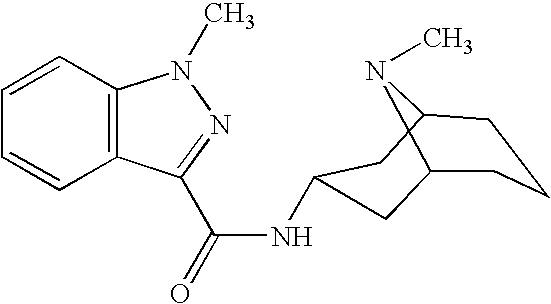
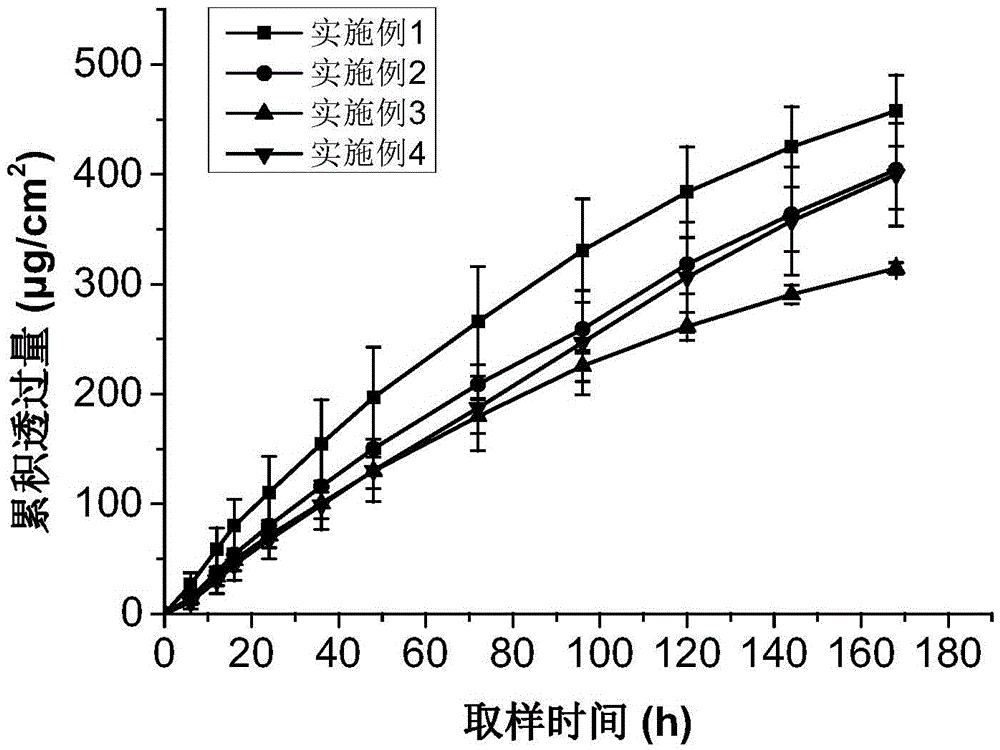
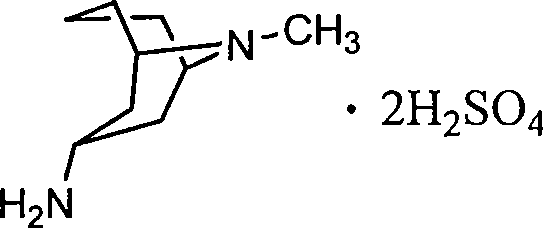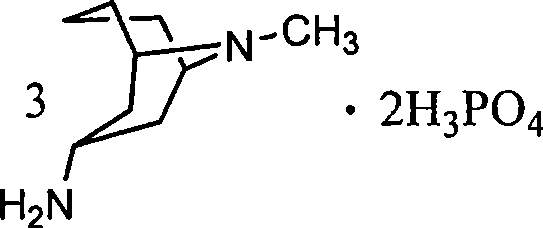Patents
Literature
57 results about "Granisetron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Granisetron is used to help prevent nausea and vomiting caused by cancer chemotherapy.
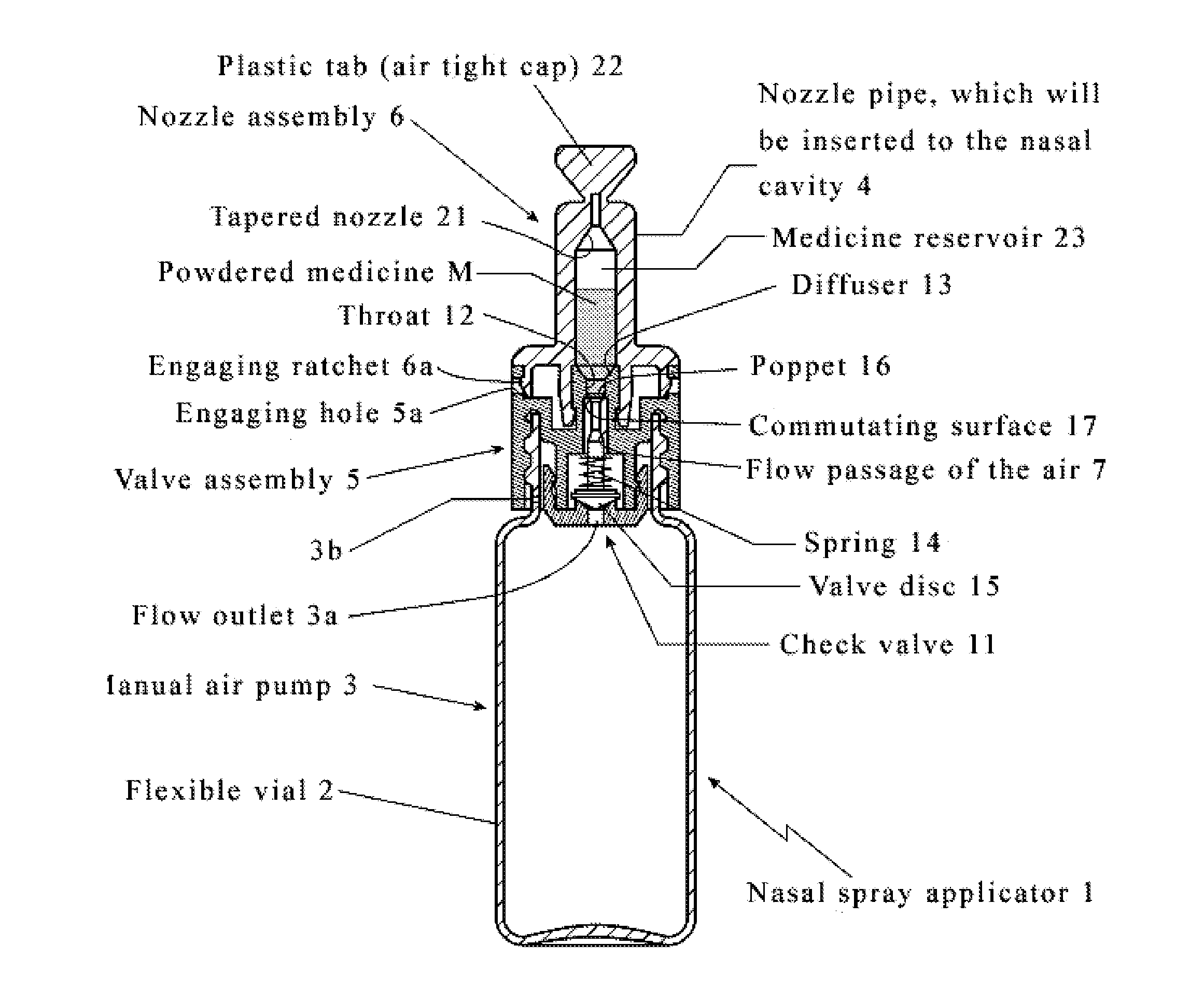
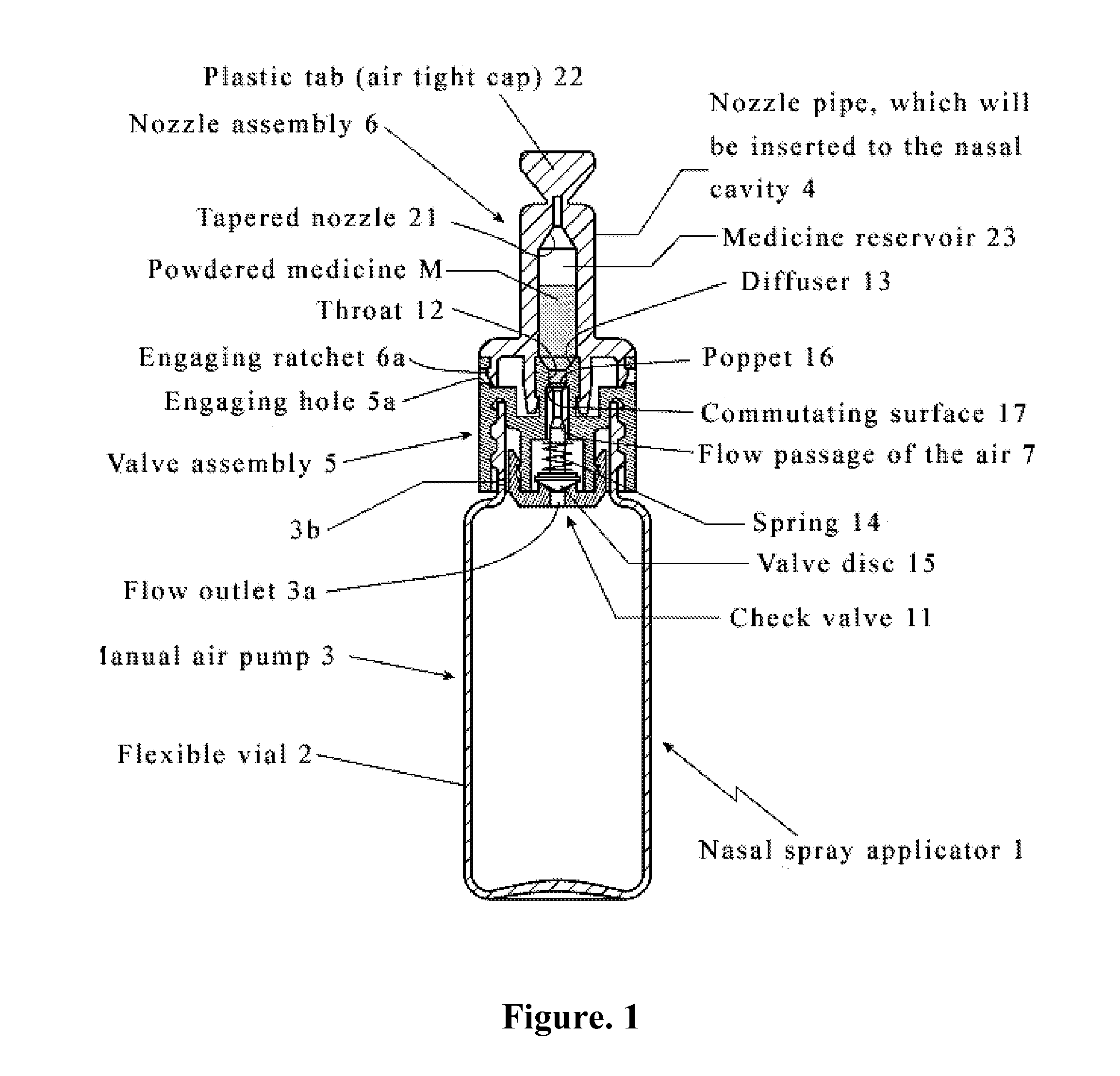
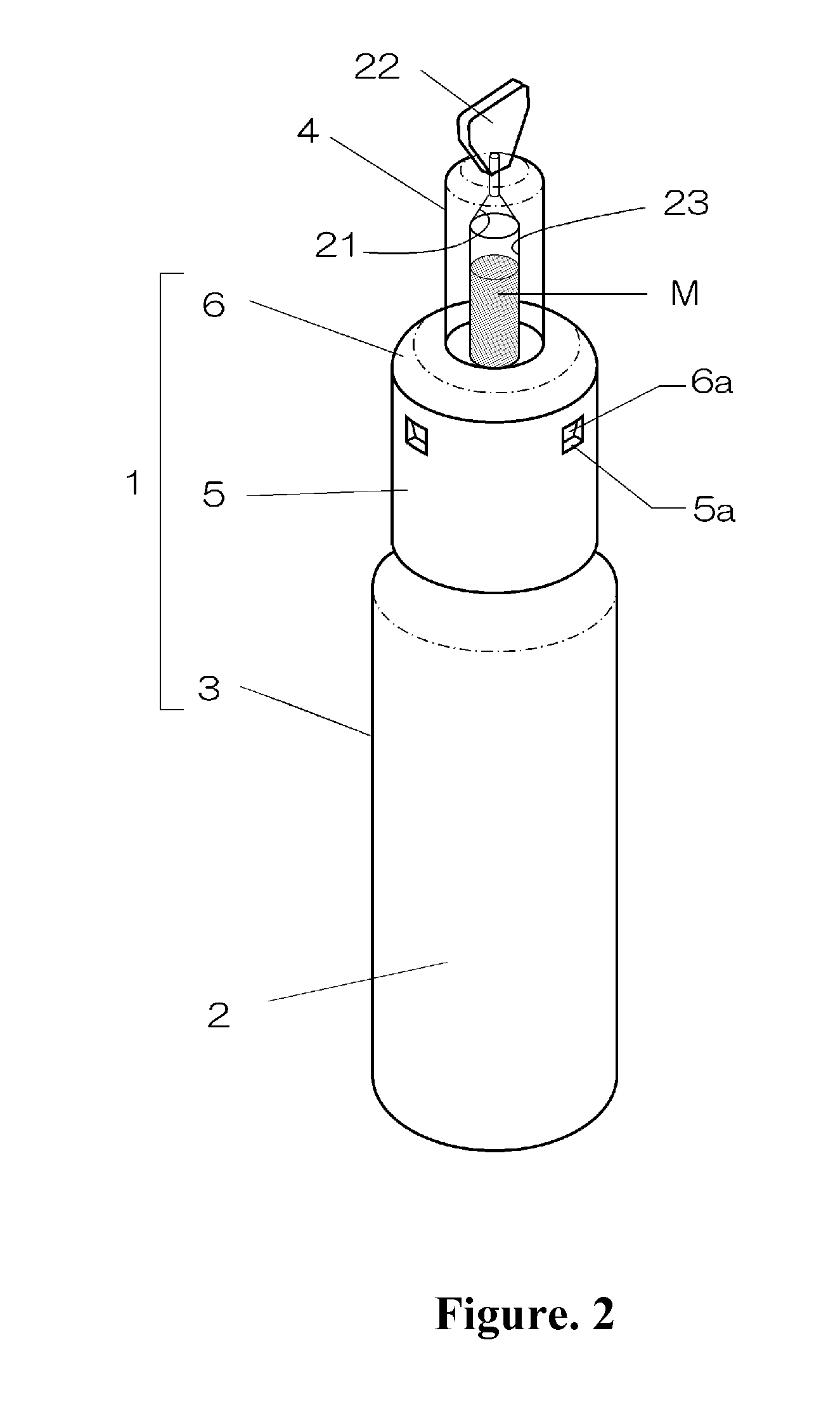
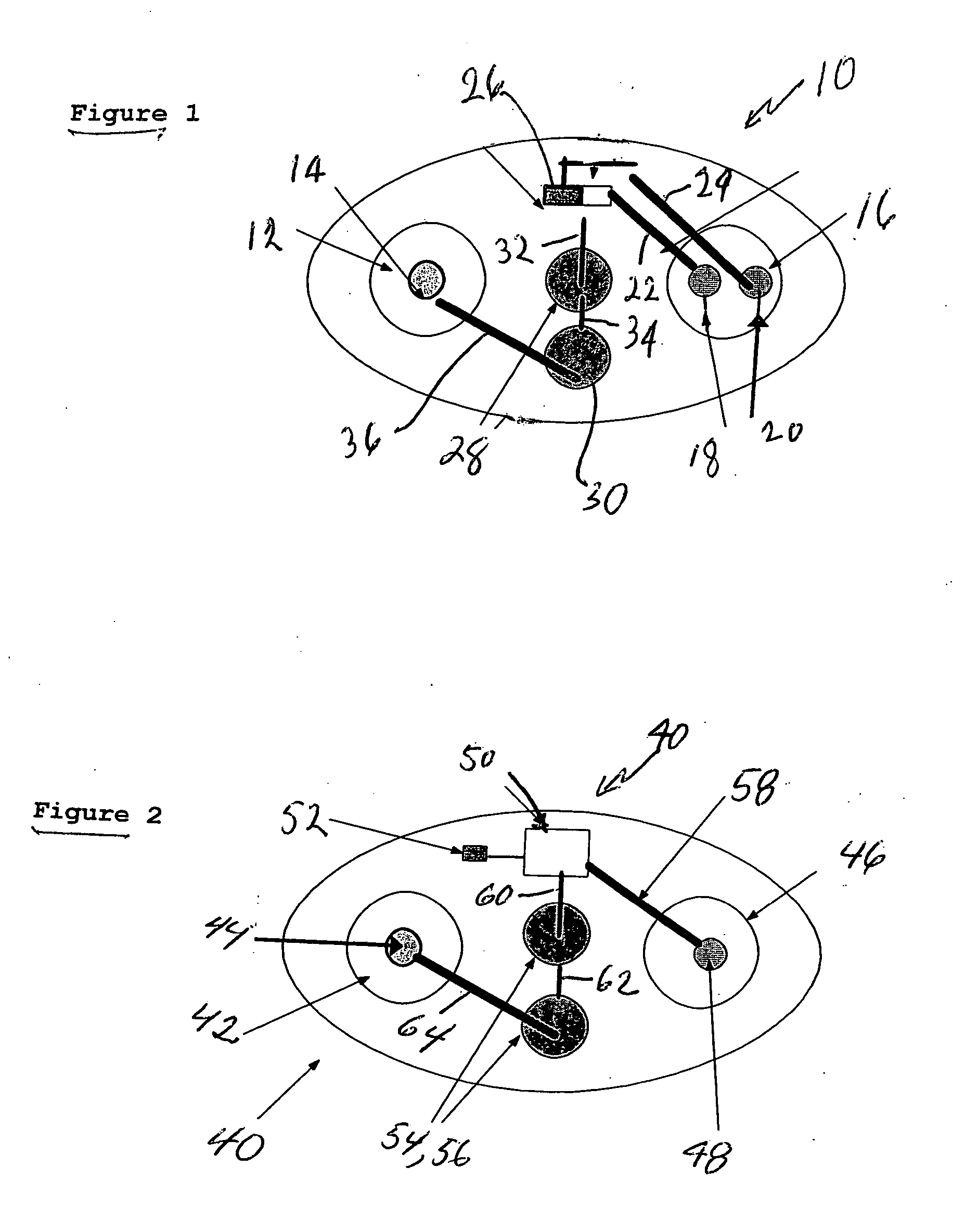
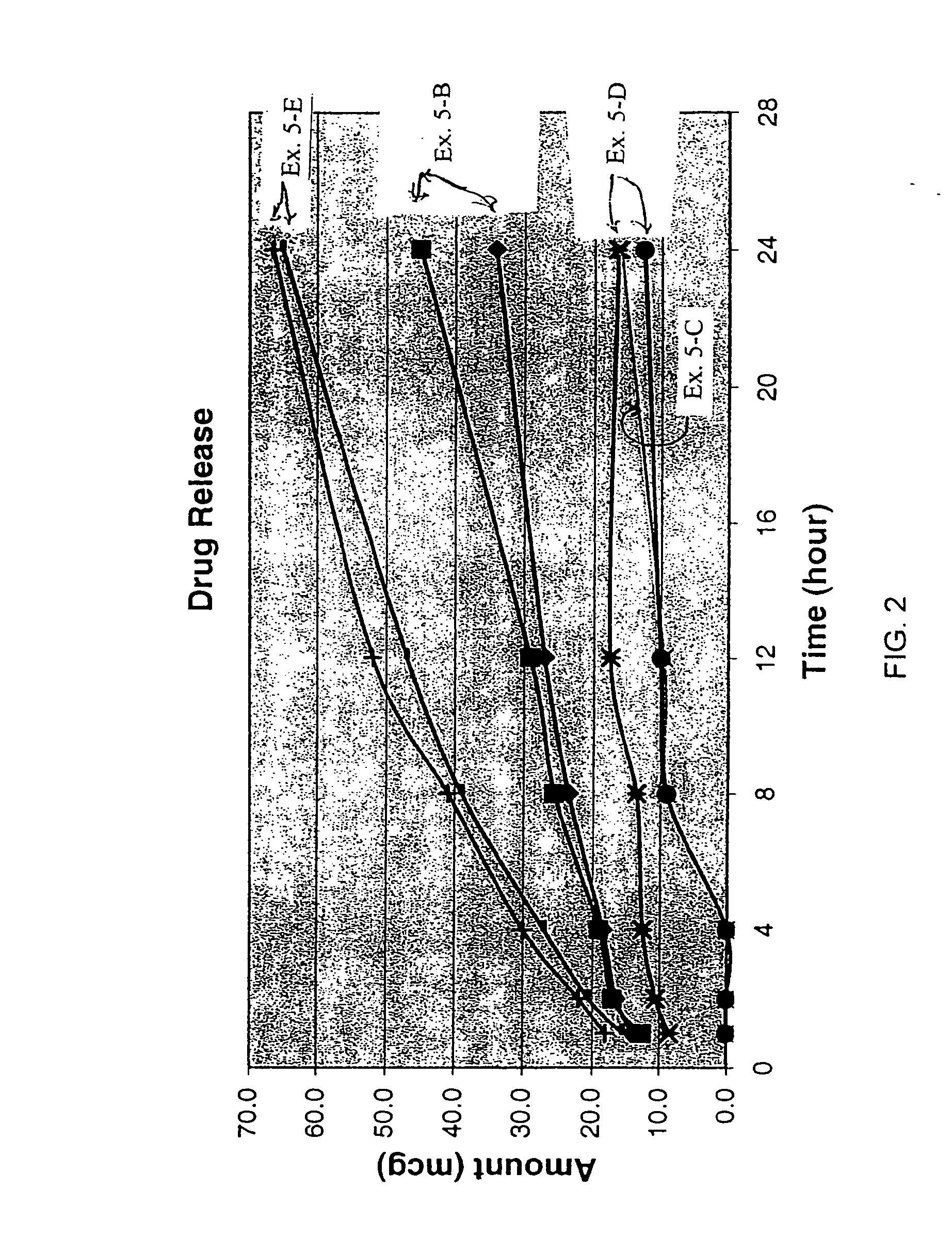
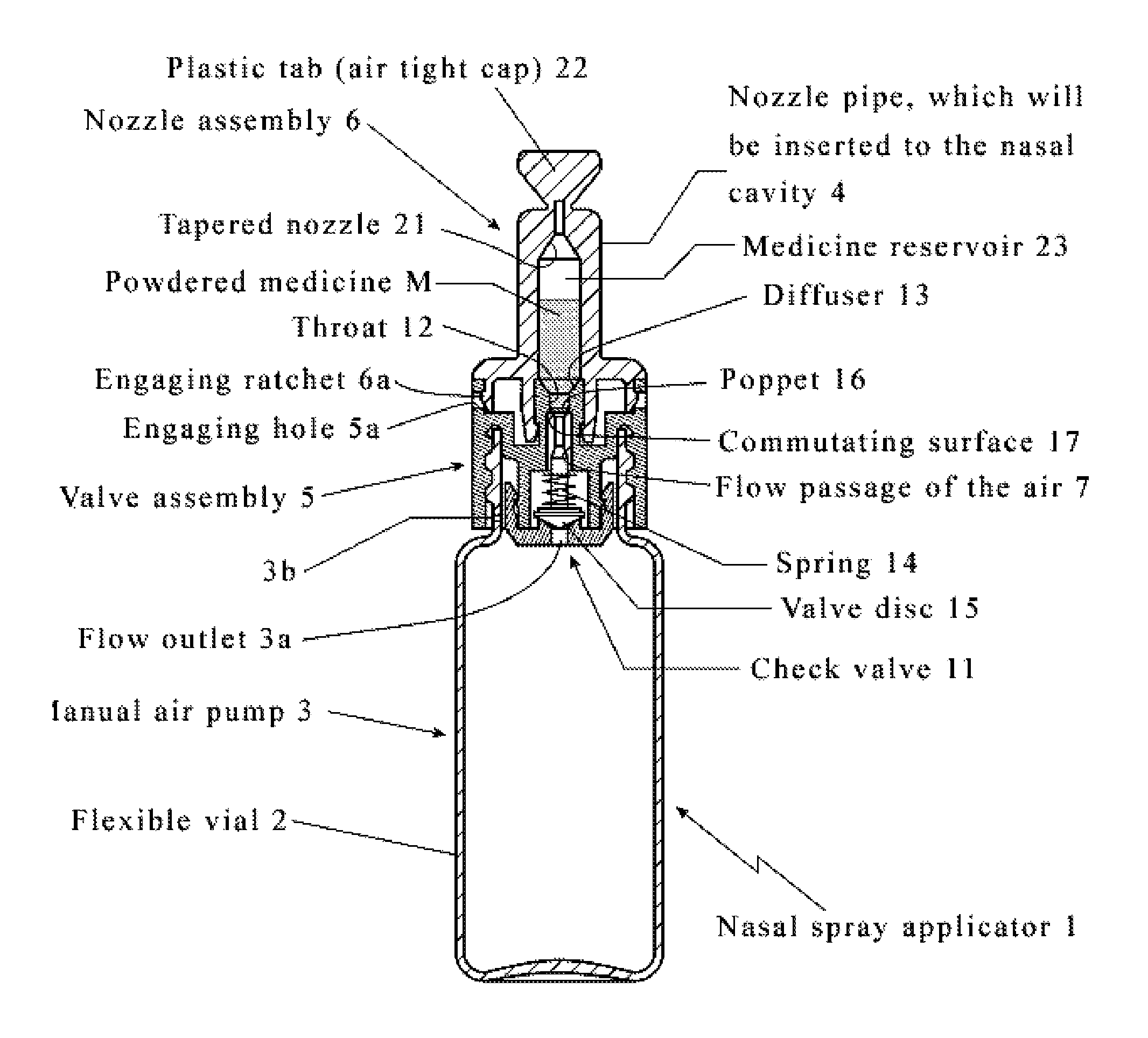
Intranasal granisetron and nasal applicator
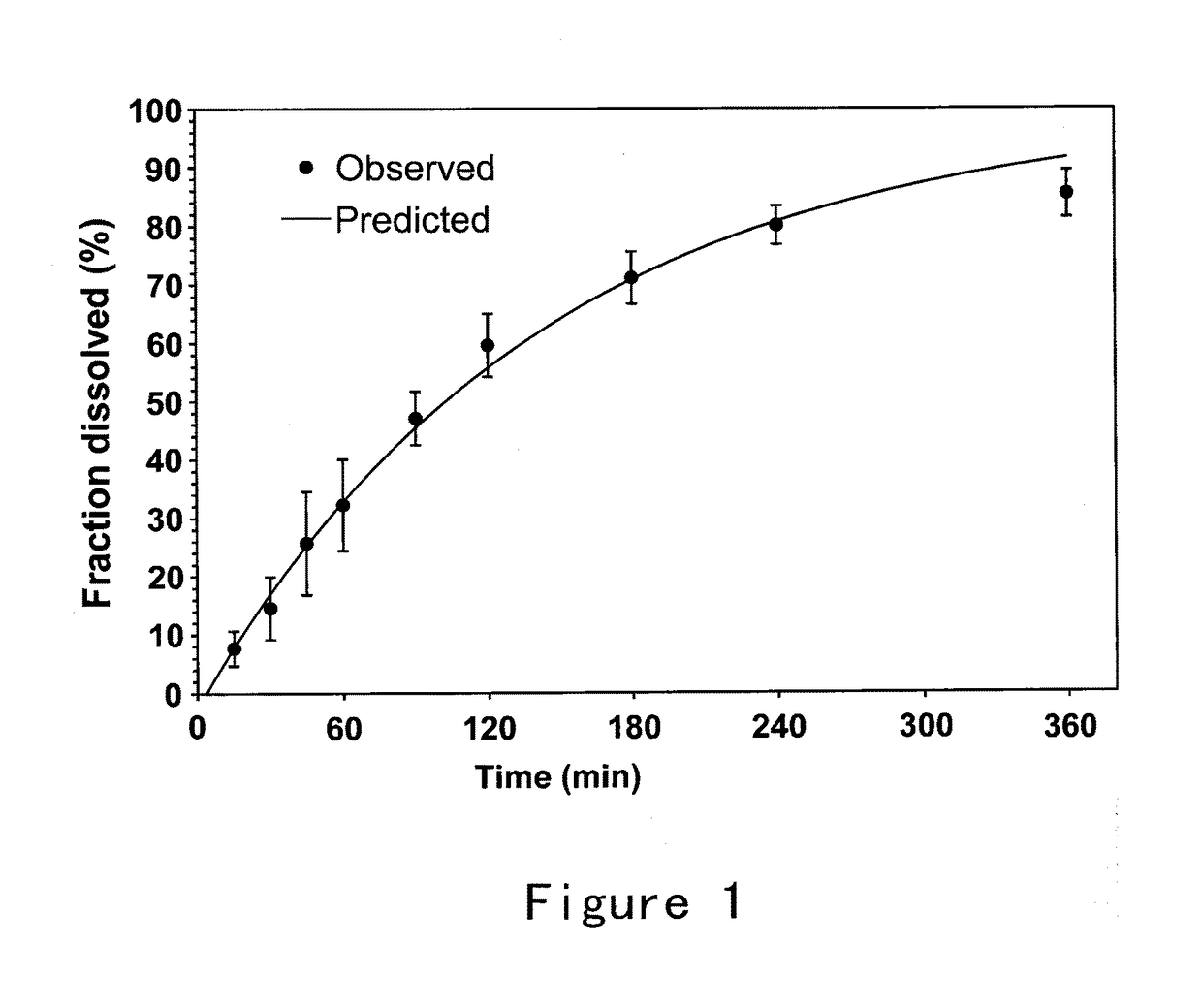
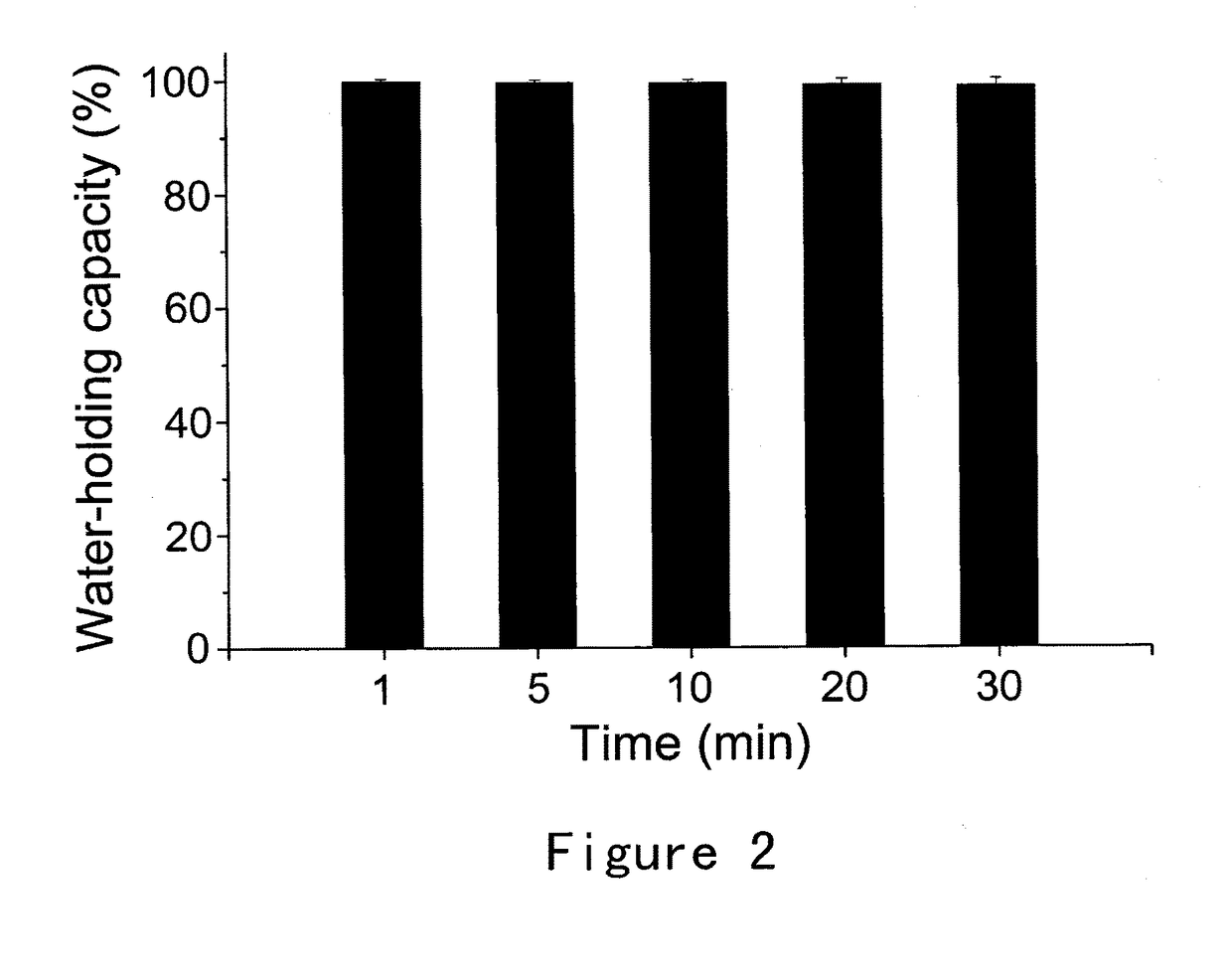
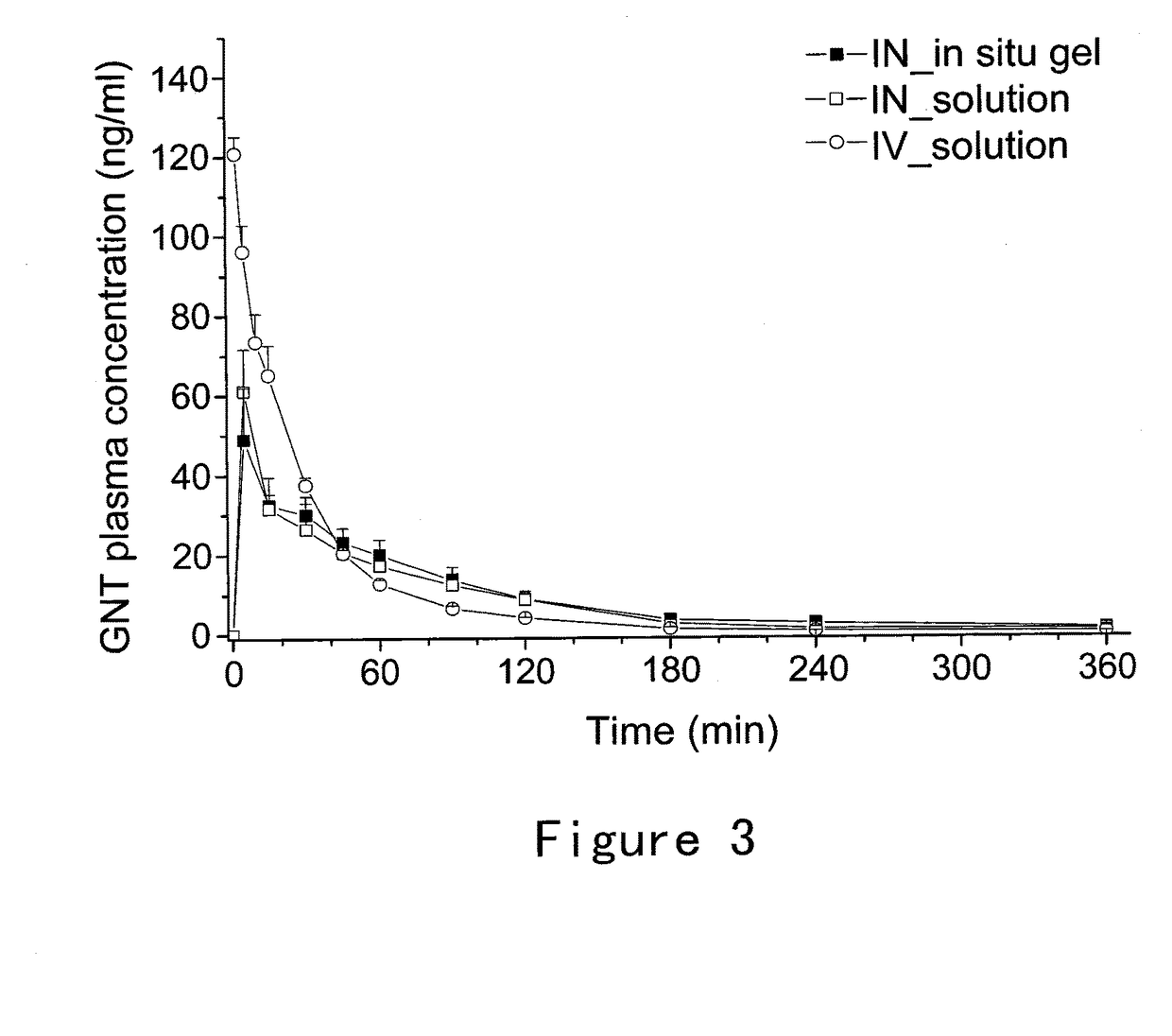
Formulations and methods of manufacture are provided for granisetron dry powder compositions suitable for intranasal administration. Also provided are methods of use for preventing or controlling emesis and other diseases and disorders and devices, compositions, and methods for nasal delivery of therapeutic formulations. Devices for delivery of dry powder formulations are also provided. Devices can be single-use devices.
