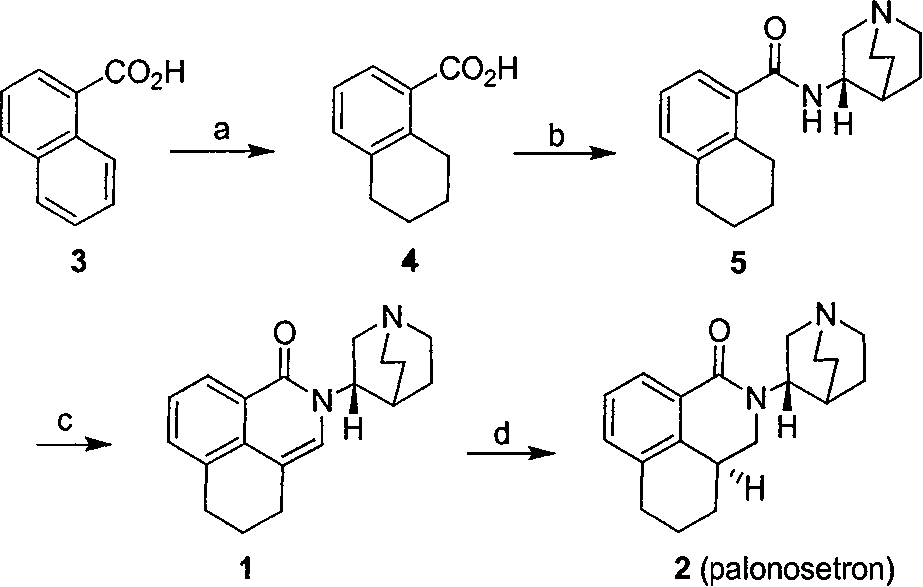
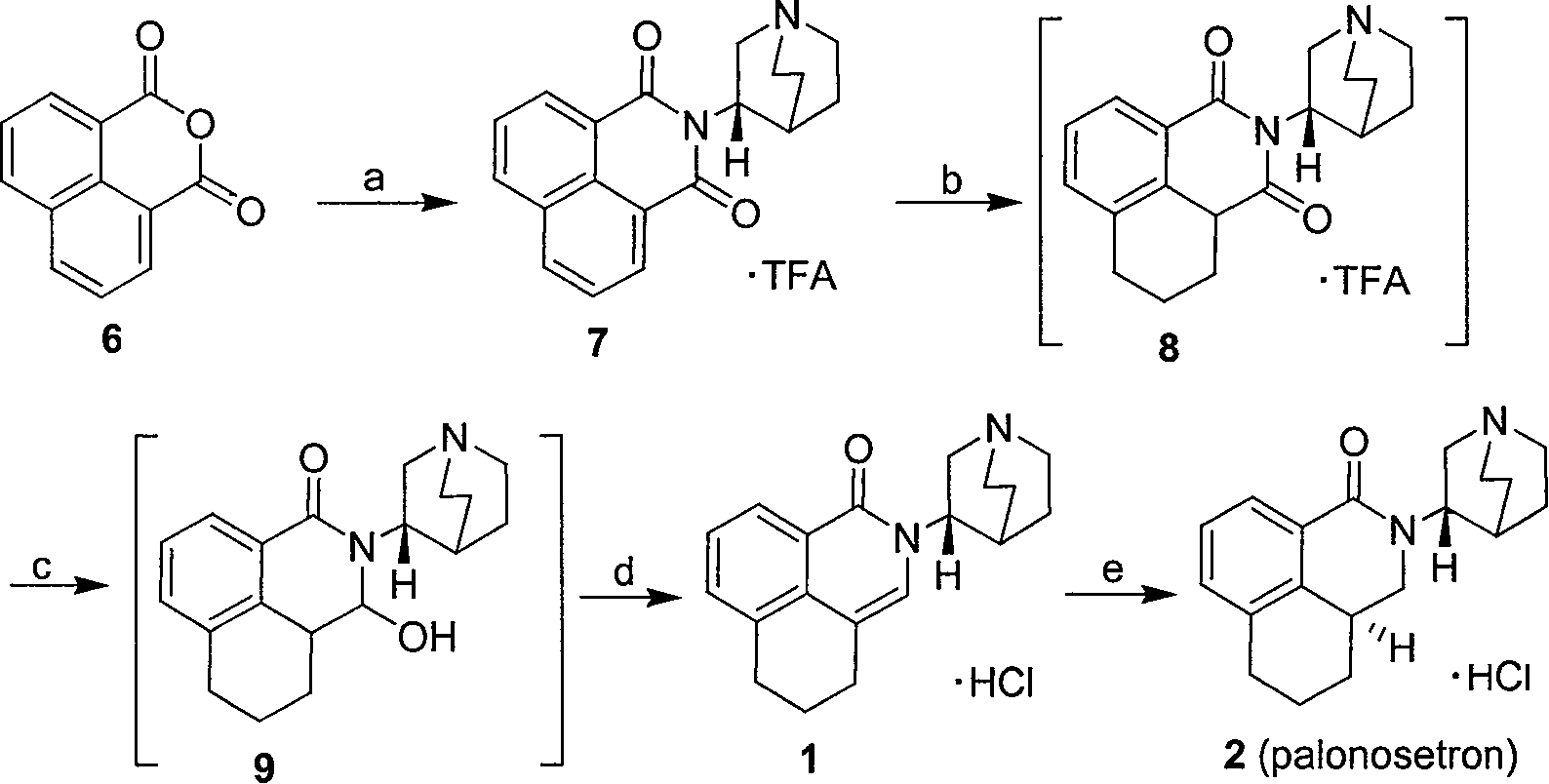
Patents
Literature
70 results about "Palonosetron Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
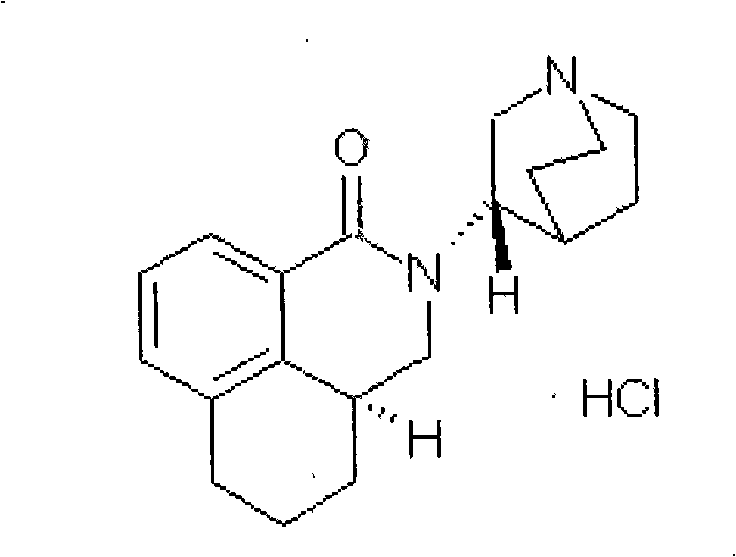
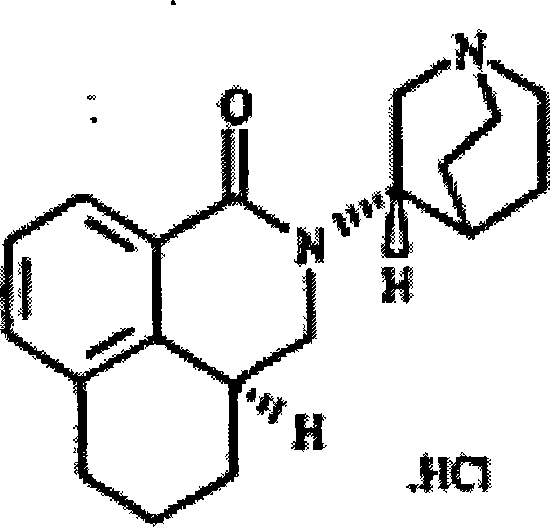
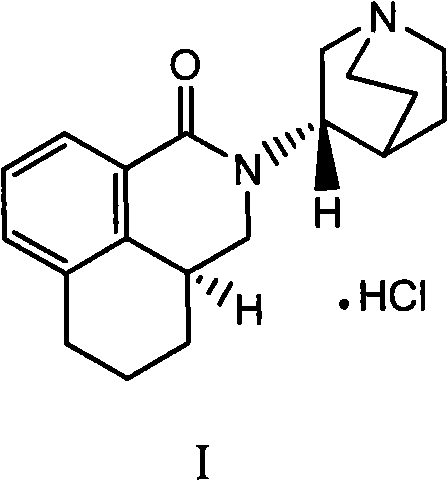
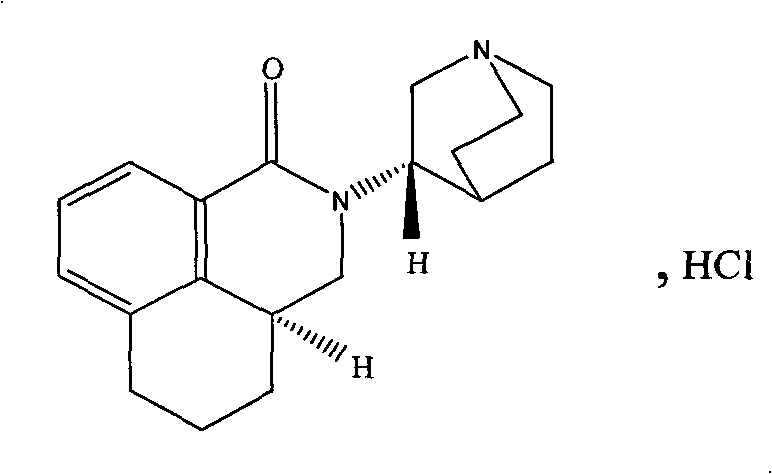
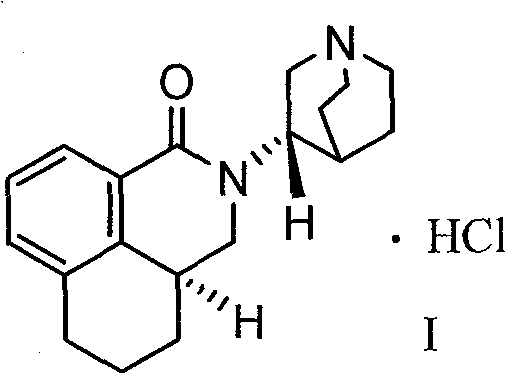
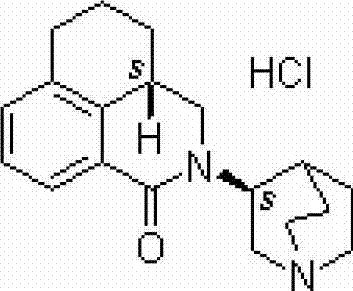
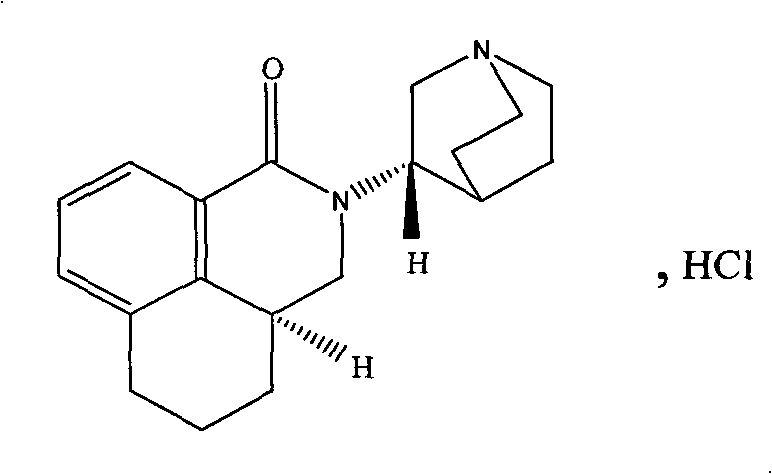
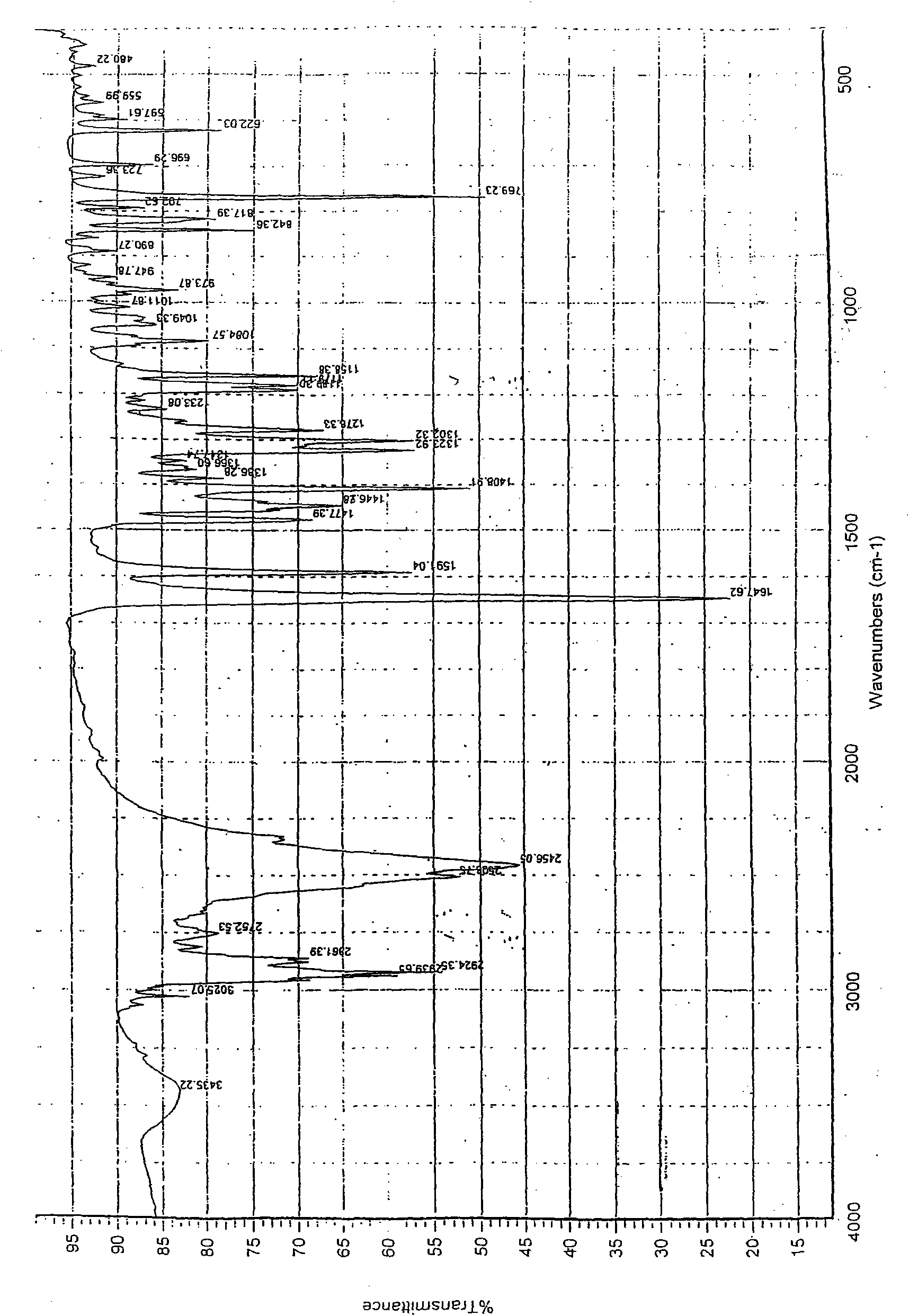
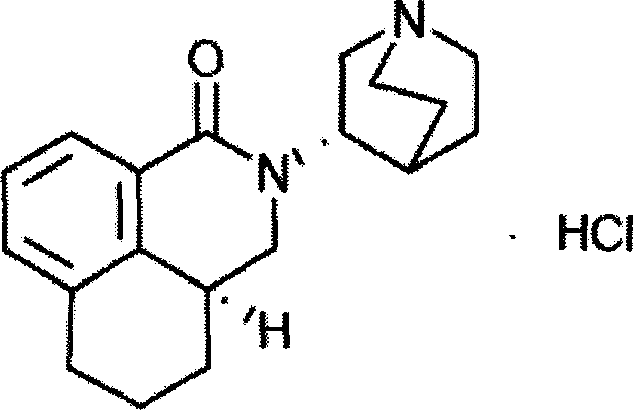
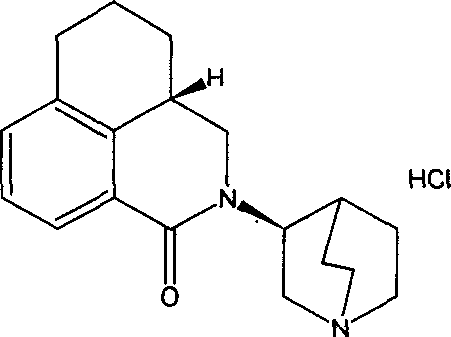
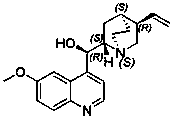
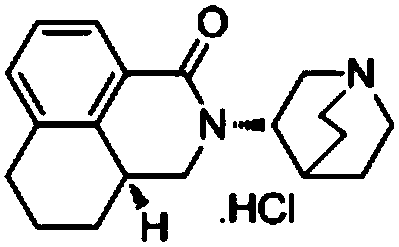
The hydrochloride salt of palonosetron, a carbazole derivative and a selective serotonin receptor antagonist with antiemetic activity. Palonosetron competitively blocks the action of serotonin at 5-hydroxytryptamine type 3 (5-HT3) receptors located on vagal afferents in the chemoreceptor trigger zone (CTZ), resulting in suppression of chemotherapy-induced nausea and vomiting. The CTZ is located in the area postrema on the dorsal surface of the medulla oblongata at the caudal end of the fourth ventricle and outside the blood-brain barrier (BBB).
