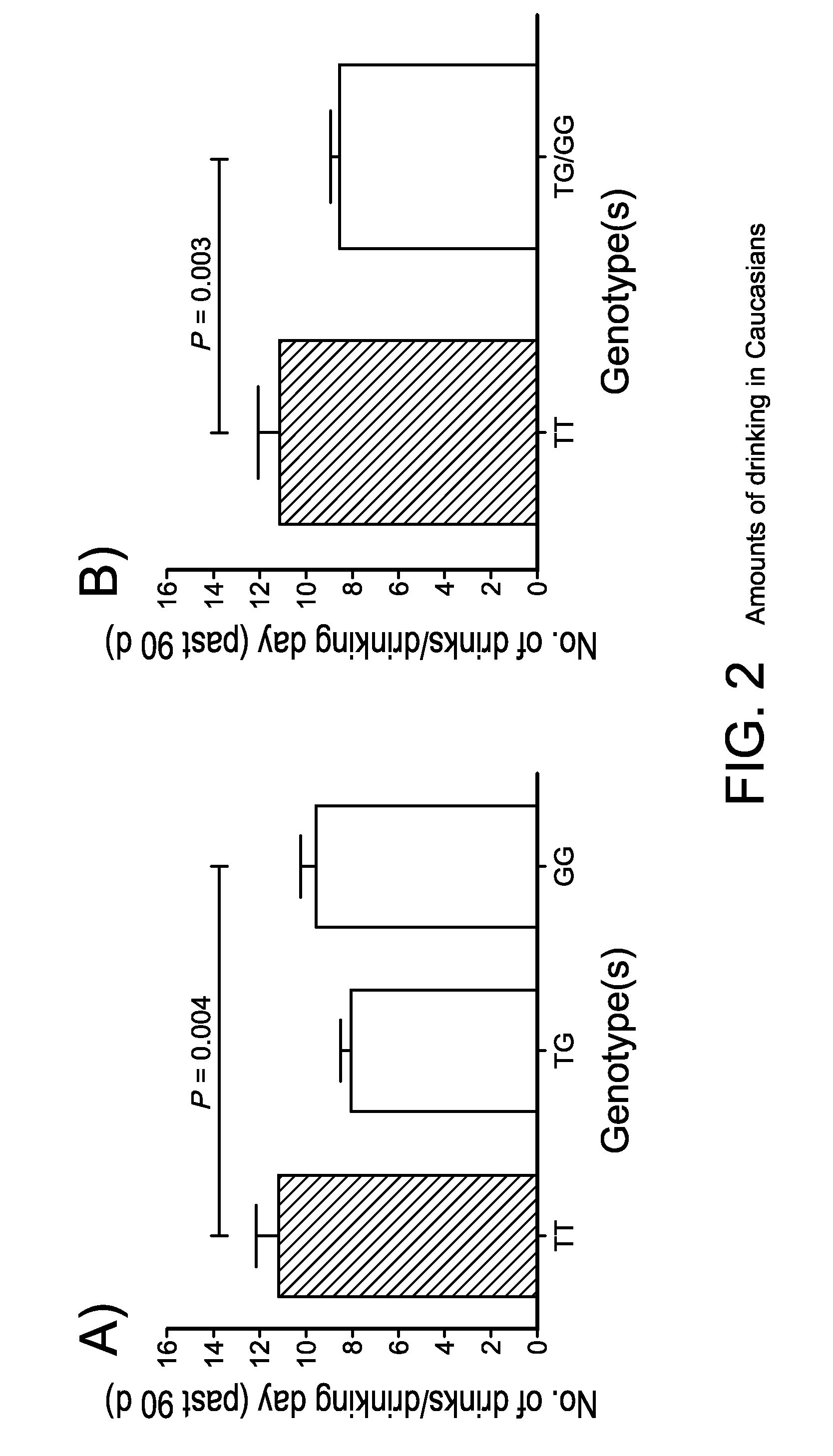
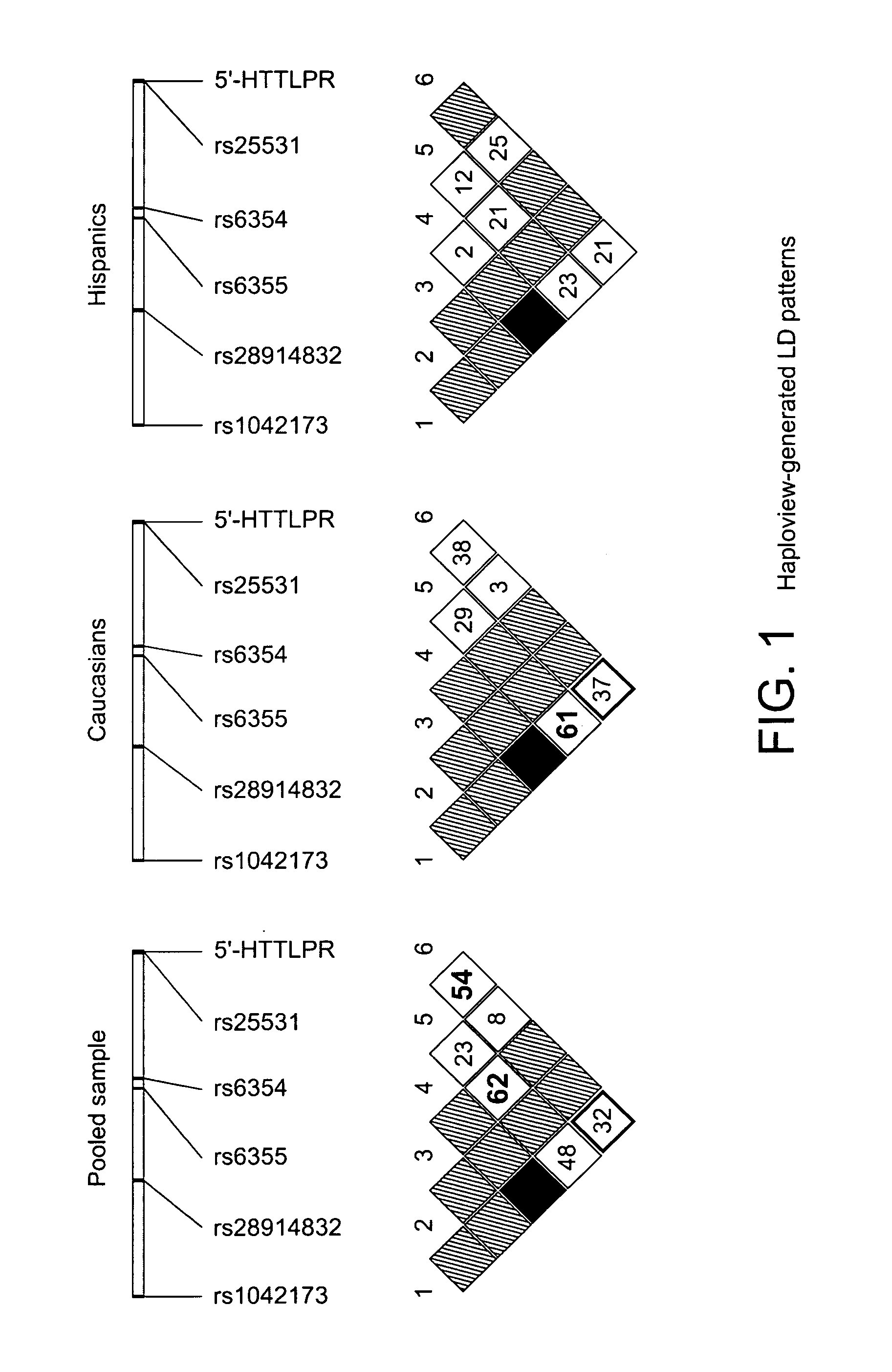
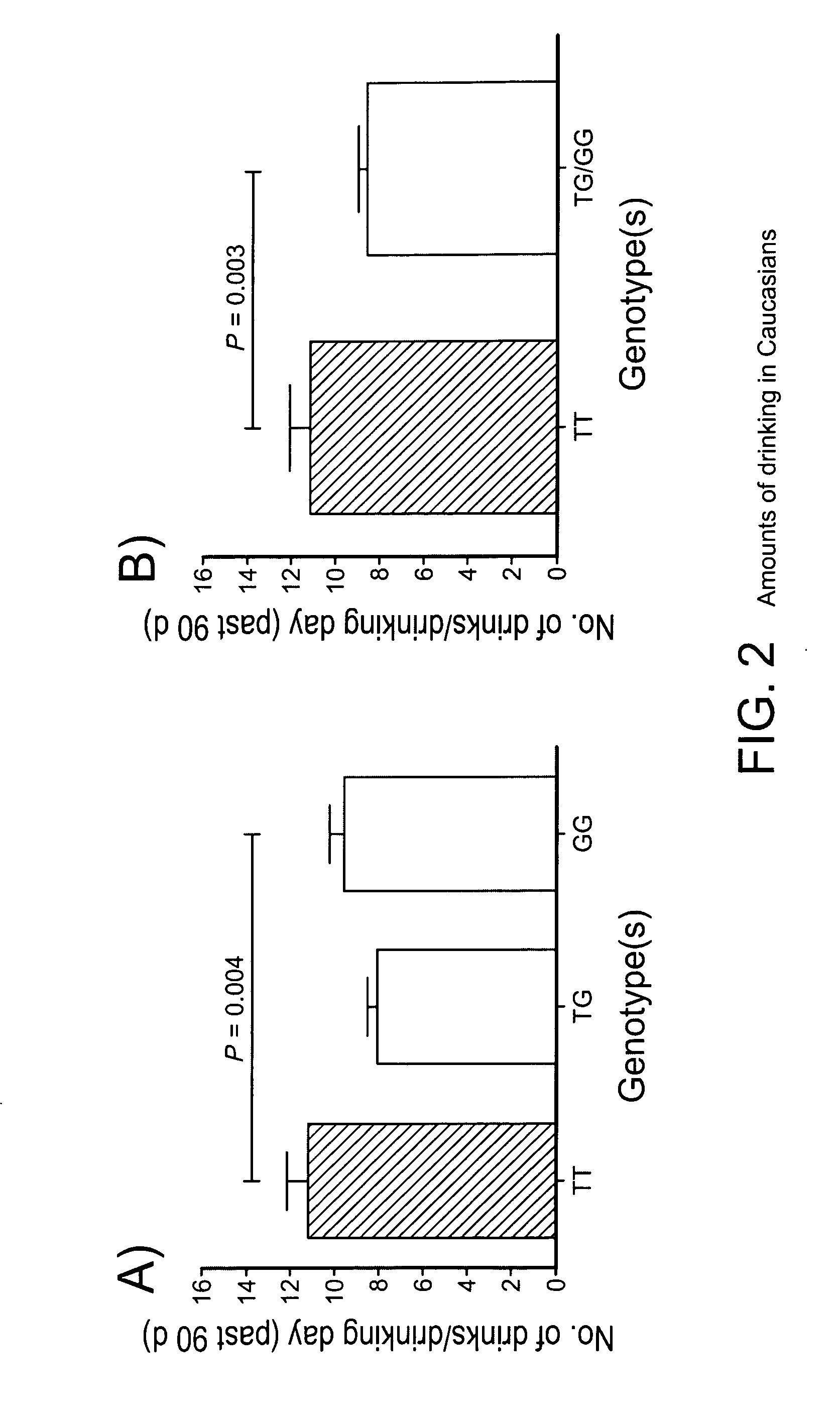
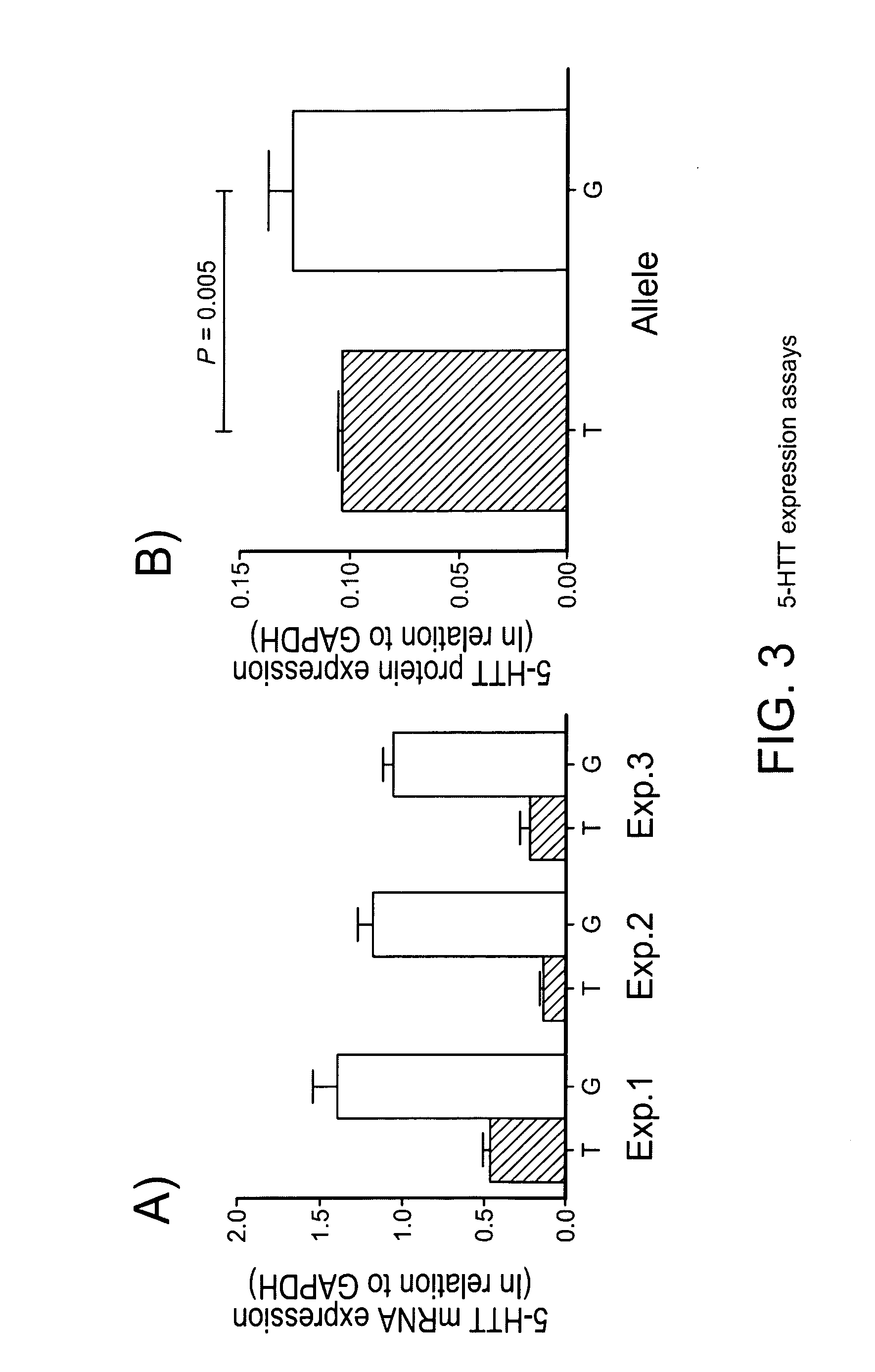
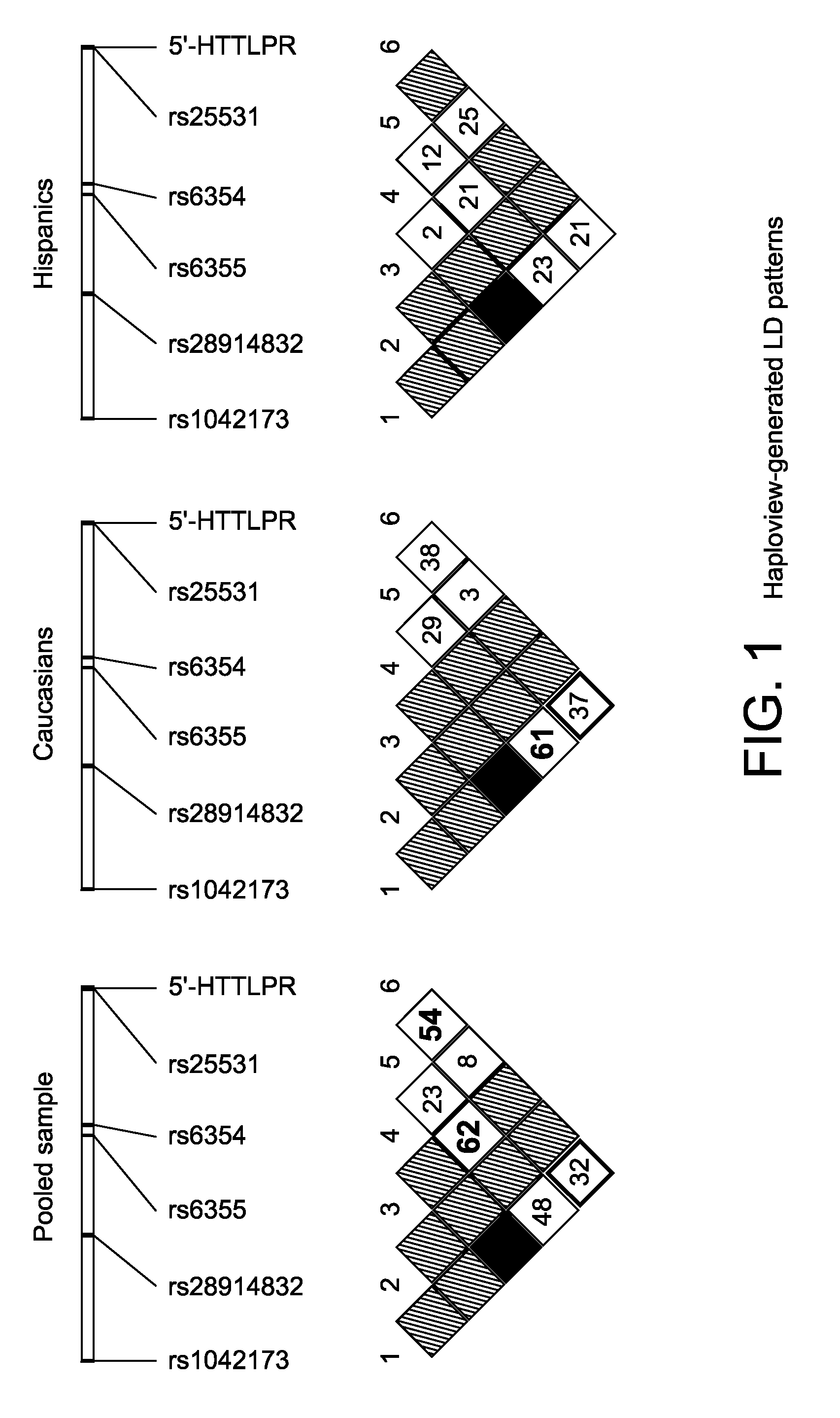
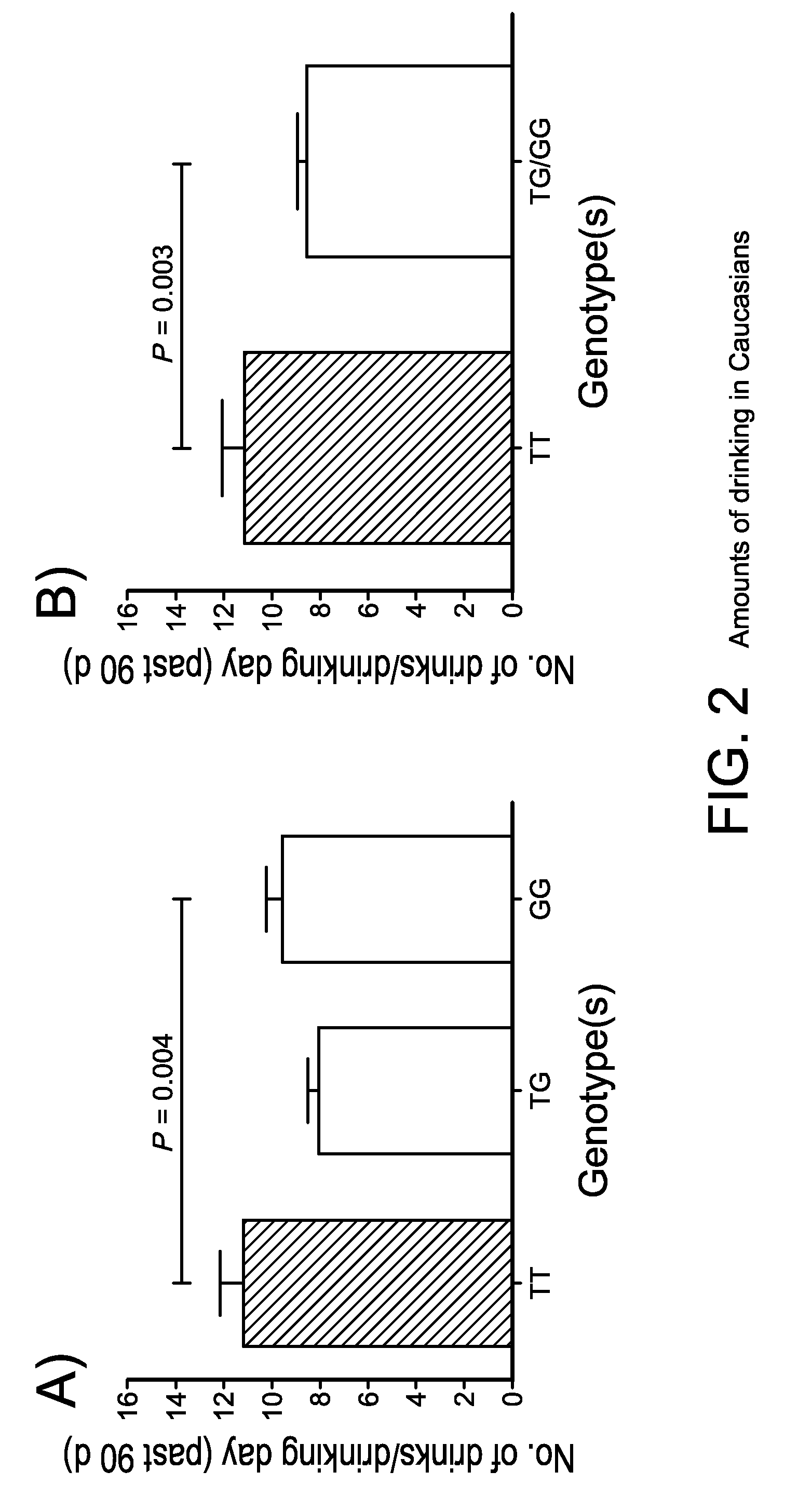
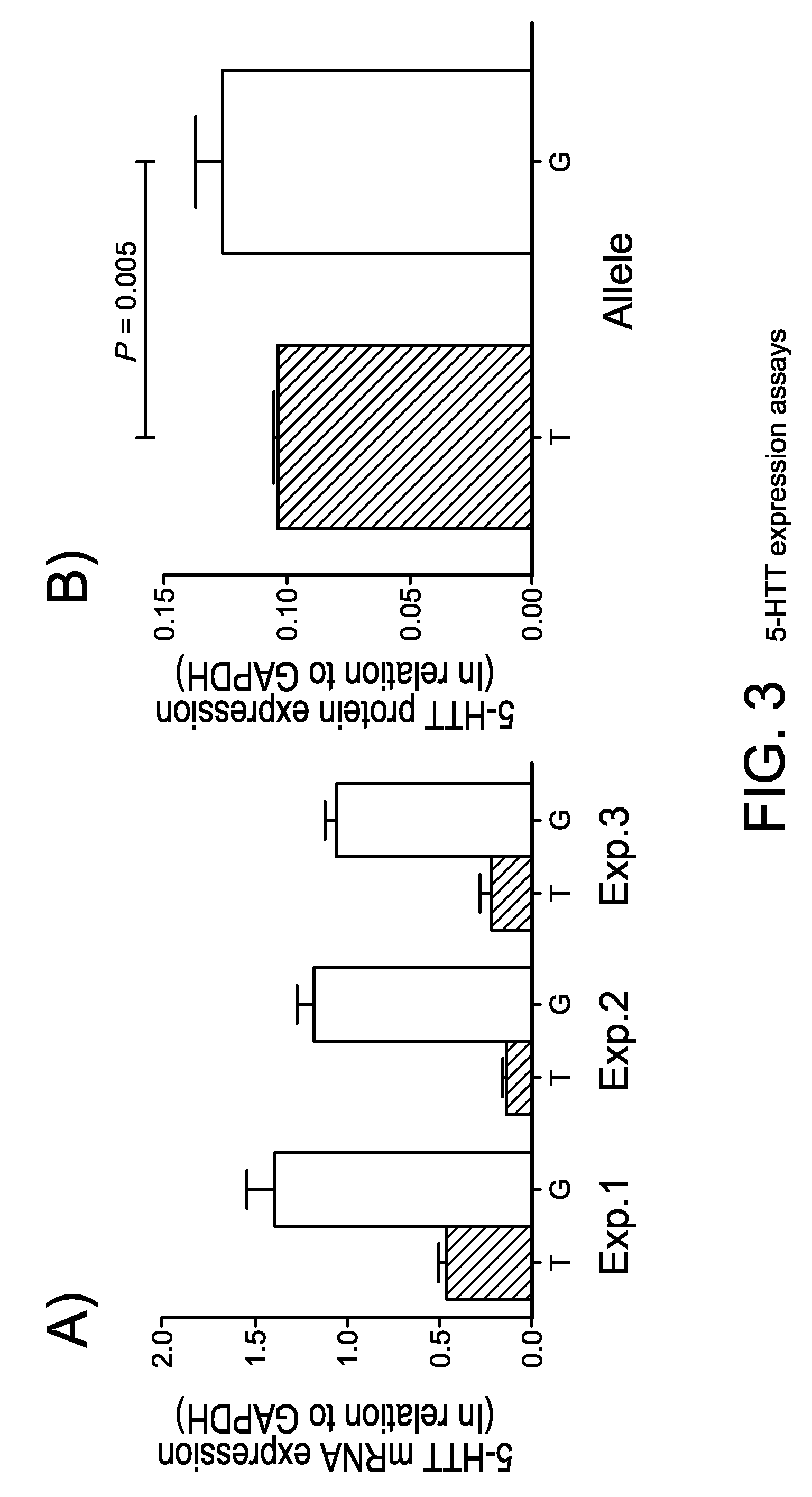
The gene responsible for encoding SERT has a functional polymorphism at the 5′-regulatory promoter region, which results in two forms, long (L) and short (S). The LL-genotype is hypothesized to play a key role in the early onset of alcohol use. The present invention discloses the differences in treatment and diagnosis based on the L or short genotypes as well