Owner:SHIN NIPPON BIOMEDICAL LAB
Transdermal systems for the delivery of therapeutic agents including granisetron using iontophoresis
InactiveUS20060253061A1Quick cureAvoid accidental activationElectrotherapyMedical devicesControl systemControl circuit
A disposable skin-worn device for the transdermal delivery at least one dose of charged therapeutic substances, including granisetron, by iontophoresis, the device comprising a donor reservoir containing an amount of a therapeutic substance to be delivered transdermally by iontophoresis, a counter reservoir, a source of electric power connected in a circuit between the donor reservoir and the counter reservoir and a control system for controlling current flow in the circuit to enable at least one dose of the therapeutic substance to be delivered transdermally by iontophoresis and wherein the control system includes a control element selected from the group consisting of a sensor activated by an external signal and a switch.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Transdermal Antiemesis Delivery System, Method and Composition Therefor
A transdermal antiemesis system, preferably in the form of a drug-in-adhesive matrix patch, is disclosed comprising an effective antiemesis amount of a selective serotonin (5-HT3) receptor antagonist as an antiemetic incorporated in a skin adhesive composition containing at least one permeation enhancer and a water-insoluble pressure-sensitive adhesive. A preferred antiemetic is granisetron base. The transdermal antiemesis system of this invention provides controlled release of the active antiemetic ingredient from the skin-contacting adhesive formulation of a transderrnal patch, and maintains a sustained transdermal delivery of the antiemetic.
Owner:NEXMED HLDG INC
Intranasal granisetron and nasal applicator
Formulations and methods of manufacture are provided for granisetron dry powder compositions suitable for intranasal administration. Also provided are methods of use for preventing or controlling emesis and other diseases and disorders and devices, compositions, and methods for nasal delivery of therapeutic formulations. Devices for delivery of dry powder formulations are also provided. Devices can be single-use devices.