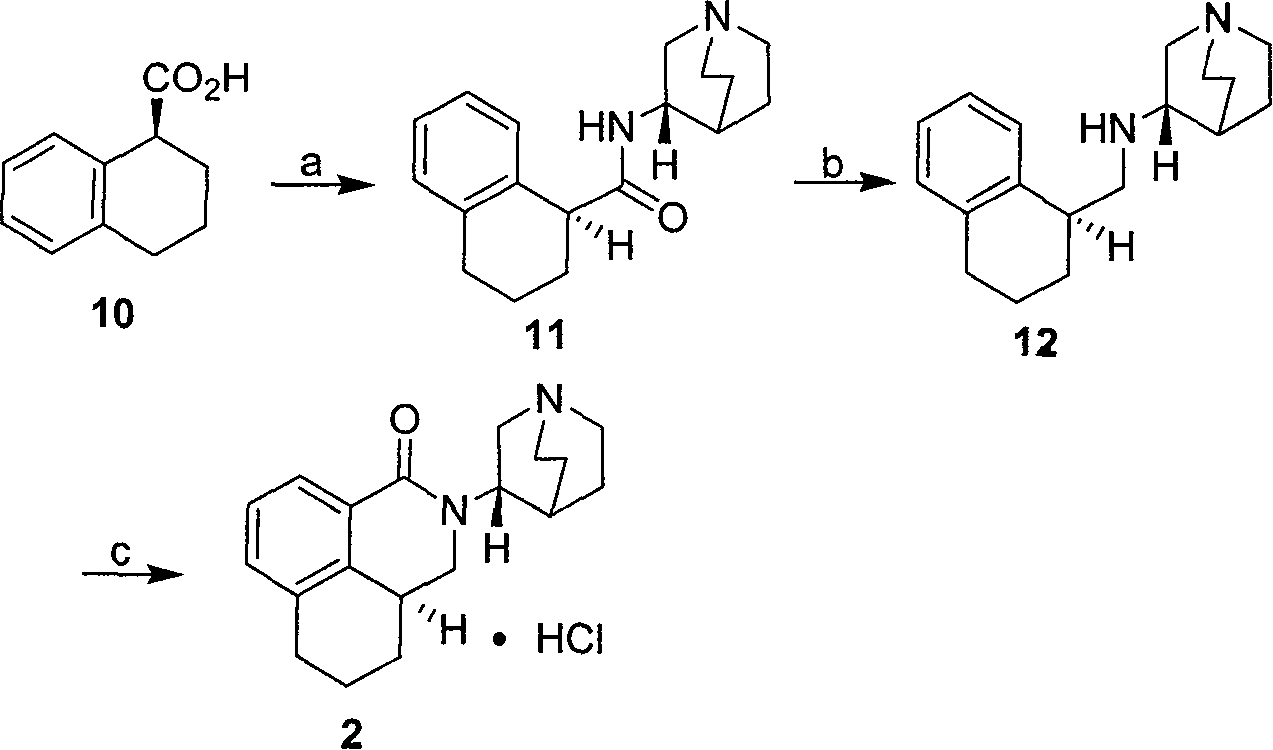
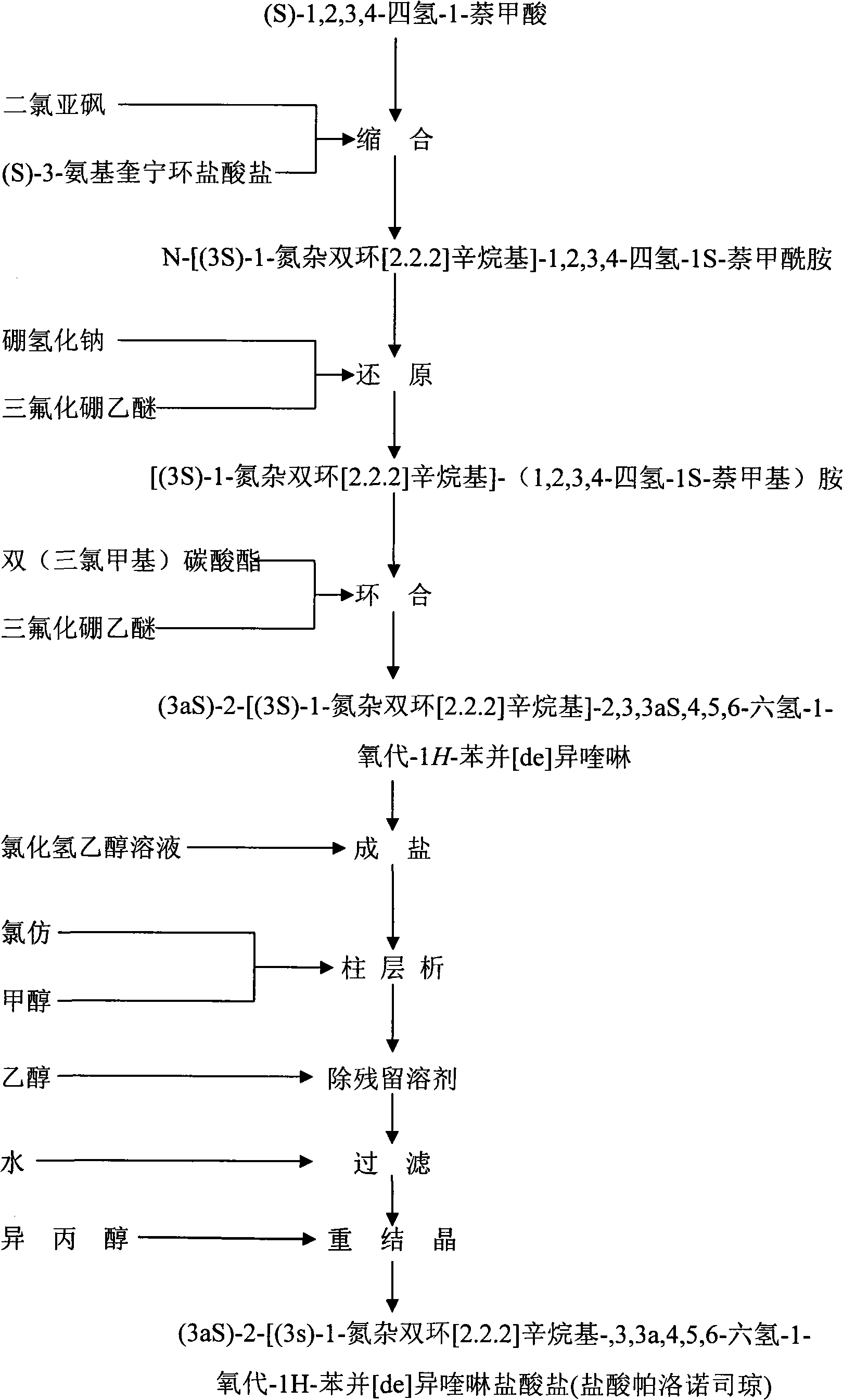
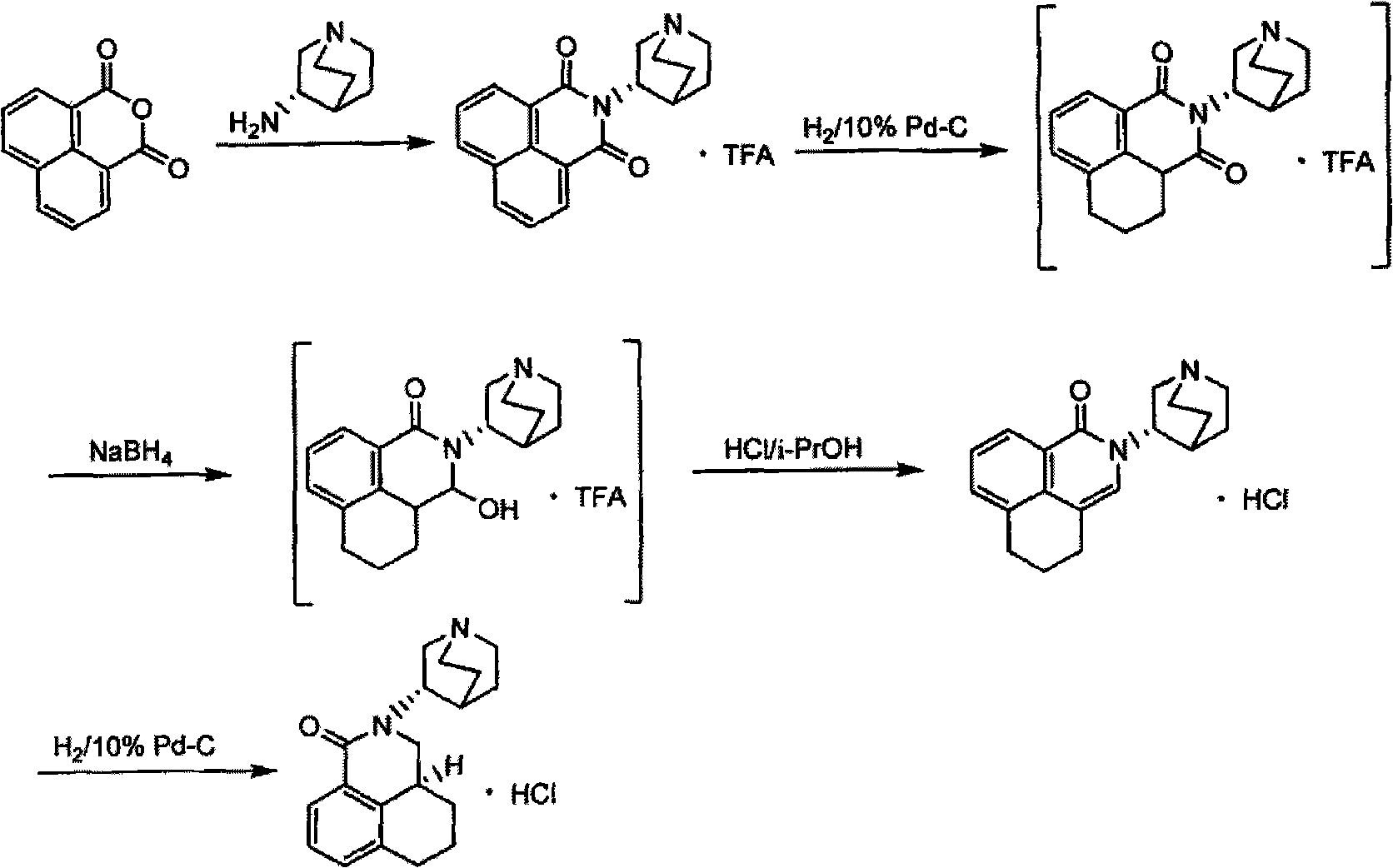
Method for synthesizing palonosetron hydrochloride
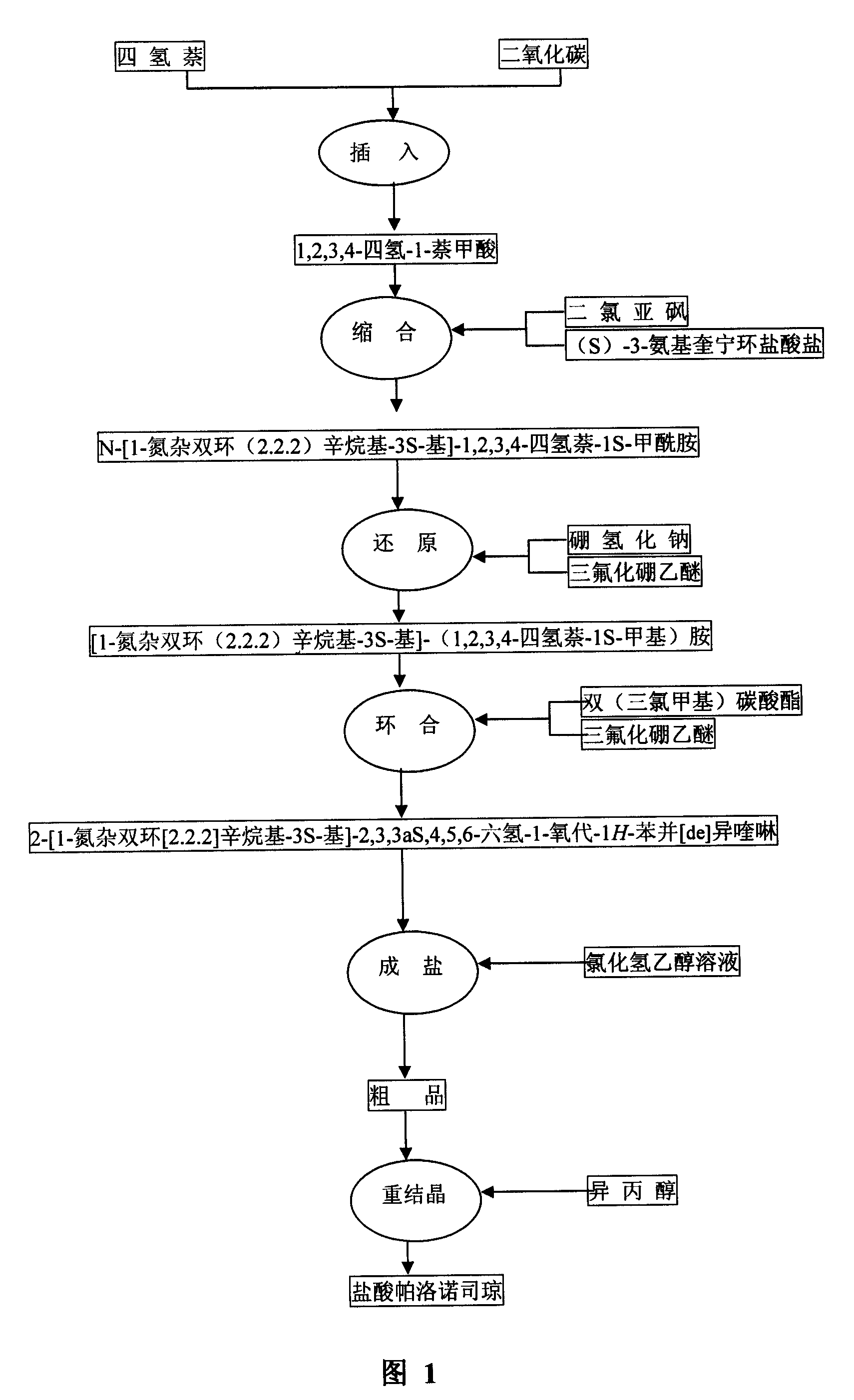
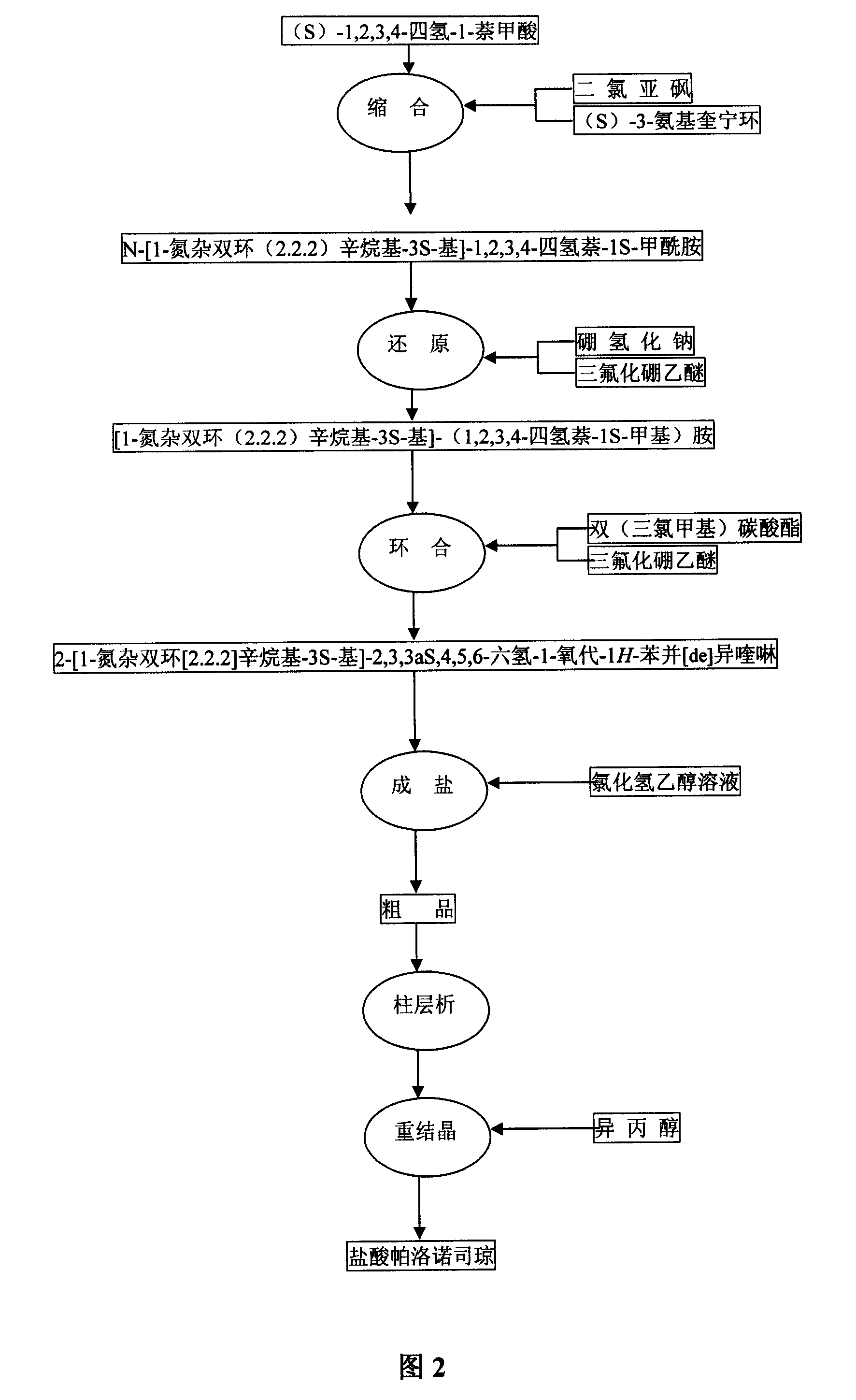
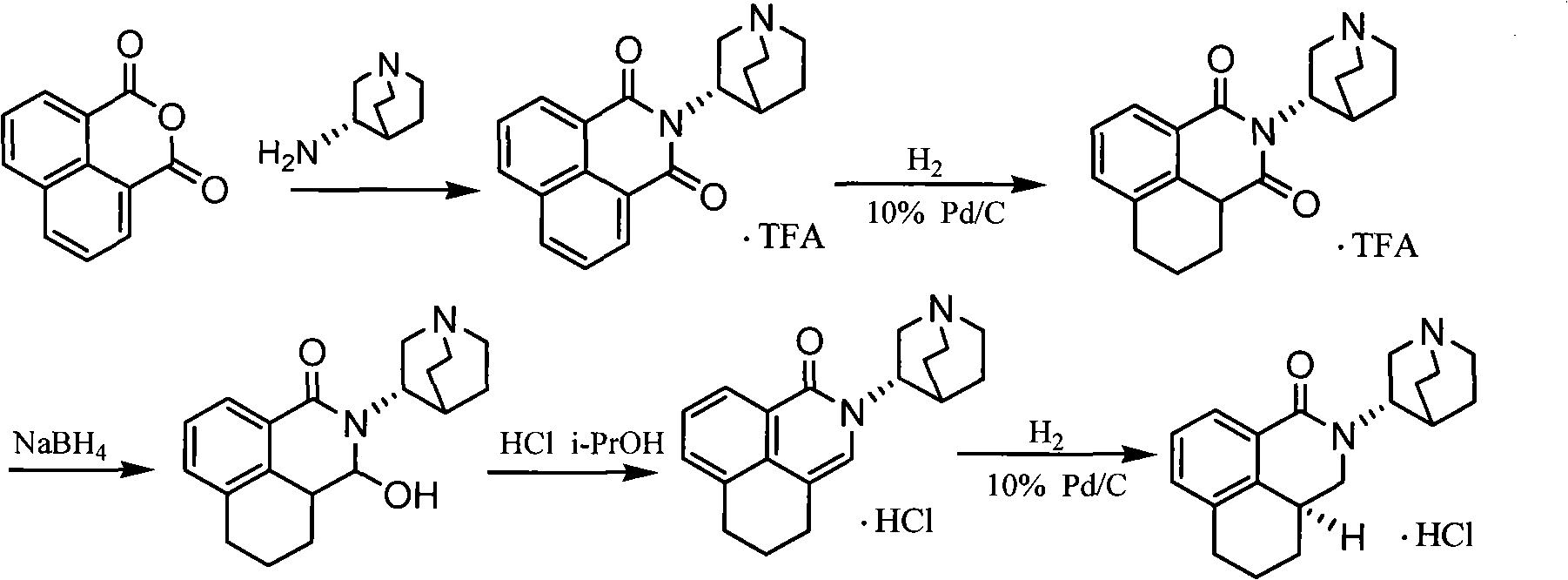
The invention discloses a novel synthesis method of palonosetron hydrochloride, which comprises that (1) (S)-tetralin formic acid is reacted with thionyl chloride and (S)-3-amido-quinine cyclic amine, to obtain (S, S)-quinuclidine tetralin formamide, (2), (S, S)-quinuclidine tetralin formamide is reacted with reductant and boron trifluoride diethyl etherate, to obtain (S, S)-tetralin methyl quinine cyclic amine, (3), (S, S)-tetralin methyl quinine cyclic amine is reacted with diphosgene to be added and reacted in boron trifluoride diethyl etherate solution, while the product is added and reacted with alcaine and water, to obtain palonosetron hydrochloride. And the synthesis route is represented as above: a: SOCI2, (S)-3-aminoquinuclidine, b: NaBH4, BF3OEt2, c: BF3OEt2, CICO2CCI3.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Method for separating and measuring palonosetron hydrochloride optical isomer
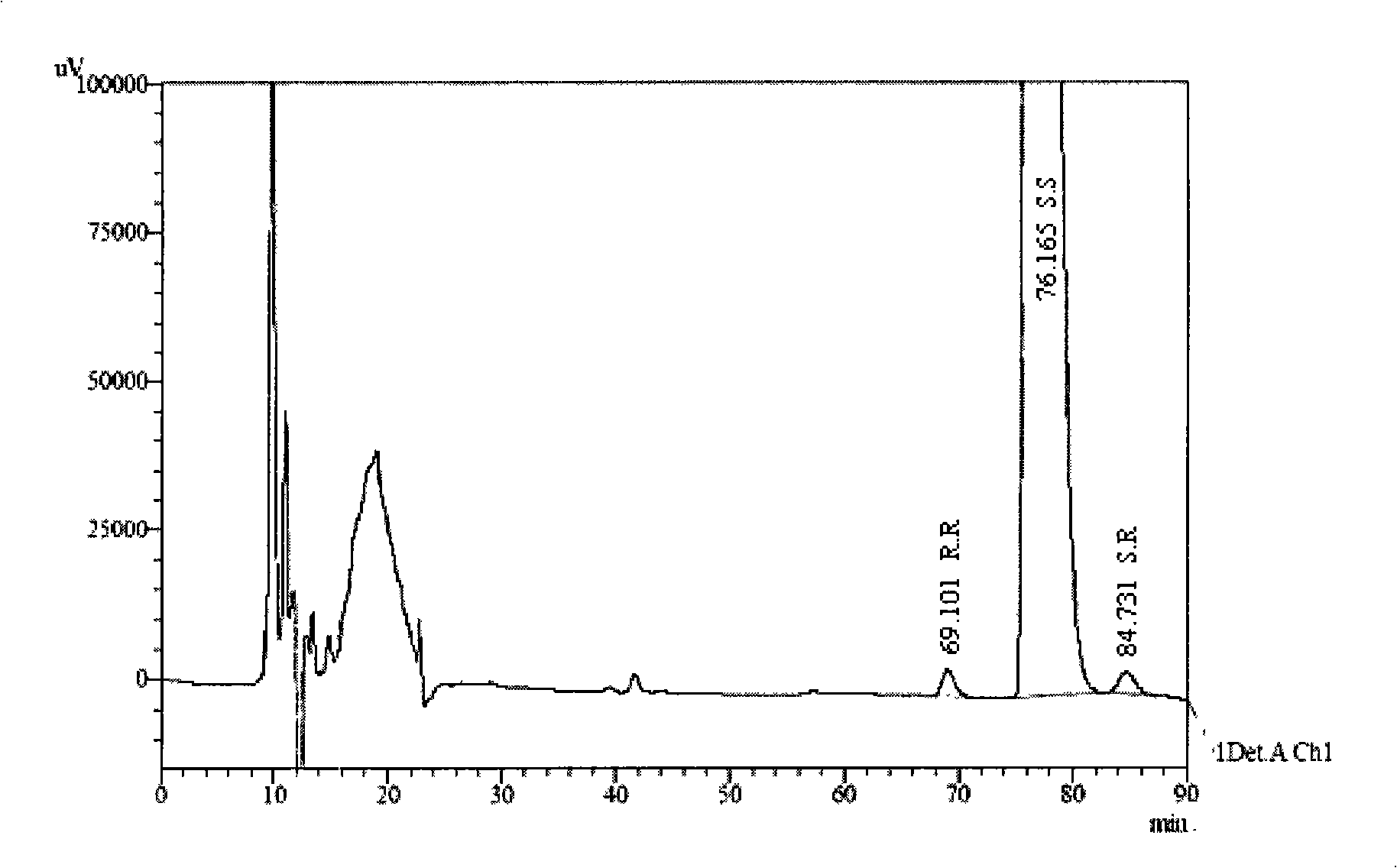
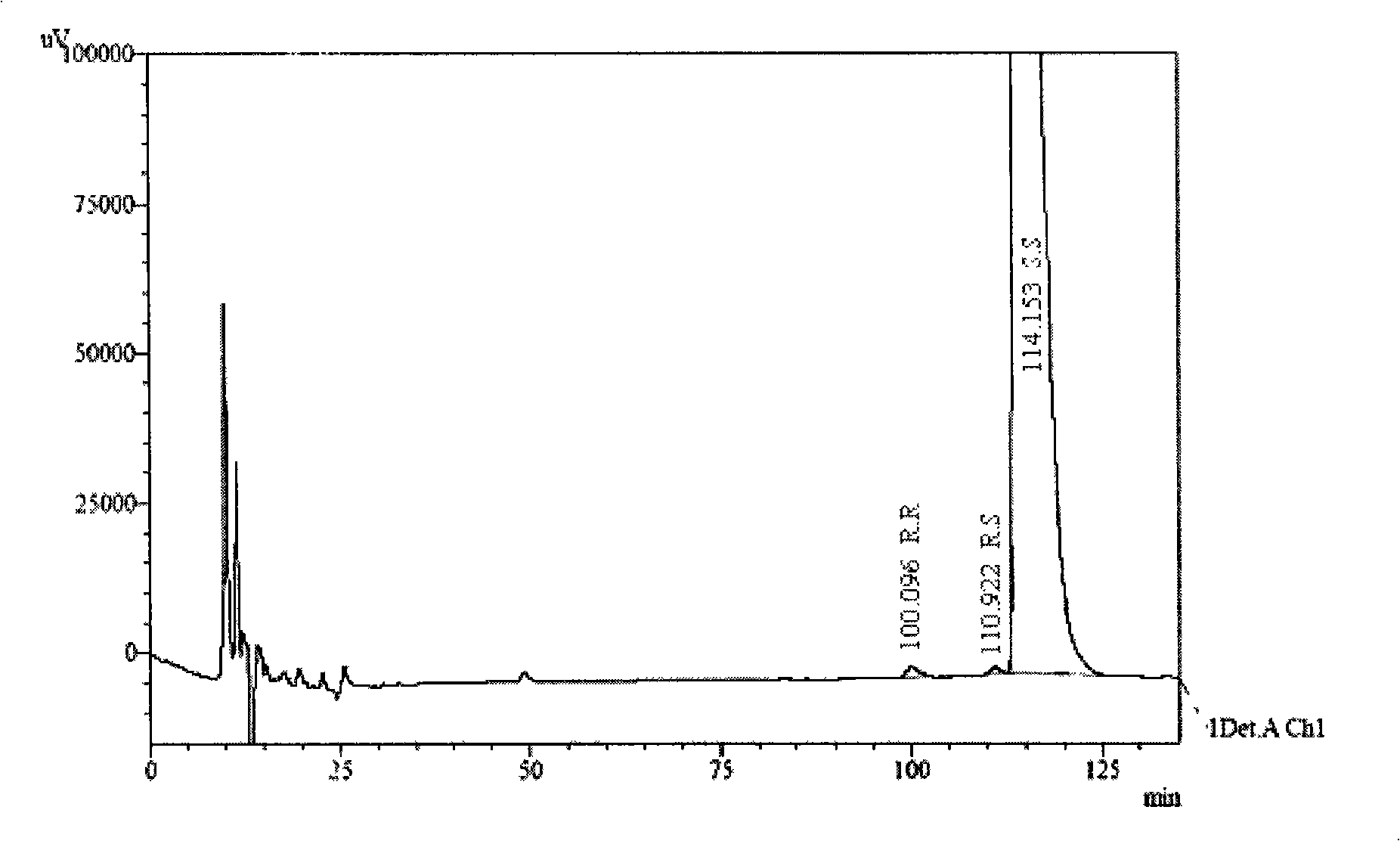
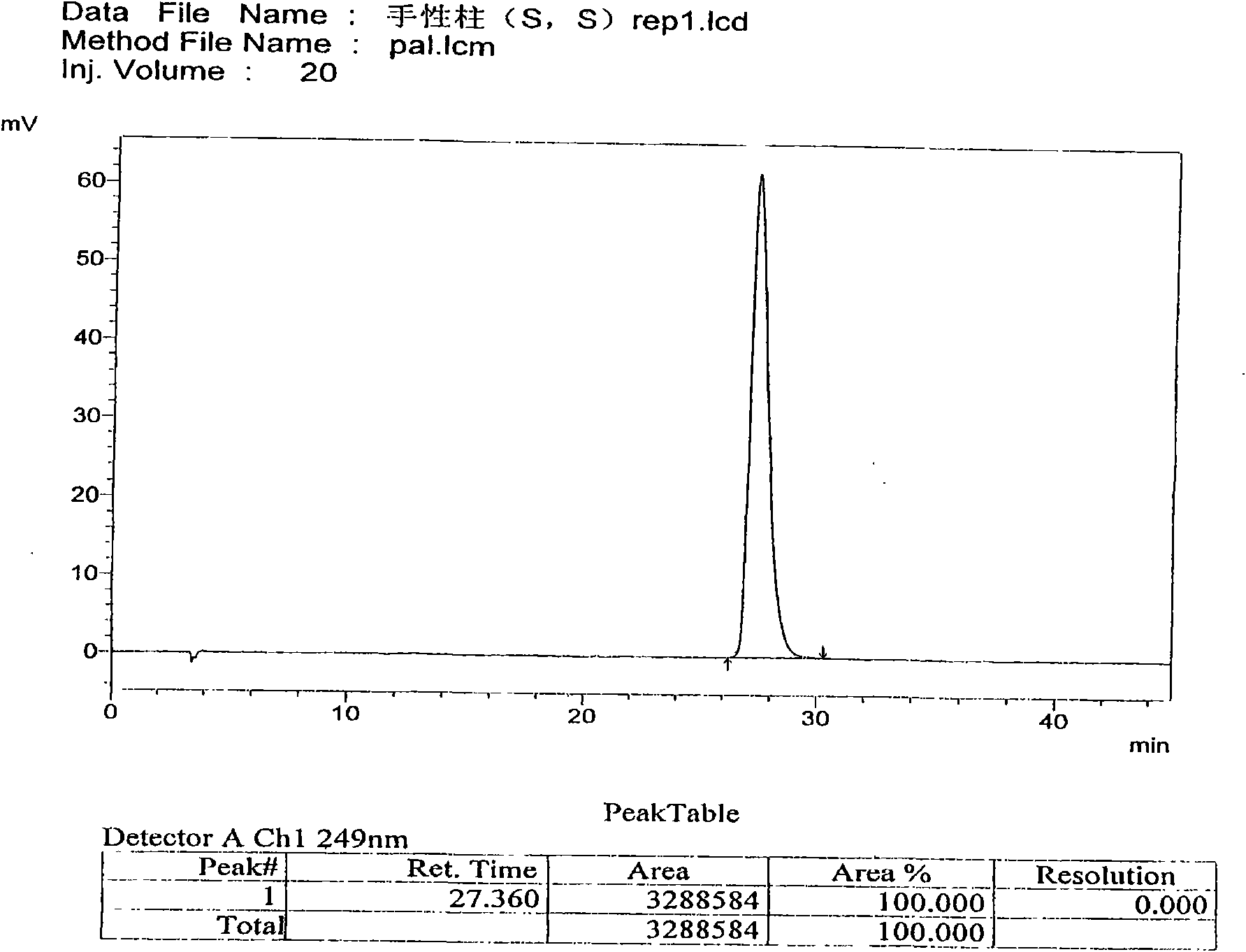
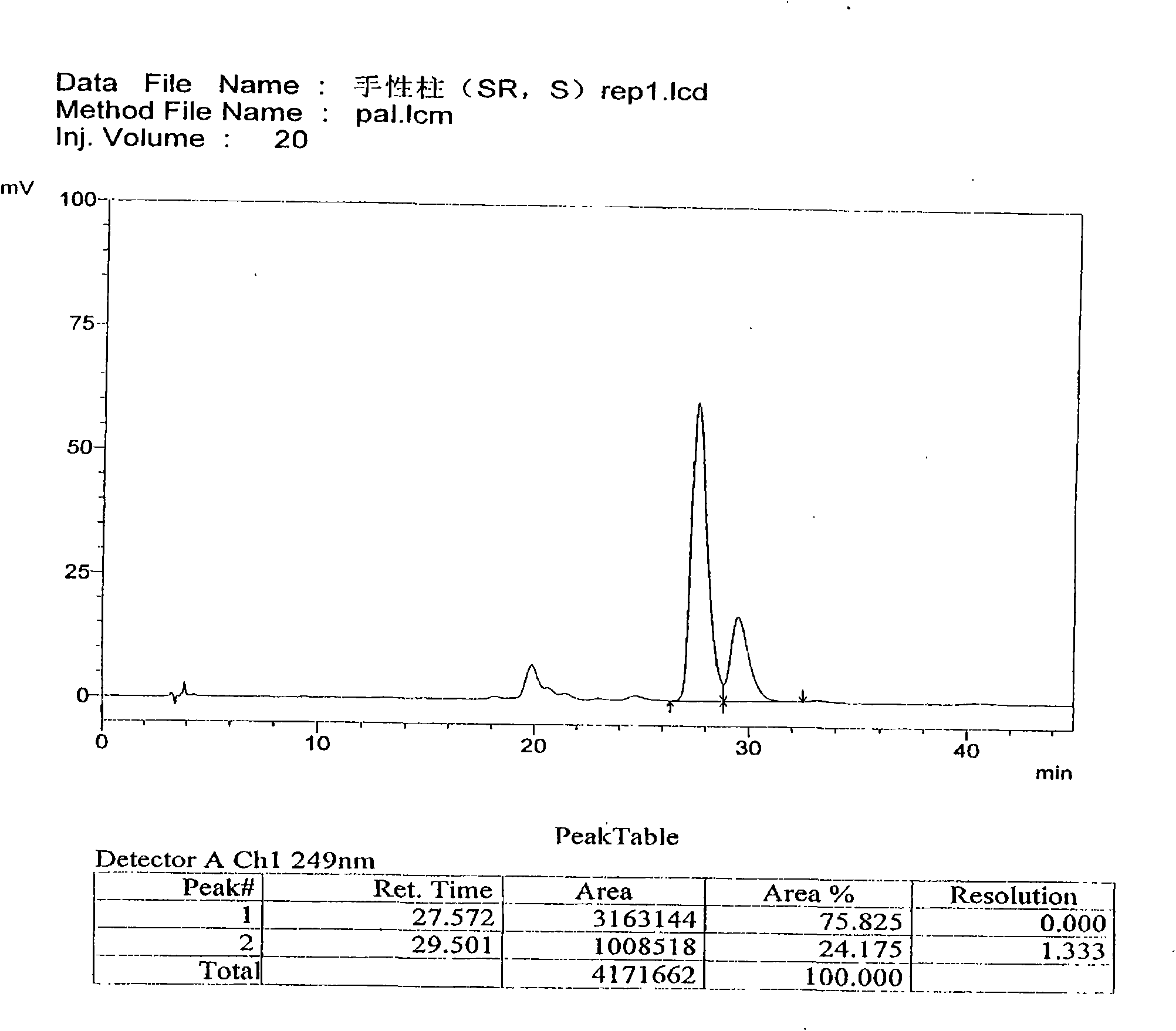
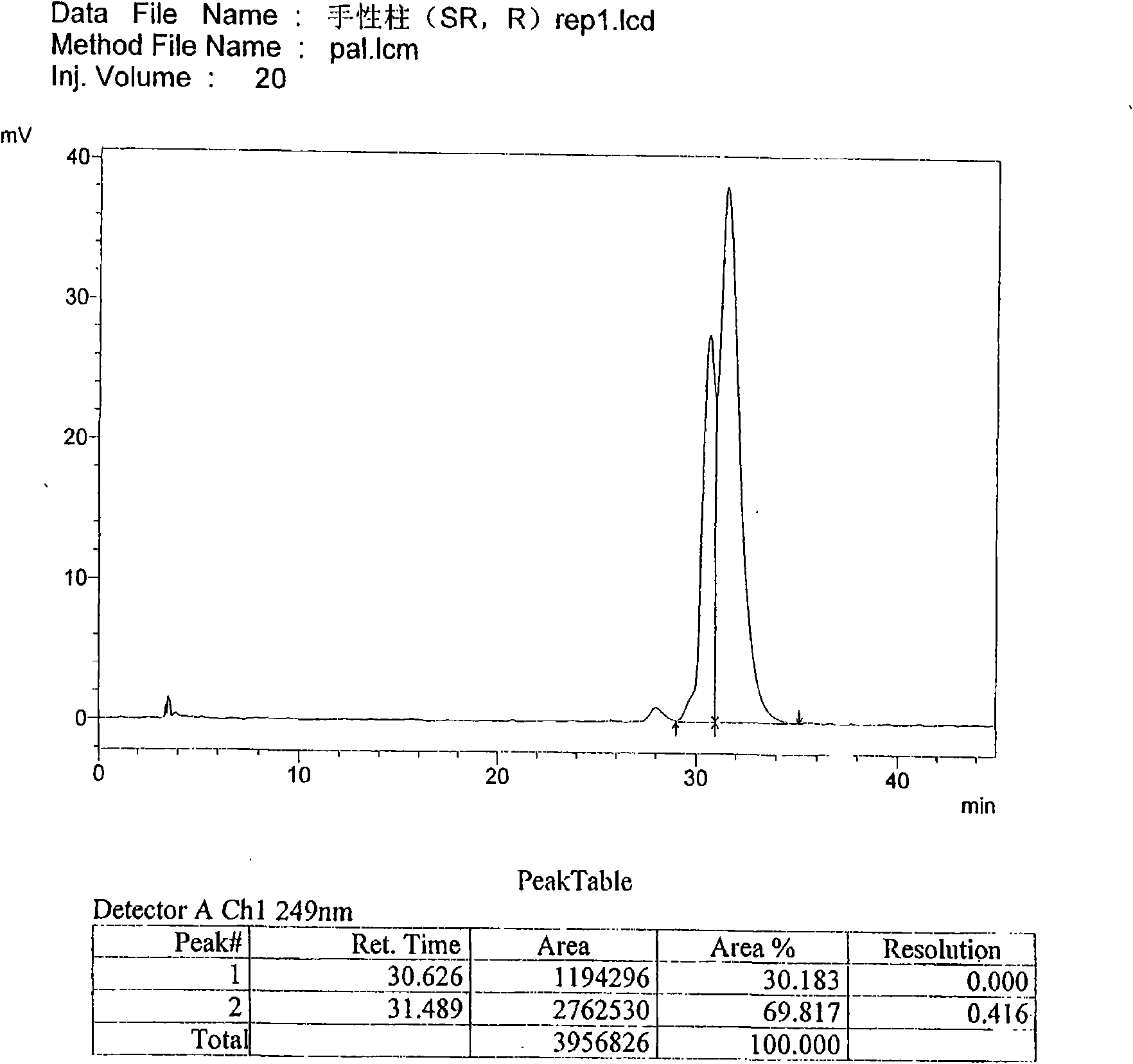
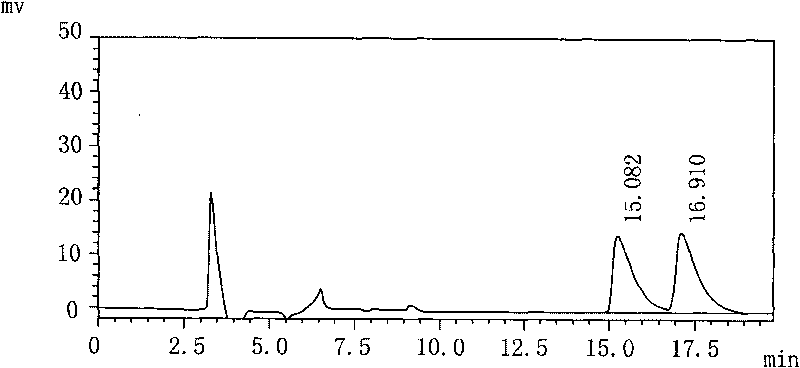
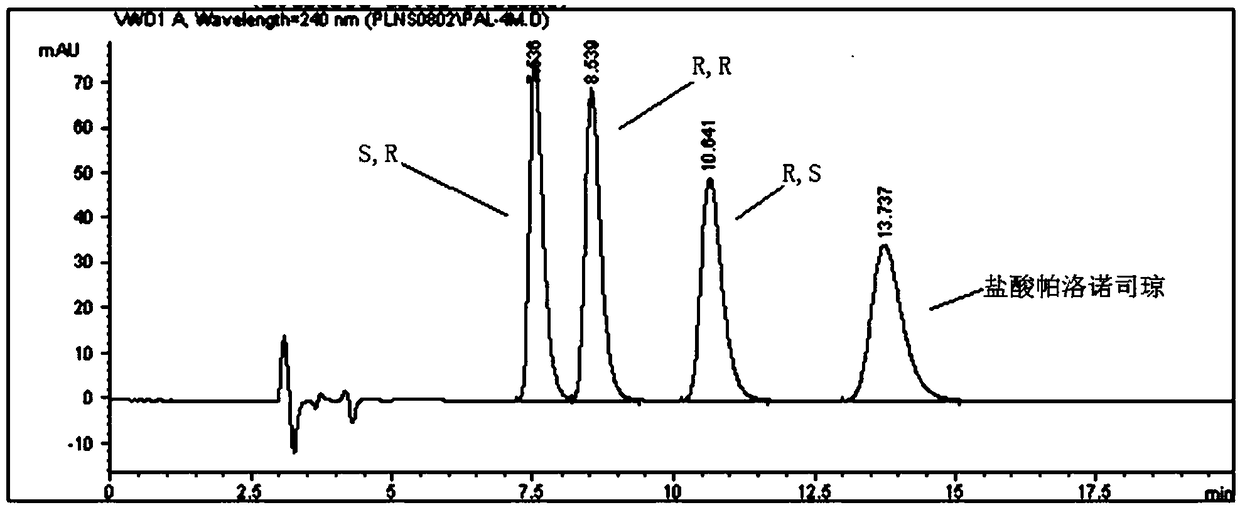
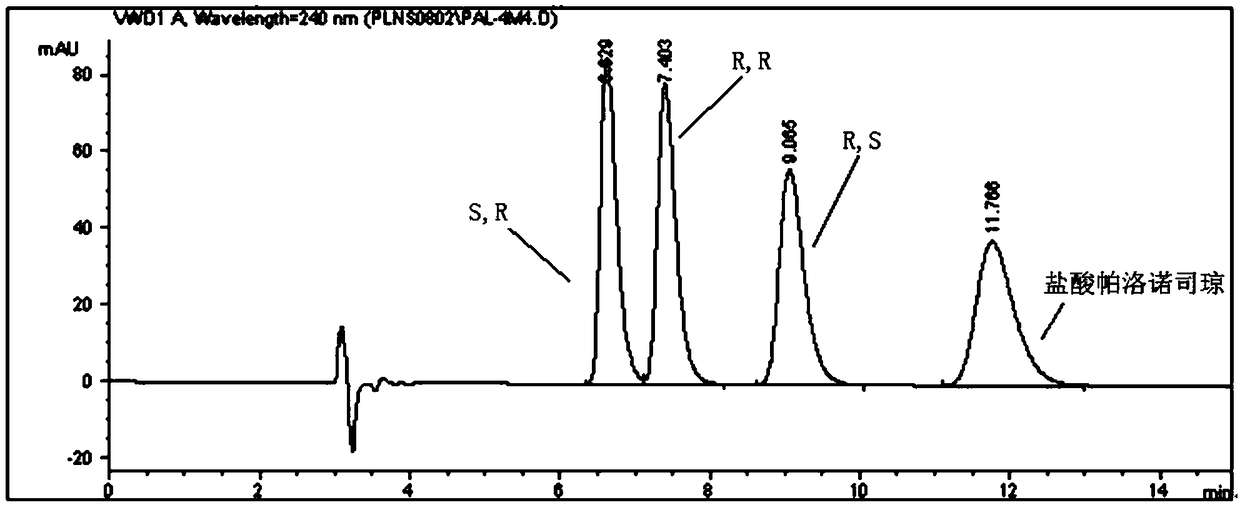
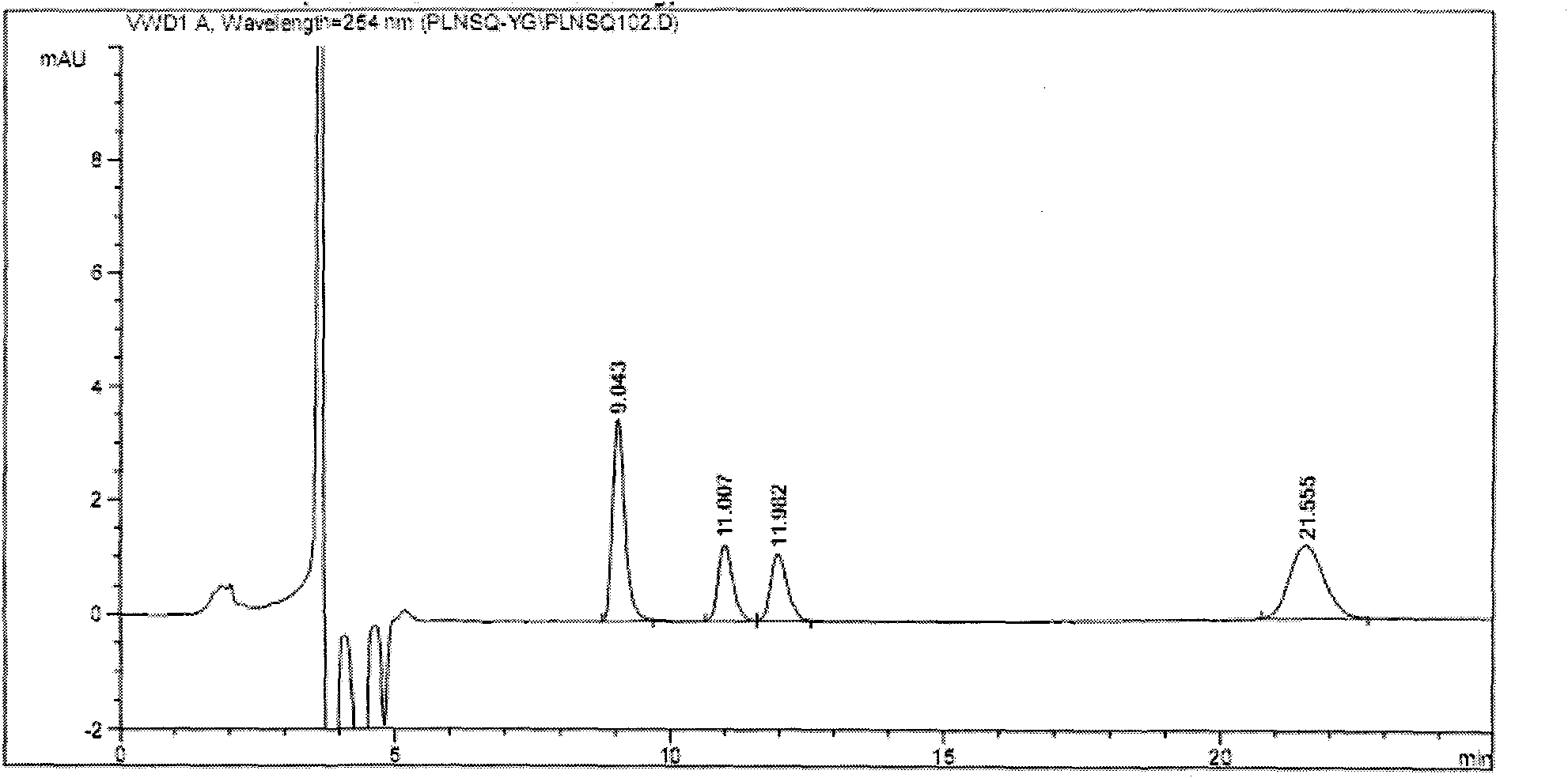
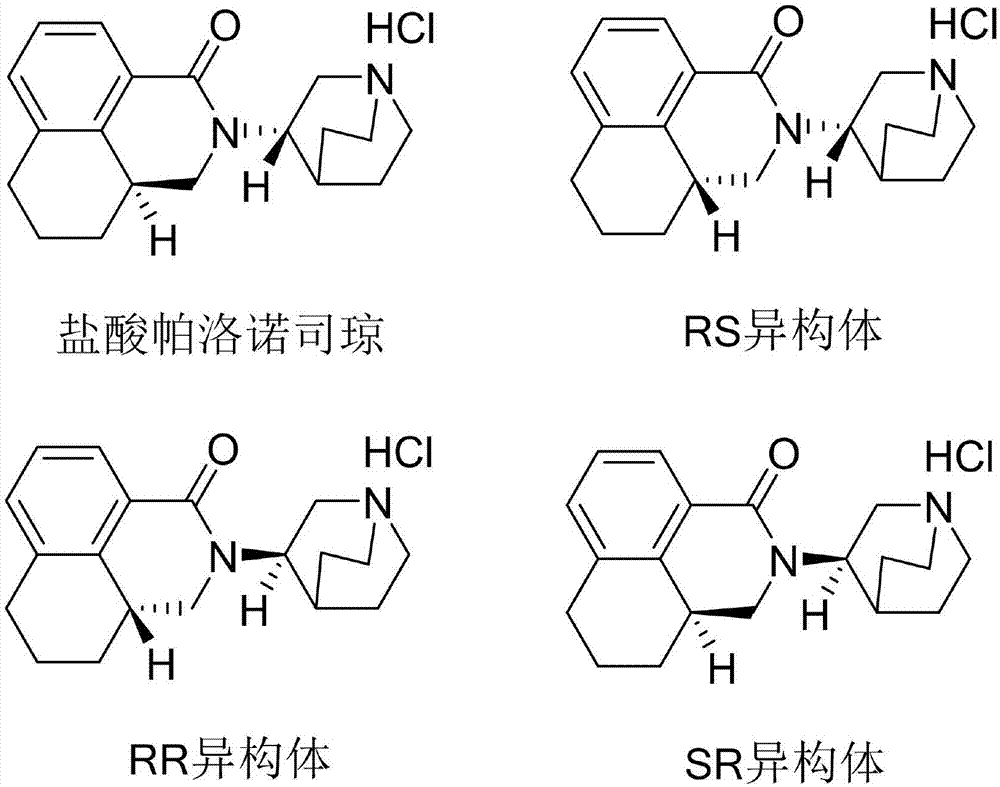
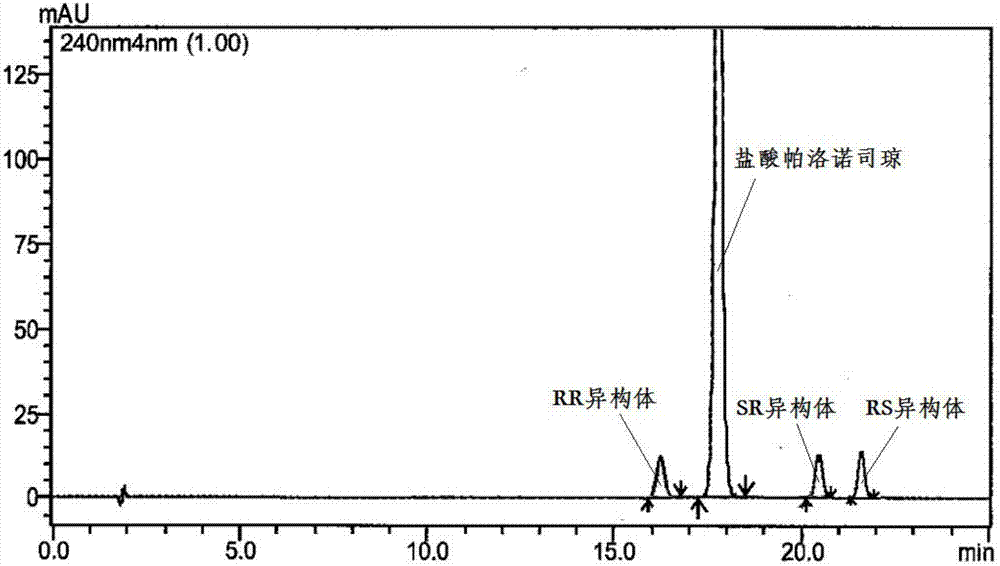
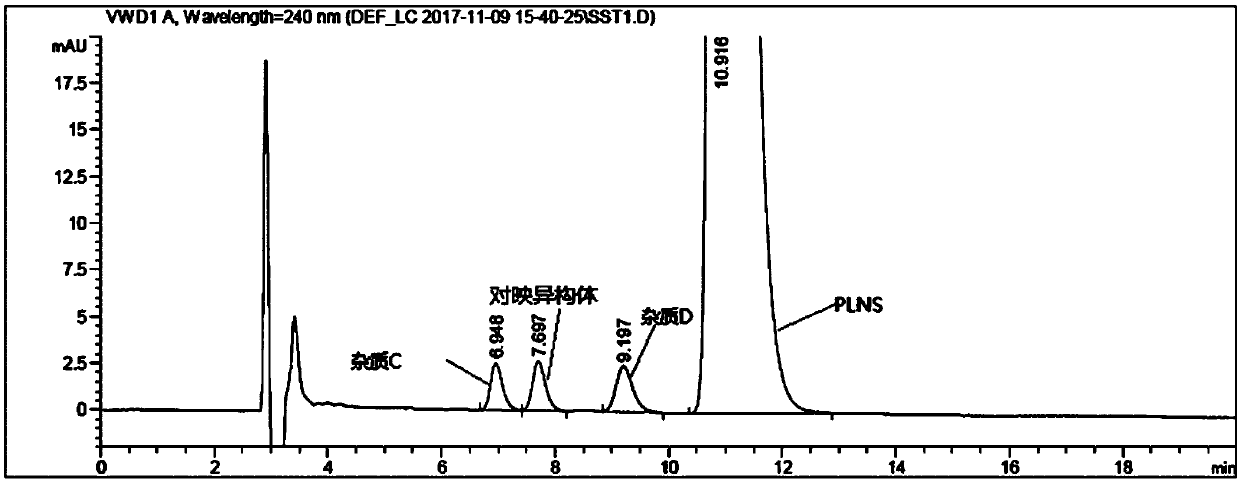
