Transdermal composition of antivomiting agent and preparation containing the same
A skin and ingredient technology, applied in the field of transdermal antiemetic ingredients and preparations containing the same ingredients, can solve the problem that effective antiemetic formulations have not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
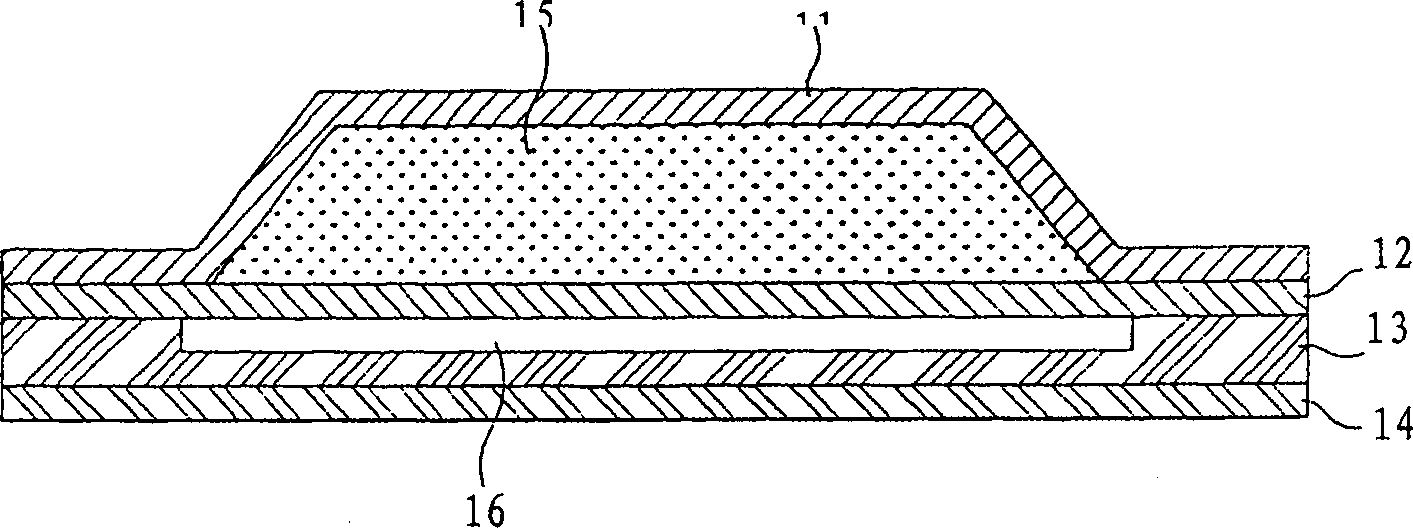
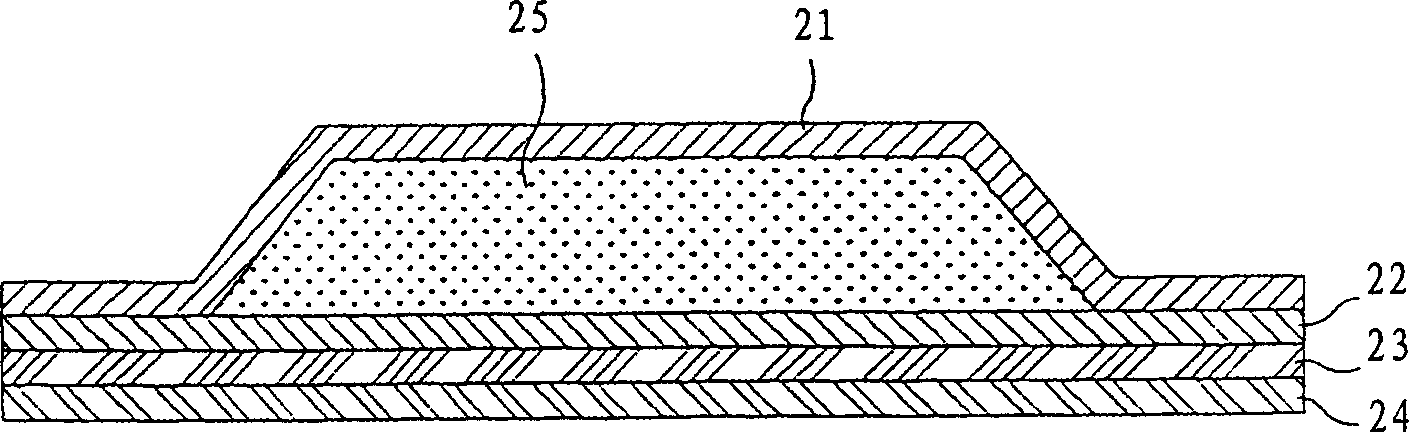
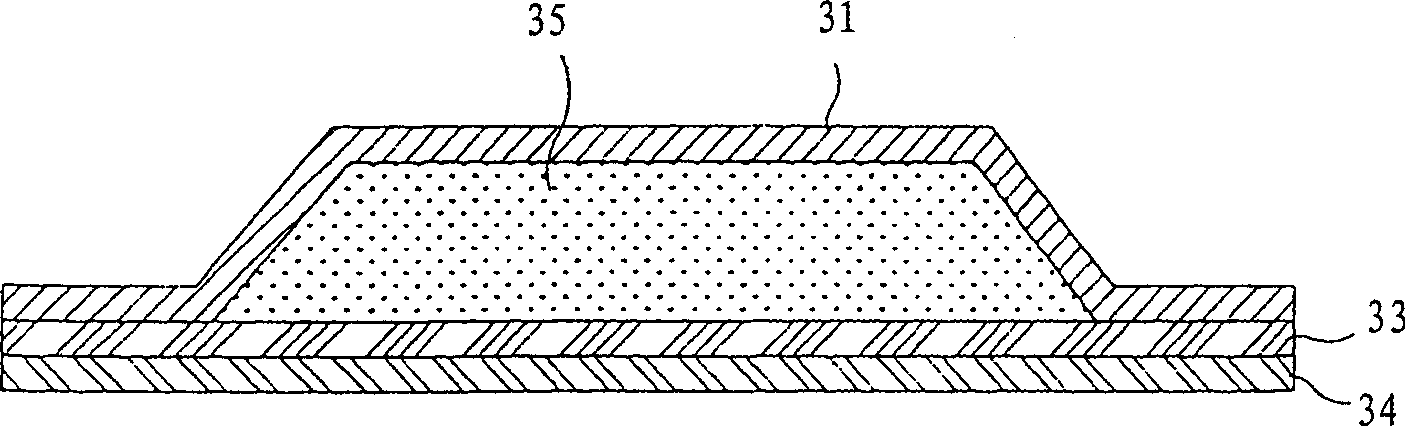
Image
Examples
example 1
[0062] Among them, Js and A have the same meaning as defined in the previous formula, and (dQ / dt)ss is the amount of drug that penetrates the skin per unit time under steady state conditions. Example 1: Preparation of Composition for Transdermal Delivery
[0063] The ethanol of 30% by weight percentage, the propylene glycol of 27% by weight percentage, the oleic acid of 30% by weight percentage and the water of 40% by weight percentage mix, then add the ondansetron of 3% by weight percentage in the mixture, obtain a transdermal Passed ingredients (ingredient 1).
[0064] The skin penetration of ondansetron was determined to be 71.4 μg / cm using the procedure of the reference example 2 / h. Comparative Example 1: Preparation of Comparative Components.
example 2
[0070]As we can see from the results of Example 1 and Comparative Examples 1 to 6, the composition 1 with the skin penetration enhancer is better than the comparative ingredients 1-7 without the skin penetration enhancer (sic-translation Author's note) showed higher skin penetration of ondansetron. Example 2: Preparation of Composition for Transdermal Delivery
