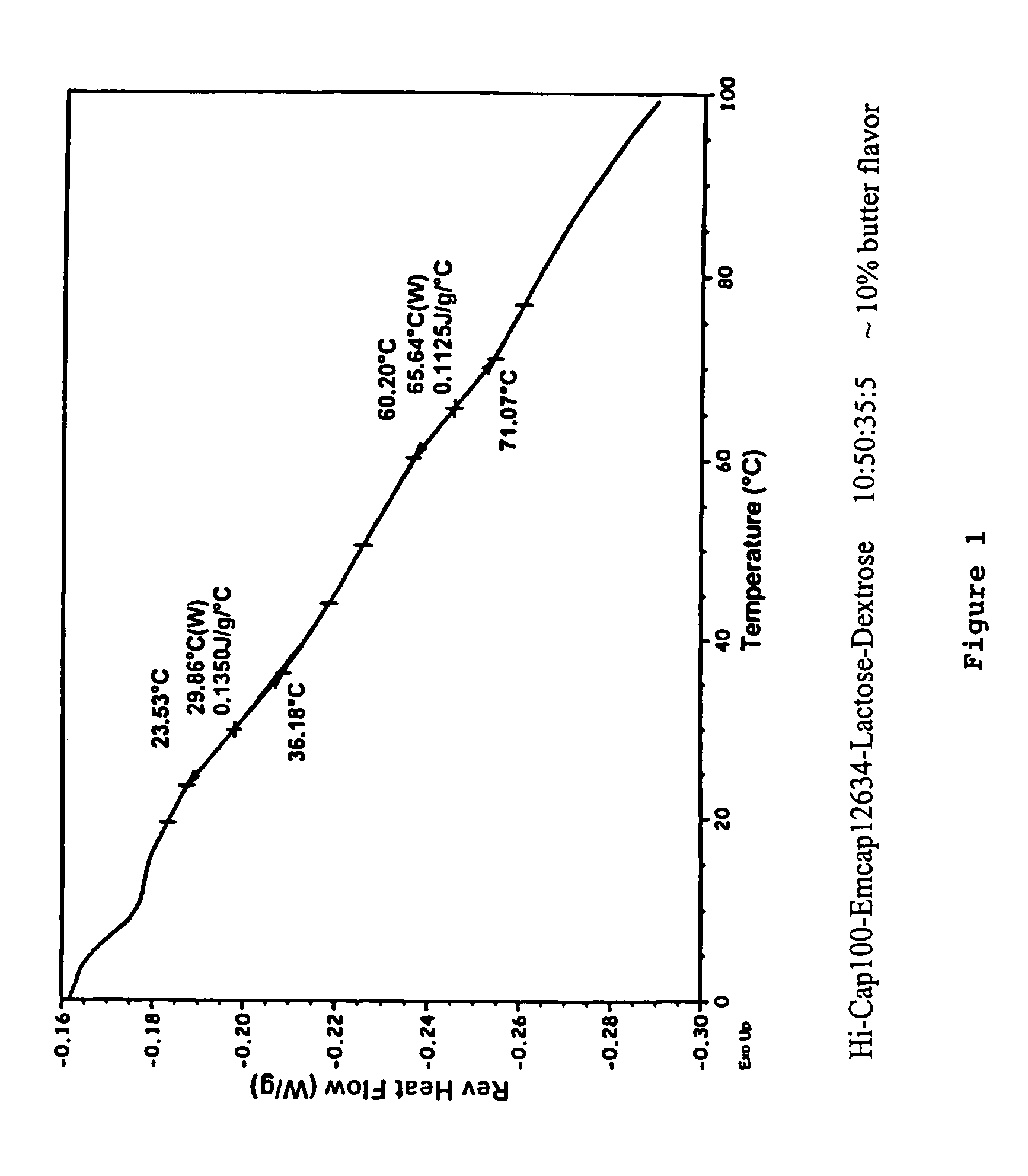
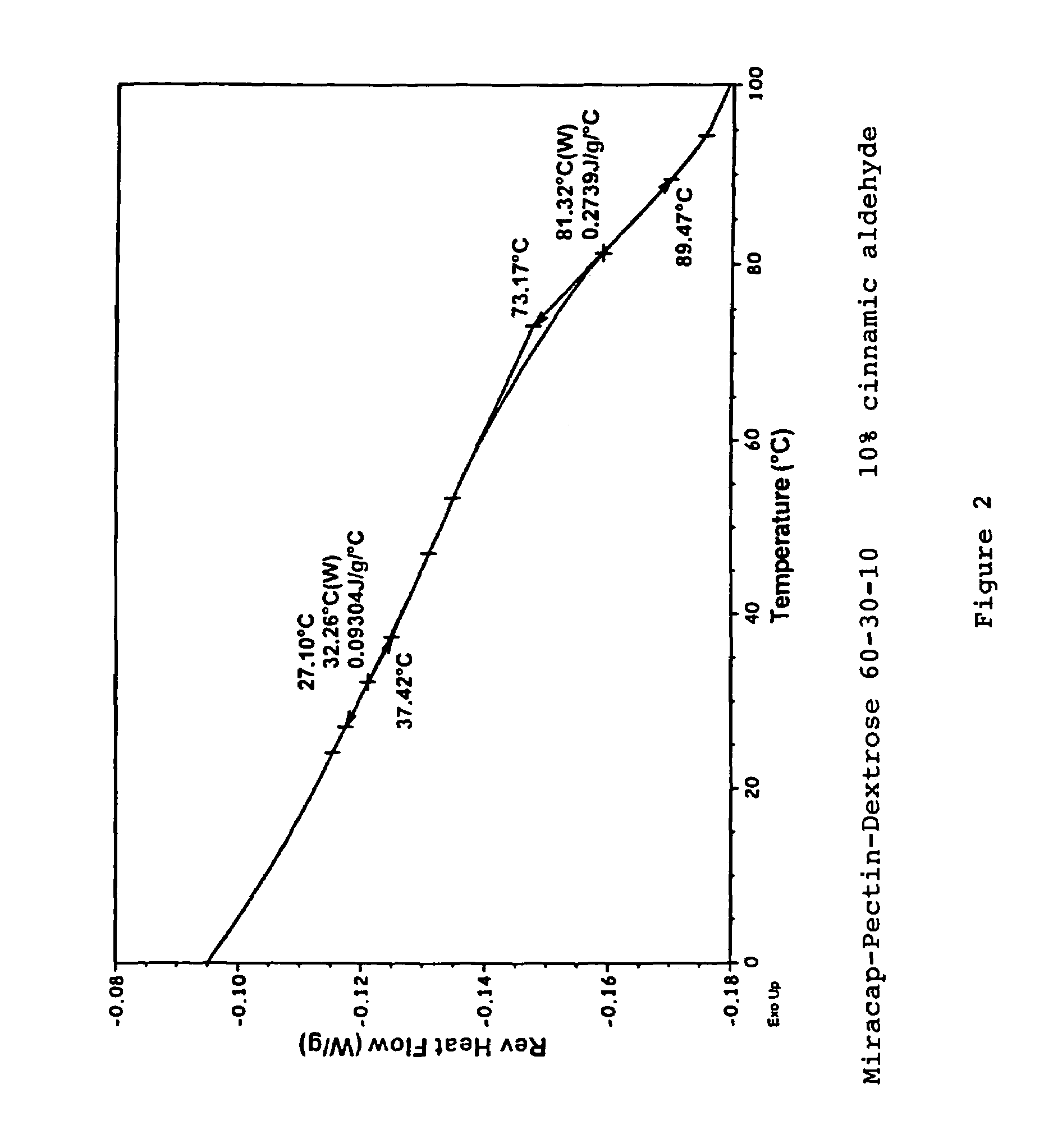
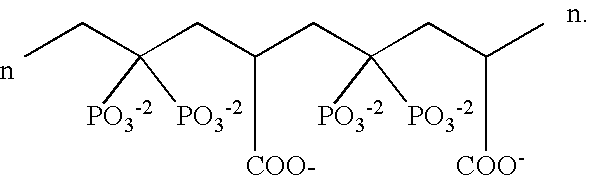
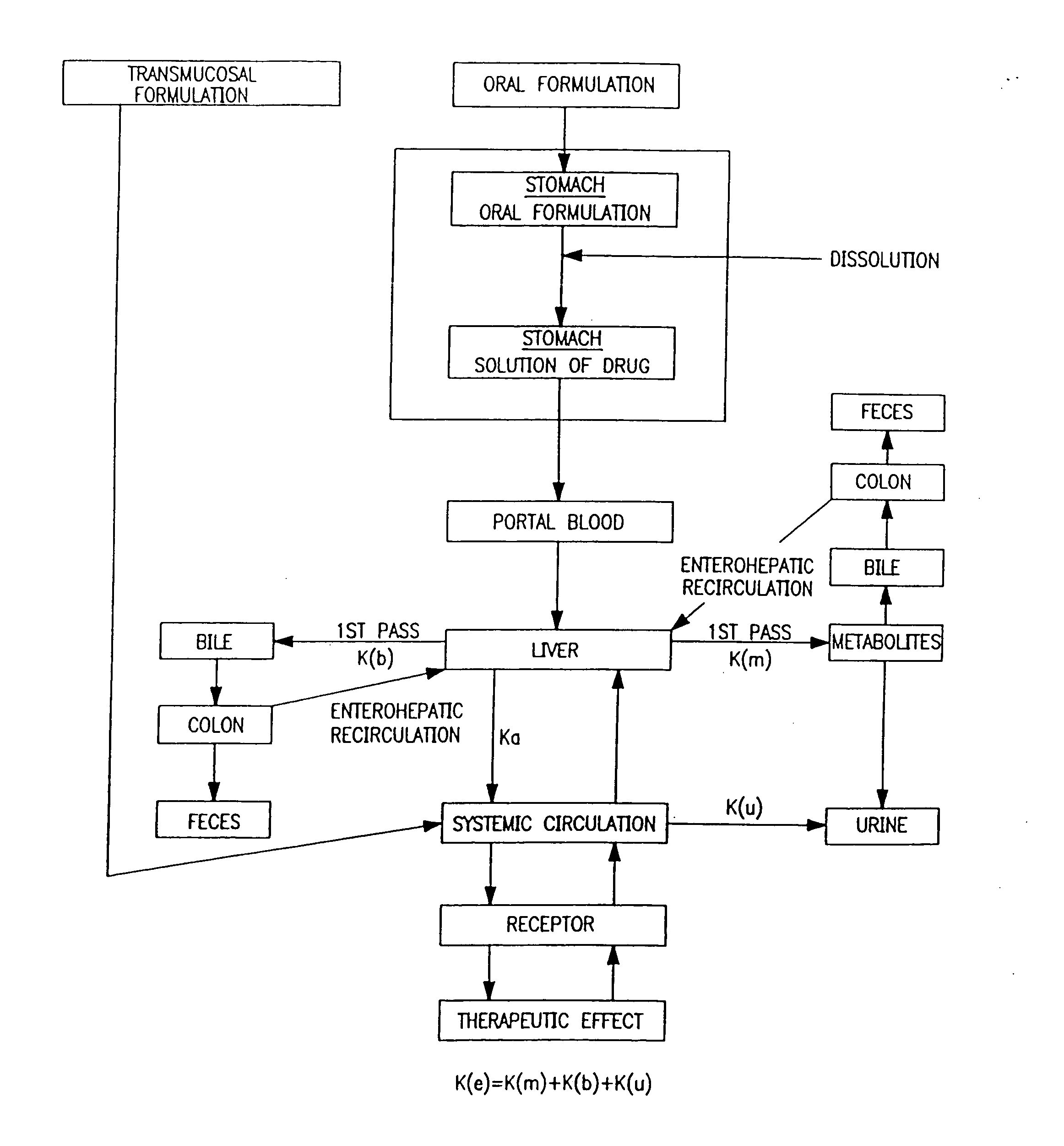
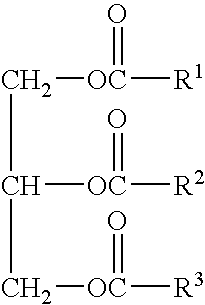
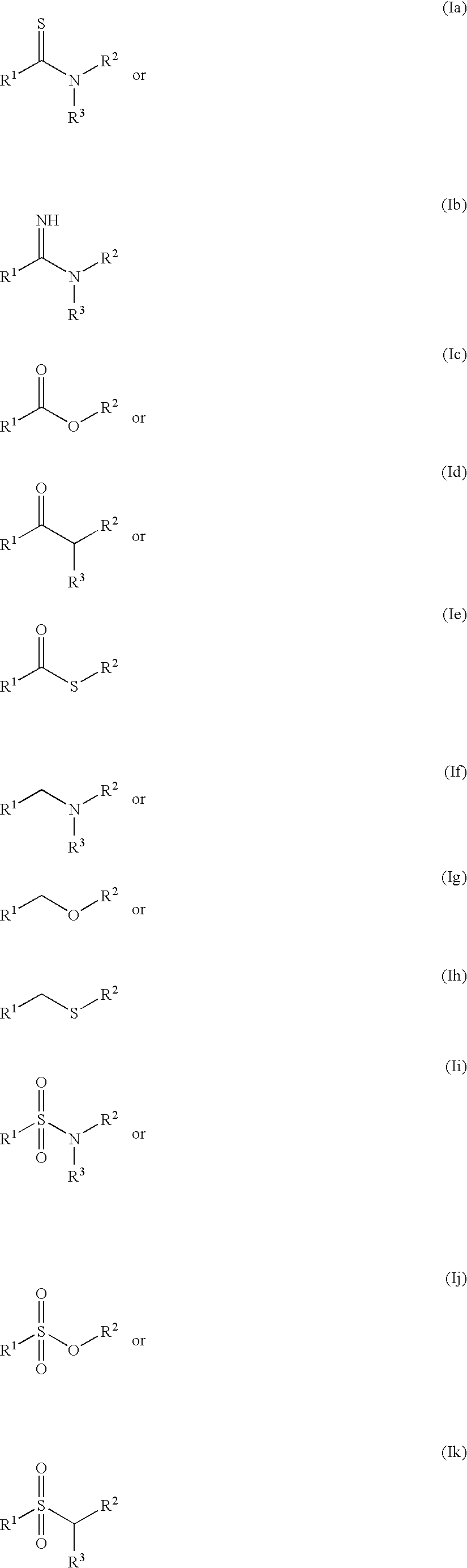
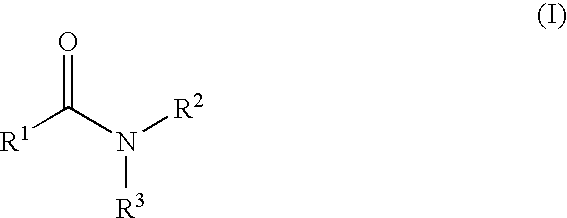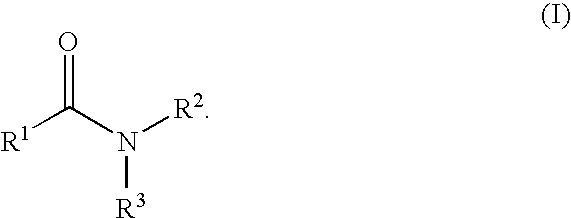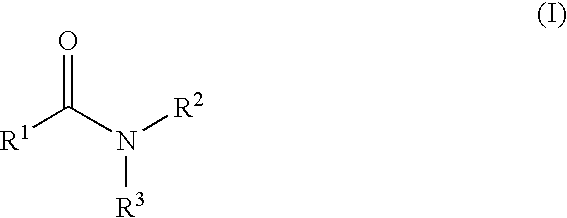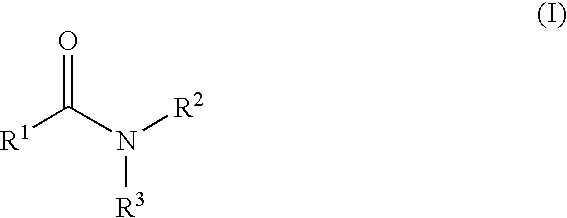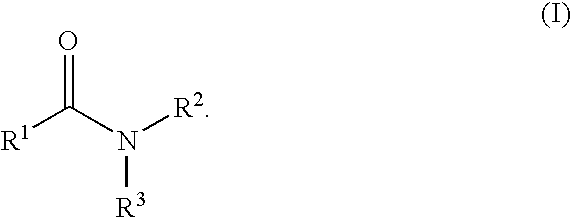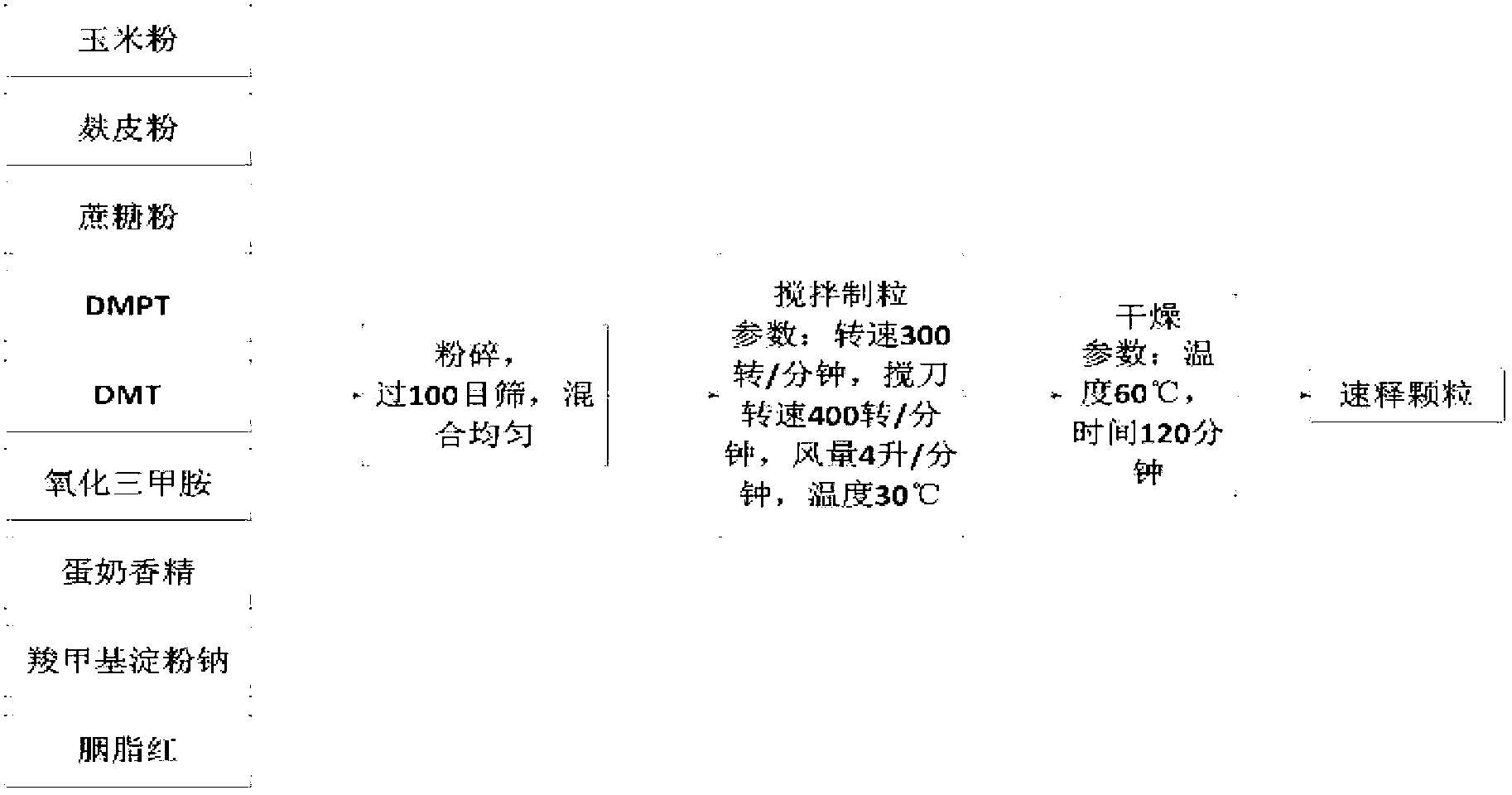Patents
Literature
4681 results about "Flavoring Agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Substances added to foods and medicine to improve the taste.