The invention discloses a separation and determination method of the optical isomer of Palonosetron HCl; the separation and determination method comprises the resolution of the optical isomer of the Palonosetron HCl by high performance liquid chromatography, wherein, amylose-three (3,5-xylyl methyl carbamate) is used as the filler by normal phase chromatographic columns, the volume ratio of mobile phase n-hexane: alcohol: diethylamine is 75-95: 5-25: 0.1-0.5, the column temperature for separating R. R isomer, R. S isomer and S. S isomer is 15 to 25 DEG C, while the column temperature for separating R. R isomer, S. S isomer and S. R isomer is 35 to 45 DEG C. The method of the invention provides an important basis for the quality monitoring of Palonosetron HCl material.
Owner:深圳万乐药业有限公司
Production technique of hydrochloric acid palonosetron
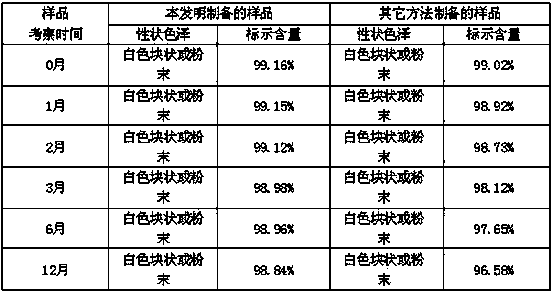
The invention relates to a new production process of palonosetron hydrochloride with high purity. The final product of palonosetron hydrochloride can be obtained by that the synthesized crude palonosetron hydrochloride is dissolved and is carried out the silica gel column chromatography, the target components are collected after a plurality of elutions, and the target components are carried out the recrystallization to get the final product. The HPLC detection shows that the purity of the product can achieve more than 99.5 percent, and a single impurity is below 0.1 percent. The new production process can improve the yield of palonosetron hydrochloride to more than 20 percent, but the synthesis yield of an existing process is only 6 percent, so the invention has significant progress by comparison.
Owner:HANGZHOU JIUYUAN GENE ENG
Palonosetron oral cavity film agent and preparation method thereof
ActiveCN105997955AFacilitated releaseImprove bioavailabilityOrganic active ingredientsDigestive systemPharmacologyOral cavity
Owner:LP PHARM (XIAMEN) CO LTD
Mouth spray for preventing and treating nausea and emesis after tumor chemotherapy and radiotheraphy and preparation method thereof