as on a single nucleotide polymorphism of the SERT gene, the 3′ UTR SNP rs 1042173. The present invention demonstrates the efficacy of using the drug ondansetron and similar drugs for treatment based on variations in the polymorphisms of the SERT gene as well as methods for diagnosing susceptibility to abuse of alcohol and other addiction-related diseases and disorders.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
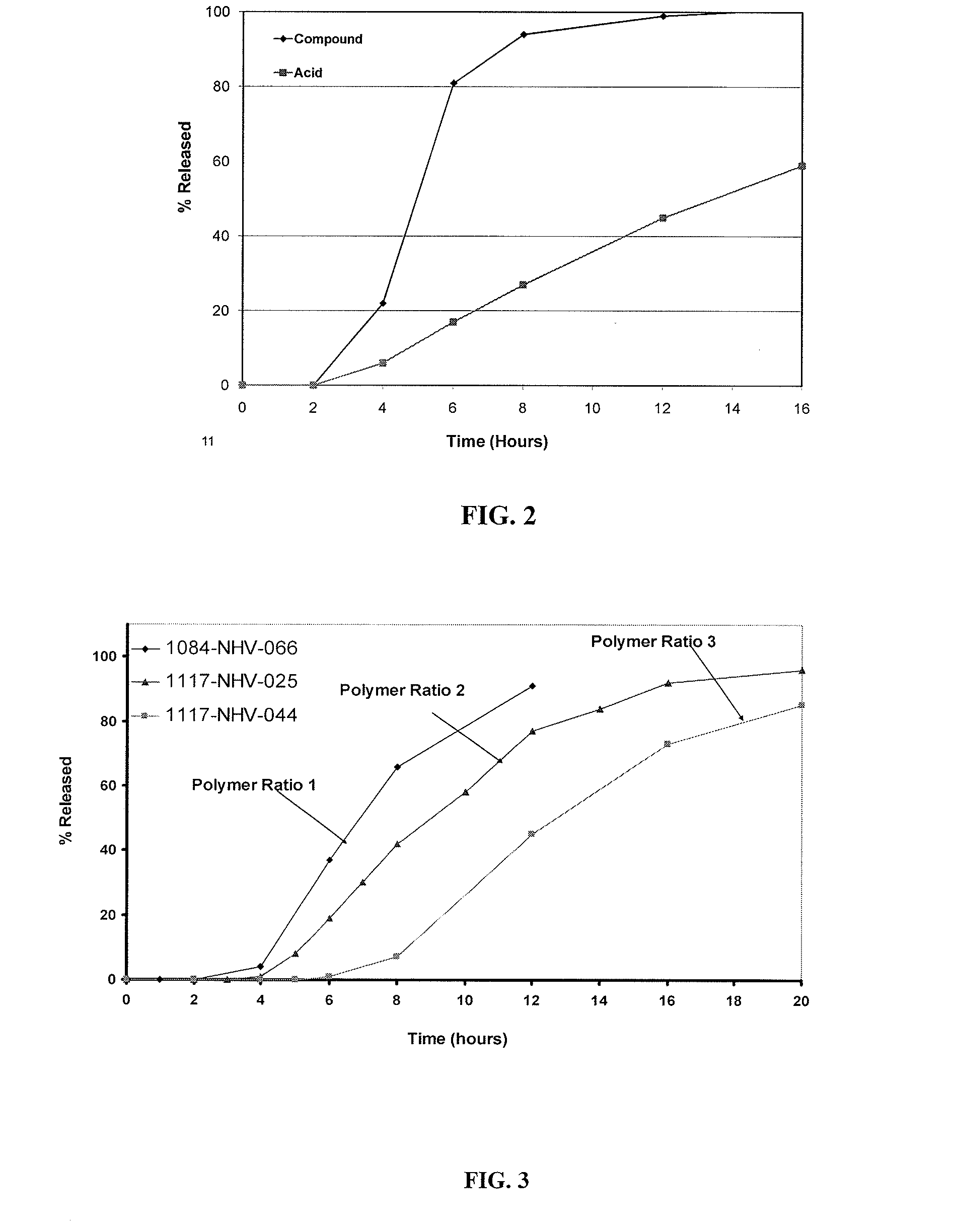
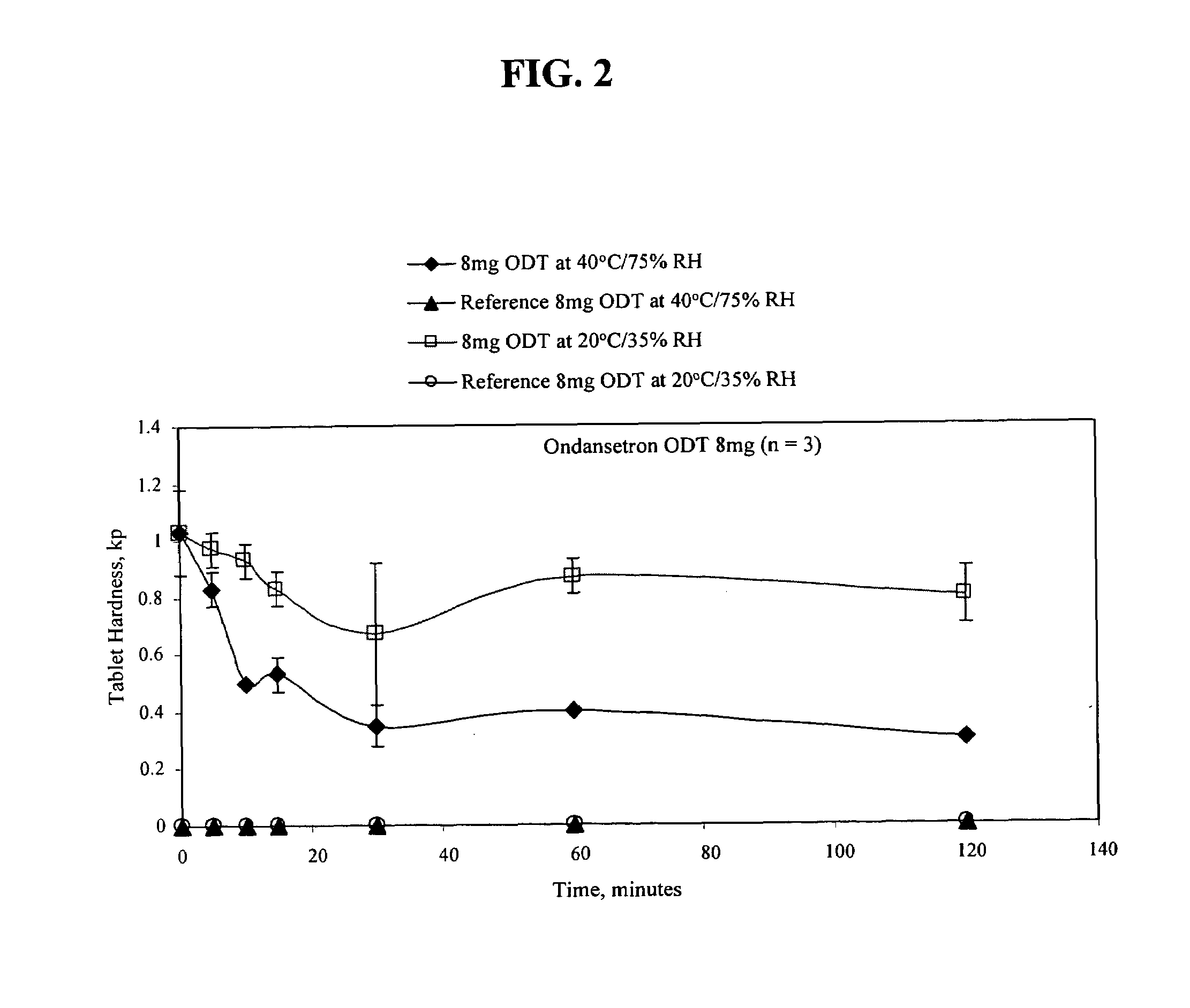
Ondansetron Orally Disintegrating Tablet Compositions for Prevention of Nausea and Vomiting