Owner:SHIN NIPPON BIOMEDICAL LAB
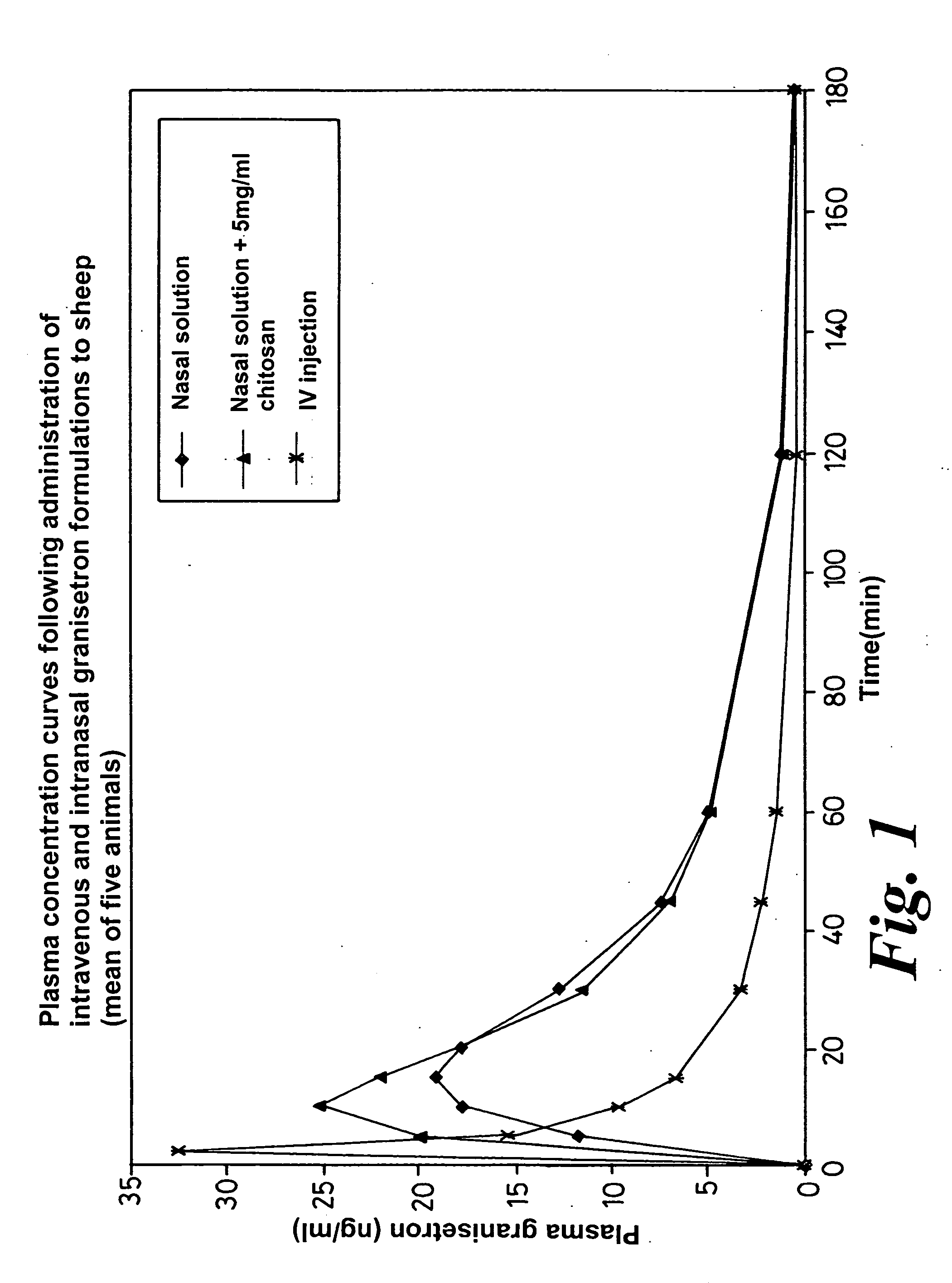
Intranasal compositions
InactiveUS20050142073A1Significant comprehensive benefitsPrevent goodBiocidePowder deliveryNausea sicknessAqueous solution
Compositions are provided for the intranasal administration of granisetron or a pharmaceutically acceptable salt thereof. Preferred compositions are in the form of an aqueous solution. Optionally, the compositions comprise chitosan, a salt or derivative thereof or a salt of a derivative of chitosan. The compositions can be used for the treatment or prevention of nausea and / or vomiting.
Owner:ARCHIMEDES DEVMENT
Nasal spray agent
The present invention is nasal spray of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron, osmotic pressure regulator and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
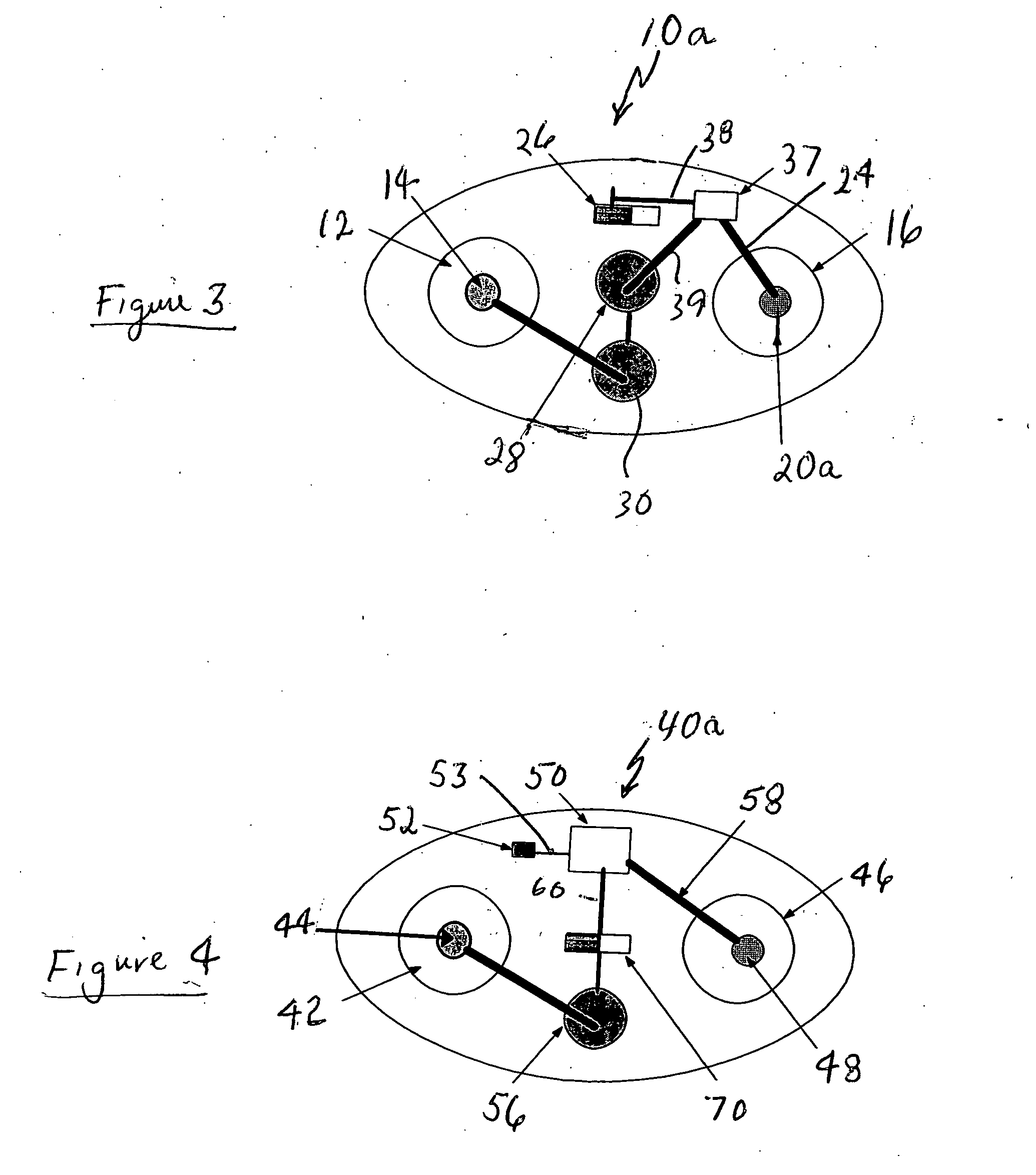
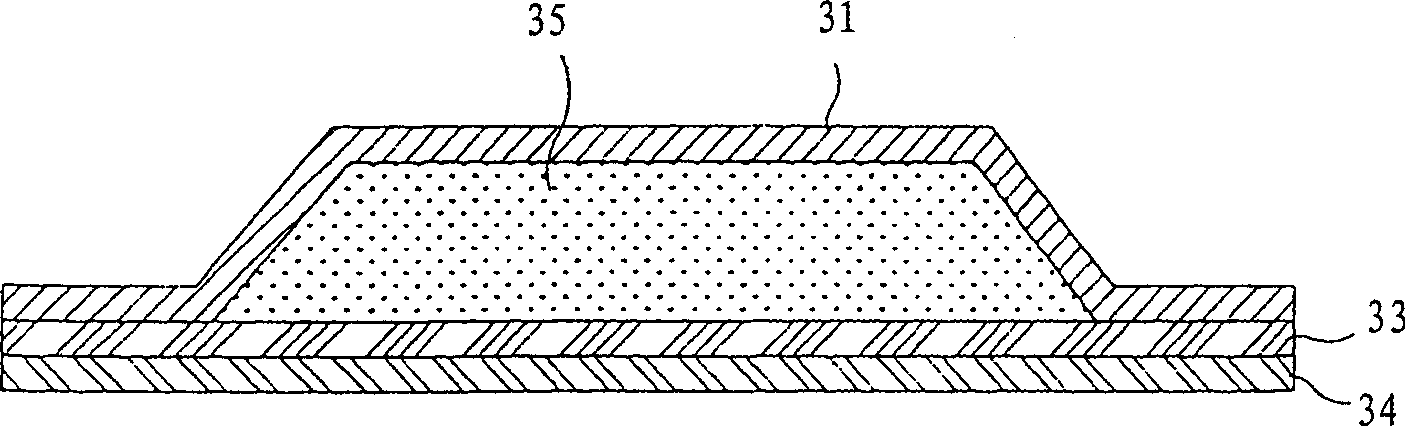
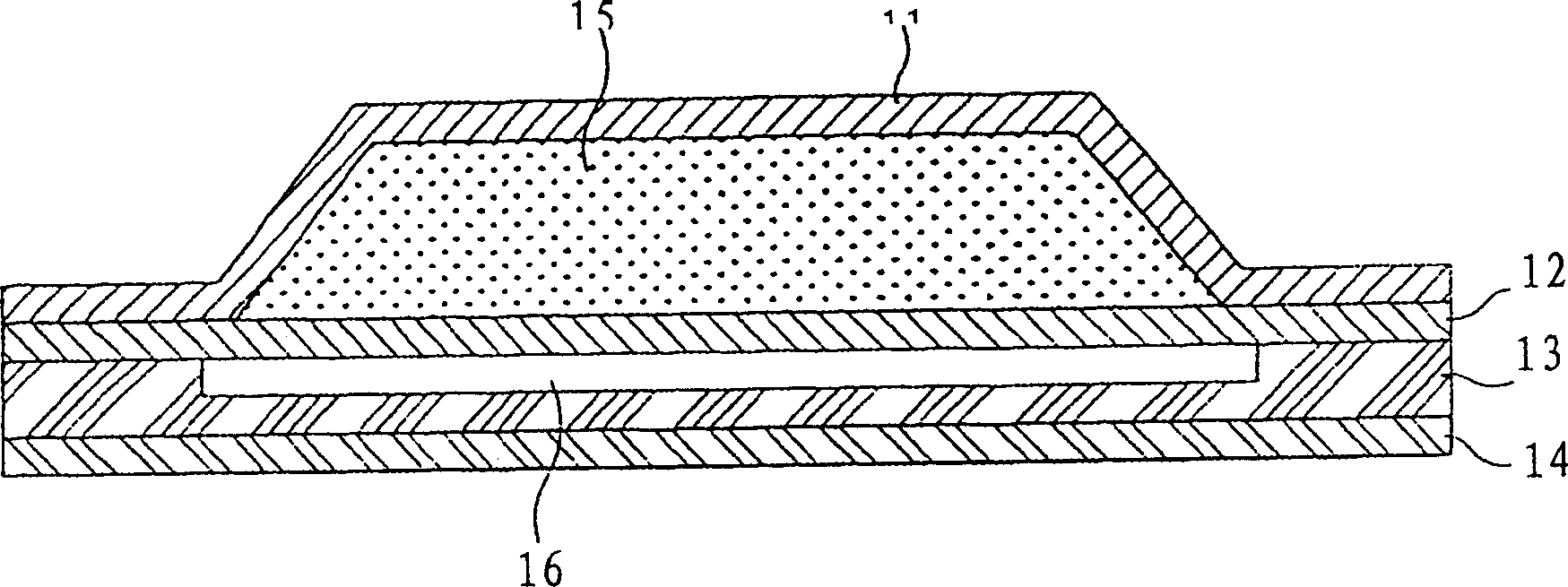
Percutaneous absorption patch and its production method
ActiveCN101455650AEasy to dissolve and disperseLess irritatingOrganic active ingredientsDigestive systemPercutaneous absorptionSkin irritant
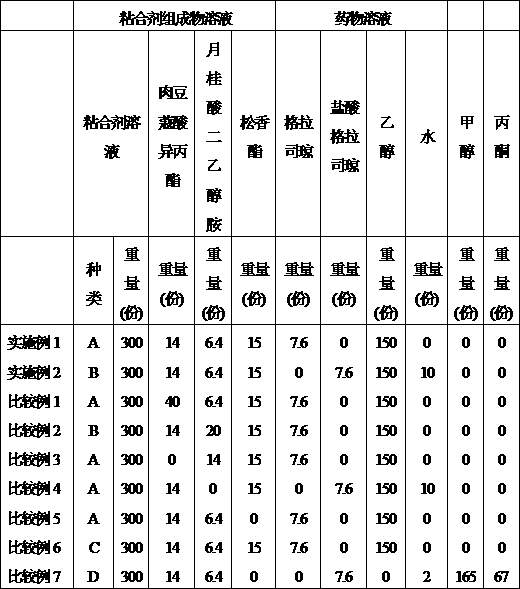
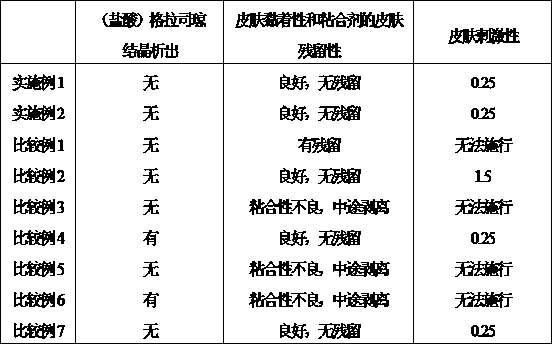
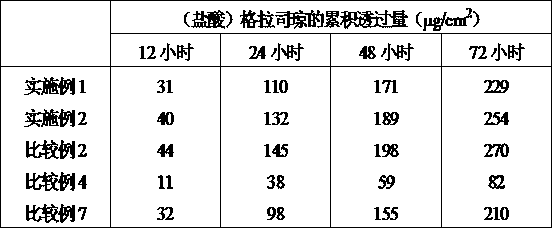
The invention provides a granisetron transdermic absorption plaster with small skin irritation, proper adhesion performance, no crystallization due to granisetron at dissolved state, suitable for long-time proper-amount transdermic administration and preparation method thereof. The the copolyers of 60-70weight% of acrylic acid 2-ethylhexyl ester and 40-30weight% of vinyl-pyrrolidone, a transdermic absorption preparation layer composed of isopropyl myristate, lauric acid diethanolamine and granisetron according the weight ratio of 100:5 - 20:1 - 10:5 - 10 are laminated on one side of a backing layer without permeability to the medicine.