InactiveCN101385712AEasy to increase or stop doseReduce efficacyAerosol deliveryPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention relates to an oral spray for controlling nausea and vomit of chemotherapy and radiation therapy, the formula of the oral spray is composed of ingredients with the following parts by weight: 5-50 parts of drug absorption enhancer, 2-20 parts of drug active ingredient and 30-90 parts of buffer, the drug absorption enhancer can be any one or the combination of more of the following ingredients: azone, propylene glycol, polysorbate (Tween), ethylene glycol deoxycholic acid sodium salt, brij, sodium decanoate, lauric acid, stearic acid, sodium lauryl sulphate, stearyl alcohol sodium sulfate, dioctyl succinate sodium sulfonate, oleic acid, GK2, menthol and borneol; the drug active ingredient can be any one combination of the following ingredients: palonosetron hydrochloride, granisetron, ondansetron, azasetron and tropisetron; and the ingredients of the buffer are sodium citrate buffer solution and phosphate buffer solution. The oral spray provides a formulation which is safer, painless and convenient for patients with advanced tumor, elderly and weak patients, children patients and the patients who suffer from the metal illness and do not obey the oral administration or the injection drug administration.
Owner:陆飚 +1
Palonosetron injection and its preparation method
InactiveCN101057827AImprove stabilityLow costOrganic active ingredientsDigestive systemPorous membraneGlucose polymers
The invention discloses a palonosetron injection and the preparation method. The essential component of the injection comprises: hydrochloric acid palonosetron 0. 01-0. 05 mg / ml, glucose 45-55 mg / ml, aminoacetic acid 0. 2-10 mg / ml. The preparation method comprises following steps: (1) dissolving hydrochloric acid, glucose, and aminoacetic acid in proper amount of water for injection, moderating pH value, adding water for injection to meet the demand; (2) filtering with micro-porous membrane; (3) packing filtered solution, aerating inert gas or carbon dioxide, pressing, covering. The product is characterized by low production cost and good stability.
