Palonosetron injection and its preparation method
A technology of palonosetron and injection, applied in the field of preparation of palonosetron injection formula and palonosetron injection formula, which can solve the problems of high price, high production cost, and large amount of excipients, etc. , to achieve the effect of low cost, good stability and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
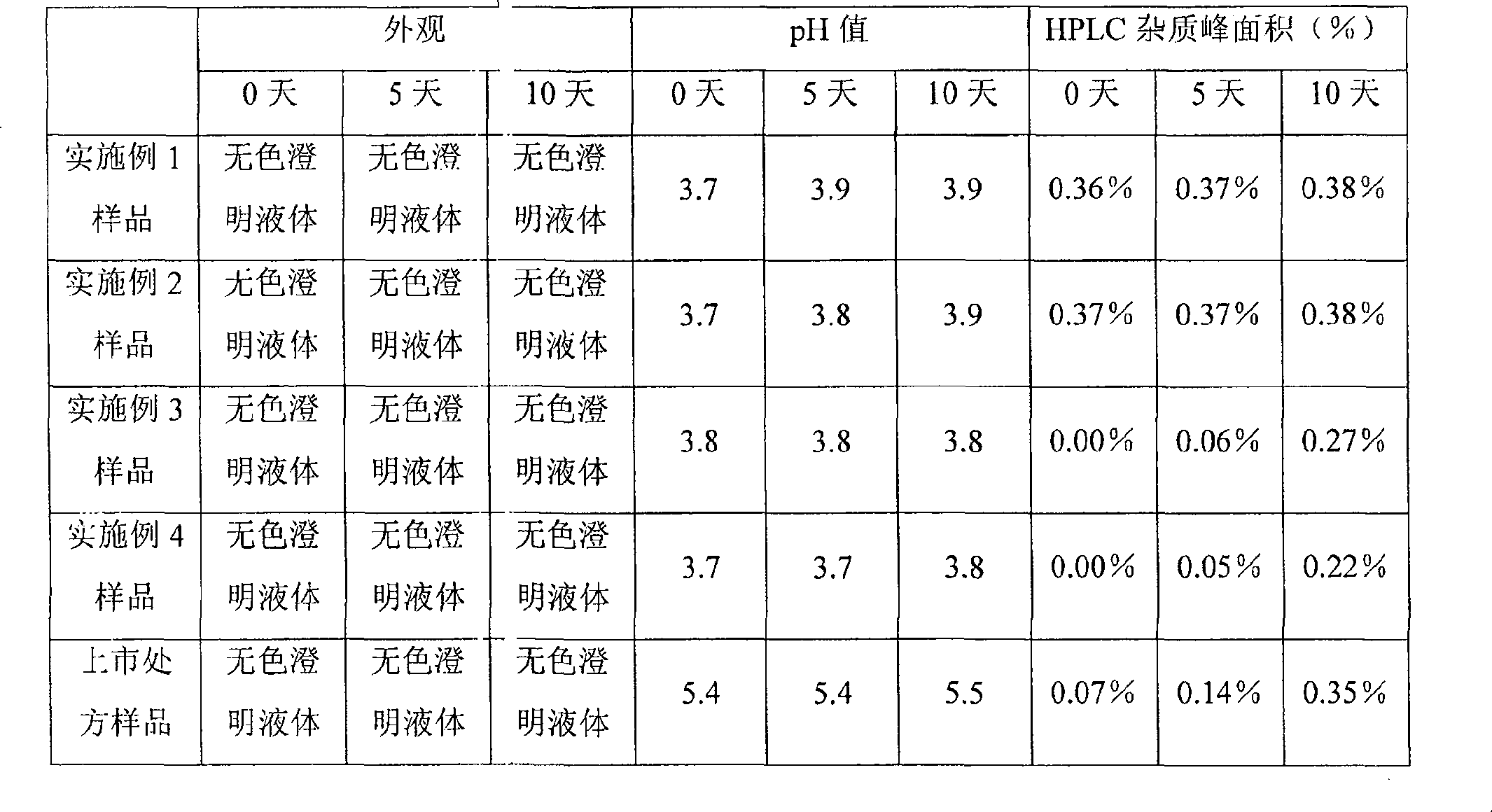
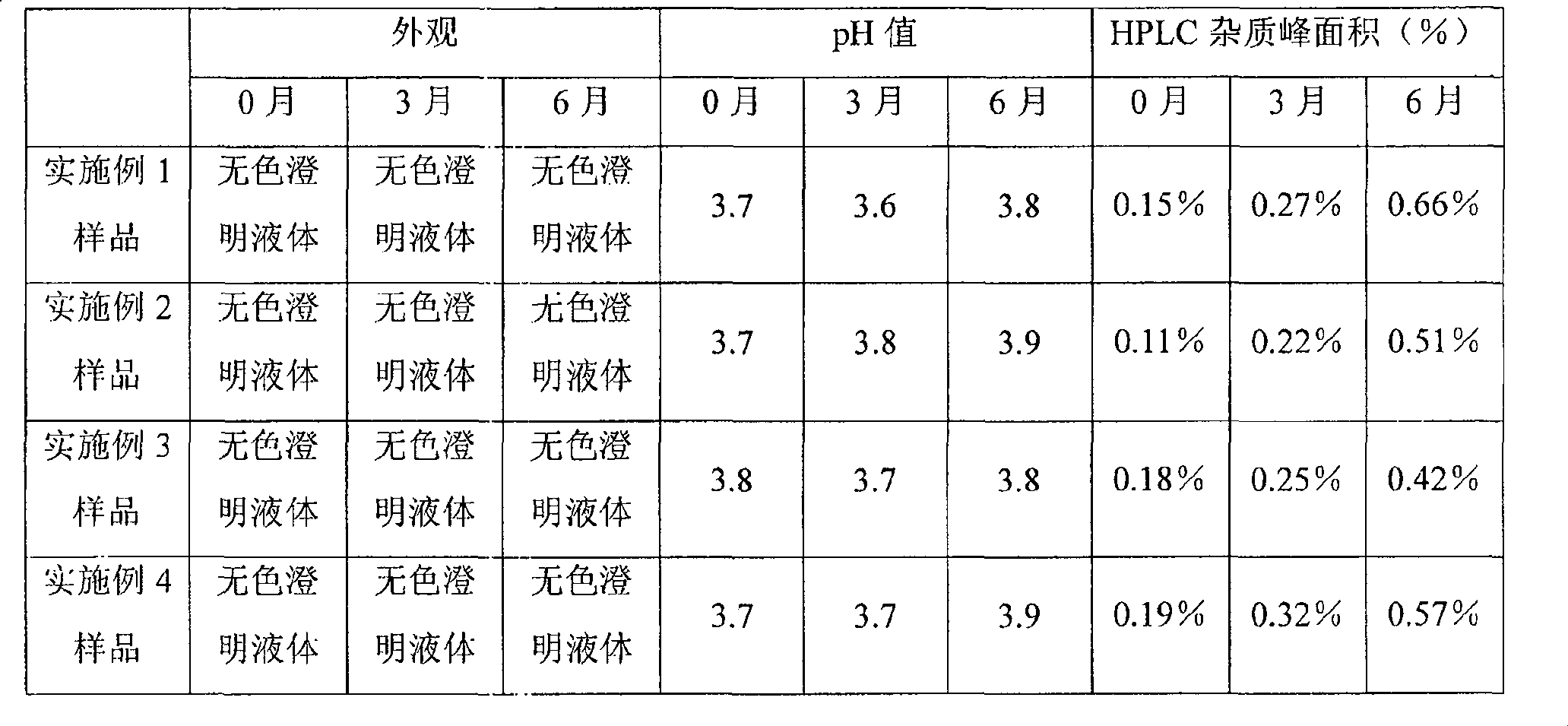
Examples
Embodiment 1
[0026] Specification 10ml: 0.25mg
[0027] Palonosetron Hydrochloride 0.25mg
[0028] Glucose 500mg
[0029] Glycine 10mg
[0030] Water for injection to 10ml
[0031] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients, add water for injection and stir to dissolve, adjust the pH to 3.7 with hydrochloric acid and sodium hydroxide, and filter through a 0.22um microporous membrane , packed in 10ml vials, filled with nitrogen gas in the bottle, corked and capped.
Embodiment 2
[0033] Specification 10ml: 0.25mg
[0034] Palonosetron Hydrochloride 0.25mg
[0035] Glucose 500mg
[0036] Glycine 20mg
[0037] Water for injection to 10ml
[0038] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients, add water for injection and stir to dissolve, adjust the pH to 3.7 with hydrochloric acid and sodium hydroxide, and filter through a 0.22um microporous membrane , divided into 10ml vials, filled with carbon dioxide, corked and capped.
Embodiment 3
[0040] Specification 5ml: 0.25mg
[0041] Palonosetron Hydrochloride 0.25mg
[0042] Glucose 250mg
[0043] Glycine 15mg
[0044] Water for injection to 5ml
[0045] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients, add water for injection and stir to dissolve, adjust the pH to 3.7 with hydrochloric acid and sodium hydroxide, and filter through a 0.22um microporous membrane , packed in 5ml vials, filled with nitrogen gas in the bottle, capped and pressed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com