Owner:COSMED PHARMA
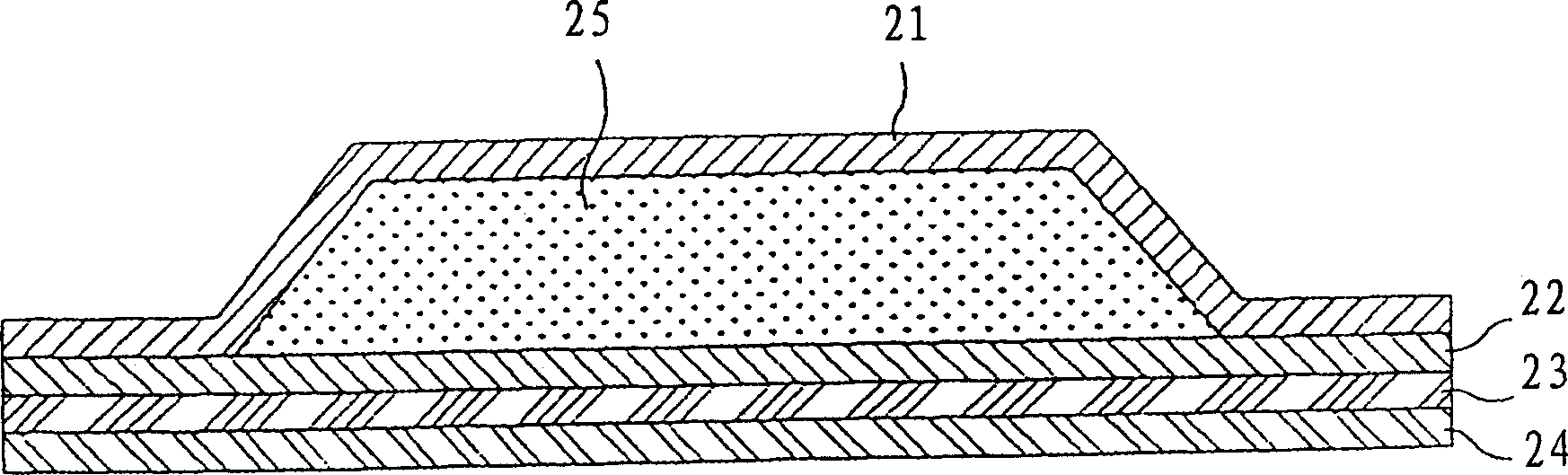
Granisetron and/or hydrochloride patch thereof
ActiveCN101721394AIncreased percutaneous penetration rateImprove toleranceOrganic active ingredientsDigestive systemOrganic acidMedicine
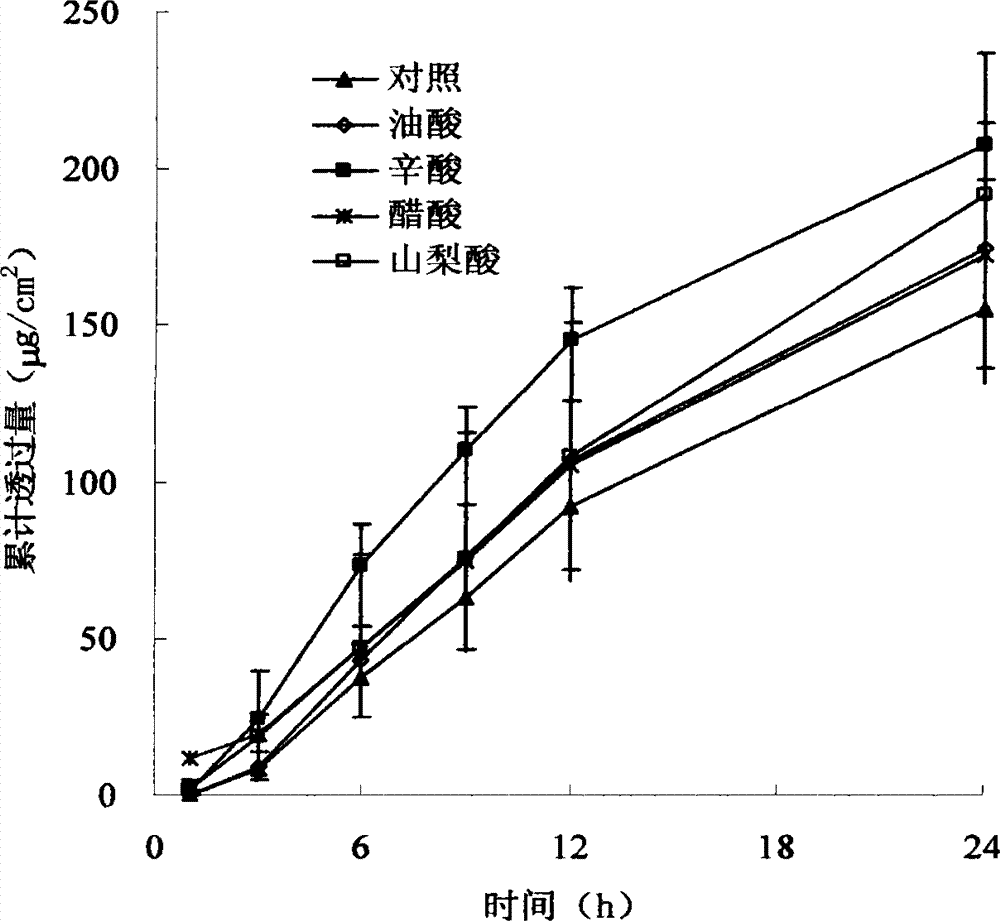
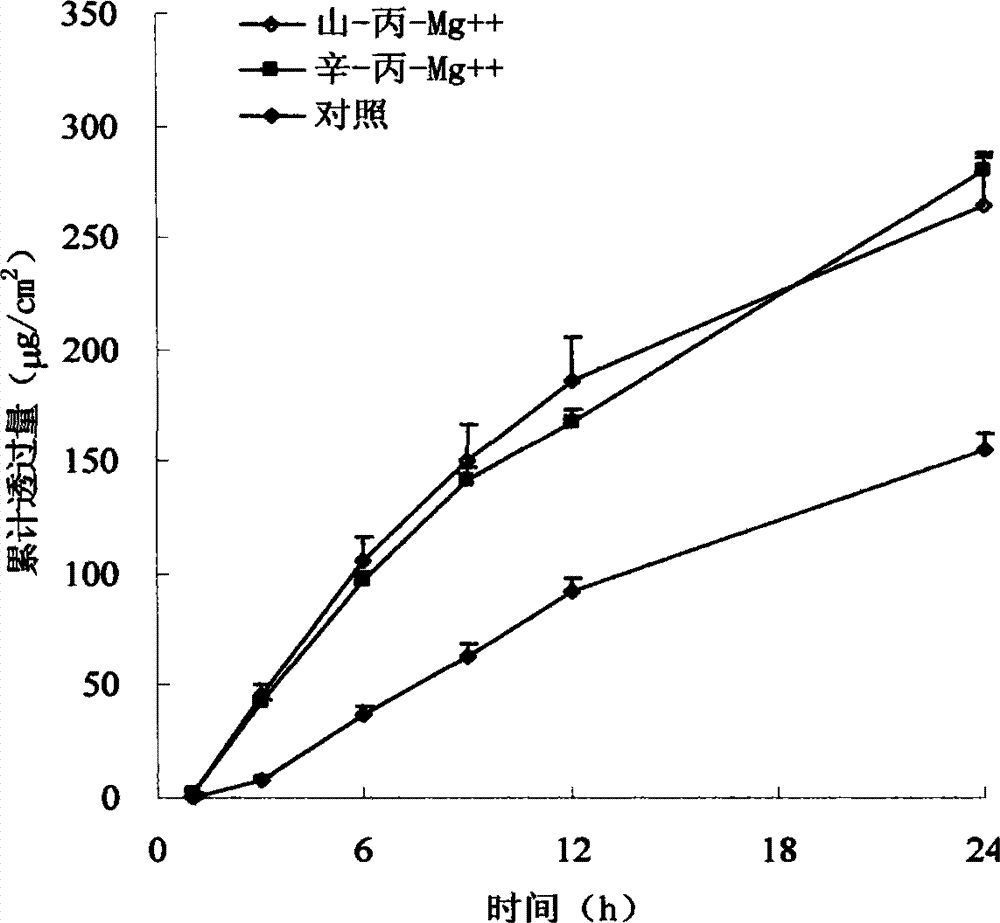
The invention relates to a Granisetron and / or a hydrochloride patch thereof, which comprises a lining layer, a medicine carrying pressure sensitive adhesive layer containing the Granisetron or / and the hydrochloride thereof, and an anti-sticking layer, wherein the medicine carrying pressure sensitive adhesive layer is characterized in that pressure sensitive adhesive contains carboxylic groups; the content of the Granisetron or / and the hydrochloride thereof in percentage by weight is 1%-12%; the medicine carrying pressure sensitive adhesive layer contains metal ions capable of enabling the pressure sensitive adhesive to generate cross linking, and the content of the metal ions in the medicine carrying pressure sensitive adhesive layer in percentage by weight is 0.05%-2%; and the medicine carrying pressure sensitive adhesive layer contains organic acids with 2-18 carbon atoms, and the molar ratio of the adding amount of the organic acids to the adding amount of the Granisetron or / and the hydrochloride thereof is (1-5):1. The patch of the invention can obviously increase the percutaneous infiltration capacity of the Granisetron and improve the solvent resistant and ageing resistant action of the substrate, can be applied to preventing and treating the symptoms of nausea, emesis and the like caused by radiotherapy, chemotherapy and operations, and has great foreseeable economic value and social value.
Owner:DALIAN UNIV OF TECH +1
Snuff
The present invention is snuff of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron; and medicine carrier selected from glucose, mannitol, lactose, sorbitol, microcrystalline cellulose and beta-cyclodextrin.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method of intranasal administration of granisetron
InactiveUS7947257B2Prevent goodConvenient treatmentPowder deliveryOrganic active ingredientsAqueous solutionPolysaccharide
Compositions are provided for the intranasal administration of granisetron or a pharmaceutically acceptable salt thereof. Preferred compositions are in the form of an aqueous solution. Optionally, the compositions comprise chitosan, a salt or derivative thereof or a salt of a derivative of chitosan. The compositions can be used for the treatment or prevention of nausea and / or vomiting.
Owner:ARCHIMEDES DEVMENT
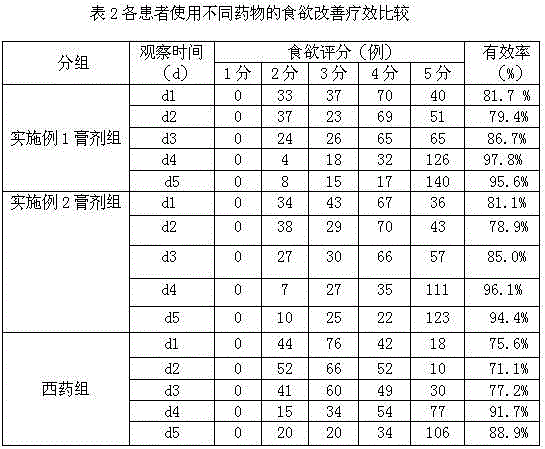
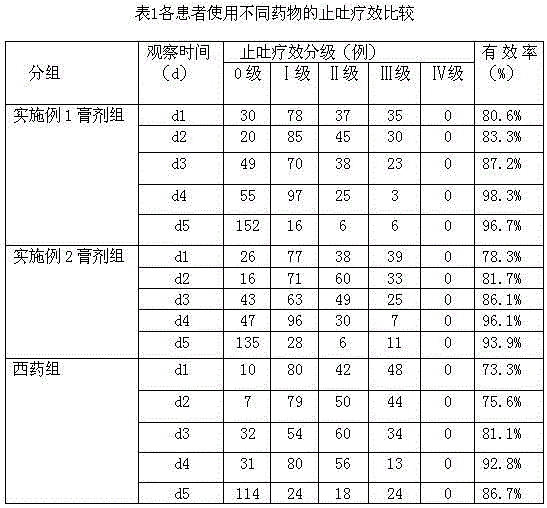
Preparation method and application of traditional Chinese medicine ointment for preventing and treating delayed nausea and vomiting caused by chemotherapy
ActiveCN104906540AEasy to acceptGood control effectHydroxy compound active ingredientsDigestive systemNausea sicknessPinellia
The invention discloses a preparation method of a traditional Chinese medicine ointment for preventing and treating delayed nausea and vomiting caused by chemotherapy. The preparation method comprises the steps that raw materials are smashed; smashed amomum villosum is fetched for extracting amomum villosum volatile oil by a steam distillation method; fresh ginger is juiced to obtain ginger juice; and smashed pinellia rhizoma prepared by ginger, cassia twigs, agastache rugosus, fructus evodiae, inula flower, pericarpium citri reticulatae, ligusticum wallichii, menthol, folia perillae acutae, endothelium corneum gigeriae galli, sea-buckthorn, the amomum villosum volatile oil and the ginger juice are evenly mixed and added with honey to be stirred in a paste shape, and the traditional Chinese medicine ointment is obtained. The preparation method has the advantages that the composition is rigorous, preparation is easy, irritation is avoided, the price is low, operation is simple and convenient, the ointment can be accepted by patients easily, and the ointment has a wide clinical application prospect. The invention further discloses application of the traditional Chinese medicine ointment for treating delayed nausea and vomiting combined with granisetron and dexamethasone. The traditional Chinese medicine ointment can well treat delayed nausea and vomiting and inappetence caused by cis-platinum, also can relieve the side effects, like constipation of patient, caused by the usage of the granisetron and dexamethasone, has good compliance, and reaches the international leading level.
Owner:山东省医学科学院附属医院
Granisetron membrane preparation and preparation method
The invention discloses a granisetron membrane preparation and a preparation method of the granisetron membrane preparation. Granisetron, membrane-forming material, water, plasticizers, surface active agents, preservatives, coloring agents and flavoring agents are used for preparing membrane agents. The granisetron can be uniformly dispersed in the membrane material in a molecule state, in an amorphous or micelle mode, the membrane agents can be rapidly melted when contacting with water. The granisetron membrane preparation has the advantages that the dissolving is quick and the action is rapid.