Owner:深圳万乐药业有限公司
Method for separating and measuring Palonosetron hydrochloride and optical isomers thereof
ActiveCN101661019AQuantitative determination of contentComponent separationSolid sorbent liquid separationOrganic solventGlycopeptide
The invention relates to a method for separating and measuring content of Palonosetron hydrochloride and optical isomers thereof by high performance liquid chromatography, which is characterized in that: a chromatographic column packing material used in the high performance liquid chromatography is macrocyclic glycopeptides and teicoplanin bonded silica gel, and a mobile phase consists of aqueoussolution of triethylamine and organic solvent.
Owner:CHONGQING HUAPONT PHARMA
Method for testing isomer of palonosetron hydrochloride injection solution
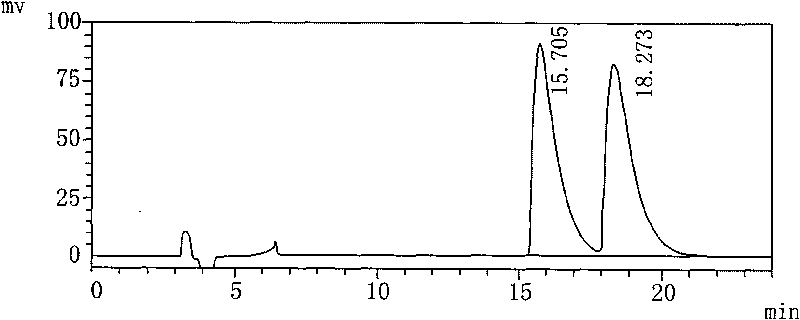
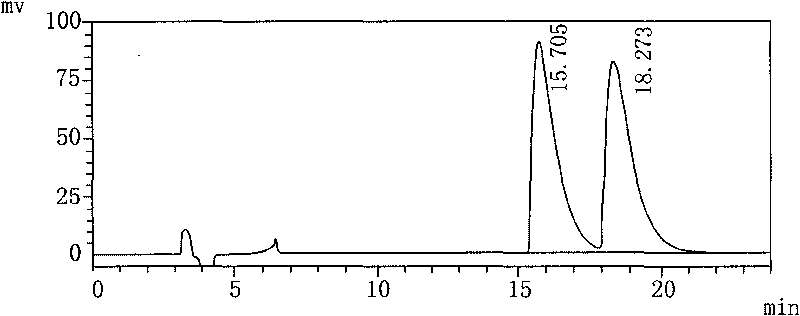
The invention relates to a method for testing medicine, in particular to a method for testing isomer of palonosetron hydrochloride injection solution. The method comprises the following steps: a. preparing test solution; b. preparing mapping isomer finished solution; c. preparing mixed pretest solution, mixing the test solution and the mapping isomer finished solution with the same volume and taking the mixture as the mixed pretest solution; and d. testing the mixed pretest solution by adopting high performance liquid chromatography. The method takes isopropanol as solvent with good dissolubility and separating effect, adopts an AS-H chiral column and the mixture of normal hexane, isopropyl alcohol and diethylamine as mobile phase when testing, separates the chromatographic peak of the mapping isomer by means of completely reaching baseline, wherein the separation degree is larger than 1.5, and plays an important role in controlling the quality of the 'palonosetron hydrochloride injection solution'.