InactiveUS20110135724A1Disperse fastEasy to swallowOrganic active ingredientsBiocideSerotoninRegimen
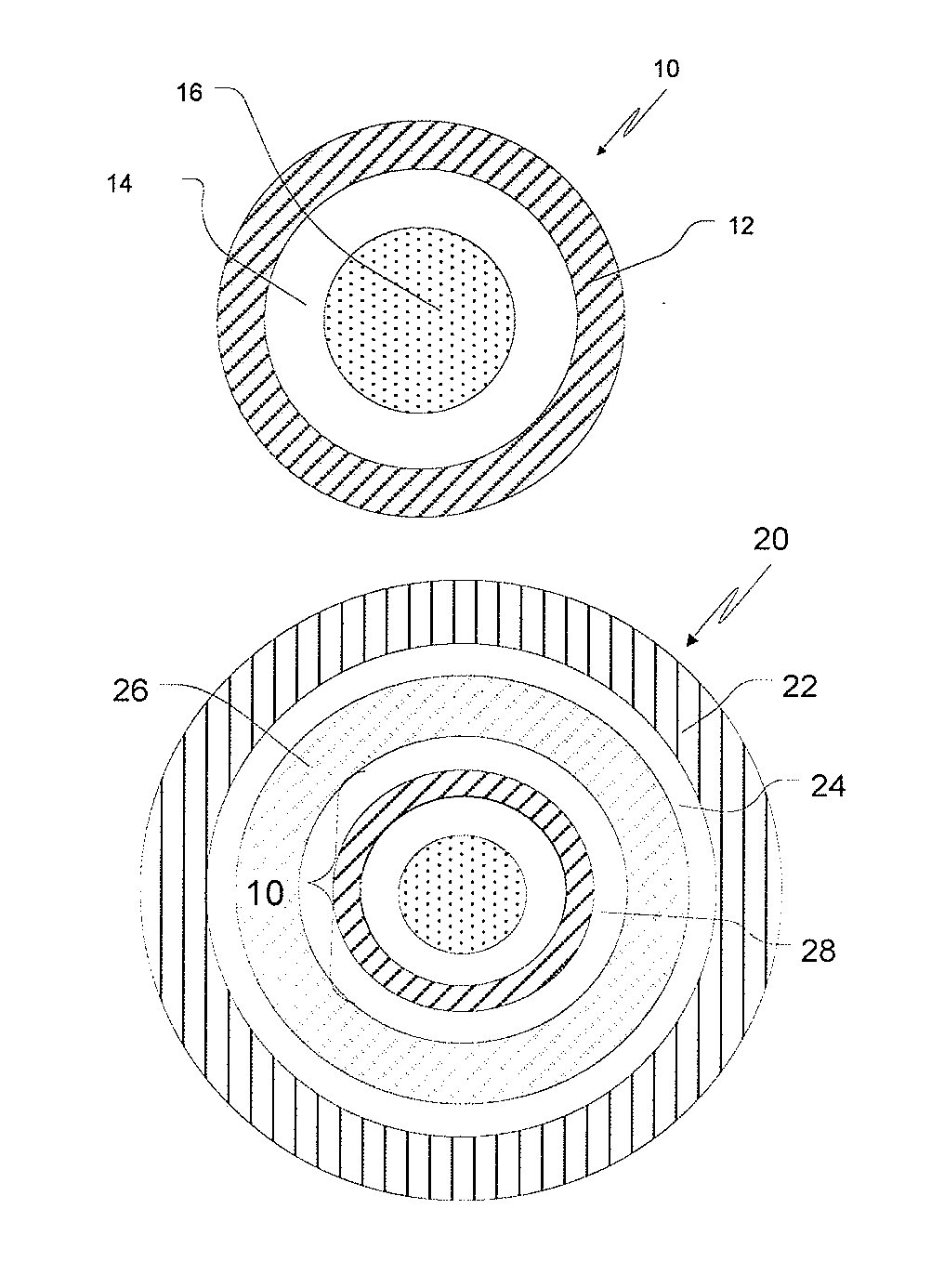
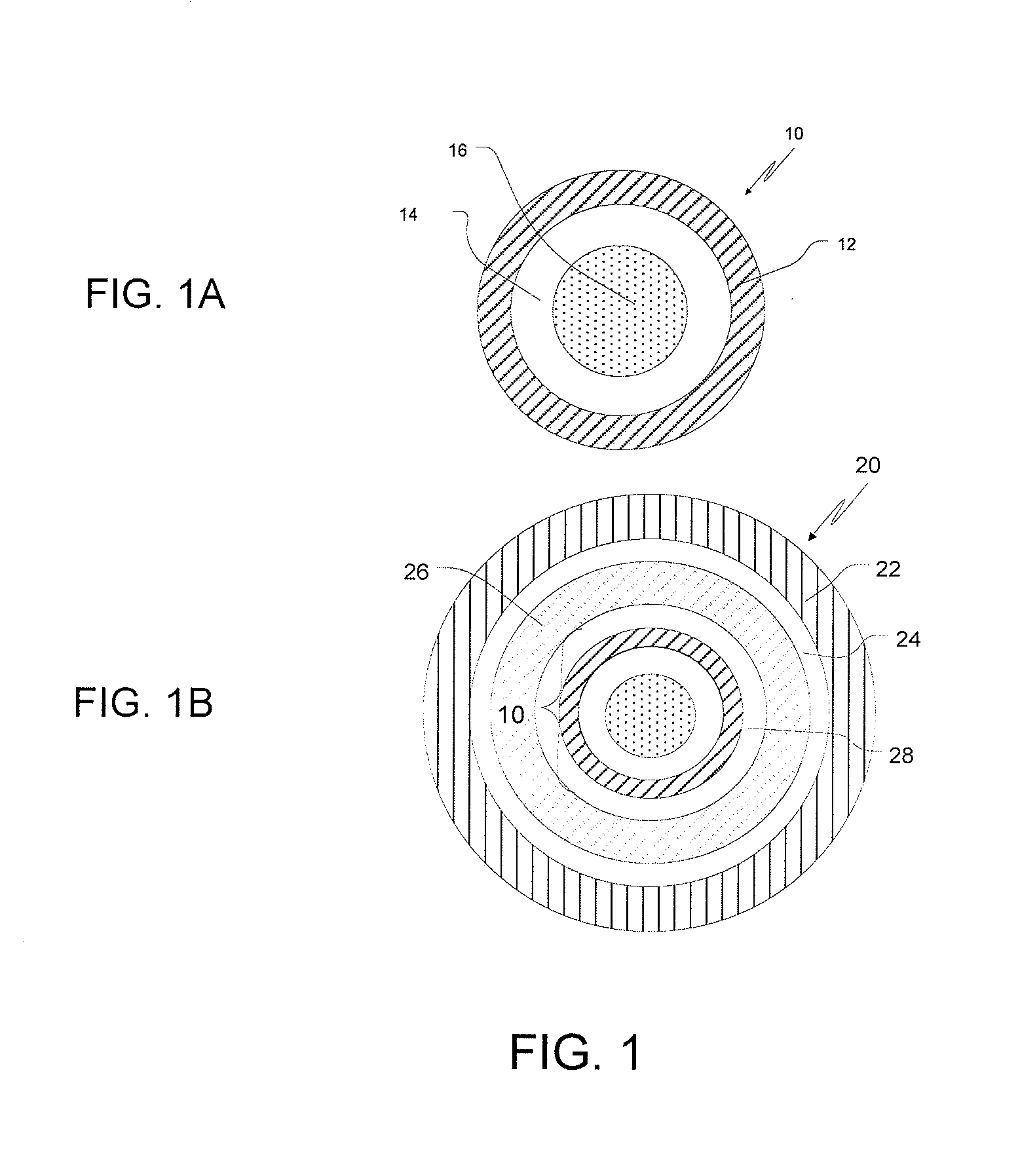
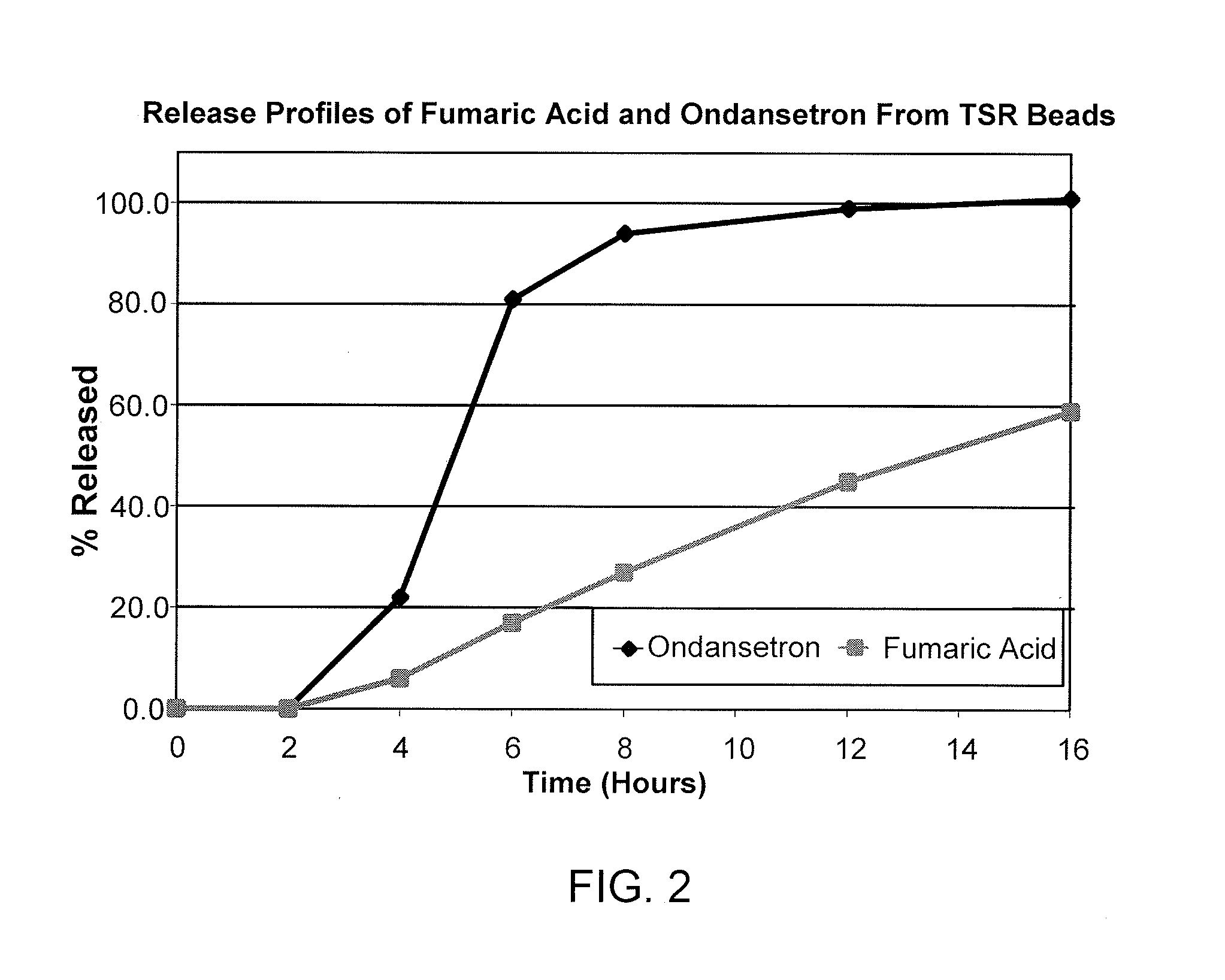
This invention is related to a pharmaceutical composition in the patient-friendly orally disintegrating tablet form comprising a weakly basic, selective serotonin 5-HT3 blocking agent for the prevention of nausea and / or vomiting for up to 24 hrs postdosing in cancer patients prior to undergoing moderately emetogenic chemotherapy or partial or whole body radiotherapy or in subjects at moderate to high risk of postoperative or postdischarge nausea and / or vomiting prior to inpatient or outpatient ambulatory surgery. The unit dosage form comprising a multitude of immediate-release drug particles providing dissolution profiles similar to that of reference drug product, and one or more timed, pulsatile-release bead populations, comprising at least one organic acid, which solubilizes said weakly basic selective serotonin 5-HT3 blocking agent prior to releasing it into the hostile intestinal environment, wherein the blocking agent is practically insoluble, is capable of delivering said antiemetic agent in patients in need thereof in a sustained-released fashion to be suitable for a once-daily dosing regimen.