Novel flavors, flavor modifiers, tastants, taste enhancers, umami or sweet tastants, and/or enhancers and use thereof
ActiveUS20050084506A1Enhancing savory tasteIncrease sweet tasteBiocideCosmetic preparationsMonosodium glutamateSalty taste
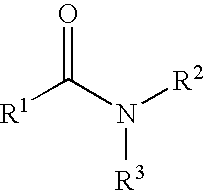
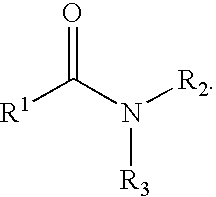
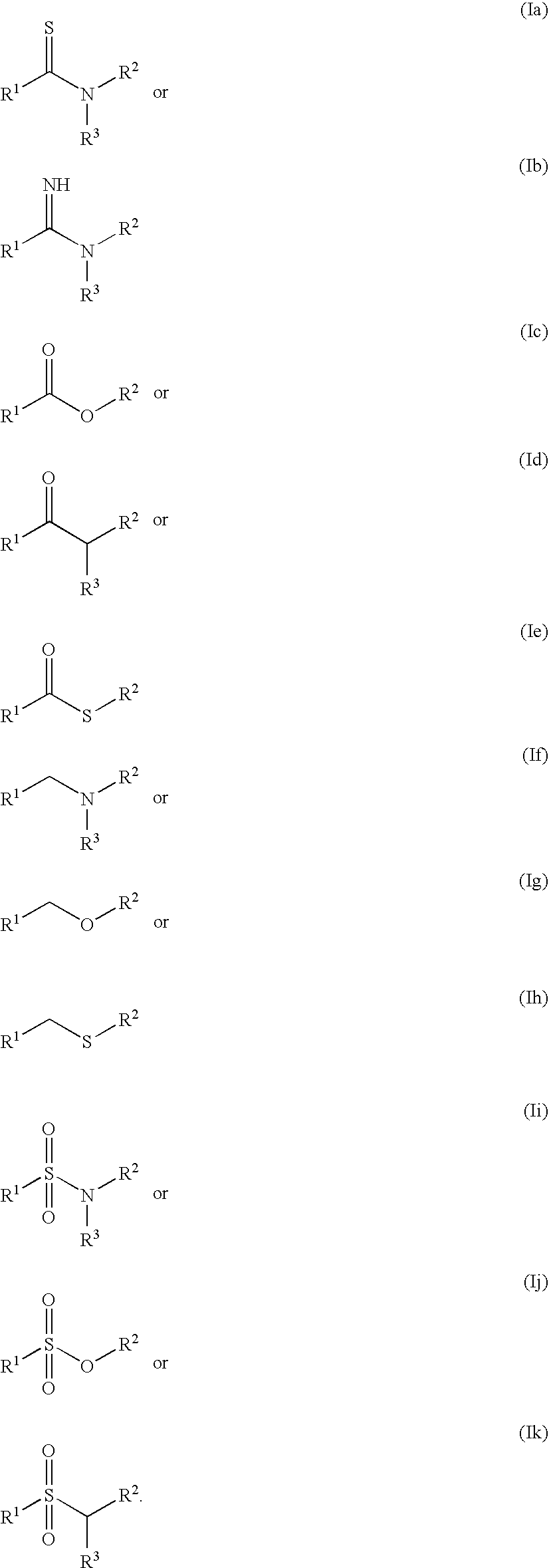
The present invention relates to the discovery that certain non-naturally occurring, non-peptide amide compounds and amide derivatives, such as oxalamides, ureas, and acrylamides, are useful flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancer, more particularly, savory (the “umami” taste of monosodium glutamate) or sweet taste modifiers,—savory or sweet flavoring agents and savory or sweet flavor enhancers, for food, beverages, and other comestible or orally administered medicinal products or compositions.
Owner:SENOMYX INC
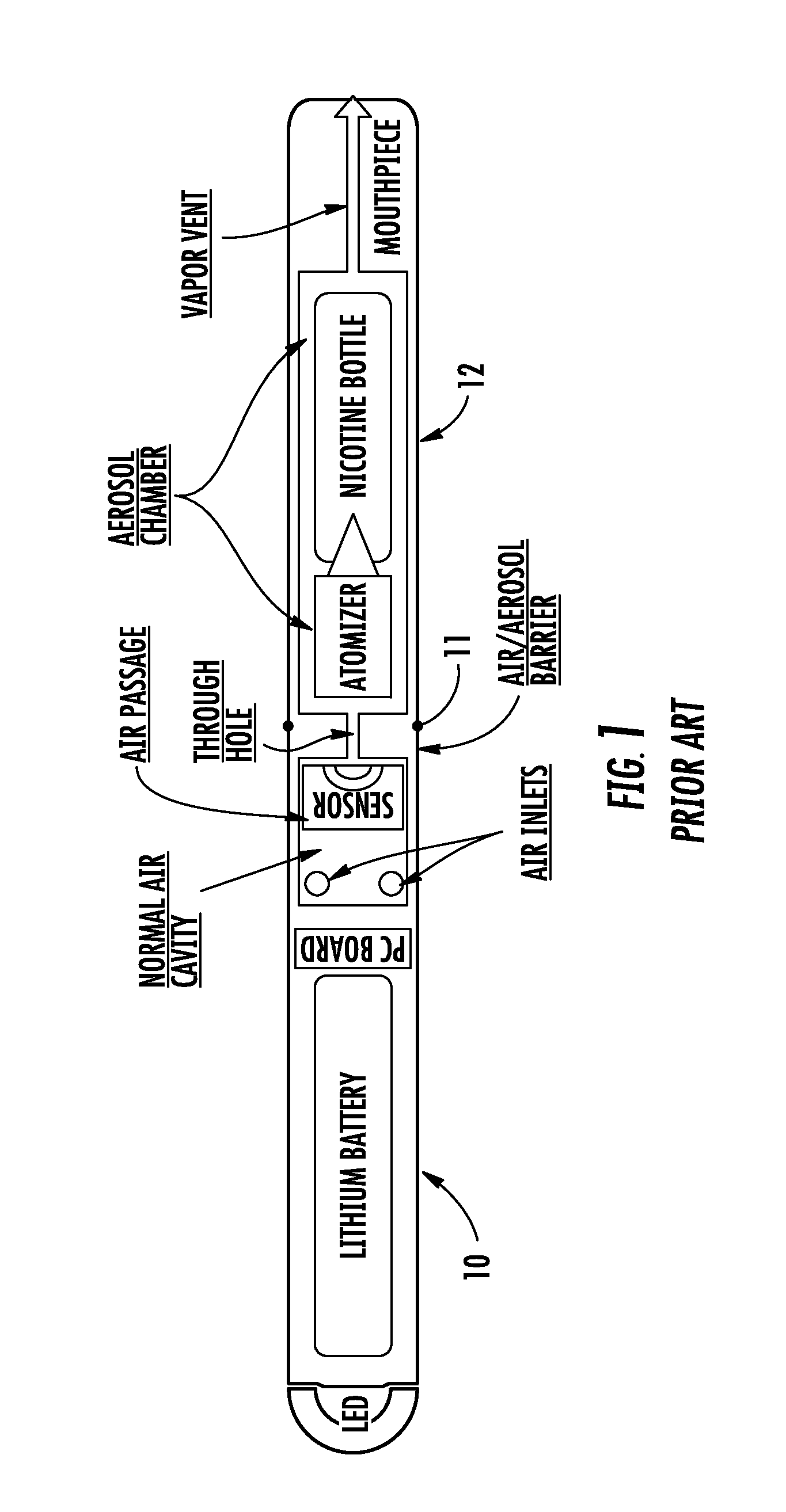
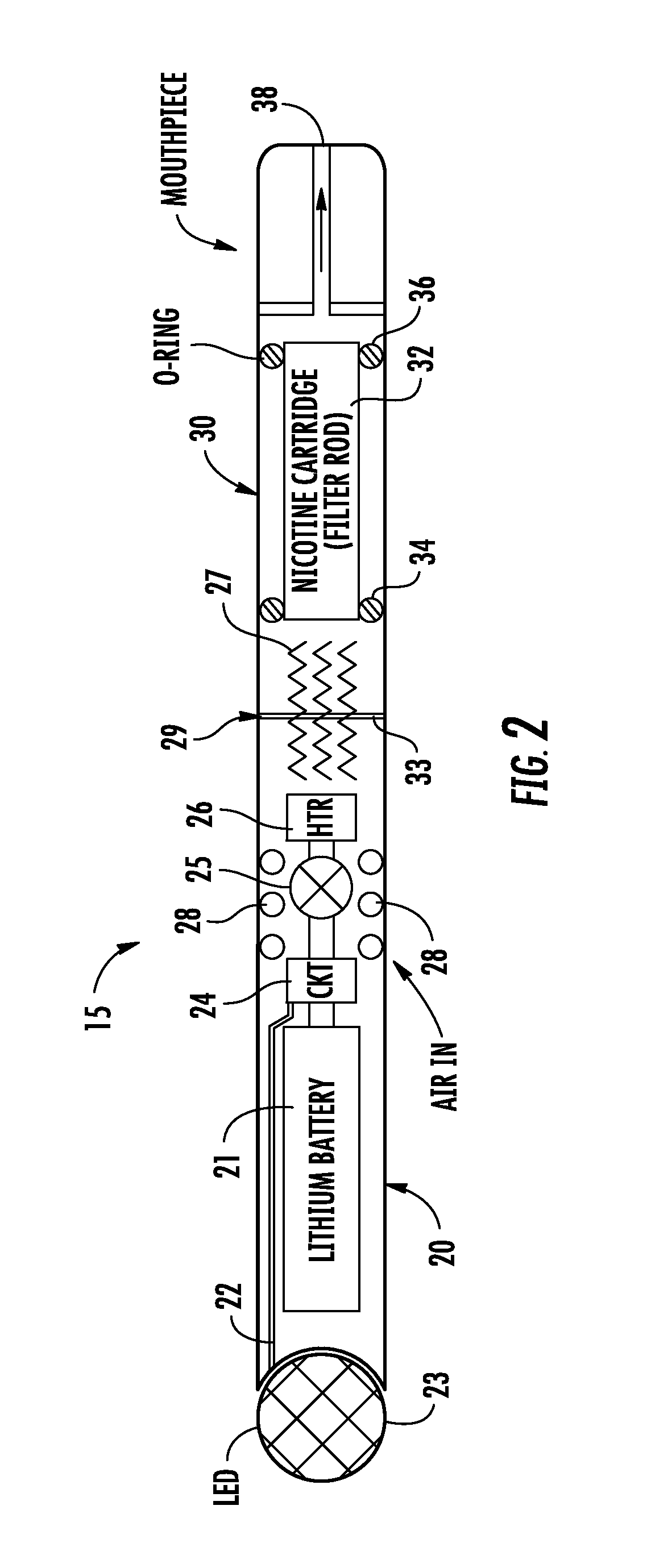
Electronic cigarette
InactiveUS20150351456A1Reduce manufacturing costImprove efficiencyTobacco pipesTobacco devicesRechargeable cellElectronic cigarette
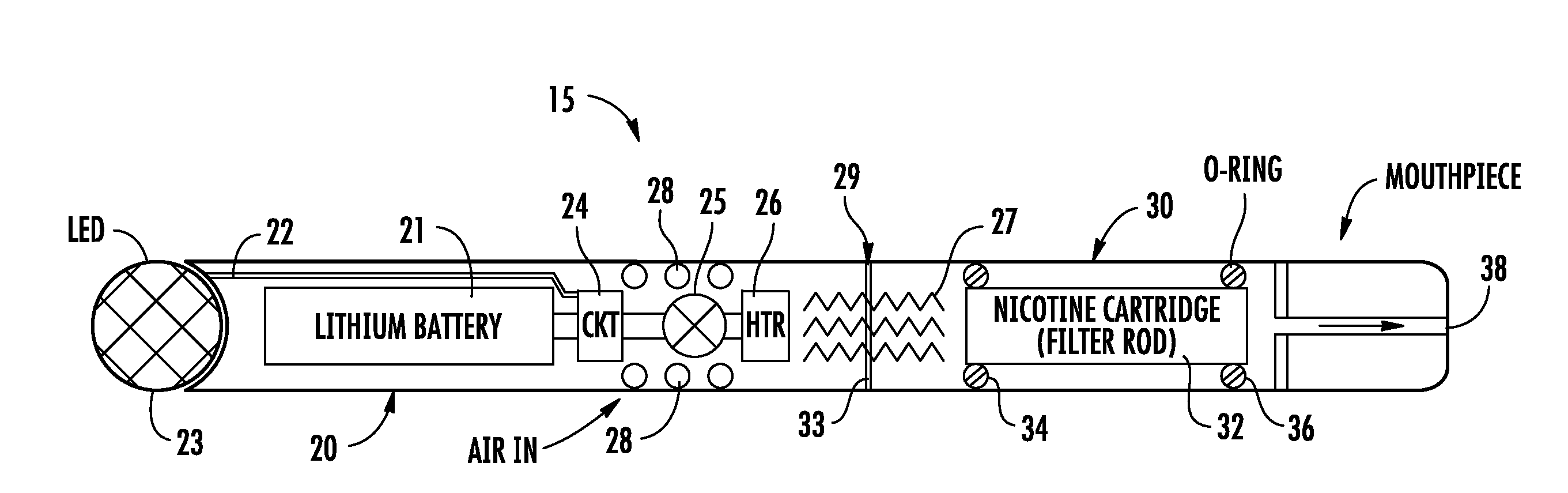
An electronic cigarette is provided in two or three sections. One section is a reusable barrel containing the electrical components, including a rechargeable battery and a heater or atomizer. Another section may include the atomizer and a replaceable and disposable cartridge having the desired volatile substance, such as nicotine, a medicament, and / or a flavoring agent.