Owner:YINCHUAN TIANCHENGJIAN PHARMA RES
Preparation process of high-purity palonosetron Hcl
ActiveCN101314601AQuality is easy to controlImprove product qualityOrganic chemistryDigestive systemWater insolubleSilica gel
The invention relates to a novel preparation method of high-purity (3aS)-2-[(3S)-1-azabicyclo[2.2.2]oct-3-yl]-2,3,3aS,4,5,6-hexahydro-1H-benz[de]isoquinolin-1-one Hydrochloride (namely, Palonosetron hydrochloride) which comprises the three steps as follows: (1) separating the Palonosetron hydrochloride by silica gel column chromatography to obtain Palonosetron hydrochloride coarse product, dissolving in ethanol, concentrating and drying; (2) dissolving in water, filtering to remove water-insoluble impurities, concentrating, and drying; and (3) re-crystallizing.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
Method for simultaneously determining four optical isomers of palonosetron hydrochloride
ActiveCN102207494AComponent separationOptically-active compound separationQuality controlColumn temperature
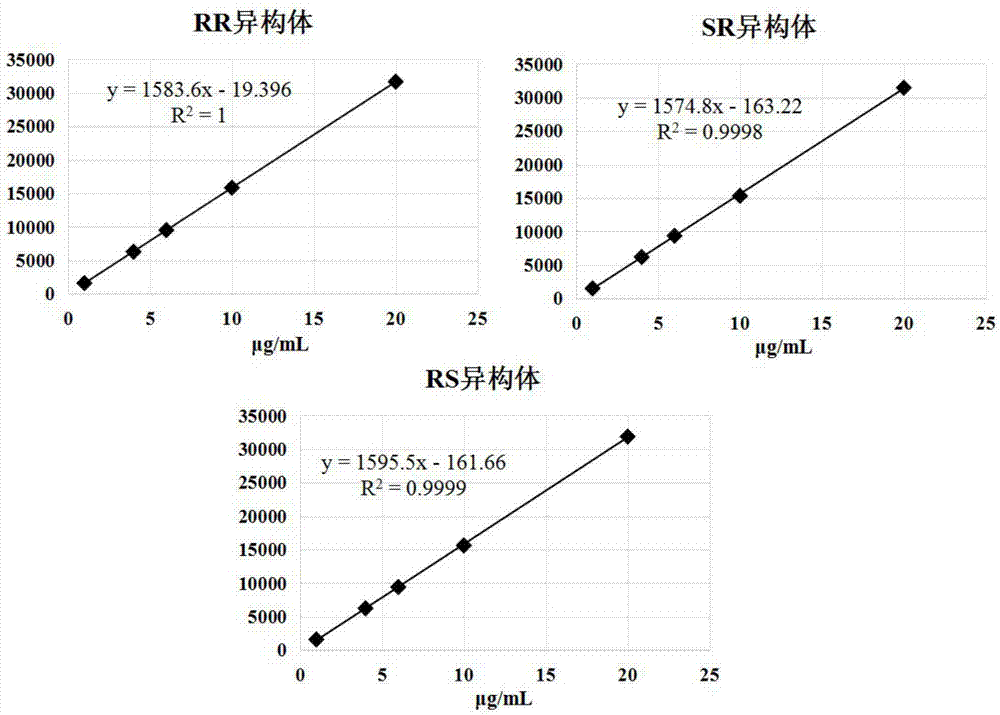
The invention discloses a method for simultaneously determining four optical isomers of palonosetron hydrochloride. The four optical isomers are S.S, S.R, R.S, R.R respectively; and the method is characterized by comprising the following step of splitting the optical isomers of the palonosetron hydrochloride by using efficient liquid chromatography, wherein an OD-H chiral chromatographic column is included, and a mobile phase consists of acetonitrile, isopropanol and diethylamine in a volume ratio of (82-92): (18-8): (0.1-0.2), and the column temperature is 30 to 40 DEG C. A flow rate in the efficient liquid chromatography is 0.3 to 1.2 ml / min. The detection wavelength in the efficient liquid chromatography is 220nm. The method is simple, convenient and practical. The purpose of simultaneously determining and separating the four optical isomers can be rapidly achieved, a basis is provided for quality control of palonosetron hydrochloride raw materials and preparations, and the quality control of a new medicine is facilitated.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Compound preparation containing fosaprepitant and palonosetron hydrochloride, and preparation method thereof
InactiveCN102755338AMeet clinical needsImprove compliancePowder deliveryOrganic active ingredientsPatient complianceFosaprepitant dimeglumine
The invention provides a sterile lyophilized compound preparation containing fosaprepitant and palonosetron hydrochloride, and a preparation method thereof. According to the invention, effective dosages of fosaprepitant dimeglumine and palonosetron hydrochloride are adopted as effective components, and auxiliary materials such as a solubilizer, a complexing agent, an excipient, and an acidity adjusting agent are contained in the preparation. The preparation is filtered, sterilized, sub-packaged, and lyophilized. When in use, the sterile lyophilized compound preparation provided by the invention is added into a solvent, such that injection solution with an appropriate concentration is prepared and can be used. With one time of medication, clinical demands of two treatment medicines can be satisfied, such that patient compliance is improved. The preparation method of the compound preparation is simple, and the method is suitable for industrialized productions.
Owner:QILU PHARMA
Method for determination of optical isomers in palonosetron hydrochloride composition
The invention provides a method for detecting palonosetron hydrochloride isomers in a palonosetron hydrochloride composition. The method adopts a chiral chromatographic column with amylose tris(3,5-dimethylphenylcarbamate) as filler; n-hexane / absolute ethyl alcohol / diethylamine as a mobile phase; and a detection wavelength of 240nm. The method comprises the following steps of preparing 0.5mug / ml of a solution containing palonosetron hydrochloride isomers with the mobile phase as a reference solution, performing determination and recording the spectra. The method provided by the invention can effectively separate three optical isomers of palonosetron hydrochloride under the same chromatographic conditions, and the separation degree between palonosetron hydrochloride and each of the isomers of palonosetron hydrochloride can be up to 1.5 to 2.0, which is in line with the requirements of Chinese Pharmacopoeia. The method provided by the invention can also be used for quantitative determination of palonosetron hydrochloride isomers.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
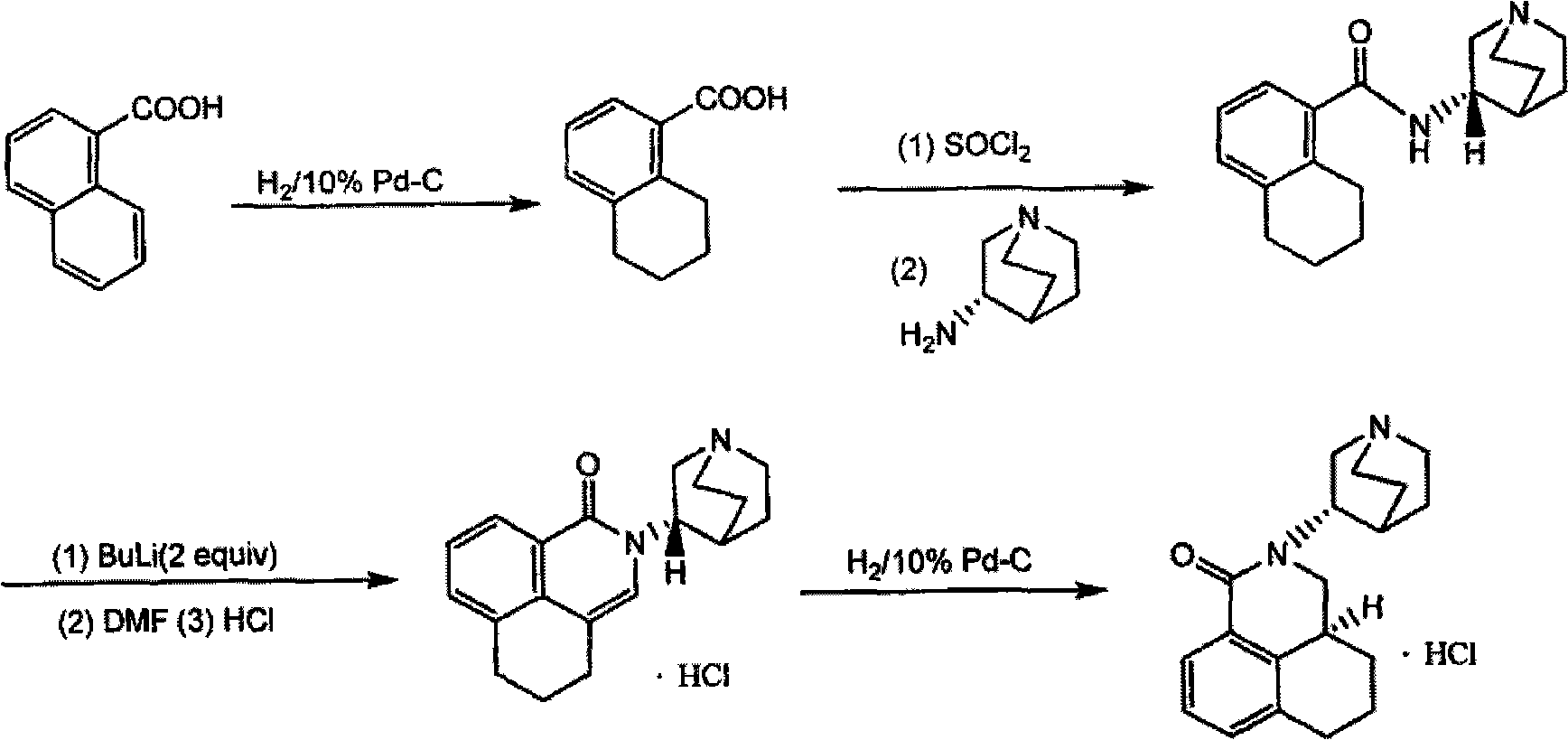
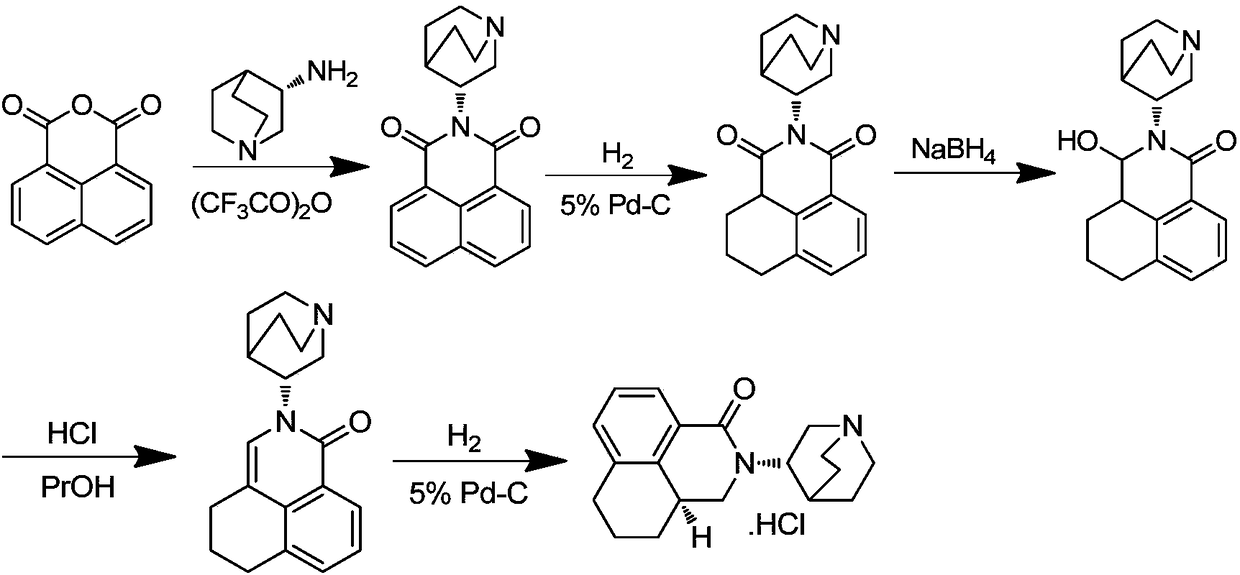
Palonosetron hydrochloride, and precursor compound and preparation method thereof
The invention discloses a palonosetron hydrochloride, and a precursor compound and a preparation method thereof. Referring to the formula (3), the structure of the precursor compound is as follows: 8-(1-azabicyclo [2, 2, 2] capryl-3S-yl)-aminomethyl-1-naphthoic acid compound. The preparation method of the precursor compound comprises the following steps of: reacting 1-naphthoic acid or ester compound thereof with haloid acid and methylating reagent under the catalysis of Lewis acid to obtain midbody 8-chloromethyl-1-naphthoic acid; further reacting with (S)-1-azabicyclo [2, 2, 2] capryl-3-yl-amine under the alkaline condition; cyclizing in molecule to obtain 2-(1-azabicyclo [2, 2, 2] capryl-3S-yl)-2, 3-dihydro-1H-benzo [de] isoquinoline-1-ketone compounds; and further performing selectivecatalytic hydrogenation reaction to obtain the object product.
Owner:SICHUAN DIHON MEDICAL DEV +1
Palonosetron preparation for injection and preparation method thereof
InactiveCN103845295AFlat surfaceNot crackedPowder deliveryOrganic active ingredientsMedicineFreeze-drying
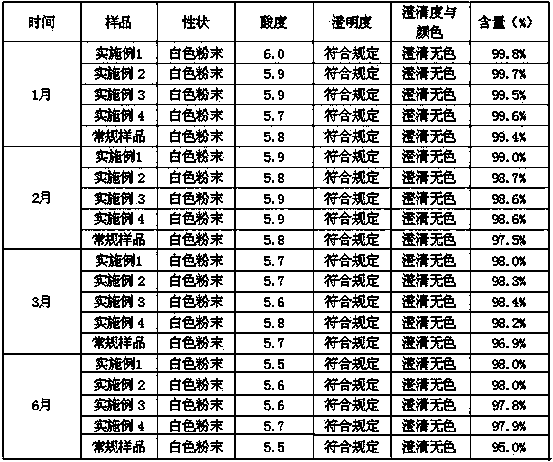
The invention belongs to the technical field of medicines, and particularly relates to palonosetron hydrochloride freeze-dried powder injection. The invention also relates to preparation of the freeze-dried powder injection. By adding beta-cyclodextrin or hydroxypropyl beta-cyclodextrin and mannitol, the palonosetron preparation has the characteristics of being smooth in surface, delicate, uniform, free of cracks, breakage and adhesion to a bottle, white in color, shaped like a loose piece, good in formation, also good in dissolubility, quite easy to dissolve, clear and transparent in solution and stable in product quality.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for separating and detection palonosetron hydrochloride and impurity
ActiveCN104764840AQuality is easy to controlSolving Separation Assay ProblemsComponent separationInorganic saltsOrganic solvent
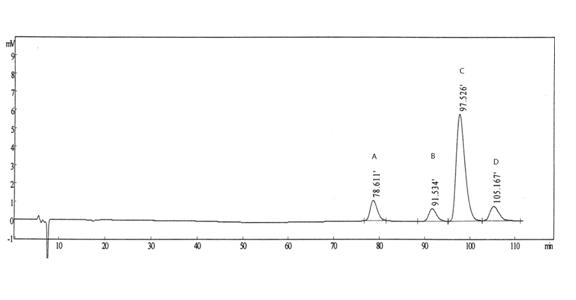
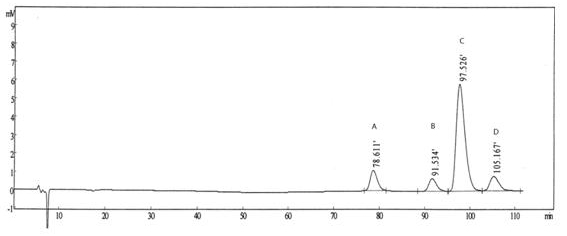
The invention belongs to the field of analytical chemistry, relates to a method for the simultaneous determination of palonosetron hydrochloride and four impurities. In particular, a high performance liquid chromatography method is employed for the simultaneous separation and determination of palonosetron hydrochloride and four process impurities A-D (including a degradation product). The column used in the method employs octadecyl silane bonded silica gel as a filler, and the method employs an inorganic salt buffer system supplemented with a certain proportion of an ion pair reagent and an organic solvent as the mobile phases for gradient elution. The method can simultaneously fully separate the process impurities and degradation products of palonosetron hydrochloride, has the advanategs of simpleness, feasibility, good reproducibility and strong specificity, and can effectively determinw palonosetron hydrochloride bulk drugs and medicine and the content of the relevant materials in the preparation.