Owner:APTALIS PHARMATECH
Nasal spray agent
The present invention is nasal spray of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron, osmotic pressure regulator and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Methods for treating apnea and apnea disorders using optically pure R(+) ondansetron
Methods for the treatment, management, or prevention of apnea and apnea disorders, or symptoms thereof, using a therapeutically effective amount of substantially optically pure R(+) ondansetron, or a pharmaceutically acceptable salt thereof, substantially free of its S(-) stereoisomer.
Owner:SEPACOR INC
Serotonin transporter gene and treatment of alcoholism
InactiveUS20120115149A1Physical improvementImproving psychological sequelaOrganic active ingredientsMicrobiological testing/measurementAlcoholismsNucleotide
The gene (SLC6A4) responsible for encoding the serotonin transporter (SERT) has a functional polymorphism at the 5′-regulatory promoter region, which results in two forms, long (L) and short (S). The LL-genotype is hypothesized to play a key role in the early onset of alcohol use. The present invention discloses the differences in treatment and diagnosis based on the L or short genotypes as well as on a single nucleotide polymorphism of the SERT gene, the 3′ UTR SNP rs1042173. The present invention demonstrates the efficacy of using the drug ondansetron and similar drugs for treatment based on diagnosing variations in the polymorphisms of the SERT gene and expression and activity of the SERT gene, as well as methods for diagnosing susceptibility to abuse of alcohol and other addiction-related diseases and disorders, for monitoring treatment and / or abuse (addictive behavior), and for determining which treatment should be used.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Orally administrable film dosage forms containing ondansetron
The invention relates to orally administrable, disintegrating film dosage forms which include ondansetron and methods of orally administering the film dosage forms.
Owner:MONOSOL RX
Mouth spray for preventing and treating nausea and emesis after tumor chemotherapy and radiotheraphy and preparation method thereof
InactiveCN101385712AEasy to increase or stop doseReduce efficacyAerosol deliveryPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention relates to an oral spray for controlling nausea and vomit of chemotherapy and radiation therapy, the formula of the oral spray is composed of ingredients with the following parts by weight: 5-50 parts of drug absorption enhancer, 2-20 parts of drug active ingredient and 30-90 parts of buffer, the drug absorption enhancer can be any one or the combination of more of the following ingredients: azone, propylene glycol, polysorbate (Tween), ethylene glycol deoxycholic acid sodium salt, brij, sodium decanoate, lauric acid, stearic acid, sodium lauryl sulphate, stearyl alcohol sodium sulfate, dioctyl succinate sodium sulfonate, oleic acid, GK2, menthol and borneol; the drug active ingredient can be any one combination of the following ingredients: palonosetron hydrochloride, granisetron, ondansetron, azasetron and tropisetron; and the ingredients of the buffer are sodium citrate buffer solution and phosphate buffer solution. The oral spray provides a formulation which is safer, painless and convenient for patients with advanced tumor, elderly and weak patients, children patients and the patients who suffer from the metal illness and do not obey the oral administration or the injection drug administration.
Owner:陆飚 +1
Serotonin transporter gene and treatment of alcoholism
ActiveUS8697361B2Long durationHigher paroxetine binding (density of SERT)Organic active ingredientsBiocideAlcoholismsNucleotide
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Ondansetron film compositions
The present invention relates to products and methods of making products having a taste masked active component. In particular, the present invention relates to taste-masked film dosage forms including at least one active component and a slow dissolving basic composition.
Owner:MONOSOL RX
Snuff
The present invention is snuff of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron; and medicine carrier selected from glucose, mannitol, lactose, sorbitol, microcrystalline cellulose and beta-cyclodextrin.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Orally administrable film dosage forms containing ondansetron
The invention relates to orally administrable, disintegrating film dosage forms which include ondansetron and methods of orally administering the film dosage forms.
Owner:MONOSOL RX
Transdermal composition of antivomiting agent and preparation containing the same
InactiveCN1364083AHydrocarbon active ingredientsHydroxy compound active ingredientsAlcoholOndansetron
A transdermal composition of the present invention comprises (a) a matrix containing (i) 20 to 80 % by weight of an alcohol, (ii) 1 to 50 % by weight of a skin penetration enhancer selected from the group consisting of a fatty acid and a derivative thereof, a fatty alcohol and a derivative thereof, an amide, a terpene, a surfactant and a mixture thereof, and (iii) 15 to 80 % by weight of water; and (b) 1 to 15 % by weight, based on the weight of the matrix, of an antivomiting agent selected from the group consisting of tropisetron, ondansetron, granisetron and pharmaceutically acceptable salts thereof, which is capable of delivering the antivomiting agent efficiently over a period of a day or more without skin irritation.
Owner:SAMYANG BIOPHARMLS CORP
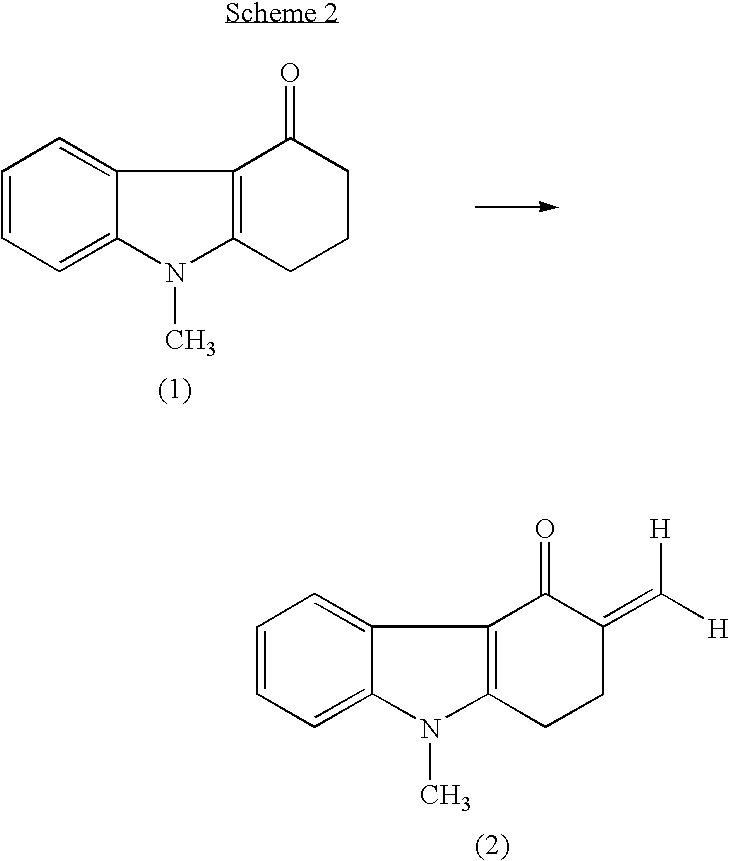
Process for preparing 1,2,3,9-tetrahydro-9-methyl-3-methylene-4H-carbazol-4-one and ondansetron therefrom
The present invention provides a rapid, high-yielding process for preparing 1,2,3,9-tetrahydro-9-methyl-3-methylene-4H-carbazol-4-one from 1,2,3,9-tetrahydro-9-methyl-4H-carbazol-4-one without using a secondary amine as a catalyst, and without using glacial acetic acid as a solvent. The present invention further provides a rapid, high-yielding process for preparing ondansetron from 1,2,3,9-tetrahydro-9-methyl-3-methylene-4H-carbazol-4-one without using alumina as a catalyst.