Owner:L PERRIGO
Universal protein formulation meeting multiple dietary needs for optimal health and enhancing the human immune system
InactiveUS20060280840A1Weight controlGood for healthVitamin food ingredientsInorganic compound food ingredientsWeight gainingAdditive ingredient
The invention includes a protein rich, dry dietary supplement comprising a blend of legume protein, whey protein, egg white, calcium caseinate and powdered skim milk that is specifically formulated for weight control without the use of artificial appetite suppressants, but instead provides beneficial nutrients and supplements that naturally curb the appetite for specified periods of time. A preferred set of ingredients includes a protein blend combined with additional nutrients, vitamins, minerals and flavorings to enhance taste and further control the need for caloric intake. Various other preferred forms of the invention provide for implementation to allow for weight gain or weight maintenance of individuals desirous of the use of the beverage as a dietary supplement for those purposes.
Owner:ROBERTSON MARION G
Aromatic amides and ureas and their uses as sweet and/or umami flavor modifiers, tastants and taste enhancers
InactiveUS20060045953A1Improve responseInduce savory taste perceptionFood preparationMonosodium glutamateSalty taste
The inventions disclosed herein relate to non-naturally occurring amide compounds that are capable, when contacted with comestible food or drinks or pharmaceutical compositions at concentrations preferably on the order of about 100 ppm or lower, of serving as savory (“umami”) or sweet taste modifiers, savory or sweet flavoring agents and savory or sweet flavor enhancers, for