Owner:HEBEI AOXING GROUP PHARMA
A long-acting transdermal patch containing granisetron
ActiveCN105520921ARelease stabilitySkin irritationOrganic active ingredientsDigestive systemTransdermal patchMedicine
The invention discloses a long-acting transdermal patch containing granisetron. The transdermal patch comprises a lining membrane, a pressure-sensitive adhesive skeleton medicine-storing layer and an antisticking layer. The pressure-sensitive adhesive skeleton medicine-storing layer comprises mixed pressure-sensitive adhesives and the granisetron, wherein the mixed pressure-sensitive adhesives comprise a hydroxy-containing acrylate pressure-sensitive adhesive and a carboxyl-containing acrylate pressure-sensitive adhesive in a weight ratio of 1:5-2:1. The long-acting transdermal patch adopts the mixed pressure-sensitive adhesives as a skeleton is provided. The granisetron can exist in the pressure-sensitive adhesive skeleton stably in a dissolved state, does not crystallize or precipitate, and can be stably released on skin because of unique infiltration promoting effects of the transdermal patch. The transdermal patch is advantaged by low skin irritation.
Owner:BEIJING CAS MICRONEEDLE TECH LTD
Transdermal drug delivery system containing granisetron
Disclosed herein is a transdermal system in a matrix form capable of enhancing granisetron carrying efficiency and improving transdermal absorption while inhibiting recrystallization, which comprises: at least one transdermal enhancer selected from a group consisting of polyglyceryl-3 oleate, polyethyleneglycol-20 almond glyceride, polyethyleneglycol-12 palm kernel glyceride, isopropyl myristate and oleyl alcohol; and an acrylate polymer.
Owner:TAHO PHARMA
Transdermal composition of antivomiting agent and preparation containing the same
InactiveCN1364083AHydrocarbon active ingredientsHydroxy compound active ingredientsAlcoholOndansetron
A transdermal composition of the present invention comprises (a) a matrix containing (i) 20 to 80 % by weight of an alcohol, (ii) 1 to 50 % by weight of a skin penetration enhancer selected from the group consisting of a fatty acid and a derivative thereof, a fatty alcohol and a derivative thereof, an amide, a terpene, a surfactant and a mixture thereof, and (iii) 15 to 80 % by weight of water; and (b) 1 to 15 % by weight, based on the weight of the matrix, of an antivomiting agent selected from the group consisting of tropisetron, ondansetron, granisetron and pharmaceutically acceptable salts thereof, which is capable of delivering the antivomiting agent efficiently over a period of a day or more without skin irritation.
Owner:SAMYANG BIOPHARMLS CORP
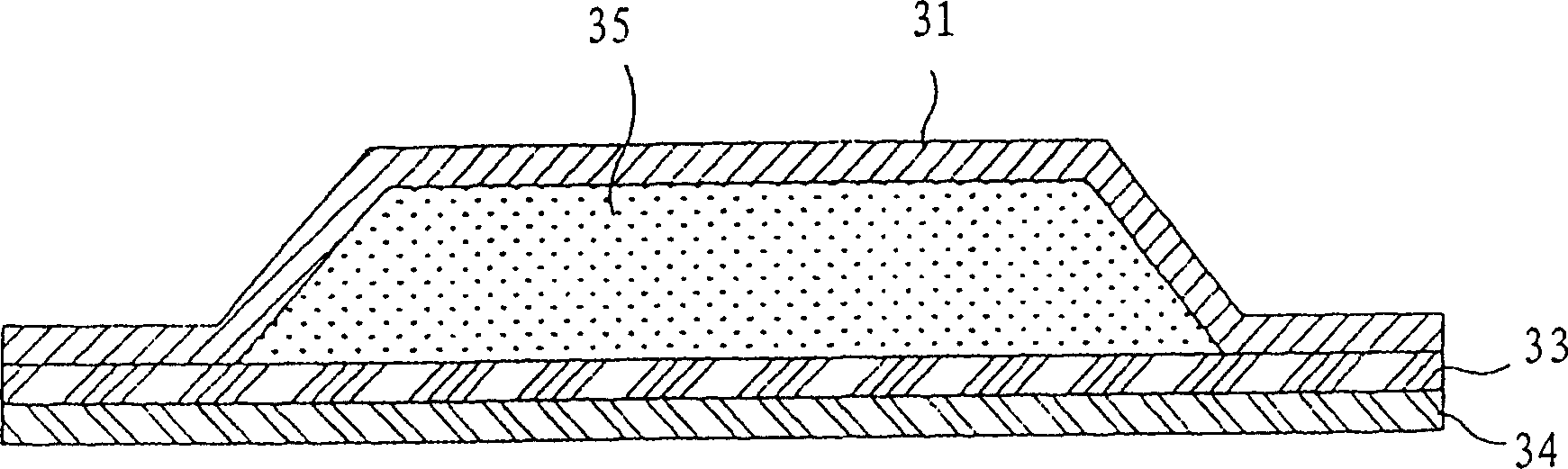
Patch used for treating nausea and vomiting, and preparation method thereof
InactiveCN103211800AEasy to dissolve and disperseOrganic active ingredientsDigestive systemTransdermal patchAcrylic adhesive
The invention relates to a transdermal patch used for treating nausea and vomiting, and a preparation method thereof. The invention provides a transdermic-absorption frame-work patch. A ransdermic-absorption preparation layer composed of an acrylic adhesive, isopropyl myristate, diethanolamine laurate, rosin ester, and granisetron with a weight ratio of 40-70:5-30:2-10:5-24:5-15 is laminated on one side of a backing layer which is non-permeable to medicine.
Owner:HENAN LINGRUI PHARMA +1
Adhesive skin patch containing serotonin receptor antagonist drug
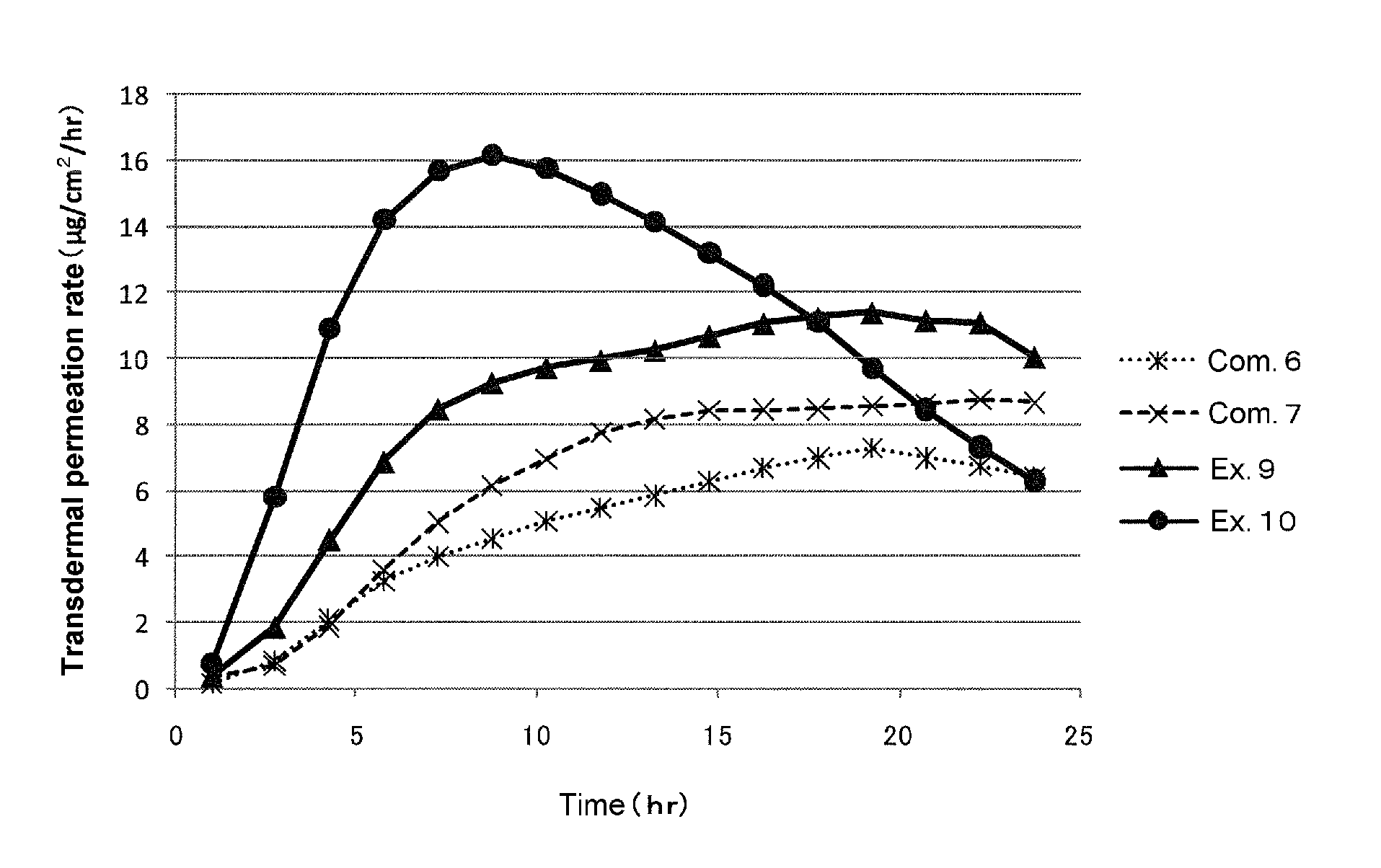
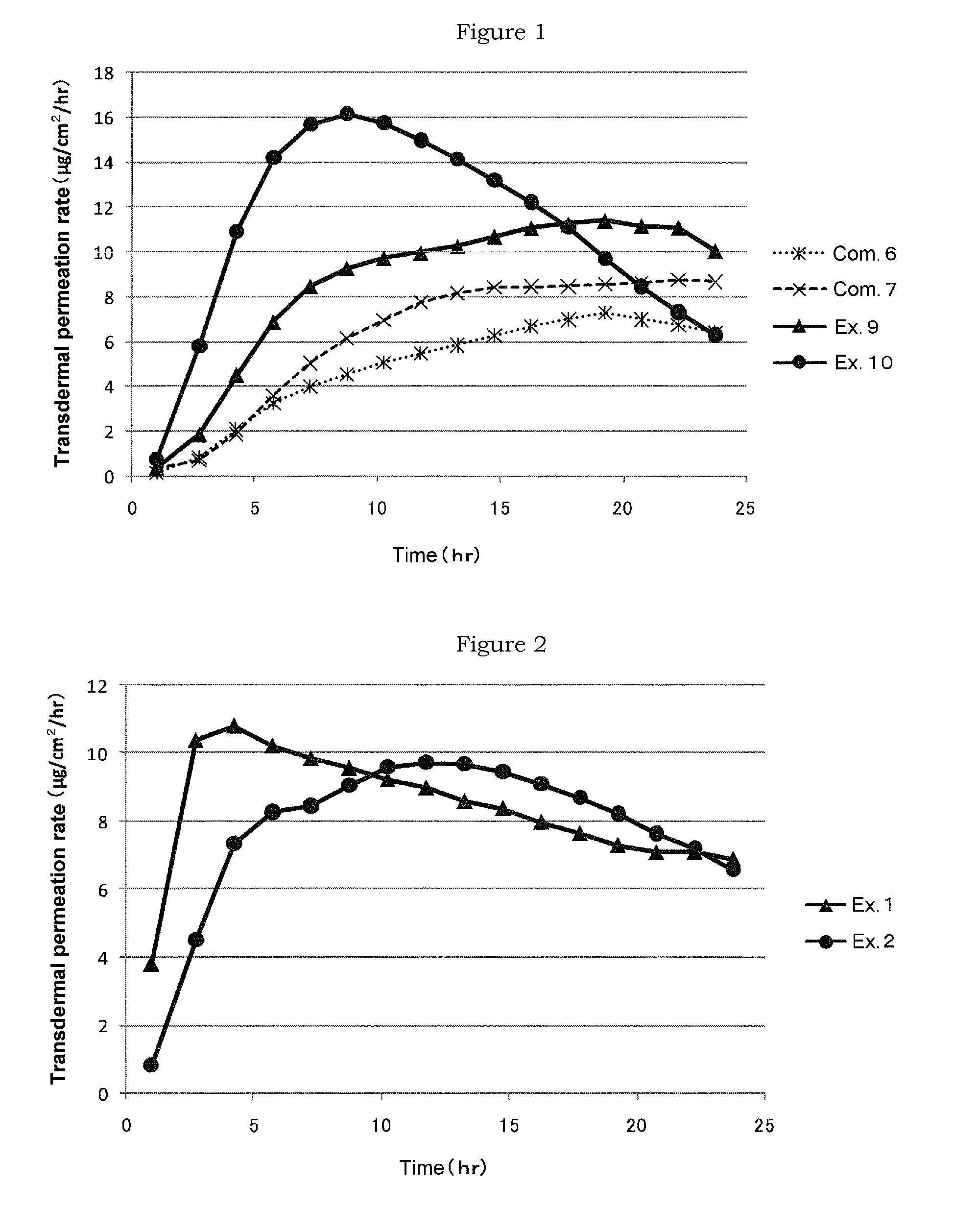
ActiveUS9205060B2Efficient transdermal absorptionMaximum transdermal permeation rateOrganic active ingredientsDigestive systemNK1 receptor antagonistBULK ACTIVE INGREDIENT
Provided is a transdermal absorption-type patch containing a serotonin receptor antagonist, which has a short transdermal absorption delay time (lag time), and in which the maximum transdermal permeation rate of the drug can be reached within a short time and the drug can disappear from circulating blood rapidly. That is, provided is a patch containing a serotonin receptor antagonist, wherein the patch contains an adhesive layer comprising a non-functional acrylic adhesive, a water-soluble organic amine, a fatty acid ester, and a serotonin receptor antagonist such as granisetron as an active ingredient.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Traditional Chinese medicine combination for preventing and treating delayed nausea and vomiting caused by chemotherapy and application thereof
ActiveCN104906539AEasy to acceptGood control effectHydroxy compound active ingredientsDigestive systemNausea sicknessHave Nausea
The invention discloses a traditional Chinese medicine combination for preventing and treating delayed nausea and vomiting caused by chemotherapy. The traditional Chinese medicine combination comprises, by weight, 20-25 parts of pinellia rhizoma prepared by ginger, 15-20 parts of cassia twigs, 10-15 parts of agastache rugosus, 10-15 parts of fructus amomi, 8-12 parts of fructus evodiae, 4-10 parts of inula flower, 4-6 parts of pericarpium citri reticulatae, 10-16 parts of ginger juice and 1-3 parts of menthol. The traditional Chinese medicine combination has the advantages of being rigorous in composition, easy to prepare, free of stimulus, low in cost, easy to operate and capable of being accepted by patients easily. The traditional Chinese medicine combination is combined with granisetron and dexamethasone for use, can well treat delayed nausea and vomiting and loss of appetite caused by cis-platinum, also can relieve the side effects, like constipation of patient, caused by the usage of the granisetron and dexamethasone, provides a novel way for delayed nausea and vomiting patients, fills the blank of treating the delayed nausea and vomiting through traditional Chinese medicine at home and abroad, and reaches the international leading level.