Owner:TARO PHARMA INDS
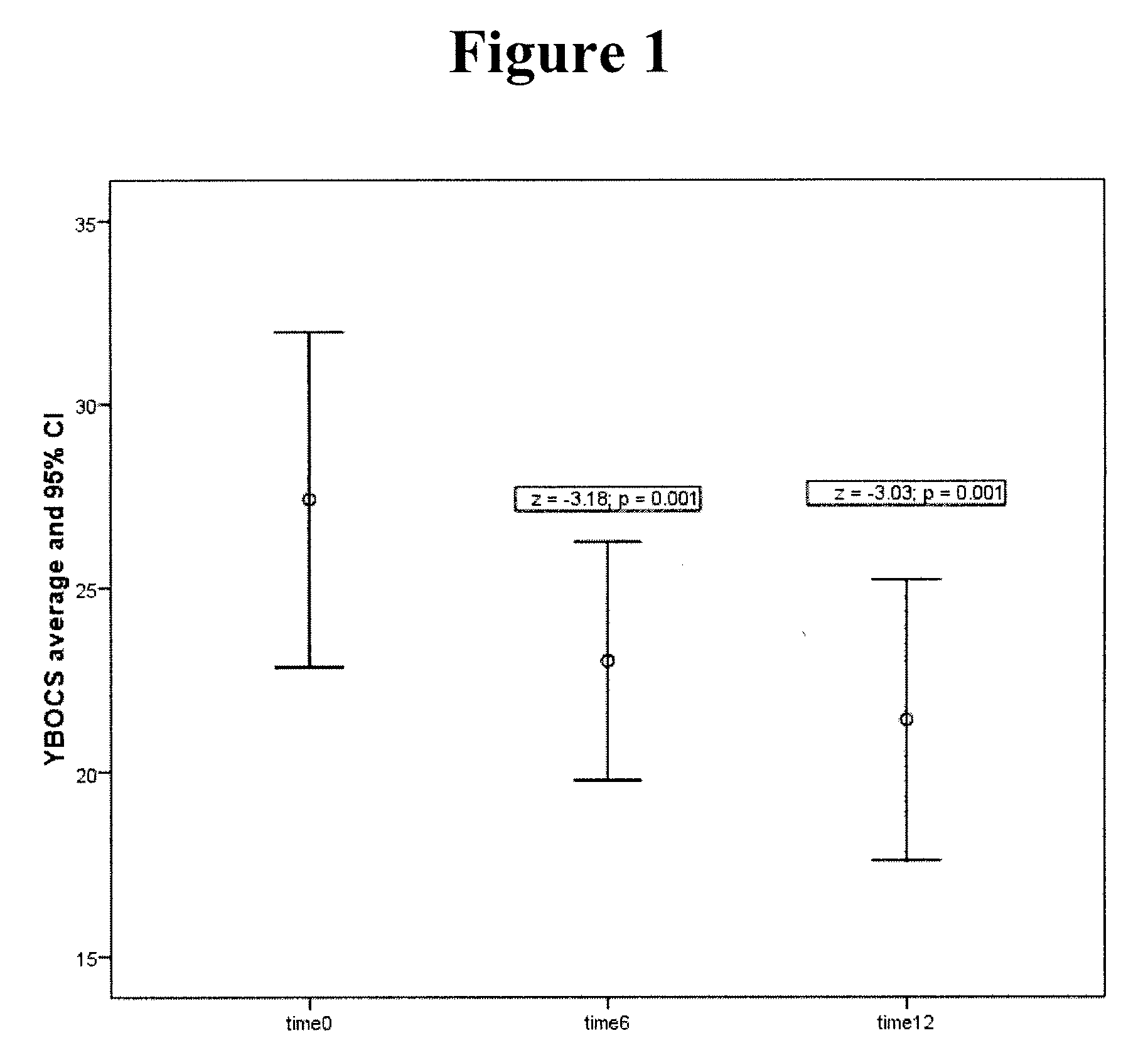
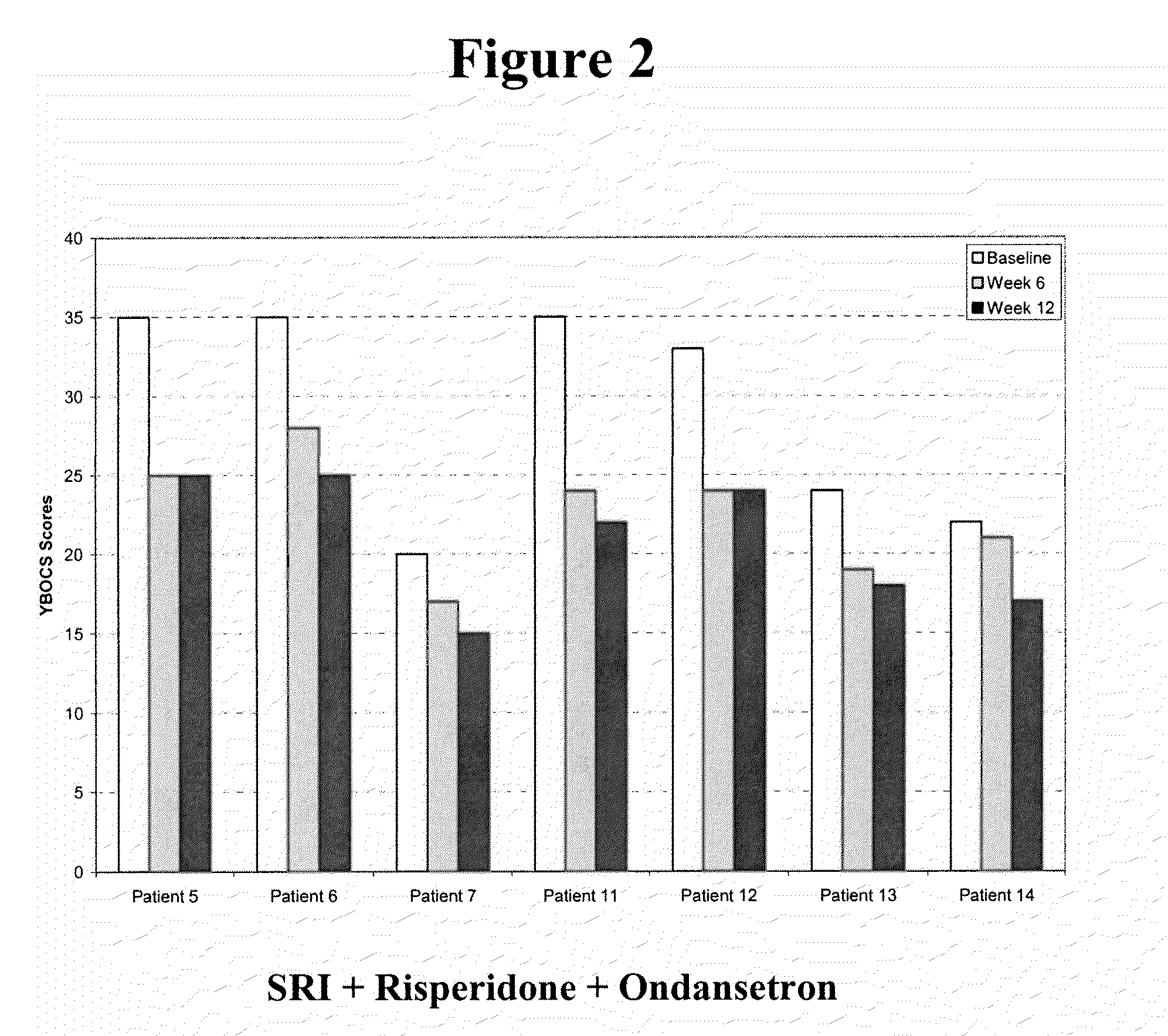
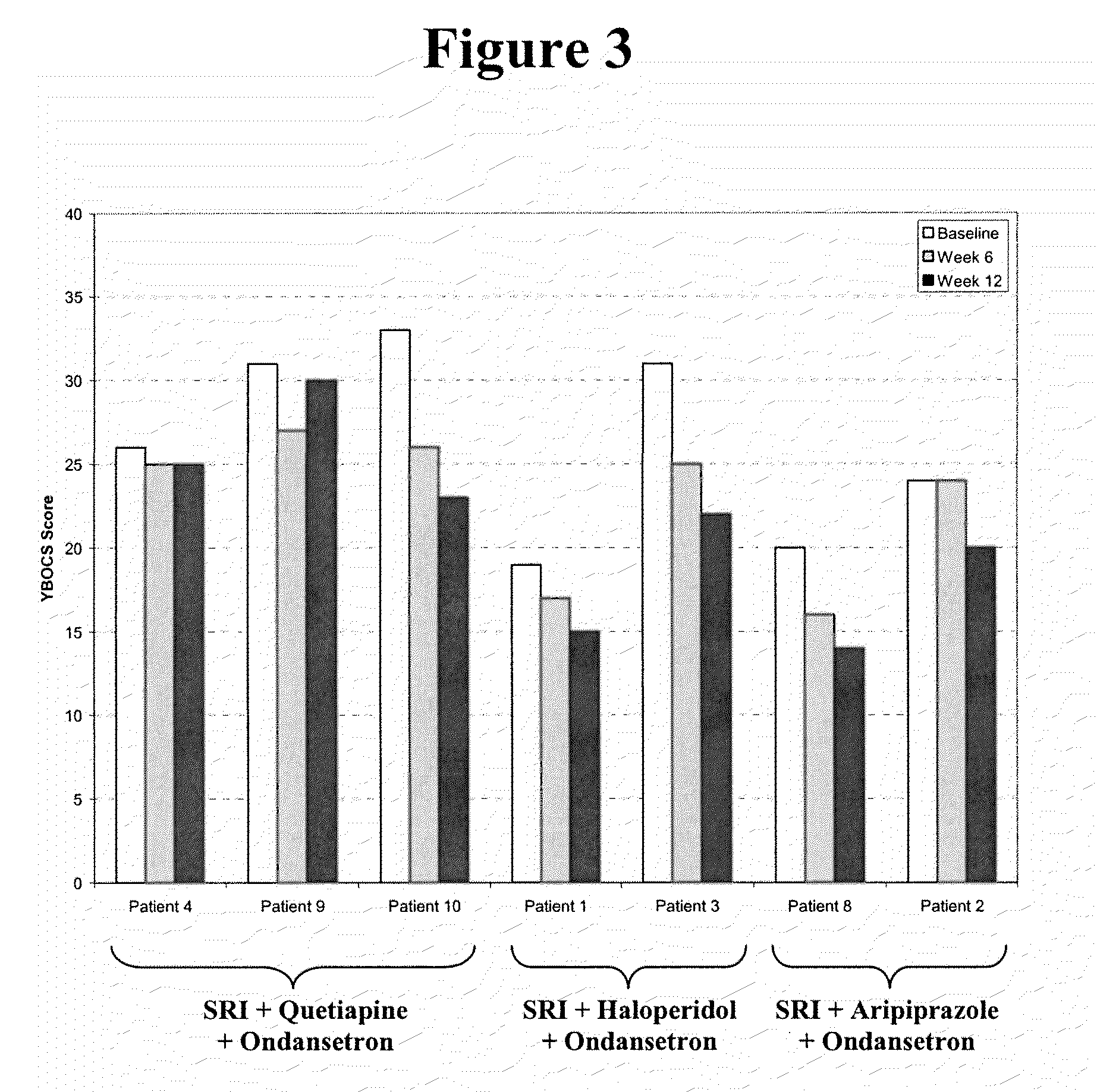
Method of treatment of obsessive compulsive disorder with ondansetron
InactiveUS20100298397A1Symptoms improvedBiocideOrganic active ingredientsObsessive compulsiveDepressant
Methods for treating obsessive compulsive disorder are described. In one method, a serotonin reuptake inhibitor (SRI) and ondansetron or a pharmaceutically acceptable salt thereof is administered to a patient suffering from obsessive compulsive disorder. The step of administering the SRI and the ondansetron is then repeated for more than seven days. In another method, an SRI, a neuroleptic, and ondansetron or a pharmaceutically acceptable salt thereof is administered to a patient suffering from obsessive compulsive disorder. The step of administering the SRI, the neuroleptic, and the ondansetron is then repeated for more than seven days. In another method, ondansetron or a pharmaceutically acceptable salt thereof is administered to a patient suffering from obsessive compulsive disorder for more than seven days. The ondansetron or pharmaceutically acceptable salt thereof may be administered as a pharmaceutically effective dose up to about 1.5 mg (free-base equivalent).