use in foods, beverages, and other comestible or orally administered medicinal products or compositions, optionally in the presence of or in mixtures with conventional flavoring agents such as monosodium glutamate or known natural and artificial sweeteners.
Owner:SENOMYX INC
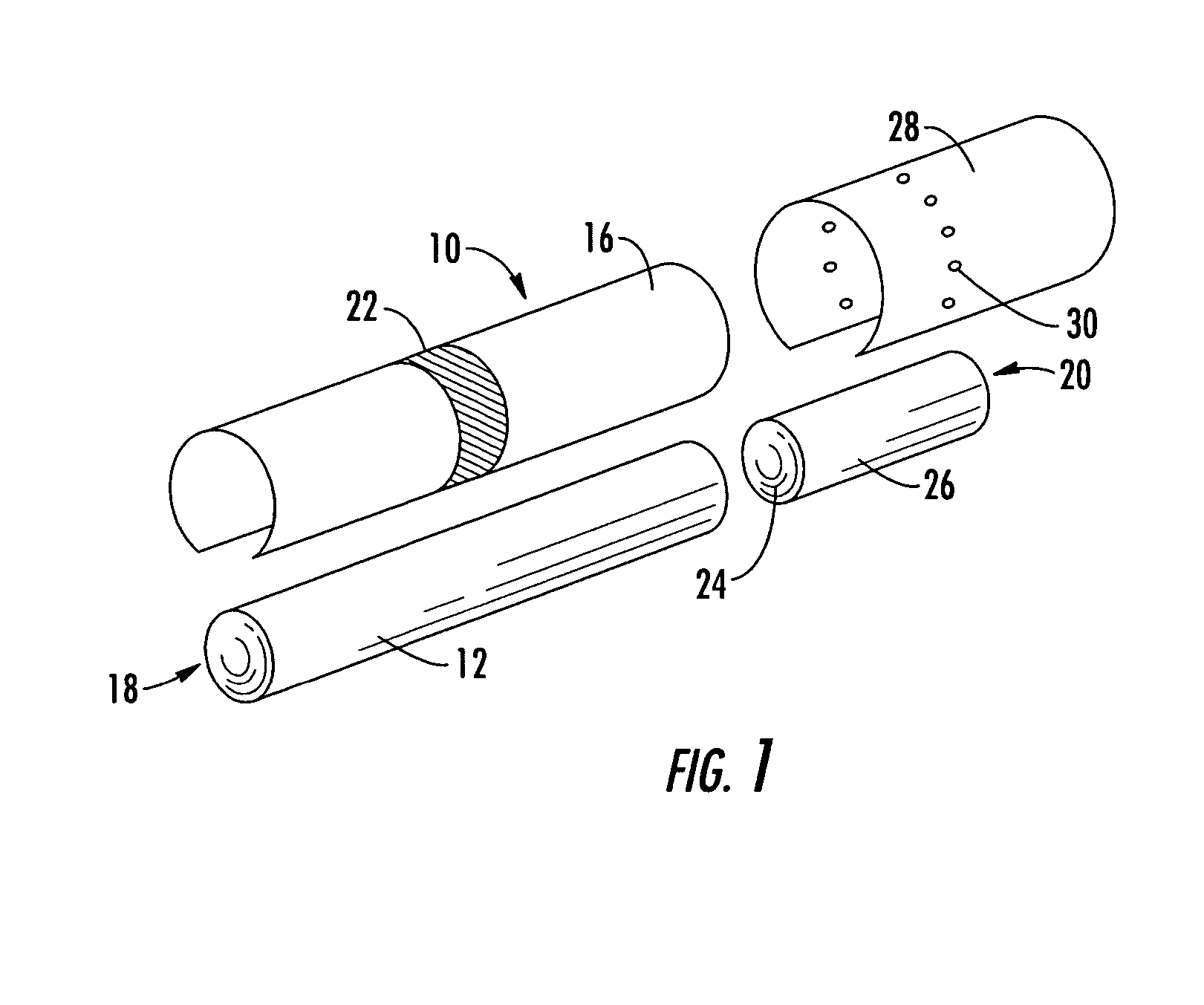
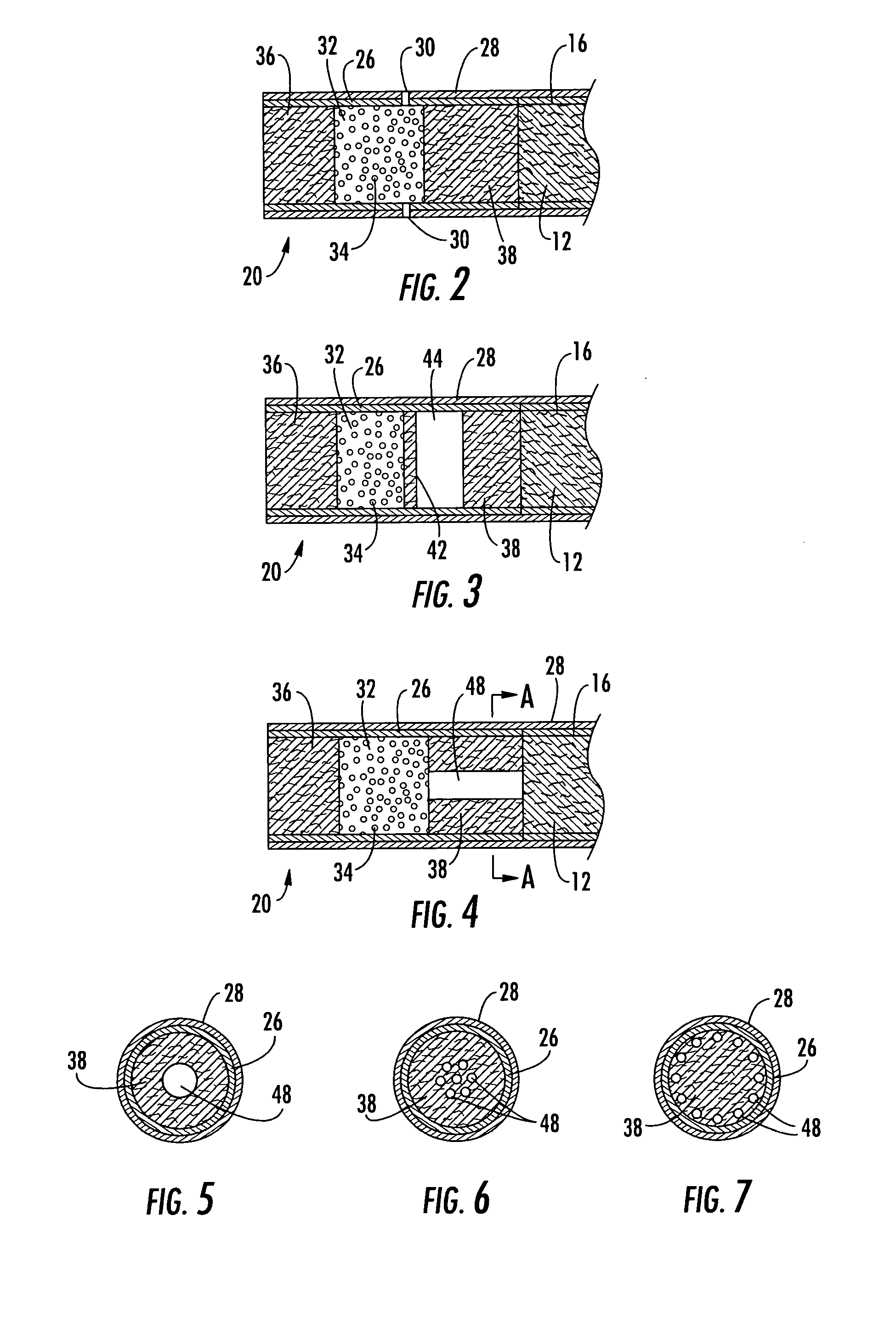
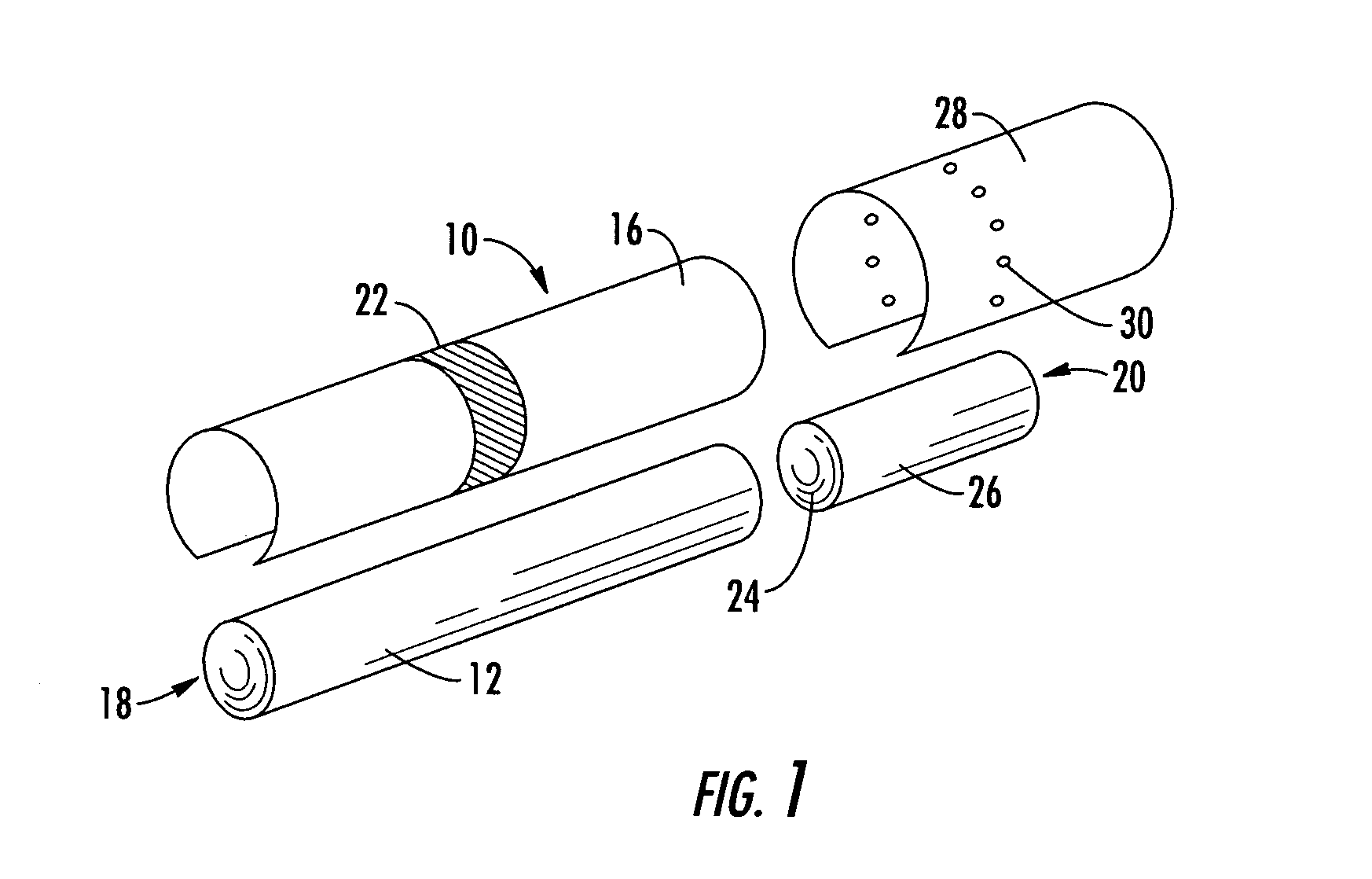
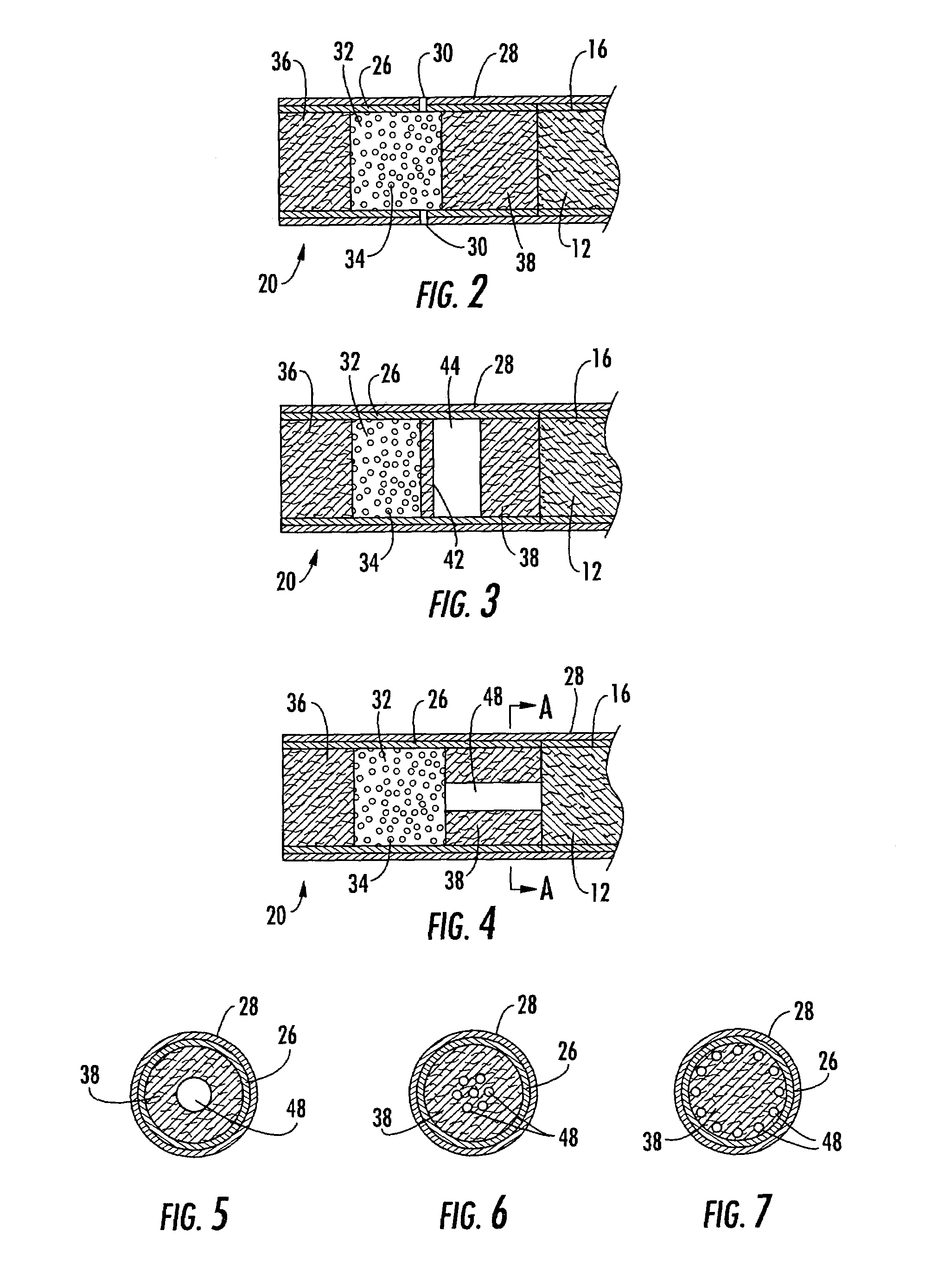
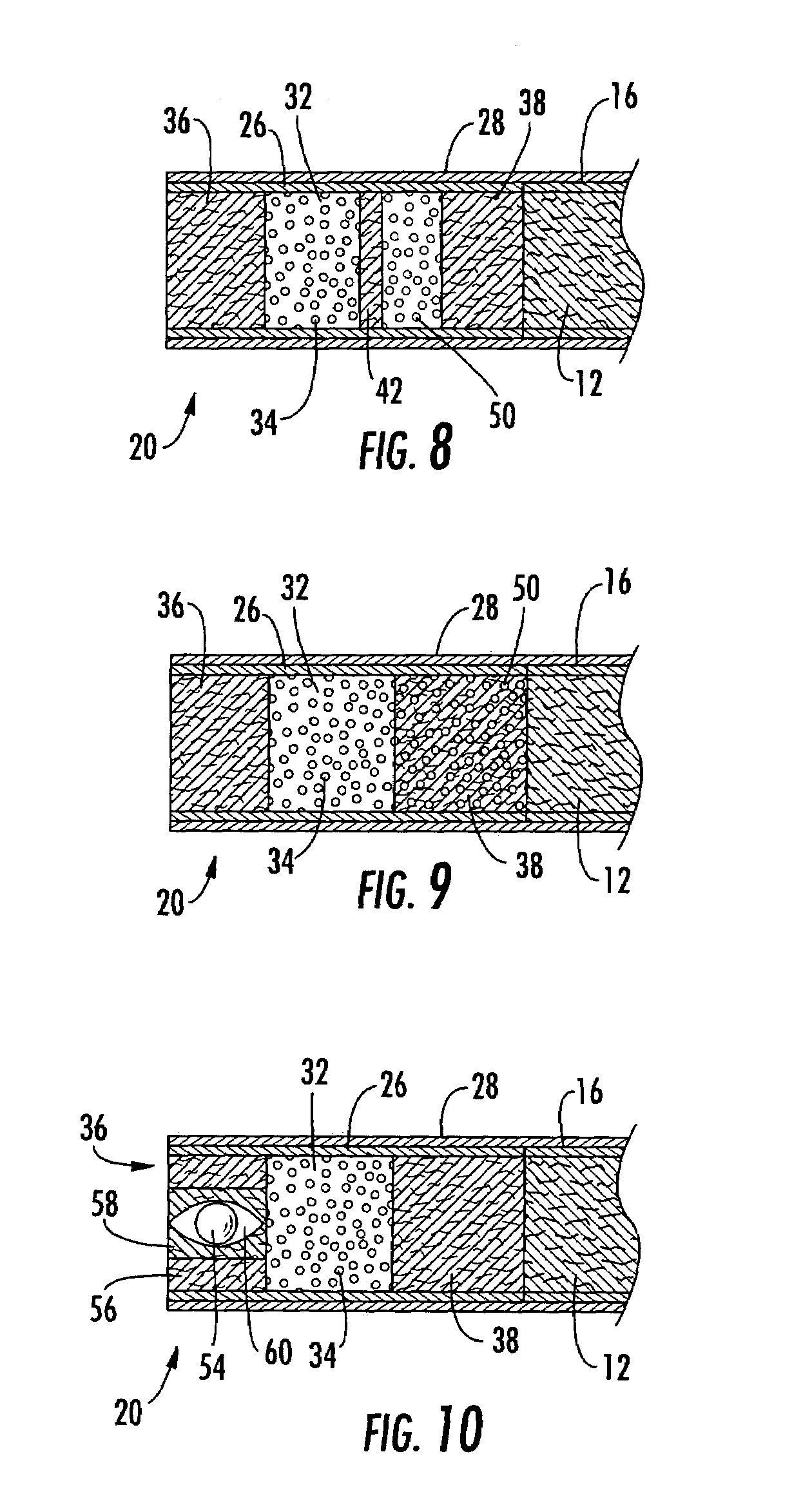
Filtered cigarette incorporating an adsorbent material
ActiveUS20050066984A1Enhanced mixing processTobacco treatmentTobacco smoke filtersFilter materialIon-exchange resin
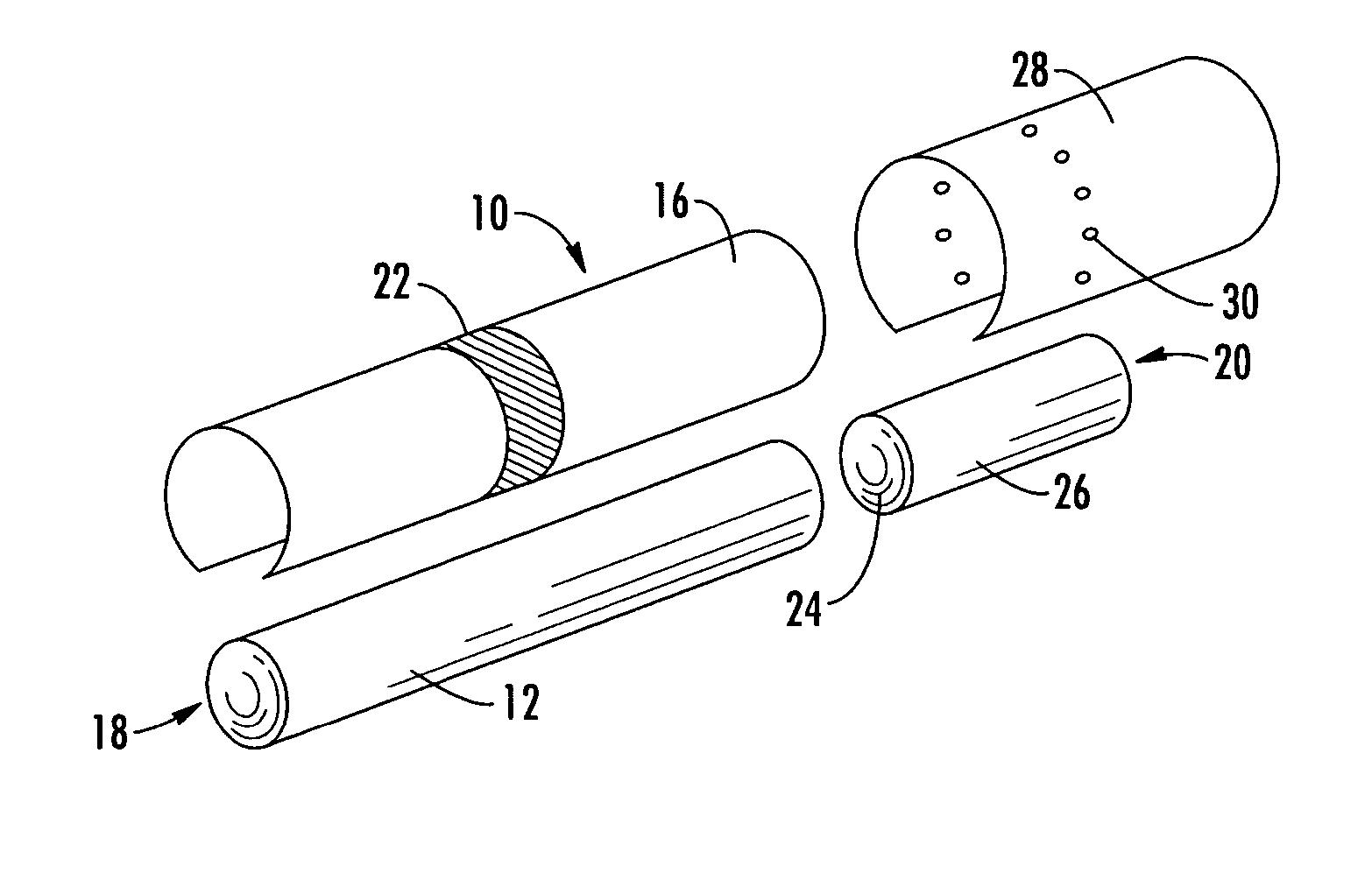
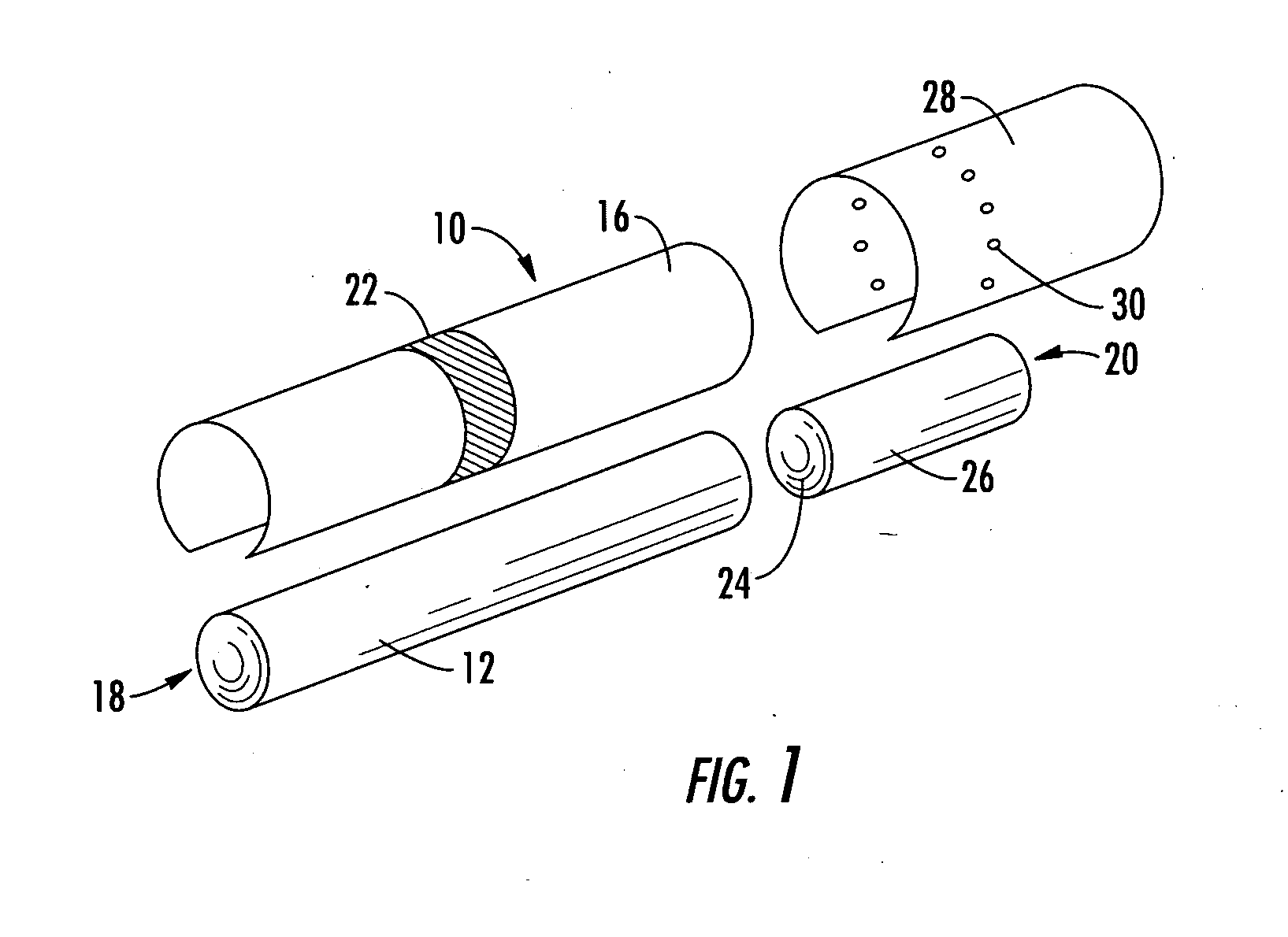
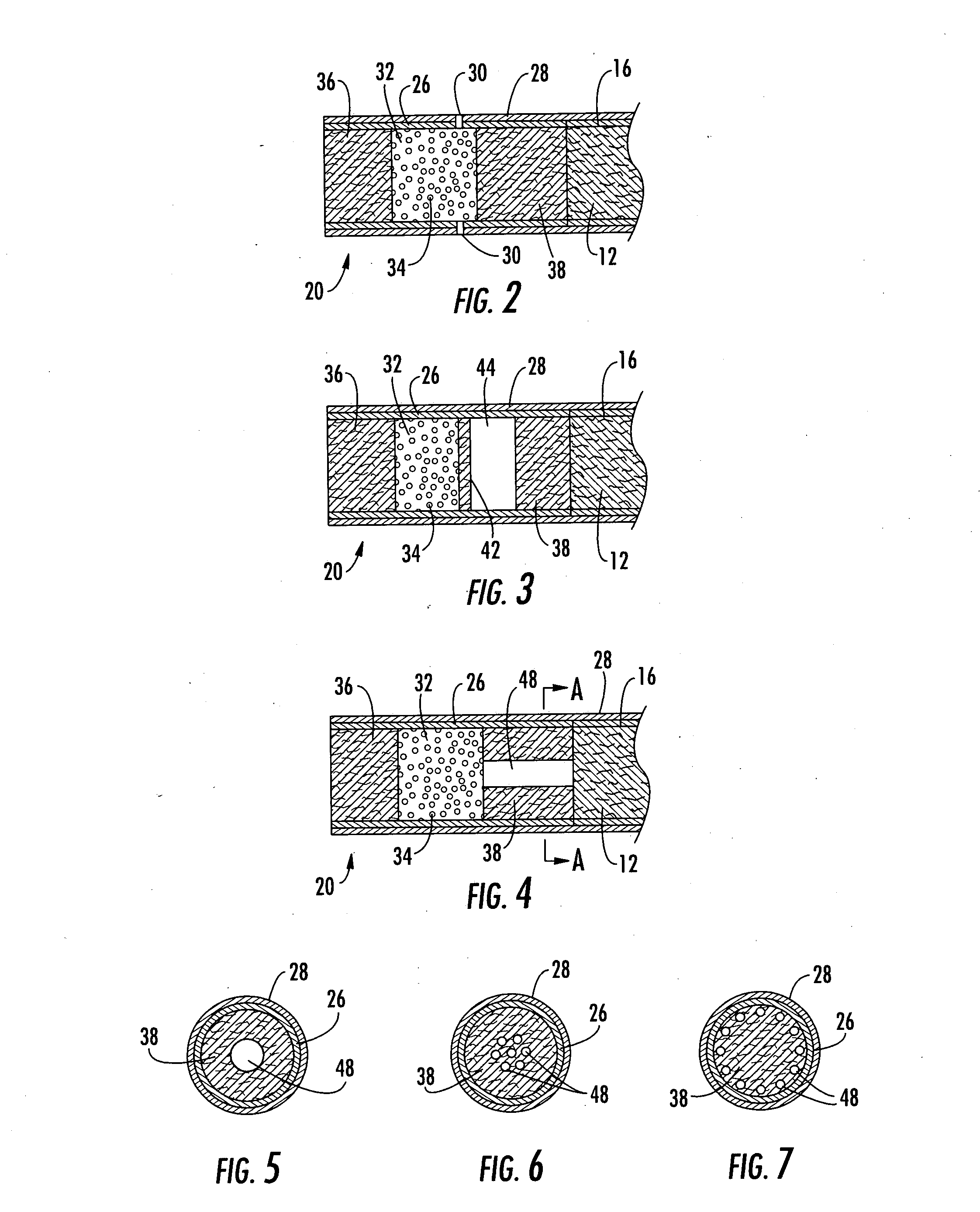
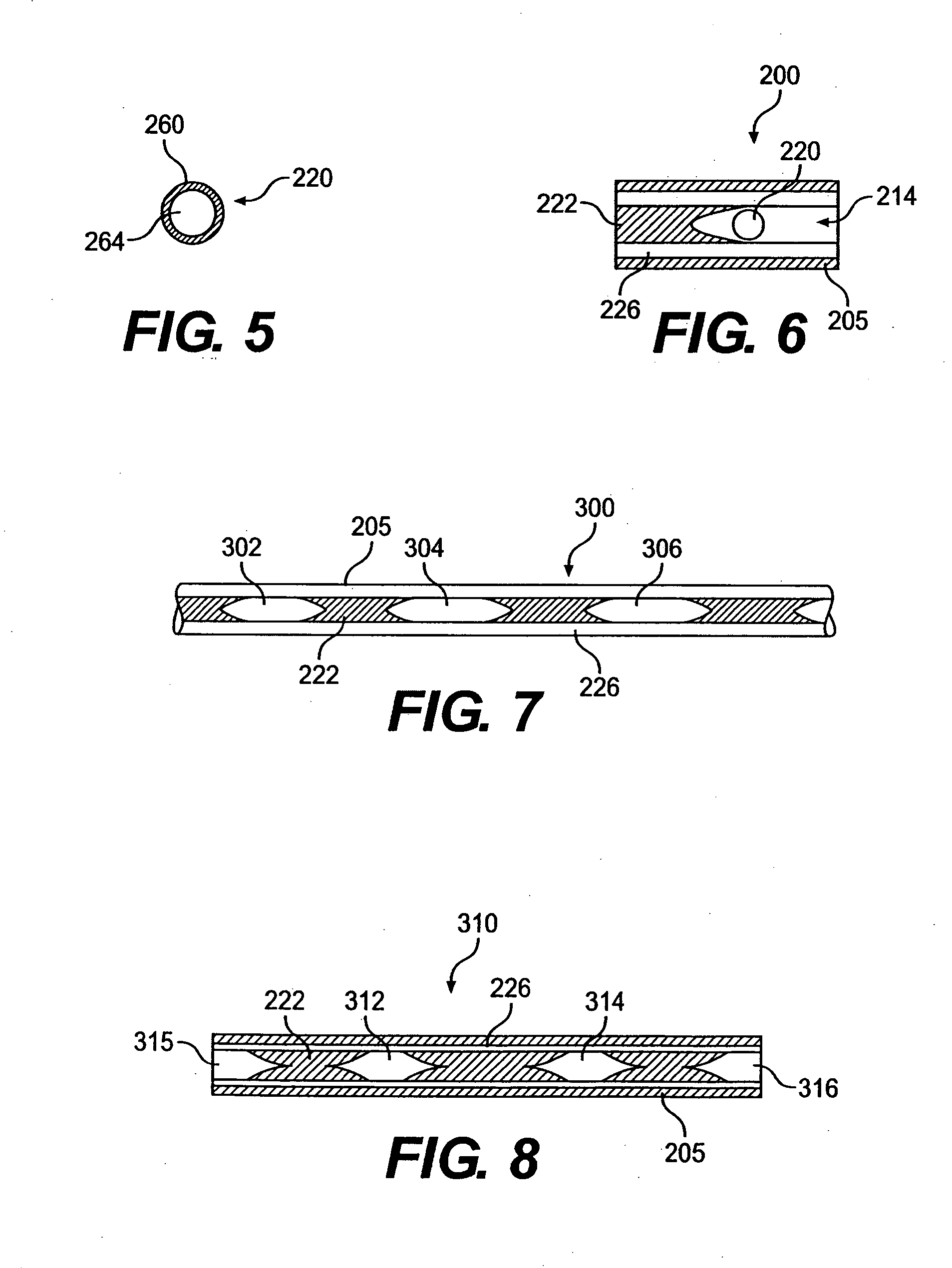
A filter element incorporating an adsorbent material is provided. The filter element may comprise a first section of filter material and a second section of filter material spaced apart to form a compartment therebetween. The compartment may be filled with one or more adsorbents or the compartment may be divided into two regions, wherein one compartment region is filled with an adsorbent and the other compartment region is either filled with an ion-exchange resin or remains empty. The section of filter material adjacent to the tobacco rod may include one or more channels therethrough for passaging smoke directly from the tobacco rod into the adsorbent-filled compartment. The mouth end section of filter material may contain a breakable capsule, wherein the breakable capsule is filled with a flavoring agent capable of altering the taste characteristics of mainstream smoke.