Owner:山东省医学科学院附属医院
Adhesive skin patch containing serotonin receptor antagonist drug
ActiveUS20140179734A1Efficient transdermal absorptionReduce latencyBiocideDigestive systemBULK ACTIVE INGREDIENTSkin patch
Provided is a transdermal absorption-type patch containing a serotonin receptor antagonist, which has a short transdermal absorption delay time (lag time), and in which the maximum transdermal permeation rate of the drug can be reached within a short time and the drug can disappear from circulating blood rapidly. That is, provided is a patch containing a serotonin receptor antagonist, wherein the patch contains an adhesive layer comprising a non-functional acrylic adhesive, a water-soluble organic amine, a fatty acid ester, and a serotonin receptor antagonist such as granisetron as an active ingredient.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Granisetron sustained-release microspheres and preparation method thereof
ActiveCN109718225AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsDigestive systemPoor complianceOrganic solvent
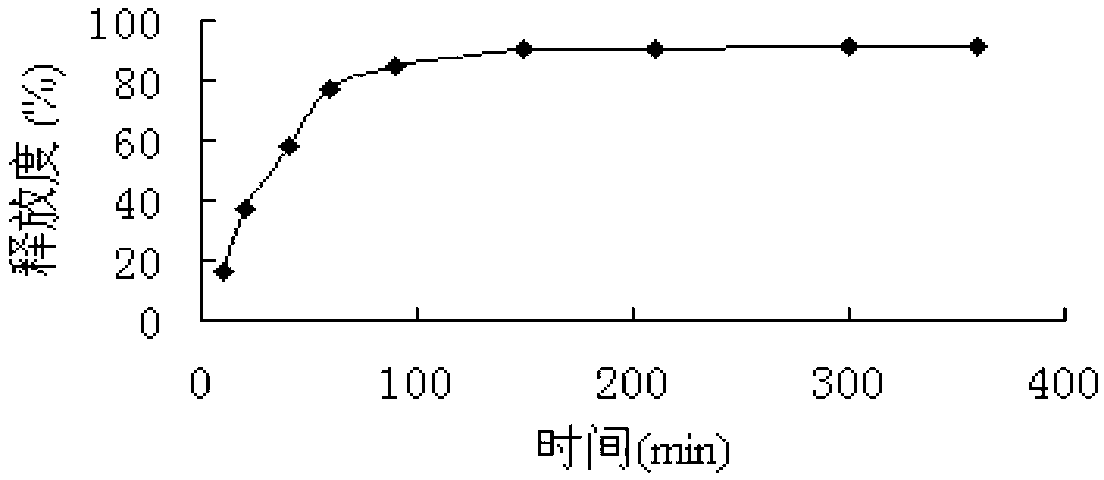
The invention relates to a preparation method of granisetron sustained-release microspheres. The method comprises the following steps: dissolving granisetron and a degradable polymer containing a hydrophobic chain segment in an organic solvent to obtain a drug-containing polymer solution; adding the drug-containing polymer solution to an aqueous phase with stirring to form O / W emulsion, and removing the organic solvent from the emulsion, wherein the aqueous phase contains a stabilizer, and the ratio of the stabilizer to the aqueous phase is 0.1-5% (w / v); and adding the obtained dispersion system into water for solidification under the condition of stirring to obtain the granisetron sustained-release microspheres. The granisetron sustained-release microspheres disclosed by the invention aresuitable in particle size, round in shape, high in encapsulation rate, good in drug loading rate, free from sudden release phenomenon, and capable of effectively solving the problems of short releasetime of a preparation and poor compliance of a patient.
Owner:SUZHOU UNIV
Transdermal systems for the delivery of therapeutic agents including granisetron using iontophoresis
A disposable skin-worn device for the transdermal delivery at least one dose of charged therapeutic substances, including granisetron, by iontophoresis, the device comprising a donor reservoir containing an amount of a therapeutic substance to be delivered transdermally by iontophoresis, a counter reservoir, a source of electric power connected in a circuit between the donor reservoir and the counter reservoir and a control system for controlling current flow in the circuit to enable at least one dose of the therapeutic substance to be delivered transdermally by iontophoresis and wherein the control system includes a control element selected from the group consisting of a sensor activated by an external signal and a switch.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Granisetron and/or hydrochloride patch thereof
ActiveCN101721394BIncreased percutaneous penetration rateImprove toleranceOrganic active ingredientsDigestive systemOrganic acidMedicine
The invention relates to a Granisetron and / or a hydrochloride patch thereof, which comprises a lining layer, a medicine carrying pressure sensitive adhesive layer containing the Granisetron or / and the hydrochloride thereof, and an anti-sticking layer, wherein the medicine carrying pressure sensitive adhesive layer is characterized in that pressure sensitive adhesive contains carboxylic groups; the content of the Granisetron or / and the hydrochloride thereof in percentage by weight is 1%-12%; the medicine carrying pressure sensitive adhesive layer contains metal ions capable of enabling the pressure sensitive adhesive to generate cross linking, and the content of the metal ions in the medicine carrying pressure sensitive adhesive layer in percentage by weight is 0.05%-2%; and the medicine carrying pressure sensitive adhesive layer contains organic acids with 2-18 carbon atoms, and the molar ratio of the adding amount of the organic acids to the adding amount of the Granisetron or / and the hydrochloride thereof is (1-5):1. The patch of the invention can obviously increase the percutaneous infiltration capacity of the Granisetron and improve the solvent resistant and ageing resistant action of the substrate, can be applied to preventing and treating the symptoms of nausea, emesis and the like caused by radiotherapy, chemotherapy and operations, and has great foreseeable economic value and social value.
Owner:DALIAN UNIV OF TECH +1
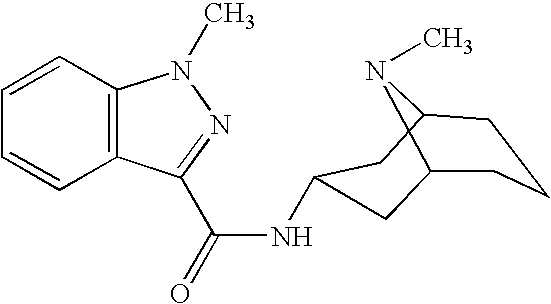
3 alpha-high tropine aliphatic amine salt, crystal formation and preparation method
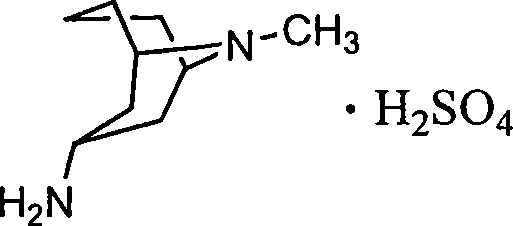
The invention relates to a 3 alpha-high tropine aliphatic amine salt, crystal formation and preparation method, which is to resolve problems of poor stability for preparing midbody of Granisetron, inconvenience for storage and transportation and low purity of final products. The 3 alpha-high tropine aliphatic amine is dissolved in one or more organic solvents and 1-10 equivalent hydrochloric acid, sulphuric acid or phosphoric acid are added in for crystallization and filtration after mixing to cool. 1 to 20-fold water and 10 to 100-fold alcohols solvent are used for recrystallization for cooling, crystallization, filtration and vacuum drying for 12 hours.
Owner:SINOPHARM CHUANKANG PHARMACEUTICAL CO LTD
Bioadhesive compositions for intranasal administration of granistron
InactiveUS20180117019A1Rapid and prolonged absorptionReduced nasal stinging sensationOrganic active ingredientsDigestive systemBioadhesiveIon
Sprayable aqueous pharmaceutical compositions containing granisetron or a pharmaceutically salt thereof, and pharmaceutically acceptable inactive ingredients, including tonicity agents, preservatives, and water soluble polymers with bioadhesive properties and / or capable of changing the rheological behavior in relation to ions, pH and temperature. The compositions are intranasally administered to a subject in need thereof in the rapid management and or prevention of nausea and / or vomiting induced by cytotoxic chemotherapy, radiation, or surgery. The composition has the advantages of rapid absorption and onset of action, prolonged drug plasma concentration and pharmacological effects comparable to intravenous infusion, as well as reduced nasal stinging sensation.
Owner:MAXINASE LIFE SCI LTD
Granisetron and dexamethasone compound percutaneous controlled release patch and preparation method thereof
InactiveCN103222977AGreat penetration promoting effectGood penetration enhancing effectOrganic active ingredientsDigestive systemCross-linkTectorial membrane
The invention relates to the field of pharmaceutic preparations and in particular relates to a granisetron and dexamethasone compound percutaneous controlled release patch and a preparation method thereof. The granisetron and dexamethasone compound percutaneous controlled release patch is characterized by being composed of a lining layer, a pressure-sensitive adhesive skeleton pattern medicine-containing storage layer and a protective film, wherein the pressure-sensitive adhesive skeleton pattern medicine-containing storage layer contains granisetron, dexamethasone, a transdermal penetration enhancer, a cross-linking agent, a plasticizer and pressure-sensitive adhesive, the transdermal penetration enhancer is azone preferably, and the content of azone is 2-5wt% based on the total weight of the pressure-sensitive adhesive skeleton pattern medicine-containing storage layer. The granisetron and dexamethasone compound percutaneous controlled release patch overcomes the defect that osmolality is unstable as difference between oil-water partition coefficients of granisetron and dexamethasone is large, and two medicines, namely granisetron and dexamethasone with different physical and chemical properties, have high osmolality and also maintain high blood concentration in a long time.
Owner:NANJING UNIV OF TECH
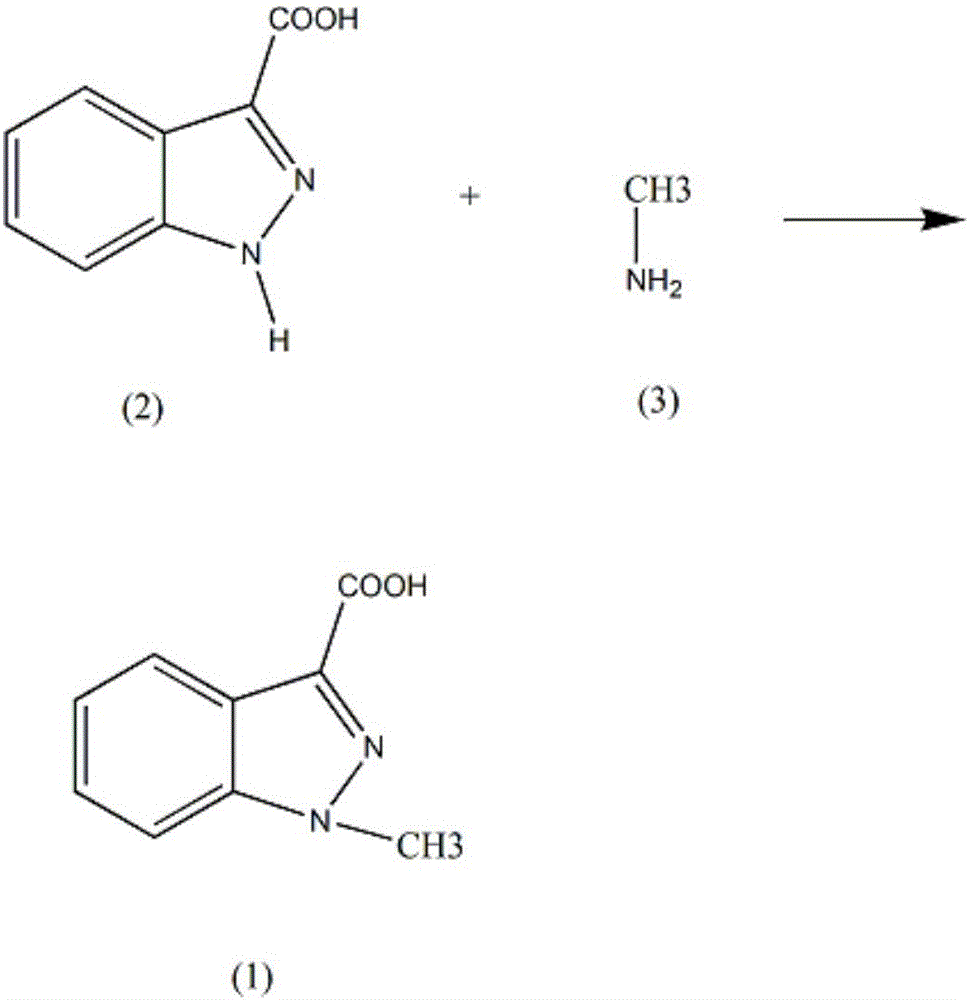
Synthesis method of intermediate 1-methylindole-3-carboxylic acid of granisetron drug
InactiveCN106432044AReduce intermediate linksLow reaction temperatureOrganic chemistryNitromethaneSodium sulfate
A synthesis method of intermediate 1-methylindole-3-carboxylic acid of a granisetron drug comprises the steps as follows: adding 130 ml of acrylonitrile and 0.32 mol of stannous chloride to a reaction vessel, controlling the stirring speed at 130 to 170 rpm, adding 0.053 mol of 1H-indazole-3-formic acid, increasing the temperature of the solution to 50-55 DEG C, preforming reflux for 5-6 h, slowly adding 0.061-0.063 mol of amine methane, performing reflux for 8-9 h, reducing the temperature of the solution to 5-9 DEG C, leaving the solution to stand for 30-32 h, separating a solid out, performing filtration, dissolving the solid in 150 ml of a potassium bromide solution, performing filtration, adding an oxalic acid solution to adjust the pH of the solution to 3-4, separating a solid out, performing suction filtration, performing washing with a saline solution and washing with nitromethane, performing dehydration with a dehydrating agent, and performing recrystallization in ethyl acetate to obtain 1-methylindole-3-carboxylic acid crystals; the saline solution in the step is any one of sodium sulfate and potassium nitrate.