[0071] Ethanol containing 20% by weight, 15% by weight of propylene glycol, 3% by weight of glyceryl monooleate, 2% by weight of polyoxyethylene (n=10) oleyl ether and 60% by weight of Tropisetron was added to an aqueous solution of water to a concentration of 5% by weight to obtain a transdermal component (component 2). The skin penetration rate of its tropisetron is 30.8μg / cm 2 / h. Examples 3 to 42: Preparation of Compositions for Transdermal Delivery
[0072] Using the ingredients shown in Table 1, repeat the steps of Example 1 or 2 to obtain the medicament components (components 3 to 42) delivered through the...
example 2
[0080] The skin penetration rate of tropisetron in this poultice is 47.2 μg / cm 2 / h. Preparation example 2: single-piece base material plaster
[0081] 20% by weight of ethanol, 15% by weight of polypropylene glycol, 2% by weight of oleic acid, 3% by weight of octanol, 5% by weight of octylphenoxypolyethoxyethanol and 55% The water by weight was mixed, and 8% by weight of polyvinylpyrrolidone and 4% by weight of polyvinyl alcohol dissolved in distilled water were added to the mixture. Vigorously stir the obtained solution to make it uniform, add 4% by weight hydroxyethyl cellulose and 6% by weight tropisetron to the solution, and stir the obtained mixture evenly.
[0082] The solution thus obtained was placed in a mold to a depth of 2 mm and kept at a temperature of 4°C for one day to obtain a hydrogel.
[0083] The hydrogel was placed in a layer on an impermeable protective film pre-coated with acrylate (Durotak 87-2196 National Starch Company), and then a silicone-coated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com