Owner:R J REYNOLDS TOBACCO COMPANY
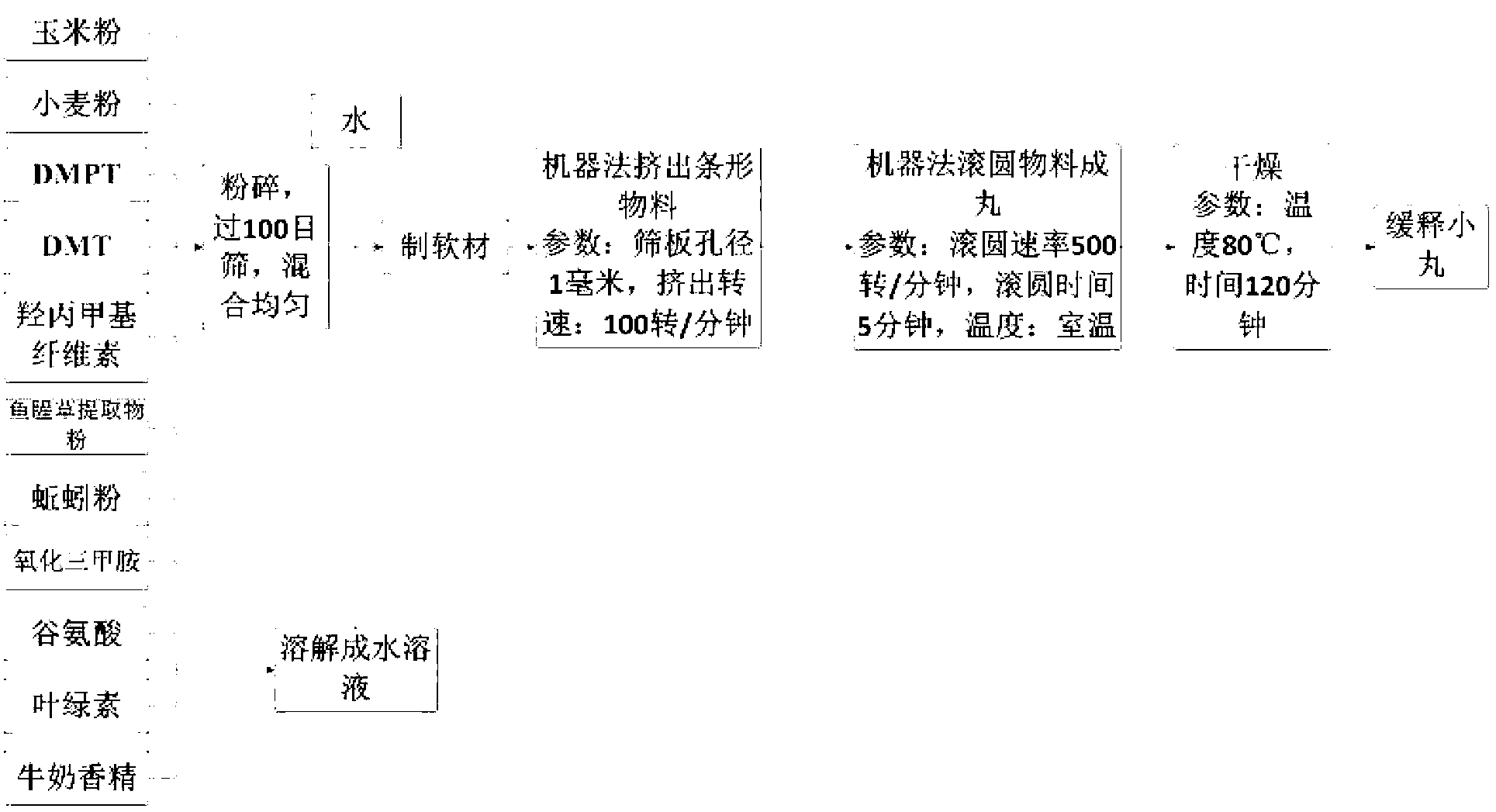
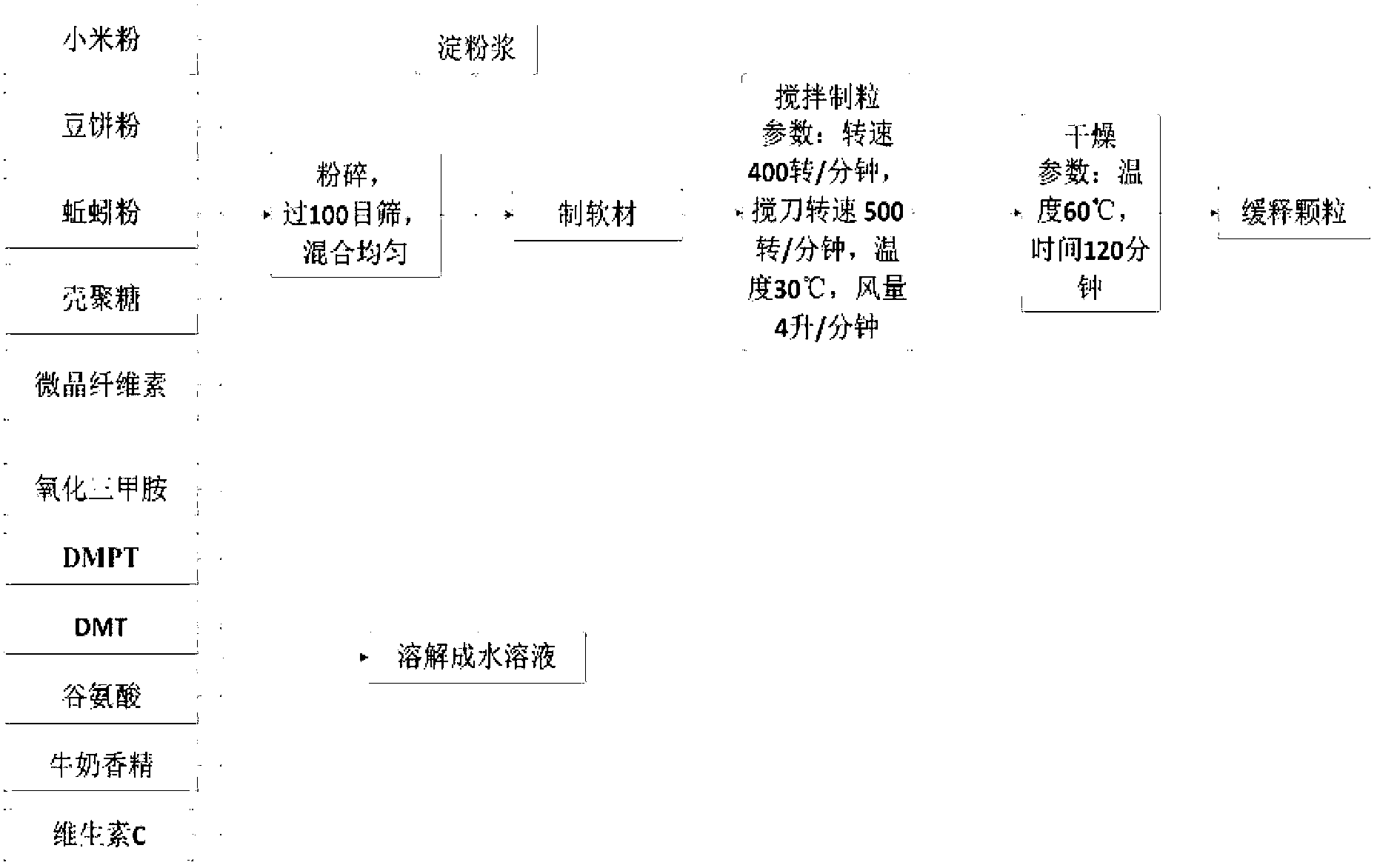
Controlled release bait material and preparation method thereof
InactiveCN103004717AQuick and long-lasting releaseFast and long-lasting effectOther angling devicesWater insolubleMicrosphere
The invention relates to a controlled release bait material and a preparation method thereof. The controlled release bait material refers to small pills, micro-pills, microcapsules, microspheres, particles or sticks prepared from raw materials including a basic material, a feed attractant, a bonding agent, a hydrophilic high-polymer material, a water-insoluble high-polymer material, a quick-release material, a filler, a colorant, a flavoring agent and the like through a preparation means; and the controlled release bait material can be coated for improving the controlled release effect. The invention further discloses a preparation method of the bait material. The controlled release material has rapid and lasting attracting force on fishes, the fish attracting time can be adjusted according to the habit of a fisherman and the habits of fishes, the food ration and frequency of fishes are increased, the fishing success rate can be increased, the food intake of fishes in cultivation of fishes can be increased, the corrosion ratios of remnant feeds and feeds in water bodies are reduced, the utilization ratios of feeds is increased, and the water body environmental pollution is lowered. The preparation method provided by the invention is suitable for industrial production.
Owner:李群益 +2
Method of enhancing salty taste, salty taste enhancer, salty taste seasoning agent and salty taste-enhanced foods
The present invention relates to a method of enhancing the salty taste of a food or beverage containing salt which comprises adding an acidic peptide or a peptide obtained by subjecting a protein to hydrolysis and deamidation to the food or beverage, a salty taste enhancer comprising the peptide as an active ingredient, a salty taste seasoning agent comprising the peptide and salt, and a food or beverage comprising the salty taste enhancer or the salty taste seasoning agent.
Owner:KYOWA HAKKO FOOD SPECIALTIES +1
Encapsulation compositions and processes for preparing the same
Matrix compositions which contain a mixture of two different food polymers are useful for encapsulating encapsulates, such as flavoring agents.
Owner:MCCORMICK & CO INC
Filtered cigarette incorporating an adsorbent material
ActiveUS20050066981A1Enhanced mixing processCigar manufactureTobacco smoke filtersFilter materialIon-exchange resin
A filter element incorporating an adsorbent material is provided. The filter element may comprise a first section of filter material and a second section of filter material spaced apart to form a compartment therebetween. The compartment may be filled with one or more adsorbents or the compartment may be divided into two regions, wherein one compartment region is filled with an adsorbent and the other compartment region is either filled with an ion-exchange resin or remains empty. The section of filter material adjacent to the tobacco rod may include one or more channels therethrough for passaging smoke directly from the tobacco rod into the adsorbent-filled compartment. The mouth end section of filter material may contain a breakable capsule, wherein the breakable capsule is filled with a flavoring agent capable of altering the taste characteristics of mainstream smoke.
Owner:R J REYNOLDS TOBACCO COMPANY
Composition of menthol and menthyl lactate, its preparation method and its applications as a cooling agent
InactiveUS6897195B2Small smellReduce volatilityCosmetic preparationsToilet preparationsShaving creamAdditive ingredient
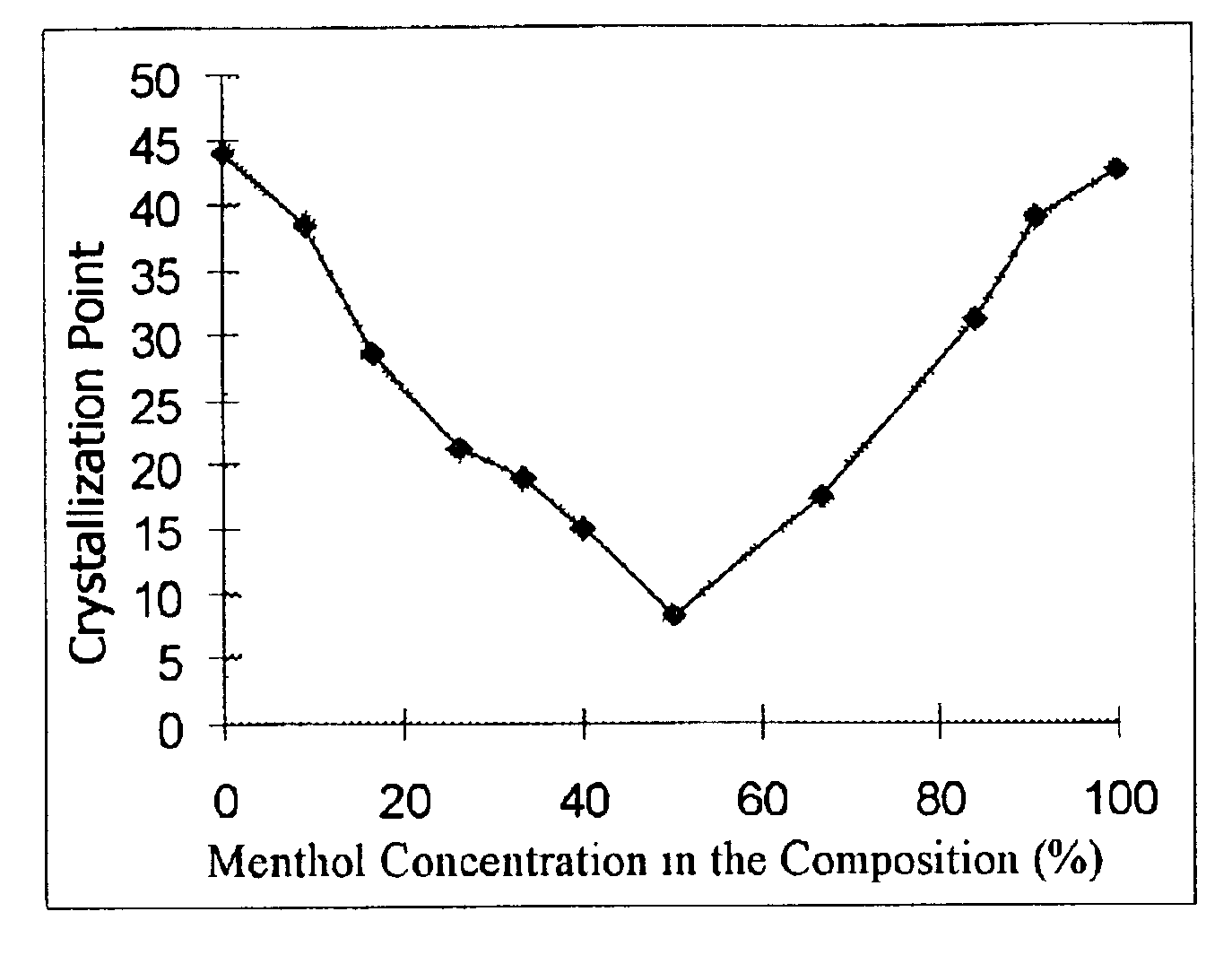
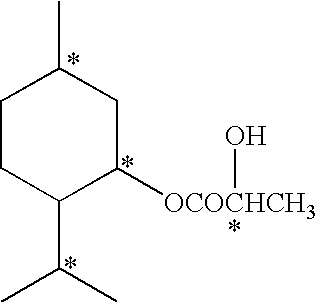
Disclosed here is a composition containing menthol and menthyl lactate, and its preparation method and its applications as a cooling agent and a flavoring agent.The present invention provides a composition characterized in that it comprises menthol and menthyl lactate in a ratio by weight in the range of 1:4˜4:1 and the corresponding crystallization point is below room temperature of 25° C. Such composition has the advantages of being liquid at room temperature; easy to use as a cooling agent or a flavoring agent; no need to use heat to melt menthol and menthyl lactate, which not only saves time, money and heating equipment, but also simplifies manufacturing process and can be used in cold processes at room temperature. The composition can be used as a cooling and / or flavoring agent and / or a fragrance ingredient in toothpaste, mouthwash, fragrance, cleansers, shaving cream, after shave products; shampoo, deodorant, antiperspirant, bath products, drinks, confectionary products, tobacco, pharmaceutical, foods, flavoring and fragrance products.