Owner:XIAMEN KAI ER LI INFORMATION TECH CO LTD
Trans-dermal composition of anti-vomiting agent and preparation containing the same
InactiveCN1209108CHydrocarbon active ingredientsHydroxy compound active ingredientsFatty acidFatty alcohol
A transdermal composition of the present invention comprises (a) a matrix containing (i) 20 to 80 % by weight of an alcohol, (ii) 1 to 50 % by weight of a skin penetration enhancer selected from the group consisting of a fatty acid and a derivative thereof, a fatty alcohol and a derivative thereof, an amide, a terpene, a surfactant and a mixture thereof, and (iii) 15 to 80 % by weight of water; and (b) 1 to 15 % by weight, based on the weight of the matrix, of an antivomiting agent selected from the group consisting of tropisetron, ondansetron, granisetron and pharmaceutically acceptable salts thereof, which is capable of delivering the antivomiting agent efficiently over a period of a day or more without skin irritation.
Owner:SAMYANG BIOPHARMLS CORP
Compound preparation used for treating melancholia and senile dementia
ActiveCN101850119AEffective therapeutic effectNervous disorderHeterocyclic compound active ingredientsHeadachesAprepitant
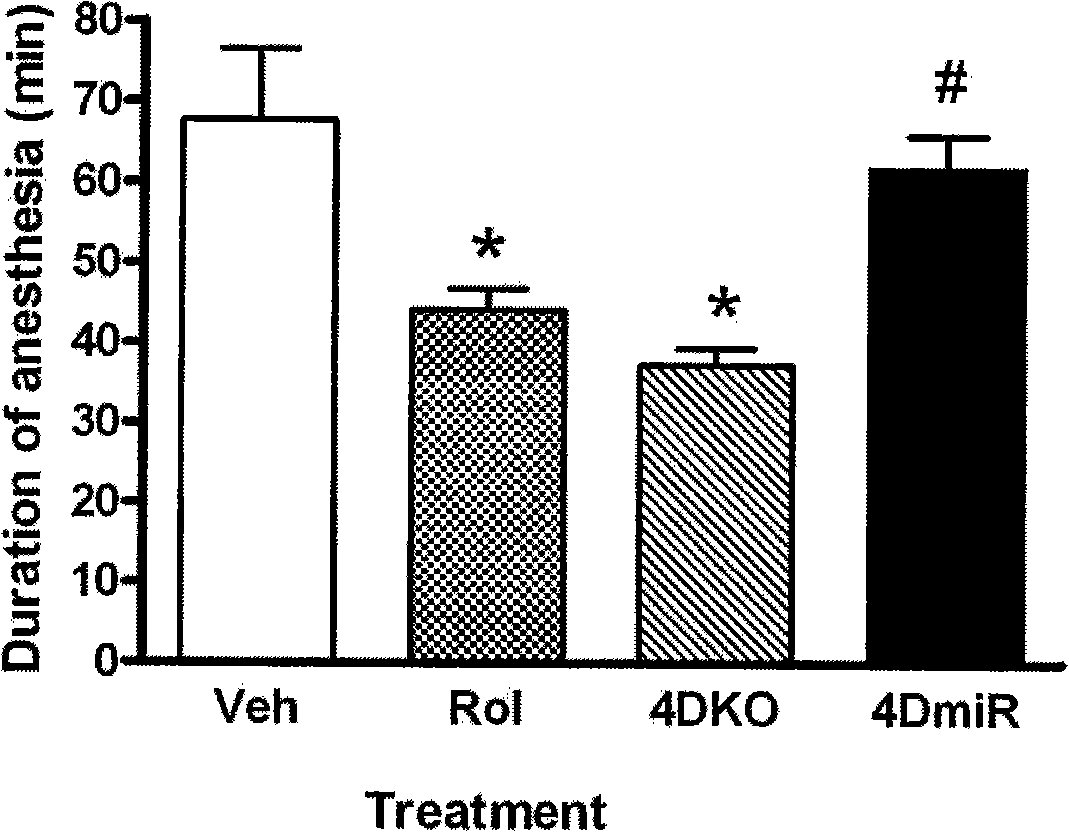
The invention discloses a compound preparation used for treating depression and senile dementia. The compound preparation comprises the following components in part by weight: 1 part of rolipram and 0.25 to 200 parts of antiemetic medicament, wherein the antiemetic medicament is granisetron, ondansetron, tropisetron, aprepitant or metoclopramide. The compound preparation is prepared from the rolipram and the granisetron, the ondansetron, the tropisetron, the aprepitant or the metoclopramide. The compound preparation has an effective curative effect of treating the melancholia and the senile dementia, and simultaneously solves the technical problems that in the prior art, the rolipram causes adverse reactions such as vomition, sicchasia, headache and the like.
Owner:兰晟生物医药(苏州)有限公司
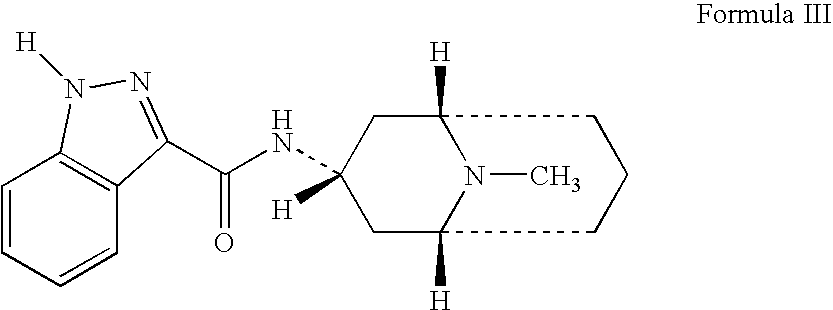
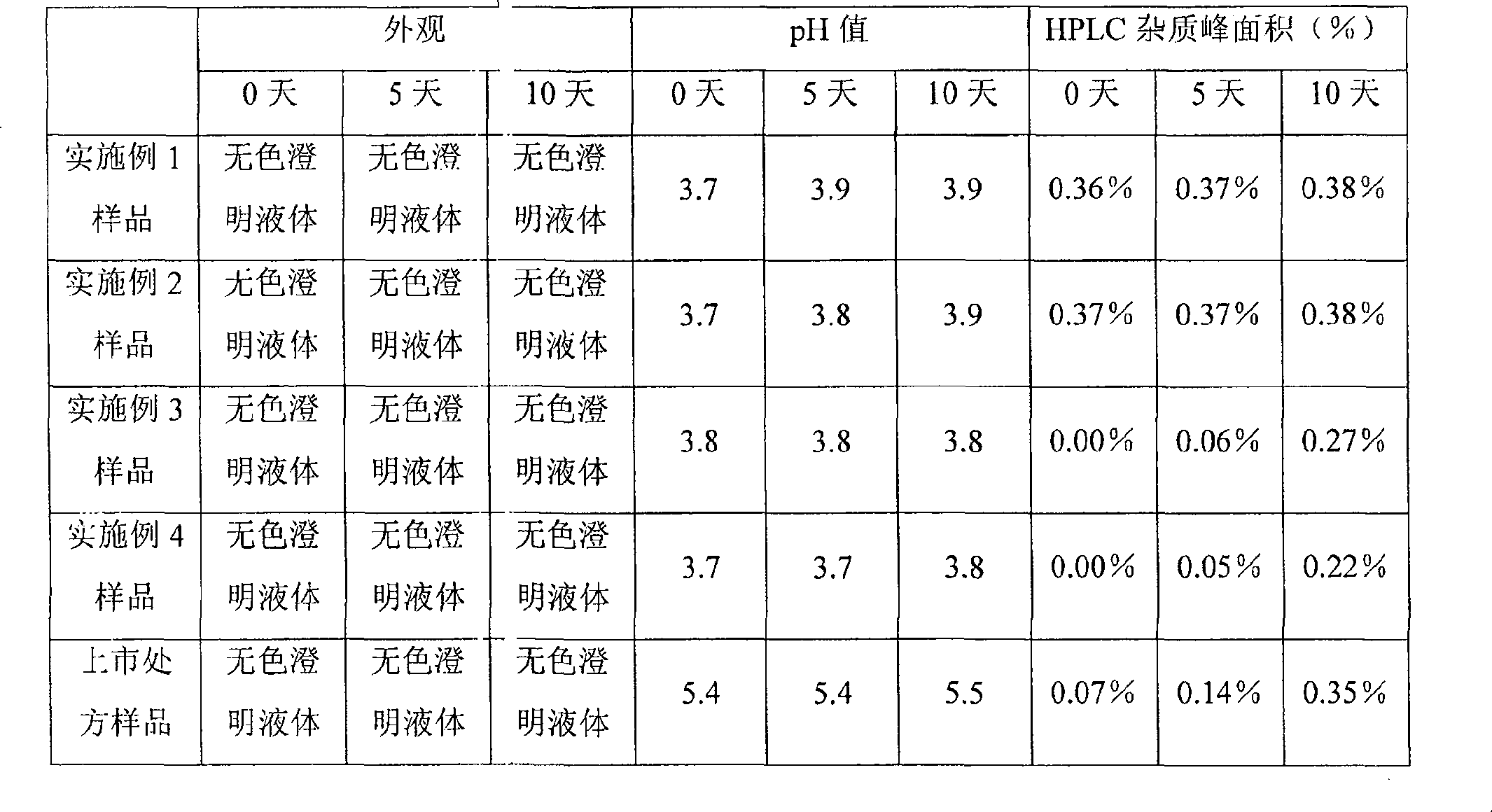
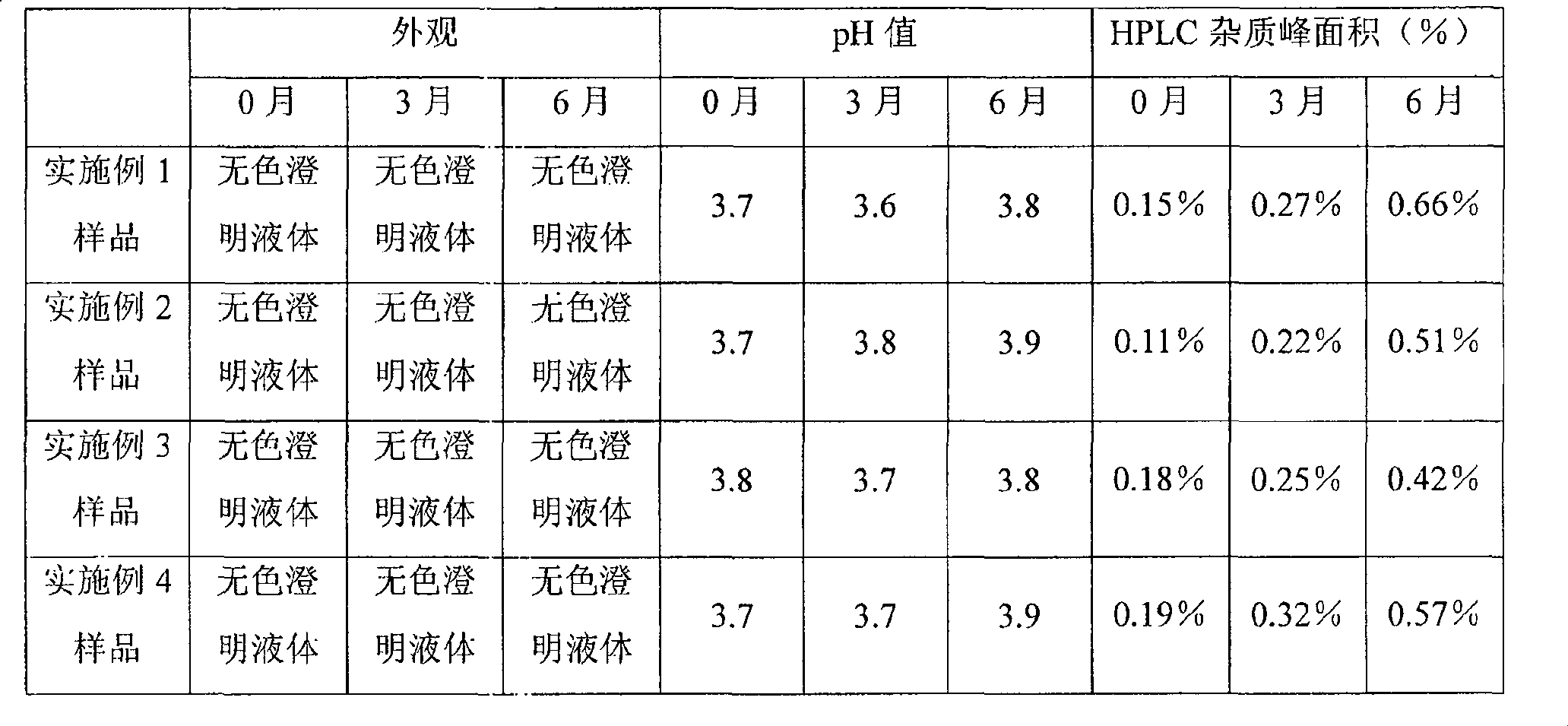
Granisetron compositions
Stable liquid pharmaceutical compositions comprise granisetron or a pharmaceutically acceptable salt thereof.
Owner:DR REDDYS LAB LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com