Owner:CHONGQING HUAPONT PHARMA
Palonosetron hydrochloride orally disintegrating tablet and preparation method thereof
ActiveCN102048705ALow friabilitySimple preparation processOrganic active ingredientsDigestive systemManufacturing technologyOrally disintegrating tablet
The invention belongs to the field of pharmacy and medical technology, and relates to a palonosetron hydrochloride orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet comprises the following components in percentage by mass: palonosetron hydrochloride 0.16 to 1.5, mannite 70 to 95, disintegrating agent 1.5 to 15, taste correcting agent 1 to 3, lubricating agent 0.5 to 2, and other auxiliary materials 0 to 15. The manufacturing technology can adopt dry direct tablet compressing. The palonosetron hydrochloride orally disintegrating tablet has the advantages of simple manufacturing technology, low cost and quick effect on indications, is convenient to take, can be disintegrated into fine grains or powder in oral cavity quickly after oral administration, and is particularly suitable for patients who swallow hard and tumor patients with weak physical fitness. The preparation exists in a form of fine grain or powder before reaching the gastrointestinal tract, and the medicine dissolves out in an accelerating manner and is distributed in the gastrointestinal tract in a large area for more absorption points, so that the bioavailability can be improved.
Owner:QILU PHARMA HAINAN
Analysis method of chiral isomer
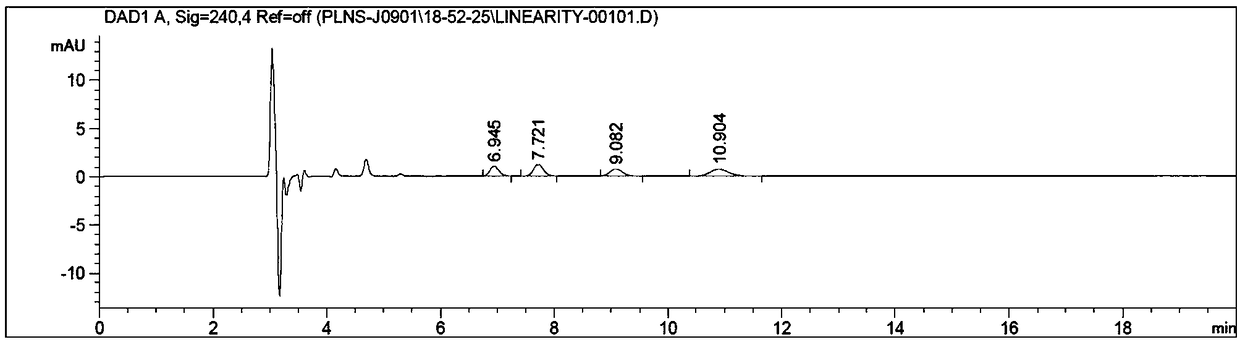
The invention discloses a method for simultaneous detection of contents of three isomers in palonosetron hydrochloride. The method includes following steps: (1) preparation of a mobile phase: mixing n-hexane with alcohol according to a volume ratio of (60-80):(40-20), adding a certain amount of trifluoroacetic acid and diethylamine, and obtaining the mobile phase; (2) preparation of a test solution: weighing the palonosetron hydrochloride, and preparing a solution with the concentration of 0.1-10 mg / ml through solvents; and (3) detection: detecting a sample by employing a high-efficiency liquid chromatography ultraviolet detector, wherein the ultraviolet detection wavelength is 230-250 nm, and a chromatographic column is a chiral column regarding amylose tris(3,5-dimethylphenylcarbamate) as a filling agent. According to the detection method, the operation is simple, the separation of major peaks and the isomers is good, the reservation is moderate, the sensitivity is high, it is verified that the accuracy and the precision of the analysis method are high, the specificity and the reproducibility of the method are good, and standardized operation is facilitated.
Owner:药源生物科技(启东)有限公司
Palonosetron hydrochloride lipidosome injection
InactiveCN103040746AImprove stabilityImprove qualityOrganic active ingredientsDigestive systemSide effectRetention time
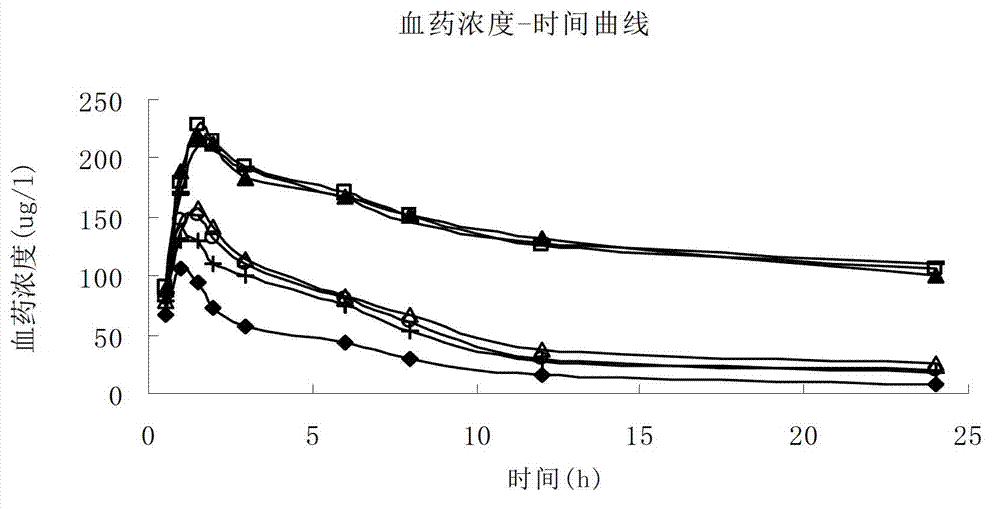
The invention discloses a palonosetron hydrochloride lipidosome injection and a preparation method thereof. The lipidosome injection is prepared by using palonosetron hydrochloride, soybean phosphatidylserine, phosphatidyl glycerol, soyasterol, Tween 80 and trehalose according to a specific weight ratio. The hydrochloride thiamphenicol glycinate lipidosome injection disclosed by the invention has the advantage of good preparation stability and a lipidosome cannot be cracked due to melting, ice crystals and the like in the refrigerating process; after the hydrochloride thiamphenicol glycinate lipidosome injection is stored for a long time, the lipidosome is also kept with good encapsulation efficiency; the quality of a preparation product is improved, the retention time of a drug in systemic circulation is prolonged, the biological availability of the drug is increased, toxic and side effects are reduced and a curative effect is obviously improved; and moreover, the palonosetron hydrochloride lipidosome injection disclosed by the invention has the advantage of simple preparation method and is suitable for large-scale industrial production.
Owner:海南路易丹尼生物科技有限公司
Preparation method of palonosetron and palonosetron hydrochloride and injection
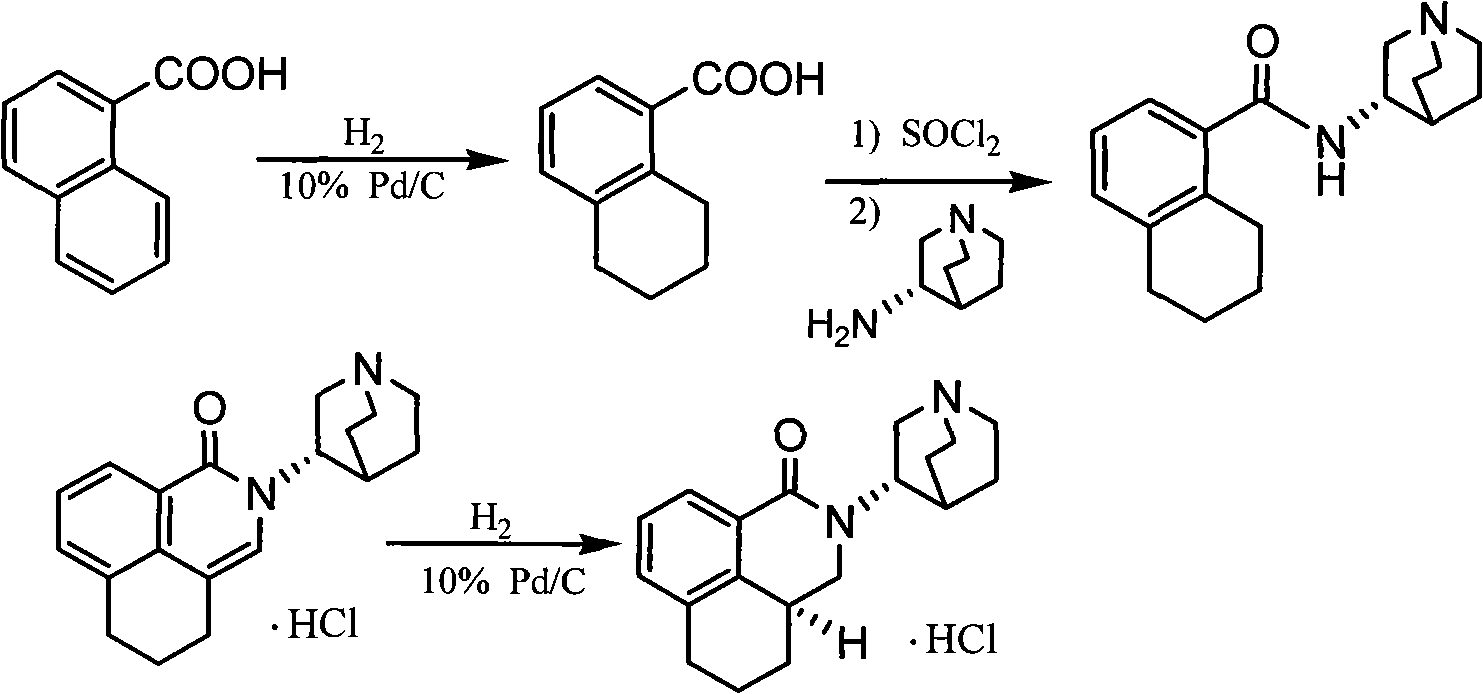
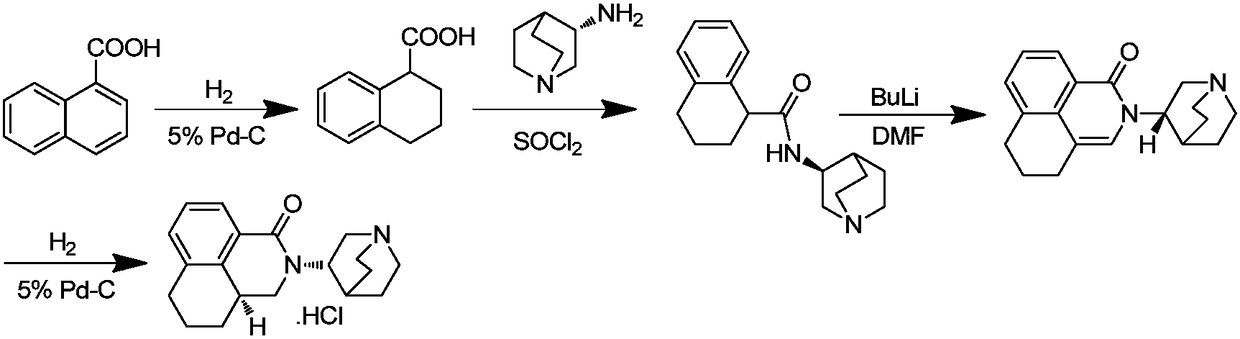
ActiveCN101633657AMild reaction conditionsSimple processOrganic active ingredientsOrganic chemistryTetralinBoron trifluoride
The invention relates to a preparation method of palonosetron and palonosetron hydrochloride and an injection, which belongs to the pharmaceutical chemical field. The invention provides the preparation method of the palonosetron, and the preparation method comprises the steps of reacting (1-aza-3(S)-bicyclo [2,2,2] octane base)-(1,2,3,4-tetralin-1S-methyl) amine with Bis(trichloromethyl)carbonate, and adding boron trifluoride to perform the reflux reaction to obtain the palonosetron. The preparation method has the advantages of simple process as well as high yield, good purity and low cost of the obtained products.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +2
Method for simultaneously determining four optical isomers of palonosetron hydrochloride
ActiveCN102207494BComponent separationOptically-active compound separationQuality controlColumn temperature
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Crystal forms of palonosetron hydrochloride and preparation method thereof
The invention relates to two crystal forms of palonosetron hydrochloride, i.e., A and B crystal forms. The X-ray diffraction for the powder of A and B crystal forms has characteristic diffraction peaks under the condition of Cu-Kalpha radiation when the Bragg angle 2 x theta is 0-50 degrees. The results of the differential thermal analysis of the A and B crystal forms show that the A crystal form has an endothermic peak at a temperature of about 320.678 DEG C, and the B crystal form has an endothermic peak at a temperature of about 320.68 DEG C. In addition, the invention also relates to a method for preparing the crystal forms and a drug composition containing the crystal forms.
Owner:HANGZHOU JIUYUAN GENE ENG +1
Compositions for treating centrally mediated nausea and vomiting
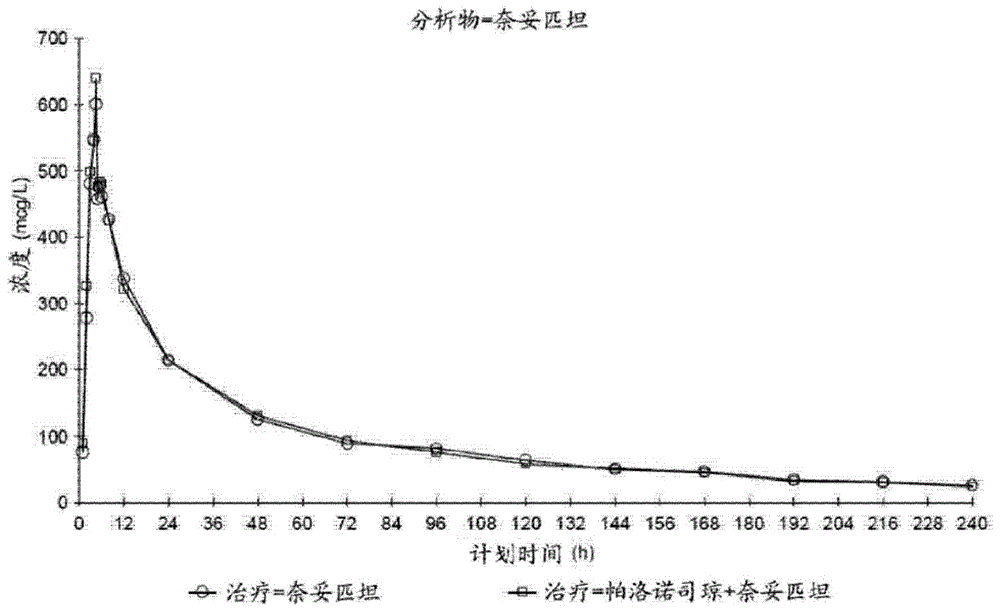
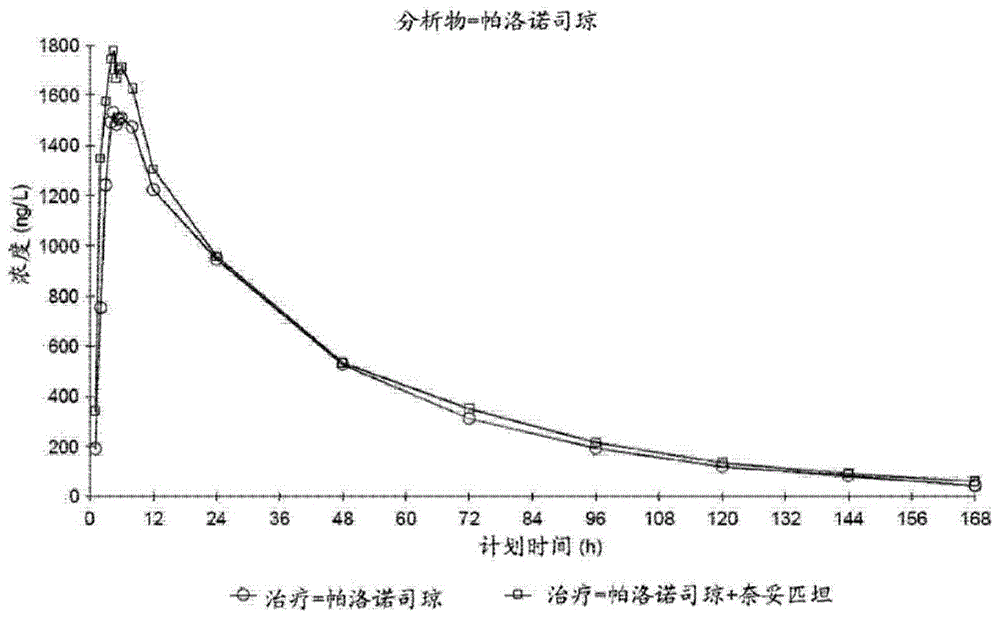
Provided are orally administrable dosage forms for use in treating chemotherapy induced nausea and vomiting (CINV), radiation therapy induced nausea and vomiting (RINV), or post operative nausea and vomiting (PONV), comprising combination of palonosetron and netupitant.