Owner:NANJING ZHONGSHI CHEM CO LTD
Filtered cigarette incorporating an adsorbent material
ActiveUS20050066982A1Enhanced mixing processTobacco treatmentTobacco smoke filtersEngineeringFilter material
A filter element incorporating an adsorbent material is provided. The filter element may comprise a first section of filter material and a second section of filter material spaced apart to form a compartment therebetween. The compartment may be filled with one or more adsorbents or the compartment may be divided into two regions, wherein one compartment region is filled with an adsorbent and the other compartment region is either filled with an ion-exchange resin or remains empty. The section of filter material adjacent to the tobacco rod may include one or more channels therethrough for passaging smoke directly from the tobacco rod into the adsorbent-filled compartment. The mouth end section of filter material may contain a breakable capsule, wherein the breakable capsule is filled with a flavoring agent capable of altering the taste characteristics of mainstream smoke.
Owner:R J REYNOLDS TOBACCO COMPANY
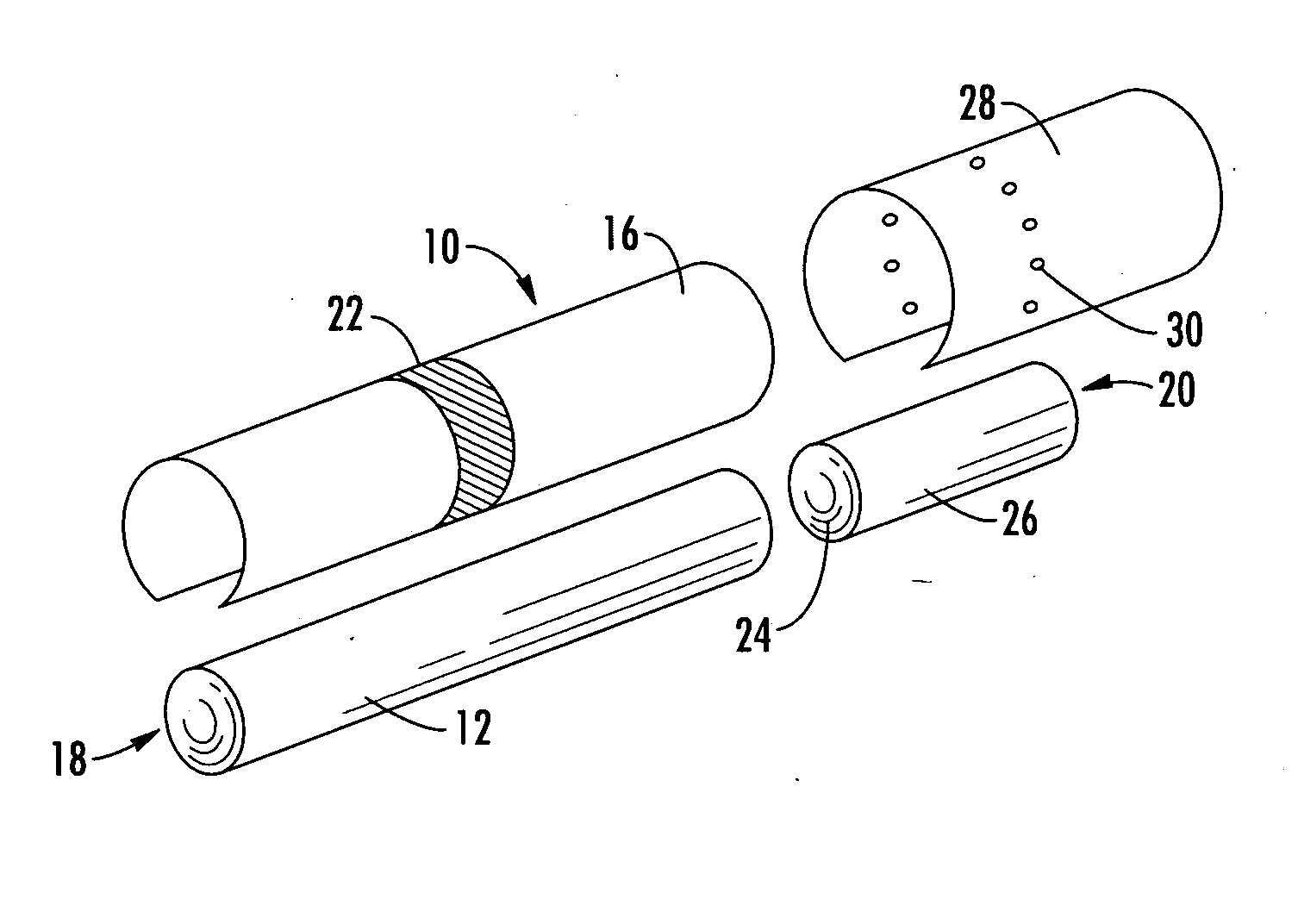
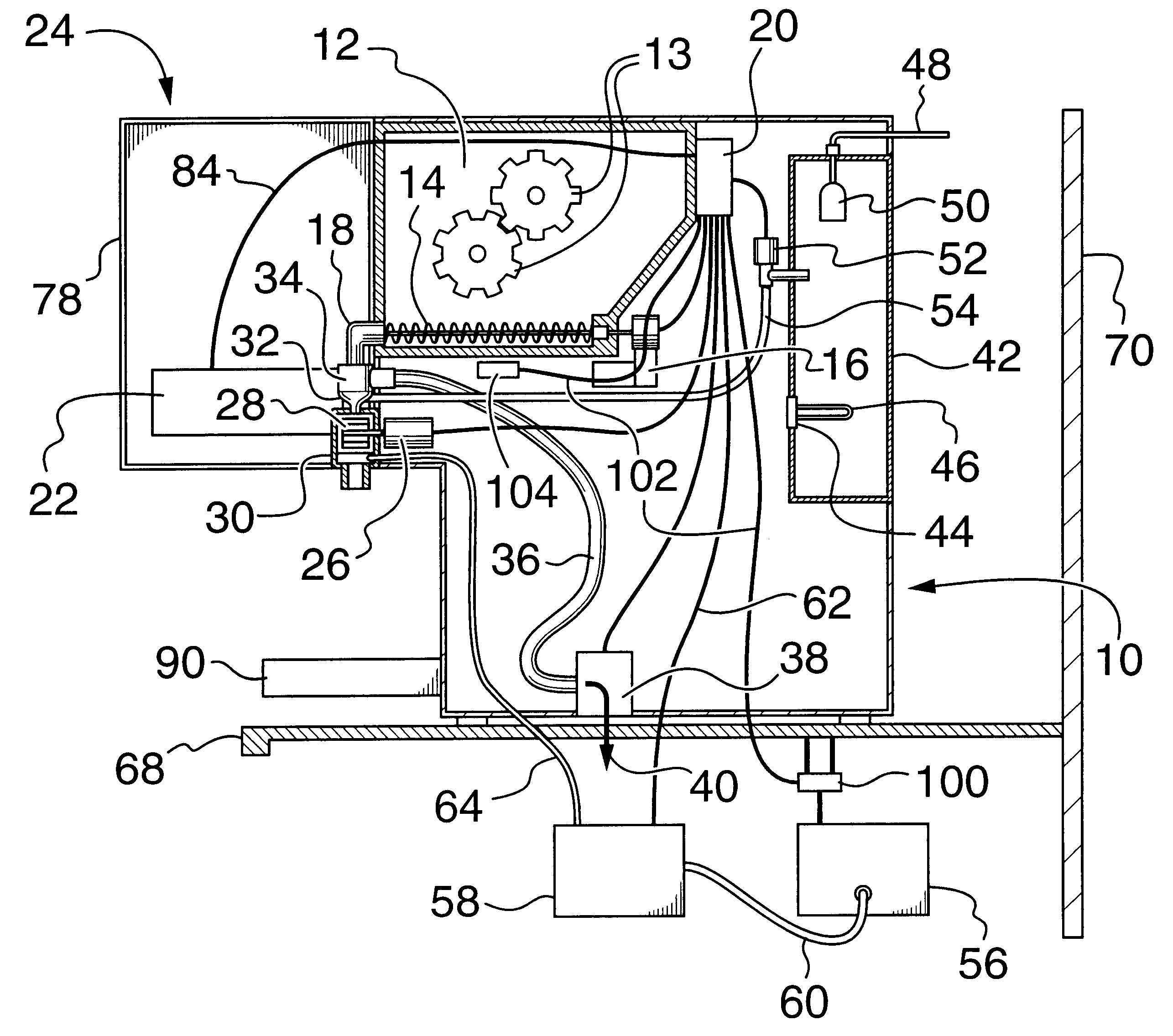
Filtered cigarette incorporating a breakable capsule
ActiveUS20060272663A1Ensure maintenanceTobacco treatmentPaper/cardboard wound articlesBiomedical engineeringFilter element
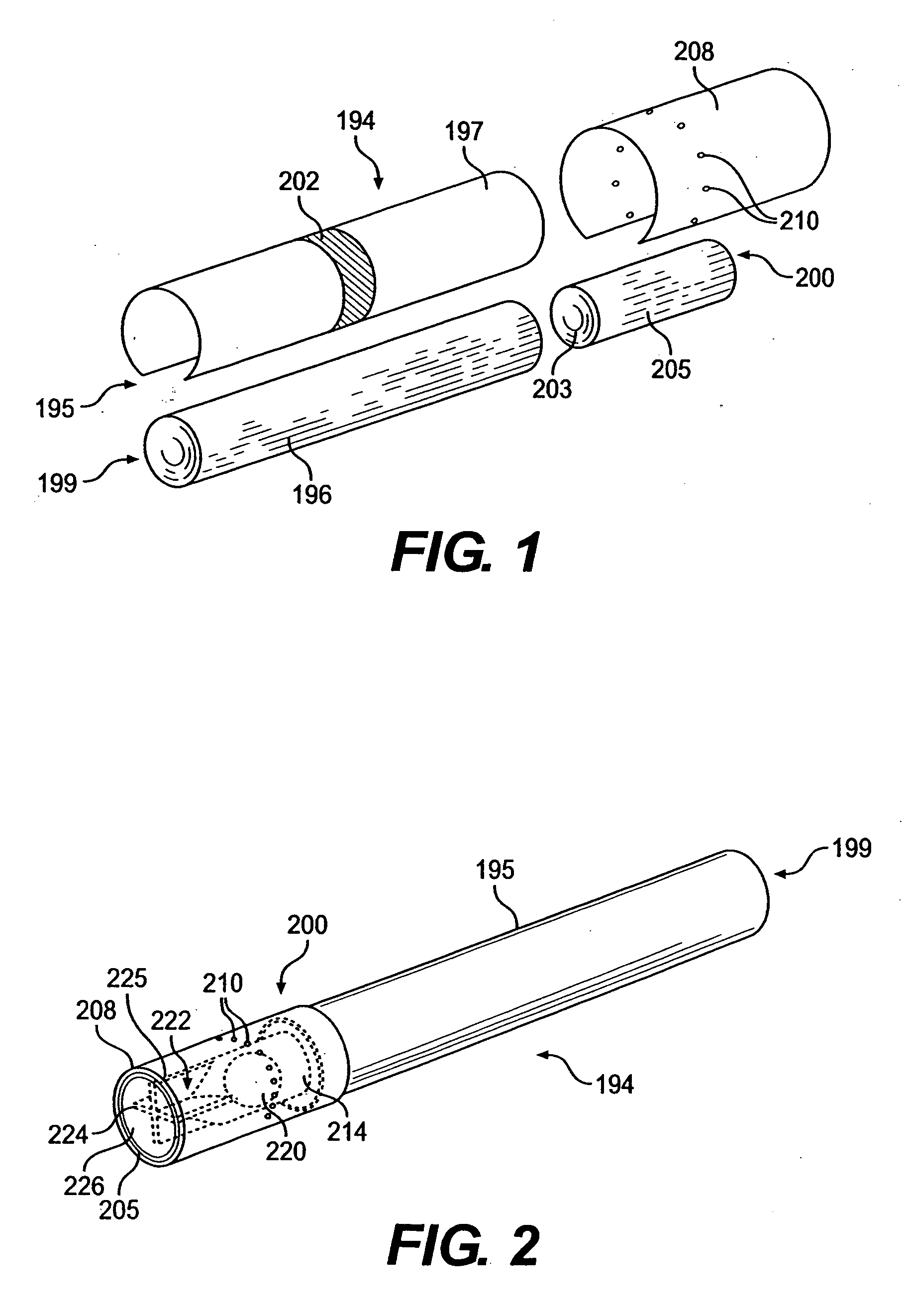
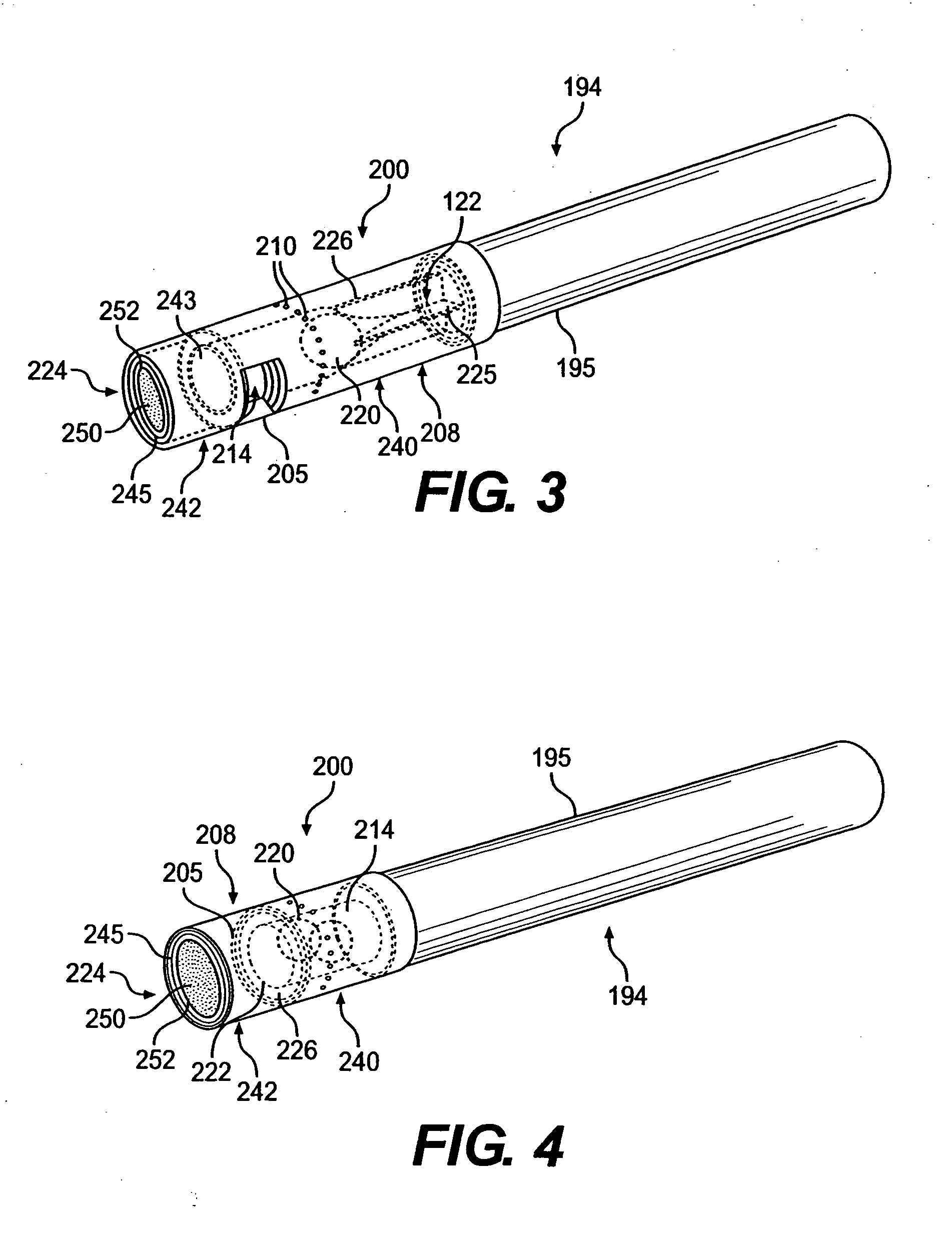
A filtered cigarette possesses at least one breakable capsule in its filter element. The filter element can possess a central cavity extending from the cigarette tobacco rod towards the middle of the filter element. The central cavity may be defined by an inner filter portion. The inner filter portion can be surrounded by an outer filter portion comprised of filter tow material that is generally permeable to the smoke generated by the cigarette. At least one breakable capsule is disposed in the central cavity of the filter element. The breakable capsules are spherical in shape, and are composed of a gelatin outer shell that encloses a payload of triglycerides and flavoring agents. The breakable capsules are adapted to rupture in response to pressure applied by the smoker to the outside region of the filter element.