Owner:TRANSCEPT PHARMA
Medicament for treating nausea emesis caused by radiotherapy and chemotherapy
InactiveCN101214368ASo slackReduce irritation damageDigestive systemPlant ingredientsDiseaseSide effect
The present invention relates to a medicine for curing nausea and vomit caused by radiotherapy and chemotherapy, including 10 to 90 portions of clematis root and 10 to 90 portions of ginger. The medicine provided by the present invention can effectively cure and prevent vomit caused by radiotherapy and chemotherapy and intractable naupathia and vomit caused by various reasons. Compared with medicines for curing vomit such as ondansetron, the present invention with quick effect, high curing effect and more than 95 percent curing rate has obvious curing effect on various acute and slow vomit, which can increase the appetite of patients, strengthen body resistance and improve disease conditions. In addition, the present invention has small side effect, convenient use and low curing expense.
Owner:张旭梅
Ondansetron oral cavity instant membrane capable of sheltering taste and preparation method thereof
InactiveCN102860997AGreat tasteFast releaseOrganic active ingredientsDigestive systemPharmacyEconomic benefits
The invention provides an ondansetron oral cavity instant membrane capable of sheltering bad medicine taste and a preparation method thereof. The oral cavity instant membrane comprises active pharmaceutical ingredients for sheltering the bad taste and auxiliary materials suitable for pharmacy, the active pharmaceutical ingredients include ondansetron or salts acceptable in the pharmacy, and the auxiliary materials suitable for the pharmacy comprise a membrane-forming material, a sweetening agent and a filling agent. The oral cavity instant membrane has the advantages that the taste is good, the oral cavity instant membrane can be rapidly dissolved in the oral cavity without water, the bioavailability is high, the usage is convenient, the processing technology is simple, convenient and practical, the related auxiliary materials suitable for the pharmacy are easy to purchase, and good social and economic benefits are achieved.
Owner:天津市聚星康华医药科技有限公司
Fast Acting Orally Disintegrating Film
ActiveUS20170056374A1Act quicklyOrganic active ingredientsDigestive systemOral medication5-HT3 receptor
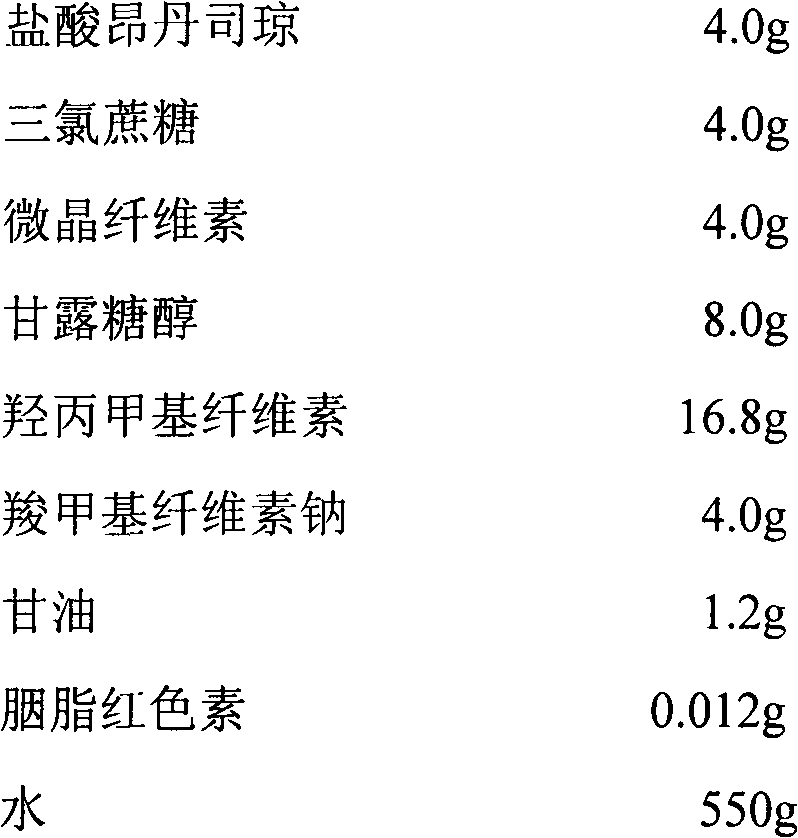
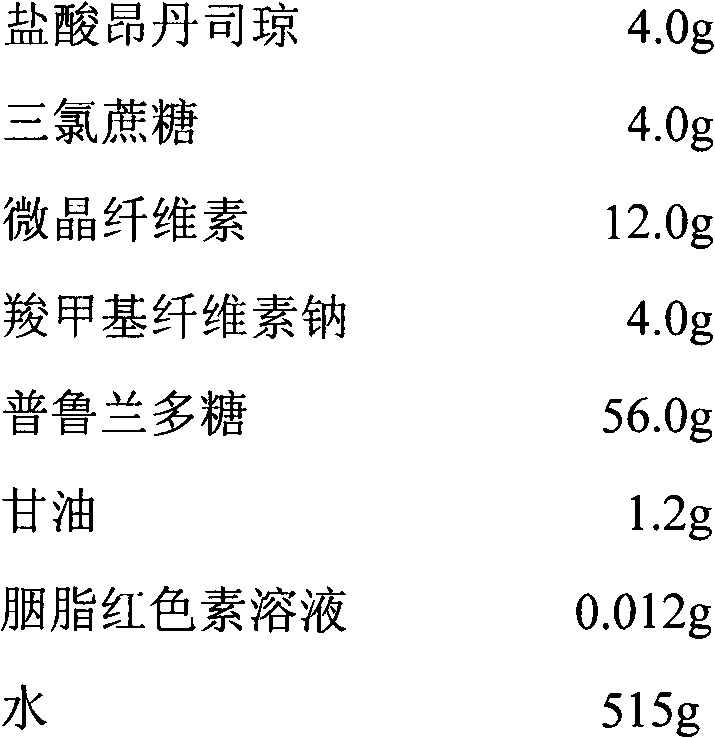
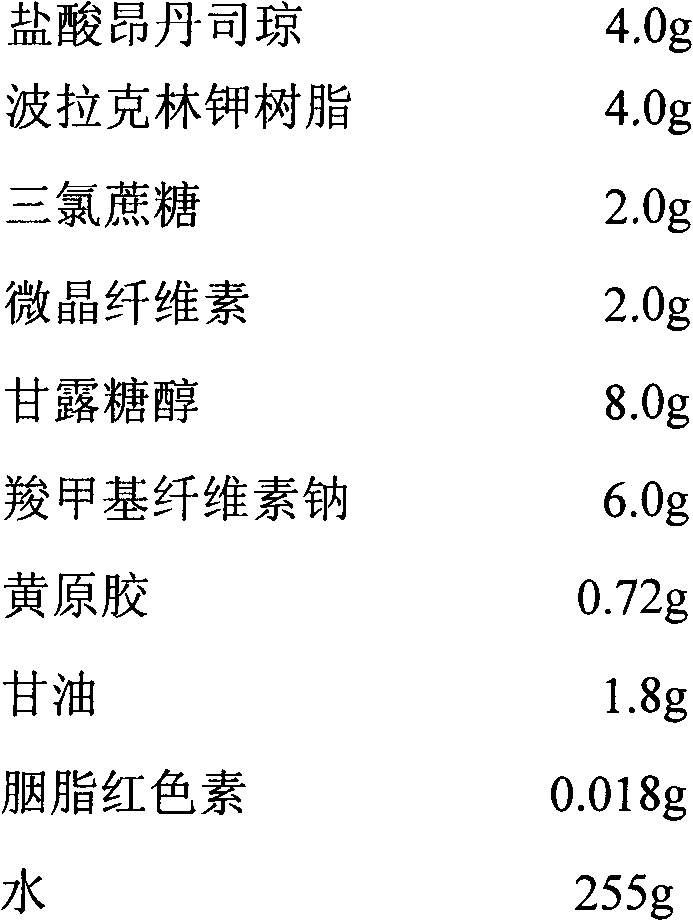
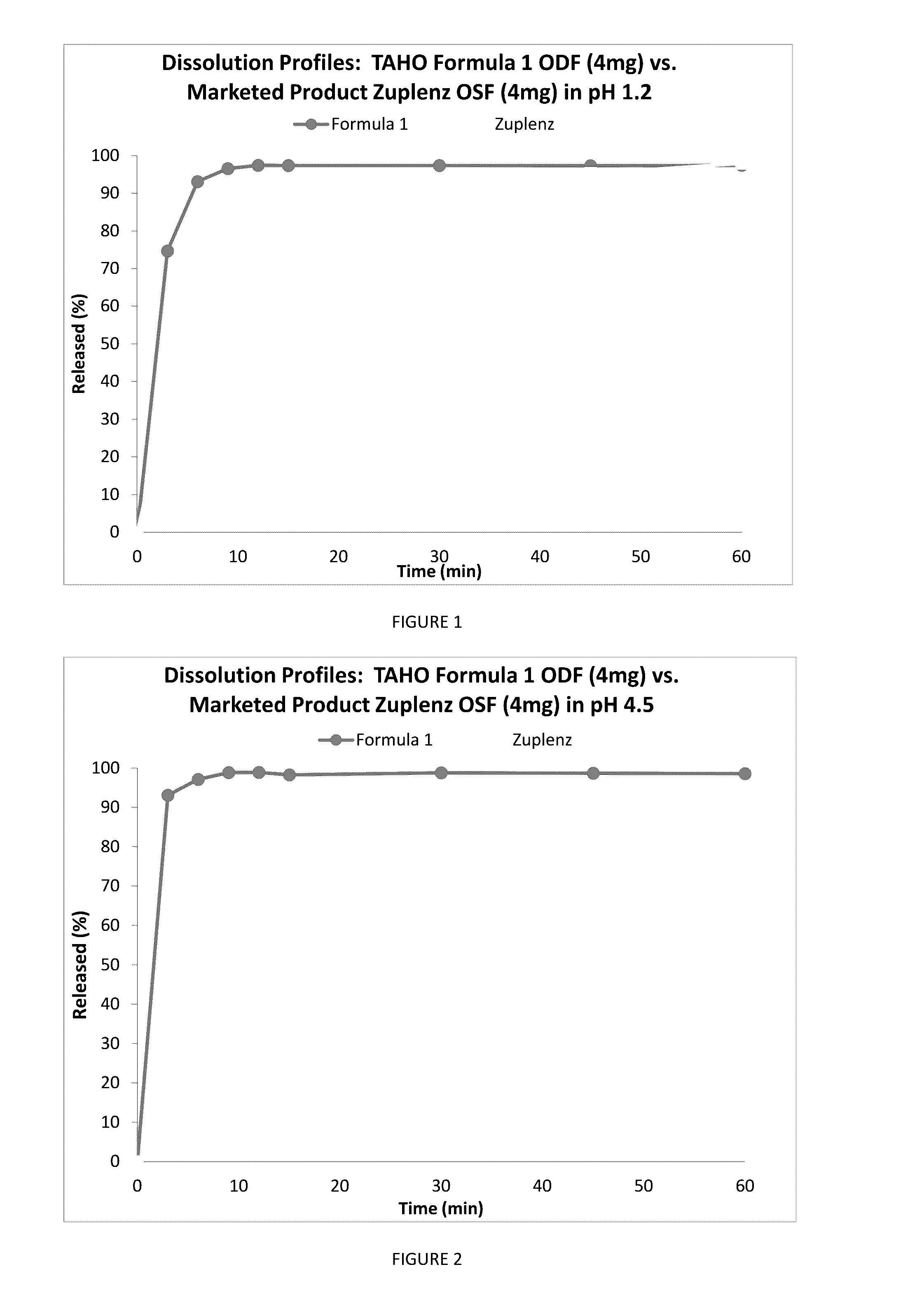
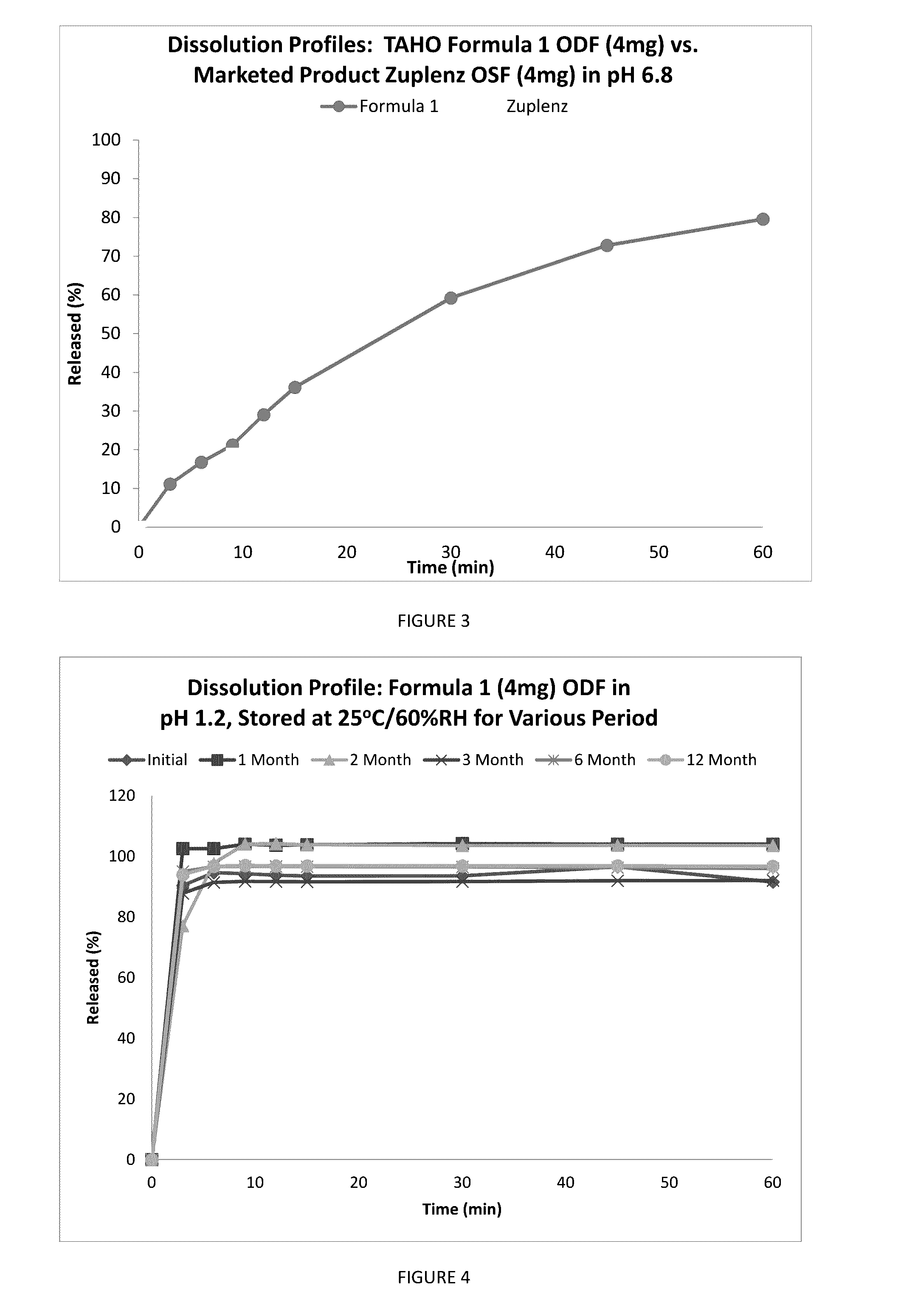
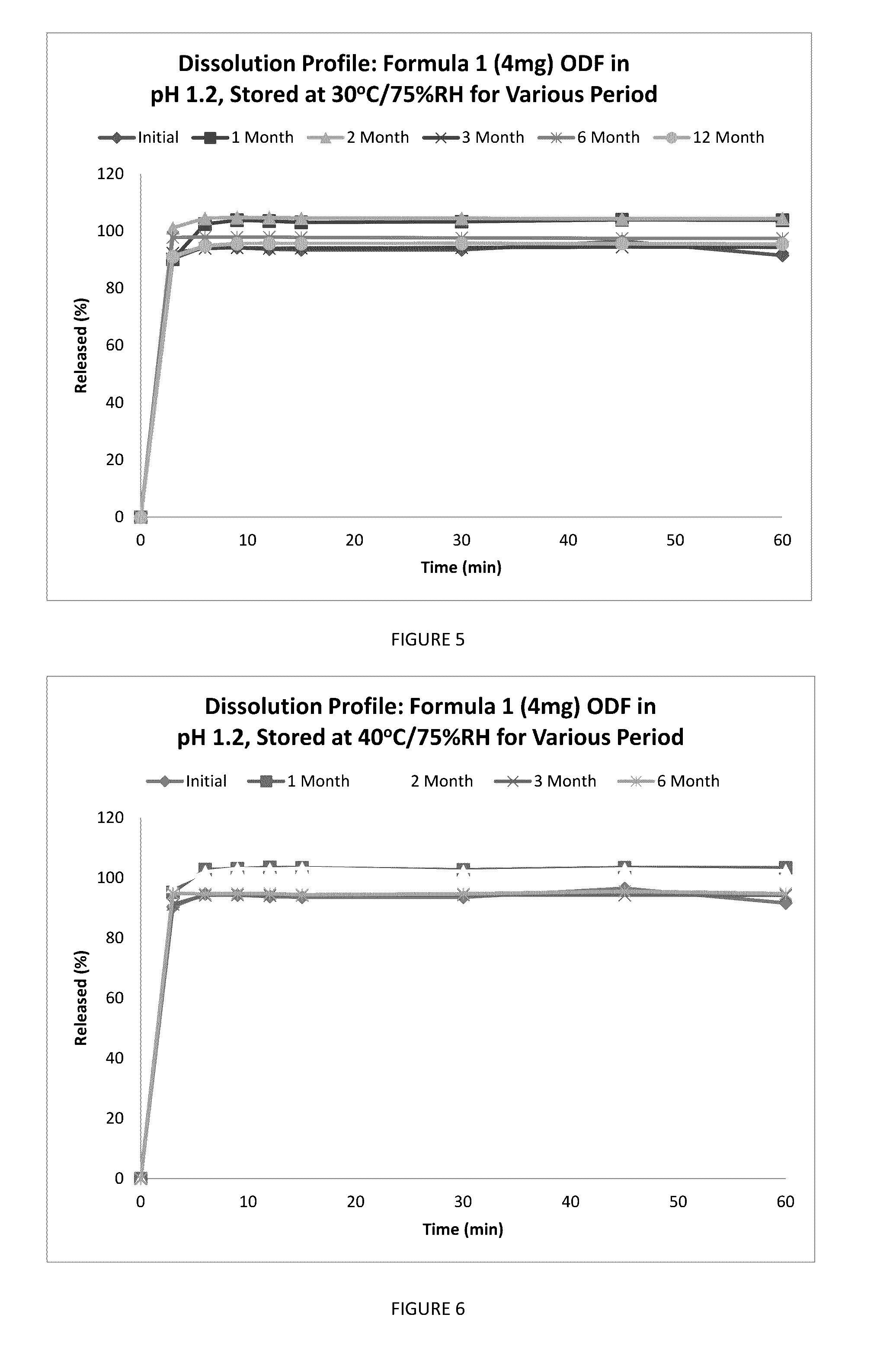