Owner:HELSINN HEALTHCARE SA
Resolution agent of palonosetron hydrochloride optical isomer and separation and detection method
ActiveCN107328874AReduce consumable costsEfficient separationComponent separationStationary phaseIsoamyl alcohol
The invention discloses a resolution agent of a palonosetron hydrochloride optical isomer and a separation and detection method. The palonosetron hydrochloride optical isomer is prepared from palonosetron hydrochloride as well as an RS isomer, an RR isomer and an SR isomer of the palonosetron hydrochloride; liquid chromatography is adopted, a stationary phase of the liquid chromatography is octadecyl silane bonded silica gel, a mobile phase is methyl alcohol-water, and isoamyl alcohol and oxalic acid which are in effective concentrations are added; the volume percentage concentration of isopropanol in the mobile phase is 0.4 to 0.8 percent, the mass volume concentration of the oxalic acid is 0.4 to 0.6 percent, and the isopropanol and the oxalic acid are added in methyl alcohol or water or a mixed solvent of the methyl alcohol and water. By adopting the separation and detection method provided by the invention, effective separation of the palonosetron hydrochloride optical isomer can be realized on a common C18 chromatographic column, the application cost is remarkably reduced, and the cost of a large of supplies of the chromatographic column can be saved.
Owner:康龙化成(南京)临床医学研究有限公司
Palonosetron hydrochloride intravenous injection medicinal composition and preparation method thereof
The invention discloses a drug compound of stable palonosetron hydrochloride for injection, characterized in that the compound contains diamine tetraacetic acid and or the ramification of the diamine tetraacetic acid as complexing agent of metal ion, pH regulator and osmotic pressure regulator.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Palonosetron lyophilized formulation and its preparation method
The invention relates to the freeze-dried preparation of Palonosetron or its medicinal salts, in particular Palonosetron hydrochloride freeze-dried preparation and its preparing process, which can improve the preparation stability.
Owner:CHONGQING PHARMA RES INST
Crystal system of chlorhydric acid palonosetron and preparation method thereof
The invention relates to two novel crystal forms of palonosetron hydrochloride that are A and B crystal forms. The A and B crystal forms have characteristic diffraction peaks when x-ray diffraction is used on the powder under Cu-K alpha radiation conditional (lamed=1. 54059) and the bragg 2theta angle between 0 and 50 degree C. The A crystal form has aendothermic peak near 320. 678 degree C, and the B crystal form has a endothermic peak near 320. 68 degree C after performed differential thermal analysis. Further the invention also relates to a process for preparing the crystal forms and pharmaceutical compositions containing the crystal forms.
Owner:HANGZHOU JIUYUAN GENE ENG +1
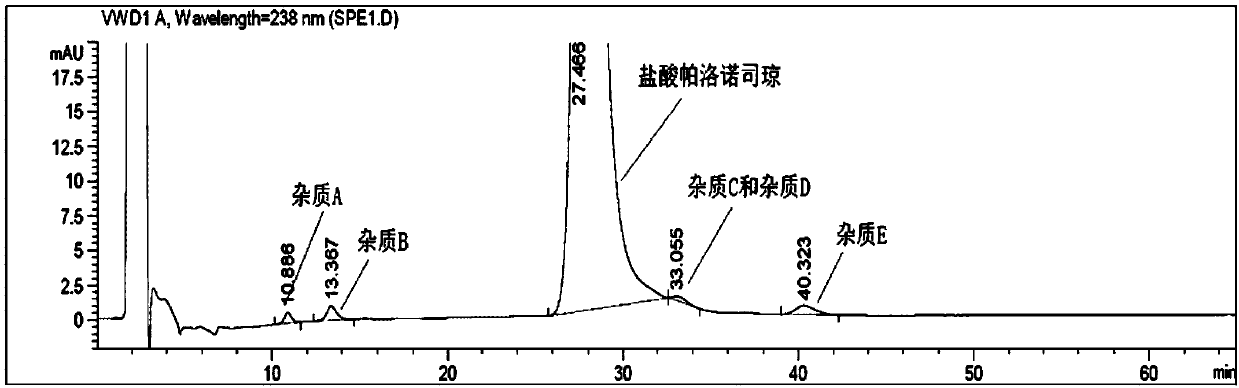
Method for separating relevant substances of hydrochloric acid palonosetron injection by virtue of reversion phase chromatography
ActiveCN107328880AThe method is simpleGood reproducibilityComponent separationEnantiomerColumn temperature
The invention relates to a high performance liquid chromatography for reverse-phase separation of a hydrochloric acid palonosetron injection and specific impurities of the injection. According to the chromatography, a macrocyclic glycopeptides chiral column is adopted, in which a volume ratio of a flow phase (a ratio of methanol to acetonitrile is 90 to 10) to 0.5% diethylamine water solution is (40 to 60)-(50 to 50), a detection wavelength is 238nm, a column temperature is 35-40 DEG C, and a flow speed is 0.8ml / min-1.2ml / min. By virtue of the chromatography, the specific impurities A, B, E, enantiomers and diastereoisomers collected in America pharmacopeia USP39 can be completely separated; and furthermore, the chromatography is simple, accurate and high in sensitivity, and can be used for detecting relevant substances of the hydrochloric acid palonosetron injection.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Recovering and reusing method of resolving agent quinine
InactiveCN107814708ASolve wasteReduce the amount of feedOrganic compound preparationCarboxylic compound preparationNitrogenRaw material
The invention relates to a recovering and reusing method of a resolving agent quinine for preparing a key intermediate S-1,2,3,4-tetrahydro-1-naphthoic acid of palonosetron hydrochloride. The recovering and reusing method comprises the following steps: taking a resolving and crystallizing mother solution of racemic 1,2,3,4-tetrahydronaphthoic acid as a raw material; acidifying and carrying out extracting separation; then alkalinizing and separating out an organic phase; finally, concentrating and recrystallizing to obtain quinine with qualified optical rotation, wherein the quinine can be usedfor resolving the 1,2,3,4-tetrahydronaphthoic acid again. According to the recovering and reusing method disclosed by the invention, by recovering and reusing the resolving agent quinine in reactingwaste solution generated by preparing an S-1,2,3,4-tetrahydro-1-naphthoic acid, the production cost is greatly reduced, discharge of a nitrogen-containing waste solution is reduced and environmental protection is benefited; in addition, the recovering and reusing method is simple in industrial operation and easy for industrialization realization.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Preparation method of high-purity palonosetron hydrochloride
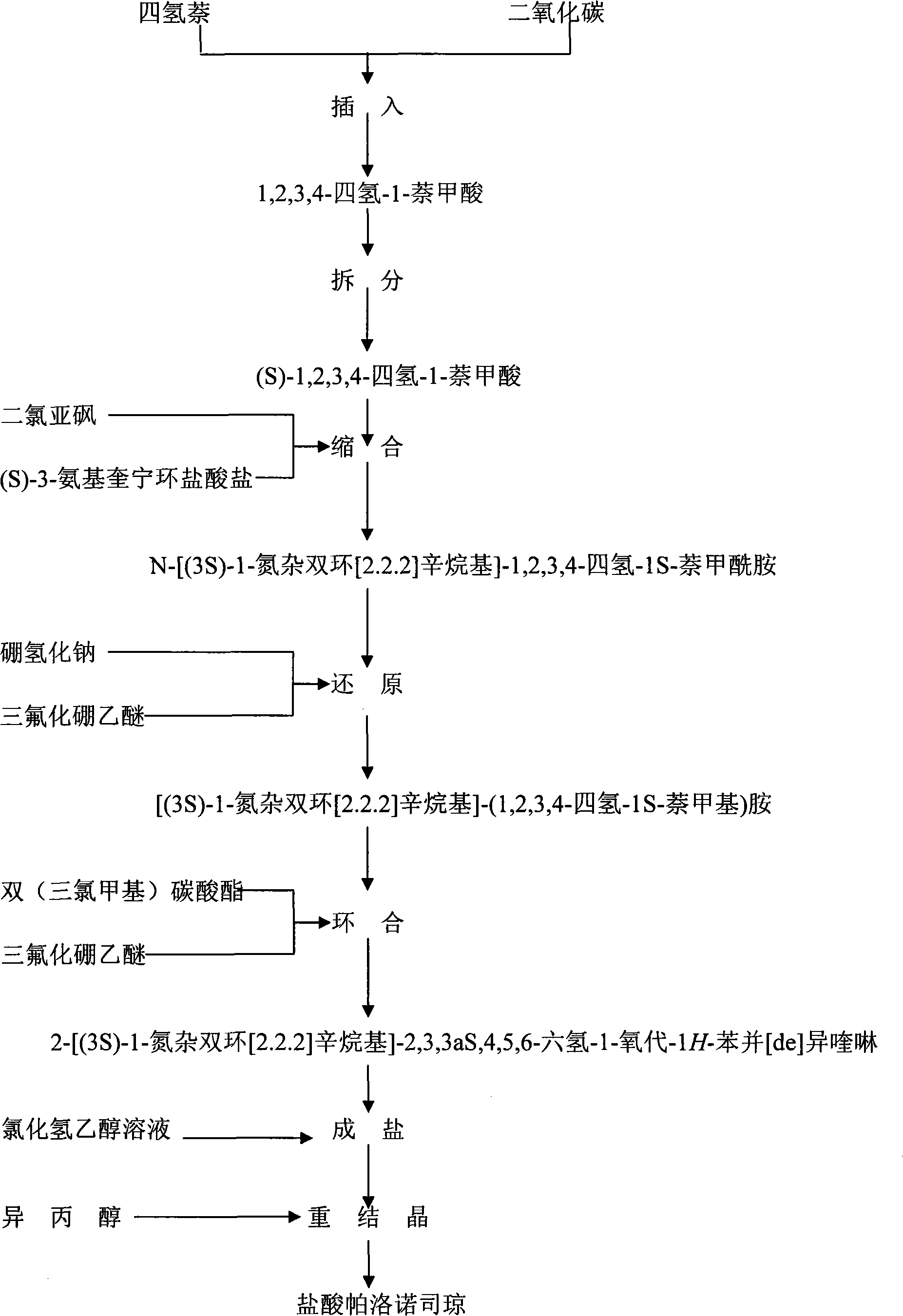
The invention discloses a preparation method of high-purity palonosetron hydrochloride. A synthesis method is as follows: taking 1,2,3,4-tetrahedro-naphthoic acid as a starting raw material, taking quinine as a resolving agent to obtain chiral(S)-1,2,3,4-tetrahedro-naphthoic acid, acylating the chiral(S)-1,2,3,4-tetrahedro-naphthoic acid by using oxalyl chloride, and then reacting with (S)-3-aminoquinuclidine ammonia salt to obtain amide compound; concentrating, adding water to dilute, adding alkali to adjust pH value to obtain (R)-N-((S)-3-quinine)-1,2,3,4-tetralyl-1-formamide; performing reduction reaction on an intermediate under the existence of the sodium borohydride and boron trifluoride diethyl etherate so as to obtain (R)-N-(1-((S)-1,2,3,4-tetralyl)methyl)-3-quinine amine, continuously reacting with the triphosgene and boron trifluoride diethyl etherate, salting, extracting and regulating the pH value to obtain a crude product of the Palonosetron after the reaction is completed; and salting and refining the crude product in isopropanol so as to obtain the Palonosetron Hydrochloride with high purity. The preparation method not only solves the problem that the palonosetron hydrochloride is low in yield and unstable in process for a long time, but also adopts class-three solvent ethyl acetate to extract in the synthesis of each step, the invention is in favor of the environmental protection, and convenient for solvent recycling and cost saving, and is suitable for industrial production.
Owner:KUNMING YUANRUI PHARMA
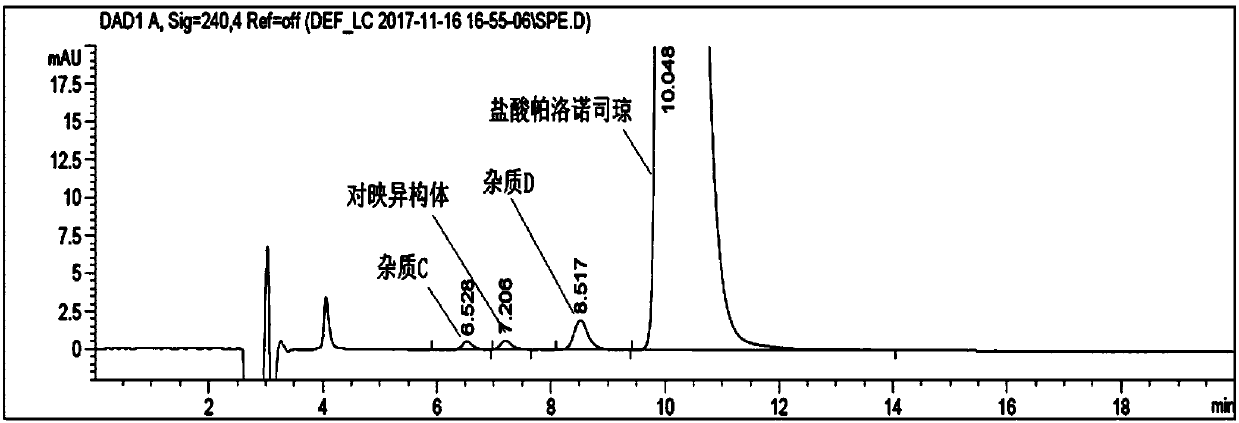
Analysis method of impurity C, impurity D and enantiomer in palonosetron hydrochloride injection or bulk drug
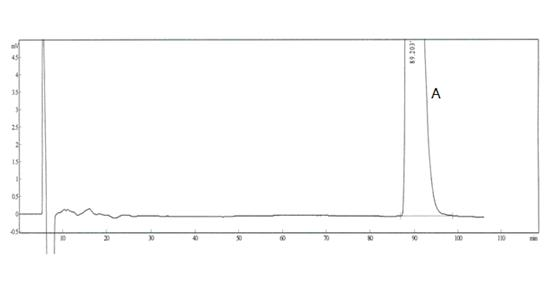
The invention discloses an analysis method of an impurity C, an impurity D and an enantiomer in the palonosetron hydrochloride injection or a bulk drug. According to the invention, the chromatographicconditions are optimized, a normal phase chromatographic column is adopted, and the normal phase HPLC systems are used to complete the separation of the impurity C, the impurity D and the enantiomer.By carrying out alkali treatment on a to-be-detected sample, the palonosetron hydrochloride is dissociated and is extracted by using normal hexane, the upper-layer n-hexane is taken as a sample solution for test, and the n-hexane-methanol-diethylamine (70: 30: 0.1) is used as a flowing phase, a polysaccharide coating chiral column is adopted for separation, and the detection wavelength is 240 nm,and finally the impurity C, the impurity D and the enantiomer are completely separated. According to the present invention, the separation degree is greater than 1.5, the analysis time is only 20 min, and the time and reagent cost are reduced.
Owner:WUXI KAIFU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com