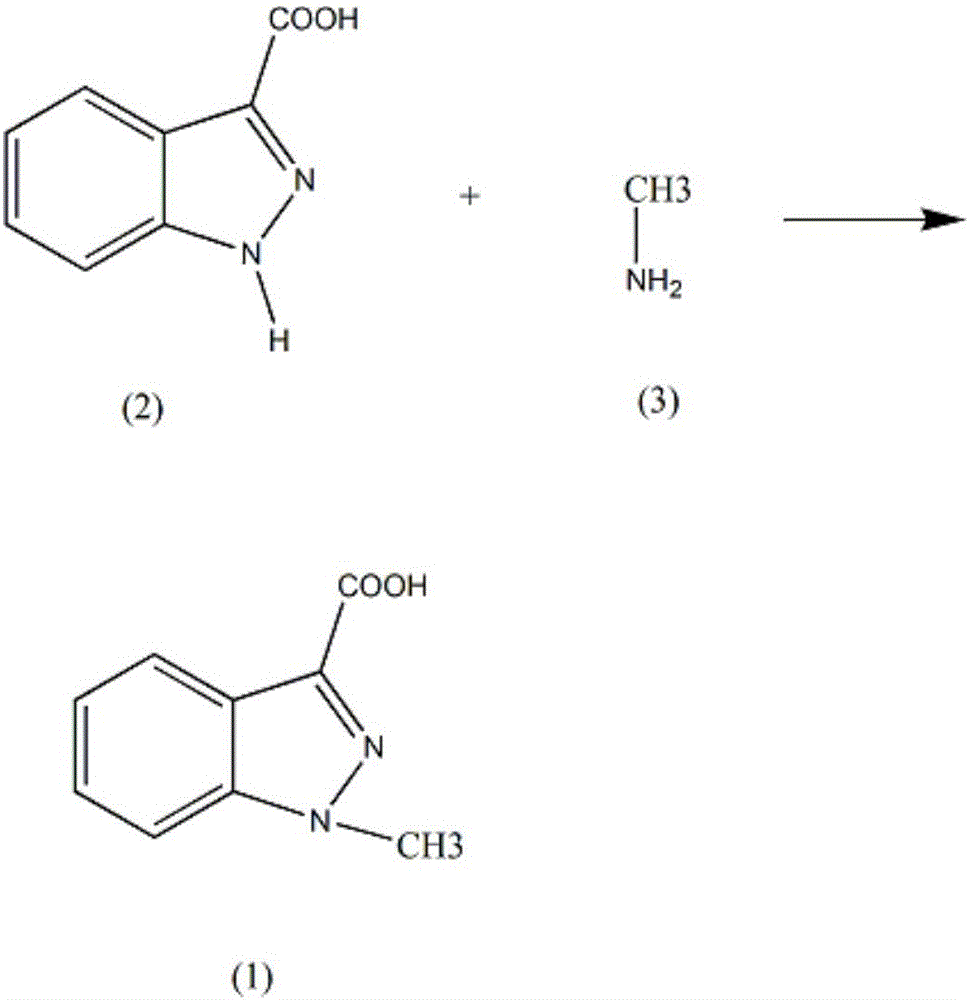
Synthesis method of intermediate 1-methylindole-3-carboxylic acid of granisetron drug
A technology of methyl indole and granisetron, applied in the field of synthesis of granisetron drug intermediate 1-methyl indole-3-carboxylic acid, to reduce reaction temperature and reaction time, increase reaction yield, reduce The effect of the middle link
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0011] Add 130ml of propionitrile and 0.32mol of stannous chloride into the reaction vessel, control the stirring speed at 130rpm, add 0.053mol of 1H-indazole-3-carboxylic acid (2), raise the temperature of the solution to 50°C, reflux for 5h, and slowly add the amine Methane (3) 0.061mol, reflux for 8h, lower the solution temperature to 5°C, let stand for 30h, precipitate solid, filter, dissolve the solid in 150ml of 15% potassium bromide solution, filter and add 30% oxalic acid The pH of the solution was adjusted to 3, and the solid was precipitated, filtered with suction, washed with sodium sulfate solution, washed with nitromethane, dehydrated with anhydrous magnesium sulfate, and recrystallized in 90% ethyl acetate to obtain crystalline 1-methylindole -3-carboxylic acid 8.02g, yield 86%.
example 2
[0013] Add 130ml of propionitrile and 0.32mol of stannous chloride into the reaction vessel, control the stirring speed at 150rpm, add 0.053mol of 1H-indazole-3-carboxylic acid (2), raise the temperature of the solution to 53°C, reflux for 5h, and slowly add the amine Methane (3) 0.062mol, reflux for 8h, lower the solution temperature to 5°C, let it stand for 31h, precipitate solid, filter, dissolve the solid in 150ml of 17% potassium bromide solution with a mass fraction of 17%, add oxalic acid with a mass fraction of 32% after filtration The pH of the solution was adjusted to 3, and a solid was precipitated, filtered with suction, washed with potassium nitrate solution, washed with 82% nitromethane, dehydrated with phosphorus pentoxide, and recrystallized in 92% ethyl acetate to obtain crystal 1 -Methylindole-3-carboxylic acid 8.30g, yield 89%.
example 3
[0015] Add 130ml of propionitrile and 0.32mol of stannous chloride into the reaction vessel, control the stirring speed at 170rpm, add 0.053mol of 1H-indazole-3-carboxylic acid (2), raise the temperature of the solution to 55°C, reflux for 6h, and slowly add the amine Methane (3) 0.063mol, reflux for 9h, reduce the solution temperature to 9°C, let it stand for 32h, precipitate solid, filter, dissolve the solid in 150ml of 20% potassium bromide solution, filter and add 35% oxalic acid The pH of the solution was adjusted to 4, and a solid was precipitated, suction filtered, washed with sodium sulfate solution, washed with 85% nitromethane, dehydrated with anhydrous magnesium sulfate, and recrystallized in 95% ethyl acetate to obtain crystal 1 -Methylindole-3-carboxylic acid 8.68g, yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com