Owner:R J REYNOLDS TOBACCO COMPANY
Personal Care Compositions Providing Enhanced Cooling Sensation
ActiveUS20100086498A1High activityEncouraging complianceCosmetic preparationsToilet preparationsPersonal careTransport agent
Disclosed are personal care compositions for use on hair, skin, oral cavity, throat and other mucosal surfaces containing a flavor / perfume system comprising one or more coolants, wherein the pleasant cool and refreshing sensation provided by the coolant is enhanced in terms of quicker onset, greater intensity or impact and / or longer duration, thereby improving appeal and acceptability of the compositions to consumers. In one embodiment the invention provides oral care compositions comprising(a) a flavor composition comprising one or more non-menthol coolants and optionally one or more additional flavor ingredients,(b) a calcium ion source and / or a calcium transport agent sufficient to potentiate and / or modulate the cooling and refreshing sensation provided by the coolant(s), and(c) an orally-acceptable carrier.Upon application to the oral cavity, the compositions provide an immediate onset of cooling sensation which lasts longer than about 15 minutes thereby providing long-lasting clean and fresh mouth impression and encouraging user compliance and repeated use of the compositions.
Owner:THE PROCTER & GAMBLE COMPANY
Therapeutic and protective dental device useful as an intra-oral delivery system
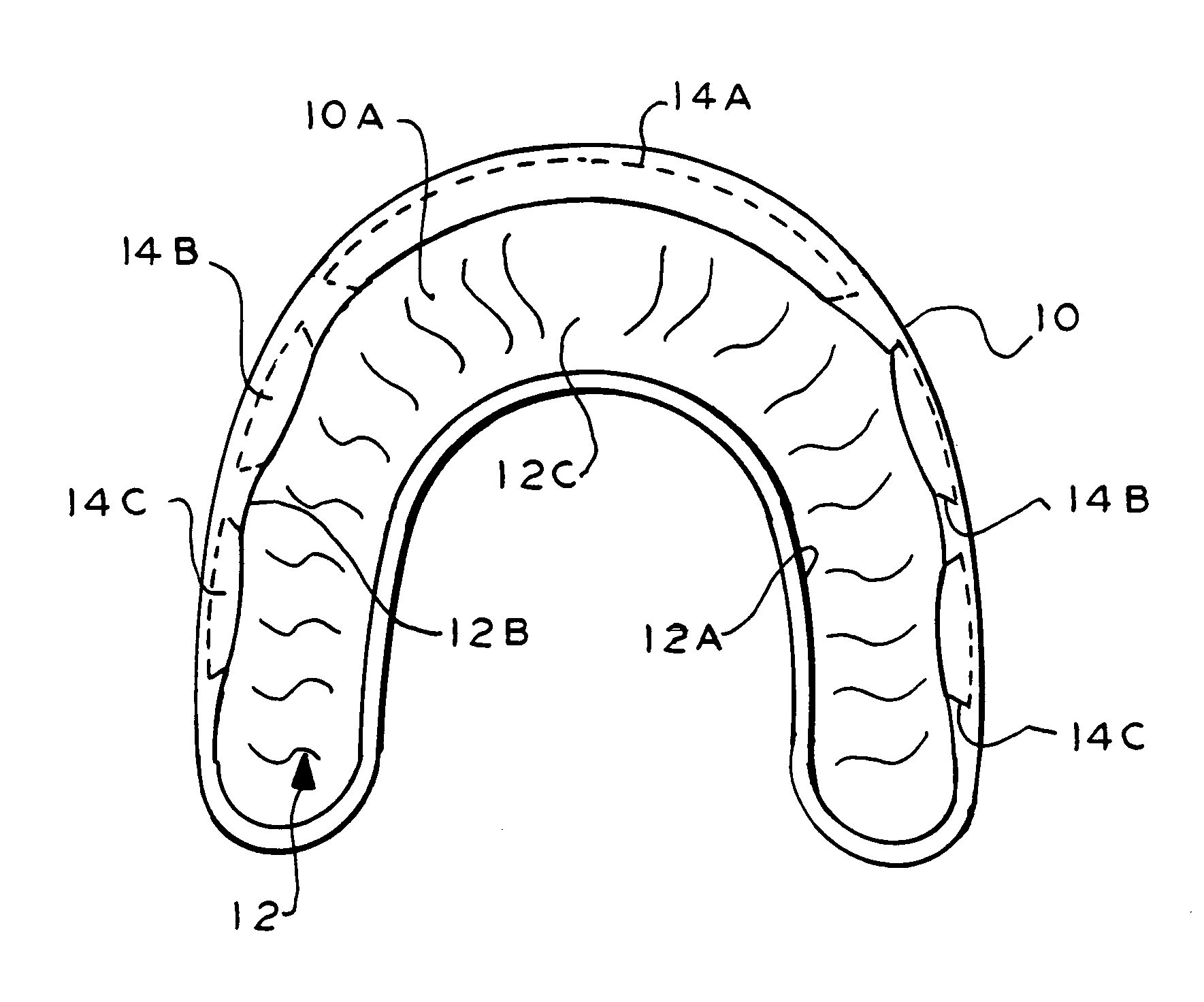
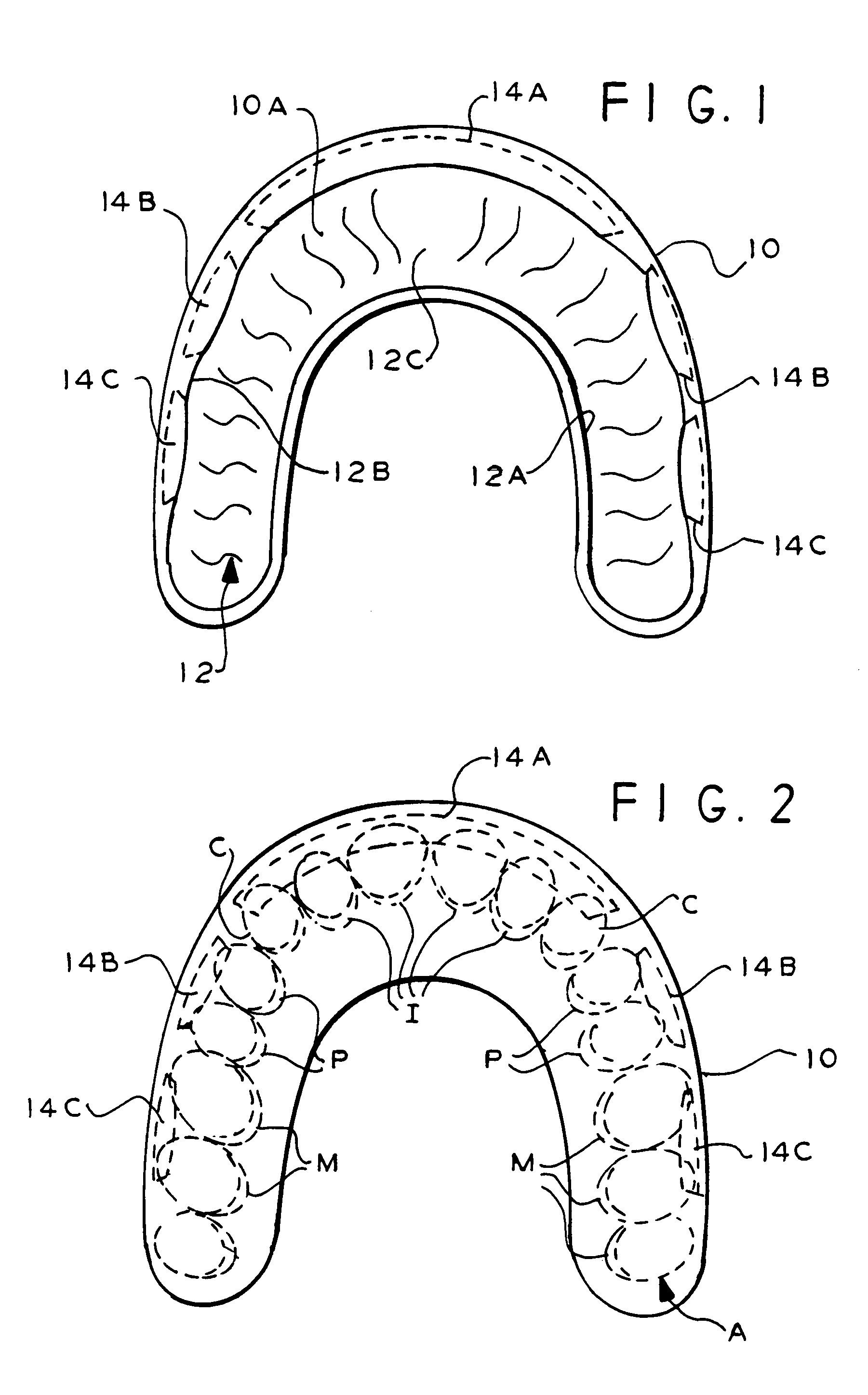
InactiveUS7328706B2Promote absorptionMaximize of contactCosmetic preparationsImpression capsOral regionMouthguard
A dental device has a U-shaped carrier with at least one channel for embracing an arch of teeth. The carrier has recessed insets in the channel. Discrete inserts carrying a beneficial agent can fit into the insets and release the agent gradually. When the device is used in a primarily therapeutic application, the inserts may be installed into all or less than all of the insets to form various insert patterns. Thus, different oral regions can be affected by different insert patterns. When the device is used as an athletic mouthguard, temporary blanks may be initially fitted in the insets, while a portion of the mouthguard is softened before an arch of teeth is pressed into the channel to make a custom impression. The inserts that are later installed in the insets possess different physical properties than the carrier and may be positioned and shaped to mechanically buffer teeth of the arch from mechanical shocks as well as release beneficial agents. The inserts may be replaced or refreshed to maintain the beneficial agent, which may be xylitol, remineralizing agents, moisturizing agents, desensitizing agents, flavoring agents, breath fresheners, chemical and biological indicators, nutraceuticals, antibiotics, probiotics, other medications and chemotherapeutics, or other agents.
Owner:DYNAMIC MOUTH DEVICES
Melting Vegetable Protein Based Substitute Cheese
This invention is directed to a molded, pressed, low animal fat substitute cheese composition, comprising;moisture in an amount that is at least about 50% by weight of the cheese composition, and(A) a vegetable protein material;(B) a vegetable oil triglyceride; and(C) a hydrocolloid.In another embodiment, the molded, pressed, low animal fat substitute cheese compositions further comprises at least one component selected from the group consisting of(D) a cheese flavorant and(E) a starch.
Owner:SOLAE LLC
Filtered cigarette incorporating an adsorbent material
ActiveUS7240678B2Enhanced mixing processCigar manufactureTobacco smoke filtersFilter materialIon-exchange resin
A filter element incorporating an adsorbent material is provided. The filter element may comprise a first section of filter material and a second section of filter material spaced apart to form a compartment therebetween. The compartment may be filled with one or more adsorbents or the compartment may be divided into two regions, wherein one compartment region is filled with an adsorbent and the other compartment region is either filled with an ion-exchange resin or remains empty. The section of filter material adjacent to the tobacco rod may include one or more channels therethrough for passaging smoke directly from the tobacco rod into the adsorbent-filled compartment. The mouth end section of filter material may contain a breakable capsule, wherein the breakable capsule is filled with a flavoring agent capable of altering the taste characteristics of mainstream smoke.
Owner:R J REYNOLDS TOBACCO COMPANY
Flavoring of drug-containing chewing gums
ActiveUS20060275344A1Avoid problemsEnhanced release controlNervous disorderContainers for annular articlesAdditive ingredientPolymer thin films
A chewing gum comprising at least one active pharmaceutical ingredient (API) with a core onto which is applied at least one inner polymer film coating and thereafter onto which is applied at least one outer hard coating. A preferred API is nicotine. Flavoring agents may be incorporated in the core, in the at least one inner polymer film coating and / or in the at least one outer hard coating. The gums formed exhibit a long lasting effect of flavoring agent(s) and result in the domination of flavoring agents in the coating(s) over flavoring agent(s) in the core, thereby (a) avoiding problems of chemical or pharmaceutical incompatibility between an API in the core and flavoring agent(s) in the coating(s) and (b) achieving an increased control of the release of the API and of non-active excipients.