A fast acting orally disintegrating film (ODF) for treatment of various medical conditions including emesis that has a simple formulation, easy to manufacture and has similar pharmacokinetic profile to currently commercially available orally administered drug products is provided. The ODF comprises an active pharmaceutical ingredient such as ondansetron or a pharmaceutical acceptable salt thereof in an amount of 2 to 24 mg, at least one hydrophilic film forming polymer in an amount of at least 8% by weight of the film, wherein the at least one hydrophilic film forming polymer is characterized by having a molecular weight of 5000 to 50000 Da, and a water soluble excipient in an amount of 10 to 30% by weight of the film. The present invention also provides a method for preparing the ODF that remains stable over a period of time under a normal pharmacologically storage condition and a method for treating or preventing various medical conditions such as nausea or vomiting or a treatment method mediated through antagonizing action of 5HT at 5-HT3 receptor by administering the ODF to a patient in need thereof.
Owner:TAHO PHARMA
Sustained releasing talbets for radioactive and chemical therpay and preparation thereof
InactiveCN1530104AMaintain blood drug concentrationOrganic active ingredientsDigestive systemTreatment effectAcrylic resin
A slow-releasing micropill 'Angdansiqiong' for treating the vomiting caused by chemicotherapy or radiotherapy is composed of pill core, enclosing layer, slow-releasing layer and surficial enclosing layer. It is prepared from Angdansiqiong as core (6.4-7.8 wt.%), acrylic resin (3.1-3.8), pore-forming agent (0.5-1.1), antisticking agent (0.6-1.1) and filler (86.2-89.3). Its advantages are durable action (more than 24 hr) and high curative effect.
Owner:潘伟
Pharmaceutical film composition
InactiveUS20170290807A1Simple processOrganic active ingredientsPharmaceutical delivery mechanismOndansetronDosage form
The present invention relates to Film Compositions of Ondansetron or its pharmaceutically acceptable salt thereof, which dosage forms are useful for the treatment of various medical conditions.
Owner:SHILPA MEDICARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O](https://images-eureka.patsnap.com/patent_img/8248cb64-9651-4786-8c08-49e307ca69de/US20080009635A1-20080110-C00001.png)
![One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O](https://images-eureka.patsnap.com/patent_img/8248cb64-9651-4786-8c08-49e307ca69de/US20080009635A1-20080110-C00002.png)
![One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O One-Pot Process for the Preparation of Antiemetic Agent, 1,2,3,9-Tetrahydro-9-Methyl-3[(2-Methyl)-1H-Imidazole-1-Yl)Methyl]-4H-Carbazol-4-O](https://images-eureka.patsnap.com/patent_img/8248cb64-9651-4786-8c08-49e307ca69de/US20080009635A1-20080110-C00003.png)