Owner:MCNEIL AB
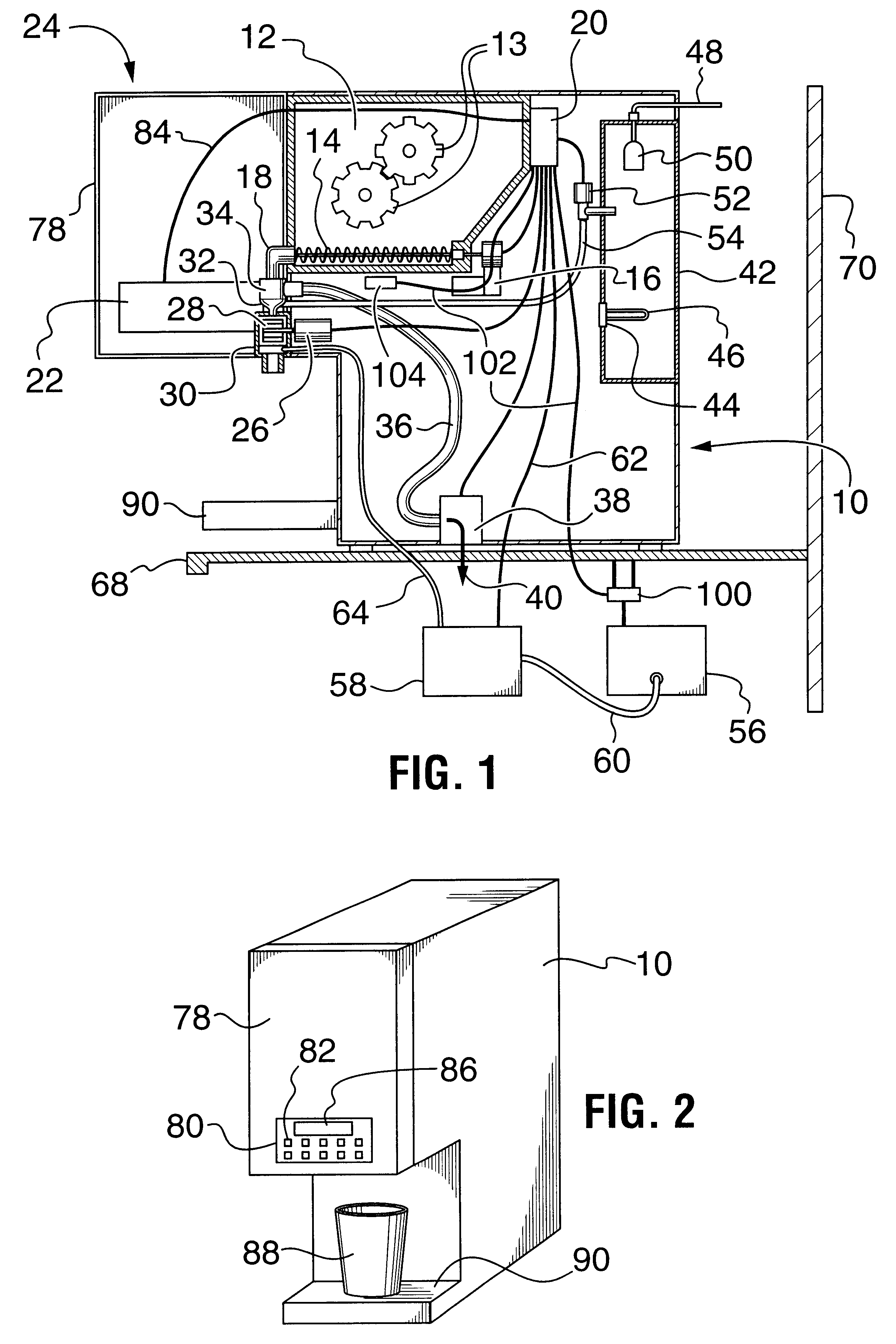
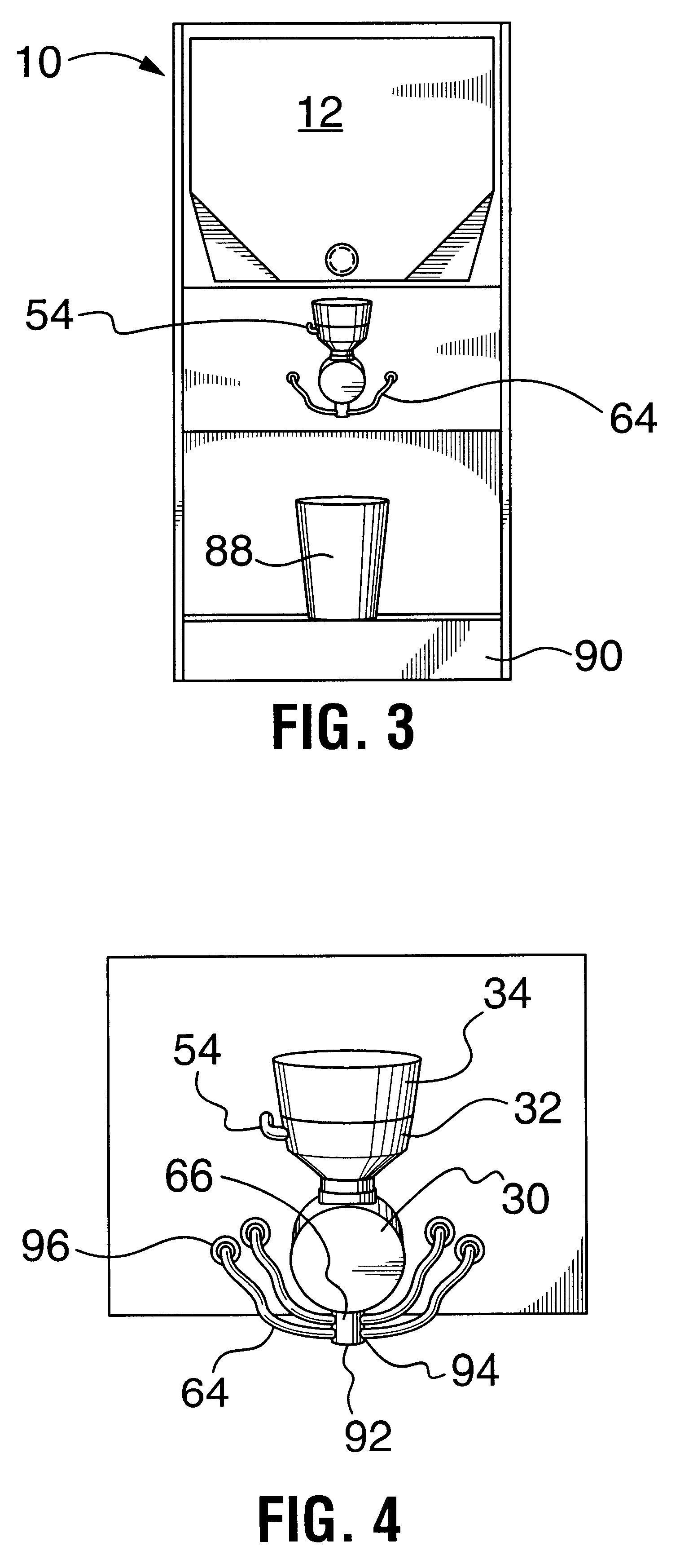
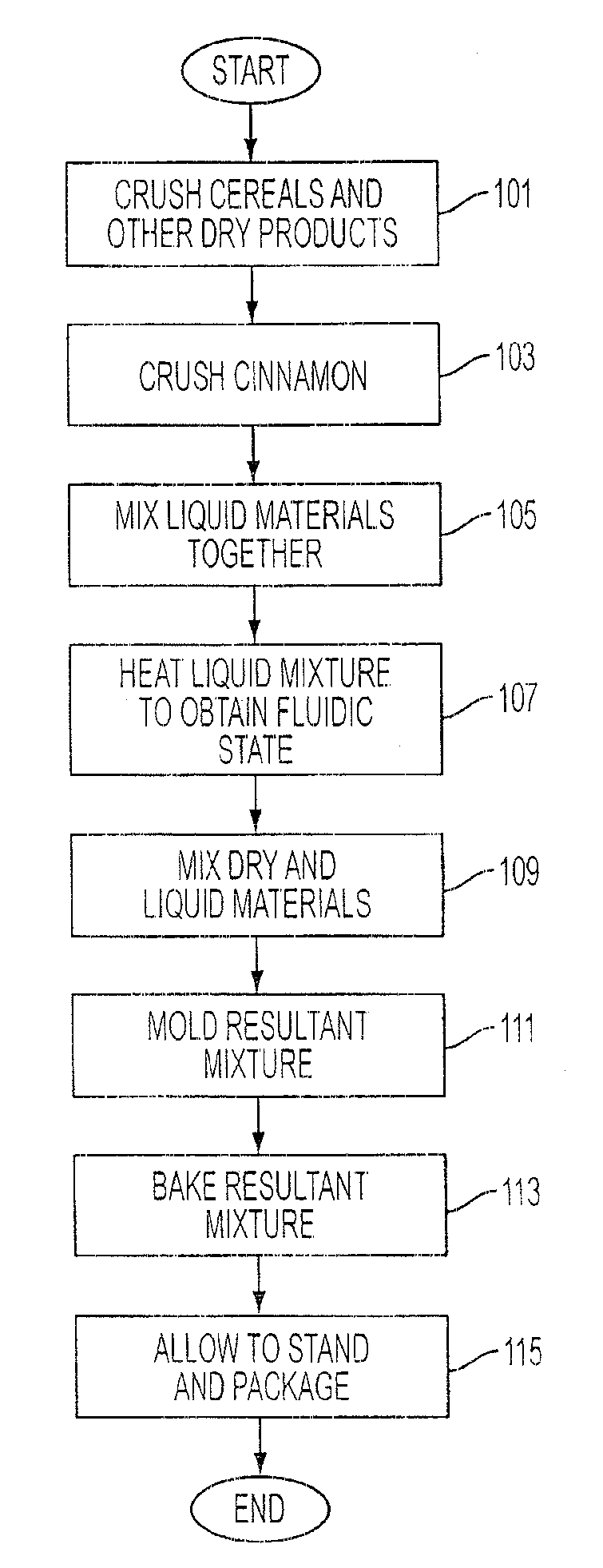
Multi-flavored hot beverage dispenser
Discloses a multi-flavour hot beverage dispenser apparatus adapted to dispense a selected flavour hot drink. The dispenser apparatus has hopper to hold a base powder. The hopper includes a hopper dispenser outlet including a motor driven auger to dispense the base powder from the hopper and a hot water boiler and hot water dispense valve. The hot water boiler has controlled heating means to heat the water to the desired temperature. A plurality of liquid flavour dispensers is provided with one or more dispensers selectively activatable to dispense a flavour syrup to flavour the dispensed hot drink to the desired flavour. The liquid flavouring supply is remote from the beverage dispenser allowing a compact sized dispenser which requires minimal counter space that does not increase with increasing flavour selection offerings. A mixer mixes the hot drink constituents to produce the selected flavour of hot drink indicated by a user by depressing a dispense key corresponding to the desired size and flavour of drink to be dispensed. A portion controlled or push and hold dispense cycle is described. In addition, drink constituent supply sensors are disclosed which operate to disable hot drink dispensing when a supply is low.
Owner:BERTONE HLDG
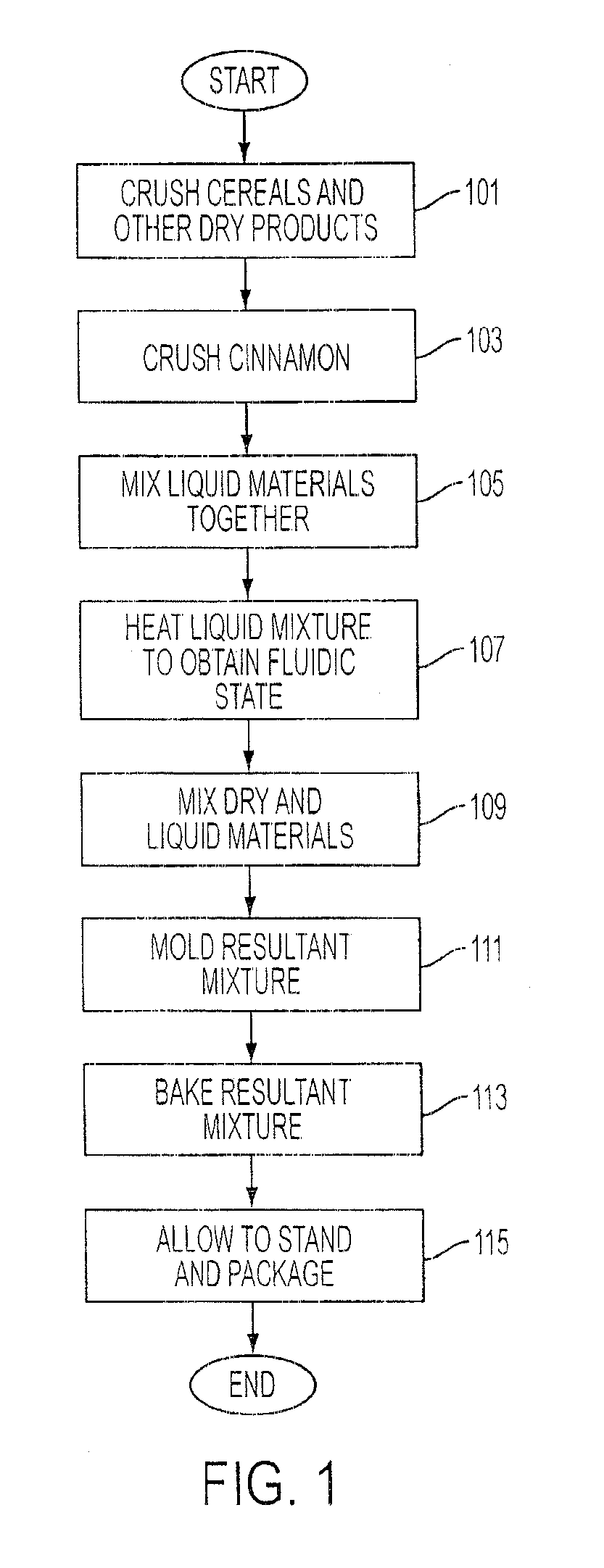
Food-based Supplement Delivery System
InactiveUS20100178413A1Total calories lowEnjoyable eating experienceFood preparationPlant ingredientsHuman useLarge dose
A cookie or other food product which is designed to deliver a larger dose of cinnamon or other additive such as fruit extract or rind to a human user without significant introduction of food items detrimental to additive(s)'s expected medicinal action and without an unpleasant taste sensation. The cookie is designed to be chewed as opposed to swallowed and the flavoring of the additive(s) is intended to enhance the cookie as opposed to the flavoring being covered up or concealed by other flavorings.
Owner:GORRIS MARK
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the central nervous system
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Buccal, polar and non-polar spray or capsule
InactiveUS6998110B2Rapid onsetFast absorptionBatteries circuit arrangementsAerosol deliverySolventPharmacology
Owner:SUDA
Chewing gum containing physiological cooling agents
A method for producing a chewing gum, as well as the chewing gum so produced, incorporates a physiological cooling agent, such as acyclic carboxamide, or combinations of physiological cooling agents. In another embodiment a combination of physiological cooling agents is made in a modified release structure. The modified release / cooling agents combination is preferably obtained by physically modifying the properties of the combination of cooling agents by coating and drying. When incorporated into gum, these particles are adapted to enhance the shelf stability of the flavor and / or produce a modified release when the gum is chewed. In another embodiment, the physiological cooling agent is present with menthol and menthone. In another embodiment, coated chewing gum has a coating that comprises a physiological cooling agent. The preferred inventive chewing gum provides a high flavor impact in which the harsh notes normally associated with such a high flavor impact have been reduced or eliminated. In addition, the preferred inventive gum provides a clean, high-quality, cooling chewing gum coating with xylitol or other polyols where xylitol has been reduced in concentration or eliminated.
Owner:WM WRIGLEY JR CO
Buccal, polar and non-polar spray or capsule containing drugs for treating pain
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Oral care compositions having improved consumer aesthetics and taste
ActiveUS20070231278A1Reduced stabilityStable and well-rounded flavor profileCosmetic preparationsToilet preparationsMentholDiol
The present invention relates to peroxide containing oral care compositions containing a flavor system that effectively masks the undesirable taste and sensations due to peroxide. The flavor system comprises menthol, at least one other secondary coolant and selected flavor chemicals that together provide a stable flavor profile and a high impact refreshing taste and sensation. The secondary coolant is selected from carboxamides, ketals, diols, menthyl esters and mixtures thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Buccal, polar and non-polar spray or capsule containing cardiovascular or renal drugs
InactiveUS20050025713A1Fast absorptionRapid onsetElcosanoid active ingredientsAerosol deliverySolventBioactive compound
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Soy protein containing food product and process for preparing same
This invention relates to a soy protein containing food product comprising; (A) a soy protein material selected from the group consisting of a soy protein flour, a soy protein concentrate, a soy protein isolate and mixtures thereof; (B) a humectant comprising (i) a colorant and at least one selected from the group consisting of (ii) a flavoring agent, (iii) a triglyceride, (iv) a food grade acid or acidic salt, (v) a food grade base or basic salt, and (vi) a food grade emulsion; and (C) water. In another embodiment, the invention is directed to a process for preparing a soy protein containing food product.
Owner:SOLAE LLC
Molecules comprising linked organic moieties as flavor modifiers for comestible compositions
InactiveUS20060257543A1Enhancing savory tasteIncrease sweet tasteFood preparationMonosodium glutamatePharmacy medicine
The inventions disclosed herein relate to genuses of non-naturally occurring small molecule compounds which comprise two or optionally three organic moieties of limited size “linked” by certain structurally related “linker” functional groups. Suitable linker groups include ester, amine, ether, keto, imino, thioamide, thioether, sulfonamide, sulfonate ester, sulfone, guanidine, and thiourea groups. The compounds are capable, when contacted with comestible food or drinks or pharmaceutical compositions, at concentrations preferably on the order of about 100 ppm or lower, of serving as savory (“umami”) or sweet taste modifiers, savory or sweet flavoring agents and savory or sweet flavor enhancers, for use in foods, beverages, and other comestible or orally administered medicinal products or compositions, optionally in the presence of or in mixtures with conventional flavoring agents such as monosodium glutamate or known natural or artificial sweeteners.
Owner:SENOMYX INC
Freezing beverage containing isomalt oligosaccharide and its preparation method
InactiveCN101057628AImprove gastrointestinal environmentHas a comprehensive nutritional effectFrozen sweetsIsomaltooligosaccharideFlavoring essences
The invention discloses a freeze beverage containing isomaltose hypgather and process for preparation, wherein the raw materials comprise the following constituents (by weight ratio): cow's milk and / or dairy product and / or dairy product analogues 0-90%, isomaltose hypgather 0. 01-10. 0%, sugar 4. 0-16. 0%, stabilizing agent 0. 1-1. 0%, sour flavor agent 0-0. 7%, edible flavoring essence 0. 01-0. 4% and balancing water.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Pharmaceutical formulation for sulfur-containing drugs in liquid dosage forms
InactiveUS20070134277A1Improve stabilityMask odorBiocidePeptide/protein ingredientsActive agentFood flavor
The pharmaceutical formulations of the invention for masking the odor from the sulfur-containing active agent comprise at least one sulfur-containing active agent, an effective amount of at least one flavoring agent. Any flavoring agent or combinations of flavoring agents may be used in the pharmaceutical formulation of the invention. The flavoring agent may be natural flavors, natural fruit flavors, artificial flavors, and mixtures thereof. The pharmaceutical formulation may contain an artificial sweetener, a natural sweetener or mixtures thereof. The pharmaceutical formulations are provided in liquid dosage form or a dry powder dosage form for reconstitution in water. Stabilizer added as one of expicients can extend the stability of the pharmaceutical formulation liquid dosage form for a period of at least 30 days when the formulation is stored below room temperature. The pharmaceutical formulations of the invention are palatable and particularly useful for the administration of sulfur-containing drugs to very small children that are in need of such medications. Methods of forming a liquid dosage form of pharmaceutical formulation by adding water to the dry powder form, methods to prepare an odor-masking pharmaceutical formulation and methods for treating lead poisoning or Wilson's disease using the odor-masking pharmaceutical formulation are also provided.
Owner:CHILDRENS MEDICAL CENT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com